Abstract
Previous results have indicated that incorporation of surface glycoprotein into retroviral particles is not a specific process and that many heterologous viral and cellular glycoproteins can be incorporated as long as they do not have long cytoplasmic C-terminal regions which were presumed to be sterically inhibitory. In this study, this concept has been directly examined by analyzing the incorporation of the wild-type human epidermal growth factor receptor (Wt-EGFR) and of a C-terminally truncated mutant of Wt-EGFR (Tr-EGFR) into human immunodeficiency virus (HIV)-like particles. Incorporation was directly analyzed at the protein level and by immunogold labelling of enriched HIV-like particles. In agreement with the above concept, Tr-EGFR, with only 7 C-terminal amino acids (aa), was efficiently incorporated into HIV-like particles. Incorporation of the Wt-EGFR species, with 542 C-terminal cytoplasmic aa, was reduced by a factor of about 5 in comparison to that of the Tr-EGFR species. However, the Wt-EGFR species was still very significantly present in the HIV-like particles. A series of control experiments verified that this represents genuine incorporation of Wt-EGFR into the membrane of HIV-like particles. These observations allow further speculation as to the processes governing glycoprotein incorporation into retroviral particles and indicate that the internal virus structure of HIV (in particular the matrix layer [MA]) can accommodate much larger heterologous cytoplasmic domains in incorporated glycoproteins than previously assumed.
The incorporation of surface glycoprotein into retroviral particles is not restricted to the homologous glycoprotein. Pseudotyping with heterologous viral glycoproteins has been possible in many combinations, e.g., influenza virus hemagglutinin in Rous sarcoma virus particles (6), vesicular stomatitis virus (VSV) G protein in murine retroviral and in lentivirus vector particles (e.g., reference 16), and others. Furthermore, several reports have demonstrated that cellular proteins can be incorporated into retroviral particles. This has been demonstrated for human immunodeficiency virus type 1 (HIV-1), released from infected T-lymphocytes, generally by indirect methods whereby the putatively incorporated protein species were not visualized (summarized in reference 1). Nearly a decade ago, Young et al. (28) directly demonstrated the incorporation of the human CD4 protein into avian leukosis virus particles expressed in quail cells. Since then, many reports have demonstrated that CD4 is incorporated into the membrane of rhabdoviruses and retroviruses, and, in fact, in the situation in which HIV coreceptor (CXCR-4 or CCR-5) is also incorporated, the resulting pseudotyped viruses are infectious for HIV-infected cells (7, 15, 22).
In contrast to the glycoproteins of other membrane viruses, lentiviral glycoproteins are an exception in that they carry very long C-terminal cytoplasmic regions (about 150 to 200 amino acids [aa] long). Evidence from genetic analyses and from in vitro assays (4) indicates that, in the case of the wild-type HIV glycoprotein, there is a specific interaction between the C-terminal domain and the viral matrix (MA) protein. Despite this, neither the cytoplasmic C terminus nor the homologous membrane anchor of HIV Env is necessary for glycoprotein incorporation, and virions encoding glycoproteins in which these domains are lacking or have been replaced are infectious (8, 13, 26, 27). In fact, as mentioned above, several heterologous viral glycoprotein species can be incorporated into HIV particles and mediate infectivity (vector transduction). However, it was not possible to achieve the reverse situation, i.e., pseudotyping of heterologous membrane viruses with wild-type HIV Env. Incorporation and pseudotyping could be achieved only by either removing or replacing the cytoplasmic C terminus (e.g., in murine leukemia virus) particles (14, 23). Furthermore, incorporation of HIV Env carrying a long heterologous cytoplasmic C terminus (of the cellular glycoprotein CD22) could not be detected (27). These results have led to the concepts that a long cytoplasmic C terminus, e.g., that of lentivirus Env, has to fit to the viral MA layer and that long heterologous C termini result in steric inhibition of incorporation.
Further proof for this concept of steric inhibition has been obtained by a recent analysis of CD4 incorporation into HIV-like particles in the presence and absence of p56lck. p56lck binds to the cytoplasmic C terminus of wild-type CD4 (Wt-CD4) but cannot bind to mutant CD4 (CD4cyt−) lacking this region. We have demonstrated that both CD4 and CD4cyt− were incorporated into HIV-like particles. However, on coexpression of p56lck, incorporation of Wt-CD4 was inhibited but incorporation of CD4cyt− remained unaffected (9). This was interpreted to be due to steric inhibition at the C terminus, in this case, by the presence of the bulky p56lck molecule interacting with Wt-CD4.
In order to directly test the concept that a cellular surface glycoprotein with a long cytoplasmic C terminus would be excluded from incorporation into retroviral particles but that incorporation would become possible on removal of the cytoplasmic C terminus, we have chosen to study incorporation of the wild-type human epidermal growth factor receptor (Wt-EGFR), which is a type 1 glycoprotein carrying 542 cytoplasmic aa, into HIV-like particles. We have generated a C-terminally truncated mutant of Wt-EGFR (Tr-EGFR), carrying only seven cytoplasmic C-terminal aa and, in agreement with our anticipation, this latter molecule was efficiently incorporated into HIV-like particles. However, surprisingly, the wild-type molecule was also present in enriched HIV-like particles, albeit in amounts that were reduced compared to those of the truncated form. We describe the experiments verifying the incorporation of Wt-EGFR and conclude that the internal structure of HIV particles, and in particular the matrix layer directly underlying the lipid membrane, can accommodate bulky structures at the C terminus of incorporated glycoproteins to a greater extent than anticipated.
MATERIALS AND METHODS
Constructs.
pKEx-Wt-EGFR is a eucaryotic expression vector (based on pKEx [20]) for the human EGFR (24). pKEx-Tr-EGFR was generated by standard PCR technology by introducing a premature stop codon into the EGFR gene which then encoded a truncated glycoprotein with only 7, instead of 542, cytoplasmic C-terminal aa. Specifically, the mutagenizing oligonucleotide (nucleotide positions 2201 to 2229 in the EGFR sequence [24], ACATCGTTCGGTAGCGCACAGCTGCGGAGG, with the nucleotide changes underlined) changed nucleotide 2212 from A to T (codon AAG for lysine to TAG) and inserted a nucleotide (A) between positions 2219 and 2220, thus generating a PvuII site for easy identification of mutant plasmids. pKEx-HIVΔEnv3 encodes all of the HIV-1 genes except nef and env and leads to the release of HIV-like particles lacking glycoprotein (described in reference 9).
Analysis of expression of Wt-EGFR and Tr-EGFR.
pKEx-Wt-EGFR and pKEx-Tr-EGFR with and without pKEx-HIVΔEnv3 were transfected into 293T cells by standard calcium phosphate procedures. Expression of EGFR was analyzed with culture supernatant (EGFR antibody) from H-EGFR-RI mouse hybridoma cells secreting antibody directed against an epitope in the extracellular domain of EGFR (25). Expression of viral proteins from pKEx-HIVΔEnv3 was analyzed with rabbit antiserum directed against the HIV-1 capsid protein (CA), p24 (12). The amounts of virus particles released into the culture supernatants were quantitated by enzyme-linked immunosorbent assay detecting HIV-1 CA (Medipro, Ketsch, Germany).
For indirect immunofluorescence of cell-surface EGFR, intact transfected 293T cells were aspirated off the dish, collected by low-speed centrifugation, and incubated for 30 min with undiluted EGFR antibody and 0.02% azide at low temperatures. After washing and incubation with fluorescein-labelled anti-mouse immunoglobulin G (IgG) (both steps performed with 0.02% azide at low temperatures), the labelled cells were either smeared on a glass slide, air-dried, and embedded for microscopy or suspended in phosphate-buffered saline (PBS)–3% fetal calf serum–0.02% azide for flow cytometric analysis.
Analyses of EGFR in cell, medium, and viral lysates were performed by radioimmunoprecipitation. For this purpose, transfected cells were metabolically labelled for different time periods with 100 μCi of [35S]methionine and [35S]cysteine (Pro-mix; Amersham, Braunschweig, Germany) per ml. Labelling of cells for analysis of the intracellular distribution of Wt-EGFR and Tr-EGFR was for 5 h at 48 h posttransfection (p.t.). Preparation of the membrane and cytosolic fractions was performed essentially as described previously (19). Briefly, labelled cells were scraped off the dish and broken by douncing in hypotonic buffer and, after removal of nuclei and large debris by low-speed centrifugation, the cellular membranes were separated from the cytosol by ultracentrifugation at 100,000 × g.
For labelling of released HIV-like particles, metabolic labelling of transfected cells was from 32 to 48 h p.t. The resultant culture supernatants were clarified by filtering through a 45-μm filter and centrifuged through a cushion of 32% sucrose for 3 h at 200,000 × g. The pellets, consisting of enriched HIV-like particle material, were either analyzed by gel electrophoresis directly, further fractionated by velocity centrifugation (see below), or subjected to immunoprecipitation. Prior to immunoprecipitation, the total cells, the cellular membrane fractions, or the pelleted HIV-like particle preparations were lysed in RIPA buffer (1% Triton, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS] in PBS). The cytosolic fractions and uncentrifuged cell culture supernatants were adjusted to 1% Triton–0.5% deoxycholate–0.1% SDS. Immunoprecipitations of appropriate aliquots of these lysates were performed with 1 ml of undiluted culture supernatant containing EGFR antibody (containing approximately 10 μg of mouse IgG) or 10 μl of rabbit anti-CA serum plus protein G-Sepharose (Pharmacia, Uppsala, Sweden) and analyzed by polyacrylamide gel electrophoresis (PAGE) and autoradiography as described previously (17). Quantitation of radioactivity in specific bands was performed by direct measurement of counts in specific bands of the polyacrylamide gel as described previously (2).
Velocity centrifugation of HIV-like particles in OptiPrep gradients.
HIV-like particles which had been concentrated by centrifugation through 32% sucrose were resuspended in PBS and analyzed on OptiPrep (6 to 18% iodixanol; Gibco, Life Technology) velocity gradients. These gradients were prepared and centrifuged as recently described (5). Eleven fractions were collected from the top and, after addition of 10 μg of nonradioactive carrier protein (bovine serum albumin [BSA]), radioactive proteins were precipitated with 20% trichloroacetic acid at low temperatures and analyzed directly by gel electrophoresis and autoradiography.
Immunoprecipitation of intact HIV-like particles.
The culture supernatants of transfected cells which had been metabolically labelled from 32 to 48 h p.t. were filtered through a 45-μm filter. As a control, the supernatants of cells expressing Wt-EGFR or Tr-EGFR alone were mixed with the medium of cells expressing HIV-like particles alone. These mixed supernatants were further incubated for 1 h at 37°C. Unmixed and mixed culture supernatants were incubated, in the presence of protease inhibitor cocktail (Boehringer, Mannheim, Germany), with 1 ml of culture supernatant containing EGFR antibody and protein G-Sepharose for 2 h at low temperatures. The protein G-Sepharose was subsequently carefully washed with PBS, in the absence of detergent, and the immunoprecipitated material was analyzed by gel electrophoresis and autoradiography.
Immunoelectron microscopy.
In order to obtain sufficient material for immunoelectron microscopy, five 10-cm-diameter dishes of transfected 293T cells were employed for each sample. In experiments in which culture supernatants were mixed, five dishes of each type of transfected cells were employed and further incubated for 1 h at 37°C. The unmixed and mixed supernatants were centrifuged through a sucrose cushion, and the resulting HIV-like particles were taken up in 100 μl of PBS. Aliquots (20 μl) were placed for 1 min on glow-discharged carbon-coated copper grids and subsequently washed with 20 drops of double-distilled water. The following sequence of incubations was performed facedown on top of 50- to 100-μl drops placed on a sheet of Parafilm: 30 min in undiluted culture supernatant containing EGFR antibody; three sequential incubations, each for 5 min, in PBS–1% BSA; 60 min of incubation in a suspension of 5-nm gold spheres coated with antibody against mouse IgG-IgM (Amersham) diluted in PBS–1% BSA (1:10); three sequential incubations, each for 5 min, in PBS–1% BSA. Finally, the grids were washed again with 20 drops of double-distilled water, stained with 2% uranyl acetate, and air-dried. Grids were examined in a Zeiss 10A electron microscope at 80 kV. The magnification indicator of the microscope was routinely controlled with a grading replica.
RESULTS
Expression of Wt-EGFR and Tr-EGFR.
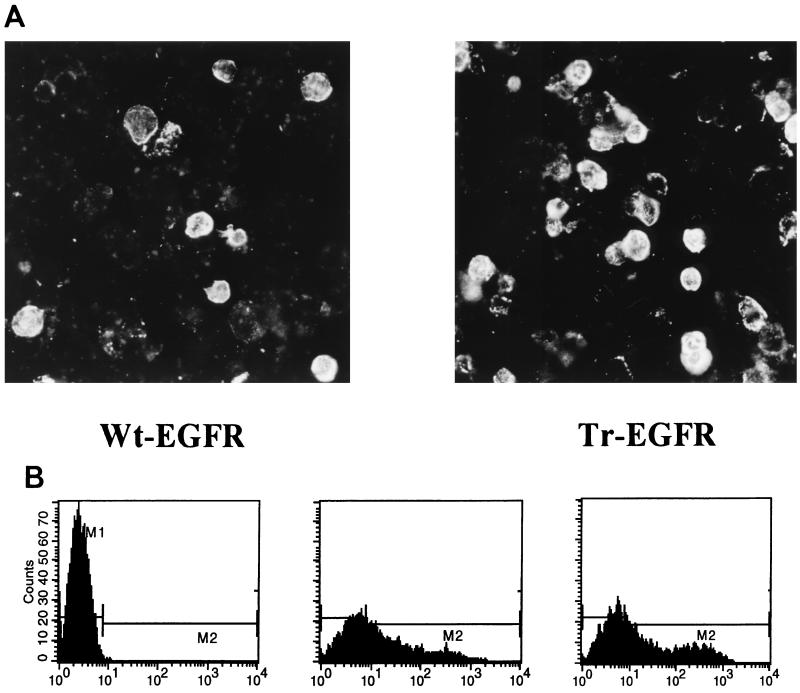
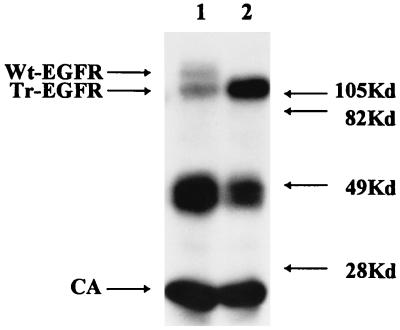
The EGFR molecule is a type I transmembrane glycoprotein consisting of 647 extracellular aa (including the signal peptide), a membrane anchor of 21 aa and a cytoplasmic C-terminal region of 542 aa (24). Our aim was to examine if, and to what extent, incorporation of Wt-EGFR into HIV-like particles occurs and to determine if this would be influenced by removal of the cytoplasmic C-terminal region. For this purpose, an expression vector encoding a truncated EGFR molecule (Tr-EGFR), with only 7 instead of 542 cytoplasmic aa, was generated (Fig. 1). Indirect immunofluorescence analysis of permeabilized 293T cells transfected with pKEx-Tr-EGFR or the wild-type construct, pKEx-Wt-EGFR, and immunoprecipitation of metabolically labelled lysates of transfected cells confirmed expression in both cases (data not shown). Since most of its cytoplasmic C terminus had been removed, it was important to confirm that Tr-EGFR was still an integral membrane protein and was transported to the cell surface. Metabolically labelled, transfected 293T cells were separated into membrane and cytosolic fractions, and these were subjected to immunoprecipitation employing monoclonal antibody specific for an epitope in the extracellular domain of EGFR. Figure 1 shows gel electrophoresis of the immunoprecipitated components. In both cases, specific bands of comparable intensities were observed only in the membrane fractions, consistent with both Wt-EGFR and Tr-EGFR being integral membrane proteins. The mobilities are consistant with the calculated molecular masses of approximately 166,000 Da for Wt-EGFR and 108,000 Da for Tr-EGFR (in each case without signal peptide and assuming glycosylation at each potential N-glycosylation site in the extracellular domain). Cell-surface staining of intact transfected cells was bright in both cases (Fig. 2A). Flow cytometric analyses (Fig. 2B) revealed that about 50% of the cells transiently transfected with Wt-EGFR or Tr-EGFR had fluorescence levels above the cutoff for the control untransfected cells. The mean cellular fluorescence was 75.34 in the case of Wt-EGFR and 131.65 in the case of Tr-EGFR. This means that, although both EGFR species are clearly present at the cell surface, the total cellular and/or surface expression of Tr-EGFR may be somewhat higher than that of Wt-EGFR.
FIG. 1.
Expression of Wt-EGFR and Tr-EGFR in cellular membranes. Upper panel: Schematic representation of Wt-EGFR (extracellular domain, 647 aa; membrane anchor, 21 aa, cytoplasmic domain, 542 aa) and Tr-EGFR (extracellular domain, 647 aa; membrane anchor, 21 aa; cytoplasmic domain, 7 aa). Lower panel: Autoradiogram of a PAGE analysis of immunoprecipitates obtained with EGFR antibody from the membrane (M) and cytosolic (C) fractions of metabolically labelled 293T cells transfected with pKEx-Wt-EGFR (Wt) or pKEx-Tr-EGFR (Tr). The positions of molecular mass markers are shown on the left.
FIG. 2.
Cell surface immunofluorescence. Intact 293T cells expressing Wt-EGFR or Tr-EGFR (as indicated) were probed with EGFR antibody plus fluorescein-labelled secondary antibody. (A) Microscopic analysis. (B) Flow cytometric analysis. From left to right, graphs are for untransfected 293T cells (the cutoff has been set such that less than 0.1% positive cells are within the region M2), 293T cells transiently transfected with pKEx-Wt-EGFR (M2 contains 51.5% positive cells with a mean fluorescence of 75.34), and 293T cells transiently transfected with pKEx-Tr-EGFR (M2 contains 53.21% positive cells with a mean fluorescence of 131.65), respectively.
Incorporation of EGFR into HIV-like particles.
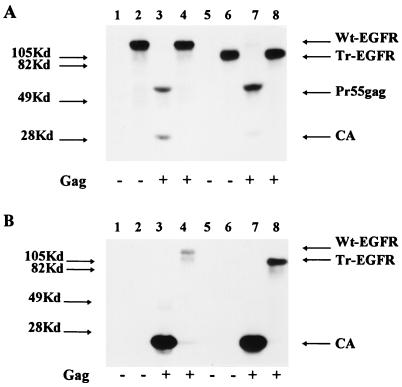
pKEx-HIVΔEnv3 encodes all HIV-1 gene products except Nef and Env and leads to the release of HIV-like particles lacking glycoprotein (9, 27). pKEx-Wt-EGFR and pKEx-Tr-EGFR were transfected either alone or together with pKEx-HIVΔEnv3 into 293T cells. Cells were metabolically labelled with [35S]methionine plus [35S]cysteine from 30 to 44 h p.t., and subsequently, radioactively labelled particle-like materials were concentrated from the culture supernatants by centrifugation through a sucrose cushion. Figure 3A shows the immunoprecipitated products from the cell lysates and confirms that the glycoproteins Wt-EGFR and Tr-EGFR were being expressed in similar amounts and that this was not influenced by the coexpression of pKEx-HIVΔEnv3. Immunoprecipitation with anti-CA serum reveals Pr55gag and p24 in the lysates of pKEx-HIVΔEnv3-expressing cells. The amounts of HIV-like particles released into the media were quantitated by enzyme-linked immunosorbent assay for CA and were approximately equivalent in individual transfections and not negatively influenced by the coexpression of EGFR. Figure 3B shows analysis of the pellets obtained after centrifugation of the transfected culture supernatants through sucrose cushions. Immunoprecipitation with anti-EGFR antibody revealed the presence of both Wt-EGFR and Tr-EGFR glycoproteins only in those cases in which pKEx-HIVΔEnv3 had been coexpressed, i.e., only when HIV-like particles were present (Fig. 3B, lanes 4 and 8). No radioactive glycoprotein could be detected in the centrifuged supernatants from cells transfected with either pKEx-Wt-EGFR or pKEx-Tr-EGFR alone (this was repeated in more than 10 experiments), confirming that neither protein was released into the culture medium in a pelletable form (e.g., membrane vesicles) in the absence of virus-like particles. In contrast to the predominant single band representing full-length Wt-EGFR in the cell lysates, the glycoprotein immunoprecipitated from the HIV-like particles was present as two bands, one migrating at the position of the full-length molecule and one smaller. The identity of this lower band, which was present in varying amounts, was not determined, but it most likely represents proteolytically degraded Wt-EGFR preferentially present in the HIV-like particles. However, it should be stressed here that EGFR species migrating at the position of the full-length Wt-EGFR molecule, and thus carrying very long cytoplasmic C termini, make up about 50% of the total EGFR species in the enriched HIV-like particles.
FIG. 3.
Analysis of the incorporation of Wt-EGFR and Tr-EGFR into HIV-like particles. (A) PAGE and autoradiography of the immunoprecipitates, obtained with anti-CA serum (lanes 1, 3, 5, and 7) or EGFR antibody (lanes 2, 4, 6, and 8), from metabolically labelled 293T cells expressing Wt-EGFR (lanes 1 to 4) or Tr-EGFR (lanes 5 to 8) without (lanes 1, 2, 5, and 6) or with (lanes 3, 4, 7, and 8) coexpression of HIV-like particles (the presence or absence of HIV-like particles [Gag] is indicated below each lane). (B) As described for panel A, but immunoprecipitates were of lysates of enriched HIV-like particles obtained by centrifugation of culture supernatants through sucrose. The positions of molecular mass markers are indicated on the left and the positions of Wt-EGFR, Tr-EGFR, Pr55gag, and CA are indicated on the right.
In order to compare the relative extents of putative incorporation of Wt-EGFR and Tr-EGFR into HIV-like particles, the amounts of radioactivity in the immunoprecipitated bands in the cell and viral lysates were quantitated. The percentage of glycoprotein present in the pelleted HIV-like particles in comparison to the total (i.e., cell plus virus) was 3.1% for Wt-EGFR (taking only the upper, full-length band into account), and 16.6% for Tr-EGFR. These values fluctuated slightly from experiment to experiment, with the relative amount (i.e., the percentage of the total amount) of Tr-EGFR always exceeding that of full-length Wt-EGFR by a factor of about 5. After correction for the number of methionines plus cysteines, this means that there are, in fact, about 7.5 times fewer Wt-EGFR molecules than there are Tr-EGFR molecules in the enriched HIV-like particle fraction. However, in a very large number of experiments and with no exception, these significant amounts of both full-length Wt-EGFR and Tr-EGFR were clearly detectable in the pelleted HIV-like particles.
These results point to both Wt-EGFR and Tr-EGFR being incorporated into HIV-like particles. In the case of Tr-EGFR, this is perhaps not surprising, since Tr-EGFR is present at the cell surface and carries only 7 cytoplasmic aa, which should not sterically interfere with the HIV-1 matrix layer, while the result that Wt-EGFR, with 542 cytoplasmic aa, was incorporated into HIV-like particles was contrary to our expectations. Thus the further experiments described below were aimed at confirming or refuting this result.
Analysis of total cell culture supernatants from transfected cells.
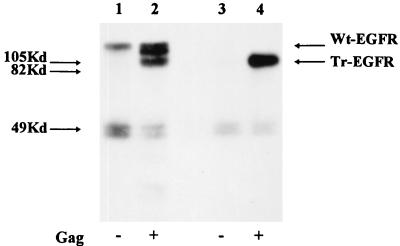
As a first step, we wished to analyze the total cell culture supernatants before enrichment of particles by centrifugation through sucrose. 293T cells were transfected and metabolically labelled as described above. The supernatants were centrifuged, filtered, adjusted to 1% Triton X-100–0.5% deoxycholate–0.1% SDS, and immunoprecipitated directly with EGFR antibodies. As shown in Fig. 4, radioactive Wt-EGFR was detected in the supernatants of cells expressing this glycoprotein alone (Fig. 4, lane 1). It is important to remember that, as indicated above, this material could not be pelleted through a sucrose cushion. In the presence of HIV-like particles, several different species derived from Wt-EGFR could be detected in the culture supernatant in amounts significantly higher than those detected in the absence of HIV-like particles (Fig. 4, lane 2). In addition to a band representing the full-length molecule, at least two further faster-migrating species were present, one at the position of truncated EGFR and a further species migrating between the positions of Wt-EGFR and Tr-EGFR. Additionally, in all immunoprecipitations of total supernatants, radioactive bands of about 50 kDa, which were interpreted to represent soluble proteolytic digestion products of the extracellular domain of EGFR, were observed despite the presence of protease inhibitor during the analyses. In the case of Tr-EGFR, no glycoprotein of 108 kDa was observed in the absence of HIV-like particles but a strong band could be detected in the supernatant of cells coexpressing HIV-like particles. This latter result further supports the conclusion drawn from results shown in Fig. 3, namely, that Tr-EGFR can truly be incorporated into HIV-like particles.
FIG. 4.
Analysis of total cell culture supernatants. PAGE and autoradiography of immunoprecipitates (with EGFR antibody) from lysates of HIV-like particles generated from the culture supernatants of metabolically labelled 293T cells expressing Wt-EGFR (lanes 1 and 2) or Tr-EGFR (lanes 3 and 4) without (lanes 1 and 3) or with (lanes 2 and 4) HIV-like particles (Gag) as indicated below each lane. The positions of molecular mass markers are indicated on the left, and the positions of Wt-EGFR and Tr-EGFR are indicated on the right.
Concerning Wt-EGFR, although pelletable glycoprotein was present in the culture supernatant only when HIV-like particles were coexpressed, its presence in the absence of HIV-like particles prompted us to perform further control experiments.
Further analyses of incorporation of Wt-EGFR and Tr-EGFR HIV-like particles.
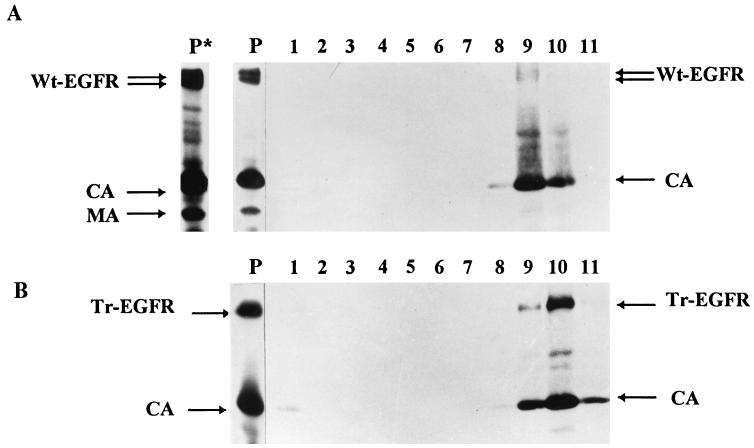
We considered the possibility that Wt-EGFR, which as shown above can be released into the culture supernatant independently of the presence of HIV-like particles, could subsequently nonspecifically associate with these and, as a result, be pelleted through a sucrose cushion. We thus transfected cells with pKEx-Wt-EGFR, pKEx-Tr-EGFR, and pKEx-HIVΔEnv3 alone and in combination and, after metabolic labelling, harvested the cell culture supernatants. The supernatants from cells expressing the EGFR glycoproteins alone (and thus, in the case of Wt-EGFR, containing released radioactive Wt-EGFR) were mixed with the supernatant of cells expressing pKEx-HIVΔEnv3 alone (and thus containing radioactive HIV-like particles) and incubated further for 1 h at 37°C. Subsequently, the mixed media, and media from cells coexpressing Wt-EGFR or Tr-EGFR plus HIV-like particles, were centrifuged through a sucrose cushion. In this experiment, the resulting HIV-like particles were analyzed directly, i.e., without immunoprecipitation. As shown in Fig. 5, the particles derived from the cultures coexpressing pKEx-Wt-EGFR or pKEx-Tr-EGFR plus pKEx-HIVΔEnv3 contained, as major components, only CA and either Wt-EGFR or Tr-EGFR. However, the particles derived from centrifugation of the mixed culture supernatants contained only CA. No EGFR components, and in particular no Wt-EGFR, could be detected even with a very long exposure of the gel. This makes it unlikely that the presence of Wt-EGFR in the HIV-like particles is due to nonspecific association of released Wt-EGFR with independently released particles but rather points to genuine incorporation, i.e., to Wt-EGFR being an integral component of the viral membrane. To gain further support for this view, the pelleted HIV-like particles containing Wt-EGFR or Tr-EGFR (i.e., material as in Fig. 5, lanes 1 and 2) were further analyzed by velocity gradient centrifugation (OptiPrep gradients), a procedure which has been reported to yield retroviral particles of superior purity compared to those obtained with conventional gradients (5). Gel electrophoretic analysis of the radioactive proteins in the gradient fractions revealed comigration of all EGFR components (Wt-EGFR and Tr-EGFR) and HIV CA to a single region of the gradient (Fig. 6). This provides further proof that these components are truly present in HIV-like particles and not in contaminating vesicular material which would migrate differently in the OptiPrep gradient.
FIG. 5.
Direct PAGE (no immunoprecipitation) of HIV-like particles. HIV-like particles, concentrated by centrifugation through a sucrose cushion, have been derived from the culture supernatants of metabolically labelled 293T cells coexpressing (Co) Wt-EGFR (lane 1) or Tr-EGFR (lane 2) plus HIV-like particles or from culture supernatants of 293T cells expressing HIV-like particles alone mixed (Mix) with the supernatant of cells expressing Wt-EGFR alone (lane 3) or Tr-EGFR alone (lane 4).
FIG. 6.
OptiPrep velocity gradient centrifugation. HIV-like particles concentrated from the culture supernatants of metabolically labelled 293T cells coexpressing Wt-EGFR plus HIV-like particles (A) or Tr-EGFR plus HIV-like particles (B) were centrifuged on 6 to 18% iodixanol gradients as described previously (5). Lanes P, gel electrophoresis of the starting materials; lane P*, a longer exposure of lane P, done to visualize more weakly labelled bands at the positions of HIV MA and reverse transcriptase; lanes 1 to 11 (from the top to the bottom of the gradient), gel electrophoresis of the radioactive proteins in the gradient fractions. The positions of Wt-EGFR, Tr-EGFR, CA, and MA are shown.
As additional proof of incorporation of Wt-EGFR and Tr-EGFR into HIV-like particles, we analyzed whether immunoprecipitation with EGFR antibody under native conditions would result in precipitation of intact HIV-like particles and thus coimmunoprecipitation of radioactive CA. Radioactively labelled supernatants from cells coexpressing Wt-EGFR or Tr-EGFR plus pKEx-HIVΔEnv3 were clarified by filtration and subsequently immunoprecipitated, in the absence of detergent, with EGFR antibody. As shown in Fig. 7, in both cases this led to coimmunoprecipitation of radioactive CA. In addition to the specific EGFR products of high molecular weight, the 50-kDa EGFR species observed previously could again be seen. The fact that CA was immunoprecipitated with EGFR antibody indicates that intact particles were immunoprecipitated and is further proof of incorporation of Wt-EGFR and Tr-EGFR into the viral membrane. In summary, all of the experiments described so far point to the presence of Wt-EGFR and Tr-EGFR in HIV-like particles demonstrating genuine incorporation.
FIG. 7.
Immunoprecipitation of native HIV-like particles with EGFR antibody. PAGE and autoradiography of immunoprecipitates derived, in the absence of detergent, from the supernatants of metabolically labelled 293T cells coexpressing Wt-EGFR (lane 1) or Tr-EGFR (lane 2) plus HIV-like particles. The positions of molecular mass markers are indicated on the right, and the positions of Wt-EGFR, Tr-EGFR, and CA are indicated on the left.
Immunogold labelling of HIV-like particles with incorporated Wt-EGFR and Tr-EGFR.
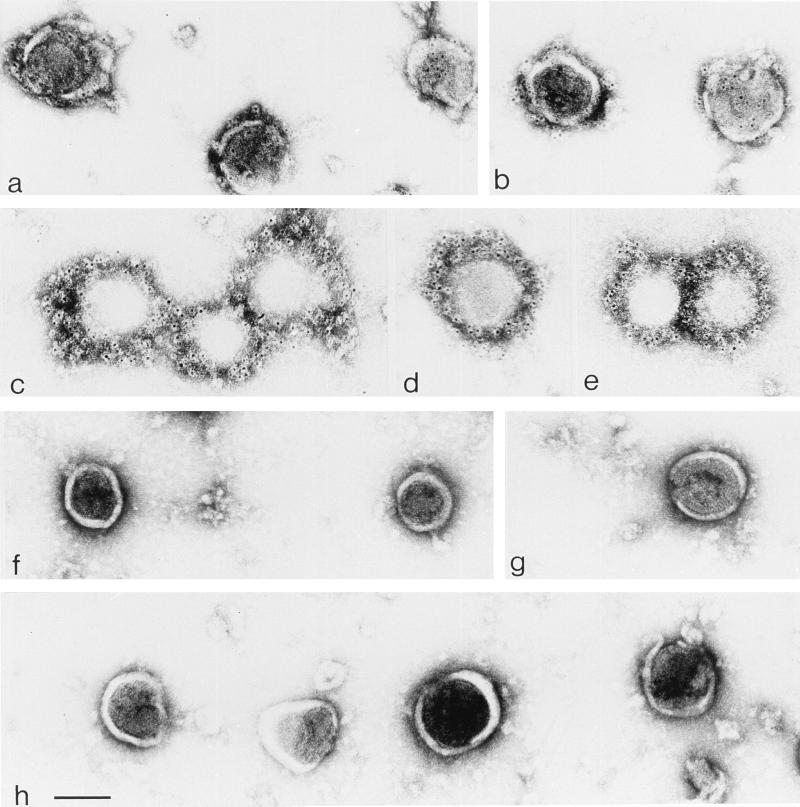
We wanted to confirm that the Wt-EGFR and Tr-EGFR components detected in the preparations of HIV-like particles and interpreted to represent incorporation were spatially tightly associated with the surface of these particles. For this purpose, HIV-like particle preparations were labelled with EGFR antibody and secondary gold-labelled antibodies and analyzed by electron microscopy. The visualized particles, which had remained unfixed and untreated during the labelling procedure, were of relatively uniform round size and not observed at all in samples of centrifuged supernatants from cells expressing Wt-EGFR or Tr-EGFR alone. Particles from supernatants of cells coexpressing pKEx-Wt-EGFR and pKEx-HIVΔEnv (Fig. 8, panels a and b) or from cells coexpressing pKEx-Tr-EGFR and pKEx-HIVΔEnv (Fig. 8, panels c through e) showed very significant gold labelling. The labelling was exclusively on, or in the vicinity of, the HIV-like particles, and virtually no gold particles could be observed in any other areas of the preparation. The negative controls were HIV-like particles from culture supernatants mixed and incubated as described above (Fig. 8, panels f and g) and HIV-like particles from cells expressing HIV-like particles in the absence of any EGFR species (Fig. 8, panel h). In these latter cases, no gold particles could be observed on or in the vicinity of the HIV-like particles.
FIG. 8.
Immunogold labelling of HIV-like particles. HIV-like particles derived from supernatants of cells coexpressing pKEx-Wt-EGFR and pKEx-HIVΔEnv (a and b), from supernatants of cells coexpressing pKEx-Tr-EGFR and pKEx-HIVΔEnv (c through e), from culture supernatant of cells expressing pKEx-Wt-EGFR alone mixed and incubated with the supernatant of cells expressing pKEx-HIVΔEnv alone (f and g), and from the culture supernatant of cells expressing pKEx-HIVΔEnv alone (h) were labelled with EGFR antibody and secondary antibody coupled to gold particles. Bar, 100 nm.
The immunogold labelling of the particles that had incorporated Tr-EGFR was dense, reflecting the very significant incorporation of this component, as illustrated by the results already described. The labelling of the HIV-like particles that had incorporated Wt-EGFR, although very significant, was clearly less dense than that of particles with Tr-EGFR. Presumably, this reflects the fact that, as indicated from the results in Fig. 3, in comparison to Tr-EGFR, incorporation of Wt-EGFR was reduced by a factor of about 5 and the actual number of incorporated Wt-EGFR molecules was reduced by a factor of about 7.5. Assuming that about half of the signal in the HIV-like particles with Wt-EGFR may be due to proteolytically degraded EGFR components, the immunolabelling of the full-length Wt-EGFR in these particles was still very significant, verifying the incorporation of this component into the HIV-like particles.
DISCUSSION
In this paper, we have addressed the question of whether a bulky foreign C terminus on a heterologous cell-surface glycoprotein sterically inhibits its incorporation into the membranes of HIV particles and, if so, whether this inhibition can be alleviated by truncation of the C-terminal domain. Wt-EGFR is a type 1 surface glycoprotein, as is HIV Env. It has a long cytoplasmic domain of 542 aa. Wt-EGFR and a truncated version with only 7 C-terminal aa (Tr-EGFR) were thus chosen as suitable molecules to address this question.
We could establish very significant incorporation of Tr-EGFR into HIV-like particles. Incorporation of Wt-EGFR was significantly decreased (by a factor of about 5 in comparison to that of Tr-EGFR) but, to our surprise, was clearly detectable. The negative controls were the centrifuged materials derived from the culture supernatants of cells expressing the glycoprotein species alone. Without exception (well over 10 experiments were performed), no Wt-EGFR or Tr-EGFR material could be detected in these samples. It is important to emphasize this fact, since in the case of certain other viral and chimeric glycoproteins we have observed that the glycoprotein of interest may be detectable to varying extents in the centrifuged materials obtained in the absence of virus-like particles (unpublished data), a situation which has also been described and further analyzed for VSV G glycoprotein (21). In these cases, and in the absence of further assays (e.g., infectivity conferred by the putatively incorporated glycoprotein), it is virtually impossible to reach a conclusion as to whether incorporation into particles has occurred or not. Importantly, as mentioned above, this was never observed with Wt-EGFR or Tr-EGFR, so that the finding of pelletable glycoprotein pointed to genuine incorporation of these components into the membranes of the HIV-like particles. This conclusion was further verified by a number of control experiments and, most convincingly, by immunoelectron microscopy. EGFR antibody-coated gold particles significantly labelled only the surface of the HIV-like particles with incorporated Wt-EGFR or Tr-EGFR, and virtually no gold particles were detected anywhere else in the purified virus preparation. In fact, the labelling of the HIV-like particles which had incorporated Tr-EGFR was very dense, pointing to the incorporation of this moiety being efficient. In a direct comparison of metabolically labelled virus particles, the number of Tr-EGFR molecules in HIV-like particles exceeded the number of gp120 molecules present in wild-type particles, expressed from a proviral construct, by a factor of about 5 (data not shown). Although detection of gp120, which can be lost from particles by shedding, gives an underestimate of the extent of HIV glycoprotein incorporation, this comparison nevertheless indicates that the incorporation of the overexpressed Tr-EGFR into HIV-like particles is presumably at least as efficient as that of the homologous viral glycoprotein. This lends further support to the notion that heterologous viral or cellular surface glycoproteins are incorporated into HIV-like particles (and presumably into retroviral particles in general) more or less passively, i.e., when circumstances which do not prevent incorporation are present. These inhibitory circumstances include interactions with other cellular proteins which prevent incorporation either sterically or by sequestering in subcellular regions different from the virus assembly site. Members of our group have previously demonstrated that this situation can apply to the incorporation of Wt-CD4 into HIV-like particles. Coexpression of its cellular interaction partner, p56lck, which binds to the C terminus of CD4, inhibited incorporation (9). Since we were unable to demonstrate a different subcellular localization of CD4 in the presence or absence of p56lck, we concluded that there had been steric inhibition of incorporation due to the presence of the bulky p56lck molecule at the C terminus of CD4. However, it cannot be strictly ruled out that, in the presence of p56lck, CD4 is sequestered away from the virus assembly site and that this could not be detected by the methods employed. Recently, evidence has been reported that HIV-1 glycoprotein, which is not incorporated into VSV particles, localized to plasma membrane domains distinct from the VSV budding sites. This distinct localization was mediated by a defined region in the cytoplasmic C terminus (11) and could account for the lack of incorporation, independent of a possible additional inhibitory role of the large size of the HIV glycoprotein C terminus. It is likely that in a differentiated cell, most cellular proteins will have functional interaction partners and thus be excluded from the membrane of retroviral particles. This would result in a passive enrichment of those cell-surface components lacking cellular interaction partners in the viral, as compared to the cellular, membrane. This situation would apply for Tr-EGFR expressed in 293T cells, and, in fact, the dense immunogold labelling of the HIV-like particles having incorporated this component strongly speaks for its enrichment in the viral membrane.
The demonstration of incorporation of Wt-EGFR into HIV-like particles was a surprising result since we had anticipated that the long cytoplasmic domain, which is foreign with respect to the viral core structure, would be sterically inhibitory. This anticipation was based on the fact that, in several cases, viral glycoproteins with long heterologous C termini have been excluded from incorporation into retroviral particles (14, 23, 27). However, in a recent report it was shown that murine leukemia virus vector particles pseudotyped with wild-type simian immunodeficiency virus Env, which has a long C-terminal domain, could mediate vector transduction into CD4+ cells (10). It is not clear how many incorporated Env molecules are required for infectivity, and in that report, neither the extent of incorporation nor the identity of the Env components was analyzed. As shown here for Wt-EGFR, proteolytically processed components may become preferentially incorporated, a possibility which was not analyzed in the case of wild-type simian immunodeficiency virus Env. In the present study, we directly visualized and quantitated incorporation of the expressed EGFR components and established that, in the case of the Wt-EGFR, the full-length molecule, and not exclusively a processed product, was incorporated into the HIV-like particles. Wt-EGFR is expressed slightly less at the cell surface than Tr-EGFR (Fig. 2B), which may be one of the factors contributing to its decreased incorporation. It is also not known to what extent Wt-EGFR and, in particular, its C-terminal domain may encounter interaction partners in 293T cells in which it is normally not expressed and how this may be influenced by natural ligands present in the medium (fetal calf serum). Thus the fivefold reduction in the extent of incorporation of Wt-EGFR in comparison to that of Tr-EGFR may be due to interactions with further cellular factors which themselves result in steric inhibition or altered subcellular localization away from the virus assembly site. Alternatively, steric inhibition by the very long Wt-EGFR C terminus itself may result in reduced but not completely abolished incorporation. Based on crystallographic data, it has been proposed that retroviral matrix proteins form a network of trimers with saucer-like depressions between neighboring trimers which is proposed to accommodate the cytoplasmic tail of gp41 (18; reviewed in reference 3). It is possible that the 542 C-terminal aa of the incorporated Wt-EGFR are somehow accommodated in this region. Alternatively, the matrix layer may not exclusively and tightly occupy the total space underlying the viral membrane, with the result that space could be available to accommodate this bulky C-terminal region.
In conclusion, the information gained from these analyses allows further refinement of models concerning the processes governing glycoprotein incorporation into retroviral particles. A hypothesis which is compatible with the data available at present is that viral and cellular glycoproteins are incorporated into retroviral particles when inhibitory circumstances do not prevail. Circumstances preventing incorporation depend on both the structure of the particular glycoprotein and the cell in which it is expressed. Surface proteins which interact with further cellular proteins may be excluded from incorporation either sterically or by sequestering to subcellular regions distinct from the viral assembly site. However, surface glycoproteins lacking cellular interaction partners would presumably be able to passively diffuse to the viral assembly site and potentially be incorporated. Although the results of previous analyses had suggested that the presence of a long heterologous C terminus per se would sterically inhibit incorporation, the possibility should now be considered that this may not, or may only partially, be the case. Further experiments should be aimed at distinguishing between the role of C-terminal regions in mediating distinct subcellular localizations as compared to direct steric inhibition at the viral assembly site. On the more practical side, these ongoing analyses provide information relevant to achieving incorporation of targeting molecules of interest into retroviral particles and indicate also that incorporation of those molecules, whose function may depend on a long cytoplasmic region, may be possible.
ACKNOWLEDGMENTS
We thank Khashayarsha Khazaie for supplying human EGFR DNA and EGFR antibody and Michael Pawlita and Thomas Wilk for fruitful discussions.
This work was supported by grant 01-KI-9412 from the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie.
REFERENCES
- 1.Bastiani L, Laal S, Kim M, Zolla-Pazner S. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J Virol. 1997;71:3444–3450. doi: 10.1128/jvi.71.5.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch V, Schwarz R T. Processing of gPr92env, the precursor to the glycoproteins of Rous sarcoma virus: use of inhibitors of oligosaccharide trimming and glycoprotein transport. Virology. 1984;132:95–109. doi: 10.1016/0042-6822(84)90094-1. [DOI] [PubMed] [Google Scholar]
- 3.Conte M, Matthews S. Retroviral matrix proteins: a structural perspective. Virology. 1998;246:191–198. doi: 10.1006/viro.1998.9206. [DOI] [PubMed] [Google Scholar]
- 4.Cosson P. Direct interaction between the envelope and matrix protein of HIV-1. EMBO J. 1996;15:5783–5788. [PMC free article] [PubMed] [Google Scholar]
- 5.Dettenhofer M, Yu X F. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J Virol. 1999;73:1460–1470. doi: 10.1128/jvi.73.2.1460-1467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong J, Roth M G, Hunter E. A chimeric avian retrovirus containing the influenza virus hemagglutinin gene has an expanded host range. J Virol. 1992;66:7374–7382. doi: 10.1128/jvi.66.12.7374-7382.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endres M J, Jaffer S, Haggerty B, Turner J D, Doranz B J, O’Brian P J, Kolson D L, Hoxie J A. Targeting of HIV- and SIV-infected cells by CD4-chemokine receptor pseudotypes. Science. 1997;278:1462–1464. doi: 10.1126/science.278.5342.1462. [DOI] [PubMed] [Google Scholar]
- 8.Freed E O, Martin M A. Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J Virol. 1995;69:1984–1989. doi: 10.1128/jvi.69.3.1984-1989.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henriksson P, Bosch V. Inhibition of cellular glycoprotein incorporation into human immunodeficiency virus-like particles by coexpression of additional cellular interaction partner. Virology. 1998;251:16–21. doi: 10.1006/viro.1998.9403. [DOI] [PubMed] [Google Scholar]
- 10.Indraccolo S, Minuzzo S, Feroli F, Mammano F, Calderazzo F, Chieco-Bianchi L, Amadori A. Pseudotyping of Moloney leukemia virus-based retroviral vectors with simian immunodeficiency virus envelope leads to targeted infection of human CD4+ lymphoid cells. Gene Ther. 1998;5:209–217. doi: 10.1038/sj.gt.3300603. [DOI] [PubMed] [Google Scholar]
- 11.Johnson J E, Rodgers W, Rose J K. A plasma membrane localisation signal in the HIV-1 envelope cytoplasmic domain prevents localisation at sites of vesicular stomatitis virus budding and incorporation into VSV virions. Virology. 1998;251:244–252. doi: 10.1006/viro.1998.9429. [DOI] [PubMed] [Google Scholar]
- 12.Kräusslich H-G, Ochsenbauer C, Traencker A-M, Mergener K, Fäcke M, Gelderblom H R, Bosch V. Analysis of protein expression and virus-like particle formation in mammalian cells lines stably expressing HIV-1 gag and env gene products with or without active HIV proteinase. Virology. 1993;192:605–617. doi: 10.1006/viro.1993.1077. [DOI] [PubMed] [Google Scholar]
- 13.Mammano F, Kondo E, Sodroski J, Bukovsky A, Göttlinger H G. Rescue of human immunodeficiency virus type 1 matrix protein mutants by envelope glycoproteins with short cytoplasmic domains. J Virol. 1995;69:3824–3830. doi: 10.1128/jvi.69.6.3824-3830.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mammano F, Salvatori F, Indraccolo S, De Rossi A, Chieco-Bianchi L, Göttlinger H. Truncation of the human immunodeficiency virus type 1 envelope glycoprotein allows efficient pseudotyping of Moloney murine leukemia virus particles and gene transfer into CD4+ cells. J Virol. 1997;71:3341–3345. doi: 10.1128/jvi.71.4.3341-3345.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mebatsion T, Finke S, Weiland F, Conzelmann K-K. A CXCR4/CD4 pseudotype rhabdovirus that selectively infects HIV-1 envelope protein-expressing cells. Cell. 1997;90:841–847. doi: 10.1016/s0092-8674(00)80349-9. [DOI] [PubMed] [Google Scholar]
- 16.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of non-dividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 17.Pfeiffer T, Zentgraf H, Freyaldenhoven B, Bosch V. Transfer of endoplasmic reticulum and Golgi retention signals to human immunodeficiency virus type 1 gp160 inhibits intracellular transport and proteolytic processing of viral glycoprotein but does not influence the cellular site of virus particle budding. J Gen Virol. 1997;78:1745–1753. doi: 10.1099/0022-1317-78-7-1745. [DOI] [PubMed] [Google Scholar]
- 18.Rao T, Belyaev A S, Fry E, Roy P, Jones I M, Stuart D I. Crystal structure of SIV matrix antigen and implications for virus assembly. Nature. 1995;378:743–747. doi: 10.1038/378743a0. [DOI] [PubMed] [Google Scholar]
- 19.Resh M D, Erikson R L. Highly specific antibody to Rous sarcoma virus src gene product recognises a novel population of pp60v-src and pp60c-src molecules. J Cell Biol. 1985;100:409–417. doi: 10.1083/jcb.100.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rittner C, Stöppler H, Pawlita M, Sczakiel G. Versatile eucaryotic vectors for strong and constitutive transient and stable gene expression. Methods Mol Cell Biol. 1991;2:176–181. [Google Scholar]
- 21.Rolls M M, Webster P, Balba N H, Rose J K. Novel infectious particles generated by expression of the vesicular stomatitis virus glycoprotein from a self-replicating RNA. Cell. 1994;79:497–506. doi: 10.1016/0092-8674(94)90258-5. [DOI] [PubMed] [Google Scholar]
- 22.Schnell M J, Johnson J E, Buonocore L, Rose J K. Construction of a novel virus that targets HIV-1 infected cells and controls HIV-1 infection. Cell. 1997;90:849–857. doi: 10.1016/s0092-8674(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 23.Schnierle B S, Stitz J, Bosch V, Nocken F, Merget-Millitzer H, Engelstädter M, Kurth R, Groner B, Cichutek K. Pseudotyping of murine leukemia virus with the envelope glycoproteins of HIV generates a retroviral vector with specificity of infection for CD4-expressing cells. Proc Natl Acad Sci USA. 1997;94:8640–8645. doi: 10.1073/pnas.94.16.8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ullrich A, Coussens L, Hayflick J S, Dull T J, Gray A, Tam A W, Lee J, Yarden Y, Libermann T A, Sclessinger J, Downward J, Mayes E L, Whittle N, Waterfield M D, Seeburg P H. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- 25.Waterfield M D, Mayes E L, Stroobant P, Bennet P L, Young S, Goodfellow P N, Banting G S, Ozanne B. A monoclonal antibody to the human epidermal growth factor receptor. J Cell Biochem. 1982;20:149–161. doi: 10.1002/jcb.240200207. [DOI] [PubMed] [Google Scholar]
- 26.Wilk T, Pfeiffer T, Bosch V. Retained in vitro infectivity and cytopathogenicity of HIV-1 despite truncation of the C-terminal tail of the env gene product. Virology. 1992;189:167–177. doi: 10.1016/0042-6822(92)90692-i. [DOI] [PubMed] [Google Scholar]
- 27.Wilk T, Pfeiffer T, Bukovsky A, Moldenhauer G, Bosch V. Glycoprotein incorporation and HIV-1 infectivity despite exchange of the gp160 membrane-spanning domain. Virology. 1996;218:269–274. doi: 10.1006/viro.1996.0190. [DOI] [PubMed] [Google Scholar]
- 28.Young J A T, Bates P, Willert K, Varmus H E. Efficient incorporation of human CD4 protein into avian leukosis virus particles. Science. 1990;250:1421–1423. doi: 10.1126/science.2175047. [DOI] [PubMed] [Google Scholar]