Abstract
We have investigated the ability of anti-CD28 antibody costimulation to induce resistance to macrophage (M)-tropic strains of human immunodeficiency virus type 1 (HIV-1) in vitro. Our results confirm the observations of Levine et al. (15) that stimulation of CD4 T cells with anti-CD3/anti-CD28 antibodies coimmobilized on magnetic beads renders the cells resistant to infection by M-tropic strains of HIV-1. The resistance was strongest when the beads were left in the cultures throughout the experiment. In contrast, stimulation of CD4 T cells with the same antibodies immobilized on the surface of plastic culture dishes failed to induce resistance and resulted in high levels of p24 production. This was true even if the cells were passaged continuously on freshly coated plates. If the beads were removed after initial stimulation, p24 production increased over time and produced a result intermediate to the other forms of stimulation. For beads-in, beads-out, and one-time plate stimulated cultures, resistance to infection correlated with down-regulation of CCR5 expression at the cell surface and with increased production of β-chemokines. However, cultures of CD4 T cells continuously passaged on anti-CD3/anti-CD28-coated plates produced large amounts of p24 despite decreased levels of CCR5 expression and increasing production of β-chemokines. Expression of the T-cell activation markers CD25 and CD69 and production of gamma interferon further supported the differences in plate versus bead stimulation. Our results explain the apparent contradiction between the ability of anti-CD28 antibody costimulation to induce resistance to HIV infection when presented on magnetic beads and the increased ability to recover virus from the cells of HIV-positive donors who are on highly active antiretroviral therapy when cells are stimulated by anti-CD3/anti-CD28 immobilized on plastic dishes.
The inability to grow autologous T cells ex vivo, in particular CD4 T cells, from human immunodeficiency virus (HIV)-positive donors has been a major stumbling block for the development of T-cell replacement therapies for AIDS. Recently, Levine et al. developed a method for expanding CD4 T cells from HIV-positive donors in vitro in the absence of antiretroviral drugs with minimal viral replication (15, 16). Their method uses stimulation of highly purified CD4 T cells with anti-CD3 and anti-CD28 antibodies coimmobilized on magnetic beads. They have further shown that costimulation of CD4 T cells by anti-CD28-coated beads renders the cells resistant to infection by macrophage (M)-tropic strains of HIV type 1 (HIV-1) in vitro (5, 15, 20). HIV production is negligible after the first 2 weeks of culture in the absence of antiviral drugs, and proviral DNA is nearly undetectable. The mechanism by which CD28 costimulation induces resistance appears to have two components. The first is by inducing the production of high levels of β-chemokines (MIP-1α, MIP-1β, RANTES) which can block access to CCR5, the coreceptor for M-tropic strains of HIV-1 (5, 20). This component is independent of CD28 and can be achieved by costimulation with other T-cell surface receptors such as CD2, CD4, CD5, or CD8 (20). The second component, which is dependent on costimulation by CD28, is the down-regulation of CCR5 expression at the RNA level (20).
In contrast, other groups have reported that costimulation with anti-CD3/anti-CD28 can result in increased virus production (2, 21, 24). In these reports, costimulation with anti-CD28 by antibodies immobilized on plastic dishes or provided by B7 expression on fixed antigen-presenting cells resulted in increased p24 production by primary CD4 T cells compared to that resulting from stimulation by phytohemagglutinin or anti-CD3 alone. However, both groups used T-tropic viruses, which were not inhibited by CD3/CD28 bead stimulation in the studies of Levine et al. (15). Recently, costimulation of patient T cells by anti-CD3 and anti-CD28 antibodies immobilized on plastic dishes was demonstrated to be a highly sensitive technique for recovery of HIV from the cells of patients on highly active antiretroviral therapy with no detectable virus load (27). This observation is particularly significant since most primary isolates of HIV-1 are M-tropic CCR5-dependent viruses (7, 18, 19). Together these results show that costimulation with anti-CD3/anti-CD28, under certain circumstances, can result in enhanced replication of M-tropic as well as T-tropic strains of HIV.
To more closely examine the issue of resistance to HIV infection, we stimulated highly enriched populations of primary human CD4 T cells with anti-CD3/anti-CD28 antibodies immobilized on magnetic beads or on the surface of plastic culture dishes. Our experiments confirmed that costimulation with anti-CD3/anti-CD28 beads reduces p24 production but that the mode and duration of exposure to anti-CD28 have a significant impact on the extent of resistance to M-tropic strains of HIV-1 in vitro. Whereas stimulation of CD4 T cells with anti-CD3/anti-CD28 beads almost completely inhibited replication of HIV as measured by p24 production, stimulation by antibodies immobilized on plastic dishes resulted in high levels of p24 production. This was true even with continuous passage of cells on freshly coated plates. Increased production of β-chemokines and decreased CCR5 coreceptor expression correlated with the induction of resistance to HIV infection denoted by decreased p24 production. The exception was continuous plate stimulation which led to high levels of p24 production despite down-regulation of CCR5 and increased production of β-chemokines. Anti-CD3/anti-CD28 bead stimulation further gave rise to increased expression of the T-cell activation markers CD25 and CD69 and also with increased production of gamma interferon (IFN-γ) in comparison to that resulting from stimulation with antibodies immobilized on plates. These results suggest that the strength and duration of the activation signal(s) play a role in the ability of CD28 costimulation to induce resistance and further explain how CD28 costimulation can induce resistance to HIV infection under certain conditions and result in increased virus production in others.
MATERIALS AND METHODS
Normal donor cells and culture conditions.
Fresh normal donor peripheral blood mononuclear cells isolated by Ficoll-Hypaque centrifugation were enriched for CD4+ T cells by depletion of CD8+ and CD14+ cells by using antibody-coated magnetic beads (Dynabeads M-450; Dynal, Lake Success, N.Y.) in accordance with the manufacturer’s instructions. The resulting T-cell population typically contained 60 to 85% CD4+ T cells, with <10% CD8+ and the remainder being CD4− CD8−. All cells were diluted to a concentration of 106/ml by using fresh AR medium (a 1:1 mixture of AIM V medium [GIBCO, Grand Island, N.Y.] supplemented with 4 mM l-glutamine and RPMI 1640 medium [JRH Biosciences, Lenexa, Kans.] supplemented with 10% heat-inactivated fetal bovine serum [HyClone Laboratories, Inc., Logan, Utah], 4 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10 mM HEPES, giving a final concentration of 5% fetal bovine serum). Cultures were split and fed with fresh AR medium supplemented with 100 IU of recombinant human interleukin-2 (IL-2) (Proleukin; Chiron Therapeutics, Emeryville, Calif.) on days 3, 5, 7, 10, and 13 poststimulation to maintain a concentration of 106 cells/ml.
Stimulation.
CD4-enriched T-cell populations were stimulated with anti-CD3 (OKT3; Ortho Diagnostics, Raritan, N.J.) and anti-CD28 (Leu-28; Becton Dickinson Immunocytometry Systems, San Jose, Calif.) monoclonal antibodies either directly coated on plastic tissue culture plates or immobilized on sheep anti-mouse immunoglobulin G-coated magnetic beads (Dynabeads M-450; Dynal) with a concentration of 150 fg of each antibody per bead (17). In a typical experiment, 500 ng of each antibody per ml was used to coat plates while bead stimulation was performed with a bead/cell ratio of 3:1. On day 3 poststimulation, T cells were removed from the coated plates while bead-stimulated cells were split into two cultures; beads were either removed (beads-out) or left in the culture for the duration of the experiment (beads-in). For continuous plate stimulation, the day 3 plate-stimulated culture was split into two subcultures, one of which was transferred to a fresh plate coated with anti-CD3 and anti-CD28 antibodies and continuously passaged on fresh anti-CD3/anti-CD28-coated plates (continuous plate) and the other of which was maintained by using uncoated plates (one-time plate).
HIV infections.
On days 3 and 7 poststimulation, cells were infected with the M-tropic strains of HIV-1, JRCSF or SF162, by resuspending 106 cells directly in supernatant containing approximately 50 ng of p24 for 4 h at 37°C, after which excess virus was removed by centrifugation and 106 infected cells were cultured in 2 ml of AR medium with 100 IU of IL-2 per ml. To minimize the potential stearic inhibition of infection by beads bound to the cells in bead-stimulated cultures, the clumps of beads and cells were disrupted as much as possible by vigorous pipetting during resuspension with the virus-containing supernatant. This procedure results in a near-single-cell suspension, with none to a few beads bound to each cell as observed microscopically. Aliquots of supernatant from the infected cultures were removed on days 3 and 7 postinfection and analyzed for p24 by enzyme-linked immunosorbent assay (ELISA) (p24 ELISA kit; DuPont-NEN, Boston, Mass.). Where indicated, infected plate-stimulated or beads-out cells were resuspended in 100% conditioned medium (CM) from a noninfected beads-in culture harvested the same day as the day of infection and spiked with 100 IU of IL-2 per ml.
Surface antigen expression and cytokine production.
The composition of the enriched cell populations was assessed by directly staining the cells with fluorochrome-conjugated anti-CD3, -CD4, -CD8, and -CD14 antibodies (Dako, Carpinteria, Calif., or Coulter/Immunotech, Miami, Fla.) by using standard methods and analyzed by flow cytometry with a Becton-Dickinson FACScan instrument and CellQuest analysis software. At indicated times poststimulation, noninfected cells were surface stained for the expression of the coreceptor CCR5 by using a phycoerythrin-conjugated monoclonal antibody to CCR5 (clone no. 2D7; Pharmingen, San Diego, Calif.) and for the T-cell activation markers CD25 and CD69 (anti-IL-2 receptor–fluorescein isothiocyanate-FITC and Leu-23-fluorescein isothiocyanate, respectively; Becton Dickinson Immunocytometry Systems, San Jose, Calif.). For β-chemokine and cytokine production, aliquots of media were removed at the indicated times poststimulation and analyzed for the presence of RANTES, MIP-1α, MIP-1β, and IFN-γ by ELISA (Quantikine kits; R&D Systems, Minneapolis, Minn.).
RESULTS
Resistance to HIV infection is induced by bead stimulation but not by plate-bound anti-CD3/anti-CD28.
To determine if alternative forms of anti-CD3/anti-CD28 antibody costimulation had equal abilities to render cultured T cells resistant to infection with M-tropic strains of HIV-1, we stimulated enriched CD4 T cells (60 to 85% CD4+) with antibodies bound to magnetic beads or immobilized on plastic culture dishes. Initially, three culture conditions were compared: (i) antibody-coated magnetic beads left in the cultures throughout the experiment (beads in) analogous to the method of Levine et al. (15, 16); (ii) beads removed on day 3 poststimulation prior to infection (beads out); and (iii) antibodies immobilized on plastic culture plates with cells removed from the plates on day 3 poststimulation, prior to infection (plate). On day 3 or day 7 poststimulation, the T cells were infected with the M-tropic strains of HIV-1, JRCSF or SF162, as described in Materials and Methods. HIV replication in the cultures was measured on day 3 and day 7 postinfection by p24 ELISA. In cultures of CD4 T cells stimulated with anti-CD3/anti-CD28 beads, little or no p24 was produced in the 7 days following infection with either of the M-tropic virus strains (Table 1). This was true if cells were infected on either day 3 or day 7 poststimulation but only if the beads were left in the culture. In contrast, cultures of cells stimulated by CD3/CD28 bound to plastic dishes produced high levels of p24 following infection. Production of p24 correlated directly with the growth rate of the cells, which varied from donor to donor. Supernatant p24 was detectable by day 3 postinfection and increased as much as 10-fold by day 7 postinfection. However, in some experiments, p24 levels were lower on day 7 postinfection. This is most likely due to the cytopathic effects of high amounts of virus replication at early times postinfection. In the majority of experiments, p24 production was lower in beads-out cultures than in plate-stimulated cultures following infection on day 3 poststimulation (Table 1). This was true for both JRCSF and SF162 and was more pronounced on day 3 postinfection than on day 7 postinfection. For infections on day 7 poststimulation, p24 production in beads-out cultures is much more vigorous and often higher than in plate-stimulated cultures. Again, this reflects a higher rate of cell growth in the beads-out cultures and also the lower levels of virus replication at early times postinfection. These results suggest that there is some resistance to infection in beads-out cultures when infections are performed immediately following bead removal, but the effect is lost over time. Thus CD3/CD28 costimulation has completely opposite effects on the replication of M-tropic strains of HIV-1 depending on how the antibodies are immobilized and how long the cells are exposed to the stimulation. The inability of these viruses to propagate in cultures of bead-stimulated cells so long as the beads remain present suggests that they are resistant to further infection. Furthermore, the observation that beads-in stimulated cells infected on day 7 poststimulation, when the bead/cell ratio has become less than 1:1 (by dilution), still fail to generate significant virus replication argues against stearic inhibition of infection by the beads.
TABLE 1.
Resistance of CD4+ T cells to infection with M-tropic HIV-1 is dependent on the method of stimulationa
| Donor | Strain | Stimulation | p24 (ng/ml)
|
|||
|---|---|---|---|---|---|---|
| Day 3 infection
|
Day 7 infection
|
|||||
| Day 3 | Day 7 | Day 3 | Day 7 | |||
| M75 | JRCSF | Plate | 272 | 2,020 | 270 | 1,524 |
| Beads out | 3.8 | 1,624 | 123 | 2,044 | ||
| Beads in | 0.5 | 2.0 | 0.2 | 1.6 | ||
| M83 | JRCSF | Plate | 24 | 520 | 3.0 | 404 |
| Beads out | 0.4 | 38 | 76 | 1,664 | ||
| Beads in | 0.3 | 0.3 | 0.2 | 0.1 | ||
| T95 | JRCSF | Plate | 31 | 2.8 | 454 | 86 |
| Beads out | 1.5 | 0.9 | 98 | 194 | ||
| Beads in | 0.6 | 0.3 | 1.1 | 0.4 | ||
| T48 | SF162 | Plate | 322 | 2,012 | 7.3 | 122 |
| Beads out | 0.9 | 150 | 11 | 510 | ||
| Beads in | 0.3 | 0.8 | 5.0 | 5.6 | ||
Enriched primary CD4 T cells (60 to 85% CD4+) were stimulated with anti-CD3 and anti-CD28 antibodies immobilized on magnetic beads or on plastic tissue culture dishes and infected on either day 3 or day 7 poststimulation with HIV-1 JRCSF or SF162 at 50 ng of p24/106 cells. For plate and beads-out cultures, the source of stimulation was removed on day 3 prior to infection. Supernatants were harvested on day 3 and day 7 postinfection and analyzed for p24 by ELISA.
Down-regulation of CCR5 surface expression correlates with resistance induced by CD3/CD28 beads.
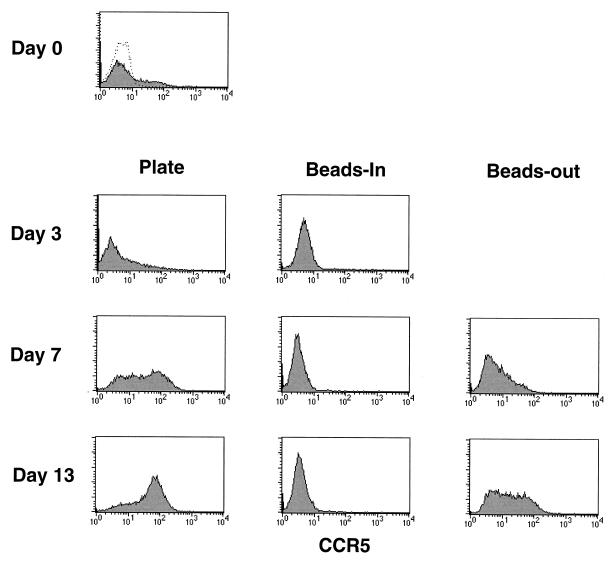
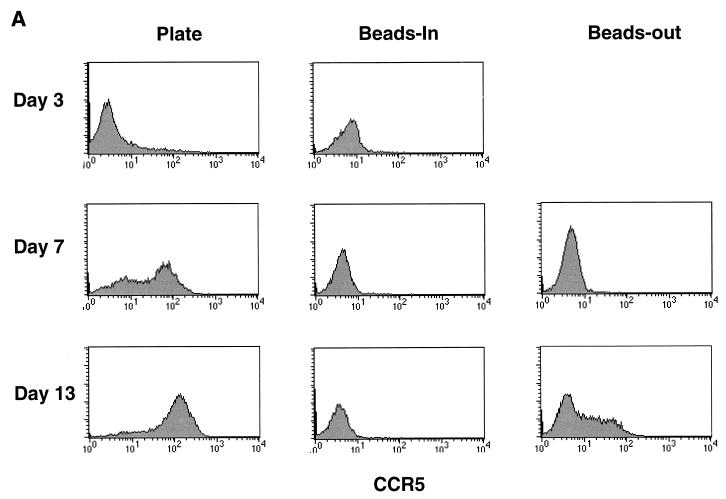
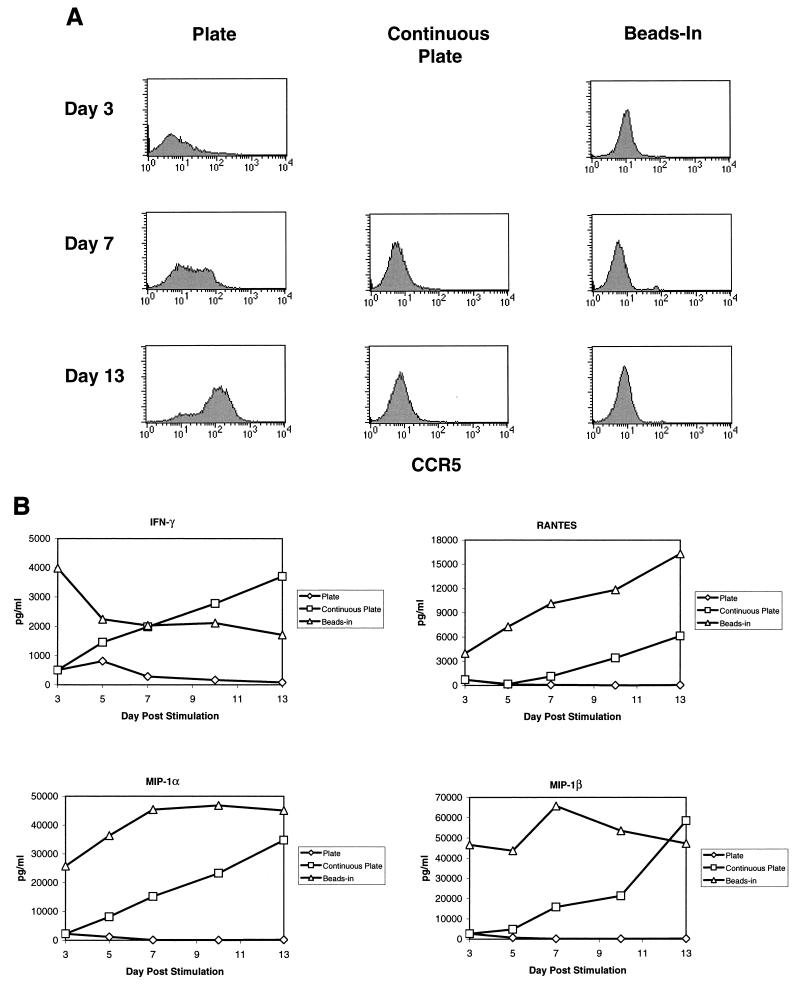
Since resistance to HIV infection was reported to involve down-regulation of CCR5 mRNA (5), we examined our cultures to see if the differences in p24 production by cells stimulated by CD3/CD28 on beads or plates could be attributed to differences in CCR5 expression on the cell surface. Noninfected enriched CD4 T cells were stained for CCR5 expression and analyzed by flow cytometry. CCR5 expression was analyzed in eight donors over several independent experiments with similar results in each case. Representative data is shown in Fig. 1. Initially, only a small percentage of resting CD4 cells had CCR5 expression detectable by fluorescence-activated cell sorting (Fig. 1, day 0). Following bead stimulation, CCR5 expression disappeared from the cell surface and was not detectable for at least 13 days poststimulation so long as the beads remained present in cultures. If the beads were removed on day 3 poststimulation, CCR5 expression gradually returned, reaching detectable levels by day 7 to 10 poststimulation. In contrast, CCR5 expression was significantly increased after stimulation by antibodies immobilized on plastic dishes, with the majority of the cells being positive by day 13 poststimulation and some expressing relatively high levels of CCR5. Our results using fluorescence-activated cell sorting to detect cell surface expression are consistent with the observations of Carroll et al. (5) that CCR5 expression is down-regulated by stimulation with anti-CD3/anti-CD28-coated beads but further show that plate-immobilized antibody stimulation has the opposite effect, significantly increasing expression of CCR5.
FIG. 1.
Surface expression of CCR5 increases over time in plate and beads-out cultures but remains undetectable in the continuous presence of CD3/CD28 beads. Noninfected CD4-enriched T cells (60 to 85% CD4+) stimulated with anti-CD3 and anti-CD28 antibodies immobilized on magnetic beads or on plastic tissue culture plates as described in the legend for Table 1 were collected at various times poststimulation, stained with anti-CCR5 phycoerythrin, and analyzed by flow cytometry. The day 0 point shows CCR5 expression before stimulation (dotted line, isotype control). Data are shown for days 0, 3, 7, and 13 representing the beginning and end of the cultures and the days on which infections were performed in Table 1.
Stimulation of β-chemokine production correlates with resistance to HIV infection.
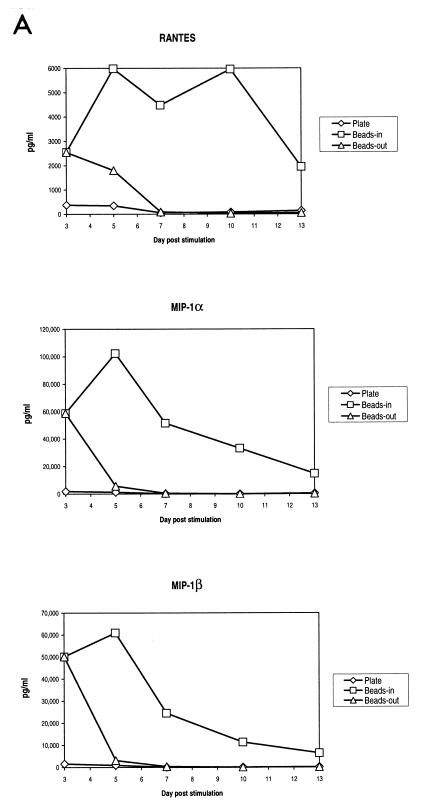
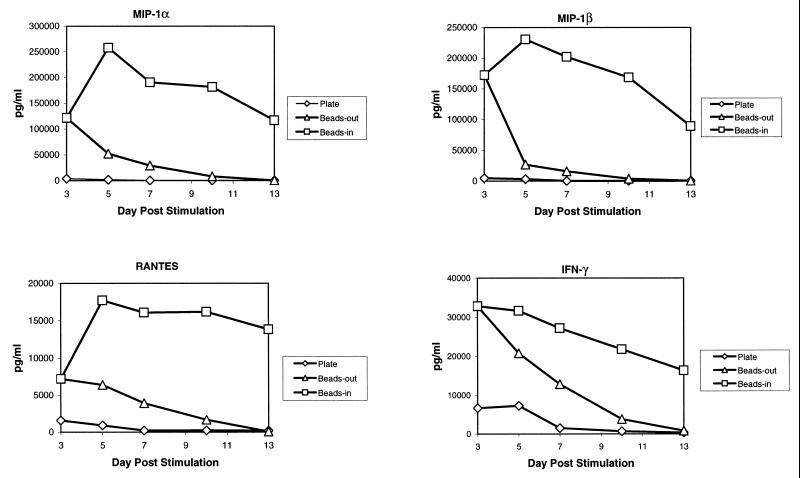
The presence of β-chemokines in the culture supernatant of stimulated T cells could interfere with infection by competing for binding to the chemokine receptors. This has been reported as a mechanism for inhibiting HIV infection (1, 6, 8, 9, 23, 26, 28). Riley et al. (20) also reported that one factor in the induction of HIV resistance induced by costimulation was the induction of β-chemokine secretion, although this mechanism was not dependent on CD28 per se. When we assayed our cultures for the presence of the β-chemokines MIP-1α, MIP-1β, and RANTES, we found that bead-stimulated cells produced high levels of all three chemokines while plate-stimulated cells produced markedly lower levels of β-chemokines (Fig. 2A). The differences in β-chemokine production were most pronounced at later times poststimulation. In bead-stimulated cultures, the levels of all three chemokines remained elevated for the entire culture period while declining rapidly in plate-stimulated cultures. In cultures of cells where the beads were removed on day 3 poststimulation, there was an initial burst of chemokine production which then rapidly declined following bead removal. A similar pattern of β-chemokine production was observed in five different donors. The latter observation indicates that continuous presence of CD3/CD28 stimulation is necessary for maintaining high levels of β-chemokine production.
FIG. 2.
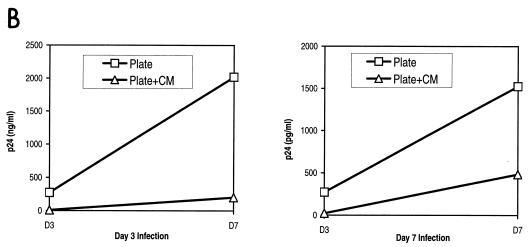
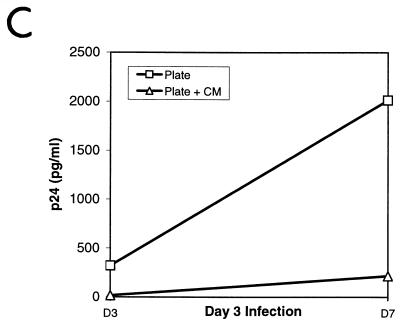
β-Chemokine production varies with the method of CD3/CD28 stimulation. (A) Production of β-chemokines by noninfected cells. Aliquots of culture supernatants from noninfected CD4-enriched primary T cells were collected on days 3, 5, 7, 10, and 13 poststimulation and assayed for the presence of RANTES, MIP-1α, and MIP-1β by ELISA. Results of a representative experiment are shown. (B and C) CM from beads-in cultures inhibits replication of M-tropic viruses. Cultures were infected on day 3 or day 7 poststimulation with 50 ng of p24/106 cells of HIV-1 JRCSF (B) or SF162 (C), washed, and resuspended in either fresh medium or 100% CM from the beads-in culture collected on the same day poststimulation. All cultures were supplemented with 100 IU of IL-2 per ml. Panel B shows the inhibition of JRCSF replication in plate-stimulated cultures following infection on day 3 or day 7 poststimulation. Panel C shows inhibition of SF162 replication in plate-stimulated cultures following infection on day 3 poststimulation.
To further demonstrate the importance of chemokines in inhibiting HIV infection, we added CM collected from the bead-stimulated (beads-in) cultures to cultures of cells where the beads had been removed or where cells were stimulated by plate-bound antibodies. The CM was harvested the same day poststimulation on which the infections were performed (day 3 or day 7). Figure 2B shows that the presence of CM from the beads-in cultures greatly inhibited p24 production in cultures of plate-stimulated cells or cultures where the beads were removed, even when added after the infection had occurred. Replication of both JRCSF and SF162 (Fig. 2C) was inhibited in the presence of CM in independent experiments with two different donors. This inhibition is presumably because of competition for coreceptor binding, down-regulation of the coreceptor from the surface as a result of chemokine binding, or both. Together these data indicate that the availability of the HIV coreceptor plays a critical role in the propagation of HIV infection in vitro.
Higher and prolonged expression of T-cell activation markers in bead-stimulated cultures.
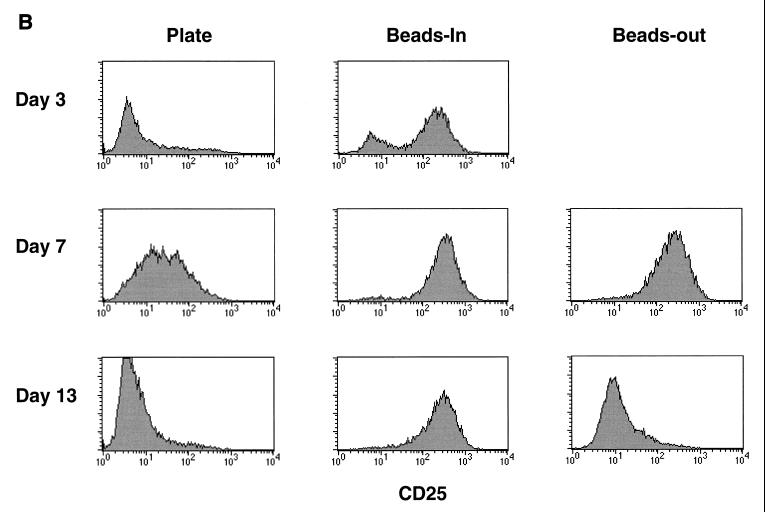
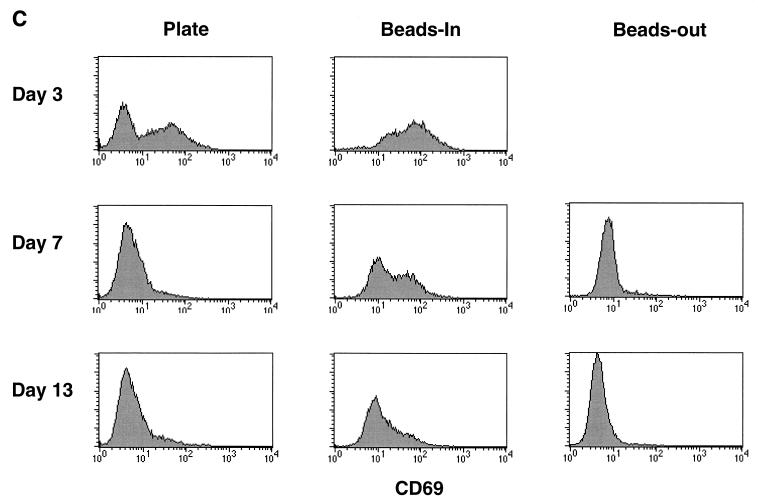
The data presented above indicate that the duration of exposure to CD3/CD28 stimulation is important for maintaining a state of resistance to HIV infection in vitro. The observation that there are differences in CCR5 expression and β-chemokine production evident on day 3 poststimulation further suggests that there may be some intrinsic differences in bead versus plate stimulation. One possible explanation for the differences in the effects of CD3/CD28 immobilized on magnetic beads versus those on plastic dishes is that the quality or strength of the signal differs between the two methods. We addressed this indirectly by looking at the expression of T-cell activation markers following stimulation by either CD3/CD28 beads or plate-bound antibodies. CD69 is the earliest known activation marker to appear on the cell surface following stimulation (25). CD25, the IL-2 receptor α-chain, appears shortly after CD69 and declines as cell proliferation decreases (4, 11). We compared CCR5, CD25, and CD69 expression levels in our enriched CD4 T-cell populations (60 to 85% CD4+) following various forms of CD3/CD28 stimulation. The data shown in Fig. 3 is from one of three different donors where a direct comparison of all three markers was performed. CCR5 expression remained absent from bead-stimulated cells in the continuous presence of beads, gradually returned when beads were removed, and was up-regulated in plate-stimulated cultures as described above (Fig. 3A). Both CD69 and CD25 expression levels were induced following stimulation with anti-CD3/anti-CD28 regardless of which method was used, although the induction was more profound on day 3 following bead stimulation (Fig. 3B and C). By day 5 poststimulation, CD69 levels had returned to baseline in the plate-stimulated cultures and in cultures where the beads were removed. In cultures where beads remained present (beads-in), CD69 expression remained elevated for at least 13 days poststimulation. CD25 expression remained elevated throughout the culture period when the beads were left in. Expression gradually returned to baseline levels in plate-stimulated cultures and in cultures where the beads were removed but remained elevated longer in the beads-out cultures. We also looked at production of IFN-γ as an indicator of the level of T-cell activation in the same three donors. Representative results from one donor are shown in Fig. 4. The levels of IFN-γ produced as a result of various forms of CD3/CD28 stimulation followed the same pattern as the production of β-chemokines. The production of IFN-γ was higher and lasted longer in bead-stimulated cultures where the beads were left in and dropped steadily in cultures of plate-stimulated cells or cultures where the beads were removed, although the drop seemed more precipitous in the plate-stimulated cultures. A similar pattern was observed in the other two donors. These data indicate that prolonged expression of T-cell activation markers correlates with a decrease in CCR5 expression and increased production of β-chemokines and IFN-γ and further demonstrates that duration of exposure to anti-CD3/anti-CD28 stimulation is important for maintaining a high level of T-cell activation. The differences in CD25 and CD69 expression on day 3 poststimulation suggest that immobilizing the antibodies on beads provides a more potent signal than the same antibodies immobilized on the surface of a culture dish.
FIG. 3.
Increased expression of CD69 and CD25 and decreased expression of CCR5 in bead-stimulated cells. Noninfected CD4-enriched T cells (60 to 85% CD4+) were stimulated as indicated and stained for the expression of CCR5, CD25, and CD69 at various times poststimulation as described in the legend to Fig. 1. (A) CCR5 expression; (B) CD25 expression; (C) CD69 expression.
FIG. 4.
Production of IFN-γ correlates with production of β-chemokines in anti-CD3/anti-CD28-stimulated cultures. Aliquots of culture supernatants from noninfected CD4-enriched T cells were collected on days 3, 5, 7, 10, and 13 poststimulation by the indicated methods and assayed for the presence of IFN-γ as well as RANTES, MIP-1α, and MIP-1β by ELISA. Results of a representative experiment are shown.
Continuous passage on anti-CD3/anti-CD28-coated plates also down-regulates CCR5.
To further address the effect of signal duration on the induction of resistance, we compared beads-in stimulation with continuous passage on anti-CD3/anti-CD28-coated plates by using cells from two different donors. For these experiments, noninfected enriched CD4 T cells were stimulated with anti-CD3/anti-CD28 antibodies on beads (beads in) or on plastic dishes where the cells were transferred to uncoated plates on day 3 or passaged continuously on freshly coated plates. In the latter case, plate-stimulated cells were harvested on day 3 poststimulation and replated on fresh antibody-coated plates. This procedure was repeated for each split of the culture. In these experiments, continuous passage on fresh anti-CD3/anti-CD28-coated plates resulted in prolonged down-regulation of CCR5 similar to that seen with continuous bead stimulation (Fig. 5A). Induction levels of CD25 and CD69 expression were also analyzed. Both markers were induced to a greater degree by bead stimulation than by plate stimulation, as previously noted. Continuous passage on fresh antibody-coated plates also had an effect on CD25 expression, maintaining it at higher levels than those seen with one-time plate stimulation, but still lower than those seen in the bead-stimulated cultures. CD69 expression also was higher in bead-stimulated cultures and was not dramatically affected by continuous plate stimulation (data not shown).
FIG. 5.
Effect of continuous plate stimulation on CCR5 expression and β-chemokine production. (A) Noninfected CD4-enriched T cells (60 to 85% CD4+) were stimulated as indicated and stained for the expression of CCR5 at various times poststimulation as described in the legend to Fig. 1. (B) Aliquots of culture supernatants from the same CD4-enriched T cells were collected on days 3, 5, 7, 10, and 13 poststimulation and assayed for the presence of RANTES, MIP-1α, MIP-1β, and IFN-γ by ELISA. The data in Fig. 5 is from one of two donors yielding similar results.
Increased production of β-chemokines and IFN-γ following continuous plate stimulation.
In the same experiment, we also asked whether continuous plate stimulation would increase production of β-chemokines and IFN-γ similar to continuous bead stimulation. At the indicated times postinfection, supernatants were assayed for the presence of MIP-1α, MIP-1β, RANTES, and IFN-γ by ELISA. Results from one of two donors are shown in Fig. 5B. Bead-stimulated cells produced relatively high levels of all four cytokines as expected. On day 3 poststimulation, plate-stimulated cultures contained low levels of all four cytokines. When the cells were transferred to uncoated plates, there was little to no increase in cytokine production. However, when the cells were repeatedly transferred to fresh anti-CD3/anti-CD28-coated plates, the levels of all cytokines rose continually over the next 10 days, reaching levels similar to those in bead-stimulated cultures.
Continuous stimulation on anti-CD3/anti-CD28-coated plates does not induce resistance to HIV infection.
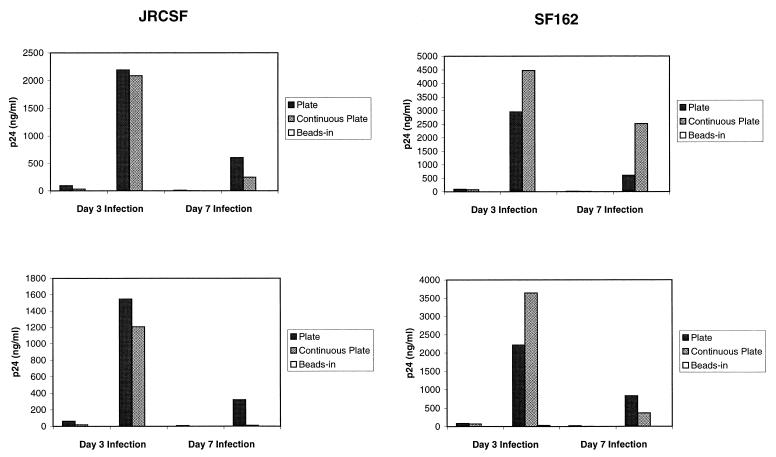
Since continuous plate stimulation caused the down-regulation of CCR5 and increased secretion of β-chemokines, we expected that this method of stimulation also would induce resistance to infection by M-tropic strains of HIV-1. To test this hypothesis, enriched CD4 T cells were infected with HIV-1 JRCSF and SF162 on day 3 and day 7 poststimulation and the cultures were assayed for p24. Cells from two different donors were tested with both viruses. The results are shown in Fig. 6. Continuous bead stimulation almost completely inhibited virus replication, and one-time plate stimulation led to high levels of p24 production, as observed in previous experiments (Table 1; Fig. 2). Surprisingly, continuous plate stimulation gave rise to high levels of p24 production relative to those resulting from one-time plate stimulation, in most cases, and sometimes higher. The lower levels of p24 production following infections on day 7 poststimulation are probably due to decreased cell growth at this point in the cultures. The fact that neither one-time nor continuous plate stimulation consistently leads to lower p24 production suggests that there is no real difference in these two methods of stimulation in terms of resistance. To ensure that CCR5 expression was down-regulated during this experiment, we stained an aliquot of cells from each culture for CCR5 on day 7 poststimulation. The cells from the continuous plate-stimulated culture showed barely detectable levels of CCR5 expression, as did bead-stimulated cells (data not shown). In contrast, one-time plate-stimulated cells had elevated levels of CCR5 expression, as observed in previous experiments. Thus, continuous plate stimulation does not protect CD4 T cells from HIV replication despite down-regulation of CCR5 and production of β-chemokines. These results suggest that down-regulation of CCR5 and production of β-chemokines may not be the only factors that contribute to the induction of resistance by anti-CD28 costimulation.
FIG. 6.
Resistance of T cells to infection following continuous plate stimulation. Enriched primary CD4 T cells were stimulated with anti-CD3/anti-CD28 antibodies immobilized on magnetic beads or on plastic tissue culture plates and infected on either day 3 or day 7 poststimulation with HIV-1 JRCSF or SF162 at 50 ng of p24/106 cells as described in Materials and Methods. Supernatants were harvested on day 3 and day 7 postinfection and analyzed for HIV-1 p24 by ELISA. Data from two different donors is shown.
DISCUSSION
Many laboratories are currently using the combination of anti-CD3 plus anti-CD28 costimulation to recover infectious HIV from cells of HIV-positive donors with low or undetectable viral loads. Yet anti-CD3/anti-CD28 costimulation has also been reported to generate resistance to infection by M-tropic strains of HIV-1 in vitro. In the studies presented here, we have investigated the ability of anti-CD28 costimulation to induce resistance to HIV infection in vitro. Our results show that the mode in which anti-CD28 and anti-CD3 are coimmobilized and the duration of exposure to the stimulation determine the generation of resistance and provide an explanation for the apparently contradictory results regarding the effect of anti-CD28 costimulation on HIV replication in vitro. Our results with beads-in and beads-out stimulation are consistent with those of Barker et al. (2), who found that replication of M-tropic viruses was blunted in the presence of CD3/CD28 beads but rebounded when the beads were removed. In our hands, in cultures of T cells stimulated by different methods of presentation of anti-CD3/anti-CD28 (beads-in, beads-out, and one-time plate stimulation), resistance to infection correlates with a decrease in surface expression of CCR5 coreceptor and with production of the β-chemokines MIP-1α, MIP-1β, and RANTES. Cultures which produce little p24 (high level of resistance) also have low to undetectable levels of CCR5 expression and produce the greatest amounts of β-chemokines, and vice versa. Furthermore, CM from beads-in cultures, which contains a high level of β-chemokines, augments resistance to HIV infection in beads-out and one-time plate-stimulated cultures. Thus, the mechanism of CD28-induced resistance appears to center around the availability of the CCR5 coreceptor.
One caveat of our surface staining experiments is that we did not start with a pure population of CD4 T cells. Thus, observed changes in surface expression of the various markers may reflect changes in cell types other than CD4 T cells. However, we do not believe that this factor has a significant effect on our results since the pattern of surface marker expression was remarkably consistent from experiment to experiment (donor to donor), and within a given experiment the same starting cell population was used for every culture. Moreover, CCR5 expression in the entire cell population was uniformly affected by a given treatment, indicating that all CD4 T cells in the population were likewise uniformly affected.
If the apparent resistance to infection is due solely to the inhibition of coreceptor usage, the effect of anti-CD3/anti-CD28 bead stimulation is most likely to block subsequent rounds of infection rather than by “curing” cells already infected. This hypothesis is supported by the observations of Barker et al. (2) and Levine et al. (15) that there is an initial burst of p24 production and detection of gag DNA by PCR which disappears after the first 2 weeks of culture as the infected cells are eliminated. Furthermore, the failure to develop resistance to T-tropic strains of HIV-1 might be due to the inability to down-regulate or block the availability of the T-tropic coreceptor CXCR4 (3, 10). This hypothesis also is consistent with the findings of Levine et al. (15), who saw no resistance to T-tropic strains of HIV-1 and found that CXCR4 expression was up-regulated by CD3/CD28 costimulation (5).
Stimulation by continuous passage on anti-CD3/CD28-coated plates provides an exception to the above rule. In our hands, CD4 T cells stimulated by continuous passage on anti-CD3/CD28-coated plates produced high levels of p24 and therefore were not resistant to infection despite down-regulated CCR5 expression and increasing production of β-chemokines. At present, we have no definitive explanation for this observation. No significant difference in growth rate or behavior of the cells under the different culture conditions was noted. One possibility is that the down-regulation of CCR5 by plate stimulation is not as complete as down-regulation by bead stimulation resulting in a low level of CCR5 on the cell surface sufficient to propagate HIV infection in vitro. Alternatively, factors other than the availability of CCR5 may play a role in generating resistance to HIV infection. For example, other coreceptors may be able to support infection by HIV-1 JRCSF and SF162 in plate-stimulated cells, or repeated stimulation by anti-CD3 and anti-CD28 antibodies coimmobilized on the surface of a tissue culture dish may increase the potential for infection by CCR5-independent mechanisms.
CD28 costimulation causes a bimodal down-regulation of CCR5 expression, both decreasing the amount of CCR5 message (5) and reducing surface expression (this report). With regard to surface expression, we cannot rule out that the apparent down-regulation is due to inhibition of staining by the presence of high levels of β-chemokines. However, several lines of evidence suggest that the presence of β-chemokines alone cannot account for the complete absence of CCR5 surface expression among bead-stimulated T cells. First, the reduction in CCR5 mRNA levels induced by bead stimulation is consistent with a concomitant decrease in surface expression; second, in one-time plate-stimulated cultures, there is clearly an increase in CCR5 expression; third, CCR5 expression remains undetectable even when chemokine levels are low or decreasing (early in plate-stimulated cultures, late in beads-in cultures); and finally, other groups studying the inhibition of infectivity by chemokines have observed only a modest decrease in staining in the presence of chemokines (26).
One issue raised by these studies is whether there is an intrinsic difference between stimulation by anti-CD3/anti-CD28 antibodies immobilized on beads and that on the surface of a tissue culture plate, or if the effects are simply due to the time of exposure to the stimulation. Clearly, both CCR5 expression and β-chemokine production are influenced by the time of exposure to anti-CD3/CD28 beads. In cultures where the beads were not removed, CCR5 expression remained undetectable throughout the culture period and production of MIP-1α, MIP-1β, and RANTES remained relatively high. When the beads were removed on day 3 poststimulation, β-chemokine production dropped off and CCR5 was slowly reexpressed. These changes correlated with a breakdown in resistance to HIV infection and resulted in significant levels of p24 production by day 7 postinfection. Thus, the characteristics of the beads-out cultures were similar to those of the one-time plate-stimulated cultures, where the cells were removed from the antibody-coated plates on day 3 poststimulation, but not entirely analogous. Bead-stimulated cultures showed higher levels of β-chemokine production on day 3 than plate-stimulated cultures despite continuous stimulation during that time. Furthermore, although CCR5 expression was not significantly different on day 3, one-time plate-stimulated cells expressed high levels by day 5 to 7 poststimulation, whereas expression only slowly increased after removal of beads on day 3 and did not reach the levels in plate-stimulated cultures by day 13. Other differences between bead and plate stimulation were observed by evaluating T-cell activation marker expression. Bead-stimulated cells expressed higher levels of CD69 and CD25 on day 3 poststimulation. While CD69 expression dropped rapidly to baseline following removal of beads, CD25 expression remained elevated significantly longer in beads-out cultures than in one-time plate-stimulated cultures. The latter observations suggest that there may be some intrinsic differences between bead and plate stimulation. The most striking evidence in support of such a difference is in the case of continuous plate stimulation, where resistance to infection does not correlate with CCR5 down-regulation and β-chemokine production. This is in contrast to continuous bead stimulation (beads in), in which the cells are resistant to infection. One possible explanation for these differences is that the beads are able to bind and present more antibody per unit area than the plate. We found no significant difference in the response of cells when we used five times the amount of antibody (2.5 μg/ml) to coat the plates, either directly or indirectly by first coating with goat anti-mouse IgG (data not shown). This could be interpreted to suggest that increasing the ligand density on the plates is not sufficient to overcome an intrinsic difference in bead versus plate stimulation or simply that the ligand density on the plate is still not equivalent to that displayed on the beads. More direct measurement of the amount of antibody bound on the plate versus that on the beads is required to resolve this issue. However, it would still be difficult to rule out an effect of other factors such as surface geometry.
CD28 has been postulated to have multiple signaling pathways associated with it which can be delineated by using different cell culture systems or different degrees of antibody cross-linking (12, 13, 22). Differential signal strength and usage of B7-1 versus B7-2 have also been reported to correlate with development of Th1 versus Th2 responses in vitro and in vivo (14). Thus, the signals generated by anti-CD3/anti-CD28 coimmobilized on beads and plates may result in signals that are qualitatively or quantitatively different or both. The fact that there are significant differences in the induction of T-cell activation markers by plate and bead stimulation even when five times the amount of coating antibody is used suggests that there may be intrinsic biochemical differences in the signals generated by each of these methods that are dictated by surface geometry. This hypothesis is supported by the failure of plate stimulation to induce resistance to HIV infection. Further investigation of these methods of anti-CD3/anti-CD28 costimulation could provide insights into the mechanism of CD28 signal transduction.
Finally, we have identified a method for inducing high levels of CCR5 coreceptor expression in vitro. Transient exposure (3 days) of CD4 T cells to anti-CD3/anti-CD28 antibodies immobilized on the surface of a tissue culture dish leads to the dramatic up-regulation of CCR5 expression once the cells are removed from the original stimulation. This observation offers at least a partial explanation for the success that has been achieved in using this method of stimulation to rescue virus from the cells of AIDS patients with no dectable viral load as a result of highly active antiretroviral therapy. Furthermore, we demonstrate a method for dramatically reducing the level of CCR5 expression in vitro and maintaining expression at low to undectable levels by using continuous anti-CD3/anti-CD28 stimulation by two means which may have different outcomes in terms of virus replication. These experimental systems may be useful for further studies of the role of coreceptor usage in HIV infection.
ACKNOWLEDGMENTS
We thank Otto Yang and Bruce Walker, AIDS Research Center, Massachusetts General Hospital and Harvard Medical School, Charlestown, Mass., for providing the M-tropic strains of HIV-1 and Gib Otten and Carl June for helpful discussions and critical review of the manuscript.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Barker E, Bossart K N, Levy J A. Differential effects of CD28 co-stimulation on HIV production by CD4+ T cells. J Immunol. 1998;161:6223–6227. [PubMed] [Google Scholar]
- 3.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantrell D A, Smith K A. The interleukin-2 T cell system: a new cell growth model. Science. 1984;224:1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- 5.Carroll R G, Riley J L, Levine B L, Feng Y, Kaushal S, Ritchey D W, Bernstein W, Weislow O S, Brown C R, Berger E A, June C H, St. Louis D C. Differential regulation of HIV-1 fusion cofactor expression by CD28 costimulation of CD4+ T cells. Science. 1997;276:273–276. doi: 10.1126/science.276.5310.273. [DOI] [PubMed] [Google Scholar]
- 6.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 7.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1 infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng H K, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 9.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 11.Greene W C, Leonard W J. The human interleukin 2 receptor. Annu Rev Immunol. 1986;4:69–96. doi: 10.1146/annurev.iy.04.040186.000441. [DOI] [PubMed] [Google Scholar]
- 12.June C H, Bluestone J A, Nadler L M, Thompson C B. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 13.Ledbetter J A, Imboden J B, Schieven G L, Grosmaire L S, Rabinovitch P S, Lindsten T, Thompson C B, June C H. CD28 ligation in T cell activation: evidence for two signal transduction pathways. Blood. 1990;75:1531–1539. [PubMed] [Google Scholar]
- 14.Lenschow D J, Walunas T L, Bluestone J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 15.Levine B L, Mosca J D, Riley J L, Carroll R G, Vahey M T, Jagodinski L L, Wagner K F, Mayers D L, Burke D S, Weislow O S, St. Louis D C, June C H. Antiviral effect and ex vivo CD4+ T cell proliferation in HIV-positive patients as a result of CD28 costimulation. Science. 1996;272:1939–1943. doi: 10.1126/science.272.5270.1939. [DOI] [PubMed] [Google Scholar]
- 16.Levine B L, Bernstein W B, Connors M, Craighead N, Lindsten T, Thompson C B, June C H. Effects of CD28 co-stimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159:5921–5930. [PubMed] [Google Scholar]
- 17.Levine B L, Ueda Y, Craighead N, Huang M L, June C H. CD28 ligands CD80 (B7-1) and CD86 (B7-2) induce long-term autocrine growth of CD4+ T cells and induce similar patterns of cytokine production in vitro. Int Immunol. 1995;7:891–904. doi: 10.1093/intimm/7.6.891. [DOI] [PubMed] [Google Scholar]
- 18.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 19.Paxton W A, Martin S R, Tse D, O’Brien T R, Skurnick J, VanDeventer N L, Padian N, Braun J F, Kotler D P, Wolinsky S M, Koup R A. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposures. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 20.Riley J L, Carroll R G, Levine B L, Bernstein W, St. Louis D C, Weislow O S, June C H. Intrinsic resistance to T cell infection with HIV type 1 induced by CD28 costimulation. J Immunol. 1997;158:5545–5553. [PubMed] [Google Scholar]
- 21.Roederer M, Raju P A, Mitra D K, Herzenberg L A, Herzenberg L A. HIV does not replicate in naïve CD4 T cells stimulated with CD3/CD28. J Clin Invest. 1997;99:1555–1564. doi: 10.1172/JCI119318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudd C. Upstream-downstream: CD28 cosignaling pathways and T cell function. Immunity. 1996;4:527–534. doi: 10.1016/s1074-7613(00)80479-3. [DOI] [PubMed] [Google Scholar]
- 23.Simmons G, Clapham P R, Picard L, Offord R E, Rosenkilde M M, Schwartz T W, Buser R, Wells T N C, Proudfoot A E I. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 24.Spina C A, Prince H E, Richman D D. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J Clin Invest. 1997;99:1774–1785. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Testi R, Philips J H, Lanier L L. Leu-23 induction as an early activation marker for functional CD3/T cell antigen receptor triggering: requirement for receptor cross-linking, prolonged elevation of intracellular Ca++, and stimulation of protein kinase C. J Immunol. 1989;142:1854–1860. [PubMed] [Google Scholar]
- 26.Trkola A, Paxton W A, Monard S P, Hoxie J A, Siani M A, Thompson D A, Wu L, Mackay C R, Horuk R, Moore J P. Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J Virol. 1998;72:396–404. doi: 10.1128/jvi.72.1.396-404.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong J K, Hezareth M, Gunthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 28.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]