Abstract
Venom systems are complex traits that have independently emerged multiple times in diverse plant and animal phyla. Within each venomous lineage there typically exists interspecific variation in venom composition where several factors have been proposed as drivers of variation, including phylogeny and diet. Understanding these factors is of broad biological interest and has implications for the development of antivenom therapies and venom-based drug discovery. Because of their high species richness and the presence of several major evolutionary prey shifts, venomous marine cone snails (genus Conus) provide an ideal system to investigate drivers of interspecific venom variation. Here, by analyzing the venom gland expression profiles of ∼3,000 toxin genes from 42 species of cone snail, we elucidate the role of prey-specific selection pressures in shaping venom variation. By analyzing overall venom composition and individual toxin structures, we demonstrate that the shifts from vermivory to piscivory in Conus are complemented by distinct changes in venom composition independent of phylogeny. In vivo injections of venom from piscivorous cone snails in fish further showed a higher potency compared with venom of nonpiscivores demonstrating a selective advantage. Together, our findings provide compelling evidence for the role of prey shifts in directing the venom composition of cone snails and expand our understanding of the mechanisms of venom variation and diversification.
Keywords: venom, venom evolution, cone snail, predator–prey interactions, conotoxins
Introduction
Venoms are mixtures of bioactive compounds that are used by many organisms to incapacitate prey or defend against predators. Venom systems have independently evolved multiple times in diverse lineages (Casewell et al. 2013). The diversity of venomous organisms highlights the evolutionary significance of venom as a highly successful trait.
Interspecific variation in venom composition is common within lineages; even closely related species can have different venom compositions (Calvete et al. 2007; Li et al. 2017; van Thiel et al. 2023). Understanding the factors driving interspecific venom variation has implications for deciphering the evolutionary dynamics of adaptation and for the development of antivenoms and venom-based drug leads.
Two primary hypotheses have been proposed to explain interspecific variation in venom composition. The first hypothesis proposes that venom composition is driven by a shared phylogenetic ancestry, wherein initial genetic variation emerges primarily through random mutation and recombination events. Subsequently, this variation is modulated at the population level by genetic drift and gene flow, which alter allele frequencies, thereby influencing the observed patterns of venom composition across species (Williams et al. 1988; Mebs 2001). Conversely, the second hypothesis proposes that venom is selected for efficacy in prey and/or predator species. This hypothesis proposes that venom components are subject to positive and negative selection to enhance their efficacy for specific taxa of prey and/or predators (Duda and Palumbi 2004; Casewell et al. 2013). While common phylogenetic ancestry is almost certainly a driver of similarity in venom composition, several recent studies have provided compelling evidence for the role of prey-associated selection in shaping venom composition in snakes (Daltry et al. 1996; Jorge da Silva and Aird 2001; Creer et al. 2003; Barlow et al. 2009; Pawlak et al. 2006, 2009; Jackson et al. 2016), cone snails (Duda and Palumbi 1999, 2000, 2004), sea anemones (Columbus-Shenkar et al. 2018), and spiders (Pekár et al. 2018). In addition to the type of prey, dietary breadth—defined as the diversity of species an organism preys upon—also appears to correlate with venom complexity in some species of snakes (Lyons et al. 2020; Schaeffer et al. 2023) and cone snails (Phuong et al. 2016). While these studies support the role of diet in driving venom diversification, each was limited to small numbers of species or subsets of toxin genes or protein families.
Cone snails (genus Conus) are a large lineage of marine gastropods that use venoms primarily for prey capture. The ∼850 extant species of cone snails are currently classified into 57 subgenera (or clades) (Puillandre et al. 2015; WoRMS 2024), which, based on their prey preference as adults, can be divided into those that prey on annelid worms, mollusks, or fish (Duda et al. 2001). Species belonging to the same clade typically share the same prey preference (e.g. species of the Gastridium clade prey on fish while those belonging to Cylinder clade feed on mollusks). All early diverging clades of Conus are believed to be worm-hunting (vermivorous) with fish-hunting (piscivory) and mollusk-hunting (molluscivory) evolving in some of the more recently diverging lineages (Duda et al. 2001; Uribe et al. 2017). Little is known about the hunting strategies of vermivorous and molluscivorous Conus, but piscivores can be divided into “taser-and-tether,” “net,” and “ambush-and-assess” hunters (Ramiro et al. 2022), where the latter two have a comparably slower mode of hunting suggesting the use of different classes of toxins.
Each cone snail venom consists of a mixture of structurally and pharmacologically diverse bioactive peptides, known as conopeptides or conotoxins (Olivera 2002). Conotoxins have traditionally been grouped into conotoxin “gene superfamilies,” based on similarity in their N-terminal signal sequence (Woodward et al. 1990), with ∼70 conotoxin gene superfamilies described to date. The toxins of each superfamily evolved from a common ancestor and typically have similar function, such that each superfamily can be associated with a particular structural and pharmacological “class” of mature peptide (Olivera 2002). However, some conotoxin gene superfamilies include multiple peptide classes. For example, the A-superfamily includes α-conotoxins, which are inhibitors of nicotinic acetylcholine receptors (nAChRs), and conotoxins that inhibit vertebrate voltage-gated potassium (KV) channels and sodium (NaV) channels (Olivera 2002).
Cone snails represent an ideal system to investigate drivers of interspecific venom variation. In this study, we tested the hypothesis that prey preference shifts have shaped venom composition in cone snails. We analyzed the complete suite of toxin genes from a broad range of cone snail species with different prey preferences, assessed the efficacy of venom from multiple cone snails with different prey preferences, and using a comparative biology approach, we tested for correlations in venom composition and efficacy with phylogeny and prey preference. We demonstrate that the evolutionary shifts from vermivory to piscivory in cone snails are accompanied by changes in venom composition, expression, and efficacy, independent of phylogenetic affinity.
Results
Phylogenetic Reconstruction Supports Three Independent Origins of Piscivory and a Single Shift to Molluscivory in Conus
We reconstructed the phylogeny of Conus using both maximum likelihood (ML) and Bayesian inference (Fig. 1, supplementary fig. S1, Supplementary Material online) based on a multilocus concatenated 11,533 nucleotide alignment of 12 conserved housekeeping genes from 62 phylogenetically diverse cone snails with known prey preferences (supplementary table S1 and file S1, Supplementary Material online). Both methods generated similar tree topologies with high statistical support in the form of bootstrap values and posterior probability, and largely consistent with previously reported relationships within Conus (Phuong et al. 2019). Several branches of the species trees have relatively low gene and site concordance factors (supplementary fig. S2, Supplementary Material online). This is not surprising given the very short branch lengths which can cause discordant topologies in single gene trees. While the constructed species trees represent our best estimate of the true phylogeny of Conus, it should be noted that additional data could alter the observed relationships.
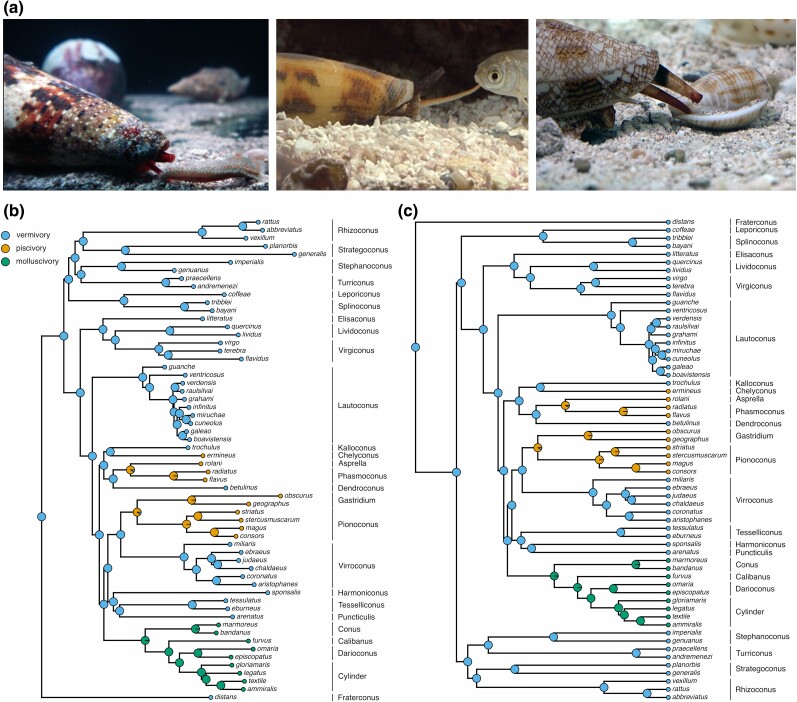
Fig. 1.
Phylogenetic reconstruction supports three independent origins of piscivory and a single shift to molluscivory in Conus. a) Example images of prey capture by vermivorous, piscivorous, and molluscivorous cone snails (from left to right). b) and c) Ancestral state reconstruction of prey preference from 1,000 stochastic character mappings using model weights from irreversible 1 Mk-trait model of evolution (see Methods and supplementary table S2, Supplementary Material online). ML tree and Bayesian tree are shown in panels (b) and (c), respectively. The terminal nodes show the prey preference of the extant cone snail species, whereas the internal nodes show the posterior probabilities of the ancestral states. Subgenera information is provided next to species names. Phylogenetic reconstruction of Conus was built using a concatenated alignment of 12 conserved housekeeping genes found in all species (see supplementary fig. S1, Supplementary Material online for unpruned tree with branch support).
Conus distans was found to represent an ancestral divergence, followed by several clades of early branching vermivores, and eventually leading to the emergence of the derived vermivores as well as piscivores and molluscivores. Based on the inferred species tree, we reconstructed the ancestral states of prey preference in Conus. Our inferred species trees strongly supported a vermivorous stem ancestor of Conus, a single transition from vermivory to molluscivory, and three independent transitions to piscivory. These latter transitions gave rise to multiple clades of fish-hunting cones: (i) Chelyconus, (ii) Asprella and Phasmoconus, and (iii) Gastridium and Pionoconus (Phuong et al. 2019). While no fish hunters from the Textilia or Afonsoconus subgenera were included here, previously published trees support these two clades in a monophyletic clade with Pionoconus (Puillandre et al. 2014; Phuong et al. 2019). Similarly, species of Embrikena were not included but have been suggested to form a sister group to Asprella (Phuong et al. 2019).
Conotoxins Group into Superfamilies With Complex Patterns of Similarity
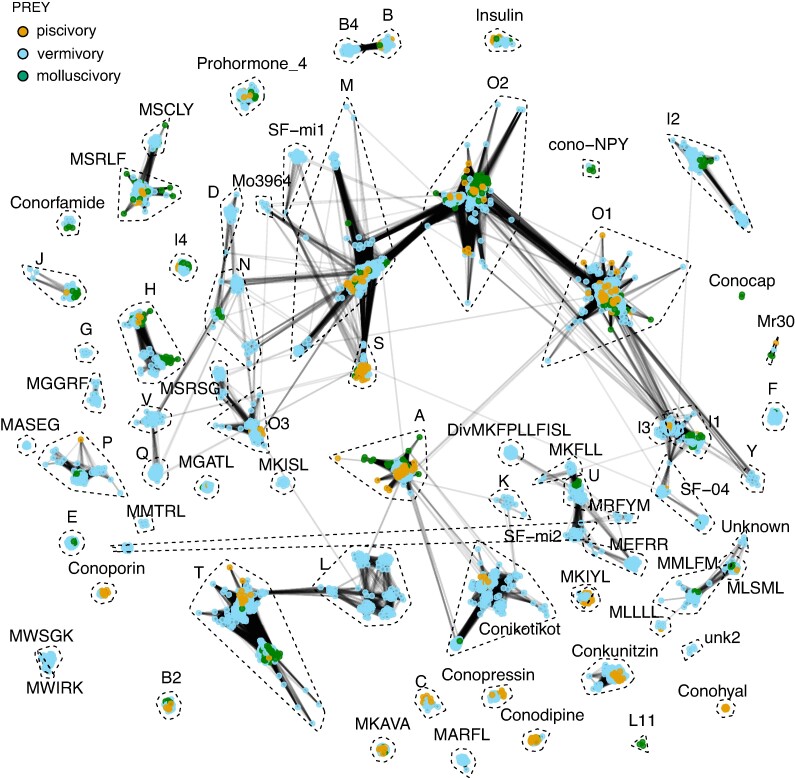
Having established a phylogeny for all species used here, we next defined metrics of venom composition. Cone snail venoms are cocktails of peptide toxins (known as conotoxins) that have historically been grouped into superfamilies based on similarity of the precursor signal sequences. We assembled venom gland RNA-seq data from 42 cone snail species (supplementary table S3, Supplementary Material online) from which we extracted a nonredundant set of 3,412 conotoxin precursors. We clustered the toxin precursor sequences into groups using sequence-based clustering with the program CLANS (Frickey and Lupas 2004; Zimmermann et al. 2018; Fig. 2, supplementary figs. S3 and S4, Supplementary Material online for clustering of conotoxin signal sequences and precursors without signal sequences, respectively). From this analysis and later manual validation, 29 conotoxin sequences that were initially misannotated were reannotated to a different superfamily.
Fig. 2.
BLOSUM62 CLANS cluster map of conotoxin gene superfamilies generated using conotoxin precursor sequences. Colored circles represent individual precursor sequences, with edges connecting the circles corresponds to psi-blast P-values >1e−12. The superfamilies are circled by dashed lines except for clusters with 2 or fewer sequences. Individual conotoxins are color-coded according to the prey preference of the species they were identified from (orange: piscivorous species; green: molluscivorous species; and blue: vermivorous species).
With the exceptions of the SF-mi2 superfamily, the clusters resulting from the CLANS analysis generally corresponded to the traditional conotoxin gene superfamilies with several superfamilies showing high similarity. Many gene superfamilies formed unique clusters with no connection to other conotoxin superfamilies (e.g. Conkunitzin, Con-insulin, and Conopressin); several of the major conotoxin superfamilies (e.g. M, O1, and O2) formed strong connections to one another; several exhibited overlapping clusters (e.g. I1 and I3; MWSGK and MWIRK); and some were split into groups with no connection between each other (e.g. SF-mi2).
Color coding of the clustering map in Fig. 2 according to prey preference revealed that some conotoxin gene superfamilies are restricted to cone snail species of a certain prey preference. For example, the conohyal, L11, and L-superfamilies are found only in piscivores, molluscivores, and vermivores, respectively.
For downstream expression analyses, we grouped toxins based on their assigned superfamily following reassignment of misannotated conotoxins and splitting of the SF-mi2 superfamily. Figure 2 demonstrates that the superfamilies consist of evolutionarily related toxins, which generally conserved pharmacological effects. While some superfamilies show distinct subgroups, it is not trivial to apply an unbiased and stringent splitting protocol across all superfamilies, leading us to use the established superfamilies as the basis for our analyses.
Venom Composition Varies in Different Species of Cone Snails
It has previously been reported that different species of cone snails vary widely in the number of conotoxins expressed and the overall composition of their venom (Phuong et al. 2016). Across the species included in this study, the number of conotoxins ranged from 31 for the vermivore Conus bayani to 236 for the vermivore Conus sponsalis, with the median being 70.5 (supplementary fig. S5, Supplementary Material online). There was high variation in the number of conotoxin gene superfamilies across species, ranging from 16 for Conus striatus to 43 for the vermivore Conus ventricosus, with a median of 27.5 superfamilies. Only the M and O1 superfamilies were detected in every species in the study. In 12 species, the O1 superfamily was the most highly expressed conotoxin superfamily; in 5 species, it was the O2, T and M in 6, A in 3, B and Q in 2, and B2, F, L, con-ikot-ikot, and V were the most highly expressed conotoxin superfamilies for a single species. For most superfamilies, we observed a strong correlation between the number of expressed conotoxin genes and the expression levels (supplementary fig. S6, Supplementary Material online), suggesting that gene duplication is a major mechanism shaping venom composition.
Analysis of venom diversity using the Shannon and Simpson metrics of diversity revealed a relatively uniform distribution of venom diversity among the included species with the vermivore Conus aristophanes having the lowest venom diversity (supplementary fig. S7, Supplementary Material online).
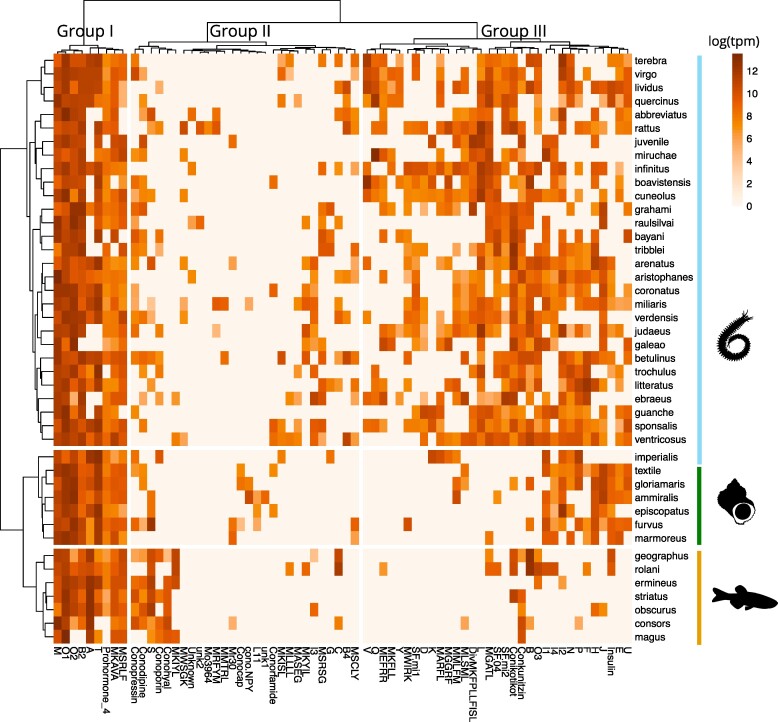
We used hierarchical clustering of conotoxin gene superfamily expression to identify groups of cone snails with similar venom composition and superfamilies that tend to co-express across species. The heat map resulting from this analysis revealed distinct clusters of cone snail species with similar conotoxin gene superfamily expression (Fig. 3). Piscivorous cone snail species form a distinct cluster despite the phylogenetic reconstruction showing independent origins of three lineages of piscivores. Within the piscivores, Conus geographus, and Conus rolani, species that use “net” and “ambush-and-assess” hunting strategies, respectively (Ramiro et al. 2022), formed a smaller subcluster distinct from the “taser-and-tether” hunters (subgenera Cheliconus and Pionoconus). Both molluscivorous and vermivorous species also formed distinct clusters except for the vermivorous Conus imperialis, which grouped with the molluscivores. We included conotoxin expression data from juvenile Conus magus, a species from the Pionoconus clade of which the adults are piscivorous, while juveniles are vermivorous (Nybakken and Perron 1988; Rogalski et al. 2023). The juvenile C. magus grouped with vermivorous snails from the Lautoconus clade and C. bayani, showing that the C. magus venom changes ontogenetically in accordance with its shifting prey preference.
Fig. 3.
Heat map and two-dimensional hierarchical clustering of toxin superfamily expression in cone snail species. Log of the transcripts per million (tpm) per conotoxin gene superfamily was calculated for each cone snail species and used for hierarchical clustering using Ward's method implemented in the R package pheatmap.
The analysis further revealed three major groups of conotoxins that tend to co-express (Fig. 3). The nine conotoxin superfamilies of Group I (A, Prohormone 4, MKAVA, MSRLF, M, O1, T, O2, B2) are expressed at high levels in all major lineages of cone snails included here. Group II conotoxins are typically found only in few species (though a subgroup of these is widely expressed in piscivores), whereas Group III conotoxins are expressed in most vermivorous species and to a lesser extent in molluscivorous and piscivorous species.
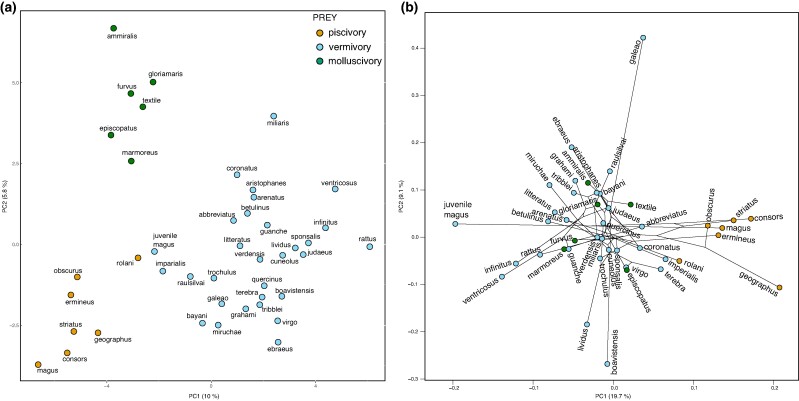
Venom Composition Correlates With Prey Type
Principal component analysis (PCA) is a statistical tool to reduce the dimensionality of a dataset while retaining as much statistical information as possible. Using superfamily expression data in a PCA analysis, we observed a clear separation of all three prey types (Fig. 4a). The variables driving the separation of piscivores are primarily driven by expression of conohyals, conoporins, conodipines, conopressin, and MKYIL. The loading variables that separate molluscivorous species in this plot are doppelganger toxins (conotoxin families that were recruited from endogenous signaling peptides to mimic the peptides of their prey), including insulins, elevenin (L11), NPY, and conocap (Safavi-Hemami et al. 2016; Robinson et al. 2017; supplementary fig. S8B, Supplementary Material online).
Fig. 4.
PCA and phylogenetic PCAs of conotoxin superfamily expression. a) PCA shows that venom composition correlates with prey type. b) pPCA of venom composition based on Bayesian tree demonstrates prey-driven evolution of venom composition in piscivorous cone snail species. However, the separation of vermivores and molluscivores does not hold up when adjusted for phylogeny. Bayesian tree topology is depicted by black lines connecting individual points. Prey preference is indicated by the color of the points.
To account for phylogenetic signal, we performed phylogenetic PCA (pPCA) of conotoxin gene superfamily expression using both ML and Bayesian trees. The plots obtained from adjusting with the two trees are highly similar, and Fig. 4b shows the results using the Bayesian tree (supplementary fig. S9, Supplementary Material online for ML tree). The first three principal components explain 19.7%, 9.1%, and 8.3% of the variance using the Bayesian tree and 20%, 9.5%, and 8% using the ML tree. The pPCA plot retained a distinct cluster of piscivorous cone snail species on the first principal components, confirming that the shift to piscivory strongly influences venom composition independent of phylogeny. Moreover, the venom composition of the vermivorous juvenile C. magus grouped more closely with the vermivores. The loadings of the pPCA show that a relatively small number of conotoxin gene superfamilies carry most of the weight in the first principal component (supplementary fig. S8a, Supplementary Material online). The positive loading variables were the A, M, MKAVA, conohyal, conopressin, and conoporins. A, M, and MKAVA conotoxins belong to Group I in the heat map and are especially highly expressed in piscivores, whereas conohyal, conopressins, and conoporins are a part of Group II, which is mostly observed in piscivores (Fig. 3).
The molluscivores group within the vermivores in the pPCA plot and we can therefore not rule out the possibility that the venom composition of these species is primarily influenced by shared evolution rather than by prey. However, the presence of a phylogenetic signal in the venom composition does not rule out the possibility of a concurrent selection imposed by diet. There is a large spread of vermivorous species in the plot, where the closely related species from the Lautoconus clade are highly dispersed (see supplementary table S1, Supplementary Material online for information on species and clades used here) due to variation in venom composition of the Lautoconus species despite short phylogenetic distances.
Prey Shifts are Associated With Variation Within Conotoxin Gene Superfamilies
Certain conotoxin gene superfamilies were expressed at high levels in all major lineages of cone snails included here, i.e. Group I of Fig. 3. It is well-established that distinct structural and pharmacological classes of conotoxin can be encoded within a single superfamily (Robinson and Norton 2014). We therefore expanded our analysis to test whether prey shifts are also associated with differences in conotoxin classes. We analyzed three conotoxin gene superfamilies (A, M, and O1), for which distinct pharmacological classes are well-established. We surmised that pharmacological classes and toxin function would be associated with differences in physiochemical features of the toxins. For each of the three toxin superfamilies, we therefore obtained physiochemical properties of the toxins as a whole and for individual amino acids in an alignment as inputs for PCA.
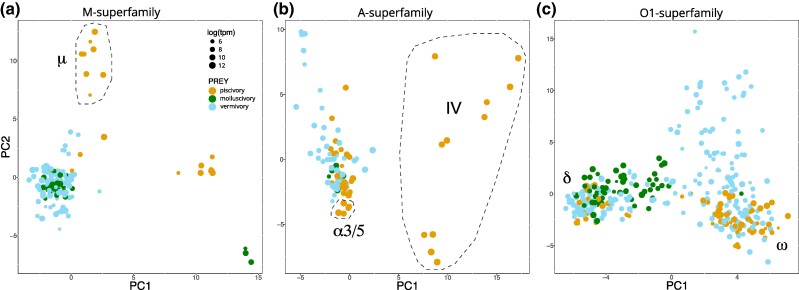
For the M-superfamily, the majority of conotoxins from vermivorous and molluscivorous species grouped together in a single major cluster (Fig. 5a). In contrast, the M-superfamily conotoxins from piscivorous species formed two distinct clusters, which were separated from the major cluster. One of these contained μ-conotoxins which are blockers of vertebrate muscle sodium channels (Cruz et al. 1985; Yanagawa et al. 1987). The second piscivore-specific cluster included several uncharacterized conotoxins.
Fig. 5.
Prey shifts are associated with variation within conotoxin gene superfamilies. a) PCA of physiochemical properties of M-superfamily conotoxins (mature peptides). The cluster comprising μ-conotoxins is labeled. b) PCA of physiochemical properties of A-superfamily conotoxins (mature peptides). The clusters comprising α3/5-conotoxins and conotoxins with a cysteine framework IV are labeled. c) PCA of physiochemical properties of O1-superfamily conotoxins (mature peptides). The clusters containing described δ- and ω-conotoxins are labeled.
For the A superfamily, there did not appear to be any separation between conotoxins from the venoms of vermivores and molluscivores, which formed a major cluster along with several conotoxins from piscivores (Fig. 5b). This main cluster included α-conotoxins that are inhibitors of neuronal-type nAChRs. In contrast, most conotoxins from piscivore venoms deviated from this main cluster. These included the cysteine framework IV conotoxins, which are inhibitors of vertebrate nAChRs, KV, and NaV channels (Craig et al. 1998; Le Gall et al. 1999), and a subgroup of α-conotoxins (α3/5) that are selective for vertebrate muscle-type nAChRs; both unique to piscivores.
O1-superfamily conotoxins grouped into three clusters in the PCA (Fig. 5c). One cluster was unique to the venoms of vermivorous species. Another cluster comprised conotoxins from vermivore and piscivore venoms including ω-conotoxins, which block voltage-gated calcium (CaV) channels. The third cluster comprised toxins from all three prey preferences (including almost all molluscivore toxins) and contained δ-conotoxins, which are modulators of NaV channels (Heinemann and Leipold 2007).
In summary, our PCAs resolved distinct pharmacological classes of conotoxins within the three superfamilies analyzed, and revealed that while A-, M-, and O1-superfamily conotoxins are found in the venoms of vermivorous, piscivorous, and molluscivorous species, there are major differences in the distribution of mature conotoxin pharmacological classes encoded within each superfamily. This was particularly evident in the venoms of piscivores where the dominant A- and M-superfamily conotoxins are pharmacologically distinct from those of vermivores and molluscivores.
Cone Snail Venom Efficacy Correlates With Prey Preference
If the dietary shifts from vermivory to piscivory or molluscivory are associated with specific changes in venom composition, these changes could be expected to have provided adaptive advantages against this prey. In other words, venom from piscivorous cone snail species could be expected to exhibit greater efficacy against fish compared with venom from molluscivorous and vermivorous cone snail species and vice versa for the other prey types. To test this hypothesis, we measured the effects of injections of crude venom from the piscivorous C. geographus and C. striatus, the molluscivorous Conus textile and Conus marmoreus, and the vermivorous Conus virgo and C. imperialis in the goldfish Carassius auratus and the sea hare Aplysia californica (a mollusk). Due to difficulties with establishing a robust behavioral assay for annelids we did not perform venom injections in annelid worms.
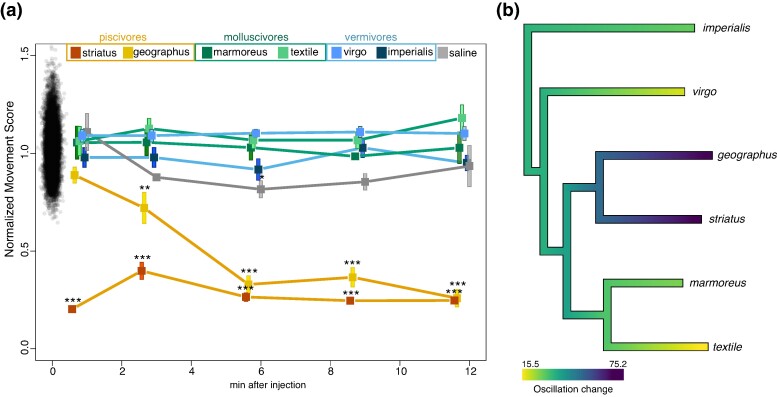
To assess the effect of venom injection in Ca. auratus, we conducted a swimming challenge assay, inspired by the swim tunnel assay (Burris et al. 2021; Lucon-Xiccato et al. 2021) and previous toxin injection studies (Lluisma et al. 2008; Rybin et al. 2020), and counted the number of tail bends as a measure of swimming performance before and after intramuscular injection of venom. First, baseline behavior for every fish was established by analyzing the tail bends before venom injection. The baseline was compared with swimming patterns of fish after venom injection to calculate a normalized movement score over time (see Methods for details; Fig. 6a). Injections from molluscivorous and vermivorous cone snail species did not reduce movement scores in fish. In contrast, fish injected with C. geographus venom exhibited decreased movement (P < 0.01 [1 min], P < 0.001 [3 to 12 min], permutation test). Similarly, fish injected with C. striatus venom were quickly paralyzed, resulting in a reduction of movement compared with the baseline behaviors (P < 0.001 [3 to 12 min], permutation test, Fig. 6a).
Fig. 6.
Effect of venom injection on the swimming behavior of the fish Ca. auratus. a) For each fish, the number of oscillations was normalized to the preinjection value (normalized movement score) and the mean was calculated for each time point (n = 4 to 5). Using the pool of preinjection values, we obtained an empirical null distribution of normal fish movement by dividing each preinjection recording by all other preinjection values. The baseline null distribution is plotted at time point 0. Statistical significance is indicated above each point (**P < 0.01; ***P < 0.001; permutation test). b) Ancestral state reconstruction of venom effect. Mean change in swimming oscillation 12 min after venom injection is shown at the terminal tree nodes. The internal edges of the tree show an ancestral state reconstruction of the effect of venom injection on fish behavior based on the observed changes in oscillation postinjection.
Phylogenetic least squares showed a difference (P = 0.0011, pgls) in the effect of injecting venom from piscivorous versus nonpiscivorous cone snails (Fig. 6b). At doses of 150 ng/g, injection of venom from C. geographus and C. striatus was lethal, whereas no lethality was observed from injections of other venoms at the highest dose tested (300 ng/g). We also observed a phenotypic difference between injection of venom from the two piscivores C. geographus and C. striatus. Immediately after injection, C. striatus venom caused strong muscle contractions at the site of injection accompanied by rapid paralysis, while injection of C. geographus venom caused a flaccid paralysis about 5 min postinjection. These effects mirror those observed for fish envenomated by these two species when maintained in aquaria (Aman et al. 2015; Olivera et al. 2017). Together, these data confirm that prey (and possibly hunting strategy)-specific adaptations in venom composition are reflected in phenotypic prey response to the venom.
To assess the effect of venom injection in the mollusk A. californica, we measured the climbing speed of the A. californica individuals over a 20 min period before and immediately after injection of 300 ng/g of venom. The relative difference in speed before and after injection was used for statistical analysis. Conus striatus, C. imperialis, C. virgo, and C. textile venom all caused a reduction in speed compared with controls, while injections of venom from neither C. geographus nor C. marmoreus were different from controls (supplementary fig. S10, Supplementary Material online). Phylogenetic least squares did not show a significantly different effect of injection of molluscivore venom compared with nonmolluscivore venom (P = 0.76, pgls). However, in two cases of injection of C. textile venom the A. californica individuals were still strongly affected many hours after injection curling up with limited movement.
Discussion
Dietary shifts have long been proposed as important factors in shaping venom evolution (Daltry et al. 1996; Duda and Palumbi 1999, 2000, 2004; Jorge da Silva and Aird 2001; Creer et al. 2003; Pawlak et al. 2006, 2009; Barlow et al. 2009; Jackson et al. 2016; Columbus-Shenkar et al. 2018; Pekár et al. 2018). In this study, we provide compelling evidence that dietary shifts have influenced the evolution of venom composition and efficacy in Conus beyond the constraints of shared phylogeny.
As part of this study, we established a phylogeny and ancestral state reconstruction of prey preference for Conus. At least two independent shifts from vermivory to piscivory have previously been proposed (Kelley et al. 2006). Our data supported three shifts, which is consistent with a recent exon-capture-based phylogeny (Phuong et al. 2019). Our data also supported a single origin of molluscivory, which are consistent with previous studies (Duda et al. 2001; Phuong et al. 2019).
Despite the independent origins of piscivory, piscivorous species exhibited a high degree of similarity in their venom composition. This convergence of venom phenotypes among distantly related species suggests that the transition to piscivory imposed selective pressures that favor specific venom profiles. In contrast, the lack of distinctive venom signatures for molluscivorous and vermivorous species when adjusted for phylogeny implies a more flexible or subtle response to the varying prey types encountered within these dietary niches. Alternatively, the methodologies and data applied were not able to capture prey-associated adaptations of venom in these clades. In this context, it is important to consider that hierarchical clustering and nonphylogeny-adjusted PCA revealed clear grouping of all three different prey types but that this pattern did not withstand phylogenetic adjustment. For molluscivores, this is likely due to the monophyletic origin of all extant molluscivorous species making it intrinsically difficult to observe prey-associated adaptations when adjusting for phylogeny. While we included 70 distinct conotoxin gene superfamilies from 42 species of cone snails in our analyses, the incorporation of additional species and gene superfamilies could provide further insights into the evolutionary patterns of venom composition. Ongoing research continually discovers new conotoxin superfamilies, some of which appear to be specific to particular lineages of cone snails. For instance, the recent discovery of new hormone-like toxins that are specifically found in molluscivores (Koch et al. 2023) suggests that the inclusion of these and other newly discovered toxins in future analyses may alter the clustering patterns among molluscivorous cone snails. While not done in this study, it is also likely that incorporating existing and emerging information on superfamily subdivisions into the clustering and expression analysis could confirm our findings regarding the correlation between venom variation and prey shifts to piscivory and potentially reveal similar patterns for the shift to molluscivory. We therefore propose that shifts to piscivory unambiguously drive venom adaptation and that better methodologies and more refined data (e.g. additional toxin superfamilies, comprehensive pharmacological information, and potential subdivisions of superfamilies) are needed to address whether similar patterns exist for the evolution of vermivory and mollscivory. Additionally, it is possible that shifts to more distantly related prey taxa (i.e. vermivory to piscivory vs. vermivory to molluscivory) required more dramatic changes in venom composition to enable targeting of more distantly related molecular targets and physiological circuits.
It is noteworthy that our detection of prey-specific venom composition in cone snails contradicts previous findings (Phuong et al. 2016; Phuong and Mahardika 2018), which did not observe such patterns. The discrepancy can likely be attributed to two main factors: the earlier study by Phuong et al. (2016) excluded piscivorous cone snails, where we observed the most pronounced signal, and only included two molluscivorous species. The later study (Phuong and Mahardika 2018) used a targeted enrichment strategy of conotoxin-encoding genes from genomic DNA. However, as recently revealed by Rogalski et al. (2023), some species undergo an ontogenetic shift in dietary preference during their lifecycle. While transcriptomic data only capture toxins expressed at a specific time, genomic data encompass a combination of juvenile- and adult-specific toxins. Thus, to elucidate the potential correlation between diet and toxin gene expression, we advocate for the transcriptomic approach used in our study, as it more accurately reflects active venom composition at a given life stage.
Other studies have investigated the evolution of venom composition in sea anemones (Smith et al. 2023) and snakes (Barua and Mikheyev 2019). While these studies did not investigate dietary shifts per se, similar convergent shifts in venom composition were observed, suggesting a common phenomenon across venomous lineages. As for sea anemones (Smith et al. 2023), we also report a strong correlation between the number of expressed toxin genes and the expression level for most superfamilies. While additional genomes would be required to confirm this, we confirm that gene duplication is one of the major mechanisms underlying shifts in venom composition in cone snails.
It is well-established that certain conotoxin gene superfamilies encode multiple distinct structural and pharmacological classes of conotoxins. We hypothesized that while all dietary groups of Conus shared certain conotoxin gene superfamilies (e.g. A, M, and O1) that differences may exist in the pharmacological class of conotoxin encoded. Our data confirmed that several pharmacological classes of conotoxin were restricted to, and highly expressed in, the venoms of piscivorous species of cone snail. These included the so-called α3/5-conotoxins (A-superfamily) which are potent inhibitors of the vertebrate muscle nAChR (Azam and McIntosh 2009); A-superfamily conotoxins with cysteine framework type IV, which are inhibitors of vertebrate nAChRs or KV/NaV channels (Le Gall et al. 1999); and the μ-conotoxins (M-superfamily) which are potent blockers of vertebrate NaV channels (Cruz et al. 1985; Yanagawa et al. 1987). These classes of conotoxin cause paralysis and death in vertebrates including fish (Kelley et al. 2006). It is clear that the presence of these pharmacological classes of conotoxins in the venoms of piscivorous cone snail species contributes to the observed efficacy of their venoms on fish and other vertebrates. It seems likely that similar pharmacological subdivisions also exist in other, less well-studied superfamilies. For example, we recently showed that molluscivorous species express a pharmacological family of T-superfamily conotoxins (referred to as χ-conotoxins), that induce a hyperactive phenotype in their gastropod prey thereby forcing the animal out of its protective shell (Espino S, Watkins M, Probst R, Koch TL, Chase K, Imperial J, Robinson SD, Flórez Salcedo P, Taylor D, Gajewiak J, Yandell M, Safavi-Hemami H, Olivera BM, unpublished data). χ-Conotoxins appear to be absent from piscivorous and vermivorous species and likely evolved as an adaptation to the molluscivorous lifestyle.
In our hierarchical clustering analysis, we identified several conotoxin gene superfamilies that exhibited frequent co-expression. While some of these superfamilies were widely distributed across Conus species, others showed a more restricted distribution, primarily occurring in specific clades. This pattern likely arises from the independent origins of novel toxins within certain lineages, suggesting both common and lineage-specific evolution of venom components. Future studies are needed to investigate whether some of these co-expressed superfamilies form functional cabals—groups of conotoxins that act synergistically on the same biological system (Olivera 1997; Olivera et al. 2017). These cabals could reveal novel insight into predation strategies used by different species to incapacitate their prey and help prioritize toxin superfamilies with therapeutic potential.
Conclusion
Our study contributes significant insights into the role of dietary shifts in shaping the evolution of venom composition in cone snails. The convergence of piscivorous strategies among distantly related species and the diverse venom profiles observed in molluscivorous and vermivorous cone snails highlight the adaptive nature of venoms in response to specific dietary niches.
Methods
Phylogenetic Reconstruction
The comparative biological methods are a suite of statistical approaches that accounts for shared ancestry through extrapolating evolutionary relationships of traits using phylogenetic trees. As such, it relies on the availability of robust phylogenetic data. We constructed a phylogeny of Conus using conserved housekeeping genes from transcriptomic data of 62 phylogenetically diverse cone snails with known prey preferences (supplementary table S1, Supplementary Material online). Considering the ancestral whole genome duplication in Neogastropoda which introduced paralogs of nearly all genes (Pardos-Blas et al. 2021; Farhat et al. 2023), we implemented a “clans check” approach to filter out genes with paralogs or weak phylogenetic signal. A common set of 71 housekeeping genes found in all transcriptomes were extracted for phylogenetic reconstruction. The nucleotide sequences of each gene were aligned using mafft v7.490 (-maxiterate 1000 -localpair) and trimmed using trimAl v1.2.59 (-gt 0.5 -cons 60). For each gene, we generated an ML tree using IQ-tree 2.2.0.3, where the model of evolution was chosen from the Bayesian information criteria. Following single gene tree reconstruction, we performed a “clans check,” where trees violating so-called “uncontestable” clades were discarded from further analyses (Siu-Ting et al. 2019). This approach is used to filter out gene sets containing paralogs and genes violating the outgroup as monophyletic (Siu-Ting et al. 2019). We selected the following clans: (i) Drillia regia, Californiconus californicus, Conasprella coriolis; (ii) Cal. californicus, Cona. coriolisi; (iii) C. geographus, Conus obscurus; (iv) Conus boavistensis, Conus galeao, Conus infinitus, Conus cuneolus, Conus miruchae, Conus raulsilvai, Conus verdensis, Conus grahami; (v) Conus consors, C. magus, Conus stercusmuscarum, C. striatus. It is essential that all gene trees have the same outgroup, i.e. a 100% concordance. For this reason, we selected clans 1 and 2 to have the intended outgroup in all gene trees. We noticed that several gene trees showed signs of including several paralogous genes (very long branches containing highly unanticipated species with no prior support for being monophyletic). To exclude those in a reproducible manner, we included clan 3 (representing piscivorous Gastridium), clan 4 (monophyletic vermivorous Lautoconus), and clan 5 (represents monophyletic piscivorous Pionoconus). All these clans are strongly supported to be monophyletic in previous studies (Puillandre et al. 2014; Phuong et al. 2019) and allowed us to filter out genes with paralogs in our phylogenetic reconstruction. Twelve genes passed all restraints (Uniprot IDs: O57592, O77302, P27659, P30151, P36241, P42577, Q3SZ71, Q62425, Q8ISN9, Q90Z10, Q9N1Q0, Q9UDW1) and the alignments were combined into a single supermatrix (supplementary file S1, Supplementary Material online).
We constructed an ML species tree using the supermatrix of 11,533 sites with partitioning for each gene (models of evolutions were chosen based on the Bayesian information criteria to be TIM3 + F + I + R3, TNe + R2, TIM2e + R3, TIM2 + F + I + R3, TN + F + I + R3, K2P + I + R3, TNe + R3, HKY + F + R3, K2P + R3, TIM2e + I + R2, TPM3 + R3, HKY + F + G4, respectively) using IQ-tree v2.2.0.3 run on a single thread on an M1 chip with 8 cores @ 3.2 GHz with seed 495731. The tree was bootstrapped with IQ-tree's UFboot algorithm with 1,000 replicates and rooted using D. regia.
We further build an alternative species tree with the supermatrix using Bayesian reconstruction in the program BEAST v2.7.3. We selected an HKY site substitution model estimating kappa and frequencies, a strict clock with uniform priors and a Yule tree model for 60,000,000 generations sampling every 10,000 generation. The first 10% of the resulting 6,001 trees were discarded as burn-in, and the remaining trees were used to build a maximum clade credibility tree.
Gene and site concordance factors for both the ML and Bayesian species trees were calculated with IQ-tree from the alignment and single gene trees. The gene concordance factor measures the proportion of single gene trees that match the topology of a given branch. While most branches have high gene concordance, it is low for several of the branches (supplementary fig. S2, Supplementary Material online). This is likely due to combination of a limited ability to resolve the gene trees because of the short branches, and from genuine conflicting signal. The site concordance factors measure the proportion of sites in the combined alignment that support a given tree topology. Most site concordance factors are high, but several branches are again relatively low. Even though some branches have low concordance factors, the species trees constructed here are the best estimate though additional data might support a different topology in the future.
Ancestral State Reconstruction
All analyses were performed using ML and Bayesian trees generated above. We collated data on confirmed prey of cone snail species from published literature (supplementary table S1, Supplementary Material online). To estimate the transition rate matrix between different prey preferences, we compared a suite of discrete trait Markov models. Discrete ancestral state reconstruction was performed with phytools (Revell 2012) using the function fitMk to identify the transitions between vermivory, molluscivory, and piscivory. We fitted the models: equal rates, all-rates-different, and models where the transition from vermivory to molluscivory and piscivory is irreversible (irreversible 1 for identical rates to molluscivory and piscivory, and irreversible 2 for different rates to molluscivory and piscivory). The models were compared with analysis of variance and prioritized using Akaike information criterion (AIC). Both irreversible 1 (equal transition rates from vermivory to molluscivory and piscivory) and irreversible 2 (distinct transition rates from vermivory to molluscivory and piscivory) had high support based on the AIC in both trees (49.5 and 32.9 AIC weight for ML tree, and 54.2 and 25.1 AIC weight for Bayesian tree; supplementary table S2, Supplementary Material online). Using the model with the highest support, irreversible 1, we simulated 1,000 stochastic prey character histories onto the Conus phylogeny to calculate the ancestral state probabilities (Fig. 1).
Datasets
Publicly available RNA-seq dataset were downloaded from NCBI and China National GeneBank (supplementary table S3, Supplementary Material online). Only a subset of the species used for phylogenetic reconstruction had available venom gland RNA-seq data of high quality. The venom gland of a single specimen of C. obscurus, Conus ermineus, and C. consors was dissected for sequencing. Total RNA was extracted using the Direct-zol RNA extraction kit (Zymo Research), with on-column DNase treatment and an additional wash step after the first purification, according to the manufacturer's instructions. Library preparation and sequencing were performed by the University of Utah High Throughput Genomics Core Facility. For assembly adapter, trimming of de-multiplexed raw reads was performed using fqtrim (Pertea 2015), followed by quality trimming and filtering using prinseq-lite (Schmieder and Edwards 2011). Error correction was performed using the BBnorm ecc tool, part of the BBtools package. Trimmed and error-corrected reads were assembled using Trinity (version 2.2.1; Haas et al. 2013) with k-mer length of 31 and minimum k-mer coverage of 10. Assembled transcripts were annotated using a blastx (Altschul et al. 1990) search (E-value setting of 1e−3) against a combined database derived from UniProt and conoserver (Kaas et al. 2010). Transcripts per million (TPM) counts were generated using the Trinity RSEM (Li and Dewey 2011) plugin (align_and_estimate_abundance) and expression data analyzed using the trinity utilities abundance_estimates_to_matrix and contig_ExN50_statistic.
Toxin Clustering
All conotoxin-encoding transcripts were extracted from each transcriptome dataset, trimmed to open-reading frame and redundant, partial and/or poorly expressed transcripts (<1 TPM) discarded. The fidelity of each assembled conotoxin transcript was then manually checked using the Map-to-Reference tool of Geneious, version 8.1.7 (Kearse et al. 2012). Erroneous transcripts were discarded, while unassembled variants were manually rebuilt and added back to the final conotoxin dataset. Relative TPM counts were generated using the Trinity RSEM plugin (align_and_estimate_abundance) and expression data analyzed using the trinity utility abundance_estimates_to_matrix.
The resulting 2,768 remapped toxins were translated to amino acid sequences using transeq. The toxin sequences were clustered using CLANS, where we performed an all-against-all psi-blast of the sequences (Frickey and Lupas 2004; Zimmermann et al. 2018). The clustering creates a map of points representing individual sequences with connections, which represents sequence similarities. The minus log blast P-values were used as attractive forces in a uniformly repulsive force field to group similar sequences. The clustering file can be found in supplementary file S2, Supplementary Material online. From this analysis and later manual validation, 29 conotoxin sequences that were initially misannotated were reannotated to a different superfamily.
Conotoxin gene superfamilies have traditionally been assigned based on signal sequence similarity. We therefore separated all conotoxin sequences into signal sequences and the remaining precursor (pro-peptide and mature peptide). The signal sequences were identified using SignalP v6.0. The conotoxin signal sequences and remaining precursors were clustered as for the complete precursors, respectively. The coordinates and attractive values were exported and visualized in R (supplementary figs. S3 and S4, Supplementary Material online).
Gene Count and Expression Correlation
We plotted the number of expressed toxin genes and expression level in each cone snail for all superfamilies. Correlations were visualized and calculated using the library ggplot's stat_smooth using a linear model and the library ggpmisc's stat_correlations with Pearson correlation and P-values implemented in R.
Venom Diversity
The venom diversity for each species was calculated using the Simpson and Shannon diversity indexes (Shannon: and Simpson: , where pi is the proportion of conotoxin gene superfamily i) on the total number of conotoxin genes for each superfamily. The diversity indexes and gene numbers were mapped onto the phylogenies.
Hierarchical Clustering and PCAs of Superfamily Expression
We calculated the total TPM and number of genes per conotoxin gene superfamily for each species included in the analysis and constructed a heat map using hieratical clustering with Ward's D of the Euclidean distance of the natural logarithm of conotoxin gene superfamily expression. The correlation matrix was calculated with the base R function “cor().”
Traditional PCA was calculated with the function “PCA()” implemented in the R library FactoMineR on the log of total toxin superfamily expression for each of the cone snail species. We further performed phylogenetic PCA (pPCA). pPCA belongs to a class of methods that takes the phylogenetic covariance into account when performing comparative analyses. We used the phyl.pca function implemented in the R library phytools on the same dataset. To compare the groupings, we obtained from the species trees generated here, and we also performed the same analysis using a previously published phylogeny (Phuong et al. 2019). The resulting clustering (supplementary fig. S11, Supplementary Material online) also showed grouping of fish hunters separate from mollusk and worm hunters.
O1, M, and A Superfamily PCA
We extracted the mature toxin regions of the annotated O1, M, and A superfamily precursors based on known toxin sequences. The mature toxins were subsequently aligned using mafft v7.490 (-maxiterate 1000 -localpair) and trimmed using trimal (-gt 0.9 -cons 60). For each of the sequences we calculated the total length, molecular weight, isoelectric point, gravy score, percentage aliphatic, aromatic, polar, unique (G and P), positive, and negative amino acids. For each amino acid in the alignment, we further calculated the charge, disorder, hydropathy, cysteine (binary), and gap (binary). The data were scaled to a mean 0 and standard deviation 1, and PCA was performed as implemented in sklearn.
Fish Injections
Intramuscular fish injections of venom were performed in adult goldfish (Ca. auratus), as previously described (López-Vera et al. 2007). Initially, 2 mg aliquots of dried venom from each species of cone snail were reconstituted in sterile 0.9% normal saline solution. The solution was subsequently diluted to 300 ng venom/gram of fish in 20 μL saline. Every fish was injected in the caudal end under the dorsal fin using a 29 G needle. Twenty microliters of saline solution were injected as control.
We employed a swimming trial to assess the effects of the venom in the kinematics of swimming. The swimming challenge was done in an open circular area with a stirring bar to induce a vortex compelling the fish to engage in swimming activity. Before injection, the fish were subject to a 1 min swimming trial to obtain baseline values. Following the injection, the fish were promptly transferred to the container. At 1, 3, 6, 9, and 12 min postinjection, the fish were subjected to one min swimming challenges with the vortex, interspersed with a 2 min rest interval between consecutive trials. The trials were recorded and used to calculate the number of tail bends (oscillations) per min using ZebraZoom (Mirat et al. 2013).
To test for significance, the preinjection oscillations were used as null values. The number of oscillations during the swimming trials was divided by the baseline value for every fish to generate a normalized movement score. A baseline behavior, or a null distribution, was established by dividing every preinjection oscillation by all other preinjection values. At each time point, the mean was calculated (n = 4 or 5). With the established null distribution of preinjection values, we used bootstrapping to extract subsets of 4 to 5 values and calculate the mean for each subset. This process creates an empirical distribution of the averages.
The injection data (oscillation change from preinjection to 12 min postinjection) were mapped onto the pruned Conus phylogeny at the terminal nodes. Using the phytools function contMap, we estimated the ancestral states at every internal point of the tree. We performed a phylogenetic generalized least squares model on the data (oscillation change from preinjection to 12 min postinjection) using binary prey classifications (i.e. piscivores compared with nonpiscivores).
Aplysia Injections
Aplysia californica (∼5 g) were obtained from The National Resource for Aplysia at the University of Miami. Venom was prepared as described for the fish injections. To assess the effect of each venom, we measured the climbing speed of the A. californica individuals over a 20 min period in a 1,000 mL glass container before and immediately after injection of 300 ng/g of venom. The relative difference in speed before and after injection was used for statistical analysis by comparing to saline control by Student's t-test. Phylogenetic least squares and ancestral state reconstruction were performed as described for the fish injections using the relative difference in speed before and after injection.
Supplementary Material
Acknowledgments
The authors thank the High Throughput Genomics Core Facility at the University of Utah for transcriptome sequencing. The support and resources from the Center for High Performance Computing at the University of Utah are gratefully acknowledged.
Contributor Information
Thomas Lund Koch, Department of Biochemistry, University of Utah, Salt Lake City, UT 84112, USA.
Samuel D Robinson, School of Biological Sciences, University of Utah, Salt Lake City, UT 84112, USA; Institute for Molecular Bioscience, The University of Queensland, Brisbane, QLD 4072, Australia.
Paula Flórez Salcedo, Department of Neurobiology, University of Utah, Salt Lake City, UT 84112, USA.
Kevin Chase, School of Biological Sciences, University of Utah, Salt Lake City, UT 84112, USA.
Jason Biggs, Division of Aquatic and Wildlife Resources, Department of Agriculture, Mangilao, GU 96913, USA.
Alexander E Fedosov, Swedish Museum of Natural History, Department of Zoology, Stockholm 114 18, Sweden.
Mark Yandell, Department of Human Genetics, Utah Center for Genetic Discovery, University of Utah, Salt Lake City, UT 84112, USA.
Baldomero M Olivera, School of Biological Sciences, University of Utah, Salt Lake City, UT 84112, USA.
Helena Safavi-Hemami, Department of Biochemistry, University of Utah, Salt Lake City, UT 84112, USA; School of Biological Sciences, University of Utah, Salt Lake City, UT 84112, USA; Department of Biomedical Sciences, University of Copenhagen, Copenhagen N 2200, Denmark.
Supplementary Material
Supplementary material is available at Molecular Biology and Evolution online.
Funding
This work was supported by a Villum Fonden Grant (19063 to H.S.-H.) and a National Institutes of Health Grant (GM122869 to M.Y. and B.M.O.).
Data Availability
All accession numbers used in this study are available in supplementary table S2, Supplementary Material online. The data underlying this article are available in NCBI Sequence Read Archive, and raw reads of C. obscurus, C. consors, and C. ermineus venom gland transcriptomes can be accessed with accession numbers SRR27489861, SRR27489862, and SRR28746436.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990:215(3):403–410. 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Aman JW, Imperial JS, Ueberheide B, Zhang MM, Aguilar M, Taylor D, Watkins M, Yoshikami D, Showers-Corneli P, Safavi-Hemami H, et al. . Insights into the origins of fish hunting in venomous cone snails from studies of Conus tessulatus. Proc Natl Acad Sci U S A. 2015:112(16):5087–5092. 10.1073/pnas.1424435112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, McIntosh JM. Alpha-conotoxins as pharmacological probes of nicotinic acetylcholine receptors. Acta Pharmacol Sin. 2009:30(6):771–783. 10.1038/aps.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow A, Pook CE, Harrison RA, Wuster W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc Biol Sci. 2009:276(1666):2443–2449. 10.1098/rspb.2009.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua A, Mikheyev AS. Many options, few solutions: over 60 my snakes converged on a few optimal venom formulations. Mol Biol Evol. 2019:36(9):1964–1974. 10.1093/molbev/msz125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris B, Jensen N, Mokalled MH. Assessment of swim endurance and swim behavior in adult zebrafish. J Vis Exp. 2021:177:e63240. 10.3791/63240-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete JJ, Escolano J, Sanz L. Snake venomics of Bitis species reveals large intragenus venom toxin composition variation: application to taxonomy of congeneric taxa. J Proteome Res. 2007:6(7):2732–2745. 10.1021/pr0701714. [DOI] [PubMed] [Google Scholar]
- Casewell NR, Wuster W, Vonk FJ, Harrison RA, Fry BG. Complex cocktails: the evolutionary novelty of venoms. Trends Ecol Evol. 2013:28(4):219–229. 10.1016/j.tree.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Columbus-Shenkar YY, Sachkova MY, Macrander J, Fridrich A, Modepalli V, Reitzel AM, Sunagar K, Moran Y. Dynamics of venom composition across a complex life cycle. Elife. 2018:7:e35014. 10.7554/eLife.35014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AG, Zafaralla G, Cruz LJ, Santos AD, Hillyard DR, Dykert J, Rivier JE, Gray WR, Imperial J, DelaCruz RG, et al. . An O-glycosylated neuroexcitatory Conus peptide. Biochemistry. 1998:37(46):16019–16025. 10.1021/bi981690a. [DOI] [PubMed] [Google Scholar]
- Creer S, Malhotra A, Thorpe RS, Stocklin R, Favreau P, Chou WH. Genetic and ecological correlates of intraspecific variation in pitviper venom composition detected using matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF-MS) and isoelectric focusing. J Mol Evol. 2003:56(3):317–329. 10.1007/s00239-002-2403-4. [DOI] [PubMed] [Google Scholar]
- Cruz LJ, Gray WR, Olivera BM, Zeikus RD, Kerr L, Yoshikami D, Moczydlowski E. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J Biol Chem. 1985:260(16):9280–9288. 10.1016/S0021-9258(17)39364-X. [DOI] [PubMed] [Google Scholar]
- Daltry JC, Wuster W, Thorpe RS. Diet and snake venom evolution. Nature. 1996:379(6565):537–540. 10.1038/379537a0. [DOI] [PubMed] [Google Scholar]
- Duda TF Jr, Kohn AJ, Palumbi S. Origins of diverse feeding ecologies within Conus, a genus of venomous marine gastropods. Biol J Linn Soc. 2001:73(4):391–409. 10.1111/j.1095-8312.2001.tb01369.x. [DOI] [Google Scholar]
- Duda TF, Palumbi SR. Molecular genetics of ecological diversification: duplication and rapid evolution of toxin genes of the venomous gastropod Conus. Proc Natl Acad Sci U S A. 1999:96(12):6820–6823. 10.1073/pnas.96.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda TF Jr, Palumbi SR. Evolutionary diversification of multigene families: allelic selection of toxins in predatory cone snails. Mol Biol Evol. 2000:17(9):1286–1293. 10.1093/oxfordjournals.molbev.a026412. [DOI] [PubMed] [Google Scholar]
- Duda TF, Palumbi SR. Gene expression and feeding ecology: evolution of piscivory in the venomous gastropod genus Conus. Proc R Soc London B Biol Sci. 2004:271(1544):1165–1174. 10.1098/rspb.2004.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat S, Modica MV, Puillandre N. Whole genome duplication and gene evolution in the hyperdiverse venomous gastropods. Mol Biol Evol. 2023:40(8):msad171. 10.1093/molbev/msad171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frickey T, Lupas A. CLANS: a Java application for visualizing protein families based on pairwise similarity. Bioinformatics. 2004:20(18):3702–3704. 10.1093/bioinformatics/bth444. [DOI] [PubMed] [Google Scholar]
- Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M, et al. . De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc. 2013:8(8):1494–1512. 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann SH, Leipold E. Conotoxins of the O-superfamily affecting voltage-gated sodium channels. Cell Mol Life Sci. 2007:64(11):1329–1340. 10.1007/s00018-007-6565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson TN, Koludarov I, Ali SA, Dobson J, Zdenek CN, Dashevsky D, Op den Brouw B, Masci PP, Nouwens A, Josh P, et al. . Rapid radiations and the race to redundancy: an investigation of the evolution of Australian elapid snake venoms. Toxins (Basel). 2016:8(11):309. 10.3390/toxins8110309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge da Silva N Jr, Aird SD. Prey specificity, comparative lethality and compositional differences of coral snake venoms. Comp Biochem Physiol Toxicol Pharmacol. 2001:128(3):425–456. 10.1016/S1532-0456(00)00215-5. [DOI] [PubMed] [Google Scholar]
- Kaas Q, Westermann JC, Craik DJ. Conopeptide characterization and classifications: an analysis using ConoServer. Toxicon. 2010:55(8):1491–1509. 10.1016/j.toxicon.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. . Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012:28(12):1647–1649. 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WP, Schulz JR, Jakubowski JA, Gilly WF, Sweedler JV. Two toxins from Conus striatus that individually induce tetanic paralysis. Biochemistry. 2006:45(47):14212–14222. 10.1021/bi061485s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch TL, Torres JP, Baskin RP, Salcedo PF, Chase K, Olivera BM, Safavi-Hemami H. A toxin-based approach to neuropeptide and peptide hormone discovery. Front Mol Neurosci. 2023:16:1176662. 10.3389/fnmol.2023.1176662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall F, Favreau P, Benoit E, Mattei C, Bouet F, Menou J-L, Ménez A, Letourneux Y, Molgó J. A new conotoxin isolated from Conus consors venom acting selectively on axons and motor nerve terminals through a Na+-dependent mechanism. Eur J Neuroscience. 1999:11(9):3134–3142. 10.1046/j.1460-9568.1999.00732.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Barghi N, Lu A, Fedosov AE, Bandyopadhyay PK, Lluisma AO, Concepcion GP, Yandell M, Olivera BM, Safavi-Hemami H. Divergence of the venom exogene repertoire in two sister species of Turriconus. Genome Biol Evol. 2017:9(9):2211–2225. 10.1093/gbe/evx157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011:12(1):323. 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lluisma AO, López-Vera E, Bulaj G, Watkins M, Olivera BM. Characterization of a novel ψ-conotoxin from Conus parius Reeve. Toxicon. 2008:51(2):174–180. 10.1016/j.toxicon.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Vera E, Jacobsen RB, Ellison M, Olivera BM, Teichert RW. A novel alpha conotoxin (α-PIB) isolated from C. purpurascens is selective for skeletal muscle nicotinic acetylcholine receptors. Toxicon. 2007:49(8):1193–1199. 10.1016/j.toxicon.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Lucon-Xiccato T, Bella L, Mainardi E, Baraldi M, Bottarelli M, Sandonà D, Bertolucci C. An automated low-cost swim tunnel for measuring swimming performance in fish. Zebrafish. 2021:18(3):231–234. 10.1089/zeb.2020.1975. [DOI] [PubMed] [Google Scholar]
- Lyons K, Dugon MM, Healy K. Diet breadth mediates the prey specificity of venom potency in snakes. Toxins (Basel). 2020:12(2):74. 10.3390/toxins12020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebs D. Toxicity in animals. Trends in evolution? Toxicon. 2001:39(1):87–96. 10.1016/S0041-0101(00)00155-0. [DOI] [PubMed] [Google Scholar]
- Mirat O, Sternberg J, Severi K, Wyart C. ZebraZoom: an automated program for high-throughput behavioral analysis and categorization. Front Neural Circuits. 2013:7:103. 10.3389/fncir.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybakken J, Perron FE. Ontogenetic change in the radula of Conus magus (Gastropoda). Mar Biol. 1988:98(2):239–242. 10.1007/BF00391200. [DOI] [Google Scholar]
- Olivera BM. E.E. Just Lecture, 1996. Conus venom peptides, receptor and ion channel targets, and drug design: 50 million years of neuropharmacology. Mol Biol Cell. 1997:8(11):2101–2109. 10.1091/mbc.8.11.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera BM. Conus venom peptide: reflections from the biology of clades and species. Annu Rev Ecol Syst. 2002:33(1):25–47. 10.1146/annurev.ecolsys.33.010802.150424. [DOI] [Google Scholar]
- Olivera BM, Raghuraman S, Schmidt EW, Safavi-Hemami H. Linking neuroethology to the chemical biology of natural products: interactions between cone snails and their fish prey, a case study. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017:203(9):717–735. 10.1007/s00359-017-1183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardos-Blas JR, Irisarri I, Abalde S, Afonso CML, Tenorio MJ, Zardoya R. The genome of the venomous snail Lautoconus ventricosus sheds light on the origin of conotoxin diversity. GigaScience. 2021:10(5):giab037. 10.1093/gigascience/giab037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak J, Mackessy SP, Fry BG, Bhatia M, Mourier G, Fruchart-Gaillard C, Servent D, Menez R, Stura E, Menez A, et al. . Denmotoxin, a three-finger toxin from the colubrid snake Boiga dendrophila (Mangrove catsnake) with bird-specific activity. J Biol Chem. 2006:281(39):29030–29041. 10.1074/jbc.M605850200. [DOI] [PubMed] [Google Scholar]
- Pawlak J, Mackessy SP, Sixberry NM, Stura EA, Le Du MH, Ménez R, Foo CS, Ménez A, Nirthanan S, Kini RM. Irditoxin, a novel covalently linked heterodimeric three-finger toxin with high taxon-specific neurotoxicity. FASEB J. 2009:23(2):534–545. 10.1096/fj.08-113555. [DOI] [PubMed] [Google Scholar]
- Pekár S, Líznarová E, Bočánek O, Zdráhal Z. Venom of prey-specialized spiders is more toxic to their preferred prey: a result of prey-specific toxins. J Anim Ecol. 2018:87(6):1639–1652. 10.1111/1365-2656.12900. [DOI] [PubMed] [Google Scholar]
- Pertea G. fqtrim: v0.9.4 release; 2015. https://zenodo.org/records/20552
- Phuong MA, Alfaro ME, Mahardika GN, Marwoto RM, Prabowo RE, von Rintelen T, Vogt PWH, Hendricks JR, Puillandre N. Lack of signal for the impact of conotoxin gene diversity on speciation rates in cone snails. Syst Biol. 2019:68(5):781–796. 10.1093/sysbio/syz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuong MA, Mahardika GN. Targeted sequencing of venom genes from cone snail genomes reveals coupling between dietary breadth and conotoxin diversity. Mol Biol Evol. 2018:35(5):1210–1224. 10.1093/molbev/msy034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuong MA, Mahardika GN, Alfaro ME. Dietary breadth is positively correlated with venom complexity in cone snails. BMC Genomics. 2016:17(1):401. 10.1186/s12864-016-2755-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puillandre N, Bouchet P, Duda TF Jr, Kauferstein S, Kohn AJ, Olivera BM, Watkins M, Meyer C. Molecular phylogeny and evolution of the cone snails (Gastropoda, Conoidea). Mol Phylogenet Evol. 2014:78:290–303. 10.1016/j.ympev.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puillandre N, Duda TF, Meyer C, Olivera BM, Bouchet P. One, four or 100 genera? A new classification of the cone snails. J Molluscan Stud. 2015:81(1):1–23. 10.1093/mollus/eyu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiro IBL, Bjorn-Yoshimoto WE, Imperial JS, Gajewiak J, Salcedo PF, Watkins M, Taylor D, Resager W, Ueberheide B, Brauner-Osborne H, et al. . Somatostatin venom analogs evolved by fish-hunting cone snails: from prey capture behavior to identifying drug leads. Sci Adv. 2022:8(12):eabk1410. 10.1126/sciadv.abk1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell LJ. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol. 2012:3(2):217–223. 10.1111/j.2041-210X.2011.00169.x. [DOI] [Google Scholar]
- Robinson SD, Li Q, Bandyopadhyay PK, Gajewiak J, Yandell M, Papenfuss AT, Purcell AW, Norton RS, Safavi-Hemami H. Hormone-like peptides in the venoms of marine cone snails. Gen Comp Endocrinol. 2017:244:11–18. 10.1016/j.ygcen.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SD, Norton RS. Conotoxin gene superfamilies. Mar Drugs. 2014:12(12):6058–6101. 10.3390/md12126058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski A, Himaya SWA, Lewis RJ. Coordinated adaptations define the ontogenetic shift from worm- to fish-hunting in a venomous cone snail. Nat Commun. 2023:14(1):3287. 10.1038/s41467-023-38924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybin MJ, O'Brien H, Ramiro IBL, Azam L, McIntosh JM, Olivera BM, Safavi-Hemami H, Yoshikami D. αM-Conotoxin MIIIJ blocks nicotinic acetylcholine receptors at neuromuscular junctions of frog and fish. Toxins (Basel). 2020:12(3):197. 10.3390/toxins12030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavi-Hemami H, Lu A, Li Q, Fedosov AE, Biggs J, Showers Corneli P, Seger J, Yandell M, Olivera BM. Venom insulins of cone snails diversify rapidly and track prey taxa. Mol Biol Evol. 2016:33(11):2924–2934. 10.1093/molbev/msw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer R, Pascolutti VJ, Jackson TNW, Arbuckle K. Diversity begets diversity when diet drives snake venom evolution, but evenness rather than richness is what counts. Toxins (Basel). 2023:15(4):251. 10.3390/toxins15040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieder R, Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics. 2011:27(6):863–864. 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu-Ting K, Torres-Sánchez M, San Mauro D, Wilcockson D, Wilkinson M, Pisani D, O'Connell MJ, Creevey CJ. Inadvertent paralog inclusion drives artifactual topologies and timetree estimates in phylogenomics. Mol Biol Evol. 2019:36(6):1344–1356. 10.1093/molbev/msz067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EG, Surm JM, Macrander J, Simhi A, Amir G, Sachkova MY, Lewandowska M, Reitzel AM, Moran Y. Micro and macroevolution of sea anemone venom phenotype. Nat Commun. 2023:14(1):249. 10.1038/s41467-023-35794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe JE, Puillandre N, Zardoya R. Beyond Conus: phylogenetic relationships of Conidae based on complete mitochondrial genomes. Mol Phylogenet Evol. 2017:107:142–151. 10.1016/j.ympev.2016.10.008. [DOI] [PubMed] [Google Scholar]
- van Thiel J, Alonso LL, Slagboom J, Dunstan N, Wouters RM, Modahl CM, Vonk FJ, Jackson TNW, Kool J. Highly evolvable: investigating interspecific and intraspecific venom variation in taipans (Oxyuranus spp.) and brown snakes (Pseudonaja spp.). Toxins (Basel). 2023:15(1):74. 10.3390/toxins15010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams V, White J, Schwaner TD, Sparrow A. Variation in venom proteins from isolated populations of tiger snakes (Notechis ater niger, N. scutatus) in South Australia. Toxicon. 1988:26(11):1067–1075. 10.1016/0041-0101(88)90205-X. [DOI] [PubMed] [Google Scholar]
- Woodward SR, Cruz LJ, Olivera BM, Hillyard DR. Constant and hypervariable regions in conotoxin propeptides. EMBO J. 1990:9(4):1015–1020. 10.1002/j.1460-2075.1990.tb08204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WoRMS Editorial Board . 2024. World register of marine species. [accessed 2020 Jul 14]. https://www.marinespecies.org at VLIZ. 10.14284/170. [DOI]
- Yanagawa Y, Abe T, Satake M. µ-Conotoxins share a common binding site with tetrodotoxin/saxitoxin on eel electroplax Na channels. J Neurosci. 1987:7(5):1498–1502. 10.1523/JNEUROSCI.07-05-01498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann L, Stephens A, Nam SZ, Rau D, Kübler J, Lozajic M, Gabler F, Söding J, Lupas AN, Alva V. A completely reimplemented MPI bioinformatics toolkit with a new HHpred server at its core. J Mol Biol. 2018:430(15):2237–2243. 10.1016/j.jmb.2017.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All accession numbers used in this study are available in supplementary table S2, Supplementary Material online. The data underlying this article are available in NCBI Sequence Read Archive, and raw reads of C. obscurus, C. consors, and C. ermineus venom gland transcriptomes can be accessed with accession numbers SRR27489861, SRR27489862, and SRR28746436.