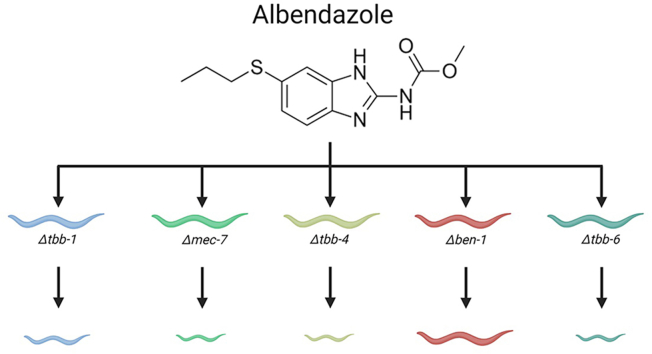
Abstract
Benzimidazole (BZ) anthelmintics are among the most important treatments for parasitic nematode infections in the developing world. Widespread BZ resistance in veterinary parasites and emerging resistance in human parasites raise major concerns for the continued use of BZs. Knowledge of the mechanisms of resistance is necessary to make informed treatment decisions and circumvent resistance. Benzimidazole resistance has traditionally been associated with mutations and natural variants in the C. elegans beta-tubulin gene ben-1 and orthologs in parasitic species. However, variants in ben-1 alone do not explain the differences in BZ responses across parasite populations. Here, we examined the roles of five C. elegans beta-tubulin genes (tbb-1, mec-7, tbb-4, ben-1, and tbb-6) in the BZ response as well as to determine if another beta-tubulin acts redundantly with ben-1. We generated C. elegans strains with a loss of each beta-tubulin gene, as well as strains with a loss of tbb-1, mec-7, tbb-4, or tbb-6 in a genetic background that also lacks ben-1. We found that the loss of ben-1 conferred the maximum level of resistance following exposure to a single concentration of albendazole, and the loss of a second beta-tubulin gene did not alter the level of resistance. However, additional traits other than larval development could be affected by the loss of additional beta-tubulins, and the roles of other beta-tubulin genes might be revealed at different albendazole concentrations. Therefore, further work is needed to fully define the possible roles of other beta-tubulin genes in the BZ response.
Keywords: Beta-tubulin, Benzimidazole, Anthelmintic resistance, C. elegans
Graphical abstract
Highlights
-
•
Loss of ben-1 causes almost complete abrogation of sensitivity to albendazole (ABZ).
-
•
Loss of other beta-tubulin genes does not confer ABZ resistance.
-
•
Loss of ben-1 and a second beta-tubulin gene does not enhance the ben-1 level of ABZ resistance.
1. Introduction
Parasitic nematode infections are among the most common infectious diseases of humans and pose significant health and socioeconomic risks for endemic regions. Upwards of 1.5 billion people are estimated to be infected with at least one parasitic nematode species globally, with infections causing anemia, impaired cognitive development, reduced growth, diarrheal disease, intestinal obstructions, and lymph edema (Salikin et al., 2020). Anti-helminth drugs, or anthelmintics, are used in endemic areas to control infections and limit adverse health effects caused by parasitic nematodes. Anthelmintics are often delivered through mass drug administration (MDA) programs designed to deliver essential medicines to regions with infected populations.
One of the most common anthelmintics delivered in MDA programs is albendazole (ABZ), a drug belonging to the benzimidazole (BZ) class of anthelmintics. The BZ drug class is included in many MDA programs because of its broad-spectrum activity, capable of treating a wide variety of intestinal helminths, as well as being safe and affordable to easily deliver to large populations (Banerjee et al., 2023). Studies of the mode of BZ action have found that they inhibit the polymerization of microtubules by targeting beta-tubulin (Hastie and Georgopoulos, 1971; Sheir-Neiss et al., 1978). A study of BZ response in the free-living model nematode Caenorhabditis elegans found that larvae exposed to BZs were developmentally impaired and uncoordinated in locomotion (Chalfie and Thomson, 1982). Subsequent experiments showed that animals with loss-of-function mutations in the beta-tubulin gene ben-1 were found to exhibit wild-type growth and movement in the presence of BZs (Driscoll et al., 1989). Wild-type growth, despite the loss of ben-1, is thought to be possible because another beta-tubulin gene acts redundantly and compensates for the loss of ben-1. The C. elegans genome contains five additional beta-tubulin genes (tbb-1, tbb-2, mec-7, tbb-4, and tbb-6) that are differentially expressed in various tissues and are thought to supply beta-tubulin function when ben-1 is lost (Hurd, 2018).
Orthologs of ben-1 were found to be the target of BZs in parasitic nematodes. A beta-tubulin gene (tbb-isotype-1) from Haemonchus contortus, a small-ruminant parasite, was found to rescue BZ susceptibility when expressed in a C. elegans strain that lacked ben-1 (Kwa et al., 1993, 1994, 1995). Unlike C. elegans, the H. contortus genome contains only four genes encoding beta-tubulins (tbb-isotype-1, tbb-isotype-2, tbb-isotype-3, and tbb-isotype-4) (Saunders et al., 2013). A smaller complement of beta-tubulin genes, combined with expression differences between each of the four genes has led to the conclusion that loss of tbb-isotype-1 likely causes lethality, indicating that BZ resistance in parasites is probably dependent on altered function variants in tbb-isotype-1 (Saunders et al., 2013). However, parasitic nematodes currently lack the genetic tools, such as genome editing, to validate resistance genes using targeted mutations. Exploration of anthelmintic resistance is dependent on C. elegans as a complement to research in parasites, and a cycle of discovery has been proposed to explore and validate the mechanisms of BZ resistance using both free-living and parasitic nematodes (Wit et al., 2021).
Anthelmintic resistance is a major concern in the control of parasites. Resistance to the BZ drug class has become nearly ubiquitous in many nematode species of veterinary importance and is now an emerging problem in nematode infections of humans (Kaplan, 2004; Howell et al., 2008; Krücken et al., 2017). The development of resistance to BZs makes the control of infections difficult and costly. To address the emergence of BZ resistance, it is necessary to understand the underlying genetics contributing to resistance. After suspected resistance-associated variants are identified in parasites, they can be validated in C. elegans using CRISPR-Cas9 genome editing. Studies of BZ resistance have identified non-synonymous variants at codons 134, 167, 198, and 200 of ben-1 orthologs in parasites (Kwa et al., 1994; Avramenko et al., 2019; Mohammedsalih et al., 2020; Venkatesan et al., 2023). Every known beta-tubulin variant associated with BZ resistance in parasitic nematodes has been shown to cause resistance in C. elegans by the introduction of the variant into the ben-1 gene (Kwa et al., 1994; Kitchen et al., 2019; Dilks et al., 2020, 2021; Venkatesan et al., 2023). These variants in parasite beta-tubulin genes are hypothesized to alter a putative BZ binding site, preventing BZs from inhibiting beta-tubulin, preserving the normal formation of microtubules, and allowing nematodes to survive and develop normally in the presence of BZ treatment.
Despite the validation of variants in ben-1 orthologs as a mechanism of resistance to BZs, ben-1 is not the only gene involved in BZ resistance. Genome-wide association studies in wild populations of C. elegans have identified multiple genomic loci independent of ben-1 that are associated with BZ resistance (Hahnel et al., 2018; Zamanian et al., 2018). Fully understanding the genetics of resistance is necessary to inform strategic decisions that improve the efficacy of existing treatments, as well as lead to the development of new treatments and control strategies. Thus, it is imperative to identify all genes associated with BZ resistance. Here, we explored how the loss of each beta-tubulin gene affects BZ resistance in C. elegans. The gene ben-1 has been extensively studied and confers the greatest level of BZ resistance. However, the roles of and potential redundancies with the other C. elegans beta-tubulin genes (tbb-1, tbb-2, mec-7, tbb-4, and tbb-6) in BZ resistance are not well understood. The known resistance mutation F200Y is present in both tbb-1 and tbb-2. However, this mutation is hypothesized to cause a change in BZ binding and not loss of gene function. All of the other beta-tubulin genes lack known resistance-associated alleles and should be inhibited by BZs. So far, validated ben-1 resistance mutations have been phenotypically equivalent to a loss of ben-1 in C. elegans, so we generated loss-of-function deletions of each beta-tubulin. We compared the effects of single beta-tubulin deletions on nematode development when exposed to a single concentration of ABZ that previously was found to confer a significant impact on the larval development of the wild-type N2 strain of C. elegans (Dilks et al., 2020, 2021; Pallotto et al., 2022). We found that the loss of ben-1 conferred the highest level of resistance and the loss of tbb-1 conferred moderate resistance. To test for genetic redundancy among beta-tubulin genes, we used CRISPR-Cas9 genome editing to delete each beta-tubulin gene in a genetic background that already has lost ben-1 function. The loss of each beta-tubulin gene in the ben-1 deletion background did not confer a detectable change in ABZ resistance compared to the loss of ben-1 alone. Overall, we find that the loss of ben-1 alone is sufficient to confer the maximum level of ABZ resistance at the concentration tested, with development equivalent to the wild-type strain in control conditions.
2. Materials and methods
2.1. C. elegans strains and maintenance
Nematodes were grown on plates of modified nematode growth media (NGMA) containing 1% agar and 0.7% agarose and seeded with the Escherichia coli strain OP50 (Andersen et al., 2014). Plates were maintained at 20 °C for the duration of all experiments. Before each assay, animals were grown for three generations to reduce the multigenerational effects of starvation.
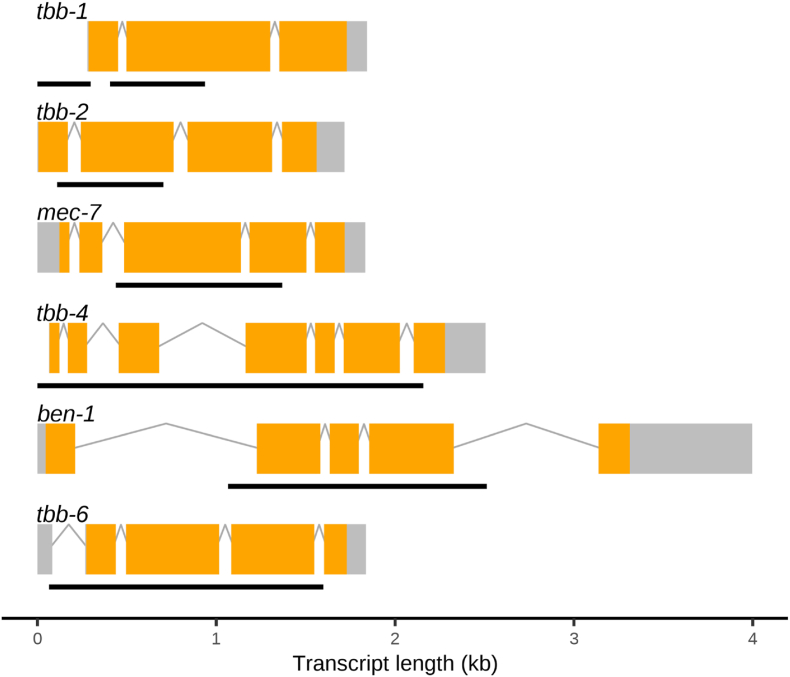
CRISPR-Cas9-edited strains were generated as previously described (Hahnel et al., 2018; Dilks et al., 2020) (S1 Table), except for VC364 tbb-1(gk207), which was acquired from the Caenorhabditis Genetics Center (Minneapolis, MN). All single deletions were generated in the reference N2 genetic background. All double deletions were generated in the ECA882 ben-1(ean64) genetic background (Dilks et al., 2020, 2021). Progeny from injected animals (F1) were individually placed onto NGMA plates to reproduce and then sequenced using Sanger sequencing to confirm the presence of the desired edit. At least two generations of animals after single-animal passage were Sanger sequenced to confirm successful genome edits. Two independent strains of each deletion and pair of deletions were generated to control for any potential off-target effects caused by CRISPR-Cas9 (Fig. 1).
Fig. 1.
Gene models and locations of deletion alleles generated in C. elegans beta-tubulin genes. Gene models of the longest isoforms are presented for each C. elegans beta-tubulin gene, with exons (orange), introns (gray lines), and untranslated regions (gray boxes) shown. Regions that were deleted using CRISPR-Cas9 genome editing are shown as black lines under each model. Deleted regions of tbb-1 are shown as two black lines because strains with two independent deletion alleles were used. Gene model data were obtained from WormBase (WS279). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.2. Nematode food preparation
The OP50 strain of E. coli was used as a nematode food source on NGMA plates. Bacterial food for the liquid-based high-throughput assay was prepared as previously described (Widmayer et al., 2022). Briefly, a frozen stock of the HB101 strain of E. coli was used to inoculate and grow a 1 L culture to an OD600 value of 0.001. HB101 was used for all liquid-based imaging assays because it grows faster than OP50 and should provide better nutrition for C. elegans (Fox et al., 2022). Six cultures containing 1 L of pre-warmed 1x Horvitz Super Broth (HSB) and an OD600 inoculum grew for 15 h at 37 °C until cultures were in the late log growth phase. After 15 h, flasks were removed from the incubator and transferred to 4 °C to halt bacterial growth. Cultures were pelleted using centrifugation, the supernatant removed, and washed with K medium (51 mM NaCl, 32 mM KCl, 3 mM CaCl2, and 3 mM MgSO4 in distilled water) (Boyd et al., 2012). Bacteria were resuspended in K medium, and the OD600 value was determined. The bacterial suspension was diluted to a final concentration of OD600100 before being aliquoted to 30 mL and frozen at -80 °C.
2.3. Albendazole stock preparation
A 100 μM stock solution of albendazole (Fluka, Catalog #: A4673-10G) was prepared in dimethyl sulfoxide (DMSO), aliquoted, and stored at -20 °C. A frozen ABZ aliquot was thawed shortly before adding the drug to the assay plates.
2.4. High-throughput phenotyping assay (HTA)
A previously described HTA was used for all ABZ response phenotyping assays (Shaver et al., 2023). Two independent assays made up of three bleaches each were performed. Strains underwent three generations of growth to control for any starvation effects and were then bleach synchronized in triplicate to control for variation caused by bleach effects. Embryos were concentrated at 0.6 embryos/μL in 50 μL of K medium (Boyd et al., 2012). A volume of 50 μL of the embryo solution was dispensed into each well of a 96-well plate. Both DMSO and ABZ conditions contained 48 wells of N2 and ECA882, and 24 wells of each of the other tested strains for each replicate bleach. Embryos were allowed to hatch overnight at 20 °C with constant shaking at 180 rpm. The following morning, HB101 aliquots were thawed at room temperature, combined, and diluted to OD60030 with K medium, and kanamycin was added at a concentration of 150 μM to inhibit further bacterial growth and prevent contamination. The final well concentration of HB101 was OD60010, and the final concentration of kanamycin was 50 μM. Each well was treated with a final concentration of either 1% DMSO or 30 μM ABZ in 1% DMSO. Animals were grown for 48 h with constant shaking at 180 rpm, after which, animals were treated with 50 mM sodium azide in M9 buffer to straighten and paralyze the animals for imaging. Following 10 min of exposure to sodium azide, each plate was imaged using a Molecular Devices ImageXpress Nano microscope (Molecular Devices, San Jose, CA) with a 2X objective (Shaver et al., 2023).
Independent assays included identical strain sets except as follows: Strains with a deletion of tbb-2 were found to be too developmentally delayed to use in these assays. The ECA3726 ben-1(ean64); mec-7(ean257) strain was removed from assay one because of an insufficient quantity of embryos after bleach synchronization. Smaller significant effects on animal development were observed for some single deletions in control conditions of assay one but not in assay two, indicating that significance assigned to the observed small effects could be the result of high levels of replication, making even small differences significant.
2.5. Data cleaning and analysis
High-throughput assay images were processed using CellProfiler (https://github.com/AndersenLab/CellProfiler). Processed image data were cleaned and processed using the easyXpress (Nyaanga et al., 2021) R package as previously described (Shaver et al., 2024). The two assays were cleaned and processed independently. All statistical comparisons and figure generation were performed in R(4.1.2) (R Core Team, 2020). Median animal lengths were calculated from each well of an assay plate and normalized across independent growths, plates, and bleaches. Deletion of each beta-tubulin gene in the same genetic background enables the determination of the quantitative effects that each gene has on BZ response, as well as to determine if the loss of each beta-tubulin gene impacts development in control conditions. Median animal length after 48 h of exposure was normalized to control conditions, and then statistical comparisons were made between N2 and each strain. We used the Rstatix package tukeyHSD function on an ANOVA model generated with the formula phenotype ∼ strain to calculate differences in the responses of the strains. Fig. 2 was generated using data from assay one because of the large amount of variation shown in animal response for the VC364 tbb-1(gk207) strain in assay two (S1 Figure), thought to be caused by human error. Fig. 3 was generated using data from assay two, because of the loss of the ECA3746 strain in assay one. All data are presented in supplemental figures.
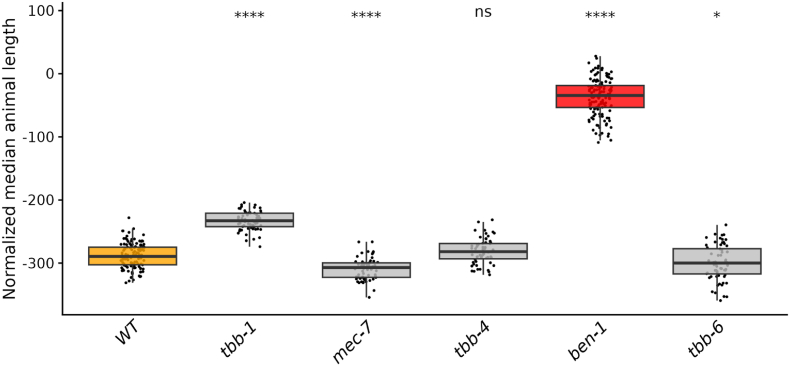
Fig. 2.
Only loss of ben-1 causes high levels of resistance to ABZ. Median animal lengths of strains grown in 30 μM ABZ that have been regressed for bleach effects and then normalized to the mean of all median animal lengths from the control condition are shown. Each point represents the summarized measurements of an individual well containing five to 30 animals. Data are shown as box plots with the median as a solid horizontal line and the 75th and 25th quartiles on the top and bottom of the box, respectively. The top and bottom whiskers extend to the maximum point within the 1.5 interquartile range from the 75th and 25th quartiles, respectively. Statistical significance compared to the wild-type strain is shown above each strain (p < 0.05 = *, p < 0.0001 = ****, ANOVA with Tukey HSD).
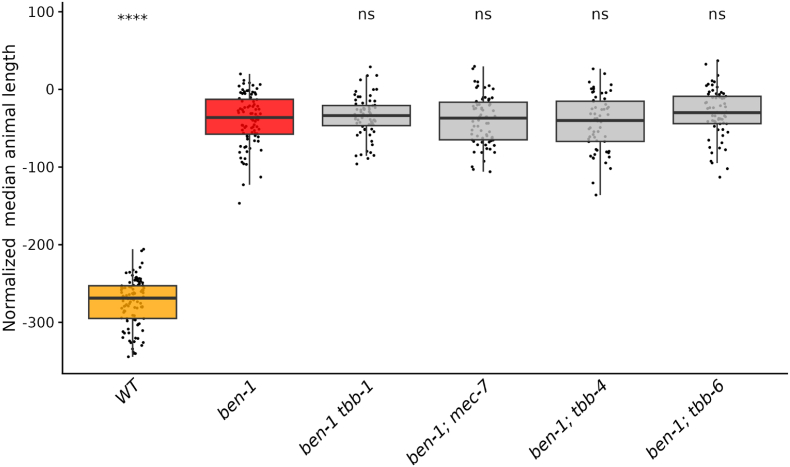
Fig. 3.
None of the other beta-tubulin genes act redundantly with ben-1 in ABZ response. Median animal lengths of strains grown in 30 μM ABZ that have been regressed for bleach effects and then normalized to the mean of all median animal lengths from the control condition are shown. Each point represents the summarized measurements of an individual well containing five to 30 animals. Data are shown as box plots with the median as a solid horizontal line and the 75th and 25th quartiles on the top and bottom of the box, respectively. The top and bottom whiskers extend to the maximum point within the 1.5 interquartile range from the 75th and 25th quartiles, respectively. Statistical significance compared to the ben-1 deletion strain is shown above each strain (p < 0.05 = *, p < 0.0001 = ****, ANOVA with Tukey HSD).
3. Results
3.1. The loss of ben-1 is the only beta-tubulin gene to confer high levels of ABZ resistance
CRISPR-Cas9 genome editing was used to generate deletions of each beta-tubulin gene in the N2 laboratory strain genetic background (Fig. 1). Edited strains with single deletions of each beta-tubulin gene were phenotyped in DMSO (control) and ABZ using a previously described high-throughput assay (HTA) that quantitatively measures nematode development (Widmayer et al., 2022; Shaver et al., 2023). The loss of tbb-1 had the most significant impact on development in control conditions, indicating that the loss of tbb-1 is possibly detrimental (S2 Figure). The loss of ben-1 was the only strain to confer high levels of resistance to ABZ, with development in ABZ conditions equivalent to that of the wild-type strain in control conditions (Fig. 2, S3 Figure). The loss of tbb-1 was found to confer a moderate level of resistance, with animal development significantly less affected than the wild-type strain but still heavily affected by ABZ as compared to growth in control conditions.
3.2. The loss of ben-1 confers the highest level of ABZ resistance compared to other beta-tubulin mutants
To determine if other beta-tubulin genes play a redundant role in ABZ resistance with ben-1, we generated individual deletions of tbb-1, mec-7, tbb-4, and tbb-6 in the ben-1(ean64) genetic background. We exposed these double beta-tubulin mutants to DMSO and ABZ in the same high-throughput development assay described above to determine if the loss of a second beta-tubulin alters the levels of BZ resistance observed in the single ben-1 mutant. Similarly to the single deletion assay, small significant differences were observed for multiple strains compared to the wild-type strain in control conditions, except for the strain ECA3628 ben-1(ean64); tbb-4(ean282) (S4 Figure), which was found to be more heavily impacted in both replicate assays. Strains with the loss of a second beta-tubulin were found to be equally resistant when compared to the loss of ben-1 alone (Fig. 3, S5 Figure). As previously noted, the loss of ben-1 almost fully rescued development at 30 μM ABZ compared to the control strain, possibly preventing any small effects conferred by the loss of a second beta-tubulin from being observed.
4. Discussion
Despite the established role variants in ben-1 orthologs have in BZ resistance, differential responses of C. elegans wild strains highlight that additional genes must be involved in BZ response (Hahnel et al., 2018; Zamanian et al., 2018). Fully understanding the mechanisms of BZ resistance is imperative to the development of strategies to promote the sustainability of BZ anthelmintics, because an understanding of all genes involved provides multiple potential targets of treatment, limiting the development of resistance. Here, we took an important first step to test additional beta-tubulin genes in BZ resistance.
4.1. ben-1 plays the largest role in ABZ resistance in C. elegans
We examined the role that five of the six C. elegans beta-tubulin genes play in ABZ resistance by generating strains with a loss of each gene, as well as strains with a loss of an additional beta-tubulin in a ben-1 mutant background. Because of detrimental effects on development, strains with a loss of tbb-2 could not be measured for responses to ABZ. Consistent with previous studies, the loss of ben-1 was sufficient to confer the maximum level of ABZ resistance at a concentration of 30 μM. Although tbb-1(gk207) demonstrated a lower level of resistance compared to the ben-1 deletion strain, and was negatively impacted in control conditions, it is possible that other background mutations could cause resistance (and slower growth in control conditions) because this strain was not backcrossed. Loss of a second beta-tubulin in a strain with a loss of ben-1 did not confer a detectable enhancement of resistance, and with the exception of the ECA3628 ben-1(ean64); tbb-4(ean282) strain, did not confer severe detriment in control conditions. The ECA3628 strain could have unintended off-target effects caused by gene editing that reduced fitness, because the effect is seen in both replicate assays but is not seen in the second independent edit strain with deletions of both ben-1 and tbb-4. Despite observing no effects from the deletion of a second beta-tubulin, we cannot definitively conclude if any other beta-tubulin gene acts redundantly with ben-1 in ABZ resistance. The assay that we used to measure ABZ resistance uses one concentration that previously was found to differentiate susceptible strains from ben-1 mutant strains (Dilks et al., 2020, 2021). It remains possible that enhancement of ABZ resistance could be detected at higher ABZ concentrations where the single contribution of ben-1 might not be sufficient to cause resistance alone. Another caveat is that only a single trait, development, was measured. ABZ affects multiple traits, including fecundity and competitive fitness over multiple generations (Shaver et al., 2024). Future studies should investigate multiple traits at different ABZ concentrations to fully understand the role of all beta-tubulin genes in the ABZ response.
4.2. BZ resistance is complicated by differences in beta-tubulin copy number, levels of expression, and resistance alleles
We tested the role of each beta-tubulin gene in ABZ response by deleting much of the coding sequence. Therefore, these results are binary for the presence or absence of each beta-tubulin gene. Amino-acid altering variants from parasites have been validated in ABZ resistance using C. elegans and shown to cause ABZ resistance equivalent to a strain with a loss of ben-1 (Dilks et al., 2020, 2021; Venkatesan et al., 2023). However, these variants likely do not cause loss of tbb-isotype-1 function in parasites (Saunders et al., 2013). What could be causing this discrepancy between loss-of-function variants in C. elegans and potential altered function variants in parasitic nematodes? In species with highly expressed beta-tubulin genes that have BZ-sensitive alleles, loss-of-function alleles would cause fitness defects, similar to what we see with the deletion of tbb-1 and tbb-2. In these species, BZ resistance must be mediated by altered function variants. In species with less highly expressed (or tissue-specific) beta-tubulin genes that have BZ-sensitive alleles, loss-of-function alleles could cause BZ resistance because other beta-tubulin genes can substitute for essential functions, similar to what is observed in C. elegans with ben-1. Interestingly, although loss-of-function alleles in tbb-isotype-1 are thought to be lethal, the loss of the H. contortus beta-tubulin gene tbb-isotype-2 has been documented in some highly resistant H. contortus populations, possibly amplifying the resistance conferred by mutations in tbb-isotype-1 alone (Saunders et al., 2013). However, variation in tbb-isotype-2 alone is likely not sufficient to cause resistance, and to our knowledge, resistance associated with variation in tbb-isotype-2 has not been documented independent of variation in tbb-isotype-1. The whole-organism expression levels of tbb-isotype-2 are less than half that of tbb-isotype-1 likely explaining why variation in tbb-isotype-1 is necessary for resistance and further highlighting the role that differences in expression can play when combined when change- or loss-of-function variants. Additionally, the phenotypic classification of BZ-resistance phenotypes differs between these two species and can be explained by differences in loss-of-function vs. altered function mutations. In C. elegans where ben-1 variants or mutations can cause loss of function, the BZ-resistance phenotype is recessive (Dilks et al., 2021). By contrast, putative BZ-resistance alleles in H. contortus are hypothesized to confer incomplete dominance where heterozygotes are somewhat resistant and homozygotes are fully resistant (Silvestre et al., 2001).
Beyond coding variants or mutations in beta-tubulin genes, changes in the levels and tissue-specific expression can alter BZ resistance. Previously, we found that some C. elegans wild strains with clear ABZ resistance, when compared to the laboratory N2 strain, do not have variants that alter the coding sequence of ben-1 but instead have much lower expression levels of ben-1 as compared to the rest of the population (Zhang et al., 2022). These strains are resistant because the susceptible beta-tubulin protein is not expressed. Additionally, we found that the expression of ben-1 in cholinergic neurons alone is sufficient to confer susceptibility to ABZ (Gibson et al., 2022), highlighting that variants modifying expression in specific tissues could confer resistance in a unique way independent of the beta-tubulin coding sequence. These observations from both C. elegans and H. contortus demonstrate that more attention should be paid to the number of beta-tubulin genes, their levels of expression, the sites of expression, and the putative BZ-resistance alleles found in each beta-tubulin gene. To definitively understand BZ resistance mediated by beta-tubulin genes, we must also drastically improve parasitic nematode genomes and gene models because most species lack full descriptions of their beta-tubulin complement.
5. Future directions
The role of ben-1 and tbb-isotype-1 beta-tubulins in BZ resistance has been thought to be similar and established C. elegans as an essential model for parasite BZ resistance research. However, BZ treatment is typically fatal in susceptible parasites (Prichard, 1988), as well as documented ovicidal effects of BZs against parasite embryos (Boes et al., 1998). Conversely, the same effects are not typically seen in C. elegans where the most significant impact is often on the developmental rate (Shaver et al., 2023). The loss of tbb-1 or tbb-2 was deleterious and loss-of-function mutations in either gene would likely be rapidly selected against in the wild (i.e., no variants are observed in natural C. elegans strains) (Crombie et al., 2024), similarly to the predicted loss of tbb-isotype-1. It is important to note that tbb-1 and tbb-2 have known resistance alleles at amino acid position 200, and future studies should edit both genes to make them harbor BZ-sensitive alleles to more closely approximate the beta-tubulin complement and alleles in H. contortus. Such studies could offer an improved model system for investigating BZ resistance. However, studies of BZ resistance need to investigate variants beyond single amino-acid alterations. Our results from previous publications demonstrate that a variety of factors such as copy number, expression, and tissue-specific function can all affect BZ resistance. To continue to broaden our understanding of BZ resistance, we must expand to a whole-genome approach that investigates variants across every single beta-tubulin gene and beyond that single class of genes.
Data availability
All code and data are available at https://github.com/AndersenLab/2024_beta_tubulin_manuscript.
Funding sources
Skyler Stone, Fiona Shao, and Gracie Paredes were funded by Northwestern University Summer Undergraduate Research Grants. Skyler Stone was also funded by the Posner Research Program at Northwestern University. This work was supported by the National Institutes of Health NIAID grant R01AI153088 to ECA.
CRediT authorship contribution statement
J.B. Collins: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Skyler A. Stone: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Emily J. Koury: Resources, Methodology, Investigation. Anna G. Paredes: Resources, Investigation. Fiona Shao: Resources, Investigation. Crystal Lovato: Resources, Investigation. Michael Chen: Resources, Investigation. Richelle Shi: Resources, Investigation. Anwyn Y. Li: Resources, Investigation. Isa Candal: Resources, Investigation. Khadija Al Moutaa: Resources, Investigation. Nicolas D. Moya: Methodology, Investigation, Formal analysis. Erik C. Andersen: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
None.
Acknowledgments
We would like to thank members of the Andersen lab for their feedback in the preparsation of this manuscript. We thank the Caenorhabditis Natural Diversity Resource (NSF Capacity Grant 2224885) and the Caenorhabditis Genetics Center (NIH Office of Research Infrastructure Programs, P40 OD010440) for providing the strains used in this study. Additionally, we would also like to thank WormBase for providing an essential resource for genetic and genomic data used in this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2024.100556.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Andersen E.C., Bloom J.S., Gerke J.P., Kruglyak L. A variant in the neuropeptide receptor npr-1 is a major determinant of Caenorhabditis elegans growth and physiology. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramenko R.W., Redman E.M., Melville L., Bartley Y., Wit J., Queiroz C., Bartley D.J., Gilleard J.S. Deep amplicon sequencing as a powerful new tool to screen for sequence polymorphisms associated with anthelmintic resistance in parasitic nematode populations. Int. J. Parasitol. 2019;49:13–26. doi: 10.1016/j.ijpara.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Banerjee S., Mukherjee S., Nath P., Mukherjee A., Mukherjee S., Ashok Kumar S.K., De S., Banerjee S. A critical review of benzimidazole: sky-high objectives towards the lead molecule to predict the future in medicinal chemistry. Results in Chemistry. 2023;6 [Google Scholar]
- Boes J., Eriksen L., Nansen P. Embryonated and infectivity of Ascaris suum eggs isolated from worms expelled by pigs treated with albendazole , pyrantel pamoate, ivermectin or piperazine dihydrochloride. Vet. Parasitol. 1998;75:181–190. doi: 10.1016/s0304-4017(97)00197-0. [DOI] [PubMed] [Google Scholar]
- Boyd W.A., Smith M.V., Freedman J.H. In: Developmental Toxicology: Methods and Protocols. Harris C., Hansen J.M., editors. Humana Press; Totowa, NJ: 2012. Caenorhabditis elegans as a model in developmental toxicology; pp. 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Thomson J.N. Structural and functional diversity in the neuronal microtubules of Caenorhabditis elegans. J. Cell Biol. 1982;93:15–23. doi: 10.1083/jcb.93.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombie T.A., McKeown R., Moya N.D., Evans K.S., Widmayer S.J., LaGrassa V., Roman N., Tursunova O., Zhang G., Gibson S.B., Buchanan C.M., Roberto N.M., Vieira R., Tanny R.E., Andersen E.C. CaeNDR, the Caenorhabditis natural diversity resource. Nucleic Acids Res. 2024;52:D850–D858. doi: 10.1093/nar/gkad887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilks C.M., Hahnel S.R., Sheng Q., Long L., McGrath P.T., Andersen E.C. Quantitative benzimidazole resistance and fitness effects of parasitic nematode beta-tubulin alleles. Int. J. Parasitol. Drugs Drug Resist. 2020;14:28–36. doi: 10.1016/j.ijpddr.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilks C.M., Koury E.J., Buchanan C.M., Andersen E.C. Newly identified parasitic nematode beta-tubulin alleles confer resistance to benzimidazoles. Int. J. Parasitol. Drugs Drug Resist. 2021;17:168–175. doi: 10.1016/j.ijpddr.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll M., Dean E., Reilly E., Bergholz E., Chalfie M. Genetic and molecular analysis of a Caenorhabditis elegans beta-tubulin that conveys benzimidazole sensitivity. J. Cell Biol. 1989;109:2993–3003. doi: 10.1083/jcb.109.6.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox B.W., Ponomarova O., Lee Y.-U., Zhang G., Giese G.E., Walker M., Roberto N.M., Na H., Rodrigues P.R., Curtis B.J., Kolodziej A.R., Crombie T.A., Zdraljevic S., Yilmaz L.S., Andersen E.C., Schroeder F.C., Walhout A.J.M. C. elegans as a model for inter-individual variation in metabolism. Nature. 2022;607:571–577. doi: 10.1038/s41586-022-04951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S.B., Ness-Cohn E., Andersen E.C. Benzimidazoles cause lethality by inhibiting the function of Caenorhabditis elegans neuronal beta-tubulin. Int. J. Parasitol. Drugs Drug Resist. 2022;20:89–96. doi: 10.1016/j.ijpddr.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnel S.R., Zdraljevic S., Rodriguez B.C., Zhao Y., McGrath P.T., Andersen E.C. Extreme allelic heterogeneity at a Caenorhabditis elegans beta-tubulin locus explains natural resistance to benzimidazoles. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie A.C., Georgopoulos S.G. Mutational resistance to fungitoxic benzimidazole derivatives in Aspergillus nidulans. J. Gen. Microbiol. 1971;67:371–373. doi: 10.1099/00221287-67-3-371. [DOI] [PubMed] [Google Scholar]
- Howell S.B., Burke J.M., Miller J.E., Terrill T.H., Valencia E., Williams M.J., Williamson L.H., Zajac A.M., Kaplan R.M. Prevalence of anthelmintic resistance on sheep and goat farms in the southeastern United States. J. Am. Vet. Med. Assoc. 2008;233:1913–1919. doi: 10.2460/javma.233.12.1913. [DOI] [PubMed] [Google Scholar]
- Hurd D.D. WormBook; 2018. Tubulins in C. elegans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kitchen S., Ratnappan R., Han S., Leasure C., Grill E., Iqbal Z., Granger O., O'Halloran D.M., Hawdon J.M. Isolation and characterization of a naturally occurring multidrug-resistant strain of the canine hookworm, Ancylostoma caninum. Int. J. Parasitol. 2019;49:397–406. doi: 10.1016/j.ijpara.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krücken J., Fraundorfer K., Mugisha J.C., Ramünke S., Sifft K.C., Geus D., Habarugira F., Ndoli J., Sendegeya A., Mukampunga C., Bayingana C., Aebischer T., Demeler J., Gahutu J.B., Mockenhaupt F.P., von Samson-Himmelstjerna G. Reduced efficacy of albendazole against Ascaris lumbricoides in Rwandan schoolchildren. Int. J. Parasitol. Drugs Drug Resist. 2017;7:262–271. doi: 10.1016/j.ijpddr.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwa M.S., Kooyman F.N., Boersema J.H., Roos M.H. Effect of selection for benzimidazole resistance in Haemonchus contortus on beta-tubulin isotype 1 and isotype 2 genes. Biochem. Biophys. Res. Commun. 1993;191:413–419. doi: 10.1006/bbrc.1993.1233. [DOI] [PubMed] [Google Scholar]
- Kwa M.S., Veenstra J.G., Roos M.H. Benzimidazole resistance in Haemonchus contortus is correlated with a conserved mutation at amino acid 200 in beta-tubulin isotype 1. Mol. Biochem. Parasitol. 1994;63:299–303. doi: 10.1016/0166-6851(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Kwa M.S., Veenstra J.G., Van Dijk M., Roos M.H. Beta-tubulin genes from the parasitic nematode Haemonchus contortus modulate drug resistance in Caenorhabditis elegans. J. Mol. Biol. 1995;246:500–510. doi: 10.1006/jmbi.1994.0102. [DOI] [PubMed] [Google Scholar]
- Mohammedsalih K.M., Krücken J., Khalafalla A., Bashar A., Juma F.-R., Abakar A., Abdalmalaik A.A.H., Coles G., von Samson-Himmelstjerna G. New codon 198 β-tubulin polymorphisms in highly benzimidazole resistant Haemonchus contortus from goats in three different states in Sudan. Parasit. Vectors. 2020;13:114. doi: 10.1186/s13071-020-3978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyaanga J., Crombie T.A., Widmayer S.J., Andersen E.C. easyXpress: an R package to analyze and visualize high-throughput C. elegans microscopy data generated using CellProfiler. PLoS One. 2021;16 doi: 10.1371/journal.pone.0252000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotto L.M., Dilks C.M., Park Y.J., Smit R.B., Lu B.T., Gopalakrishnan C., Gilleard J.S., Andersen E.C., Mains P.E. Interactions of Caenorhabditis elegans β-tubulins with the microtubule inhibitor and anthelmintic drug albendazole. Genetics. 2022;221(4):iyac093. doi: 10.1093/genetics/iyac093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard R.K. Anthelmintics and control. Vet. Parasitol. 1988;27:97–109. doi: 10.1016/0304-4017(88)90066-0. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2020. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Salikin N.H., Nappi J., Majzoub M.E., Egan S. Combating parasitic nematode infections, newly discovered antinematode compounds from marine epiphytic bacteria. Microorganisms. 2020;8 doi: 10.3390/microorganisms8121963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders G.I., Wasmuth J.D., Beech R., Laing R., Hunt M., Naghra H., Cotton J.A., Berriman M., Britton C., Gilleard J.S. Characterization and comparative analysis of the complete Haemonchus contortus beta-tubulin gene family and implications for benzimidazole resistance in strongylid nematodes. Int. J. Parasitol. 2013;43:465–475. doi: 10.1016/j.ijpara.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Shaver A.O., Miller I.R., Schaye E.S., Moya N.D., Collins J.B., Wit J., Blanco A.H., Shao F.M., Andersen E.J., Khan S.A., Paredes G., Andersen E.C. Quantifying the fitness effects of resistance alleles with and without anthelmintic selection pressure using Caenorhabditis elegans. PLoS Pathog. 2024;20(5) doi: 10.1371/journal.ppat.1012245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaver A.O., Wit J., Dilks C.M., Crombie T.A., Li H., Aroian R.V., Andersen E.C. Variation in anthelmintic responses are driven by genetic differences among diverse C. elegans wild strains. PLoS Pathog. 2023;19 doi: 10.1371/journal.ppat.1011285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheir-Neiss G., Lai M.H., Morris N.R. Identification of a gene for beta-tubulin in Aspergillus nidulans. Cell. 1978;15:639–647. doi: 10.1016/0092-8674(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Silvestre A., Cabaret J., Humbert J.F. Effect of benzimidazole under-dosing on the resistant allele frequency in Teladorsagia circumcincta (Nematoda) Parasitology. 2001;123:103–111. doi: 10.1017/s0031182001008009. [DOI] [PubMed] [Google Scholar]
- Venkatesan A., Jimenez Castro P.D., Morosetti A., Horvath H., Chen R., Redman E., Dunn K., Collins J.B., Fraser J.S., Andersen E.C., Kaplan R.M., Gilleard J.S. Molecular evidence of widespread benzimidazole drug resistance in Ancylostoma caninum from domestic dogs throughout the USA and discovery of a novel β-tubulin benzimidazole resistance mutation. PLoS Pathog. 2023;19 doi: 10.1371/journal.ppat.1011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmayer S.J., Crombie T.A., Nyaanga J.N., Evans K.S., Andersen E.C. C. elegans toxicant responses vary among genetically diverse individuals. Toxicology. 2022;479 doi: 10.1016/j.tox.2022.153292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wit J., Dilks C.M., Andersen E.C. Complementary approaches with free-living and parasitic nematodes to understanding anthelmintic resistance. Trends Parasitol. 2021;37:240–250. doi: 10.1016/j.pt.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian M., Cook D.E., Zdraljevic S., Brady S.C., Lee D., Lee J., Andersen E.C. Discovery of genomic intervals that underlie nematode responses to benzimidazoles. PLoS Neglected Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Roberto N.M., Lee D., Hahnel S.R., Andersen E.C. The impact of species-wide gene expression variation on Caenorhabditis elegans complex traits. Nat. Commun. 2022;13:3462. doi: 10.1038/s41467-022-31208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All code and data are available at https://github.com/AndersenLab/2024_beta_tubulin_manuscript.