Abstract
The predation-driven Mesozoic marine revolution (MMR) is believed to have induced a dramatic change in the bathymetric distribution of many shallow marine invertebrates since the late Mesozoic. For instance, stalked crinoids – isocrinids (Isocrinida) have undergone a striking decline in shallow-sea environments and today they are restricted to deep-sea settings (below 100 m depth). However, the timing and synchronicity of this shift are a matter of debate. A delayed onset of MMR and/or shifts to a retrograde, low-predation community structure during the Paleogene in the Southern Ocean were invoked. In particular, recent data from the Southern Hemisphere suggest that the environmental restriction of isocrinids to the deep-sea settings may have occurred at the end of the Eocene around Antarctica and Australia, and later in the early Miocene in New Zealand. Here, we report the anomalous occurrence of the isocrinids in shallow nearshore marine facies from the middle Miocene of Poland (Northern Hemisphere, Central Paratethys). Thus, globally, this is the youngest record of shallow-sea stalked crinoids. This finding suggests that some relict stalked crinoids may have been able to live in the shallow-water environments by the middle Miocene, and further confirms that the depth restriction of isocrinids to offshore environments was not synchronous on a global scale.
Keywords: Echinoderms, Sea lilies, Predation, Neogene, Cenozoic
Subject terms: Palaeontology, Evolutionary ecology
Introduction
Today, stalked crinoids, including isocrinids (Isocrinida), are restricted to the depth below 100 m (e.g. Refs.1,2). However, in the geologic past, especially during the Triassic and Jurassic, they were abundant in shallow-sea (nearshore and inner shelf) environments (e.g. Refs.3). In a seminal paper on bathymetric distribution of fossil isocrinids from Euramerica, Bottjer and Jablonski4 showed that the shift to deeper environments started by the Cretaceous and was completed by the Eocene. A variety of causal mechanisms for this onshore-offshore pattern has been suggested including bias toward onshore deposits of Mesozoic age5. Nevertheless, the most likely driver was increased diversification of durophagous predators especially fishes and grazing echinoids. This has been supported by observations of Recent crinoids, which show increased frequency of regenerated arms in shallower than in deeper sea settings6. Evidence of numerous bite marks and arm/pinnule regenerations on shallow-sea Mesozoic crinoids ascribed to predation is also consistent with this hypothesis (e.g. Refs.7–9).
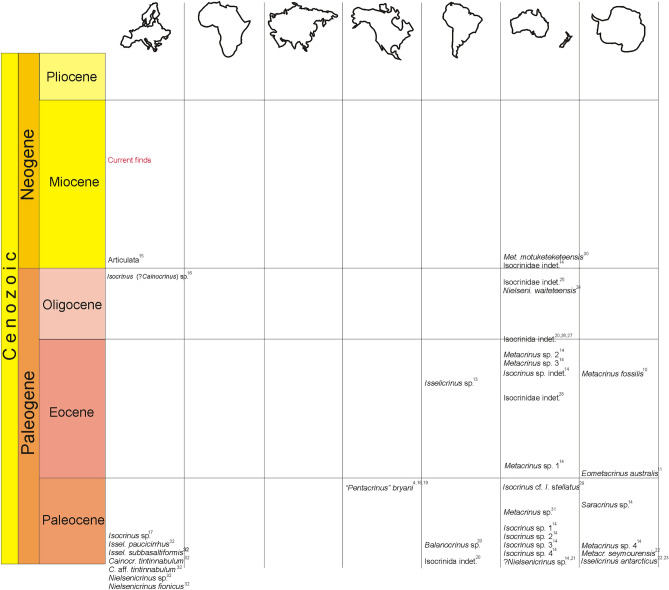
Admittedly, however, there are at least a few records of shallow-sea isocrinids from the Cenozoic, especially from the Southern Hemisphere (Fig. 1). Among the most spectacular occurrences are dense assemblages of ophiuroids and isocrinids in a late Eocene, shallow-marine setting of the La Meseta Formation on Seymour Island, Antarctic Peninsula10–13. This anomalous record has been referred by the latter authors to as the retrograde community structure that might have developed due to a combination of reduced predation pressure, a favorable temperature regime and increased upwelling in Antarctic surface waters. Nevertheless, a recent compilation on the Cenozoic occurrences of isocrinids from the Southern Hemisphere implies a continuous record of shallow marine isocrinids from the Cretaceous to the late Eocene, rather than temporal reversions14, suggesting that the timing of the onshore-offshore shift was not synchronous globally.
Figure 1.
Cenozoic occurrences of shallow-sea stalked crinoids in different continents: Europe (Refs.15–18), North America (Refs.4,19,20), South America (Refs.13,21), Australia (Refs.14,21–30), and Antarctica (Refs.10,11,14,31,32). The depth of inner shelf is understood here as approximately 40 m (for details see Fig. 2.1. in Ribeiro33).
In this paper, we describe isocrinid ossicles from the middle Miocene shallow marine facies exposed in two localities in Poland. These findings not only represent the youngest global fossil record of shallow-sea isocrinids, but also support the view that occasionally, some stalked crinoids might have remained in the onshore environments long time after the initiation of the Mesozoic marine revolution.
Geological settings and stratigraphy
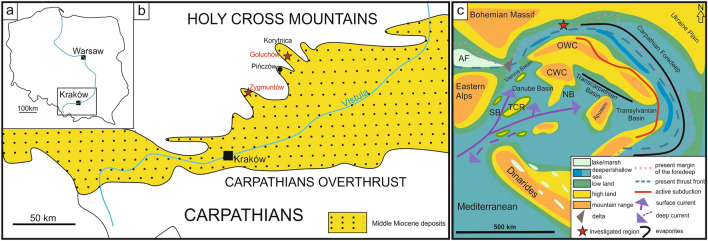
Field works aimed at collecting crinoid material were conducted in two localities in the southern Poland (Fig. 2), namely Gołuchów and Zygmuntów (near Książ Wielki). Gołuchów is located on the southern edge of the Holy Cross Mountains, approximately 30 km south of Kielce city and approximately 20 km east of Jędrzejów city. The outcrop is located in a disused quarry (coordinates: 50.622703 N, 20.615972 E). Zygmuntów profile (near Książ Wielki) is located approximately 40 km southwest of Gołuchów and approximately 30 km south of Jędrzejów city. Herein, as a result of the construction of the S7 Cracow-Kielce expressway, Miocene sediments are exposed (coordinates: 50.26339 N, 20.11198 E).
Figure 2.
Location of the investigated outcrops. On the left, location sketch of the Gołuchów and Zygmuntów on the background of middle Miocene deposits in the southern Poland (a,b). On the right, palaeogeographic location of the studied outcrops ((AF) Alpine Foredeep; (CWC) Central Western Carpathians; (NB) Novohrad-Nógrád Basin; (OWC) Outer Western Carpathians; (SB) Styrian Basin; (TCR) Transdanubian High; (VB)) (c). Redrawn and modified after Studencka et al.34; Kováč et al.35.
Both localities are located in the marginal, northern part of the Carpathian Foredeep, on the southern foreland of the Holy Cross Mountains36,37 (Fig. 2). In the Miocene, this region was located in the northern part of Central Parathetys, which was characterized by the presence of numerous shallow marine bays34,36,38–43 (Fig. 2).
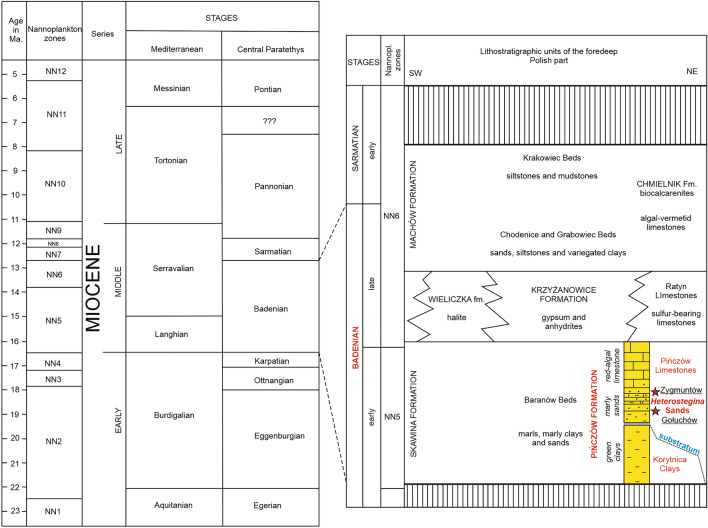
Stratigraphically, sediments exposed here belong to the lower part of the middle Miocene Mediterranean stages (Langhian and Serravalian) corresponding to the Central Paratethys Badenian stage (e.g. Ref.35; Fig. 3). In the local lithostratigraphic division, the studied sites are classified to the so-called Pińczów formation42–45, that is represented by siliciclastic, siliciclastic-carbonate and carbonate lithoral and sublithoral facies36,38,39,42,45–47(Fig. 3). Similar facies of this age continue in the eastwards towards Ukraine34,47.
Figure 3.
Stratigraphic scheme of the Miocene (after Steininger et al.48) and stratigraphic schemes for the foredeep depozone of the Fore-Carpathian Basin in Poland (modified after Jasionowski49; Oszczypko et al.37).
In the studied area, depending on the palaeomorphology of the basement, various clays, sands, marly sands, sandy limestones and limestones overlie abrasive Upper Jurassic (Kimmeridgian) or Upper Cretaceous (Campanian–Maastrichtian) substrate38,50 (Fig. 3). According to the local lithostratigraphic unites, the sediments exposed in both localities belong to the so-called Heterostegina Sands (Fig. 3). Both with the so-called Korytnica Clays, these levels are included in the Badenian dinocyst Zone Unipontidinium aquaeductum equivalent of Nannoplankton Zone NN5-645,51–54.
Within the sedimentary succession of the Heterostegina Sands, marly or calcareous sands and sandy limestone units yielding abundant fossils may be distinguished38,39,41,45,46. The most characteristic features for Heterostegina Sands are large benthic foraminifers (Heterostegina and Amphistegina)53, along with oval rhodoids and isolated colonies of red-algae (Lithothamnium in older literature) 42,43,46. Small and large-sized pelecypods, lingulid brachiopods, echinoids, starfish and large gastropods are abundant in these sediments38,41,46,55. Large oysters, commonly found herein, are typically encrusted by serpulids.
Gołuchów quarry
At the base of the quarry, strongly lithified lower Kimmeridgian oolitic-bioclastic limestone facies are exposed38. Directly above the Kimmeridgian rocks, Badenian sediments, mostly represented by fine-grained red-algal sandy limestones with pebbles of the Kimmeridgian oolitic limestone, are present38 (Fig. 4a) Above, fine detritical sands and poorly lithified limy or marly sandstones38 of Heterostegina Sands are developed. Herein, abundant fossils (Fig. 4b) are present, including foraminifers, molluscs, bryozoans, serpulids, echinoderms (asteroids, echinoids and stalked crinoids, the latter group described herein), and teeth of fish.
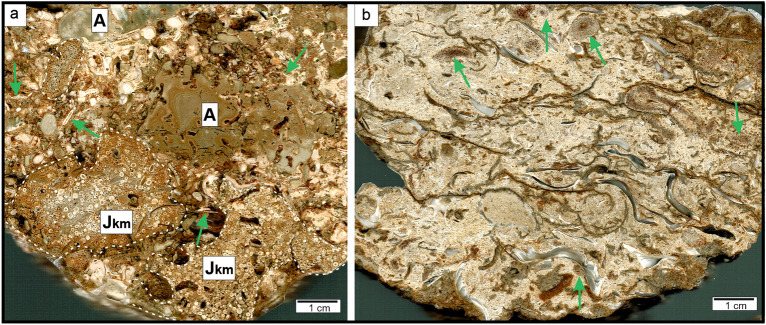
Figure 4.
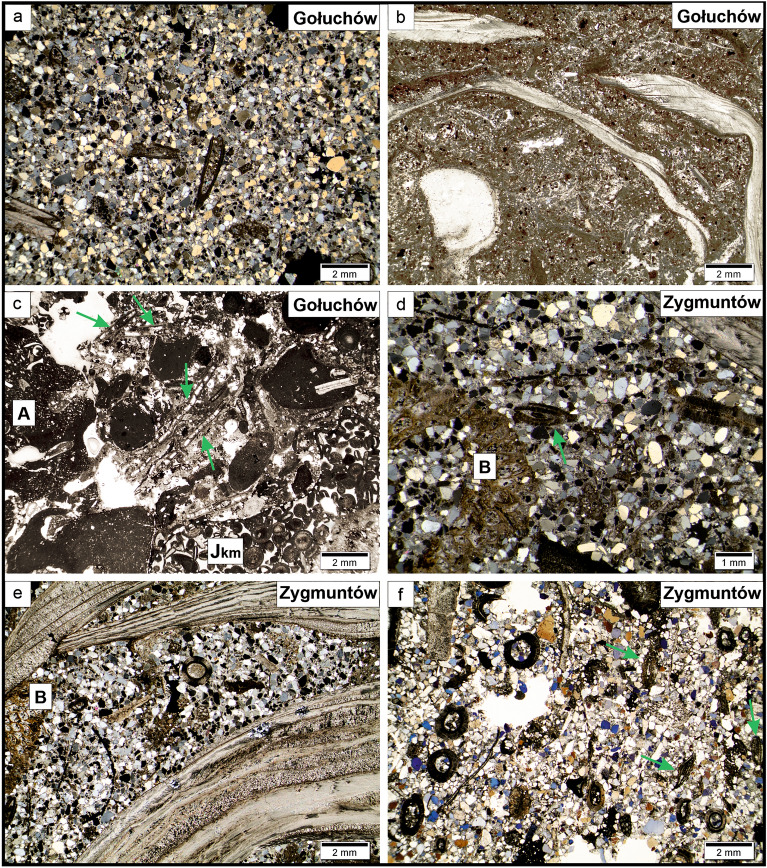
Polished slabs from Gołuchów Quarry. (a) Bioclastic floatstone/rudstone with numerous large Badenian benthic foraminifers (arrows), bivalve shells, bored algae (A) and oolitic clasts (Jkm). (b) bioclastic-bivalve floatstone with thick-shelled parallel or chaotic distributed bivalves and bryozoans (arrows). In calcareous matrix visible numerous small quartz grains.
Our analyses of thin sections (Fig. 5a–c) taken from these rock levels suggest the presence of two facies types: (i) sandy limestone (FT 1-facies type 1) with numerous bioclasts (Fig. 5b,c) and (ii) sand and/or poorly lithified (sporadically marly) sandstone with numerous bioclasts (FT 2-facies type 2) (Fig. 5a). Numerous quartz grains and exclusively Miocene bioclasts embedded in the carbonate matrix are observed herein. FT 1 facies type is mostly represented by sandy limestone (rudstone-floatstone), within each numerous bored fragments of algal colony are present (Fig. 5b,c). Among bioclasts, abundant and well-preserved small and large benthic foraminifers (Amphistegina sp. and Heterostegina costata), molluscs, bryozoans and echinoderms were observed. The matrix is mostly composed of carbonates though small quartz grains are also observed. In the FT 2 sharp-edged quartz grains with a diameter of up to 1 mm, most often ~ 0.5 mm (Fig. 5a) are abundant. Glauconite grains are also common in these sediments. Among the bioclasts, Badenian small and large benthic foraminifers and polychaetes (Fig. 5a) were observed. Calcareous clasts up to 3 mm thick were also observed, in which fragments of red-algae and micritic sediments with small quartz grains resembling FT 1 can be distinguished.
Figure 5.
Microfacies from study oucrops. (a) bioclastic sandstone; visible mainly quartz grains, calcareous matrix and fragments of bivalves and polychaetes. (b) bivalve-bioclastic floatstone. (c) foraminifer-algae floatstone/rudstone with numerous large benthic foraminifers (arrows) and bored algaes (A); in the lower part clast of ooid limestone (Jkm). (d) bioclastic-reach sandstone with large benthic foraminifers (arrows), bryozoans (B), bivalves. (e, f) calcareous sandstone with numerous benthic foraminifers (arrows), bryozoans (B), shells of bivalves and polychaetes.
Zygmuntów (near Książ Wielki)
In the newely exposed outcrop, sands with gravels embedded with two layers of clays, each with a thickness of about 0.6 m, and three layers of limestones, each 0.4 m thick, are present. Herein, within the lenses of sands and gravels, abundant benthic invertebrate fossils and fish teeth can be found. Crinoids were recorded in marly sands of the middle part of the section. Grill56 and Studencka55 based on characteristic bivalve assemblage suggested that these sediments belong to the Lagenidae Zone (early Badenian).
Our analyses of thin sections indicated that the bioclastic-reach sand and/or poorly lithified sandstone facies have limy matrix (Fig. 5d). Quartz grains typically have diameters of ~ 0.5 mm. Smaller grains are sharp-edged (Fig. 5e,f). Glauconite grains are also noted. Among numerous, exclusively Miocene bioclasts, we observed thick-shelled bivalves, bryozoans, serpulids, echinoids and fragments algal colonies (Fig. 5d–f). Abundant and well-preserved benthic foraminifers of various size (such as Amphistegina sp.) (Fig. 2f) are the most characteristic. Overall, this facies is similar to FT 1 observed in Gołuchów.
Palaeoenvironmental interpretation
Facies and microfacies (Figs. 4, 5) recorded in both localities can be ascribed to the so-called Heterostegina Sands. Facies type 2 belongs to algal-amphisteginid/heterosteginid facies44 with numerous bryozoans and large benthic foraminifers. In general, these facies have been developed in near-shore shallow marine environment of moderate energy, that was occasionally subjected to storms, which resulted in faunal accumulations with coquina lags41,46. Mass occurance of well-preserved large foraminifers (Amphistegina and Heterostegina) are typical for shallow marine early Badenian Paratethys deposits. It is noteworthy that Recent representatives of Amphistegina prefer shallow-waters at a depth below 20 m57. No linear current sedimentary structures were observed, which is indicative of higher turbulance during storms. Previous studies based on analyses of the shallow-water fauna determined the palaeodepth of these sedimentary environment to be several to several dozen meters36,38,39,42,46.
Results
Fish fossil assemblage
Apart from collected crinoid fossils, a number of fish teeth were recorded (they will be described in a separate paper elsewhere). In the Gołuchów quarry, quite numerous fish teeth were recorded, most of which belong to teleost fish (above 70% collected specimens). All of them belong to the family Sparidae (stratigraphic range Eocene–Recent58). Less numerous are shark teeth, mainly belonging to the family Odontaspididae, including Carcharias acutissima (Oligocene–Pliocene59,60) and Araloselachus cf. vorax (Miocene61).
Assemblage of fish teeth from Zygmuntów (near Książ Wielki) is less abundant but more taxonomically diverse. It is dominated by shark teeth (68% of all specimens) belonging to at least four families. Teeth of Otodus megalodon (Miocene–Pliocene62), Cosmopolitodus hastalis (Miocene–early Pleistocene59,60), Isurus (Paleocene–Recent59) and Galeocerdo (Eocene–Recent59) were found here. Myliobatoid teeth representing the genus Aetobatus (Paleocene–Recent5) are occasionally found. Teleost fish teeth and tooth plates constitute 24% of collected teeth specimens, and are represented only by Sparidae.
No fish teeth from Mesozoic taxa were found at both sites. The stratigraphic ranges of all elasmobranch genera and teleost Sparidae are Cenozoic. Sparid fishes, whose teeth are present in Gołuchów are typically reef-associated forms and prefer warm and shallow water environments63. Sparid teeth are adapted to a durophagous diet and indicate feeding on shelled invertebrate fauna58,60,63.
Crinoid description
In both localities fragments of stems and isolated brachial of isocrinids were recorded (Fig. 6). Their detailed description is given below. Systematic description and terminology follows Hess and Messing64.
Figure 6.
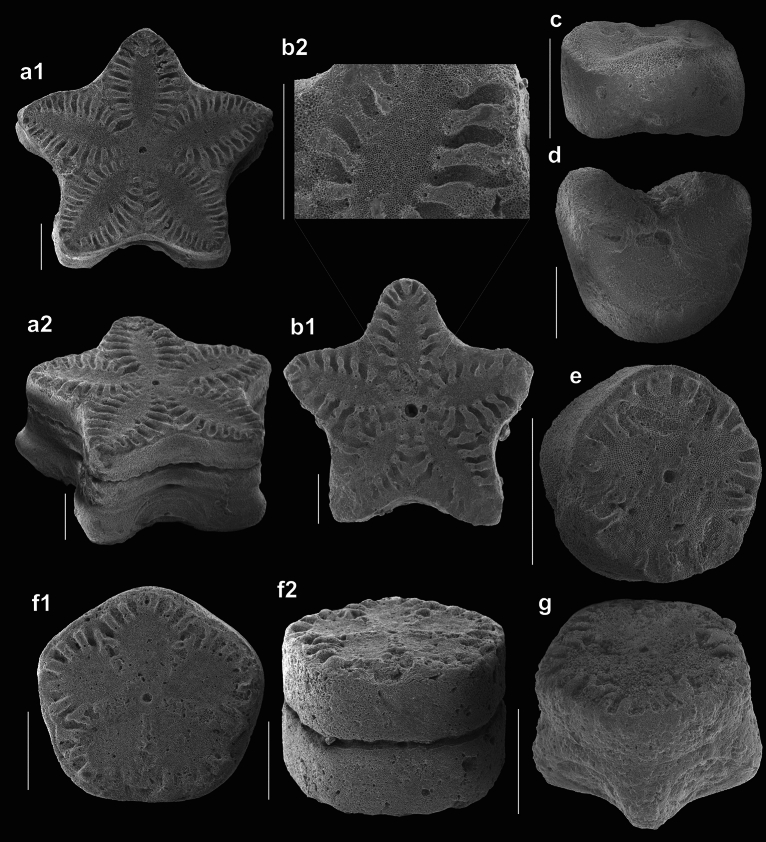
Metacrininae gen. et sp. indet. from Miocene (Badenian) strata of southern Poland. Scale bar equals 1 mm. (a) articular facet of proximal columnal (a1) and oblique view of proximal pluricolumnal (a2). Zygmuntów locality. GIUS 12–1475/8. (b) Articular facet of proximal columnal (b1) and enlargement of petal floor (b2). Zygmuntów locality. GIUS 12-1475/9. (c) oblique view of cirral. Gołuchów locality. GIUS 12-1475/3. (d) Proximal facet of secundibrachial. Gołuchów locality. GIUS 12-1475/4. (e) oblique view of distal columnal. Gołuchów locality. GIUS 12-1475/1. (f) articular facet of distal/medial columnal (f1) and oblique view of distal/medial pluricolumnal (f2). Gołuchów locality. GIUS 12-1475/5. (g) oblique view of distal/medial pluricolumnal. Gołuchów locality. GIUS 12-1475/7.
Systematic palaeontology
Order Isocrinida Sieverts-Doreck, in Moore et al.65.
Suborder Isocrinina Sieverts-Doreck, in Ubaghs66.
Family Isselicrinidae Klikushin67.
Subfamily Metacrininae Klikushin67.
Metacrininae gen. et sp. indet.
Material
25 columnals and pluricolumnals consisting of up to 3 columnals, 1 secundibrachial and 1 cirral from Gołuchów locality, 5 columnals and 2 pluricolumnals consisting of up to 3 columnals from Zygmuntów (near Książ Wielki) locality (Fig. 6). The Miocene (Badenian) crinoids from southern Poland are housed at the University of Silesia in Katowice, Faculty of Natural Sciences, Institute of Earth Sciences (Poland), under the catalogue number GIUS 12-1475.
Measurements
Columnal diameter varies from 1.2 up to 4.7 mm; columnal height varies from 0.39 up to 1.1 mm. Cirral diameter is 1.42 mm and its length 1.82 mm. The brachial is 0.45 mm-wide and 2.18 mm-high.
Description
Distal columnals are circular and high, medial columnals are pentagonal to pentalobate, and proximal columnals are stellate in outline. Nodal columnals are distinctly higher than internodals. All nodal columnals are pentagonal with swollen interradii. Nodal diameter ranges from 1.22 to 1.49 of internodal diameter, and nodal height ranges from 14 to 36% of internodal diameter. Internodal columnals display symplectial facets with distinct marginal crenulae that may be V-shaped and fused adradially. Petal floors are rhombic in case of distal and medial columnals. They are discoidal and tear-shaped in proximal columnals. They are separated from each other by adradial crenulae belts, consisting of two parallel sets of very minute tubercles. Number of both marginal and adradial crenulae varies from 24 in circular distal columnals up to 80 in star-shaped proximal columnals. Almost all columnals possess large radial pores. They are circular, and typically surround petal floors. Latera is smooth and straight in distal and medial columnals; it is smooth and distinctly convex in proximal columnals. Lumen is small and circular. Cirrus scars of nodal columnals are of similar diameter, with a width ranging from 33 to 46% of the nodal diameter. Cirrus scar height ranges from 72 to 87% of nodal height. They are oval and may be proximally short. Secundibrachial is muscular and rounded aborally. Pinnule socket is visible. Cirrus is oval and its latera is smooth.
Discussion
The material described herein is strikingly similar to those described from the Miocene of France and ascribed to metacrinid crinoids68. Among the subfamily Metacrininae, three genera (Metacrinus, Eometacrinus, Saracrinus) are known according to Hess and Messing64, but Améziane et al.69 distinguished two genera (Metacrinus, Saracrinus) within this subfamily. Although Saracrinus is closely related to Metacrinus, Hess and Messing 64 based on opinions of Meyer and Oji10, Ameziane70 and Roux et al.71 decided to separate it from Metacrinus.
Typical Saracrinus has 4 primibrachials, among which primibrachials 1 and 2 are united by cryptosyzygy. Similarly in the case of Metacrinus, cryptosyzygy occurs between primibrachials 1 and 2. In the case of species with 7 primibrachials, cryptosyzygy also occurs between primibrachials 4 and 5 or 5 and 6. In the case of secundibrachials cryptosyzygy occur between secundibrachials 2 and 3 or 3 and 4 and also in more distal parts of arms. Other articulations in brachials are muscular. In the case of Eometacrinus, 5 primibrachials are known and synarthry is known between primibrachials 1 and 2, and cryptosyzygy between primibrachials 4 and 5. All of the above indicate that without an access to more complete (articulated) arms of Metacrininae, it is not possible to classify isolated metacrinitid stem material at the genus level. This led to the inclusion of the current material in Metacrininae.
It should be stressed that the material at hand differs strongly from the previously recorded crinoid taxa in the Mesozoic of Poland72–78.
Distribution
Paleogene (Eocene)–Holocene.
Discussion
Discovery of isocrinid crinoids from the shallow marine facies of the middle Miocene age in the southern Poland (Northern Hemisphere, Central Paratethys) has a number of palaeoecologic implications. Although, the presence of isocrinid columnals from this region (Książ Wielki) has been already noted in 1934 by Krach79, they were never described nor illustrated, and the scientific value of their occurrences was not appreciated. Subsequently, for a long time the outcrop has been overgrown and was not assessable to palaeontological investigations. However, recent roadworks, which enabled us to collect new samples containing isocrinid columnals, allowed us to thoroughly describe these important fossils.
Although our material is mostly disarticulated (Fig. 6), and represented predominantly by stem fragments, its morphologic (resemblance to other well known Miocene isocrinid taxa) and taphonomic features (some of the material represented by articulated non-abraded pluricolumnals showing ornamentation and delicate stereom microstructure), combined with the sedimentologic and palaeogeographic context (restricted nearshore bay situated far away from the deeper slopes; see Fig. 2) suggest that these crinoids were paraautochthonous, i.e. they were not subject to significant transport and/or redeposition before final burial. Indeed, in-situ observations and tumbling experiments on Recent crinoids demonstrated that taphonomic features are a useful proxy in assessing autochthonous and allochthonous ossicles80,81. Those, which are subject to significant transport typically reveal altered (thinned or rounded) shape and broken stereom trabeculae with numerous shallow abrasion-induced grooves (wear scars). At least some of the material, especially from the Zygmuntów locality, does not reveal any of these features. Although the ossicles from Gołuchów, are not so well preserved – their disarticulation gradient is higher and some of them are slightly bioeroded/abraded – such features are typical in such high energy environment4,82. Finally, no evidence of post-diagenetic breakage on cleavage planes, which are indicative of reworking and redeposition from older rocks, were noted.
Overall, these data suggest that some relic isocrinid fauna might have been able to live in shallow marine environments by the middle Miocene. These findings expand the number of isocrinid occurrences in the Cenozoic shallow marine facies. Importantly, our compilation and critical evaluation of published data (Fig. 1) on the Cenozoic shallow marine isocrinids, suggest that isocrinids from Poland, were actually the latest stalked crinoids which were able to live in the nearshore environment. This is quite surprising given the fact that in such environment, a variety of predators are common. Indeed, from both localities numerous teeth of fish predators (see above) and cidaroid echinoids were recorded [represented by common, shallow water, species Eucidaris zeamays83]. One may speculate that these crinoids might have not been a preferential prey for some of these predators at that time – today, at least some representatives of fish groups recorded in our localities (Sparidae) are known to prey upon crinoids84. One the other hand, fossil isocrinids, like their Recent descendants, are known to possess a number of anti-predatory adaptations (regeneration, autotomy and crawling abilities, among the others)85–88, which, to some extent, might help them survive in such an environment.
Notwithstanding, the results presented here suggest that the disappearance of isocrinids from shallow marine environments was asynchronous on a global scale. Not only in the Gondwana Realm they remained in shallow water long after the initiation of the Mesozoic marine revolution, but in Euramerica, some relic populations of isocrinid fauna might have been also able to inhabit such environments.
Materials and methods
Field works have been carried out in 2021 in Zygmuntów, and in 2023 in Gołuchów. Fossils, in particular crinoids, but also fish teeth, were initially searched in the field. At this stage, few of them have been collected. Additionally, bulk samples weighing 30 to 40 kg were taken from each lithological levels. In Gołuchów these were: red-algal sandy limestones with pebbles and fine-detrital sands or weakly compact calcareous and marly sandstones representing Heterostegina Sands, whereas in Zygmuntów, sands with gravel, clays and limestones were taken. Further work was carried out in the Palaeontological Laboratory of the Institute of Earth Sciences of the University of Silesia in Katowice. The marly samples were washed only with a stream of hot water and sieved through sieves with mesh diameters of 1.00 mm and 0.315 mm. Limestone samples were boiled in a Glauberian salt solution; after cooling, they were frozen. This process was repeated twice. The macerated rock was washed with hot water, sieved through sieves and dried at 180 °C. The obtained residue was examined for the presence of fauna using an Olympus SZX7-TR30 binocular. Crinoids were documented only in finely grained marly sands.
Acknowledgements
Two reviewers are acknowledged for improving the quality of this paper. Special thanks are also due to Michał Rakociński (University of Silesia in Katowice), Damian Kuźma (Katowice) and Michał Bugajski (Woźniki) for help during field works. This research project has been supported by the National Science Centre, Poland (www.ncn.gov.pl), Grant No. 2020/39/B/ST10/00006 to MAS and AGH-UST Grant No. 16.16.140.315 to MK.
Author contributions
MAS designed research. MAS, KP, TB conducted field works. MAS, UR, KP, MK, TB, RN, PG performed research and contributed to writing this paper.
Data availability
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Messing, C.G. Submersible observations of deep-water crinoid assemblages in the tropical western Atlantic Ocean. In Presented at 5th International Echinoderm Conference, Galway (1984).
- 2.Oji, T. Miocene Isocrinidae (stalked crinoids) from Japan and their biogeographic implication. Trans. Proc. Palaeontol. Soc. Jpn.157, 412–429 (1990). [Google Scholar]
- 3.Ausich, W. I., Donovan, S. K., Hess, H. & Simms, M. J. Fossil Crinoids 1–275 (Cambridge University Press, 1999). [Google Scholar]
- 4.Bottjer, D. J. & Jablonski, D. Paleoenvironmental patterns in the evolution of post-Paleozoic benthic marine invertebrates. Palaios3, 540–560 (1988). 10.2307/3514444 [DOI] [Google Scholar]
- 5.Smith, A. B. Systematics and the Fossil Record 1–223 (Blackwell, 1994). [Google Scholar]
- 6.Oji, T. Is predation intensity reduced with increasing depth? Evidence from the West Atlantic stalked crinoid Endoxocrinus parrae (Gervais) and implications for the Mesozoic marine revolution. Paleobiology22, 339–351 (1996). 10.1017/S0094837300016328 [DOI] [Google Scholar]
- 7.Gorzelak, P., Salamon, M. A. & Baumiller, T. Predator-induced macroevolutionary trends in Mesozoic crinoids. Proc. Natl. Acad. Sci. U. S. A.109, 7004–7007 (2012). 10.1073/pnas.1201573109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Penas, A., Baumiller, T. K., Aurell, M. & Zamora, S. Intact stalked crinoids from the late Aptian of NE Spain offer insights into the Mesozoic Marine Revolution in the Tethys. Geology 10.1130/G52179.1 (2024). 10.1130/G52179.1 [DOI]
- 9.Salamon, M. A. et al.Ausichicrinites zelenskyyi gen. et sp. nov., a first nearly complete feather star (Crinoidea) from the Upper Jurassic of Africa. R. Soc. Open Sci.9, 9220345 (2022). 10.1098/rsos.220345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer, D. L. & Oji, T. Eocene crinoids from Seymour Island, Antarctic Peninsula: Paleobiogeographic and paleoecologic implications. J. Paleontol.67, 250–257 (1993). 10.1017/S0022336000032170 [DOI] [Google Scholar]
- 11.Baumiller, T. K. & Gaździcki, A. New crinoids from the Eocene La Meseta Formation of Seymour Island, Antarctic Peninsula. Palaeontol. Pol.55, 101–116 (1996). [Google Scholar]
- 12.Aronson, R. B., Blake, D. B. & Oji, T. Retrograde community structure in the late Eocene of Antarctica. Geology25, 903–906 (1997). [DOI] [Google Scholar]
- 13.Malumián, N. & Olivero, E. B. Shallow-water late middle Eocene crinoids from Tierra del Fuego: A new southern record of a retrograde community structure. Sci. Mar.69, 349–353 (2005). 10.3989/scimar.2005.69s2349 [DOI] [Google Scholar]
- 14.Whittle, R. J., Hunter, A. W., Cantrill, D. J. & McNamara, K. J. Globally discordant Isocrinida (Crinoidea) migration confirms asynchronous Marine Mesozoic Revolution. Commun. Biol.1, 1–10 (2018). 10.1038/s42003-018-0048-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroh, A. & Harzhauser, M. An Echinoderm Fauna from the Lower Miocene of Austria: Paleoecology and implications for central Paratethys paleobiogeography. Ann. Naturhistorischen Museums Wien101A, 145–191 (1999). [Google Scholar]
- 16.Kutscher, M. Die Echinodermen des Oberoligozans von Sternberg. Z. Geol. Wissenschaften Berlin10, 221–239 (1981). [Google Scholar]
- 17.Sekuła, M., Gorzelak, P., Konieczyński, K. & Salamon, M. A. Liliowce z pogranicza kredy i paleogenu z doliny środkowej Wisły – dane wstępne. Przegląd Geologiczny56, 38–41 (2008). [Google Scholar]
- 18.Hutton, F.W. Catalogue of the Tertiary Mollusca and Echinodermata of New Zealand in the Collection of the Colonial Museum. New Zealand Geological Survey, Wellington, 1–48 (1873).
- 19.Rasmussen, H. W. Lower Tertiary Crinoidea, Asteroidea and Ophiuroidea from Northern Europe and Greenland. Kongelige Danske Videnskabers Selskabs Skrifter19, 1–83 (1972). [Google Scholar]
- 20.Weller, S. A report on the Cretaceous of New Jersey 1–871 (Geological Survey of New Jersey, Paleontology Series, 1907). [Google Scholar]
- 21.Greacen, K. F. The stratigraphy, fauna, and correlation of the Vincentown Formation. New Jersey Dept. Conserv. Geol. Ser. Bull.52, 83–85 (1941). [Google Scholar]
- 22.Charrier, R. & Lahsen, A. Stratigraphy of Late Cretaceous -Early Eocene, Seno Skyring-Strait of Magellan area, Magallanes Province, Chile. Am. Assoc. Petrol. Geol. Bull.53, 568–533 (1969). [Google Scholar]
- 23.Eagle, M. K. New fossil crinoids (Articulata: Comatulida) from the Late Oligocene of the Pentland Hills and Hurstlea, South Island. Rec. Auckl. Inst. Museum44, 85–110 (2007). [Google Scholar]
- 24.Kelly, M., Lee, D., Kelly, S. & Buckeridge, J. S. A recent sponge, Pleroma aotea Kelly (“Order” Lithistida: Family Pleromidae), in the late Eocene Ototara Limestone of Otago, New Zealand. N. Zeal. J. Mar. Freshw. Res.37, 129–148 (2003). 10.1080/00288330.2003.9517152 [DOI] [Google Scholar]
- 25.Robinson, J. H. & Lee, D. E. A shallow, warm-water calcitic molluscan fauna from an Early Oligocene seamount, North Otago, New Zealand. N. Zeal. J. Geol. Geophys.54, 135–147 (2011). 10.1080/00288306.2011.537612 [DOI] [Google Scholar]
- 26.Feldmann, R. M. & Maxwell, P. A. Late Eocene decapod Crustacea from North Westland, South Island, New Zealand. J. Paleontol.64, 779–797 (1990). 10.1017/S0022336000018989 [DOI] [Google Scholar]
- 27.Eagle, M. K. A new genus of fossil crinoid (Cyrtocrinidia: Sclerocrinidae) from Chatham Island, New Zealand. Rec. Auckl. Inst. Museum42, 35–47 (2005). [Google Scholar]
- 28.Eagle, M. K. Saracrinus (Crinoidea: Metacrininae) from the Early Miocene of Motuketekete Island, Hauraki Gulf, Auckland, New Zealand. Rec. Auckl. Inst. Museum41, 5–12 (2004). [Google Scholar]
- 29.Stilwell, J. D., Fordyce, R. E. & Rolfe, P. J. Paleocene Isocrinids (Echinodermata: Crinoidea) from the Kauru formation, South Island, New Zealand. J. Paleontol.68, 135–141 (1994). 10.1017/S0022336000025658 [DOI] [Google Scholar]
- 30.Rasmussen, H. W. Crinoideos del Cretacico superior y del Terciario inferior de la Isla Vicecomodoro Marambio (Seymour Island), Antartida. Contrib. El Instituto Antártico Argentino4, 79–97 (1979). [Google Scholar]
- 31.Milner, G. J. The first record of an isocrinid crinoid from the Tertiary of Australia. West. Aust. Museum Rec.14, 385–389 (1989). [Google Scholar]
- 32.Eagle, M. K. A new fossil isocrinid crinoid from the Late Oligocene of Waitete Bay, Northern Coromandel. Rec. Auckl. Inst. Museum30, 1–12 (1993). [Google Scholar]
- 33.Ribeiro, M. Headland Sediment Bypassing Processes. Ph.D. thesis, Universidade de Lisboa, 1–235 (2017).
- 34.Studencka, B., Gontsharova, I. A. & Popov, S. V. The bivalve faunas as a basis for reconstruction of the Middle Miocene history of the Paratethys. Acta Geol. Pol.48, 285–342 (1998). [Google Scholar]
- 35.Kovač, M. et al. Badenian evolution of the Central Paratethys Sea: Paleogeography, climate and eustatic sea-level changes. Geol. Carpath.58, 579–606 (2007). [Google Scholar]
- 36.Alexandrowicz, S. W. Middle Miocene (Badenian) sequence at Górki, southern part of the Korytnica Bay (Holy Cross Mountains, Central Poland). Acta Geol. Pol.29, 353–361 (1979). [Google Scholar]
- 37.Oszczypko, N., Krzywiec, P., Popadyuk, I. & Peryt, T. Carpathian Foredeep Basin (Poland and Ukraine)–its sedimentary, structural and geodynamic evolution. In The Carpathians and Their Foreland: Geology and Hydrocarbon Resources Vol. 84 (eds Golonka, J. & Picha, F. J.) 293–350 (American Association of Petroleum Geologists, 2006). [Google Scholar]
- 38.Radwański, A. Transgresja dolnego tortonu na południowo-wschodnich i wschodnich stokach Gór Świętokrzyskich. Acta Geol. Pol.23, 375–434 (1973). [Google Scholar]
- 39.Rutkowski, J. Detrytyczne osady sarmatu na południowym obrzeżeniu Gór Świętokrzyskich. Prace Geol. PAN100, 1–77 (1976). [Google Scholar]
- 40.Bałuk, W. & Radwański, A. Organic communities and facies development of the Korytnica basin (Middle Miocene; Holy Cross Mountains, Central Poland). Acta Geol. Pol.27, 85–123 (1977). [Google Scholar]
- 41.Studencki, W. Facies and sedimentary environment of the Pinczow Limestones (Middle Miocene; Holy Cross Mountains, Central Poland). Facies18, 1–26 (1988). 10.1007/BF02536793 [DOI] [Google Scholar]
- 42.Radwański, A. Lower Tortonian transgression onto the southern slopes of the Holy Cross Mts. Acta Geol. Pol.19, 1–164 (1969). [Google Scholar]
- 43.Wysocka, A. Clastic Badenian deposits and sedimentary environments of the Roztocze Hills across the Polish- Ukrainian border. Acta Geol. Pol.52, 535–561 (2002). [Google Scholar]
- 44.Studencki, W. Red-algal limestones in the Middle Miocene of the Carpathian Foredeep in Poland: facies variability and palaeoclimate implications. Geol. Q.43, 395–404 (1999). [Google Scholar]
- 45.Stachacz, M., Jurkowska, A. & Machaniec, E. Górna kreda niecki miechowskiej i miocen północnej części zapadliska przedkarpackiego. Paleo Tyniec2013, 75–87 (2013). [Google Scholar]
- 46.Gutowski, J. Sedimentary environment and synecology of macrobenthic assemblages of the marly sands and red-algal limestones in the Korytnica Basin (Middle Miocene; Holy Cross Mountains, Central Poland). Acta Geol. Pol.34, 324–340 (1984). [Google Scholar]
- 47.Wysocka, A. et al. The Middle Miocene of the Fore-Carpathian Basin (Poland, Ukraine and Moldova). Acta Geol. Pol.66, 351–401 (2016). [Google Scholar]
- 48.Steininger, F. F., Bernor, R. L. & Fahlbusch, V. European Neogene Marine/Continental Chronologic Correlations 15–46 (Plenum, 1989). [Google Scholar]
- 49.Jasionowski, M. Lithostratigraphy of the Miocene deposits in the eastern part of the Carpathian Foredeep. Biuletyn Państwowego Instytutu Geologicznego375, 43–60 (1997). [Google Scholar]
- 50.Górka, M. The Lower Badenian (Middle Miocene) coral patch reef at Grobie (southern slopes of the Holy Cross Mountains, Central Poland), its origin, development and demise. Acta Geol. Pol.52, 521–534 (2002). [Google Scholar]
- 51.Martini, E. Calcareous nannoplankton from the Korytnica Basin (Middle Miocene; Holy Cross Mountains, Central Poland). Acta Geol. Pol.27, 125–133 (1977). [Google Scholar]
- 52.Gedl, P. Middle Miocene dinoflagellate cysts from the Korytnica clays (Góry Świętokrzyskie Mountains, Poland). Ann. Soc. Geol. Pol.66, 191–218 (1996). [Google Scholar]
- 53.Rögl, F. & Brandstatter, F. The foraminifera genus Amphistegina from the Korytnica Clays (Holy Cross Mts., Central Poland) and its significance in the Miocene of Parathetys. Acta Geol. Pol.43, 121–146 (1993). [Google Scholar]
- 54.Olszewska, B. Biostratigraphy of Neogene in the Carpathian Foredeep in the light of new micropalaeontological data. Prace Państw. Inst. Geol.168, 9–28 (1999). [Google Scholar]
- 55.Studencka, B. Remarks on Miocene bivalve zonation in the Polish part of the Carpathian Foredeep. Geol. Q.43, 467–477 (1999). [Google Scholar]
- 56.Grill, R. Ober mikropaliiontologische Gliederungsmoglichkeiten in Mioziin des Wiener Beckens. Mitteilungen des Reichsamts für Bodenforschung, Zweigstelle Wien6, 33–44 (1943). [Google Scholar]
- 57.Cosentino, C., Guastella, R., Mancin, N. & Caruso, A. Spatial and vertical distribution of the genus Amphistegina and its relationship with the indigenous benthic foraminiferal assemblages in the Pelagian Archipelago (Central Mediterranean Sea). Mar. Micropaleontol.188, 102344 (2024). 10.1016/j.marmicro.2024.102344 [DOI] [Google Scholar]
- 58.Day, J. Evolutionary relationships of the Sparidae (Teleostei: Percoidei): Integrating fossil and recent data. Trans. R. Soc. Edinb. Earth Sci.93, 333–353 (2003). 10.1017/S0263593300000468 [DOI] [Google Scholar]
- 59.Cappetta, H. Chondrichthyes II. Mesozoic and Cenozoic Elasmobranchii 1–193 (Gustav Fischer Verlag, 1987). [Google Scholar]
- 60.Marsili, S., Carnevale, G., Danese, E., Bianucci, G. & Landini, W. Early Miocene vertebrates from Montagnadella Maiella Italy. Ann. Paléontol.93, 27–66 (2007). 10.1016/j.annpal.2007.01.001 [DOI] [Google Scholar]
- 61.Szabó, M. Middle Miocene (Badenian) chondrichthyan and osteichthyan remains from St. Margarethen (eastern Austria) in the vertebrate palaeontological collection of the Hungarian Natural HistoryMuseum. Fragm. Palaeontol. Hung.36, 1–38 (2019). 10.17111/FragmPalHung.2019.36.53 [DOI] [Google Scholar]
- 62.Boessenecker, R. W. et al. The early Pliocene extinction of the mega-toothed shark Otodus megalodon: A view from the eastern North Pacific. PeerJ7, e6088 (2019). 10.7717/peerj.6088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szabó, M., Kocsis, L., Bosnakoff, M. & Sebe, K. A diverse Miocene fish assemblage (Chondrichthyes and Osteichthyes) from the Pécs-Danitzpuszta sand pit (Mecsek Mts., Hungary). Földtani Közlöny151, 363–410 (2021). 10.23928/foldt.kozl.2021.151.4.363 [DOI] [Google Scholar]
- 64.Hess, H. & Messing, C. G. Treatise on Invertebrate Paleontology, part T, Echinodermata 2, Crinoidea Vol. 3, 1–261 (The University of Kansas, 2011). [Google Scholar]
- 65.Moore, R. C., Lalicker, C. G. & Fischer, A. G. Invertebrate Fossils 1–766 (McGraw-Hill Book Company, 1952). [Google Scholar]
- 66.Ubaghs, G. Sous-Classe 4. Articulata J.S. Miller. In Traité de Paléontologie (ed. Piveteau, J.) 756–765 (Masson, 1953). [Google Scholar]
- 67.Klikushin, V. G. Sea lilies of the genus Isselicrinus. Paleontol. J.11, 87–95 (1977). [Google Scholar]
- 68.Roux, M. & Philippe, M. Early Miocene stalked crinoids (Echinodermata) from the southern Rhodanian basin (southeastern France). Paleoenvironments and taxonomy. Zootaxa5052, 301–331 (2021). 10.11646/zootaxa.5052.3.1 [DOI] [PubMed] [Google Scholar]
- 69.Améziane, N., Eléaume, M. & Roux, M. Classification of Isocrinida (Echinodermata: Crinoidea) with the description of a new extant genus and species from the western Pacific. Zool. J. Linn. Soc.200, 994–1012 (2014). 10.1093/zoolinnean/zlad101 [DOI] [Google Scholar]
- 70.Améziane, N. Echinodermata crinoidea: Les pentacrinesrécoltéslors de la campagne KARUBAR en Indonésie. Mémoires du Muséum National d’Histoire Naturelle172, 627–667 (1997). [Google Scholar]
- 71.Roux, M., Messing, Ch. G. & Améziane, N. Artificial keys to the genera of living stalked crinoids (Echinodermata). Bull. Mar. Sci.70, 799–830 (2002). [Google Scholar]
- 72.Radawańska, U. Lower Kimmeridgian comatulid crinoids of the Holy Cross Mountains, Central Poland. Acta Geol. Pol.55, 269–282 (2005). [Google Scholar]
- 73.Borszcz, T., Gorzelak, P., Konieczyński, K., Łukowiak, M. & Salamon, M. A. Upper Jurassic (Kimmeridgian) crinoids from the southern Poland. Freib. Forschungshefte Paläontol. Stratigr. Fazies C532, 83–93 (2009). [Google Scholar]
- 74.Gorzelak, P. & Salamon, M. A. Signs of benthic predation on Late Jurassic stalked crinoids, preliminary data. Palaios24, 70–73 (2009). 10.2110/palo.2008.p08-032r [DOI] [Google Scholar]
- 75.Salamon, M. A. & Gorzelak, P. Late Cretaceous crinoids (Crinoidea) from Eastern Poland. Palaeontogr. Abt.A291, 1–43 (2010). [Google Scholar]
- 76.Lach, R. Late Cretaceous sea lilies (Crinoida, Crinoidea) from the Miechów through, Southern Poland. Palaeontogr. Abt. A305, 91–133 (2016). 10.1127/pala/305/2016/91 [DOI] [Google Scholar]
- 77.Salamon, M. A. Late Cretaceous echinoderms (crinoids and echinoids) from Chełm Quarry (eastern Poland). Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen287, 153–166 (2018). 10.1127/njgpa/2018/0710 [DOI] [Google Scholar]
- 78.Krajewski, M., Olchowy, P. & Salamon, M. A. Late Jurassic (Kimmeridgian) sea lilies (Crinoidea) from central Poland (Łódź Depression). Ann. Paléontol.105, 63–73 (2019). 10.1016/j.annpal.2018.12.001 [DOI] [Google Scholar]
- 79.Krach, W. Miocen okolic Miechowa. Stratygrafia i paleontologia. Biuletyn Państwowego Instytutu Geologicznego43, 1–100 (1947). [Google Scholar]
- 80.Gorzelak, P. & Salamon, M. A. Experimental tumbling of echinoderms-taphonomic patterns and implications. Palaeogeogr. Palaeoclimatol. Palaeoecol.386, 569–574 (2013). 10.1016/j.palaeo.2013.06.023 [DOI] [Google Scholar]
- 81.Llewellyn, G. & Messing, C. G. Compositional and taphonomic variations in modern crinoid-rich sediments from the deep-water margin of a carbonate bank. Palaios8, 554–573 (1993). 10.2307/3515032 [DOI] [Google Scholar]
- 82.Brett, C. E., Moffat, H. A. & Taylor, W. L. Echinoderm taphonomy, taphofacies, and Lagerstätten. Paleontol. Soc. Pap.3, 147–190 (1997). 10.1017/S1089332600000243 [DOI] [Google Scholar]
- 83.Sismonda, E. Appendice alla Monografia degli Echinidi del Piemonte. Memorie della Reale accademia delle scienze di Torino4, 385–394 (1842). [Google Scholar]
- 84.Buxton, C. D. Feeding biology of the roman Chrysoblephus laticeps (Pisces: Sparidae). S. Afr. J. Mar. Sci.2, 33–42 (1984). 10.2989/02577618409504356 [DOI] [Google Scholar]
- 85.Oji, T. & Okamoto, T. Arm autotomy and arm branching pattern as antipredatory adaptations in stalked and stalkless crinoids. Paleobiology20, 27–39 (1994). 10.1017/S0094837300011118 [DOI] [Google Scholar]
- 86.Baumiller, T. K. Crinoid ecological morphology. Annu. Rev. Earth Planet. Sci.36, 221–249 (2008). 10.1146/annurev.earth.36.031207.124116 [DOI] [Google Scholar]
- 87.Gorzelak, P. Microstructural evidence for stalk autotomy in Holocrinus–the oldest stem-group isocrinid. Palaeogeogr. Palaeoclimatol. Palaeoecol.506, 202–207 (2018). 10.1016/j.palaeo.2018.06.036 [DOI] [Google Scholar]
- 88.Gorzelak, P. et al. Experimental neoichnology of post-autotomy arm movements of sea lilies and possible evidence of thrashing behaviour in Triassic holocrinids. Sci. Rep.10, 15147 (2020). 10.1038/s41598-020-72116-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- García-Penas, A., Baumiller, T. K., Aurell, M. & Zamora, S. Intact stalked crinoids from the late Aptian of NE Spain offer insights into the Mesozoic Marine Revolution in the Tethys. Geology 10.1130/G52179.1 (2024). 10.1130/G52179.1 [DOI]
Data Availability Statement
All data generated or analysed during this study are included in this published article.