Abstract
Background
Mastitis can be caused by ineffective positioning of the baby at the breast or restricted feeding. Infective mastitis is commonly caused by Staphylococcus aureus. The prevalence of mastitis in breastfeeding women may reach 33%. Effective milk removal, pain medication and antibiotic therapy have been the mainstays of treatment.
Objectives
This review aims to examine the effectiveness of antibiotic therapies in relieving symptoms for breastfeeding women with mastitis with or without laboratory investigation.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 September 2012), contacted investigators and other content experts known to us for unpublished trials and scanned the reference lists of retrieved articles.
Selection criteria
We selected randomised controlled trials (RCTs) and quasi‐RCTs comparing the effectiveness of various types of antibiotic therapies or antibiotic therapy versus alternative therapies for the treatment of mastitis.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. When in dispute, we consulted a third author.
Main results
Two trials met the inclusion criteria. One small trial (n = 25) compared amoxicillin with cephradine and found no significant difference between the two antibiotics in terms of symptom relief and abscess formation. Another, older study compared breast emptying alone as 'supportive therapy' versus antibiotic therapy plus supportive therapy, and no therapy. The findings of the latter study suggested faster clearance of symptoms for women using antibiotics, although the study design was problematic.
Authors' conclusions
There is insufficient evidence to confirm or refute the effectiveness of antibiotic therapy for the treatment of lactational mastitis. There is an urgent need to conduct high‐quality, double‐blinded RCTs to determine whether antibiotics should be used in this common postpartum condition.
Keywords: Female, Humans, Breast Milk Expression, Amoxicillin, Amoxicillin/therapeutic use, Anti‐Bacterial Agents, Anti‐Bacterial Agents/therapeutic use, Breast Feeding, Breast Feeding/adverse effects, Cephradine, Cephradine/therapeutic use, Mastitis, Mastitis/etiology, Mastitis/therapy, Randomized Controlled Trials as Topic
Plain language summary
Antibiotics for mastitis in breastfeeding women
Inflammation of the breast, or mastitis, can be infective or non‐infective. Infective mastitis is one of the most common infections experienced by breastfeeding women. The condition (infective or not) varies in severity, ranging from mild symptoms with some local inflammation, redness, warmth and tenderness in the affected breast through to more serious symptoms including fever, abscess and septicaemia, which may require hospitalisation. Recovery can take time, and there may be substantial discomfort for the affected mother and her baby. Mastitis usually occurs during the first three months after birth and results in the mother being confined to bed for one day, followed by restricted activity. The condition is associated with decreased milk secretion, decreased productivity, and in difficulties caring for the baby. This burden to mothers, along with the cost of care, the potential negative impact on continuation of breastfeeding, and the danger of serious complications such as septicaemia, makes mastitis a serious condition which warrants early diagnosis and effective therapy. The review included two studies and approximately 125 women. One study compared two different antibiotics, and there were no differences between the two antibiotics for symptom relief. A second study comparing no treatment, breast emptying, and antibiotic therapy, with breast emptying suggested more rapid symptom relief with antibiotics. There is very little evidence on the effectiveness of antibiotic therapy, and more research is needed.
Background
Description of the condition
Mastitis is an inflammatory condition of the breast, usually associated with lactation (WHO 2000). Hence, it is also known as lactational mastitis (Hughes 1989) or puerperal mastitis (Editorial 1976). An estimated 2% to 33% of breastfeeding women develop lactational mastitis (Buescher 2001; Fetherston 1998; Foxman 2002; Jonsson 1994; Kaufmann 1991; Kinlay 1998; Marshall 1975; Riordan 1990; Vogel 1999). Population‐based studies in Australia reported that 15% to 20% of women during the first six months after delivery were affected, while a cohort study of American women reported that 10% of women experienced mastitis during the three months following the birth. Mastitis may recur: a New Zealand study of 350 lactating mothers showed a 8.5% recurrence rate.
The primary cause of mastitis is milk stasis (Hughes 1989), which may or may not be associated with infection. Mastitis can be caused by ineffective positioning of the baby at the breast, limited feeding (in cases where the mother is introducing formula feeding), or restricted feeding, all of which may result in milk stasis and mastitis. In infective mastitis, Staphylococcus aureus and Staphylococcus albus are the commonest organisms found on laboratory investigation (Novy 1984; Riordan 1990). Escherichia coli (Lawrence 1999) and streptococci are found less frequently (Novy 1984). Delayed, inappropriate or inadequate treatment may result in unnecessary discontinuation of breastfeeding, breast tissue damage, recurrence, and substantial cost (Evans 1995).
Clinical symptoms of mastitis include unilateral breast pain, redness (erythema) and swelling, and may be associated with flu‐like symptoms (fever, chills and aches). Unilateral erythema, oedema and tenderness of the affected breast are usually present on examination. In contrast, engorgement of the breast is normally bilateral and uncomfortable rather than acutely painful; and in cases of breast abscess, a fluctuating, tender and hard breast mass is found with overlying erythema (Bedinghaus 1997; Hager 1992; Ogle 1988). Milk leucocyte count, bacteria colony count and culture may be useful investigations to differentiate infective from non‐infective mastitis (Thomsen 1984).
Description of the intervention
The principles of treating mastitis include supportive counselling and supportive therapy (bed rest, increased fluids), effective milk removal (by encouraging the continuation of breastfeeding and assessing how the baby is feeding; helping the mother to adjust positioning and attachment if necessary; and milk expression), symptomatic treatment (pain medication, use of anti‐inflammatory agents), antibiotic therapy (Walker 1999), probiotic therapy (Jimenez 2008) and other agents such as nisin (Fernandez 2008). Although efficient milk removal is the mainstay of treatment, antibiotics are usually prescribed to cover possible bacterial infections. These include penicillin, dicloxacillin and cephalosporins (Hager 1992; Marchant 2002) for staphylococcal and streptococcal infections; for gram‐negative organisms, cephalexin or amoxicillin may be appropriate (Olsen 1990).
The use of antibiotics in the treatment of mastitis varies worldwide, and researchers have been unable to reach a consensus on whether to prescribe antibiotics for women with lactational mastitis. Osterman 2000 underscored the benefit of prescribing both antibiotic therapy and supportive treatment in the presence of infective agents. They prescribed antibiotics after bacterial cultivation for 61% of participants in a cohort of lactational women with mastitis. The authors argued that since breast milk potentially contained pathogenic bacteria, the majority of the mothers should be treated with antibiotics. However, Kvist 2004 only treated 9% of breast inflammation with antibiotics. A study in Western Australia treated 85% of participants with inflammatory breast symptoms with antibiotics (Kvist 2005).
Matheson 1988 concluded that phenoxymethylpenicillin failed to stop abscess formation in 20% of mothers suffering from mastitis and that the majority of them recovered without antibiotics. Amir 2004a stated that low incidence of abscess formation (0.1% in their study) raised the question as to whether antibiotic therapy is appropriate for all mothers with symptoms of breast inflammation. They reported that only 2.9% (95% confidence interval 1.0 to 6.7) of women who took antibiotics for mastitis developed abscesses.
There has also been argument about the type of antibiotic chosen for breastfeeding women suffering from mastitis. Practice relating to the choice of antibiotic therapy has varied widely. An audit of the management of mastitis in the emergency department of Melbourne Hospital, Australia, showed that the majority of women with mastitis received flucloxacillin (91 women out of 111), a beta‐lactamase stable penicillin closely related to cloxacillin, as recommended by Australian Antibiotic Guideline (1996) (Amir 2004b). Amir reported that, due to adverse hepatic events, dicloxacillin should replace flucloxacillin (Amir 2004b). A prospective study (n = 840) conducted in the US between 1994 and1998 reported that 86% of women with mastitis received antibiotics, most of whom were on cephalexin (46%). The rest received amoxicillin (7%), ampicillin (7%) and amdinocillin clavulanate (7%). No cultures were performed because of cost restrictions (Foxman 2002). Another recent publication (Eglash 2006) reported a chart review of 64 women with lactational mastitis presenting to a lactation specialist between 1997 and 2002; these women received routine antibiotic therapy at the time inclusive of cephalexin, dicloxacillin, erythromycin, amoxicillin and clindamycin. The choice of antibiotics was based on the mother's and her baby's records of allergies and intolerances; mother's preference regarding the frequency of antibiotic administration; bacterial culture and sensitivities, and medication cost.
Why it is important to do this review
There is little consensus on who should be prescribed antibiotics, the most appropriate antibiotic to use, the best time to begin treatment and how long the treatment should continue. Most studies have focused on the effectiveness of emptying the breast and the timing of treatment, rather than on the type of antibiotics used (Crepinsek 2010; Devereux 1970; Kinlay 1998; Thomsen 1984). Use of laboratory investigation before antibiotic therapy is not consistent, and type of antibiotic chosen depends on physician choice rather than scientific proof. There is also little information on the cost‐effectiveness of different therapies.
Objectives
The objective of this review is to examine the effectiveness of antibiotic therapies in relieving symptoms for breastfeeding women who have mastitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐RCTs.
Types of participants
Lactating women who were diagnosed with mastitis, with or without laboratory investigations. We have excluded breast engorgement and breast abscess from this review.
Types of interventions
Intervention
Antibiotic therapy (various routes of administration, dosages, durations or timing of administration).
Control
Placebo, no treatment, other supportive treatments such as breast emptying or another antibiotic of a different class.
Types of outcome measures
Primary outcomes
Symptom improvement reported by women
Symptom improvement by clinical assessment
Continued breastfeeding
Resolution of infection as confirmed by laboratory test
Secondary outcomes
Adverse drug reactions following antibiotic therapy
Neonatal complications (e.g. neonatal colitis)
Hospitalisation
Costs
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 September 2012).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
For details of additional searching carried out in the initial version of the review, see:Appendix 1.
Searching other resources
We contacted investigators (named in the retrieved articles) and other content experts known to us for unpublished trials. In addition, we looked for relevant trials in the references of the retrieved articles.
We did not apply any language restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Appendix 3.
For this update, two review authors independently assessed for inclusion the two reports that were identified as a result of the updated search. We did not include either. If we identify new trials for inclusion in future updates of this review, we will use the methods described in Appendix 4.
Results
Description of studies
Two studies met the pre‐stated inclusion criteria in this review. One study had two treatment arms (amoxicillin versus cephradine) for the treatment of mastitis (Hager 1996). Another study compared antibiotic therapy versus no therapy and/or non‐pharmacological therapy (breast emptying) and the unit of analysis was the breast rather than the woman (Thomsen 1984).
Results of the search
In the initial version of the review, we identified 11 references (10 through the database search, and one through handsearching) (Amir 2004b). We reviewed titles and inspected abstracts. We excluded seven studies at initial screening for one or more of the following reasons: not related to mastitis, not a RCT, intervention of interest was not used or no relevant outcome was reported. We considered four studies potentially eligible for inclusion, but, after inspection of the full paper, excluded two of them. We have provided the reasons for exclusion in the Characteristics of excluded studies table.
Following an updated search in March 2010, we excluded a further two reports (Fernandez 2008; Jimenez 2008).
Following an updated search in September 2012, we excluded one report (Arroyo 2010) and one is awaiting translation from Chinese (Zhou 2009), see Characteristics of studies awaiting classification.
Included studies
Participants
Hager 1996 included 13 participants in the amoxicillin group and 12 in the cephradine group. Thomsen 1984 included 55 'cases' (individual breasts) in each of three arms (antibiotic plus breast emptying, breast emptying alone and no treatment). We have assumed that the number of women included in the analysis was approximately 100, although the actual number of women suffering from infective mastitis is not provided in the paper. (The paper described findings for women with both infective and non‐infective mastitis (n = 213). The total number of affected breasts was 339, so overall, approximately 60% of the women had both breasts affected. The number of infected breasts was 165 (55 in each of three treatment groups); assuming that the same proportions of women with infective versus non‐infective mastitis had both breasts affected, this would mean that approximately 100 women had infective mastitis and are included in the analysis in this review.)
Participants included in these trials were lactating mothers with symptoms of mastitis such as persistent tenderness of breast, swelling, redness, decreased milk secretion, fever or breast discomfort. Leucocyte count was also used in Thomsen 1984 as an inclusion criterion for treatment.
Intervention
Hager 1996 did not include any placebo or non‐treatment control group. The treatment regimens compared in this trial were oral amoxicillin, 500 mg every eight hours for seven days versus oral cephradine, 500 mg every eight hours for seven days. Participants in both groups were advised to continue breastfeeding and to apply warm, moist compresses every four to six hours (Hager 1996). Thomsen 1984 included two control groups, one where women received no treatment and a second where there was breast emptying. The treatment group in this study received the following antibiotics: penicillin 500,000 IU three per day for six days, oral ampicillin 500 mg, four per day for six days and erythromycin 500 mg twice per day for six days (Thomsen 1984).
Outcomes
In both included studies, resolution of symptoms (fever, erythema and tenderness) was the main outcome measure. Thomsen 1984 reported on continuation of normal lactation in a follow‐up visit two weeks after treatment. Both studies measured several negative outcomes, including persistence of symptoms, impaired milk secretion and recurrence of infection. Duration of follow‐up was 30 days for Hager 1996 and 14 days for Thomsen 1984.
Excluded studies
We excluded seven studies at initial screening for one or more of the following reasons: not related to mastitis, not a RCT, intervention of interest was not used or no relevant outcome was reported. We considered four studies potentially eligible for inclusion, but, after inspection of the full paper, excluded two of them.
Following updated searches in March 2010, and September 2012, we excluded a further three reports (Arroyo 2010; Fernandez 2008; Jimenez 2008). One study report is awaiting classification (Zhou 2009) as it needs to be translated.
We have provided the reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
SeeCharacteristics of included studies table.
Allocation
Both included studies were RCTs. However, only one of the studies adequately described the method of allocation concealment. Generation of randomisation sequence was not reported in either study, but one study reported concealment of allocation using pre‐sealed opaque envelopes (Hager 1996).
Blinding
Investigators were blinded in Hager 1996 and the method of blinding was considered adequate The other study did not describe the method of blinding (Thomsen 1984).
Incomplete outcome data
The assessment of pre‐determined variables in each study was based primarily on follow‐up. There was no reported loss to follow‐up in either of these studies.
Selective reporting
All expected outcomes were reported in one study (Hager 1996). In the other study, it was unclear whether or not outcomes had been selectively reported (Thomsen 1984).
Other potential sources of bias
Baseline characteristics were balanced in one study (Hager 1996), and in the other study it was not possible to tell whether other sources of bias were present (Thomsen 1984).
Effects of interventions
As the studies identified were not sufficiently similar and not of sufficient quality, we did not do a meta‐analysis and have therefore presented data, and discussed results, separately for the two included studies. In future updates of the review, as new studies emerge, it may be possible to add further comparisons and, where appropriate, combine findings in a meta‐analysis.
Primary outcomes
Proportion of participants with resolution of symptoms or improvement
Symptom improvement reported by women (outcome number 1) was not measured in either of the included studies. Both studies included findings relating to the second primary outcome (symptom improvement assessed by clinicians). However, comparison between the two studies was impossible since one was comparing antibiotic therapy with breast emptying versus breast emptying or no treatment, while the other was comparing two different types of antibiotic therapy.
One study assessed symptom improvement assessed by clinicians after seven days, but did not provide information on the continuation of breastfeeding (Hager 1996). In this study all women prescribed cephradine and most of those prescribed amoxicillin had symptom improvement after seven days; there were no statistically significant differences between groups (risk ratio (RR) 0.85, 95% confidence interval (CI) 0.65 to 1.12) (Analysis 1.1).
1.1. Analysis.
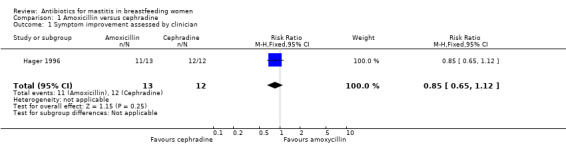
Comparison 1 Amoxicillin versus cephradine, Outcome 1 Symptom improvement assessed by clinician.
Thomsen 1984 assessed continuation of breastfeeding after 14 days. In this study, analysis was carried out on cases (breasts) rather than a participant basis. Each case was a breast with mastitis symptoms. Cases were divided into three groups depending on laboratory investigations; cases with milk stasis only (n = 126), cases with non‐infective inflammation (n = 48), and cases with infective mastitis (n = 165). Only cases in the third group (those with infective disease) were considered relevant to this review. This infective mastitis group was divided into three subgroups: cases receiving no treatment (n = 55), breast emptying only with no antibiotic therapy (n = 55), or receiving antibiotic therapy plus emptying the breast (n = 55). We carried out two separate comparisons: first women receiving no treatment versus women receiving antibiotics with breast emptying; and second, breast emptying alone versus antibiotics with breast emptying.
For cases with infective mastitis, the outcome was good in 15% of cases if there was no treatment and in most cases (96%) for women undergoing antibiotic therapy with breast emptying (RR 6.63, 95% CI 3.48 to 12.60) (Analysis 2.1). (The outcome was considered either as good if inflammatory symptoms disappeared followed by normal lactation two weeks after the initial diagnosis; or bad when symptoms persisted for more than 14 days. Impaired milk secretion, recurrence of infection, or progression of the symptoms to sepsis or breast abscesses were also considered as bad outcomes.) When antibiotic therapy with breast emptying was compared with breast emptying alone, again those receiving antibiotic therapy were more likely to have a good outcome (RR 1.89, 95% CI 1.45 to 2.47) (Analysis 3.1).
2.1. Analysis.
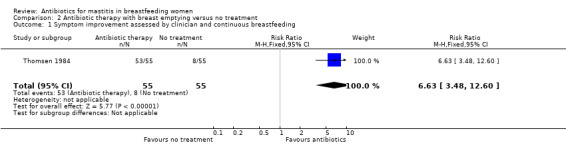
Comparison 2 Antibiotic therapy with breast emptying versus no treatment, Outcome 1 Symptom improvement assessed by clinician and continuous breastfeeding.
3.1. Analysis.
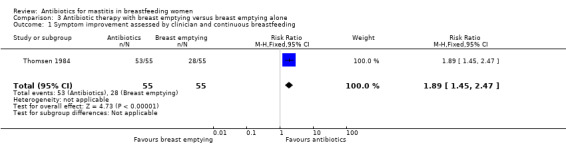
Comparison 3 Antibiotic therapy with breast emptying versus breast emptying alone, Outcome 1 Symptom improvement assessed by clinician and continuous breastfeeding.
Women were also likely to recover more quickly if they received antibiotics with a mean duration of symptoms of 6.7 days in the no‐treatment group, 4.2 days in the breast emptying group and 2.1 days in the antibiotic therapy with breast emptying group.
Sensitivity analysis
In the Thomsen 1984 study, the unit of analyses was breasts rather than individual women, so for women who contributed two infected breasts to the analysis, the response to treatment in each breast was unlikely to have been independent. We therefore conducted a series of sensitivity analyses where we made several different assumptions. For example, we assumed that 28 women (in both groups) each had two infected breasts; this effectively reduced the sample size to half the original size. The results of the sensitivity analyses (based on differing assumptions) made little difference to the results, although the smaller sample sizes (when women rather than single breasts were the unit of analysis) resulted in wider confidence intervals.
Secondary outcomes
Thomsen 1984 did not report whether or not there were any adverse events or drug reactions following antibiotic therapy. Hager 1996, however, mentioned that there were no adverse side effects to the antibiotics administered. The women were asked about compliance with dosing at the return visit, and all indicated that they had taken their medication as prescribed with no complications. Neonatal complications, hospitalisation and costs were not reported in either study.
Relapse rate
Recurrence within 30 days was reported for one woman (7.6%) in the amoxicillin arm and two women (16.6%) in the cephradine arm (Hager 1996). Thomsen 1984 had an overall recurrence of 12 cases in the infective mastitis arm. The recurrence rate for the antibiotic therapy group was not stated.
Discussion
The main finding of the review is that there is insufficient evidence available to confidently evaluate the effect of antibiotic therapy on mastitis. Controlled scientific studies such as randomised controlled trials (RCTs) are lacking in this field. Observational studies suggest that the type of antibiotic prescribed depends on physician preference, without any scientific proof. Various classes of antibiotics are prescribed without laboratory investigation. These include penicillins and cephalosporins (Amir 2004b; Marchant 2002; Olsen 1990). Antibiotic resistance may arise as a result of tendency to prescribe broad‐spectrum antibiotics. It is possible that lower‐cost, narrower‐spectrum antibiotics based on bacterial culture might be as effective as the use of higher cost, broad‐spectrum antibiotics. The findings of this review were not able to shed light on these questions.
Antibiotic therapy can be directed by leucocyte count and the susceptibility tests of isolated bacteria (Hager 1996). This approach was used by Thomsen 1984, who examined the effect of antibiotic therapy versus supportive therapy. They categorised 213 women with mastitis into three groups: milk stasis (with bacteria < 103/mL; leucocytes < 106/mL), non‐infective inflammation (bacteria < 103/mL; leucocytes > 106/mL), and infective mastitis (bacteria > 103/mL; leucocytes > 106/mL). For those in the latter group, antibiotic therapy was based on sensitivity cultivation. Women who received antibiotic therapy achieved the fastest symptom clearance of 2.1 days, as opposed to the other two groups (6.7 days if under no treatment and 4.2 days if under supportive therapy). Moreover, 11% of cases with no intervention developed an abscess, while none in the group treated with antibiotic therapy suffered from any abscess, indicating a better outcome if antibiotics were used in cases with mastitis. However, there were limitations in the Thomsen 1984 study. Although it was a RCT, the study was conducted 25 years ago and it lacked several features of a well‐designed trial: the process of concealment was not mentioned and there was no placebo used. Current practice for treating mastitis varies widely. There is a lack of properly‐designed RCTs to evaluate the best antibiotic therapy for treating mastitis. Hager 1996 is the only RCT suitable for review that compared two types of antibiotics (amoxicillin, cephradine) in a small group of 25 women with mastitis. The author‐calculated sample size for each arm of this study was 72; however, the number of included women was 13 for the amoxicillin group and 12 in the cephradine group. Therefore, this study was underpowered and was unable to detect differences in the predetermined treatment outcome. The authors were unable to run a Chi² test as the number of women in cross‐tabulated cells was less than five. Therefore, the Fisher Exact test was used and there was no significant difference between the two arms in terms of treatment failure.
Hager 1996 suggested that both oral antibiotics appeared equally effective in the treatment of sporadic acute puerperal mastitis. Moreover, there is a lack of information on the possible side effects of antibiotics on neonates when they are used for treatment of mastitis.
Our updated literature search was unfruitful in finding any studies that looked at some of the pre‐determined outcomes of this review, such as hospitalisation and costs. We found two new studies (Fernandez 2008; Jimenez 2008) focusing on other methods of treatment using probiotics and other agents such as nisin. These studies introduced lactobacillus strains and bacteriocin nisin as the potential and effective alternative therapy for mastitis. However, since the focus of our study is antibiotic therapy we did not include them in our review. It is recommended that a new title would specifically look into the effectiveness of these alternative therapies. The need for comprehensive RCTs on mastitis and antibiotic therapy still remains.
Authors' conclusions
Implications for practice.
There is little evidence from the RCTs currently available to evaluate the effect of antibiotic therapy on mastitis. The included trials failed to meet some of the criteria for methodological quality, and the outcome measures used were too varied for comparisons to be made between studies.
Implications for research.
There is an urgent need for high‐quality, large randomised placebo‐controlled trials. Future research should be designed so as to have adequate power (sample size), adequate allocation concealment, blinding of outcome assessors, and clear description of follow‐up, to allow appropriate comparisons between various antibiotic therapies or placebo groups, or both. Primary outcomes of this review, including symptom improvement reported by women or found by clinical assessment, the effect of continued breastfeeding versus no breastfeeding and the result of treating the infective mastitis based on laboratory investigation, should be further investigated. Secondary outcomes such as neonatal complications, hospitalisation, cost of treatment and adverse reactions following antibiotic therapy, should also be considered. Each and every one of these variables are important in terms of maternal and child health. We recommend a comprehensive RCT to investigate all of the above mentioned variables.
Symptom improvement reported by women.
Symptom improvement by clinical assessment.
Continued breastfeeding.
Resolution of infection as confirmed by laboratory test.
What's new
| Date | Event | Description |
|---|---|---|
| 23 November 2012 | New citation required but conclusions have not changed | Two trials identified from an updated search in September 2012 ‐ one excluded (Arroyo 2010) and one awaiting translation (Zhou 2009). |
| 30 September 2012 | New search has been performed | Search updated in September 2012. |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 19 March 2010 | New search has been performed | Search updated. Two new studies identified but excluded (Fernandez 2008; Jimenez 2008). |
| 4 February 2009 | Amended | Corrected typographical error. |
Acknowledgements
We are grateful to Professor Jackie Ho who provided us with her constructive comments.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Search strategy
This strategy was run in CENTRAL (The Cochrane Library 2007, Issue 4), MEDLINE (January 1966 to 2007) and EMBASE (January 1985 to 2007)
#1 MASTITIS (subject heading) #2 mastitis #3 puerper* #4 breastfeeding #5 (breast next feeding) #6 breast‐feeding #7 lactation* #8 nursing #9 antibio* #10 treatment* #11 ANTI‐BACTERIAL AGENTS (MeSH) (Note: we used Antibiotic agent in EMBASE) #12 therap* #13 (#1 or #2) #14 (#3 or #4 or #5 or #6 or #7 or #8) #15 (#9 or #10 or #11 or #12) #16 (#13 and #14 and #15)
Appendix 2. 2 Search strategy (June 20th 2012)
1 exp Mastitis/ or mastitis.mp. or exp Granulomatous Mastitis/ (10755)
2 breastfeeding.mp. or exp Breast Feeding/ (27103)
3 exp Breast Feeding/ or breast next feeding.mp. or exp Lactation/ (52662)
4 breast‐feeding.mp. or exp Breast Feeding/ (27418)
5 exp Lactation Disorders/ or Lactation/ or lactation.mp. (42383)
6 nursing.mp. (426127)
7 exp Anti‐Bacterial Agents/ or antibio.mp. (487245)
8 treatment.mp. or exp Therapeutics/ (5009754)
9 therap.mp. (25)
10 2 or 3 or 4 or 5 or 6 (491228)
11 7 or 8 or 9 (5304972)
12 1 and 10 and 11 (983)
13 exp Random Allocation/ or exp Research Design/ or randomized control trial.mp. or exp Clinical Trials as Topic/ (503055)
14 12 and 13 (67)
15 limit 14 to (humans and yr="2010 ‐Current") (2)
Appendix 3. Data collection and analysis for previous versions
Selection of studies
Two review authors independently assessed the titles and abstracts of identified studies. Where we could not make a clear decision on the basis of the title or abstract, we considered the study to be relevant. We retrieved the full text of relevant studies. If the retrieved articles were not written in English, we obtained a translation. Two review authors independently examined the retrieved articles to assess whether they satisfied the inclusion criteria. We resolved disagreement by consensus with a third review author.
Two review authors independently double entered data. A third review author checked to ensure concordance.
Assessment of methodological quality of included studies
We assessed the quality of included studies using the criteria in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We assessed each study for quality in terms of sequence generation, allocation sequence concealment, blinding, incomplete outcome data, selective outcome reporting and ‘other’ potential sources of bias. We reported methods used for the generation of the randomisation sequence for each trial.
(1) Sequence generation
We considered the sequence generation to be adequate if the following or similar methods were used: repeated coin tossing, throwing dice or dealing previously shuffled cards or computer generation of random numbers. We considered any other non‐random type of sequence generation inadequate.
(2) Allocation concealment
We assigned a quality score for concealment to each trial, using the following criteria:
adequate concealment of allocation, such as telephone randomisation, consecutively numbered sealed opaque envelopes;
unclear whether adequate concealment of allocation;
inadequate concealment of allocation, such as random number tables, sealed envelopes.
Where the method of allocation was unclear, we had planned to contact study authors to provide further details.
(3) Blinding (blinding of participants, researchers and outcome assessment)
We assessed blinding using the following criteria:
blinding of participants (yes/no/unclear);
blinding of caregiver (yes/no/unclear);
blinding of outcome assessment (yes/no/unclear).
(4) Incomplete outcome data (loss of participants, e.g. withdrawals, drop‐outs, protocol deviations)
We assessed completeness to follow‐up using the following criteria:
less than 5% of participants excluded;
5% to 10% of participants excluded;
more than 10% and up to and including 20% of participants excluded.
We planned to exclude studies from analysis, because of the risk of bias, if:
(A) more than 20% of participants were lost to follow‐up; (B) more than 20% of participants were not analysed according to randomisation groups and where it was not possible to restore participants to the correct group; (C) where there was a large difference (more than 10%) in withdrawal of participants between groups.
We also considered selective outcome reporting and other potential sources of bias such as time lag, location bias and language bias.
Data extraction and management
We designed a form to extract data. Two review authors extracted the data independently onto standardised, structured tables. We resolved differences in opinion as above. We performed data entry using the Review Manager software (RevMan 2008). If data were unclear, we tried to contact authors of the original reports to provide further details.
Data analysis
We used the Cochrane Review Manager software (RevMan 2008) to analyse the data.
Dichotomous data
For dichotomous data, we have reported results as risk ratios with 95% confidence intervals.
Continuous data
For continuous data, we planned to calculate the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference with 95% confidence interval to combine trials measuring the same outcome, but using different methods. We recorded evidence of skewness.
Measures of treatment effect
We used a fixed‐effect model for combining data in the absence of significant heterogeneity if trials were sufficiently similar. In the case of heterogeneity, we planned to use a random‐effects model.
Assessment of heterogeneity
We would have applied tests of heterogeneity between trials, if appropriate, using the I² test. If we identified high levels of heterogeneity among the trials (I² exceeding 50%), we would have explored it by prespecified subgroup analysis and by performing sensitivity analysis. We would have used a random‐effects meta‐analysis as an overall summary if this had been considered appropriate.
Sensitivity analyses
We would have carried out sensitivity analysis to explore the effect of trial quality. We would have excluded studies of poor quality (with poor allocation concealment or levels of attrition above 20%) in the analysis in order to assess for any substantive difference to the overall results.
The analysed units in Thomsen 1984 study were breasts, rather than women (213 women or 339 breasts). The sample would have included women with either both or only one breast affected, and where both breasts were infected the treatment effect in each breast would not be independent. Therefore, we carried out an exploratory sensitivity analysis to look at various different scenarios, for example, where we assumed that all women had two affected breasts, or where half had only one breast affected.
Subgroup analyses
We had planned subgroup analyses classifying whole trials by interaction tests as described by Deeks 2001. We planned to carry out the following subgroup analyses:
dosage of antibiotics used (e.g. antibiotic A dosage 250 mg versus antibiotic A dosage 400 mg);
drug reaction: (maternal drug reaction, infant/child drug reaction).
Appendix 4. Data collection and analysis for future updates
Selection of studies
Two review authors will independently assess for inclusion all the potential studies we identify as a result of the search strategy. We will resolve any disagreement through discussion or, if required, we will consult the third review author.
We will design a form to extract data. For eligible studies, two review authors will extract the data using the agreed form. We will resolve discrepancies through discussion or, if required, we will consult the third review author. Data will be entered into Review Manager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above is unclear, we will attempt to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors will independently assess risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement will be resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We will describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We will assess the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We will describe for each included study the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We will assess the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We will describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will consider that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We will describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We will describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We will state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we will re‐include missing data in the analyses which we undertake.
We will assess methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We will describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We will assess the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We will describe for each included study any important concerns we have about other possible sources of bias.
(7) Overall risk of bias
We will make explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we will assess the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we will present results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We will include cluster‐randomised trials in the analyses along with individually‐randomised trials. Their sample sizes or standard errors will be adjusted using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, this will be reported and sensitivity analyses conducted to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a separate meta‐analysis.
Cross‐over trials
Cross‐over trials will be excluded from this review.
Dealing with missing data
For included studies, levels of attrition will be noted. The impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses will be carried out, as far as possible, on an intention‐to‐treat basis i.e. we will attempt to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We will assess statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We will regard heterogeneity as substantial if I² is greater than 50% and either T² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identify substantial heterogeneity (above 50%), we will explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
If there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually, and use formal tests for funnel plot asymmetry. For continuous outcomes we will use the test proposed by Egger 1997, and for dichotomous outcomes we will use the test proposed by Harbord 2006. If asymmetry is detected in any of these tests or is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We will carry out statistical analysis using the Review Manager software (RevMan 2011). We will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
If we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses:
dosage of antibiotics used (e.g. antibiotic A dosage 250 mg versus antibiotic A dosage 400 mg);
drug reaction: (maternal drug reaction, infant/child drug reaction).
Subgroup analyses will be restricted to the review's primary outcomes.
We will assess differences between subgroups by interaction tests available within RevMan (RevMan 2011).
Sensitivity analysis
We plan to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Data and analyses
Comparison 1. Amoxicillin versus cephradine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom improvement assessed by clinician | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.65, 1.12] |
Comparison 2. Antibiotic therapy with breast emptying versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom improvement assessed by clinician and continuous breastfeeding | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.63 [3.48, 12.60] |
Comparison 3. Antibiotic therapy with breast emptying versus breast emptying alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom improvement assessed by clinician and continuous breastfeeding | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.45, 2.47] |
Comparison 4. Sensitivity analyses: antibiotic therapy versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Assuming that in both groups both breasts are infected: symptom improvement assessed by a clinician | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.75 [2.72, 16.77] |
| 2 Assuming that in both groups 60% of women have 2 breasts infected and 40% 1 infected | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.4 [2.84, 14.44] |
4.1. Analysis.
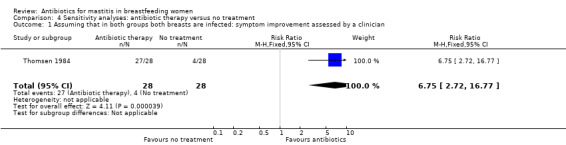
Comparison 4 Sensitivity analyses: antibiotic therapy versus no treatment, Outcome 1 Assuming that in both groups both breasts are infected: symptom improvement assessed by a clinician.
4.2. Analysis.
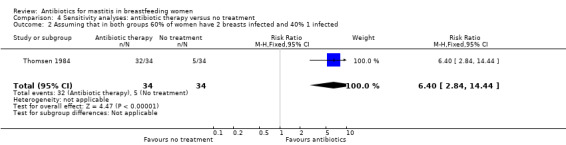
Comparison 4 Sensitivity analyses: antibiotic therapy versus no treatment, Outcome 2 Assuming that in both groups 60% of women have 2 breasts infected and 40% 1 infected.
Comparison 5. Sensitivity analyses: antibiotic therapy versus breast emptying.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Assuming that in both groups both breasts are infected: symptom improvement assessed by a clinician | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.32, 2.81] |
| 2 Assuming that in both groups 60% of women have 2 breasts infected and 40% 1 infected | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.33, 2.66] |
5.1. Analysis.
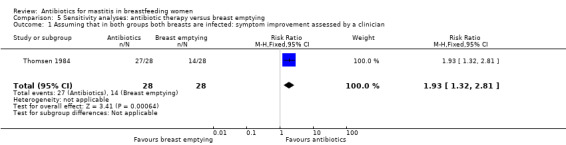
Comparison 5 Sensitivity analyses: antibiotic therapy versus breast emptying, Outcome 1 Assuming that in both groups both breasts are infected: symptom improvement assessed by a clinician.
5.2. Analysis.
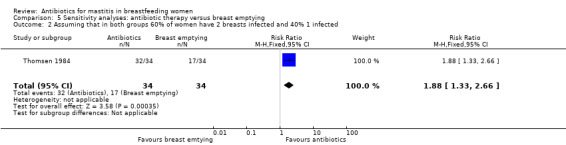
Comparison 5 Sensitivity analyses: antibiotic therapy versus breast emptying, Outcome 2 Assuming that in both groups 60% of women have 2 breasts infected and 40% 1 infected.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hager 1996.
| Methods | Randomised: method of randomisation is not mentioned. Adequate concealment of allocation is mentioned inclusive of using pre‐sealed, opaque envelopes. Double blind: no. It was mentioned that investigators were blinded. Intention‐to‐treat not mentioned. Follow‐up is described. Patients were seen for follow‐up visits in 7 days. 25 recruited and no drop‐outs. Design: parallel. | |
| Participants | 25 lactating mothers with Sporadic Acute Puerperal Mastitis (SAPM) were recruited for this study. Inclusion criteria: 3 criteria of oral temperature of 37.56ºC, tenderness on palpation of the breast and segmental erythema was needed to include a participant. Exclusion criteria: maternal age of < 18 years, documented allergy to penicillins or cephalosporins, and antibiotic therapy within the previous 30 days. Baseline characteristics such as age, parity, history of mastitis, or history of diabetes mellitus was similar between the 2 groups. | |
| Interventions | The treatment regimens were oral amoxicillin, 500 mg every 8 h for 7 days, or oral cephradine, 500 mg every 8 h for 7 days. Continuation of breastfeeding and usage of warm and moist compresses to the involved breast every 4‐6 h was recommended for all patients. All patients presented to outpatient clinic and visited by single physician. Patients were instructed to notify the physician if their temperature remained > 37.56ºC (> 99.6ºF) after 48 hrs or if they were unable to comply with the antibiotic regimen. |
|
| Outcomes | Outcomes were inclusive of resolution of mastitis, namely fever, erythema and tenderness. | |
| Notes | University of Kentucky Medical Center Outpatient Clinic patients enrolled from July 1991 until December 1993. Informed consent signed by all patients. Historical information and study data were recorded on pre‐coded data sheet. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method is not mentioned. |
| Allocation concealment (selection bias) | Low risk | Adequate concealment of allocation is mentioned inclusive of using pre‐sealed, opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It was mentioned that investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up is described. Patients were seen for follow‐up visits in 7 days. 25 recruited and no drop‐outs. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes appear to have been reported. |
| Other bias | Low risk | Groups appear balanced for baseline characteristics (apart from duration of symptoms ‐ see Table 1, page 99). |
Thomsen 1984.
| Methods | Randomised: method of randomisation is not mentioned. Allocation concealment: there is no mention of patient allocation. Antibiotic therapy was rather directed by susceptibility tests of the isolated bacteria. Unit of allocations was "mothers" but the unit analysed in the results was shown as "breasts". Double blind: no. It is unclear whether patient or outcome assessor was blinded. Intention‐to‐treat not mentioned. Follow‐up is not described clearly. From definition of bad outcome it can be implied that patients have been visited 2 weeks after the attack. No drop‐outs. Design: parallel. | |
| Participants | 213 nursing women with the diagnosis of infective mastitis (presence and persistence of tenderness, swelling, redness, heat, and decreased milk secretion) were recruited for this study. Diagnosis was made based on clinical symptoms, leucocyte count and anaerobic/aerobic bacteria cultivation. The unit of analysis in this study was not women but rather single breasts (339 breasts). There were 3 groups. Group 1 included those with milk stasis (< 106 leucocytes and < 103 bacteria) (number of breasts = 126) and group 2 were inclusive of non‐infective mastitis (> 106 leucocytes and < 103 bacteria) (number of = 48). Group 3 consisted of 165 inflammatory breasts with proven infective mastitis (> 106 leucocytes and > 103 bacteria). This final group (included in this review) was then randomly assigned to 3 subgroups with 55 cases in each group: Those with no therapy (subgroup 1), standard of care therapy (subgroup 2) and finally cases of antibiotic therapy (subgroup 3). Inclusion criteria: the presence and persistence of tenderness, swelling, redness, heat, and decreased milk secretion. Exclusion criteria were not mentioned. Baseline characteristics were not mentioned. All patients presented to Kommune hospital, Aarhus in Denmark. There is no mention if patients were visited in outpatient clinic. |
|
| Interventions | The treatment regimens contained penicillin 500,000 IU 3 per day for 6 days, oral ampicillin 500 mg, 4 per day for 6 days or erythromycin 500 mg 2 times per day for 6 days. In control group non‐intervention therapy was adopted which consisted of emptying the breast every 6 h by nursing the baby followed by expression by hand or mechanical suction. | |
| Outcomes | Outcome was considered as good if symptoms of mastitis disappeared followed by normal lactation throughout 2 weeks after the attack. | |
| Notes | Study was done in 1983 (no exact date is mentioned) in Department of Obstetrics and Gynecology, Kommunehospital, Aarhus in Denmark. There is no mention if patient signed any consent form. Study had been approved by local ethical committee. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method is not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Antibiotic therapy was rather directed by susceptibility tests of the isolated bacteria. |
| Blinding (performance bias and detection bias) All outcomes | High risk | It is unclear whether women or outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Follow‐up is not described clearly. From the definition of bad outcome it can be implied that patients were visited 2 weeks after the attack. There is no report of drop‐outs or for any of the subgroups. |
| Selective reporting (reporting bias) | Unclear risk | Unclear from study report. No study protocol. |
| Other bias | Unclear risk | No baseline characteristics table. |
h: hours IU: international units SAPM: sporadic acute puerperal mastitis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amir 2004b | Allocation is not clear. Randomisation: yes. Participants: 10 randomised breastfeeding women with cracked nipples colonised with Staphylococcus aureus were recruited out of 135 women who were originally found to be eligible. In the placebo arm, 2 out of 5 women continued to take the capsules. Intervention: 7‐day course of either an oral antibiotic (flucloxacillin) or identical placebo capsules. Researcher did not complete the study due to logistic problems. This study is therefore an unfinished RCT. |
| Arroyo 2010 | One study arm used antibiotic but the two control groups used different forms of oral lactobacilli and did not use placebo, no treatment, or other supportive treatments such as breast emptying or another antibiotic of a different class. |
| Fernandez 2008 | Bacteriocin nisin was used for treatment group and no report of antibiotic usage was studied. |
| Gerstner 1987 | Information in this briefly presented study is more concentrated on postpartum infections in general rather than mastitis. |
| Jimenez 2008 | Lactobacillus strains isolated from breast milk were used as a mode of treatment and there was no report of antibiotic therapy. |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Zhou 2009.
| Methods | Randomised controlled trial. Parallel. |
| Participants | 198 women with acute mastitis for Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, China. 99 cases in each group. |
| Interventions | Treatment group: oral cefradine. Control group: kneading and dispersing manipulation. |
| Outcomes | Local breast lump size; clinical symptoms; adverse reactions. |
| Notes | Information available from English abstract, full report in Chinese and currently being translated. |
Contributions of authors
Chirk Jenn Ng conceived and wrote the protocol. His co‐authors Shayesteh Jahanfar and Cheong Lieng Teng helped to write the background and methods respectively.
Shayesteh Jahanfar wrote the review. Chirk Jenn Ng and Cheong Lieng Teng commented and corrected drafts and provided a translation of one of the studies.
Shayesteh Jahanfar is the guarantor of the review.
Sources of support
Internal sources
University of Malaya, Malaysia.
Royal Perak Medical College, Malaysia.
International Medical University, Malaysia.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Hager 1996 {published data only}
- Hager DW, Barton RJ. Treatment of sporadic acute puerperal mastitis. Infectious Diseases in Obstetrics and Gynecology 1996;4(2):97‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomsen 1984 {published data only}
- Thomsen AC, Espersen T, Maigaard S. Course and treatment of milk stasis, noninfectious inflammation of the breast, and infectious mastitis in nursing women. American Journal of Obstetrics and Gynecology 1984;149:492‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amir 2004b {published data only}
- Amir LH, Lumley J, Garland S. A failed RCT to determine if antibiotics prevent mastitis: cracked nipples colonized with staphylococcus aureus: a randomised treatment trial. BMC Pregnancy and Childbirth 2004;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arroyo 2010 {published data only}
- Arroyo R, Martin V, Maldonado A, Jimenez E, Fernandez L, Rodriguez JM. Treatment of infectious mastitis during lactation: antibiotics versus oral administration of lactobacilli isolated from breast milk. Clinical Infectious Diseases 2010;50(12):1551‐8. [DOI] [PubMed] [Google Scholar]
Fernandez 2008 {published data only}
- Fernandez L, Delgado S, Herrero H, Maldonado A, Rodriguez JM. The bacteriocin nisin, an effective agent for the treatment of staphylococcal mastitis during lactation. Journal of Human Lactation 2008;24(3):311‐6. [DOI] [PubMed] [Google Scholar]
Gerstner 1987 {published data only}
- Gerstner GJ. Single dose Ceftriaxon (1g) vs Cefotaxim (three 1g doses) for OB/GYN infections ‐ a randomised trial. 12th FIGO World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 300, 1988.
Jimenez 2008 {published data only}
- Jimenez E, Fernandez L, Maldonado A, Martin R, Olivares M, Xaus J, et al. Oral administration of lactobacillus strains isolated from breast milk as an alternative for the treatment of infectious mastitis during lactation. Applied and Environmental Microbiology 2008;74(15):4650‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Zhou 2009 {published data only}
- Zhou M, Li X, Cheng YQ, Shen R, Zhao Y, Zhao HZ, et al. Kneading and dispersing manipulation in treatment of early‐stage acute mastitis: A randomized controlled trial. Journal of Chinese Integrative Medicine 2009;7(12):1130‐3. [DOI] [PubMed] [Google Scholar]
Additional references
Amir 2004a
- Amir LH, Foster D, McLachlan H, Lumley J. Incidence of breast abscess in lactating women: report from an Australian cohort. BJOG: an international journal of obstetrics and gynaecology 2004;111(12):1378‐81. [DOI] [PubMed] [Google Scholar]
Bedinghaus 1997
- Bedinghaus JM. Care of the breast and support of breastfeeding. Primary Care 1997;24:147‐60. [PubMed] [Google Scholar]
Buescher 2001
- Buescher ES, Hair PS. Human milk anti‐inflammatory component contents during acute mastitis. Cell Immunology 2001;210:87‐95. [DOI] [PubMed] [Google Scholar]
Crepinsek 2010
- Crepinsek MA, Crowe L, Michener K, Smart NA. Interventions for preventing mastitis after childbirth. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD007239.pub2] [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. London: BMJ Books, 2001. [Google Scholar]
Devereux 1970
- Devereux WP. Acute puerperal mastitis: evaluation of its management. American Journal of Obstetrics and Gynecology 1970;108:78‐81. [DOI] [PubMed] [Google Scholar]
Editorial 1976
- Anonymous. Puerperal mastitis. British Medical Journal 1976;1:920‐1. [PMC free article] [PubMed] [Google Scholar]
Eglash 2006
- Eglash A, Plane MB, Mundt M. History, physical and laboratory findings, and clinical outcomes of lactating women treated with antibiotics for chronic breast and/or nipple pain. Journal of Human Lactation 2006;22:429‐33. [DOI] [PubMed] [Google Scholar]
Evans 1995
- Evans M, Head J. Mastitis: incidence, prevalence and cost. Breastfeeding Reviews 1995;3:65‐72. [Google Scholar]
Fetherston 1998
- Fetherston C. Risk factors for lactation mastitis. Journal of Human Lactation 1998;14:101‐9. [DOI] [PubMed] [Google Scholar]
Foxman 2002
- Foxman B, D'Arcy H, Gillespie B, Bobo J, Longeway M. Lactation mastitis: occurrence and medical management among 946 breastfeeding women in the United States. American Journal of Epidemiology 2002;155:103‐14. [DOI] [PubMed] [Google Scholar]
Hager 1992
- Hager WD. Mastitis. In: Mead PB, Hager WD editor(s). Infectious Protocols for Obstetrics and Gynaecology. Montvale, NJ: Medical Economics Publishing, 1992:27‐32. [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.0 [updated February 2008]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2008.
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hughes 1989
- Huges LE, Mensel RE, Webster DJT. Infection of the breast. Benign Disorders and Diseases of the Breast. London: Bailliere Tindall, 1989:143‐9. [Google Scholar]
Jonsson 1994
- Jonsson S, Pulkkinen MO. Mastitis today: incidence, prevention and treatment. Annales Chirurgiae et Gynaecologiae, Supplementum 1994;208(83):84‐7. [PubMed] [Google Scholar]
Kaufmann 1991
- Kaufmann R, Foxman B. Mastitis among lactating women: occurrence and risk factors. Social Science and Medicine 1991;33:701‐5. [DOI] [PubMed] [Google Scholar]
Kinlay 1998
- Kinlay JR, O'Connell DL, Kinlay S. Incidence of mastitis in breastfeeding women during the six months after delivery: a prospective cohort study. Medical Journal of Australia 1998;169:310‐2. [DOI] [PubMed] [Google Scholar]
Kvist 2004
- Kvist LJ, Wilde Larsson B, Hall‐Lord ML. Effects of acupuncture and care interventions on the outcome of inflammatory symptoms of the breast in lactating women. International Nursing Review 2004;51:56‐64. [DOI] [PubMed] [Google Scholar]
Kvist 2005
- Kvist LJ, Rydhstroem H. Factors related to breast abscess after delivery: a population‐based study. BJOG: an international journal of obstetrics and gynaecology 2005;112:1070‐4. [DOI] [PubMed] [Google Scholar]
Lawrence 1999
- Lawrence RA. Breastfeeding ‐ a Guide for the Medical Profession. 5th Edition. St. Louis: CV Mosby, 1999. [Google Scholar]
Marchant 2002
- Marchant DJ. Inflammation of the breast. Obstetrics and Gynecology Clinics of North America 2002;29:89‐102. [DOI] [PubMed] [Google Scholar]
Marshall 1975
- Marshall BR, Hepper JK, Zirbel CC. Sporadic puerperal mastitis: an infection that need not interrupt lactation. JAMA 1975;233:1377‐9. [DOI] [PubMed] [Google Scholar]
Matheson 1988
- Matheson I, Aursnes I, Horgen M, Aobo O, Melby K. Bacteriological findings and clinical symptoms in relation to outcome in puerperal mastitis. Acta Obstetricia et Gynecologica Scandinavica 1988;67:723‐6. [DOI] [PubMed] [Google Scholar]
Novy 1984
- Novy MJ. Disorders of lactation. In: Benson RC editor(s). Obstetric and Gynaecologic Diagnosis and Treatment. Los Altos: Lange Medical Publications, 1984:864‐7. [Google Scholar]
Ogle 1988
- Ogle KS, Davis S. Mastitis in lactating women. Journal of Family Practice 1988;26:139‐44. [PubMed] [Google Scholar]
Olsen 1990
- Olsen CG, Gordon RE Jr. Breast disorders in nursing mothers. American Family Physician 1990;41:1509‐16. [PubMed] [Google Scholar]
Osterman 2000
- Osterman K, Rahm V‐A. Lactation mastitis: bacterial cultivation of breast milk, symptoms, treatment and outcome. Journal of Human Lactation 2000;16:297‐302. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Riordan 1990
- Riordan JM, Nichols FH. A descriptive study of lactation mastitis in long‐term breastfeeding women. Journal of Human Lactation 1990;6:53‐8. [DOI] [PubMed] [Google Scholar]
Vogel 1999
- Vogel A, Hutchinson BL, Mitchell EA. Mastitis in the first year postpartum. Birth 1999;26:218‐25. [DOI] [PubMed] [Google Scholar]
Walker 1999
- Walker M. Mastitis. Lactation Consultant series 2, No. 298‐2. Illinois USA: La Leche League International, 1999. [Google Scholar]
WHO 2000
- World Health Organization. Mastitis: causes and management. WHO/FCH/CAH/00.13. Geneva: WHO, 2000. [Google Scholar]
References to other published versions of this review
Jahanfar 2009
- Jahanfar S, Ng CJ, Teng CL. Antibiotics for mastitis in breastfeeding women. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005458.pub2] [DOI] [PubMed] [Google Scholar]
Ng 2005
- Ng C, Jahanfar S, Teng CL. Antibiotics for mastitis in breastfeeding women. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005458] [DOI] [Google Scholar]


