Abstract
Breast cancer with brain metastasis accounts for the second largest number of brain metastases among solid malignancies. Despite advances in HER2-targeted therapy, 50% of patients with human epidermal growth factor receptor 2-positive (HER2+) breast cancer develop brain metastases and are associated with poor outcomes. In this article, we report the case of a patient with HER2+ metastatic breast cancer who developed brain metastases, despite experiencing a durable effect on extracranial metastases after treatment with trastuzumab and pertuzumab. The patient exhibited intracranial progression while receiving treatment with trastuzumab deruxtecan monotherapy after secondary brain radiotherapy and multiple lines of therapy with anti-HER2 agents, such as pyrotinib, lapatinib, tucatinib, and ado-trastuzumab emtansine. However, the administration of anlotinib (an antiangiogenesis medication) and trastuzumab deruxtecan resulted in intracranial and extracranial partial response and was linked to manageable side effects. The present case indicates that the combination of anlotinib and trastuzumab deruxtecan may be a promising treatment option for patients with HER2+ breast cancer with brain metastasis. Nevertheless, further studies are warranted to verify the present findings.
Keywords: HER2 positive, breast cancer, brain metastases, anlotinib, trastuzumab deruxtecan
Introduction
Breast cancer (BC) is the most common type of cancer in women and the second leading cause of cancer-related deaths after lung cancer.1 The progress achieved in the development of diagnostic technologies and systemic therapies, has significantly improved the outcomes of metastatic BC. However, 10–30% of patients with BC develop brain metastases (BMs), which are associated with poor prognosis and impaired cognitive and sensory functions. Patients with human epidermal growth factor receptor 2-positive (HER2+) BC have a higher incidence of BMs compared with those with HER2-negative (HER2−) BC.2
Local intervention, such as brain radiotherapy and neurosurgery, is currently the initial treatment for BMs. However, the rate of intracranial progression in patients receiving these therapies is high.3 The development of HER2-targeted therapies has prolonged the survival of patients with HER2+ breast cancer with brain metastasis (BCBM). Small-molecule tyrosine-kinase inhibitors (TKIs), including pylotinib, lapatinib, neratinib, and tucatinib, have exhibited good blood–brain barrier(BBB) penetration and efficacy in patients with BMs.4 Antibody–drug conjugates, such as ado-trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd) are also efficacious against BMs.5,6 Despite advancements in the treatment of HER2+ BCBM with both TKIs and antibody–drug conjugates, the prognosis for these patients remains unfavorable.
In this article, we report the case of a patient with BCBM who experienced intracranial progression while receiving monotherapy with T-DXd after secondary brain radiotherapy and multiple lines of treatment with anti-HER2 agents, such as pyrotinib, lapatinib, tucatinib, and T-DM1. Subsequently, the patient received anlotinib plus T-DXd and achieved partial response. The treatment resulted in > 13-month progression-free survival (PFS).
Case Presentation
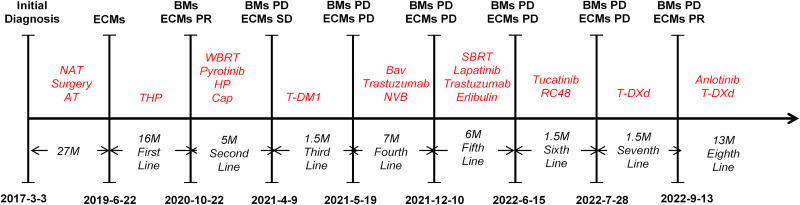
A 37-year-old primigravid woman at 25 weeks of gestation presented with a self-detected left-sided breast mass in the upper inner quadrant. Ultrasound-guided core biopsies of the breast mass showed grade 3 invasive ductal carcinoma of the left breast, and immunohistochemistry staining revealed positivity for estrogen receptor (ER;50%+), progesterone receptor (PR;50%+), HER2(3+), and Ki-67(30%+). The patient was diagnosed with left-side BC during pregnancy (cT3N2Mx luminal B2 subtype), and received four cycles of treatment with epirubicin and cyclophosphamide from March 6, 2017 to May 9, 2017. She underwent cesarean section surgery at 37 weeks of gestation. Subsequently, she received four cycles of therapy with docetaxel and trastuzumab. On September 12, 2017, she underwent a modified radical mastectomy, followed by postoperative adjuvant radiotherapy, targeted therapy with trastuzumab for 1 year, and endocrine therapy with goserelin and letrozole. Unfortunately, on June 22, 2019, chest and abdominal enhanced computed tomography(CT) revealed the presence of multiple extracranial metastases in the vertebral bones, lungs, live, and bilateral supraclavicular mediastinum. Bronchial brushing under bronchoscopy confirmed metastatic BC. She received 21 cycles of therapy with albumin-bound paclitaxel, trastuzumab, and pertuzumab every 3 weeks, with routine follow-up using CT.
Due to complaints of dizziness and an unsteady gait, a brain contrast-enhanced magnetic resonance imaging (MRI) was conducted on October 22, 2020. The results revealed the presence of multiple BMs in the frontal and parietal lobes, as well as the left temporal lobe. We administered whole brain radiotherapy (30GY/10F) along with combination therapy with pyrotinib, trastuzumab, pertuzumab, and capecitabine. On April 9, 2021, brain MRI and chest CT images revealed progressive disease (PD) of BMs and stable disease of extracranial metastases, she received two cycles of treatment with T-DM1. The patient experienced an epileptic episode on May 19, 2021. Hence, she underwent brain-enhanced MRI which further indicated intracranial PD. In response to this observation, she was treated with oral navelbine chemotherapy, bevacizumab, trastuzumab and anti-epilepsy therapy.
On December 10, 2021 examinations using MRI and CT revealed disease progression within BMs and lung metastases. Therefore, the patient received stereotactic body radiotherapy, as well as systemic therapy with lapatinib, trastuzumab, and eribulin. On June 15, 2022, administration of tucatinib and disitamab vedotin (RC48) was initiated because of intracranial and extracranial PD. On July 28, 2022, brain MRI showed enlargement of some BMs, while CT scans revealed enlargement of the former lung lesions. Thus, we recommended switching the treatment with two cycles of T-DXd.
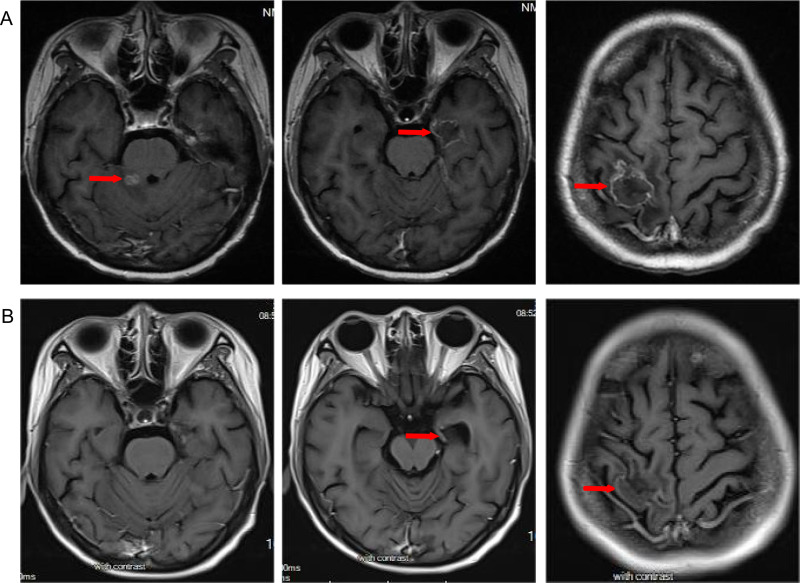
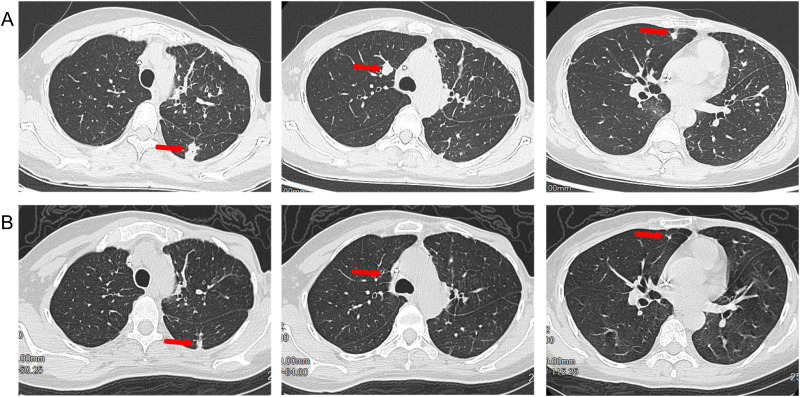
On September 13, 2022, brain MRI indicated new metastatic lesion in the left basal ganglia area and enlargement of lesions in the left frontal lobe and right parietal lobe (Figure 1A), and chest CT showed a reduction in the size of lung lesions (Figure 2A). Hence, we administered anlotinib to control the BMs. After two cycles of treatment with anlotinib and T-DXd, the patient achieved partial response of BMs (Figure 1B) and lung metastases (Figure 2B), with tolerable side effects including grade 2 thrombocytopenia and grade 2 leukopenia. The PFS of the patient exceeded 13 months. The patient continues to receive treatment with anlotinib plus T-DXd. Figure 3 outlines the medical history of this patient.
Figure 1.
Brain contrast-enhanced magnetic resonance imaging (MRI) scans show brain metastases before and after anlotinib and T-DXd therapy. (A) brain metastases before anlotinib and T-DXd therapy; (B) partial response in brain metastases after anlotinib and T-DXd treatment.
Figure 2.
Chest and abdominal enhanced computed tomography(CT) scans show lung metastases before and after anlotinib and T-DXd therapy. (A) lung metastases before anlotinib and T-DXd therapy; (B) partial response in lung metastases after anlotinib and T-DXd treatment.
Figure 3.
Timeline of the clinical course in this patient.
Abbreviations: NAT, Neoadjuvant therapy; AT, adjuvant therapy; M, months; ECMs, extracranial metastases; BMs, brain metastases; PR, partial response; PD, progress disease; SD, stable disease; THP, albumin-bound paclitaxel, trastuzumab and pertuzumab; WBRT, whole brain radiotherapy; HP, trastuzumab and pertuzumab; Cap, capecitabine; Bav, bevacizumab; NVB, navelbine; SBRT, stereotactic radiotherapy; RC48, disitamab vedotin; T-DXd, trastuzumab deruxtecan.
Discussion
BCBM accounts for the second largest number of BMs among solid malignancies. The occurrence of BMs in BC is closely linked to a grim prognosis, as it often results in neurological deterioration, thereby significantly reducing the quality of life and shortening the average survival time7. Up to 50% of all patients with HER2+ metastatic BC develop BM despite achieving control of their extracranial lesions.8 At the time of detection, >80% of patients have multiple parenchymal BMs, thus missing the opportunity for surgical treatment.9 Whole-brain radiotherapy yields symptomatic and clinical responses in BC patients with multiple BMs, though survival prognosis remains dismal.9 As a systemic treatment, HER2-targeted therapy has become an increasingly important part option for patients with HER2+ BCBM. However, traditional anti-HER2 agents such as trastuzumab and pertuzumab, are considered ineffective against BCBM because they are unable to cross the BBB.10
As small molecules, TKIs offer the advantage of effective BBB penetration, and have demonstrated efficacy in patients with HER-2+ BCBM. Lapatinib, as a small dual TKI targeting HER1 and HER2, brought CNS objective responses in 6% of patients with HER2+ BCBM.11 When combined with capecitabine, lapatinib can enhance the objective response rate (ORR) of CNS and extend the median PFS in patients with HER2+ BCBM.12,13 Tucatinib monotherapy or in combination with trastuzumab and capecitabine improved the survival of patients with HER2+ BCBM.14,15 Additionally, pyrotinib-based therapies have shown potential benefits in the treatment of patients with HER2+ BCBM.16–18 Neratinib-containing regimens have demonstrated relatively favorable efficacy in terms of prolonging PFS.19–21
Antibody-drug conjugates have shown promising therapeutic prospects in patients with HER2+ BCBM. The KAMILLA trial revealed that treatment with T-DM1 exerted beneficial effects on the central nervous system in patients with HER-2+ BCBM.5 Subgroup analysis in the DESTINY-Breast01 trial revealed that treatment with T-DXd brought intracranial ORR of 58.3%, and offered a notable advantage in patients with stable BM.22 DEBBRAH and TUXEDO-1 trials showed T-DXd also exhibited promising effectiveness in treating active HER2+ BCBM patients, with promising intracranial ORR and generally manageable side effects.8,23 The present patient had both intracranial and extracranial PD after six lines of therapy. After two cycles of treatment with T-DXd, we recorded therapeutic efficacy against lung metastases and lack of efficacy against BMs. The summary of current clinical trials using targeted agents in Her2+ BCBM was listed in Table 1.
Table 1.
Summary of Current Clinical Trials Using Targeted Agents in Her2+ BCBM
| Author & Published Time | Study | Therapy | Phase | Intracranial ORR | mPFS (Months) | mOS (Months) |
|---|---|---|---|---|---|---|
| Guy Jerusalem et al 2022 Oct22 | DESTINY-Breast01 NCT03248492 | T-DXd | II | 58.3% | 18.1 | NA |
| José Manuel Pérez-García et al 2023. Jan8 | DEBBRAH NCT04420598 | T-DXd | II | 46.2% | NA | NA |
| Rupert Bartsch et al 2022. Sep23 | TUXEDO-1 NCT04752059 | T-DXd | II | 73.3% | 14 | NA |
| F Montemurro et al 2020 Oct5 | KAMILLA NCT01702571 | T-DM1 | IIIb | 21.4% | 5.5 | 18.9 |
| Nancy U Lin et al 2020 Aug14 | HER2CLIMB NCT02614794 | Tucatinib versus Placebo+Trastuzumab + Capecitabine | III | 47.3% versus 20.0% (P=0.03) | 9.9 versus 4.2 (P<0.0001) | 18.1 versus 12.0 (P=0.005) |
| Min Yan et al 2022 Mar16 | PERMEATE NCT03691051 | Pyrotinib + capecitabine | II | 74·6% in cohort A and 42·1% in cohort B | NA | NA |
| Sara A Hurvitz et al 2021 Aug20 | NALA NCT01808573 | Neratinib + capecitabine versus lapatinib + capecitabine | III | 26.3% versus 15.4% | 7.8 versus 5.5 (P=0.074) | 16.4 versus 15.4 (p=0.635) |
| Thomas Bachelot et al 2013 Jan12 | LANDSCAPE NCT00967031 | Lapatinib + capecitabine | II | 65·9% | 5·5 | 17·0 |
| Nancy U. Lin et al 2009 Feb11 | NCT00263588 | Lapatinib | II | 6% | 2.40 | 6.37 |
| Rachel A Freedman et al 2019 May19 | TBCRC 022 NCT01494662 | Neratinib and Capecitabine | II | 49% in cohort 3A and 33% in cohort 3B | 5.5 in cohort 3A and 3.1 in cohort 3B | 13.3 in cohort 3A and 15.1 in cohort 3B |
Notes: cohort A: radiotherapy-naive HER2-positive brain metastases; cohort B: progressive disease after radiotherapy; cohort 3A: Lapatinib-naïve, cohort 3B: lapatinib-treated.
Abbreviations: ORR, objective response rate; mPFS, median progression-free survival; mOS, median overall survival; NA, not available; T-DXd, trastuzumab deruxtecan; T-DM1, ado-trastuzumab emtansine.
Therefore, it is necessary to utilize new drugs that can cross the BBB. Anlotinib (an oral TKI), targets several receptors, including vascular endothelial growth factor receptor (VEGFR), platelet-derived24 growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), MET, RET, and C-Kit. At present, it has been approved for the treatment of non-small cell lung cancer.24,25 Pharmacokinetic study reveals that Anlotinib exhibits favorable tissue distribution, with concentrations in the central nervous system (CNS) paralleling those in plasma.26 This characteristic is particularly significant for cancers with CNS metastases, such as glioblastoma and non-small cell lung cancer with brain metastases, where Anlotinib has shown promising antitumor activity.27,28 Furthermore, anlotinib has demonstrated objective efficacy with manageable toxicity in advanced/ metastatic triple-negative or HER2− BC.29,30 The combined administration of anlotinib and T-DXd resulted in significant efficacy against both intracranial and extracranial areas of the patient with HER2+ BCBM. Importantly, this treatment was associated with acceptable toxicity. This case study is limited by the absence of histopathological confirmation for the brain metastases in the patient.
Conclusion
Despite advancements in anti-HER therapy may significantly prolong the survival of patients with HER2+ BCBM, the prognosis for these patients remains unfavorable. Combination therapy with anlotinib and T-DXd is available for patients with HER2+ BCBM. However, studies are required to assess the efficacy and toxicity of this combination regimen in this setting.
Acknowledgments
We owe thanks to the patient and her family.
Ethics Statement
This research was reviewed and approved by the Ethics Committee for Research in Ganzhou People’s Hospital. The patient provided her written informed consent for participation in this study and publication of the case.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Zhang Q, He P, Tian T, et al. Real-world efficacy and safety of pyrotinib in patients with HER2-positive metastatic breast cancer: a prospective real-world study. Article. Front Pharmacol. 2023:141100556. doi: 10.3389/fphar.2023.1100556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun H, Xu J, Dai S, Ma Y, Sun T. Breast cancer brain metastasis: current evidence and future directions. Review. Cancer Med. 2023;12(2):1007–1024. doi: 10.1002/cam4.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertolini F, Spallanzani A, Fontana A, Depenni R, Luppi G. Brain metastases: an overview; Review. CNS Oncology. 2015;4(1):37–46. doi: 10.2217/cns.14.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynn CS, Tang S-C. Anti-HER2 therapy in metastatic breast cancer: many choices and future directions. Review. Cancer Metastasis Rev. 2022;41(1):193–209. doi: 10.1007/s10555-022-10021-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montemurro F, Delaloge S, Barrios CH, et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Article. Ann Oncol. 2020;31(10):1350–1358. doi: 10.1016/j.annonc.2020.06.020 [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Liu K, Wang M, Wang K, Zhu H. Trastuzumab deruxtecan versus trastuzumab emtansine for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: a cost-effectiveness analysis. Article. Breast. 2022;66:191–198. doi: 10.1016/j.breast.2022.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witzel I, Oliveira-Ferrer L, Pantel K, Mueller V, Wikman H. Breast cancer brain metastases: biology and new clinical perspectives. Review. Breast Cancer Res. 2016;188. doi: 10.1186/s13058-015-0665-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Garcia JM, Vaz Batista M, Cortez P, et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: the DEBBRAH trial. Article. Neuro-Oncology. 2023;25(1):157–166. doi: 10.1093/neuonc/noac144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Custodio-Santos T, Videira M, Brito MA. Brain metastasization of breast cancer. Review. Biochim Et Biophys Acta Rev Cancer. 2017;1868(1):132–147. doi: 10.1016/j.bbcan.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Xu H, Han Y, Wu Y, Wang J. Comparative efficacy of tyrosine kinase inhibitors and antibody-drug conjugates in HER2-positive metastatic breast cancer patients with brain metastases: a systematic review and network meta-analysis. Review. Cancers. 2022;14(14):3372. doi: 10.3390/cancers14143372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin NU, Diéras V, Paul D, et al. Multicenter Phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15(4):1452–1459. doi: 10.1158/1078-0432.Ccr-08-1080 [DOI] [PubMed] [Google Scholar]
- 12.Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group Phase 2 study. Lancet Oncol. 2013;14(1):64–71. doi: 10.1016/S1470-2045(12)70432-1 [DOI] [PubMed] [Google Scholar]
- 13.Saleem A, Searle GE, Kenny LM, et al. Lapatinib access into normal brain and brain metastases in patients with Her-2 overexpressing breast cancer (vol 5, 30, 2015). EJNMMI Res. 2017:774. doi: 10.1186/s13550-017-0323-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin NU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB Trial. Article. J Clin Oncol. 2020;38(23):2610–2619. doi: 10.1200/jco.20.00775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khatri VM, Mills MN, Oliver DE, et al. Tucatinib and stereotactic radiosurgery in the management of HER2 positive breast cancer brain metastases. Article; Early Access. J Neuro Oncol. 2023. doi: 10.1007/s11060-023-04402-7 [DOI] [PubMed] [Google Scholar]
- 16.Yan M, Ouyang QC, Sun T, et al. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): a multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol. 2022;23(3):353–361. doi: 10.1016/S1470-2045(21)00716-6 [DOI] [PubMed] [Google Scholar]
- 17.Tian W, Hao S, Wang L, Chen Y, Li Z, Luo D. Pyrotinib treatment enhances the radiosensitivity in HER2-positive brain metastatic breast cancer patients. Article. Anti-Cancer Drugs. 2022;33(1):E622–E627. doi: 10.1097/cad.0000000000001199 [DOI] [PubMed] [Google Scholar]
- 18.Ma X, Li Y, Li L, et al. Pyrotinib-based treatments in HER2-positive breast cancer patients with brain metastases. Article. Annals of Medicine. 2022;54(1):3085–3095. doi: 10.1080/07853890.2022.2139411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman RA, Gelman RS, Anders CK, et al. TBCRC 022: a Phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2019;37(13):1081–1089. doi: 10.1200/Jco.18.01511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurvitz SA, Saura C, Oliveira M, et al. Efficacy of neratinib plus capecitabine in the subgroup of patients with central nervous system involvement from the NALA trial. Oncologist. 2021;26(8):E1327–E1338. doi: 10.1002/onco.13830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nasrazadani A, Brufsky A. Neratinib: the emergence of a new player in the management of HER2+breast cancer brain metastasis. Article. Future Oncol. 2020;16(7):247–254. doi: 10.2217/fon-2019-0719 [DOI] [PubMed] [Google Scholar]
- 22.Jerusalem G, Park YH, Yamashita T, et al. CNS metastases in HER2-positive metastatic breast cancer treated with trastuzumab deruxtecan: DESTINY-Breast01 subgroup analyses. Meeting Abstract. Ann Oncol. 2020:31:S63–S64. doi: 10.1016/j.annonc.2020.03.239 [DOI] [Google Scholar]
- 23.Bartsch R, Berghoff AS, Furtner J, et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Article. Nature Med. 2022;28(9):1840–1847. doi: 10.1038/s41591-022-01935-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han B, Li K, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 Phase 3 randomized clinical trial. JAMA Oncol. 2018;4(11):1569–1575. doi: 10.1001/jamaoncol.2018.3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou M, Chen X, Zhang H, et al. China National Medical Products Administration approval summary: anlotinib for the treatment of advanced non-small cell lung cancer after two lines of chemotherapy. Review. Cancer Commun. 2019:3936. doi: 10.1186/s40880-019-0383-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong CC, Chen F, Yang JL, et al. Pharmacokinetics and disposition of anlotinib, an oral tyrosine kinase inhibitor, in experimental animal species. Acta Pharmacol Sin. 2018;39(6):1048–1063. doi: 10.1038/aps.2017.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Z, Liu J, Ma Y, et al. Anlotinib combined with cranial radiotherapy for non-small cell lung cancer patients with brain metastasis: a retrospectively, control study. Article. Cancer Manage Res. 2021;13:6101–6111. doi: 10.2147/cmar.S319650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Q, Huang K, Meng X, et al. Safety and efficacy of anlotinib hydrochloride plus temozolomide in patients with recurrent glioblastoma. Clin Cancer Res. 2023;29(19):3859–3866. doi: 10.1158/1078-0432.Ccr-23-0388 [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Zhi W, Zhang L. Efficacy of anlotinib in Chinese patients with metastatic breast cancer: a retrospective observational study. Article; Early Access. J Cell Mol Med. 2023. doi: 10.1111/jcmm.18008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao Y, Luo Z, Yu Y, et al. A real-world study of anlotinib as third-line or above therapy in patients with her-2 negative metastatic breast cancer. Article. Front Oncol. 2022:12939343. doi: 10.3389/fonc.2022.939343 [DOI] [PMC free article] [PubMed] [Google Scholar]