Abstract
Helicobacter pylori (H. pylori) is responsible for various chronic or acute diseases, such as stomach ulcers, dyspepsia, peptic ulcers, gastroesophageal reflux, gastritis, lymphoma, and stomach cancers. Although specific drugs are available to treat the bacterium's harmful effects, there is an urgent need to develop a preventive or therapeutic vaccine. Therefore, the current study aims to create a multi-epitope vaccine against H. pylori using lipid nanoparticles. Five epitopes from five target proteins of H. pylori, namely, Urease, CagA, HopE, SabA, and BabA, were used. Immunogenicity, MHC (Major Histocompatibility Complex) bonding, allergenicity, toxicity, physicochemical analysis, and global population coverage of the entire epitopes and final construct were carefully examined. The study involved using various bioinformatic web tools to accomplish the following tasks: modeling the three-dimensional structure of a set of epitopes and the final construct and docking them with Toll-Like Receptor 4 (TLR4). In the experimental phase, the final multi-epitope construct was synthesized using the solid phase method, and it was then enclosed in lipid nanoparticles. After synthesizing the construct, its loading, average size distribution, and nanoliposome shape were checked using Nanodrop at 280 nm, dynamic light scattering (DLS), and atomic force microscope (AFM). The designed vaccine has been confirmed to be non-toxic and anti-allergic. It can bind with different MHC alleles at a rate of 99.05%. The construct loading was determined to be about 91%, with an average size of 54 nm. Spherical shapes were also observed in the AFM images. Further laboratory tests are necessary to confirm the safety and immunogenicity of the multi-epitope vaccine.
Keywords: Helicobacter pylori, Immunoinformatics, Multi-epitope, Vaccine
Subject terms: Immunology, Vaccines, Peptide vaccines
Introduction
Helicobacter pylori (H. pylori) is considered one of the most common sources of infection in humans1. The relationship between H. pylori and different acute or chronic diseases, e.g., ulcers, dyspepsia, gastroesophageal reflux, gastritis, and stomach cancer, is quite well-known and documented2,3. Moreover, the bacterium genus is associated with various extra-gastric disorders, including coronary artery diseases, strokes, peripheral artery conditions, skin disorders, chronic kidney disease, respiratory diseases, and insulin resistance2. The bacterium H. pylori is usually found in the gastric mucosa in childhood, remaining there throughout one’s life. Per one estimate, while half of the world’s population is exposed and infected with H. pylori, levels of infection are significantly higher in countries with low socio-economic standings4.
So, a vaccine to fight against this pathogenic agent has to be robust to provide prevention against different gastric and extra-gastric disorders5. Even though designing and developing a vaccine to prevent or even eradicate H. pylori infections and complications presents a challenging task, numerous studies have shown the possibility of developing one using a wide range of antigens and adjuvants. In this regard, the important point is to choose the right protein target in designing the vaccine. It is thought that both virulent factors and surface proteins are suitable targets. For example, CagA is one of the main virulence factors of H. pylori. As some previous studies report, CagA-positive strains are significantly present in populations with gastric ulcers and precancerous lesions6. The urease enzyme is used to fight the acidic environment of the stomach by producing ammonia7. VacA exotoxin causes damage to the mucosal membrane8. Outer proteins such as BabA, Oip, SabA, and other adhesins adhere to the host cells9. The H. pylori genome encodes several adhesive proteins facilitating strong contact between H. pylori and gastric epithelial cells. These proteins include the blood group antigen-binding adhesin and the sialic acid-binding adhesin10. Urease, CagA, HopE, BabA, and SabA are antigen determinants recognized explicitly by antibodies, B cells, and/or T cells, capable of stimulating an immune response11.
Since H. pylori eludes the immune system, it is impossible to identify. Using the H. pylori vaccine can help the immune system do the identification task better and sooner. We think the developed vaccines have both preventive and therapeutic natures. Further, it is predicted that humans will probably use such vaccines in the near future. In this study, our goal was to develop a multi-epitope vaccine against H. pylori using lipid nanoparticles. We utilized five epitopes from five target proteins (Urease, CagA, HopE, BabA, and SabA). We investigated the immunogenicity, MHC (Major Histocompatibility Complex) binding, allergenicity, toxicity, physicochemical properties, and global population coverage of the individual epitopes and the final construct using various bioinformatics web tools.
Results
Immunoinformatic results
Based on the sequence of target proteins- including Urease, CagA, HopE, SabA, and BabA (Supplementary 1)—the linear B-cell epitopes were predicted for each target by the ABCpred web server (Supplementary 2–6). The best linear epitopes with the highest score were selected for each protein target. In addition, an analysis was made of antigenicity, allergenicity, toxicity, and MHC alleles of all epitopes. Finally, five epitopes were selected for five target proteins (Table 1). Further, the ability of the best epitope to induce Interleukin 4 (IL4), Interleukin 10 (IL10), and Interferon gamma (IFN-γ) was examined (Table 2). Also, the interaction between antigenic peptides and TLR4 molecules was done using docking method (Table 3 and Supplementary 7).
Table 1.
The best epitope for five target proteins of Helicobacter pylori.
| Target | Sequence | Antigenicity | Allergenicity | Toxicity | MHC I alleles | MHC II alleles |
|---|---|---|---|---|---|---|
| Urease | HIHFISPQQI | ANTIGEN | Non-allergen | Non-Toxin | 5 | 5 |
| CagA | NLSEKEKEKF | ANTIGEN | Non-allergen | Non-Toxin | 3 | 1 |
| HopE | SWGVGSDLLA | ANTIGEN | Non-allergen | Non-Toxin | 3 | 4 |
| BabA | EKSTSSTTIF | ANTIGEN | Non-allergen | Non-Toxin | 5 | 2 |
| SabA | NVQGNPPFKT | ANTIGEN | Non-allergen | Non-Toxin | 5 | 1 |
Table 2.
The ability of the best epitope to induce IL4, IL10, and IFN-γ.
| IL4 | IL10 | IFN-γ | |
|---|---|---|---|
| Urease | POSITIVE | NEGATIVE | NEGATIVE |
| CagA | POSITIVE | NEGATIVE | POSITIVE |
| HopE | POSITIVE | POSITIVE | POSITIVE |
| BabA | NEGATIVE | NEGATIVE | NEGATIVE |
| SabA | POSITIVE | NEGATIVE | NEGATIVE |
Table 3.
Docking of the best epitopes and final construct with TLR4.
| Docking score (kcal/mol) | RMSD (Angstrom) | |
|---|---|---|
| Urease | − 155 | 10 |
| CagA | − 157 | 1214 |
| HopE | − 154 | 11 |
| BabA | − 146 | 12 |
| SabA | − 154 | 15 |
The final multi-epitope construct was analyzed after selecting the epitope for each target. All the steps mentioned above were executed for the final construct, including antigenicity, allergenicity, toxicity, MHC alleles, cytokine secretions, physicochemical properties, and solubility (Table 4). The secondary and tertiary structures of the selected epitopes and the final structure (Supplementary 8 and Supplementary 9) were also designed and validated by PROSA and ERRAT.
Table 4.
The characterization of final construct of multi-epitope vaccine.
| Sequence | HIHFISPQQIKKKNLSEKEKEKFKKKSWGVGSDLLAKKKEKSTSSTTIFKKKNVQGNPPFKT |
| Antigenicity | Antigenic |
| Allergenicity | Non-allergen |
| Toxicity | Non-Toxin |
| MHC I alleles | 38 |
| MHC II alleles | 32 |
| IL4 | Inducer |
| IL10 | Inducer |
| IFN-γ | Inducer |
| Docking score (kcal/mol) | − 187 |
| RMSD (Angstrom) | 11 |
| Hydrophobicity | − 0.3 |
| Steric hindrance | 0.61 |
| Sidebulk | 0.61 |
| Hydropathicity | − 1.07 |
| Amphipathicity | 1.19 |
| Hydrophilicity | 0.59 |
| Net Hydrogen | 1 |
| PI | 7 |
| Mol wt | 5675 |
| coverage rate | 99.05% |
Experimental results
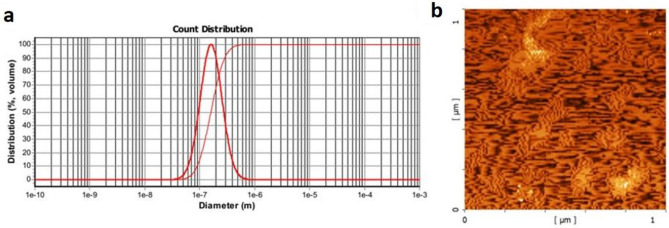
As is seen in Fig. 1a, the size distribution of lipid nanoparticles of the final construct was 32.4–862 nm, with an average size of 154 nm. AFM images showed the spherical shapes of lipid nanoparticles (Fig. 1b). Also, the loading test revealed that 91% of the final construct entered the lipid nanoparticles, and only 9% were free. Here, alum 2% was used as a vaccine adjuvant since alum induces a Th2 response by improving the attraction and uptake of antigen by antigen-presenting cells (APCs) 18.
Figure 1.
Characterization of lipid nanoparticles containing the final construct of multi-epitope vaccine by DLS (a) and AFM (b) results. As is seen, the size distribution of the lipid nanoparticle of the final construct was 32.4–862 nm, with an average size being 154 nm. AFM images clearly show the spherical shapes.
Discussion
This study set out with the aim of developing a multi-epitope peptide vaccine against H. pylori. The bacterium is usually transmitted to humans orally-anally from the mother to the newborn at birth. After the baby is born, the likelihood of transmission increases. Researchers are searching for novel ways to create conditions through vaccination and immunotherapy so that the body can fight the pernicious bacterium and eliminate it from the immune system. The idea of designing a vaccine to combat H. pylori has been around for almost 40 years12. Unfortunately, an acceptable, practical, low-risk vaccine has not yet entered the market for public use. The current issue with H. pylori is that it hinders the immune system’s ability to fight the microorganism by hiding itself in the underlying layers of the mucosa13. This pathogen can remain hidden from the immune system, making it difficult for immune system cells to identify the bacterium. This could explain why H. pylori can stay in the body for years without encountering an immune response. Moreover, as H. pylori is present in newborns at birth, the immune system perceives its antigens as its own since they appear during the body's immune system development. Consequently, the body recognizes the bacterium as beneficial or as part of its natural flora and does not produce antibodies to fight against it. This lack of reaction is also evident in the body’s response to intestinal bacteria.
Vaccination affects the elimination of H. pylori from the body by introducing the most important surface proteins to the immune cells. This triggers the immune response to produce antibodies. Alternatively, mRNA or DNA vaccine can express target proteins to induce the immune system to respond. Antigen-presenting cells (APCs) process the expressed proteins, and the necessary immune response is eventually produced. The immune system recognizes the bacterium’s antigens by creating a suitable vaccine against H. pylori. This study considered five important epitopes, i.e., Urease, CagA, HopE, BabA, and SabA, as important protein targets for designing vaccines. A careful investigation was made of immunogenicity, MHC bonding, allergenicity, toxicity, physicochemical analysis, and global population coverage of the whole set of epitopes and the final construct. The final multi-epitopes were synthesized using the solid phase method and placed inside lipid nanoparticles. Additional laboratory tests and experiments are required to establish the safety and immunogenicity of the multi-epitope vaccine. It is recommended to identify other proteins of H. pylori as potential. Alternatively, in another independent study, other bacteria and viruses of the digestive system can be identified, and a multi-epitope vaccine can be designed for several types of gastrointestinal pathogens. Additional complementary laboratory tests can be conducted at animal and human levels. In previous research studies, various multi-epitope vaccines are proposed to fight against H. pylori. For example, Zhou et al., showed the therapeutic efficacy of a multi-epitope vaccine against H. pylori in a BALB/c mice model. Oral therapeutic immunization with HUepi-LTB significantly decreased H. pylori colonization compared to oral immunization with PBS (phosphate-buffered saline). The protection was correlated with antigen-specific CD4+ T cells and IgG and mucosal IgA antibody responses14. Meza et al., worked on a novel design of a multi-antigenic, multistage, and multi-epitope vaccine against H. pylori. Silico—techniques were exploited to design a chimeric construct consisting of cholera toxin B subunit fused to multi-epitope of urease B (residue 148–158), cytotoxin-associated gene A (residue 584–602), neutrophil-activating protein (residue 4–28), vacuolating cytotoxin gene A (residue 63–81), H. pylori adhesive A (residue77–99), heat shock protein A (residue 32–54), and gamma-glutamyl transpeptidase (residue 271–293)15 Guo et al., demonstrated the immunological features and efficacy of a multi-epitope vaccine CTB-UE against H. pylori in the BALB/c mice model. The experimental results indicated that CTB-UE could induce comparatively high levels of specific antibodies against native H. pylori urease, UreA, UreB, or the selected B cell epitopes UreA183–203 and UreB327–334 involved in the active site of urease16. Nezafat et al., designed an efficient multi-epitope oral vaccine against H. pylori using immunoinformatics and structural vaccinology approaches. They believed their vaccine candidate could induce mucosal sIgA and IgG antibodies as well as Th1/Th2/Th17-mediated protective immunity, which is crucial for eradicating H. pylori infection17. Rahman et al., worked on core proteome-mediated therapeutic target mining and multi-epitope vaccine design for H. pylori. They selected B and T-Cell overlapped epitopes to design 9 vaccine constructs using linkers and adjuvants. The least allergenic and most antigenic construct (C-8) was selected as a promiscuous vaccine to elicit a host immune response18. Jafari et al., designed a multi-epitope vaccine against H. pylori based on Melittin as an adjuvant. The final construct was estimated as antigenic (71 and 74%) and non-allergenic with a molecular weight of 36.785KD. The instability index (II) and codon frequency distribution were predicted to be 26.5% and 92%, respectively. The pET-23a vector changed into E. coli BL21 (DE3) strain. The evaluation of expression by SDS-PAGE analysis showed that the optimized expression is in SOB medium 8 h after induction by 0.5 mM IPTG19. Ru et al., designed a multi-epitope vaccine with CTLA-4 extracellular domain to combat H. pylori. Appropriate linkers sequentially linked the dominant epitopes, and the cytotoxic T lymphocyte-associated antigen 4 extracellular domain was attached to the N-terminal of the epitope sequence. Meanwhile, molecular docking, molecular dynamics simulations, and principal component analysis were performed to show that the multi-epitope vaccine structure had strong interactions with B7 (B7-1, B7-2) and Toll-like receptors20. Ma et al., designed a multi-epitope vaccine against H. pylori using an Immunoinformatics Approach. In the final stage, Construct S1 was selected, and the molecular docking showed that it had the potential to bind TLR2, TLR4, and TLR9 to stimulate a robust immune response21. Khan et al., worked on immunoinformatics approaches to explore H. pylori proteome (Virulence Factors) to design B and T cell multi-epitope subunit vaccine. 7 CTL and 12 HTL antigenic epitopes (based on c-terminal cleavage and MHC binding scores) were predicted from the four selected proteins (CagA, OipA, GroEL, and cagA). The predicted epitopes were joined by AYY and GPGPG linkers. Β-defensins adjuvant was added to the N-terminus of the vaccine22. Wang et al., investigated the immunological response of recombinant H. pylori multi-epitope vaccine with different vaccination strategies. Their vaccine showed good intramolecular/extramolecular immune adjuvant effects. The vaccine’s intramolecular immune adjuvant effect worked better. Both intramolecular injection and oral administration of rBIB have an immune protective effect against H. pylori challenges. Further, oral administration of rBIB provided better immune protective effects23. Yang et al., showed protection against H. pylori infection in BALB/c mice by oral administration of a multi-epitope vaccine of CTB-UreI-UreB. They constructed a multi-epitope vaccine by linking cholera toxin B subunit (CTB), two antigenic fragments of H. pylori urease I subunit (UreI20–29, UreI98–107) and four antigenic fragments of H. pylori urease B subunit (UreB12–23, UreB229–251, UreB327–400, UreB515–561), resulting in the recombinant CTB-UreI-UreB (BIB)24. Ghosh et al., designed an interestingly new Multi-Epitopic Peptide Vaccine Candidate Against H. pylori. They developed a novel vaccine construct using B-cell-derived T-cell epitopes from four target antigenic proteins (HpaA, FlaA, FlaB, and Omp18). They found a way to induce possible immune responses using advanced immunoinformatics approaches25. Urrutia et al., designed a novel epitope-based oral vaccine against H. pylori. Their multiepitope vaccine was composed of cholera toxin subunit B (CTB) and used as a mucosal adjuvant to enhance vaccine immunogenicity for oral immunization. CTB fused to 11 epitopes predicted of pathogenic (UreB170–189, VacA459–478, CagA1103–1122, GGT106–126, NapA30–44, and OipA211–230) and colonization (HpaA33–52, FlaA487–506, FecA437–456, BabA129–149, and SabA540–559) proteins from H. pylori26. Guo et al., showed immunological properties and therapeutic efficacy of a multivalent epitope-based vaccine against four H. pylori adhesins (urease, Lpp20, HpaA, and CagL) in Mongolian gerbils. The results indicated that CFAdE could induce comparatively high levels of specific antibodies against urease, Lpp20, HpaA, and CagL. Additionally, oral therapeutic immunization with CFAdE plus polysaccharide adjuvant (PA) significantly decreased H. pylori colonization compared with oral immunization with urease plus PA. The protection was correlated with IgG and sIgA antibodies and antigen-specific CD4+ T cells27. Altogether, the literature review shows that designing a multi-epitope vaccine for any target28–33 by resorting to different molecular software or databases34–37 is not limited.
Methods
Immunoinformatic section
Determining the sequence of protein targets
The sequence of H. pylori’s protein targets was extracted from the NCBI database (https://www.ncbi.nlm.nih.gov/PROTEIN). Our selected protein targets were: urease enzyme beta subunit (WP_000724308.1), CagA (WP_209612238.1), HopE (WP_000395382.1), BabA (WP_209611253.1), and SabA (WP_209612393.1). The sequence of each target protein is seen in Supplementary 1.
Linear epitope prediction
We utilized the ABCpred server (https://webs.iiitd.edu.in/raghava/abcpred/ABC_submission.html) to predict linear epitopes of target proteins. In this server, the threshold was set at 0.5, and the length of antigenic fragments was set at 10 amino acids.
Predicting immunogenicity, MHC binding, allergenicity, and toxicity of epitopes
The immunogenicity, MHC binding, allergenicity, and toxicity were respectively checked by Vaxijen (http://www.ddgpharmfac.net/vaxijen/VaxiJen/VaxiJen.html), Vaxitop (http://www.violinet.org/vaxign/vaxitop/index.php), AllerTOP (https://www.ddg-pharmfac.net/AllerTOP), and ToxinPred (https://webs.iiitd.edu.in/raghava/toxinpred/design.php) servers.
Interaction of selected epitopes and TLR4
The three-dimensional structure of selected peptides was modeled by Ascalaph Designer software under NVT (Number of atoms, Volume, Temperature) conditions at 310 degrees Kelvin in a maximum of 10,000 picoseconds (Supplementary 7). Further, the PDB file of TLR4 was extracted from the PDB site (https://www.rcsb.org/). Then, the HDOCK interacted with selected peptides with TLR4 molecules (http://hdock.phys.hust.edu.cn/).
Prediction of cytokine secretion
We evaluated the ability of selected epitopes to secrete interferon-gamma (http://crdd.osdd.net/raghava/ifnepitope/predict.php), interleukin4 (https://webs.iiitd.edu.in/raghava/il4pred/predict.php), and interleukin 10 (https://webs.iiitd.edu.in/raghava/il10pred/predict3.php).
Characterization of the final construct of multi-epitope vaccine
After making epitope predictions for each target, the final multi-epitope construct was set up using the KKK linker. Then, we checked the antigenicity, allergenicity, toxicity, MHC alleles, cytokine secretions, physicochemical properties, and solubility for the final construct. The secondary and tertiary structures of the final structure were also designed. The 3D structure of the selected epitope and final construct were validated by the PROCHECK server (https://saves.mbi.ucla.edu/) UCLA-DOE LAB—SAVES v6.0.
Experimental section
Synthesizing and characterizing lipid nanoparticles
The final construct of the multi-epitope vaccine was synthesized using the solid phase method (Proteogenix, France). Then, 2 mL PBS was added to the vial of synthesized peptide (10 mg) and mixed vigorously for 5 min. Next, 1 mL of lecithin (100 mg/mL), 1 mL of olive oil, and one drop of 2% alum solution were added to the peptide solution and mixed for 5 min at room temperature. The mixture was then sonicated for 10 s and centrifuged at 10,000 RPM for 10 min. Finally, the supernatant was discarded, and 3 mL of PBS was added to precipitate. After synthesizing the nanoliposomes, the construct loading, the size distribution, and the shape of nanoliposomes were checked by Nanodrop at 280 nm, DLS, and AFM, respectively. The loading percentage was calculated using the following formula.
Supplementary Information
Acknowledgements
We extend our appreciations and thanks to the staff of Faculty of Advanced Sciences and Technology, Tehran Medical Science, Islamic Azad University, Tehran, Iran for their unflinching support to implement the present project.
Author contributions
(I) Conception and design: A.J, A.E, M.K.E, S.S (II) Provision of study: A.J, A.E, M.KE, S.S (III) Collection, and assembly of data: A.J, A.E, (IV) Data analysis and interpretation: A.J,A.E (V) Manuscript writing and final approval of manuscript: A.J, A.E, M.K.E, S.S.
Funding
This article was financially supported in full by Azam Esmaeilzadeh from the Faculty of Advanced Sciences and Technology, Tehran Medical Science, Islamic Azad University, Tehran, Iran.
Data availability
All data within this article are available on the official request of honorable researchers.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-68947-x.
References
- 1.Denic, M., Touati, E. & De Reuse, H. Pathogenesis of Helicobacter pylori infection. Helicobacter25, e12736 (2020). 10.1111/hel.12736 [DOI] [PubMed] [Google Scholar]
- 2.Gravina, A. G. et al.Helicobacter pylori and extragastric diseases: A review. World J. Gastroenterol.24, 3204 (2018). 10.3748/wjg.v24.i29.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malfertheiner, P. et al.Helicobacter pylori infection. Nat. Rev. Dis. Primers.9, 19 (2023). 10.1038/s41572-023-00431-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowada, A. & Asaka, M. Economic and health impacts of Helicobacter pylori eradication strategy for the treatment of peptic ulcer disease: A cost-effectiveness analysis. Helicobacter27, e12886 (2022). 10.1111/hel.12886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dos-Santos-Viana, I. et al. Vaccine development against Helicobacter pylori: From ideal antigens to the current landscape. Expert Rev. Vacc.20, 989–999 (2021). 10.1080/14760584.2021.1945450 [DOI] [PubMed] [Google Scholar]
- 6.Takahashi-Kanemitsu, A., Knight, C. T. & Hatakeyama, M. Molecular anatomy and pathogenic actions of Helicobacter pylori CagA that underpin gastric carcinogenesis. Cell. Mol. Immunol.17, 50–63 (2020). 10.1038/s41423-019-0339-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uberti, A. F. et al.Helicobacter pylori urease: Potential contributions to Alzheimer’s disease. Int. J. Mol. Sci.23, 3091 (2022). 10.3390/ijms23063091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim, J. M. Helicobacter pylori 89–102 (Springer, 2024). [Google Scholar]
- 9.Xu, C., Soyfoo, D. M., Wu, Y. & Xu, S. Virulence of Helicobacter pylori outer membrane proteins: An updated review. Eur. J. Clin. Microbiol. Infect. Dis.39, 1821–1830 (2020). 10.1007/s10096-020-03948-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doohan, D., Rezkitha, Y. A. A., Waskito, L. A., Yamaoka, Y. & Miftahussurur, M. Helicobacter pylori BabA–SabA key roles in the adherence phase: The synergic mechanism for successful colonization and disease development. Toxins13, 485 (2021). 10.3390/toxins13070485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalali, B., Mejías-Luque, R., Javaheri, A. & Gerhard, M. H. pylori virulence factors: Influence on immune system and pathology. Mediat. Inflamm.2014, 12 (2014). 10.1155/2014/426309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutton, P. & Boag, J. M. Status of vaccine research and development for Helicobacter pylori. Vaccine37, 7295–7299 (2019). 10.1016/j.vaccine.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smoot, D. T. How does Helicobacter pylori cause mucosal damage? Direct mechanisms. Gastroenterology113, S31–S34 (1997). 10.1016/S0016-5085(97)80008-X [DOI] [PubMed] [Google Scholar]
- 14.Zhou, W.-Y. et al. Therapeutic efficacy of a multi-epitope vaccine against Helicobacter pylori infection in BALB/c mice model. Vaccine27, 5013–5019 (2009). 10.1016/j.vaccine.2009.05.009 [DOI] [PubMed] [Google Scholar]
- 15.Meza, B., Ascencio, F., Sierra-Beltrán, A. P., Torres, J. & Angulo, C. A novel design of a multi-antigenic, multistage and multi-epitope vaccine against Helicobacter pylori: An in silico approach. Infect. Genet. Evol.49, 309–317 (2017). 10.1016/j.meegid.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 16.Guo, L. et al. Immunological features and efficacy of a multi-epitope vaccine CTB-UE against H. pylori in BALB/c mice model. Appl. Microbiol. Biotechnol.98, 3495–3507 (2014). 10.1007/s00253-013-5408-6 [DOI] [PubMed] [Google Scholar]
- 17.Nezafat, N., Eslami, M., Negahdaripour, M., Rahbar, M. R. & Ghasemi, Y. Designing an efficient multi-epitope oral vaccine against Helicobacter pylori using immunoinformatics and structural vaccinology approaches. Mol. BioSyst.13, 699–713 (2017). 10.1039/C6MB00772D [DOI] [PubMed] [Google Scholar]
- 18.Rahman, N., Ajmal, A., Ali, F. & Rastrelli, L. Core proteome mediated therapeutic target mining and multi-epitope vaccine design for Helicobacter pylori. Genomics112, 3473–3483 (2020). 10.1016/j.ygeno.2020.06.026 [DOI] [PubMed] [Google Scholar]
- 19.Jafari, E. & Mahmoodi, S. Design, expression, and purification of a multi-epitope vaccine against Helicobacter pylori based on Melittin as an adjuvant. Microb. Pathogenesis157, 104970 (2021). 10.1016/j.micpath.2021.104970 [DOI] [PubMed] [Google Scholar]
- 20.Ru, Z. et al. Immmunoinformatics-based design of a multi-epitope vaccine with CTLA-4 extracellular domain to combat Helicobacter pylori. FASEB J.36, e22252 (2022). 10.1096/fj.202101538RR [DOI] [PubMed] [Google Scholar]
- 21.Ma, J. et al. A novel design of multi-epitope vaccine against Helicobacter pylori by immunoinformatics approach. Int. J. Peptide Res. Therapeut.27, 1027–1042 (2021). 10.1007/s10989-020-10148-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan, M. et al. Immunoinformatics approaches to explore Helicobacter Pylori proteome (Virulence Factors) to design B and T cell multi-epitope subunit vaccine. Sci. Rep.9, 13321 (2019). 10.1038/s41598-019-49354-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang, B. et al. Immunological response of recombinant H. pylori multi-epitope vaccine with different vaccination strategies. Int. J. Clin. Exp. Pathology7, 6559 (2014). [PMC free article] [PubMed] [Google Scholar]
- 24.Yang, J. et al. Protection against Helicobacter pylori infection in BALB/c mice by oral administration of multi-epitope vaccine of CTB-UreI-UreB. Pathogens Dis.73, ftv026 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Ghosh, P. et al. A novel multi-epitopic peptide vaccine candidate against Helicobacter pylori: In-silico identification, design, cloning and validation through molecular dynamics. Int. J. Peptide Res. Therapeut.27, 1149–1166 (2021). 10.1007/s10989-020-10157-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urrutia-Baca, V. H. et al. Immunoinformatics approach to design a novel epitope-based oral vaccine against Helicobacter pylori. J. Comput. Biol.26, 1177–1190 (2019). 10.1089/cmb.2019.0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo, L. et al. Immunologic properties and therapeutic efficacy of a multivalent epitope-based vaccine against four Helicobacter pylori adhesins (urease, Lpp20, HpaA, and CagL) in Mongolian gerbils. Helicobacter22, e12428 (2017). 10.1111/hel.12428 [DOI] [PubMed] [Google Scholar]
- 28.Dey, J., Mahapatra, S. R., Raj, T. K., Misra, N. & Suar, M. Identification of potential flavonoid compounds as antibacterial therapeutics against Klebsiella pneumoniae infection using structure-based virtual screening and molecular dynamics simulation. Mol. Divers.2023, 1–18 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Dey, J. et al. Designing of multi-epitope peptide vaccine against Acinetobacter baumannii through combined immunoinformatics and protein interaction–based approaches. Immunol. Res.71, 639–662 (2023). 10.1007/s12026-023-09374-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dey, J. et al. Designing a novel multi-epitope vaccine to evoke a robust immune response against pathogenic multidrug-resistant Enterococcus faecium bacterium. Gut Pathogens14, 21 (2022). 10.1186/s13099-022-00495-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dey, J. et al. Exploring Klebsiella pneumoniae capsule polysaccharide proteins to design multiepitope subunit vaccine to fight against pneumonia. Expert Rev Vacc.21, 569–587 (2022). 10.1080/14760584.2022.2021882 [DOI] [PubMed] [Google Scholar]
- 32.Mahapatra, S. R. et al. Immunoinformatics-guided designing of epitope-based subunit vaccine from Pilus assembly protein of Acinetobacter baumannii bacteria. J. Immunol. Methods508, 113325 (2022). 10.1016/j.jim.2022.113325 [DOI] [PubMed] [Google Scholar]
- 33.Mahapatra, S. R., Dey, J., Raj, T. K., Misra, N. & Suar, M. Designing a next-generation multiepitope-based vaccine against Staphylococcus aureus using reverse vaccinology approaches. Pathogens12, 376 (2023). 10.3390/pathogens12030376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahapatra, S. R. et al. The potential of plant-derived secondary metabolites as novel drug candidates against Klebsiella pneumoniae: Molecular docking and simulation investigation. South Afr. J. Botany149, 789–797 (2022). 10.1016/j.sajb.2022.04.043 [DOI] [Google Scholar]
- 35.Narang, P. K. et al. Functional annotation and sequence-structure characterization of a hypothetical protein putatively involved in carotenoid biosynthesis in microalgae. South Afr. J. Botany141, 219–226 (2021). 10.1016/j.sajb.2021.04.014 [DOI] [Google Scholar]
- 36.Sudeshna-Panda, S. et al. Investigation on structural prediction of pectate lyase enzymes from different microbes and comparative docking studies with pectin: The economical waste from food industry. Geomicrobiol. J.39, 294–305 (2022). 10.1080/01490451.2021.1992042 [DOI] [Google Scholar]
- 37.Margreitter, C., Mayrhofer, P., Kunert, R. & Oostenbrink, C. Antibody humanization by molecular dynamics simulations—in-silico guided selection of critical backmutations. J. Mol. Recogn.29, 266–275 (2016). 10.1002/jmr.2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data within this article are available on the official request of honorable researchers.