Abstract
Study Design.
A retrospective cohort study.
Objective.
The aim of this study was to identify an association between preoperative opioid use and reoperations rates.
Summary of Background Data.
Chronic opioid use is a public health crisis in the United States and has been linked to worse outcomes after lumbar spine surgery. However, no studies have identified an association between preoperative opioid use and reoperations rates.
Methods.
A retrospective cohort study was conducted using patients from one private insurance database who underwent primary lumbar decompression/discectomy (LDD) or posterior/transforaminal lumbar interbody fusion (PLIF/TLIF). Preoperative use of five specific opioid medications (tramadol, hydromorphone, oxycodone, hydromorphone, and extended-release oxycodone) was categorized as acute (within 3 months), subacute (acute use and use between 3 and 6 months), or chronic (subacute use and use before 6 months). Multivariate regression, controlling for multilevel surgery, age, sex, and Charlson Comorbidity Index, was used to determine the association of each medication on reoperations within 5 years.
Results.
A total of 11,551 patients undergoing LDD and 3291 patients undergoing PLIF/TLIF without previous lumbar spine surgery were identified. In the LDD group, opioid-naïve patients had a 5-year reoperation rate of 2.8%, compared with 25.0% and 8.0 with chronic preoperative use of hydromorphone and oxycodone, respectively. In multivariate analysis, any preoperative use of oxycodone was associated with increased reoperations (odds ratios [OR] = 1.4, 2.0, and 2.3, for acute, subacute, and chronic use; P < 0.01). Chronic use of hydromorphone was also associated with increased reoperations (OR = 7.5, P < 0.01). In the PLIF/TLIF group, opioid-naïve patients had a 5-year reoperation rate of 11.3%, compared with 66.7% and 16.8% with chronic preoperative use of hydromorphone and oxycodone, respectively. In multivariate analysis, any preoperative use of hydromorphone was associated with increased reoperations (OR = 2.9, 4.0, and 14.0, for acute, subacute, and chronic use; P < 0.05).
Conclusion.
Preoperative use of the higher-potency opioid medications is associated with increased reoperations after LDD and PLIF/TLIF in a dose-dependent manner. Surgeons should use this data for preoperative opioid cessation counseling and individualized risk stratification.
Keywords: chronic opioid use, hydrocodone, hydromorphone, lumbar decompression, lumbar discectomy, opioid crisis, opioids, oxycodone, posterior lumbar interbody fusion, reoperation, transforaminal lumbar interbody fusion
Excessive prescription opioid use is an epidemic in the United States with medical sales of opioids increasing by a factor of 10 over 5 years between 1997 and 2002.1 This increase in opioid sales corresponded with an almost 400% increase in unintentional opioid related deaths from 3000 to 11,000 over from 1999 to 2007.1 As a result, opioid use accounted for 55.7 billion USD in societal costs in 2007 and 78 billion USD in 2013.2,3
Patients with spine-related complaints are particularly vulnerable to opioid misuse, due to the association of back pain with chronic opioid therapy and also the painful nature of spine surgery.4 In fact, patients experiencing back pain are often prescribed opioid medications before their initial visit with the surgeon.5,6 In one large retrospective review of a managed care insurance cohort, 61% of patients received a prescription of opioids from primary care physicians within 6 months of their index presentation for back pain.5 In addition, a nationwide Danish registry study of patients purchasing tramadol found that back/spine pain was a significant risk factor for chronic use compared to other noncancer diagnoses, such as cardiac disease or severe migranes.6
Although the general morbidity and mortality of chronic opioid abuse have been well established, there are also negative opioid-related effects on outcomes following spine surgery. Preoperative opioid use before lumbar spine surgery has been strongly associated with chronic postoperative opioid use in a number of prior studies.7-17 Furthermore, recent studies now demonstrate worse post-operative patient-reported outcomes in patients with preoperative opioid use.7,17-22 However, despite the association with outcomes, no studies have demonstrated an association between preoperative opioid use and long-term rates of revision lumbar spine surgery after common degenerative lumbar spine procedures, including lumbar discectomy/decompression surgery or lumbar interbody fusion. This association, if present, could be invaluable for both preoperative opioid cessation counseling and risk stratification.
METHODS
Patient Population
A retrospective cohort study was conducted using the Pearl-Diver Patient Records Database (www.pearldiverinc.com, Fort Wayne, IN). The Humana private health insurance data set from years 2007 to 2016, which includes >20 million patients, was queried. Two separate cohorts were created. First, all adult patients who underwent lumbar discectomy or decompression (LDD) were included if they were without previous lumbar spine surgery and had at least 5 years of postoperative follow-up within the dataset. These patients were identified using Current Procedural Terminology (CPT) codes 63047 and 63030. Second, all adult patients undergoing posterior lumbar interbody fusion or transforaminal lumbar interbody fusion (PLIF/TLIF) were included if they also were without previous lumbar spine surgery and had at least 5 years of postoperative follow-up within the dataset. These patients were identified using CPT code 22630.
Experimental Variables
Preoperative opiate use was then assessed based on preoperative outpatient prescription data. The five different opiate medications of various strengths were assessed: tramadol (0.1 morphine milligram equivalents [MME]), hydrocodone (1.0 MME), oxycodone (1.5 MME), hydromorphone (4.0 MME), and extended release (ER) oxycodone (1.5 MME).23 Acute preoperative use was defined as a preoperative opiate prescription filled within 3 months before surgery. Subacute preoperative use was defined as acute use within 3 months in addition to use between 3 and 6 months preoperatively. Chronic preoperative use was defined as subacute preoperative use (within 3 months and use between 3 and 6 months) in addition to use before 6 months preoperatively.
Outcome Variables
The primary outcome measure was reoperation of the lumbar spine within 5 years of surgery (either decompression or fusion) identified by CPT codes 22612, 22630, 22558, 22533, 22800, 22802, 22804, 22808, 22810, 22812, 63047, 63030, 63042. Secondary outcomes measures were revision lumbar spine surgery at earlier time intervals of 1, 2, 3, and 4 years. Additional confounding variables assessed were age, sex, Charlson Comorbidity Index (CCI),24 and multilevel disease.
Statistical Analysis
Statistical analysis was performed using PearlDiver Bell-weather statistical software. Multivariate logistic regression, controlling for multilevel surgery, age, sex, and Charlson Comorbidity Index was then used to identify any association of acute, subacute, and chronic preoperative opiate use of the five different opioid medications with 5-year reoperation rates. All statistical tests were two-tailed and an alpha level of 0.05 was taken as statistically significant.
RESULTS
A total of 11,370 patients undergoing LDD without prior lumbar spine surgery were identified (Table 1). Of those 55.4% were multilevel and 44.6% were single level. A total of 4865 patients (42.8%) were opioid-naïve, preoperatively. The most commonly used preoperative opioid medication was hydrocodone (Table 2; 43.6% acute preoperative use, 18.4% chronic use), followed by oxycodone (15.5% acute preoperative use, 4.7% chronic preoperative use), and tramadol (11.3% acute preoperative use, 3.5% chronic preoperative use).
TABLE 1.
Group LDD and Group PLIF/TLIF Demographics
| Group LDD (N = 11,551) | Group PLIF/TLIF (N = 3291) | |||
|---|---|---|---|---|
| Sex | ||||
| Male | 5399 | 46.7% | 1261 | 38.3% |
| Female | 6152 | 53.3% | 2030 | 61.7% |
| Age | ||||
| <50 | 867 | 7.5% | 255 | 7.7% |
| 50–59 | 1396 | 12.1% | 573 | 17.4% |
| 60–69 | 3735 | 32.3% | 1247 | 37.9% |
| 70–79 | 4345 | 37.6% | 1048 | 31.8% |
| 80 + | 1208 | 10.5% | 168 | 5.1% |
| Charlson Comorbiditv Index | ||||
| 0 | 3433 | 29.7% | 991 | 30.1% |
| 1 | 2744 | 23.8% | 828 | 25.2% |
| 2 | 1906 | 16.5% | 605 | 18.4% |
| 3 | 1296 | 11.2% | 378 | 11.5% |
| 4 + | 2172 | 18.8% | 569 | 17.3% |
| Levels | ||||
| Single-level | 5471 | 47.4% | 1745 | 53.0% |
| Multilevel | 6080 | 52.6% | 1546 | 47.0% |
LDD indicates lumbar decompression/discectomy; PLIF/TLIF, posterior/transforaminal lumbar interbody fusion.
Table 2.
Group LDD Preoperative Opioid Use
| Lumbar Decompression With 5-year Follow up (N = 11,551) | ||||||
|---|---|---|---|---|---|---|
| Acute Use | Subacute Use | Chronic Use | ||||
| Hydromorphone | 162 | (1.4%) | 23 | (0.2%) | 12 | (0.1%) |
| Oxycodone | 1822 | (15.8%) | 569 | (4.9%) | 485 | (4.2%) |
| Hydrocodone | 5028 | (43.5%) | 2329 | (20.2%) | 2133 | (18.5%) |
| Tramadol | 1307 | (11.3%) | 522 | (4.5%) | 406 | (3.5%) |
| ER oxycodone | 65 | (0.6%) | 26 | (0.2%) | 12 | (0.1%) |
ER indicates extended release; LDD, lumbar decompression/discectomy.
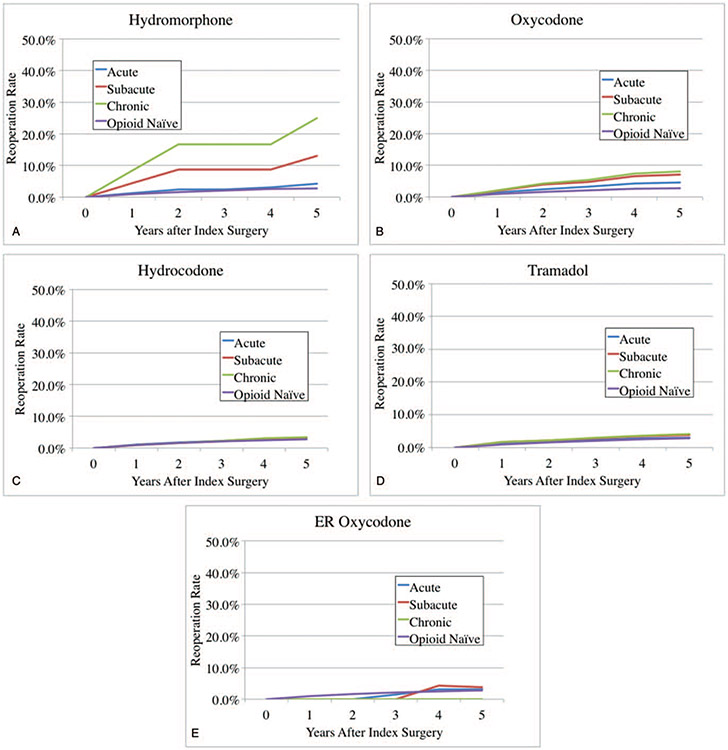
In this group a total of 773 patients (6.8%) underwent reoperation in 5 years postoperatively. Opioid-naïve patïents had a 5-year reoperation rate of 5.8%. In patients taking hydromorphone preoperatively, 5-year reoperation rates were 8.9%, 21.7%, and 38.5% with acute, subacute, and chronic preoperative use (Figure 1A). In patients taking oxycodone preoperatively, 5-year reoperation rates were 8.6%, 10.4%, and 12.6% with acute, subacute, and chronic preoperative use (Figure 1B). In patients taking hydrocodone preoperatively, 5-year reoperation rates were 7.6%, 7.5%, and 8.0% with acute, subacute, and chronic preoperative use (Figure 1C). In patients taking tramadol preoperatively, 5-year reoperation rates were 6.3%, 7.1%, and 8.3% with acute, subacute, and chronic preoperative use (Figure 1D). In patients taking ER-oxycodone preoperatively, 5-year reoperation rates were 4.9%, 4.3%, and 0.0% with acute, subacute, and chronic preoperative use (Figure 1E).
Figure 1.
(A) Preoperative hydromorphone use and reoperation rates after lumbar decompression/discectomy. (B) Preoperative oxycodone use and reoperation rates after lumbar decompression/discectomy. (C) Preoperative hydrocodone use and reoperation rates after lumbar decompression/discectomy. (D) Preoperative tramadol use and reoperation rates after lumbar decompression/discectomy. (E) Preoperative ER oxycodone use and reoperation rates after lumbar decompression/discectomy.
In multivariate analysis of group LDD (Table 3), controlling for multilevel surgery, age, gender, and CCI, any preoperative use of oxycodone was associated with increased reoperations (odds ratio [OR] = 1.2, 1.5, and 1.8, for acute, subacute, and chronic use; P < 0.05). Subacute and chronic use of hydromorphone was also associated with increased reoperations (OR = 2.9 and 6.8, for subacute and chronic use; P < 0.05).
TABLE 3.
Group LDD Multivariate Regression of Risk Factors for 5-year Reoperation
| Acute Preoperative Opiate Use | Lumbar Decompression With 5-year Follow-up (N = 11,551) | |||
|---|---|---|---|---|
| Odds Ratio* | (97.5% Confidence Interval) | P | ||
| Tramadol | 0.96 | 0.68 | 1.33 | 0.83 |
| Hydrocodone | 0.94 | 0.76 | 1.16 | 0.58 |
| Oxycodone | 1.42 | 1.09 | 1.82 | <0.01 |
| Hydromorphone | 1.10 | 0.46 | 2.21 | 0.81 |
| ER oxycodone | 0.68 | 0.11 | 2.23 | 0.60 |
| Subacute preoperative opiate use | Odds Ratio* | (97.5% Confidence Interval) | P | |
| Tramadol | 1.17 | 0.71 | 1.81 | 0.51 |
| Hydrocodone | 0.91 | 0.70 | 1.18 | 0.49 |
| Oxycodone | 1.97 | 1.37 | 2.76 | <0.01 |
| Hydromorphone | 3.19 | 0.74 | 9.58 | 0.07 |
| ER Oxycodone | 0.67 | 0.04 | 3.28 | 0.70 |
| Chronic preoperative opiate use | Odds Ratio* | (97.5% Confidence Interval) | P | |
| Tramadol | 1.25 | 0.73 | 2.01 | 0.38 |
| Hydrocodone | 0.97 | 0.74 | 1.26 | 0.84 |
| Oxycodone | 2.30 | 1.59 | 3.24 | <0.01 |
| Hydromorphone | 7.48 | 1.64 | 25.53 | <0.01 |
| ER oxycodone | 0.00 | < 0.01 | 1.21E + 16 | 0.98 |
Multivariate logistic regression controlling for multilevel surgery, patient age, sex, and Charlson Comorbidity index. ER indicates extended release; LDD, lumbar decompression/discectomy.
A total of 3291 patients undergoing PLIF/TLIF without previous lumbar spine surgery were identified (Table 4). Of those, 47.0% were multilevel and 53.0% were single level. A total of 1360 patients (41.3%) were opioid-naïve preoperatively. The most commonly used preoperative opioid medication was hydrocodone (Table 5; 43.9% acute preoperative use, 23.1% chronic use), followed by oxycodone (16.6% acute preoperative use, 5.8% chronic preoperative use), and tramadol (11.2% acute preoperative use, 3.9% chronic preoperative use). In this group a total of 418 patients (12.7%) underwent reoperation in 5 years postoperatively.
TABLE 4.
Group PLIF/TLIF Preoperative Opioid Use
| TLIF/PLIF With 5-year Follow-up (N = 3291) | ||||||
|---|---|---|---|---|---|---|
| Acute Use | Subacute Use | Chronic Use | ||||
| Hydromorphone | 50 | (1.5%) | 14 | (0.4%) | 6 | (0.2%) |
| Oxycodone | 547 | (16.6%) | 220 | (6.7%) | 191 | (5.8%) |
| Hydrocodone | 1446 | (43.9%) | 803 | (24.4%) | 759 | (23.1%) |
| Tramadol | 368 | (11.2%) | 173 | (5.3%) | 129 | (3.9%) |
| ER Oxycodone | 33 | (0.1%) | 16 | (0.5%) | 8 | (0.2%) |
ER indicates extended release; PLiF/TLiF, posterior/transforaminal lumbar interbody fusion.
TABLE 5.
Group PLIF/TLIF Multivariate Regression of Risk Factors for Reoperation
| Acute Preoperative Opiate Use | PLIF/TLIF With 5-year Follow-up (N = 3291) | |||
|---|---|---|---|---|
| Adjusted Odds Ratio* | (97.5% Confidence Interval) | P | ||
| Tramadol | 1.09 | 0.78 | 1.49 | 0.60 |
| Rocodone | 1.10 | 0.89 | 1.35 | 0.38 |
| Oxycodone | 1.07 | 0.81 | 1.40 | 0.64 |
| Hydromorphone | 2.88 | 1.49 | 5.32 | <0.01 |
| ER Oxycodone | 1.65 | 0.64 | 3.76 | 0.26 |
| Subacute Preoperative Opiate Use | Adjusted Odds Ratio* | (97.5% Confidence Interval) | P | |
| Tramadol | 1.27 | 0.81 | 1.92 | 0.28 |
| Hydrocodone | 0.90 | 0.70 | 1.14 | 0.39 |
| Oxycodone | 1.11 | 0.74 | 1.63 | 0.60 |
| Hydromorphone | 4.05 | 1.20 | 12.29 | 0.02 |
| ER Oxycodone | 1.44 | 0.32 | 4.66 | 0.58 |
| Chronic Preoperative Opiate Use | Adjusted Odds Ratio* | (97.5% Confidence Interval) | P | |
| Tramadol | 1.48 | 0.90 | 2.33 | 0.11 |
| Hydrocodone | 0.95 | 0.73 | 1.21 | 0.66 |
| Oxycodone | 1.27 | 0.83 | 1.90 | 0.25 |
| Hydromorphone | 13.97 | 2.50 | 53.48 | <0.01 |
| ER oxycodone | 0.85 | 0.11 | 5.13 | 0.88 |
Multivariate logistic regression controlling for multilevel surgery, patient age, gender and Charlson Comorbidity index.
ER indicates extended release; PLIF/TLIF, posterior/transforaminal lumbar interbody fusion.
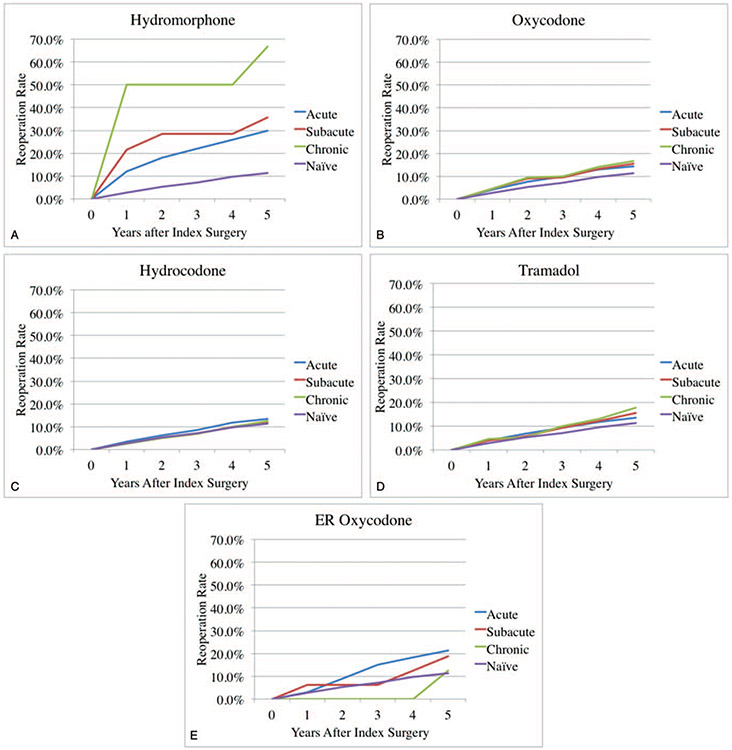
Opioid-naïve patients had a 5-year reoperation rate of 11.3%. In patients taking hydromorphone preoperatively, 5-year reoperation rates were 30.0%, 35.7%, and 66.7% with acute, subacute, and chronic preoperative use (Figure 2A). In patients taking oxycodone preoperatively, 5-year reoperation rates were 14.2%, 15.5%, and 16.8% with acute, subacute, and chronic preoperative use (Figure 2B). In patients taking hydrocodone preoperatively, 5-year reoperation rates were 13.3%, 12.1%, and 12.4% withacute, subacute, and chronic preoperative use (Figure 2C). In patients taking tramadol preoperatively, 5-year reoperation rates were 13.6%, 15.6%, and 17.8% with acute, subacute, and chronic preoperative use (Figure 2D). In patients taking ER-oxycodone preoperatively, 5-year reoperation rates were 21.2%, 18.8%, and 12.5% with acute, subacute, and chronic preoperative use (Figure 2E).
Figure 2.
(A) Preoperative hydromorphone use and reoperation rates after posterior lumbar interbody fusion. (B) Preoperative oxycodone use and reoperation rates after posterior lumbar interbody fusion. (C) Preoperative hydrocodone use and reoperation rates after posterior lumbar interbody fusion. (D) Preoperative tramadol use and reoperation rates after posterior lumbar interbody fusion. (E) Preoperative ER oxycodone use and reoperation rates after posterior lumbar interbody fusion.
In multivariate analysis of group PLIF/TLIF (Table 5), controlling for multilevel surgery, age, gender, and CCI, any preoperative use of hydromorphone was associated with increased reoperations (OR = 2.9, 4.0, and 14.0, for acute, subacute, and chronic use; P < 0.05).
DISCUSSION
tSpine-related pathology has been repeatedly associated with chronic opioid use.
In one series of 583 elective spine surgery patients, >50% reported preoperative opioid use.16 A Danish nationwide registry study demonstrated that, among patients with initial opioid prescriptions, back and spinal pain was a significant risk factor for chronic opioid use compared to other pain diagnoses. Back pain patients are also often already taking opioids before presentation to a spine surgeon. For example, in one large retrospective review of a managed care insurance cohort, of 26,000 patients with an index presentation for back pain, 61% of patients received a prescription of opioids from primary care physicians within 6 months.5 However, despite the association between preoperative opioids and worse preoperative outcomes,7,18-22 no studies have specifically demonstrated an association between preoperative opioid use and long-term rates of reoperation after lumbar decompression/discectomy or PLIF/TLIF surgery.
The present study is the first to demonstrate an association between preoperative use of specific opioid medications and long-term rates of revision lumbar spine surgery in both lumbar discectomy/decompression and TLIF/PLIF populations. In the LDD cohort, chronic hydromorphone use was associated with 6.8 × increased odds of reoperation, whereas the in the PLIF/TLIF population, chronic hydro-morphone use was associated with >14.0× increased odds of reoperation. Additionally, in the LDD population, any preoperative oxycodone use, even acutely, was associated with reoperation (up to 80% increased odds with chronic use). These are novel findings that may be invaluable for preoperative opioid cessation counseling. Increased risk of additional surgery and the associated postoperative morbidity and rehabilitation may be a useful motivator for patients to enter an opioid cessation program.25-28
A few previous studies have looked at the effect of opioids on early revision surgeries in select spine surgery populations. Notably, Jain et al recently demonstrated increased rates of early revision surgery within 1 year after posterolateral lumbar fusion (10,681 patients), total hip replacement, and total knee replacement with opioid medications use >6 months preoperatively.14,29 However, this study included only same-level revision surgery, and only within 1 year postoperatively without comparison of different opioid medications or lumbar decompression or interbody fusions. Same-level reoperation during this early period most likely relates to pseudoarthrosis or inadequate decompression. Our present study includes all additional lumbar spine surgery and follows patients out to 5 years postoperatively, including degeneration of additional levels and extension of the previous fusion. The same group also performed an analysis of cervical fusion, concluding that chronic opioid (>6 months) abuse was associated with increased odds of additional cervical fusion surgery within 1 year of surgery.30
The etiology of this association between preoperative opioid use and reoperation after lumbar spine surgery is not well understood. Chronic opioid users may simply have more severe “pain generators” such as severe spinal deformity requiring more extensive surgical treatment with higher associated reoperation rates.31 However, opioid use patterns have been shown to be highly variable among patients, subject to influence by a number of factors.32 From a pathophysiological basis, chronic opioid use has been shown to lead to hypersensitivity, known as opioid-induced hyperalgesia.33,34 Chronic uncontrolled pain could possibly lead surgeons into pursuing surgical intervention once other options are exhausted. However, opioid use has also been associated with various biopsychosocial factors, such as pain catastrophizing and objective pain tolerance, traits that may have confounding relationships with revision surgery.35-37 Finally, there is some early evidence in rabbit models, that systemic opioids delay both maturation and remodeling of spinal fusion.38 If this finding translates to humans, opioids may predispose lumbar fusion patients to pseudoarthroses requiring revision fusion surgery.
These possible etiologies also potentially explain the stronger association of opioids with reoperations in patients undergoing lumbar fusion compared with lumbar decompression surgery. For example, neurogenic leg pain from nerve root compression may be more easily diagnosed by surgeons and more effectively treated with lumbar decompression surgery. However, patients with back pain requiring lumbar fusion may have multifactorial source of their pain, including opioid-induced hyperalgesia. Similarly, the alteration of spinal fusion in rabbit models suggests pseudoarthrosis may be another source of reoperation in lumbar fusion patients but not lumbar decompression patients.3,8
The association with reoperation, specifically in the TLIF/PLIF and LDD populations, are a novel finding. Previous studies have demonstrated an association of opioid use with poor postoperative patient-reported outcomes.7,18-22,39 In one series of 2128 elective cervical or lumbar spine surgery patients, any preoperative opioid use was found to be associated with lack of meaningful improvement in pain, function, and quality of life.7 However, the authors did not report reoperation rates. In another series of 1836 elective cervical or lumbar spine surgery patients, preoperative opiate use >47.8 MME per day was associated with lower odds of achieving minimum clinically important difference in outcomes.20 More recently, Zakaria et al demonstrated that chronic preoperative opioid (>6 months) use was associated with decreased Oswestry Disability Index (ODI) improvement in a large series of 8600 lumbar fusion patients.18 This study also did not consider reoperations and followed patients for only 2 years postoperatively. Finally, in a smaller series of 93 one- and two-level TLIF patients, any amount of preoperative opioid use was associated with worse back pain, ODI, and 12-item Short Form Health Survey Physical Component Score (SF-12 PCS) at 12 months.19 Although these recent studies all show a clear association with outcomes, most lacked the appropriate sample size or follow-up period to determine an association with reoperations. The present study addresses these limitations by identifying a substantially larger patient sample and following patients for a total of 5 years postoperatively.
In addition to patient-reported outcomes, a number of studies have described an association between preoperative opioid intake and postoperative complications. In a study of >2000 spine surgery patients, chronic preoperative opioid use was associated with increased postoperative medical complications, including pneumonia, myocardial infarction, and postoperative lieus.7 In two recent large series of cervical fusion patients, each over 20,000 patients, preoperative opioid use was associated with both increased wound complications and postoperative emergency department visits.30,40 Finally, a nationwide study of all orthopedic surgery, including spine surgery, found that a diagnosis of preoperative opioid abuse was associated with increased postoperative complications, including mortality, respiratory failure, surgical site infection, pneumonia, myocardial infarction, and postoperative lieus.21 Although less clearly linked with 5-year reoperations, postoperative complications may lead to poor patient-reported outcomes.
The present study is also novel in comparing the associations of the five most-commonly prescribed opioid medications of varying strengths, with varying duration of preoperative use (acute, subacute, or chronic), in lumbar decompression and lumbar interbody fusion populations. Few other studies have considered the strength or dosage of preoperative opioids taken. Wick et al used Bayesian inference and Markov Chain Monte Carlo methods to identify 47.8 MME as the threshold dosage associated with worse postoperative outcomes after elective spine surgery.20 This is equivalent to 12.0 mg of hydromorphone, 31.9 mg of oxycodone, and 47.8 mg of hydrocodone. A smaller study of 93 TLIF patients found no dose-related effect of preoperative opioids with patient-reported outcomes.19 However, when measured in aggregate, preoperative opioid users had worse postoperative back pain, disability, and physical function. One previous study of only cervical spine patients demonstrated no differences in patient-reported outcomes in 1000 patients were identified between patients taking weak opioids (codeine, propoxyphene, hydrocodone) versus strong opioids (oxycodone, morphine, and meperidine) preoperatively.41 The authors concluded that strength of opioid medication was not associated with outcomes. However, hydromorphone was not considered in this study. This is important because in the present study preoperative hydromorphone had the strongest associations with reoperation after lumbar decompression and interbody fusion surgery. This medication is increasingly prescribed for acute pain, accounting for 36% of emergency department opioid prescriptions in 2014 in the United States.42 However, based on the current findings, hydromorphone use should likely be avoided for treatment back or spine-related pain.
The present study also demonstrates that, in general, longer-term preoperative hydromorphone and oxycodone use (<6 months before surgery) had stronger associations with reoperations, compared with more acute use, in a dose-dependent fashion. In general, previous studies demonstrate that more chronic opiate use is associated with worse overall outcomes. Zakaria et al18 found that opioid use >6 months was associated with worse patient-reported outcomes after lumbar fusion. However, new (<6 weeks) and short-term (<3 months) opioid use were actually associated with improved outcomes compared to opioid-naïve patients. In a series of >10,000 lumbar decompression patients, long-term use (>3 months) and medium-term use (>2 weeks) was associated with worse outcomes, but not short-term use (<2 weeks).15 In addition, only long-term use was associated with chronic opioid use and failed back syndrome. Yet another study of 140 lumbar decompression-only patients found that short-term preoperative opioid use (<3 months) was associated with increased return to work compared with long-term use (>3 months).43 Specifically, Oliesky et al compared a number of definitions of chronic opioid use and found that >120 MME for >91 days had the strongest association with chronic postoperative use.12
Based on the findings of the present study, efforts to reduce preoperative opioid use are warranted. There are currently very few reports of preoperative opioid reduction strategies before spine surgery. The Enhanced Recovery After Surgery (ERAS) protocol is a multidisciplinary approach to optimizing surgical outcomes and reducing costs, including reduction of preoperative opioid utilization.25 These outpatient programs involve not only gentle opioid weaning/cessation, but also regular psychological counseling focused on self-efficacy, resilience, and pain coping. Simple opioid reduction without multimodal alter-natives for pain control and psychosocial therapy are largely ineffective, without focus on preventing pain catastrophizing, hypersensitivity, and improving resilience.25 One preliminary report of 5 patients who completed a 6- to 7-week multidisciplinary preoperative reduction program before spine surgery demonstrated reduction in opioid use from 238 MME at initial presentation to 157 MME at the time of surgery.26 This involved regular outpatient biophyschosocial counseling and opioid monitoring. In a larger population of lumbar spine fusion patients, the ERAS protocol resulted in no change in acute postoperative opioid use, but a significant reduction in use of long-acting opioids post-operatively, from 14.6% to 5.2%.27 A similar study in the total joint arthroplasty literature demonstrated that preoperative opioid reduction in chronic users, resulted in improved clinical outcomes, similar to opioid-naïve patients.28 This suggests that active preoperative opioid reduction programs in chronic users may improve long-term outcomes such as reoperations.28 Therefore, further investigation and implementation of these types of preoperative opioid reduction programs is necessary for patients indicated for spine surgery.
The present study does have several limitations that must be considered. First, this is a retrospective study of an insurance cohort and the associations identified cannot necessarily be assumed to indicate a causal relationship or be extrapolated to the general population. Given this is a privately insured population, there is likely to be restricted clinical and sociodemographic variation to some degree. The factors considered for adjustment do not adjust for similarly important confounders, including behavioral health and socioeconomic factors. Nevertheless, the association between opioids and reoperation in this population is novel and important to understand. Next, the present study is not able to determine the amount of opioid medication that is actually taken by patients after filling a prescription, as these data are not contained in the database. However, previous studies have demonstrated that the amount of opioid medication taken preoperatively is proportional to the size of opioid prescription given.44 Another limitation is the limited number of patients identified with preoperative use of hydromorphone and ER oxycodone. The limited numbers of patients limits the ability of the current analysis to draw statistically significant conclusions. Nevertheless, a significant association with hydromorphone was still identified, despite acute use in only 1.4% to 1.5% of each population. This suggests that there is indeed a strong relationship between hydromorphone and reoperations. Finally the present study does not distinguish patients based on preoperative diagnosis. This is difficult as diagnosis can only be determined based on International Classification of Disease (ICD) coding, which has been shown to have poor sensitivity for identifying medical diagnoses in research studies.45 Nevertheless, the present study attempts to control for patient factors by including age, sex, Charleston Comorbidity Index, and multilevel surgery in the multivariate logistic regression analysis.
In conclusion, the present study demonstrates that in lumbar decompression or posterior interbody fusion populations, even acute preoperative use of hydromorphone and oxycodone are associated with increased reoperation rates at 5-year follow-up, with a clear dose-dependent effect. As this has not been previously demonstrated in these populations, this association will be valuable for preoperative opioid cessation counseling and for individualized risk stratification. In addition, further investigation of preoperative opioid cessation strategies any possible beneficial effect of cessation on reoperation rates and outcomes is warranted.
Key Points.
❑ No studies have identified an association between preoperative opioid use and reoperation rates after lumbar decompression or interbody fusion surgeries.
❑ In a cohort of 11,551 patients undergoing LDD, opioid-naïve patients had a 5-year reoperation rate of 2.8%, compared with 25.0% and 8.0% with chronic preoperative use of hydromorphone and oxycodone, respectively.
❑ In multivariate analysis of this LDD population, acute, subacute, and chronic preoperative use of oxycodone and chronic preoperative use of hydromorphone were associated with increased risk of reoperation within 5years of index surgery.
❑ In a cohort of 3291 patients undergoing PLIF/TLIF, opioid-naïve patients had a 5-year reoperation rate of 11.3%, compared with 66.7% and 16.8% with chronic preoperative use of hydromorphone and oxycodone.
❑ In multivariate analysis of this PLIF/TLIF population, acute, subacute, and chronic preoperative use of hydromorphone had increasing associations with 5-year reoperations.
Footnotes
Level of Evidence: 3
References
- 1.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med 2010;363:1981–5. [DOI] [PubMed] [Google Scholar]
- 2.Birnbaum HG, White AG, Schiller M, et al. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med 2011;12:657–67. [DOI] [PubMed] [Google Scholar]
- 3.Florence CS, Zhou C, Luo F, et al. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care 2016;54:901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerbershagen HJ, Aduckathil S, van Wijck AJ, et al. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology 2013;118:934–44. [DOI] [PubMed] [Google Scholar]
- 5.Deyo RA, Smith DH, Johnson ES, et al. Opioids for back pain patients: primary care prescribing patterns and use of services. J Am Board Fam Med 2011;24:717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen CA, Ernst MT, Stougaard M, et al. Tramadol prescribed use in general and chronic noncancer pain: a nationwide register-based cohort study of all patients above 16 years. Scand J Pain 2019; Sep 17. Epub 2019/09/19. [DOI] [PubMed] [Google Scholar]
- 7.Hills JM, Pennings JS, Archer KR, et al. Preoperative opioids and 1-year patient-reported outcomes after spine surgery. Spine (Phila Pa 1976) 2019;44:887–95. [DOI] [PubMed] [Google Scholar]
- 8.Hockley A, Ge D, Vasquez-Montes D, et al. Minimally invasive versus open transforaminal lumbar interbody fusion surgery: an analysis of opioids, nonopioid analgesics, and perioperative characteristics. Global Spine J 2019;9:624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adogwa O, Davison MA, Vuong VD, et al. Reduction in narcotic use after lumbar decompression and fusion in patients with symptomatic lumbar stenosis or spondylolisthesis. Global Spine J 2019;9:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitter FT, Sikora M, Lindberg-Larsen M, et al. Use of opioids and other analgesics before and after primary surgery for adult spinal deformity: a 10-year nationwide study. Neurospine 2019;17:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adogwa O, Davison MA, Vuong V, et al. Sex differences in opioid use in patients with symptomatic lumbar stenosis or spondylolisthesis undergoing lumbar decompression and fusion. Spine (Phila Pa 1976) 2019;44:E800–7. [DOI] [PubMed] [Google Scholar]
- 12.Oleisky ER, Pennings JS, Hills J, et al. Comparing different chronic preoperative opioid use definitions on outcomes after spine surgery. Spine J 2019;19:984–94. [DOI] [PubMed] [Google Scholar]
- 13.Schoenfeld AJ, Belmont PJ Jr, Blucher JA, et al. Sustained preoperative opioid use is a predictor of continued use following spine surgery. J Bone Joint Surg Am 2018;100:914–21. [DOI] [PubMed] [Google Scholar]
- 14.Jain N, Phillips FM, Weaver T, et al. Preoperative chronic opioid therapy: a risk factor for complications, readmission, continued opioid use and increased costs after one- and two-level posterior lumbar fusion. Spine (Phila Pa 1976) 2018;43:1331–8. [DOI] [PubMed] [Google Scholar]
- 15.O’Donnell JA, Anderson JT, Haas AR, et al. Preoperative opioid use is a predictor of poor return to work in workers’ compensation patients after lumbar diskectomy. Spine (Phila Pa 1976) 2018;43: 594–602. [DOI] [PubMed] [Google Scholar]
- 16.Armaghani SJ, Lee DS, Bible JE, et al. Preoperative opioid use and its association with perioperative opioid demand and postoperative opioid independence in patients undergoing spine surgery. Spine (Phila Pa 1976) 2014;39:E1524–30. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence JT, London N, Bohlman HH, et al. Preoperative narcotic use as a predictor of clinical outcome: results following anterior cervical arthrodesis. Spine (Phila Pa 1976) 2008;33:2074–8. [DOI] [PubMed] [Google Scholar]
- 18.Zakaria HM, Mansour TR, Telemi E, et al. The association of preoperative opioid usage with patient-reported outcomes, adverse events, and return to work after lumbar fusion: analysis from the Michigan Spine Surgery Improvement Collaborative (MSSIC). Neurosurgery 2019. [DOI] [PubMed] [Google Scholar]
- 19.Villavicencio AT, Nelson EL, Kantha V, et al. Prediction based on preoperative opioid use of clinical outcomes after transforaminal lumbar interbody fusions. J Neurosurg Spine 2017;26:144–9. [DOI] [PubMed] [Google Scholar]
- 20.Wick JB, Sivaganesan A, Chotai S, et al. Is there a preoperative morphine equianalgesic dose that predicts ability to achieve a clinically meaningful improvement following spine surgery?. Neurosurgery 2018;83:245–51. [DOI] [PubMed] [Google Scholar]
- 21.Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res 2015;473:2402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee D, Armaghani S, Archer KR, et al. Preoperative opioid use as a predictor of adverse postoperative self-reported outcomes in patients undergoing spine surgery. J Bone Joint Surg Am 2014;96:e89; Epub 2014/06/05. [DOI] [PubMed] [Google Scholar]
- 23.Opioid Oral Morphine Milligram Equivalent (MME) Conversion Factors. Baltimore, MD: U.S. Centers for Medicare and Medicaid Services; 2017. [cited 2019]; Available from: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. [Google Scholar]
- 24.D’Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol 1996;49:1429–33. [DOI] [PubMed] [Google Scholar]
- 25.McAnally H. Rationale for and approach to preoperative opioid weaning: a preoperative optimization protocol. Perioper Med 2017;6:19; Epub 2017/12/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassamal S, Haglund M, Wittnebel K, et al. A preoperative interdisciplinary biopsychosocial opioid reduction program in patients on chronic opioid analgesia prior to spine surgery: a preliminary report and case series. Scand J Pain 2016;13:27–31. [DOI] [PubMed] [Google Scholar]
- 27.Smith J, Probst S, Calandra C, et al. Enhanced recovery after surgery (ERAS) program for lumbar spine fusion. Perioperative medicine (London, England) 2019;8:4; Epub 2019/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen LC, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty 2016;31 (9 suppl):282–7. [DOI] [PubMed] [Google Scholar]
- 29.Jain N, Brock JL, Malik AT, et al. Prediction of complications, readmission, and revision surgery based on duration of preoperative opioid use: analysis of major joint replacement and lumbar fusion. J Bone Joint Surg Am 2019;101:384–91. [DOI] [PubMed] [Google Scholar]
- 30.Jain N, Brock JL, Phillips FM, et al. Chronic preoperative opioid use is a risk factor for increased complications, resource use, and costs after cervical fusion. Spine J 2018;18:1989–98. [DOI] [PubMed] [Google Scholar]
- 31.Scheer JK, Tang JA, Smith JS, et al. Reoperation rates and impact on outcome in a large, prospective, multicenter, adult spinal deformity database: clinical article. J Neurosurg Spine 2013;19:464–70. [DOI] [PubMed] [Google Scholar]
- 32.Lovecchio F, Premkumar A, Stepan JG, et al. Opioid consumption patterns after lumbar microdiscectomy or decompression. Spine (Phila Pa 1976) 2019;44:1599–605. [DOI] [PubMed] [Google Scholar]
- 33.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006;104:570–87. [DOI] [PubMed] [Google Scholar]
- 34.Lee M, Silverman SM, Hansen H, et al. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011;14:145–61. [PubMed] [Google Scholar]
- 35.Teunis T, Stoop N, Park CJ, et al. What factors are associated with a second opioid prescription after treatment of distal radius fractures with a volar locking plate?. Hand (N Y) 2015;10:639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nickel BT, Klement MR, Byrd WA, et al. Rand young investigator’s award: battling the opioid epidemic with prospective pain threshold measurement. J Arthroplasty 2018;33 (7S):S3–7. [DOI] [PubMed] [Google Scholar]
- 37.Lovecchio F, Derman P, Stepan J, et al. Support for safer opioid prescribing practices: a catalog of published use after orthopaedic surgery. J Bone Joint Surg Am 2017;99:1945–55. [DOI] [PubMed] [Google Scholar]
- 38.Jain N, Himed K, Toth JM, et al. Opioids delay healing of spinal fusion: a rabbit posterolateral lumbar fusion model. Spine J 2018;18:1659–68. [DOI] [PubMed] [Google Scholar]
- 39.Anderson PA, Subach BR, Riew KD. Predictors of outcome after anterior cervical discectomy and fusion: a multivariate analysis. Spine (Phila Pa 1976) 2009;34:161–6. [DOI] [PubMed] [Google Scholar]
- 40.Kalakoti P, Volkmar AJ, Bedard NA, et al. Preoperative chronic opioid therapy negatively impacts long-term outcomes following cervical fusion surgery. Spine (Phila Pa 1976) 2019;44:1279–86. [DOI] [PubMed] [Google Scholar]
- 41.Kelly MP, Anderson PA, Sasso RC, et al. Preoperative opioid strength may not affect outcomes of anterior cervical procedures: a post hoc analysis of 2 prospective, randomized trials. J Neurosurg Spine 2015;23:484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazer-Amirshahi M, Ladkany D, Mullins PM, et al. Trends in and predictors of hydromorphone administration in US emergency departments (2007–2014). J Opioid Manag 2018;14:265–72. [DOI] [PubMed] [Google Scholar]
- 43.Tye EY, Anderson JT, Faour M, et al. Prolonged preoperative opioid therapy in patients with degenerative lumbar stenosis in a workers’ compensation setting. Spine (Phila Pa 1976) 2017;42: E1140–6. [DOI] [PubMed] [Google Scholar]
- 44.Larach DB, Sahara MJ, As-Sanie S, et al. Patient factors associated with opioid consumption in the month following major surgery. Ann Surg 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samuel AM, Lukasiewicz AM, Webb ML, et al. ICD-9 diagnosis codes have poor sensitivity for identification of preexisting comorbidities in traumatic fracture patients: A study of the National Trauma Data Bank. J Trauma Acute Care Surg 2015;79:622–30. [DOI] [PubMed] [Google Scholar]