Abstract
Objective:
Alveolar echinococcosis is caused by Echinococcus multilocularis, a parasite of zoonotic significance with a wide range of intermediate and final hosts, and the parasite survives successfully in diversified conditions. Plentiful studies have been done to study the genetic structure of the population of the parasite and the level of intimate kinship using mitochondrial (mt) DNA. The present study was conducted to investigate the population structure, genetic variation, and phylogenetic relationship of various isolates of E. multiocularis submitted to GenBank worldwide. Sequences of mt genes (mt-cytochrome c oxidase (cox1), mt-NADH dehydrogenase (nad1)) of E. multilocularis were analyzed to achieve the set goals.
Materials and Methods:
A total of 275 and 124 gene sequences of mt-cox1 and mt-nad1 belonging to E. multilocularis, respectively, were retrieved from the National Center for Biotechnology Information GenBank. The retrieved sequences were subjected to alignment with respective reference sequences using MEGA software. The PopArt software was used to establish median-joining networks, while DnaSp was used to calculate neutrality and diversity indices. MrBayes software was used to investigate the phylogenetic association between haplotypes based on Bayesian phylogeny.
Results:
Approximately 13 and 20 distinctive haplotypes of nad1 and cox1 genes, respectively, were observed in the present study. In both of the mt genes, diversity indices indicated low haplotype (mt-cox1 = 0.140; mt-nad1 = 0.374) and nucleotide (mt-cox1 = 0.00111; mt-nad1 = 0.00287) diversities. The values of Tajima’s D and Fu Fs for a population of both of the genes under study were found to be negative.
Conclusion:
This study is a maiden attempt to provide insights into the population structure and genetic variation of E. multilocularis on a global scale. However, it is suggested that to better understand the population structure and genetic diversity of E. multilocularis, more geographical locations and amplifications of full-length gene sequences should be considered, which could be helpful in widening the insights into the genetic diversity of E. multilocularis.
Keywords: Echinococcus multilocularis, cox1, nad1, genetic variability
Introduction
Parasitic infections causing infectious diseases are gaining significant importance nowadays in both the veterinary and public health sectors, resulting in both economic losses and serious illness [1–4]. Alveolar echinococcosis (AE), which is thought to be among the most dangerous zoonotic diseases in nontropical areas, is caused by a form of parasitic tapeworm called Echinococcus multilocularis [5], which occurs predominantly in the northern half of the globe and can cause hyperplasia, fibrosis, putrefaction of liver tissues, and hepatic fibrosis [6]. Human AE disease is brought about by the accidental ingestion of food sullied with eggs, which form microcystic metacestode vesicles in the liver [7].
Chiefly, E. multilocularis maintains a sylvatic life cycle in which small mammals, i.e., arvicoline rodents, serve as intermediate hosts and wild canids, i.e., foxes (Vulpes vulpes) and coyotes (Canis latrans), as final hosts. However, a synanthropic cycle aided by domestic carnivores, i.e., dogs, could be expected, which can be quite important in some regions for the spread of the parasite [8]. Echinococcus multilocularis adult forms could be found in the small intestine of the final hosts, ranging in size from 1.2 to 4.5 mm [9]. The proglottids are secreted in the environment through the feces of specific hosts, which contain embryonated eggs. The eggs are distinguished by a thick and extremely tough keratinized embryophore layer, which enables them to endure in the environment for a long time while being vulnerable to desiccation and higher temperatures [10]. The eggs are ingested by intermediate hosts. Upon ingestion, the oncosphere (larvae) is released from the eggs. The larvae pierce the intestine to enter the bloodstream and reach the liver (the primary target). In the liver, the metacestodal stage of the parasite grows and multiplies asexually, resulting in the production of protoscolices. These protoscolices get themselves attached to the intestinal wall of the intermediate hosts, where they convert into adult worms. Finally, the intermediate host is consumed by the final host, starting a new life cycle [11].
The geographic distribution and prevalence of the parasite in wild hosts are expanding worldwide because of anthropogenic activities [12]. Echinococcus multilocularis has been reported to cycle throughout many cities in Europe [13] and Japan over the past ten years, following the colonization of urban areas by red fox populations [14]. In addition, the recent growth of coyote populations in North America and the high prevalence of the parasite in coyote populations in urban areas [15] may pose a high risk of exposure to humans, as has been seen, for instance, in the extensively populated prairies of the Tibetan plateau and China, where the rise in the population of definitive hosts led to a high prevalence [16].
Frequent examination of mitochondrial (mt) DNA sequences was done to determine the population structure and degree of close kinship due to their high mutation rates and maternal inheritance [17]. Because of their vast variety of hosts and ecological diversification, molecular studies can play a critical role in determining the distribution of E. multilocularis. The diversity indices indicate that forecasting antigenic variation and phylogenetic association could be better understood by employing the main population in defined habitats. Moreover, numerous investigations have been done to identify the genetic variations in various strains of E. multiloclaris through the use of partial sequences of mt-cytochrome c oxidase (cox1) and mt- NADH dehydrogenase (nad1) mt genes. Therefore, to better understand the population dynamics of E. multilocularis, it is vital to investigate the genetic diversity of E. multilocularis isolates on a global scale. Furthermore, there is very little data available regarding the epidemiology and population structure of E. multilocularis. To address the identified research gap, the existing study aimed to examine the population structure, genomic variation, and phylogenetic association of E. multilocularis using mt gene sequences of mt-cox1 and mt-nad1 submitted to the National Center for Biotechnology Information database from various geographical locations around the world.
Materials and Methods
Collection of data
A dataset of 399 gene sequences was created after filtering the obtained mt-cox1 (n = 275) and mt-nad1 (n = 124) gene sequences of E. multilocularis submitted to the NCBI database by February 10, 2023.
Alignment and phylogenetic analysis
The MEGA software version 11 was used to import the FASTA format of all the gene sequences [18]. First, all the sequences were subjected to cutting from both ends by using reference sequences of both the genes mt-cox1 (accession no. MZ026358) and mt-nad1 (accession no. AB018440). A total of 399 gene sequences, i.e., 497 bp mt-cox1 = 275 and 285 bp mt-nad1 = 124, were subjected to bioinformatic analyses after the elimination of short gene sequences upon filtration. The phylogenetic association between different haplotypes of cox1 and nad1 was inferred through the application of the Bayesian approach and MrBayes v.3.1.1 software [19]. The parameters were lodged in every 1,000 states using a length of 5,000,000 states. 25% of the data was deleted as a “burn-in.” The posterior distribution of the parameters was evaluated using the MCMC sampling method.
Analyses of haplotypes and networking
Haplotype analyses were done through the examination of sequences in FASTA format using the DnaSP tool [20]. Neutrality indices, numbers of haplotypes and nucleotides, and nucleotide and haplotype change values were used to determine the genetic makeup of both genes. After the conversion of sequences to Nexus format [22], a haplotype network was created using PopArt [21].
Results
A total of 399 gene sequences from E. multilocularis were examined during the present study. The gene isolates of E. multilocularis were obtained from the NCBI database. A total of 275 gene sequences of mt-cox1 gene and 124 of mt-nad1 gene were obtained from 13 countries (Table 1).
Table 1. Accession number of mt-cox1 and mt-nad1 gene fragments of E. multilocularis isolates used in the study.
| mt-cox1 | mt-nad1 | ||||
|---|---|---|---|---|---|
| Origin | No. of isolates | Accession numbers | Origin | No. of isolates | Accession numbers |
| Japan | 1 | AB385610 | Japan | 2 | AB018440, NC000928 |
| Russia | 6 | AB688128-29/32, AB777915/17/19 | Iran | 32 | AB617846-47-48/50-51-52-53-54-55, AB621793-94-95-96-97-98-99-00-01, KX186699-KX186700-01-02/04-05, AB720065-66-67-68-69, KT318129-30, KT033489 |
| Poland | 30 | KY205679-80-81-82-83/85/87-88-89-90-91, MW255900-01-02/04/06/07-08/10-11/13-14-15/92-93-94-95-96-97, MN444798 | Poland | 12 | AJ132907-08-09-10, MH986749-50-51, JX266825-26, MN444804-05, AJ237639 |
| mt-CO1 | mt-ND1 | ||||
|---|---|---|---|---|---|
| Origin | No. of isolates | Accession numbers | Origin | No. of isolates | Accession numbers |
| Mongolia | 1 | KC893696 | Sweden | 4 | KX384668-69-70-71 |
| China | 193 | AB461417, AB477010-11-12, AB491457-58-59-60-61, JF906152-53, MN251846-47/49, MH259764-65-66-67-68-69-70/72/74, KY446474-75-76-77-78-79-80-81-82-83-84-85-86-87-88-89-90-91-92-93-94-95-96-97-98-99, KY446501-02-03-04-05-06-07, KY354083-84-85-86-87-88-89-90-91-92-93-94-95, KY328672-73-74-75-76-77-78-79-80-81-82-83-84-85-86-87-88-89-90-91-92-93/94/96-97, KY062624-25-26-27-28-29-30-31-32-33-34, MK598850, KX685923-24-25-26, MZ026301-02-03-04-05-06-07-08-09-10-11-12-13-14-15-16-17-18-19-20-21-22-23-24-25-26-27-28-29-30-31-32-33-34-35-36-37-38-39-40-41-42-43-44-45-46-47-48-49-50-51-52-53-54-55-56-57-58-59-60-61-62-63-64, KT965438-39-40-41-42, MH211144-45-46-47-48/50-51-52-53/55-56-57-58-59 | China | 12 | KY094609, EU704122-23-24, KU723572, AY389984, MH259775-76-77-78, MN448476/78, |
| France | 1 | AB461413 | Turkey | 6 | MK248696, MK248702-03-04-05-06 |
| Slovakia | 19 | AB461414, OP225830, OP225946-47-48-49-50-51-52-53-54, OP225398, OP225402/45/48/55, OP225644, MN444797-98 | Slovakia | 13 | MW326786-87, MN444801-02-03, MW343787-88-89, MW357715, MW366778-79, MW384819-20 |
| USA | 4 | OK330092, AB461418, LC380931, NC000928 | Germany | 2 | AJ237640, AB668376 |
| Austria | 1 | AB461412 | Austria | 2 | MN251882, MN444806 |
| Hungary | 1 | MN444795 | Hungary | 2 | MN444799, MN444800 |
| Switzerland | 2 | MT461410-11 | Switzerland | 1 | KR870967 |
| Kyrgyzstan | 4 | MN829532/36-37-38 | Estonia | 1 | AY855918 |
| Slovenia | 1 | MW560731 | |||
| Kazakhstan | 1 | OM640356 | |||
Analyses of polymorphism and haplotypes
Distinct mutations were found in both genes. Approximately 24 distinct mutations were present in mt-cox1 gene, and 14 distinct mutations were present in mt-nad1 gene. After analyses of 275 gene sequences of mt-cox1 gene, 20 distinct haplotypes were identified (Table 2). Out of 275 gene sequences, 175 were associated with a single haplotype, the Hap01, which acts as the dominant haplotype. Whereas, upon analysis of 124 mt-nad1 gene sequences, 13 distinct haplotypes were identified (Table 3). Out of 97 gene sequences, 32 were present as a single haplotype, with Hap01 acting as the dominant haplotype among these.
Table 2. Haplotypes of mt-cox1 sequences of E. multilocularis and accession numbers of isolates forming groups.
| Name of haplotype | No. of Isolates | Accession number | Name of haplotype | No. of Isolates | Accession number |
|---|---|---|---|---|---|
| Hap01 | 255 | OK330092-USA, AB461418-USA, AB461417-China, KY205687-Poland, KY205688-Poland, KY205689-Poland, KY205690-Poland, KY205691-Poland, KY205679-Poland, KY205680-Poland, KY205681-Poland, KY205682-Poland, KY205683-Poland, KY205685-Poland, AB461414-Slovakia, AB688128-Russia, AB688129-Russia, AB688132-Russia, AB777915-Russia, AB777917-Russia, AB777919-Russia, AB461412-Austria, AB461413-France, AB477010-China, | Hap02 | 1 | KC550004-Canada |
| AB477011-China, AB477012-China, MN251846-China, MN251847-China, MN251849-China, MW255892-Poland, MW255893-Poland, MW255894-Poland, MH259764-China, MN829532-Kyrgyzstan, MN829536-Kyrgyzstan, MN829537-Kyrgyzstan, MN829538-Kyrgystan, MW255900-Poland, MW255901-Poland, MW255902-Poland, MW255904-Poland, MW255906-Poland, MW255907-Poland, MW255908-Poland, MW255910-Poland, MW255911-Poland, MW255913-Poland, MW255914-Poland, MW255915-Poland, | Hap03 | 1 | MH259772-China | ||
| MK843308-Canada, MK843309-Canada, MT461409-Canada, MT461410-Switzerland, MT461411-Switzerland, MW255895-Poland, MW255896-Poland, MW255897-Poland, KC550007-Canada, MH259765-China, MH259766-China, MH259767-China, MH259768-China, MH259769-China, MH259770-China, MH259774-China, AB385610-Japan, LC380931-USA, NC000928-USA, KY446479-China, KY446490-China, | Hap04 | 1 | KY446478-China | ||
| KY446501-China, KY446502-China, KY354084-China, KY446475-China, KY446476-China, KY446482-China, KY446487-China, KY446491-China, KY354083-China, KY354085-China, KY446474-China, KY446477-China, KY446483-China, KY446484-China, KY446485-China, KY446492-China, KY446493-China, KY446494-China, KY446495-China, KY446496-China, KY446497-China, KY446499-China, KY446504-China, | Hap05 | 1 | KY446480-China | ||
| KY446505-China, KY446506-China, KY354087-China, KY328674-China, KY328694-China, KC550001-Canada, KY354093-China, KY328673-China, KY328675-China, KY328678-China, KY328679-China, KY328685-China, KY328689-China, KY328672-China, KY328683-China, KY328686-China, KY328691-China, KY328692-China, KY328693-China, KY328680-China, KY328684-China, KY354088-China, KY328690-China, | Hap06 | 1 | KY446481-China | ||
| KY354094-China, KY062624-China, KY354092-China, KY062633-China, KY062634-China, KY062628-China, KY062632-China, MK598850-China, KY062625-China, KY062631-China, KY354095-China, KY354090-China, KY328676-China, KY328687-China, KY328688-China, KX685923-China, KX685924-China, KX685925-China, KX685926-China, AB491457-China, AB491458-China, AB491459-China, AB491460-China, | Hap07 | 1 | KY446503-China | ||
| AB491461-China, JF906152-China, JF906153-China, KC582621-Canada, KC582623-Canada, KC582624-Canada, KC582625-Canada, KC582627-Canada, OP225830-Solvakia, OP225948-Solvakia, OP225949-Solvakia, OP225950-Solvakia, OP225951-Solvakia, OP225952-Solvakia, OP225953-Solvakia, OP225954-Solvakia, OP225398-Solvakia, OP225402-Solvakia, OP225448-Solvakia, OP225555-Solvakia, OP225945-Solvakia, OP225946-Solvakia, OP225947-Solvakia, MN444795-Hungary, | Hap08 | 1 | KY354086-China | ||
| MN444796-Solvakia, MN444797-Solvakia, MN444798-Poland, KY062627-China, KY354091-China, KY328681-China, KC893696-Mongolia, KY062629-China, KY354096-China, KY354097-China, KY062626-China, MZ026309-China, MZ026319-China, MZ026320-China, MZ026321-China, MZ026322-China, MZ026323-China, MZ026324-China, MZ026325-China, MZ026326-China, MZ026327-China, MZ026328-China, MZ026329-China, MZ026330-China, | Hap09 | 1 | KY446486-China | ||
| MZ026331-China, MZ026332-China, MZ026333-China, MZ026334-China, MZ026335-China, MZ026336-China, MZ026337-China, MZ026338-China, MZ026339-China, MZ026340-China, MZ026341-China, MZ026342-China, MZ026343-China, MZ026344-China, MZ026345-China, MZ026346-China, MZ026347-China, MZ026348-China, MZ026349-China, MZ026350-China, MZ026351-China, | Hap10 | 1 | KY446488-China | ||
| MZ026352-China, MZ026353-China, MZ026363-China, OP225644-Slovakia, MZ026301-China, MZ026302-China, MZ026303-China, MZ026304-China, MZ026305-China, MZ026306-China, MZ026307-China, MZ026308-China, MZ026310-China, MZ026311-China, MZ026312-China, MZ026313-China, MZ026314-China, MZ026315-China, MZ026316-China, MZ026317-China, MZ026318-China, MZ026354-China, MZ026355-China, MZ026356-China, | Hap11 | 1 | KY446489-China | ||
| MZ026357-China, MZ026358-China, MZ026359-China, MZ026360-China, MZ026361-China, MZ026362-China, MZ026364-China, KY328677-China, KY328682-China, KY354089-China, KY062630-China, KT965438-China, KT965440-China, KT965439-China, KT965441-China, KT965442-China, MH211144-China, MH211145-China, MH211147-China, MH211148-China, MH211150-China, MH211151-China, MH211152-China | Hap12 | 1 | KY446498-China | ||
| Hap13 | 1 | KY446507-China | |||
| Hap14 | 1 | KC582626-Canada | |||
| Hap15 | 2 | MH211146-China,MH211158-China | |||
| Hap16 | 1 | MH211153-China | |||
| Hap17 | 1 | MH211155-China | |||
| Hap18 | 1 | MH211156-China | |||
| Hap19 | 1 | MH211157-China | |||
| Hap20 | 1 | MH211159-China |
Table 3. Haplotypes of mt-nad1 sequences of E. multilocularis and accession numbers of isolates forming groups.
| Name haplotype | No. of isolates | Accession numbers |
|---|---|---|
| Hap01 | 97 | AB018440-Japan, NC000928-Japan, AB617846-Iran, AB617847-Iran, AB617848-Iran, AB617850-Iran, AB617851-Iran, AB617852-Iran, AB617853-Iran, AB617854-Iran, AB617855-Iran, AB621793-Iran, AB621794-Iran, AB621795-Iran, AB621796-Iran, KX186700-Iran, AB621797-Iran, AB621798-Iran, AB621799-Iran, KX186701-Iran, KX186702-Iran, AB621800-Iran, AB720065-Iran, AB62180-Iran, AB720067-Iran, AB720068-Iran, AB720066-Iran, KX186705-Iran, AB720069-Iran, KT318130-Iran, KX186704-Iran, KX186699-Iran, KT033489-Iran, KT318129-Iran, AJ132907-Poland, AJ132908-Poland, AJ132909-Poland, AJ132910-Poland, MH986751-Poland, JX266825-Poland, MN444805-Poland, JX266826-Poland, MH986750-Poland, MH986749-Poland, HAJ237639-Poland, MN444804-Poland, JF751034-Canada, KC848475-Canada, KC848476-Canada, KC848477-Canada, KF962559-Canada, KF962566-Canada, KF962567-Canada, KF962568-Canada, OK095088-Canada, KX384668-Sweden, KX384669-Sweden, KX384670-Sweden, KX384671-Sweden, KY094609-China, EU704123-China, KU723572-China, MH259775-China, AY389984-China, EU704122-China, EU704124-China, MH259776-China, MH259777-China, MH259778-China, MK248696-Turkey, MK248702-Turkey, MK248703-Turkey, MK248704-Turkey, MK248705-Turkey, MK248706-Turkey, MW326786-Slovakia, MW326787-Slovakia, MN444803-Slovakia, MW343787-Slovakia, MW343788-Slovakia, MN444802-Slovakia, MN444801-Slovakia, MW343789-Slovakia, MW357715-Slovakia, MW366778-Slovakia, MW366779-Slovakia, MW384819-Slovakia, MW384820-Slovakia, AB668376-Germany, MN251882-Austria, MN444806-Austria, MN444799-Hungary, MN444800-Hungary, KR870967-Switzerland, AY855918-Estonia, MW560731-Slovenia, OM640356-Kazakhstan |
| Hap02 | 16 | KC848462-Canada, KC848464-Canada, KC848465-Canada, KC848466-Canada, KC848467-Canada, KC848468-Canada, KC848469-Canada, KC848470-Canada, KC848471-Canada, KC848472-Canada, KC848473-Canada, KF962555-Canada, KF962562-Canada, KF962563-Canada, KF962564-Canada, KF962565-Canada |
| Hap03 | 1 | KF962556-Canada |
| Hap04 | 1 | KF962557-Canada |
| Hap05 | 1 | KF962558-Canada |
| Hap06 | 1 | KF962560-Canada |
| Hap07 | 1 | KF962561-Canada |
| Hap08 | 1 | KF962569-Canada |
| Hap09 | 1 | KF962570-Canada |
| Hap10 | 1 | KF962571-Canada |
| Hap11 | 1 | MN448475-China |
| Hap12 | 1 | MN448476-China |
| Hap13 | 1 | AJ237640-Germany |
Haplotype network
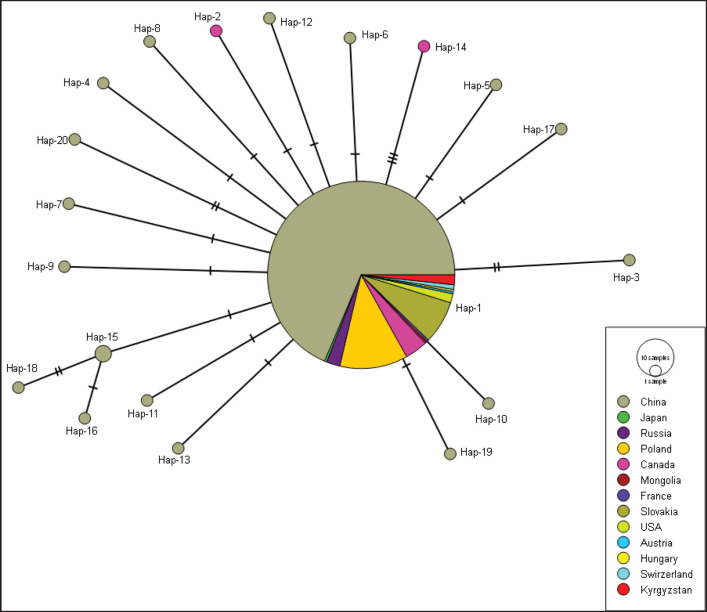
In mt-cox1 gene network, 20 different haplotypes were present (Table 2). As per the analyses, the difference between the primary haplotype and the other haplotypes was 1–3 mutations. Hap01 was identified as one of the most prevalent haplotypes, which accounted for 92.72% (255/275) of the total. Hap15 came in second with 0.72% (2/275). 90% (18/20) of the network’s haplotypes were all distinct single haplotypes. Canada (n = 2) and China (n = 16) each contributed a single haplotype (Fig. 1).
Figure 1. Appearance of mt-cox1 (497 bp) haplotypes E. multilocularis sequences.
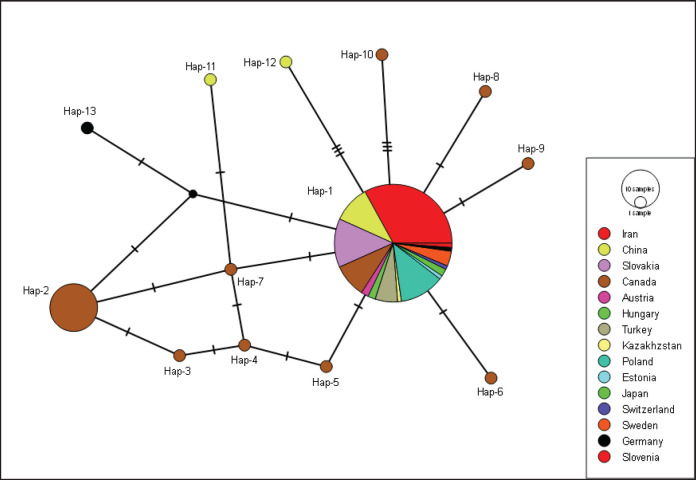
In mt-nad1 gene network, there were 13 haplotypes (Table 3). The difference between the primary haplotypes and the other haplotypes was 1–4 in this network. The most prevalent haplotype, Hap01, made up 78.22% (97/124) of the haplotype network, whereas Hap02 made up 12.90% (16/124). 84.61% (11/13) of the haplotype network was made up of a single distinct haplotype. Germany (n = 1), China (n = 2), and Canada (n = 8) each had a single haplotype (Fig. 2).
Figure 2. Appearance of mt-nad1 (285 bp) haplotypes E. multilocularis sequences.
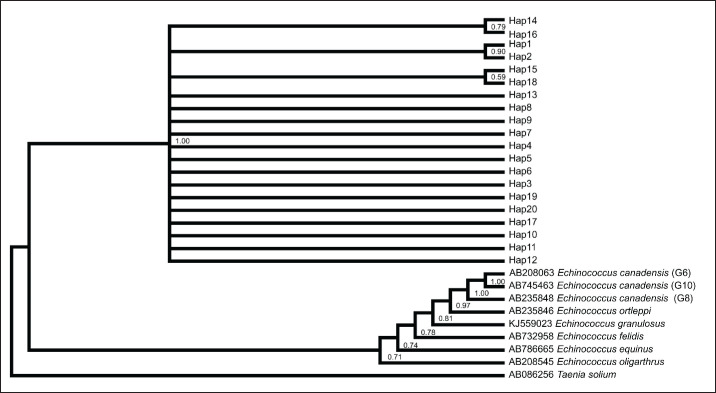
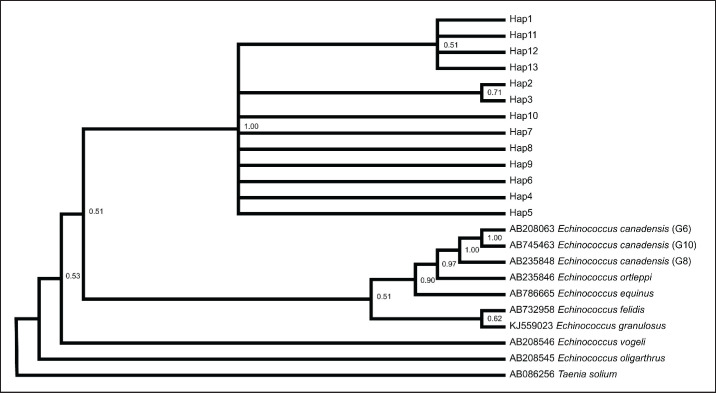
The findings of the haplotype network and phylogenetic analyses were consistent with each other. In the construction of both phylogenetic trees, Taenia solium was added as an outgroup. After the construction of the tree, sister relationships have been found between Elodea. canadensis and Echinococcus ortleppi while Echinococcus oligarthra occupies the basal side of the tree. The tree was generated after the alignment of the gene sequences of mt-cox1 and mt-nad1 gene sequences, as shown in Figures 3 and 4, respectively. The haplotypes, i.e., Hap03, Hap16, and Hap18 of mt-cox1 gene were found farther apart with mutations at three different points, while, in the case of mt-nad1 gene, only Hap02 was found farther apart, having mutations at four points.
Figure 3. Phylogenetic tree view of E. multilocularis sequences using mt-cox1 (497 bp).
Figure 4. Phylogenetic tree view of E. multilocularis sequences using mt-nad1 (285 bp).
Analyses of neutrality, diversity, and gene flow
Table 4 depicts the values of neutrality and diversity indices for both genes, i.e., mt-cox1 and mt-nad1. Tajima’s D and Fu’s FS were computed to determine the selection pressure on the population of the parasite. The presence of a higher number of alleles was depicted through negative values in the mt-cox1 and mt-nad1 areas obtained from Tajima D and Fu’s FS.
Table 4. Diversity and neutrality indices were obtained using nucleotide data of the mt-cox1 (497 bp) and mt-nad1 (285 bp) genes of E. multilocularis.
| Indices | nad1 (285 bp) | cox1 (497 bp) |
|---|---|---|
| No. of sequences | 124 | 275 |
| No. of mutations | 14 | 24 |
| Parsimony informative sites | 3 | 1 |
| No. of haplotypes | 13 | 20 |
| Haplotype diversity (Hd) | 0.374 ± 0.052 | 0.140 ± 0.029 |
| Nucleotide diversity (π) | 0.00287 ± 0.00045 | 0.00111 ± 0.00026 |
| Tajima’s D | −1.89245 | −2.53266 |
| Fu’s Fs | −9.568 | −41.675 |
| FLD | −4.94806 | −9.15087 |
| FLF | −4.56645 | −7.75910 |
Discussion
Animals throughout the world are vulnerable to parasite-borne acute, chronic, and severe illnesses [23–25]. Parasitic diseases are responsible for huge economic losses in terms of loss of production and medicinal costs [26,27]. In modern-day science, where parasites are believed to have evolved according to their respective habitat and ecosystem, it is becoming important to have knowledge about the genetic diversity of the parasites for a better understanding of the transmission dynamics of parasites so that effective monitoring and control measures may be devised [28,29].
Population structure and genetic diversity of E. multiloclaris were examined during the study using two specialized genes, i.e., mt-cox1 and mt-nad1, which are frequently used to distinguish among the species of Echinococcus parasite. The nucleotide sequences of these genes were downloaded from GenBank. The present study revealed the global epidemicity of infections caused by Echinococcus, along with the genetic diversity, population structure, and gene flow of the parasite. For this purpose, a total of 275 mt-cox1 genes (497 bp) and 124 mt-nad1 (285 bp) gene sequences that have already been registered in the NCBI database were used.
The major factor behind the haplotype variation between different researchers is the length of the gene sequence selected for the study of the parasite. The longer the length of the genes selected for genetic studies, the greater the number of haplotypes identified upon mt gene sequencing. Out of a total of 399 nucleotide sequences, 20 haplotypes of mt-cox1 gene and 13 mt-nad1 genes were examined. A similar study has been conducted by Kinkar et al. [30] that revealed high genetic diversity among E. granulosus. The scientist has identified 171 haplotypes of E. granulosus among 212 samples (haplotype diversity = 0.994), which shows almost the entire mt sequence [30].
Neutrality indices were computed to evaluate population growth and nucleotide variability [31]. The Tajima’s D model measures divergence in populations from the conventional neutral model. Where a positive value indicates heterozygosity (having selected advantage), while the negative Tajima’s D value indicates that one allele has a selective advantage over the other allele and significant growth in population [32]. In the present study, the values of Tajima’s D were found to be very low for both of the genes, i.e., mt-cox1 and mt-nad1, which indicate a maximum likelihood for future population expansion. The value of Tajima’s D for mt-nad1 gene was lower (−1.89245) than that of mt-cox1 (−2.53266), which depicts the rapid increase in the population of the former gene. The negative values of neutrality indices indicate migration across different countries, which, according to Tajima’s D, indicates more population expansion in the coming years.
Another marker for population growth sensitivity is Fu’s FS. Through this method, we can identify whether the gene pool of populations of different parasite species is the same and reveal identical tendencies in growth or not [33,34]. The values of Fu’s FS for both mt-cox1 and mt-nad1 genes were found to be extremely low, which shows that worldwide expansion in the population of parasites could be expected.
Nucleotide diversity was used to assess the polymorphism in the population. The mean nucleotide difference of mt-nad1 gene (0.00287) was found to be greater than that of mt-cox1 gene (0.00111). Haplotype diversity was also calculated to identify the uniqueness of the haplotypes within the populations. In the present investigation, marginal differences were identified in the gene sequences of mt-nad1 gene (0.374) and mt-cox1 gene (0.140). A total of 20 haplotypes were discovered in the mt-cox1 gene. Out of these, 18 haplotypes were identified as distinct haplotypes, accounting for 92.72% of the network. The examination of mt-nad1 gene sequences revealed 13 distinct haplotypes having 78.82% as the dominant primary haplotype of the network. Furthermore, major haplotypes represented a single ancestor. Mutation rates of both genes, i.e., mt-cox1 and mt-nad1 were analyzed, which revealed 24 mutations in mt-cox1 (497 bp) and 14 mutations in mt-nad1 gene (285 bp). The higher mutation rates in both genes indicate the extensive and complicated evolutionary history of E. multilocularis. Extensive diversity in the genetic makeup of E. multilocularis has been reported worldwide. Furthermore, the complex phylogenetic associations identified through geographic and phylogenetic analyses highlighted the significant role of animal trade in the present distribution of E. granulosus [30].
Due to a lack of investigations into the population structure of E. multilocularis, an extensive comparative study was conducted by comparing the results of this study with those reported by other scientists worldwide. However, it is a fact that the length of the selected gene sequences has influenced the genetic variation of the parasite, e.g., E. granulosus [35,36]. It is further supported by a study that selected 223 European isolates and 89 Italian isolates from the same gene, mt-cox1. The higher number of isolates revealed 24 haplotypes, while the lower number of isolates revealed seven haplotypes [37]. Similarly, seven haplotypes were discovered in 69 Argentinian isolates of cox1 gene [38]. Another study, which was conducted in the Sindh province of Pakistan, revealed five haplotypes out of 112 isolates of cox1 gene sequence [39]. Furthermore, findings from another Pakistani study that included the full-length cox1 and nad1 genes were much more illustrative than those studying fragmentary sequences [40]. The entire sequence may provide more effective evidence to verify the validity of E. granulosus genotypes. Based on these observations, it is recommended that future studies about E. multilocularis employ full-length gene amplification rather than using a partial segment of the genes.
Acknowledgment
The authors are thankful to the laboratory staff of our institutes who helped with data collection.
Conclusion
Despite the fact that multiple molecular investigations have been carried out, the present study is the maiden attempt to investigate the population structure of E. multilocularis. Low diversity between the selected genes of E. multilocularis, i.e., mt-cox1 and mt-nad1 was found during the present study. For the whole population, the values of Tajima’s D and Fu’s FS were found to be negative, though statistically insignificant. The findings of the study will add to the existing bank of knowledge about the genetic diversity of E. multilocularis and also provide baseline data for future epidemiological insights into the population structure of E. multilocularis.
List of Abbreviations
AE, Alveolar echinococcosis; cox1, cytochrome c oxidase I; nad1, NADH dehydrogenase; NCBI, National Center for Biotechnology Information; Hap, Haplotype; MEGA, Molecular Evolutionary Genetics Analysis Software; MCMC, Markov Chain Monte Carlo; mt, Mitochondrial; NCBI, National Center for Biotechnology Information
Conflict of interest
The authors have declared that they have no competing interests.
Authors’ contributions
Conceptualization—MAA, MIS, MHW, and MS; Data analysis—MAM, AA, AAMA, MIS, and WQ; Manuscript writing (original draft)—MAA, AA, and MIS; Review and editing—SH, AAMA MS, and MHW.
References
- [1].Mahmood Q, Younus M, Sadiq S, Iqbal S, Idrees A, Khan S, et al. Prevalence and associated risk factors of cystic echinococcosis in food animals—a neglected and prevailing zoonosis. Pak Vet J. 2022;42:59–64. [Google Scholar]
- [2].Rafique A, Nasir S, Ashraf A, Nawaz Z, Zahid FM, Abbas A, et al. Sero-surveillance and risk factors analysis of caprine toxoplasmosis in Faisalabad Punjab, Pakistan. Pak Vet J. 2022;42:102–6. [Google Scholar]
- [3].Pinilla JC, Gutierrez A, Florez AA. Canine visceral leishmaniasis in Colombia resistant to treatment of choice (meglumine antimoniate plus allopurinol) Int J Vet Sci. 2022;11:117–20. https://doi.org/10.47278/journal.ijvs/2021.103. [Google Scholar]
- [4].Nawaz M, Zhou J, Khalid I, Shamim A, Hussain A, Ahmed Z, et al. Antiparasitic activity of plants extract against gastrointestinal nematodes and Rhipicephalus microplus. Int J Vet Sci. 2022;11:474–8. https://doi.org/10.47278/journal.ijvs/2022.147. [Google Scholar]
- [5].Baumann S, Shi R, Liu W, Bao H, Schmidberger J, Kratzer W, et al. Interdisciplinary echinococcosis working group ulm. Worldwide literature on epidemiology of human alveolar echinococcosis: a systematic review of research published in the twenty-first century. Infection. 2019;47:703–727. doi: 10.1007/s15010-019-01325-2. https://doi.org/10.1007/s15010-019-01325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Torgerson PR, Keller K, Magnotta M, Ragland N. The global burden of alveolar echinococcosis. PLoS Negl Trop Dis. 2010;4:e722. doi: 10.1371/journal.pntd.0000722. https://doi.org/10.1371/journal.pntd.0000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vuitton DA, McManus DP, Rogan MT, Romig T, Gottstein B, Naidich A, et al. International consensus on terminology to be used in the field of echinococcoses. Parasite. 2020;27:41. doi: 10.1051/parasite/2020024. https://doi.org/10.1051/parasite/2020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Weng X, Mu Z, Wei X, Wang X, Zuo Q, Ma S, et al. The effects of dog management on Echinococcus spp. prevalence in villages on the eastern Tibetan Plateau, China. Parasit Vectors. 2020;13:207. doi: 10.1186/s13071-020-04082-6. https://doi.org/10.1186/s13071-020-04082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lymbery AJ. Phylogenetic pattern, evolutionary processes and species delimitation in the genus Echinococcus. Adv Parasitol. 2017;95:111–145. doi: 10.1016/bs.apar.2016.07.002. https://doi.org/10.1016/bs.apar.2016.07.002. [DOI] [PubMed] [Google Scholar]
- [10].Thompson RCA, Deplazes P, Lymbery AJ. Echinococcus and Echinococcosis, Part A. In: Thompson RCA, Deplazes P, Lymbery AJ, editors. Advances in Parasitology. Vol. 95. Cambridge, MA: Academic Press; 2017. p. 525. [Google Scholar]
- [11].Eckert J, Gemmell MA, Meslin FO-X, Pawlowski ZS, Organization WH. WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. In: Eckert J, Gemmell MA, Meslin FO-X, Pawlowski ZS, editors. Paris, France: Organization WH World Organisation for Animal Health; 2001. [Google Scholar]
- [12].Davidson RK, Romig T, Jenkins E, Tryland M, Robertson LJ. The impact of globalisation on the distribution of Echinococcus multilocularis. Trends Parasitol. 2012;28:239–47. doi: 10.1016/j.pt.2012.03.004. https://doi.org/10.1016/j.pt.2012.03.004. [DOI] [PubMed] [Google Scholar]
- [13].Deplazes P, Hegglin D, Gloor S, Romig T. Wilderness in the city: the urbanization of Echinococcus multilocularis. Trends Parasitol. 2004;20:77–84. doi: 10.1016/j.pt.2003.11.011. https://doi.org/10.1016/j.pt.2003.11.011. [DOI] [PubMed] [Google Scholar]
- [14].Yimam AE, Nonaka N, Oku Y, Kamiya M. Prevalence and intensity of Echinococcus multilocularis in red foxes (Vulpes vulpes schrencki) and raccoon dogs (Nyctereutes procyonoides albus) in Otaru City, Hokkaido, Japan. Jpn J Vet Res. 2002;49:287–96. [PubMed] [Google Scholar]
- [15].Luong LT, Chambers JL, Moizis A, Stock TM, Clair CS. Helminth parasites and zoonotic risk associated with urban coyotes (Canis latrans) in Alberta, Canada. J Helminthol. 2020;94:e25. doi: 10.1017/S0022149X1800113X. https://doi.org/10.1017/S0022149X1800113X. [DOI] [PubMed] [Google Scholar]
- [16].Tiaoying L, Jiamin Q, Wen Y, Craig PS, Xingwang C, Ning X, et al. Echinococcosis in Tibetan populations, western Sichuan province, China. Emerg Infect Dis. 2005;11:1866. doi: 10.3201/eid1112.050079. https://doi.org/10.3201/eid1112.050079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jia W, Yan H, Lou Z, Ni X, Dyachenko V, Li H, et al. Mitochondrial genes and genomes support a cryptic species of tapeworm within Taenia taeniaeformis. Acta Trop. 2012;123:154–63. doi: 10.1016/j.actatropica.2012.04.006. https://doi.org/10.1016/j.actatropica.2012.04.006. [DOI] [PubMed] [Google Scholar]
- [18].Knudsen B, Knudsen T, Flensborg M, Sandmann H, Heltzen M, Andersen A, et al. CLC main workbench: version 5.5. Aarhus: CLC Bio. 2007 [Google Scholar]
- [19].Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–5. doi: 10.1093/bioinformatics/17.8.754. https://doi.org/10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- [20].Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol. 2017;34:3299–302. doi: 10.1093/molbev/msx248. https://doi.org/10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- [21].Leigh JW, Bryant D. POPART: full-feature software for haplotype network construction. Methods Ecol Evol. 2015;6:1110–16. https://doi.org/10.1111/2041-210X.12410. [Google Scholar]
- [22].Maddison DR, Swofford DL, Maddison WP. NEXUS: an extensible file format for systematic information. Syst Biol. 1997;46:590–621. doi: 10.1093/sysbio/46.4.590. https://doi.org/10.1093/sysbio/46.4.590. [DOI] [PubMed] [Google Scholar]
- [23].Alvi MA, Li L, Bahadur SUK, Saqib M, Ohiolei JA, Ali RMA, et al. First comparative biochemical profile analysis of cystic fluids of Taenia hydatigena and Echinococcus granulosus obtained from slaughtered sheep and goats. Pak Vet J. 2022;42:215–21. [Google Scholar]
- [24].Qamar W, Zaman MA, Faheem M, Ahmed I, Ali K, Qamar MF, et al. Molecular confirmation and genetic characterization of Haemonchus contortus isolates at the nuclear ribosomal ITS2 region: First update from Jhang region of Pakistan. Pak Vet J. 2022;42:251–5. [Google Scholar]
- [25].Alberfkani MI, Albarwary AJS, Jaafar GM, Zubair AI, Abdullah RY. Molecular characterization and phylogenetic analysis of cox1 and ITS 1 gene fragments of Moniezia species isolated from sheep. Pak Vet J. 2022;42:566–70. https://doi.org/10.29261/pakvetj/2022.073. [Google Scholar]
- [26].Mo’awad HFM, Sobhy MM, Ismail TF, Enbaawy M. Seroprevalence of Coxiella burnetti (Q fever) in cows and buffaloes in Egypt. Int J Vet Sci. 2022;11:16–22. https://doi.org/10.47278/journal.ijvs/2021.068. [Google Scholar]
- [27].Mahmoud HYAH, Ali AAA, Khalil AM, Amin YA, Ali AO. The infection rate of Fasciola and Anaplasma in cattle and buffaloes in Qena, Egypt. Int J Vet Sci. 2022;11:308–14. https://doi.org/10.47278/journal.ijvs/2021.110. [Google Scholar]
- [28].Spotin A, Karamat M, Mahami-Oskouei M, Shahbazi A, Ahmadpour E, Galeh TM, et al. Genetic variability and transcontinental sharing of Giardia duodenalis infrapopulations determined by glutamate dehydrogenase gene. Acta Trop. 2018;177:146–56. doi: 10.1016/j.actatropica.2017.10.001. https://doi.org/10.1016/j.actatropica.2017.10.001. [DOI] [PubMed] [Google Scholar]
- [29].Spotin A, Mahami-Oskouei M, Harandi MF, Baratchian M, Bordbar A, Ahmadpour E, et al. Genetic variability of Echinococcus granulosus complex in various geographical populations of Iran inferred by mitochondrial DNA sequences. Acta Trop. 2017;165:10–6. doi: 10.1016/j.actatropica.2016.03.002. https://doi.org/10.1016/j.actatropica.2016.03.002. [DOI] [PubMed] [Google Scholar]
- [30].Kinkar L, Laurimäe T, Acosta-Jamett G, Andresiuk V, Balkaya I, Casulli A, et al. Global phylogeography and genetic diversity of the zoonotic tapeworm Echinococcus granulosus sensu stricto genotype G1. Int J Parasitol. 2018;48:729–42. doi: 10.1016/j.ijpara.2018.03.006. https://doi.org/10.1016/j.ijpara.2018.03.006. [DOI] [PubMed] [Google Scholar]
- [31].Ramos-Onsins SE, Rozas J. Statistical properties of new neutrality tests against population growth. Mol Biol Evol. 2002;19:2092–100. doi: 10.1093/oxfordjournals.molbev.a004034. https://doi.org/10.1093/oxfordjournals.molbev.a004034. [DOI] [PubMed] [Google Scholar]
- [32].Vamathevan JJ, Hasan S, Emes RD, Amrine-Madsen H, Rajagopalan D, Topp SD, et al. The role of positive selection in determining the molecular cause of species differences in disease. BMC Evol Biol. 2008;8:273. doi: 10.1186/1471-2148-8-273. https://doi.org/10.1186/1471-2148-8-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fu YX. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics. 1997;147:915–25. doi: 10.1093/genetics/147.2.915. https://doi.org/10.1093/genetics/147.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li YL, Kong XY, Yu ZN, Kong J, Ma S, Chen LM. Genetic diversity and historical demography of Chinese shrimp Feneropenaeus chinensis in Yellow Sea and Bohai Sea based on mitochondrial DNA analysis. Afr J Biotechnol. 2009;8(7):1193–1202. [Google Scholar]
- [35].Romig T, Ebi D, Wassermann M. Taxonomy and molecular epidemiology of Echinococcus granulosus sensu lato. Vet Parasitol. 2015;213:76–84. doi: 10.1016/j.vetpar.2015.07.035. https://doi.org/10.1016/j.vetpar.2015.07.035. [DOI] [PubMed] [Google Scholar]
- [36].Yanagida T, Mohammadzadeh T, Kamhawi S, Nakao M, Sadjjadi SM, Hijjawi N, et al. Genetic polymorphisms of Echinococcus granulosus sensu stricto in the Middle East. Parasitol Int. 2012;61:599–603. doi: 10.1016/j.parint.2012.05.014. https://doi.org/10.1016/j.parint.2012.05.014. [DOI] [PubMed] [Google Scholar]
- [37].Casulli A, Interisano M, Sreter T, Chitimia L, Kirkova Z, La Rosa G, et al. Genetic variability of Echinococcus granulosus sensu stricto in Europe inferred by mitochondrial DNA sequences. Infect Genet Evol. 2012;12:377–83. doi: 10.1016/j.meegid.2011.12.014. https://doi.org/10.1016/j.meegid.2011.12.014. [DOI] [PubMed] [Google Scholar]
- [38].Andresiuk MV, Gordo FP, Saarma M, Elissondo MC, Taraborelli A, Casa longue C, et al. Echinococcus granulosus genotype G1 dominated in cattle and sheep during 2003–2006 in Buenos Aires province, an endemic area for cystic echinococcosis in Argentina. Acta Trop. 2013;127:136–42. doi: 10.1016/j.actatropica.2013.04.008. https://doi.org/10.1016/j.actatropica.2013.04.008. [DOI] [PubMed] [Google Scholar]
- [39].Ehsan M, Akhter N, Bhutto B, Arijo A, Ali Gadahi J. Prevalence and genotypic characterization of bovine Echinococcus granulosus isolates by using cytochrome oxidase 1 (Co1) gene in Hyderabad, Pakistan. Vet Parasitol. 2017;239:80–5. doi: 10.1016/j.vetpar.2017.04.006. https://doi.org/10.1016/j.vetpar.2017.04.006. [DOI] [PubMed] [Google Scholar]
- [40].Alvi MA, Ohiolei JA, Saqib M, Li L, Tayyab MH, Alvi AA, et al. Echinococcus granulosus (Sensu stricto) (G1, G3) and E. ortleppi (G5) in Pakistan: phylogeny, genetic diversity and population structural analysis based on mitochondrial DNA. Parasit Vectors. 2020;13:347. doi: 10.1186/s13071-020-04199-8. https://doi.org/10.1186/s13071-020-04199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]