Key Points
Question
Is preoperative chemoradiotherapy (CRT) or preoperative and/or perioperative chemotherapy associated with improved outcomes in patients with adenocarcinoma of the esophagus and esophagogastric junction (AEG)?
Findings
In this network meta-analysis consisting of individual data from 2549 patients in 17 studies, both preoperative CRT plus surgery and preoperative and/or perioperative chemotherapy plus surgery were associated with longer overall survival compared with surgery alone in patients with AEG.
Meaning
These results suggest that preoperative CRT and preoperative and/or perioperative chemotherapy are both associated with longer survival for patients with AEG.
This individual patient data network meta-analysis assesses the association between preoperative chemoradiotherapy and preoperative and/or perioperative chemotherapy compared with surgery alone in patients with adenocarcinoma of the esophagus and esophagogastric junction.
Abstract
Importance
The prognosis of patients with adenocarcinoma of the esophagus and esophagogastric junction (AEG) is poor. From current evidence, it remains unclear to what extent preoperative chemoradiotherapy (CRT) or preoperative and/or perioperative chemotherapy achieve better outcomes than surgery alone.
Objective
To assess the association of preoperative CRT and preoperative and/or perioperative chemotherapy in patients with AEG with overall survival and other outcomes.
Data Sources
Literature search in PubMed, Cochrane Library, Cumulative Index to Nursing and Allied Health Literature, ClinicalTrials.gov, and International Clinical Trials Registry Platform was performed from inception to April 21, 2023.
Study Selection
Two blinded reviewers screened for randomized clinical trials comparing preoperative CRT plus surgery with preoperative and/or perioperative chemotherapy plus surgery, 1 intervention with surgery alone, or all 3 treatments. Only data from participants with AEG were included from trials that encompassed mixed histology or gastric cancer. Among 2768 initially identified studies, 17 (0.6%) met the selection criteria.
Data Extraction and Synthesis
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines were followed for extracting data and assessing data quality by 2 independent extractors. A bayesian network meta-analysis was conducted using the 2-stage approach.
Main Outcomes and Measures
Overall and disease-free survival, postoperative morbidity, and mortality.
Results
The analyses included 2549 patients (2206 [86.5%] male; mean [SD] age, 61.0 [9.4] years) from 17 trials (conducted from 1989-2016). Both preoperative CRT plus surgery (hazard ratio [HR], 0.75 [95% credible interval (CrI), 0.62-0.90]; 3-year difference, 105 deaths per 1000 patients) and preoperative and/or perioperative chemotherapy plus surgery (HR, 0.78 [95% CrI, 0.64-0.91]; 3-year difference, 90 deaths per 1000 patients) showed longer overall survival than surgery alone. Comparing the 2 modalities yielded similar overall survival (HR, 1.04 [95% CrI], 0.83-1.28]; 3-year difference, 15 deaths per 1000 patients fewer for CRT). Similarly, disease-free survival was longer for both modalities compared with surgery alone. Postoperative morbidity was more frequent after CRT plus surgery (odds ratio [OR], 2.94 [95% CrI, 1.01-8.59]) than surgery alone. Postoperative mortality was not significantly more frequent after CRT plus surgery than surgery alone (OR, 2.50 [95% CrI, 0.66-10.56]) or after chemotherapy plus surgery than CRT plus surgery (OR, 0.44 [95% CrI, 0.08-2.00]).
Conclusions and Relevance
In this meta-analysis of patients with AEG, both preoperative CRT and preoperative and/or perioperative chemotherapy were associated with longer survival without relevant differences between the 2 modalities. Thus, either of the 2 treatments may be recommended to patients.
Introduction
In 2020, esophageal cancer ranked sixth in mortality (544 100 deaths) worldwide.1,2 It consists of squamous cell carcinoma and adenocarcinoma. Esophageal and esophagogastric junction adenocarcinoma are considered a single entity, adenocarcinoma of the esophagogastric junction (AEG). The prognosis for patients with AEG undergoing upfront surgery has been poor. Five-year survival ranged from 36.9% for patients with node-negative disease to 9.6% for those with node-positive disease.2
Substantial evidence suggests that preoperative chemotherapy or chemoradiotherapy (CRT) prolongs overall survival (OS) compared with surgery alone.3,4,5 While preoperative CRT is usually not continued postoperatively, chemotherapy is given preoperatively and postoperatively (perioperative chemotherapy). Preoperative is preferred to mere postoperative treatment because it increases the likelihood of complete resection. In addition, many patients are unable to begin or sustain postoperative treatment due to complications or deterioration.6,7
The available evidence does not allow a conclusion on whether preoperative CRT or preoperative and/or perioperative chemotherapy has better outcomes for AEG. Both have shown prolonged survival compared with surgery alone in randomized clinical trials (RCTs)4,8,9,10,11,12 and meta-analyses.3,5,13 A randomized head-to-head comparison has been performed in 4 trials, with 3 showing inconclusive results,14,15,16 and the large Neo-AEGIS trial (Neoadjuvant Trial in Adenocarcinoma of the Oesophagus and Oesophagogastric Junction International Study)17 reporting similar survival and quality of life between treatment groups, thus suggesting equipoise. Three other RCTs directly comparing the modalities18,19,20 have not reported results yet. In summary, it remains unclear which is the best multimodal approach for treating AEG. To integrate the evidence comparing preoperative CRT, preoperative and/or perioperative chemotherapy, and surgery alone with regard to relevant outcomes in patients with AEG, we performed an individual patient data (IPD) network meta-analysis (NMA) including data from all pertinent RCTs.
Methods
The work was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines. It was registered and the protocol published in the Cochrane Library.21 It was approved by the ethics committee of the Medical Faculty, Martin-Luther-University Halle-Wittenberg, Halle, Germany, with a waiver of informed consent because data were provided anonymously.
Inclusion Criteria and Literature Search
We included patients from RCTs comparing at least 2 of the following: preoperative CRT plus surgery, preoperative and/or perioperative chemotherapy plus surgery, or surgery alone. Participants needed to have nonmetastatic, untreated, resectable AEG. There were no restrictions regarding blinding, follow-up, study size, and language. We searched the following databases from inception to April 21, 2023, using a predefined search strategy (eAppendix 1 in Supplement 1): PubMed, Cochrane Library, Cumulative Index to Nursing and Allied Health Literature, ClinicalTrials.gov, and International Clinical Trials Registry Platform. We checked reference lists of included studies for additional references.
Literature Screening and Data Collection
Two reviewers (U.R. and J.F.) independently screened titles, abstracts, and, if potentially eligible, full texts for inclusion. Disagreement was resolved by a third reviewer (J.K.). Individual patient data were requested from all trials for all randomized participants fulfilling inclusion criteria. For trials not providing IPD, aggregate data (AD) were extracted by 2 researchers independently (U.R. and J.F.).
Data Quality and Risk of Bias
Data quality checks were performed (eAppendix 2 in Supplement 1). Two researchers (U.R. and J.F.) independently assessed risk of bias for each included study using criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions22 and version 2 of the Cochrane Risk of Bias 2 tool23 (eAppendix 3 in Supplement 1).
Variables
Individual patient data were requested or AD were retrieved for patient and trial characteristics (eAppendix 4 in Supplement 1). Outcomes included OS (randomization until death), disease-free survival (DFS; from a landmark 6 months after randomization until recurrence or death), local recurrence-free survival (RFS; from a landmark 6 months after randomization until local recurrence), distant RFS (from a landmark 6 months after randomization until distant recurrence), toxicity, postoperative mortality or morbidity, microscopically tumor-free (R0) resection margin, pT category at resection, pathological complete response (pCR), and quality of life. Information on race and ethnicity was not available from IPD or AD.
Statistical Analysis
Network graphs were created with nodes representing interventions, edges representing treatment comparisons, and line thickness proportional to the number of trials comparing 2 treatments. The NMAs were conducted using the 2-stage approach.24,25 In the first stage, relative treatment effects were estimated from IPD, or AD if IPD were unavailable, for each study separately. For survival, the log–hazard ratio (HR) with SE was calculated per study applying a Cox proportional hazards regression model with the log-HR adjusted for age and sex. Prior death, recurrence, or failure to become disease free were regarded as events at the landmark used for DFS and local and distant RFS analyses. A logistic regression model was applied for estimating the log–odds ratio (OR) with SE of the binary outcomes for each study. Unadjusted ORs were estimated, because reported ORs of the studies not providing IPD were not adjusted. For the analyses of postoperative mortality, morbidity, and tumor stage, only patients who underwent surgery were included.
In the second stage, the estimated treatment effects were combined by applying a bayesian random-effects model using weakly informative half-normal priors for heterogeneity and a vague prior for treatment effects (eAppendix 5 in Supplement 1).26,27,28 Computations were done on the log scale, and results were transformed back for presenting pooled HRs and ORs with 95% credible intervals (CrIs). For OS and DFS, anticipated absolute effects were computed as absolute risk for an event occurring within 3 years using the estimated HRs.
For each comparison, consistency of the evidence was assessed by the node-splitting approach.29,30 The available evidence in the network was split at a node and the direct and indirect estimates of the treatment effect were assessed for agreement (eAppendix 5 in Supplement 1). Heterogeneity was measured as a τ value representing the SD of the underlying effects across studies. Treatment ranking was performed by calculating the surface under the cumulative ranking (SUCRA) curve from the posterior probability of being the most successful treatment.31 A SUCRA value of 1.00 indicates a treatment certain to be the best; a value of 0, certain to be the worst.31 Additionally, the median rank and 95% CrI of the posterior distribution for the rank were calculated.
Subgroup analyses for OS and DFS were conducted by using the bayesian NMA approach described earlier (eAppendix 6 in Supplement 1). Sensitivity analyses were conducted for all outcomes with respect to model assumptions and the choice of priors to investigate robustness of network results.32 All analyses were performed using R, version 4.4.0 (R Project for Statistical Computing)33 and JAGS, version 4.3.1 (SourceForce).34 For inconsistency tests, 2-sided P < .05 indicated statistical significance.
Results
The analyses included 2549 patients (mean [SD] age, 61.0 [9.4] years; 2206 (86.5%) male and 343 [13.5%] female). A total of 1255 patients (68.5% of those with information available) had an Eastern Cooperative Oncology Group performance status of 0, and 1314 (65.1% of those with information available) had AEG type I.
Study Selection and Study Details
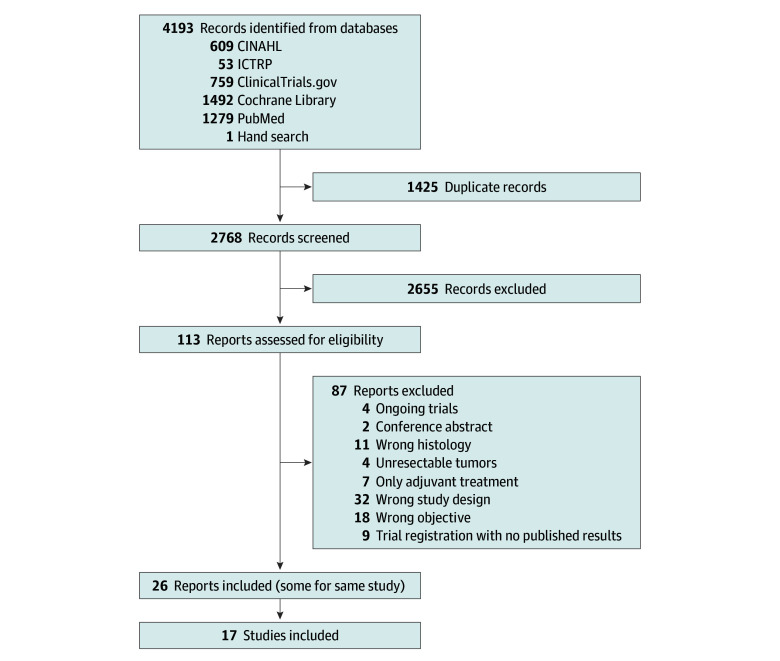
The literature search yielded 4193 records (Figure 1). After excluding duplicates, 2768 records were screened. Seventeen trials4,8,9,10,11,12,14,15,16,19,35,36,37,38,39,40,41 were included in the analyses (relevant excluded trials are listed in the eResults in Supplement 1). Eight trials4,8,10,16,35,36,37,40 included both squamous cell carcinoma and adenocarcinoma. From these, only participants with AEG were included. Five trials8,9,12,36,38 compared preoperative and/or perioperative chemotherapy plus surgery with surgery alone; 8 trials4,10,11,35,37,39,40,41 compared preoperative CRT plus surgery with surgery alone; and 4 trials14,15,16,19 compared preoperative and/or perioperative chemotherapy plus surgery with preoperative CRT plus surgery. In the trials with respective information available,4,8,9 87.9% to 91.8% of randomized patients completed all planned preoperative CRT or chemotherapy cycles, whereas only 38.5% completed all planned perioperative chemotherapy cycles. Individual patient data were available from 14 trials4,8,9,10,11,12,14,15,16,35,36,37,38,40 and unavailable from 3 trials,19,39,41 of which 2 trials19,41 had not yet reported OS. Main trial characteristics are displayed in Table 1. Network graphs are shown in eFigure 1 in Supplement 1.
Figure 1. PRISMA Flow Diagram.
CINAHL indicates Cumulative Index to Nursing and Allied Health Literature; ICTRP, International Clinical Trials Registry Platform.
Table 1. Characteristics of the 17 Included Trials.
| Trial | Recruitment period and location | Included histology | Treatment scheme per group (No. of patients with AEG per trial group included in meta-analysis) |
|---|---|---|---|
| IPD available | |||
| Ychou et al,12 2011 (ACCORD) | 1995-2003, France | Adenocarcinoma | Group A: cisplatin, 100 mg/m2, on day 1, fluorouracil, 800 mg/m2/d, on days 1-5, 2-3 preoperative and 3-4 postoperative cycles (n = 85); group B: surgery alone (n = 84) |
| Tepper et al,10 2008 (CALGB 9781) | 1997-2000, US | Adenocarcinoma and squamous cell carcinoma | Group A: preoperative simultaneous CRT, 50.4 Gy, cisplatin, 100 mg/m2, on days 1 and 29, fluorouracil, 1000 mg/m2, on days 1-4 and d 29-32 (n = 23); group B: surgery alone (n = 19) |
| Eyck et al,42021 (CROSS) | 2004-2008, the Netherlands | Adenocarcinoma and squamous cell carcinoma | Group A: preoperative simultaneous CRT, 41.4 Gy, and paclitaxel, 50 mg/m2, carboplatin (area under the curve, 2 mg/mL/min) (n = 134); group B: surgery alone (n = 141) |
| Schuhmacher et al,38 2010 (EORTC 40954) | 1999-2004, several European countries | Adenocarcinoma | Group A: cisplatin, 50 mg/m2, on days 1, 15, and 29, fluorouracil, 2000 mg/m2, on days 1, 8, 15, 22, and 36, 2 preoperative cycles (n = 37); group B: surgery alone (n = 39) |
| Mariette et al,37 2014 (FFCD 9901) | 2000-2009, France | Adenocarcinoma and squamous cell carcinoma | Group A: preoperative simultaneous CRT with 45 Gy, cisplatin, 75 mg/m2, on days 1 or 2 and 29 or 30, fluorouracil, 800 mg/m2, on days 1-4 and 29-32 (n = 30); group B: surgery alone (n = 27) |
| Cunningham et al,9 2006 (MAGIC) | 1994-2002, United Kingdom, the Netherlands, Germany, Singapore, New Zealand, and Brazil | Adenocarcinoma | Group A: epirubicin, 50 mg/m2, on day 1, cisplatin, 60 mg/m2, on day 1, fluorouracil, 200 mg/m2, on days 1 to 21, 3 preoperative and 3-4 postoperative cycles (n = 65); group B: surgery alone (n = 66) |
| von Döbeln et al,16 2019 (NeoRes) | 2006-2013, Sweden and Norway | Adenocarcinoma and squamous cell carcinoma | Group A: cisplatin, 100 mg/m2, day 1, fluorouracil, 750 mg/m2, days 1-5, 3 preoperative cycles (n = 66); group B: preoperative simultaneous CRT, 40 Gy, cisplatin, 100 mg/m2 on day 1, fluorouracil, 750 mg/m2, on days 1-5, 3 preoperative cycles (n = 65) |
| Allum et al,8 2009 (OE02) | 1992-1998, European countries | Adenocarcinoma and squamous cell carcinoma | Group A: cisplatin, 80 mg/m2, on day 1, fluorouracil, 1000 mg/m2, on days 1-4, 2 preoperative cycles (n = 265); group B: surgery alone (n = 268) |
| Stahl et al,15 2017 (POET) | 2000-2005, Germany | Adenocarcinoma | Group A: cisplatin, 50 mg/m2, biweekly, fluorouracil, 2000 mg/m2, weekly, 2.5 preoperative cycles (n = 59); group B: cisplatin, 50 mg/m2, biweekly, fluorouracil, 2000 mg/m2, weekly, 2 preoperative cycles followed by preoperative simultaneous CRT, 30 Gy, cisplatin, 50 mg/m2, on days 1-8, etoposide, 80 mg/m2, on days 3-5 (n = 60) |
| Kelsen et al,36 2007 (RTOG 8911) | 1990-1995, US and Canada | Adenocarcinoma and squamous cell carcinoma | Group A: cisplatin,100 mg/m2, on day 1, fluorouracil, 1000 mg/m2, on days 1-5, 2 preoperative cycles (n = 121); group B: surgery alone (n = 126) |
| Burmeister et al,14 2011 (TROG) | 2000-2006, Australia and New Zealand | Adenocarcinoma | Group A: cisplatin, 80 mg/m2, on day 1, fluorouracil, 1000 mg/m2, on days 1-4, 2 preoperative cycles (n = 38); group B: cisplatin, 80 mg/m2, on day 1, fluorouracil, 1000 mg/m2, on days 1-4, 2 preoperative cycles, simultaneous radiotherapy, 35 Gy, 15 fractions, with second cycle with fluorouracil reduced to 800 mg/m2 (n = 39) |
| Burmeister et al,35 2005 (TROG AGITG) | 2000-2006, Australia and New Zealand | Adenocarcinoma and squamous cell carcinoma | Group A: preoperative simultaneous CRT, 35 Gy, cisplatin, 80 mg/m2, on day 1, fluorouracil, 800 mg/m2, on days 1-4, 2 preoperative cycles (n = 80); group B: surgery alone (n = 78) |
| Urba et al,40 2001 | 1989-1994, US | Adenocarcinoma and squamous cell carcinoma | Group A: preoperative simultaneous CRT, 45 Gy, cisplatin, 20 mg/m2, on days 1-5 and 17-21, fluorouracil, 300 mg/m2, on days 1-21, vinblastine, 1 mg/m2, on days 1-4 and 17-21 (n = 37); group B: surgery alone (n = 39) |
| Walsh et al,11 1996 | 1990-1995, Ireland | Adenocarcinoma | Group A: preoperative simultaneous CRT, 40 Gy, cisplatin, 75 mg/m2, on days 7 and 49, fluorouracil, 15 mg/kg on days 1-5 and 42-47 (n = 58); group B: surgery alone (n = 55) |
| IPD unavailable | |||
| Tian et al,39 2021 | 2012-2016, China | Adenocarcinoma | Group A: preoperative simultaneous CRT, 45 Gy, capecitabine, 1000 mg/m2, twice daily on days 1-14, oxaliplatin, 130 mg/m2, on day 1, 2 preoperative cycles, 6 postoperative cycles (n = 76); group B: surgery alone (n = 73) |
| Leong et al,19 2017 | 2009-2014, Australia, New Zealand, and Belgium, Germany, Canada | Adenocarcinoma | Group A: epirubicin, 50 mg/m2, on day 1, cisplatin, 60 mg/m2, on day 1, fluorouracil, 200 mg/m2 for 21-d continuous infusion, 3 preoperative cycles, 3 postoperative cycles (n = 60); group B: epirubicin, 50 mg/m2, on day 1, cisplatin, 60 mg/m2, on day 1, fluorouracil, 200 mg/m2, for 21-d continuous infusion, 2 preoperative cycles, simultaneous CRT, 45 Gy, continuous fluorouracil, 200 mg/m2, on days 1-25, epirubicin, 50 mg/m2, on day 1, cisplatin, 60 mg/m2, on day 1, fluorouracil, 200 mg/m2, 21-d continuous infusion, 3 postoperative cycles (n = 60) |
| Zhao et al,41 2015 | 2012-2013, China | Adenocarcinoma | Group A: preoperative simultaneous CRT, 45 Gy, oxaliplatin, 130 mg/m2, on day 1, capecitabine, 2000 mg/m2, on days 1-14 (n = 36); group B: surgery alone (n = 40) |
Abbreviations: ACCORD, Actions Concertées dans les Cancers Colo-Rectaux et Digestifs; AEG, adenocarcinoma of the esophagus or esophageal junction; AGITG, Australasian Gastro-Intestinal Trials Group; CALGB, Cancer and Leukemia Group B; CROSS, Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study; CRT, chemoradiotherapy; EORTC, European Organisation for Research and Treatment of Cancer; FFCD, Federation Francophone de Cancerologie Digestive; IPD, individual patient data; MAGIC, Medical Research Council Adjuvant Gastric Infusional Chemotherapy; NeoRes, Neoadjuvant Chemotherapy Versus Radiochemotherapy for Cancer of the Esophagus or Cardia; POET, Preoperative Therapy in Esophagogastric Adenocarcinoma Trial; RTOG, Radiation Therapy Oncology Group; TROG, Trans Tasman Radiation Oncology Group.
Risk of Bias and Data Quality
Overall risk of bias was low in 12 trials4,8,9,10,11,12,15,19,36,37,38,39 and moderate in 5 trials14,16,35,40,41 (eFigures 2 and 3 in Supplement 1). Comparison-adjusted funnel plots for each outcome showed no small-study effect (eFigure 4 in Supplement 1). No implausible outliers were identified. Any differences between IPD and published results were small (eResults in Supplement 1).
Associations of Treatments With Outcomes
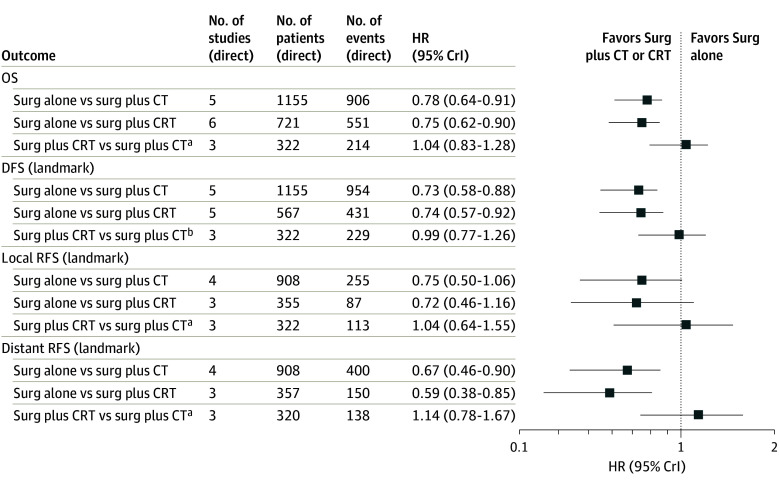
Associations of treatments with outcomes were first assessed by calculating network estimates of the age- and sex-adjusted HRs. A corresponding summary forest plot for the survival outcomes is shown in Figure 2. Overall survival results favored preoperative and/or perioperative chemotherapy plus surgery (HR, 0.78 [95% CrI, 0.64-0.91]; 3-year difference, 90 deaths per 1000 patients) and preoperative CRT plus surgery (HR, 0.75 [95% CrI, 0.62-0.90]; 3-year difference: 105 deaths per 1000 patients) over surgery alone. The 2-stage bayesian NMA estimated an HR of 1.04 (95% CrI, 0.83-1.28; 3-year difference, 15 deaths per 1000 patients) in favor of preoperative CRT plus surgery vs preoperative and/or perioperative chemotherapy plus surgery with low between-study heterogeneity (τ = 0.12). For DFS, results favored preoperative and/or perioperative chemotherapy plus surgery over surgery (HR, 0.73 [95% CrI, 0.58-0.88]) and preoperative CRT plus surgery over surgery (HR, 0.74 [95% CrI, 0.57-0.92]). The 2-stage bayesian network estimated an HR of 0.99 (95% CrI, 0.77-1.26) for preoperative and/or perioperative chemotherapy plus surgery vs preoperative CRT plus surgery with low heterogeneity (τ = 0.17). Both preoperative and/or perioperative chemotherapy plus surgery (HR, 0.67 [95% CrI, 0.46-0.90]) and preoperative CRT plus surgery (HR, 0.59 [95% CrI, 0.38-0.85]) were associated with longer distant RFS compared with surgery alone. For local RFS, 95% CrIs included 1.
Figure 2. Summary Forest Plot of Survival Outcomes.
The plot displays the network estimates of the age- and sex-adjusted hazard ratios (HRs) and the 95% credible interval (CrI) for each survival outcome. The numbers of studies, patients, and events are related to the direct comparison through the HR and 95% CrI, estimated from the network using direct and indirect evidence for each outcome. CRT indicates chemoradiotherapy; CT, chemotherapy; DFS, disease-free survival; OS, overall survival; RFS, recurrence-free survival; and surg, surgery.
aFavors surgery plus CRT.
bFavors surgery plus CT.
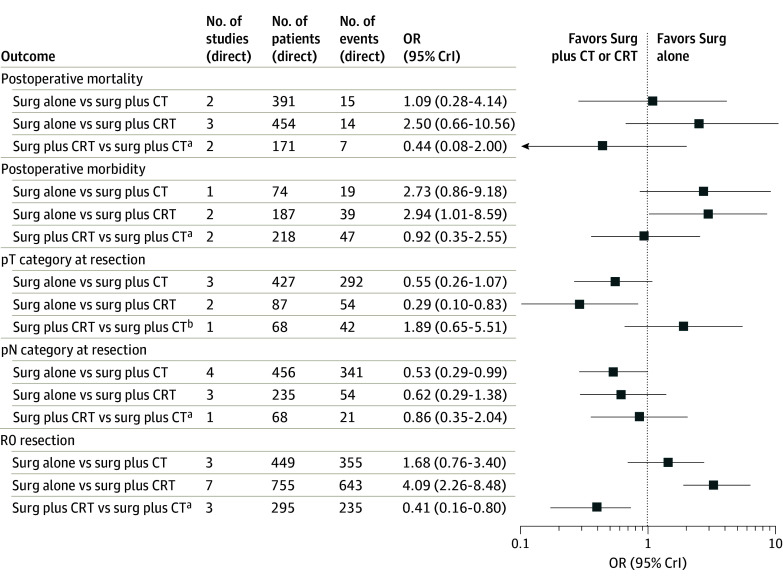
Results for the binary outcomes are shown in Figure 3. Postoperative mortality was not significantly more frequent after CRT plus surgery compared with surgery alone (OR, 2.50 [95% CrI, 0.66-10.56]) or after chemotherapy plus surgery compared with CRT plus surgery (OR, 0.44 [95% CrI, 0.08-2.00]). Postoperative morbidity was significantly higher after preoperative CRT compared with surgery alone (OR, 2.94 [95% CrI, 1.01-8.59]). The pT category was lower for patients after preoperative CRT plus surgery compared with surgery alone (OR, 0.29 [95% CrI, 0.10-0.83]), while the pN category was lower after preoperative chemotherapy plus surgery compared with surgery alone (OR, 0.53 [95% CrI, 0.29-0.99]). R0 resection was more frequent after preoperative CRT than surgery alone (OR, 4.09 [95% CrI, 2.26-8.48]) and less frequent after preoperative chemotherapy than preoperative CRT (OR, 0.41 [95% CrI, 0.16-0.80]).
Figure 3. Summary Forest Plot of Binary Outcomes.
The plot displays the network estimates of the odds ratios (ORs) and the 95% credible interval (CrI) for each binary outcome. The numbers of studies, patients, and events are related to the direct comparison through the OR and 95% CrI, estimated from the network using direct and indirect evidence for each outcome. CRT indicates chemoradiotherapy; CT, chemotherapy; and surg, surgery.
aFavors surgery plus CT.
bFavors surgery plus CRT.
Subsequently, a comparison of direct and indirect estimates was made. It showed no inconsistencies for any outcome and largely matched the network estimates (eFigure 5 in Supplement 1). The certainty of the evidence according to the Grading of Recommendations Assessment, Development and Evaluation system was high for OS, DFS, and RFS and moderate for morbidity and mortality.
Last, modalities were ranked by calculating SUCRA scores and median ranks (Table 2). For all survival outcomes, R0 resection, and pT category, preoperative CRT plus surgery had the highest and surgery alone had the lowest probability of being the best treatment. For pN category, preoperative and/or perioperative chemotherapy plus surgery had the highest and surgery alone had the lowest probability of being the best treatment. For postoperative morbidity and mortality, surgery alone had the highest and preoperative CRT plus surgery had the lowest probability of being the best treatment.
Table 2. Surface Under the Cumulative Ranking Scores for All Outcomes.
| Outcome | Score by treatment group (median rank [95% CrI])a | ||
|---|---|---|---|
| Surgery plus CRT | Surgery plus chemotherapy | Surgery alone | |
| OS | 0.82 (1 [1-2]) | 0.68 (2 [1-2]) | <0.01 (3 [3-3]) |
| DFS | 0.72 (2 [1-2]) | 0.78 (1 [1-2]) | <0.01 (3 [3-3]) |
| Local RFS | 0.75 (1 [1-3]) | 0.69 (2 [1-3]) | 0.06 (3 [2-3]) |
| Distant RFS | 0.88 (1 [1-2]) | 0.61 (2 [1-2]) | 0.01 (3 [3-3]) |
| Postoperative mortality | 0.10 (3 [1-3]) | 0.66 (2 [1-3]) | 0.74 (1 [1-3]) |
| Postoperative morbidity | 0.22 (3 [2-3]) | 0.31 (2 [1-3]) | 0.97 (1 [1-2]) |
| R0 resectability | 1.00 (1 [1-1]) | 0.47 (2 [2-3]) | 0.04 (3 [2-3]) |
| pT category on resection | 0.94 (1 [1-2]) | 0.54 (2 [1-3]) | 0.02 (3 [2-3]) |
| pN category on resection | 0.62 (2 [1-3]) | 0.82 (1 [1-2]) | 0.06 (3 [2-3]) |
Abbreviations: CrI, credible interval; CRT, chemoradiotherapy; DFS, disease-free survival; OS, overall survival; RFS, recurrence-free survival.
A value of 1.00 indicates a treatment certain to be the best; 0, certain to be the worst.
A single study assessed pCR in both treatment groups with only 5 events (1 of 36 after chemotherapy and 4 of 35 after CRT).36 As the MAGIC (Medical Research Council Adjuvant Gastric Infusional Chemotherapy) study9 and the study by Tian et al39 compared chemotherapy plus surgery with surgery, a relative treatment effect could not be calculated. Thirteen of 130 participants in these studies achieved pCR.
Toxicity and quality of life were assessed descriptively given that no meta-analysis was possible. Five studies4,10,12,37,41 reported toxicity results. In all these, toxicity was assessed in only 1 treatment arm, because the comparator was surgery alone. Individual study results are reported in the eTable in Supplement 1. A total of 227 of 402 participants (56.4%) experienced toxic effects of any grade. Quality of life was reported by 4 trials4,14,16,35 with IPD available only from one.4
Subgroup and Sensitivity Analyses
A summary forest plot with the network estimates of the subgroup analyses for OS is shown in eFigure 6 in Supplement 1. A forest plot with NMA results of the subgroup analyses for DFS is shown in eFigure 7 in Supplement 1. The estimates resemble those of the analyses of the overall study populations for most subgroups, with wider 95% CrIs due to fewer patients in the respective subgroups. Sensitivity analyses for all outcomes suggested robustness of the models with respect to the choice of the priors (eFigure 8 in Supplement 1).
Discussion
This IPD NMA compared preoperative CRT plus surgery, preoperative and/or perioperative chemotherapy plus surgery, and surgery alone in 2549 patients with AEG from 17 RCTs. Risk of bias was low in most studies and moderate in the remainder of the trials, with selective reporting being the most frequent reason for moderate risk. While treatment adherence was high for preoperative CRT and chemotherapy, it was considerably lower for postoperative chemotherapy.
The NMA shows that both preoperative CRT plus surgery and preoperative and/or perioperative chemotherapy plus surgery are associated with longer OS, DFS, and distant RFS compared with surgery alone. The association with OS was consistent throughout most subgroups. In some subgroups, results were inconclusive, probably due to lower statistical power. The comparisons between preoperative and/or perioperative chemotherapy plus surgery and preoperative CRT plus surgery showed no differences regarding survival. Following surgery after either modality, pT and pN categories were lower than following surgery alone, which reflects downstaging by preoperative treatments.42 R0 resection was more frequent after preoperative treatment than upfront surgery, but the difference was more pronounced after CRT than chemotherapy. Downstaging and R0 resection are associated with survival42,43; therefore, these findings constitute a relationship between the treatments and their survival outcome. Pathological complete response, which is another surrogate,44,45 was only assessed in 3 RCTs9,36,39 and could therefore not be validly analyzed. Both preoperative modalities appeared to have higher postoperative morbidity compared with surgery alone, although there was an association only for preoperative CRT. Postoperative mortality was higher after CRT than after chemotherapy or surgery alone, but results were not statistically significant. Overall, the risk of postoperative complications was slightly elevated after preoperative treatment and especially CRT. Possible mechanisms comprise immunosuppression and tissue vulnerability,46 and special attention is warranted to prevent, detect, and treat complications early in patients who underwent preoperative therapy.
A recently updated IPD NMA chose a different approach and included patients with esophageal carcinoma regardless of histology.47 The included trials overlapped with ours, and survival results were strikingly similar. Of note, histology was not identified as an effect modifier. Previous meta-analyses included both patients with AEG and esophageal squamous cell carcinoma,5,48,49 only 1 of the 2 modalities preoperative chemotherapy or CRT,50 or studies using chemotherapy as well as those using CRT without being able to compare the 2 modalities by integrating direct and indirect evidence in an NMA.7,13,51 In line with our results, they consistently showed the advantage of preoperative CRT plus surgery or preoperative and/or perioperative chemotherapy plus surgery over surgery alone with regard to survival and surrogate outcomes like downstaging and complete resection.5,7,13,47,48,50 Postoperative morbidity and mortality were also assessed, and no relevant differences between the modalities were found. One meta-analysis51 exclusively assessed safety and found no differences between preoperative therapy plus surgery and surgery alone but did not include data pertaining only to patients with AEG. These findings are different from ours, which showed safety concerns after preoperative CRT.
Our analyses could not demonstrate a clear survival advantage for 1 of the 2 multimodal approaches. After our search, results of the Neo-AEGIS trial, which compared preoperative CRT with perioperative chemotherapy, were published.17 It closed prematurely following futility analyses, and no survival or mortality differences were found, which is in line with our findings. Ongoing RCTs comparing the 2 approaches will further add to the evidence.18,19,20
The observation that preoperative treatment is completed by a much higher proportion of patients than the postoperative part of perioperative treatment is in agreement with previous evidence.6 It probably reflects both the fact that more patients are unable to initiate chemotherapy following extensive surgery and that toxicity is more often treatment limiting in the postoperative setting. It underscores the importance of administering a sufficient dose of preoperative chemotherapy. A possible incremental benefit of postoperative continuation of chemotherapy could not be assessed in our analyses.
The included RCTs were conducted over a wide time range and included various treatments, which makes it difficult to discern treatment effects of specific drug combinations. The duration of preoperative chemotherapy was heterogenous and might have modulated single-trial effects. The notion that longer chemotherapy is associated with longer survival was not supported by the OE05 trial (United Kingdom Medical Research Council esophageal cancer trial),52 which compared 2 and 4 cycles of preoperative chemotherapy with no survival difference, and by another RCT53 that showed no survival difference for CRT with or without induction chemotherapy. Although perioperative chemotherapy usually consists of longer and more dose-intense chemotherapy than CRT, this does not translate into longer survival.
Recently, checkpoint inhibitors have become standard of care in certain treatment lines for many solid tumors. Besides, ERBB2 (formerly HER2) blockade is established for overexpressing AEG in metastatic settings. These treatments were not in the scope of our analyses. Surrogate end point results from RCTs on preoperative checkpoint inhibition are promising.54,55,56 In 1 trial,56 however, this did not translate into longer event-free survival or OS, while other trials are still to report survival.54,55 Perioperative anti-ERBB2 treatment has shown benefits for pCR.57 Notwithstanding, in a phase 3 trial, the addition of ERBB2 blockade to preoperative CRT did not prolong survival, which questions pCR as a surrogate marker.58
Limitations
This meta-analysis has limitations. By combining data from trials with different inclusion and exclusion criteria and treatments, heterogeneity was inevitable. While internal validity was lower than in a single RCT, external validity was higher. In light of the sensitive search strategy in multiple databases and reference lists and the possible inclusion of non–English language publications, we are confident that no relevant trials were missed. An IPD NMA allowed for a valid comparison of all 3 modalities. This distinguishes our NMA from previous meta-analyses that had to rely on AD, could not perform specific analyses for AEG and other subgroups, and were thus prone to bias.5,49 No evidence of inconsistency of the NMA was found in a node-splitting model,30 in which each treatment comparison was split into direct and indirect evidence. The sensitivity analyses revealed robust results with regard to the priors for the bayesian NMA models. For few comparisons of binary end points, where the 95% CrI barely excluded or included 1, the choice of prior changed the significance of the pooled effect. Therefore, more studies would reduce the influence of the prior and enhance the certainty of the results. Some included trials consisted of patients with AEG and squamous cell carcinoma. Thanks to IPD or stratified AD, we included only patients with AEG, rendering results specific to that population. Inclusion criteria of single RCTs regarding tumor location, stage, and resectability were strict, thus minimizing the likelihood that patients who had gastric, metastatic, or irresectable tumors were included. In the single trials, different treatments were used. This made it difficult to apply the results to all existing preoperative regimens or to recommend a specific regimen. Although this IPD NMA includes all available evidence from RCTs published during the search period, the overall number of included trials and thus patients, specifically in some subgroups, is still limited, requiring more evidence for some comparisons to be able to draw definite conclusions.
Conclusions
Findings of this IPD NMA suggest that both preoperative CRT plus surgery and preoperative and/or perioperative chemotherapy plus surgery are associated with longer survival of patients with AEG compared with surgery alone. No differences between the effect of the 2 modalities could be found. The association might be mediated through tumor downstaging and a higher probability of complete resection. Future research should focus on identifying specific groups of patients in whom 1 of the 2 modalities could be more effective, and on the integration of checkpoint inhibitors and targeted therapies into preoperative treatment schemes.
eAppendix 1. Predefined Search Strategy
eAppendix 2. Data Quality Checks
eAppendix 3. Assessment of Risk of Bias in Included Studies
eAppendix 4. Variables, Eligibility, and Missing Data
eAppendix 5. Statistical Methods
eAppendix 6. Subgroup Analyses and Certainty Assessment
eResults. Excluded Studies, Differences Between IPD Datasets and Published Results, and Sensitivity Analyses
eFigure 1. Network Graphs for Survival Outcomes and Binary Outcomes
eFigure 2. Risk of Bias per Domain and Trials
eFigure 3. Risk of Bias Summary Across Trials
eFigure 4. Comparison-Adjusted Funnel Plots of Each Outcome
eTable. Frequency of Toxicity Events (Any Grade) in the Single Trials Reporting This Outcome
eFigure 5. Assessment of Inconsistency by the Node-Splitting Approach
eFigure 6. NMA Results of the Subgroup Analyses for OS
eFigure 7. NMA Results of the Subgroup Analyses for DFS
eFigure 8. Results of the Sensitivity Analyses
eReferences.
Data Sharing Statement
References
- 1.Morgan E, Soerjomataram I, Rumgay H, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163(3):649-658.e2. doi: 10.1053/j.gastro.2022.05.054 [DOI] [PubMed] [Google Scholar]
- 2.Gavin AT, Francisci S, Foschi R, et al. ; EUROCARE-4 Working Group . Oesophageal cancer survival in Europe: a EUROCARE-4 study. Cancer Epidemiol. 2012;36(6):505-512. doi: 10.1016/j.canep.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 3.Ronellenfitsch U, Schwarzbach M, Hofheinz R, et al. Preoperative chemo(radio)therapy versus primary surgery for gastroesophageal adenocarcinoma: systematic review with meta-analysis combining individual patient and aggregate data. Eur J Cancer. 2013;49(15):3149-3158. doi: 10.1016/j.ejca.2013.05.029 [DOI] [PubMed] [Google Scholar]
- 4.Eyck BM, van Lanschot JJB, Hulshof MCCM, et al. ; CROSS Study Group . Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. 2021;39(18):1995-2004. doi: 10.1200/JCO.20.03614 [DOI] [PubMed] [Google Scholar]
- 5.Sjoquist KM, Burmeister BH, Smithers BM, et al. ; Australasian Gastro-Intestinal Trials Group . Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681-692. doi: 10.1016/S1470-2045(11)70142-5 [DOI] [PubMed] [Google Scholar]
- 6.Fazio N, Biffi R, Maibach R, et al. ; Swiss Group for Clinical Cancer Research SAKK; European Institute of Oncology, Milan, Italy . Preoperative versus postoperative docetaxel-cisplatin-fluorouracil (TCF) chemotherapy in locally advanced resectable gastric carcinoma: 10-year follow-up of the SAKK 43/99 phase III trial. Ann Oncol. 2016;27(4):668-673. doi: 10.1093/annonc/mdv620 [DOI] [PubMed] [Google Scholar]
- 7.Ronellenfitsch U, Schwarzbach M, Hofheinz R, et al. ; GE Adenocarcinoma Meta-analysis Group . Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst Rev. 2013;(5):CD008107. doi: 10.1002/14651858.CD008107.pub2 [DOI] [PubMed] [Google Scholar]
- 8.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27(30):5062-5067. doi: 10.1200/JCO.2009.22.2083 [DOI] [PubMed] [Google Scholar]
- 9.Cunningham D, Allum WH, Stenning SP, et al. ; MAGIC Trial Participants . Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11-20. doi: 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 10.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086-1092. doi: 10.1200/JCO.2007.12.9593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335(7):462-467. doi: 10.1056/NEJM199608153350702 [DOI] [PubMed] [Google Scholar]
- 12.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715-1721. doi: 10.1200/JCO.2010.33.0597 [DOI] [PubMed] [Google Scholar]
- 13.Fu T, Bu ZD, Li ZY, et al. Neoadjuvant chemoradiation therapy for resectable esophago-gastric adenocarcinoma: a meta-analysis of randomized clinical trials. BMC Cancer. 2015;15:322. doi: 10.1186/s12885-015-1341-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burmeister BH, Thomas JM, Burmeister EA, et al. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? a randomised phase II trial. Eur J Cancer. 2011;47(3):354-360. doi: 10.1016/j.ejca.2010.09.009 [DOI] [PubMed] [Google Scholar]
- 15.Stahl M, Walz MK, Riera-Knorrenschild J, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): long-term results of a controlled randomised trial. Eur J Cancer. 2017;81:183-190. doi: 10.1016/j.ejca.2017.04.027 [DOI] [PubMed] [Google Scholar]
- 16.von Döbeln GA, Klevebro F, Jacobsen AB, et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: long-term results of a randomized clinical trial. Dis Esophagus. 2019;32(2). doi: 10.1093/dote/doy078 [DOI] [PubMed] [Google Scholar]
- 17.Reynolds JV, Preston SR, O’Neill B, et al. ; Neo-AEGIS Investigators and Trial Group . Trimodality therapy versus perioperative chemotherapy in the management of locally advanced adenocarcinoma of the oesophagus and oesophagogastric junction (Neo-AEGIS): an open-label, randomised, phase 3 trial. Lancet Gastroenterol Hepatol. 2023;8(11):1015-1027. doi: 10.1016/S2468-1253(23)00243-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoeppner J, Lordick F, Brunner T, et al. ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer. 2016;16(1):503. doi: 10.1186/s12885-016-2564-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leong T, Smithers BM, Haustermans K, et al. TOPGEAR: a randomized, phase III trial of perioperative ECF chemotherapy with or without preoperative chemoradiation for resectable gastric cancer: interim results from an international, intergroup trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol. 2017;24(8):2252-2258. doi: 10.1245/s10434-017-5830-6 [DOI] [PubMed] [Google Scholar]
- 20.Lorenzen S, Biederstädt A, Ronellenfitsch U, et al. RACE-trial: neoadjuvant radiochemotherapy versus chemotherapy for patients with locally advanced, potentially resectable adenocarcinoma of the gastroesophageal junction—a randomized phase III joint study of the AIO, ARO and DGAV. BMC Cancer. 2020;20(1):886. doi: 10.1186/s12885-020-07388-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronellenfitsch U, Friedrichs J, Grilli M, et al. Preoperative chemoradiotherapy versus chemotherapy for adenocarcinoma of the esophagus and esophagogastric junction (AEG): systematic review with individual participant data (IPD) network meta-analysis (NMA). Cochrane Database Syst Rev. 2021;(5):CD014748. doi: 10.1002/14651858.CD014748 [DOI] [Google Scholar]
- 22.Higgins H, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions, version 6.4. Updated August 2023. Cochrane; Accessed July 15, 2024. https://www.training.cochrane.org/handbook
- 23.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 24.Riley RD, Simmonds MC, Look MP. Evidence synthesis combining individual patient data and aggregate data: a systematic review identified current practice and possible methods. J Clin Epidemiol. 2007;60(5):431-439. doi: 10.1016/j.jclinepi.2006.09.009 [DOI] [PubMed] [Google Scholar]
- 25.Simmonds MC, Higgins JP, Stewart LA, Tierney JF, Clarke MJ, Thompson SG. Meta-analysis of individual patient data from randomized trials: a review of methods used in practice. Clin Trials. 2005;2(3):209-217. doi: 10.1191/1740774505cn087oa [DOI] [PubMed] [Google Scholar]
- 26.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607-617. doi: 10.1177/0272989X12458724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: inconsistency in networks of evidence based on randomised controlled trials. NICE Decision Support Unit Technical Support Documents. National Institute for Health and Care Excellence. May 2011. Updated April 2014. Accessed May 17, 2024. https://www.ncbi.nlm.nih.gov/books/NBK310372/pdf/Bookshelf_NBK310372.pdf [PubMed]
- 28.van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012;3(4):285-299. doi: 10.1002/jrsm.1054 [DOI] [PubMed] [Google Scholar]
- 29.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7-8):932-944. doi: 10.1002/sim.3767 [DOI] [PubMed] [Google Scholar]
- 30.van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. 2016;7(1):80-93. doi: 10.1002/jrsm.1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163-171. doi: 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 32.Bafeta A, Trinquart L, Seror R, Ravaud P. Reporting of results from network meta-analyses: methodological systematic review. BMJ. 2014;348:g1741. doi: 10.1136/bmj.g1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R: A Language and Environment for Statistical Computing. Version 4.4.0. R Foundation for Statistical Computing. 2024. Accessed April 4, 2024. https://cran.r-project.org/src/base/R-4/
- 34.SourceForce . JAGS: just another Gibbs sampler. September 8, 2006. Accessed December 5, 2023. https://sourceforge.net/projects/mcmc-jags/
- 35.Burmeister BH, Smithers BM, Gebski V, et al. ; Trans-Tasman Radiation Oncology Group; Australasian Gastro-Intestinal Trials Group . Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6(9):659-668. doi: 10.1016/S1470-2045(05)70288-6 [DOI] [PubMed] [Google Scholar]
- 36.Kelsen DP, Winter KA, Gunderson LL, et al. ; Radiation Therapy Oncology Group; USA Intergroup . Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25(24):3719-3725. doi: 10.1200/JCO.2006.10.4760 [DOI] [PubMed] [Google Scholar]
- 37.Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32(23):2416-2422. doi: 10.1200/JCO.2013.53.6532 [DOI] [PubMed] [Google Scholar]
- 38.Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28(35):5210-5218. doi: 10.1200/JCO.2009.26.6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian Y, Wang J, Qiao X, et al. Long-term efficacy of neoadjuvant concurrent chemoradiotherapy for potentially resectable advanced Siewert type II and III adenocarcinomas of the esophagogastric junction. Front Oncol. 2021;11:756440. doi: 10.3389/fonc.2021.756440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19(2):305-313. doi: 10.1200/JCO.2001.19.2.305 [DOI] [PubMed] [Google Scholar]
- 41.Zhao Q, Li Y, Wang J, et al. Concurrent neoadjuvant chemoradiotherapy for Siewert II and III adenocarcinoma at gastroesophageal junction. Am J Med Sci. 2015;349(6):472-476. doi: 10.1097/MAJ.0000000000000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilke TJ, Bhirud AR, Lin C. A review of the impact of preoperative chemoradiotherapy on outcome and postoperative complications in esophageal cancer patients. Am J Clin Oncol. 2015;38(4):415-421. doi: 10.1097/COC.0000000000000021 [DOI] [PubMed] [Google Scholar]
- 43.Ronellenfitsch U, Schwarzbach M, Hofheinz R, et al. Predictors of overall and recurrence-free survival after neoadjuvant chemotherapy for gastroesophageal adenocarcinoma: pooled analysis of individual patient data (IPD) from randomized controlled trials (RCTs). Eur J Surg Oncol. 2017;43(8):1550-1558. doi: 10.1016/j.ejso.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 44.Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23(19):4330-4337. doi: 10.1200/JCO.2005.05.017 [DOI] [PubMed] [Google Scholar]
- 45.Hipp J, Kuvendjiska J, Hillebrecht HC, et al. Pathological complete response in multimodal treatment of esophageal cancer: a retrospective cohort study. Dis Esophagus. 2023;36(7):doac095. doi: 10.1093/dote/doac095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang W, Feng M, Zheng J, et al. Association of the collagen score with anastomotic leakage in rectal cancer patients after neoadjuvant chemoradiotherapy. Surgery. 2021;170(5):1331-1341. doi: 10.1016/j.surg.2021.05.023 [DOI] [PubMed] [Google Scholar]
- 47.Faron M, Cheugoua-Zanetsie M, Tierney J, et al. ; MANATEC-02 Collaborative Group . Individual participant data network meta-analysis of neoadjuvant chemotherapy or chemoradiotherapy in esophageal or gastroesophageal junction carcinoma. J Clin Oncol. 2023;41(28):4535-4547. doi: 10.1200/JCO.22.02279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kidane B, Coughlin S, Vogt K, Malthaner R. Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database Syst Rev. 2015;2015(5):CD001556. doi: 10.1002/14651858.CD001556.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao X, Ren Y, Hu Y, Cui N, Wang X, Cui Y. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or the gastroesophageal junction: a meta-analysis based on clinical trials. PLoS One. 2018;13(8):e0202185. doi: 10.1371/journal.pone.0202185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faron M, Cheugoua-Zanetsie AM, Thirion P, et al. ; MANATEC-02 collaborative group . Individual patient data meta-analysis of neoadjuvant chemotherapy followed by surgery versus upfront surgery for carcinoma of the oesophagus or the gastro-oesophageal junction. Eur J Cancer. 2021;157:278-290. doi: 10.1016/j.ejca.2021.08.014 [DOI] [PubMed] [Google Scholar]
- 51.Kumagai K, Rouvelas I, Tsai JA, et al. Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. Br J Surg. 2014;101(4):321-338. doi: 10.1002/bjs.9418 [DOI] [PubMed] [Google Scholar]
- 52.Alderson D, Cunningham D, Nankivell M, et al. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): an open-label, randomised phase 3 trial. Lancet Oncol. 2017;18(9):1249-1260. doi: 10.1016/S1470-2045(17)30447-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ajani JA, Xiao L, Roth JA, et al. A phase II randomized trial of induction chemotherapy versus no induction chemotherapy followed by preoperative chemoradiation in patients with esophageal cancer. Ann Oncol. 2013;24(11):2844-2849. doi: 10.1093/annonc/mdt339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janjigian YY, Al-Batran SE, Wainberg ZA, et al. LBA73 Pathological complete response (pCR) to durvalumab plus 5-fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) in resectable gastric and gastroesophageal junction cancer (GC/GEJC): interim results of the global, phase III MATTERHORN study. Ann Oncol. 2023;34:S1315-S1316. doi: 10.1016/j.annonc.2023.10.074 [DOI] [Google Scholar]
- 55.Lorenzen S, Götze TO, Thuss-Patience P, et al. ; AIO and SAKK Study Working Groups . Perioperative atezolizumab plus fluorouracil, leucovorin, oxaliplatin, and docetaxel for resectable esophagogastric cancer: interim results from the randomized, multicenter, phase II/III DANTE/IKF-s633 trial. J Clin Oncol. 2024;42(4):410-420. doi: 10.1200/JCO.23.00975 [DOI] [PubMed] [Google Scholar]
- 56.Shitara K, Rha SY, Wyrwicz LS, et al. ; KEYNOTE-585 investigators . Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. 2024;25(2):212-224. doi: 10.1016/S1470-2045(23)00541-7 [DOI] [PubMed] [Google Scholar]
- 57.Hofheinz RD, Merx K, Haag GM, et al. FLOT Versus FLOT/trastuzumab/pertuzumab perioperative therapy of human epidermal growth factor receptor 2–positive resectable esophagogastric adenocarcinoma: a randomized phase II trial of the AIO EGA Study Group. J Clin Oncol. 2022;40(32):3750-3761. doi: 10.1200/JCO.22.00380 [DOI] [PubMed] [Google Scholar]
- 58.Safran HP, Winter K, Ilson DH, et al. Trastuzumab with trimodality treatment for oesophageal adenocarcinoma with HER2 overexpression (NRG Oncology/RTOG 1010): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2022;23(2):259-269. doi: 10.1016/S1470-2045(21)00718-X [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Predefined Search Strategy
eAppendix 2. Data Quality Checks
eAppendix 3. Assessment of Risk of Bias in Included Studies
eAppendix 4. Variables, Eligibility, and Missing Data
eAppendix 5. Statistical Methods
eAppendix 6. Subgroup Analyses and Certainty Assessment
eResults. Excluded Studies, Differences Between IPD Datasets and Published Results, and Sensitivity Analyses
eFigure 1. Network Graphs for Survival Outcomes and Binary Outcomes
eFigure 2. Risk of Bias per Domain and Trials
eFigure 3. Risk of Bias Summary Across Trials
eFigure 4. Comparison-Adjusted Funnel Plots of Each Outcome
eTable. Frequency of Toxicity Events (Any Grade) in the Single Trials Reporting This Outcome
eFigure 5. Assessment of Inconsistency by the Node-Splitting Approach
eFigure 6. NMA Results of the Subgroup Analyses for OS
eFigure 7. NMA Results of the Subgroup Analyses for DFS
eFigure 8. Results of the Sensitivity Analyses
eReferences.
Data Sharing Statement