Abstract
Background
Psychosocial impacts of lung cancer screening (LCS) can cause both harm to individuals and serve as barriers to screening participation and adherence. Early data suggest that the psychosocial impacts of LCS are moderated by certain factors (e.g. sociodemographic characteristics and beliefs), but evidence synthesis is lacking. This systematic review aimed to understand individual‐level risk factors for psychosocial burden during LCS as a precursor to developing strategies to identify and support participants, and improve LCS engagement.
Methods
Four databases were searched for full‐text articles published in English reporting any association between participant factors and psychosocial outcomes experienced during LCS. Study quality was assessed by two independent investigators; findings were synthesised narratively. The review was pre‐registered with PROSPERO and adhered to PRISMA guidelines.
Results
Thirty‐five articles were included; most (33/35) studies were assessed at high or moderate risk of bias. Study designs were pre‐post (n = 13), cross‐sectional (n = 13), qualitative (n = 8) and mixed‐methods (n = 1) and conducted primarily in the United States (n = 17). Psychological burden in LCS varied, and was often associated with younger age, female gender, current smoking status or increased smoking history, lower education, lower socio‐economic group, not being married or co‐habiting and experience with cancer. However, results were mixed, and non‐significant associations were also reported across all factors. Beliefs (e.g. fatalism, stigma and expectation of LDCT results) and comorbid psychological burden were also linked to psychosocial outcomes, but evidence was sparse. Associations between risk perception, other participant factors and other psychosocial outcomes was inconclusive, likely reflecting individual biases in risk conceptualisation.
Conclusion(s)
Several participant factors are consistently reported to be associated with psychosocial impacts of LCS, though study heterogeneity and high risk of bias necessitate more robust evaluation. Further research on how perceptions, beliefs and expectations can be used to improve psychosocial outcomes during LCS is needed.
1. INTRODUCTION
Globally, lung cancer is the leading cause of cancer mortality. 1 This is generally attributed to advanced‐stage diagnoses, making earlier detection of lung cancer a worldwide priority for cancer control. Low‐dose computed tomography (LDCT) screening for lung cancer, evaluated in landmark trials in the United States and Europe, can achieve a 20%–24% reduction in lung cancer mortality in high‐risk populations. 2 , 3 There is, however, concern that lung cancer screening (LCS) can induce psychological harm. Some psychological burden for participants is seen across most cancer screening programmes, primarily presenting as anxiety or distress to the individual or their family/carers, with consequences for screening participation, adherence and other medical help‐seeking behaviours as well. 4 , 5 For LCS, there are additional unique considerations for psychosocial outcomes. These include the potential for shame, regret or stigma around smoking behaviour, high chance of finding indeterminate nodules requiring surveillance and potential for overdiagnosis. 6 , 7
Current consensus is that LCS is unlikely to result in any clinically significant psychological burden for participants, and if it does, adverse effects will not persist long term. 4 , 8 However, evidence from real‐world LCS programmes is sparse, and most trial samples significantly under‐represent priority groups. 4 , 8 For example, participants in the Danish Lung Cancer Screening Trial were identified as likely being more ‘psychologically robust’ than a LCS population in a real‐world setting. 9 Measuring psychosocial outcomes in communities where LCS would take place is particularly important for socially driven factors and outcomes (e.g. risk perception, stigma and peer pressure as motivation to quit smoking), 10 given the difference in social processes and engagement between controlled trials and real‐world practice. Additionally, enrolment in an organised LCS programme would entail ongoing surveillance and/or regular screening, and so there may be changing or compounding psychosocial impacts over time. Measurement across these routine and follow‐up scans is limited as yet, and some study authors have acknowledged that long‐term psychosocial impacts may have been overlooked. 5 , 9
In addition to these considerations, evidence suggests that certain participant‐level risk factors significantly mediate or moderate psychosocial burden during LCS. 4 , 5 , 11 These include sociodemographic characteristics, smoking status and history, and health beliefs, but a robust synthesis of the literature is needed. The aim of this systematic review is therefore to synthesise the evidence for individual factors associated with psychosocial outcomes of LCS. This would inform development of strategies for identifying and supporting participants who experience psychosocial harm through the LCS pathway. Additionally, psychological barriers to uptake have been connected to low rates of LCS, 12 , 13 , 14 so a better understanding of related risk factors could support improved participation and adherence.
2. METHODS
2.1. Search strategy
Databases searched were Medline, Embase, PsycINFO and Cumulative Index of Nursing and Allied Health Literature (CINAHL) from inception to 15 June 2022. An update of the search was performed on 12 July 2023. In consultation with a research librarian, a search strategy for Medline was developed then modified to suit the required syntax for other databases (Table S1). No search limitations for date, language or geography were used. Searches of references and cited articles for all included studies were also conducted.
2.2. Study inclusion and exclusion criteria
Studies were included if they were original, full‐text articles reporting quantitative or qualitative psychosocial outcomes. Studies were excluded if they were reviews, case studies, case reports, opinions, comments or editorials. Participants in LCS via LDCT across the entire screening continuum (including enrolment, recruitment and follow‐up) were included; though studies where participants were only eligible for LCS (and had not engaged in the LCS pathway) were not.
Included factors and outcomes are presented in Box 1. Any psychosocial outcome was considered, including those related to decision‐making and smoking cessation (e.g. motivation and readiness to quit), though behavioural smoking outcomes (e.g. quit rates) were excluded. Only experienced outcomes were included, thus studies which reported on anticipated impacts of LCS were excluded. Relevant factors were any participant‐level predictor, moderator, mediator or covariate of an outcome of interest. Broadly, these were categorised into sociodemographic factors (e.g. age, gender and education level), health‐related factors (e.g. smoking history, experience with cancer and pre‐existing psychological burden), beliefs (e.g. risk perception, fatalism and stigma) and other factors. Stigma was included as a belief outcome, though noting that it has social aspects as well. There was cross‐over between factors and outcomes, with some categorised as both (e.g. certain beliefs).
BOX 1. List of factors and outcomes examined in identified studies.
| Factors | Outcomes |
|---|---|
|
Sociodemographic factors
Health‐related factors
Beliefs
Other
|
Psychological
Beliefs
Decision‐related
Smoking‐related
Social
|
All relevant analyses within each study were reported; some studies examined multiple outcomes under a single category (e.g. anxiety and HRQoL under psychological outcomes), therefore results from a single study were sometimes conflicting within category summaries. Where a factor of interest was examined as a modifier or covariate, studies were included if the relationship between the factor of interest and the outcome was directly reported.
2.3. Data extraction and synthesis
Procedures for data synthesis and analysis were determined a priori. The lead investigator (KM) undertook title, abstract and full‐text screening, with a second investigator (BN or AS) independently assessing a 20% subset at both stages of screening to ensure agreement and consistency. Any discrepancies were discussed with reference to the pre‐defined inclusion and exclusion criteria, with consultation by a third investigator if required. Data extraction of study characteristics was performed by the lead investigator (KM) and checked for accuracy by another investigator (TL or CJJ). Results were extracted independently by two investigators (KM and either TL or CJJ). Evidence was summarised by factor and outcome of interest; given the heterogeneity across studies, meta‐analysis and subgroup analysis were not considered appropriate and instead results were synthesised narratively.
2.4. Quality assessment
Quality assessment was performed by two investigators independently (KM and either TL or CJJ), with any discrepancies resolved via discussion or via an additional investigator (BN). Validated tools from the Joanna Briggs Institute (JBI) were used and an overall assessment (low, moderate or high risk of bias) was given based on pre‐determined criteria about the number of appraisal checklist items fulfilled. 15 Studies were not excluded from the review based on methodological quality, but this was considered in the interpretation of findings.
2.5. Protocol and registration
This systematic review presents the participant factors associated with psychosocial outcomes of LCS as part of a larger review (PROSPERO registration: CRD42022334634). A companion systematic review will report on the programme factors (service delivery aspects and interventions).
3. RESULTS
3.1. Study characteristics
Figure 1 presents the PRISMA flow diagram of search results. 16 Key characteristics of the 35 included studies are presented in Table 1. Study designs included pre‐post studies (n = 13), cross‐sectional studies (n = 13), qualitative studies (n = 8) and mixed‐methods studies (n = 1), conducted in Australia (n = 1), Canada (n = 2), Denmark (n = 1), Netherlands/Belgium (n = 5), South Korea (n = 1), United Kingdom (n = 8) and United States (n = 17). Articles were published between 2001 and 2022.
FIGURE 1.
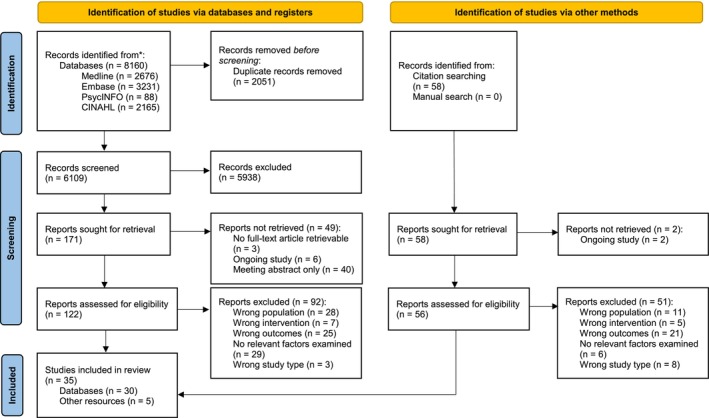
Results of search—PRISMA flow diagram.
TABLE 1.
Key characteristics of included studies.
| Author, year | Location (trial/programme) | Study design | Study aim | Participant description, N | Measurement point(s) | Factors tested | Outcomes of interest | Measurement tool | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Balata et al., 2020 43 | England (Manchester LHC pilot) | Pre‐post (cross‐sectional for outcomes of interest) | To determine if attendance at a Lung Health Check (LHC) and participation in LDCT influenced smoking behaviour and attitudes to smoking in a deprived population |
Participants who enrolled in LHC pilot and attended a second LDCT N = 919 Currently smoking at T0 and T1: N = 415 Currently smoking at T0 and no longer smoking at T1: N = 47 No longer smoking at T0 and T1: N = 433 No longer smoking at T0 and currently smoking at T1: N = 24 |
|
|
Smoking worry (measured at T1) | 3 items | Moderate |
| Intention to quit smoking (measured at T1) | 1 item | ||||||||
| Confidence/self‐efficacy in quitting smoking (measured at T1) | 2 items | ||||||||
| Barta et al., 2021 38 | United States | Cross‐sectional | To identify racial differences in attitudes and beliefs towards lung cancer and LCS, and determine whether sociodemographic factors/screening beliefs are associated with adherence rates to screening follow‐up |
New LCS participants arriving for an SDM office visit prior to LDCT Study cohort: N = 269 HINTS cohort: N = 2235 (used only for cancer beliefs outcomes) |
After recruitment, prior to SDM and LDCT |
|
Cancer beliefs (fatalism) | 4 items taken from HINTS | High |
| Risk perception | 1 item | ||||||||
|
Lung cancer worry |
3 items adapted from CWS | ||||||||
| LDCT worry | 3 items | ||||||||
| Bold et al., 2022 34 | United States | Cross‐sectional | To investigate patient characteristics, treatment perceptions and potential barriers to quitting smoking |
Attendees for a lung cancer screening visit who reported current cigarette smoking N = 147 |
At time of LCS visit |
|
Motivation/intention to quit smoking | 1 item | High |
| Concerns about quitting smoking | 6 items | ||||||||
| Brain et al., 2016 20 | England (UKLS) | Pre‐post | To measure the effects of UKLS trial participation on short‐term and long‐term psychosocial outcomes |
High‐risk individuals (≥5% over 5 years using the LLPv2 risk prediction model) randomly allocated to LDCT or control group At T0: N = 4055 Screening arm: N = 2018 Control arm: N = 2019 |
|
|
Cancer distress (measured at T1, T2) | CWS | Moderate |
| Bunge et al., 2008 29 | Netherlands/Belgium (NELSON) | Pre‐post | To evaluate how many participants in a LCS trial had a low or high affective risk perception of developing lung cancer, and whether participants with a high affective risk perception showed a higher level of lung cancer‐specific distress during LCS |
Participants who had a baseline LDCT appointment as part of the NELSON trial and received a negative or indeterminate scan result (positive results excluded from analysis) N = 351 T1 respondents: N = 324 T2 respondents: N = 288 |
|
|
HRQoL | SF‐12 | High |
| Affective risk perception (feeling about risk) | 1 item | ||||||||
| Lung cancer‐specific distress | IES | ||||||||
| Buttery et al., 2022 45 | England (Lung Health Check) | RCT (qualitative for outcome of interest) | To compare the effectiveness of two approaches to smoking cessation support for people who smoke attending a lung health screening service |
Participants attending a targeted LHC randomised into either immediate smoking cessation support (with pharmacotherapy), or usual care (very brief advice to quit and signposting to local services) N = 84 (at follow‐up) Intervention: N = 48 Usual care: N = 36 |
3 months post intervention |
|
Motivation to quit | Interviews | Moderate |
| Byrne et al., 2008 19 | United States (PLuSS) | Pre‐post | To measure the effect of screening outcomes on anxiety levels, fear of cancer and perceived risk of lung cancer for participants in three different screening outcome categories |
Individuals recruited into the PLuSS trial T0 = 400 T3 = 341 |
|
|
Perceived risk of lung cancer | 1 item | Moderate |
| Anxiety (state, trait) | STAI | ||||||||
| Fear of lung cancer | 3 items adapted from PCQ | ||||||||
| Byrne et al., 2019 27 | United States | Cross‐sectional | To describe the characteristics of people being screened in community settings, including factors influencing screening decisions and the level of information sought prior to screening |
Individuals undergoing LDCT‐based LCS in community‐based radiology clinics N = 27 |
|
|
Fear of being diagnosed with lung cancer | 1 item per outcome (measured as importance to LCS decision‐making) | High |
| Reassurance seeking | |||||||||
| Perceived efficacy of LCS | |||||||||
| Decisional conflict | DCS | ||||||||
| Decisional satisfaction | Satisfaction with Decision Scale | ||||||||
| Decisional regret | Decision Regret Scale | ||||||||
| Cordon et al., 2021 49 | United States (LSTH) | Mixed‐methods (qualitative and quantitative interview) | To explore the effects of COVID‐19 on older people who smoke enrolled in the Lung Screening, Tobacco and Health (LSTH) trial |
Participants who had completed LDCT and were enrolled or had started a smoking cessation intervention (8 counselling sessions + 8 weeks of nicotine patches) or usual care (3 sessions + 2 weeks of patches) N = 30 Intervention: N = 21 UC: N = 9 |
|
|
Motivation to quit |
|
High (High for quantitative; Moderate for qualitative) |
| Dunn et al., 2017 23 | England (UKLS) | Pre‐post | To examine the role of screening expectations in modifying psychological responses to screening results among high‐risk individuals receiving LCS |
Participants in UKLS who had completed LDCT N = 1589 |
|
|
Concern about LDCT result | 1 item | High |
| Eberth et al., 2022 32 | United States | Cross‐sectional | To explore how patients who have been referred for LCS by their healthcare provider describe the SDM visit, including what information they learned about screening and their level of certainty about their screening decision |
Patients completing LDCT screening N = 75 |
|
|
Decisional conflict | Items adapted from the SURE scale | High |
| Golden et al., 2020 44 | United States | Qualitative | To use the LCS decision discussion as a case study to understand possible underlying components of a teachable moment to enhance motivation for smoking cessation |
Patients who had completed SDM for LCS during routine care N = 51 Elected LDCT: N = 43 Declined LDCT: N = 8 |
|
|
|
Semi‐structured interviews | Moderate |
| Golden et al., 2022 51 | United States | Qualitative | To (1) determine whether teachable moments for smoking cessation occur downstream from the initial LCS decision‐making interaction, (2) investigate patient experiences with smoking cessation and recommendations for improving cessation rates within LCS |
Patients who had completed SDM for LCS during routine care N = 39 (61 interviews) T1 = 32 T2 = 29 Elected LDCT: N = 32 Declined LDCT: N = 7 |
|
|
Motivation to quit | Semi‐structured interviews | Moderate |
| Greene et al., 2019 40 | United States | Qualitative | To explore how those at highest risk for lung cancer (people who currently smoke) experienced, understood and made decisions about participation in LCS after being offered during routine primary care visits |
Individuals who had been offered screening at a routine primary care visit N = 37 Elected LDCT: N = 33 Declined/delayed LDCT: N = 4 |
|
|
|
Semi‐structured interviews | Moderate |
| Hall et al., 2018 41 | United States | Cross‐sectional | To (1) quantify referral clarity and perceived accuracy during LCS; (2) identify medical, sociodemographic, smoking, behaviour and numeracy correlates to LCS uncertainty; (3) demonstrate associations between LCS uncertainty and emotional functioning |
Patients who had recently completed LDCT N = 169 |
|
|
Perceived stress | PSS‐4 | High |
| Anxiety | GAD‐2 | ||||||||
| Referral clarity | 1 item | ||||||||
| Perceived accuracy of LCS | 1 item | ||||||||
| Han et al., 2019 31 | United States | Mixed‐methods (pre‐post surveys for outcomes of interest) | To evaluate the effects of providing personalized cancer risk information (PCRI) to patients referred for LCS |
Patients referred for LDCT who received PCRI N = 60 |
|
|
|
|
High |
| Kaerlev et al., 2012 26 | Denmark (DLCST) | Cross‐sectional | To examine psychological adverse effects in LCS participants with the calculation of risk ratios for prescription of anti‐depressive or anxiolytic medication |
Participants randomised to an intervention group (CT scan) or control group in the DLCST N = 4104 Intervention: N = 2052 Control: N = 2052 |
|
|
Use of antidepressant or axiolytic medication | Prescription of antidepressant or axiolytic medication | Moderate |
| Kathuria et al., 2020 48 | United States | Qualitative | To characterise perspectives of physicians and LCS participants on communication and perceived utility of LCS integrated with smoking cessation |
Patients who underwent LCS in the previous year N = 28 |
|
|
Motivation to quit | Semi‐structured interviews and focus groups | Moderate |
| Kummer et al., 2020a 17 | England (LSUT) | Qualitative | To explore the spectrum of psychological and behavioural responses among individuals with indeterminate and incidental LDCT results |
Patients who had received a Lung Health Check as part of the LSUT trial N = 28 |
|
|
|
Semi‐structured interviews | Moderate |
| Kummer et al., 2020b 42 | England (LSUT) | Pre‐post | To (1) investigate sociodemographic and smoking‐related characteristics associated with psychological outcomes following LCS, and (2) compare psychological outcomes for screened vs. ‘screening unaware’ (community comparison sample) |
Patients undergoing a LDCT pilot trial N = 787 |
|
|
Cancer worry |
Adapted version of CWS | Moderate |
| Anxiety | HADS | ||||||||
| Depression | HADS | ||||||||
| Lebrett et al., 2022 22 | England (Manchester LHC) | Cross‐sectional | To examine lung cancer risk perception, disease knowledge and lung cancer‐specific worry in attendees of a community‐based LCS programme and explore association between these measures and lung cancer risk scores, screening eligibility and other variables |
Individuals attending for a Lung Health Check who consented for linkage of responses with clinical data N = 243 |
|
|
Perceived risk (absolute and comparative) | 2 items | Moderate |
| Lung cancer worry (frequency and impact) | 2 items adapted from CWS | ||||||||
| Anxiety | PHQ‐4 | ||||||||
| Depression | PHQ‐4 | ||||||||
| Lee et al., 2021 28 | Korea (K‐LUCAS) | Pre‐post | To report the interim results of baseline screening during the K‐LUCAS trial (to December 2017) |
K‐LUCAS trial participants (people who currently or formerly smoke at high‐risk aged 55–74 years with an at least 30‐pack‐year smoking history) N = 5597 |
|
|
|
1 item per outcome | High |
| Lillie et al., 2017 39 | United States | Cross‐sectional | To (1) identify which factors people consider most important in making LCS decisions; (2) explore whether factors considered important vary by individual characteristics; (3) detect whether perceived importance of benefits and harms of screening varied by LCS completion |
Veterans randomised to receive direct LCS invitation with decision aid or usual care N = 588 Intervention (direct LCS invitation): N = 384 Usual care (provider referral for LCS): N = 204 |
|
|
|
1 item per outcome (measured as % of participants rating certain decision‐making factors as important) | High |
| Nishi et al., 2021 33 | United States | Cross‐sectional | To describe the quality of SDM among people who recently received LCS |
Patients who had completed LDCT within 12 months N = 266 |
|
|
Decisional conflict | SURE scale (4‐item short version of DCS) | High |
| Olson et al., 2022 37 | Australia (ILST) | Qualitative | To examine participants' emotionally imbued experiences of LCS |
Individuals who currently smoke undergoing LDCT as part of the ILST trial N = 27 |
|
|
|
Semi‐structured interviews | Low |
| Ostroff et al., 2001 46 | United States (ELCAP) | Cross‐sectional | To (1) describe self‐reported changes in smoking behaviour following enrolment in a LCS programme and (2) examine potential predictors and covariates of change in smoking behaviour |
Participants enrolled in an LCS programme for high‐risk people who smoke who had undergone baseline LDCT N = 134 |
|
|
Motivation to quit | Telephone survey interview | Moderate |
| Quaife et al., 2021 4 | United Kingdom (SUMMIT study) | Prospective cohort (cross‐sectional for outcomes of interest) | To test whether (and which) psychological factors are associated with screening uptake behaviour prospectively using a longitudinal cohort design embedded within a multicentre LCS implementation trial |
Participants invited for a Lung Health Check N = 7730 |
|
|
|
SRQ‐LCS, 25 | Low |
| Taghizadeh et al., 2019 25 | Canada (Pan‐Can study) | Pre‐post |
To describe changes in anxiety and HRQoL in a high‐risk Canadian cohort undergoing LCS |
Individuals undergoing LDCT as part of the PanCan study N = 1237 T0 = 1237 T1 = 953 T2 = 1066 |
|
|
Anxiety | STAI | High |
| Turner et al., 2021 30 | Canada (Pan‐Can study) | Cross‐sectional | To clarify the determinants of lung cancer risk perception and its role in LCS programmes and recruitment |
Participants undergoing an LDCT scan as part of the PanCan study N = 2514 |
|
|
|
1 item per outcome | Moderate |
| Van den Bergh et al., 2008 24 | Netherlands (NELSON) | Pre‐post | To assess discomfort experienced by subjects during LDCT and while waiting for results and explore the impact of LCS on HRQoL over time |
Participants in the NELSON trial who received a negative or indeterminate baseline LDCT result (positive results excluded from analysis) N = 324 (returned T1 questionnaire) N = 270 (returned all questionnaires) |
|
|
Anxiety | STAI‐6 | Moderate |
| Lung cancer‐specific distress | IES | ||||||||
| HRQoL |
|
||||||||
| Discomfort experienced during LDCT | Multiple items | ||||||||
| Van den Bergh et al., 2010a 36 | Netherlands/Belgium (NELSON) | Pre‐post | To assess changes in generic and lung cancer‐specific HRQoL changes over time among participants undergoing LCS in the short term |
Patients in the NELSON trial who received a negative or indeterminate baseline LDCT result (positive results excluded from analysis) N = 630 (returned T0 questionnaire) N = 494 (returned all questionnaires) |
|
|
Anxiety | STAI‐6 | High |
| Lung cancer‐specific distress | IES | ||||||||
| HRQoL |
EQ‐5D SF‐12 (T0, T3 only) |
||||||||
| Van den Bergh et al., 2010b 50 | Netherlands/Belgium (NELSON) | Pre‐post | To evaluate whether LCS participants who made an informed decision had better HRQoL than those who did not make an informed decision, especially those receiving an indeterminate test result which required a follow‐up CT scan |
Patients in the NELSON trial who made or made not an informed decision to participate in LCS N = 288 Informed decision: N = 155 No informed decision: N = 133 |
|
|
Anxiety | STAI‐6 | High |
| Lung cancer‐specific distress | IES | ||||||||
| HRQoL |
|
||||||||
| Psychological consequences of LCS (T2 only) | Part 1 of COS‐LC | ||||||||
| Risk perception (cognitive) | 1 item | ||||||||
| Risk perception (affective) | 1 item | ||||||||
| Van den Bergh et al., 2011 18 | Netherlands/Belgium (NELSON) | Pre‐post |
To 1) compare HRQoL in a LCS and control group over 2 years; 2) explore the short‐term effects on HRQoL of an indeterminate result at second‐round screening; 3) evaluate the long‐term effects of an indeterminate baseline result; and 4) evaluate the differences between getting a negative follow‐up scan and getting at least one indeterminate or positive result at follow‐up |
Patients in the NELSON trial, randomised to either receive LCS or control. N = 1288 (returned T0 questionnaire) LCS: N = 658 Control: N = 630 |
|
|
Anxiety | STAI‐6 | Moderate |
| Lung cancer‐specific distress | IES | ||||||||
| HRQoL |
|
||||||||
| Williams et al., 2022 35 | USA (LSTH) | Pre‐post | To (1) examine changes in readiness to quit, motivation to quit, and cigarettes per day from before screening to after the receipt of lung screening results, and (2) examine the extent to which the teachable moment domains of perceived risk for lung cancer and lung cancer worry were associated with changes in these smoking‐related attitudes and behaviours |
Patients completing baseline or annual LDCT scan N = 843 |
|
|
Readiness to quit |
Contemplation Ladder |
Moderate |
| Motivation to quit | 1 item | ||||||||
| Zeliadt et al., 2015 47 | United States | Qualitative | To understand views on smoking cessation from people who currently smoke in the context of being offered LCS as a routine service in primary care |
Patients who had been offered LCS as part of a LCS Clinical Demonstration Project N = 37 |
(8 interviewed at both T0 and T1) |
|
Relief | Interviews | High |
Abbreviations: CES‐D, Center for Epidemiologic Studies Depression Scale; CI, confidence interval; COS‐LC, Consequences of Screening in Lung Cancer questionnaire; CWS, Cancer Worry Scale; DCS, decisional conflict scale; DLCST, Danish Lung Cancer Screening Trial; ECLS, Early Diagnosis of Lung Cancer Scotland trial; ELCAP, Early Lung Cancer Action Project; EQ‐5D, EuroQoL 5 dimension health‐related quality of life measure; GAD‐2, Generalised Anxiety Disorder‐2 scale; HADS, Hospital Anxiety and Depression Scale; HRQoL, health‐related quality of life; IES, Impact of Events Scale; IQR, interquartile range; K‐LUCAS, Korean Lung Cancer Screening Project; LCS, lung cancer screening; LDCT, low‐dose CT scan; LHC, Lung Health Check; LSTH, Lung Screening, Tobacco, and Health trial; LSUT, Lung Screen Uptake Trial; LUSI, German Lung Cancer Screening Intervention trial; MD, mean difference; MID, minimal important difference; NELSON, Dutch–Belgian Randomised Controlled Lung Cancer Screening Trial; OR, odds ratio; PanCan, Pan‐Canadian Early Detection of Lung Cancer Study; PCQ, Psychological Consequences Questionnaire; PANAS, Positive and Negative Affect Schedule; PCRI, personalised cancer risk information; PHQ‐4, Patient Health Questionnaire‐4; PLuSS, Pittsburgh Lung Screening Study; PSS‐4, 4‐item Perceived Stress Scale; RR, relative risk; SDM, shared decision‐making; SF‐12, 12‐Item Short Form Health Survey; SRQ‐LCS 25, self‐regulatory questionnaire for lung cancer screening; STAI, State–Trait Anxiety Inventory; UKLS, United Kingdom Lung Cancer Screening Trial.
Risk of bias was assessed as high (n = 16), moderate (n = 17) and low (n = 2). Quality ratings for quantitative studies included in this review were commonly hindered by possible selection bias and coverage bias, and sample sizes were also often small and/or unjustified. Quasi‐experimental study designs frequently lacked control groups, and cross‐sectional designs had low response rates. Full quality appraisals according to the JBI critical appraisal tool checklists are provided in Tables S3–S5.
A summary of the factor–outcome combinations examined is provided in Table S2. Key results for each factor–outcome combination are summarised in Table 2 and are presented narratively below by broad outcome categories: psychological outcomes (both general and lung cancer or LCS‐specific; e.g. anxiety and lung cancer‐specific worry), beliefs (e.g. risk perception and fatalism), decision‐related (e.g. decisional conflict), and smoking‐related (e.g. motivation to quit smoking).
TABLE 2.
Summary of key results.
| Factor (# of studies) | Outcome of interest | Results |
|---|---|---|
| Sociodemographic factors | ||
| Age (n = 19) | Psychological (n = 12) | Younger age was associated with worse psychological outcomes in seven studies. 17 , 18 , 19 , 20 , 21 , 22 , 23 The most commonly found association was with lung cancer or LDCT‐specific cancer distress or concern (n = 4). 18 , 19 , 20 , 21 However, most (n = 9) analyses found no significant differences in psychological outcomes by age, 17 , 18 , 19 , 22 , 24 , 25 , 26 , 27 , 28 including for anxiety 18 , 22 , 25 , 28 (n = 4) HRQoL 18 , 24 (n = 2), cancer worry 17 , 22 (n = 2) and fear of lung cancer 19 , 27 (n = 2) |
| Beliefs (n = 7) | Younger age was associated with higher perceived lung cancer risk in three studies, 21 , 29 , 30 but not in three others. 19 , 22 , 31 In a high‐quality cross‐sectional study, older age was associated with more perceived control over lung cancer treatment, more positive perception of consequences of lung cancer, and less perceived stigma. 21 There was no significant association between age and the importance of perceived efficacy of LCS for making a LCS decision in one study 27 | |
| Decision‐related (n = 3) | Associations between age and decisional outcomes was measured in three studies; 27 , 32 , 33 two studies found no significant difference in decisional conflict by age 32 , 33 | |
| Smoking‐related (n = 5) | Age and smoking‐related psychosocial outcomes evidence was mixed, with most studies (n = 3) reporting no significant relationship between age and interest, readiness, motivation or concerns about quitting smoking. 31 , 34 , 35 One study reported that increasing age was significantly associated with less perceived benefit of smoking cessation. 21 | |
| Gender (n = 20) | Psychological (n = 12) | Across 10 studies, 17 , 18 , 19 , 20 , 21 , 22 , 24 , 25 , 26 , 36 women were consistently reported to have worse psychological outcomes, including cancer‐related distress, fear or worry 17 , 18 , 19 , 20 , 21 , 22 , 36 (n = 7) and anxiety 17 , 18 , 22 , 25 , 36 (n = 5). However, these findings were not always clinically meaningful. Six studies 17 , 19 , 22 , 23 , 24 , 27 found no significant relationship between psychological outcomes and gender, including for cancer‐related distress, fear or worry 17 , 23 , 24 , 27 (n = 4), depression 17 , 22 (n = 2) and anxiety 19 , 24 (n = 2). |
| Beliefs (n = 8) | Some analyses (n = 3) reported women to have higher perceived risk of lung cancer; 21 , 22 , 30 while others (n = 4) reported no difference by gender. 19 , 22 , 29 , 31 In a high‐quality cross‐sectional study, women were reported to have less feelings of personal and treatment control. 21 Two studies (one qualitative and one quantitative) each reported stigma was more common in women. 21 , 37 Women rated the perceived efficacy of LCS as significantly more important for making a LCS decision in one study. 27 | |
| Decision‐related (n = 3) | Associations between gender and decisional outcomes was measured in three studies. 27 , 32 , 33 Two studies found no difference in decisional conflict by gender, 32 , 33 one study reported women having significantly lower decisional regret than men. 27 | |
| Smoking‐related (n = 4) | Studies mostly reported no significant association between gender‐ and smoking‐related psychosocial outcomes (n = 4), 21 , 31 , 34 , 35 except women being more likely to endorse concerns about weight gain after quitting in one study. 34 | |
| Race/ethnicity (n = 9) | Psychological (n = 5) | Four studies reported no differences in psychological outcomes across race or ethnicity, 17 , 19 , 20 , 38 primarily for lung cancer or LDCT distress or fear. Two others reported worse outcomes for participants with African American 38 (n = 1) or Asian 21 (n = 1) ethnic backgrounds. |
| Beliefs (n = 3) | Three studies measured risk perception and found no significant relationship with race. 19 , 21 , 38 Higher fatalism and more negative perceptions of personal control were reported for participants with African American 38 or Asian 21 ethnic backgrounds in one study each. Though in one study, both participants with Black and Asian ethnic backgrounds had significantly more positive perceptions of the consequences of lung cancer and perceptions of treatment control, while White participants reported higher perceived stigma. 21 | |
| Decision‐related (n = 2) | Across two studies, White 32 , 33 (n = 2) and Hispanic 33 (n = 1) participants were significantly less likely to experience decisional conflict about LCS than other ethnicities. | |
| Smoking‐related (n = 3) | There was no significant relationship between race/ethnicity and motivation to quit smoking 31 , 34 , 35 (n = 2), readiness to quit smoking 35 (n = 1) or perceived efficacy of smoking cessation 21 (n = 1). In one study, those who identified as non‐Hispanic Black had significantly more concerns about withdrawal symptoms and were more likely to believe that they do not need to make smoking changes. 34 | |
| Education (n = 11) | Psychological (n = 7) | Higher levels of education were associated with better psychological outcomes in four studies, 17 , 18 , 19 , 22 including for cancer‐related distress or fear 18 , 19 , 22 (n = 3) and anxiety 18 , 19 (n = 2). However, analyses in another four studies 17 , 20 , 22 , 39 reported no relationship, specifically with cancer distress, fear, worry or impact on mood (n = 4) 17 , 20 , 22 , 39 and anxiety (n = 4). 17 , 22 , 39 Results from the K‐LUCAS trial was the only study to show minimally higher anxiety in those with higher education levels, though this study had a high risk of bias and significance between groups was not calculated. 28 |
| Beliefs (n = 5) | The association between risk perception and education was varied, with two studies reporting no relationship, 22 , 39 and one study reporting participants with higher education having lower perception of lung cancer risk. 19 Higher objective numeracy scores, but not subjective numeracy or education level, were associated with the importance of perceived efficacy of LCS for making LCS decisions in one study. 27 | |
| Decision‐related (n = 2) | Two studies found no significant relationship between education level and decisional conflict about LCS. 32 , 33 | |
| Smoking‐related (n = 1) | The K‐LUCAS trial reported higher motivation to quit smoking in the higher education group, however between‐group analysis was not completed. 28 | |
| Income, employment, insurance and deprivation (n = 9) | Psychological (n = 7) | Seven studies examined the relationship between income, insurance, deprivation, and employment status with psychological outcomes. Five studies 17 , 20 , 23 , 26 , 28 found worse psychological outcomes for those who had higher levels of deprivation, lower income or were unemployed; however, relationships in two of these studies were no longer significant after multivariate analysis. 17 , 20 In addition, three studies 17 , 21 , 39 reported no significant association between psychological outcomes, with one of these being the only high‐quality (low risk of bias) quantitative study included. 21 |
| Beliefs (n = 3) | Two studies found no significant relationship between income or deprivation level, and risk perception. 21 , 39 One high quality study reported that greater affluence was significantly associated with more negative perceptions of consequences and higher perceived stigma, but more positive perceptions of personal control. 21 There was no association between fatalism and perception of treatment control. 21 There was no significant association between insurance and the importance of perceived efficacy of LCS for making a LCS decision in one study. 27 | |
| Decision‐related (n = 1) | One study examined decisional conflict and employment, finding no significant association. 32 | |
| Smoking‐related (n = 2) | Greater affluence was significantly associated with lower perceived efficacy of smoking cessation in a high‐quality cross‐sectional study. 21 The K‐LUCAS trial reported slightly higher motivation to quit smoking for those with lower income; however, between‐group analysis was not completed. 28 | |
| Relationship status (n = 6) | Psychological (n = 6) | Married or co‐habiting participants were reported to have significantly better psychological outcomes in four studies, 17 , 19 , 20 , 26 however analyses across another four studies reported no association between relationship status and psychological burden, 17 , 19 , 21 , 23 with one of these being the only high‐quality (low risk of bias) quantitative study included. 21 |
| Beliefs (n = 2) | Two studies reported no significant relationship between relationship or marital status and risk perception. 19 , 21 In one high quality cross‐sectional study, those who were in a partnership or co‐habiting had more positive perceptions of treatment control and fatalism, but there was no relationship with the consequences of lung cancer, perceptions of personal control or stigma. 21 | |
| Smoking‐related (n = 1) | One study found no significant relationship between perceived efficacy of smoking cessation and relationship status. 21 | |
| Health‐related factors | ||
| Health status (n = 5) | Psychological (n = 3) | Alcohol consumption 25 and Body Mass Index (BMI) 22 were not significantly associated with psychological outcomes across one study each. In another study, a participant's number of comorbidities was reportedly associated with antidepressive or anxiolytic use. 26 |
| Beliefs (n = 3) | Having asthma, 30 COPD 30 and symptoms (such as dyspnoea or cough), 30 but not BMI 22 or the number of comorbidities, 30 were associated with lung cancer risk perception in one study each. Medical conditions were unrelated to the importance of perceived efficacy of LCS for making LCS decisions in one study. 27 | |
| Experience with cancer, lung cancer or LCS (n = 9) | Psychological (n = 6) | Personal or family experience with cancer (including lung cancer) was associated with higher lung cancer distress, concern about LDCT result or fear in three studies, 20 , 23 , 40 but had no links to psychological burden in three other studies. 22 , 24 , 25 |
| Beliefs (n = 2) | Family history of lung cancer was associated with comparative risk perception in two studies, 22 , 30 but not absolute risk perception in one. 22 Previous cancer was not associated with risk perception in two studies. 22 , 30 One study reported an associated between the number of previous chest radiographs, but not CT scans, with risk perception. 30 | |
| Decision‐related (n = 1) | The number of times someone was previously screened for lung cancer, or the time since previous scan, were not related to decisional outcomes in one study. 33 | |
| Smoking‐related (n = 1) | In one study, those attending for a baseline scan (rather than an annual follow‐up scan) reported higher readiness and motivation to quit smoking. 35 | |
| Smoking status and history (n = 21) | Psychological (n = 16) | Most analyses (n = 9) reported no significant association between smoking status (i.e. current versus former smoking) and outcomes such as anxiety 17 , 18 , 22 , 25 , 39 (n = 5) and lung cancer‐specific fear, worry or concern (n = 4). 17 , 21 , 23 , 27 Seven studies reported worse psychological outcomes for participants who reported current smoking, 18 , 19 , 20 , 22 , 36 , 39 , 41 though these was not always clinically important. Four studies reported that more pack years were associated with increased psychological burden, 18 , 22 , 28 , 36 but three studies also reported no significant relationship. 18 , 22 , 25 In two qualitative studies, more pronounced smoking history was often linked to increased worry, guilt and shame in the context of LCS. 40 , 42 |
| Beliefs (n = 10) | The relationship between smoking status and risk perception was varied, reported as worse for people currently smoking (n = 4), 19 , 21 , 22 , 39 worse for people who had quit smoking (n = 1), 30 and not significantly different (n = 2). 22 , 29 Higher number of pack years was associated with higher perceived risk in one study, 30 but not in two others. 22 , 30 Younger smoking start age and higher average cigarettes smoked per day were also associated with higher risk perception in one study. 30 Stigma was described by people currently smoking in two qualitative studies, 40 , 42 with significantly higher perceived stigma reported by this group in another quantitative study. 21 Higher fatalism and reduced perceptions of control were also higher in those who were currently smoking. 21 Two studies indicated no difference in perceived efficacy or accuracy of LCS by smoking status. 27 , 41 | |
| Decision‐related (n = 2) | People who formerly smoked were more likely to report worse decisional outcomes compared with those currently smoking in two studies, 27 , 33 though a significant relationship was only reported in one. 33 Pack years was not significantly associated with decisional conflict in one study. 33 | |
| Smoking‐related (n = 5) | Findings on the impact of smoking status on smoking‐related psychosocial outcomes were mixed. Current smoking status was associated with more smoking worry 43 (n = 1) and a higher intention to quit 43 (n = 1), but lower confidence in quitting 43 (n = 1) and lower perceived efficacy of smoking cessation 21 (n = 1), in conjunction with LCS. Another study reported that participants with greater nicotine dependence were significantly more likely to endorse concerns about quitting smoking. 34 There was no difference in motivation to quit by pack years in two studies, 28 , 35 though readiness and motivation to quit was associated with being less likely to smoke every day and having made a 24‐hour quit attempt in the last 7 days in one study. 35 | |
| Pre‐ or co‐existing psychological burden (n = 9) | Psychological (n = 5) | One study reported that for those with low distress at baseline, LDCT participants (versus non‐participants) had significantly higher distress 2 weeks post‐results. 20 However, for those with initial high distress, completing LDCT was not impactful, and overall distress remained high. 20 Pre‐existing concern about lung cancer was associated with anxiety about LCS (and consequential relief following LDCT results) in one qualitative study. 42 Three studies indicated that pre‐existing levels of anxiety and depression were associated with worse harm in other psychological outcomes at different points during LCS. 22 , 25 , 26 |
| Beliefs (n = 2) | Two studies reported a significant relationship between worse psychological outcomes (anxiety, depression and HRQoL) and higher absolute and comparative risk perception. 22 , 30 However, one study also reported no association between lung cancer worry and risk perception. 22 | |
| Smoking‐related (n = 5) | Worry or concern about lung cancer was significantly associated with readiness or motivation to quit smoking in two quantitative studies. 30 , 35 This relationship was qualitatively described in three studies; 42 , 44 , 45 though, the reported association was more varied. Psychological harm experienced during LCS appeared to motivate quitting smoking for some participants, while it had no effect for others 42 , 44 , 45 | |
| Calculated risk of lung cancer (n = 3) | Psychological (n = 2) | Calculated lung cancer risk had varying impacts on psychological outcomes across two studies, but primarily results indicated no significant relationship. 22 , 30 |
| Beliefs (n = 3) | Calculated lung cancer risk via PLCOm2012 was not associated with absolute or comparative risk in two studies, 22 , 31 but was associated with comparative risk in one study. 30 | |
| Beliefs | ||
| Lung cancer risk perception (n = 7) | Psychological (n = 4) | Two quantitative studies found that both comparative risk perception and affective risk perception (feelings about risk) resulted in more psychological burden. 22 , 29 Two qualitative studies, however, suggested that even though participants sometimes overestimated their risk of lung cancer, this did not cause distress (but noted that there were differences among participants). 42 , 44 |
| Beliefs (n = 1) | The only study looking at the relationships between absolute and comparative risk reported a significant association, where participants with higher perceived absolute risk were more likely to also perceive themselves to be at ‘higher’ comparative risk. 22 | |
| Smoking‐related (n = 4) | Two studies reported that those with higher perceived risk showed more motivation or intention to quit smoking (n = 2), 30 , 46 though one study suggested the inverse 35 and two studies suggested no relationship between risk perception and motivation to quit. 35 , 44 | |
|
Expectation of LDCT result (n = 4) |
Psychological (n = 3) | One study specifically measured how LDCT result expectations impacted psychological outcomes during LCS. 23 Participants who expected and received a negative (‘normal’) result had significantly lower concern than any other result expectation group 2 weeks after receiving results. 23 There was no significant difference in concern for those with an unexpected versus an expected ‘positive’ scan (i.e. requiring follow‐up). 23 Across qualitative studies, participants who received an unexpected abnormal result reported more psychological burden, 42 participants who received an unexpected ‘negative’ result felt relief and reduced stress 47 and being ‘psychologically prepared’ for a possible indeterminate result appeared to provide an emotional buffer. 42 |
| Smoking‐related (n = 1) | One qualitative study reported that having an unexpected ‘negative’ scan reportedly made participants ‘feel it was worth making a change’ and provided motivation to quit smoking. 45 | |
| Perception of health (n = 3) | Psychological (n = 1) | Lower perception of health was non‐significantly associated with fear of lung cancer, and significantly associated with anxiety of waiting for LDCT results in one study. 39 |
| Beliefs (n = 1) | There was no association between perception of health and lung cancer risk perception in one study. 39 | |
| Smoking‐related (n = 2) | Health perception was only significantly associated with motivation to quit in one study, but not readiness to quit. 35 In a qualitative study, those who downplayed the impact of tobacco use on health appeared to have lower motivation to quit smoking in the context of LCS. 48 | |
| Fatalism (n = 1) | Psychological (n = 1) | Qualitatively in one study, higher fatalism was reported to result in relief and other positive psychological responses following any type of LDCT result. 42 |
| Social (n = 1) | One qualitative study suggested that having a fatalistic outlook reduced seeking of social support. 42 | |
| Stigma (n = 1) | Psychological (n = 1) | Stigma from smoking appeared to drive higher worry and lack of reassurance from a ‘normal’ LDCT scan result in one qualitative study. 42 |
| Beliefs (n = 1) | A qualitative study reported a relationship between perceived stigma and fatalism, indicating that some participants felt ‘lucky’, while others felt that it was ‘only a matter of time’. 42 | |
| Perception of LCS (n = 1) | Psychological (n = 1) | One study found that perceived accuracy of LCS was not associated with perceived stress or anxiety. 41 |
| Other factors | ||
| Individual responses to COVID‐19 (n = 2) | Smoking‐related (n = 2) | Two qualitative studies described the pandemic as creating additional stress and anxiety that reduced motivation or readiness to quit smoking, 45 , 49 this was reported as both specific to smoking cessation interventions during LCS 45 and more generally. 49 However, for a minority, COVID‐19 increased motivation as people were more conscious of their smoking habits and could make changing smoking behaviour a priority. 49 |
| Informed decision‐making and knowledge (n = 2) | Psychological (n = 1) | One study reported that participants who did not make an informed decision to participate in LCS experienced no worse anxiety, HRQoL or lung cancer specific distress than subjects who did make an informed decision (including after receiving an indeterminate result). 50 |
| Beliefs (n = 1) | One study examined the impact of informed decision making on risk perception, finding that those with an informed decision had more accurate cognitive perceptions of risk, though there was no relationship with affective risk perception 50 | |
| Decision‐related (n = 1) | One study found a significant correlation between knowledge and decisional conflict. 32 | |
| Social factors (n = 2) | Psychological (n = 2) | Two qualitative studies reported on social factors. One suggested that social support provided an important ‘buffer’ of emotional support throughout the screening pathway. 42 Another suggested that having to disclose results to family members resulted in guilt and shame (possibly driven by internalised blame or stigma around smoking). 37 |
3.2. Psychological outcomes
Twenty‐four studies measured psychological outcomes. Across 10 studies, women were reported to have worse psychological outcomes than men during LCS, but these were not always clinically meaningful, 17 , 18 , 19 , 20 , 21 , 22 , 24 , 25 , 26 , 36 and some analyses (n = 6) reported no significant relationship with gender. 17 , 19 , 22 , 23 , 24 , 27 Younger age was associated with worse psychological outcomes in seven studies, 17 , 18 , 19 , 20 , 21 , 22 , 23 though most analyses (n = 9) found no significant differences by age. 17 , 18 , 19 , 22 , 24 , 25 , 26 , 27 , 28 Better psychological outcomes were found across four studies for those with higher levels of education 17 , 18 , 19 , 22 and married and co‐habiting participants, 17 , 19 , 20 , 26 though non‐significant associations were also reported in four studies for each factor. 17 , 19 , 20 , 21 , 22 , 23 , 39 Four studies found no differences in outcomes across race or ethnicity, 17 , 19 , 20 , 38 while worse outcomes for participants from African American 38 or Asian 21 ethnic backgrounds were reported in one study each.
Across 16 studies examining associations between smoking characteristics and psychological outcomes, seven studies reported worse outcomes for people who were currently smoking, 18 , 19 , 20 , 22 , 36 , 39 , 41 while nine studies reported no significant association with smoking status. 17 , 18 , 21 , 22 , 23 , 24 , 25 , 27 , 39 Similarly, higher pack years and a more pronounced smoking history were linked to increased psychological burden in some cases (n = 6), 18 , 22 , 28 , 36 , 40 , 42 but not others (n = 3). 18 , 22 , 25 Personal or family experience with cancer (including lung cancer) was significantly associated with worse psychological outcomes in half of the studies which examined the factor (n = 3). 20 , 23 , 40 Five studies reported the impact of pre‐existing or comorbid psychological burden, all of which indicated an association with psychological harm during LCS. 20 , 22 , 25 , 26 , 42 One study also suggested that for those with low distress at baseline, LDCT participants had higher distress than non‐participants after receiving results. 20
Findings on the impact of risk perception on psychological outcomes were mixed and appear to be individualised (n = 4). 22 , 29 , 42 , 44 Expectation of LDCT results impacted subsequent psychological outcomes differently across three studies. 23 , 42 , 47 One study suggested preparation for an ‘indeterminate’ or ‘abnormal’ LDCT result could reduce psychological burden, 42 but this had no reported effect in another study. 23 Those who received an expected ‘normal’ result reported the lowest concern in one study; 23 another described that if a ‘normal’ result was unexpected then this also led to relief and reduced stress. 47 The impact of perceived stigma and fatalistic health beliefs were examined in one qualitative study; while higher fatalism led to positive psychological outcomes after receiving results (relief and reassurance), smoking‐related stigma appeared to have the opposite effect. 42
One study reported that making an informed decision to participate in LCS had no impact on psychological outcomes, including after receiving an indeterminate result. 50 Two qualitative studies examined social factors, with one suggesting that social support provided a ‘buffer’ of emotional support throughout the LCS pathway, 42 and the other describing feelings of guilt and shame in having to inform family members (possibly driven by internalised blame or stigma around smoking). 37
3.3. Beliefs
3.3.1. Lung cancer risk perception
Risk perception was measured as an outcome in nine studies. Higher perceived lung cancer risk was associated with younger age 21 , 29 , 30 and female gender 21 , 22 , 30 in three studies each, though no relationship was reported in three 19 , 22 , 31 and four 19 , 22 , 29 , 31 studies, respectively. No association was reported between risk perception and race or ethnicity 19 , 21 , 38 (n = 3), income or deprivation level 21 , 39 (n = 2) or relationship status 19 , 21 (n = 2). Most studies reported no relationship with education level 22 , 39 (n = 2) as well, though one study reported those with higher education having lower risk perception. 19 Family history of lung cancer was associated with comparative risk perception (risk compared to others) in two studies, 22 , 30 but not absolute risk perception in one study. 22 Previous cancer was also not associated with risk perception in two studies. 22 , 30 The association between smoking status and risk perception varied, reported as worse for those currently smoking (n = 4), 19 , 21 , 22 , 39 those who had quit smoking (n = 1) 30 and not significantly different (n = 2). 22 , 29 Higher number of pack years was associated with higher perceived risk in one study, 30 but not in two others. 22 , 29
Two studies reported a significant relationship between increased risk perception and worse psychological outcomes (anxiety, depression and HRQoL), 22 , 30 but not lung cancer worry. 22 Calculated lung cancer risk via PLCOm2012 was not associated with risk perception in two studies, 22 , 31 but was in another. 30 One study examined the impact of informed decision‐making on risk perception, finding that those with an informed decision had more accurate cognitive perceptions of risk, though there was no relationship with perceived affective (feelings about) this risk. 50
3.3.2. Fatalism and perceptions of control
Three studies measured health beliefs related to fatalism and perceptions of control. Higher fatalism was linked to not being in a relationship or co‐habiting (n = 1), 21 being of African American (n = 1) 38 or Asian (n = 1) 21 ethnic backgrounds, and current smoking status (n = 1). 21 One high‐quality cross‐sectional study specifically measured other health beliefs, finding that more negative perceptions of personal control were associated with female gender, less affluence, current smoking status and Asian ethnicity. 21 The same study found that participants with Black and Asian ethnic backgrounds had more positive perceptions of the efficacy of lung cancer treatment, as did men, those who were older, and those who were married, co‐habiting or in a partnership. 21 Differences in perceptions of stigma were described in one qualitative study as moderating fatalistic outlooks. 42
3.3.3. Stigma
Four studies examined stigma, with two reporting that perceived stigma was more pronounced for women. 21 , 37 Younger age and White ethnicity were also associated with higher perceived stigma in one study. 21 Stigma related to current smoking status was described in two qualitative studies. 40 , 42 Further, significantly higher perceived stigma was reported by people who were currently smoking in another quantitative study. 21
3.4. Decision‐related psychosocial outcomes
Three studies measured decision‐related psychosocial outcomes. Most reported no significant difference in outcomes for age (n = 2), 32 , 33 level of education (n = 2), 32 , 33 employment status (n = 1) 32 or gender (n = 2). 32 , 33 People who had quit smoking were more likely to report worse decisional outcomes compared with people who were currently smoking in two studies, 27 , 33 though a significant relationship was only reported in one. 33 White 32 , 33 (n = 2) and Hispanic 33 (n = 1) participants were reported as being less likely to experience decisional conflict about LCS than other ethnicities. One study reported that the number of times screened for lung cancer, or time since last scan, was not related to decisional outcomes. 32
3.5. Smoking‐related psychosocial outcomes
Smoking‐related psychosocial outcomes were assessed in 14 studies. Most analyses reported no significant relationship between smoking‐related outcomes and age (n = 3), 31 , 34 , 35 gender (n = 4) 21 , 31 , 34 , 35 or race/ethnicity (n = 3), 21 , 34 , 35 except women being more likely to endorse concerns about weight gain after quitting and African American participants being less likely to endorse concerns about nicotine withdrawal symptoms in one study. 34 Current smoking status was associated with more smoking worry 43 (n = 1) and a higher intention to quit 43 (n = 1), but lower confidence in quitting 43 (n = 1) and lower perceived efficacy of smoking cessation 21 (n = 1), in conjunction with LCS. There was no difference in motivation to quit by pack years in two studies. 28 , 35
Worry or concern about lung cancer was associated with readiness or motivation to quit smoking in five studies, 30 , 35 , 42 , 45 , 51 though with varying impacts. For some participants, psychological harm experienced during LCS appeared to motivate quitting smoking, 30 , 35 , 45 while it had no (or the opposite) effect for others. 42 , 44 , 45 Two studies reported that those with higher perceived risk showed more motivation or intention to quit smoking (n = 2), 30 , 46 though one study suggested the inverse 35 and another two studies suggested no relationship between risk perception and motivation to quit. 35 , 44 Two qualitative studies described the COVID‐19 pandemic as creating additional stress and anxiety that reduced motivation or readiness to quit smoking; 45 , 49 this was reported as both specific to smoking cessation interventions during LCS 45 and more generally. 49
3.6. Social outcomes
Social outcomes were only described in one qualitative study, which reported that a fatalistic outlook reduced seeking of social support. 42
4. DISCUSSION
This review found that several participant factors were consistently reported to be associated with psychosocial outcomes in LCS. However, associations were not consistent across studies and non‐significant findings also frequently reported. This may be due to bias in study designs and lack of power to show associations; indeed, small and/or unjustified sample sizes were an issue across many quantitative studies in risk of bias assessment. Many of the factors and outcomes examined were also interrelated, which should be considered in interpretation of findings. Sociodemographic and smoking‐related characteristics were the most frequently examined factors. Correlations between younger age, female gender, and current smoking status, and increased psychological burden were commonly reported. Similar findings were reported recently in the Watch the Spot trial (which included those with both screening and incidentally detected nodules). 52 Links with pre‐existing or comorbid psychological harm, expectation of LDCT results and other beliefs were also found, though were examined in fewer studies and were more nuanced. While this reviews' results are limited by study quality (as systematically assessed in our work), they provide a basis for understanding who may be at risk of psychological harm during LCS based on all available evidence. These findings are also useful when considering psychological barriers to LCS uptake and developing campaigns or strategies to engage certain groups. 5
Considering the evidence for risk factors associated with psychological harm found in this review, interventions to manage psychosocial experiences during LCS should not be ‘one size fits all’. Different assessment, referral and intervention pathways which target specific factors or at‐risk groups should be considered in LCS programme design. For example, people who live alone or do not have support systems could be assessed and referred to an intervention focused at providing social support, which have been shown to improve quality of life for lung cancer patients. 53 Acknowledging that most participants in LCS do not experience clinically significant psychological burden, avoiding a blanket universal intervention approach may also have cost‐effectiveness benefits, 54 , 55 especially considering participation volumes in larger screening programmes.
A key factor identified in our review was pre‐existing or comorbid psychological burden, which appears to significantly predict other psychological outcomes during LCS. Measuring participants' anxiety or distress at baseline participation in a LCS programme could therefore be critical in identifying participants who may experience psychological harm. For those with a cancer diagnosis, distress screening is part of the standard of care and there is a myriad of practice guidelines, 56 though none extend to cancer screening. While measuring distress at every LCS visit may not be feasible or useful with the number of participants in a screening programme, strategies to enable participants to easily communicate distress, and for providers to easily identify it, at every touchpoint could be considered. As providers are often time‐poor during shared decision‐making discussions, 57 approaches to assess participant distress without adding to clinician time burden may be useful. Pre‐completion of patient‐reported outcome or experience measures, or assessment of psychological outcomes by a nurse prior could be beneficial. Programme coordinators or ‘navigators’ have also been successful in providing engagement and support for participants during LCS, so involving them in distress screening and management could also be an option. 58 , 59 Appropriate triage and referral pathways for those experiencing psychosocial harm are also needed, which would require programme‐level set‐up and resourcing. 56
Findings on factors and outcomes associated with risk perception in this review were varied. This likely reflects the complexity of risk comprehension and the integral role of personal, cultural and social biases. With this in mind, it is important to delineate between absolute (how likely are you to get lung cancer?) and comparative (how likely are you to get lung cancer compared to someone similar to you?) risk perception. 60 Comparative risk perception has a direct social focus and therefore may be more tied up in cognitive biases and have other consequences for psychosocial outcomes. 61 This difference was often not articulated in studies included in this review, with ‘risk perception’ used as an umbrella term for both concepts. Further research should investigate the differences in absolute and comparative risk perception on psychosocial outcomes specifically for LCS, especially considering the unique personal and social complexities around smoking behaviour and stigma in LCS. Findings from the Manchester Lung Health Check pilot suggest that provision of comparative (rather than absolute) risk could support better risk understanding, however it was flagged that further research was required to determine the optimal approach to risk communication in the LCS setting. 22 The general population almost always significantly overestimate their cancer risk, and while evidence shows that risk information can help align perceptions closer to actual estimates, overestimations remain. 31 , 61 In addition, there is evidence that people who have stopped smoking underestimate their lung cancer risk due to perceived protective health benefits of having quit. 62 These enduring inaccurate conceptualisations of risk are possible drivers behind the nil or positive impacts of personalised cancer risk information (PCRI) seen for psychological outcomes in the broader literature. 61 This further necessitates research on how to effectively communicate risk in LCS, and to understand the true psychosocial effects.
The harms of stigma in cancer and non‐communicable respiratory diseases are well‐documented and include psychosocial burden, delays in medical help‐seeking behaviours and reduced participation in early detection activities. 63 , 64 , 65 Lung cancer stigma is especially pervasive owing to decades of public focus on its links with smoking, 63 , 66 and the narrative of smoking being an individual ‘choice’ rather than an addiction. Most work to date on stigma in lung cancer has focused on those with a diagnosis, with less evidence on the impact of stigma in earlier stages of the lung cancer continuum or as early as the LCS pathway. Recent qualitative studies suggest that stigma is present during LCS and that it can act as a barrier to participation in screening, 37 , 67 , 68 but there is limited evidence on the psychosocial consequences of stigma on screening, as identified by this review. Some studies referenced internalised shame, blame and guilt around smoking behaviour, 40 , 42 but there was little exploration of the impacts of interpersonal or societal level stigma. 63 A detailed understanding of how and where stigma emerges during the LCS pathway is needed to design appropriate strategies to address it.
4.1. Limitations
There are limitations to address in this review. Critically, the findings from this review are based on a heterogeneous group of studies with varying methodological quality, with risk of bias assessed as moderate or high in 33 of 35 included studies. There was variation in study design and measurement of factors and outcomes of interest. Most studies were completed in trial settings or at a single site, limiting generalisability of conclusions to real‐world groups who would be participating in LCS. Selection bias and coverage bias were also common across quantitative studies.
5. CONCLUSION
This review provides the first comprehensive synthesis of participant‐level factors associated with psychological burden for LCS participants. These findings, despite the acknowledged limitations of the existing evidence, point to a clear association between certain individual factors and psychosocial impacts during LCS. Sociodemographic and health risk factors—for example, age, gender and smoking status—were the most examined in this review and are easily identifiable among participants in an LCS programme context. However, the evidence for these factors was mixed in terms of significant and non‐significant relationships reported. Some factors—particularly beliefs and expectations—were examined less frequently, and these factors may require more time and effort to elucidate during LCS, but could serve as more refined predictors of psychological harm and warrant further research. Importantly, the interplay of different participant factors in the LCS context should be considered when designing strategies to manage psychosocial experiences.
AUTHOR CONTRIBUTIONS
Kathleen McFadden: Conceptualization (equal); data curation (lead); formal analysis (lead); investigation (equal); methodology (equal); project administration (lead); writing – original draft (lead). Brooke Nickel: Conceptualization (equal); data curation (equal); methodology (equal); supervision (equal); writing – review and editing (equal). Nicole M. Rankin: Conceptualization (equal); methodology (equal); supervision (equal); writing – review and editing (equal). Tong Li: Data curation (equal). Chloe J. Jennett: Data curation (equal). Ashleigh Sharman: Data curation (equal). Samantha L. Quaife: Conceptualization (equal); writing – review and editing (equal). Nehmat Houssami: Conceptualization (equal); methodology (equal); supervision (lead); writing – review and editing (equal). Rachael H. Dodd: Conceptualization (equal); methodology (equal); supervision (lead); writing – review and editing (equal).
FUNDING INFORMATION
KM is supported by The Daffodil Centre Postgraduate Research Scholarship. NH is supported by the National Breast Cancer Foundation (NBCF) Chair in Breast Cancer Prevention grant (EC‐21‐001) and a NHMRC Investigator (Leader) grant (1194410). BN is supported by a NHMRC Investigator Emerging Leader Research Fellowship (1194108). TL is supported by a Cancer Institute NSW Early Career Fellowship (#2022/ECF1420). CJJ is supported by The Daffodil Centre Postgraduate Research Scholarship. SLQ is supported by a Barts Charity programme grant (G‐001522, MGU0461).
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Supporting information
Data S1.
ACKNOWLEDGMENTS
The funder did not play a role in the design of the study; the collection, analysis and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
McFadden K, Nickel B, Rankin NM, et al. Participant factors associated with psychosocial impacts of lung cancer screening: A systematic review. Cancer Med. 2024;13:e70054. doi: 10.1002/cam4.70054
Rachael H. Dodd and Nehmat Houssami should be considered joint senior authors.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. doi: 10.3322/CAAC.21660 [DOI] [PubMed] [Google Scholar]
- 2. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung‐cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503‐513. doi: 10.1056/NEJMOA1911793/SUPPL_FILE/NEJMOA1911793_DATA-SHARING.PDF [DOI] [PubMed] [Google Scholar]
- 3. National Lung Screening Trial Research Team , Aberle DR, Adams AM, et al. Reduced lung‐cancer mortality with low‐dose computed tomographic screening. N Engl J Med. 2011;365(5):395‐409. doi: 10.1056/NEJMOA1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quaife SL, Janes SM, Brain KE. The person behind the nodule: a narrative review of the psychological impact of lung cancer screening. Transl Lung Cancer Res. 2021;10(5):2427‐2440. doi: 10.21037/TLCR-20-1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carter‐Harris L. Hidden in plain sight: psychological barriers to participation in lung cancer screening. Thorax. 2020;75(12):1033‐1034. doi: 10.1136/thoraxjnl-2020-216191 [DOI] [PubMed] [Google Scholar]
- 6. Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307(22):2418‐2429. doi: 10.1001/JAMA.2012.5521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carter‐Harris L, Ostroff J. In: Breitbart W, Butow P, Jacobsen P, Lam W, Lazenby M, Loscalzo M, eds. Psycho‐Oncology. Oxford University Press; 2021. doi: 10.1093/med/9780190097653.001.0001 [DOI] [Google Scholar]
- 8. Wu GX, Raz DJ, Brown L, Sun V. Psychological burden associated with lung cancer screening: a systematic review. Clin Lung Cancer. 2016;17(5):315‐324. doi: 10.1016/J.CLLC.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rasmussen JF, Siersma V, Malmqvist J, Brodersen J. Psychosocial consequences of false positives in the Danish lung cancer CT screening trial: a nested matched cohort study. BMJ Open. 2020;10(6):e034682. doi: 10.1136/BMJOPEN-2019-034682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clark ME, Young B, Bedford LE, et al. Lung cancer screening: does pulmonary nodule detection affect a range of smoking behaviours? J Public Health (Bangkok). 2019;41(3):600‐608. doi: 10.1093/PUBMED/FDY158 [DOI] [PubMed] [Google Scholar]
- 11. Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153(4):1004‐1015. doi: 10.1016/J.CHEST.2017.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ali N, Lifford KJ, Carter B, et al. Barriers to uptake among high‐risk individuals declining participation in lung cancer screening: a mixed methods analysis of the UK lung cancer screening (UKLS) trial. BMJ Open. 2015;5(7):e008254. doi: 10.1136/BMJOPEN-2015-008254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quaife SL, Marlow LAV, McEwen A, Janes SM, Wardle J. Attitudes towards lung cancer screening in socioeconomically deprived and heavy smoking communities: informing screening communication. Health Expect. 2017;20(4):563‐573. doi: 10.1111/HEX.12481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cavers D, Nelson M, Rostron J, et al. Understanding patient barriers and facilitators to uptake of lung screening using low dose computed tomography: a mixed methods scoping review of the current literature. Respir Res. 2022;23(1):1‐18. doi: 10.1186/S12931-022-02255-8/TABLES/2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Joanna Briggs Institute . Critical appraisal tools. 2022. Accessed December 7, 2022. https://jbi.global/critical‐appraisal‐tools.
- 16. Page MJ, McKenzie JE, Bossuyt PM, et al. Statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:372. doi: 10.1136/BMJ.N71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kummer S, Waller J, Ruparel M, Duffy SW, Janes SM, Quaife SL. Psychological outcomes of low‐dose CT lung cancer screening in a multisite demonstration screening pilot: the lung screen uptake trial (LSUT). Thorax. 2020;75(12):1065‐1073. doi: 10.1136/thoraxjnl-2020-215054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Den Bergh KAM, Essink‐Bot ML, Borsboom GJJM, Scholten ET, Van Klaveren RJ, De Koning HJ. Long‐term effects of lung cancer computed tomography screening on health‐related quality of life: the NELSON trial. Eur Respir J. 2011;38(1):154‐161. doi: 10.1183/09031936.00123410 [DOI] [PubMed] [Google Scholar]
- 19. Byrne MM, Weissfeld J, Roberts MS. Anxiety, fear of cancer, and perceived risk of cancer following lung cancer screening. Med Decis Mak. 2008;28(6):917‐925. doi: 10.1177/0272989X08322013 [DOI] [PubMed] [Google Scholar]
- 20. Brain K, Lifford KJ, Carter B, et al. Long‐term psychosocial outcomes of low‐dose CT screening: results of the UK lung cancer screening randomised controlled trial. Thorax. 2016;71(11):996‐1005. doi: 10.1136/THORAXJNL-2016-208283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quaife SL, Waller J, Dickson JL, et al. Psychological targets for lung cancer screening uptake: a prospective longitudinal cohort study. J Thorac Oncol. 2021;16(12):2016‐2028. doi: 10.1016/j.jtho.2021.07.025 [DOI] [PubMed] [Google Scholar]
- 22. Lebrett MB, Crosbie EJ, Yorke J, et al. Risk perception and disease knowledge in attendees of a community‐based lung cancer screening programme. Lung Cancer. 2022;168:1‐9. doi: 10.1016/J.LUNGCAN.2022.04.003 [DOI] [PubMed] [Google Scholar]
- 23. Dunn CE, Edwards A, Carter B, Field JK, Brain K, Lifford KJ. The role of screening expectations in modifying short–term psychological responses to low‐dose computed tomography lung cancer screening among high‐risk individuals. Patient Educ Couns. 2017;100(8):1572‐1579. doi: 10.1016/J.PEC.2017.02.024 [DOI] [PubMed] [Google Scholar]
- 24. Van Den Bergh KAM, Essink‐Bot ML, Bunge EM, et al. Impact of computed tomography screening for lung cancer on participants in a randomized controlled trial (NELSON trial). Cancer. 2008;113(2):396‐404. doi: 10.1002/CNCR.23590 [DOI] [PubMed] [Google Scholar]
- 25. Taghizadeh N, Tremblay A, Cressman S, et al. Health‐related quality of life and anxiety in the PAN‐CAN lung cancer screening cohort. BMJ Open. 2019;9(1):e024719. doi: 10.1136/BMJOPEN-2018-024719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaerlev L, Iachina M, Pedersen JH, Green A, Nørgård BM. CT‐screening for lung cancer does not increase the use of anxiolytic or antidepressant medication. BMC Cancer. 2012;12:12. doi: 10.1186/1471-2407-12-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Byrne MM, Lillie SE, Studts JL. Lung cancer screening in a community setting: characteristics, motivations, and attitudes of individuals being screened. Health Psychol Open. 2019;6(1):205510291881916. doi: 10.1177/2055102918819163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee J, Kim Y, Kim HY, et al. Feasibility of implementing a national lung cancer screening program: interim results from the Korean lung cancer screening project (K‐LUCAS). Transl Lung Cancer Res. 2021;10(2):723‐736. doi: 10.21037/TLCR-20-700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bunge EM, van den Bergh KAM, Essink‐Bot ML, van Klaveren RJ, de Koning HJ. High affective risk perception is associated with more lung cancer‐specific distress in CT screening for lung cancer. Lung Cancer. 2008;62(3):385‐390. doi: 10.1016/J.LUNGCAN.2008.03.029 [DOI] [PubMed] [Google Scholar]
- 30. Turner J, Pond GR, Tremblay A, et al. Risk perception among a lung cancer screening population. Chest. 2021;160(2):718‐730. doi: 10.1016/J.CHEST.2021.02.050 [DOI] [PubMed] [Google Scholar]
- 31. Han PKJ, Lary C, Black A, et al. Effects of personalized risk information on patients referred for lung cancer screening with low‐dose CT. Med Decis Mak. 2019;39(8):950‐961. doi: 10.1177/0272989X19875966/ASSET/IMAGES/LARGE/10.1177_0272989X19875966-FIG2.JPEG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eberth JM, Zgodic A, Pelland SC, Wang SY, Miller DP. Outcomes of shared decision‐making for low‐dose screening for lung cancer in an Academic Medical Center. J Cancer Educ. 2022;30:1‐16. doi: 10.1007/S13187-022-02148-W/TABLES/5 [DOI] [PubMed] [Google Scholar]
- 33. Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision‐making for lung cancer screening: how well are we “Sharing”? Chest. 2021;160(1):330‐340. doi: 10.1016/J.CHEST.2021.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bold KW, Cannon S, Ford BB, et al. Examining tobacco treatment perceptions and barriers among Black versus non‐Black adults attending lung cancer screening. Cancer Prev Res. 2022;15:327‐334. doi: 10.1158/1940-6207.CAPR-21-0398/675657/AM/EXAMINING-TOBACCO-TREATMENT-PERCEPTIONS-AND [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Williams RM, Cordon M, Eyestone E, et al. Improved motivation and readiness to quit shortly after lung cancer screening: evidence for a teachable moment. Cancer. 2022;128(10):1976‐1986. doi: 10.1002/CNCR.34133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Den Bergh KAM, Essink‐Bot ML, Borsboom GJJM, et al. Short‐term health‐related quality of life consequences in a lung cancer CT screening trial (NELSON). Br J Cancer. 2010;102(1):27‐34. doi: 10.1038/SJ.BJC.6605459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olson RE, Goldsmith L, Winter S, et al. Emotions and lung cancer screening: Prioritising a humanistic approach to care. Health Soc Care Community. 2022;30(6):e5259‐e5269. doi: 10.1111/HSC.13945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barta JA, Shusted CS, Ruane B, et al. Racial differences in lung cancer screening beliefs and screening adherence. Clin Lung Cancer. 2021;22(6):570‐578. doi: 10.1016/J.CLLC.2021.06.003 [DOI] [PubMed] [Google Scholar]
- 39. Lillie SE, Fu SS, Fabbrini AE, et al. What factors do patients consider most important in making lung cancer screening decisions? Findings from a demonstration project conducted in the veterans health administration. Lung Cancer. 2017;104:38‐44. doi: 10.1016/J.LUNGCAN.2016.11.021 [DOI] [PubMed] [Google Scholar]
- 40. Greene PA, Sayre G, Heffner JL, et al. Challenges to educating smokers about lung cancer screening: a qualitative study of decision making experiences in primary care. J Cancer Educ. 2019;34(6):1142‐1149. doi: 10.1007/S13187-018-1420-Y [DOI] [PubMed] [Google Scholar]
- 41. Hall DL, Lennes IT, Carr A, Eusebio JR, Yeh GY, Park ER. Lung cancer screening uncertainty among patients undergoing LDCT. Am J Health Behav. 2018;42(1):69‐76. doi: 10.5993/AJHB.42.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kummer S, Waller J, Ruparel M, Cass J, Janes SM, Quaife SL. Mapping the spectrum of psychological and behavioural responses to low‐dose CT lung cancer screening offered within a lung health check. Health Expect. 2020;23(2):433‐441. doi: 10.1111/hex.13030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Balata H, Traverse‐Healy L, Blandin‐Knight S, et al. Attending community‐based lung cancer screening influences smoking behaviour in deprived populations. Lung Cancer. 2020;139:41‐46. doi: 10.1016/J.LUNGCAN.2019.10.025 [DOI] [PubMed] [Google Scholar]
- 44. Golden SE, Ono SS, Melzer A, et al. “I already know that smoking ain't good for me”: patient and clinician perspectives on lung cancer screening decision‐making discussions as a teachable moment. Chest. 2020;158(3):1250‐1259. doi: 10.1016/J.CHEST.2020.03.061 [DOI] [PubMed] [Google Scholar]
- 45. Buttery SC, Williams P, Mweseli R, et al. Immediate smoking cessation support versus usual care in smokers attending a targeted lung health check: the QuLIT trial. BMJ Open Respir Res. 2022;9(1):e001030. doi: 10.1136/BMJRESP-2021-001030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ostroff JS, Buckshee N, Mancuso CA, Yankelevitz DF, Henschke CI. Smoking cessation following CT screening for early detection of lung cancer. Prev Med (Baltim). 2001;33(6):613‐621. doi: 10.1006/PMED.2001.0935 [DOI] [PubMed] [Google Scholar]
- 47. Zeliadt SB, Heffner JL, Sayre G, et al. Attitudes and perceptions about smoking cessation in the context of lung cancer screening. JAMA Intern Med. 2015;175(9):1530‐1537. doi: 10.1001/JAMAINTERNMED.2015.3558 [DOI] [PubMed] [Google Scholar]
- 48. Kathuria H, Koppelman E, Borrelli B, et al. Patient‐physician discussions on lung cancer screening: a missed teachable moment to promote smoking cessation. Nicotine Tob Res. 2020;22(3):431‐439. doi: 10.1093/NTR/NTY254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cordon M, Eyestone E, Hutchison S, et al. A qualitative study exploring older smokers' attitudes and motivation toward quitting during the COVID‐19 pandemic. Prev Med Rep. 2021;22:101359. doi: 10.1016/J.PMEDR.2021.101359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van Den Bergh KAM, Essink‐Bot ML, Van Klaveren RJ, De Koning HJ. Informed decision making does not affect health‐related quality of life in lung cancer screening (NELSON trial). Eur J Cancer. 2010;46(18):3300‐3306. doi: 10.1016/J.EJCA.2010.05.030 [DOI] [PubMed] [Google Scholar]
- 51. Golden SE, Schweiger L, Melzer AC, et al. “It's a decision I have to make”: patient perspectives on smoking and cessation after lung cancer screening decisions. Prev Med Rep. 2022;30:102014. doi: 10.1016/J.PMEDR.2022.102014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gould MK, Creekmur B, Qi L, et al. Emotional distress, anxiety and general health status in patients with small pulmonary nodules shortly after their identification: results from the watch the spot trial. Chest. 2023;164:1560‐1571. doi: 10.1016/j.chest.2023.06.022 [DOI] [PubMed] [Google Scholar]
- 53. Hofman A, Zajdel N, Klekowski J, Chabowski M. Improving social support to increase Qo, in lung cancer patients. Cancer Manag Res. 2021;13:2319‐2327. doi: 10.2147/CMAR.S278087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jansen F, van Zwieten V, Coupé VM, Leemans CR, Verdonck‐de Leeuw IM. A review on cost‐effectiveness and cost‐utility of psychosocial care in cancer patients. Asia Pac J Oncol Nurs. 2016;3(2):125‐136. doi: 10.4103/2347-5625.182930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Le LKD, Esturas AC, Mihalopoulos C, et al. Cost‐effectiveness evidence of mental health prevention and promotion interventions: a systematic review of economic evaluations. PLoS Med. 2021;18(5):e1003606. doi: 10.1371/JOURNAL.PMED.1003606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Donovan KA, Grassi L, Deshields TL, Corbett C, Riba MB. Advancing the science of distress screening and management in cancer care. Epidemiol Psychiatr Sci. 2020;29:e85. doi: 10.1017/S2045796019000799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tanner NT, Silvestri GA. Shared decision‐making and lung cancer screening: Let's get the conversation started. Chest. 2019;155(1):21‐24. doi: 10.1016/J.CHEST.2018.10.013 [DOI] [PubMed] [Google Scholar]
- 58. Baggett TP, Barbosa Teixeira J, Rodriguez EC, et al. Patient navigation to promote lung cancer screening in a community health center for people experiencing homelessness: protocol for a pragmatic randomized controlled trial. Contemp Clin Trials. 2022;113:106666. doi: 10.1016/J.CCT.2021.106666 [DOI] [PubMed] [Google Scholar]
- 59. Darling GE, Tammemägi MC, Schmidt H, et al. Organized lung cancer screening pilot: informing a province‐wide program in Ontario, Canada. Ann Thorac Surg. 2021;111(6):1805‐1811. doi: 10.1016/J.ATHORACSUR.2020.07.051 [DOI] [PubMed] [Google Scholar]
- 60. Zajac LE, Klein WMP, McCaul KD. Absolute and comparative risk perceptions as predictors of cancer worry: moderating effects of gender and psychological distress. J Health Commun. 2007;11(SUPPL. 1):37‐49. doi: 10.1080/10810730600637301 [DOI] [PubMed] [Google Scholar]
- 61. Bayne M, Fairey M, Silarova B, et al. Effect of interventions including provision of personalised cancer risk information on accuracy of risk perception and psychological responses: a systematic review and meta‐analysis. Patient Educ Couns. 2020;103(1):83‐95. doi: 10.1016/J.PEC.2019.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Park ER, Streck JM, Gareen IF, et al. A qualitative study of lung cancer risk perceptions and smoking beliefs among national lung screening trial participants. Nicotine Tob Res. 2014;16(2):166‐173. doi: 10.1093/NTR/NTT133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hamann HA, Ver Hoeve ES, Carter‐Harris L, Studts JL, Ostroff JS. Multilevel opportunities to address lung cancer stigma across the cancer control continuum. J Thorac Oncol. 2018;13(8):1062‐1075. doi: 10.1016/J.JTHO.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rose S, Paul C, Boyes A, Kelly B, Roach D. Stigma‐related experiences in non‐communicable respiratory diseases: a systematic review. Chron Respir Dis. 2017;14(3):199‐216. doi: 10.1177/1479972316680847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Akin‐Odanye EO, Husman AJ. Impact of stigma and stigma‐focused interventions on screening and treatment outcomes in cancer patients. Ecancermedicalscience. 2021;15:15. doi: 10.3332/ECANCER.2021.1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Marlow LAV, Waller J, Wardle J. Does lung cancer attract greater stigma than other cancer types? Lung Cancer. 2015;88(1):104‐107. doi: 10.1016/J.LUNGCAN.2015.01.024 [DOI] [PubMed] [Google Scholar]
- 67. Carter‐Harris L, Ceppa DKP, Hanna N, Rawl SM. Lung cancer screening: what do long‐term smokers know and believe? Health Expect. 2017;20(1):59‐68. doi: 10.1111/HEX.12433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dunlop KLA, Marshall HM, Stone E, et al. Motivation is not enough: a qualitative study of lung cancer screening uptake in Australia to inform future implementation. PLoS One. 2022;17(9):e0275361. doi: 10.1371/JOURNAL.PONE.0275361 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


