Abstract
Objective:
Research has demonstrated that Leptospermum scoparium possesses various therapeutic benefits. This study set out to determine whether or not L. scoparium extracts had any effect on the ability of HepG2 and MCF-7 breast cancer cells to survive.
Materials and Methods:
The antiproliferative activity of L. scoparium extracts was explored using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and lactate dehydrogenase assays. The most active fraction was selected to investigate its effects on apoptosis induction using flow cytometry and quantitative real-time polymerase chain reaction. The constituents of this fraction were characterized using GC-MS analysis.
Results:
Research demonstrated that the chloroform fraction of L. scoparium (LSCF) significantly impacted the HepG2 and MCF-7 cancer cell lines. Treatment with LSCF led to a notable rise in both early and late apoptotic cells. Furthermore, there was an upregulation in the mRNA levels of P53, Bax, and caspases, while the expression of Bcl-2 mRNA saw a decrease. The analysis of LSCF revealed the primary components to be cis-calamenene, beta-eudesmol, cyclododecane, and alpha-muurolene.
Conclusion:
The study showed the promising antiproliferative activity of L. scoparium, suggesting its potential application for cancer treatment.
Keywords: Antiproliferative, apoptosis, Leptospermum scoparium, MTT, LDH, MCF-7, GC-MS
Introduction
Cancer is a major concern in public health since it is the main killer on a global scale. The initiation of cancer is marked by changes to the genetic material in normal cells, which interfere with the cellular signaling pathways that control cell growth and programmed cell death [1,2]. Conventional cancer treatments often have adverse side effects, making alternative therapies necessary [3]. Natural products, such as chemotherapeutic agents derived from plants, are gaining popularity due to their relative safety.
Leptospermum scoparium, commonly referred to as manuka or tea tree, is a tree of varying heights originating from New Zealand and is part of the Myrtaceae family (Fig. 1). This family is found across various regions, such as Australia and Southeast Asia, encompassing around 133 genera and over 3,800 species. Manuka has a long history of traditional use, including the boiling of its leaves for tea and using manuka oil as an antiseptic [4]. The essential oil produced by L. scoparium has antimicrobial and antioxidant properties [5]. However, manuka honey (MH), made from the nectar of its flowers, is the most prized product of the species, as it has numerous therapeutic properties, including wound healing and treatment of fungal/bacterial infections, skin ulcers, and gastrointestinal diseases [6].
Figure 1. Manuka tree, L. scoparium (Accessed on July 2019).
Numerous laboratory and animal studies indicate that MH can suppress the growth of different cancer cell types [7]. This anti-cancer activity is attributed to its ability to promote apoptosis and cause DNA fragmentation [8]. Its therapeutic properties are due to the components of plant origin, such as flavonoids and polyphenols. Although MH is widely used for medical purposes [9], its most significant application is its anticancer activity [10,11]. The use of manuka leaf extract in treating breast and liver cancer cell lines shows promise [12], but more research is needed to establish its safety and efficacy.
Published research on the cytotoxic effects of L. scoparium species is scarce. Consequently, this research seeks to explore the cytotoxic and apoptotic effects of extracts from the manuka plant on MCF-7 and HepG2 cancer cell lines, as well as to determine the specific phytochemicals that may contribute to these effects.
Materials and Methods
Collecting plants and processing their extracts
Leptospermum scoparium samples were collected between June and July 2019 from the South Island of New Zealand. The identification of the L. scoparium specimen was confirmed by a taxonomist from King Saud University’s. Next, the plant matter was finely powdered. It was then extracted using the Soxhlet device from 50 gm of this powdered substance. Hexane, chloroform, ethyl acetate, and methanol were among the solvents used for this task. The extract was concentrated by running it through a rotary evaporator set to 45°C.
Cell viability test using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
Cell survival following the methodology previously detailed [13], the antiproliferative properties of L. scoparium extracts were assessed using the MTT reduction test. Afterward, a microplate reader calibrated to 540 nm was used to quantify the optical density.
Release assay of lactate dehydrogenase (LDH)
LDH kits are being used to assess the release of LDH from cells. The levels of LDH in the treated samples were compared to those in the control samples, following the procedure outlined in the kit’s instructions.
Apoptosis detection
After being treated for 24 h with a kit from BioLegend, USA, HepG2, and MCF-7 cell lines were subjected to an apoptotic staining experiment utilizing FITC Annexin V and PI. The flow cytometry system was used for the analysis, and the results were interpreted by CXP software.
Quantitative real-time PCR (qRT-PCR)
Following exposure to IC50 and 2x IC50 of L. scoparium chloroform fraction (LSCF), RNA was extracted from both control and treated cells using Trizol TM reagent [14]. Afterward, cDNA was produced using a specialized kit (Invitrogen™, USA) called the SuperScript™ VILO™ Synthesis Kit. After that, we followed the protocol from the previous work [15] and ran qRT-PCR using a reaction mixture that included cDNA, SYBR Green, and particular primers. ΔCT = (CT gene of interest – CT internal control) was the formula used to determine the mRNA expression level, with glyceraldehyde-3-phosphate dehydrogenase serving as the internal reference.
Mass spectrometry analysis (GC/MS)
GC/MS analysis of the L. scoparium chloroform extract. Spectra were compared between the extracts and libraries maintained by the NIST and WILEY Spectral to determine the components.
Data analysis
The information is shown as the average ± standard deviation, based on a minimum of three measurements. The comparison of datasets was conducted using the t-test.
Results
MTT proliferation assay
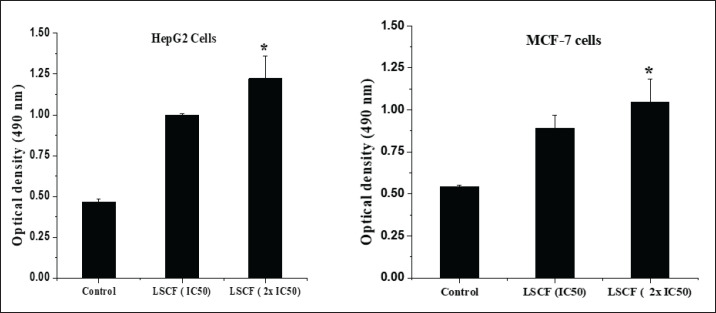
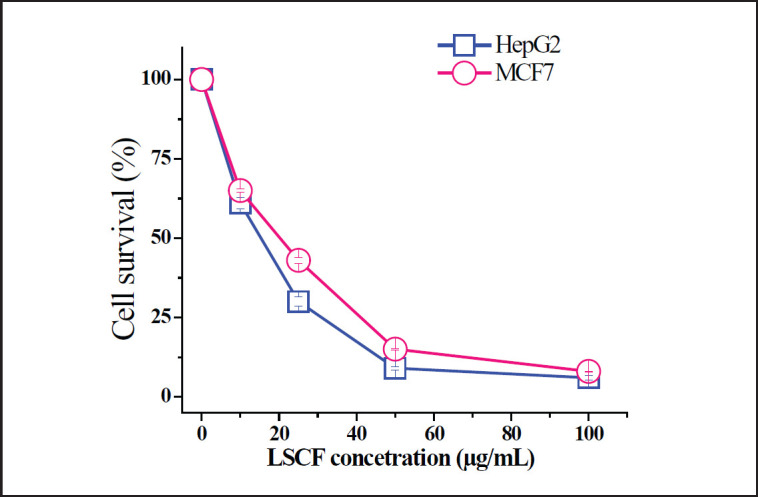
The cytotoxic effect of various solvent fractions of L. scoparium was assayed on HepG2 and MCF-7 cells. The various concentrations were checked using an MTT assay and all fractions showed variable effects (Table 1). In comparison to all tested fractions, LSCF was the most active fraction with more adverse effects on HepG2 cells (IC50 = 15 µg/ml) followed by MCF-7 (IC50 = 20 µg/ml). Since LSCF exerted a remarkable anti-proliferation effect against both cells (Fig. 2), it was selected for further assays.
Table 1. IC50 values of L. scoparium different fractions against HepG2 and MCF-7 cancer cells.
| Fraction | IC50 (µg/ml) | |
|---|---|---|
| HepG2 | MCF-7 | |
| Hexane | 59.5 ± 1.5 | 49.1 ± 1.1 |
| Chloroform | 15 ± 0.3 | 20 ± 0.5 |
| Ethyl acetate | 188.8 ± 3.6 | 191.6 ± 2.2 |
| Methanol | 173 ± 3.3 | 162.5 ± 2.5 |
| Doxorubicin | 1.5 ± 0.2 | 1.4 ± 0.1 |
Figure 2. Effect of LSCF on HepG2 and MCF-7 cells using MTT assay. Cells were treated with different concentrations of extract for 48 h and the absorbance of the MTT formazan was determined at 540 nm in an ELISA reader. Data represent means ± SD.
LDH assay
LDH assay was employed to confirm the cytotoxic effect of LSCF. The results disclosed that LSCF treatments caused a higher release of LDH than non-treated cells. Figure 3 shows that the LCSF’s cytotoxicity was concentration-dependent and that both cell lines were significantly LDH-released at the IC50 concentration of the extract (p < 0.05).
Figure 3. Cytotoxicity of LCSF on HepG2 and MCF-7 cells evaluated by LDH assay. LDH activity was determined at 490 nm in an ELISA reader. Values represent means ± SD (*p < 0.05) was considered significant compared to control) of three independent experiments carried out in triplicates.
LCSF induces apoptosis in HepG2 and MCF-7 cells
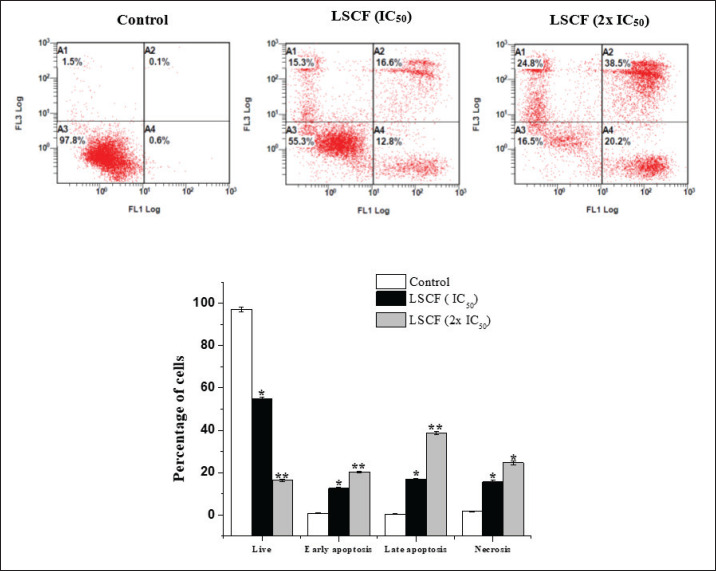
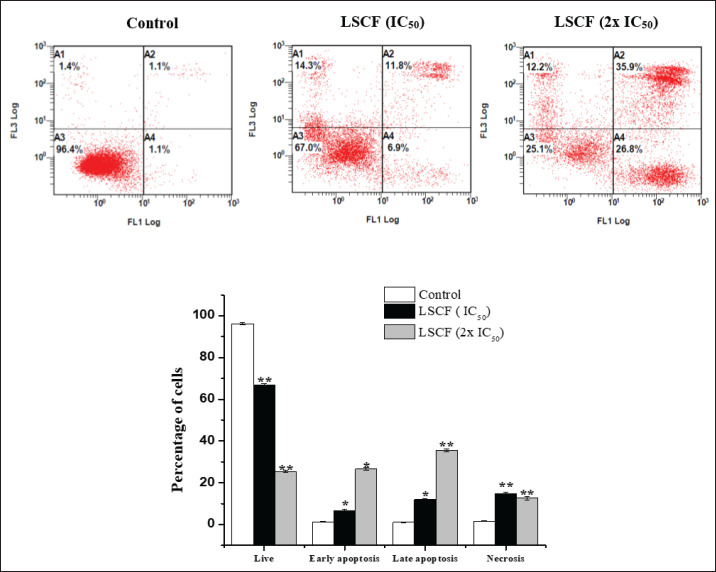
The association between the decrease in cell viability and the induction of apoptosis was examined in HepG2 and MCF-7 cells using FITC-Annexin V and PI labeling, respectively. According to our findings, LCSF caused cell death with only a small percentage of dead cells (Figs. 4 and 5). As shown in (Fig. 4), HepG2 cells treated with LCSF at15 and 30 µg/ml (IC50 and 2x IC50) displayed a dose-dependent increase of early (12.8% ± 0.4% and 20.2% ± 0.5%) and late apoptosis (16.6% ± 0.6% and 38.5% ± 0.9%) within 24 h of treatment, relative to the control. The same results were shown in MCF-7 (Fig. 5), when compared to control cells, early and late apoptotic cells increased dramatically to 6.9% ± 0.5% and 26.8% ± 0.7%, and 11.8% ± 0.4% and 35.9% ± 0.6%, respectively.
Figure 4. Effect of LSCF on HepG2 cells using annexin V-FITC/PI double staining. HepG2 cells were treated with 15 and 30 µg/ml for 24 h. (A) Hepg2 cells in different stages (A4) Early-stage apoptotic cells, (A3) viable cells (A2) cells in the late stage of apoptosis (A1) cells undergoing necrosis. (B) Apoptotic rate (%) of cell populations in different stages. Results are presented as the mean ± SD.
Figure 5. Effect of LSCF on MCF-7 cells using annexin V-FITC/PI double staining. MCF-7 cells were treated with 20 and 40 µg/ml) for 24 h. (A) (A4) Early-stage apoptotic cells, (A3) viable cells (A2) cells in late stage of apoptosis (A1) cells undergoing necrosis. (B) Apoptotic rate (%) of cell populations in different stages. Results are presented as the mean ± SD.
Effect of LSCF on apoptosis-related gene expression levels
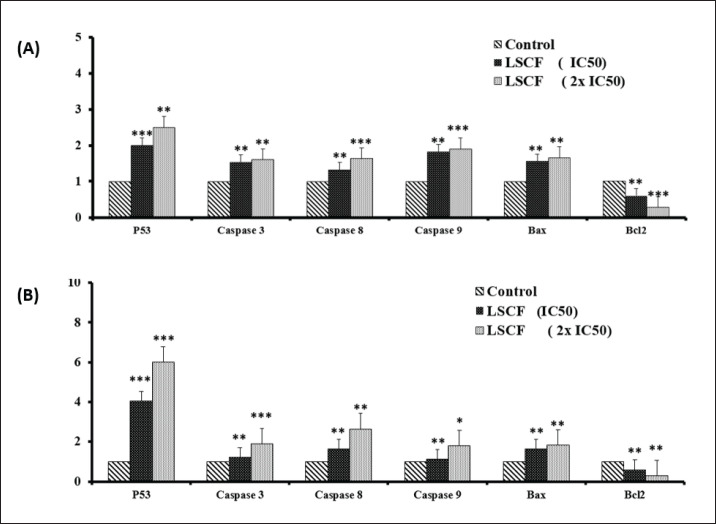
Levels of gene expression associated with apoptotic induction were also evaluated in HepG2 and MCF-7 cells treated with LSCF. Quantitative real-time polymerase chain reaction (RT-PCR) showed that levels of pro-apoptotic genes P53 and Bax rose dose-dependently while levels of anti-apoptotic gene Bcl-2 fell dramatically when treated cells were compared to untreated controls (Fig. 6). In addition, LCFS treatments resulted in up-regulation of caspases3, 8 and 9 mRNA expression levels compared to untreated cells (Fig. 6). Overall, our data indicated that LSCF inhibits HepG2 and MCF-7 cells proliferation through apoptosis pathway.
Figure 6. Effect of LSCF on the expression of apoptosis-associated genes in HepG2 and MCF-7 cells. (A) Relative expression of p53, and caspases 3, 8, 9, Bax, and Bcl2 genes in HepG2 cancer cells treated with 15 and 30 µg/ml concentrations for 24 h compared with the control (untreated cells). (B) Relative expression of the same genes in MCF-7 cancer cells treated with 20 and 40 µg/ml concentrations for 48 h. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the control (untreated cells). Data are presented as the mean ± standard deviation.
LSCF GC-MS analysis
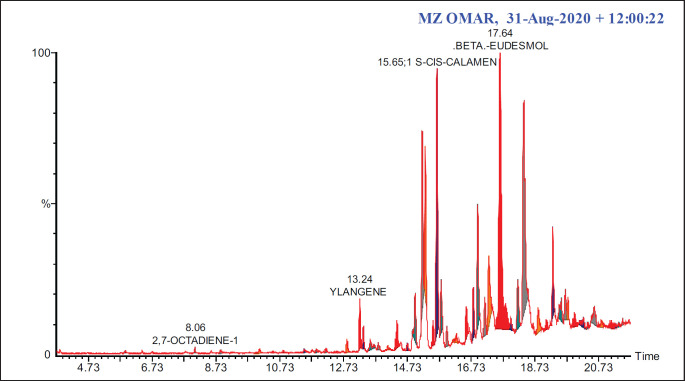
In the LSCF, 25 primary components were identified using GC-MS data. Table 2 displays the retention time (RT), chemical formula, and molecular weight of these compounds. The peaks that showed the presence of some compounds are displayed in (Fig. 7). The major bioactive constituents identified were cis-calamenene (36.44%), beta-eudesmol (18.21%), cyclododecane (5.560%) and alpha-muurolene (3.62%) (Table 2).
Table 2. Summary of primary components identified in LSCF using GC-MS.
| Compound name | Chemical formula | MW (gm/mol) |
RT (min) | Area% |
|---|---|---|---|---|
| Styrene | C8H8 | 104.15 | 5.87 | 0.160 |
| 2,7-Octadiene-1,6-diol | C8H14O2 | 142.2 | 8.06 | 0.380 |
| Terpinyl acetate | C12H20O2 | 196.29 | 10.84 | 0.200 |
| 4-Phenyl-2-butanone (benzyl acetone) | C10H12O | 148.205 | 11.49 | 0.420 |
| 2-Phenylethyl ester of acetic acid (phenethyl acetate) | C10H12O2 | 164.204 | 11.87 | 0.270 |
| Dihydro-alpha-ionone | C13H22O | 194.31 | 12.84 | 0.950 |
| Ylangene | C15H24 | 204.35 | 13.23 | 1.450 |
| Alpha-copaene (copaene) | C15H24 | 204.35 | 13.35 | 0.990 |
| Hexyl Ester of benzenepropanoic acid | C15H22O2 | 234.33 | 13.58 | 3.520 |
| Trans-Caryophyllene | C15H24 | 204.35 | 14.10 | 0.120 |
| Alloaromadendrene | C15H24 | 204.35 | 14.39 | 1.110 |
| Alpha-muurolene | C15H24 | 204.35 | 14.98 | 3.620 |
| Beta-selinene | C15H24 | 204.35 | 15.20 | 1.580 |
| Alpha-selinene | C15H24 | 204.35 | 15.29 | 2.400 |
| 1 s-cis-calamenene | C15H22 | 202.3352 | 15.65 | 36.440 |
| Cadina-1,4-diene | C15H22 | 204.35 | 15.79 | 2.540 |
| Calacorene | C15H20 | 200.32 | 15.98 | 2.190 |
| (-)-Caryophyllene oxide | C15H24O | 220.35 | 16.59 | 1.620 |
| 2,4,6,8-Tetramethyl-1-nonene | C13H26 | 182.35 | 16.93 | 3.330 |
| delta cadinol | C15H26O | 222.37 | 17.16 | 2.300 |
| Beta-eudesmol | C15H26O | 222.37 | 17.64 | 18.210 |
| Cyclododecane | C12H24 | 168.32 | 18.37 | 5.560 |
| Neophytadiene | C20H38 | 278.5 | 19.30 | 2.560 |
| 2-methyl-6-methylene-octa-1,7-dien-3-ol | C10H16O | 152.23 | 20.59 | 3.170 |
| 9-octadecenoic acid | C18H34O2 | 282.5 | 21.48 | 0.540 |
The bold numbers in the table refer to the most significant compounds in the extract.
Figure 7. GC-MS spectra of LSCF.
Discussion
There is increasing curiosity about the possibility of using natural products generated from plants to create new therapeutic cancer treatments, which is a major obstacle in modern medicine [16]. Examining the potential cytotoxic effects of L. scoparium fractions on HepG2 and MCF-7 cancer cell lines was the primary objective of this investigation. The ability of these fractions to induce apoptosis was verified using flow cytometry and quantitative real-time PCR. All tested fractions demonstrated an anti-proliferative effect that increased with concentration in both breast and liver cancer cells [17]. Strong inhibitory activity is indicated by an IC50 value of 30 µg/ml or lower, as per the criteria established by the ANC Institute [18,19]19]. The first experiments demonstrated that fractions of L. scoparium had strong inhibitory effects, with IC50 values of 15 µg/ml for HepG2 cells and 20 µg/ml for MCF-7 cells. Additionally, the LDH assay was used to evaluate the extent of cell membrane damage. This assay quantifies the release of this enzyme from the cytosol into the extracellular space upon cell death. The findings from both the LDH and MTT assays suggest that L. scoparium fractions possess notable cytotoxic properties [20,21]21]. Comparable results were also observed in cell lines of the lung cancerous cell treated with fractions from Leptospermum flavescens, a related species, in a similar experimental setup [22].
According to the study referenced by Afrin et al. [14], the analysis indicates that Strawberry tree honey (STH) possesses elevated concentrations of phenolics, flavonoids, amino acids, and proteins, along with stronger antioxidant activity, when compared to MH. Furthermore, both types of honey exhibit cytotoxic effects on colon cancer cells, which vary depending on the dose and duration of treatment. Nevertheless, STH’s impact becomes more noticeable at lower doses, indicating that it may have chemopreventive qualities in colon cancer. Research on breast and lung cancer cells has shown that MH can inhibit the activity of the cancer-promoting transcription factor p-STAT3. The IL-6 receptor (IL-6R) signaling cascade relies on two key components, gp130 and p-JAK2, which we lower to achieve this. Furthermore, MH and its flavonoid components have been found to bind directly to IL-6Rα. This binding impedes the receptor’s interaction with the IL-6 ligand, highlighting the blockade of IL-6R as a promising therapeutic approach for cancers dependent on IL-6 signaling [15]. Halwani [23] investigates the antimicrobial and anticancer properties of Shaoka and MH on HepG2 and MCF-7 cells. The antibacterial properties of Shaoka honey are comparable to those of phenol on ESBL Escherichia coli, and it is especially effective against multi-drug-resistant strains. This honey also has potent apoptotic effects on cancerous cell lines, which raises the possibility that it could be utilized therapeutically to treat cancer and infectious diseases.
The apoptosis pathway is a well-studied mechanism that regulates cell death [24,25]. Cancer cells that avoid apoptosis tend to grow uncontrollably and develop resistance to anti-cancer therapies [26,27]. The majority of chemotherapeutic drugs trigger cell death in cancer by way of the apoptotic pathway, which is regulated by genes belonging to the Bcl-2 family. These genes include both pro- and anti-apoptotic variants, such as Bcl-2 and Bax. The current study showed that LSCF treatment activated apoptosis by increasing Bax expression and decreasing Bcl-2 levels.
Efficient apoptosis requires upregulation of the caspase gene family, and LSCF treatment was found to dose-dependently induce the expression of different caspases, confirming apoptosis activation [28]. These findings are consistent with previous research that showed L. flavescens extract induces apoptosis and activates caspase-3 in lung cells [29]. Recent research indicates that MH could possess anti-cancer qualities. It appears to modulate levels of reactive oxygen species within cells and alter intracellular calcium levels, which can lead to the programmed death of cancer cells. This potential anti-cancer mechanism is linked to MH’s distinctive capacity to effectively regulate hydrogen peroxide transfer via aquaporin-3 [30].
The major phytoconstituents found in LSCF were analyzed using GC-MS analysis, which revealed that some of the compounds have anticancer activity. Beta-eudesmol is one of the major components present in LSCF, as shown in Table 2. Many studies have shown that beta-eudesmol triggers caspase 3/7, increases Bax levels, and decreases Bcl-2 levels, leading to cytotoxicity and death in different types of cancer cells [31–33]. This study suggests that these components in CMCF could collaboratively enhance the anti-proliferative properties of L. scoparium.
This discovery aligns with Alsaud et al. [5] extraction of β-caryophyllene from manuka leaves using a hydrophobic deep eutectic solvent (HDES) made from menthol and lactic acid. The ethanol extracts had greater quantities of total phenolic components and antioxidant capabilities, according to the research. In contrast, manuka leaf extracts produced by HDES demonstrated significant antibacterial activity, which may have practical applications in industry. Research indicates that deep eutectic solvents (DESs) with a 1:2 molar ratio of menthol to lactic acid extract β-caryophyllene from manuka leaves in New Zealand are better than normal solvents, and environmentally friendly. This extraction method was fine-tuned to the point where it could be reused as a solvent, and under certain conditions, it yielded 10.25 mg of β-caryophyllene per gram of manuka leaf [34].
However, the limitation of this study is that it did not conduct proteomic and metabolomics analysis to investigate the pathway of the anticancer effect of the manuka leaf extract.
Conclusion
Scientific studies have shown that L. scoparium chloroform extract can inhibit the development and spread of cancer cells. The extract’s anti-cancer effects are amplified by its association with the induction of apoptosis, which is concentration-dependent. Also, this extract changes a lot of genes that have to do with cell death in these cells. Overall, these results suggest that L. scoparium may serve as a valuable resource for developing treatments targeting cancer through apoptotic mechanisms.
Acknowledgment
Imam Mohammad Ibn Saud Islamic University (IMSIU) supported this research. Fahd A. Nasr from Imam Mohammad ibn Saud Islamic University’s Biology Department deserves thanks. Please also thank Wajhul Qamar and Omar M. Noman from King Saud University College of Pharmacy for their assistance with the analysis.
List of Abbreviations
Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; CXP, Cytomics Flow Cytometry (Software by Beckman Coulter); DMEM, dulbecco minimal essential medium; DNA, Deoxyribonucleic Acid; FITC, Fluorescein Isothiocyanate; GCMS, Gas Chromatography-Mass Spectrometry; IC50, inhibitory concentration at 50%; LDH, lactate dehydrogenase; LSCF, L. scoparium chloroform fraction; mRNA, Messenger Ribonucleic Acid; MCF, Mean Channel Fluorescence; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NIST, National Institute of Standards and Technology; PI, Propidium Iodide; RNA, Ribonucleic Acid; SYBR, SYBR Green (a fluorescent dye used in molecular biology).
Conflict of interest
The author declares that there is no conflict of interest.
Authors’ contributions
Mohammed Al-Zharani makes the conceptualization, data analysis, resource, visualization, supervision, writing, and revision.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. https://doi.org/10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- [2].Mamdouh AM, Khodeer DM, Tantawy MA, Moustafa YM. In-vitro and in-vivo investigation of amygdalin, metformin, and combination of both against doxorubicin on hepatocellular carcinoma. Life Sci. 2021;285:119961. doi: 10.1016/j.lfs.2021.119961. https://doi.org/10.1016/j.lfs.2021.119961. [DOI] [PubMed] [Google Scholar]
- [3].Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan T, et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther. 2021;6:1–48. doi: 10.1038/s41392-021-00572-w. https://doi.org/10.1038/s41392-021-00572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mathew C, Tesfaye W, Rasmussen P, Peterson GM, Bartholomaeus A, Sharma M, et al. Mānuka oil—a review of antimicrobial and other medicinal properties. Pharmaceuticals. 2020;13:343. doi: 10.3390/ph13110343. https://doi.org/10.3390/ph13110343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alsaud N, Shahbaz K, Farid M. Antioxidant and antibacterial evaluation of manuka leaves (Leptospermum scoparium) extracted by hydrophobic deep eutectic solvent. Chem Eng Res Des. 2021;174:96–106. https://doi.org/10.1016/j.cherd.2021.08.004. [Google Scholar]
- [6].Almasaudi S. The antibacterial activities of honey. Saudi J Biol Sci. 2021;28:2188–96. doi: 10.1016/j.sjbs.2020.10.017. https://doi.org/10.1016/j.sjbs.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tashkandi H. Honey in wound healing: an updated review. Open Life Sci. 2021;16:1091–100. doi: 10.1515/biol-2021-0084. https://doi.org/10.1515/biol-2021-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Paunică-Panea G, Teodorescu S, Preda A, Gligor L, Silaghi A, Constantin VD. Chronic wound management; SURGICAL therapy and complementary nursing with manuka honey. J Mind Med Sci. 2023;10:139–47. https://doi.org/10.22543/2392-7674.1387. [Google Scholar]
- [9].Al-Ghamdi AA, Ansari MJ. Biological and therapeutic roles of Saudi Arabian honey: a comparative review. J King Saud Univ Sci. 2021;33:101329. https://doi.org/10.1016/j.jksus.2020.101329. [Google Scholar]
- [10].Al-Khalaf AA, Alabdelkareem I, Al-Rejaie SS, Mohany M, Hozzein WN. Molecular docking studies on methanolic propolis extracts collected from different regions in Saudi Arabia as a potential inhibitor of topoisomerase IIβ. Separations. 2022;9:392. https://doi.org/10.3390/separations9120392. [Google Scholar]
- [11].Mohamed HK, Mobasher MA, Ebiya RA, Hassen MT, Hagag HM, Sayed RE, et al. Anti-inflammatory, anti-apoptotic, and antioxidant roles of honey, royal jelly, and propolis in suppressing Nephrotoxicity induced by Doxorubicin in male Albino rats. Antioxidants. 2022;11:1029. doi: 10.3390/antiox11051029. https://doi.org/10.3390/antiox11051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Al Refaey HR, Newairy ASA, Wahby MM, Albanese C, Elkewedi M, Chaudry MU, et al. manuka honey enhanced sensitivity of HepG2, hepatocellular carcinoma cells, for Doxorubicin and induced Apoptosis through inhibition of Wnt/β-Catenin and ERK1/2. Biol Res. 2021;54:16. doi: 10.1186/s40659-021-00339-1. https://doi.org/10.1186/s40659-021-00339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nasr FA, Shahat AA, Alqahtani AS, Ahmed MZ, Qamar W, Mishari AA, et al. Centaurea bruguierana inhibits cell proliferation, causes cell cycle arhourrest, and induces apoptosis in Human MCF-7 Breast carcinoma cells. Mol Biol Rep. 2020;47:6043–51. doi: 10.1007/s11033-020-05679-x. https://doi.org/10.1007/s11033-020-05679-x. [DOI] [PubMed] [Google Scholar]
- [14].Afrin S, Hernandez TYF, Gasparrini M, Bompadre S, Quiles JL, Sanna G, et al. Strawberry-tree honey induces growth inhibition of human colon cancer cells and increases ROS generation: a comparison with manuka honey. Int J Mol Sci. 2017;18:613. doi: 10.3390/ijms18030613. https://doi.org/10.3390/ijms18030613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aryappalli P, Shabbiri K, Masad RJ, Al-Marri RH, Haneefa SM, Mohamed YA, et al. Inhibition of tyrosine-phosphorylated STAT3 in human breast and lung cancer cells by manuka honey is mediated by selective antagonism of the IL-6 receptor. Int J Mol Sci. 2019;20:4340. doi: 10.3390/ijms20184340. https://doi.org/10.3390/ijms20184340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rio DC, Ares M, Hannon GJ, Nilsen TW. Purification of RNA using TRIzol (TRI Reagent) Cold Spring Harb Protoc. 2010;2010:5439. doi: 10.1101/pdb.prot5439. https://doi.org/10.1101/pdb.prot5439. [DOI] [PubMed] [Google Scholar]
- [17].Abutaha N, Nasr FA, Al-zharani M, Alqahtani AS, Noman OM, Abdelhabib S, et al. Effects of hexane root extract of Ferula Hermonis Boiss on human breast and colon cancer cells: an in vitro and in vivo study. BioMed Res Int. 2019;2019:e3079895. doi: 10.1155/2019/3079895. https://doi.org/10.1155/2019/3079895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hashem S, Ali TA, Akhtar S, Nisar S, Sageena G, Ali S, et al. Targeting cancer signaling pathways by natural products: exploring promising anti-cancer agents. Biomed Pharmacother. 2022;150:113054. doi: 10.1016/j.biopha.2022.113054. https://doi.org/10.1016/j.biopha.2022.113054. [DOI] [PubMed] [Google Scholar]
- [19].Canga I, Vita P, Oliveira AI, Castro MÁ, Pinho C. In vitro cytotoxic activity of African plants: a review. Molecules. 2022;27:4989. doi: 10.3390/molecules27154989. https://doi.org/10.3390/molecules27154989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Al-Abkal F, Abdel-Wahab BA, El-Kareem HFA, Moustafa YM, Khodeer DM. Protective effect of pycnogenol against methotrexate-induced hepatic, renal, and cardiac toxicity: an in vivo study. Pharmaceuticals. 2022;15:674. doi: 10.3390/ph15060674. https://doi.org/10.3390/ph15060674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li H, Wang Z, Fang X, Zeng W, Yang Y, Jin L, et al. Poroptosis: a form of cell death depending on plasma membrane nanopores formation. Science. 2022;25:104481. doi: 10.1016/j.isci.2022.104481. https://doi.org/10.1016/j.isci.2022.104481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Taher M, Susanti D, Haris MS, Rushdan AA, Widodo RT, Syukri Y, et al. Gylated liposomes enhance the effect of cytotoxic drug: a review. Heliyon. 2023;9:e13823. doi: 10.1016/j.heliyon.2023.e13823. https://doi.org/10.1016/j.heliyon.2023.e13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Halawani EM. Potential effects of Saudi Shaoka (Fagonia bruguieri) honey against multi-drug-resistant bacteria and cancer cells in comparison to manuka honey. Saudi J Biol Sci. 2021;28:7379–89. doi: 10.1016/j.sjbs.2021.08.055. https://doi.org/10.1016/j.sjbs.2021.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tong X, Tang R, Xiao M, Xu J, Wang W, Zhang B, et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15:174. doi: 10.1186/s13045-022-01392-3. https://doi.org/10.1186/s13045-022-01392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kari S, Subramanian K, Altomonte IA, Murugesan A, Yli-Harja O, Kandhavelu M. Programmed cell death detection methods: a systematic review and a categorical comparison. Apoptosis. 2022;27:482–508. doi: 10.1007/s10495-022-01735-y. https://doi.org/10.1007/s10495-022-01735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Perini GF, Ribeiro GN, Pinto Neto JV, Campos LT, Hamerschlak N. BCL-2 as therapeutic target for hematological malignancies. J Hematol Oncol. 2018;11:65. doi: 10.1186/s13045-018-0608-2. https://doi.org/10.1186/s13045-018-0608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Qian S, Wei Z, Yang W, Huang J, Yang Y, Wang J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front Oncol. 2022;12:985363. doi: 10.3389/fonc.2022.985363. https://doi.org/10.3389/fonc.2022.985363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20:175–93. doi: 10.1038/s41580-018-0089-8. https://doi.org/10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Navanesan S, Wahab NA, Manickam S, Sim KS. Leptospermum flavescens constituent-LF1 causes cell death through the induction of cell cycle arrest and apoptosis in human lung carcinoma cells. PLOS One. 2015;10:e0135995. doi: 10.1371/journal.pone.0135995. https://doi.org/10.1371/journal.pone.0135995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Martinotti S, Pellavio G, Patrone M, Laforenza U, Ranzato E. manuka honey induces apoptosis of epithelial cancer cells through aquaporin-3 and calcium signaling. Life. 2020;10:256. doi: 10.3390/life10110256. https://doi.org/10.3390/life10110256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Srijiwangsa P, Ponnikorn S, Na-Bangchang K. Effect of β-eudesmol on NQO1 suppression-enhanced sensitivity of cholangiocarcinoma cells to chemotherapeutic agents. BMC Pharmacol Toxicol. 2018;19:32. doi: 10.1186/s40360-018-0223-4. https://doi.org/10.1186/s40360-018-0223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tomko AM, Whynot EG, Ellis LD, Dupré DJ. Anti-cancer potential of cannabinoids, terpenes, and flavonoids present in cannabis. Cancers. 2020;12:1985. doi: 10.3390/cancers12071985. https://doi.org/10.3390/cancers12071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Latif FMA, Ainane A, Aboubaker IH, Mohamed J, Ainane T. Exploring the potent anticancer activity of essential oils and their bioactive compounds: mechanisms and prospects for future cancer therapy. Pharmaceuticals. 2023;16:1086. doi: 10.3390/ph16081086. https://doi.org/10.3390/ph16081086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Alsaud N, Shahbaz K, Farid M. Evaluation of deep eutectic solvents in the extraction of β-caryophyllene from New Zealand manuka leaves (Leptospermum scoparium) Chem Eng Res Des. 2021;166:97–108. https://doi.org/10.1016/j.cherd.2020.11.028. [Google Scholar]