Abstract
Purpose
There is no agreed-upon standard option for patients with locally advanced head and neck squamous cell carcinoma (LA HNSCC) unfit for cisplatin-based regimens. Therefore, we performed a systematic review to explore alternative options for this population.
Methods
We searched PubMed, Cochrane, and Embase databases for observational studies and clinical trials (CTs) assessing treatment options for LA HNSCC cisplatin-ineligible patients. This study was registered in PROSPERO under the number CRD42023483156.
Results
This systematic review included 24 studies (18 observational studies and 6 CTs), comprising 4450 LA HNSCC cisplatin-ineligible patients. Most patients were treated with cetuximab-radiotherapy [RT] (50.3%), followed by carboplatin-RT (31.7%). In seven studies reporting median overall survival (OS) in patients treated with cetuximab-RT, it ranged from 12.8 to 46 months. The median OS was superior to 40 months in two studies assessing carboplatin-RT, and superior to 15 months in two studies assessing RT alone. For other regimens such as nimotuzumab-RT, docetaxel-RT, and carboplatin-RT plus paclitaxel the median OS was 21, 25.5, and 28 months, respectively.
Conclusions
Our systematic review supports the use of a variety of therapy combinations for LA HNSCC cisplatin-ineligible patients. We highlight the urgent need for clinical studies assessing treatment approaches in this population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-024-05887-z.
Keywords: Head and neck squamous cell carcinoma, HNSCC, Cisplatin, Ineligible, Chemotherapy
Introduction
Head and neck cancer (HNC) is among the ten most common cancers globally (Barsouk et al. 2023). Each year about 4.5% of all cancer diagnoses and deaths are accounted for HNC (Barsouk et al. 2023). Roughly 90% of HNC are squamous cell carcinomas (SCC) and a great percentage of patients are diagnosed with locally advanced disease (Dhull et al. 2018; Haddad et al. 2023).
The mainstay of treatment for locally advanced head and neck squamous cell carcinoma (LA HNSCC) is surgery followed by adjuvant chemoradiotherapy (CRT) or CRT alone (Machiels et al. 2020; Pfister et al. 2020). For unresectable tumors or patients with poor functional status and contraindications to surgery, CRT is the recommended option (Machiels et al. 2020; Pfister et al. 2020). Regardless of tumor location, concomitant CRT with high-dose cisplatin (> 200 mg/m3) has been proved superior to RT alone (Machiels et al. 2020; Porceddu et al. 2020). The benefit is consistent whether using standard or altered fractionation RT regimens (Machiels et al. 2020). Among cisplatin schedules, the 3-weekly regimen is the preferred option.
Nevertheless, CRT accounts for important treatment-related morbidity. Among the several complications of RT, swallowing impairment and aspiration in HNC patients notably impact overall survival (De Ruysscher et al. 2019; Trotti et al. 2003). As to cisplatin, the most common and debilitating adverse events include gastrointestinal symptoms, nephrotoxicity, and peripheral neuropathy (Barabas et al. 2008). Accordingly, the study by Espeli et al. estimated that about 50% of patients on high-dose cisplatin were unable to complete the planned treatment schedule due to its toxicity (Espeli et al. 2012).
Given the complexity of HNSCC clinicopathological features, a multidisciplinary approach is essential (De Felice et al. 2019). To optimize clinical decisions, multidisciplinary programs assess all individual features implicated in treatment strategies (Ang 2008; De Felice et al. 2019). Additionally, these programs cover a variety of supportive measures and services that are crucial in maintaining quality of life and treatment adherence, including nutritional assessment, pain management, and rehabilitation (Ang 2008; De Felice et al. 2019). This is of special relevance in patients unable to tolerate cisplatin-based treatments and who often present with a high burden of frailty and comorbidities.
A retrospective study conducted in a cohort of veterans in the United States estimated that about one-third of HNSCC patients were classified as ineligible for cisplatin and were treated with alternative regimens (Sun et al. 2022). Yet, international guidelines do not support specific regimens due to the scarce evidence for HNSCC cisplatin-unfit patients (Koyfman et al. 2019; Machiels et al. 2020). A combination of docetaxel (DTX), cetuximab (CTX), and RT is outlined in the National Comprehensive Cancer Network (NCCN) guidelines as alternative agents for this population (NCCN Clinical Practice Guidelines in Oncology. Head and Neck Cancer., n.d.). Some authors support the use of carboplatin (CB)-based regimens, 5-fluorouracil, and RT alone as feasible options (Haddad et al. 2023; Sun et al. 2022). In contrast, studies have shown that CTX-RT is superior to RT alone, whereas retrospective data support CB-based regimens over CTX (Bonner et al. 2006; Sun et al. 2022).
Other regimens currently being assessed include DTX plus RT and the combination of immune checkpoint inhibitors and RT (Patil et al. 2023; Tao et al. 2023). However, there is a lack of consensus concerning the optimal agent for this population. In light of heterogeneous and limited evidence for this group of patients, we performed a systematic review to compile all available information on treatment options for cisplatin-ineligible patients with LA HNSCC within clinical trials and in the real-world setting.
Materials and methods
This study was performed and reported according to the guidelines from the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (Page et al. 2021). It was registered in the International Prospective Register of Systematic Reviews (PROSPERO [CRD42023483156]). Initially, this study was planned as a systematic review and meta-analysis. However, due to important differences in the design of studies and outcomes, only a systematic review was performed. The PRISMA checklist for the abstract and the manuscript is available in Supplementary Table S1.
Data source and search strategy
The initial systematic search was conducted on PubMed, Embase, and Cochrane databases on September 20th, 2023, and it was last updated on November 16, 2023. The following combination of medical subject headings (MeSH) terms and boolean connectors were used: “head and neck” AND “cisplatin” AND “ineligible”. The full search strategy used on each database is available for reference in Supplementary Table S2.
Eligibility criteria
The main eligibility criteria consisted of published phase II or III clinical trials (CTs) and prospective or retrospective cohort studies evaluating treatment options in patients with LA HNSCC and contraindications for cisplatin. We included studies on squamous cell cancers mainly located in the oral cavity, oropharynx, hypopharynx, and larynx. To be eligible for inclusion, studies were required to report at least one survival or response outcome as listed below. No specific restrictions were made as to the publication date, presence of a control/comparator group, number of patients, or the treatment regimen assessed.
The main exclusion criteria were as follows: (1) unclear or no information if patients were cisplatin-ineligible; (2) metastatic/recurrent HNSCC patients; (3) cancers located in the brain, eye, esophagus, thyroid or skin of the head and neck or non-squamous cell cancers; (4) patients on induction chemotherapy regimens; (5) only safety data reported; (6) abstracts from conferences and not original studies; and (8) patients who switched from standard cisplatin regimens to low-dose cisplatin. Due to differences in outcomes and prognosis, studies analyzing exclusively squamous cell nasopharyngeal carcinomas or SCC of paranasal sinuses were excluded.
Data collection and outcomes
Three authors (IM, GCN, MID) independently screened the studies by title and abstract, selected the articles for full-text review, and extracted data from included studies. Other authors (DM and RGL) were consulted in case of inconsistencies. Data was collected from individual studies on the study design, study location, number of patients, and patients' baseline characteristics (e.g., sex, age, tumor stage).
Our primary outcome of interest was overall survival (OS). Secondary outcomes include (1) progression-free survival (PFS); (2) locoregional control (LRC); (3) disease-free survival (DFS); (4) incidence of distant metastases or recurrence; (5) locoregional failure (LRF); (6) objective response rate (ORR); (7) disease control rate (DCR); and (8) adverse events (AEs). All outcomes were presented in tables according to the information available in individual studies.
Considering that some outcome definitions and treatment schedules varied across studies, supplemental tables with the eligibility criteria and treatment details of each included study (Supplementary Table S3) and outcome definitions (Supplementary Table S4) are available for reference. Other responses and survival outcomes not included in main tables are described in Supplementary Table S5. Details about the included population are presented in Supplementary Table S6.
Quality assessment
Three authors (IM, GCN, and MID) independently conducted the risk of bias assessment. Inconsistencies were resolved by consensus or by consulting other authors (DM and RGL). The risk of bias in non-randomized studies was explored using ROBINS-I, and for randomized studies, RoB 2 tool was used (Sterne et al. 2016, 2019).
Results
Baseline characteristics
The screening process held 1270 results, of which 234 were selected for full review. Most studies lacked the population of interest or information as to cisplatin ineligibility. A list with all publications assessed can be found in Supplementary Table S7. Finally, 24 studies (18 observational studies and 6 CTs) met our eligibility criteria (Fig. 1) (Addeo et al. 2019; Agarwal et al. 2011; Beckham et al. 2020; Corry et al. 2017; Fung et al. 2020; Hamauchi et al. 2019; Han et al. 2023; Imai et al. 2022; Magnes et al. 2021; Maring et al. 2018, p. 201; Nassif et al. 2022; Patil et al. 2023; Pryor et al. 2009; Rades et al. 2023; Rambeau et al. 2017; Saigal et al. 2014; Srinivas et al. 2020; Sun et al. 2022, p. 20; Swiecicki et al. 2020; Tao et al. 2023; Ueki et al. 2023; Van Der Linden et al. 2014; Weiss et al. 2020; Ye et al. 2013).
Fig. 1.
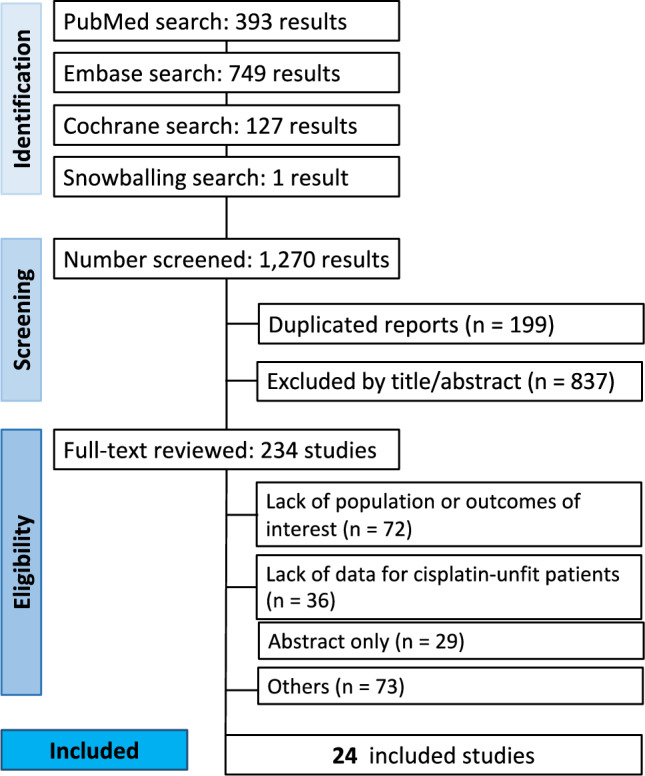
PRISMA flow diagram of study screening and selection. Blue vertical boxes indicate each stage of the screening, and the horizontal boxes present more detailed information about the process, including the steps performed in each stage
Overall, 4450 LA HNSCC cisplatin-ineligible patients were included. Most patients were treated with cetuximab-RT (2238 patients; 50.3%), followed by carboplatin-RT (1409 patients; 31.7%). Other regimens include RT alone (312 patients; 7%); docetaxel-RT (180 patients; 4%); carboplatin-RT plus paclitaxel (118 patients; 2.7%); nimotuzumab [nimo]-RT (21 patients; 5%), pembrolizumab [pembro]-RT (96 patients; 2.1%), and carboplatin-RT combined with cetuximab (76 patients; 1.7%) (Table 1).
Table 1.
Baseline characteristics of included studies in the systematic review
| Study | Design | Location | Treatment regimen | N | Median age (range) | Male N (%) | ECOG PS scalem N (%) | Tumor location N (%) | HPV + N (%) | Smokers N (%) | Median follow-up time (range) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–1 | ≥ 2 | Oral cavity | Oropharynx | Larynx | Hypopharynx | Othersa | ||||||||||
| Addeo et al. (2019) | Retrospective cohort | Italy | CTX-RT | 64 | 73.7 (69–87) | 46 (72) | 16 (25) | 48 (75) | 0 | 31 (49) | 22 (34) | 11 (17) | 0 | 5 (8) | 24 (37.5) | 41 (31.1–36.8) |
| Agarwal et al. (2011) | Retrospective cohort | India | CTX-RT | 37 | 59 (35–87) | 33 (89.2) | 37 (100) | 0 | 10 (27) | 19 (51.3) | 0 | 0 | 8 (21.6) | NA | 27 (73) | 16 (IQR: 12–23) |
| Beckham et al. (2020) | Retrospective cohort | USA |
CB-RT (N = 74) CTX-RT (N = 61) |
135 |
CB-RT:: 63 (44–87) CTX-RT: 66 (46–88) |
CB-RT: 54 (73) CTX-RT: 36 (59) |
NA | NA | 0 |
CB-RT: 22 (29.7%) CTX-RT: 16 (26.2%) |
CB-RT: 41 (55.4) CTX-RT: 35 (57.4) |
CB-RT: 11 (14.9) CTX-RT: 10 (16.4) |
0 | 0 |
CB-CRT: 57 (77)b CTX-RT: 49 (80.3)b |
NA |
| Corry et al. (2017) | Phase I/II CT | Australia | CB-RT + CTX | 60 | 66 (42–87) | 57 (95) | 58 (96.7) | 2 (3.3) | 0 | 38 (63.3) | 16 (26.7) | 6 (10) | 0 | 29 (83) | NA | 48 (NA) |
| Fung et al. (2020) | Retrospective cohort | Canada | CTX-RT | 115 | 64.5 (SD, 9.3)c | 92 (80) | NA | NA | 0 | 115 (100) | 0 | 0 | 0 | 100 (87) | 77 (67) | NA |
| Hamauchi et al. (2019) | Retrospective cohort | Japan |
CB-RT (N = 29) CTX-RT (N = 18) |
47 |
CB-RT: 74 (54–82) CTX-RT: 75 (56–83) |
CB-RT: 27 (93.1) CTX-RT: 15 (83.3) |
CB-RT: 26 (89.7) CTX-RT: 18 (100) |
CB-RT: 3 (10.3) CTX-RT: 0 |
CB-RT: 2 (6.9) CTX-RT: 0 |
CB-RT: 11 (37.9) CTX-RT: 7 (38.9) |
CB-RT: 1 (3.4) CTX-RT: 6 (33.3) |
CB-RT: 15 (51.7) CTX-RT: 5 (27.8) |
0 | NA |
CB-RT: 26 (89.7)b CTX-RT: 13 (86.7)b |
CB-RT: 60 (13.2–94.2) CTX-RT: 53.6 (25.5–62.5) |
| Han et al. (2023) | Retrospective cohort | USA | CB-RT + paclitaxel | 65 | 71 (44–85) | 58 (89.2) | NA | NA | 0 | 43 (66.2) | 17 (26.2) | 4 (6.2) | 1 (1.5) | 37 (86) | 49 (75.4) | 29 (5–91) |
| Imai et al. (2022) | Retrospective cohort | Japan | CTX-RT | 88 | 69 (48–84) | 78 (89) | 84 (95.5) | 4 (4.5) | 0 | 37 (42) | 51 (58)d | d | 0 | 10 (27) | NA | 35.9 (NA) |
| Magnes et al. (2021) | Retrospective cohort | Austria |
CTX-RT (N = 26) RT (N = 56) |
82 |
CTX-RT: 71.5 (56–89) RT: 58.5 (43–91) |
CTX-RT: 23 (88.5) RT: 45 (80.4) |
CTX-RT: 17 (65.4) RT: 33 (59) |
CTX-RT: 9 (34.6) RT: 5 (8.9) |
NA | NA | NA | NA | NA | NA | NA | 60.9 (NA) |
| Maring et al. (2018) | Retrospective cohort | Germany | CB-RT + paclitaxel | 23 | 63 (43–75) | 19 (82.6) | NA | NA | 0 | 16 (70) | 0 | 7 (30) | 0 | 7 (30) | NA | 27 (NA) |
| Nassif et al. (2022) | Retrospective cohort | Germany | CB-RT + paclitaxel | 30 | NA | 22 (73.3) | NA | NA | 3 (10) | 9 (30) | 5 (16.7) | 8 (26.7) | 5 (16.7) | NA | NA | 41 (1–97) |
| Patil et al. (2023) | Phase II/III CT | India |
RT (N = 176) DTX-RT (N = 180) |
356 |
RT: 63 (26–83) DTX-RT: 61 (23–83) |
RT: 153 (85) DTX-RT: 144 (81.8) |
RT: 105 (59.7) DTX-RT: 91 (50.6) |
RT: 71 (40.3) DTX-RT: 89 (49.4) |
RT: 60 (34.1) DTX-RT: 73 (40.6) |
RT: 53 (30.1) DTX-RT: 48 (26.7) |
RT: 27 (15.3) DTX-RT: 24 (13.3) |
RT: 30 (17) DTX-RT: 31 (17.2) |
RT: 6 (2.4) DTX-RT: 4 (2.2) |
RT: 2 (3.8) DTX-RT: 2 (4.2) |
RT: 78 (44.3) DTX-RT: 78 (43.3) |
32.4 (IQR: 26.3–42.1) |
| Pryor et al. (2009) | Prospective cohort | Australia | CTX-RT | 13 | 68 (52–82) | 10 (77) | NA | NA | 0 | 5 (39) | 0 | 5 (39) | 3 (23.1) | NA | NA | NA |
| Rades et al. (2023) | Retrospective cohort | Germany | CB-RT | 45 | NA | 33 (73) | NA | NA | e | 26 (58)e | 13 (29)e | e | 6 (13)e | 10 (29) | 37 (82) | 24 (0–71) |
| Rambeau et al. (2017) | Retrospective cohort | France | CTX-RT | 88 | NA | 74 (84.1) | 55 (62.5) | 32 (36.4) | 8 (9) | 51 (58) | 13 (14.8) | 13 (14.8) | 3 (3.4) | NA | 83 (94.3) | 9.9 (NA) |
| Saigal et al. (2014) | Retrospective cohort | USA | CB-RT + CTX | 16 | 71.5 (57–90) | 15 (93.7) | 16 (100) | 0 | 0 | 9 (56.2) | 4 (25) | 1 (6.25) | 2 (12.5) | NA | NA | 24 (1–69) |
| Srinivas et al. (2020) | Retrospective cohort | India | Nimo-RT | 21 | 55 (28‐72) | 17 (81) | 14 (66.7) | 7 (33.3) | 10 (47.6) | 2 (9.5) | 4 (19) | 3 (14.3) | 2 (9.5) | NA | NA | NA |
| Sun et al. (2022) | Retrospective cohort | USA |
CB-RT (N = 1231) CTX-RT (N = 1439) |
2724 |
CB-RT: 64 (59–69) CTX-RT: 66 (61–72) |
CB-RT: 1216 (98.8) CTX-RT: 1485 (99.5) |
CB-RT: 534 (43.4) CTX-RT: 733 (49) |
CB-RT: 101 (8.2) CTX-RT: 146 (9.8) |
0 |
CB-RT: 676 (54.9) CTX-RT: 882 (59.1) |
NA | NA |
CB-RT: 1033 (83.9)f CTX-RT: 1190 (79.7)f |
NA |
CB-RT: 549 (44.6) CTX-RT: 738 (49.3) |
62 (57–66) |
| Swiecicki et al. (2020) | Phase II CT | USA | CTX-RT | 21 | 65.6 (39–85) | 17 (81) | 17 (81) | 4 (19) | 2 (9.5) | 16 (76,2) | 0 | 1 (4.8%) | 2 (10) | 10 (48) | 20 (95.2) | 48 (NA) |
| Tao et al. (2023) | Phase II trial | Multicenter |
CTX-RT (N = 66) Pembro-RT (N = 67) |
133 |
CTX-RT: 67 (47–81) Pembro-RT: 65 (48–79) |
CTX-RT: 53 (82) Pembro-RT: 59 (89) |
CTX-RT: 66 (100) Pembro-RT: 67 (100) |
0 |
CTX-RT: 5 (8) Pembro-RT: 4 (6) |
CTX-RT: 40 (62) Pembro-RT: 39 (59) |
CTX-RT: 9 (14) Pembro-RT: 5 (8) |
CTX-RT: 11 (16.7) Pembro-RT: 18 (26.9) |
0 |
CTX-RT: 18 (45) Pembro-RT: 18 (46) |
Cet-RT: 59 (89.4) Pembro-RT: 62 (92.5) |
CTX-RT: 5.8 (IQR 24.8- 26.2) Pembro-RT: 25.6 (IQR: 24.8–26.8) |
| Van Der Linden et al. (2014) | Retrospective cohort | NL |
CTX-RT (N = 61) RT (N = 80) |
141 |
CTX-RT: 65 (42–83) RT: 62 (40–87) |
RT-CTX: 37 (60.7) RT: 61 (76.2) |
CTX-RT: 27 (82)g RT: 37 (95)g |
CTX-RT: 6 (18%)h RT: 2 (5)h |
0 |
CTX-RT: 32 (52.5) RT: 20 (25) |
CTX-RT: 6 (9.8) RT: 56 (70) |
CTX-RT: 23 (37.7) RT: 4 (5) |
0 | NA | NA | 29 (20–38) |
| Ueki et al. (2023) | Phase II CT | Japan | CB-RT | 30 | 73.5 (46–85) | 28 (93.3) | 30 (100) | 0 | 0 | 9 (30) | 3 (10) | 14 (47) | 4 (1.3) | 3 (33.3) | NA | NA |
| Weiss et al. (2020) | Phase II CT | Multicenter | Pembro-RT | 29 | 63.1 (range, 39–86)c | 28 (97) | 29 (100) | 0 | 1 (3.4) | 20 (69) | 3 (10.3) | 2 (6.9) | 3 (10.3) | 14 (48.3) | 18 (62) | 21 (10–40) |
| Ye et al. (2013) | Retrospective cohort | Canada | CTX-RT | 87 | 62 (40–89) | 75 (86.2) | 79 (91) | 8 (9) | 7 (8) | 51 (59) | 14 (16) | 5 (6) | 10 (11.5) | NA | NA | 16 (NA) |
Studies informing patients´ p16 + status were considered in the HPV + column; median age is given in years; median follow-up is given in months and range is presented between parenthesis unless indicated otherwise
CB carboplatin; CTX cetuximab; CT clinical trial; DTX docetaxel; ECOG PS Eastern Cooperative Oncology Group Perfomance Status; HPV + human papillomavirus positive; IQR interquartile range; NA not available; NL Netherlands; N Number of patients; Nimo nimotuzumab; Pembro Pembrolizumab; RT radiotherapy; SD standard deviation; USA United States
aother locations include unknown primary, multiple localization, and node without primitive, supraglottic larynx, oro or hypopharynx cancers, nose, paranasal sinuses, salivary glands, and lymph node metastasis
bdata was used considering patients who smoked more than 10 packages-year
cvalues are given in mean (SD or range)
dthis study includes patients with larynx or hypopharynx cancers
erefers to patients classified as non-oropharyngeal carcinoma and mixed (both oropharyngeal and non-oropharyngeal)
fin this study, patients' tumor site were classified as oropharynx/oral cavity, hypopharynx/larynx, or both
gindicates ECOG PS ≤ 1
hindicates ECOG PS 1–2
The majority of patients were male (4110 patients; 92.4%) and had oropharyngeal carcinoma (2343 patients; 52.7%). Tumor staging and other details regarding included population are available in Supplementary Table S6. Cisplatin-ineligibility criteria differed among studies. Common causes to consider patients unfit for cisplatin were renal and hearing impairment and poor performance status. We did not quantify the number of patients who fit each category since in many studies this information was not presented. A full description of cisplatin-ineligibility criteria is presented in Supplementary Table S8.
Survival and response outcomes
Data for OS is presented in Table 2. In seven studies reporting median OS in patients treated with cetuximab plus RT, it ranged from 12.8 to 46 months. Median OS was superior to 43 months in two studies assessing carboplatin-RT and superior to 15 months in two studies assessing RT alone. Most studies included a limited number of patients. Nevertheless, Sun et al. 2022, the study with the greatest sample size (1493 patients on CTX-RT and 1231 on CB-RT), found significantly higher survival rates for carboplatin-based regimens compared to cetuximab. For other regimens such as nimotuzumab-RT, docetaxel-RT, and carboplatin-RT combined with paclitaxel the median OS was 21, 25.5, and 28 months, respectively. The OS at one, two, three, four, and five years according to the information available is presented in the same table.
Table 2.
Overall survival (OS) according to the treatment regimen
| (a) Median OS | |||
|---|---|---|---|
| Study | Median OS (95% CI) in months | Total (N) | Treatment regimen |
| Addeo et al. (2019) | 34 (31.1–36.8) | 64 | CTX-RT |
| Sun et al. (2022) | 31.1 (12.4–87.8) | 1493 | CTX-RT |
| Rambeau et al. (2017) | 12.8 (8.7–20.2) | 88 | CTX-RT |
| Magnes et al. (2021) | 46.0 (9.5–82.5) | 26 | CTX-RT |
| Imai et al. (2022 | 25.6 (NA) | 88 | CTX-RT |
| Fung et al. (2020 | 34 (19–48) | 115 | CTX-RT |
| Hamauchi et al. (2019) | 35.5 (NA) | 18 | CTX-RT |
| Hamauchi et al. (2019) | 91.9 (NA) | 29 | CB-RT |
| Sun et al. (2022) | 43.4 (15.3–123.8) | 1231 | CB-RT |
| Srinivas et al. (2020) | 21 (NA) | 21 | Nimo-RT |
| Magnes et al. (2021) | 58.3 (36.0–80.7) | 56 | RT alone |
| Patil et al. (2023) | 15.3 (13.1–22.0) | 176 | RT alone |
| Maring et al. (2018) | 28 (NA) | 23 | CB-RT + paclitaxel |
| Patil et al. (2023) | 25.5 (17.6–32.5) | 180 | DTX-RT |
| (b) 1-year OS | ||||
|---|---|---|---|---|
| Study | N | Total | Proportion | Treatment regimen |
| Ye et al. (2013) | 70 | 87 | 80.5% | CTX-RT |
| Swiecicki et al. (2020) | 15 | 21 | 71.4% | CTX-RT |
| Srinivas et al. (2020) | 13 | 21 | 63.7% | Nimo-RT |
| Weiss et al. (2020) | 25 | 29 | 86.2% | Pembro-RT |
| Rades et al. (2023) | 40 | 45 | 89% | CB-RT |
| (c) 2-year OS | ||||
|---|---|---|---|---|
| Study | N | Total | Proportion | Treatment regimen |
| Agarwal et al. (2011) | 16 | 37 | 44.4% | CTX-RT |
| Tao et al. (2023) | 36 | 65 | 55.4% | CTX-RT |
| Swiecicki et al. (2020) | 10 | 21 | 47.6% | CTX-RT |
| Han et al. (2023) | 58 | 65 | 88.7% | CB-RT + paclitaxel |
| Tao et al. (2023) | 41 | 66 | 62.1% | Pembro-RT |
| Weiss et al. (2020) | 22 | 29 | 75.9% | Pembro-RT |
| Patil et al. (2023) | 73 | 176 | 41.7% | RT alone |
| Patil et al. (2023) | 91 | 180 | 50.8% | DTX-RT |
| Rades et al. (2023) | 37 | 45 | 83% | CB-RT |
| (d) 3-year OS | ||||
|---|---|---|---|---|
| Study | N | Total | Proportion | Treatment regimen |
| Magnes et al. (2021) | 16 | 26 | 61.3% | CTX-RT |
| Rades et al. (2023) | 34 | 45 | 75% | CB-RT |
| Nassif et al. (2022) | 17 | 30 | 56.3% | CB-RT + paclitaxel |
| Magnes et al. (2021) | 37 | 56 | 66.1% | RT alone |
| Saigal et al. (2014 ) | 11 | 16 | 71.4% | CB-RT + CTX |
| (e) 4-year OS | ||||
|---|---|---|---|---|
| Study | N | Total | Proportion | Treatment regimen |
| Corry et al. (2017) | 46 | 60 | 76.7% | CTX-RT |
| (f) 5-year OS | ||||
|---|---|---|---|---|
| Study | N | Total | Proportion | Treatment regimen |
| Sun et al. (2022) | 528 | 1231 | 42.9% | CB-RT |
| Sun et al. (2022) | 507 | 1493 | 34.0% | CTX-RT |
| Magnes et al. (2021) | 12 | 26 | 46.6% | CTX-RT |
| Magnes et al. (2021) | 26 | 56 | 47.1% | RT alone |
OS overall survival; CI confidence interval; N number of patients; NA not available; CTX cetuximab; RT radiotherapy; CB carboplatin; DTX docetaxel; Nimo nimotuzumab; Pembro pembrolizumab; Total indicates the total number of patients included in the study
Median PFS was reported mostly for patients on cetuximab-RT, ranging from 6.5 to 17 months across four studies (Table 3). Median PFS was 42.7 months in 29 patients on carboplatin-RT (one study), 30 months in 23 patients on carboplatin-RT combined with paclitaxel (one study), and 28.5 months in 56 patients receiving RT (one study). At 2 years, the PFS of 65 patients on carboplatin-RT combined with paclitaxel described in one study was 72.3%. For 65 patients receiving cetuximab-RT, it was 40% (one study). In two studies with 66 and 29 patients treated with pembro-RT, the PFS ranged from 42.4% to 72.4%, respectively. The PFS at 1 and 3 years is presented in Table 3.
Table 3.
Progression-free survival (PFS) according to the treatment regimen
| (a) Median PFS | |||
|---|---|---|---|
| Study | Median PFS (95% CI) in months | Total (N) | Treatment regimen |
| Addeo et al. (2019) | 14.8 (13.9–15.5) | 64 | CTX-RT |
| Hamauchi et al. (2019) | 11.6 (NA) | 18 | CTX-RT |
| Rambeau et al. (2017) | 6.5 (6.0–10.1) | 88 | CTX-RT |
| Magnes et al. (2021) | 17 (0.0–60.3) | 26 | CTX-RT |
| Hamauchi et al. (2019) | 42.7 (NA) | 29 | CB-RT |
| Maring et al. (2018) | 30 (NA) | 23 | CB-RT + paclitaxel |
| Magnes et al. (2021) | 28.5 (17.1–39.4) | 56 | RT alone |
| (b) 1-year PFS | ||||
|---|---|---|---|---|
| Study | N | Total | Proportion | Treatment regimen |
| Weiss et al. (2020) | 22 | 29 | 75.9% | Pembro-RT |
| (c) 2-year PFS | ||||
|---|---|---|---|---|
| Study | N | Total | Proportion | Treatment regimen |
| Han et al. (2023) | 47 | 65 | 72.3% | CB-RT + paclitaxel |
| Tao et al. (2023) | 26 | 65 | 40% | CTX-RT |
| Tao et al. (2023) | 28 | 66 | 42.4% | Pembro-RT |
| Weiss et al. (2020) | 21 | 29 | 72.4% | Pembro-RT |
| (d) 3-year PFS | ||||
|---|---|---|---|---|
| Study | N | Total | Proportion | Treatment regimen |
| Nassif et al. (2022) | 21 | 30 | 68.9% | CB-RT + paclitaxel |
| Saigal et al. (2014) | 6 | 16 | 39.7% | CB-RT + CTX |
PFS progression-free survival; CI confidence interval; N number of patients; NA not available; CTX cetuximab; RT radiotherapy; CB carboplatin; Pembro pembrolizumab; Total indicates the total number of patients included in the study
The 1-year DFS was reported in two studies (21 and 87 patients in each) assessing cetuximab-RT and was superior to 45% in both studies (Supplementary Table S9). At two years, two studies with 21 and 37 patients reported the DFS, varying between 29.5% and 38.1%. For 176 patients treated with RT alone, it was 30.3% (one study). In one study, the 2-year DFS of 180 patients on docetaxel-RT was 42.2% (Supplementary Table S9).
The LRC at one year or 15 months was described in three studies including the following regimens: cetuximab-RT (143 patients), pembro-RT (57 patients), and carboplatin-RT (45 patients) (Supplementary Table S10). A rate of 78% was seen for patients on carboplatin-RT. Patients on pembro-RT had a 59.6% LRC. The group on CTX-RT presented with rates varying between 58.9% and 71.3%. At two years, one study with 45 patients on carboplatin-RT reported a higher rate of 69% compared to 35.5% for 37 patients treated with cetuximab-RT (Supplementary Table S10). The 3-year LRC was higher for 30 patients on carboplatin-RT combined with paclitaxel compared with other regimens, as shown in Supplementary Table S10.
The ORR, DCR, and the incidence of distant recurrence and locoregional failure according to the regimen used are presented in Tables S11 and S12, respectively.
Adverse events
For two studies on carboplatin-based regimens, the most common AEs were dry mouth, RT dermatitis, and mucositis. The majority of them were grade 1–2, except for mucositis, in which both studies reported high rates of grade 3 events (Supplementary Table S13A).
Eight studies reported skin toxicity in patients treated with cetuximab-RT, most of them grade 1–2. Four studies described important rates of grade 3 or higher RT dermatitis. In some of them, grade 3 AEs accounted for more than 50% of all events. Dysphagia was described in four studies, with most events being grade 1–2. Among eight studies exploring CTX-associated mucositis, three reported a high incidence (above 50%) of grade 3 AEs (Supplementary Table S13B).
One study explored AEs of 179 patients on docetaxel-RT. Skin toxicity, mucositis, and dysphagia were often reported. For skin toxicity, most events were grade 1–2. However, important rates of grade 3 or higher mucositis and dysphagia were observed (Supplementary Table S13C).
Pembro-RT and nimo-RT were assessed in two and one studies, respectively (Supplementary Table S13D e S13E). Anemia, mucositis, dysphagia, and diarrhea were frequently observed in patients receiving either regimen. For the group receiving pembro-RT, most AEs were grade 1–2. No grade 3 or higher AEs were observed in patients on nimo-RT. For other regimens, the toxicity profile is shown in Supplementary Table S13.
Quality assessment
Overall, six out of 22 non-randomized studies were judged to be at high risk of bias (Supplementary Table S14) (Addeo et al. 2019; Hamauchi et al. 2019; Rambeau et al. 2017; Saigal et al. 2014; Srinivas et al. 2020; Van Der Linden et al. 2014). Most of them lacked adjustments for confounding factors, failing to meet the criteria for the first domain. All other observational studies were adjusted for cofounders and judged at moderate risk of bias, as well as the phase II CTs (Agarwal et al. 2011; Beckham et al. 2020; Corry et al. 2017; Fung et al. 2020; Han et al. 2023; Imai et al. 2022; Magnes et al. 2021; Maring et al. 2018, p. 201; Nassif et al. 2022; Pryor et al. 2009; Rades et al. 2023; Sun et al. 2022; Swiecicki et al. 2020; Ueki et al. 2023; Weiss et al. 2020; Ye et al. 2013). The two randomized clinical trials met most criteria for all domains and were determined to be at low risk of bias (Supplementary Table S14) (Patil et al. 2023; Tao et al. 2023).
Discussion
To our knowledge, this is the first systematic review evaluating treatment alternatives for cisplatin-ineligible LA HNSCC patients. Median OS ranged from 12.8 to 46 months in seven studies including patients treated with cetuximab-RT. The median OS was superior to 40 months in two studies assessing carboplatin-RT and superior to 15 months in two studies assessing RT alone. For other regimens such as nimotuzumab-RT, docetaxel-RT, and carboplatin-RT combined with paclitaxel, the median OS was superior to 21 months. Median PFS was reported in four studies on patients on cetuximab-RT and it ranged from 6.5 to 17 months. Median PFS was superior to 20 months in patients treated with carboplatin-RT, carboplatin-RT combined with paclitaxel, or RT alone.
Cisplatin-ineligibility can be classified into two groups: absolute and relative contraindications (Szturz et al. 2019). Some of the absolute contraindications include impaired renal and hearing function and peripheral neuropathy (Kim et al. 2023; Szturz et al. 2019). Relative contraindications comprise patients who may not derive benefit from cisplatin due to individual factors (e.g. age, performance status, weight loss, previous cardiovascular disease) (Kim et al. 2023; Szturz et al. 2019). Reflecting the universal lack of consensus regarding cisplatin-ineligibility criteria, the studies included in this systematic review were heterogeneous in classifying patients as non-eligible for cisplatin.
International guidelines are vague in recommending treatment options for the LA HNSCC cisplatin-unfit population due to little evidence to support alternative regimens (Koyfman et al. 2019; Machiels et al. 2020). Patients unsuitable for cisplatin are frequently treated based on extrapolated data from studies assessing non-cisplatin regimens in populations eligible for cisplatin and who do not express the large comorbidity burden of unfit patients (Haddad et al. 2023). Accordingly, cetuximab raised as an alternative regimen over the standard of care (SOC) cisplatin-based CRT since its approval in the US in 2011 (Porceddu et al. 2020). The addition of CTX to RT was investigated in a phase III trial conducted by Bonner et al., showing a greater benefit for patients in the combination group compared to RT alone (Bonner et al. 2006). However, some authors support RT alone, especially intensity-modulated radiotherapy, as an alternative for such a population (Haddad et al. 2023). Multiple trials have shown the inferior efficacy of cetuximab-RT compared to SOC (Machiels et al. 2020; NCCN Clinical Practice Guidelines in Oncology. Head and Neck Cancer., n.d.). Yet, with a more acceptable AE profile compared to cisplatin, the combination of cetuximab and RT remains a reasonable option for LA HNSCC patients with contraindications to cisplatin (Addeo et al. 2019; Rambeau et al. 2017).
Carboplatin plus RT is another alternative for non-eligible patients. Although there is no phase III trial assessing CTX-RT versus CB-RT in cisplatin-unfit patients, retrospective studies support carboplatin over cetuximab (Sun et al. 2022). In a large US veteran cohort study conducted in 1231 patients treated with carboplatin and 1493 with cetuximab regimens, carboplatin was associated with a 15% OS benefit compared to cetuximab (Sun et al. 2022). In this study, most patients on carboplatin received a combination of carboplatin-RT and docetaxel, a derivative of paclitaxel. Accordingly, the phase III study conducted by Patil et al. on 180 patients treated with docetaxel plus RT compared to 176 patients on RT alone found an improvement in OS, DFS, and LRC in favor of the docetaxel-arm (Patil et al. 2023). Furthermore, the use of docetaxel plus RT and cetuximab as adjuvant treatment is recommended with a category 2B by NCCN guidelines for the LA HNSCC cisplatin-ineligible population (NCCN Clinical Practice Guidelines in Oncology. Head and Neck Cancer., n.d.).
Immunotherapy agents are currently emerging for the treatment of HNC in various stages (Tao et al. 2023; Weiss et al. 2020). Previous studies support the benefit of anti-PD1 agents in the recurrent or metastatic setting (Burtness et al. 2019). In the cisplatin-ineligibility scenario, a phase III trial recently explored the role of ICI combined with RT for LA HNSCC (Tao et al. 2023). The study conducted by Tao et al. compared 66 patients on Pembro-RT with 65 patients on cetuximab-RT (Tao et al. 2023). The OS, PFS, and LRC were similar between groups (Tao et al. 2023). Nevertheless, the AEs rates were significantly lower in the pembro-arm, suggesting that this combination may be as efficient as cetuximab-based regimens with a milder toxicity profile. Data from ongoing studies analyzing other combinations are awaited, such as the phase III REACH studying avelumab plus CTX plus RT versus SOC (NCT02999087) and NANORAY-312, a phase III trial assessing NBTXR3 in combination with RT and CTX for cisplatin-unfit patients (NCT04892173).
It is noteworthy to mention that all these regimens may have different responses and tolerability profiles according to tumor location, risk stratification, and individual factors (Haddad et al. 2023; Porceddu et al. 2020). For instance, human papillomavirus (HPV)-negative patients are known to present with poor outcomes, both on cisplatin-based or alternative regimens (Haddad et al. 2023; Kim et al. 2023, p. 2; Porceddu et al. 2020). These patients are often older and present with an even higher burden of comorbidities. Therefore, different factors such as tumor location and risk, sociodemographic characteristics, and adverse-events profile should be carefully considered in treatment decisions for HNSCC cisplatin non-eligible patients.
This study has limitations. First, network meta-analysis is the preferred method to compare multiple interventions in a specific population (Faltinsen et al. 2018). The limited high-quality evidence and important heterogeneity among studies prevented us from applying meta-analyses. The study’s design, follow-up time, cisplatin ineligibility criteria, outcomes and ‘locally advanced head and neck cancer’ definition, and treatment schedules also differed in included studies. For some treatment regimens, a limited number of studies were available. Due to the unavailability of data from most studies, data stratified on tumor location and risk stratification could not be presented.
This large systematic review includes over 4000 cisplatin-unfit LA HNSCC patients and covers a range of alternative therapies for this population, including cetuximab-RT, carboplatin-RT, carboplatin-RT combined with cetuximab or paclitaxel, RT alone, docetaxel-RT, and pembro or nimo-RT. The response, survival, and safety data presented offer essential insights and guidance for clinicians treating this group. Yet, the lack of consensus on cisplatin ineligibility criteria adds important complexity to the management of these patients. Thus, future studies should focus on establishing cisplatin ineligibility criteria and exploring tolerable but effective alternative strategies for this population. Importantly, patients should be stratified according to relevant clinicopathological factors (i.e. tumor location, HPV status, patient’s age, and performance status).
Conclusions
To the best of our knowledge, this is the first systematic review evaluating treatment alternatives for cisplatin-ineligible LA HNSCC patients. This study gathers extensive information regarding treatment strategies available for cisplatin-non-eligible LA HNSCC patients within CTs and in the real-world setting. Our findings support the use of several available options such as cetuximab-RT, carboplatin-RT, carboplatin-RT combined with cetuximab or paclitaxel, RT alone, docetaxel-RT, and pembro or nimo-RT for cisplatin-unfit LA HNSCC patients. Nonetheless, we highlight the limited evidence to guide treatment choice and the urgent need for clinical studies assessing new treatment approaches in this population.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization, IM, DM; Methodology, IM, GCN, MID; Software, IM, MID; Validation, IM, GCN, MID, DM; Formal analysis, IM, GCN, MID, RGL, DM; Investigation, IM, GCN, MID, ACBKM, MM, LSP, CTC, RGL, DM; Data Curation, IM, GCN, MID, RGL, DM; Resources, IM, GCN, MID, ACBKM, MM, LSP, CTC, RGL, DM; Visualization, IM, GCN, MID, ACBKM, MM, LSP, CTC, RGL, DM; Writing original draft, IM, GCN, MID, ACBKM, MM, LSP, CTC, RGL, DM; Writing – review and editing, IM, GCN, MID, ACBKM, MM, LSP, CTC, RGL, DM; Project Administration, IM, CTC, RGL, DM; Supervision, CTC, RGL, DM. All authors critically reviewed the manuscript and approved its current version for submission.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
All research data presented in this study is accessible upon request to the corresponding author.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Addeo R, Caraglia M, Vincenzi B, Luce A, Montella L, Mastella A, Mazzone S, Ricciardiello F, Carraturo M, Del Prete S, Sperlongano P (2019) Efficacy and safety of cetuximab plus radiotherapy in cisplatin-unfit elderly patients with advanced squamous cell head and neck carcinoma a retrospective study. Chemotherapy 64(1):48–56. 10.1159/000500714 [DOI] [PubMed] [Google Scholar]
- Agarwal JP, Gupta T, Budrukkar NA, Laskar SG, Murthy V, Kumar P, Narohna V, Pai P, Chaturvedi P, Dcruz AK (2011) Cetuximab with radiotherapy in patients with loco-regionally advanced squamous cell carcinoma of head and neck unsuitable or ineligible for concurrent platinum-based chemo-radiotherapy: Ready for routine clinical practice. Indian J Cancer 48(2):148–153. 10.4103/0019-509X.82872 [DOI] [PubMed] [Google Scholar]
- Ang KK (2008) Multidisciplinary management of locally advanced SCCHN: optimizing treatment outcomes. Oncologist 13(8):899–910. 10.1634/theoncologist.2007-0157 [DOI] [PubMed] [Google Scholar]
- Barabas K, Milner R, Lurie D, Adin C (2008) Cisplatin: a review of toxicities and therapeutic applications. Vet Comp Oncol 6(1):1–18. 10.1111/j.1476-5829.2007.00142.x [DOI] [PubMed] [Google Scholar]
- Barsouk A, Aluru JS, Rawla P, Saginala K, Barsouk A (2023) Epidemiology, risk factors, and prevention of head and neck squamous cell carcinoma. Med Sci 11(2):42. 10.3390/medsci11020042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham TH, Barney C, Healy E, Wolfe AR, Branstetter A, Yaney A, Riaz N, McBride SM, Tsai CJ, Kang J, Yu Y, Chen L, Sherman E, Dunn L, Pfister DG, Tan J, Rupert R, Bonomi M, Zhang Z, Bhatt AD (2020) Platinum-based regimens versus cetuximab in definitive chemoradiation for human papillomavirus-unrelated head and neck cancer. Int J Cancer 147(1):107–115. 10.1002/ijc.32736 [DOI] [PubMed] [Google Scholar]
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354(6):567–578. 10.1056/NEJMoa053422 [DOI] [PubMed] [Google Scholar]
- Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, De Castro G, Psyrri A, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesía R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Hong R-L, González Mendoza R, Roy A, Yorio J (2019) Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394(10212):1915–1928. 10.1016/S0140-6736(19)32591-7 [DOI] [PubMed] [Google Scholar]
- Corry J, Bressel M, Fua T, Herschtal A, Solomon B, Porceddu SV, Wratten C, Rischin D (2017) Prospective study of cetuximab, carboplatin, and radiation therapy for patients with locally advanced head and neck squamous cell cancer unfit for cisplatin. Int J Radiat Oncol Biol Phys 98(4):948–954. 10.1016/j.ijrobp.2017.02.088 [DOI] [PubMed] [Google Scholar]
- De Felice F, Tombolini V, De Vincentiis M, Magliulo G, Greco A, Valentini V, Polimeni A (2019) Multidisciplinary team in head and neck cancer: amanagement model. Med Oncol 36(1):2. 10.1007/s12032-018-1227-z [DOI] [PubMed] [Google Scholar]
- De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AWM, Hegi-Johnson F (2019) Radiotherapy toxicity. Nat Rev Dis Prim 5(1):13. 10.1038/s41572-019-0064-5 [DOI] [PubMed] [Google Scholar]
- Dhull AK, Atri R, Dhankhar R, Chauhan AK, Kaushal V (2018) Major risk factors in head and neck cancer a retrospective analysis of 12-year experiences. World J Oncol 9(3):80–84. 10.14740/wjon1104w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeli V, Zucca E, Ghielmini M, Giannini O, Salatino A, Martucci F, Richetti A (2012) Weekly and 3-weekly cisplatin concurrent with intensity-modulated radiotherapy in locally advanced head and neck squamous cell cancer. Oral Oncol 48(3):266–271. 10.1016/j.oraloncology.2011.10.005 [DOI] [PubMed] [Google Scholar]
- Faltinsen EG, Storebø OJ, Jakobsen JC, Boesen K, Lange T, Gluud C (2018) Network meta-analysis: the highest level of medical evidence? BMJ Evid-Based Med 23(2):56–59. 10.1136/bmjebm-2017-110887 [DOI] [PubMed] [Google Scholar]
- Fung AS, Afzal A, Banerjee R, Debenham B, Hao D (2020) A real-world comparison of cisplatin versus cetuximab used concurrently with radiation in the treatment of locally advanced oropharyngeal carcinoma: updated results. Curr Oncol 27(4):e416 [DOI] [PubMed] [Google Scholar]
- Haddad RI, Harrington K, Tahara M, Szturz P, Le Tourneau C, Salmio S, Bajars M, Lee NY (2023) Managing cisplatin-ineligible patients with resected, high-risk, locally advanced squamous cell carcinoma of the head and neck: Is there a standard of care? Cancer Treat Rev 119:102585. 10.1016/j.ctrv.2023.102585 [DOI] [PubMed] [Google Scholar]
- Hamauchi S, Yokota T, Mizumachi T, Onozawa Y, Ogawa H, Onoe T, Kamijo T, Iida Y, Nishimura T, Onitsuka T, Yasui H, Homma A (2019) Safety and efficacy of concurrent carboplatin or cetuximab plus radiotherapy for locally advanced head and neck cancer patients ineligible for treatment with cisplatin. Int Clin Oncol 24(5):468–475. 10.1007/s10147-018-01392-9 [DOI] [PubMed] [Google Scholar]
- Han J, Zakeri K, Raab G, Hesse J, Shamseddine A, Chen L, Yu Y, Kang JJ, McBride SM, Riaz N, Tsai CJ, Gelblum D, Sherman EJ, Wong RJ, Michel L, Lee NY (2023) Concurrent carboplatin and paclitaxel definitive radiation therapy for locally advanced head and neck cancer. Head Neck. 10.1002/hed.27456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai C, Saeki H, Yamamoto K, Ichikawa A, Arai M, Tawada A, Suzuki T, Takiguchi Y, Hanazawa T, Ishii I (2022) Radiotherapy plus cetuximab for locally advanced squamous cell head and neck cancer in patients with cisplatin-ineligible renal dysfunction: a retrospective study. Oncol Lett. 10.3892/ol.2022.13271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Liu HC, Mell LK (2023) Treatment considerations for patients with locoregionally advanced head and neck cancer with a contraindication to cisplatin. Curr Treat Options Oncol 24(3):147–161. 10.1007/s11864-023-01051-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyfman SA, Ismaila N, Crook D, D’Cruz A, Rodriguez CP, Sher DJ, Silbermins D, Sturgis EM, Tsue TT, Weiss J, Yom SS, Holsinger FC (2019) Management of the neck in squamous cell carcinoma of the oral cavity and oropharynx: ASCO clinical practice guideline. J Clin Oncol 37(20):1753–1774. 10.1200/JCO.18.01921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiels J-P, René Leemans C, Golusinski W, Grau C, Licitra L, Gregoire V (2020) Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS–ESMO–ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 31(11):1462–1475. 10.1016/j.annonc.2020.07.011 [DOI] [PubMed] [Google Scholar]
- Magnes T, Wagner SM, Melchardt T, Weiss L, Rinnerthaler G, Huemer F, Kopp M, Gampenrieder SP, Mayrbäurl B, Füreder T, Lenger D, Andel J, Egle A, Greil R (2021) Postoperative chemoradiotherapy with cisplatin is superior to radioimmunotherapy with cetuximab and radiotherapy alone: analysis of the Austrian head and neck cancer registry of the AGMT. Wien Klin Wochenschr 133(21–22):1131–1136. 10.1007/s00508-021-01939-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maring S, Elsayad K, Stenner M, Rudack C, Haverkamp U, Rehkämper J, Wardelmann E, Eich HT (2018) Efficacy of carboplatin/paclitaxel-based radiochemotherapy in locally advanced squamous cell carcinoma of head and neck. Oncol Res Treat 41(12):736–742. 10.1159/000494031 [DOI] [PubMed] [Google Scholar]
- Nassif S, Wichmann J, Strube D, Vassis S, Christiansen H, Steinmann D (2022) Cisplatin versus carboplatin and paclitaxel in radiochemotherapy for patients with locally advanced head and neck squamous cell carcinoma. In Vivo (athens, Greece) 36(2):821–832. 10.21873/invivo.12769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCCN Clinical Practice Guidelines in Oncology. Head and neck cancer. (n.d.). Retrieved December 6, 2023, from https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1437
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil VM, Noronha V, Menon N, Singh A, Ghosh-Laskar S, Budrukkar A, Bhattacharjee A, Swain M, Mathrudev V, Nawale K, Balaji A, Peelay Z, Alone M, Pathak S, Mahajan A, Kumar S, Purandare N, Agarwal A, Puranik A, Prabhash K (2023) Results of phase III randomized trial for use of docetaxel as a radiosensitizer in patients with head and neck cancer, unsuitable for cisplatin-based chemoradiation. J Clin Oncol 41(13):2350–2361. 10.1200/JCO.22.00980 [DOI] [PubMed] [Google Scholar]
- Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, Bruce JY, Busse PM, Caudell JJ, Cmelak AJ, Colevas AD, Eisele DW, Fenton M, Foote RL, Galloway T, Gillison ML, Haddad RI, Hicks WL, Hitchcock YJ, Darlow SD (2020) Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 18(7):873–898. 10.6004/jnccn.2020.0031 [DOI] [PubMed] [Google Scholar]
- Porceddu SV, Scotté F, Aapro M, Salmio S, Castro A, Launay-Vacher V, Licitra L (2020) Treating patients with locally advanced squamous cell carcinoma of the head and neck unsuitable to receive cisplatin-based therapy. Front Oncol. 10.3389/fonc.2019.01522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor DI, Porceddu SV, Burmeister BH, Guminski A, Thomson DB, Shepherdson K, Poulsen M (2009) Enhanced toxicity with concurrent cetuximab and radiotherapy in head and neck cancer. Radiother Oncol 90(2):172–176. 10.1016/j.radonc.2008.09.018 [DOI] [PubMed] [Google Scholar]
- Rades D, Zwaan I, Soror T, Idel C, Pries R, Bruchhage KL, Hakim SG, Yu NY (2023) Chemoradiation with cisplatin vs carboplatin for squamous cell carcinoma of the head and neck (SCCHN). Cancers 15(13):3278. 10.3390/cancers15133278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambeau A, Gervais R, De Raucourt D, Babin E, Dugué AE, Florescu C, Blanchard D, Gery B (2017) Retrospective evaluation of concomitant cetuximab and radiotherapy tolerance for locoregional advanced head and neck squamous cell carcinoma treatment in patients unfit for platinum-based chemotherapy. Eur Arch Oto-Rhino-Laryngol 274(7):2883–2889. 10.1007/s00405-017-4550-7 [DOI] [PubMed] [Google Scholar]
- Saigal K, Santos ES, Tolba K, Kwon D, Elsayyad N, Abramowitz MC, Mandalia A, Samuels MA (2014) Concurrent radiotherapy with carboplatin and cetuximab for the treatment of medically compromised patients with locoregionally advanced head and neck squamous cell carcinoma. Front Oncol. 10.3389/fonc.2014.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas K, Sundaram R, Divyambika C, Chaudhari S (2020) Nimotuzumab with intensity-modulated radiation therapy in unresectable and platinum-ineligible locally advanced head-and-neck cancer. South Asian J Cancer 9(1):43–46. 10.4103/sajc.sajc_29_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan A-W, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Higgins JP (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, Higgins JPT (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- Sun L, Candelieri-Surette D, Anglin-Foote T, Lynch JA, Maxwell KN, D’Avella C, Singh A, Aakhus E, Cohen RB, Brody RM (2022) Cetuximab-based vs carboplatin-based chemoradiotherapy for patients with head and neck cancer. JAMA Otolaryngol - Head Neck Surg 148(11):1022–1028. 10.1001/jamaoto.2022.2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiecicki PL, Li P, Bellile E, Stucken C, Malloy K, Shuman A, Spector ME, Chinn S, Casper K, McLean S, Moyer J, Chepeha D, Wolf GT, Prince M, Bradford C, Nyati M, Eisbruch A, Worden FP, Jolly S, Mierzwa M (2020) Paired phase II trials evaluating cetuximab and radiotherapy for low risk HPV associated oropharyngeal cancer and locoregionally advanced squamous cell carcinoma of the head and neck in patients not eligible for cisplatin. Head Neck 42(8):1728–1737. 10.1002/hed.26085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szturz P, Cristina V, Herrera Gómez RG, Bourhis J, Simon C, Vermorken JB (2019) Cisplatin eligibility issues and alternative regimens in locoregionally advanced head and neck cancer: recommendations for clinical practice. Front Oncol 9:464. 10.3389/fonc.2019.00464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Biau J, Sun XS, Sire C, Martin L, Alfonsi M, Prevost JB, Modesto A, Lafond C, Tourani JM, Miroir J, Kaminsky MC, Coutte A, Liem X, Chautard E, Vauleon E, Drouet F, Ruffier A, Ramee JF, Bourhis J (2023) Pembrolizumab versus cetuximab concurrent with radiotherapy in patients with locally advanced squamous cell carcinoma of head and neck unfit for cisplatin (GORTEC 2015–01 PembroRad): a multicenter, randomized, phase II trial. Ann Oncol 34(1):101–110. 10.1016/j.annonc.2022.10.006 [DOI] [PubMed] [Google Scholar]
- Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, Komaroff E, Nalysnyk L, Zilberberg MD (2003) Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 66(3):253–262. 10.1016/S0167-8140(02)00404-8 [DOI] [PubMed] [Google Scholar]
- Ueki Y, Ohshima S, Yokoyama Y, Takahashi T, Shodo R, Yamazaki K, Ohtaki K, Saijo K, Tanaka R, Togashi T, Sato Y, Takano S, Omata J, Takahashi N, Okabe R, Horii A (2023) Multicenter prospective phase II trial of concurrent chemoradiotherapy with weekly low-dose carboplatin for cisplatin-ineligible patients with advanced head and neck squamous cell carcinoma. Int J Clin Oncol. 10.1007/s10147-023-02423-w [DOI] [PubMed] [Google Scholar]
- Van Der Linden N, Van Gils CWM, Pescott CP, Buter J, Uyl-De Groot CA (2014) Cetuximab in locally advanced squamous cell carcinoma of the head and neck: generalizability of EMR 062202–006 trial results. Euro Arch Oto-Rhino-Laryngol 271(6):1673–1678. 10.1007/s00405-013-2646-2 [DOI] [PubMed] [Google Scholar]
- Weiss J, Sheth S, Deal AM, Grilley Olson JE, Patel S, Hackman TG, Blumberg JM, Galloway TJ, Patel S, Zanation AM, Shen CJ, Hayes DN, Hilliard C, Mehra R, McKinnon KP, Wang H-H, Weissler MC, Bauman JR, Chera BS, Vincent BG (2020) Concurrent definitive immunoradiotherapy for patients with stage III–IV head and neck cancer and cisplatin contraindication. Clin Cancer Res 26(16):4260–4267. 10.1158/1078-0432.CCR-20-0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye A, Hay J, Laskin J, Wu J, Ho C (2013) Toxicity and outcomes in combined modality treatment of head and neck squamous cell carcinoma: cisplatin versus cetuximab. J Cancer Res Ther 9(4):607. 10.4103/0973-1482.126455 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All research data presented in this study is accessible upon request to the corresponding author.


