Abstract
Introduction
Pulmonary mucosa-associated lymphoid tissue (MALT) lymphoma progresses with advancing disease stage. However, no standard treatment approach has been established. This single-center retrospective study evaluated clinical and radiological characteristics, treatment modalities, and long-term prognosis of pulmonary MALT lymphoma.
Methods
The study included 42 patients diagnosed with pulmonary MALT lymphoma between October 2004 and July 2019. Primary therapeutic modalities were determined using modified Ann Arbor staging. Therapeutic response was evaluated via computed tomography and laboratory analyses every 6 months for 5 years. Radiological findings were categorized based on the Lugano classification as complete response (CR), partial response, stable disease (SD), or progressive disease.
Results
Initial treatment included observation (n = 2), surgical resection (n = 6), or systemic chemotherapy (n = 34). Patients treated surgically had localized disease and achieved initial and long-term CR. Of the 34 patients who underwent chemotherapy, 30 achieved CR, 2 achieved SD, and 2 died. Overall and progression-free survival (PFS) rates were 93.9% and 54.3%, respectively. Multivariate analysis indicated that PFS was lower in patients with modified Ann Arbor stage III-IV lymphoma and those who did not achieve CR.
Conclusions
Optimized treatment based on anatomical location, pulmonary function, and disease stage can improve long-term survival in patients with pulmonary MALT lymphoma.
Keywords: Lung cancer, Radiology, Treatment outcome, Prognosis, Lymphoma of mucosa-associated lymphoid tissue, Long-term treatment
Introduction
Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT), also known as MALT lymphoma, accounts for 6–8% of all non-Hodgkin lymphomas (NHLs) [1]. Although primary NHL of the lung is very rare, accounting for 0.5–1.0% of all lung malignancies, the most frequent subset of primary lymphoma of the lung is MALT lymphoma [2], which frequently arises from bronchial-associated lymphoid tissue [1, 3] and is related to continuous antigen stimulation associated with chronic inflammation, smoking, and autoimmune diseases [4, 5]. Although respiratory symptoms are usually present at diagnosis, they are not specific to pulmonary MALT lymphoma [6]. However, various lung abnormalities can be observed on radiological imaging, including bilateral and multiple lesions on computed tomography (CT) in approximately 70% of patients [6, 7]. The median age at diagnosis is 50–60 years, and few cases are observed in patients under 30 years of age [2]. Pulmonary MALT lymphoma usually has an indolent course and remains localized in the lung for long periods before dissemination, and most patients have a favorable prognosis, with a 5-year overall survival (OS) rate of 90% [6, 8].
Available treatment modalities for pulmonary MALT lymphoma include surgical resection, systemic chemotherapy, radiotherapy, and observation [8–10]. Notably, pulmonary MALT lymphoma is often diagnosed via surgical resection, but lung cancer was initially highly suspected. Specifically, if the morphology of the lung mass suggests a high likelihood of primary lung cancer based on imaging findings and is resectable, clinicians often opt for video-assisted thoracoscopic surgical (VATS) lobectomy or wedge resection for both diagnostic and treatment purposes. Therefore, surgical resection can serve a dual purpose: diagnosing localized pulmonary MALT lymphoma, possibly leading to long-term remission, while ruling out lung cancer [8, 10]. Furthermore, less invasive diagnostic and therapeutic approaches, such as limited resection by thoracoscopy, have advanced in recent years, and some patients with localized disease may benefit from an observational approach (watch-and-wait) [10]. Given the indolent nature of pulmonary MALT lymphoma and the lack of uniform treatment approaches, especially for localized disease, further defining the roles of surgical resection and the observational approach is essential. In this single-center retrospective study, we evaluated clinical and radiological characteristics, treatment modalities, outcomes, and long-term prognosis among patients with pulmonary MALT lymphoma.
Materials and Methods
Patient Enrollment, Diagnosis, and Staging
We included 42 patients diagnosed with pulmonary MALT lymphoma at our institute between October 2004 and July 2019. All enrolled patients underwent pathological confirmation by two independent pathologists and were diagnosed using the morphological and immunophenotypic features defined by the 2017 World Health Organization classification [11].
In all patients, immunohistochemical staining was positive for CD20 and CD79a, and levels of CD5, CD10, CD23, cyclin D1, and Ki-67 were also evaluated to exclude patients with other types of low-grade lymphoma. Comprehensive reviews of medical records were performed to obtain information on patients’ laboratory values, demographics, smoking history, symptoms at presentation, diagnosis date, treatment modality, clinical prognosis, and last follow-up date. The staging was evaluated using the modified Ann Arbor staging system based on medical history, physical examination, bone marrow biopsy, and radiological findings, including neck, chest, and abdomen/pelvis imaging using fluorodeoxyglucose-positron emission tomography/CT (FDG-PET/CT) (online suppl. Table 1; for all online suppl. material, see https://doi.org/10.1159/000535228) [2]. Specifically, bone marrow biopsy was routinely performed as a part of the staging workup, as FDG-PET/CT might have under-detected marrow involvement by the lymphoma. Furthermore, chromosomal abnormalities, such as complex karyotype, in marrow involving lymphoma cells provide additional information about the prognosis of the disease [12]. Patients were stratified using the MALT lymphoma international prognostic index (MALT-IPI), as previously described (online suppl. Table 2) [13]. We classified CT findings according to the distribution of lung lesions as follows: (1) single nodular or consolidation, (2) multiple nodular or consolidation, (3) diffuse interstitial lung disease pattern, or (4) bronchiectasis and bronchiolitis [7].
Treatment and Response Evaluation
Patients were managed by the Catholic University Lymphoma Group, a multidisciplinary team including expert hematologists, pulmonologists, hematopathologists, radiologists, and nuclear medicine specialists. Surgical procedures were performed by an experienced thoracic surgeon at our institute, and primary therapeutic modalities were determined according to the modified Ann Arbor staging [2]. Patients diagnosed with single nodular unilateral pulmonary involvement, as stage IE, generally underwent surgical resection or close observation without any intervention. However, patients diagnosed with stage IIE or stage IE lymphoma with bilateral involvement of multifocal pulmonary lesions underwent surgical resection and/or systemic chemotherapy. VATS lobectomy or wedge resection was performed when the pulmonary lesions and mediastinal lymph nodes were completely resectable. Patients with stage III or IV lymphoma underwent systemic chemotherapy. Cyclophosphamide 750 mg/m2 on day 1, vincristine 1.4 mg/m2 on day 1, and prednisone 60 mg/m2 from day 1 to 5 (CVP) with or without rituximab 375 mg/m2 on day 1; cyclophosphamide 750 mg/m2 on day 1, doxorubicin 50 mg/m2 on day 1, vincristine 1.4 mg/m2 on day 1, and prednisone 60 mg/m2 from days 1 to 5 (CHOP) with or without rituximab 375 mg/m2 on day 1; or bendamustine 90 mg/m2 from days 1 to 2 and rituximab 375 mg/m2 on day 1 (BR) were the primary systemic chemotherapy regimens by physicians’ decisions. Chronic hepatitis B reactivation due to Rituximab usage (R-CVP, R-CHOP, and BR) should be considered in chronic hepatitis B highly prevalent nations such as Korea; notably, we routinely use a prophylaxis dose of oral Entecavir 0.5 mg daily in patients with positive hepatitis B core antibody IgG (HBcAb IgG positive).
All patients were evaluated for a therapeutic response using neck, chest, abdominal, and pelvic CT scans with FDG-PET and laboratory testing every 6 months over 5 years. Radiological findings were categorized based on the Lugano classification: complete response (CR), partial response, stable disease (SD), or progressive disease (PD) [14]. If patients presented with PD or relapsed after first-line treatment, surgical resection was performed for entirely resectable tumors. Salvage chemotherapy was administered in patients with advanced disease status or tumors that progressed following initial chemotherapy.
Statistical Analysis
Categorical variables were compared using χ2 and Fisher’s exact tests. Continuous variables were assessed using the Student’s t test and Mann-Whitney U-test. Progression-free survival (PFS) was defined as the time from diagnosis to PD, relapse after CR, or death, whichever occurred first. OS was defined as the time from diagnosis to death due to any cause. PFS and OS were estimated using the Kaplan-Meier method and compared using the log-rank test. Treatment-related mortality (TRM) was defined as death due to disease, considering patients who experienced relapse or unrelated deaths as competing risks. The cumulative incidence of relapse (CIR) was defined as relapse, considering TRM as a competing risk. TRM and CIR were calculated using cumulative incidence estimation, and comparisons between the groups were based on Gray’s competing risk methods. Univariate and multivariate analyses based on the Cox proportional regression model were used to calculate survival hazard ratios (HRs), and the Fine-Gray proportional hazard regression model was used to calculate the HRs for cumulative incidences. Multivariate modes were derived using stepwise selection among candidate variables with the Wald test for an overall p value for factors with more than two levels and a p value <0.05 to warrant inclusion in the model. Statistical significance was determined based on a two-tailed p value <0.05. All statistical analyses were conducted using SPSS (version 13.0, SPSS Inc., Chicago, IL, USA) and R-software (version 3.4.1, R Foundation for Statistical Computing, 2017).
Results
Patient Characteristics
Baseline characteristics for the 42 patients with pulmonary MALT lymphoma are provided in Table 1. The median age was 63 years (range: 39–87 years), with a slight male predominance (male: 54.8%; female: 45.2%). To obtain biopsy specimens, VATS lobectomy or wedge resection, CT-guided percutaneous core-needle biopsy, and transbronchial lung biopsy were used in 50.0%, 26.2%, and 23.8% of patients, respectively. During the diagnostic process, leukopenia, anemia, and thrombocytopenia were detected in 5 (11.9%), 13 (30.9%), and 2 (4.8%) patients, respectively. Cough (42.9%) was the most common initial symptom, followed by sputum (38.1%), dyspnea (9.5%), chest tightness (7.1%), and hemoptysis (4.8%). Approximately 38.1% of patients had a history of smoking, with a median of 15 packs per year (range: 2.0–40.0). The most common comorbidity was hypertension (33.3%), followed by old pulmonary tuberculosis sequelae (23.8%), previous malignancies (16.7%; two cases of early gastric cancer and thyroid cancer, and one each of renal cell carcinoma, supraglottic squamous cell carcinoma, and multiple myeloma), autoimmune disease or chronic obstructive pulmonary disease (7.1%; two cases of rheumatoid arthritis and one of systemic lupus erythematosus), and type 2 diabetes mellitus and tuberculosis-destroyed lung (4.8%). None of the patients had a history of Sjogren’s syndrome. Among 42 patients, 21 (50.0%) tested positive for HBcAb IgG, either with or without hepatitis B surface antibody (HBsAb) and received a prophylaxis dose of oral Entecavir 0.5 mg daily for 1 year following rituximab-containing chemotherapy. Notably, only 2 patients (4.8%) were positive for anti-hepatitis C antibodies, suggesting chronic hepatitis C infection. Initial or routine hepatitis B virus DNA or hepatitis C virus RNA real-time reverse transcriptase-quantitative polymerase chain reaction analyses were not conducted unless there was clinical evidence of hepatitis reactivation.
Table 1.
Clinical characteristics of patients with primary pulmonary MALT lymphoma (total n = 42)
| Characteristics | Values |
|---|---|
| Age, years, median (range) | 63.0 (39.0–87.0) |
| Age ≥70, n (%) | 11 (26.2) |
| Sex, n (%) | |
| Male | 23 (54.8) |
| Female | 19 (45.2) |
| Diagnostic methodology, n (%) | |
| VATS surgery | 21 (50.0) |
| VATS wedge resection | 12 (28.6) |
| VATS lobectomy | 9 (21.4) |
| CT-guided percutaneous lung biopsy | 11 (26.2) |
| Transbronchial lung biopsy | 10 (23.8) |
| ECOG performance status, n (%) | |
| 0–1 | 40 (93.2) |
| 2–4 | 2 (4.8) |
| Lactate dehydrogenase, IU/L, median (range) | 383.0 (143.0–550.0) |
| Normal, n (%) | 31 (73.8) |
| Elevated, n (%) | 11 (26.2) |
| B symptom, n (%) | |
| No | 33 (78.6) |
| Yes | 9 (21.4) |
| Initial cytopenia, n (%) | |
| Leukopenia | 5 (11.9) |
| Anemia | 13 (30.9) |
| Thrombocytopenia | 2 (4.8) |
| Clinical symptom at presentation, n (%) | |
| Cough | 18 (42.9) |
| Sputum | 16 (38.1) |
| Dyspnea | 4 (9.5) |
| Chest tightness | 3 (7.1) |
| Hemoptysis | 2 (4.8) |
| Smoking status, n (%) | |
| Never smoker | 26 (61.9) |
| Smoker | 16 (38.1) |
| Peak-per-year, median (range) | 15.0 (2.0–40.0) |
| Comorbidities, n (%) | |
| Hypertension | 14 (33.3) |
| Old pulmonary TB sequelae | 10 (23.8) |
| Previous malignancya | 7 (16.7) |
| Autoimmune diseaseb | 3 (7.1) |
| Chronic obstructive pulmonary disease | 3 (7.1) |
| Diabetes mellitus, type 2 | 2 (4.8) |
| TB destroyed lung | 2 (4.8) |
| Hepatitis B profile, n (%) | |
| HBsAb(−) HBsAg(+) HBcAb IgG(+) | 3 (7.2) |
| HBsAb(−) HBsAg(−) HBcAb IgG (+) | 5 (11.9) |
| HBsAb(+) HBsAg(−) HBcAb IgG(+) | 13 (30.9) |
| HBsAb(+) HBsAg(−) HBcAb IgG (−) | 17 (40.5) |
| HBsAb(−) HBsAg(−) HBcAb IgG (−) | 4 (9.5) |
| Anti-HCV Ab, n (%) | 2 (4.8) |
| MALT-IPI score, n (%) | |
| Low risk | 14 (33.3) |
| Intermediate risk | 20 (47.6) |
| High risk | 8 (19.1) |
| Radiological findings, n (%) | |
| Single nodular or consolidation | 19 (45.2) |
| Multiple nodular or consolidation | 16 (38.1) |
| Diffuse interstitial lung disease | 4 (9.5) |
| Bronchiectasis and bronchiolitis | 3 (7.1) |
| Modified Ann Arbor stage | |
| Localized stage, n (%) | |
| IE | 14 (33.3) |
| IIE | 11 (26.2) |
| Advanced stage, n (%) | |
| III | 7 (16.7) |
| IVc | 10 (23.8) |
Anti-HCV Ab, anti-hepatitis C virus antibody; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; HBcAb, hepatitis B core antibody; HBsAb, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; IU, international unit; MALT, mucosa-associated lymphoid tissue; MALT-IPI, marginal zone lymphoma of MALT-international prognostic index; TB, tuberculosis; VATS, video-assisted thoracic surgery.
aTwo early cases of gastric cancer and thyroid cancer, and each one of renal cell carcinoma, supraglottic squamous cell carcinoma, and multiple myeloma.
bTwo cases of rheumatoid arthritis and one case of systemic lupus erythematosus.
cAll stage IV diagnosed patients presented with extra-lymphatic organ or tissue involvement (4 of bone marrow, 2 of tonsils, 2 of nasopharynx, 1 of thyroid, and 1 of perirenal mass) without related symptoms. Biopsies of these extra-lymphatic involved organs or tissues confirmed them as MALT lymphoma, with no evidence of high-grade transformation.
At the time of diagnosis, 19.1% of patients had high-risk MALT-IPI scores. The radiological manifestations of all cases were reviewed and categorized into four different patterns (shown in Fig. 1; Table 1). Lung involvement in the CT scan was classified as single nodule/consolidation in 45.2%, multiple nodules/consolidations in 38.1%, diffuse interstitial lung disease in 9.5%, and bronchiectasis/bronchiolitis pattern in 7.1% of patients. Twenty-five patients had lung presentation only (n = 14, 33.3%) or lung with hilar, mediastinal lymph node, chest wall, or diaphragm involvement (n = 11, 26.2%) represented as stages IE-IIE (localized stage). Seventeen patients had extrapulmonary marginal zone lymphoma in the abdominal lymph node (n = 7, 16.7%) or extra-lymphatic organ or tissue involvement (n = 10, 23.8%) recognized after initial diagnosis (stages III-IV, advanced stage). Ten patients with stage IV lymphoma presented with extra-lymphatic organ or tissue involvement (4 patients with the involvement of bone marrow, 2 of tonsils, 2 of nasopharynx, 1 of thyroid, and 1 of perirenal mass) without any related symptoms. These lesions were biopsied and confirmed as MALT lymphoma without evidence of high-grade transformation.
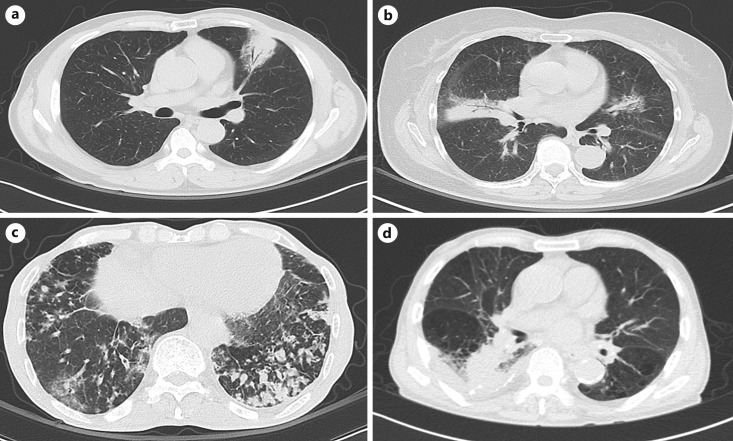
Fig. 1.
Radiological manifestations of primary pulmonary MALT lymphoma. a An oval mass-like consolidation with surrounding ground-glass opacities in the anterior segment of the left upper lobe with favorable contrast enhancement. b Multiple poorly marginated nodules with air bronchograms and nodules distributed along with the bronchovascular bundle. c Multifocal patchy ground-glass opacities and consolidations in both lungs, increased number of ill-defined nodules in bilateral lower lobes, combined with underlying bronchiectasis with diffuse centrilobular ill-defined nodules in both lungs with lower lobe predominance. d Irregular mass-like consolidation in the superior segment of the right lower lobe with obliteration of the superior segmental bronchus with small right pleural effusion and bronchiectasis in underlying pulmonary emphysema, combined with diffuse bronchial wall thickening.
Treatment Modalities and Responses
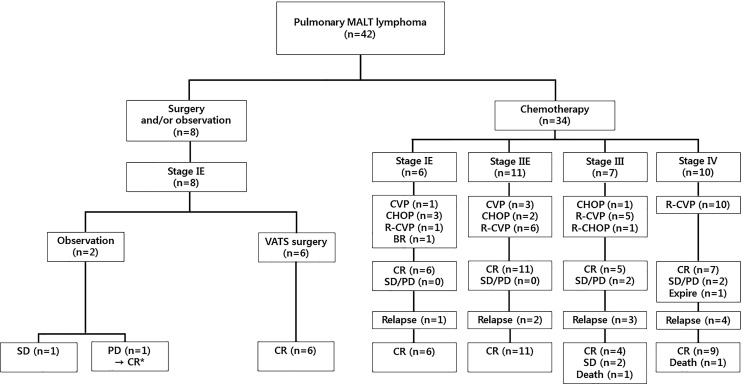
Initial treatment at the time of pulmonary MALT lymphoma diagnosis included a watch-and-wait strategy (n = 2); VATS surgery and observation (n = 6); systemic chemotherapy with CHOP (n = 6) and CVP (n = 4); or chemoimmunotherapy with R-CHOP (n = 1), R-CVP (n = 22), or BR (n = 1), as presented in Figure 2 and Table 2. The initial treatment response was evaluated based on findings from neck, chest, and abdomen/pelvic CT with FDG-PET-CT after six cycles of BR, CHOP, or R-CHOP and eight cycles of CVP or R-CVP. The same imaging modalities were utilized 6 months after surgery for patients treated with VATS lobectomy or wedge resection, and all patients treated with surgical resection had localized disease. All 6 patients who underwent VATS surgery (either lobectomy or wedge resection) achieved initial CR and remained in long-term remission. Two patients were diagnosed with pulmonary MALT lymphoma by transbronchial lung biopsy. Despite remnant lesions, the watch-and-wait strategy was adopted until PD. Of these, 1 patient remained in the SD category (follow-up duration, 24.1 months), and the other achieved CR after undergoing eight cycles of R-CVP due to disease progression.
Fig. 2.
Flowchart of pulmonary MALT lymphoma treatment based on therapeutic modality and modified Ann Arbor staging. *This patient achieved complete remission after undergoing eight cycles of R-CVP due to disease progression. MALT, mucosa-associated lymphoid tissue; VATS, video-assisted thoracoscopic surgery; CVO, central venous occlusion; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CVP, rituximab, cyclophosphamide, vincristine, and prednisone; BR, bendamustine and rituximab; CR, complete response; SD, stable disease; PD, progressive disease.
Table 2.
First-line treatment and clinical response stratified according to treatment modalities
| Modality | Number of patients | CR, n (%) | PR, n (%) | SD, n (%) | PD, n (%) | Death, n (%) | Relapse, n (%) | Final outcomes |
|---|---|---|---|---|---|---|---|---|
| Watch and wait | 2 | 0 | 0 | 2 (100)a | 0 | 0 | 0 | CR (1)/SD (1) |
| Surgery | ||||||||
| VATS lobectomy | 4 | 4 (100) | 0 | 0 | 0 | 0 | 0 | CR (4) |
| VATS wedge resection | 2 | 2 (100) | 0 | 0 | 0 | 0 | 0 | CR (2) |
| Systemic chemotherapy | ||||||||
| CHOP | 6 | 5 (83.3) | 1 (16.7)b | 0 | 0 | 1 (16.7) | 2 (33.3) | CR (5)/death (1) |
| CVP | 4 | 3 (75.0) | 1 (25.0)c | 0 | 0 | 0 | 2 (50.0) | CR (4) |
| R-CHOP | 1 | 1 (100) | 0 | 0 | 0 | 0 | 0 | CR (1) |
| R-CVP | 22 | 15 (68.2) | 5 (22.7)d | 0 | 2 (9.1) | 1 (4.6) | 7 (31.8) | CR (19)/SD (2)/death (1) |
| BR | 1 | 1 (100) | 0 | 0 | 0 | 0 | CR (1) | |
| Treatment response | 42 | 31 (73.8) | 7 (16.7) | 2 (4.8) | 2 (4.8) | 2 (4.8) | 11 (28.6) | CR (37)/SD (3)/death (2) |
BR, bendamustine and rituximab; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete remission; CVP, cyclophosphamide, vincristine, and prednisone; MALT, mucosa-associated lymphoid tissue, PR, partial remission; PD, progressive disease; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CVP, rituximab, cyclophosphamide, vincristine, and prednisone; SD, stable disease; VATS, video-assisted thoracic surgery.
aTwo patients were diagnosed with pulmonary MALT lymphoma via transbronchial lung biopsy and, despite having remnant lesions, opted for a watch-and-wait approach. One patient exhibited SD with a follow-up duration of 24.1 months, while the other achieved complete remission after undergoing eight cycles of R-CVP due to disease progression.
bThis patient relapsed 35.4 months after attaining CR and received supportive care due to age and low-performance status, ultimately succumbing to disease progression.
cThis patient finally achieved CR after six cycles of CVP and has maintained CR since.
dOne of the patients who achieved PR after four cycles of R-CVP died during the fifth cycle of R-CVP due to an underlying chronic hepatitis B flare-up.
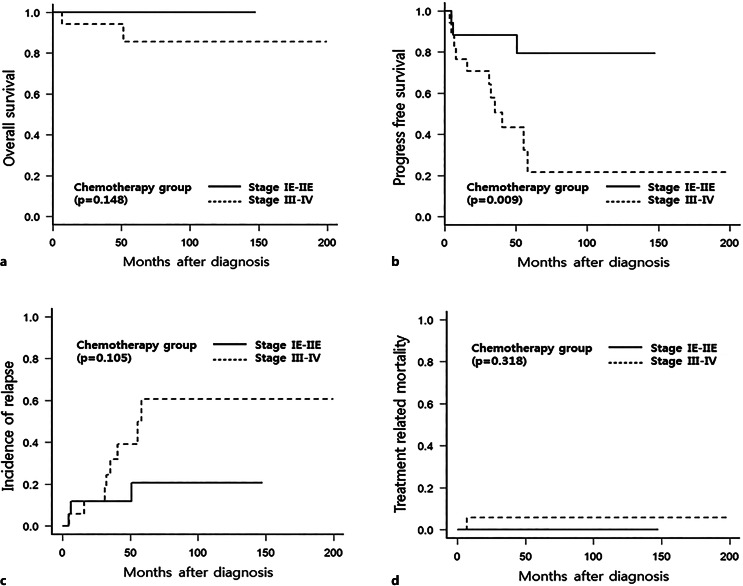
Among the 34 patients who underwent systemic chemotherapy, 30 achieved CR, 2 achieved SD, and 2 died (one due to disease progression and another due to a flare-up of underlying chronic hepatitis B during R-CVP treatment). No patient experienced a high-grade transformation of MALT lymphoma in this study. The detailed clinical characteristics, treatment history, and outcomes of the 17 patients with disease progression or relapse are presented in Table 3. In the subgroup analysis (shown in Fig. 3), chemotherapy outcomes demonstrated a trend of greater CIR in patients with advanced-stage disease. Furthermore, significantly higher PFS was observed in patients with localized-stage pulmonary MALT lymphoma, although patients who underwent systemic chemotherapy exhibited no difference in OS or TRM when those with localized- and advanced-stage disease were compared.
Table 3.
Detailed clinical characteristics of 17 patients with disease progression or relapsed primary pulmonary MALT lymphoma
| Patient | Modified Ann Arbor stage | Initial treatment | Initial treatment response | Follow-up | Salvage treatment | Final outcomes | Clinical outcomes |
|---|---|---|---|---|---|---|---|
| 1 | Stage IE | Observation | SD | Disease progression | R-CVP | CR | Alive |
| 2 | Stage IE | Observation | SD | N/A | N/A | N/A | Remains in SD (follow-up duration of 24.1 months) |
| 3 | Stage IIE | CHOP | CR | Relapse (lung, RLL, and LUL) | BR | CR | Alive |
| 4 | Stage III | CHOP | PR | Relapse (mediastinum and gastric) | BSC | PD | Expired due to disease progression |
| 5 | Stage IE | CVP | CR | Relapse (lung, RLL) | CHOP | CR | Alive |
| 6 | Stage IIE | CVP | CR | Relapse (mediastinum and liver) | CHOP and ASCT | CR | Alive |
| 7 | Stage IV | R-CVP | PR | N/A | N/A | N/A | Expired due to CHB aggravation |
| 8 | Stage III | R-CVP | PR | Disease progression | BR | CR | Alive |
| 9 | Stage IV | R-CVP | PD | Disease progression | DLICE→ESHAP and ASCT | CR | Alive |
| 10 | Stage III | R-CVP | CR | Relapse (mediastinum) | N/A | N/A | Remains in SD (follow-up duration of 28.8 months) |
| 11 | Stage IV | R-CVP | CR | Relapse (lung, LLL, and LNs) | BR | CR | Alive |
| 12 | Stage IV | R-CVP | CR | Relapse (lung, RLL) | VATS wedge resection | CR | Alive |
| 13 | Stage IV | R-CVP | CR | Relapse (both lung and tonsil) | DLICE | CR | Alive |
| 14 | Stage III | R-CVP | PR | Relapse (lung, RUL) | N/A | SD | Alive |
| 15 | Stage IV | R-CVP | PR | Relapse (lung, LLL) | VATS wedge resection | CR | Alive |
| 16 | Stage IV | R-CVP | PR | Disease progression | DHAP→Zevalin | CR | Alive |
| 17 | Stage III | R-CVP | PD | Disease progression | DLICE→DHAP→BR→ESHAP→EPOCH | SD | Alive |
ASCT, autologous hematopoietic stem cell transplantation; BR, bendamustine and rituximab; BSC, best supportive care; CHB, chronic hepatitis B infection; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete remission; CVP, cyclophosphamide, vincristine, and prednisone; DHAP, dexamethasone, high-dose cytarabine, cisplatin; DLICE, dexamethasone, l-asparaginase, ifosfamide, carboplatin, etoposide; EPOCH, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; ESHAP, etoposide, methylprednisolone, high-dose cytarabine, cisplatin; PR, partial remission; PD, progressive disease; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CVP, rituximab, cyclophosphamide, vincristine, and prednisone; SD, stable disease; VATS, video-assisted thoracic surgery.
Fig. 3.
OS, PFS, CIR, and TRM in patients with pulmonary MALT lymphoma who received systemic chemotherapy. Kaplan-Meier curve of OS (a) and PFS (b) and cumulative incidence of competing risk curve of CIR (c) and TRM (d).
Patients with modified Ann Arbor stage IE lymphoma and single nodular or consolidation pulmonary MALT lymphoma detected on CT underwent surgical resection or the watch-and-wait approach (observation/resection group, n = 8). As shown in Table 4, there were no significant differences associated with sex, MALT-IPI risk classification, diagnostic methods, relapse rate, or last follow-up treatment response, although the observation/resection group included a greater percentage of older adults (71.1 ± 2.9% vs. 61.0 ± 2.0%, p = 0.019) and demonstrated better initial treatment responses (CR, partial response, SD, and PD of 75.0%, 0%, 25.0%, and 0% vs. 80.6%, 20.6%, 0%, and 5.9%, p = 0.014). Differences between localized and advanced-stage clinical characteristics and treatment outcomes are presented in online supplementary Table 3.
Table 4.
Treatment modalities and responses in patients with pulmonary MALT lymphoma
| Characteristics | Observation/resection (n = 8) | Chemotherapy (n = 34) | p value |
|---|---|---|---|
| Age, mean, standard deviation | 71.1±8.1 | 61.0±11.5 | 0.019 |
| Male sex, n (%) | 4 (50.0) | 19 (55.9) | 1.000 |
| MALT-IPI score, n (%) | 0.533 | ||
| Low risk | 4 (50.0) | 10 (29.4) | |
| Intermediate risk | 3 (37.5) | 17 (50.0) | |
| High risk | 1 (12.5) | 7 (20.6) | |
| Modified Ann Arbor stage, n (%) | <0.001 | ||
| Stage IE | 8 (100.0) | 6 (17.6) | |
| Stage IIE | 0 (0) | 11 (32.4) | |
| Stage III | 0 (0) | 7 (20.6) | |
| Stage IV | 0 (0) | 10 (29.4) | |
| Radiological findings, n (%) | 0.008 | ||
| Single nodular or consolidation | 8 (100.0) | 11 (32.4) | |
| Multiple nodular or consolidation | 0 (0) | 16 (47.1) | |
| Diffuse interstitial lung disease | 0 (0) | 4 (11.8) | |
| Bronchiectasis and bronchiolitis | 0 (0) | 3 (8.8) | |
| Diagnostic method, n (%) | 0.094 | ||
| VATS lobectomy | 4 (50.0) | 5 (14.7)b | |
| VATS wedge resection | 2 (25.0) | 10 (29.4) | |
| CT-guided percutaneous lung biopsy | 0 (0.0) | 11 (32.4) | |
| Transbronchial lung biopsy | 2 (25.0)a | 8 (23.5) | |
| Initial treatment response, n (%) | 0.014 | ||
| CR | 6 (75.0) | 25 (80.6) | |
| Partial response | 0 (0.0) | 7 (20.6) | |
| SD | 2 (25.0) | 0 (0.0) | |
| PD | 0 (0.0) | 2 (5.9) | |
| Relapse of disease, n (%) | 0 (0.0) | 11 (32.4) | 0.086 |
| PD, n (%) | 1 (12.5) | 3 (8.8) | 1.000 |
| Treatment response, last f/u, n (%) | 0.648 | ||
| CR | 7 (87.5) | 30 (88.2) | |
| SD | 1 (12.5) | 2 (5.9) | |
| Died | 0 (0.0) | 2 (5.9) | |
| Follow-up duration, months, mean, standard deviation | 53.1±30.0 | 72.4±41.7 | 0.226 |
| Time to progression, months, mean, standard deviation | 43.2±14.7 | 50.9±45.3 | 0.419 |
CR, complete remission; CT, computed tomography; f/u, follow-up; MALT, mucosa-associated lymphoid tissue; MALT-IPI, marginal zone lymphoma of MALT-international prognostic index; PD, progressive disease; SD, stable disease; VATS, video-assisted thoracic surgery.
aTwo patients diagnosed with pulmonary MALT lymphoma by transbronchial lung biopsy decided not to receive further treatment due to refusal of treatment.
bAlthough two of the VATS lobectomy patients were in stage IE, patients required additional chemotherapy as consolidation.
Survival Outcomes and Prognostic Factors
Over a median follow-up period of 61.1 months (range: 6.7–199.4 months), OS and PFS were 93.9% (95% confidence interval [CI]: 76.9–98.5%) and 54.3% (95% CI: 34.8–70.3), respectively. CIR and TRM after completing first-line treatment were 38.5% (95% CI: 20.7–56.1) and 2.4% (95% CI: 0.2–11.0), respectively. We analyzed prognostic factors associated with survival outcomes. Associations between age, sex, stage, MALT-IPI risk classification, smoking status, bulky mass formation (≥3 cm), radiological findings at diagnosis and treatment modalities, initial treatment response, and PFS were evaluated to identify prognostic factors (Table 5). Multivariate analysis revealed that PFS was significantly lower in patients with modified Ann Arbor stage III-IV lymphoma (advanced-stage) (HR: 3.72; 95% CI: 1.11–12.39; p = 0.033) and in those who did not achieve initial CR (HR: 3.14; 95% CI: 1.08–9.18; p = 0.037). The results of the univariate analyses of survival outcomes in the entire cohort are presented in online supplementary Table 4.
Table 5.
Prognostic factors affecting PFS and relapse incidence in patients with primary pulmonary MALT lymphomaa
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| PFS (95% CI) | p value | HR (95% CI) | p value | |
| Age, years | 0.453 | |||
| <70 (n = 31) | 96.8% (79.2–99.5) | |||
| ≥70 (n = 11) | 85.7% (33.4–97.9) | |||
| Sex | 0.785 | |||
| Male (n = 23) | 54.7% (26.1–76.3) | |||
| Female (n = 19) | 55.1% (27.7–75.8) | |||
| Modified Ann Arbor stage | 0.002 | 0.033 | ||
| Localized stage (stages IE-IIE) (n = 25) | 78.7% (51.2–91.8) | 1.00 | ||
| Advanced stage (stages III-IV) (n = 17) | 21.7% (4.0–48.3) | 3.72 (1.11–12.39) | ||
| LDH | 0.778 | |||
| Normal LDH (n = 31) | 52.8% (29.4–71.7) | |||
| Elevated LDH (n = 11) | 54.5% (18.0–80.7) | |||
| B symptom | 0.376 | |||
| No (n = 33) | 62.6% (39.9–78.7) | |||
| Yes (n = 9) | 31.7% (4.9–64.7) | |||
| Smoking status | 0.656 | |||
| Never smoker (n = 26) | 52.6% (27.1–72.9) | |||
| Smoker (n = 16) | 57.1% (27.1–78.7) | |||
| Mass formation ≥3 cm | 0.398 | |||
| No (n = 26) | 54.5% (27.9–75.0) | |||
| Yes (n = 16) | 51.6% (22.9–74.2) | |||
| Radiological findings | 0.225 | |||
| Single consolidation (n = 19) | 69.5% (40.5–86.4) | |||
| Multiple consolidation (n = 16) | 39.4% (12.5–65.8) | |||
| Diffuse interstitial lung disease (n = 4) | 75.0% (12.8–96.1) | |||
| Bronchiectasis and bronchiolitis (n = 3) | 33.3% (0.9–77.4) | |||
| Initial treatment modality | 0.212 | |||
| Resection + observation (n = 8) | 80.0% (20.4–96.9) | |||
| Chemotherapy (n = 34) | 50.0% (29.6–67.4) | |||
| Initial treatment response | 0.001 | 0.037 | ||
| CR (n = 31) | 69.8% (45.6–84.8) | 1.00 | ||
| PR/SD/PD (n = 11) | 15.9% (1.1–47.5) | 3.14 (1.08–9.18) | ||
CR, complete remission; HR, hazard ratio; IPI, international prognostic index; LDH, lactate dehydrogenase; MALT-IPI, marginal zone lymphoma of MALT-international prognostic index; PD, progressive disease; PFS, progression-free survival; PR, partial remission; SD, stable disease.
aUnivariate analysis variables were selected based on prior literature on the currently known prognostic factors, which included advanced-stage disease, elevated LDH, failure to achieve CR after frontline therapy, or multifocal lung lesions. Multivariate modes were derived using stepwise selection among candidate variables with the Wald test for an overall p value for factors with more than two levels and a p value <0.05 to warrant inclusion in the model.
Discussion
We examined clinical characteristics, radiological findings, and treatment responses associated with survival outcomes in pulmonary MALT lymphoma in a sufficient number of patients from a single institute over a long follow-up period. Pulmonary MALT lymphoma is a rare disease, usually occurring in patients aged ≥50 years, and is characterized by indolent and favorable outcomes, but disease progression increases significantly as the disease stage advances [5, 7, 13, 15]. Our findings were in agreement with those of previous studies, in which the majority of patients (50–86%) were diagnosed with localized-stage disease (stages IE-IIE) and rarely had constitutional symptoms [8, 10]. Cough was generally the most frequent symptom, followed by sputum and dyspnea; however, it should be noted that symptoms or physical signs are usually nonspecific to pulmonary MALT lymphoma [6, 8, 10, 15]. As the disease progressed, the proportion of symptomatic patients increased. Chronic inflammation in the lungs, which may be associated with smoking, is believed to be the etiological cause of the development of bronchial-associated lymphoid tissue and subsequent pulmonary MALT lymphoma [1, 3]. In this study, 38.1% of patients were smokers, and the proportions of patients with a history of tuberculosis, comorbid chronic obstructive disease, and autoimmune disease were 23.8%, 7.1%, and 7.1%, respectively.
Due to its indolent characteristics, the overall outcomes of pulmonary MALT lymphoma in this study were excellent, with only two deaths occurring. During the nadir period of the 4th R-CVP, one case of TRM involved a hepatitis B flare-up that progressed to fulminant liver failure. The other case was due to disease progression in relapsed, refractory pulmonary MALT lymphoma. Importantly, relapse events were relatively frequent (11 relapse events and a CIR of 38.5% among patients). Specifically, 11 patients showed disease relapse; of these, 1 and 2 were stages IE and IIE, respectively. All three of these initial localized-stage patients experienced local relapse, with solitary relapsed lesions present in the right lower lobe. Moreover, 3 and 5 relapsed patients were at stages III and IV, respectively; only one stage III patient exhibited distant relapse, manifested as left inguinal lymphadenopathies. Overall, 10 out of 11 patients experienced localized relapse. In contrast to those included in previously published studies, most patients in our study were older (median, 63 years), with normal Eastern Cooperative Oncology Group status (93.2%) and lactate dehydrogenase (LDH; 73.8%), and an absence of B symptoms (78.6%). However, mediastinal lymphangitis spread, massive mass formation with or without cavitation, and advanced-stage (overall, 40.5%) disease with extra-lymphatic organ or tissue involvement (23.8%) were more frequent in our cohort. Furthermore, in this study, re-biopsy during relapse to identify pathological relapse and/or high-grade transformation was performed as frequently as possible; however, no cases of high-grade transformation were found. The incidence of high-grade transformation of pulmonary MALT lymphoma is unknown, and previous studies found no high-grade transformations among pulmonary MALT lymphoma patients, as corroborated by our cohort [16]. Among the 42 patients in this study, no secondary malignancies were reported during the prolonged follow-up. However, 4 patients had previously had thyroid cancer (n = 2), renal cell carcinoma (n = 1), and supraglottic squamous cell carcinoma (n = 2) before pulmonary MALT lymphoma diagnosis. This prior cancer history led to a more aggressive diagnostic process, yet no cases of relapse or other secondary malignancies unrelated to pulmonary MALT lymphoma were identified.
Established treatment options for pulmonary MALT lymphoma include observation, surgical resection, radiotherapy, and systemic chemotherapy. For patients with localized pulmonary MALT lymphoma, surgical resection results in an excellent long-term prognosis [2, 6, 8–10, 17]. Radiation is another reasonable treatment option for localized lesions, and excellent outcomes have been observed in patients treated with radiation therapy at a median dose of 3,400 cGy (range: 3,000–3,600 cGy) [18]. However, we did not routinely use radiation therapy for localized pulmonary or mediastinal lesions due to potential complications such as pulmonary fibrosis or cardiac toxicity. Notably, surgical resection for pulmonary MALT lymphoma is controversial, as several experts have suggested that surgical resection should not be the treatment of choice due to the risks of complications and a relatively high relapse rate. One study found no difference in the survival rate between patients who underwent surgical resection and those who underwent chemotherapy [15]. Another study reported the successful diagnosis of pulmonary NHL by either open thoracotomy or limited VATS surgery; however, no survival benefits were observed for complete versus incomplete resection [2]. More recently, studies have shown that, despite no difference in OS, chemotherapy provides longer PFS than does surgical resection, even in patients with limited-stage pulmonary MALT lymphoma [15, 19, 20]. In contrast, surgical resection has been considered a suitable treatment for pulmonary MALT lymphoma limited to one side of the lung and is associated with long-term CR and minimal postoperative complications [2, 8–10, 17, 18, 21]. Our study suggests that surgical resection is an effective treatment modality, although its use should be limited to managing localized, single lesions in stage IE pulmonary MALT lymphoma.
Surgery is not a primary option in the case of stomach MALT lymphoma, and radiation therapy is considered mainly after the failure of Helicobacter pylori eradication treatment or locally advanced disease involving regional lymph nodes [22]. However, radiotherapy has only limited indications as an initial treatment in pulmonary cases. Therefore, if surgical resection is selected as a treatment modality in pulmonary MALT lymphoma, the validity and scope of the operation should be thoroughly determined through the multidisciplinary care service. Accurate evaluation using a multidisciplinary approach should be emphasized to identify resectable lesions in these patients. Patient age, comorbidities, and lung function should be carefully considered when establishing surgical margins to ensure complete resection while preserving lung function and quality of life. With surgical resection, patients with lymphoma lesions that are limited to a single isolated nodule or consolidated in the unilateral lung field may achieve long-term complete remission. However, when repeated recurrence is followed by repeated surgeries, pulmonary function cannot be preserved, and there is an increased risk of adverse events related to surgery, especially in older patients. Therefore, the application and scope of surgical treatment should be carefully selected based on a thorough evaluation of the tumor characteristics. If patients are asymptomatic or unable to receive treatment, an observational strategy may be another suitable option for an indolent disease course.
In accordance with our institution’s treatment policy, we chose to pursue an aggressive approach for pulmonary MALT lymphoma using surgical resection and/or chemotherapy, even though low-grade lymphoma progresses slowly and expectant monitoring is acceptable. Our aim was to achieve superior survival outcomes and completely rule out the possibility of other aggressive malignancies, such as lung cancer. Consequently, complete surgical resection using VATS was performed when feasible, yielding excellent long-term PFS and potentially curative outcomes in patients with localized pulmonary MALT lymphoma, as supported by several prior studies [2, 8–10, 18, 19, 21]. Regardless of subtype, the standard chemotherapy regimen for all indolent lymphomas in Korea is R-CVP, with national insurance covering only stage III or IV. Although we considered CHOP, an anthracycline-included regimen, we preferred initiating treatment with a Rituximab-included regimen and reserving anthracycline for salvage purposes. The CVP regimen was used for patients who, preferring treatment to a watch-and-wait approach, mostly presented with lymphoma-related symptoms. R-CHOP and BR, which are not chemotherapy regimens reimbursed by national insurance, were recommended at the patient’s own expense for advanced-stage pulmonary MALT lymphoma or when a high-grade transformation could not be ruled out. Overall, 17 of 25 patients with localized-stage pulmonary MALT lymphoma opted for systemic chemotherapy due to lymphoma-related symptoms such as chronic cough (n = 8), sputum (n = 8), dyspnea (n = 1), chest tightness (n = 2), and hemoptysis (n = 2), which persisted for more than 3 months and caused anxiety. Furthermore, if the pulmonary lesions displayed a dispersed pattern and were unresectable, or if patients had underlying cardiopulmonary diseases such as chronic obstructive pulmonary disease or congestive heart failure that could significantly impair lung function after surgery, localized-stage patients choosing lymphoma treatment typically underwent systemic chemotherapy.
Currently known prognostic factors for MALT lymphoma include advanced-stage disease, elevated LDH, failure to achieve CR after frontline therapy, or multifocal lung lesions [6, 8, 10, 17, 18]. Similarly, advanced-stage disease and inadequate initial treatment responses were associated with lower PFS in the current study. Multivariate analyses revealed that no factors were associated with a shorter OS (online suppl. Table 2).
There are several limitations to this study. Due to the indolent nature of pulmonary MALT lymphoma with a long time-to-event, relatively rare events occurred in many censored patients, which may have introduced some bias in the study. Furthermore, we could not analyze in-depth treatment outcomes for surgical resection of multifocal lesions in the unilateral lung or compare lobectomy versus partial wedge resection due to the limited number of patients. We were also unable to evaluate radiotherapy outcomes when compared with other modalities. This was a single-institution retrospective study, and many previously known negative prognostic variables were not reproducible because of the relatively small number of patients, which may have led to errors and possible bias. Furthermore, we did not analyze the prevalence of Achromobacter xylosoxidans, which has been associated with the incidence of pulmonary MALT lymphoma, especially in European countries [23]. However, prevalence rates may differ significantly according to country or race, and this controversial issue warrants further investigation within Asian populations.
At our institution, we routinely administer systemic chemotherapy for enhanced clinical outcomes when indicated, and indolent lymphoma is no exception. Owing to this therapeutic approach, half of the patients with localized disease (n = 17, 50% of chemotherapy-treated patients) underwent systemic chemotherapy as the first-line treatment, potentially introducing selection bias. We discussed the treatment decision with the patient, explained the risks of treatment-related complications, and informed them that “watch-and-wait” is a worldwide consensus for indolent lymphoma. Additionally, we elucidated the benefits of treatment, highlighting the possibility of long-term remission and control of lymphoma-related symptoms. However, while we cited several expert opinions suggesting that surgical resection is an effective treatment modality specifically for managing single lesions [2, 6, 8–10, 17], aggressive intervention in indolent lymphoma could not be entirely justified. Moreover, while we chose chemotherapy for localized-stage pulmonary MALT lymphoma, the PFS benefits were not as significant as those observed in advanced-stage patients.
In conclusion, the current results indicate that pulmonary MALT lymphoma tends to be an indolent disease with favorable long-term treatment outcomes and frequent relapses, especially in patients with advanced-stage disease. Given that disease stage and initial treatment responses were associated with lower PFS, large-scale multicenter studies are required to further evaluate appropriate therapeutic modalities for pulmonary MALT lymphoma and associated prognostic factors.
Acknowledgments
We wish to thank the members of the Catholic University Lymphoma Group network for their valuable contribution to gathering patient information and the care of these patients in multidisciplinary care service. The authors have no financial or proprietary disclosure concerning this article.
Statement of Ethics
The study protocol was approved according to the guidelines of the Institutional Review Board (IRB) and Ethics Committee of the Catholic Medical Center, Republic of Korea (KC21RASI0635) and conducted according to the tenets of the Helsinki Declaration. Because this research involved minimal risk and it was not possible to conduct without a waiver of informed consent, obtaining consent from participants was not applicable to this study. The waiver of informed consent did not adversely affect the rights and welfare of the study participants and was approved by the IRB.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research was supported by Catholic University of Korea Open Access Agreements.
Author Contributions
G.-J.M. performed the research, collected and analyzed the data, and wrote the manuscript. C.K.R. provided patients and reviewed the manuscript. Y-W.J., J.H.O., B.-O.C., and G.S.P. provided the materials and reviewed the manuscript. T.Y.K. reviewed the manuscript and analyzed the data. S.-G.C. designed and conducted the study, provided patients and materials, analyzed data, and wrote the manuscript. All authors read and approved the final manuscript.
Funding Statement
This research was supported by Catholic University of Korea Open Access Agreements.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.
Supplementary Material
References
- 1. Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52(8):1410–6. [DOI] [PubMed] [Google Scholar]
- 2. Ferraro P, Trastek VF, Adlakha H, Deschamps C, Allen MS, Pairolero PC. Primary non-Hodgkin’s lymphoma of the lung. Ann Thorac Surg. 2000;69(4):993–7. [DOI] [PubMed] [Google Scholar]
- 3. Li G, Hansmann ML, Zwingers T, Lennert K. Primary lymphomas of the lung: morphological, immunohistochemical and clinical features. Histopathology. 1990;16(6):519–31. [DOI] [PubMed] [Google Scholar]
- 4. Zucca E, Bertoni F. The spectrum of MALT lymphoma at different sites: biological and therapeutic relevance. Blood. 2016;127(17):2082–92. [DOI] [PubMed] [Google Scholar]
- 5. Deng W, Wan Y, Yu JQ. Pulmonary MALT Lymphoma has variable features on CT. Sci Rep. 2019;9(1):8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borie R, Wislez M, Thabut G, Antoine M, Rabbat A, Couderc LJ, et al. Clinical characteristics and prognostic factors of pulmonary MALT lymphoma. Eur Respir J. 2009;34(6):1408–16. [DOI] [PubMed] [Google Scholar]
- 7. Bae YA, Lee KS, Han J, Ko YH, Kim BT, Chung MJ, et al. Marginal zone B-cell lymphoma of bronchus-associated lymphoid tissue: imaging findings in 21 patients. Chest. 2008;133(2):433–40. [DOI] [PubMed] [Google Scholar]
- 8. Stefanovic A, Morgensztern D, Fong T, Lossos IS. Pulmonary marginal zone lymphoma: a single centre experience and review of the SEER database. Leuk Lymphoma. 2008;49(7):1311–20. [DOI] [PubMed] [Google Scholar]
- 9. Ahmed S, Kussick SJ, Siddiqui AK, Bhuiya TA, Khan A, Sarewitz S, et al. Bronchial-associated lymphoid tissue lymphoma: a clinical study of a rare disease. Eur J Cancer. 2004;40(9):1320–6. [DOI] [PubMed] [Google Scholar]
- 10. Sammassimo S, Pruneri G, Andreola G, Montoro J, Steffanoni S, Nowakowski GS, et al. A retrospective international study on primary extranodal marginal zone lymphoma of the lung (BALT lymphoma) on behalf of International Extranodal Lymphoma Study Group (IELSG). Hematol Oncol. 2016;34(4):177–83. [DOI] [PubMed] [Google Scholar]
- 11. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed, Vol. 2. Lyon, France: IARC Press; 2017. [Google Scholar]
- 12. Min GJ, Jeon YW, Park SS, Shin SH, Yahng SA, Yoon JH, et al. Poor prognosis in patients with diffuse large B cell lymphomas with bone marrow involvement possessing chromosomal abnormalities, despite aggressive treatment. Ann Hematol. 2020;99(3):557–70. [DOI] [PubMed] [Google Scholar]
- 13. Thieblemont C, Cascione L, Conconi A, Kiesewetter B, Raderer M, Gaidano G, et al. A MALT lymphoma prognostic index. Blood. 2017;130(12):1409–17. [DOI] [PubMed] [Google Scholar]
- 14. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oh SY, Kim WS, Kim JS, Kim SJ, Kwon HC, Lee DH, et al. Pulmonary marginal zone B-cell lymphoma of MALT type--what is a prognostic factor and which is the optimal treatment, operation, or chemotherapy? consortium for Improving Survival of Lymphoma (CISL) study. Ann Hematol. 2010;89(6):563–8. [DOI] [PubMed] [Google Scholar]
- 16. Maeshima AM, Taniguchi H, Toyoda K, Yamauchi N, Makita S, Fukuhara S, et al. Clinicopathological features of histological transformation from extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue to diffuse large B-cell lymphoma: an analysis of 467 patients. Br J Haematol. 2016;174(6):923–31. [DOI] [PubMed] [Google Scholar]
- 17. Lee H, Yang B, Nam B, Jeong BH, Shin S, Zo JI, et al. Treatment outcomes in patients with extranodal marginal zone B-cell lymphoma of the lung. J Thorac Cardiovasc Surg. 2017;154(1):342–9. [DOI] [PubMed] [Google Scholar]
- 18. Husnain M, Kuker R, Reis IM, Iyer SG, Zhao W, Chapman JR, et al. Clinical and radiological characteristics of patients with pulmonary marginal zone lymphoma: a single center analysis. Cancer Med. 2020;9(14):5051–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang L, Xia ZJ, Zhang YJ, Huang HQ, Lin TY, Lu Y. Radical surgery may be not an optimal treatment approach for pulmonary MALT lymphoma. Tumour Biol. 2015;36(8):6409–16. [DOI] [PubMed] [Google Scholar]
- 20. Zinzani PL, Pellegrini C, Gandolfi L, Casadei B, Derenzini E, Broccoli A, et al. Extranodal marginal zone B-cell lymphoma of the lung: experience with fludarabine and mitoxantrone-containing regimens. Hematol Oncol. 2013;31(4):183–8. [DOI] [PubMed] [Google Scholar]
- 21. Imai H, Sunaga N, Kaira K, Kawashima O, Yanagitani N, Sato K, et al. Clinicopathological features of patients with bronchial-associated lymphoid tissue lymphoma. Intern Med. 2009;48(5):301–6. [DOI] [PubMed] [Google Scholar]
- 22. Matysiak-Budnik T, Fabiani B, Hennequin C, Thieblemont C, Malamut G, Cadiot G, et al. Gastrointestinal lymphomas: French Intergroup clinical practice recommendations for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFH). Dig Liver Dis. 2018;50(2):124–31. [DOI] [PubMed] [Google Scholar]
- 23. Adam P, Czapiewski P, Colak S, Kosmidis P, Tousseyn T, Sagaert X, et al. Prevalence of Achromobacter xylosoxidans in pulmonary mucosa-associated lymphoid tissue lymphoma in different regions of Europe. Br J Haematol. 2014;164(6):804–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.