Abstract
Purpose
Elective open incisional hernia operations are a frequently performed and complex procedure. Prophylactic drainage is widely practised to prevent local complications, but nevertheless the benefit of surgical drain placement remains a controversially discussed subject. Objective of this analysis was to evaluate the current status of patient care in clinical routine and outcome in this regard.
Methods
The study based on prospectively collected data of the Herniamed Register. Included were all patients with elective open incisional hernia between 1/2005 and 12/2020 and completed 1-year follow-up. Multiple linear and logistic regression analysis was performed to assess the relation of individual factors to the outcome variables.
Results
Analysed were data from 39,523 patients (28,182 with drain, 11,341 without). Patients with drain placement were significantly older, had a higher BMI, more preoperative risk factors, and a larger defect size. Drained patients furthermore showed a significant disadvantage in the outcome parameters intraoperative complications, general complications, postoperative complications, complication-related reoperations, and pain at the 1-year follow-up. No significant difference was observed with respect to the recurrent rate.
Conclusion
With 71.3%, the use of surgical drainages has a high level of acceptance in elective open incisional hernia operations. The worse outcome of patients is associated with the use of drains, independent of other influencing factors in the model such as patient or surgical characteristics. The use of drains may be a surrogate parameter for other unobserved confounders.
Keywords: Drain, Open incisional hernia repair, Outcome, Elective hernia operations
Introduction
Surgical drains have a long history in medicine as an integral part of the therapeutic concept [1]. Already since the mid-1800s, the use of drains in gastrointestinal surgery has widely been practised. Lawson Tait, a nineteenth century surgeon, even coined the dictum “When in doubt, drain” [2], but in practice the situation turns out to be much more complex and leaves the decision of drain usage to the surgeon's perception of the overall situation. In open ventral hernia repair, drains are traditionally placed to avoid seroma and hematoma formation by facilitating fluid drainage [3]. The prophylactic placement of drains has, however, aroused much controversy as studies have been published indicating that drains often fail to protect against seromas and may even contribute to infectious complications [4]. Traditional intra-abdominal and subcutaneous drains were also assessed within the context of optimizing perioperative management which began with the fast-track concept of Kehlet in 1995 for colon surgery and the ERAS (enhanced recovery after surgery) management in 2005, and their avoidance was recommended in the case of questionable protective effects [5–7]. But what are the consequences for clinical practice? Already in the past, differences between the current status of research and patient care in clinical routine [8] have been observed, leading to a more differentiated view concerning the interpretation of the respective results. Accordingly, a thorough assessment of the quality of care in hernia surgery within the framework of clinical health services research is a prerequisite that contributes an essential element to the further development of optimized therapies in everyday clinical practice. Based on data from the Herniamed Hernia registry, we evaluated the reality of care in elective open incisional hernia operations, with a particular focus on the utilization of drains in this study.
Material and methods
We evaluated prospectively collected data from 836 centres in Germany, Austria and Switzerland from the internet-based Herniamed Hernia registry and included operated patients from January 5, 2009 to December 31, 2020 with completed 1-year follow-up visit in this evaluation. The inclusion criteria were elective incisional hernia operations with open procedures (open direct suture, open onlay, open sublay, open intraperitoneal onlay mesh (IPOM), component separation). Exclusion criteria were incompletely documented cases, invalid age information, patients under the age of 16, and the use of non-approved meshes. Senior or high-risk patients were not excluded. All patients signed a consent form agreeing to the processing of their data [9]. Baseline demographic data included age, gender, BMI (body mass index), and ASA (American Society of Anesthesiologists) score. In addition to the surgical methods mentioned above, the use of drains, EHS (European Hernia Society) classification, mesh implantation, pre- and postoperative pain, and recurrences were recorded. Single outcome and influencing variables (risk factors, complications) were summarized as global variables. A general, intra- or postoperative complication or risk factor was considered present if at least one single item applied.
Plausibility assessment
A plausibility check was performed to confirm the presence of a correct data set with patient master and operation data. Furthermore, the plausibility of length-of-stay data, information on surgery time and mesh size, age, weight, height, BMI, and follow-up data was verified.
Statistical analysis
All analyses were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA). A p-value of ≤ 0.05 was considered statistically significant. Univariate descriptive statistics were performed for the comparison of drain use (yes vs. no). All categorical patient data are presented in absolute and relative counts, while mean and standard deviation (SD) are shown for continuous data. Unadjusted analyses were carried out to assess the effect of individual influencing variables on an outcome parameter. The Chi-square test was used for categorical target variables, and the robust t-test (Satterthwaite) was used for continuous variables. Multivariable analyses were performed using the binary logistic regression model. All pair-wise odds ratios are given with the corresponding 95% confidence intervals. To rule out a potential bias in the selection of the analysis population (patients with 1-year follow-up) compared to patients without follow-up, standardized differences were estimated for the two populations.
Results
Patient and operation characteristics
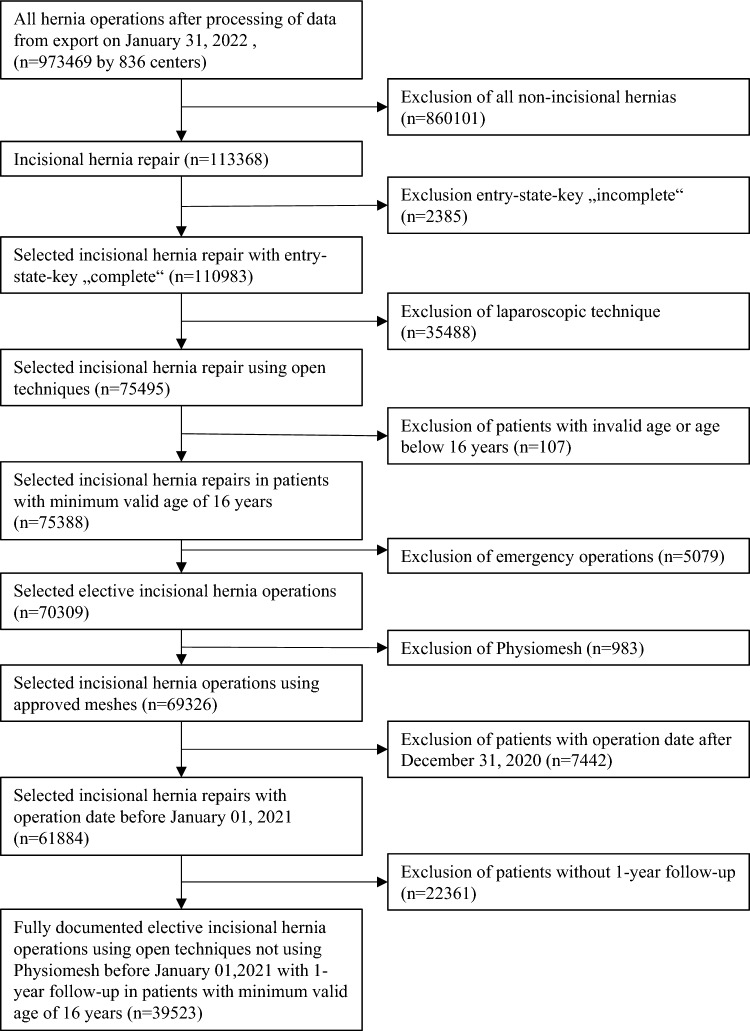
Between January 5, 2009 and December 31, 2020, data from 39,523 patients who underwent elective open incisional hernia surgery with completed 1-year follow-up were entered into the Herniamed Registry (Fig. 1). Drains were used in 28,182 patients (71.31%) undergoing elective surgery, while 11,341 patients (28.69%) did not receive a drain. Drained patients had an average age of 63.6 ± 12.8 years (mean ± SD) and were thus significantly older than patients without drain use who had an average age of 59.8 ± 15.1 years (p < 0.001). Additionally, the BMI was significantly higher in patients with compared to patients without drains (29.8 ± 5.9 vs. 27.9 ± 5.4, p < 0.001) (Table 1).
Fig. 1.
Flowchart of patient inclusion
Table 1.
Unadjusted analysis results for homogeneity between drain use (yes vs. no) and age and BMI, respectively; descriptive statistics and results of unadjusted analysis for homogeneity between the comparison groups (drainage yes vs. no) and categorical influencing variables
| Drainage | ||||||
|---|---|---|---|---|---|---|
| Yes | No | p | ||||
| n | Mean ± SD | n | Mean ± SD | |||
| Age (years) | 28,182 | 63.6 ± 12.8 | 11,341 | 59.8 ± 15.1 | < 0.001 | |
| BMI (kg/m2) | 28,080 | 29.8 ± 5.9 | 11,305 | 27.9 ± 5.4 | < 0.001 | |
| n | % | n | % | |||
| Gender | Male | 14,377 | 51.0 | 5737 | 50.6 | 0.441 |
| Female | 13,805 | 49.0 | 5604 | 49.4 | ||
| ASA | I | 2261 | 8.0 | 2102 | 18.5 | < 0.001 |
| II | 15,539 | 55.1 | 6434 | 56.7 | ||
| III/IV | 10,382 | 36.8 | 2805 | 24.7 | ||
| Operation technique | Open—onlay | 2324 | 8.2 | 518 | 4.6 | < 0.001 |
| Open—sublay | 18,748 | 66.5 | 3693 | 32.6 | ||
| Open—IPOM | 4004 | 14.2 | 3122 | 27.5 | ||
| Component separation | 1441 | 5.1 | 163 | 1.4 | ||
| Open—direct suture | 1665 | 5.9 | 3845 | 33.9 | ||
| Defect size | I (< 4 cm) | 6365 | 22.6 | 7696 | 67.9 | < 0.001 |
| II (4–10 cm) | 14,991 | 53.2 | 2904 | 25.6 | ||
| III (> 10 cm) | 6826 | 24.2 | 741 | 6.5 | ||
| EHS classification | Medial | 21,913 | 77.8 | 8672 | 76.5 | < 0.001 |
| Lateral | 4039 | 14.3 | 1921 | 16.9 | ||
| Combined | 2230 | 7.9 | 748 | 6.6 | ||
| Preoperative pain | No | 9415 | 33.4 | 3599 | 31.7 | 0.006 |
| Yes | 16,433 | 58.3 | 6777 | 59.8 | ||
| Unknown | 2334 | 8.3 | 965 | 8.5 | ||
| Mesh | Yes | 26,191 | 92.9 | 7324 | 64.6 | < 0.001 |
| No | 1991 | 7.1 | 4017 | 35.4 | ||
| Recurrent operation | Yes | 5873 | 20.8 | 1904 | 16.8 | < 0.001 |
| No | 22,309 | 79.2 | 9437 | 83.2 | ||
| Chronic obstructive pulmonary disease (COPD) | Yes | 3227 | 11.5 | 977 | 8.6 | < 0.001 |
| No | 24,955 | 885 | 10,364 | 91.4 | ||
| Diabetes | Yes | 4033 | 14.3 | 1025 | 9.0 | < 0.001 |
| No | 24,149 | 85.7 | 10,316 | 91.0 | ||
| Aortic aneurysm | Yes | 540 | 1.9 | 117 | 1.0 | < 0.001 |
| No | 27,642 | 98.1 | 11,224 | 99.0 | ||
| Immunosuppression | Yes | 599 | 2.1 | 217 | 1.9 | 0.180 |
| No | 27,583 | 97.9 | 11,124 | 98.1 | ||
| Corticoids | Yes | 500 | 1.8 | 178 | 1.6 | 0.156 |
| No | 27,682 | 98.2 | 11,163 | 98.4 | ||
| Smoking | Yes | 3605 | 12.8 | 1372 | 12.1 | 0.060 |
| No | 24,577 | 87.2 | 9969 | 87.9 | ||
| Coagulopathy | Yes | 747 | 2.7 | 193 | 1.7 | < 0.001 |
| No | 27,435 | 97.3 | 11,148 | 98.3 | ||
| Antithrombotic medication | Yes | 3665 | 13.0 | 1141 | 10.1 | < 0.001 |
| No | 24,517 | 87.0 | 10,200 | 89.9 | ||
| Anticoagulant medication | Yes | 969 | 3.4 | 286 | 2.5 | < 0.001 |
| No | 27,213 | 96.6 | 11,055 | 97.5 | ||
In the unadjusted analysis of the relationship between the two patient groups (drain vs. no drain) with respect to patient and operation characteristics, the expression of almost all variables differed significantly. Only with respect to gender, no statistically significant difference could be observed (p = 0.441) (Table 1). In the detailed evaluation of the unadjusted analyses concerning items relevant for general complications, significant differences between the two patient groups for fever (p < 0.001) and pulmonary embolism (p 0.007) were detected. For thrombosis, p = 0.059. The unadjusted analysis results of the relationship between postoperative complications and drain use are presented in Table 2. No significant differences in the topic-specific items seroma, wound healing disorder, and infection were observed (p < 0.001 each).
Table 2.
Descriptive statistics and results of unadjusted analysis for homogeneity between comparison groups (drainage yes vs. no) and outcome variables as well as individual items of postoperative complications
| Drainage | ||||||
|---|---|---|---|---|---|---|
| Yes | No | p | ||||
| n | % | n | % | |||
| Intraoperative complications—total | Yes | 551 | 2.0 | 94 | 0.8 | < 0.001 |
| No | 27,631 | 98.0 | 11,247 | 99.2 | ||
| General complications—total | Yes | 1269 | 4.5 | 220 | 1.9 | < 0.001 |
| No | 26,913 | 95.5 | 11,121 | 98.1 | ||
| Fever | Yes | 139 | 0.5 | 21 | 0.2 | < 0.001 |
| No | 28,043 | 99.5 | 11,320 | 99.8 | ||
| Pulmonary embolism | Yes | 53 | 0.2 | 8 | < 0.1 | 0.007 |
| No | 28,129 | 99.8 | 11,333 | > 99.1 | ||
| Thrombosis | Yes | 30 | 0.1 | 5 | < 0.1 | 0.059 |
| No | 28,152 | 99.1 | 11,336 | > 99.9 | ||
| Postoperative complications—total | Yes | 3041 | 10.8 | 562 | 5.0 | < 0.001 |
| No | 25,141 | 89.2 | 10,779 | 95.0 | ||
| Complication-related reoperations | Yes | 1387 | 4.9 | 204 | 1.8 | < 0.001 |
| No | 26,795 | 95.1 | 11,137 | 98.2 | ||
| Recurrence at 1-year follow-up | Yes | 1422 | 5.0 | 607 | 5.4 | 0.212 |
| No | 26,760 | 95.0 | 10,734 | 94.6 | ||
| Pain on exertion at 1-year follow-up | Yes | 5494 | 19.5 | 1824 | 16.1 | < 0.001 |
| No | 22,688 | 80.5 | 9517 | 83.9 | ||
| Pain at rest at 1-year follow-up | Yes | 3148 | 11.2 | 1008 | 8.9 | < 0.001 |
| No | 25,034 | 88.8 | 10,333 | 91.1 | ||
| Pain requiring treatment at 1-year follow-up | Yes | 2472 | 8.8 | 766 | 6.8 | < 0.001 |
| No | 25,710 | 91.2 | 10,575 | 93.2 | ||
| Bleeding | Yes | 748 | 2.7 | 138 | 1.2 | < 0.001 |
| No | 27,434 | 97.3 | 11,203 | 98.8 | ||
| Seroma | Yes | 1353 | 4.8 | 255 | 2.2 | < 0.001 |
| No | 26,829 | 95.2 | 11,086 | 97.8 | ||
| Prolonged ileus or obstruction | Yes | 188 | 0.7 | 30 | 0.3 | < 0.001 |
| No | 27,994 | 99.3 | 11,311 | 99.7 | ||
| Bowel injury/anastomotic insufficiency | Yes | 77 | 0.3 | 25 | 0.2 | 0.349 |
| No | 28,105 | 99.7 | 11,316 | 99.8 | ||
| Wound healing disorder | Yes | 889 | 3.2 | 142 | 1.3 | < 0.001 |
| No | 27,293 | 96.8 | 11,199 | 98.7 | ||
| Infection | Yes | 478 | 1.7 | 75 | 0.7 | < 0.001 |
| No | 27,704 | 98.3 | 11,266 | 99.3 | ||
Intraoperative complications in logistic regression analyses
The risk of intraoperative complications was significantly associated with defect size, surgical procedure, drain use, age (p < 0.001 each), and recurrence (p = 0.006). Specifically, intraoperative complications occurred more frequently in larger defects, the surgical procedures open-direct suture and open-IPOM, drained patients (OR odds ratio = 1902 [1483; 2438]), elderly patients, and patients with recurrences (Table 3).
Table 3.
Results of the multivariable analysis for intraoperative complications including odds ratios with corresponding 95% confidence interval
| Variable | p-value | Categories | Odds ratio | LCL | UCL | p-value (pair-wise) |
|---|---|---|---|---|---|---|
| Defect size | < 0.001 | III (> 10 cm) vs. I (< 4 cm) | 4.905 | 3.669 | 6.556 | < 0.001 |
| II (4–10 cm) vs. I (< 4 cm) | 3.261 | 2.501 | 4.253 | < 0.001 | ||
| III (> 10 cm) vs. II (4–10 cm) | 1.504 | 1.258 | 1.798 | < 0.001 | ||
| Operation technique | < 0.001 | Open—direct suture vs. Open—sublay | 4.097 | 2.414 | 6.954 | < 0.001 |
| Open—IPOM vs. open—sublay | 1.570 | 1.272 | 1.936 | < 0.001 | ||
| Open—direct suture vs. open—onlay | 3.344 | 1.854 | 6.031 | < 0.001 | ||
| Open—direct suture vs. component separation | 3.054 | 1.667 | 5.593 | < 0.001 | ||
| Open—direct suture vs. open—IPOM | 2.610 | 1.519 | 4.487 | < 0.001 | ||
| Component separation vs. open—sublay | 1.342 | 0.962 | 1.871 | 0.083 | ||
| Open—IPOM vs. open—onlay | 1.281 | 0.907 | 1.809 | 0.160 | ||
| Open—onlay vs. open—sublay | 1.225 | 0.892 | 1.682 | 0.209 | ||
| Component separation vs. open—IPOM | 0.855 | 0.598 | 1.222 | 0.389 | ||
| Component separation vs. open—onlay | 1.095 | 0.710 | 1.688 | 0.681 | ||
| Drainage | < 0.001 | Yes vs. no | 1.902 | 1.483 | 2.438 | |
| Age [10-years-OR] | < 0.001 | 1.166 | 1.088 | 1.250 | ||
| Recurrent operation | 0.006 | Yes vs. no | 1.293 | 1.075 | 1.554 | |
| BMI [5-points-OR] | 0.120 | 1.056 | 0.986 | 1.130 | ||
| Risk factors | 0.296 | Yes vs. no | 1.092 | 0.926 | 1.287 | |
| EHS classification | 0.473 | Medial vs. lateral | 1.158 | 0.912 | 1.471 | 0.227 |
| Lateral vs. combined | 0.857 | 0.609 | 1.205 | 0.374 | ||
| Medial vs. combined | 0.992 | 0.755 | 1.305 | 0.956 | ||
| Gender | 0.486 | Female vs. male | 1.059 | 0.902 | 1.242 | |
| ASA | 0.851 | II vs. I | 1.092 | 0.781 | 1.527 | 0.606 |
| III/IV vs. I | 1.064 | 0.742 | 1.525 | 0.736 | ||
| III/IV vs. II | 0.974 | 0.818 | 1.161 | 0.770 | ||
| Preoperative pain | 0.900 | Yes vs. no | 0.985 | 0.830 | 1.169 | 0.860 |
| Unknown vs. no | 0.930 | 0.682 | 1.268 | 0.646 | ||
| Yes vs. unknown | 1.059 | 0.787 | 1.425 | 0.707 | ||
| Mesh | 0.998 | Yes vs. no | 1.001 | 0.600 | 1.671 |
General complications in logistic regression analyses
The general complications were significantly related to defect size, EHS classification (lateral), the need for drains (p < 0.001 each), and tendentially also BMI (p = 0.077). The risk of general complications was increased by larger defects, higher ASA score, older age, the presence of risk factors, component separation, and drain use (OR = 1421 [1209; 1670]) (Table 4).
Table 4.
Results of the multivariable analysis for general complications including odds ratios with corresponding 95% confidence interval
| Variable | p-value | Categories | Odds ratio | LCL | UCL | p-value (pair-wise) |
|---|---|---|---|---|---|---|
| Defect size | < 0.001 | III (> 10 cm) vs. I (< 4 cm) | 2.862 | 2.397 | 3.418 | < 0.001 |
| III (> 10 cm) vs. II (4–10 cm) | 1.633 | 1.449 | 1.841 | < 0.001 | ||
| II (4–10 cm) vs. I (< 4 cm) | 1.752 | 1.486 | 2.067 | < 0.001 | ||
| ASA | < 0.001 | III/IV vs. II | 1.582 | 1.409 | 1.776 | < 0.001 |
| III/IV vs. I | 1.952 | 1.478 | 2.578 | < 0.001 | ||
| II vs. I | 1.234 | 0.943 | 1.614 | 0.125 | ||
| Age [10-years-OR] | < 0.001 | 1.197 | 1.141 | 1.256 | ||
| Risk factors | < 0.001 | Yes vs. no | 1.429 | 1.279 | 1.596 | |
| Operation technique | < 0.001 | Component separation vs. open—sublay | 1.684 | 1.389 | 2.041 | < 0.001 |
| Component separation vs. open—onlay | 2.177 | 1.632 | 2.903 | < 0.001 | ||
| Component separation vs. open—IPOM | 1.581 | 1.271 | 1.968 | < 0.001 | ||
| Open—direct suture vs. component separation | 0.466 | 0.300 | 0.724 | < 0.001 | ||
| Open—IPOM vs. open—onlay | 1.376 | 1.065 | 1.778 | 0.015 | ||
| Open—onlay vs. open—sublay | 0.774 | 0.612 | 0.977 | 0.031 | ||
| Open—direct suture vs. open—IPOM | 0.737 | 0.486 | 1.117 | 0.150 | ||
| Open—direct suture vs. open—sublay | 0.785 | 0.522 | 1.179 | 0.243 | ||
| Open—IPOM vs. open—sublay | 1.065 | 0.922 | 1.230 | 0.394 | ||
| Open—direct suture vs. open—onlay | 1.014 | 0.644 | 1.598 | 0.951 | ||
| EHS classification | < 0.001 | Medial vs. lateral | 1.565 | 1.307 | 1.875 | < 0.001 |
| Lateral vs. combined | 0.619 | 0.486 | 0.789 | < 0.001 | ||
| Medial vs. combined | 0.969 | 0.809 | 1.161 | 0.736 | ||
| Drainage | < 0.001 | Yes vs. no | 1.421 | 1.209 | 1.670 | |
| BMI [5-points-OR] | 0.077 | 1.042 | 0.996 | 1.091 | ||
| Gender | 0.248 | Female vs. male | 1.065 | 0.957 | 1.186 | |
| Mesh | 0.272 | Yes vs. no | 0.817 | 0.570 | 1.172 | |
| Preoperative pain | 0.455 | Yes vs. no | 1.077 | 0.959 | 1.209 | 0.210 |
| Unknown vs. no | 1.047 | 0.852 | 1.287 | 0.664 | ||
| Yes vs. unknown | 1.029 | 0.845 | 1.253 | 0.778 | ||
| Recurrent operation | 0.704 | Yes vs. no | 1.026 | 0.900 | 1.169 |
Postoperative complications in logistic regression analyses
The occurrence of postoperative complications was significantly associated with the defect size, BMI, the presence of risk factors, surgical method, EHS classification, the use of drains, ASA classification, and age (p < 0.001 each). A larger defect, a higher BMI, the presence of at least one risk factor, component separation and open-IPOM, the use of drains (OR = 1366 [1230; 1517]), a higher ASA score, and older age increased the risk for postoperative complications (Table 5).
Table 5.
Results of the multivariable analysis for postoperative complications including odds ratios with corresponding 95% confidence interval
| Variable | p-value | Categories | Odds ratio | LCL | UCL | p-value (pair-wise) |
|---|---|---|---|---|---|---|
| Defect size | < 0.001 | III (> 10 cm) vs. I (< 4 cm) | 2.592 | 2.314 | 2.903 | < 0.001 |
| III (> 10 cm) vs. II (4–10 cm) | 1.616 | 1.489 | 1.753 | < 0.001 | ||
| II (4–10 cm) vs. I (< 4 cm) | 1.604 | 1.447 | 1.779 | < 0.001 | ||
| BMI [5-points-OR] | < 0.001 | 1.145 | 1.112 | 1.180 | ||
| Risk factors | < 0.001 | Yes vs. no | 1.364 | 1.268 | 1.468 | |
| Operation technique | < 0.001 | Component separation vs. open—IPOM | 1.788 | 1.526 | 2.094 | < 0.001 |
| Component separation vs. open—sublay | 1.496 | 1.302 | 1.718 | < 0.001 | ||
| Component separation vs. open—onlay | 1.559 | 1.294 | 1.879 | < 0.001 | ||
| Open—direct suture vs. component separation | 0.571 | 0.416 | 0.783 | < 0.001 | ||
| Open—IPOM vs. open—sublay | 0.837 | 0.757 | 0.925 | < 0.001 | ||
| Open—IPOM vs. open—onlay | 0.872 | 0.744 | 1.023 | 0.093 | ||
| Open—direct suture vs. open—sublay | 0.853 | 0.638 | 1.141 | 0.285 | ||
| Open—direct suture vs. open—onlay | 0.890 | 0.651 | 1.216 | 0.463 | ||
| Open—onlay vs. open—sublay | 0.959 | 0.835 | 1.102 | 0.558 | ||
| Open—direct suture vs. open—IPOM | 1.020 | 0.757 | 1.373 | 0.897 | ||
| EHS classification | < 0.001 | Medial vs. lateral | 1.483 | 1.321 | 1.666 | < 0.001 |
| Lateral vs. combined | 0.712 | 0.605 | 0.838 | < 0.001 | ||
| Medial vs. combined | 1.057 | 0.931 | 1.200 | 0.392 | ||
| Drainage | < 0.001 | Yes vs. no | 1.366 | 1.230 | 1.517 | |
| ASA | < 0.001 | III/IV vs. II | 1.212 | 1.121 | 1.310 | < 0.001 |
| III/IV vs. I | 1.264 | 1.080 | 1.480 | 0.004 | ||
| II vs. I | 1.043 | 0.901 | 1.208 | 0.571 | ||
| Age [10-years-OR] | < 0.001 | 1.059 | 1.028 | 1.092 | ||
| Recurrent operation | 0.164 | Yes vs. no | 1.063 | 0.975 | 1.158 | |
| Mesh | 0.265 | Yes vs. no | 1.163 | 0.892 | 1.516 | |
| Preoperative pain | 0.465 | Yes vs. no | 1.048 | 0.970 | 1.132 | 0.235 |
| Yes vs. unknown | 1.040 | 0.912 | 1.187 | 0.557 | ||
| Unknown vs. no | 1.007 | 0.878 | 1.156 | 0.919 | ||
| Gender | 0.466 | Female vs. male | 0.974 | 0.907 | 1.046 |
Complication-related reoperations in logistic regression analyses
The risk of reoperation was significantly associated with defect size, the presence of risk factors, the use of drains, EHS classification, BMI, surgical method and ASA classification (p < 0.001 each). The complication-related reoperation rate was significantly higher when drains were used (OR = 1632 [1385, 1924]). In addition, a larger defect, the presence of a risk factor, a higher BMI, component separation, and a higher ASA score also increased the risk of reoperation (Table 6).
Table 6.
Results of the multivariable analysis for complication-related reoperations including odds ratios with corresponding 95% confidence interval
| Variable | p-value | Categories | Odds ratio | LCL | UCL | p-value (pair-wise) |
|---|---|---|---|---|---|---|
| Defect size | < 0.001 | III (> 10 cm) vs. I (< 4 cm) | 2.609 | 2.201 | 3.093 | < 0.001 |
| III (> 10 cm) vs. II (4–10 cm) | 1.553 | 1.382 | 1.744 | < 0.001 | ||
| II (4–10 cm) vs. I (< 4 cm) | 1.681 | 1.436 | 1.967 | < 0.001 | ||
| Risk factors | < 0.001 | Yes vs. no | 1.394 | 1.253 | 1.551 | |
| Drainage | < 0.001 | Yes vs. no | 1.632 | 1.385 | 1.924 | |
| EHS classification | < 0.001 | Medial vs. lateral | 1.780 | 1.479 | 2.142 | < 0.001 |
| Lateral vs. combined | 0.563 | 0.441 | 0.719 | < 0.001 | ||
| Medial vs. combined | 1.003 | 0.840 | 1.197 | 0.975 | ||
| BMI [5-points-OR] | < 0.001 | 1.115 | 1.068 | 1.164 | ||
| Operation technique | < 0.001 | Component separation vs. open—IPOM | 1.774 | 1.427 | 2.205 | < 0.001 |
| Component separation vs. open—sublay | 1.587 | 1.316 | 1.914 | < 0.001 | ||
| Component separation vs. open—onlay | 1.593 | 1.228 | 2.067 | < 0.001 | ||
| Open—direct suture vs. component separation | 0.440 | 0.277 | 0.697 | < 0.001 | ||
| Open—direct suture vs. open—sublay | 0.698 | 0.454 | 1.073 | 0.101 | ||
| Open—direct suture vs. open—onlay | 0.700 | 0.441 | 1.111 | 0.131 | ||
| Open—IPOM vs. open—sublay | 0.895 | 0.773 | 1.035 | 0.135 | ||
| Open—direct suture vs. open—IPOM | 0.780 | 0.502 | 1.211 | 0.268 | ||
| Open—IPOM vs. open—onlay | 0.898 | 0.713 | 1.131 | 0.361 | ||
| Open—onlay vs. open—sublay | 0.996 | 0.815 | 1.218 | 0.970 | ||
| ASA | < 0.001 | III/IV vs. II | 1.336 | 1.193 | 1.495 | < 0.001 |
| III/IV vs. I | 1.347 | 1.066 | 1.702 | 0.013 | ||
| II vs. I | 1.009 | 0.809 | 1.258 | 0.940 | ||
| Preoperative pain | 0.105 | Yes vs. no | 1.106 | 0.987 | 1.238 | 0.082 |
| Unknown vs. no | 1.193 | 0.983 | 1.447 | 0.074 | ||
| Yes vs. unknown | 0.927 | 0.772 | 1.113 | 0.417 | ||
| Age [10-years-OR] | 0.107 | 1.037 | 0.992 | 1.084 | ||
| Gender | 0.139 | Female vs. male | 0.924 | 0.833 | 1.026 | |
| Recurrent operation | 0.194 | Yes vs. no | 1.085 | 0.959 | 1.227 | |
| Mesh | 0.812 | Yes vs. no | 1.047 | 0.717 | 1.530 |
Results of the 1-year follow-up in logistic regression analyses
The risk of recurrences at the 1-year follow-up was strongly related to previous recurrences, the surgical method (e.g. open-onlay), EHS classification, higher BMI, larger defect size (p < 0.001 each), the use of meshes (p = 0.001), the ASA score (p = 0.002), female gender (p = 0.004), higher age (p = 0.031), and preoperative pain (p = 0.050). No significant relation could be shown for the use of drains (p = 0.650) (Table 7). Pain at rest at the 1-year follow-up was significantly associated with higher age, preoperative pain, female gender, postoperative complications, EHS classification, higher BMI, prior surgeries, drain use, larger defect size, and ASA score (p = 0.001 each). The risk of pain at rest increased with drain use (OR = 1174 [1075; 1282]) (Table 8). The pain on exertion at the 1-year follow-up was significantly dependent on age, gender, preoperative pain, postoperative complications, EHS classification, defect size, BMI, use of drains, presence of recurrences (p = 0.001 each), surgical method (p = 0.001), presence of risk factors (p = 0.008) and ASA score (p = 0.020). Drain use increased the risk of pain on exertion (OR = 1.173 [1.094; 1.258]) (Table 9). Pain requiring treatment at the 1-year follow-up was significantly related to age, preoperative pain, gender, postoperative complications, EHS classification, recurrent interventions, ASA score, defect size, use of drains, presence of risk factors (p < 0.001 each), BMI (p = 0.011), and surgical method (p = 0.023). The use of drains was furthermore associated with a higher risk of pain requiring treatment (OR = 1211 [1097; 1338]) (Table 10).
Table 7.
Results of the multivariable analysis for recurrence in the follow-up including odds ratios with corresponding 95% confidence interval
| Variable | p-value | Categories | Odds ratio | LCL | UCL | p-value (pair-wise) |
|---|---|---|---|---|---|---|
| Recurrent operation | < 0.001 | Yes vs. no | 1.489 | 1.342 | 1.652 | |
| Operation technique | < 0.001 | Open—onlay vs. open—sublay | 1.609 | 1.369 | 1.891 | < 0.001 |
| Open—IPOM vs. open—sublay | 1.379 | 1.218 | 1.561 | < 0.001 | ||
| Component separation vs. open—onlay | 0.608 | 0.458 | 0.808 | < 0.001 | ||
| Open—direct suture vs. open—sublay | 1.505 | 1.120 | 2.024 | 0.007 | ||
| Component separation vs. open—IPOM | 0.710 | 0.546 | 0.922 | 0.010 | ||
| Open—direct suture vs. component separation | 1.538 | 1.055 | 2.243 | 0.025 | ||
| Open—IPOM vs. open—onlay | 0.857 | 0.715 | 1.026 | 0.094 | ||
| Open—direct suture vs. open—IPOM | 1.092 | 0.808 | 1.475 | 0.568 | ||
| Open—direct suture vs. open—onlay | 0.935 | 0.680 | 1.288 | 0.682 | ||
| Component separation vs. open—sublay | 0.979 | 0.762 | 1.256 | 0.865 | ||
| EHS classification | < 0.001 | Medial vs. lateral | 0.685 | 0.610 | 0.769 | < 0.001 |
| Lateral vs. combined | 1.305 | 1.079 | 1.579 | 0.006 | ||
| Medial vs. combined | 0.894 | 0.756 | 1.057 | 0.190 | ||
| BMI [5-points-OR] | < 0.001 | 1.097 | 1.056 | 1.140 | ||
| Defect size | < 0.001 | II (4–10 cm) vs. I (< 4 cm) | 1.298 | 1.152 | 1.462 | < 0.001 |
| III (> 10 cm) vs. I (< 4 cm) | 1.343 | 1.158 | 1.558 | < 0.001 | ||
| III (> 10 cm) vs. II (4–10 cm) | 1.035 | 0.915 | 1.171 | 0.583 | ||
| Mesh | 0.001 | Yes vs. no | 0.633 | 0.480 | 0.835 | |
| ASA | 0.002 | III/IV vs. II | 1.174 | 1.059 | 1.303 | 0.002 |
| III/IV vs. I | 1.332 | 1.107 | 1.603 | 0.002 | ||
| II vs. I | 1.134 | 0.961 | 1.338 | 0.137 | ||
| Gender | 0.004 | Female vs. male | 0.873 | 0.796 | 0.956 | |
| Age [10-years-OR] | 0.031 | 0.960 | 0.925 | 0.996 | ||
| Preoperative pain | 0.050 | Yes vs. no | 1.089 | 0.984 | 1.205 | 0.100 |
| Unknown vs. no | 1.220 | 1.031 | 1.444 | 0.020 | ||
| Yes vs. unknown | 0.892 | 0.762 | 1.045 | 0.157 | ||
| Risk factors | 0.243 | Yes vs. no | 1.059 | 0.962 | 1.165 | |
| Drainage | 0.650 | Yes vs. no | 1.027 | 0.914 | 1.155 |
Table 8.
Results of the multivariable analysis for pain at rest in the follow-up including odds ratios with corresponding 95% confidence interval
| Variable | p-value | Categories | Odds ratio | LCL | UCL | p-value (pair-wise) |
|---|---|---|---|---|---|---|
| Age [10-years-OR] | < 0.001 | 0.822 | 0.801 | 0.844 | ||
| Preoperative pain | < 0.001 | Yes vs. no | 1.682 | 1.555 | 1.820 | < 0.001 |
| Unknown vs. no | 1.413 | 1.238 | 1.613 | < 0.001 | ||
| Yes vs. unknown | 1.190 | 1.055 | 1.343 | 0.005 | ||
| Gender | < 0.001 | Female vs. male | 1.544 | 1.444 | 1.650 | |
| Postoperative complications | < 0.001 | Yes vs. no | 1.775 | 1.609 | 1.958 | |
| EHS classification | < 0.001 | Medial vs. lateral | 0.674 | 0.618 | 0.735 | < 0.001 |
| Lateral vs. combined | 1.264 | 1.099 | 1.453 | < 0.001 | ||
| Medial vs. combined | 0.852 | 0.754 | 0.962 | 0.010 | ||
| BMI [5-points-OR] | < 0.001 | 0.931 | 0.905 | 0.958 | ||
| Recurrent operation | < 0.001 | Yes vs. no | 1.180 | 1.090 | 1.277 | |
| Drainage | < 0.001 | Yes vs. no | 1.174 | 1.075 | 1.282 | |
| Defect size | < 0.001 | III (> 10 cm) vs. I (< 4 cm) | 1.227 | 1.104 | 1.363 | < 0.001 |
| II (4—10 cm) vs. I (< 4 cm) | 1.136 | 1.043 | 1.237 | 0.003 | ||
| III (> 10 cm) vs. II (4–10 cm) | 1.080 | 0.989 | 1.179 | 0.086 | ||
| ASA | < 0.001 | III/IV vs. I | 1.297 | 1.136 | 1.481 | < 0.001 |
| II vs. I | 1.215 | 1.082 | 1.365 | < 0.001 | ||
| III/IV vs. II | 1.067 | 0.988 | 1.152 | 0.099 | ||
| Mesh | 0.151 | Yes vs. no | 1.198 | 0.936 | 1.532 | |
| Risk factors | 0.165 | Yes vs. no | 1.051 | 0.980 | 1.127 | |
| Operation technique | 0.378 | Component separation vs. open—IPOM | 1.147 | 0.966 | 1.362 | 0.118 |
| Open—IPOM vs. open—onlay | 0.902 | 0.783 | 1.040 | 0.156 | ||
| Component separation vs. open—sublay | 1.117 | 0.954 | 1.307 | 0.169 | ||
| Open—onlay vs. open—sublay | 1.079 | 0.953 | 1.222 | 0.228 | ||
| Open—direct suture vs. Component separation | 0.841 | 0.623 | 1.133 | 0.255 | ||
| Open—direct suture vs. open—onlay | 0.870 | 0.658 | 1.150 | 0.328 | ||
| Open—IPOM vs. open—sublay | 0.974 | 0.888 | 1.068 | 0.573 | ||
| Open—direct suture vs. open—sublay | 0.939 | 0.724 | 1.218 | 0.634 | ||
| Component separation vs. open—onlay | 1.035 | 0.854 | 1.254 | 0.727 | ||
| Open—direct suture vs. open—IPOM | 0.964 | 0.739 | 1.257 | 0.787 |
Table 9.
Results of the multivariable analysis for pain on exertion in the follow-up including odds ratios with corresponding 95% confidence interval
| Variable | p-value | Categories | Odds ratio | LCL | UCL | p-value (pair-wise) |
|---|---|---|---|---|---|---|
| Age [10-years-OR] | < 0.001 | 0.769 | 0.753 | 0.785 | ||
| Gender | < 0.001 | Female vs. male | 1.596 | 1.514 | 1.683 | |
| Preoperative pain | < 0.001 | Yes vs. no | 1.616 | 1.521 | 1.718 | < 0.001 |
| Unknown vs. no | 1.322 | 1.191 | 1.468 | < 0.001 | ||
| Yes vs. unknown | 1.223 | 1.110 | 1.347 | < 0.001 | ||
| Postoperative complications | < 0.001 | Yes vs. no | 1.546 | 1.422 | 1.680 | |
| EHS classification | < 0.001 | Medial vs. lateral | 0.698 | 0.650 | 0.749 | < 0.001 |
| Medial vs. combined | 0.807 | 0.733 | 0.889 | < 0.001 | ||
| Lateral vs. combined | 1.156 | 1.034 | 1.293 | 0.011 | ||
| Defect size | < 0.001 | III (> 10 cm) vs. I (< 4 cm) | 1.318 | 1.211 | 1.433 | < 0.001 |
| II (4–10 cm) vs. I (< 4 cm) | 1.204 | 1.125 | 1.288 | < 0.001 | ||
| III (> 10 cm) vs. II (4–10 cm) | 1.095 | 1.020 | 1.174 | 0.012 | ||
| BMI [5-points-OR] | < 0.001 | 0.945 | 0.924 | 0.967 | ||
| Drainage | < 0.001 | Yes vs. no | 1.173 | 1.094 | 1.258 | |
| Recurrent operation | < 0.001 | Yes vs. no | 1.150 | 1.079 | 1.226 | |
| Operation technique | 0.001 | Open—onlay vs. open—sublay | 1.187 | 1.076 | 1.309 | < 0.001 |
| Open—IPOM vs. open—onlay | 0.837 | 0.748 | 0.937 | 0.002 | ||
| Open—direct suture vs. open—onlay | 0.754 | 0.606 | 0.938 | 0.011 | ||
| Component separation vs. open—sublay | 1.151 | 1.014 | 1.307 | 0.030 | ||
| Open—direct suture vs. Component separation | 0.777 | 0.614 | 0.984 | 0.036 | ||
| Component separation vs. open—IPOM | 1.159 | 1.009 | 1.331 | 0.037 | ||
| Open—direct suture vs. open—sublay | 0.895 | 0.730 | 1.097 | 0.285 | ||
| Open—direct suture vs. open—IPOM | 0.901 | 0.732 | 1.109 | 0.324 | ||
| Component separation vs. open—onlay | 0.970 | 0.831 | 1.132 | 0.698 | ||
| Open—IPOM vs. open—sublay | 0.994 | 0.923 | 1.069 | 0.862 | ||
| Risk factors | 0.008 | Yes vs. no | 1.078 | 1.019 | 1.140 | |
| ASA | 0.020 | II vs. I | 1.131 | 1.034 | 1.236 | 0.007 |
| III/IV vs. I | 1.148 | 1.035 | 1.273 | 0.009 | ||
| III/IV vs. II | 1.015 | 0.954 | 1.080 | 0.638 | ||
| Mesh | 0.175 | Yes vs. no | 1.142 | 0.943 | 1.384 |
Table 10.
Results of the multivariable analysis for pain requiring treatment in the follow-up including odds ratios with corresponding 95% confidence interval
| Variable | p-value | Categories | Odds ratio | LCL | UCL | p-value (pair-wise) |
|---|---|---|---|---|---|---|
| Age [10-years-OR] | < 0.001 | 0.787 | 0.764 | 0.810 | ||
| Preoperative pain | < 0.001 | Yes vs. no | 1.941 | 1.770 | 2.127 | < 0.001 |
| Unknown vs. no | 1.670 | 1.439 | 1.938 | < 0.001 | ||
| Yes vs. unknown | 1.162 | 1.017 | 1.327 | 0.027 | ||
| Gender | < 0.001 | Female vs. male | 1.622 | 1.504 | 1.749 | |
| Postoperative complications | < 0.001 | Yes vs. no | 1.910 | 1.718 | 2.125 | |
| EHS classification | < 0.001 | Medial vs. lateral | 0.676 | 0.613 | 0.745 | < 0.001 |
| Medial vs. combined | 0.819 | 0.717 | 0.937 | 0.004 | ||
| Lateral vs. combined | 1.212 | 1.039 | 1.415 | 0.014 | ||
| Recurrent operation | < 0.001 | Yes vs. no | 1.286 | 1.180 | 1.402 | |
| ASA | < 0.001 | III/IV vs. I | 1.493 | 1.283 | 1.736 | < 0.001 |
| II vs. I | 1.307 | 1.144 | 1.494 | < 0.001 | ||
| III/IV vs. II | 1.142 | 1.048 | 1.244 | 0.002 | ||
| Defect size | < 0.001 | III (> 10 cm) vs. I (< 4 cm) | 1.296 | 1.152 | 1.458 | < 0.001 |
| II (4–10 cm) vs. I (< 4 cm) | 1.169 | 1.061 | 1.287 | 0.002 | ||
| III (> 10 cm) vs. II (4–10 cm) | 1.109 | 1.007 | 1.223 | 0.037 | ||
| Drainage | < 0.001 | Yes vs. no | 1.211 | 1.097 | 1.338 | |
| Risk factors | < 0.001 | Yes vs. no | 1.153 | 1.067 | 1.246 | |
| BMI [5-points-OR] | 0.011 | 0.961 | 0.931 | 0.991 | ||
| Operation technique | 0.023 | Open—direct suture vs. open—onlay | 0.696 | 0.517 | 0.936 | 0.017 |
| Open—direct suture vs. component separation | 0.694 | 0.504 | 0.954 | 0.025 | ||
| Open—onlay vs. open—sublay | 1.166 | 1.017 | 1.337 | 0.028 | ||
| Open—direct suture vs. open—IPOM | 0.757 | 0.571 | 1.003 | 0.052 | ||
| Component separation vs. open—sublay | 1.169 | 0.983 | 1.390 | 0.077 | ||
| Open—direct suture vs. open—sublay | 0.811 | 0.616 | 1.069 | 0.137 | ||
| Open—IPOM vs. open—sublay | 1.072 | 0.968 | 1.187 | 0.184 | ||
| Open—IPOM vs. open—onlay | 0.919 | 0.786 | 1.074 | 0.289 | ||
| Component separation vs. open—IPOM | 1.091 | 0.904 | 1.317 | 0.365 | ||
| Component separation vs. open—onlay | 1.003 | 0.812 | 1.238 | 0.980 | ||
| Mesh | 0.388 | Yes vs. no | 0.893 | 0.691 | 1.154 |
Standardized differences for patients with and without follow-up
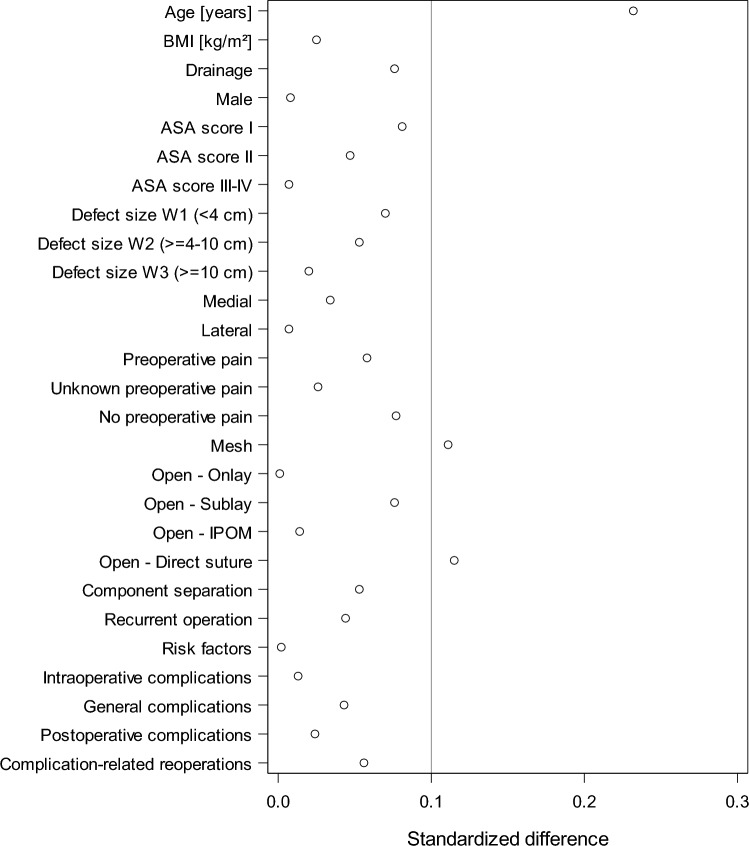
The results of the standardized differences for patients with (n = 39,523) and without (n = 22,361) follow-up verified that there was no bias in the patient selection of the analysis population. Patients in the analysis population were on average 3.3 years older, received more often a mesh and were less frequently operated with direct sutures. The standardized difference was above the reference value of 10%. For all other variables, including the complication rates, standardized differences of less than 0.1 were found, thus indicating no bias in patient selection.
Discussion
Should surgeons in case of doubt use drains or not in elective open incisional hernia surgery? It is beyond dispute that surgical drains help to remove access fluid which is assumed to reduce wound-related complications and seroma formation, but these advantages may nevertheless be counterbalanced with certain downsides like an increased risk of infections and postoperative pain. To shed more light on this question, we performed a Herniamed registry-based evaluation of prospectively collected data of 39,523 patients which is so far the most comprehensive quality assurance study in Germany. The influence of drains on the outcome of hernia operations has already been examined in several controlled randomized trials and meta-analyses in the past [10, 11]. A registry analysis, however, enables an analysis of the clinical results as part of health services research and points out possible differences between the current status of research and patient care in clinical routine. This analysis of a large clinical data basis is thus an important contribution to understand the “real world” effect of a treatment outside the tightly controlled environment of randomized trials [8].
Our investigations covered the period from 2009 to 2020 with 39,523 elective open incisional hernia operations, during which 28,182 patients (71.31%) received a drain. The high frequency of drain use in more than 2/3 of the patient collective clearly mirrors the high acceptance of drainages in clinical routine. The unadjusted analysis of the relationship between drain use, patient variables, and operation characteristic shows that the expression of almost all features differed significantly. Only with respect to the gender, no difference was observed. Drained patients had a significantly higher age (63.6 vs. 59.8, p < 0.001), higher BMI (29.8 vs. 27.9, p < 0.001), higher ASA score (p < 0.001), larger hernia defects (p < 0.001), and required significantly more frequently mesh application (92.9% vs. 64.6%, p < 0.001). All in all, the clinical care situation in the drainage group shows a negative selection with regard to patient and hernia characteristics. The use of drains is typically linked with the more complex operations. In component separation, 89.8% of patients received drain, in open sublay 83.5% and in open onlay 81.8%.
Most studies analysing the influence of drains investigated similar outcome criteria like local complications, particularly bleeding and seroma formation, surgical site infections (SSI), surgical site occurrences (SSO) and surgical site occurrences requiring procedural interventions (SSOPI) [3, 10, 12, 13]. All of these studies point to the fact that single influencing factors are difficult to extract since complications in elective open incisional hernia surgery are caused by numerous parameters, which was also the case in our study. We carried out eight multivariable analyses (intraoperative complications, general complication, postoperative complications, complication-related reoperations, recurrence on 1-year follow-up, pain on exertion at 1-year follow-up, pain on rest at 1-year follow-up, pain requiring treatment at 1-year follow-up). With the exception of recurrences in the follow-up, the use of drains was in each case associated with a significantly higher incidence of complications and higher pain rates. The multivariable analyses also showed a significant association of defect size, ASA and EHS classification in all cases, and for the items operation technique and age in seven of the eight analyses performed.
Placing the focus on subject-specific criteria for drain use such as the influence of local complications, the data situation remains quite heterogeneous in the literature and reveals no clear evidence of a protective effect of drains on seroma formation. Miller et al. compared the outcome of 580 patients each with or without drainage, similar hernia size and robotic surgery with respect to seroma formation at 30 days and found a significantly decreased postoperative seroma occurrence of 3.8% in the group with drainage vs. 15.2% in the group without (p < 0.0001) [12]. No significant difference with respect to the use of drains observed Westphalen et al. [11] who assessed the seroma frequency in 21 patients per group with non-significant hernia defect size difference and the exclusion of ASA III–IV patients at three different postoperative ultrasound (US) time points and with seroma frequencies between 19.0 and 52.4% with drain vs. 28.6–57.1% without drain (p = 0.469 for early postoperative US; p = 0.852 for late US). In a RCT by Willemin et al. [10], fluid collection at 30 days was reported in 60.3% of the drain group patients vs. 62.0% (p = 0.844) without drain after open mesh repair, indicating that drains failed to reduce the rate of postoperative fluid collections that might contribute to seroma formation. In our analysis of the clinical care situation with negative selection of the patient population and hernia characteristics as well as more complex hernia surgeries, we observed significantly more seromas when drains were used, even though the rate of seroma formation was generally low (4.8 vs. 2.2% without drain, p < 0.001).
In addition to SSOs like seroma formation, also the effect of drain use on SSIs and SSOPI was investigated as decisive factor. Several studies suggest that the use of drains increases the risk of SSIs, while others found no significant difference in infection rates with or without drains. This became particularly evident in data of the Americas Hernia Society Quality Collaborative [12, 13] and in a recent RCS reporting comparable site infection rates in both groups [10]. Westphalen et al. reported no significant difference with or without drain use concerning surgical wound infections [11]. Even in the most recent literature, the data situation shows a heterogeneous picture. In a meta-analysis of ventral hernia repair by Mohamedahmed et al. (2023), drained patient groups had higher SSI rates and longer total operation times in eight studies involving 2568 patients, but no significant advantage was seen in terms of wound-related complications [14]. Marcolin et al. (2023) published a meta-analysis for retromuscular ventral hernia repair with four studies involving 1,724 patients and found no differences in SSI, hematoma, SSO, or SSO-requiring procedural intervention, but the group with drain placement had significantly fewer seromas [15]. Our evaluation of the care situation, however, revealed a significant difference in the patient group with drain vs. without concerning SSI (1.7% vs. 0.7%, p < 0.001) and SSO (3.2% vs. 1.3%, p < 0.001). In addition, the complication-related reoperation rate was significantly increased when drains were used (OR = 1632 [1385; 1924]).
Relationships not evaluated in our analysis are the influence of the time point of drain removal or the prolonged prophylactic use of antibiotics on the SSI and SSO. Plymate et al. showed a linear, non-significant increase of SSO depending on the drain duration [16]. Only a BMI of > 35 represented a predictor of wound occurrence in their study. Other authors found only little persuasive evidence for a prolonged antibiotic use to reduce SSI and SSO [17, 18].
Drains were used in 71.3% of elective open incisional hernia operations between 1/2009 and 12/2020 which indicates a high level of acceptance in the clinical care situation. We assume that a less favourable risk profile of patient and hernias characteristics leads to a negative selection when drains are used. In the following, a significant association with a higher risk of complications and pain is observed for all target parameters with the exception of recurrences. Similar results were also reported in a registry-based multivariable analysis by Schaaf et al. who observed more intraoperative complications, general complications, and complication-related reoperations in patients with drains. In their study, also larger defect size and BMI were unfavourably associated with postoperative complications, recurrences and pain [19]. From a clinical point of view, it is difficult to extract the separate effect of drainages on the complications, as the multivariable analyses showed that these were significantly influenced in all outcome measures by numerous other variables such as defect size, ASA classification, and EHS classification. Apparent in our analysis became however that the use of drains is significantly associated with a higher occurrence of SSI and SSO in the clinical routine, especially if patients with higher BMI and larger defects are concerned.
Taken together, drains are currently used in over 70% of elective open incisional hernia surgeries, based on various criteria such as the complexity of the procedures, hernia characteristics, or patient constitution. Despite adjusting for other influencing variables in the model (independent of patient and surgical characteristics), we observed a significant association between outcomes and drain usage. The poorer patient outcomes are associated with the use of drains, regardless of other factors in the model such as patient or surgical characteristics. However, the use of drains may serve as a surrogate parameter for other unobserved confounding factors. These results should prompt a re-evaluation of the predominantly "traditional" use of drainage and encourage careful case-by-case assessment. Further investigations are required as the data situation still remains heterogeneous.
Limitations
Our study has a number of important strengths. The data used in this article are the largest quality-assured data pool in Germany, Austria and Switzerland covering the period from 2009 to 2020; the statistical power to detect changes is thus high. In general, it should be noted that effects that have been proven to be significant do not necessarily have to be also clinically relevant, since even very small differences can be statistically significant due to relatively large number of cases. A limitation of this study is the rate of missing follow-up examinations. In accordance with the selection criteria of the Herniamed registry (see flowchart in Fig. 1), patients with non-incisional hernias, entry-state-key incomplete, operations performed using laparoscopic technique, patients under 16 years of age, emergency operations, patients with physiomesh or operation dates after December 31, 2020, and patients without 1-year follow-up were excluded. The lack of follow-ups (drop out) for a relevant proportion is another limitation of the registry, but the subgroup analysis does not show any selection bias (Fig. 2).
Fig. 2.
Standardized differences for patients with and without follow-up
Our analysis was a project in clinical health services research.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Conflict of interest
Dr. Köckerling reports grants to fund Herniamed from Johnson&Johnson, Norderstedt, Karl Storz, Tuttlingen, MenkeMed, Munich, and DB Karlsruhe, as well as personal fees from DB Karlsruhe. All other authors have nothing to disclose.
Ethical approval
Only cases of routine hernia surgery were documented in the Herniamed Registry and all patients have signed a special informed consent declaration agreeing to participate. The Herniamed Registry has ethical approval (BASEC Nr. 2016 – 00123; 287/2017 BO2; F-2022–111).
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
All patients with routine hernia surgery documented in the Herniamed Registry have signed an informed consent declaration agreeing to participate.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Samaiya A (2015) To drain or not to drain after colorectal surgery. Indian J Surg 77(3):1363–1368. 10.1007/s12262-015-1259-y 10.1007/s12262-015-1259-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dougherty SH, Simmons RL (1992) The biology and practice of surgical drains. Part II Curr Probl Surg 29:633–730. 10.1016/0011-3840(92)90028-2 10.1016/0011-3840(92)90028-2 [DOI] [PubMed] [Google Scholar]
- 3.Wouters D, Cavallaro G, Jensen KK et al (2022) The European hernia society prehabilitation project: a systematic review of intra-operative prevention strategies for surgical site occurrences in ventral hernia surgery. Front Surg 9:847279. 10.3389/fsurg.2022.847279 10.3389/fsurg.2022.847279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramshaw B, Dean J, Forman B et al (2016) Can abdominal wall reconstruction be safely performed without drains? Am Surg 82(8):707–712. 10.1177/000313481608200829 10.1177/000313481608200829 [DOI] [PubMed] [Google Scholar]
- 5.Schwenk W (2022) Optimized perioperative management (fast-track, ERAS) to enhance postoperative recovery in elective colorectal surgery. GMS Hyg Infect Control. 10.3205/dgkh000413 10.3205/dgkh000413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bardram L, Funch-Jensen P, Jensen P et al (1995) Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet 345(8952):763–764. 10.1016/s0140-6736(95)90643-6 10.1016/s0140-6736(95)90643-6 [DOI] [PubMed] [Google Scholar]
- 7.Fearon KC, Ljungqvist O, Von Meyenfeldt M et al (2005) Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 24(3):466–477. 10.1016/j.clnu.2005.02.002 10.1016/j.clnu.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 8.Sahm M, Pross M, Otto R et al (2015) Clinical health service research on the surgical therapy of acute appendicitis: comparison of outcomes based on 3 german multicenter quality assurance studies over 21 years. Ann Surg 262(2):338–346. 10.1097/SLA.0000000000001115 10.1097/SLA.0000000000001115 [DOI] [PubMed] [Google Scholar]
- 9.Köckerling F, Heine T, Ado D et al (2021) Trends in emergent groin hernia repair-an analysis from the herniamed registry. Front Surg 8:655755. 10.3389/fsurg.2021.655755 10.3389/fsurg.2021.655755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willemin M, Schaffer C, Kefleyesus A et al (2022) Drain versus no drain in open mesh repair for incisional hernia, results of a prospective randomized controlled trial. World J Surg. 10.1007/s00268-022-06725-4 10.1007/s00268-022-06725-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westphalen AP, Araújo AC, Zacharias P (2015) Repair of large incisional hernias. To drain or not to drain. Randomized clinical trial. Acta Cir Bras 30(12):844–851. 10.1590/S0102-865020150120000009 10.1590/S0102-865020150120000009 [DOI] [PubMed] [Google Scholar]
- 12.Miller BT, Tamer R, Petro CC et al (2022) Retromuscular drain versus no drain in robotic retromuscular ventral hernia repair: a propensity score-matched analysis of the abdominal core health quality collaborative. Hernia. 10.1007/s10029-022-02696-6 10.1007/s10029-022-02696-6 [DOI] [PubMed] [Google Scholar]
- 13.Krpata DM, Prabhu AS, Carbonell AM et al (2017) Drain placement does not increase infectious complications after retromuscular ventral hernia repair with synthetic mesh: an AHSQC analysis. J Gastrointest Surg 21(12):2083–2089. 10.1007/s11605-017-3601-0 10.1007/s11605-017-3601-0 [DOI] [PubMed] [Google Scholar]
- 14.Mohamedahmed AYY, Zaman S, Ghassemi N et al (2023) Should routine surgical wound drainage after ventral hernia repair be avoided? A systematic review and meta-analysis. Hernia. 10.1007/s10029-023-02804-0 10.1007/s10029-023-02804-0 [DOI] [PubMed] [Google Scholar]
- 15.Marcolin P, de Figueiredo SMP, Constante MM et al (2023) Drain placement in retromuscular ventral hernia repair: a systematic review and meta-analysis. Hernia 27(3):519–526. 10.1007/s10029-023-02792-1 10.1007/s10029-023-02792-1 [DOI] [PubMed] [Google Scholar]
- 16.Plymale MA, Harris JW, Davenport DL et al (2016) Abdominal wall reconstruction: the uncertainty of the impact of drain duration upon outcomes. Am Surg 82(3):207–211. 10.1177/000313481608200312 10.1177/000313481608200312 [DOI] [PubMed] [Google Scholar]
- 17.Wong A, Lee S, Nathan NS et al (2016) Postoperative prophylactic antibiotic use following ventral hernia repair with placement of surgical drains reduces the postoperative surgical-site infection rate. Plast Reconstr Surg 137(1):285–294. 10.1097/PRS.0000000000001925 10.1097/PRS.0000000000001925 [DOI] [PubMed] [Google Scholar]
- 18.Weiss E, McClelland P, Krupp J et al (2019) Use of prolonged prophylactic antibiotics with closed suction drains in ventral abdominal hernia repair. Am Surg 85(4):403–408 10.1177/000313481908500432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaaf S, Willms A, Adolf D et al (2022) What are the influencing factors on the outcome in lateral incisional hernia repair? A registry-based multivariable analysis. Hernia. 10.1007/s10029-022-02690-y 10.1007/s10029-022-02690-y [DOI] [PMC free article] [PubMed] [Google Scholar]