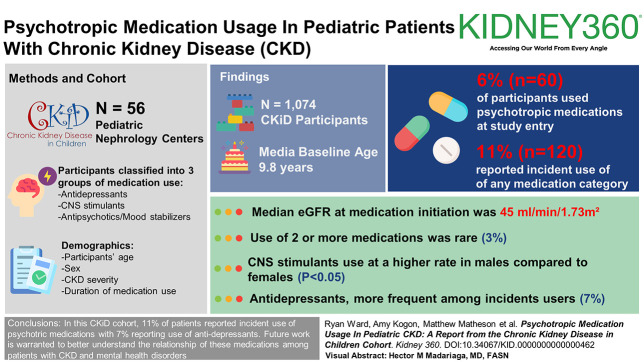
Visual Abstract
Keywords: CKD, pediatric nephrology, quality of Life
Abstract
Key Points
Psychotropic medication use is prevalent in the pediatric CKD population.
Central nervous system stimulant usage was more common in male patients, and antidepressant usage was more frequently reported at follow-up visits during teenage years.
Background
Mental health disorders within the pediatric CKD population are prevalent. The frequency is unknown with which psychotropic medications that commonly treat these conditions are used in this population.
Methods
Data from the CKD in Children (CKiD) cohort study were used to describe the use of psychotropic medications and patient-related characteristics of use. Medications were classified into three groups: antidepressants, central nervous system (CNS) stimulants, and antipsychotic/mood stabilizing medications. Participant age, sex, CKD severity, and duration of medication use were ascertained. Medication use was evaluated in parallel with CKD disease type, presence of urological comorbidity, and hypertension. Chi-square tests compared subgroup medication use.
Results
Among 1074 CKiD participants (median baseline age 9.8 years), 6% (n=60) used psychotropic medications at study entry with 11% reporting incident use of any medication category (n=120). CNS stimulants were most common at baseline. Antidepressants were more frequent among incident users at 7%. Use of two or more medications was rare (3%). Median eGFR at medication initiation was 45 ml/min per 1.73 m2. CNS stimulants were reported at a higher rate in male compared with female participants (P < 0.05).
Conclusions
Eleven percent of CKiD patients report incident use of any psychotropic medication, with 7% reporting incident use of antidepressants. Future work is warranted to better ascertain the frequency, safety, and efficacy of psychotropic medication usage in relationship to formal mental health disorder diagnoses in the pediatric CKD population.
Introduction
Pediatric chronic disease contributes to experiencing mental health symptoms and/or disorders, including anxiety, depression, and attention deficit hyperactivity disorder (ADHD).1 This is seen in pediatric patients with CKD who experience their physical, behavioral, social, and psychological development within the context of a host of CKD-related factors. While the prevalence of mental health disorders (MHDs) and associated symptoms within the pediatric CKD population have been described,2–5 there are no published data to establish the frequency and type of psychotropic medications used in this population.
Documenting psychotropic medication use in patients with CKD is important in that kidney impairment can affect the pharmacokinetics of psychotropic medications. In both adult and pediatric general populations, selective serotonin reuptake inhibitors (SSRIs) are the most prescribed medication to treat mood disorders.6 However, SSRIs are protein-bound and have limited ability to be removed by dialysis.1 Providers, therefore, may be reluctant to prescribe SSRIs to patients with CKD. Other medications used in the treatment of MHDs, including mood-stabilizing medications (e.g., lithium), can cause kidney impairment and damage in and of themselves and may require caution with use in patients with CKD.7 Moreover, central nervous system (CNS) stimulants are used frequently to treat ADHD, yet side effect considerations (e.g., hypertension, appetite suppression) may limit their use in the pediatric CKD population. As such, there is a need to understand the type and frequency of psychotropic medications used in pediatric patients with CKD. Finally, and owing to this lack of pharmacological data, the characteristics of medications used and pertinent patient-related characteristics (e.g., patient age, sex, duration of use, and CKD type and severity) are largely undescribed in the pediatric CKD population.
The primary objective of this study was to characterize the frequency of psychotropic pharmacotherapies within the CKD in Children (CKiD) cohort. Furthermore, medication use characteristics were evaluated to ascertain the category of agents used, duration of use, and the age/CKD stage at prescription. We hypothesized that antidepressant medications would comprise the most used category of psychotropic medications and that medication use would increase with both disease duration and severity. Finally, we sought to identify differences in psychotropic medication use on the basis of specific patient-level characteristics, including disease etiology and CKD-associated comorbidities. Hypertension was a variable of interest given its association with attention dysregulation.8 We hypothesized that children with non-glomerular CKD would experience more frequent use of psychotropic medications given the longer duration of CKD experienced by this subgroup. Finally, because CKD-associated comorbidities—specifically, need for urinary catheterization—has been associated with school absenteeism, we hypothesized that regular urinary catheterization would predict psychotropic medication usage given the potential for interrupted daily routines and separation from peers.9 Given the lack of literature on this topic and data available within the CKiD cohort study, our aims and hypotheses reflected an observational and descriptive study of psychotropic medication use independent of the presence of formal psychiatric diagnoses.
Methods
The CKiD study is a multicenter study involving 56 pediatric nephrology centers in North America since study inception in 2003.10,11 Details of study design, inclusion and exclusion criteria, and methods have been published.11 As part of CKiD study participation, all patients/parents record medication use—including non-CKD medications—at each annual visit. For this analysis, study inclusion required (1) completion of a CKiD visit with documentation of medications and (2) documentation of kidney function (eGFR).12 Children with severe intellectual disabilities and those with genetic syndromes with CNS manifestations were not eligible for study enrollment on the basis of exclusion criteria. The CKiD protocol adhered to the Declaration of Helsinki and was independently approved by each center's institutional review board.
Demographic Data
Demographic data are obtained/confirmed at each CKiD study visit and include patient age, sex, disease etiology (i.e., glomerular or non-glomerular), urinary catheterization use versus non-catheterization use, presence of hypertension, and household income. Race was not included in this analysis given its nature as a social construct; however, household income was included as an indicator to infer how this aspect of social determinants of health factored into psychotropic medication use. Families reported household income in the following discrete ranges: $0–$5999, $6000–$11,999, $12,000–$17,999, $18,000–$23,999, $24,000–$29,999, $30,000–$34,999, $36,000–$74,999, or ≥$75,000.
Psychotropic Medication Use
CKiD participants and/or parents were asked about all medication usage, including psychotropic agents. Specifically, participants/parents were asked, “Has your child taken any medication in the last 30 days?” Further pharmacological descriptive data were then attained, including medication name, route of administration, frequency, and dosage. Notably, the reason for prescription of a certain medication (e.g., psychiatric and mental health diagnoses) was not collected as part of CKiD study procedures. Psychotropic medications were categorized by the study team into three groups: antidepressants, CNS stimulants, and antipsychotics (including mood-stabilizing agents). Because clonidine and guanfacine can be used as antihypertensive agents, psychotropic medications, or both and data regarding the indication for their use were not collected as part of the study protocol, they were not included in analyses for this study.
Assessment of CKD
Creatinine- and cystatin-C–based estimations of kidney function were obtained at each study visit in addition to other CKD progression measures, including assessment of proteinuria as defined by a first-morning urine protein-to-creatinine ratio. From these values, the under age 25 eGFR was calculated.12
Statistical Analysis
To characterize the frequency of psychotropic pharmacotherapies within the CKiD cohort, data from both baseline and follow-up visits were compiled to establish the number of participants reporting medication use, the classes of medications used, participant age at time of report, duration of medication use, and CKD severity at time of report. Duration of usage was calculated as time (years) from first report of use until time medication was no longer reported. CKD stage at follow-up (incident) visit reporting was assessed via under age 25 eGFR. Both baseline medication use (medication use from the first CKiD study visit) and incident medication use (medication use at CKiD follow-up visits not reported at baseline) were included in the analyses.
We sought to detect differences in psychotropic medication use on the basis of patient-specific characteristics. CKiD cohort participants were divided into the following subgroups: sex (male versus female), disease origin (glomerular versus non-glomerular disease), urologic comorbidity (catheterization use versus non-catheterization use), presence of hypertension (on the basis of study visit BP categorized as elevated BP or stage 1–2 hypertension, or self-reported diagnosis of hypertension with antihypertensive medication use as per CKiD study protocol),11,13 and household income. We created a binary variable for household income which separated the six lesser categories and two larger categories (<$36,000/yr versus ≥$36,000/yr). $36,000 does not indicate a specific poverty level, as poverty is determined by individuals per household, but does represent financial difficulty for families with children with chronic illnesses. Participant sex was chosen given differences in predisposition to conditions (e.g., ADHD in males and depression in females). Since socioeconomic status (SES) is a strong factor involved in the social determinants of health, we selected household income as an indicator of SES for comparison. Chi-square tests compared medication usage among the various subgroups. For subgroup comparisons, we compared rates of ever-use (baseline or incident) to maximize data availability. To account for multiple subgroup comparisons, Holm method was used to adjust P values.
Results
Data were available for 1074 CKiD participants. Table 1 displays descriptive statistics for the entire cohort. The median age at baseline entry into the study was 9.8 years and median age of CKD onset was at 0 years. Table 2 illustrates the pharmacotherapy and patient-related characteristics for CKiD participants at study entry (baseline use) and follow-up visits (incident use). At baseline, 6% (n=60) of participants reported use of psychotropic medications, with CNS stimulants being the most prescribed category. Incident antidepressant use was reported for 7% (n=74) of participants and was the most frequently initiated medication during follow-up.
Table 1.
Descriptive statistics of the CKD in Children cohort (N=1074)
| Characteristic | Median (IQR) or n (%) |
|---|---|
| Age at baseline | 9.8 (4.9–14.2) |
| Age at CKD onset | 0.0 (0.0–1.5) |
| Duration of follow-up | 8.4 (5.4–10.8) |
| U25eGFR, ml/min per 1.73 m2 | 50 (36–64) |
| Male sex | 690 (64) |
| Black race | 235 (22) |
| Hispanic ethnicity | 158 (15) |
| Household income ≥$36,000/yr | 626 (60) |
| Maternal education | 398 (38) |
| High school or less some college | 281 (27) |
| College or more | 374 (36) |
| Glomerular etiology | 274 (26) |
| Catheter use | 71 (10) |
| Hypertension | 637 (59) |
| ADD or ADHD | 98 (9) |
| Depression | 51 (5) |
| Learning disability | 110 (10) |
| Anxiety | 36 (3) |
| Other neuropsychiatric condition | 48 (5) |
Other neuropsychiatric condition comprised collective free responses from CKD in Children participants/caregivers that did not correspond to preceding neuropsychiatric conditions. ADD, attention deficit disorder; ADHD, attention deficit hyperactivity disorder; IQR, interquartile range; U25eGFR, under age 25 eGFR.
Table 2.
Pharmacotherapy for mental health disorders within the CKD in Children Cohort (n=1074)
| Medication | Baseline Users, N (%) | Baseline Median Age (IQR) | Incident Users, N (%) | Incident Use Median Age (IQR) | Median Years (IQR) to Discontinuation | Median U25eGFR (IQR) at Incident Use |
|---|---|---|---|---|---|---|
| Antidepressants | 22 (2) | 15.4 (14.0–15.9) | 74 (7) | 17.4 (14.6–19.8) | 2.1 (0.9–5.8) | 44 (30–63) |
| CNS stimulants | 41 (4) | 13.0 (10.3–15.6) | 67 (6) | 12.8 (9.1–14.9) | 1.2 (0.5–4.6) | 49 (38–63) |
| Antipsychotics | 9 (1) | 14.9 (13.0–14.9) | 20 (2) | 17.3 (14.8–18.4) | 1.1 (0.8–2.5) | 41 (36–62) |
| Any one of the above | 60 (6) | 14.0 (10.9–15.7) | 120 (11) | 14.6 (11.4–18.0) | 47 (34–63) | |
| Any two of the above | 11 (1) | 14.9 (13.1–15.9) | 33 (3) | 15.5 (12.4–18.2) | 45 (34–64) |
CNS, central nervous system; IQR, interquartile range; U25eGFR, under age 25 eGFR.
Use of multiple medication categories was less common in the cohort, with 1% (n=11) and 3% (n=33) reporting two or more categories at baseline and incident visits, respectively. In the entire cohort, CNS stimulant use at baseline was reported at a median age of 13 years (interquartile range [IQR], 10.3–15.6) and at 12.8 years (IQR, 9.1–14.9) for incident users. Medications from other categories were reported later in adolescence with incident antidepressant use being reported at a median age 17.4 years (IQR, 14.6–19.8). For all incident users, the median eGFR at time of reporting medication use was 45 ml/min per 1.73 m2. Antidepressants had the greatest median years to discontinuation (2.1 years, IQR, 0.9–5.8), whereas antipsychotics had the fewest (1.1 years, IQR, 0.8–2.5).
Differences in psychotropic medication ever-use on the basis of CKiD subgroups are displayed in Table 3. A greater proportion of males compared with females reported use of CNS stimulants (P = 0.003). There were no differences in medication use on the basis of disease origin, catheterization use, baseline hypertension status, or household income.
Table 3.
Ever use of pharmacotherapy for mental health disorders among subgroups
| Medication Type | Sex | P Value | |
|---|---|---|---|
| Male (n=690) | Female (n=384) | ||
| Antidepressants | 58 (8%) | 38 (10%) | 1.00 |
| CNS stimulants | 87 (13%) | 21 (5%) | 0.003a |
| Antipsychotics | 19 (3%) | 10 (3%) | 1.00 |
| CKD Diagnosis | P Value | ||
|---|---|---|---|
| Non-Glomerular (n=800) | Glomerular (n=274) | ||
| Antidepressants | 68 (9%) | 28 (10%) | 1.00 |
| CNS stimulants | 86 (11%) | 22 (8%) | 1.00 |
| Antipsychotics | 22 (3%) | 7 (3%) | 1.00 |
| Urologic Comorbidity | P Value | ||
|---|---|---|---|
| Non-Catheterization (n=616) | Catheterization (n=71) | ||
| Antidepressants | 48 (8%) | 6 (9%) | 1.00 |
| CNS stimulants | 56 (9%) | 6 (8%) | 1.00 |
| Antipsychotics | 14 (2%) | 5 (7%) | 0.28 |
| Hypertension Status | P Value | ||
|---|---|---|---|
| Non-Hypertensive (n=434) | Hypertensive (n=637) | ||
| Antidepressants | 45 (10%) | 51 (8%) | 1.00 |
| CNS stimulants | 33 (9%) | 69 (11%) | 1.00 |
| Antipsychotics | 12 (3%) | 17 (3%) | 1.00 |
| Household Income | P Value | ||
|---|---|---|---|
| <$36,000/yr (n=409) | ≥$36,000/yr (n=626) | ||
| Antidepressants | 38 (9%) | 56 (9%) | 1.00 |
| CNS stimulants | 43 (11%) | 61 (10%) | 1.00 |
| Antipsychotics | 13 (3%) | 16 (3%) | 1.00 |
Hypertension status defined as elevated BP or stage 1/2 hypertension or self-reported diagnosis of hypertension with antihypertensive medication use. P values adjusted for 15 comparisons using the Holm method. CNS, central nervous system.
Denotes significance at P < 0.05.
Discussion
This is the first study to report frequencies of psychotropic pharmacotherapies in children and adolescents with CKD. CNS stimulants were more commonly reported by male patients and corresponded with a younger age at first report. Antidepressants were more commonly reported at incident visits and had the longest duration of use. Incident reporting of any medication initiation aligned with CKD stage 3.
The baseline CNS stimulant usage rates, age at report, and participant sex differences findings within our cohort were not unexpected. The National Survey of Children's Health during the previous decade estimated that upwards of 11% of youth had been diagnosed with ADHD, with male patients being more likely diagnosed than female patients and that 6.1% of respondents received medication for treatment.14 Consistent with previous CKiD results indicating that children with CKD are at increased risk of executive function deficits, nearly 10% of our cohort reported ever-use of CNS stimulants.10
Eleven percent of patients in the CKiD cohort reported incident use of any psychotropic medication during study participation, with 7% reporting incident use of antidepressants. According to Centers for Disease Control and Prevention data, 10.9% of adolescents in the general pediatric population aged 12–17 years received medication for any MHD,15 with 3.4% of adolescents specifically using antidepressants.16 In comparison with children with other chronic diseases, one single-center study reported antidepressant usage in 10.2% of oncology patients,17 whereas nearly 20% of patients with congenital heart disease are prescribed antidepressants in adulthood.18 Reporting from the United Kingdom indicated that among youth prescribed an antidepressant, 4.7% were prescribed a second psychotropic medication19 akin to the number our CKiD participants were prescribed multiple medications. Regarding duration of use, antidepressants were reported to have the longest duration of use. However, as previously mentioned, the reason for prescription, and by extension, discontinuation, were not obtained as part of CKiD study procedures.
The rate of use of antidepressants in our cohort (approximately 7%) appears equivalent to the previously reported rates of depression and depressive symptoms in the CKiD cohort.20 The potential pharmacokinetic properties of commonly used psychotropic agents may make some clinicians hesitant to prescribe them for patients with CKD.1 Thus, it is possible that psychotherapy is preferentially elected, rather than pharmacotherapy, which has the benefit in limiting their child's medications. Indeed, CKiD data demonstrated associations between number of CKD-related medications prescribed and worse quality of life.21
We evaluated associations of CKD severity, etiology, and comorbidities on the use of psychotropic medications. Our cohort reported incident use of medications at CKD stage 3, rather than at greater stages of CKD. Theoretically, decreased kidney function could predispose one to MHDs/symptoms due to physical ailments, interrupted daily routines caused by health care visits, and possibly altered neurocognition.1 Some reports indicate greater psychiatric burden with progressing kidney disease. For example, in pediatric patients with glomerular-origin disease, increasing proteinuria predicted mental health diagnoses.22 Another study suggested higher depression rates in adult patients on dialysis compared with pre-ESKD patients.23 Conversely, other studies suggest that pediatric CKD severity and duration do not lead to increased psychiatric diagnoses or worsened MHD symptoms.3,5,24 Previous CKiD cohort work has not found associations between depressive symptoms, eGFR, and/or disease duration.3 One explanation posited could be that most CKiD participants have kidney function consistent with CKD stage 3, indicating that reports of medication usage and CKD severity may simply reflect average CKiD participant statistics.
We found no difference in medication usage rates between patients with glomerular and non-glomerular disease. While much of our cohort comprised patients with non-glomerular etiologies, which have onset early in life relative to glomerular causes, a large subset of CKiD participants were diagnosed later in life. Theoretically, non-glomerular causes of CKD, such as vesicoureteral reflux, could increase the risk of MHDs and reliance upon pharmacotherapy because of their lifelong and unremitting treatment courses. Conversely, in those with glomerular disease, worse proteinuria has been associated with greater rates of psychiatric illness,22 which could potentially translate to more MHD-specific prescriptions for this patient population. Furthermore, glomerular diseases are often treated with corticosteroids, which affect mood. Opposite of our hypothesis, patients requiring urinary catheterization were not more likely to report use of psychotropic medications. It should be noted that catheterization use does not encompass all patients with CKD with urologic comorbidity because some with congenital anomalies have surgical interventions (i.e., vesicostomy) leading to incontinent drainage. Therefore, further studies including children with incontinent drainage may provide more details into psychotropic medication use in children with both CKD and urological comorbidities.
CKiD participants did not differ from peers without hypertension in psychotropic medication usage. In the general pediatrics literature, limited data suggest an association between hypertension and attention dysregulation,8 potentially predisposing children with high BP to require attention/behavior-modifying medications. Because stimulant ADHD medications may increase heart rate and BP, providers may hesitate to prescribe these to pediatric patients with CKD with or at risk of hypertension. However, National Health and Nutrition Examination Survey data and a recent meta-analysis suggest that stimulant medications do not increase the risk of hypertension in children and adolescents.25,26 Thus, provided that these medications are dosed appropriately, they appear to remain a viable option for attention regulation in those with CKD.
No significant associations between household income and medication usage were observed, despite strong associations between SES and MHDs.27 Possibly, the lack of association between household income and medication usage among households with lower income reflects a lack of access to treatment. Further research into the social determinants of health is required to investigate reasons for our findings.
This study does have several limitations. The observational nature of the CKiD cohort study prevents any causality determinations. Next, we used self-reported medication use, which can introduce recall bias. Another limitation is the possibility of forgetting to report a medication and/or inaccuracies in correctly reporting psychotropic medications by the caregiver/patient. To counter this, participants and/or caregivers are asked to bring medications and/or prescriptions to study visits for accurate documentation. As mentioned, indication for medication prescription was not obtained during CKiD study visits. Along these lines, our determination of duration of medication use was limited in this measure. Caregiver/patient report of medication discontinuation in between annual CKiD visits is not queried as part of the CKiD study. Thus, duration of use was calculated on the basis of the first CKiD study visit in which the medication was no longer reported. It is possible that medications were mistakenly not reported and subsequently counted as discontinued in our study, influencing the integrity of this variable. Furthermore, our dataset did not include provider-confirmed MHD diagnoses, preventing us from commenting on medication use in association with diagnosis. As indicated previously, an analysis accounting for MHD diagnoses lies outside the scope of this study. Moreover, participation in CKiD involves commitment to annual lengthy study visits. CKiD participants are likely to have strong family and social support to accommodate repeated study participation, which may not be representative of social support across the pediatric CKD population as a whole. Finally, while the CKiD cohort is the largest described cohort of pediatric patients with CKD worldwide, non-North American patients are not included. Furthermore, data regarding psychotropic medication use are lacking among other international registries, limiting comparisons with other populations. In addition, given the limitations in the collected data, our analytical model was unable to account for the numerous societal factors that may influence psychotropic medication use. Given our ever-use methodology to allow broadest inclusion of medication use, we leveraged data from multiple years for each patient, constraining analyses by age distribution or CKD stage. Finally, while all participants were enrolled before 2020, some data acquired for these analyses were captured during the severe acute respiratory syndrome coronavirus 2 outbreak. Because the pandemic could be a confounding variable in the rates of medication usage, we performed a post hoc sensitivity analyses with pre-2020 data. The results from this analysis were not different from those reported in this study. It should be mentioned that changes in psychotropic medication usage, such as prescribing practices and alterations to psychiatric diagnoses, have occurred since the CKiD study began and could also potentially confound medication usage. However, the coronavirus disease 2019 pandemic was unprecedented in its association with MHDs, hence our decision for this specific post hoc analysis.
Despite these limitations, our study has multiple, notable strengths. The CKiD study is a robust, diverse, North American wide cohort study with extensive kidney function and comorbidity-related data collected over more than 15 years. The robustness of this prospective research study allows us to provide the first report into the frequency of psychotropic pharmacotherapies within the pediatric CKD population. Importantly, documenting the use of psychotropic medications allows for tracking over time to evaluate prevalence of use as this population ages.
In summary, psychotropic medication use is prevalent within the pediatric CKD population. Our objectives were specific in their observational nature and lay the foundation for follow-up studies that should include thorough psychiatric evaluations, documented MHD diagnoses, family history, specific indications for medication prescription, and elucidation of usage patterns with disease etiologies, aging, and/or CKD progression.
Acknowledgments
Data in this manuscript were collected by the CKD in Children (CKiD) prospective cohort study with clinical coordinating centers (Principal Investigators) at Children's Mercy Hospital and the University of Missouri—Kansas City (Bradley Warady, MD) and Children's Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD and Derek Ng, PhD) at the Johns Hopkins Bloomberg School of Public Health. The CKiD website is located at https://statepi.jhsph.edu/ckid, and a list of CKiD collaborators can be found at https://statepi.jhsph.edu/ckid/site-investigators/.
Disclosures
Disclosure forms, as provided by each author, are available with the online version of the article at http://links.lww.com/KN9/A520.
Funding
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Diabetes and Digestive and Kidney Diseases (UO1 DK066143, U01 DK066174, U24 DK082194, and U24 DK066116), and National Heart, Lung, and Blood Institute.
Author Contributions
Conceptualization: Lyndsay A. Harshman, Stephen R. Hooper, Amy J. Kogon, Matthew B. Matheson, Ryan C. Ward.
Data curation: Susan L. Furth, Bradley A. Warady.
Formal analysis: Matthew B. Matheson, Ryan C. Ward.
Funding acquisition: Susan L. Furth, Bradley A. Warady.
Investigation: Susan L. Furth, Bradley A. Warady.
Methodology: Stephen R. Hooper.
Supervision: Lyndsay A. Harshman.
Writing – original draft: Lyndsay A. Harshman, Stephen R. Hooper, Amy J. Kogon, Matthew B. Matheson, Ryan C. Ward.
Writing – review & editing: Anne Dawson, Susan L. Furth, Stephen R. Hooper, Amy J. Kogon, Matthew B. Matheson, Stephen Molitor, Bradley A. Warady, Ryan C. Ward, Cynthia Wong.
Data Sharing Statement
Anonymized data created for the study are or will be available in a persistent repository upon publication. Aggregated Data. NIDDK Repository. The datasets generated during and/or analyzed during the current study are available in the NIDDK repository, https://repository.niddk.nih.gov/studies/ckid/.
References
- 1.Simões E Silva AC, Miranda AS, Rocha NP, Teixeira AL, Teixeira AL. Neuropsychiatric disorders in chronic kidney disease. Front Pharmacol. 2019;10:932. doi: 10.3389/fphar.2019.00932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreira JM, Bouissou Morais Soares CM, Teixeira AL, Simões E Silva AC, Kummer AM, Kummer AM. Anxiety, depression, resilience and quality of life in children and adolescents with pre-dialysis chronic kidney disease. Pediatr Nephrol. 2015;30(12):2153–2162. doi: 10.1007/s00467-015-3159-6 [DOI] [PubMed] [Google Scholar]
- 3.Kogon AJ Kim JY Laney N, et al. Depression and neurocognitive dysfunction in pediatric and young adult chronic kidney disease. Pediatr Nephrol. 2019;34(9):1575–1582. doi: 10.1007/s00467-019-04265-z [DOI] [PubMed] [Google Scholar]
- 4.Hooper SR Gerson AC Johnson RJ, et al. Neurocognitive, social-behavioral, and adaptive functioning in preschool children with mild to moderate kidney disease. J Dev Behav Pediatr. 2016;37(3):231–238. doi: 10.1097/DBP.0000000000000267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stahl JL, Wightman AG, Flythe JE, Weiss NS, Hingorani SR, Stoep AV. Psychiatric diagnoses in children with CKD compared to the general population. Kidney Med. 2022;4(6):100451. doi: 10.1016/j.xkme.2022.100451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forman-Hoffman VL, Viswanathan M. Screening for depression in pediatric primary care. Curr Psychiatry Rep. 2018;20(8):62. doi: 10.1007/s11920-018-0926-7 [DOI] [PubMed] [Google Scholar]
- 7.Gong R, Wang P, Dworkin L. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168–F1171. doi: 10.1152/ajprenal.00145.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuemmeler BF, Østbye T, Yang C, McClernon FJ, Kollins SH. Association between attention-deficit/hyperactivity disorder symptoms and obesity and hypertension in early adulthood: a population-based study. Int J Obes. 2011;35(6):852–862. doi: 10.1038/ijo.2010.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson KL, Weiss NS, Halbach S. Chronic school absenteeism of children with chronic kidney disease. J Pediatr. 2018;199:267–271. doi: 10.1016/j.jpeds.2018.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hooper SR Gerson AC Butler RW, et al. Neurocognitive functioning of children and adolescents with mild-to-moderate chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(8):1824–1830. doi: 10.2215/CJN.09751110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furth SL Cole SR Moxey-Mims M, et al. Design and methods of the chronic kidney disease in children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1(5):1006–1015. doi: 10.2215/CJN.01941205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce CB, Muñoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 2021;99(4):948–956. doi: 10.1016/j.kint.2020.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn JT Mitsnefes M Pierce C, et al. Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52(4):631–637. doi: 10.1161/HYPERTENSIONAHA.108.110635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visser SN Danielson ML Bitsko RH, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry. 2014;53(1):34–46.e2. doi: 10.1016/j.jaac.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black LI, Zablotsky B. Urgent care center and retail health clinic utilization among children: United States, 2019. NCHS Data Brief. 2020;381(393):1–8. PMID: 33270552 [PubMed] [Google Scholar]
- 16.Pratt LA, Brody DJ, Gu Q. Antidepressant use among persons aged 12 and over:United States,2011-2014. NCHS Data Brief. 2017;(283):1–8. PMID: 29155679 [PubMed] [Google Scholar]
- 17.Portteus A, Ahmad N, Tobey D, Leavey P. The prevalence and use of antidepressant medication in pediatric cancer patients. J Child Adolesc Psychopharmacol. 2006;16(4):467–473. doi: 10.1089/cap.2006.16.467 [DOI] [PubMed] [Google Scholar]
- 18.Kovacs AH Brouillette J Ibeziako P, et al. Psychological outcomes and interventions for individuals with congenital heart disease: a scientific statement from the American heart association. Circ Cardiovasc Qual Outcomes. 2022;15(8):e000110. doi: 10.1161/HCQ.0000000000000110 [DOI] [PubMed] [Google Scholar]
- 19.Cao TXD Fraga LFC Fergusson E, et al. Prescribing trends of antidepressants and psychotropic coprescription for youths in UK primary care, 2000-2018. J Affect Disord. 2021;287:19–25. doi: 10.1016/j.jad.2021.03.022 [DOI] [PubMed] [Google Scholar]
- 20.Kogon AJ Matheson MB Flynn JT, et al. Depressive symptoms in children with chronic kidney disease. J Pediatr. 2016;168:164–170.e1. doi: 10.1016/j.jpeds.2015.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Díaz-González de Ferris ME Pierce CB Gipson DS, et al. Health-related quality of life in children with chronic kidney disease is affected by the number of medications. Pediatr Nephrol. 2021;36(5):1307–1310. doi: 10.1007/s00467-021-04919-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desmond HE Lindner C Troost JP, et al. Association between psychiatric disorders and glomerular disease. Glomerular Dis. 2021;1(3):118–128. doi: 10.1159/000516359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer S Vecchio M Craig JC, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84(1):179–191. doi: 10.1038/ki.2013.77 [DOI] [PubMed] [Google Scholar]
- 24.Senses Dinc G, Cak T, Cengel Kultur E, Bilginer Y, Kul M, Topaloglu R. Psychiatric morbidity and different treatment modalities in children with chronic kidney disease. Arch Pediatr. 2019;26(5):263–267. doi: 10.1016/j.arcped.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 25.Hailpern SM Egan BM Lewis KD, et al. Blood pressure, heart rate, and CNS stimulant medication use in children with and without ADHD: analysis of NHANES data. Front Pediatr. 2014;2:100. doi: 10.3389/fped.2014.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L Yao H Li L, et al. Risk of cardiovascular diseases associated with medications used in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(11):e2243597. doi: 10.1001/jamanetworkopen.2022.43597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiss F. Socioeconomic inequalities and mental health problems in children and adolescents: a systematic review. Soc Sci Med. 2013;90:24–31. doi: 10.1016/j.socscimed.2013.04.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data created for the study are or will be available in a persistent repository upon publication. Aggregated Data. NIDDK Repository. The datasets generated during and/or analyzed during the current study are available in the NIDDK repository, https://repository.niddk.nih.gov/studies/ckid/.