Abstract
Over the past decade, the emergence of patient-derived tumor organoids (PDTOs) has broadened the repertoire of preclinical models and progressively revolutionized three-dimensional cell culture in oncology. PDTO can be grown from patient tumor samples with high efficiency and faithfully recapitulates the histological and molecular characteristics of the original tumor. Therefore, PDTOs can serve as invaluable tools in oncology research, and their translation to clinical practice is exciting for the future of precision medicine in oncology. In this review, we provide an overview of methods for establishing PDTOs and their various applications in cancer research, starting with basic research and ending with the identification of new targets and preclinical validation of new anticancer compounds and precision medicine. Finally, we highlight the challenges associated with the clinical implementation of PDTO, such as its representativeness, success rate, assay speed, and lack of a tumor microenvironment. Technological developments and autologous cocultures of PDTOs and stromal cells are currently ongoing to meet these challenges and optimally exploit the full potential of these models. The use of PDTOs as standard tools in clinical oncology could lead to a new era of precision oncology in the coming decade.
Subject terms: Cancer models, Tumour biomarkers, Cell biology
Patient-Derived Tumor Organoids: Revolutionizing Oncology Research and Precision Medicine
The shift from 2D to 3D cell cultures has greatly improved cancer research, providing a more realistic model of tumors. Patient-Derived Tumor Organoids (PDTOs) have become a key tool in cancer research, allowing scientists to grow efficiently tumor cells from patient samples in a 3D environment that closely mirrors the original tumor. PDTOs are a major step forward in cancer research, bridging the gap between traditional cell cultures and clinical realities, with the potential for successful clinical applications despite some challenges that could be overcome by technological developments. Thus, they offer a promising platform for understanding cancer, testing drug responses, and developing personalized treatments, with the potential to greatly impact future patient care.
This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
Background
Models in oncology: from 2D to 3D culture
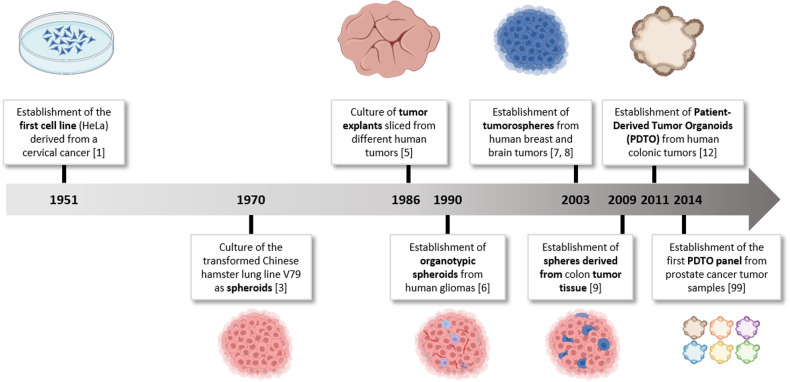
Since the establishment of the first cell line (HeLa) from a cervical cancer sample in 19511, cell lines grown in monolayer cultures have served as tools to advance the understanding of cancer biology and to develop new treatments (Fig. 1). Although their ability to accurately mimic pathology is debated, they are still widely used in research laboratories. However, it is acknowledged that their genetic drift over time often prevents them from fully simulating real human tumors2. Their ability to mimic cellular interactions and the various gradients observed in vivo (such as oxygen, nutrients, and metabolites) are also compromised, ultimately affecting important cellular processes such as intracellular signaling pathway activation, adhesion, mechanotransduction, proliferation, and response to anticancer treatments, which does not consistently reflect the physiological reality of cancer tissue.
Fig. 1. Timeline of the development of tumor cell models in oncology (created with BioRender.com).
Adapted from199.
In this context, scientists have sought to maintain or recreate tumor complexity through various three-dimensional (3D) cell culture approaches. The spheroid model was proposed in the early 1970s by radiobiologists3. These highly compact spherical structures can reach a size of over 1 mm and are primarily obtained from immortalized cell lines, thus preventing tumor cell adhesion to the culture surface by using various methods (such as rotational culture systems and use of antiadhesive substrates, among other methods) to allow for cell aggregation4. Unfortunately, these cell lines acquire irrelevant mutations over time that do not reflect the biological characteristics of the original tissue.
Other 3D tumor cell culture approaches have subsequently emerged, including tumor explants obtained from slices of cancer tissue5, organotypic spheroids from patients’ tumor fragments cultured under nonadherent conditions6, tumorospheres generated from self-renewing tumor-initiating cells7,8, and tumor spheres from partially dissociated tumor tissue9. However, these models have limitations (such as limited culture maintenance, lack of proliferation, and low establishment success rates), thus explaining their disparate use in laboratories worldwide.
Over the past decade, the emergence of patient-derived tumor organoids (PDTOs) has progressively revolutionized 3D culture in oncology. Originally, culture conditions were optimized to allow for adult “normal” stem cells, which have self-renewal and differentiation properties, to self-organize in 3D and to reproduce the microanatomy and some functions of their original organ in vitro. The self-renewal capabilities of stem cells ensure the viability of the culture.
In 2009, the laboratory of Hans Clevers (Hubrecht Institute, Netherlands), a pioneer in this field, demonstrated that a single adult intestinal stem cell expressing the LGR5 receptor, which was isolated from mice, could reform in culture and exhibit a structure and cellular diversity that are similar to the crypts and villi of the intestinal epithelium10. These principles have since been adapted to many organs11 and to the culture of PDTO, initially based on digestive origin12 and subsequently from other cancerous locations13. Thus, PDTOs derived from various cancers, such as colorectal, lung, pancreatic, breast, ovarian, and prostate cancers, have been established by various teams (Table 1).
Table 1.
Tumor types for which PDTOs have been established.
| Tumor location | Histological type | Establishment rate (%) | Number of PDTO lines generated | Source of samples | References | |
|---|---|---|---|---|---|---|
| Digestive | Appendix | High Grade and Low Grade appendiceal primary | 73.1 | 19 | S | 129 |
| High Grade and Low Grade appendiceal primary | 75 | 9 | S | 165 | ||
| Biliary Tract | IHC, gall bladder cancer, and NE carcinoma of the ampulla of Vater | 33.3 | 6 | S | 166 | |
| EHC, gall bladder cancer | 85.7 | 6 | S | 167 | ||
| Colorectal | UN | 90 | 22 | S | 18 | |
| UN | 60 | 46 | S | 168 | ||
| ADK, NE | 100 | 55 | S and biopsy | 32 | ||
| ADK | 77 | 55 | S and biopsy | 43 | ||
| UN | 76 | 13 | S (liver metastasis) | 169 | ||
| ADK | 68 | 19 | Ascite (mestastasis) | 170 | ||
| Gastric | Various | > 50 | 46 | S | 70 | |
| Various | 76.60 | 44 | S, biopsy and ascite | 171 | ||
| ADK | 92 | 11 | Ascite (metastasis) | 172 | ||
| Gastroenteropancréatic | Neuroendocrine | 64.1 | 25 | S and biopsy | 173 | |
| Neuroendocrine | 88.9 | 8 | S | 174 | ||
| Neuroendocrine | 16 | 5 | S | 175 | ||
| Liver | HCC, CC | 47 | 8 | S | 19 | |
| HCC | 26 | 10 | Biopsy | 176 | ||
| Pancreas | ADK | 75 | 103 | S and biopsy | 100 | |
| ADK | 62 | 52 | S and biopsy | 68 | ||
| IPMN | 81 | 13 | S | 177 | ||
| Gynecologic | Breast | IDC and ILC | >80 | 95 | S | 71 |
| IDC and ILC | 87.5 | UN | S and biopsy | 178 | ||
| Endometrium | Endometrioid Carcinoma | 100 | 4 | S | 179 | |
| Endometrioid, Clear Cell and Serous Carcinoma | 40 | 16 | S | 180 | ||
| Ovary | Various | 83 | 5 | S | 179 | |
| Various | 65 | 56 | S and biopsy | 22 | ||
| Head and Neck | Head and Neck | SCC | 65 | 26 | S | 93 |
| SCC | 30.2 | 13 | S | 181 | ||
| Oropharyngeal and esophagus | SCC | 71.4 | 15 | Biopsy | 182 | |
| SCC | 80 | 25 | Biopsy | 183 | ||
| ADK | 31 | 10 | S | 184 | ||
| Salivary gland | Various | 84 | 24 | S | 185 | |
| Various | 19 | 7 | S and biopsy | 186 | ||
| Thyroid | Papillary carcinoma | 7 | UN | S | 187 | |
| Papillary carcinoma | 77.6 | 38 | S | 188 | ||
| Urologic | Bladder | Urothelial carcinoma | 70 | 12 | Biopsy | 20 |
| Urothelial carcinoma | 82 | 9 | S and ascites | 189 | ||
| Kidney | Clear Cell Renal Cell Carcinoma | 74 | 25 | S | 190 | |
| Clear Cell, Papillary and Chromophobe Renal Carcinoma | 76.7 | 33 | S | 191 | ||
| Prostate | Adenocarcinoma | 15-20 | 6 | Biopsy (metastasis) | 101 | |
| NE | 16 | 4 | Biopsy (metastasis) | 192 | ||
| Others | Brain | Glioblastoma | 91.4 | 53 | S | 38 |
| Glioblastoma | 31.25 | 10 | S | 193 | ||
| Lung | NSCLC | 94 | 18 | S | 194 | |
| NSCLC and Small Cell Carcinoma | 55.5 | 20 | S and biopsy | 102 | ||
| Peritoneal | Mesothelioma | 100 | 2 | S | 195 | |
| Mesothelioma | 85.7 | 7 | S and biopsy | 196 | ||
| Skin | Melanoma | 90 | 9 | S | 128 | |
| Melanoma | 73 | 22 | S | 197 | ||
| Oral mucosal melanoma | 64 | 30 | S | 198 | ||
ADK Adenocarcninoma, CC Cholangiocarcinoma, EHC Extrahepatic Cholangiocarcinoma, HCC Hepatocellular carcinoma, IDC Invasive Ductal Carninoma, IHC Intrahepatic Cholangiocarcinoma, ILC Invasive Lobular Carcinoma, NE Neuroendocrine, NK Not Known, NSCLC Non Small Cell Carnicoma, SCC Squamous Cell Carcinoma, S Surgical specimen
This review provides an overview of the various aspects of PDTO production, their use and relevance for research and/or care in oncology, and the associated challenges.
Origin of the PDTO and methods of establishment
Patient sample type
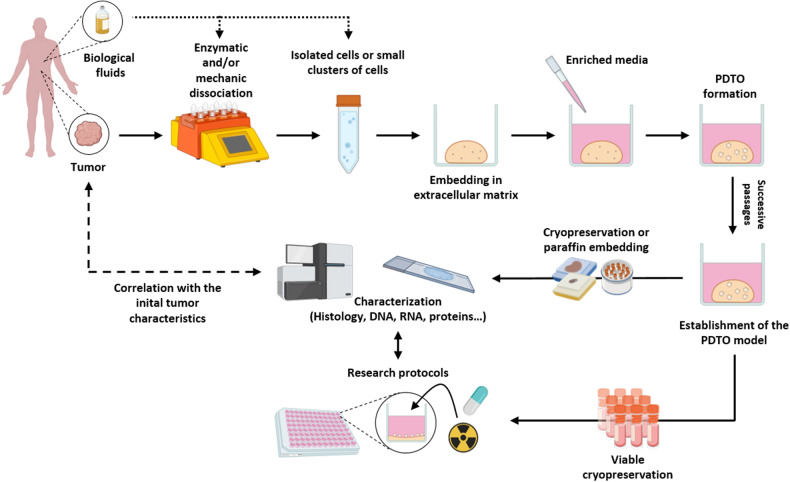
PDTOs are generated by culturing tumor cells from patient biopsies, surgical specimens, or biological fluids such as ascites and blood14,15. In most cases, obtaining PDTOs from cancer tissues involves an initial step of mechanical and/or enzymatic dissociation, thus resulting in a suspension of isolated cells or small aggregates. The cells are then embedded in an extracellular matrix (ECM) dome and cultured in specific enriched media (Fig. 2) by using the submerged culture method12. PDTO can also be obtained by introducing tumorigenic alterations via genetic engineering16 in pluripotent stem cells, induced or embryonic stem cells, tissue-specific stem cells (adult stem cells), or normal organoids17.
Fig. 2. Procedure for the generation of PDTOs.
Schematic representation of the various stages in the production of tumor organoids for research purposes (created with BioRender.com). Adapted from199.
PDTO and tumor of origin
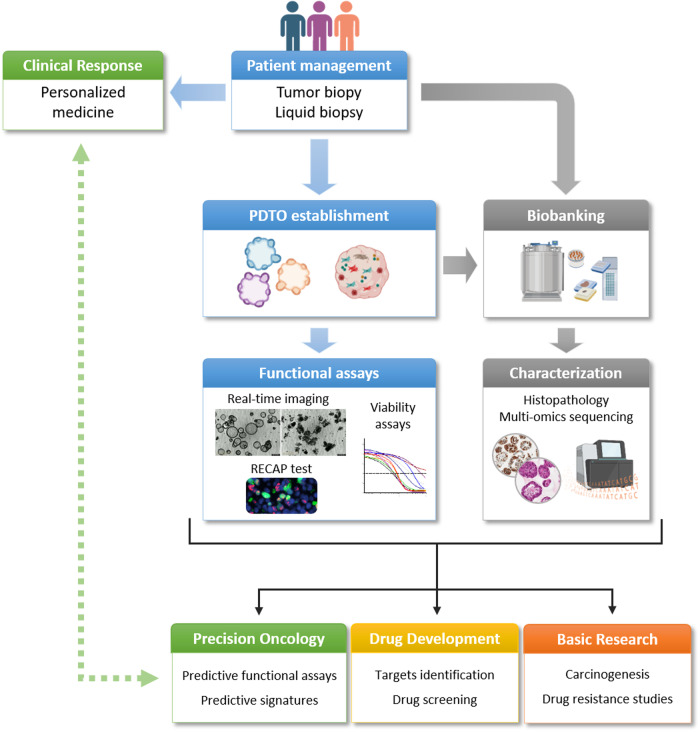
PDTOs have the advantage of being very similar to the tumor from which they are derived. For instance, PDTOs and tumor are comparable in terms of histology18 and genetics19 and display clonal evolution in culture20. However, principal component analysis (PCA) of transcriptomic data demonstrated that the parental tumors were grouped together and away from PDTOs from bladder cancer20, in contrast to PDX models, which are able to match with their tumor of origin21. These inconsistencies are mainly explained by the rapid growth of PDTOs in culture, as well as their lack of stromal components20. Overall, this resemblance remains relatively stable over time compared with that of cell lines22. Therefore, they are suitable for research and for predictive purposes in the context of precision medicine (Fig. 3). However, like any tumor sample harvested for diagnostic or predictive purposes, PDTOs represent only the tumor fraction from which they originate. Therefore, although the heterogeneity of the sample fragment is well preserved during the establishment of PDTOs (especially when they are truly generated from single cells representing the polyclonal nature of tumors in general23), other molecular characteristics that are present in another part of the tumor may be lost, thus emphasizing the importance of the quality of sampling during this process.
Fig. 3.
Contribution of PDTO to the fields of basic research and precision oncology (created with BioRender.com).
Extracellular matrix
The ECM in which PDTOs are cultured provides an essential 3D microenvironment for their growth and self-organization. The most commonly used commercial ECMs are natural hydrogels derived from decellularized murine chondrosarcomas (Engelbreth-Holm-Swarm)24 with or without growth factors (Matrigel, BME). These hydrogels are primarily composed of laminin and collagen IV. However, these ECMs have many drawbacks, including significant interbatch variability that can affect repeatability and their animal origin, which may hinder their use in clinical settings. Additionally, their composition and their subsequent mechanical and chemical properties are not finely regulated, thus preventing the reproduction of topographical constraints specific to particular organs. Therefore, numerous natural and synthetic alternatives have been developed. Natural hydrogels include pure collagen hydrogels25 that may be mixed with other proteins, such as laminin, fibronectin, or hyaluronic acid26, as well as alginate hydrogels extracted from brown algae27. Protocols to obtain hydrogels from decellularized tissues that offer the biochemical properties of the original tissue have also been approved by the Food and Drug Administration (FDA) and have been proven to be effective for organoid culture28. Some laboratories have chosen to finely regulate the composition of their ECMs and have created synthetic hydrogels. The most commonly used polymers for these synthetic ECMs are polyethylene glycol (PEG)29 or poly(lactic-co-glycolic acid) (PLGA)30. Each of these hydrogels has advantages and disadvantages, and their use depends on the type of PDTO being cultured and the tissue of origin.
Growth medium
The culture medium is supplemented with growth factors and signaling pathway inhibitors, the nature of which varies depending on the tissue’s origin to facilitate PDTO development14. Examples of routinely used media components are presented in Table 2. Two signaling pathways are essential for the growth of most types of PDTOs: activation of the EGFR pathway, which promotes cancer cell proliferation and requires supplementation with EGF in the culture medium, and stimulation of the Wnt pathway, which requires the addition of agonists (R-Spondin and Wnt3a) for LGR and Frizzled receptors, as well as their coreceptor LRP. This pathway is involved in controlling various processes, such as proliferation, adhesion, and cell differentiation, via stabilization of the β-catenin transcriptional co-factor15,31. However, although they are essential for the growth of colon organoids derived from healthy tissue12, in most colorectal cancer cells, the activation of mutations in the Wnt pathway eliminate the need to add Wnt and R-Spondin growth factors to the medium. Similarly, tumors with mutations in the EGF receptor signaling pathway are cultured in the absence of EGF32–34. Therefore, the choice of components for PDTO culture media depends on several established protocols, although additional experiments are needed to identify the optimal composition for each histological subtype of cancer.
Table 2.
Main components of the media allowing for PDTO culture.
| Tumor location | Reference | Medium Supplement | Antibiotics | Wnt pathway activator | Antioxydant | Precursor of NAD and NADP | EGFR activator | TGFß inhibitor | FGFR activator | CCK2 receptor agonist | BMPs inhibitor | ROCK Inhibitor | p38 MAPK inhibitor | Other | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Digestive | Appendix | 129 | FBS | P/S | − | − | − | − | − | − | − | − | − | − | − |
| Biliary Tract | 166 | B27, N2 | − | RSPO | NAC | Nicotinamide | EGF | A83-01 | − | Gastrin | − | Y-27632 | − | Forsokolin | |
| 167 | B27, N2 | P/S | Wnt3a, RSPO | − | − | EGF | A83-01 | FGF10 | Gastrin | Noggin | Y-27632 | − | − | ||
| Colorectal | 32 | B27 | P/S | Wnt3a, RSPO | NAC | − | EGF | A83-01 | – | Gastrin | Noggin | – | SB202190 | – | |
| 18 | B27 | Primocin | Wnt3a, RSPO | NAC | Nicotinamide | EGF | A83-01 | – | Gastrin | Noggin | Y-27632 | SB202190 | PGE2 | ||
| 168 | B27 | P/S | – | NAC | – | EGF | A83-01 | – | Gastrin | – | Y-27632 | SB202190 | – | ||
| 43 | B27, N2 | ATB-ATM | Wnt3a, RSPO | NAC | Nicotinamide | EGF | A83-01 | – | Gastrin | Noggin | – | SB202190 | – | ||
| 169 | – | P/S | Wnt3a, RSPO | – | – | EGF | - | FGF10, FGF2 | – | Noggin | Y-27632 | – | IGF-1, Endothelin 3 | ||
| 170 | B27 | ATB-ATM, Gentamicin | – | – | – | EGF | A83-01 | – | Gastrin | – | Y-27632 | SB202190 | SB431542 | ||
| Gastric | 70 | B27 | P/S, primocin | Wnt3a, RSPO | NAC | – | EGF | A83-01 | FGF10 | Gastrin | Noggin | Y-27632 | – | – | |
| 171 | B27 | P/S | Wnt3a, RSPO | NAC | – | EGF | A83-01 | FGF10 | Gastrin | Noggin | – | – | – | ||
| 172 | B27 | P/S, primocin | Wnt3a, RSPO | NAC | Nicotinamide | EGF | A83-01 | FGF10 | Gastrin | Noggin | Y-27632 | – | – | ||
| Liver | 176 | B27, N2 | – | Wnt3a, RSPO | NAC | Nicotinamide | EGF | A83-01 | FGF10 | Gastrin | – | – | – | HGF, Forsokolin | |
| 19 | B27, N2 | P/S | Wnt3a, RSPO | NAC | Nicotinamide | EGF | A83-01 | FGF10 | Gastrin | Noggin | Y-27632 | – | DXM, HGF, Forsokolin | ||
| Pancreas | 68 | B27 | P/S, primocin | Wnt3a, RSPO | NAC | Nicotinamide | EGF | A83-01 | FGF10 | Gastrin | Noggin | Y-27632 | – | PGE2 | |
| 100 | B27 | – | Wnt3a, RSPO | NAC | Nicotinamide | EGF | A83-01 | FGF10 | Gastrin | Noggin | Y-27632 | – | PGE2 | ||
| 177 | B27 | P/S, primocin | Wnt3a, RSPO | NAC | Nicotinamide | EGF | A83-01 | FGF10 | Gastrin | Noggin | – | – | – | ||
| Gynecologic | Breast | 71 | B27 | P/S, primocin | RSPO | NAC | Nicotinamide | – | A83-01 | FGF10, FGF7 | – | Noggin | Y-27632 | SB202190 | Neuregulin 1 |
| Endometrium | 179 | – | P/S, Amphotericin B | RSPO | – | – | EGF | – | – | – | Noggin | Y-27632 | – | Jagged-1 | |
| Ovary | 22 | B27 | Primocin | Wnt3a, RSPO | NAC | Nicotinamide | EGF | A83-01 | FGF10 | – | Noggin | Y-27632 | – | HCT, Neuregulin 1, Forsokolin | |
| Head and Neck | Head and Neck | 93 | B27 | P/S, primocin | RSPO | NAC | Nicotinamide | EGF | A83-01 | FGF10, FGF2 | – | Noggin | Y-27634 | – | PGE2, CHIR, Forsokolin |
| Oropharyngeal and esophagus | 182 | B27, N2 | – | Wnt3a, RSPO | NAC | Nicotinamide | EGF | A83-01 | – | Gastrin | Noggin | Y-27633 | SB202190 | – | |
| Salivary gland | 185 | B27 | P/S | RSPO | NAC | – | EGF | A83-01 | FGF10 | – | Noggin | Y-27635 | – | DXM, CHIR | |
| 186 | B27 | Primocin | RSPO | – | – | EGF | A83-01 | – | – | Noggin | Y-27636 | – | CHIR, R1881 | ||
| Thyroid | 187 | B27 | P/S | Wnt3a | – | Nicotinamide | EGF | A83-01 | FGF2 | – | Noggin | Y-27637 | – | VEGF-121 | |
| Urologic | Bladder | 20 | FBS | Primocin | – | – | – | – | – | – | – | – | Y-27632 | – | – |
| Kidney | 190 | B27 | P/S | RSPO | NAC | Nicotinamide | EGF | A83-01 | FGF10, FGF2 | – | Noggin | SB202190 | HCR, PGE2, Epinephrine | ||
| Prostate | 192 | B27 | Primocin | RSPO | NAC | Nicotinamide | EGF | A83-01 | FGF10, FGF2 | – | Noggin | – | SB202190 | PGE2, Testosterone | |
| Others | Brain | 38 | B27, N2 | P/S | – | – | – | – | – | – | – | – | – | – | 2-mercaptoethanol, Insulin, NEAAs, Neurobasal |
| Lung | 194 | B27 | Primocin | RSPO | NAC | Nicotinamide | – | A83-01 | FGF10, FGF7 | – | Noggin | Y-27638 | SB202190 | – | |
| 102 | B27, N2 | P/S | – | – | – | EGF | – | FGF2 | – | – | Y-27639 | – | – | ||
| Skin | 128 | FBS | P/S | – | – | – | – | – | – | – | – | – | – | – | |
P/S Penicillin-Streptomycin, ATB-ATM Antibiotic-Antimycotic, DXM Dexamethasone, Gmax Glutamax, HCT Hydrocortisone, L-Glu L-Glutamine, NAC N-Acetyl-L-Cysteine.
Alternate PDO cultures
PDTOs can also be cultivated by using an air-liquid interface (ALI) culture system, which has the advantages of growing epithelial cells and maintaining microenvironment components, including fibroblasts and immune cells35. In the ALI technique, the tissue is very finely sliced and subsequently coated with collagen before being deposited on a filter, after which media that is poor in growth factor is added. In this system, the microenvironment can be retained for one month36. In rarer cases, PDTOs can be cultured without the use of ECM, either to reverse the polarity (apical-out polarity) of cystic organoids grown in ECM37 or to establish patient-derived glioblastoma organoids38. In this instance, glioblastoma samples were cut into ~1 mm diameter pieces and cultured in ultralow attachment plates containing fully defined serum-free media. The plates were then placed on an orbital shaker to facilitate PDTO formation and increase nutrient and oxygen diffusion38.
Expansion and use of PDTO
Once formed, PDTOs cultivated in submerged ECM can be dissociated and reseeded for amplification for experimental use. PDTOs can also be cryopreserved for subsequent reculturing. The biobanking of these models allows for the creation of large biological collections that are useful for numerous applications in both basic and clinical research15 (Fig. 2). The establishment of extensive panels of PDTOs is a valuable way of investigating cancer heterogeneity. Furthermore, these collections can be built together with models derived from the same tumor, such as patient-derived xenografts (PDXs), thus offering a broad range of complementary experimental possibilities39,40.
PDTOs can be subjected to various treatments (chemotherapy, radiotherapy, or targeted therapies), and their responses to treatments can be evaluated. Various viability tests, such as the CellTiter-Glo41–43, CellTiter Blue44, MTS45, and CCK-8 assays46, are widely used. Cellular imaging techniques (with or without probes), as well as histology and/or immunohistochemistry, are also utilized. They can be used to study the morphology of PTDOs (including size, texture, or organelle structure47,48), metabolism (for example, by using optical imaging49 or mass spectrometry50), proliferation (Ki67 expression proportion38) or cell death (by using the viability ratio51 or caspase probes52), as well as the expression of specific proteins constituting potential therapeutic targets (such as PD-L1 for immunotherapy53). Moreover, they can be performed at the endpoint and in real time without sample deterioration by using nontoxic probes. They allow for the assessment of the intensity and/or localization of these processes within PDTOs so that the latter may be classified according to their response to treatments.
Applications in oncology
Mechanistic and basic insights
Organoids and PDTOs are increasingly being used within the scientific community, particularly for basic oncology research. Organoids have demonstrated their utility in modeling the stages of carcinogenesis in various types of tumors, including colon cancer33, breast cancer54 and pancreatic cancer55. They have been transformed into tumor organoids via the inactivation of tumor suppressor genes (such as TP53, PTEN, or APC) or the activation of oncogenes (such as KRAS) by using CRISPR/Cas9 technology. Additionally, the inhibition of gene expression via RNA interference approaches in tumor organoids has highlighted the involvement of SIRT5 in pancreatic cancer56 and ARGLU1 in gastric cancers57. The evaluation of the very early stages of transition from healthy to tumor organoids can help researchers to better understand the molecular mechanisms of tumor initiation and thereby reveal new early diagnostic biomarkers for cancers for which early diagnosis is still a challenge, such as pancreatic cancer58. Tumor organoid models may also be relevant for mimicking the genomic evolution of tumors, as was recently demonstrated by Lee et al., who studied genetic alterations occurring during bladder cancer tumor organoid culture compared with tumors developing in vivo20.
The assessment of the mechanisms of resistance to treatments is a leading area of application for tumor organoids due to initial evidence showing their potential to recapitulate the clinical response of the original tumor. Resistance mechanisms to conventional and targeted therapies are dynamic and sequential. They involve reversible phenotypic changes, such as transient senescence mechanisms59, metabolic reprogramming60, epigenetic changes61, modification of the tumor microenvironment, epithelial–mesenchymal transition62 and/or irreversible mutational changes63. These phenomena are difficult to observe in patients or animal models, as multiple sampling steps during patient management are often difficult to achieve. Tumor organoids can be used to track the sequence of resistance acquisition and identify the involved mechanisms in a reproducible and more relevant manner than can be achieved via 3D spheroid culture64. Moreover, by using imaging techniques coupled with capture systems, tumor organoids exhibiting different responses can be analyzed separately, thus enabling the assessment of the effects of a treatment on cell heterogeneity (and vice versa). Several strategies have recently been adopted to analyze resistance mechanisms by using PDTOs. One of them involves the molecular comparison of PDTOs derived from patients treated with neoadjuvant chemotherapy to PDTOs from treatment-naive tumors to identify signaling pathways that could be targeted with specific therapies65. Another strategy is to grow tumor organoids from PDXs treated with chemotherapy in mice to evaluate several parameters that are impossible to assess in vivo, including the secretion of extracellular vesicles following treatment66. Recently, we developed a model of acquired resistance to FOLFIRINOX, which is a combination of three chemotherapies, from PDTO derived from pancreatic adenocarcinoma67. We measured a set of parameters (ROS production, double-strand DNA breaks, apoptosis, mutational profiles, and stemness) throughout the process. This scenario allowed for the identification of key steps of acquired resistance to combined drugs, thus highlighting the reversible nature of these mechanisms. Finally, we demonstrated that tumor organoids are an excellent model for residual disease, which is another aspect of treatment resistance67.
Identification of efficient treatments and/or new therapeutic targets
Organoid biobanks exhibit promises for identifying new therapeutic strategies, guiding the use of molecules in development, and drug repurposing. Several groups have utilized panels of PDTOs originating from different tumor types to screen therapeutic molecules. The feasibility of medium-throughput pharmacological screening was demonstrated by exposing PDTOs derived from colorectal cancers to 83 molecules, thus highlighting the association between the efficacy of various molecules and relevant genetic alterations related to targeted pathways. Screening of molecular libraries in PDTO models has also identified MTAP as a new target in pancreatic cancer68 and SIRT1 as a new target in bladder cancer69. In another study, 9 gastric cancer PDTOs were exposed to 37 molecules that are used in clinical practice and under development, thus showing good responses to targeted therapies that are already indicated for other cancers (such as a stemness STAT-3 target inhibitor or a CDK4/CDK6 inhibitor)70. Sachs et al. also evaluated the relevance of 6 molecules (at 21 different concentrations) that act in vitro on the human epidermal growth factor receptor (HER) signaling pathway; moreover, the majority of HER2-overexpressing PDTOs were sensitive to these molecules, and those not expressing HER2 were resistant. However, some HER2-expressing lines did not meet this criterion, thus highlighting the value of functional tests to assess and predict treatment responses71. In another study, a panel of 24 pancreatic PDTOs showed variable sensitivity to 74 molecules, whether they were used in clinical practice or not; specifically, for the same PDTO model, responses to treatments targeting the same signaling pathways were similar68, thus demonstrating the consistency of the results. Another team used 6 PDTO models of rhabdoid tumors to identify, among 150 molecules, a potentially effective treatment for these rare pediatric tumors that currently have no therapeutic options. A molecule acting on neddylation (which is a posttranslational modification that adds the ubiquitin-like protein NEDD8 to substrate proteins) showed efficacy in all of the tested PDTO lines, thus indicating that NEDD8 is a promising target for further preclinical studies72. Ovarian PDTOs have also been used to validate the antitumor effect of a combination of a Bcl-xL inhibitor with an EGFR inhibitor73 or with an α1-adrenergic receptor antagonist74. UBE2N has also been identified as being a potential therapeutic target in ovarian cancers, with its inhibition sensitizing several PDTO models to carboplatin75. Finally, coculture of PDTOs from glioblastoma with chimeric antigen receptor-T (CAR-T) cells demonstrated antigen recognition, subsequent T-cell activation, and tumor cell death, thus highlighting the potential of PDTOs for testing antigen-specific CAR-T-cell treatment responses38.
By recapitulating tumor heterogeneity and imitating the characteristics of the original tumor, the PDTO model allows for high-throughput screening of numerous emerging therapeutic options, thus making it potentially possible to identify tumor subtypes that could preferentially benefit patients. However, it is important to keep in mind that PDTO media often contain numerous growth factors and compounds, which can interfere with the evaluation of sensitivity to specific targeted therapies or anticancer drugs. For instance, the presence of EGF in the media may affect the use of EGFR-targeted drugs, such as cetuximab. Thus, the addition of exogenous EGF confers cetuximab resistance to colorectal cancer cell lines and PDTO76, and EGF-depleted media is needed to assess the response to this drug77. Therefore, screening a library of potential anticancer compounds may lead to a higher rate of false-positive results than expected. Moreover, high-throughput screening of PDTO models is a particularly burdensome, time-consuming, and costly process compared to cell line screening. To generalize its use, its benefits need to be clarified (which is ongoing in relevant laboratories), and efforts should be made to automate the culture, treatment, and analysis processes.
Identification of biomarkers and predictive molecular signatures
PDTO panels can also be used to define predictive molecular signatures (genomic, transcriptomic, and proteomic signatures) of treatment response. In the context of conventional treatments, these approaches are performed directly on patients’ tumors. However, for molecules in development prior to clinical trials, it may be possible to define the sensitivity level of PDTOs to the molecules under investigation and to search for differential signatures in groups of sensitive or resistant PDTO models. This could lead to the very early development of companion tests that could support and accelerate the development of new drugs.
Several studies have established a link between response to conventional treatments and predictive signatures of various natures, thus providing perspectives for the development of innovative therapies or novel therapeutic sequences78,79. Biomarkers related to recurrence in pancreatic cancers have been identified by using a PDTO bank with established metabolic profiles. Several oncometabolites from the Krebs cycle were found to be more abundant in PDTOs from patients who experienced early recurrence. This characteristic may not only identify the most aggressive tumors but also constitute a vulnerability that could be targeted80. Machine learning analysis of pharmacogenomic data from collections of PDTOs derived from 19 colorectal cancer patients and 9 bladder cancer patients also identified specific biomarkers for sensitivity to 5-FU or cisplatin. These biomarkers subsequently demonstrated predictive value in discriminating responders and nonresponders in larger cohorts81. In-depth and exhaustive molecular characterization of extended collections of PDTOs derived from panels of tumors could efficiently enable the identification of predictive biomarkers (or predictive signatures including several of these biomarkers of different types) (Fig. 3). The value of this approach, which may include artificial intelligence techniques, will need to be confirmed by correlation studies with the clinical response of molecules in development. Nevertheless, the prospects in the field of developing new candidate drugs are immense, and such approaches could both accelerate their validation and provide a much better definition of patient subpopulations that are likely to benefit from these new therapies.
Precision medicine
In addition to the availability of conventional or innovative treatments, precision medicine requires the identification of biomarkers enabling the selection of patients who are likely to benefit from these therapeutic strategies. Currently, the evaluation of the expression of key targets or the presence of genetic abnormalities associated with the responses to different treatments helps in guiding the therapeutic management of selected patients82–84. This information is of diagnostic, prognostic and predictive interest but also has several limitations, such as the lack of selectivity of some molecular signatures85 and the limits of interpretation, such as complex mutational signatures or variants of unknown importance86. This underscores the interest in developing functional tests that are capable of providing additional high-value information for predicting the response to both conventional and innovative treatments. The progressive implementation of functional tests in oncology began from the hypothesis that exposing primary cells from the patient’s tumor to treatments (isolated or not isolated from stromal cells) could predict their response. These tests could also identify correlations between ex vivo treatment responses and the presence of predictive biomarkers of different types (such as DNA, messenger RNA, noncoding RNA, and proteins, among other biomarkers) and origins (such as tumors, blood, and urine, among other origins). Thus, they may help in identifying the tumor phenotype through functional approaches that address various parameters of treatment response, and they can lead to the identification of predictive molecular signatures, which can correspondingly support the development of new therapies. The response of PTDO to one or more molecules after exposure can thereby be used predictively to guide therapeutic decisions for the patients that they originate from in a so-called “chemogram”, or they can even be retrospectively used (once a sufficiently large collection of models is obtained) to search for predictive molecular signatures (such as genomic, transcriptomic and proteomic signatures) of treatment response87.
A growing body of evidence indicates that PDTOs can predict the responses of the tumor that they derive from to anticancer treatments88. The correlation between the response to treatments of PDTO models and the clinical response of patients, which is a crucial and essential first step for the potential future clinical use of PDTOs, is becoming increasingly evident. A review reported of a sensitivity of 81% and a specificity of 74% for predicting treatment responses by using functional tests performed on PDTOs89. These figures are difficult to compare with other tests that are currently used in personalized medicine, such as the search for predictive mutations or aberrant expression profiles of tumor markers on which most targeted treatments are based. Indeed, the latter method requires a diagnostic test with a sensitivity and specificity as close as possible to 100% to be able to reach a “mutated” or “overexpressed” status. Once this status is determined, the treatment is administered to the patient, although not all selected patients will eventually respond. This scenario is precisely what is expected from functional tests that are performed on PDTO, which seek to directly determine the effectiveness of the treatment on the patient’s tumor without using an intermediate marker, which is an approach that includes (by definition) all or most of the parameters of the response to treatments.
Biomarkers can also be used to measure the response of PDTOs to treatment. For example, an increase in c-Jun phosphorylation after treatment exposure has been observed in cisplatin-sensitive gastric cancer PDTOs90. A pioneering study demonstrated the potential benefits of using PDTO derived from metastatic gastrointestinal tumors to predict the responses of 21 patients to different chemotherapies (100% sensitivity, 93% specificity, 88% positive predictive value, and 100% negative predictive value)44. According to another study, 91% of pancreatic cancer patients responded to first-line chemotherapy, and 80% of patients responded to second-line chemotherapy from PDTOs derived from 11 chemo-naive tumors. However, lines derived from 5 pretreated tumors predicted a treatment response in only 40% of patients91. Correlation with response to radiotherapy was analyzed in 19 colorectal cancer patients, thus resulting in the establishment of a prediction model with an accuracy of 82% for sensitive patients and 92% for resistant patients92. Furthermore, the least radiosensitive PDTO derived from head and neck squamous cell carcinomas in 7 patients corresponded to those who relapsed after treatment93. Finally, a study demonstrated an accuracy of 84% (78% sensitivity and 92% specificity) in predicting the response of colorectal PDTOs to a combination of chemotherapy and radiotherapy51; this was a particularly interesting result, which was due to the frequent use of multimodal treatments in clinical practice. Other studies have reported on the responses of ovarian cancer patients to PARP protein inhibitors, which are involved in the repair of single-strand DNA breaks. The use of these molecules is relevant in tumors with deficiencies in homologous recombination (HR) DNA repair, wherein the inhibition of single-strand break repair generates an accumulation of double-strand breaks, which remain unrepaired in this context. A functional assay known as the RECAP (REpair CAPacity test) provides an overall assessment of the status of the HR pathway. Before and after DNA damage induction by irradiation, the organization of repair foci through the HR pathway was quantified by detecting the localization of the RAD51 protein in proliferating cells. It has been applied to PDTO derived from ovarian tumors with potential success in identifying patients who are likely to benefit from treatment with PARP inhibitors22,94. Several clinical trials are underway to determine whether PDTO can predict patients’ responses to treatments (Table 3). Some research teams are continuing to use the response of PDTOs to guide therapeutic decision-making (Table 3). In one such study, PDTO was used to select the molecule administered after metastasectomy for recurrent colorectal cancer, thus leading to persistent remission at 6 months (when more than 50% of patients had progressed or died at that point in time)95,96. PDTO is also being used as a tool for therapeutic decision-making in patients with metastatic cancers who do not respond to first-line treatments for breast or gastrointestinal cancers (NCT04279509; NCT04450706; NCT04611035). They are also under evaluation for their predictive value in adjuvant settings for pancreatic cancer (NCT04931394) and breast cancer (NCT05177432), as well as in neoadjuvant settings for gastric (NCT05351398) or colorectal cancer (NCT04842006). Another ongoing study is even using PDTO to determine the drug to instill locally in bladder tumors during initial management (NCT05024734). Therefore, research offers hope for the rapid introduction of PDTOs into clinical management, which could even precede their use for drug screening in the pharmaceutical industry. However, their use for predictive purposes still has some limitations. For example, a study demonstrated a lack of correlation between the response to biopsy-derived PDTO and the clinical responses of patients with metastatic colorectal cancer treated with the 5-FU/oxaliplatin combination. Conversely, a prediction rate exceeding 80% was observed in patients treated with irinotecan and the irinotecan–5-FU combination, thus suggesting that the predictive nature of PDTO could depend on the anticancer agents that are used or that it would be necessary to better adapt the doses and ratios of chemotherapy combinations applied to the PDTO for them to remain relevant in an in vitro setting97. Furthermore, in another clinical study, patients with metastatic colorectal cancer that progressed despite first-line treatments were offered the opportunity to adapt their next therapeutic line based on the response of PDTOs that were derived from their tumor98. No improvements in clinical responses were observed; however, the amount of exploitable data was limited due to the low establishment rate (57%) and the overall status of the patients, who were too compromised to continue systemic treatment.
Table 3.
Ongoing clinical trials comparing clinical and PDTO response to treatments or using PDTO to guide clinical decision-making.
| Name of the study | Identifier | Estimated study completion date | Location | Type of cancer studied | Step of the treatment | Type of treatment |
|---|---|---|---|---|---|---|
| Clinical trials in which principal outcome include comparison between PDTO and clinical response | ||||||
| Translational Analysis In Longitudinal Series of Ovarian Cancer Organoids (TAILOR) | NCT04555473 | May-23 | Roma, Italia | Ovary | NeoAdj. or Adj. | CT |
| Clinical Study on Drug Sensitivity Verification or Prediction of Therapy for Breast Cancer by Patient-Derived Organoid Model | NCT03544047 | Jul-21 | Beijing, China | Breast | NeoAdj. | CT |
| Drug Sensitivity Correlation Between Patient-Derived Organoid Model and Clinical Response in NSCLC Patients | NCT03453307 | Jul-21 | Shijazhuang, China | Lung | NeoAdj. or Adj. | CT, IT |
| Establishing Organoids From Metastatic Pancreatic Cancer Patients, the OPT-I Study | NCT03500068 | Sep-22 | Amsterdam, Netherlands | Pancreas | Pall. | CT |
| OPPOSITE: Outcome Prediction Of Systemic Treatment in Esophagogastric Carcinoma | NCT03429816 | Aug-22 | Dresden and Heidelberg, Germany | Gastric, œsophagus | NeoAdj. | CT, RT |
| Organoid Based Response Prediction in Esophageal Cancer (RARESTEM/Org) | NCT03283527 | Jan-20 | Groningen and Leewarden, Netherlands | Oeosphagus | NeoAdj. | CT, RT |
| Organoids in Predicting Chemoradiation Sensitivity on Rectal Cancer | NCT03577808 | Nov-20 | Shangai, China | CRC | NeoAdj. | CT, RT |
| Patient-derived Organoid Model and Circulating Tumor Cells for Treatment Response of Lung Cancer | NCT03655015 | Dec-22 | San Antonio, USA | Lung | Adj. | CT, IT |
| Pharmacotyping of Pancreatic Patient-derived Organoids | NCT05196334 | Dec-24 | Copenhague, Danemark | Pancreas | Prise en charge | CT |
| Cetuximab Sensitivity Correlation Between Patient-Derived Organoids and Clinical Response in Colon Cancer Patients. | NCT04906733 | Dec-23 | Shanghai, China | CRC | Adj. | CT, TT |
| Study on the Consistency Evaluation of Organoids Used in the Clinical Treatment of Ovarian Cancer With Anti-tumor Drugs | NCT05175326 | Nov-21 | Guangzhou, China | Ovary | NeoAdj. or Adj. | CT |
| Development of a Prediction Platform for Neoadjuvant Treatment and Prognosis in Pancreatic Cancer Using Organoid | NCT04777604 | Jan-26 | Seoul, Korea | Pancreas | NeoAdj. | CT |
| Organoids-on-a-chip for Colorectal Cancer and in Vitro Screening of Chemotherapeutic Drugs | NCT04996355 | May-24 | Beijing, China | CRC | Adj. | CT |
| Development of a Prediction Platform for Adjuvant Treatment and Prognosis in Resected Pancreatic Cancer Using Organoid | NCT04736043 | Jan-26 | Seoul, Korea | Pancreas | Adj. | CT |
| Study on Consistency Evaluation for Drug Sensitivity of Patient-Derived Organoid Model From Cholangiocarcinoma Patients | NCT05634694 | Dec-24 | Guangzhou, China | Cholangiocarcinoma | Adj. | CT |
| SOTO: Treatment Sensitivity of Organoids to Predict Treatment Outcome | NCT05400239 | May-23 | London, United Kingdom | HNSCC | Adj. or Pall. | CT, RT |
| The Culture of Advanced or Recurrent Ovarian Cancer Organoids and Drug Screening | NCT05290961 | Dec-24 | Chongqing, China | Ovary | Adj. | CT |
| The Culture of Ovarian Cancer Organoids and Drug Screening | NCT04768270 | Dec-24 | Chongqing, China | Ovary | Adj. | CT |
| Tailoring Treatment in Colorectal Cancer (TargetCRC) | NCT05401318 | Jan-27 | Viken, Norway | CRC | UN | CT, IT |
| 3D Bioprinted Models for Predicting Chemotherapy Response in Colorectal Cancer With/Without Liver Metastases | NCT04755907 | Dec-23 | Beijing, China | CRC | NeoAdj. or Adj. | CT |
| KM3D Multicenter Cancer Consortium: Predicting Patient Response Using 3D Cell Culture Models | NCT05338073 | Jan-26 | Durham, USA | Various | UN | CT |
| Clinical trials in which secondary outcome include comparison between PDTO and clinical response | ||||||
| Early-Line Anti-EGFR Therapy to Facilitate Retreatment for Select Patients With mCRC | NCT04587128 | Oct-25 | Madison, USA | CRC | Pall. | CT, TT |
| Establishment of Squamous Cell Organoids of the Head and Neck to Assess Their Response to Innovative Therapies (ORGAVADS) | NCT04261192 | Feb-25 | Caen, France | HNSCC | Adj. | CT, RT, IT |
| Feasibility Study of Multi-Platform Profiling of Resected Biliary Tract Cancer | NCT04561453 | Jun-25 | Chicago, USA | Biliary Tract | Adj. | CT |
| Novel 3D Myeloma Organoid to Study Disease Biology and Chemosensitivity (Organoid) | NCT03890614 | Apr-23 | Winston-Salem, USA | Myeloma | UN | CT |
| Patient-derived Organoids of Lung Cancer to Test Drug Response | NCT03979170 | Dec-24 | Geneva, Switzerland | Lung | Adj. | CT |
| Prediction Model of Response for CCRT in Esophageal Cancer | NCT03081988 | Dec-22 | Daegu, Korea | Esophagus | NeoAdj. | CT, RT |
| Real Time Molecular Analysis of Breast Cancer Receiving Neo-adjuvant Chemotherapy (NEO-R) | NCT04504747 | Jan-30 | Marseille, France | Breast | NeoAdj. | CT |
| Trifluridine/Tipiracil and Irinotecan for the Treatment of Advanced Refractory Biliary Tract Cancer | NCT04072445 | Jan-23 | Minessota, USA | Biliary Tract | Pall. | CT |
| Developing Breast (Cancer) Organoids | NCT05317221 | May-28 | Maastricht, Netherlands | Breast | UN | CT |
| Novel 3D Hematological Malignancy Organoid to Study Disease Biology and Chemosensitivity (Organoid) | NCT03890614 | Apr-23 | Winston-Salem, USA | Hematology | UN | CT |
| Consistency Between Treatment Responses in PDO Models and Clinical Outcomes in Gastric Cancer | NCT05203549 | Jun-23 | Shangai, China | Myeloma | NeoAdj., Adj., Pall. | CT |
| The Culture of Advanced/Recurrent/Metastatic Colorectal Cancer Organoids and Drug Screening | NCT05304741 | Dec-23 | Chongqing, China | CRC | Pall. | CT, TT |
| Establishment of an ex Vivo Tumor Collection of Triple-negative Breast Cancers in Order to Validate the Interest of Innovative Therapies and the Search for Predictive Biomarkers of Response to Treatment (TRIPLEX) | NCT05404321 | Dec-26 | Caen, France | Breast | NeoAdj. | CT |
| A Pilot Study of a Micro-Organosphere Drug Screen Platform to Lead Care in Advanced Breast Cancer | NCT04655573 | Oct-24 | Durham, USA | Breast | NaoAdj. or Pall. | CT |
| Using Ex Vivo Tumoroids To Predict Immunotherapy Response In NSCLC (TUMORIN) | NCT05332925 | Feb-24 | Kansas, USA | Lung | Pall. | IT |
| Molecular Characteristics of Gastroesophageal Adenocarcinoma (MOCHA): A Prospective Feasibility Study | NCT04219137 | Nov-21 | Toronto, Canada | Oesophagogastric | UN | UN |
| Systemic Neoadjuvant and Adjuvant Control by Precision Medicine in Rectal Cancer (SYNCOPE) | NCT04842006 | Dec-31 | Helsinki, Finlande | CRC | NeoAdj. | CT, RT |
| Clinical trials using PDTO to guide clinical decisions | ||||||
| Functional Precision Oncology for Metastatic Breast Cancer (FORESEE) | NCT04450706 | Aug-25 | Salt Lake City, USA | Breast | Pall. (2nd line) | CT |
| Q-GAIN (Using Qpop to Predict Treatment for GAstroIntestinal caNcer) | NCT04611035 | Jan-23 | Singapore | Gastrointestinal | Pall. (2nd line) | CT (14 drugs) |
| Selecting Chemotherapy With High-throughput Drug Screen Assay Using Patient Derived Organoids in Patients With Refractory Solid Tumors (SCORE) | NCT04279509 | May-22 | Singapore | HNSCC, CRC, ovary, breast | Pall. (3rd line) | CT (10+/-5 drugs) |
| Organoid-Guided Chemotherapy for Advanced Pancreatic Cancer | NCT04931381 | May-25 | Shanghai, China | Pancreas | Pall. (1st line) | CT (5 drugs) |
| Organoid-Guided Adjuvant Chemotherapy for Pancreatic Cancer | NCT04931394 | May-25 | Shanghai, China | Pancreas | Adj. | CT (5 drugs), RT |
| Guiding Instillation in Non Muscle-invasive Bladder Cancer Based on Drug Screens in Patient Derived Organoids | NCT05024734 | Nov-26 | Berne, Suisse | Bladder | Adj. | CT intravesical (4 drugs) |
| Patient-derived-organoid (PDO) Guided Versus Conventional Therapy for Advanced Inoperable Abdominal Tumors | NCT05378048 | Jul-25 | Hong Kong | Abdominal Solid Tumor | Pall. (2nd line) | CT |
| The Clinical Efficacy of Drug Sensitive Neoadjuvant Chemotherapy Based on Organoid Versus Traditional Neoadjuvant Chemotherapy in Advanced Gastric Cancer | NCT05351398 | Dec-23 | Shanghai, China | Gastric | NeoAdj. | CT |
| The Clinical Efficacy of Drug Sensitive Neoadjuvant Chemotherapy Based on Organoid Versus Traditional Neoadjuvant Chemotherapy in Advanced Rectal Cancer | NCT05352165 | Dec-25 | Shanghai, China | CRC | NeoAdj. | CT (5 protocols) |
| Quadratic Phenotypic Optimization Platform (QPOP) Utilization to Enhance Selection of Patient Therapy Through Patient Derived Organoids in Breast Cancer (QUEST) | NCT05177432 | Dec-25 | Singapore | Breast | Adj. | CT (10-12 drugs) |
| Prospective Multicenter Study Evaluating Feasibility and Efficacy of Tumor Organoid-based Precision Medicine in Patients With Advanced Refractory Cancers (ORGANOTREAT) | NCT05267912 | Jan-27 | Paris, France | CRC, rare solid tumor | Pall. | CT |
| A Study on the Potential Benefit of Neoadjuvant Therapy for AGC Patients | NCT05442138 | Sep-01 | Henan, China | Gastric | NeoAdj. | UN |
| Functional Precision Oncology to Predict, Prevent, and Treat Early Metastatic Recurrence of TNBC (TOWARDS-II) | NCT05464082 | Sep-27 | Utah, USA | Breast | Pall. (2nd line) | CT |
| Precise Therapy for Refractory HER2 Positive Advanced Breast Cancer | NCT05429684 | Feb-24 | Shaanxi, China | Breast | Pall. | CT (10 drugs) |
| Using QPOP to Predict Treatment for Sarcomas and Melanomas (Q-SAM) | NCT04986748 | Dec-28 | Singapore | Sarcoma, melanoma | Pall. | CT (14 drugs) |
| Evaluation of ex Vivo Drug Combination Optimization Platform in Recurrent High Grade Astrocytic Glioma | NCT05532397 | Dec-25 | Singapore | Glioma | Pall. | CT |
Adj. Adjuvant, CT Chemotherapy, UN Unknown, NeoAdj. Neaoadjuvant, IT Immunotherapy, Pall. Palliative, RT Radiotherapy, TT Targeted Therapy.
Challenges and issues
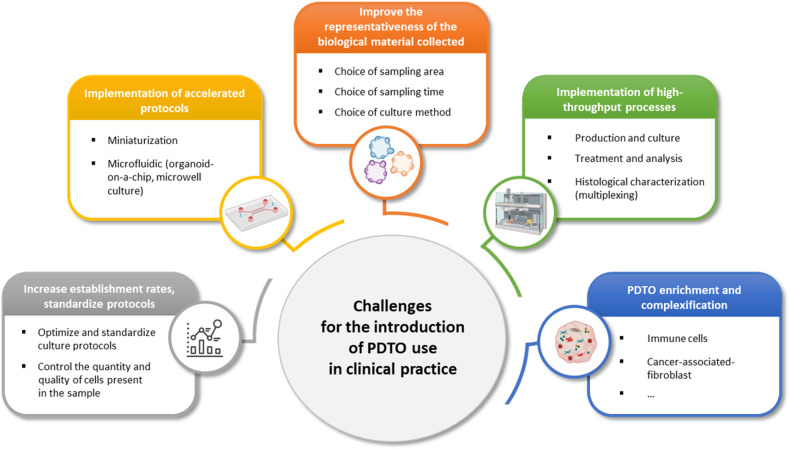
PDTO currently provides a wealth of information regarding its architectural organization, heterogeneity, molecular characteristics, and response to various treatments. The coherence of this response with clinical outcomes is gradually being confirmed by the literature. However, it is currently impossible to address all of the scientific and medical questions that have been raised by the community, due to a number of limitations of these models and/or their current culture conditions. In addition to the complexity of experimental protocols (which are difficult to routinely set up in a conventional biology laboratory) and the relative complexity of sampling circuits and associated regulatory and ethical aspects, serious limitations need to be addressed to ensure that reliable generated data are delivered to clinicians within a time frame that is compatible with clinical management. The challenges to be addressed include the representativeness of the sampled material considering the initial tumor and its polyclonal nature, the quantity of required material, the success and timing of establishment, the time needed to evaluate the response, the need to make the PDTO more complex or to at least culture them with stromal cells (such as fibroblasts and immune cells, among other cell types) to better predict the responses to some specific molecules, and the establishment of high-throughput culture processes capable of handling a large number of tumor samples (Fig. 4). Some of these points and potential solutions are discussed below.
Fig. 4. Future challenges for using PDTO in clinical practice (created with BioRender.com).
Adapted from 200.
Ensuring the representativeness of the sample
One of the challenges in personalized medicine in oncology is ensuring that the utilized tumor material (in this case, PDTO) corresponds accurately to the tumor that will receive the treatment throughout the course of patient care. It has been demonstrated that liver PDTOs derived from different regions of the original tumor showed similar responses to different therapies. In contrast, ovarian PDTOs derived from distinct intraperitoneal nodules demonstrated treatment responses that could differ from each other41. These findings suggest the need to sample tumor material from multiple areas when tumors are disseminated and to generate as many clonal PDTOs as possible to ensure the representativeness of the obtained lineages. However, this would significantly complicate both the sampling and culture procedures. This issue needs to be evaluated in large-scale cohorts. Cancer cells can also evolve over time and under the influence of treatments to which they have been exposed. Previous studies have shown that the sensitivity of PDTOs derived from ovarian41, breast99 or pancreatic100 tumor samples at different stages of treatment is likely to change. It may be necessary to repeat tumor tissue sampling during disease progression, after treatment, in cases of relapse, or when metastases appear. Paradoxically, pancreatic PDTO derived from biopsies of recurrence or progression in patients who were already treated predicted the therapeutic response only 40% of the time, whereas PDTO derived from treatment-naive patients accurately predicted not only the response to the first line treatment (91%) but also to the second line (80%) of treatment91.
Improving the success rate of establishment
The establishment rate of PDTO models varies considerably depending on the tumor location, ranging from less than 20% for prostate cancer101 to approximately 60% for ovarian cancer22 and up to over 90% for colon cancer32. The achievement of an establishment rate close to 100% will be necessary for the use of PDTOs to be feasible in a clinical context. Ooft et al.‘s study suggested that an insufficient establishment rate could be a major hindrance to the clinical use of PDTO. The proportion of tumor cells in the initial sample can influence the success rate of establishment102, thus potentially explaining the greater difficulty in obtaining a satisfactory establishment rate with small biopsies or after relapse. Furthermore, contamination by normal organoids from surrounding tumor tissues represents a real issue in some cases103. To prevent overgrowth of normal organoids, PDTOs can be selected by selective pressure based on their mutational pattern. For instance, the MDM2 antagonist Nutlin-3a can be used to select TP53-mutated PDTOs, and the withdrawal of EGF or Wnt3A can be used to select PDTOs harboring activating mutations in the EGFR and Wnt pathways, respectively104. PDTO can also be isolated from normal organoids via phenotype-based manual selection or via clonal expansion by using cell sorting. However, all of these approaches for selecting pure tumor organoids can lead to a loss of cellular heterogeneity compared to initial cultures104. Finally, for some types of tumors, such as sarcomas, the establishment of stable PDTO culture seems to be much more challenging105. Improvements in the establishment rate could involve enhancing the preparation and culture conditions (such as dissociation methods, culture substrates, and enriched or tailored media for the selection of tumor cells, among other conditions). The implementation of controlled and standardized methods is the first step in this process14. Fujii et al. achieved a 100% establishment rate by using eight different culture conditions, including various Wnt activators, p38 inhibitors, and oxygen concentrations. The varying requirements between tumors make it challenging to achieve a platform without overly costly or complex procedures32.
A platform based on more than 1000 PDTO models of different histological types was established to optimize culture conditions and analyze treatment responses52. The authors of that study showed that PDTOs can be established in both basic and enriched media (except for pancreatic tumors). However, subtle variations in the medium composition can sometimes have a significant impact on the establishment rate of tumor subtypes. It will likely be necessary to define the most suitable medium for each tumor type or subtype, such as by allowing for the best establishment rate and representativeness in a timely manner. Culture conditions could also be optimized by using finely controlled ECMs. Depending on the tumor type, the necessary biochemical and mechanical environments can vary considerably, thus suggesting the need for adaptation of the utilized matrices, as suggested by the optimization of a sliced tumor explant model106. The envisaged alternatives (whether they are natural, synthetic or a combination of both) show great potential but still require considerable developments to enable their widespread use and to completely replace current commercial matrices107,108.
Making the predictive functional assay compatible with the clinical management timeline
One of the major limitations impeding the implementation of PTDO-guided therapeutic decision-making in routine clinical practice is how quickly the results are returned to the clinician for patient treatment. In the majority of cases, it is not compatible with the timeframe of clinical practice, and further technical challenges remain to be addressed to deliver a therapeutic option to physicians in a convenient time frame. This limitation can be overcome by increasing the elapsed time between sampling and patient treatment and/or making the predictive functional assay faster. For the first strategy, one option could involve generating PDTOs from biopsy at the time of diagnosis to inform the selection of adjuvant chemotherapy. However, this would require the processing and culture of some samples that will appear to be benign. Another option would be to use PDTO from treatment-naive patients to select the therapy that will be given after a recurrence or upon progression. Nonetheless, treatments exert selective pressures that drive tumor cell evolution and favor the appearance of resistant clones, thus leading to the development of recurrence with genetic profiles that are markedly different from those of the primary tumor. Therefore, further investigations are required to determine whether the response of PDTOs derived from samples that are collected prior to standard-of-care treatment could reflect the response to recurrence. The second strategy consists of decreasing the elapsed time between sampling and the results of the functional assay. The methods for treating PDTO and analyzing the response are crucial areas in which action can be taken to reduce response times. Many teams are specifically focusing on miniaturization and microfluidics processes, which would allow for the testing of a larger number of molecules on a smaller number of PDTOs, thus ultimately enabling more work on isolated PDTO (“single PDTO”) to accelerate evaluation of the response to treatment. This scenario is even more important because the amount of tumor sample is often drastically limited. In this context, the standardization of the methods also appears to be a major challenge because the variability of the response will increase if the number of PDTOs that are used for this evaluation is low. The influence of the number and size of PDTOs per condition on the response to treatment and the processes to be implemented are particularly important for controlling and standardizing these parameters. Further correlation studies will be required for these purposes.
For instance, by developing a microwell system allowing for the analysis of approximately one hundred PDTOs (a quantity obtainable in the first passage), a team recently evaluated the response to treatments of pulmonary PDTO within one week109. A microfluidic system leading to the formation of droplets of ECM around cells has also been proposed. PDTO was generated, and a response to functional testing was obtained in under 14 days, with the first correlations with clinical responses in patients being observed110. Another team conducted high-throughput screening one week after seeding ovarian tumor cells in an ECM matrix ring system rather than a droplet system111. Microfluidic devices based on the use of micrometer-sized channels also enable the dynamic control of nutrient, oxygen, and waste flows, thus consistently producing high-quality PDTOs112. The use of methods of treatment response analysis through imaging to accelerate information processing has also been proposed113. When combined with artificial intelligence, such methods have the potential to allow for rapid and cost-effective evaluation of treatment responses, thus further reducing the required response time. The development of new equipment automating the culture or treatment of PDTO, whole organoid sorting, and high-throughput microfluidic culture, among other methods, is also a subject of ongoing research, with biologists and physicists working together on these issues. Various automated techniques have been implemented by different platforms (such as the EIPM core facility in New York, https://eipm.weill.cornell.edu/research/organoids/; ORGAPRED core facility, www.orgapred.com and the laboratory of Stem Cell Bioengineering114). The aims of these initiatives are to work faster and with a smaller quantity of PDTOs so that results can be rapidly obtained (which is crucial for clinical use) and to allow for a greater number of tests to be conducted on the available PDTOs, such as by evaluating a larger number of molecules.
Complexification of models
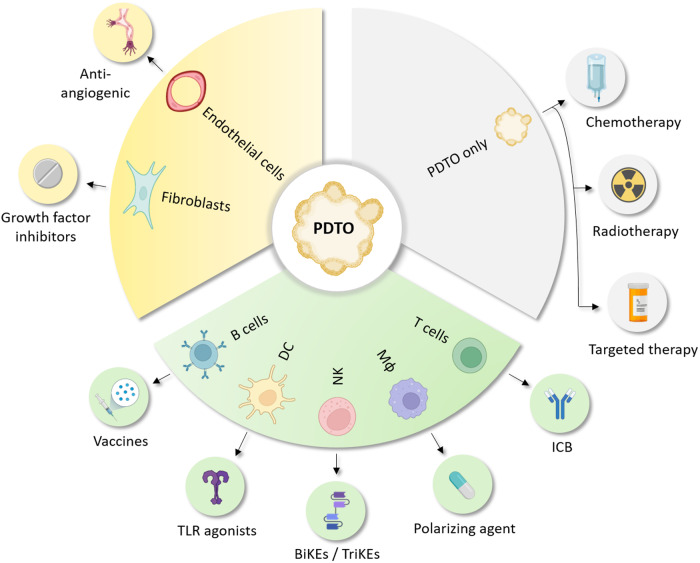
The interaction of cancer cells with cells in the tumor microenvironment, such as cancer-associated fibroblasts (CAFs), endothelial cells and immune cells, can influence treatment responses115 and/or constitute a therapeutic target. Anti-angiogenic strategies and immunotherapies are among the therapies for which it is still difficult to use PDTOs for predictive purposes. Various developments are currently underway to complexify PDTO models by coculturing them with cells from the tumor microenvironment, either directly or by using microfluidic devices enabling compartmentalized coculture of different cell types, as well as by using “vascularization”, or coculture of various types of normal cells and PDTOs (referred to as “organoids-on-chip, tumors-on-chip, organs-on-chip” approaches)116,117. These developments will accelerate preclinical evaluation and pharmacological research, in particular.
Immune cells
As described above, one of the challenges in PDTO development is to “complexify” the culture with nontumoral cells to widen the spectrum of therapies with associated predictive assays (Fig. 5). Thus, the coculture of PDTOs with autologous immune cells is a very active field of research that aims to develop relevant models to evaluate and predict the responses to immunotherapies. A major issue in developing such a model involves the source of immune cells, as this affects their phenotype, including their maturation status, metabolism, cycling activity, and migration capacity. The use of immune cells infiltrating the tumor of origin seems to be the best strategy, as was proposed in a model of tumor slices cultured in ALI in which the immune diversity of the tumor microenvironment (TME) is maintained36. In addition to representing TME diversity, these types of models are sensitive to immune checkpoint blockade (ICB) and display morphologic changes and increased cell death after treatment36,118–120. PDTO using “native” immune cells can also be obtained from dissociated tumor samples cultured in Matrigel domes121 or acoustically assembled spheroids122 and are more easily cryopreserved. Nevertheless, due to the lack of immune-specific factors, immune cells infiltrating this type of PDTO exhibit a progressive decrease in viability and almost disappear after one month of culture36. Consequently, functional assays must be quickly performed, and the reuse of these models for further tests can be complicated. An alternative is to expand tumor-infiltrating lymphocytes (TILs), as suggested by the study by Knochelmann et al., who managed to isolate and expand TILs from murine and human solid tumors by using interleukin-2 (IL-2)123. This type of strategy has been successfully used with organoids and PDTOs derived from intestinal and colorectal tissue in which organoid infiltration and killing by intraepithelial T cells were observed124–127. However, the number of expandable TILs is highly dependent on the amount of tissue sample that is available, which may explain why this type of protocol is mainly used in the intestinal tract wherein the amount of resected tissue is relatively abundant. Thus, the use of peripheral immune cells may be needed for PDTOs derived from small pieces of resected tissue or biopsies. For example, Votanopoulos et al. used immune cells isolated from lymph nodes to activate patient-matched T cells to kill PDTOs derived from melanoma128, appendiceal cancer129 and Merkel cell carcinoma130. However, access to this type of surgical sample may not always be easy, which could prevent the use of immune-enriched PDTO on a larger clinical scale. Another option would be to use immune cells isolated from peripheral blood mononuclear cells (PBMCs), which may be easier to harvest. This method provided interesting results in PDTO derived from pancreatic ductal adenocarcinoma131,132 as well as colorectal and lung cancer133. Furthermore, these immune-enriched PDTOs using PBMCs may be more suitable for clinical purposes, as they were also used in an exploratory study (NCT03026140) assessing the response to neoadjuvant immunotherapy in colorectal cancers134. In this study, the authors managed to establish 12 PDTO-PBMC cocultures derived from patients and showed that T-cell reactivity against matched PDTO was more often observed in patients responding to treatment (3/6, 50%) than in nonresponders (0/6, 0%). Nevertheless, the use of such a model can induce a nonnegligible bias, as most of the T cells that are present in PBMCs will not display antigen specificity against PDTO. A first attempt to address this challenge was the study by Dijkstra et al., who published a detailed protocol in which repeated cycles of coculture of PBMCs and PDTOs were used to induce the emergence of PDTO-specific T cells133–135. Another challenge lies in the exhaustion status of the cells. Indeed, antigen persistence induces several alterations in T cells, such as immune checkpoint expression and epigenetic modifications, which have recently been reported to increase progressively from the periphery to the tumor bed136. Thus, preactivation protocols will have to mimic this exhaustion process as much as possible to increase the relevance of the model. Finally, until recently, immune-enriched PDTOs have mainly focused on T cells for the evaluation of immune checkpoint blockade (ICB)-based immunotherapies in clinical practice (Fig. 5). Complexification with other immune cells, such as macrophages, natural killer (NK) cells, dendritic cells (DCs) and B cells, may allow for other immunotherapies, such as macrophage polarizing agents, bispecific and trispecific killer engagers (BiKEs and TriKEs), Toll-like receptor agonists and cancer vaccines, to be tested (Fig. 5). Therefore, coculture of PDTOs and immune cells faces a number of challenges, which need to be addressed before an off-the-shelf model becomes available for translational and preclinical research.
Fig. 5. Coculture of PDTOs with autologous stromal and immune cells broadens therapies that could be tested.
DC dendritic cell, NK natural killer cell, Mɸ macrophage, TLR toll-like receptor, BiKEs bispecific killer cell engagers, TriKEs trispecific killer cell engagers, ICB immune checkpoint blockade (created with BioRender.com).
Cancer-associated fibroblasts
CAFs play a significant role in tumor development and aggressiveness, which are primarily accomplished through the secretion of paracrine factors or ECM remodeling, thus providing both biochemical and mechanical support for tumor growth. However, long-term PDTO culture often leads to the gradual loss of multicellular components of the tumor microenvironment, thus limiting model accuracy137. Therefore, the development of PDTOs that can mimic in vivo cancer cell and stromal fibroblast interactions is crucial. These advances in the development of 3D coculture models of multicellular PTDO offer a deeper understanding of the cellular and molecular cues derived from both the cellular and acellular interactions provided by CAFs and their surrounding ECM. Organoids cultured in the presence of CAFs are useful for assessing complex diseases such as cancer. They can also be used to assess preclinical anticancer drugs prior to clinical trials. Recently, a 3D coculture of CAFs and oral cancer organoids was established. CAFs enhanced the organoid-forming ability of CD44+ oral cancer stem cells138. These cultures not only allowed us to evaluate the tumor-promoting effects of CAFs but also revealed the role of the NOTCH signaling pathway in the activation of CAFs139. A previous study demonstrated the relevance of incorporating CAFs in pancreatic PDTOs for the functional analysis of CAF activation. The authors identified the genesis of two CAF subpopulations, depending on the spatial localization within the pancreatic PTDO with different protein expression profiles140. CAFs may also exhibit antitumorigenic properties, as shown in lung squamous carcinoma PDTOs, in which epithelial overexpression of SOX2 is sufficient to mediate the transition from hyperplasia to dysplasia. Surprisingly, CAFs suppress the activity of high SOX2 levels, restore hyperplasia and enhance the formation of acinar-like structures, thus demonstrating that stromal factors can overcome cell-intrinsic oncogenic changes in determining the disease phenotype141. The addition of CAFs to liver PDTOs promoted tumor growth and resistance to conventional chemotherapeutic agents that are used in clinical practice. That study provided evidence for the potential clinical importance of CAFs in liver cancer142.
Vascularization
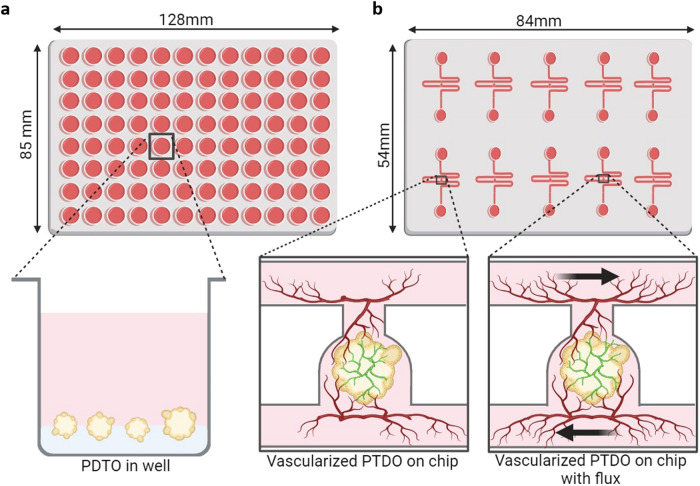
Coculturing PDTOs with endothelial cells allows for the analysis of the effectiveness of antiangiogenic molecules such as bevacizumab and sorafenib143 under conditions that are more similar to the physiology of the tumor. Indeed, as some tumors are particularly vascularized, whereas others are much less so (such as pancreatic cancer), it seems essential to consider this dimension when testing the potential efficacy of a treatment, particularly when evaluating intratumoral angiogenesis, the effect of cancer cells and the cancer microenvironment on tumor vascularization, endothelial network architecture and maturation dynamics and functionality. Microfluidics can significantly contribute to this domain, thus enabling work to be conducted under flow conditions that mimic the physiology of the tumor. This technology has been applied to several types of 3D culture, human induced pluripotent stem cells144, tumor spheroids145 and organoids146. The examination of the efficacy of a drug or immune cells on a vascularized PDTO under flow could better mimic the tumor environment, thereby improving the predictive capabilities of these models (Fig. 6).
Fig. 6. Vascularization and microfluidic applications for PDTO.
a Representation of a classical 96-well plate with PDTOs grown in a Matrigel bed. b Representation of a serpentine microfluidic chip with a single PDTO vascularized with endothelial cells and an endothelial network in the gel, thus allowing for perfusion of the medium and/or drugs in the PDTO. The addition of flow improved vascular network formation (created with BioRender.com).
These coculture models are particularly useful for some studies but necessitate modifications in the preparation of biological collections during PDTO preparation. It becomes important to preserve stromal cells from tumor dissociation as much as possible and to harvest autologous immune cells at the time of tumor sampling for subsequent coculture with autologous PDTOs. Although this procedure involves specific logistics and compliance with the rules and requirements for the use of human samples, it provides significant possibilities for applications, especially in clinical settings. Ongoing developments based on the use of coculture devices (such as organoids-on-chip) could allow for the evaluation of the response of PDTOs to a wide range of treatments, including those targeting the tumor microenvironment.
Organoids-on-chip
The integration of PDTOs into microfluidic systems has subsequently emerged as being a powerful tool in cancer research. In addition to the advantages of PDTOs (as discussed above), microfluidics also have additional benefits for tumor organoids-on-chips, including precise control of nutrient and oxygen gradients, fluid flow, spatial organization, and the incorporation of components of the microenvironment, among other benefits, as well as the ability to create microphysiological systems (MPSs) that more closely resemble human physiology.
To date, numerous researchers have developed their own organoids-on-chip systems145,147–149, whereas others have used commercially available chips150,151. The overall design typically includes one channel with organoids embedded in a hydrogel and one or more channels of culture media, thus providing lateral flow by using a peristaltic pump or pressure controllers. Although these models typically overcome the issue of media renewal that comes with classical static cultures, most designs do not address issues such as normalization of organoid size, number and localization. To overcome these challenges, several teams have developed trapping methods by pipetting a single organoid into a central chamber152, with acoustofluidics153 or the hydrodynamic trapping of organoids154. Trapping methods allow for the standardization of the localization of the organoid, thus making image acquisition and downstream analysis more reproducible. In addition, the ability to trap a single organoid makes it possible to study tumor heterogeneity and clonal evolution, which is difficult to address with typical organoid culture methods.
In terms of cancer research applications, organoids-on-chip can help with various issues that the field is experiencing. First, high-throughput screening of anticancer drugs can be facilitated by simultaneously testing multiple compounds, along with different combinations of treatments or even drug regimens155,156. These platforms also enable the development of personalized cancer therapies by culturing PDTOs and testing drug responses ex vivo. Due to the limited quantity of biological samples that are needed, the delay between the procurement of biopsies and the response to drug treatments from mature organoids can decrease, which is one of the greatest challenges in the field of personalized medicine. Additionally, organoids-on-chip better emulate the tissue microenvironment than do organoids.
The addition of flow itself is an important microenvironmental cue that benefits organoid growth over culture in well plates. For example, the size and efficiency of PDTO formation increased with the addition of flow in a colorectal cancer context157. Another team observed improvements in ovarian cancer organoid size and changes in response to drugs158. Moreover, the integration of vascular or endothelial networks inside of organoids-on-chip models represents a major advantage over classic PDTO culture and enables researchers to specifically examine intratumoral angiogenesis, the effect of cancer cells and the cancer microenvironment on tumor vascularization. The endothelial network inside of the organoids-on-chip system usually surrounds the organoid or PDTO159 and can anastomose with the 3D structure, thus providing successful intravascular perfusion (Fig. 6) and, as a consequence, better maturation of the organoid146. Moreover, the endothelial network is more developed when there is media flow and can be perfused and transport small molecules160, blood cells161 or PBMCs162. The circulation of cells and small molecules provides the opportunity to study not only cancer metastasis phenomena but also tumoral inflammation or immunotherapies. Finally, with appropriate platforms, on-chip organoids can be coupled with sensors to obtain more quantitative data and perform real-time kinetic studies on the response of PDTOs to treatments. For instance, such captors could measure oxygen levels163 or metabolites such as glucose or lactate164 to more accurately evaluate PDTO metabolism.
By combining the strengths of PDTO culture with microfluidic engineering, this innovative platform allows for the improvement of tissue differentiation and integration of microenvironmental cues into PDTOs. Overall, organoids-on- chip systems exhibit great potential for accelerating drug discovery, understanding disease mechanisms, and ultimately improving patient outcomes in oncology.
Conclusion
Organoids and PDTOs represent groundbreaking developments for both researchers and clinicians in various fields, including developmental and cancer biology, regenerative medicine, toxicology, drug development, and precision oncology. Their vast potential is waiting to be fully exploited, although numerous challenges remain for the scientific and medical communities. These challenges include an understanding of how to successfully obtain and maintain PDTOs, accelerating establishment processes and predictive testing, enhancing model complexity, and improving physiological representation via enrichment or integration into coculture devices. High-throughput culture and analysis processes, protocol standardization, and determination of the optimal sampling for obtaining reliable responses are challenges that are being actively addressed by the scientific community. With the integration of these models into routine clinical use, precision oncology can enter into a new era in the coming decade. This represents the essence of research that is being conducted worldwide and ongoing clinical trials aimed at validating the potential of this approach. The future holds considerable promise for leveraging these models to advance personalized cancer treatment and transform patient care.
Acknowledgements
The work of L.B.W. and L.P. on the PDTO and ORGAPRED core facility was supported by grants from GIS IBiSA, Cancéropôle Nord-Ouest, Ligue contre le Cancer (Calvados’s commitee), the Fondation de l’Avenir, the Fondation ARC pour la Recherche sur le Cancer, the Groupement des Entreprises Françaises dans la Lutte Contre Le Cancer (GEFLUC), the Fonds de Dotation Patrick de Brou de Laurière, La Rochambelle, the Vaincrabe Association, the Lions clubs of Normandy, the Solidarité don d’espoir Association, and the Comprehensive Cancer Center F. Baclesse. The ORGAPRED, ORGATHEREX, PLATONUS ONE, POLARIS, and EQUIP’INNOV projects were supported by the Normandy County Council and the European Union within the framework of the Operational Programme ERDF/ESF 2014-2020 and by the French state, as part of the planning contract 2015-2020 between the State and the Normandy Region. The work of A.V. on the PDTO and OrgaRES core facility (Univ. Lille, CANTHER) were supported by grants from Contrat de Plan Etat-Région CPER Cancer 2015-2020, Cancéropôle Nord-Ouest, the Fondation ARC pour la Recherche Sur Le Cancer, Ligue Contre Le Cancer and GIS IBiSA. The work of G.G. on PDTO and the 3D-Hub-O core facility were supported by research funding from the Cancéropôle Provence-Alpes-Côte d’Azur, the Institut National du Cancer INCa Pré-néo 2019-188, Région Sud and GIS IBiSA. C.G. and the 3D-Hub-S core facility were supported financially by Cancéropôle Provence-Alpes-Côte d’Azur and by the GIS IBiSA. We would like to thank Ray Cooke for his careful reading and editing.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Lucie Thorel, Marion Perréard, Romane Florent.
These authors jointly supervised this work: Louis-Bastien Weiswald, Laurent Poulain.
Contributor Information
Louis-Bastien Weiswald, Email: lb.weiswald@baclesse.unicancer.fr.
Laurent Poulain, Email: l.poulain@baclesse.unicancer.fr.
References
- 1.Scherer, W. F., Syverton, J. T. & Gey, G. O. Studies on the propagation in vitro of poliomyelitis viruses. J. Exp. Med.97, 695–710 (1953). 10.1084/jem.97.5.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrell, B. How accurate are cancer cell lines? Nature463, 858–858 (2010). 10.1038/463858a [DOI] [PubMed] [Google Scholar]
- 3.Sutherland, R. M., Inch, W. R., McCredie, J. A. & Kruuv, J. A multi-component radiation survival curve using an in vitro tumour model. Int J. Radiat. Biol. Relat. Stud. Phys. Chem. Med.18, 491–495 (1970). 10.1080/09553007014551401 [DOI] [PubMed] [Google Scholar]
- 4.Weiswald, L.-B., Bellet, D. & Dangles-Marie, V. Spherical cancer models in tumor biology. Neoplasia17, 1–15 (2015). 10.1016/j.neo.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman, A. E. & Hoffman, R. M. In vivo-like growth of human tumors in vitro. Proc. Natl Acad. Sci. USA83, 2694–2698 (1986). 10.1073/pnas.83.8.2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjerkvig, R., Tønnesen, A., Laerum, O. D. & Backlund, E. O. Multicellular tumor spheroids from human gliomas maintained in organ culture. J. Neurosurg.72, 463–475 (1990). 10.3171/jns.1990.72.3.0463 [DOI] [PubMed] [Google Scholar]
- 7.Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA100, 3983–3988 (2003). 10.1073/pnas.0530291100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh, S. K. et al. Identification of a cancer stem cell in human brain tumors. Cancer Res.63, 5821–5828 (2003). [PubMed] [Google Scholar]
- 9.Weiswald, L.-B. et al. Newly characterised ex vivo colospheres as a three-dimensional colon cancer cell model of tumour aggressiveness. Br. J. Cancer101, 473–482 (2009). 10.1038/sj.bjc.6605173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature459, 262–265 (2009). 10.1038/nature07935 [DOI] [PubMed] [Google Scholar]
- 11.Kim, J., Koo, B.-K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol.21, 571–584 (2020). 10.1038/s41580-020-0259-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology141, 1762–1772 (2011). 10.1053/j.gastro.2011.07.050 [DOI] [PubMed] [Google Scholar]
- 13.Foo, M. A. et al. Clinical translation of patient-derived tumour organoids- bottlenecks and strategies. Biomark. Res.10, 10 (2022). 10.1186/s40364-022-00356-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driehuis, E., Kretzschmar, K. & Clevers, H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat. Protoc.15, 3380–3409 (2020). 10.1038/s41596-020-0379-4 [DOI] [PubMed] [Google Scholar]
- 15.Xu, H. et al. Organoid technology and applications in cancer research. J. Hematol. Oncol.11, 116 (2018). 10.1186/s13045-018-0662-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbet, V. & Broutier, L. Future Match Making: When Pediatric Oncology Meets Organoid Technology. Front. Cell Dev. Biol.9, 674219 (2021). 10.3389/fcell.2021.674219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan, H., Demirci, U. & Chen, P. Emerging organoid models: leaping forward in cancer research. J. Hematol. Oncol.12, 142 (2019). 10.1186/s13045-019-0832-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Wetering, M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell161, 933–945 (2015). 10.1016/j.cell.2015.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broutier, L. et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med.23, 1424–1435 (2017). 10.1038/nm.4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, S. H. et al. Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell173, 515–528.e17 (2018). 10.1016/j.cell.2018.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeRose, Y. S. et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat. Med.17, 1514–1520 (2011). 10.1038/nm.2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopper, O. et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med.25, 838–849 (2019). 10.1038/s41591-019-0422-6 [DOI] [PubMed] [Google Scholar]
- 23.Dolega, M. E., Abeille, F., Picollet-D’hahan, N. & Gidrol, X. Controlled 3D culture in Matrigel microbeads to analyze clonal acinar development. Biomaterials52, 347–357 (2015). 10.1016/j.biomaterials.2015.02.042 [DOI] [PubMed] [Google Scholar]
- 24.Passaniti, A., Kleinman, H. K. & Martin, G. R. Matrigel: history/background, uses, and future applications. J. Cell Commun. Signal16, 621–626 (2022). 10.1007/s12079-021-00643-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sachs, N., Tsukamoto, Y., Kujala, P., Peters, P. J. & Clevers, H. Intestinal epithelial organoids fuse to form self-organizing tubes in floating collagen gels. Development144, 1107–1112 (2017). 10.1242/dev.143933 [DOI] [PubMed] [Google Scholar]
- 26.Sokol, E. S. et al. Growth of human breast tissues from patient cells in 3D hydrogel scaffolds. Breast Cancer Res.18, 19 (2016). 10.1186/s13058-016-0677-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capeling, M. M. et al. Nonadhesive Alginate Hydrogels Support Growth of Pluripotent Stem Cell-Derived Intestinal Organoids. Stem Cell Rep.12, 381–394 (2019). 10.1016/j.stemcr.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giobbe, G. G. et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun.10, 5658 (2019). 10.1038/s41467-019-13605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gjorevski, N. & Lutolf, M. P. Synthesis and characterization of well-defined hydrogel matrices and their application to intestinal stem cell and organoid culture. Nat. Protoc.12, 2263–2274 (2017). 10.1038/nprot.2017.095 [DOI] [PubMed] [Google Scholar]
- 30.Lancaster, M. A. et al. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol.35, 659–666 (2017). 10.1038/nbt.3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel, S., Alam, A., Pant, R. & Chattopadhyay, S. Wnt Signaling and Its Significance Within the Tumor Microenvironment: Novel Therapeutic Insights. Front. Immunol.10, 2872 (2019). 10.3389/fimmu.2019.02872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujii, M. et al. A Colorectal Tumor Organoid Library Demonstrates Progressive Loss of Niche Factor Requirements during Tumorigenesis. Cell Stem Cell18, 827–838 (2016). 10.1016/j.stem.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 33.Drost, J. et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature521, 43–47 (2015). 10.1038/nature14415 [DOI] [PubMed] [Google Scholar]
- 34.Verissimo, C. S. et al. Targeting mutant RAS in patient-derived colorectal cancer organoids by combinatorial drug screening. Elife5, e18489 (2016). 10.7554/eLife.18489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, X., Ootani, A. & Kuo, C. An Air-Liquid Interface Culture System for 3D Organoid Culture of Diverse Primary Gastrointestinal Tissues. Methods Mol. Biol.1422, 33–40 (2016). 10.1007/978-1-4939-3603-8_4 [DOI] [PubMed] [Google Scholar]
- 36.Neal, J. T. et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell175, 1972–1988.e16 (2018). 10.1016/j.cell.2018.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Co, J. Y., Margalef-Català, M., Monack, D. M. & Amieva, M. R. Controlling the polarity of human gastrointestinal organoids to investigate epithelial biology and infectious diseases. Nat. Protoc.16, 5171–5192 (2021). 10.1038/s41596-021-00607-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacob, F. et al. A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-tumoral Heterogeneity. Cell180, 188–204.e22 (2020). 10.1016/j.cell.2019.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bürtin, F. et al. Creation and Maintenance of a Living Biobank - How We Do It. J. Vis. Exp.10.3791/62065. (2021). [DOI] [PubMed]
- 40.Hoare, O. et al. Exploring the Complementarity of Pancreatic Ductal Adenocarcinoma Preclinical Models. Cancers13, 2473 (2021). 10.3390/cancers13102473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Witte, C. J. et al. Patient-Derived Ovarian Cancer Organoids Mimic Clinical Response and Exhibit Heterogeneous Inter- and Intrapatient Drug Responses. Cell Rep.31, 107762 (2020). 10.1016/j.celrep.2020.107762 [DOI] [PubMed] [Google Scholar]
- 42.Derouet, M. F. et al. Towards personalized induction therapy for esophageal adenocarcinoma: organoids derived from endoscopic biopsy recapitulate the pre-treatment tumor. Sci. Rep.10, 14514 (2020). 10.1038/s41598-020-71589-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganesh, K. et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat. Med.25, 1607–1614 (2019). 10.1038/s41591-019-0584-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vlachogiannis, G. et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science359, 920–926 (2018). 10.1126/science.aao2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorski, J. W. et al. Utilizing Patient-Derived Epithelial Ovarian Cancer Tumor Organoids to Predict Carboplatin Resistance. Biomedicines9, 1021 (2021). 10.3390/biomedicines9081021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu, H.-D., Xia, B.-R., Jin, M.-Z. & Lou, G. Organoid of ovarian cancer: genomic analysis and drug screening. Clin. Transl. Oncol.22, 1240–1251 (2020). 10.1007/s12094-019-02276-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Becker, L. et al. Data-Driven Identification of Biomarkers for In Situ Monitoring of Drug Treatment in Bladder Cancer Organoids. Int. J. Mol. Sci.23, 6956 (2022). 10.3390/ijms23136956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spiller, E. R. et al. Imaging-Based Machine Learning Analysis of Patient-Derived Tumor Organoid Drug Response. Front. Oncol.11, 771173 (2021). 10.3389/fonc.2021.771173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharick, J. T. et al. Metabolic Heterogeneity in Patient Tumor-Derived Organoids by Primary Site and Drug Treatment. Front. Oncol.10, 553 (2020). 10.3389/fonc.2020.00553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neef, S. K. et al. Metabolic Drug Response Phenotyping in Colorectal Cancer Organoids by LC-QTOF-MS. Metabolites10, 494 (2020). 10.3390/metabo10120494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao, Y. et al. Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell Stem Cell26, 17–26.e6 (2020). 10.1016/j.stem.2019.10.010 [DOI] [PubMed] [Google Scholar]
- 52.Larsen, B. M. et al. A pan-cancer organoid platform for precision medicine. Cell Rep.36, 109429 (2021). 10.1016/j.celrep.2021.109429 [DOI] [PubMed] [Google Scholar]
- 53.Scognamiglio, G. et al. Patient-derived organoids as a potential model to predict response to PD-1/PD-L1 checkpoint inhibitors. Br. J. Cancer121, 979–982 (2019). 10.1038/s41416-019-0616-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dekkers, J. F. et al. Modeling Breast Cancer Using CRISPR-Cas9-Mediated Engineering of Human Breast Organoids. J. Natl Cancer Inst.112, 540–544 (2020). 10.1093/jnci/djz196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seino, T. et al. Human Pancreatic Tumor Organoids Reveal Loss of Stem Cell Niche Factor Dependence during Disease Progression. Cell Stem Cell22, 454–467.e6 (2018). 10.1016/j.stem.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 56.Hu, T. et al. Metabolic Rewiring by Loss of Sirt5 Promotes Kras-Induced Pancreatic Cancer Progression. Gastroenterology161, 1584–1600 (2021). 10.1053/j.gastro.2021.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li, F. et al. Identification of ARGLU1 as a potential therapeutic target for gastric cancer based on genome-wide functional screening data. EBioMedicine69, 103436 (2021). 10.1016/j.ebiom.2021.103436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Picollet-D’hahan, N., Dolega, M. E., Freida, D., Martin, D. K. & Gidrol, X. Deciphering Cell Intrinsic Properties: A Key Issue for Robust Organoid Production. Trends Biotechnol.35, 1035–1048 (2017). 10.1016/j.tibtech.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 59.Guillon, J. et al. Chemotherapy-induced senescence, an adaptive mechanism driving resistance and tumor heterogeneity. Cell Cycle18, 2385–2397 (2019). 10.1080/15384101.2019.1652047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Germain, N. et al. Lipid Metabolism and Resistance to Anticancer Treatment. Biology9, 474 (2020). 10.3390/biology9120474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strauss, J. & Figg, W. D. Epigenetic approaches to overcoming chemotherapy resistance. Lancet Oncol.16, 1013–1015 (2015). 10.1016/S1470-2045(15)00231-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El Amrani, M. et al. Gemcitabine-induced epithelial-mesenchymal transition-like changes sustain chemoresistance of pancreatic cancer cells of mesenchymal-like phenotype. Mol. Carcinog.58, 1985–1997 (2019). 10.1002/mc.23090 [DOI] [PubMed] [Google Scholar]
- 63.Fernandes, M., Jamme, P., Cortot, A. B., Kherrouche, Z. & Tulasne, D. When the MET receptor kicks in to resist targeted therapies. Oncogene40, 4061–4078 (2021). 10.1038/s41388-021-01835-0 [DOI] [PubMed] [Google Scholar]
- 64.Sundar, S. J. et al. Three-dimensional organoid culture unveils resistance to clinical therapies in adult and pediatric glioblastoma. Transl. Oncol.15, 101251 (2021). 10.1016/j.tranon.2021.101251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farshadi, E. A. et al. Organoids Derived from Neoadjuvant FOLFIRINOX Patients Recapitulate Therapy Resistance in Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res.27, 6602–6612 (2021). 10.1158/1078-0432.CCR-21-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang, L. et al. PDX-derived organoids model in vivo drug response and secrete biomarkers. JCI Insight5, e135544 (2020). 10.1172/jci.insight.135544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hadj Bachir, E. et al. A new pancreatic adenocarcinoma-derived organoid model of acquired chemoresistance to FOLFIRINOX: First insight of the underlying mechanisms. Biol. Cell114, 32–55 (2022). 10.1111/boc.202100003 [DOI] [PubMed] [Google Scholar]
- 68.Driehuis, E. et al. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc. Natl Acad. Sci.116, 26580–26590 (2019). 10.1073/pnas.1911273116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan, P. et al. SRT1720 inhibits the growth of bladder cancer in organoids and murine models through the SIRT1-HIF axis. Oncogene40, 6081–6092 (2021). 10.1038/s41388-021-01999-9 [DOI] [PubMed] [Google Scholar]
- 70.Yan, H. H. N. et al. A Comprehensive Human Gastric Cancer Organoid Biobank Captures Tumor Subtype Heterogeneity and Enables Therapeutic Screening. Cell Stem Cell23, 882–897.e11 (2018). 10.1016/j.stem.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 71.Sachs, N. et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell172, 373–386.e10 (2018). 10.1016/j.cell.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 72.Calandrini, C. et al. Organoid-based drug screening reveals neddylation as therapeutic target for malignant rhabdoid tumors. Cell Rep.36, 109568 (2021). 10.1016/j.celrep.2021.109568 [DOI] [PubMed] [Google Scholar]
- 73.Vernon, M. et al. Functional miRNA Screening Identifies Wide-ranging Antitumor Properties of miR-3622b-5p and Reveals a New Therapeutic Combination Strategy in Ovarian Tumor Organoids. Mol. Cancer Therapeutics19, 1506–1519 (2020). 10.1158/1535-7163.MCT-19-0510 [DOI] [PubMed] [Google Scholar]
- 74.Florent, R. et al. Bim, Puma and Noxa upregulation by Naftopidil sensitizes ovarian cancer to the BH3-mimetic ABT-737 and the MEK inhibitor Trametinib. Cell Death Dis.11, 1–16 (2020). 10.1038/s41419-020-2588-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wambecke, A. et al. The lncRNA ‘UCA1’ modulates the response to chemotherapy of ovarian cancer through direct binding to miR‐27a‐5p and control of UBE2N levels. Mol. Oncol.15, 3659–3678 (2021). 10.1002/1878-0261.13045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garvey, C. M. et al. Anti-EGFR Therapy Induces EGF Secretion by Cancer-Associated Fibroblasts to Confer Colorectal Cancer Chemoresistance. Cancers12, 1393 (2020). 10.3390/cancers12061393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Millen, R. et al. Patient-derived head and neck cancer organoids allow treatment stratification and serve as a tool for biomarker validation and identification. Medicine4, 290–310.e12 (2023). 10.1016/j.medj.2023.04.003 [DOI] [PubMed] [Google Scholar]
- 78.Chen, G. et al. Colorectal cancer organoid models uncover oxaliplatin-resistant mechanisms at single cell resolution. Cell Oncol.45, 1155–1167 (2022). 10.1007/s13402-022-00728-y [DOI] [PubMed] [Google Scholar]
- 79.Shi, X. et al. Integrated profiling of human pancreatic cancer organoids reveals chromatin accessibility features associated with drug sensitivity. Nat. Commun.13, 2169 (2022). 10.1038/s41467-022-29857-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Braun, L. M. et al. Metabolic Profiling of Early and Late Recurrent Pancreatic Ductal Adenocarcinoma Using Patient-Derived Organoid Cultures. Cancers12, 1440 (2020). 10.3390/cancers12061440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kong, J. et al. Network-based machine learning in colorectal and bladder organoid models predicts anti-cancer drug efficacy in patients. Nat. Commun.11, 5485 (2020). 10.1038/s41467-020-19313-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ancevski Hunter, K., Socinski, M. A. & Villaruz, L. C. PD-L1 Testing in Guiding Patient Selection for PD-1/PD-L1 Inhibitor Therapy in Lung Cancer. Mol. Diagn. Ther.22, 1–10 (2018). 10.1007/s40291-017-0308-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bailey, M. H. et al. Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell173, 371–385.e18 (2018). 10.1016/j.cell.2018.02.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Letai, A., Bhola, P. & Welm, A. L. Functional precision oncology: Testing tumors with drugs to identify vulnerabilities and novel combinations. Cancer Cell40, 26–35 (2022). 10.1016/j.ccell.2021.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mirza, M. R. et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med.375, 2154–2164 (2016). 10.1056/NEJMoa1611310 [DOI] [PubMed] [Google Scholar]
- 86.Federici, G. & Soddu, S. Variants of uncertain significance in the era of high-throughput genome sequencing: a lesson from breast and ovary cancers. J. Exp. Clin. Cancer Res.39, 46 (2020). 10.1186/s13046-020-01554-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nicolle, R. et al. Relevance of biopsy-derived pancreatic organoids in the development of efficient transcriptomic signatures to predict adjuvant chemosensitivity in pancreatic cancer. Transl. Oncol.16, 101315 (2022). 10.1016/j.tranon.2021.101315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verduin, M., Hoeben, A., De Ruysscher, D. & Vooijs, M. Patient-Derived Cancer Organoids as Predictors of Treatment Response. Front. Oncol.11, 641980 (2021). 10.3389/fonc.2021.641980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wensink, G. E. et al. Patient-derived organoids as a predictive biomarker for treatment response in cancer patients. NPJ Precis Oncol.5, 30 (2021). 10.1038/s41698-021-00168-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsukamoto, Y. et al. Enhanced phosphorylation of c-Jun by cisplatin treatment as a potential predictive biomarker for cisplatin response in combination with patient-derived tumor organoids. Lab. Invest.102, 1355–1366 (2022). 10.1038/s41374-022-00827-2 [DOI] [PubMed] [Google Scholar]
- 91.Beutel, A. K. et al. A Prospective Feasibility Trial to Challenge Patient–Derived Pancreatic Cancer Organoids in Predicting Treatment Response. Cancers13, 2539 (2021). 10.3390/cancers13112539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park, M. et al. A Patient-Derived Organoid-Based Radiosensitivity Model for the Prediction of Radiation Responses in Patients with Rectal Cancer. Cancers13, 3760 (2021). 10.3390/cancers13153760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Driehuis, E. et al. Oral Mucosal Organoids as a Potential Platform for Personalized Cancer Therapy. Cancer Discov.9, 852–871 (2019). 10.1158/2159-8290.CD-18-1522 [DOI] [PubMed] [Google Scholar]
- 94.Hill, S. J. et al. Prediction of DNA Repair Inhibitor Response in Short-Term Patient-Derived Ovarian Cancer Organoids. Cancer Discov.8, 1404–1421 (2018). 10.1158/2159-8290.CD-18-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mauri, G. et al. Empowering Clinical Decision Making in Oligometastatic Colorectal Cancer: The Potential Role of Drug Screening of Patient-Derived Organoids. JCO Precis. Oncol.5, PO.21.00143 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buyse, M. et al. Progression-free survival is a surrogate for survival in advanced colorectal cancer. J. Clin. Oncol.25, 5218–5224 (2007). [DOI] [PubMed]
- 97.Ooft, S. N. et al. (2019) Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients Dishing out treatment recommendations. Sci. Transl. Med. 11, eaay2574 (2019). [DOI] [PubMed]
- 98.Ooft, S. N. et al. Prospective experimental treatment of colorectal cancer patients based on organoid drug responses. ESMO Open6, 100103 (2021). 10.1016/j.esmoop.2021.100103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu, Y. et al. Combining Organoid Models with Next-Generation Sequencing to Reveal Tumor Heterogeneity and Predict Therapeutic Response in Breast Cancer. J. Oncol.2022, 9390912 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tiriac, H. et al. Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discov.8, 1112–1129 (2018). 10.1158/2159-8290.CD-18-0349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao, D. et al. Organoid cultures derived from patients with advanced prostate cancer. Cell159, 176–187 (2014). 10.1016/j.cell.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim, M. et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun.10, 3991 (2019). 10.1038/s41467-019-11867-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dijkstra, K. K. et al. Challenges in Establishing Pure Lung Cancer Organoids Limit Their Utility for Personalized Medicine. Cell Rep.31, 107588 (2020). 10.1016/j.celrep.2020.107588 [DOI] [PubMed] [Google Scholar]
- 104.Wallaschek, N. et al. Establishing Pure Cancer Organoid Cultures: Identification, Selection and Verification of Cancer Phenotypes and Genotypes. J. Mol. Biol.431, 2884–2893 (2019). 10.1016/j.jmb.2019.05.031 [DOI] [PubMed] [Google Scholar]
- 105.Colella, G. et al. Sarcoma Spheroids and Organoids-Promising Tools in the Era of Personalized Medicine. Int J. Mol. Sci.19, 615 (2018). 10.3390/ijms19020615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Majumder, B. et al. Predicting clinical response to anticancer drugs using an ex vivo platform that captures tumour heterogeneity. Nat. Commun.6, 6169 (2015). 10.1038/ncomms7169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heo, J. H., Kang, D., Seo, S. J. & Jin, Y. Engineering the Extracellular Matrix for Organoid Culture. Int J. Stem Cells15, 60–69 (2022). 10.15283/ijsc21190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xie, Z., Wang, L. & Zhang, Y. Advances in Organoid Culture Research. Glob. Med. Genet9, 268–276 (2022). 10.1055/s-0042-1756662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hu, Y. et al. Lung cancer organoids analyzed on microwell arrays predict drug responses of patients within a week. Nat. Commun.12, 2581 (2021). 10.1038/s41467-021-22676-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ding, S. et al. Patient-derived micro-organospheres enable clinical precision oncology. Cell Stem Cell29, 905–917.e6 (2022). 10.1016/j.stem.2022.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Phan, N. et al. A simple high-throughput approach identifies actionable drug sensitivities in patient-derived tumor organoids. Commun. Biol.2, 78 (2019). 10.1038/s42003-019-0305-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim, S. K., Kim, Y. H., Park, S. & Cho, S.-W. Organoid engineering with microfluidics and biomaterials for liver, lung disease, and cancer modeling. Acta Biomater.132, 37–51 (2021). 10.1016/j.actbio.2021.03.002 [DOI] [PubMed] [Google Scholar]
- 113.Larsen, B. M., Cancino, A., Shaxted, J. M. & Salahudeen, A. A. Protocol for drug screening of patient-derived tumor organoids using high-content fluorescent imaging. STAR Protoc.3, 101407 (2022). 10.1016/j.xpro.2022.101407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brandenberg, N. et al. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat. Biomed. Eng.4, 863–874 (2020). 10.1038/s41551-020-0565-2 [DOI] [PubMed] [Google Scholar]
- 115.Wang, J. et al. Patient-Derived Tumor Organoids: New Progress and Opportunities to Facilitate Precision Cancer Immunotherapy. Front. Oncol.12, 872531 (2022). 10.3389/fonc.2022.872531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu, L., Yu, L., Li, Z., Li, W. & Huang, W. Patient-derived organoid (PDO) platforms to facilitate clinical decision making. J. Transl. Med.19, 40 (2021). 10.1186/s12967-020-02677-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qu, J., Kalyani, F. S., Liu, L., Cheng, T. & Chen, L. Tumor organoids: synergistic applications, current challenges, and future prospects in cancer therapy. Cancer Commun.41, 1331–1353 (2021). 10.1002/cac2.12224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fan, T. W. et al. Innate immune activation by checkpoint inhibition in human patient-derived lung cancer tissues. Elife10, e69578 (2021). 10.7554/eLife.69578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martin, S. Z. et al. Ex vivo tissue slice culture system to measure drug-response rates of hepatic metastatic colorectal cancer. BMC Cancer19, 1030 (2019). 10.1186/s12885-019-6270-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saleh, R., Toor, S. M., Al-Ali, D., Sasidharan Nair, V. & Elkord, E. Blockade of PD-1, PD-L1, and TIM-3 Altered Distinct Immune- and Cancer-Related Signaling Pathways in the Transcriptome of Human Breast Cancer Explants. Genes11, 703 (2020). 10.3390/genes11060703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wan, C. et al. Enhanced Efficacy of Simultaneous PD-1 and PD-L1 Immune Checkpoint Blockade in High-Grade Serous Ovarian Cancer. Cancer Res.81, 158–173 (2021). 10.1158/0008-5472.CAN-20-1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ao, Z. et al. Rapid Profiling of Tumor-Immune Interaction Using Acoustically Assembled Patient-Derived Cell Clusters. Adv. Sci.9, e2201478 (2022). 10.1002/advs.202201478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Knochelmann, H. M. et al. Modeling ex vivo tumor-infiltrating lymphocyte expansion from established solid malignancies. Oncoimmunology10, 1959101 (2021). 10.1080/2162402X.2021.1959101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bonnereau, J. et al. Autologous T cell responses to primary human colorectal cancer spheroids are enhanced by ectonucleotidase inhibition. Gut72, 699–709 (2023). 10.1136/gutjnl-2021-326553 [DOI] [PubMed] [Google Scholar]
- 125.Hammoudi, N. et al. Autologous organoid co-culture model reveals T cell-driven epithelial cell death in Crohn’s Disease. Front. Immunol.13, 1008456 (2022). 10.3389/fimmu.2022.1008456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kong, J. C. H. et al. Tumor-Infiltrating Lymphocyte Function Predicts Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. JCO Precis. Oncol.2, 1–15 (2018). 10.1200/PO.18.00075 [DOI] [PubMed] [Google Scholar]
- 127.Nozaki, K. et al. Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J. Gastroenterol.51, 206–213 (2016). 10.1007/s00535-016-1170-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Votanopoulos, K. I. et al. Model of Patient-Specific Immune-Enhanced Organoids for Immunotherapy Screening: Feasibility Study. Ann. Surg. Oncol.27, 1956–1967 (2020). 10.1245/s10434-019-08143-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Forsythe, S. D. et al. Organoid Platform in Preclinical Investigation of Personalized Immunotherapy Efficacy in Appendiceal Cancer: Feasibility Study. Clin. Cancer Res.27, 5141–5150 (2021). 10.1158/1078-0432.CCR-21-0982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Forsythe, S. D. et al. Application of immune enhanced organoids in modeling personalized Merkel cell carcinoma research. Sci. Rep.12, 13865 (2022). 10.1038/s41598-022-17921-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Holokai, L. et al. Murine- and Human-Derived Autologous Organoid/Immune Cell Co-Cultures as Pre-Clinical Models of Pancreatic Ductal Adenocarcinoma. Cancers12, 3816 (2020). 10.3390/cancers12123816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tsai, S. et al. Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer18, 335 (2018). 10.1186/s12885-018-4238-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dijkstra, K. K. et al. Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell174, 1586–1598.e12 (2018). 10.1016/j.cell.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chalabi, M. et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med.26, 566–576 (2020). 10.1038/s41591-020-0805-8 [DOI] [PubMed] [Google Scholar]
- 135.Cattaneo, C. M. et al. Tumor organoid-T-cell coculture systems. Nat. Protoc.15, 15–39 (2020). 10.1038/s41596-019-0232-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang, Q. et al. The primordial differentiation of tumor-specific memory CD8+ T cells as bona fide responders to PD-1/PD-L1 blockade in draining lymph nodes. Cell185, 4049–4066.e25 (2022). 10.1016/j.cell.2022.09.020 [DOI] [PubMed] [Google Scholar]
- 137.Clinton, J. & McWilliams-Koeppen, P. Initiation, Expansion, and Cryopreservation of Human Primary Tissue-Derived Normal and Diseased Organoids in Embedded Three-Dimensional Culture. Curr. Protoc. Cell Biol.82, e66 (2019). 10.1002/cpcb.66 [DOI] [PubMed] [Google Scholar]
- 138.Zhao, H., Jiang, E. & Shang, Z. 3D Co-culture of Cancer-Associated Fibroblast with Oral Cancer Organoids. J. Dent. Res.100, 201–208 (2021). 10.1177/0022034520956614 [DOI] [PubMed] [Google Scholar]
- 139.Chen, X. et al. Phenotype transition of fibroblasts incorporated into patient-derived oral carcinoma organoids. Oral. Dis.29, 913–922 (2023). 10.1111/odi.14071 [DOI] [PubMed] [Google Scholar]
- 140.Öhlund, D. et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med.214, 579–596 (2017). 10.1084/jem.20162024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen, S. et al. Cancer-associated fibroblasts suppress SOX2-induced dysplasia in a lung squamous cancer coculture. Proc. Natl Acad. Sci. USA115, E11671–E11680 (2018). 10.1073/pnas.1803718115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu, J. et al. Cancer-Associated Fibroblasts Provide a Stromal Niche for Liver Cancer Organoids That Confers Trophic Effects and Therapy Resistance. Cell Mol. Gastroenterol. Hepatol.11, 407–431 (2021). 10.1016/j.jcmgh.2020.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Truelsen, S. L. B. et al. The cancer angiogenesis co-culture assay: In vitro quantification of the angiogenic potential of tumoroids. PLoS One16, e0253258 (2021). 10.1371/journal.pone.0253258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Arslan, U. et al. Vascularized hiPSC-derived 3D cardiac microtissue on chip. Stem Cell Rep.18, 1394–1404 (2023). 10.1016/j.stemcr.2023.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nashimoto, Y. et al. Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials229, 119547 (2020). 10.1016/j.biomaterials.2019.119547 [DOI] [PubMed] [Google Scholar]
- 146.Quintard, C. et al. A microfluidic platform integrating functional vascularized organoids-on-chip. Nat. Commun.15, 1452 (2024). 10.1038/s41467-024-45710-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hachey, S. J. et al. An in vitro vascularized micro-tumor model of human colorectal cancer recapitulates in vivo responses to standard-of-care therapy. Lab a Chip21, 1333–1351 (2021). 10.1039/D0LC01216E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ugrinic, M. et al. Fabrication of high aspect ratio microfluidic devices for long term in vitro culture of 3D tumor models. Microelectronic Eng.267–268, 111898 (2023).
- 149.Haque, M. R. et al. Patient-derived pancreatic cancer-on-a-chip recapitulates the tumor microenvironment. Microsyst. Nanoeng.8, 36 (2022). 10.1038/s41378-022-00370-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Russell, T. et al. Cortical spheroid on perfusable microvascular network in a microfluidic device. Plos One18, 1–14 (2023). 10.1371/journal.pone.0288025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Geyer, M. et al. A microfluidic-based PDAC organoid system reveals the impact of hypoxia in response to treatment. Cell Death Discov.9, 20 (2023). 10.1038/s41420-023-01334-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee, S.-R. et al. U-IMPACT: a universal 3D microfluidic cell culture platform. Microsyst. Nanoeng.8, 126 (2022). 10.1038/s41378-022-00431-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cai, H. et al. Trapping cell spheroids and organoids using digital acoustofluidics. Biofabrication12, 035025 (2020). 10.1088/1758-5090/ab9582 [DOI] [PubMed] [Google Scholar]
- 154.Quintard, C. et al. Microfluidic device integrating a network of hyper-elastic valves for automated glucose stimulation and insulin secretion collection from a single pancreatic islet. Biosens. Bioelectron.202, 113967 (2022). 10.1016/j.bios.2022.113967 [DOI] [PubMed] [Google Scholar]
- 155.Wang, X. et al. A Tumor‐Organoid‐Based Precision Medicine Platform for the Prediction of Drug Sensitivity of Colorectal Cancer. Adv. Therapeutics5, 2200093 (2022).
- 156.Schuster, B. et al. Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nat. Commun.11, 5271 (2020). 10.1038/s41467-020-19058-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pinho, D., Santos, D., Vila, A. & Carvalho, S. Establishment of colorectal cancer organoids in microfluidic-based system. Micromachines12, 497 (2021). [DOI] [PMC free article] [PubMed]
- 158.Cavarzerani, E., Caligiuri, I., Bartoletti, M., Canzonieri, V. & Rizzolio, F. 3D dynamic cultures of HGSOC organoids to model innovative and standard therapies. Front. Bioeng. Biotechnol.11, 1–13 (2023). 10.3389/fbioe.2023.1135374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hachey, S. J. et al. A human vascularized microtumor model of patient-derived colorectal cancer recapitulates clinical disease. Transl. Res.255, 97–108 (2023). [DOI] [PMC free article] [PubMed]
- 160.Rajasekar, S. et al. IFlowPlate—A Customized 384‐Well Plate for the Culture of Perfusable Vascularized Colon Organoids. Adva. Mater.32, e2002974 (2020). [DOI] [PubMed]
- 161.Palikuqi, B. et al. Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature585, 426–432 (2020). 10.1038/s41586-020-2712-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kroll, K. T. et al. Immune-infiltrated kidney organoid-on-chip model for assessing T cell bispecific antibodies. Proc. Natl Acad. Sci.120, 2017 (2023). 10.1073/pnas.2305322120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bussooa, A. et al. Real-time monitoring of oxygen levels within thermoplastic Organ-on-Chip devices. Biosens. Bioelectron. X11, 4–11 (2022). [Google Scholar]
- 164.Dornhof, J. et al. Microfluidic organ-on-chip system for multi-analyte monitoring of metabolites in 3D cell cultures. Lab Chip22, 225–239 (2022). 10.1039/D1LC00689D [DOI] [PubMed] [Google Scholar]
- 165.Votanopoulos, K. I. et al. Appendiceal Cancer Patient-Specific Tumor Organoid Model for Predicting Chemotherapy Efficacy Prior to Initiation of Treatment: A Feasibility Study. Ann. Surg. Oncol.26, 139–147 (2019). 10.1245/s10434-018-7008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Saito, Y. et al. Establishment of Patient-Derived Organoids and Drug Screening for Biliary Tract Carcinoma. Cell Rep.27, 1265–1276.e4 (2019). 10.1016/j.celrep.2019.03.088 [DOI] [PubMed] [Google Scholar]
- 167.Wang, Z. et al. Establishment and drug screening of patient-derived extrahepatic biliary tract carcinoma organoids. Cancer Cell Int.21, 519 (2021). 10.1186/s12935-021-02219-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schütte, M. et al. Molecular dissection of colorectal cancer in pre-clinical models identifies biomarkers predicting sensitivity to EGFR inhibitors. Nat. Commun.8, 14262 (2017). 10.1038/ncomms14262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Buzzelli, J. N., Ouaret, D., Brown, G., Allen, P. D. & Muschel, R. J. Colorectal cancer liver metastases organoids retain characteristics of original tumor and acquire chemotherapy resistance. Stem Cell Res.27, 109–120 (2018). 10.1016/j.scr.2018.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Narasimhan, V. et al. Medium-throughput Drug Screening of Patient-derived Organoids from Colorectal Peritoneal Metastases to Direct Personalized Therapy. Clin. Cancer Res.26, 3662–3670 (2020). 10.1158/1078-0432.CCR-20-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nanki, K. et al. Divergent Routes toward Wnt and R-spondin Niche Independency during Human Gastric Carcinogenesis. Cell174, 856–869.e17 (2018). 10.1016/j.cell.2018.07.027 [DOI] [PubMed] [Google Scholar]
- 172.Li, J. et al. Malignant ascites-derived organoid (MADO) cultures for gastric cancer in vitro modelling and drug screening. J. Cancer Res. Clin. Oncol.145, 2637–2647 (2019). 10.1007/s00432-019-03004-z [DOI] [PubMed] [Google Scholar]
- 173.Kawasaki, K. et al. An Organoid Biobank of Neuroendocrine Neoplasms Enables Genotype-Phenotype Mapping. Cell183, 1420–1435.e21 (2020). 10.1016/j.cell.2020.10.023 [DOI] [PubMed] [Google Scholar]
- 174.D’Agosto, S. et al. Long-term organoid culture of a small intestinal neuroendocrine tumor. Front. Endocrinol.14, 999792 (2023). 10.3389/fendo.2023.999792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dijkstra, K. K. et al. Patient-Derived Organoid Models of Human Neuroendocrine Carcinoma. Front. Endocrinol.12, 627819 (2021). 10.3389/fendo.2021.627819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nuciforo, S. et al. Organoid Models of Human Liver Cancers Derived from Tumor Needle Biopsies. Cell Rep.24, 1363–1376 (2018). 10.1016/j.celrep.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Beato, F. et al. Establishing a Living Biobank of Patient-Derived Organoids of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Lab. Invest.101, 204–217 (2021). 10.1038/s41374-020-00494-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mazzucchelli, S. et al. Establishment and Morphological Characterization of Patient-Derived Organoids from Breast Cancer. Biol. Proced. Online21, 12 (2019). 10.1186/s12575-019-0099-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Maru, Y., Tanaka, N., Itami, M. & Hippo, Y. Efficient use of patient-derived organoids as a preclinical model for gynecologic tumors. Gynecol. Oncol.154, 189–198 (2019). 10.1016/j.ygyno.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 180.Boretto, M. et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol.21, 1041–1051 (2019). 10.1038/s41556-019-0360-z [DOI] [PubMed] [Google Scholar]
- 181.Tanaka, N. et al. Head and neck cancer organoids established by modification of the CTOS method can be used to predict in vivo drug sensitivity. Oral. Oncol.87, 49–57 (2018). 10.1016/j.oraloncology.2018.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kijima, T. et al. Three-Dimensional Organoids Reveal Therapy Resistance of Esophageal and Oropharyngeal Squamous Cell Carcinoma Cells. Cell Mol. Gastroenterol. Hepatol.7, 73–91 (2019). 10.1016/j.jcmgh.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Karakasheva, T. A. et al. Generation and characterization of patient-derived esophageal cancer organoids. Curr. Protoc. Stem Cell Biol.53, e109 (2020). 10.1002/cpsc.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li, X. et al. Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat. Commun.9, 2983 (2018). 10.1038/s41467-018-05190-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang, B. et al. An organoid library of salivary gland tumors reveals subtype-specific characteristics and biomarkers. J. Exp. Clin. Cancer Res.41, 350 (2022). 10.1186/s13046-022-02561-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lassche, G. et al. Development and characterization of patient-derived salivary gland cancer organoid cultures. Oral. Oncol.135, 106186 (2022). 10.1016/j.oraloncology.2022.106186 [DOI] [PubMed] [Google Scholar]
- 187.Sondorp, L. H. J. et al. Patient-Derived Papillary Thyroid Cancer Organoids for Radioactive Iodine Refractory Screening. Cancers12, 3212 (2020). 10.3390/cancers12113212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang, H. et al. Establishment of papillary thyroid cancer organoid lines from clinical specimens. Front. Endocrinol.14, 1140888 (2023). 10.3389/fendo.2023.1140888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jiang, Y. et al. Patient-derived bladder cancer organoid model to predict sensitivity and feasibility of tailored precision therapy. Curr. Urol.17, 221–228 (2023). 10.1097/CU9.0000000000000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bolck, H. A. et al. Tracing Clonal Dynamics Reveals that Two- and Three-dimensional Patient-derived Cell Models Capture Tumor Heterogeneity of Clear Cell Renal Cell Carcinoma. Eur. Urol. Focus7, 152–162 (2021). 10.1016/j.euf.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 191.Li, Z. et al. Patient‐derived renal cell carcinoma organoids for personalized cancer therapy. Clin. Transl. Med.12, e970 (2022). 10.1002/ctm2.970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Puca, L. et al. Patient derived organoids to model rare prostate cancer phenotypes. Nat. Commun.9, 2404 (2018). 10.1038/s41467-018-04495-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Verduin, M. et al. Patient-derived glioblastoma organoids reflect tumor heterogeneity and treatment sensitivity. Neuro Oncol. Adv.5, vdad152 (2023). [DOI] [PMC free article] [PubMed]
- 194.Sachs, N. et al. Long-term expanding human airway organoids for disease modeling. EMBO J.38, e100300 (2019). 10.15252/embj.2018100300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mazzocchi, A. R., Rajan, S. A. P., Votanopoulos, K. I., Hall, A. R. & Skardal, A. In vitro patient-derived 3D mesothelioma tumor organoids facilitate patient-centric therapeutic screening. Sci. Rep.8, 2886 (2018). 10.1038/s41598-018-21200-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fang, X. et al. Organoids derived from patients provide a new opportunity for research and individualized treatment of malignant peritoneal mesothelioma. Mol. Cancer23, 12 (2024). 10.1186/s12943-023-01901-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ou, L. et al. Patient-derived melanoma organoid models facilitate the assessment of immunotherapies. EBioMedicine92, 104614 (2023). 10.1016/j.ebiom.2023.104614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sun, L. et al. A human mucosal melanoma organoid platform for modeling tumor heterogeneity and exploring immunotherapy combination options. Sci. Adv.9, eadg6686 (2023). 10.1126/sciadv.adg6686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Thorel, L. et al. Patient-derived tumor organoids (or tumoroid), a growing preclinical model for oncology. Med. Sci. (Paris)38, 880–887 (2022). [DOI] [PubMed]
- 200.Perréard, M. et al. Patient-derived tumor organoids (or tumoroid) as valuable precision medicine tools. Med. Sci. (Paris)38, 888–895 (2022). [DOI] [PubMed]