Abstract
Glycogen storage, conversion and utilization in astrocytes play an important role in brain energy metabolism. The conversion of glycogen to lactate through glycolysis occurs through the coordinated activities of various enzymes and inhibition of this process can impair different brain processes including formation of long-lasting memories. To replenish depleted glycogen stores, astrocytes undergo glycogen synthesis, a cellular process that has been shown to require transcription and translation during specific stimulation paradigms. However, the detail nuclear signaling mechanisms and transcriptional regulation during glycogen synthesis in astrocytes remains to be explored. In this report, we study the molecular mechanisms of vasoactive intestinal peptide (VIP)-induced glycogen synthesis in astrocytes. VIP is a potent neuropeptide that triggers glycogenolysis followed by glycogen synthesis in astrocytes. We show evidence that VIP-induced glycogen synthesis requires CREB-mediated transcription that is calcium dependent and requires conventional Protein Kinase C but not Protein Kinase A. In parallel to CREB activation, we demonstrate that VIP also triggers nuclear accumulation of the CREB coactivator CRTC2 in astrocytic nuclei. Transcriptome profiles of VIP-induced astrocytes identified robust CREB transcription, including a subset of genes linked to glucose and glycogen metabolism. Finally, we demonstrate that VIP-induced glycogen synthesis shares similar as well as distinct molecular signatures with glucose-induced glycogen synthesis, including the requirement of CREB-mediated transcription. Overall, our data demonstrates the importance of CREB-mediated transcription in astrocytes during stimulus-driven glycogenesis.
Keywords: CREB, Astrocyte, Transcription, Vasoactive intestinal peptide, Glycogen synthesis
Subject terms: Glycobiology, Learning and memory, Molecular neuroscience
Introduction
Conversion of glycogen to lactate via glycolysis in astrocytes is an essential cellular process during memory formation1–4. The lactate generated by astrocytes and subsequently shuttled into neurons was first described in the astrocyte-neuron lactate shuttle (ANLS) model and highlights the important contribution of astrocytes in long-term plasticity5. Experiments show that disruption of glycogen conversion to lactate or its shuttling impairs long-term but not short-term memories1,2,4. Even though gains have been made in understanding lactate transport and utilization in neurons and the molecular mechanisms of ANLS in the context of long-term plasticity6–9, much less is known about how glycogen synthesis, storage and turnover are regulated in astrocytes when exposed to various stimuli including neural activity. Many questions remain unanswered: What are the astrocytic genes critical for glycogen synthesis and what are the nuclear signaling pathways that trigger gene expression? What are key enzymes that are regulated during glycogen synthesis and are they stimulus-dependent? Another area of research that deserves more attention is the contribution of neurotransmitters and gliotransmitters such as vasoactive intestinal peptide (VIP), noradrenalin (NA) or ATP toward glycogen metabolism, particularly in the context of long-term plasticity. Many of these neurotransmitters have modulatory properties and its release during memory formation can impact plasticity directly by binding to receptors on neurons, but also through regulating astrocyte metabolism10. Since there is evidence that links neurotransmitter release and glycogen turnover in astrocytes during formation of long-term memories, it is important to elucidate the molecular mechanisms that regulate neurotransmitter-mediated glycogen synthesis in the brain.
Previous work has shown the potent effects of VIP, NA, secretin and adenosine in inducing glycogenolysis in astrocytes11,12. In particular, a brief application of VIP rapidly depletes astrocytic glycogen stores, and this followed by a prolong glycogen resynthesis phase that can last for more than 24 h12. This glycogen resynthesis occurs even after removal of the original stimulus and the newly synthesized glycogen levels often exceed basal levels. Importantly, protein synthesis and transcription are required for this process12. However, beyond these initial studies, the details of the signaling pathways and transcriptional regulation of glycogen synthesis has not been extensively studied. As first steps in mapping this cellular process, our paper seeks to explore the signaling mechanisms and transcriptional changes that regulates glycogen synthesis in astrocytes.
To examine the importance of transcriptional response in astrocytes in the context of glycogen synthesis, we chose to use the peptide hormone VIP to stimulate astrocytes in cell-based and brain slices assays. This selection was done for a few reasons: first, VIP is known to trigger conversion of glycogen in astrocytes which is quickly followed glycogen synthesis and this process requires transcription. Second, VIP and its receptors are found in the brain, but beyond its expression in a select group of VIP interneurons, the impact of VIP release on astrocytes metabolism in the brain remains poorly understood. Cellular mechanisms and pathways uncovered in this study will provide a framework for understanding how neuromodulatory transmitters and peptide hormones alter astrocytic glycogen metabolism in animal models. Finally, few studies have been done to examine stimulus-dependent gene expression changes in astrocytes in the context of glycogen synthesis. Our paper aims to fill that gap in our knowledge.
The results from the study show that VIP drives glycogen synthesis through activation of a major transcription CREB (cAMP-Response Element Binding Protein). The presence of VIP induces CREB phosphorylation and CREB-regulated transcription of several target genes such as Ppp1r3c that is involved in regulating glycogen levels in astrocytes. When CREB function is inhibited, many of the cellular processes associated with glycogen production are also impaired. In addition, we also describe the robust transcriptional repression of Phkg1, a gene that encodes a catalytic subunit of phosphorylase kinase and is implicated in glycogen storage diseases13. Uniquely, this stimulus-driven resynthesis of glycogen requires protein kinase C (PKC) and not protein kinase A (PKA), though PKC activity alone is necessary but not sufficient to drive efficient glycogen synthesis. As CREB function is enhanced by the CRTC family of transcriptional coactivators, we noted that CRTC2 also responds to VIP by robustly accumulating in astrocyte but not neuronal nuclei, and this translocation parallels CREB phosphorylation and transcription. Finally, we compared glycogen synthesis mediated by changes in glucose concentrations with that of VIP and observed that while it is also CREB-dependent, glucose driven glycogen synthesis shares some but not all properties with VIP-induced glycogen synthesis.
Results
VIP-induced glycogen synthesis requires CREB transcription in astrocytes
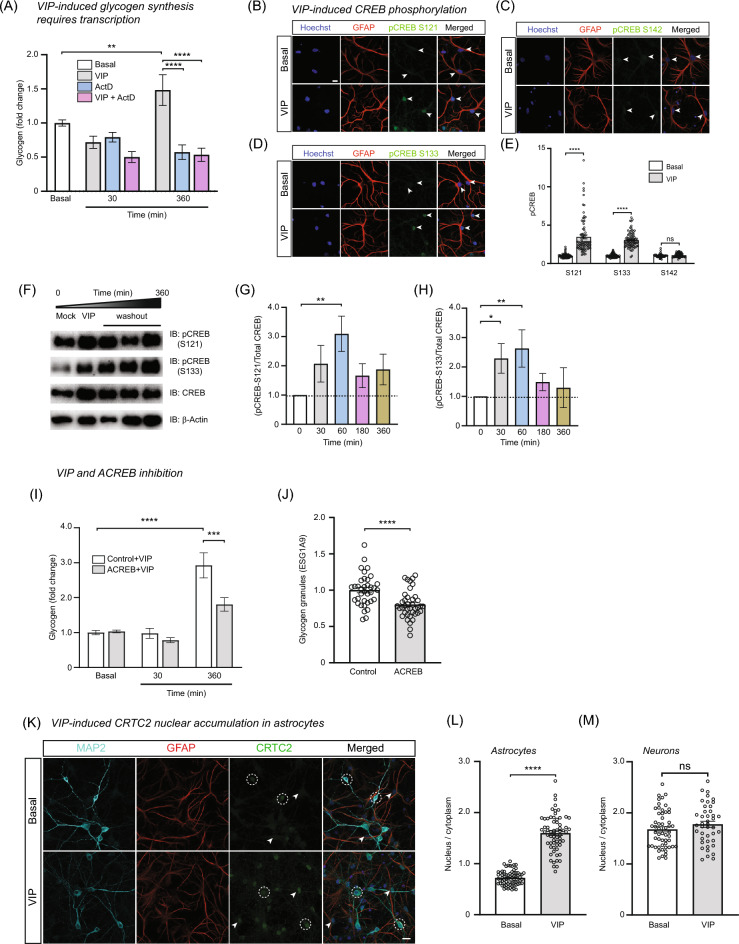
A brief exposure to VIP is reported to trigger glycogenolysis that is accompanied by sustained glycogen synthesis up to 24 h even after removal of stimulus12. To verify that this process is transcription-dependent, we cultured cortical astrocytes from mouse forebrain and briefly stimulated astrocytes with VIP in the continuous presence or absence of Actinomycin D (ActD) and harvested cells at 30 min and 6 h post-stimulation. We observed a robust synthesis and accumulation of glycogen that exceeded basal levels at 6 h post-stimulation and the presence of ActD impaired VIP-induced glycogen synthesis (Fig. 1A). In astrocytes, bicuculline-induced neural activity enhanced expression of CREB-target genes associated with the ANLS pathway, mitochondrial NADH shuttling and pyruvate uptake14. However, there is yet no direct experimental evidence that links CREB-mediated transcription with glycogen synthesis. To explore whether CREB is activated in the presence of VIP, we stimulated hippocampal cocultures and observed a robust increase in phosphorylation of astrocytic CREB specifically at serine residues S121 and S133, but not at S142 (Fig. 1B–E). Importantly, both serine residues for CREB at position 121 and 133 have been reported as phosphorylation sites for PKC while the serine residue at position 142 is reported to be target sites for CaMKII and CK-II15. To track the persistence of CREB phosphorylation during VIP stimulation, we exposed cortical astrocytes to VIP briefly (30 min) followed by wash out and recovery before harvesting lysates at different time points post-stimulation. Immunoblot data indicate that CREB phosphorylation at S121 and S133 gradually increase and peaked at 30 min after removal of VIP (60 min from start of experiment; Fig. 1F–H).
Figure 1.
VIP-induced glycogen synthesis requires CREB-dependent transcription. (A) Glycogen assay for cortical astrocytes stimulated with VIP in the absence or presence of ActD (1 µM; 30 min). Bar graph shows change in glycogen concentration relative to basal at 30 and 360 min across all replicates. Statistical tests: 2-way ANOVA with Tukey’s post-hoc test. (B–D) Cocultures were stimulated with VIP and immunolabeled with antibodies against pCREB (S121, S133 and S142; green), GFAP (red) and Hoechst dye (blue). Arrowheads indicate GFAP-positive astrocytic nuclei. Scale bar, 10 µm. (E) Group data show normalized pCREB intensities against average basal values for each independent experiment. Unpaired t-tests were performed for each individual pCREB antibody. (F) A time-course immunoblotting of pCREB (S121 and S133) on cortical astrocyte lysates after VIP stimulation followed by washout and recovery. (G, H). Bar plot show pCREB/total CREB staining normalized against mock treated sample (t = 0) for individual experiment with 1-way ANOVA with Dunnet’s multiple comparison performed on the dataset. (I) Cortical astrocytes transduced with control or ACREB expressing lentiviral vectors and stimulated with VIP and assayed for glycogen post-stimulation. Bar graphs show glycogen concentration relative to basal between control and ACREB samples across all replicates. Statistical tests: 1-way ANOVA with Tukey’s post-hoc. (J) Cortical astrocytes transduced with control or ACREB expressing lentiviral vectors were quantified for endogenous levels of glycogen granules. Bar plots shows intensity of ESG1A9 staining in GFP-positive astrocytes. Statistical test: Unpaired students’ t-test. (K) Cocultures were stimulated with VIP and labeled with antibodies against CRTC2 (green), GFAP (red) and Hoechst dye (blue). White arrowheads and dashed circles indicate astrocyte and neuronal nuclei respectively. (L,M) The nucleus to cytoplasmic ratio of CRTC2 as shown in (K) was quantified for neurons and astrocytes across all conditions and plotted as bar graphs. Statistical test: Unpaired t-test. Unless otherwise stated, for all graphs, only statistical notations relevant to the results are shown in the graphs. Experimental replicates were performed for all datasets to obtain the pooled data and p values notations are as follows (*p < 0.05; ** p < 0.01; ***p < 0.001; **** p < 0.0001; n.s. not significant). Detailed statistical outputs are listed in the Supplementary Table.
To directly investigate the role of CREB in glycogen synthesis, we inhibited CREB function in cortical astrocytes using a dominant negative construct known as ACREB which interferes with the endogenous binding of CREB on CRE-elements in the promoter region of CREB-target genes16. We transduced astrocytes with either empty (control) or ACREB-expressing lentivirus before briefly stimulating cells with VIP as previously described and measuring glycogen concentration. As expected, astrocytes expressing control vector saw a robust increase in glycogen synthesis 6 h post-stimulation while cells that express ACREB is partially impaired in glycogen synthesis (Fig. 1I). To further complement glycogen assays, we immunostained and measured glycogen granules in cortical astrocytes using ESG1A9, a monoclonal antibody that specifically detect glycogen granules in astrocytes (Fig. S1) and showed that ACREB expressing cortical astrocytes have fewer glycogen granules under basal conditions as compared to control astrocytes (Fig. 1J).
It has been shown that CREB transcription is greatly enhanced by a family of transcriptional coactivators known as CRTC (cAMP-Regulated Transcriptional Coactivator). In particular, CRTC2 translocation into the nucleus has been linked to metabolic homeostasis and regulation of gluconeogenesis in the liver17,18. In the hypothalamic neurons, CRTC2 has been linked to glucose sensing19. To examine if VIP activation of CREB concurrently drives nuclear accumulation of CRTC2, we stimulated cocultures with VIP and saw robust accumulation of CRTC2 in the nucleus (Fig. 1K–M). Interestingly, only astrocytes but not neurons responded to VIP stimulation even though CRTC2 immunoreactivity can be detected in neurons (Fig. 1K–M). To ensure that both neurons and astrocytes are capable of responding to VIP, we examined expression of VPAC2, the receptor which binds to VIP, and found it localized in both cell types (data not shown). We also performed a similar experiment in acute hippocampal slice by bath application of VIP and also observed a robust increase of CRTC2 exclusively in astrocytes, but not neurons (Fig. S2). Collectively, our experiments confirmed that CREB is activated by VIP and CREB-mediated transcription is required during glycogen synthesis.
VIP triggers CREB-mediated transcription in cortical astrocytes
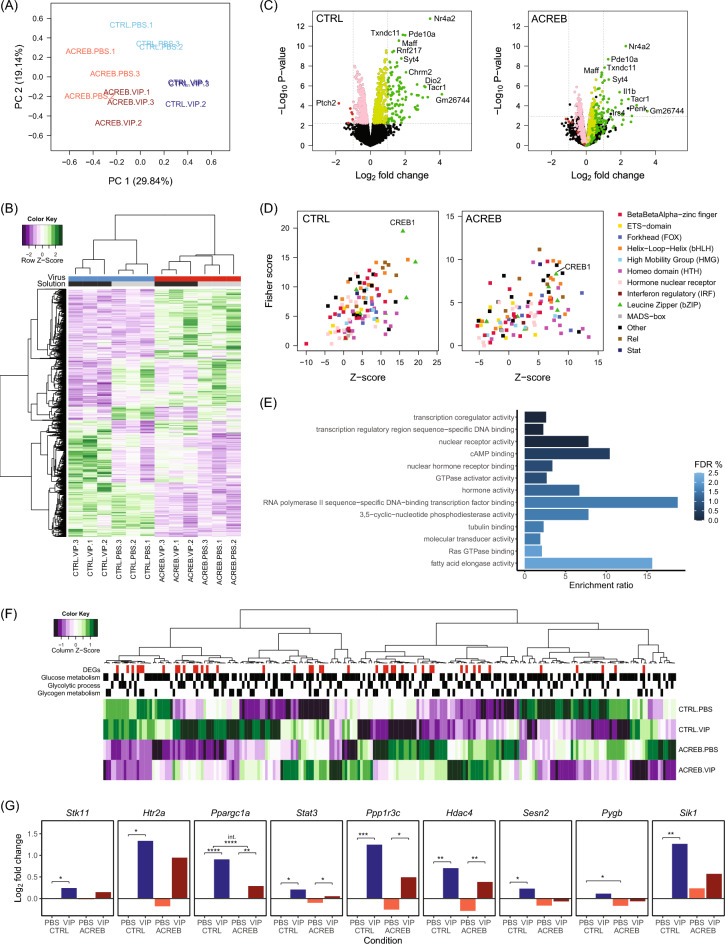
To examine the extent of CREB-mediated transcription upon VIP induction, we transduced cortical astrocytes with either control or ACREB expression vectors and exposed cells with either PBS or VIP before harvesting RNA for RNAseq (Fig. S3). PCA of samples following normalization and batch correction showed a strong effect of both virus (PC1: 29.84%) and stimulation (PC2: 19.14%; Fig. 2A). Overall, we detected 2709 differentially expressed genes (DEGs) across all conditions (FDR < 0.1; Fig. 2B). In the control condition, 884 genes were differentially expressed (DE) following VIP stimulation compared to PBS (113 LFC ≥ ± 1), while only 185 genes were DE following VIP stimulation in the presence of ACREB (27 LFC ≥ ± 1). As expected, the majority of VIP-induced DEGs were upregulated (95% of DEGs with LFC ≥ ± 1). Volcano plots show that ACREB strongly reduced the magnitude of differential expression as well as the number of DEGs induced by VIP (Fig. 2C). Only two genes were strongly upregulated (LFC > 1) by VIP when CREB was inhibited that were not DE in the control condition (Penk and Irs4). Curiously, we also noted that long non-coding RNAs (lncRNAs) were also significantly upregulated in cells expressing ACREB (Fig. S4). Taken together, our findings indicate that VIP induces transcription in cortical astrocytes, with ACREB substantially attenuating the upregulation in gene expression.
Figure 2.
Transcriptome profile of VIP-stimulated astrocytes (A) Principal component analysis (PCA) of samples following normalization & batch effect correction. PC1 corresponds to the effect of virus and accounts for 29.84% of variance between samples; PC2 corresponds to the effect of VIP and accounts for 19.14% of variance. (B) Heatmap and hierarchical clustering of differentially expressed genes (DEGs) identified in an ANOVA-like test for differential expression across all conditions (N = 2709, false discovery rate (FDR) < 0.1). Heatmap shows counts per million (CPMs) in each sample, adjusted for unwanted variation and standardized for each gene. Hierarchical clustering of samples shows that the samples cluster by virus, and the control samples further cluster by solution. (C) Volcano plots showing log2 fold changes (LFCs) in gene expression between VIP and PBS conditions plotted against log10 p values from differential expression tests. DEGs that were up- and down-regulated in response to VIP in the control condition are highlighted in green and red respectively on both plots (FDR < 0.1); DEGs with LFC ≥ ± 1 is highlighted in a darker shade. (D) OPOSSUM3 analysis of over-represented TFBSs in genes upregulated following VIP stimulation in control (N = 508) and ACREB (N = 122) conditions (FDR < 0.1). Fisher scores, indicating TF target gene enrichment, are plotted against z-scores, indicating TFBS enrichment. (E) Enriched molecular functions (FDR < 0.05) among genes upregulated in response to VIP (N = 508). (F) Heatmaps of expressed genes annotated to the GO terms glucose metabolic process (GO:0006006), glycolytic process (GO:0006096) and glycogen metabolic process (GO:0005977) or their child terms. Heatmaps show LFCs from baseline (CTRL.PBS) standardized for each gene and hierarchical clustering of genes. DEGs (FDR < 0.1) are highlighted. (G) A subset of genes identified from analyzing the GO terms in 2F with higher expression in the VIP control relative to ACREB condition. The order of plots corresponds to gene order in the heatmap. P-values for contrasts of interest are indicated (* p < 0.01; ** p < 0.001; *** p < 0.0001; **** p < 0.00001).
To characterize VIP-induced transcription of CREB target genes and to verify that ACREB expression attenuated CREB function, we analyzed enrichment of transcription factor binding sites (TFBSs) and motifs among the significantly upregulated genes following VIP stimulation in each condition (CTRL: 508 genes; ACREB: 122 genes; FDR < 0.1). Our analysis using OPOSSUM3 revealed that CREB1 (JASPAR ID: MA0018.2) target genes were the most strongly enriched among DEGs upregulated in response to VIP (Fisher score = 19.5, 336/486 detected DEGs) and CREB1 TF binding sites were also highly enriched. (z-score = 15.9, nucleotide rate = 0.00576; Fig. 2D). As expected, the over-representation of CREB1 target genes was substantially reduced in cells expressing ACREB with the gene rank dropping to 9th (Fisher score: 8.30; 84/117 hits; Fig. 2D). We also validated the VIP-induced enrichment of CREB cis-regulatory elements and inhibition by ACREB in a complementary analysis using iRegulon (Fig. S5). Collectively, both OPOSSUM3 and iRegulon analyses confirm that CREB is the primary transcription factor that responds to VIP stimulation and ACREB effectively attenuated VIP induction of CREB target genes.
We next investigated the molecular functions of the genes upregulated in response to VIP stimulation (FDR < 0.1, LFC > 0; N = 508). We performed an Overrepresentation Analysis and found that cellular processes associated with transcriptional regulation, nuclear receptor activity and G-protein coupled receptor signaling were strongly enriched (Fig. 2E). To identify VIP-responsive genes that contribute to glycogen synthesis, we specifically examined expression of genes associated with biological function annotated to glucose metabolism (GO:0005977), glycogen metabolism (GO:0006006) and glycolytic processes (GO:0006096). From all the genes collated from these GO terms (Fig. 2F), we identified a subset of genes that were strongly induced by VIP and showed an attenuated response with ACREB (Fig. 2F). The most notable candidates in this groupgroup of DEGs identified from the GO terms includes Ppp1r3c which codes for the key glycogen synthesis protein PTG (Protein Targeting to Glycogen). Ppp1r3c expression is upregulated by VIP in both control (log2FC = 1.25, p = 5.74 × 10−5) and ACREB conditions (log2FC = 0.746, p = 1.74 × 10−3), albeit at lower levels in the presence of ACREB. Similarly, we also identified Ppargc1a as another relevant target gene that responded to VIP stimulation, but with a significant interaction effect, indicating that the response to VIP was significantly reduced in the ACREB condition (log2FC = − 0.617, p = 3.78 × 10−6). We independently validated the reduction of Ppargc1a and Ppp1r3c expression in ACREB-expressing astrocytes compared to controls following VIP stimulation by qPCR on these targets (Fig. S7). Furthermore, our analysis also uncovered an additional group of VIP-induced genes that were attenuated in the presence of ACREB that are specifically associated with the negative regulation of glycolysis (GO:0045820) which includes Ppargc1a, Hdac4 and Stat3 (Fig. S6). These three genes and Ppp1r3c have highly similar expression profiles and are significantly upregulated in response to VIP in both control and ACREB conditions (Hdac4: Control: log2FC = 0.701, p = 2.28 × 10−4, ACREB: log2FC = 0.670, p = 2.15 × 10−4; Ppargc1a: Control: log2FC = 0.905, p = 2.91 × 10−10, ACREB: log2FC = 0.288, p = 3.25 × 10−4; Stat3: Control: log2FC = 0.206, p = 1.63 × 10−3, ACREB: log2FC = 0.152, p = 9.47 × 10−3; Fig. 2G). Overall, our transcriptome data supports our findings in Fig. 1, and confirms that VIP stimulation primarily trigger CREB activation and expression of CREB target genes in astrocytes. While many of the CREB target genes altered by VIP are associated with diverse signaling pathways, we have identified a subset of these CREB-sensitive genes that are linked to glycogen metabolism.
PKC is required for CREB activation and transcription for efficient glycogen synthesis
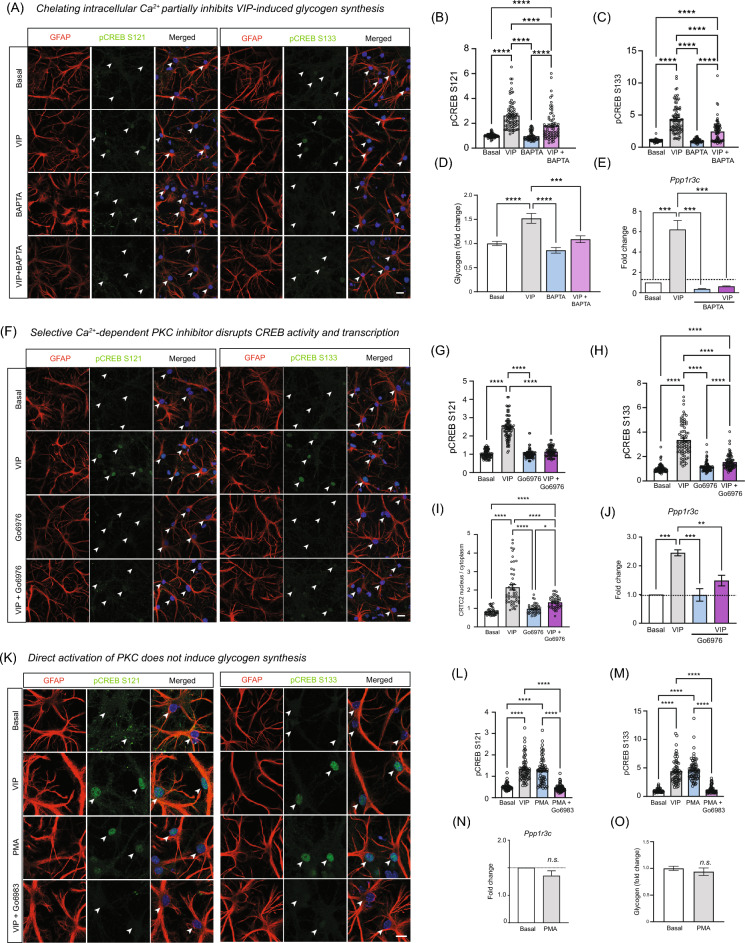
To better understand the signaling cascades in astrocytes that lead to the activation of CREB during VIP stimulation, we decided to examine the kinases that crucial for this process. In the brain, VIP binds to VPAC receptors which is detected in both neurons and astrocytes (data not shown)20–22. VPAC receptors are G-protein coupled receptors that can activate a variety of G-proteins including Gαs, Gαi and Gαq/11, which trigger downstream signaling pathways that activate Protein Kinase A (PKA) or Protein Kinase C (PKC)23–27. To examine the contribution of either PKA and PKC in CREB-driven glycogen synthesis, we first examined the phosphorylation of CREB at S121 and S133 in astrocytes with VIP in the presence of either Go6983 or Rp-cAMP (RpC), which inhibit PKC and PKA respectively. Phosphorylation of CREB at either site induced by VIP was strongly inhibited in the presence of Go6983 but not RpC (Fig. 3A–C, S15). An additional experiment using PKA inhibitor H89 also showed no effect (Fig. S16). Similarly, results from immunoblotting show reduced CREB phosphorylation at S121 and S133 after PKC but not PKA inhibition (Fig. S8). Since CREB phosphorylation is often used as a proxy for CREB activity, we exposed cortical astrocytes to VIP in the presence or absence of Go6983, to examine if PKC-induced reduction of CREB phosphorylation impacts glycogen synthesis. At 6 h post-stimulation, glycogen synthesis after induction was impaired when PKC activity is inhibited (Fig. 3D). In contrast, astrocytes exposed to RpC showed no impairment in glycogen production (Fig. 3E). Since NA binds to β2-adrenergic Gαs-coupled GPCRs on astrocytes and triggers glycogenolysis and glycogen synthesis via a cAMP/PKA pathway, we examined if PKC inhibition would also impair glycogen synthesis and interestingly, we observed that glycogen synthesis triggered by NA is also dependent on PKC (Fig. 3F)10. To complement the glycogen assays, we performed qPCR to look at pCREB-driven Pp1r3c gene expression and saw that inhibition of PKC but not PKA blocked VIP-induced transcription of the gene (Fig. 3G,H). Finally, we asked if nuclear translocation of CRTC2 required PKC activity. Just like CREB phosphorylation and CREB-mediated transcription, CRTC2 nuclear accumulation in astrocytes was also sensitive to PKC inhibitor Go6983 but not RpC following VIP stimulation (Fig. 3I,J). Collectively, our transcriptome data, glycogen assay, immunocytochemistry of CREB phosphorylation, qPCR of candidate genes and nucleocytoplasmic translocation of CRTC2 supports the hypothesis that VIP induces select CREB target gene expression in astrocytes that regulate glycogen synthesis in a PKC-dependent pathway.
Figure 3.
VIP-induced glycogen synthesis is mediated by PKC. (A) Cocultures were preincubated with Go6983 or Rp-cAMP (RpC) prior to VIP stimulation in the presence or absence of the inhibitors. Cells immunolabeled and quantified for pCREB (S121 or S133; green), GFAP (red) and Hoechst dye (blue). Arrowheads in confocal micrograph indicate GFAP-positive nuclei. Scale bar: 10 µm. (B,C) GFAP-positive nuclei were quantified for CREB S121 and S133 intensities and all group data are normalized against basal conditions across individual replicates. (D) Glycogen assay performed on cortical astrocytes stimulated with VIP (0.5 µM; 30 min) in the absence or presence of Go6983 (20 µM) for 6 h before being assayed for glycogen content. Graphs show glycogen concentration relative to basal across all independent experiments. (E) Same experiments as described in (D), except that cells were incubated with RpC (20 µM) during VIP stimulation. (F) Experiment performed similar to (D) except that astrocytes were stimulated with NA instead of VIP in the presence of Go6983 (20 µM) for 6 h before performing glycogen assays. (G,H) qPCR for Ppp1r3c in cortical astrocytes stimulated with VIP but in the presence of either (G) Go6976 or (H) RpC. Astrocytes were harvested 3h after treatment and prepared for RT-qPCR. (I) Cocultures were pretreated with Go6983 (20 µM; 30 min) or RpC (20 µM; 30 min) before being stimulated with VIP (0.5 µM; 30 min) in the presence of inhibitors. Cells were processed for immunocytochemistry and labeled with CRTC2 (green), GFAP (red) or Hoechst dye (blue). (J) The nucleus to cytoplasmic ratio of CRTC2 as shown in (H) were quantified and plotted as a bar graph. Unless otherwise stated, for all graphs, 1-way ANOVA followed by Tukey multiple comparisons tests were performed and only statistical notations relevant to the results are shown in the graphs. Experimental replicates were performed for all datasets to obtain the pooled data and P-values notations are as follows (*p < 0.05; ** p < 0.01; ***p < 0.001; **** p < 0.0001; n.s. not significant). Detailed statistical outputs are listed in the sSupplementary Table.
Since PKC plays a role in VIP-induced glycogen synthesis in astrocytes, we set out to examine which PKC isozyme might be responsible for participating in the signaling cascade responsible for initiating glycogen synthesis. There are three classes of PKC isozymes with different requirements for activation: conventional, novel and atypical. Conventional PKC requires both Ca2+ and diacylglycerol (DAG) for activation while novel PKC is activated by DAG and is Ca2+ independent. Finally, the activation of atypical PKC (aPKC) does not require Ca2+ or DAG28. Since conventional PKC activity requires Ca2+, we first asked if chelating intracellular Ca2+ with membrane permeable BAPTA-AM alters CREB-transcription or glycogen synthesis. We observed a partial but significant decrease in phosphorylation of CREB at S121 and S133 (Fig. 4A–C). Importantly, incubation of cortical astrocytes with BAPTA-AM did not impact cell viability (data not shown). Given that phosphorylation of CREB was reduced, we next performed glycogen assays on cortical astrocytes in the presence of BAPTA-AM during VIP stimulation and showed that glycogen synthesis was indeed impaired in the absence of intracellular Ca2+ (Fig. 4D). To show that transcriptional changes in gene expression critical for glycogen synthesis is suppressed, we performed qPCR and saw that VIP-induced increase in Ppp1r3c transcription was also abolished in the presence of BAPTA (Fig. 4E). As a follow up to the BAPTA experiment, we confirmed the involvement of conventional PKC by using Go6976, a selective PKC antagonist that discriminates between Ca2+ dependent and independent isozymes29. Much like Go6983, the bath application of Go6976 in cocultures strongly inhibited the phosphorylation of pCREBS133 and pCREBS121 (Fig. 4F–H) as well as the nuclear accumulation of CRTC2 in cocultured astrocytes upon VIP stimulation (Figs. 4I and Fig. S9). Furthermore, Go6976 treatment also significantly reduced VIP-induced transcription of Ppp1r3c in cortical astrocytes (Fig. 4J). Finally, we asked if atypical PKC (aPKC) is driving VIP-induced CREB activation and glycogen synthesis. There are two isoforms of atypical PKC (iota and zeta). Both PKC iota and a truncated form of PKC zeta known as PKMzeta have been strongly linked to long-term plasticity and memory formation30. However, the role of aPKC in astrocytes in not well-defined. To that end, we incubated cells with ZIP—a peptide inhibitor that blocks both PKC iota and PKMzeta—or with scrambled (SCR) peptides as controls at concentrations known to inhibit synaptic plasticity30 but does not promote excitotoxity in neurons or astrocytes31. Inhibition of aPKC did not significantly reduce VIP-driven phosphorylation of CREB at S121 and S133 either via immunoblotting (Fig. S10) or immunocytochemistry (Fig. S11). Furthermore, ZIP also did not significantly alter expression of glycogen-associated genes Ppp1r3c (Fig. S12) nor alter glycogenolysis or glycogen synthesis (Fig. S13). Collectively, these pharmacological experiments demonstrate that conventional PKC is the most likely PKC isozyme responsible for triggering VIP-induced and CREB-mediated transcription required for the replenishment of glycogen stores in astrocytes.
Figure 4.
VIP-induced glycogen synthesis requires activation of conventional PKC. (A) Astrocyte cocultures were pre-treated with BAPTA-AM (30 µM) prior to a brief stimulation with VIP (0.5 µM; 30 min). Cells were immunostained for pCREB (S121 and S133; green), GFAP (red) and Hoechst dye (blue). Scale bar, 10 µm. White arrowheads indicate GFAP-positive astrocyte nuclei. (B,C) Group data show normalized pCREB intensities against average basal values for each independent experiment. (D) Glycogen assay performed on cortical astrocytes stimulated with VIP (0.5 µM; 30 min) in the absence or presence of BAPTA-AM (30 µM) for 6 h. Graphs show glycogen concentration relative to basal across all independent experiments performed. (E) qPCR for Ppp1r3c in cortical astrocytes stimulated with VIP, but in the presence of BAPTA. (F) Astrocyte cocultures were pre-treated with Go6976 (1 µM; 30 min) prior to addition of VIP (0.5 µM; 30 min). Cell were then immunostained with pCREB (S121 or S133; green), GFAP (red) or Hoechst dye (blue). Scale bar, 10 µm. White arrowheads indicate astrocyte nuclei. (G, H) Group data show normalized pCREB intensities against average basal values for each independent experiment. (I) Cocultures were treated and processed as described in (F), except cells were immunostained with CRTC2 and quantified. (J) qPCR for Ppp1r3c stimulated cortical astrocytes with VIP, but in the presence of Go6976. mRNA was harvested 3h post-stimulation and RT-qPCR was performed. (K) Cocultures were pretreated with Go6983 before stimulated with PMA (4 nM; 30 min) or VIP (1 µM; 30 min) and immunostained with pCREB (S121 or S133; green), GFAP (red) or Hoechst dye (blue). White arrowheads indicate astrocyte nuclei. Scale bar, 10 µm. (L,M) Group data show normalized pCREB intensities against average basal values for each independent experiment. (N) qPCR for Ppp1r3c in presence of PMA for cortical astrocytes. Unpaired t-tests with Welch’s correction performed on samples. (O) Glycogen assay performed on cortical astrocytes stimulated with PMA (4 nM; 30 min). Cells were assayed for glycogen at 6 h post-recovery. Bar graph plotted to show glycogen concentration relative to basal levels. Unpaired t-tests with Welch’s corrections performed on samples. Unless otherwise stated, all experiments were performed in at least triplicates with 1-way ANOVA followed by Tukey multiple comparisons tests were performed and only relevant statistical notations are shown in the graphs. Experimental replicates were performed for all datasets to obtain the pooled data and P-values notations are as follows (*p < 0.05; ** p < 0.01; ***p < 0.001; **** p < 0.0001; n.s. not significant). Detailed statistical outputs are listed in the Supplementary Table.
While PKC is crucial for CREB-transcription linked to glycogen synthesis, its activity alone is likely insufficient to initiate glycogen synthesis in the absence of VIP ligand-receptor binding. To test this idea, we directly activated PKC by using phorbol myristate acetate (PMA), a well-characterized phorbol ester which mimics DAG, an upstream activator of PKC. In the presence of PMA, astrocytes showed robust phosphorylation of CREB at S121 and S133 comparable to cells induced by VIP (Fig. 4K–M). Moreover, PMA phosphorylation was also blocked by Go6983 (Fig. 4K–M). Despite phosphorylation of CREB, the levels of Ppp1r3c mRNA were not elevated (Fig. 4N) and PMA stimulation failed to enhance glycogen stores beyond what was already present at basal levels post-stimulation (Fig. 4O). Altogether, our results confirm the importance of conventional PKC-dependent CREB-mediated transcription during glycogen synthesis, and it is likely that VIP-VPAC ligand receptor binding is required to trigger other cellular cascades critical for glycogen synthesis.
Shared and distinct molecular signatures in glucose-driven glycogen synthesis
We have demonstrated that VIP-triggered glycogenolysis is followed by CREB-mediated glycogen synthesis in astrocytes. We next wanted to examine if there are shared or distinct cellular mechanisms in astrocytes when exposed to a more physiological stimulus such as changes in external glucose concentrations. We hypothesize that under a glucose-rich environment, astrocytes utilize glucose to help maintain glycogen stores; but when the glucose levels are low or absent, cells will rapidly convert glycogen to glucose. Once glycogen stores are depleted, astrocytes will then initiate a transcriptional program to induce glycogen synthesis while suppressing glycogenolysis.
To test this hypothesis, we performed a series of glucose depletion followed by restoration experiments in cortical astrocytes. We first incubated cortical astrocytes in media lacking extracellular glucose and observed an immediate depletion of glycogen stores (Fig. 5A) with a corresponding loss of glycogen granules (Fig. 5B,C). Subsequent restoration of glucose in the media resulted in a rebound and replenishment of glycogen stores (Fig. 5A–C). We also observed that phosphorylation of CREB at S121 and S133 correspondingly increased during glucose restoration of cells. (Fig. 5D,E). As with VIP induction, CREB phosphorylation is sensitive to PKC but not PKA inhibitors (Fig. 5D,E). Interestingly, CREB phosphorylation is also moderately elevated during glucose depletion, suggesting that the absence of external glucose or rapid depletion of glycogen is sufficient to induce CREB phosphorylation (Fig. 5D,E). Similar to CREB phosphorylation, glucose depletion also triggered nuclear entry of CRTC2, but unlike the more persistent phosphorylation of CREB during glucose depletion and restoration phases, the nuclear levels of CRTC2 rapidly returned to basal state when cells were re-exposed to glucose (Fig. 5F,G), suggesting that the kinetics of CRTC2 nuclear entry in response to glucose levels is distinct from CREB phosphorylation.
Figure 5.
Glucose depletion, restoration and CREB-driven glycogen synthesis. (A) Cortical astrocytes incubated in media containing 6 mM (basal) before being swapped with media depleted of glucose (0 mM) for 1 h. A subset of cells was recovered in basal media for 1 h (restoration). Upon recovery, glycogen assays were done on all samples, quantified. (B) Experiment was performed similar as described in (A), except cortical astrocytes were processed for immunocytochemistry with antibodies used to detect glycogen granules (ESG1A9 green, GAPDH, red). Hoechst dye (blue) was included to label the nucleus. Scale bar, 10 µm. (C) The density of ESG1A9 puncta in astrocytes as shown in (B) were quantified and plotted as a bar graph. (D,E) Experiments were carried out as described in (A) but in addition, cells were incubated with Go6983 (20 µM) or Rp-cAMP (RpC, 20 µM) throughout refeeding period. After treatment, cells were immunolabeled to detect pCREB S121 and S133 and quantified in GFAP-positive cells. Scale bar, 20 µm. (F) Cells were treated similarly as described in (A) but immunolabeled to detect CRTC2 (green) and GFAP (red) along with Hoechst dye to label the nucleus (blue). Scale bar 20 µm. (G) Nucleus to cytoplasmic ratio for CRTC2 were quantified for GFAP-positive astrocytes. (H) Cortical astrocytes were transduced with ACREB expressing lentivirus or a control pCIG vector. Cells were incubated with media depleted for glucose (0 mM; 3 h) followed by recovery in basal media (6 mM; 2 h). After recovery, glycogen assays were performed and quantified. (I–K) qPCR was performed for Ppp1r3c, C/ebp-β and Phkg1 on cortical astrocytes depleted or refed with glucose similar to that described for (A). Unless otherwise stated, for all graphs, 1-way ANOVA followed by Tukey multiple comparisons tests were performed and only relevant statistical notations are shown in the graphs. For all experiments, experimental replicates were performed to obtain the pooled data and P-values notations are as follows (*p < 0.05; ** p < 0.01; ***p < 0.001; **** p < 0.0001; n.s. not significant). Detailed statistical outputs are listed in the sSupplementary Table.
To examine if CREB activity is required for efficient glycogen synthesis in the presence of glucose, we delivered control or ACREB constructs to astrocytes and subjected the cells to glucose depletion as previously described and performed glycogen assays. The data indicate that cells expressing ACREB have impaired glycogen synthesis when glucose is reintroduced into the media (Fig. 5H). To compare changes in gene expression when cells are exposed to VIP or glucose depletion, we performed qPCR on select target genes we previously examined. Unexpectedly, we failed to detect any changes in Ppp1r3c mRNA either during glucose-depletion or when it was restored (Fig. 5I). Despite the importance of Ppp1r3c expression in promoting glycogen synthesis32,33, we speculate that other proteins in the glycogen synthesis pathway may be responsive to fluctuating glucose levels. To that end, we decided to test the expression of several candidate genes in the glycogen pathway. First, we observed that the mRNA levels for C/ebp-β was significantly elevated during glucose restoration (Fig. 5J), an observation that aligns with other studies which indicate that C/ebp-β is also playing a role during glycogen metabolism and is involved in maintaining glycogen homeostasis in cells34. In addition to upregulated genes, we also examined the suppression of genes that promotes glycogenolysis. We reasoned that downregulation of these gene responsible for breaking down glycogen along with upregulation of genes that promote glycogen synthesis should allow rapid accumulation of glycogen stores in cells. To that end, we identified a cluster of three metabolic genes (Phkg1, Pik3r1 and Soga1) that are downregulated in VIP-stimulated control samples (CTRL.PBS v. CTRL.VIP; p < 0.01; Fig. 2) in the mRNAseq experiments. Among the three metabolic genes, Phkg1 (Phosphorylase kinase catalytic subunit γ1) promotes glycogen breakdown by directly activating glycogen phosphorylase. Therefore, downregulation of Phkg1 transcription should indirectly elevate glycogen levels. Indeed, we observed a robust reduction of Phkg1 mRNA in astrocytes during glucose depletion and restoration experiments (Fig. 5K). We also verified through qPCR of VIP-treated astrocytes from mRNAseq samples that Phkg1 transcription was indeed downregulated (Fig. S14). Overall, we conclude that there are shared, and distinct cellular mechanisms associated with CREB-mediated transcription during glycogen synthesis when astrocytes are exposed to VIP or with changing levels of glucose.
Discussion
In this paper, we present evidence that CREB-mediated transcription is required for efficient glycogen synthesis in astrocytes upon VIP stimulation, and also when cells are exposed to glucose after a period of deprivation. In cultured astrocytes, VIP enhances CREB phosphorylation at specific sites and transcription of CREB target genes, a subset of which are associated with glucose and glycogen metabolic pathways. Importantly, we show that CREB-mediated transcription and glycogen synthesis are impaired when CREB function is blocked by expression of a dominant negative inhibitor, ACREB16. Among the astrocytic metabolism genes that are upregulated by VIP but attenuated in the presence of ACREB are Ppp1r3c and Ppargc1α. Ppp1r3c encodes the protein PTG (Protein Targeting to Glycogen), a subunit of protein phosphatase 1 (PP1) that binds to glycogen and regulates glycogen levels in astrocytes. A rise in PTG mRNA during stimulation will dephosphorylate and activate glycogen synthase, thus increasing glycogen stores while a deletion of the gene impaired glycogen synthesis33,35. Similarly, Ppargc1α which encodes PGC-1α, is a transcriptional coactivator and primary regulator of gluconeogenesis. Increased levels of PGC-1α in cells enhances glycogen stores and promotes glycogen synthesis in muscle36. However, unlike PTG, the role of PGC-1α in astrocytes remains poorly characterized. Not surprisingly, both these genes are predicted to have CRE binding sites in their promoter regions and are potential CREB targets for transcription (CREB Target Gene Database; Salk Institute)37. Interestingly, we observed that the mRNA of many other genes directly associated with glycogenolysis or glycogen synthesis such as glycogen synthase (Gys1), glycogen phosphorylase (Pygb), glycogen branching enzyme (Gbe) and glycogen debranching enzyme (Agl) are not altered by VIP (data not shown), suggesting the possibility that the activity of these enzymes is post-translationally regulated.
It is notable that VIP stimulation but not glucose restoration after depletion, upregulated expression of Ppp1r3c during glycogen synthesis. This is surprising given the importance of PTG in glycogen metabolism32,35,38 and it is unclear whether other parallel transcriptional outputs are activated during glucose-induced glycogen synthesis. Nonetheless, there are other complementary changes in gene expression that occur during VIP and glucose restoration. Specifically, we report for the first time, a strong transcriptional repression of Phkg1. PHKG1 is a subunit of phosphorylase kinase that activates glycogen phosphorylase to breakdown glycogen. A strong suppression of Phkg1 transcription during glycogen synthesis is therefore expected to prevent glycogen breakdown and thus, increase glycogen stores. As far as we know, the suppression of Phkg1 mRNA expression during glycogen synthesis has never been reported in astrocytes. Similarly, C/ebp-β expression is also robustly upregulated during glucose restoration. As a transcription factor, C/ebp-β has the potential to further modulate genes involved in glycogen synthesis34. Given the diversity in ligand-receptor interactions and the complexity of post-translational modifications of enzymes associated with glycogenolysis and glycogen synthesis, it is plausible, that various stimuli may activate different signaling cascades in the glycogen synthesis pathway39. This idea is supported by observations in human myoblasts where signaling pathways that upregulate glycogen synthesis during exposure to insulin is distinct from synthesis triggered after glucose deprivation40.
Perhaps far more convincingly, our collective experiments show that PKC activity is important during VIP-induced glycogen synthesis in astrocytes. Indeed, we have shown that all the associated signaling mechanisms that contribute to glycogen production require PKC and not PKA. This includes CREB phosphorylation at S121 and S133—with both serine residues being identified as PKC target sites, transcription of glycogen genes and the nuclear entry of CRTC2. Our results also align with previous work showing NA/ATP activation of CREB in astrocytes occurring via a cAMP- and calcium-independent pathway41. While VIP binds with high affinity to VPAC1 and VPAC2 G-protein coupled receptors that activate Gαs signaling23, there is also plenty of evidence that VIP-VPAC ligand receptor activation also trigger Gαi and Gαq pathways leading to diacylglycerol (DAG) and PKC activation24–27. Nevertheless, the activation of G proteins during VIP-VPAC receptor interaction in the context of glycogen metabolism is likely more nuanced. We observed that direct activation of DAG failed to trigger PTG transcription or increase in glycogen production beyond baseline levels, indicating that PKC phosphorylation of CREB alone is necessary but not to trigger glycogen synthesis and that VIP-VPAC interactions must trigger additional signaling mechanisms critical for the process. One possibility is that VIP-driven glycogen synthesis is tightly coupled to glycogenolysis and that glycogen turnover is required to trigger glycogen synthesis—a cellular event that is absent when PKC is directly activated by phorbol esters. However, at least one report has shown that these two metabolic processes in astrocytes are decoupled12.
The roles of VIP-VPAC signaling in neuronal plasticity, cognition and regulation of circadian rhythms have been described but less is known about the impact of VIP release on glia as cell-type specific studies remain lacking42–54. We and others have shown that VPAC receptors are found in astrocytes (data not shown) which argues that the cell is capable of responding to endogenous release of VIP20–22,55. While our data in this paper supports VIP-induced glycogen synthesis, glial responses to VIP are not limited to just modulating glycogen metabolism as it is reported that VIP also triggers astrocytogenesis and secretion of neurotrophic factors, cytokines and chemokines that promote reactive gliosis56–59. At present, VIP release in the hippocampus is thought to come primarily from VIP-interneurons60,61 and it is tempting to speculate that VIP release from these interneurons can concurrently modulate synaptic transmission and impact local astrocyte function, including glycogen metabolism, in a way that informs long-term plasticity. In support of this idea, Gao and colleagues have shown that binding of another neuromodulator, NA, to astrocytic β2-adrenergic receptors triggers the release of lactate crucial for memory consolidation10. In another study, evoked firing of single VIP-interneurons triggered vasodilation of surrounding microvasculature, presumably via the release and association of VIP with vascular endothelial cells62. Finally, the possibility of peripherally circulating VIP crossing the blood brain barrier and impacting glial function while controversial, cannot yet be discounted63–65. Overall, our findings solidify the important role of CREB-mediated transcription during glycogen synthesis in astrocytes and the potential function of VIP as a neuropeptide that can modulate glycogen metabolism critical for establishing long-term memories.
Methods and materials
Animals
C57BL6/J mice are housed in Animal Research Facility (ARF) in the Clinical Science Building (CSB) at the Lee Kong Chian School of Medicine. All animals had ad libitum access to food and water unless otherwise stated. Animals were maintained in 12 h light-dark cycle with relatively humidity of 70–80%. All procedures and protocols related to animal use, maintenance, euthanasia, and experimentation were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC; Protocol number: A18091) in Nanyang Technological University and is in accordance with relevant guidelines regulations as stated in the ARRIVE guidelines. Animals were euthanized with CO2 inhalation as outlined by the American Veterinary and Medical Association and ARRIVE guidelines for euthanasia of animals66.
Primary cultures
Hippocampal cocultures were prepared as previously described with modifications in media preparation67. Briefly, hippocampi from C57BL6/J mouse pups (post-natal day 0–2) were dissected in cold Hank’s Balanced Salt Solution (HBSS; Invitrogen) and pooled together, dissociated and plated on poly-DL-lysine (40 kDa PDL; 0.1 mg/ml; Sigma) coated coverslips or plates (Marienfeld Superior). Dissociated cocultures were kept in a defined serum-free media, Neurobasal A (Invitrogen) supplemented with 2% B27 (Gibco), 1% GlutaMAX (Gibco). All cultures were maintained in a 37 °C, 5% CO2 incubator and unless otherwise stated, all experiments were performed on 14–21 DIV (days in-vitro) cultures. For cortical astrocytes, mouse forebrain from C57BL6/J mouse pups (post-natal day 0–2) were dissected in cold HBSS (Invitrogen) and digested in 0.25% trypsin for 20 min in 37 °C water bath. The tissue was triturated with a glass pipette and cell suspension was sieved and resuspended in A10 astrocytes media which contains minimum essential media (MEM) (PAN-Biotech/Thermo Fisher), supplement with 10% horse serum (Gibco) and 1X Glutamax (Gibco). Cells were further processed and plated on PDL (40 kDa; 0.1 mg/ml; Sigma) coated dishes (Greiner Bio-one). All cultures were maintained in a 37 °C, 5% CO2 incubator with regular replacement of A10 media every 2 days. When cortical cultures reach confluency at DIV 7, cells were agitated to detach other glial subtypes, trypsinized and re-plated into the appropriate PDL coated tissue culture dishes to obtain astrocyte enriched cultures for further experiments.
Cell lines and lentiviruses
HEK293T cells (sourced from ATCC CRL-1573) were maintained in D10 media consisting of DMEM (Nacalai Tesque) supplemented with 10% fetal bovine serum (Hyclone) and 1% Penicillin/Streptomycin (Nacalai Tesque) in a 37 °C humidified incubator with 5% CO2. For transfection, cells were seeded in 6-well plates coated with 0.1 mg/ml PDL (40 kDa; 0.1 mg/ml; Sigma) at a density of 5 × 105 cells per well before DNA transfection using DNAfectin (ABM) following manufacturer protocols. Lentiviruses used in this study were either produced in the lab or packaged by a commercial company (Vector Builder). To produce high titer lentiviruses, HEK293T cells grown on D10 media were transfected with 3rd generation lentiviral component plasmids (RRL-vector 10 µg; pMDL gag/pol 6.5 µg; pRSV-Rev 3.5 µg and pMD-VSVG 3.5 µg) using DNAFectin for 12 h before media replacement (DMEM+1% FBS). Each viral supernatant was harvested twice at 48 h and 60 h post-transfection, pooled, filtered and ultracentrifuged (SW28; 23k rpm; 90 min) before the viral pellet was resuspended in PBS. For transduction of cortical astrocytes, lentiviral particles were diluted in serum-free MEM and incubated with cortical astrocytes in reduced volume for 24 h before being replaced with A10 media with media changes every 2 d. Cells are allowed to recover for at least 4–7 days during which they are monitored for comparable transduction efficiency prior to experimentation.
Cloning
Lentiviral backbone pCIG3 (pCMV-IRES-GFP) and CMV500 A-CREB were purchased from Addgene (#78264 and #33371) and A-CREB sequences were amplified and subcloned to generate pCIG3-ACREB (See Table 1 for primers).
Table 1.
Primers.
| Cebp/β | 5-GGTTTCGGGACTTGATGCA | 5-CAACAACCCCGCAGGAAC |
| Ppp1r3c | 5-TGCCTCTCGGTCCAATGAG | 5-GGCATGACGGAACTTGTCAA |
| Phkg1 | 5-GCTCACAGACTTCGGGTTTTCC | 5-ATGTCCACCTCCTTCCGATAGC |
| Hprt | 5-TGTTGTTGGATATGCCCTTG | 5-GGCCACAGGACTAGAACACC |
| β-actin | 5-GTGACGTTGACATCCGTAAAGA | 5-TGCTAGGAGCCAGAGCAGTAA |
| A-CREB | 5-GATTCTAGAGCCATGGACTACAAGGAC | 5-CCCGATATCTTAATCTGACTTGTGGCA |
RNA Isolation and quantitative PCR
Cortical astrocytes were washed with 1× PBS and lysed directly using 1 ml of TRIZOL. From TRIZOL mix, the total RNA was ethanol precipitated or column purified using RNeasy Kit (QIAGEN) and assayed for quality and concentration via the Nanodrop 2000c. cDNA was generated using Revert-Aid First Strand cDNA Synthesis Kit (Thermo Fisher) with polydT primers. cDNA and primers were added to SYBR® Green PCR Master Mix (Thermo Scientific) with appropriate primer pairs (see Table 1) and analyzed using One-Step Real Time PCR System (Applied Biosystem). Reactions were performed in triplicates and normalized against the ROX reference dye. Unless otherwise stated, changes in gene expression levels were normalized against the mouse β-actin and ΔΔ Ct values were obtained and normalized against baseline results.
Pharmacology and stimulation
Depending on experiments, cortical astrocytes, hippocampal cultures or acute slices were treated with various pharmacological agents with the following concentrations: bicuculline (BIC; 40 µM; Sigma), vasoactive intestinal peptide (VIP; 0.5 µM; Tocris), (R)-Adenosine, cyclic 3′,5′-(hydrogenphosphorothioate) triethylammonium (Rp-cAMP, 20 µM, Tocris), Go6983 (20 µM; Tocris), (R)-Adenosine, cyclic 3′,5′-(hydrogenphosphorothioate) triethylammonium (Rp-cAMP, 20 µM, Tocris), H89 (10 µM; Tocris), Go6976 (1 µM; Tocris), BAPTA-AM (30 µM; Tocris), ZIP/Scambled peptide (2 µM; Pepmic Co.), phorbol myristate acetate (PMA; 4 nM; Tocris), actinomycinD (ActD; 1 µM; Tocris). All pharmacological agents were diluted in the appropriate buffers compatible with the type of cellular preparations used for the study. Any preincubations and treatments for cocultures were performed in conditioned media. For glucose depletion and restoration, mature cortical cultures were preincubated in basal media for 30 min (Neurobasal A + 10% horse serum + 5.5 mM glucose) before incubation in glucose-depleted basal media for 3.5 h. Cells were then washed with PBS and replaced with basal media for 2 h before being processed.
Antibody labeling assays
For immunocytochemistry, cells on coverslips were rinsed with PBS, fixed with 3.2% PFA (EM Sciences), permeabilized with 0.1% Triton X-100 (Sigma) and blocked with 10% goat serum (Gibco). Cells were then incubated in primary antibodies diluted in blocking buffer for 3 h at RT or overnight at 4 °C. Secondary antibodies and Hoechst nuclear dye (Thermo Scientific) were incubated for 1 h at RT. Coverslips were mounted with Aqua/Poly-Mount (Polysciences) on slides and allowed to set prior to imaging. For immunohistochemistry of acute brain slices, vibratome sections are fixed with 4% PFA overnight in 4°C, washed with PBS and permeabilized in 0.4% Triton X-100 in RT before blocking with 10% goat serum (Gibco) with gentle shaking in 4 °C. Free floating sections are then incubated with primary antibodies overnight at 4 °C and secondary antibodies with Hoechst nuclear dye in RT for 1 h. All antibodies are diluted in 10% goat serum and processed sections are mounted on slides with Aqua/Poly-Mount. For immunoblotting, hippocampal tissues were dissected and homogenized in RIPA buffer [150 mM NaCl, 1.0% Triton X-100, 0.5% Sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0 with protease and phosphatase inhibitor (Pierce)]. For primary cell, adherent cells were washed with PBS, and directly lysed with 1% NP 40 lysis buffer (1% IGEPAL® CA-630 (Sigma); 10 mM Tris pH 7.5; 150 mM NaCl; protease and phosphatase inhibitor). All samples were then centrifuged, and supernatants measured for protein concentration using Pierce BCA protein assay. Equal protein amounts are loaded in each gel and SDS-PAGE performed using standard published protocols. Separated proteins on the gel were transferred to Immobilon-P membrane (Millipore), blocked with ChemiBLOCKER (Chemicon), incubated with primary and HRP-conjugated secondary antibodies before being developed with Luminata Classico or Forte HRP substrates (Merck). Bands were visualized with Gel Doc XR+ System (Bio-Rad) and quantified with Image Lab (Bio-Rad). All antibodies used in the paper include monoclonals: CREB (48H2; Cell Signaling), pCREB-S133 (87G3, Cell Signaling), β-actin (66009-1-Ig; Proteintech), GFAP (GA5; Cell Signaling), ESG1A9 (Kind gift from Hitoshi Ashida, Kobe University, Japan68), GAPDH (60004-1-Ig; Proteintech), and polyclonals: GFP (A11122; ThermoFisher), MAP2 (1100-MAP2; Phosphosolutions), CRTC2 (PA5-34547; ThermoFisher), pCREB-S142 (STJ91016; Proteintech), pCREB-S121 (STJ90463; Proteintech), VPAC1 (α-mVPAC1 pAb Kind gift from Glenn Dorsam, North Dakota State University, USA55). Unless otherwise stated, Alex Fluor conjugated secondary antibodies (ThermoFisher) and Hoechst nuclear dye (33342; Life Technologies) were used in all immunofluorescence assays.
Glycogen assay
Glycogen assays performed on cortical astrocytes where samples were stimulated and in absence or presence of different pharmacological compounds. For all inhibitor-based experiments, astrocytes were pretreated with compounds for 30 min prior to stimulation. Cells were then allowed to recover in fresh A10 media and harvested at the appropriate time points depending on experimental conditions. All glycogen assays were performed using a Glycogen Fluorometric Assay Kit (BioVision, SF, CA) based on manufacturer protocol. For equally plated cortical astrocytes on 6-well dishes, cells were washed with PBS, trypsinized and washed extensively with PBS to remove extracellular glucose, trypsin or other metabolites. Cells were then resuspended in water, boiled and the supernatant measured to obtain total glycogen and protein content.
Sequencing, alignment and DE analysis
For mRNAseq, cortical astrocytes were transduced with Control or ACREB expression vectors for 5 days. Cells were bath applied with PBS or VIP (0.5 µM) for 30 min followed by a 1.5 h recovery before harvesting RNA for mRNAseq. Strand-specific cDNA libraries (~ 250–300bp) were prepared for mRNAseq by NovogeneAIT Genomics (Singapore) and 20M 150 bp paired-end reads per sample were generated by sequencing on the Illumina Novaseq 6000 platform. Raw FASTQ files were processed using the Gekko high-performance cluster computing system at Nanyang Technological University, Singapore). First, Illumina adapters were trimmed (≤ 2 seed mismatches; paired-end alignment score ≥ 10; adapter length ≥ 3) and reads with an average quality score below 15 or length below 30 bases were removed using Trimmomatic (v0.39)69. Paired reads were then aligned to the mouse genome assembly GRCm38 (mm10) using STAR (v2.7.1a)70 and quantified using HTseq (v0.11.2)71. Analyses of gene expression count matrices were conducted in R 3.6.1 (R Core Team, 2019) using packages available through Bioconductor72. Data were filtered to retain genes with CPM > 1 in a minimum of three samples (N = 15,345) and then upper quartile normalised. Differential expression tests were conducted using the robust quasi-likelihood F-test pipeline in edgeR73–75. RUVseq76 was used to correct for batch effects and other sources of unwanted variability, by identifying the top two latent factors of variation in the expression of empirically-determined negative control genes (selected by excluding the top 5000 genes in a first-pass ANOVA-like test; N = 10,345) and incorporating these factors into the design matrix. Differential expression tests were performed for overall differential expression across conditions (ANOVA-like test) and for the following individual contrasts: “CTRL.VIP—CTRL.PBS”, “ACREB.VIP—ACREB.PBS”, “ACREB.PBS—CTRL.PBS”, and the interaction effect: “(ACREB.VIP—ACREB.PBS)—(CTRL.VIP—CTRL.PBS)”. Hierarchical clustering of DEGs was performed on batch-corrected CPMs, as well as on log2 fold changes (LFCs) from baseline for each condition obtained from edgeR analysis, using 1 − Pearson-correlation as the dissimilarity index and the average-linkage method. The R packages gplots77 and dendextend78 were used to plot dendrograms and heatmaps of per-gene z-scores. Heatmaps and dendrograms were also plotted for all expressed genes annotated to selected gene ontology (GO) terms using annotations downloaded from the Mouse Genome Database (accessed 5th May 2020)79, indicating genes that were overall differentially expressed across conditions (FDR < 0.1 in ANOVA) and with statistics from individual contrasts reported for selected genes. Sequencing datasets in this manuscript are available for download in the NCBI GEO repository (Accession: GSE243500).
TFBS, GO and cell type enrichment analyses
For TFBS analysis, DEG lists were submitted for analysis by OPOSSUM380 with default parameters (5000 bp region upstream and downstream of the transcription start site (TSS), conservation cutoff = 0.40, matrix score threshold = 85%). OPOSSUM3 uses TFBS motifs from the JASPAR database to generate Fisher scores for enrichment in the number of TF target genes and z-scores for enrichment in the number of TFBSs for each TF81. To control for GC bias, we identified genes with the top ten closest percentage GC content values for each upregulated gene in both contrasts of interest using the package genefilter82 and combined these to produce a list of 3951 background genes. We also used iRegulon (v1.3)83, an application in Cytoscape (v3.7.0)84, to identify clusters of enriched motifs and associated transcription factors: upregulated DEGs in selected contrasts were analysed against a background of all expressed genes using default parameters (10 K motif collection, 20 kb region centred on the TSS, minimum normalized enrichment score [NES] = 3.0). Data were exported to R for further analysis and plotting. For gene ontology, we used WebGestalt to identify over-represented molecular functions among genes upregulated in response to VIP stimulation (N = 508) compared to all expressed genes (FDR < .05)85,86. Redundancy between terms was reduced using affinity propagation. Lists of genes associated with specific metabolic GO terms were downloaded from the MGI database.
Imaging and statistical analysis
All confocal micrographs were obtained using a Zeiss LSM800 inverted scanning confocal with a 40× or 63× 1.4 NA oil objectives. Images are collected as a stack and maximum projected. Unless otherwise stated, micrographs were analyzed using Fiji image processing software. Mean nuclear intensity for the protein-of-interest was determined by referencing the nuclear region marked by Hoechst dye as region-of-interest (ROI). To normalize against batch-to-batch variations between astrocyte cultures, all experimental replicates were quantified and normalized against an average basal or control sample prior to pooling of group data. Unless otherwise stated, all experiments were done in triplicates and normalized individual data points were pooled and graphed. For quantification of nuclear to cytoplasmic ratio of proteins, Fiji was used to subtract nuclear signal reference based on appropriate nuclear (e.g., Hoechst) and somatic cell markers (e.g., GFAP for astrocytes). For the majority of glycogen granule measurements, confocal stacks were first maximally projected to capture all the granules in the cell. Threshold and size exclusion filtering were performed to isolate granules prior to quantification. All data in bars and texts are presented as mean ± SEM. For the majority of datasets, an analysis of variance (ANOVA) was used to compare differences between means when comparing multiple groups. Following that, Tukey's post-hoc test was performed. For measuring statistical significance between the means of two samples, a two-tailed unpaired t-test was performed with Welch’s corrections. Any statistically significant difference of p < 0.05 that was relevant to the experimental design and hypothesis was reported. Annotation for statistical significance is represented in each graph as follow: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.001). Unless otherwise stated, data is collected from triplicate experiments, with any exceptions noted in the sSupplementary Table All statistical outputs can also be found in the Supplementary Table. Finally, all graphs were generated, and statistical analyses performed, using GraphPad Prism v6.0/v8.0.
Supplementary Information
Acknowledgements
We thank Albert Chen for help with the manuscript. Glenn Dorsam, Freddy Jeanneteau and Hitoshi Ashida for generously sharing their reagents. Nicole Gunawan, Arnold Wei Jun Tan and Sophia Nguyen for their assistance on the manuscript. The authors have no conflict of interest to declare.
Author contributions
T.H.C. and L.W.L. conceptualize the research and designed the experiments. L.W.L., J.R.G., N.Z., V.A.B., J.M.T., Y.M.L. and T.H.C. performed the experiments. Supervision and funding were done by T.H.C. Writing of the manuscript and editing were done by L.W.L. and T.H.C. All authors reviewed the manuscript.
Funding
This research is supported by the Singapore Ministry of Education under its Singapore Ministry of Education Academic Research Fund Tier 3 (MOE2017-T3-1-002), Academic Research Fund Tier 1 (MOE2018-T1-002-033) and by Nanyang Assistant Professorship (NAP) from Nanyang Technological University Singapore.
Data availability
All sequencing datasets in this manuscript are available for download in the NCBI GEO repository (Accession: GSE243500). All statistical outputs analysed during this study are included in the Supplementary Information.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-67976-w.
References
- 1.Suzuki, A. et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell144, 810–823. 10.1016/j.cell.2011.02.018 (2011). 10.1016/j.cell.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman, L. A., Korol, D. L. & Gold, P. E. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS ONE6, e28427. 10.1371/journal.pone.0028427 (2011). 10.1371/journal.pone.0028427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Dowd, B. S., Gibbs, M. E., Ng, K. T., Hertz, E. & Hertz, L. Astrocytic glycogenolysis energizes memory processes in neonate chicks. Brain Res. Dev. Brain Res.78, 137–141. 10.1016/0165-3806(94)90018-3 (1994). 10.1016/0165-3806(94)90018-3 [DOI] [PubMed] [Google Scholar]
- 4.Gibbs, M. E., Anderson, D. G. & Hertz, L. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia54, 214–222. 10.1002/glia.20377 (2006). 10.1002/glia.20377 [DOI] [PubMed] [Google Scholar]
- 5.Pellerin, L. & Magistretti, P. J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. U.S.A.91, 10625–10629. 10.1073/pnas.91.22.10625 (1994). 10.1073/pnas.91.22.10625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinman, M. Q., Gao, V. & Alberini, C. M. The role of lactate-mediated metabolic coupling between astrocytes and neurons in long-term memory formation. Front. Integr. Neurosci.10, 10. 10.3389/fnint.2016.00010 (2016). 10.3389/fnint.2016.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belanger, M., Allaman, I. & Magistretti, P. J. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab.14, 724–738. 10.1016/j.cmet.2011.08.016 (2011). 10.1016/j.cmet.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 8.Calì, C., Tauffenberger, A. & Magistretti, P. The strategic location of glycogen and lactate: From body energy reserve to brain plasticity. Front. Cell. Neurosci.13, 82. 10.3389/fncel.2019.00082 (2019). 10.3389/fncel.2019.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pellerin, L. & Magistretti, P. J. Sweet sixteen for ANLS. J. Cereb. Blood Flow Metab.32, 1152–1166. 10.1038/jcbfm.2011.149 (2012). 10.1038/jcbfm.2011.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao, V. et al. Astrocytic β2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proc. Natl. Acad. Sci. U.S.A.113, 8526–8531. 10.1073/pnas.1605063113 (2016). 10.1073/pnas.1605063113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magistretti, P. J., Morrison, J. H., Shoemaker, W. J., Sapin, V. & Bloom, F. E. Vasoactive intestinal polypeptide induces glycogenolysis in mouse cortical slices: A possible regulatory mechanism for the local control of energy metabolism. Proc. Natl. Acad. Sci. U.S.A.78, 6535–6539 (1981). 10.1073/pnas.78.10.6535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorg, O. & Magistretti, P. J. Vasoactive intestinal peptide and noradrenaline exert long-term control on glycogen levels in astrocytes: Blockade by protein synthesis inhibition. J. Neurosci.12, 4923–4931 (1992). 10.1523/JNEUROSCI.12-12-04923.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma, J. et al. A splice mutation in the PHKG1 gene causes high glycogen content and low meat quality in pig skeletal muscle. PLoS Genet.10, e1004710. 10.1371/journal.pgen.1004710 (2014). 10.1371/journal.pgen.1004710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasel, P. et al. Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat. Commun.8, 15132. 10.1038/ncomms15132 (2017). 10.1038/ncomms15132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johannessen, M. & Moens, U. Multisite phosphorylation of the cAMP response element-binding protein (CREB) by a diversity of protein kinases. Front. Biosci.12, 1814–1832. 10.2741/2190 (2007). 10.2741/2190 [DOI] [PubMed] [Google Scholar]
- 16.Ahn, S. et al. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol. Cell. Biol.18, 967–977. 10.1128/mcb.18.2.967 (1998). 10.1128/mcb.18.2.967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, Y., Vera, L., Fischer, W. H. & Montminy, M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature460, 534–537. 10.1038/nature08111 (2009). 10.1038/nature08111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravnskjaer, K. et al. Cooperative interactions between CBP and TORC2 confer selectivity to CREB target gene expression. Embo J.26, 2880–2889 (2007). 10.1038/sj.emboj.7601715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerner, R. G., Depatie, C., Rutter, G. A., Screaton, R. A. & Balthasar, N. A role for the CREB co-activator CRTC2 in the hypothalamic mechanisms linking glucose sensing with gene regulation. EMBO Rep.10, 1175–1181. 10.1038/embor.2009.177 (2009). 10.1038/embor.2009.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joo, K. M. et al. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J. Comp. Neurol.476, 388–413. 10.1002/cne.20231 (2004). 10.1002/cne.20231 [DOI] [PubMed] [Google Scholar]
- 21.Grimaldi, M. & Cavallaro, S. Functional and molecular diversity of PACAP/VIP receptors in cortical neurons and type I astrocytes. Eur. J. Neurosci.11, 2767–2772. 10.1046/j.1460-9568.1999.00693.x (1999). 10.1046/j.1460-9568.1999.00693.x [DOI] [PubMed] [Google Scholar]
- 22.Magistretti, P. J., Manthorpe, M., Bloom, F. E. & Varon, S. Functional receptors for vasoactive intestinal polypeptide in cultured astroglia from neonatal rat brain. Regul. Pept.6, 71–80 (1983). 10.1016/0167-0115(83)90136-2 [DOI] [PubMed] [Google Scholar]
- 23.Le Péchon-Vallée, C., Magalon, K., Rasolonjanahary, R., Enjalbert, A. & Gérard, C. Vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptides stimulate mitogen-activated protein kinase in the pituitary cell line GH4C1 by a 3’,5’-cyclic adenosine monophosphate pathway. Neuroendocrinology72, 46–56. 10.1159/000054570 (2000). 10.1159/000054570 [DOI] [PubMed] [Google Scholar]
- 24.Langer, I. Mechanisms involved in VPAC receptors activation and regulation: Lessons from pharmacological and mutagenesis studies. Front. Endocrinol.3, 129. 10.3389/fendo.2012.00129 (2012). 10.3389/fendo.2012.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacKenzie, C. J. et al. Mechanisms of phospholipase C activation by the vasoactive intestinal polypeptide/pituitary adenylate cyclase-activating polypeptide type 2 receptor. Endocrinology142, 1209–1217. 10.1210/endo.142.3.8013 (2001). 10.1210/endo.142.3.8013 [DOI] [PubMed] [Google Scholar]
- 26.Spengler, D. et al. Differential signal transduction by five splice variants of the PACAP receptor. Nature365, 170–175. 10.1038/365170a0 (1993). 10.1038/365170a0 [DOI] [PubMed] [Google Scholar]
- 27.Straub, S. G. & Sharp, G. W. A wortmannin-sensitive signal transduction pathway is involved in the stimulation of insulin release by vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide. J. Biol. Chem.271, 1660–1668. 10.1074/jbc.271.3.1660 (1996). 10.1074/jbc.271.3.1660 [DOI] [PubMed] [Google Scholar]
- 28.Newton, A. C. Protein kinase C: perfectly balanced. Crit. Rev. Biochem. Mol. Biol.53, 208–230. 10.1080/10409238.2018.1442408 (2018). 10.1080/10409238.2018.1442408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martiny-Baron, G. et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem.268, 9194–9197 (1993). 10.1016/S0021-9258(18)98335-3 [DOI] [PubMed] [Google Scholar]
- 30.Tsokas, P. et al. Compensation for PKMζ in long-term potentiation and spatial long-term memory in mutant mice. eLife.10.7554/eLife.14846 (2016). 10.7554/eLife.14846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadeh, N., Verbitsky, S., Dudai, Y. & Segal, M. Zeta inhibitory peptide, a candidate inhibitor of protein kinase Mζ, is excitotoxic to cultured hippocampal neurons. J. Neurosci.35, 12404–12411. 10.1523/jneurosci.0976-15.2015 (2015). 10.1523/jneurosci.0976-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Printen, J. A., Brady, M. J. & Saltiel, A. R. PTG, a protein phosphatase 1-binding protein with a role in glycogen metabolism. Science275, 1475–1478. 10.1126/science.275.5305.1475 (1997). 10.1126/science.275.5305.1475 [DOI] [PubMed] [Google Scholar]
- 33.Crosson, S. M., Khan, A., Printen, J., Pessin, J. E. & Saltiel, A. R. PTG gene deletion causes impaired glycogen synthesis and developmental insulin resistance. J. Clin. Investig.111, 1423–1432. 10.1172/jci17975 (2003). 10.1172/jci17975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardinaux, J. R. & Magistretti, P. J. Vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, and noradrenaline induce the transcription factors CCAAT/enhancer binding protein (C/EBP)-beta and C/EBP delta in mouse cortical astrocytes: Involvement in cAMP-regulated glycogen metabolism. J. Neurosci.16, 919–929. 10.1523/jneurosci.16-03-00919.1996 (1996). 10.1523/jneurosci.16-03-00919.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allaman, I., Pellerin, L. & Magistretti, P. J. Protein targeting to glycogen mRNA expression is stimulated by noradrenaline in mouse cortical astrocytes. Glia30, 382–391 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Wende, A. R. et al. A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J. Biol. Chem.282, 36642–36651. 10.1074/jbc.M707006200 (2007). 10.1074/jbc.M707006200 [DOI] [PubMed] [Google Scholar]
- 37.Zhang, X. et al. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc. Natl. Acad. Sci. U.S.A.102, 4459–4464. 10.1073/pnas.0501076102 (2005). 10.1073/pnas.0501076102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brady, M. J., Printen, J. A., Mastick, C. C. & Saltiel, A. R. Role of protein targeting to glycogen (PTG) in the regulation of protein phosphatase-1 activity. J. Biol. Chem.272, 20198–20204. 10.1074/jbc.272.32.20198 (1997). 10.1074/jbc.272.32.20198 [DOI] [PubMed] [Google Scholar]
- 39.Jensen, J. & Lai, Y. C. Regulation of muscle glycogen synthase phosphorylation and kinetic properties by insulin, exercise, adrenaline and role in insulin resistance. Arch. Physiol. Biochem.115, 13–21. 10.1080/13813450902778171 (2009). 10.1080/13813450902778171 [DOI] [PubMed] [Google Scholar]
- 40.Halse, R., Bonavaud, S. M., Armstrong, J. L., McCormack, J. G. & Yeaman, S. J. Control of glycogen synthesis by glucose, glycogen, and insulin in cultured human muscle cells. Diabetes50, 720–726. 10.2337/diabetes.50.4.720 (2001). 10.2337/diabetes.50.4.720 [DOI] [PubMed] [Google Scholar]
- 41.Carriba, P. et al. ATP and noradrenaline activate CREB in astrocytes via noncanonical Ca(2+) and cyclic AMP independent pathways. Glia60, 1330–1344. 10.1002/glia.22352 (2012). 10.1002/glia.22352 [DOI] [PubMed] [Google Scholar]
- 42.Jones, J. R., Simon, T., Lones, L. & Herzog, E. D. SCN VIP neurons are essential for normal light-mediated resetting of the circadian system. J. Neurosci.38, 7986–7995. 10.1523/jneurosci.1322-18.2018 (2018). 10.1523/jneurosci.1322-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazuski, C. et al. Entrainment of circadian rhythms depends on firing rates and neuropeptide release of VIP SCN neurons. Neuron99, 555-563.e555. 10.1016/j.neuron.2018.06.029 (2018). 10.1016/j.neuron.2018.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunha-Reis, D., Aidil-Carvalho Mde, F. & Ribeiro, J. A. Endogenous inhibition of hippocampal LTD and depotentiation by vasoactive intestinal peptide VPAC1 receptors. Hippocampus24, 1353–1363. 10.1002/hipo.22316 (2014). 10.1002/hipo.22316 [DOI] [PubMed] [Google Scholar]
- 45.Cunha-Reis, D., Ribeiro, J. A. & Sebastião, A. M. VPAC2 receptor activation mediates VIP enhancement of population spikes in the CA1 area of the hippocampus. Ann. N. Y. Acad. Sci.1070, 210–214. 10.1196/annals.1317.016 (2006). 10.1196/annals.1317.016 [DOI] [PubMed] [Google Scholar]
- 46.Cunha-Reis, D., Ribeiro, J. A. & Sebastião, A. M. VIP enhances synaptic transmission to hippocampal CA1 pyramidal cells through activation of both VPAC1 and VPAC2 receptors. Brain Res.1049, 52–60. 10.1016/j.brainres.2005.04.077 (2005). 10.1016/j.brainres.2005.04.077 [DOI] [PubMed] [Google Scholar]
- 47.Cunha-Reis, D., Sebastião, A. M., Wirkner, K., Illes, P. & Ribeiro, J. A. VIP enhances both pre- and postsynaptic GABAergic transmission to hippocampal interneurones leading to increased excitatory synaptic transmission to CA1 pyramidal cells. Br. J. Pharmacol.143, 733–744. 10.1038/sj.bjp.0705989 (2004). 10.1038/sj.bjp.0705989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeuchi, S. et al. Activation of the VPAC2 receptor impairs axon outgrowth and decreases dendritic arborization in mouse cortical neurons by a PKA-dependent mechanism. Front. Neurosci.14, 521. 10.3389/fnins.2020.00521 (2020). 10.3389/fnins.2020.00521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itoh, S., Takashima, A. & Morimoto, T. Impaired spatial learning by vasoactive intestinal peptide in Morris water maze task in the rat. Can. J. Physiol. Pharmacol.72, 25–29. 10.1139/y94-005 (1994). 10.1139/y94-005 [DOI] [PubMed] [Google Scholar]
- 50.Takashima, A., Maeda, Y. & Itoh, S. Influence of chronic intracerebroventricular infusion of vasoactive intestinal peptide (VIP) on memory processes in Morris water pool test in the rat. Peptides14, 1073–1078. 10.1016/0196-9781(93)90089-y (1993). 10.1016/0196-9781(93)90089-y [DOI] [PubMed] [Google Scholar]
- 51.Takashima, A., Maeda, Y. & Itoh, S. Vasoactive intestinal peptide (VIP) causes memory impairment in passive avoidance responding of the rat. Peptides14, 1067–1071. 10.1016/0196-9781(93)90088-x (1993). 10.1016/0196-9781(93)90088-x [DOI] [PubMed] [Google Scholar]
- 52.Ivanova, M., Ternianov, A., Tashev, R., Belcheva, S. & Belcheva, I. Lateralized learning and memory effects of vasoactive intestinal peptide infused into the rat hippocampal CA1 area. Regul. Pept.156, 42–46. 10.1016/j.regpep.2009.05.009 (2009). 10.1016/j.regpep.2009.05.009 [DOI] [PubMed] [Google Scholar]
- 53.Ago, Y. et al. Impaired extinction of cued fear memory and abnormal dendritic morphology in the prelimbic and infralimbic cortices in VPAC2 receptor (VIPR2)-deficient mice. Neurobiol. Learn. Mem.145, 222–231. 10.1016/j.nlm.2017.10.010 (2017). 10.1016/j.nlm.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaudhury, D., Loh, D. H., Dragich, J. M., Hagopian, A. & Colwell, C. S. Select cognitive deficits in vasoactive intestinal peptide deficient mice. BMC Neurosci.9, 63. 10.1186/1471-2202-9-63 (2008). 10.1186/1471-2202-9-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hermann, R. J. et al. Characterization and use of a rabbit-anti-mouse VPAC1 antibody by flow cytometry. J. Immunol. Methods376, 20–31. 10.1016/j.jim.2011.10.009 (2012). 10.1016/j.jim.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zupan, V. et al. Involvement of pituitary adenylate cyclase-activating polypeptide II vasoactive intestinal peptide 2 receptor in mouse neocortical astrocytogenesis. J. Neurochem.70, 2165–2173. 10.1046/j.1471-4159.1998.70052165.x (1998). 10.1046/j.1471-4159.1998.70052165.x [DOI] [PubMed] [Google Scholar]
- 57.Brenneman, D. E. et al. Complex array of cytokines released by vasoactive intestinal peptide. Neuropeptides37, 111–119. 10.1016/s0143-4179(03)00022-2 (2003). 10.1016/s0143-4179(03)00022-2 [DOI] [PubMed] [Google Scholar]
- 58.Nishimoto, M., Miyakawa, H., Wada, K. & Furuta, A. Activation of the VIP/VPAC2 system induces reactive astrocytosis associated with increased expression of glutamate transporters. Brain Res.1383, 43–53. 10.1016/j.brainres.2011.01.082 (2011). 10.1016/j.brainres.2011.01.082 [DOI] [PubMed] [Google Scholar]
- 59.Zusev, M. & Gozes, I. Differential regulation of activity-dependent neuroprotective protein in rat astrocytes by VIP and PACAP. Regul. Pept.123, 33–41. 10.1016/j.regpep.2004.05.021 (2004). 10.1016/j.regpep.2004.05.021 [DOI] [PubMed] [Google Scholar]
- 60.Kosaka, T. et al. GABAergic neurons containing CCK-8-like and/or VIP-like immunoreactivities in the rat hippocampus and dentate gyrus. J. Comp. Neurol.239, 420–430. 10.1002/cne.902390408 (1985). 10.1002/cne.902390408 [DOI] [PubMed] [Google Scholar]
- 61.Cunha-Reis, D. & Caulino-Rocha, A. VIP modulation of hippocampal synaptic plasticity: A role for VIP receptors as therapeutic targets in cognitive decline and mesial temporal lobe epilepsy. Front. Cell. Neurosci.14, 153. 10.3389/fncel.2020.00153 (2020). 10.3389/fncel.2020.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cauli, B. et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J. Neurosci.24, 8940–8949. 10.1523/jneurosci.3065-04.2004 (2004). 10.1523/jneurosci.3065-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dufes, C. et al. Brain delivery of vasoactive intestinal peptide (VIP) following nasal administration to rats. Int. J. Pharm.255, 87–97. 10.1016/s0378-5173(03)00039-5 (2003). 10.1016/s0378-5173(03)00039-5 [DOI] [PubMed] [Google Scholar]
- 64.Dogrukol-Ak, D., Banks, W. A., Tuncel, N. & Tuncel, M. Passage of vasoactive intestinal peptide across the blood-brain barrier. Peptides24, 437–444. 10.1016/s0196-9781(03)00059-7 (2003). 10.1016/s0196-9781(03)00059-7 [DOI] [PubMed] [Google Scholar]
- 65.Jozsa, R. et al. Short-term fasting differentially alters PACAP and VIP levels in the brains of rat and chicken. Ann. N. Y. Acad. Sci.1070, 354–358. 10.1196/annals.1317.044 (2006). 10.1196/annals.1317.044 [DOI] [PubMed] [Google Scholar]
- 66.Leary, S., Underwood, U., Anthony, R., Cartner, S., Corey, D., Grandin, T., Greenacre, C., Gwaltney-Bran, S., McCrackin, M., Meyer, R., Miller, D., Shearer, J., Yanong, R., Golab, G. & Patterson-Kane, E. AVMA Guidelines for the Euthanasia of Animals: 2013 edition. https://www.avma.org/KB/Policies/Pages/Euthanasia-Guidelines.aspx (2013).
- 67.Ch’ng, T. H. et al. Activity-dependent transport of the transcriptional coactivator CRTC1 from synapse to nucleus. Cell150, 207–221. 10.1016/j.cell.2012.05.027 (2012). 10.1016/j.cell.2012.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakamura-Tsuruta, S. et al. Comparative analysis of carbohydrate-binding specificities of two anti-glycogen monoclonal antibodies using ELISA and surface plasmon resonance. Carbohydr. Res.350, 49–54. 10.1016/j.carres.2011.12.029 (2012). 10.1016/j.carres.2011.12.029 [DOI] [PubMed] [Google Scholar]
- 69.Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics30, 2114–2120 (2014). 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics29, 15–21. 10.1093/bioinformatics/bts635 (2013). 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anders, S., Pyl, P. T. & Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics31, 166–169 (2015). 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gentleman, R. C. et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol.5, R80 (2004). 10.1186/gb-2004-5-10-r80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou, X., Lindsay, H. & Robinson, M. D. Robustly detecting differential expression in RNA sequencing data using observation weights. Nucleic Acids Res.42, e91–e91 (2014). 10.1093/nar/gku310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics26, 139–140. 10.1093/bioinformatics/btp616 (2010). 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lun, A. T., Chen, Y. & Smyth, G. K. It’s DE-licious: A recipe for differential expression analyses of RNA-seq experiments using quasi-likelihood methods in edgeR. Methods Mol. Biol.1418, 391–416. 10.1007/978-1-4939-3578-9_19 (2016). 10.1007/978-1-4939-3578-9_19 [DOI] [PubMed] [Google Scholar]
- 76.Risso, D., Ngai, J., Speed, T. P. & Dudoit, S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat. Biotechnol.32, 896–902. 10.1038/nbt.2931 (2014). 10.1038/nbt.2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Warnes, G. R. et al.gplots: Various R Programming tools for Plotting Data (2015).
- 78.Galili, T. dendextend: An R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics31, 3718–3720 (2015). 10.1093/bioinformatics/btv428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bult, C. J. et al. Mouse genome database (MGD) 2019. Nucleic Acids Res.47, D801–D806. 10.1093/nar/gky1056 (2019). 10.1093/nar/gky1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kwon, A. T., Arenillas, D. J., Hunt, R. W. & Wasserman, W. W. oPOSSUM-3: Advanced analysis of regulatory motif over-representation across genes or ChIP-Seq datasets. G3 Genes Genomes Genet.2, 987–1002 (2012). 10.1534/g3.112.003202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sandelin, A., Alkema, W., Engström, P., Wasserman, W. W. & Lenhard, B. JASPAR: An open-access database for eukaryotic transcription factor binding profiles. Nucleic Acids Res.32, D91–D94 (2004). 10.1093/nar/gkh012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gentleman, R., Carey, V., Huber, W. & Hahne, F. Genefilter: Methods for filtering genes from high-throughput experiments. R package version 1.66.0 (2019).
- 83.Janky, R. S. et al. iRegulon: From a gene list to a gene regulatory network using large motif and track collections. PLoS Comput. Biol.10, e1003731. 10.1371/journal.pcbi.1003731 (2014). 10.1371/journal.pcbi.1003731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res.13, 2498–2504 (2003). 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang, B., Kirov, S. & Snoddy, J. WebGestalt: An integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res.33, W741-748. 10.1093/nar/gki475 (2005). 10.1093/nar/gki475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang, J., Duncan, D., Shi, Z. & Zhang, B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): Update 2013. Nucleic Acids Res.41, W77-83. 10.1093/nar/gkt439 (2013). 10.1093/nar/gkt439 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequencing datasets in this manuscript are available for download in the NCBI GEO repository (Accession: GSE243500). All statistical outputs analysed during this study are included in the Supplementary Information.