Abstract
Purpose of review
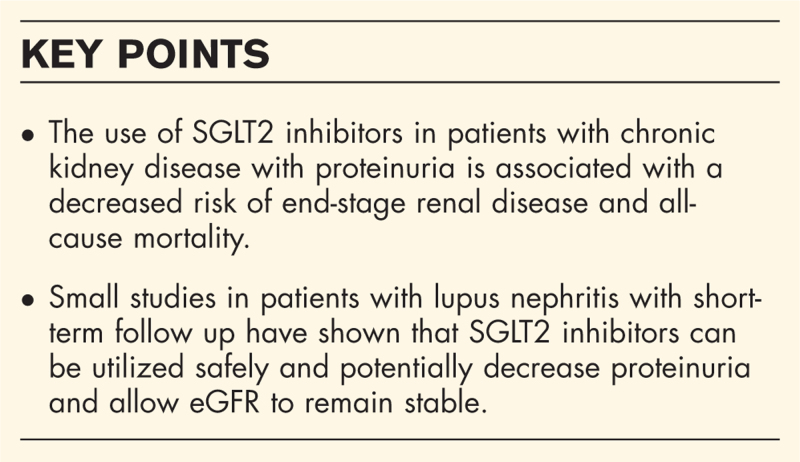
Sodium glucose cotransporter 2 (SGLT2) inhibitors are a class of medications initially developed for the treatment of diabetes, although their cardiac and renal protective benefits are far reaching. There has been marked interest in the rheumatology community to adopt these medications into our clinical practice, particularly for chronic kidney disease with persistent proteinuria.
Recent findings
SGLT2 inhibitors have been approved for patients with type 2 diabetes mellitus, heart failure with reduced or preserved ejection fraction, atherosclerotic cardiovascular disease in the setting of type 2 diabetes mellitus, as well as chronic kidney disease with proteinuria. Large studies on SGLT2 inhibitors have largely excluded patients with proteinuric chronic kidney disease due to autoimmune glomerulonephritis due to concerns for confounding from immunosuppression. The Dapagliflozin and Prevention of Adverse Outcomes in CKD Trial (DAPA-CKD) showed that SGLT2 inhibition decreased progression of renal disease in patients with IgA nephropathy. Expanding this to other autoimmune glomerulonephropathies, several small studies have shown improvements in proteinuria in patients with lupus nephritis treated with SGLT2 inhibitors. A study evaluating safety of SGLT2 inhibitors in patients with lupus identified no specific concerns even with concomitant use of immunosuppression.
Summary
Small studies have shown that SGLT2 inhibitors can been utilized safely and efficaciously in patients with lupus nephritis. Additional research is needed to identify where these medications fit into the rheumatology treatment armamentarium.
Keywords: chronic kidney disease, lupus nephritis, sodium glucose cotransporter 2 inhibitors
INTRODUCTION
Sodium glucose cotransporter 2 (SGLT2) inhibitors are a class of oral medications initially developed for treatment of diabetes. Their cardiac and renal benefits have been found to far surpass their minimal glucose lowering effects and these medications have been subsequently approved for multiple chronic medical conditions including chronic kidney disease, atherosclerotic heart disease and heart failure. These medications are now employed across multiple specialties in medicine.
Patients with autoimmune diseases, a population known to be at high risk for cardiovascular and renal-related morbidity and mortality, could also benefit from SGLT2 inhibitors. Interest in these medications within the rheumatology community has grown in recent years. Data in IgA nephropathy showed the benefits of SGLT2 inhibitors in decreasing albuminuria and preventing progression of chronic kidney disease [1▪]. Since then, interest in applying these medications to other glomerulopathies, particularly the prototypic glomerulopathy lupus nephritis, has grown. To date, small-scale studies have shown promising safety and efficacy in patients with lupus and lupus nephritis. This review seeks to highlight the useful literature for the rheumatologist, including small scales studies with these medications and future directions for the field.
Box 1.
no caption available
SGLT2 INHIBITOR DRUG MECHANISM
SGLT2 inhibitors block the action of sodium glucose cotransporter 2 (SGLT2), which is located in the early proximal tubule of the kidney. It is responsible for reabsorption of approximately 90% of glucose filtered by the kidney. Sodium glucose cotransporter 1 (SGLT1) is located later in the proximal tubule and reabsorbs most of the remaining glucose [2] but is also present in small intestine, heart and skeletal muscle making it a less ideal drug target [3].
When hyperglycemia occurs, increasing amounts of glucose are reabsorbed in the proximal tubule leading to sustained hyperglycemia in the body, which can be maladaptive in the setting of chronic hyperglycemia of diabetes. Prolonged hyperglycemia is also associated with upregulation of renal SGLT2 protein expression in rodent models, which can further perpetuate hyperglycemia, worsening long-term effects [2].
As glucose levels in the body escalate, SGLT2 works to reabsorb increasing amounts of glucose which is followed by sodium, chloride, and fluid. This decreases the levels of sodium and chloride delivered to the macula densa further through the nephron. Tubuloglomerular feedback interprets the lower concentrations of sodium and chloride as lower effective circulating volume, increases dilation of the afferent arteriole, and upregulates glomerular filtration at the level of the nephron [2,4]. It is hypothesized that this leads to hyperfiltration in the diabetic kidney, propagating long term injury [4].
SGLT2 inhibitors’ blockage of the actions of SGLT2 leads to an osmotic diuresis [3]. In addition to increased levels of glucose remaining in the urine, there is an increased delivery of sodium, chloride, and water to the macula densa with SGLT2 blockade. The kidney interprets this as increased glomerular perfusion which leads to afferent arteriolar vasoconstriction and decreases intraglomerular pressure [5] leading to less stress on the system. The decreased intraglomerular pressure also leads to a decrease in albumin lost in the urine, which is important as decreased albuminuria is associated with reduction in risk of end-stage renal disease [5]. Hypoglycemia is not typically seen with isolated use of SGLT2 inhibition because of the compensatory response of SGLT1 further downstream in the proximal tubule [6]. The glucose lower effects of SGLT2 inhibition are higher in the setting of hyperglycemia and decrease as serum glucose levels fall [6].
SGLT2 inhibitors are known to initially decrease glomerular filtration rates in the first 2–4 weeks of therapy. While this phenomenon is well documented, the mechanisms hypothesized have varied and are potentially different in patients depending on underlying physiology [6,7▪,8▪].
It has also been hypothesized that SGLT2 inhibitors may modulate pro-inflammatory cellular processes and decrease markers of oxidative stress [8▪], although mechanisms remain largely unclear.
CURRENTLY APPROVED INDICATIONS OF SGLT2 INHIBITORS
The currently approved indications for SGLT2 inhibitors, as illustrated by a review of the key articles below, include
-
(1)
Type 2 diabetes mellitus
-
(2)
Chronic kidney disease with proteinuria with or without type 2 diabetes mellitus
-
(3)
Heart failure with reduced or preserved ejection fraction
-
(4)
Atherosclerotic cardiovascular disease in the setting of type 2 diabetes mellitus
Type 2 diabetes
SGLT-2 inhibitors were initially approved for use in type 2 diabetes mellitus, as their glucosuric effects improve glycemic control, however their effects on hemoglobin A1c were found to be relatively weak [9] with a decrease by only 0.5–1% [10]. SGLT2 inhibitors are approved as second-line or third-line therapy for type 2 diabetes depending on prior medications utilized and other medication contraindications. For patients with diabetes and associated kidney disease, studies have shown SGLT2 inhibitors to decrease risks of dialysis, transplantation, and death due to chronic kidney disease in patients previously maximized on renin-angiotensin-aldosterone inhibition (RAAS) [11].
Chronic kidney disease with proteinuria with or without diabetes mellitus
SGLT2 inhibitors are approved for patients with proteinuric chronic kidney disease. The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial enrolled 4304 individuals with estimated glomerular filtration rate (eGFR) 25–75 ml/min/1.73 m2 and urinary albumin-to-creatinine 200–5000 mg/g (median 950 mg/g). One-third of enrolled patients did not have diabetes. Patients were assigned dapagliflozin 10 mg daily vs. placebo. At follow up of 2.4 years, patients in the dapagliflozin group had statistically significant decreased end stage kidney disease (5.1 vs. 7.5%) and all-cause mortality (4.7 vs. 6.8%). The risk of 50% or greater decline in eGFR decreased from 9.3 to 5.2% [12].
The Study of Heart and Kidney Protection with Empagliflozin (EMPA-KIDNEY) trial enrolled 6609 patients with either eGFR 20–44 ml/min/1.73 m2 or eGFR 45–89 ml/min/1.73 m2 and urinary albumin-to-creatinine ratio greater than 200 mg/g [13▪]. Notably, less than 50% of enrolled patients had diabetes. Patient were treated with either empagliflozin 10 mg daily or placebo. At the end of 2 years, the treatment group demonstrated a reduced incidence of end-stage renal disease (3.3 vs. 4.8%), sustained decline in eGFR to less than 10 ml/min/1.73 m2 (3.5 vs. 5.1%), and sustained decrease in eGFR of 40% or more (10.9 vs. 14.3%). The risks of all-cause mortality (4.5 vs. 5.1%) and nonfatal cardiovascular events (4.3 vs. 4.6%) were similar between the groups [13▪]. The largest benefits were noted in patients with the highest urinary albumin-to-creatinine ratios [13▪].
Heart failure with reduced or preserved ejection fraction
Multiple clinical trials also identified the benefits of SGLT2 inhibitors in patients with heart failure including the EMPEROR-reduced trial and DAPA-HF trial [14,15]. In patients with heart failure with reduced ejection fraction, SGLT2 inhibitors reduce hospitalizations, prevent worsening New York Heart Association class and decrease all-cause mortality [14–16]. This is true in both patients with and without diabetes, again arguing this is independent of the glycemic effects of these medications [14–16].
The CHIEF-HF trial included patients with preserved ejection fraction in addition to those with reduced ejection fraction and either type 2 diabetes or no diabetes. An analysis of 417 patients identified higher quality of life scores and lower symptom burden in patients treated with canagliflozin regardless of ejection fraction or presence or absence of diabetes [17▪]. The EMPEROR-preserved trial reported patients treated with empagliflozin had lower risk of heart failure hospitalizations vs. placebo [18].
Atherosclerotic cardiovascular disease in the setting of diabetes
Studies evaluating patients with diabetes with high cardiovascular risk have identified benefits with the use of SGLT2 inhibitors including lower rates of death from cardiovascular causes, nonfatal myocardial infarction and nonfatal stroke [19,20].
There are additional noted benefits of these medications outside of the typically approved indications. SLGT2 inhibitors have consistently been found to decrease blood pressure with decreases in systolic values by 3–5 mmHg and diastolic values by 2–3 mmHg [2,7▪,8▪]. In patients with diabetes, SGLT2 inhibitors have been associated with weight loss, typically around 2–3 kg over 6–12 months [7▪,21]. There are also data to suggest that SGLT2 inhibitors can decrease rates of atrial fibrillation and atrial flutter in high-risk patients with type 2 diabetes mellitus [22].
DATA TO SUPPORT USE OF SGLT2 INHIBITORS IN AUTOIMMUNE GLOMERULONEPHRITIS
The treatment of proteinuric renal disease is particularly applicable for the rheumatologist. This review seeks to summarize new data suggesting that SGLT2 inhibitors additionally have a role in the treatment of renal disease related to underlying autoimmune conditions.
Large-scale studies have repeatedly identified the renoprotective benefits of SGLT2 inhibitors when added to renin-angiotensin-aldosterone (RAAS) blockade, the current standard of care, in chronic kidney disease with proteinuria. Unfortunately, patients with autoimmune glomerulonephritis often have persistent residual proteinuria despite maximization of RAAS blockade, optimization of blood pressure and avoidance of other nephrotoxins.
Data from the Dapagliflozin and Prevention of Adverse Outcomes in CKD Trial (DAPA-CKD) included 270 patients with IgA nephropathy. The uses of dapagliflozin in these patients was associated with decreased progression of kidney disease and decreased albuminuria [1▪]. This is a particularly hopeful finding, as treatments for IgA nephropathy have been historically lacking. Rheumatologists are often called upon to manage immunosuppressive agents, although the data to guide use of these medications in IgA nephropathy remain minimal.
Interest in the use of SGLT2 inhibitors has since expanded to other glomerulonephropathies, particularly lupus nephritis, a glomerulonephropathy in which immunosuppression has a clearly defined role. A handful of small studies have identified both the safety and efficacy of SLGT2 inhibitor use in lupus nephritis and are summarized in Table 1.
Table 1.
Studies of SGLT2 inhibitors in patients with lupus and lupus nephritis
| Study | Patient population | SGLT2 inhibitor | Follow up | Conclusions |
| Wang et al. RMD Open 2022 [23▪▪] | 38 patients with lupus Included 17 patients with active lupus nephritis defined as proteinuria >0.5 g/24 h at entry |
Dapagliflozin 10 mg daily | 6 months | 12/38 patients (31.58%) had adverse events attributed to dapagliflozin, although only two adverse events were severe. No significant change in SLEDAI scores. Proteinuria in patients with lupus nephritis remained unchanged. GFR remained stable. Mean prednisone dose was decreased by approximately 30%. |
| Morales and Galindo, Ann Rheum Dis 2022 [24] | Five patients with biopsy-proven lupus nephritis | Empagliflozin 10 mg daily | 2 months | Mean proteinuria decreased from 2.2 g/day to 1.2 g/day No significant changes in GFR. |
| Zhao et al., Ann Rheum Dis 2023 [25▪▪] | Nine patients with biopsy-proven lupus nephritis | Various SGLT2 inhibitors including dapagliflozin 10 mg daily, canagliflozin 100 mg daily, ertugliflozin 5 mg daily | 2 months | Proteinuria decreased by 29.6–96.3% GFR was stable over treatment period. |
A study by Wang et al.[23▪▪] showed that the addition of dapagliflozin to standard therapy in patients with systemic lupus erythematosus (SLE) was well tolerated in a phase I/II trial. This study enrolled 38 patients with SLE, 17 (44.7%) of whom had active lupus nephritis which was defined as proteinuria more than 0.5 g/24 h. Dapagliflozin 10 mg was added to standard of care therapy and patients were monitored for the following 6 months. Eight patients (21%) experienced adverse events leading to drug discontinuation although only half of these events were attributed to the dapagliflozin. One major lupus flare was recorded. Only one lower urinary tract infection was observed in the study (2.63% of patients). Secondary end points of this study included SLE disease activity index, which decreased over the course of the study (SLEDAI 4.24→3.97) but was not found to be statistically significant. From baseline to 6-month follow up, the mean daily prednisone dose decreased by 30% (14.21 to 10.67 mg). The median 24-h urine protein remained unchanged in the patients with active lupus nephritis throughout the course of the study. It should be noted that the baseline SLEDAI score (mean 4.24) suggested that patients enrolled in this study had relatively low SLE-related disease activity upon entry [23▪▪].
In a pilot study of five patients with biopsy-proven lupus nephritis, the addition of empagliflozin 10 mg/day to their ongoing immunosuppressive therapy led to improvements in proteinuria (mean baseline 2.2 g/day with 8 week follow up mean 1.2 g/day). The authors did not note a significant change in glomerular filtration rates among these patients [24].
A study by Zhao et al.[25▪▪] also included a review of nine patients with biopsy-proven lupus nephritis who were treated with SGLT2 inhibitors for 2 months. Follow-up evaluation showed a decrease in proteinuria among all nine patients ranging from 29.6 to 96.3% with stable glomerular filtration rate over treatment period [25▪▪].
There has also been a push to use SGLT2 inhibitors in other proteinuric renal diseases such as ANCA-associated vasculitis [26▪], although to our knowledge, this has not been formally studied to date.
SGLT2 INHIBITORS AND INFLAMMATORY MODELS
SGLT2 inhibitors are also thought to have a role in mitigating inflammation, explaining in part why they may be additionally helpful in disease processes such as atherosclerosis and heart failure. Inflammation is part of the body's immune response to dealing with stress, but prolonged inflammation can lead to chronic damage and organ dysfunction.
Data suggest that SGLT2 inhibitors decrease inflammation through multiple mechanisms. In animal models, SGLT2 inhibitors have been shown to decrease polarization of macrophages and decrease infiltration into affected tissues [27▪] as well as decrease NLRP3 inflammasome activation [25▪▪,27▪]. SGLT2 inhibition has been reported to reduce oxidative stress and adipose tissue-mediated inflammation [16]. In in-vitro models, SGLT2 inhibitors have been shown to suppress secretion of pro-inflammatory cytokines from endothelial cells [28].
SGLT2 inhibitors may also have additional benefits in autoimmune diseases via altering T cell mediated processes [29,30]. It was recently reported that canagliflozin inhibits T cell activation as well as T cell proliferation via downregulation of mammalian target of rapamycin complex 1 (mTORC1). Via multiple intracellular mechanisms canagliflozin inhibits the secretion of multiple pro-inflammatory cytokines including interleukin-17 (IL-17) and interferon gamma (IFNγ). Importantly, these findings have not been confirmed with the use of dapagliflozin [29].
Of particular interest to rheumatologists, a recent study in Annals of Rheumatic Diseases reported that SGLT2 inhibitors decreased podocyte damage in experimental models of lupus nephritis. Mouse models of lupus nephritis (MRL/lpr mice) were treated with empagliflozin with significant improvements in creatinine levels as well as proteinuria. Histologic evaluation showed that empagliflozin treated mice had less foot process effacement, which the authors posit was due to less inflammasome activation and increased autophagy [25▪▪].
ADVERSE EVENTS
Despite the numerous noted benefits of SGLT2 inhibitors, there are several potential adverse events of which clinicians should be cognizant. Identification of any high-risk baseline features in a patient will allow a clinician to guide therapy accordingly. Side effects and high-risk populations are summarized in Table 2.
Table 2.
Potential side effects of SGLT2 inhibitors
| Side effect | High-risk population |
| Genitourinary infections, including urinary tract infection, candida infections and rare cases of Fournier's gangrene | Patients with known risk factors for genitourinary infections including concomitant immunosuppression |
| Diabetic ketoacidosis | Any history of diabetic ketoacidosis |
| Hypotension/volume depletion | Concomitant use of blood pressure medications and/or diuretics Population at risk for being unable to drink sufficient fluids |
| Fragility fractures | Any other risk factors for osteoporosis including high dose steroid use |
| Lower limb amputation | Avoid use in patients with severe neuropathy, peripheral vascular disease or foot ulcerations which carry higher risks for amputation |
Genitourinary tract infections
The most cited adverse events with SGLT2 inhibitors are urinary tract infections and genitourinary infections, a common issue seen in patients on chronic steroids for treatment of autoimmune diseases. By blocking glucose reabsorption in the proximal tubules, SGLT2 inhibitors increase the amount of glucose in the urinary tract, which can foster an environment for bacterial growth. A meta-analysis identified a trend toward increased UTI with SGLT2 inhibitor use [relative risk (RR) 1.07, 95% confidence interval (95% CI) 0.99–1.15] and a significant increase in the risk of genital infection (RR 3.75, 95% CI 3.00–4.67) [31▪]. The initiation of canagliflozin was associated with both increased vaginal colonization of candida species as well as increased symptomatic candida infections in women with diabetes [32]. There have also been reports of Fournier gangrene associated with SGLT2 inhibitor use [33]. Interestingly, the study by Wang et al.[23▪▪] only identified one UTI among 38 lupus patients over a 6-month period despite standard-of-care concomitant immunosuppressive agents arguing that SGLT-2 inhibitors may not add to urogenital infection risk in patients despite ongoing use of immunosuppression.
Diabetic ketoacidosis
Diabetic ketoacidosis (DKA) is a known risk with the use of SGLT2 inhibitors through the stimulation of ketogenesis as well as possibly decreased renal clearance of ketone bodies [34]. Atypically, DKA in the setting of SGLT2 inhibitor use can present with normal blood sugars [35]. As a result, any patient with symptoms of DKA such as nausea, vomiting or malaise should have SGLT2 inhibitor held until the presence of acidosis can be ruled out. These medications are typically avoided in patients with type 1 diabetes or any history of DKA; there have been successful reports of their use [4], but serious concerns remain [36]. Patients should be cautioned to skip SGLT2 inhibitor dose on days they are fasting (e.g., due to scheduled surgery or illness) [37].
Hypotension and volume depletion
With initiation of SGLT-2 inhibitors, blood pressure needs to be closely monitored. By causing osmotic diuresis [38], urine volume can increase by up to 400–500 ml [7▪,39] putting patients at risk for volume depletion [31▪] and subsequent hypotension. In some cases, diuretics and/or blood pressure medications will need to be lowered or discontinued.
Acute kidney injury
While there is an initial expected decrease in eGFR with the initiation of SGLT2 inhibitors, in general, these medications have been found to be protective against the development of acute kidney injury [31▪].
Hypoglycemia
In isolation, SGLT2 inhibitors are not thought to carry a significant risk of hypoglycemia. A meta-analysis did not show concerns for severe hypoglycemia with SGLT2 inhibitors compared to placebo [31▪]. As SGLT2 inhibitors are commonly utilized with other treatments for diabetes, however, the clinician must be cognizant of combined risks of medications. Risk is thought to be largely due to background medications [39]. This may be particularly pertinent for the use of SGLT2 inhibitors in patients with lupus, as hydroxychloroquine is also associated with a rare risk of hypoglycemia and patients should be monitored for adverse events [40,41].
Fragility fractures
A study of canagliflozin reported a higher incidence of fractures in patients on treatment [42]. This is hypothesized to be due to potential risks of orthostatic hypotension [38] as well as actual decreases in bone density [43]. A meta-analysis did identify a trend toward increased risk of fracture for SGLT2 inhibitors (RR 1.07, 95% CI 0.99–1.16) [31▪]. For the rheumatologist, this risk needs to be kept in mind, particularly in the setting of concomitant high-dose steroid use, an additional risk factor for bone loss.
Lower limb amputation
Finally, SGLT2 inhibitor use has been associated with an increased risk of lower limb amputation [20,31▪] and should be used with caution in patients with known risk factors including severe neuropathy, peripheral vascular disease or history of foot ulceration. The mechanisms for this risk remain unclear with hypotheses suggesting volume depletion leads to reduced tissue perforation predisposing to ulceration [44].
Of note for rheumatologists, there has been one case report for the development of cutaneous polyarteritis nodosa associated with the use of empagliflozin [45] and a case of anti-HMGCR positive myopathy after dapagliflozin exposure [46].
LABORATORY ABNORMALITIES WITH THE USE OF SGLT2 INHIBITORS
Clinically, SGLT-2 inhibitors are associated with an immediate decrease in eGFR around 5 ml/min/1.73 m2[7▪] over the first 2–4 weeks of treatment, but eGFR remains preserved when compared to placebo over long-term follow up of months to years [5,47,48]. Studies looking at the discontinuation of SGLT2 inhibitors have shown that eGFR rebounds to baseline value when medication is stopped even after long term use [47,48]. This detail is important for physicians, as an initial decrease in eGFR should not be caused to discontinue the SGLT2 inhibitor. This physiologic response can also make interpreting clinical responses to medications challenging, particularly if SGLT2 inhibitors are started around the same time as immunosuppression.
Additional laboratory monitoring for patients on SGLT2 inhibitors depends on their indication for use. In patients with chronic kidney disease, creatinine, eGFR and potassium should be routinely monitored. In patients with diabetes, hemoglobin A1c should be followed. Patients should be reassessed for any risk factors predisposing to known side effects of these medications throughout their treatment course.
HOW DO SGLT2 INHIBITORS DIFFER FROM ANGIOTENSIN-CONVERTING ENZYME INHIBITORS?
Angiotensin-converting enzyme (ACE) inhibitors have long been used as standard of care therapy in chronic kidney disease, hypertension and heart failure and have well defined roles in the treatment paradigm. ACE inhibitors block the conversion of angiotensin I to angiotensin II, thereby decreasing vasoconstriction of arterioles, and decreasing release of aldosterone. Normal aldosterone release causes reabsorption of sodium and chloride in the tubules followed by water. RAAS blockade leads to efferent arteriole vasodilation. By blocking aldosterone, ACE inhibitors decrease the effective circulating volume and blood pressure [49–51]. Meta-analyses have identified particular benefits in patients with proteinuric chronic kidney disease with reductions in rate of progression to end-stage renal disease [50,51]. In current practice, ACE inhibitors are standard of care for patients with proteinuric renal disease due to underlying autoimmune disease such as lupus nephritis [52].
As summarized above, the effects of SGLT2 inhibition are concentrated in the proximal tubule and downstream macula densa. SGLT2 inhibition leads the system to see a higher glomerular perfusion and as a result decreases intraglomerular pressure [5,8▪]. ACE inhibitors and SGLT2 inhibitors target separate processes integral to modulating stress on the kidney.
Mechanisms, indications and common side effects of ACE inhibitors and SGLT2 inhibitors are summarized in Table 3.
Table 3.
ACE inhibitors vs. SGLT2 inhibitors
| ACE inhibitors | SGLT2 inhibitors | |
| Approved indications | Hypertension Chronic kidney disease, most effective with proteinuria Heart failure ST-elevation myocardial infarction Patients with coronary artery disease and hypertension if diabetes mellitus, left ventricular dysfunction, or chronic kidney disease also present |
Type 2 diabetes mellitus Atherosclerotic cardiovascular disease in the setting of type 2 diabetes mellitus Chronic kidney disease with proteinuria with or without type 2 diabetes mellitus Heart failure with reduced or preserved ejection fraction |
| Mechanism of action | Inhibition of renin-angiotensin-aldosterone system (RAAS). ACE inhibitors block conversion of angiotensin I to angiotensin II promoting efferent arteriolar dilatation and decrease aldosterone secretion. |
Block sodium glucose cotransporter 2 (SGLT2) leading to decreased glucose reabsorption in the proximal tubule |
| Side effects | Dry cough Hypotension Dizziness, syncope Hyperkalemia |
Genitourinary infections including UTI and candida infections Hypotension Diabetic ketoacidosis Fragility fractures Lower limb amputations |
WHY USE SGLT2 INHIBITORS WITH ANGIOTENSIN-CONVERTING ENZYME INHIBITORS?
The combination of ACE inhibitors and SGLT2 inhibitors is hypothesized to be increasingly effective, as they target separate pathways. SGLT2 inhibition ultimately leads to afferent arteriole constriction and ACE inhibitors lead to efferent arteriole vasodilation. These combined effects ultimately decrease renal workload [8▪].
Data have also supported combination use of these medications in cardiovascular disease. A meta-analysis looking at the use of both ACE inhibitors or angiotensin II receptor blocker (ARB) plus SGLT2 inhibitors found a greater reduction in risks of major adverse cardiovascular events (MACE), cardiovascular death or hospitalization due to heart failure when compared to use of ACE inhibitor/ARB plus placebo [25▪▪].
To date, studies have focused on the use of SGLT2 inhibitors as adjunctive therapy along with standard of care ACE inhibitors. Isolated use of SGLT2 inhibitors has not been effectively evaluated to date, as greater than 97% of participants in the large renal trial DAPA-CKD were on background RAAS blockade [53]. New clinical trials are evaluating the efficacy of SGLT2 inhibition in isolation.
WHERE SHOULD SGLT-2 INHIBITORS BE USED IN THE TREATMENT PARADIGM FOR AUTOIMMUNE GLOMERULONEPHRITIS?
SGLT2 inhibitors provide a new tool for clinicians treating complex renal disease with proteinuria, yet the timing of these additional medications should be considered cautiously. In patients with new diagnosis of lupus nephritis, it is of the utmost importance to start immunosuppression in a timely manner. This should be done concomitantly with other standard of care management for proteinuria, including RAAS inhibition, strict management of blood pressure, elimination of other nephrotoxic agents and maximization of healthy practices (e.g. smoking cessation). Use of SGLT2 inhibitors should also be tailored to renal function as eGFR cut-offs and approved indications outside of diabetes vary between the multiple medications in this class.
Starting SGLT2 inhibitors at the same time as immunosuppression can lead to clinical confusion. The expectant drop in eGFR with initiation of SGLT2 inhibition may lead the clinician to erroneously believe that initial immunosuppression is not effective leading to unnecessary escalations in immunosuppressive therapy. The initiation of multiple medications, all with a myriad of side effects, can lead to confusion in teasing out any adverse events. SGLT2 inhibitors have known infectious risk, which can be compounded with concomitant immunosuppression. Finally, the clinician must be conscious of pill burden and difficulties with medication compliance with complex chronic diseases.
SGLT2 inhibitors are most appropriate for patients with mild to moderate decreases in eGFR, particularly those with high levels of proteinuria. We recommend considering use once patients have completed initial intensive immunosuppressive treatment for their glomerulonephritis and are receiving maintenance doses of immunosuppression and steroids to avoid confusion with fluctuating labs and decrease side effect risks. SGLT2 inhibitors should be started after the standard of care therapy with ACE inhibitors. Clinicians should remember that the benefits of SGLT2 inhibition are seen in the long rather than short term; while initiation of these medications is important, it should be delayed until it can be done safely with the lowest risk for side effects to ensure they can be utilized in the long term.
Hopefully upcoming studies will be able to elucidate exactly when SGLT2 inhibitors should be introduced in patients with autoimmune glomerulonephritis.
CONCLUSION
As most studies in the rheumatology community focus on the use of immunosuppression in autoimmune glomerulonephritis, the emergence of SGLT2 inhibitors has provided an exciting new treatment modality. The clear cardiorenal and renal protective effects of these medications provide a hopeful adjunctive therapy to current standard of care, particularly as chronic kidney disease and atherosclerosis are common comorbidities in our patients associated with significant morbidity and mortality.
A small body of literature has identified potential benefits and confirmed safety of the use of SGLT2 inhibitors in patients with lupus nephritis. We posit that these medications may be most effectively utilized in patients with autoimmune glomerulonephritis with mild to moderate decreases in eGFR, particularly those with high levels of proteinuria, once they are entering the maintenance phase of immunosuppressive therapy. While SGLT2 inhibitors are generally well tolerated, the clinician should keep the multiple reported side effects, particularly risk of genitourinary infections in mind. We are hopeful that use of SGLT2 inhibitors in the long term will help to mitigate cardiac and renal complications commonly seen with patients with autoimmune glomerulonephritis.
The wealth of literature on SGLT2 inhibitors has exploded in the past decade. Going forward, the literature needs to clarify exactly where these medications fit into our treatment paradigm and the full breadth of rheumatology patients for which these medications should be considered.
Acknowledgements
The authors wish to thank Dr Panduranga Rao for his assistance with the manuscript.
Financial support and sponsorship
W.J.M. was supported by the Michael and Marcia Klein Professorship and Mary Piazza lupus research fund.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Wheeler DC, Toto RD, Stefansson BV, et al. A prespecified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int 2021; 100:215–224. [DOI] [PubMed] [Google Scholar]; SGLT2 inhibitor use in 270 patients with IgA nephropathy decreased albuminuria and reduced risk of progression to chronic kidney disease while maintaining an appropriate safety profile. These data sparked interest in use of SGLT2 inhibitors in other autoimmune glomerulonephritis.
- 2.Vallon V, Verma S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol 2021; 83:503–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fonseca-Correa JI, Correa-Rotter R. Sodium-glucose cotransporter 2 inhibitors mechanisms of action: a review. Front Med (Lausanne) 2021; 8:777861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014; 129:587–597. [DOI] [PubMed] [Google Scholar]
- 5.Heerspink HJ, Desai M, Jardine M, et al. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol 2017; 28:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fattah H, Vallon V. The potential role of SGLT2 inhibitors in the treatment of Type 1 diabetes mellitus. Drugs 2018; 78:717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪.Bailey CJ, Day C, Bellary S. Renal protection with SGLT2 inhibitors: effects in acute and chronic kidney disease. Curr Diab Rep 2022; 22:39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review of the mechanisms of numerous cardiac and protective effects of SGLT2 inhibitors, which extend far beyond their glucose and blood pressure lowering abilities.
- 8▪.Lytvyn Y, Kimura K, Peter N, et al. Renal and vascular effects of combined SGLT2 and angiotensin-converting enzyme inhibition. Circulation 2022; 146:450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]; In patients with type 1 diabetes, the addition of SGLT2 inhibitors to standard of care therapy with ACE inhibitors led to expected decrease in eGFR and blood pressure. The combined effects of these medications decrease intraglomerular pressure leading to decreased long-term cardiorenal adverse effects.
- 9.Musso G, Gambino R, Cassader M, et al. A novel approach to control hyperglycemia in type 2 diabetes: sodium glucose co-transport (SGLT) inhibitors: systematic review and meta-analysis of randomized trials. Ann Med 2012; 44:375–393. [DOI] [PubMed] [Google Scholar]
- 10.Caruso I, Giorgino F. SGLT-2 inhibitors as cardio-renal protective agents. Metabolism 2022; 127:154937. [DOI] [PubMed] [Google Scholar]
- 11.Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019; 7:845–854. [DOI] [PubMed] [Google Scholar]
- 12.Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383:1436–1446. [DOI] [PubMed] [Google Scholar]
- 13▪.Herrington WG, Staplin N, Wanner C, et al. EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023; 388:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that the largest benefits of SGLT2 empagliflozin use occurred in patients with the highest urinary albumin-to-creatinine ratios.
- 14.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 15.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 16.Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol 2020; 17:761–772. [DOI] [PubMed] [Google Scholar]
- 17▪.Spertus JA, Birmingham MC, Nassif M, et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med 2022; 28:809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]; Canagliflozin use led to higher quality of life scores and lower symptom burden in patients with heart failure (both reduced and preserved ejection fraction) with or without diabetes.
- 18.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021; 385:1451–1461. [DOI] [PubMed] [Google Scholar]
- 19.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in Type 2 diabetes. N Engl J Med 2015; 373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 20.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in Type 2 diabetes. N Engl J Med 2017; 377:644–657. [DOI] [PubMed] [Google Scholar]
- 21.Brown E, Wilding JPH, Barber TM, et al. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: mechanistic possibilities. Obes Rev 2019; 20:816–828. [DOI] [PubMed] [Google Scholar]
- 22.Zelniker TA, Bonaca MP, Furtado RHM, et al. Effect of dapagliflozin on atrial fibrillation in patients with Type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 Trial. Circulation 2020; 141:1227–1234. [DOI] [PubMed] [Google Scholar]
- 23▪▪.Wang H, Li T, Sun F, et al. Safety and efficacy of the SGLT2 inhibitor dapagliflozin in patients with systemic lupus erythematosus: a phase I/II trial. RMD Open 2022; 8:e002686. [DOI] [PMC free article] [PubMed] [Google Scholar]; This phase I/II trial confirmed the safety of dapagliflozin, even in patients on immunosuppressive medications with underlying SLE. This study demonstrated similar efficacy of decreased weight, decreased SBP, and overall eGFR stability in patients with lupus and/or lupus nephritis.
- 24.Morales E, Galindo M. SGLT2 inhibitors in lupus nephropathy, a new therapeutic strategy for nephroprotection. Ann Rheum Dis 2022; 81:1337–1338. [DOI] [PubMed] [Google Scholar]
- 25▪▪.Zhao XY, Li SS, He YX, et al. SGLT2 inhibitors alleviated podocyte damage in lupus nephritis by decreasing inflammation and enhancing autophagy. Ann Rheum Dis 2023; 82:1328–1340. [DOI] [PubMed] [Google Scholar]; Empagliflozin decreased podocyte injury seen on histology in a mouse model of lupus nephritis by decreasing inflammation. The authors also analyzed data from nine lupus nephritis patients treated with 2 months of SGLT2 inhibitors and reported significant decreases in proteinuria with stable eGFR.
- 26▪.Saemann M, Kronbichler A. Call for action in ANCA-associated vasculitis and lupus nephritis: promises and challenges of SGLT-2 inhibitors. Ann Rheum Dis 2022; 81:614–617. [DOI] [PubMed] [Google Scholar]; A review of the available literature and strong push to study the potential use of SGLT2 inhibitors in lupus nephritis and ANCA-associated vasculitis, two autoimmune diseases complicated by morbidity and mortality from chronic kidney disease.
- 27▪.Feijoo-Bandin S, Aragon-Herrera A, Otero-Santiago M, et al. Role of sodium-glucose co-transporter 2 inhibitors in the regulation of inflammatory processes in animal models. Int J Mol Sci 2022; 23:5634. [DOI] [PMC free article] [PubMed] [Google Scholar]; SGLT2 inhibitors used in animal models have identified decreased polarization of macrophages and decreased infiltration into affected tissues as well as decreased NLRP3 inflammasome activation.
- 28.Abdollahi E, Keyhanfar F, Delbandi AA, et al. Dapagliflozin exerts anti-inflammatory effects via inhibition of LPS-induced TLR-4 overexpression and NF-kappaB activation in human endothelial cells and differentiated macrophages. Eur J Pharmacol 2022; 918:174715. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins BJ, Blagih J, Ponce-Garcia FM, et al. Canagliflozin impairs T cell effector function via metabolic suppression in autoimmunity. Cell Metab 2023; 35:1132–1146. e9. [DOI] [PubMed] [Google Scholar]
- 30.Certo M, Niven J, Mauro C. Repurposing SGLT2 inhibitors for autoimmune diseases? YES, WE MAY!. Cell Chem Biol 2023; 30:1009–1011. [DOI] [PubMed] [Google Scholar]
- 31▪.Qiu M, Ding LL, Zhang M, et al. Safety of four SGLT2 inhibitors in three chronic diseases: a meta-analysis of large randomized trials of SGLT2 inhibitors. Diab Vasc Dis Res 2021; 18:14791641211011016. [DOI] [PMC free article] [PubMed] [Google Scholar]; There have been numerous safety concerns with the use of SGLT2 inhibitors. This meta-analysis confirmed the increased risk of diabetic ketoacidosis, genital infection, volume depletion, fractures and amputation as well as decreased risks of acute kidney injury.
- 32.Nyirjesy P, Zhao Y, Ways K, et al. Evaluation of vulvovaginal symptoms and Candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. Curr Med Res Opin 2012; 28:1173–1178. [DOI] [PubMed] [Google Scholar]
- 33.Bersoff-Matcha SJ, Chamberlain C, Cao C, et al. Fournier Gangrene associated with sodium-glucose cotransporter-2 inhibitors: a review of spontaneous postmarketing cases. Ann Intern Med 2019; 170:764–769. [DOI] [PubMed] [Google Scholar]
- 34.Burke KR, Schumacher CA, Harpe SE. SGLT2 inhibitors: a systematic review of diabetic ketoacidosis and related risk factors in the primary literature. Pharmacotherapy 2017; 37:187–194. [DOI] [PubMed] [Google Scholar]
- 35.Goldenberg RM, Berard LD, Cheng AYY, et al. SGLT2 inhibitor-associated diabetic ketoacidosis: clinical review and recommendations for prevention and diagnosis. Clin Ther 2016; 38:2654–2664. e1. [DOI] [PubMed] [Google Scholar]
- 36.Taylor SI, Blau JE, Rother KI, et al. SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: balancing benefits and risks. Lancet Diabetes Endocrinol 2019; 7:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McQuarrie EP, Gillis KA, Mark PB. Seven suggestions for successful SGLT2i use in glomerular disease - a standalone CKD therapy? Curr Opin Nephrol Hypertens 2022; 31:272–277. [DOI] [PubMed] [Google Scholar]
- 38.Weir MR, Januszewicz A, Gilbert RE, et al. Effect of canagliflozin on blood pressure and adverse events related to osmotic diuresis and reduced intravascular volume in patients with type 2 diabetes mellitus. J Clin Hypertens (Greenwich) 2014; 16:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollack R, Cahn A. SGLT2 inhibitors and safety in older patients. Heart Fail Clin 2022; 18:635–643. [DOI] [PubMed] [Google Scholar]
- 40.Kang L, Mikuls TR, O’Dell JR. Hydroxychloroquine: a diabetic drug in disguise? BMJ Case Rep 2009; 2009:bcr0820080654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unubol M, Ayhan M, Guney E. Hypoglycemia induced by hydroxychloroquine in a patient treated for rheumatoid arthritis. J Clin Rheumatol 2011; 17:46–47. [DOI] [PubMed] [Google Scholar]
- 42.Alba M, Xie J, Fung A, et al. The effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on mineral metabolism and bone in patients with type 2 diabetes mellitus. Curr Med Res Opin 2016; 32:1375–1385. [DOI] [PubMed] [Google Scholar]
- 43.Bilezikian JP, Watts NB, Usiskin K, et al. Evaluation of bone mineral density and bone biomarkers in patients with Type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab 2016; 101:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khouri C, Cracowski JL, Roustit M. SGLT-2 inhibitors and the risk of lower-limb amputation: is this a class effect? Diabetes Obes Metab 2018; 20:1531–1534. [DOI] [PubMed] [Google Scholar]
- 45.To D, Bradshaw S, Lipson J. Case report of empagliflozin-induced cutaneous polyarteritis nodosa. J Cutan Med Surg 2018; 22:516–518. [DOI] [PubMed] [Google Scholar]
- 46.Stella M, Biassoni E, Fiorillo C, et al. A case of anti-HMGCR myopathy triggered by sodium/glucose co-transporter 2 (SGLT2) inhibitors. Neurol Sci 2022; 43:4567–4570. [DOI] [PubMed] [Google Scholar]
- 47.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in Type 2 diabetes. N Engl J Med 2016; 375:323–334. [DOI] [PubMed] [Google Scholar]
- 48.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in Type 2 diabetes and nephropathy. N Engl J Med 2019; 380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 49.Goyal A, Cusick AS, Thielemier B. ACE inhibitors. StatPearls [Internet]. Treasure Island FL: StatPearls Publishing; 2024. [PubMed] [Google Scholar]
- 50.Jafar TSC, Landa M. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. Ann Intern Med 2001; 135:73–87. [DOI] [PubMed] [Google Scholar]
- 51.Giatras I LJ, Levey AS for the Angiotensin-Converting Enzyme Inhibition and Progressive Renal Disease Study Group. Effect of angiotensin-converting enzyme inhibitors on the progression of nondiabetic renal disease: a meta-analysis of randomized trials. Ann Intern Med 1997; 127:337-345. [DOI] [PubMed] [Google Scholar]
- 52.Hahn BH, McMahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012; 64:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wheeler DC, Stefansson BV, Batiushin M, et al. The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: baseline characteristics. Nephrol Dial Transplant 2020; 35:1700–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]