Abstract
Background
Secondary peritonitis is associated with a high mortality rate and if not treated successfully leads to development of abscesses, severe sepsis and multi‐organ failure. Source control and adjunctive antibiotics are the mainstay of treatment. However, no conclusive evidence suggest that one antibiotic regimen is better than any other but at the same time have a lower toxicity.
Objectives
To ascertain the efficacy and adverse effects of different antibiotic regimens in treating intra‐abdominal infections in adults. Outcomes were divided into primary (clinical success and effectiveness in reducing mortality) and secondary (microbiological success, preventing wound infection, intra‐abdominal abscess, clinical sepsis, remote infection, superinfection, adverse reactions, duration of treatment required, effectiveness in reducing hospitalised stay, and time to defervescence).
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library, Issue 4, 2004), MEDLINE (from 1966 to November 2004), EMBASE (from 1980 to November 2004) and Cochrane Colorectal Cancer Group specialised register SR‐COLOCA. Bibliographies of identified studies were screened for further relevant trials.
Selection criteria
Randomised and quasi‐randomised controlled trials comparing different antibiotic regimens in the treatment of secondary peritonitis in adults were selected. Trials reporting gynaecological or traumatic peritonitis were excluded from this review. Ambiguity regarding suitability of trials were discussed among the review team.
Data collection and analysis
Six reviewers independently assessed trial quality and extracted data. Data collection was standardised using data collection form to ensure uniformity among reviewers. Statistical analyses were performed using the random effects model and the results expressed as odds ratio for dichotomous outcomes, or weight mean difference for continuous data with 95% confidence intervals.
Main results
Fourty studies with 5094 patients met the inclusion criteria. Sixteen different comparative antibiotic regimens were reported. All antibiotics showed equivocal comparability in terms of clinical success. Mortality did not differ between the regimens. Despite the potential high toxicity profile of regimens using aminoglycosides, this was not demonstrated in this review. The reason for this could be the inherent bias within clinical trials in the form of patient selection and stringency in monitoring drug levels.
Authors' conclusions
No specific recommendations can be made for the first line treatment of secondary peritonitis in adults with antibiotics, as all regimens showed equivocal efficacy. Other factors such as local guidelines and preferences, ease of administration, costs and availability must therefore be taken into consideration in deciding the antibiotic regimen of choice. Future trials should attempt to stratify patients and perform intention‐to‐treat analysis to allow better external validity.
Keywords: Humans, Anti‐Bacterial Agents, Anti‐Bacterial Agents/therapeutic use, Intestinal Perforation, Intestinal Perforation/complications, Peritonitis, Peritonitis/drug therapy, Peritonitis/etiology, Peritonitis/mortality, Randomized Controlled Trials as Topic
Plain language summary
Antibiotics are effective in preventing post‐operative complications following infection of the peritoneum (peritonitis), but there is no evidence to support that one regimen is superior to another, and at the same time has less side effects.
Patients with peritonitis originated from the gut will often require surgery. Antibiotics are useful in the treatment of the ongoing infection and for prevention of post‐operative complications. This review does not result in specific recommendations for any antibiotic regimen for the first line treatment of secondary peritonitis in adults, as all regimens showed equivocal efficacy. Other factors such as local guidelines and preferences, ease of administration, costs and availability must therefore be taken into consideration in deciding the antibiotic regimen of choice. More large scale trials are needed, and future trials should attempt to stratify patients and perform intention‐to‐treat analysis to allow better external validity.
Background
Secondary peritonitis, which is defined as inflammation of the peritoneum secondary to perforation of a hollow viscus or transmural necrosis of gastrointestinal tract, is associated with a high mortality rate (Wittmann 1996; Bosscha 1999; Tellado 2000). To serve as an example, patients with large bowel perforation have mortality rates varying from 20% to 60% (Wittmann 1990; Christou 1993; Ohmann 1993; Mc Lauchlan 1995; Pacelli 1996). Peritonitis is the initial phase of infection which, if not treated, is followed by formation of an abscess, as the body successfully localises peritoneal contamination. These severe abdominal infections are invariably accompanied by a high level of sepsis, endotoxin production and systemic inflammatory response syndrome (SIRS), which often results in multiple organ failure (Bohnen 1983).
Surgical eradication of the infectious focus (source control) is the most important prerequisite for a successful treatment (Tellado 2000; Schein 2002). Timely surgical intervention aims to eliminate the source of contamination, reduce the microbial inoculum and prevent the development of persistent sepsis (Bosscha 1999), and these can be achieved by drainage of all fluid collections, closure or resection of any openings from the gastrointestinal tract and resection of inflamed and necrotic tissue.
Judicious use of appropriate antibiotics in peritonitis serves as an adjunctive treatment to surgical intervention (Bohnen 1992). Antibiotic therapy was first introduced in the 1960s, however mortality did not improve following their use until better understanding of the pathophysiology of these infections, screening techniques, intensive care and resuscitation, and use of appropriate antimicrobial drugs were developed in the 1990s (Tellado 2000). Even the best antimicrobial agent, however, has little efficacy if used without an effort to gain adequate source control.
The site of the gut perforation influences which pathogens are implicated. The flora within the small bowel consist mainly of enterococci and Escherichia coli. The distal small bowel lumen contains progressively increasing number of Enterobacteriaceae and anaerobic organisms, including the Bacteroides group. Within the colon, the bacteria population is very high and anaerobes (i.e. Peptostreptococcus, Clostridium, and most commonly Bacteroides species) outnumber aerobes.
The polymicrobial nature of the gastrointestinal tract therefore demands use of antibiotics which cover aerobic, facultative anaerobic Enterobacteriaceae and anaerobic organisms, particularly Bacteroides fragilis (Nichols 1992). Antimicrobial therapy is often empirical, as treatment is started before diagnosis can be firmly established at surgery (Bohnen 1992; Holzheimer 2001; Mazuski 2002a). This has been accomplished by the use of a number of regimens either in single or combinations of antimicrobials. For many years, the antibiotic therapy of choice for patients with mixed intra‐abdominal infections and peritonitis has been a combination regimen ‐ an aminoglycoside to cover the aerobic and facultative organisms combined with an additional agent effective against anaerobic bacteria. Despite toxic drawbacks, aminoglycoside‐based combination therapy is highly successful against mixed flora in intra‐abdominal infection. However, the potential toxicity of these aminoglycosides has provided incentive for the development of alternative drug therapies using single agents. Nevertheless, combination therapy still remain a popular choice as it not only acts to broaden the antimicrobial spectrum, but also to achieve enhanced bacterial killing by synergism and to prevent the emergence of antibiotic resistance.
The challenges for adjunctive antimicrobial treatment in the surgical management of severe intra‐abdominal infections are therefore threefold ‐ to provide an effective spectrum against mixed aerobic and anaerobic pathogens; to achieve therapeutic serum concentrations before operation; and to avoid important side‐effects such as nephrotoxicity. There is, however, no strong evidence to identify one regimen as being more efficacious than another and at the same time have the least acceptable side‐effects. Recent reviews on antibiotics and intra‐abdominal infections (Holzheimer 2001; Mazuski 2002a; Mazuski 2002b) have further highlighted these problems and the inadequacies where current evidence is lacking.
Treatment failure is often associated with the cause and extent of the initial infection as well as the response of the host to that infection. Useful tools for identifying patients at increased risk of adverse outcome following the insult are the APACHE II (Knaus 1985; Mulier 2003) and POSSUM severity scoring system (Jones 1992; Copeland 2002). Identifying these high risk patients can often guide the clinician towards a more aggressive approach and use of broader spectrum antimicrobial regimens. However, the latter may put this particular group of patients at an increased risk of toxicity from the agents used.
The course of the disease is thus influenced by the physiological reserve of the patient, perioperative optimisation, the severity of the underlying pathology, success of the operation and subsequent management and complications. These factors generate further controversy concerning the optimal antibiotic therapy.
There have been a vast expansion and development in antibiotic regimens over the last decade, many of which are costly. This review is therefore strategically timed and will aim to scrutinise the clinical effectiveness and toxicity of the regimens and provide the evidence required for guiding practitioners in treating secondary peritonitis with systemic parenteral antibiotics.
Objectives
1. The primary aim of this review was to assess the adequacy of the antibiotic regimens in eradicating initial sepsis and the need for subsequent interventions to eradicate peritoneal sepsis. As part of the review, mortality associated with the initial pathology was also be assessed and correlated to the efficacy of the different antibiotic regimens.
2. Patients with peritonitis frequently undergo surgery to treat the cause of infection, and a secondary objective was to identify whether certain systemic antibiotic regimens reduce post‐operative infection rates and post‐operative stay. Wound, urinary and chest infection rates were also specifically examined, together with an evaluation of the success of antibiotic regimens in adequate source control, most specifically the need for subsequent interventions to eradicate peritoneal sepsis.
3. Certain antibiotics, particularly the aminoglycosides, have higher toxicity compared to the others. The various adverse events relating to the regimens used were elucidated and compared.
Methods
Criteria for considering studies for this review
Types of studies
Acceptable randomised controlled trials and controlled clinical trials were included (in which treatment allocations were randomised using coin flips, odd‐even numbers, case record number, days of the week, or other such pseudo‐ or quasi random processes) (Alderson 2004) in which treatment with one antibiotic agent or regimen was compared to another or placebo in patients with secondary peritonitis.
Types of participants
Trials including adult patients with secondary peritonitis diagnosed clinically or at surgery, requiring a course of antibiotic treatment were entered into the review. Patients with peritonitis were divided into aetiological or risk‐assessed subgroups, where possible (Solomkin 1984):
1. Faecal 2. Ischaemia 3. Biliary and pancreatic 4. Upper gastrointestinal 5. APACHE II / POSSUM score range
Gynaecological causes of peritonitis were not reviewed, nor will trials of antibiotics in appendicitis unless the patients presented with 2+ quadrant peritonitis. Patients with peritonitis secondary to continuous ambulatory peritoneal dialysis or peritonitis secondary to trauma were similarly excluded as these patients have different disease patterns and microbial flora.
Patients who had received more than two doses of antibiotic within the last 24 hours were also excluded from evaluation.
Types of interventions
Trials comparing one antibiotic agent or regimen versus another or placebo for treatment of secondary peritonitis were recruited for this review.
Types of outcome measures
The primary aims of the review were to assess the efficacy of the antibiotic regimens in eradicating the initial sepsis and reducing mortality.
This review also assessed these secondary aims:
1. Wound infection. 2. Post‐operative intra abdominal abscess. 3. Respiratory and urinary tract infections. 4. Adverse events related to antibiotic therapy. 5. Failure rate in terms of change of antibiotics and re‐operation. 6. Cost effectiveness.
All definitions were standardised whenever possible.
Search methods for identification of studies
See: Collaborative Review Group search strategy.
The following bibliographic databases were searched to identify relevant primary studies:
The Cochrane Central Register of Controlled Trials (CENTRAL), 2004 issue 4. MEDLINE from 1966 to November 2004. EMBASE from 1980 to November 2004. Cochrane Colorectal Cancer Group specialised register SR‐COLOCA.
The following search strategy will be used to search the databases:
#1 Periton$ #2 Abdo$ #3 Intra‐abdo$ #4 Intraabdo$ #5 #2 or #3 or #4 #6 Infect$ #7 Sep$ #8 #6 or #7 #9 #5 and #8 #10 #1 or #9 #11 Antibio$ #12 Antimicro$ #13 Anti‐infect$ #14 Drug therapy #15 #11 or #12 or #13 or #14 #16 #10 and #15
1. Trials examining treatment of primary bacterial peritonitis, antibiotics prophylaxis and peritonitis as a result of continuous ambulatory peritoneal dialysis were not included. Trials on antifungal therapies, topical antibiotics and antiseptic agents were similarly excluded.
Trials that fulfil the eligibility criteria were recruited regardless of language.
2. Two independent assessors for inclusion evaluated all identified trials from the search. Identified and included studies were further examined for additional studies from the reference list.
3. Authors of technical reports and conference proceedings, and pharmaceutical companies were contacted when indicated to seek additional unpublished studies that would potentially fulfil the eligibility criteria.
Data collection and analysis
Study selection: Two independent reviewers conducted a methodical search of the databases according to the search strategy specified. Trials were considered for inclusion if they fulfilled the following inclusion criteria:
randomised controlled trials or controlled clinical trials where one regimen of antibiotics versus another or placebo was used to treat secondary peritonitis.
trials reporting treatment of adult patients
The following exclusion criteria were used:
trials involving peritonitis as a result of spontaneous, gynaecological, traumatic and continuous ambulatory peritoneal dialysis related causes.
studies involving paediatric patients (<16 years of age ).
Two authors evaluated titles, keywords and abstracts of the identified citations for possible inclusion. A third author further assessed trials that did not fully meet the criteria of this review for possible inclusion. At this stage, any disagreement as to the suitability of the trials were resolved by discussion among all six authors. When a trial was identified, the full paper was obtained and inspected independently by two authors.
Quality assessment: The methodology of identified studies was assessed by two independent authors. Trials fulfilling the eligibility criteria were assessed for quality using the following characteristics:
concealment of allocation sequence was classified as adequate, unclear, inadequate or not used as recommended by the Cochrane Handbook (Alderson 2004).
Allocation according to computer generated numbers, sequentially numbered sealed envelopes, shuffles, etc were considered truly random, whereas, randomisation according to date of birth, case record number, day of the week, etc, were considered inadequate. When studies did not report any concealment approach, concealment was considered to be unclear.
blinding of physicians and outcome assessors
Adequacy of efforts to make treatment and control arms indistinguishable to prevent performance and detection bias was assessed.
patient attrition
Efforts were made to assess the way trials handle losses of participants (e.g. withdrawals, dropouts, protocol deviation) and the use of intention‐to‐treat analysis. Trials had to fulfil the following two criteria for intention‐to‐treat analysis: 1) trial participants should have been analysed in the groups to which they were randomised regardless of which (or how much) treatment they actually received, and regardless of other protocol irregularities, such as ineligibility; and 2) all participants should have been included regardless of whether their outcomes were actually collected (Alderson 2004).
patient stratification and external validity
Presence of patient stratification according to well established severity scores such as APACHE II and POSSUM were scrutinised to aid in facilitating the external validity of the trials (Egger 2001). Collection of data Data collection were standardised by means of specially developed data extraction forms and double checked by a second independent author. The data collected was divided into the following study characteristics:
methods
Details of the randomisation method were recorded according to the classification used in RevMan and suggested by the Cochrane Handbook (Alderson 2004). Duration of the study and follow up time, type of blinding used and methods employed to avoid attrition bias were retrieved.
participants
Data with regards to patient numbers in relation to power calculations, age, gender distribution, severity of illness and attempts at patient stratification using severity scoring systems such as APACHE II and POSSUM were recorded.
interventions
Details of blinding, type, length, dose and timing of antibiotics administration were noted. Length of antibiotic administration was documented as mean and standard deviation.
outcome measures and results
Primary outcome measures ‐ in terms of mortality rate and interventional success. Interventional success was documented as either clinical or bacteriological success. Failure rate was quantified either as re‐operation or change of antibiotic regimen. Secondary outcome measures ‐ such as wound and super‐ infection, adverse events and length of hospital stay.
Synthesis of data The data collected was analysed using intention‐to‐treat analysis. The statistical package (MetaView of RevMan) provided by the Cochrane Collaboration was used. For dichotomous outcome (death or survival), the impact of the intervention was expressed as odds ratio together with 95% confidence intervals. Continuous outcomes were compared using weighted mean difference. The following data were extracted to perform subgroup analysis:
APACHE II / POSSUM score.
duration of antibiotic administration.
aetiology of secondary peritonitis.
toxicity / side‐effects.
Antimicrobial regimens were grouped according to their molecular class. Each arm of each controlled study referred to a specific regimen / dosage pattern. All studies where the antibiotics under comparison were assigned to the same set of regimen / dosage pattern were pooled. Tables of comparison included the following outcomes: Primary aims: 1) Death for any cause. 2) Success / failure rate (in terms of re‐operation and change of antibiotics).
Secondary aims: 1) Postoperative wound infections (discharge of pus or necessity for additional interventions). 2) Postoperative intra‐abdominal infection (clinical or imaging studies). 3) Bacterial eradication (comparison of intra‐ and post‐operative cultures). 4) Adverse drug effects (this was divided into minor symptoms such as rashes, and abnormal blood results; moderate symptoms and severe symptoms such as renal failure, deafness and other complications requiring change of antibiotics).
Potential effects of publication bias on the results of the meta‐analysis were assessed from a funnel graph of the sample size plotted against the odds ratio. Heterogeneity in the results of the trials were assessed using a Chi‐square test of heterogeneity (p<0.1). Data were pooled using the random effects model.
Results
Description of studies
For a detailed description of studies see table of 'Characteristics of included studies' and 'Characteristics of excluded studies'.
148 potentially suitable titles and abstracts were identified from the search strategy and references for full paper review. Out of these, 40 studies involving 5094 evaluable patients were considered eligible for inclusion.
108 papers were excluded for various reasons.
The commonest exclusion reason being inclusion of paediatric (15 studies: Allo 1999, Arguedas 1996, Bennion 1990, Birolini 1985, Birolini 1989, Danish 1984, de Vries 1990, Dougherty 1995, Fink 1989, Hollender 1989, Huizinga 1988, Kooi 1990, Luke 1991, Mullick 1987, Raahave 1970, Sirinek 1987, Sirinek 1991, Stellato 1988, Stone 1975, Stone 1981, Stone 1982a, Stone 1982b, Stone 1983b, Tally 1981, Tally 1986), and
non‐peritonitis patients (36 studies: Andaker 1987, Baird 1983, Birolini 1985, Birolini 1989, Biron 1984, Cakmakci 1993, Christen 1987, Colardyn 1996, Collier 1981, Cometta 1994, Condon 1995, Danziger 1988, Fink 1989, Geroulanos 1995, Harding 1982, Hollender 1989, Holloway 1989, Jaspers 1998, Joshi 1986, Kasholm‐Tengve 1986, Kirkpatrick 1983, Leal del Rosal 1989, Levine 1989, Luke 1991, Marra 1998, Mehtar 1997, Ohlin 1999, Poularas 1988, Schentag 1983, Smith 1984, Solomkin 1985, Stone 1975, Stone 1984, Tally 1981, Tally 1986, Yoshioka 1991) whereby data for adult peritonitis patients were not extractable.
Other studies were excluded as a result of non‐randomisation (10 studies: Arguedas 1996, Ball 1981, Busuttil 1982, Heseltine 1986, Holloway 1989, Inthorn 1989, Lou 1982, Smith 1982, Stone 1978, Vestweber 1994),
no comparative regimens (six studies: Arguedas 1996, Ball 1981, Busuttil 1982, Smith 1982, Stone 1978, Vestweber 1994),
addition of other antibiotics (16 studies: Barie 1997, Drusano 1982, Henry 1985, Hollender 1989, Hoogkamp 1995, Jaspers 1998, Leal del Rosal 1989, Rohrborn 2000, Scheinin 1994, Solomkin 1990, Solomkin 1996, Solomkin 2003, Tally 1981, Tally 1986, Teppler 2004, Williams 1991),
peritonitis secondary to trauma (11 studies: Baird 1983, Barboza 1994, Bubrick 1990, Condon 1995, Donahue 1998, Huizinga 1988, Huizinga 1995, Luke 1991, Najem 1983, Niinikoski 1993, Niinikoski 1993),
dual publication of data (10 studies: Eklund 1993, Fink 1991, Polk 1993, Scott 1987a, Smith 1983, Stone 1982b, Tellado 2002, Teppler 2004, Walters 1999, Wilson 1997), and
administration of antibiotics > 24 prior to commencement of study drugs (5 studies: Canadian 1983, Colardyn 1996, Hoogkamp 1995, Lennard 1985, Wilson 1997).
All 40 included studies were prospective randomised controlled trials. Out of these, there were:
13 double‐blinded trials (Berne 1982, Berne 1987, Berne 1993, Berne 1996, Christou 1996, Cohn 2000, Hopkins 1994, Malangoni 1985, Smith 1980, Solomkin 2001, Study 1986, Walker 1993, Yellin 1985),
five were double blinded studies with use of placebo (Berne 1982, Malangoni 1985, Solomkin 2001, Study 1986, Yellin 1985) and
five studies were single blinded (Brismar 1992, Brismar 1995, Dupont 2000, Jaccard 1998, Swedish 1990).
Trial participants Within the 40 included studies, the age range of the participants was between 16 and 99 years old.
The breakdown of the studies according to the centres that they were performed were as follows:
Three Canadian (Christou 1996, Poenaru 1990, Smith 1980).
One Dutch (de Groot 1993).
One Finnish (Paakkonen 1991).
One French (Dupont 2000).
One German (Kempf 1996).
One Greek (Kanellakopoulou 1993).
One Italian (Basoli 1997).
Two Scandinavian (Angeras 1996, Scandinavian 1984).
One Spanish (Torres 1999).
Five Swedish (Brismar 1995, Brismar 1996, Study 1986, Swedish 1990, Tornqvist 1985).
Three Swiss (Gozenbach 1987, Jaccard 1998, Zanetti 1999).
One Taiwanese (Shyr 1995).
Two United Kingdom (Leaper 1987, Scott 1987).
12 USA (Berne 1982, Berne 1987, Berne 1993, Berne 1996, Busuttil 1984, Eckhauser 1992, Greenberg 1994, Hopkins 1994, Jauregui 1990, Malangoni 1985, Walker 1993, Yellin 1985).
One Europe and America (Brismar 1992).
Three North American (Cohn 2000, Investigators 1994, Solomkin 2001).
One Multinational (Leal del Rosal 1995).
Trial regimens 38 trials compared 2 regimens, and 2 trials (Berne 1982 & Scott 1987) included 3 regimens (Table 1).
1. Antibiotic regimens.
| Study | Antibiotic 1 | Antibiotic 2 | Antibiotic 3 | Shown difference |
| Angeras 1996 | Imipenem/cilastatin (81%) | Cefuroxime/metronidazole (86%) | No | |
| Basoli 1997 | Imipenem/cilastatin (98%) | Meropenem (95%) | No | |
| Berne 1982 | Gentamicin/clindamycin (98%) | Cefamandole (77%) | Cefoperazone (86%) | Gentamicin/clindamycin superior to cefamandole and cefoperazone. |
| Berne 1987 | Gentamicin/clindamycin (100%) | Aztreonam/clindamycin (96%) | No | |
| Berne 1993 | Gentamicin/clindamycin (83%) | Cefepime/metronidazole (94%) | No | |
| Berne 1996 | Tobramycin/clindamycin (91%) | Meropenem (92%) | Meropenem more effective at reducing postoperative stay, duration of therapy and time to defervescence. | |
| Brismar 1992 | Imipenem/cilastatin (69%) | Piperacillin/tazobactam (91%) | Piperacillin/tazobactam significantly more effective. | |
| Brismar 1995 | Imipenem/cilastatin (96%) | Meropenem (98%) | No | |
| Brismar 1996 | Biapenem (65%) | Imipenem/cilastatin (68%) | No | |
| Busuttil 1984 | Cefamandole (77%) | Gentamicin/clindamycin (82%) | No | |
| Christou 1996 | Imipenem/cilastatin (88%) | Cefoxitin (84%) | No | |
| Cohn 1990 | Piperacillin/tazobactam (63%) | Ciprofloxacin/metronidazole (74%) | Ciprofloxacin/metronidazole clinically more effective. | |
| de Groot 1993 | Imipenem/cilastatin (71%) | Aztreonam/clindamycin (64%) | No | |
| Dupont 2000 | Piperacillin/tazobactam (44%) | Piperacillin/tazobactam/amikacin (48%) | No | |
| Eckhauser 1992 | Aminoglycosides/clindamycin (77%) | Imipenem/cilastatin (89%) | No | |
| Gozenbach 1987 | Netilmicin/clindamycin (67%) | Imipenem/cilastatin (81%) | No | |
| Greenberg 1994 | Gentamicin/clindamycin (52%) | Cefoperazone/sulbactam (70%) | No | |
| Hopkins 1994 | Amikacin/clindamycin (86%) | Cefotetan (90%) | No | |
| Investigators 1994 | Gentamicin/clindamycin (72%) | Piperacillin/tazobactam (83%) | No | |
| Jaccard 1998 | Imipenem/cilastatin (93%) | Piperacillin/tazobactam (95%) | No | |
| Jauregui 1990 | Gentamicin/clindamycin (62%) | Cefoperazone/sulbactam (87%) | Cure rate for cefoperazone/sulbactam was statistically higher than gentamicin/clindamycin | |
| Kanellakopoulou 1993 | Imipenem/cilastatin (94%) | Meropenem (97%) | No | |
| Kempf 1996 | Meropenem (93%) | Cefotaxime/metronidazole (70%) | Meropenem shown to be statistically significantly more successful (clinically and microbiologically) than cefotaxime/metronidazole | |
| Leal del Rosal 1995 | Amikacin/metronidazole (94%) | Isepamicin/metronidazole (96%) | No | |
| Leaper 1987 | Imipenem/cilastatin (84%) | Ampicillin/metronidazole/gentamicin (92%) | No | |
| Malangoni 1985 | Tobramycin/clindamycin (83%) | Cefoxitin (79%) | No | |
| Paakkonen 1991 | Cefuroxime/metronidazole (64%) | Piperacillin (71%) | No | |
| Poenaru 1990 | Tobramycin/antianaerobe (79%) | Imipenem/cilastatin (67%) | No | |
| Scandinavian 1984 | Gentamicin/clindamycin (50%) | Imipenem/cilastatin (55%) | No | |
| Scott 1987 | Gentamicin/penicillin G/metronidazole (88%) | Cefotetan (87%) | Cephradine/metronidazole (66%) | No |
| Shyr 1995 | Gentamicin/clindamycin (93%) | Piperacillin/tazobactam (93%) | No | |
| Smith 1980 | Tobramycin/clindamycin (74%) | Tobramycin/metronidazole (83%) | No | |
| Solomkin 2001 | Imipenem/cilastatin (80%) | Clinafloxacin (82%) | No | |
| Study 1986 | Gentamicin/clindamycin (89%) | Ampicillin/sulbactam (78%) | No | |
| Swedish 1990 | Gentamicin/metronidazole (80%) | Pefloxacin/metronidazole (88%) | No | |
| Tornqvist 1985 | Cefuroxime | Cefuroxime/metronidazole | No. No clinical success rates documented. | |
| Torres 1999 | Gentamicin/metronidazole (92%) | Cefminox (99%) | No | |
| Walker 1993 | Ampicillin/sulbactam (88%) | Cefoxitin (79%) | No | |
| Yellin 1985 | Gentamicin/clindamycin (100%) | Ampicillin/sulbactam (88%) | Trial had shown difference in clinical success rate in favour of gentamicin/clindamycin regimen | |
| Zanetti 1999 | Imipenem/cilastatin (78%) | Meropenem (77%) | No |
The breakdown of the studies according to timing of infusion were as follows:
15 pre‐operatively (Berne 1982, Berne 1987, Berne 1993, Berne 1996, Brismar 1995, Brismar 1996, Christou 1996, Cohn 2000, Greenberg 1994, Hopkins 1994, Investigators 1994, Kanellakopoulou 1993, Paakkonen 1991, Tornqvist 1985, Yellin 1985).
two intra‐operatively (Brismar 1992, de Groot 1993).
one post‐operatively (Gozenbach 1987).
22 other studies did not explicitly illustrate the timing of antibiotic infused.
Out of the 40 included studies, the duration of antibiotics were explicitly specified as > 3 days in 28 studies (Angeras 1996, Basoli 1997, Berne 1982, Berne 1987, Berne 1996, Brismar 1992, Brismar 1995, Brismar 1996, Cohn 2000, de Groot 1993, Dupont 2000, Eckhauser 1992, Hopkins 1994, Investigators 1994, Jauregui 1990, Kanellakopoulou 1993, Kempf 1996, Leal del Rosal 1995, Leaper 1987, Paakkonen 1991, Scandinavian 1984, Smith 1980, Solomkin 2001, Study 1986, Swedish 1990, Tornqvist 1985, Walker 1993, Zanetti 1999). In the rest of the studies, the duration of antibiotic therapy was either not specified or was administered for less than 48 hours. Outcome measures The primary outcome measures of this review were two‐fold, namely effectiveness of the regimen in promoting clinical success following operative intervention and in reducing mortality from the infection. Different definitions were given for clinical success in all the trials reviewed. For the purpose of this review, the authors have utilised clinical cure as the definition of clinical success instead of satisfactory outcome.
Primary outcome: Primary outcomes in terms of clinical cure and mortality were reported as follow:
Clinical success was reported in 38 studies (Angeras 1996, Basoli 1997, Berne 1982, Berne 1987, Berne 1993, Berne 1996, Brismar 1992, Brismar 1995, Brismar 1996, Busuttil 1984, Christou 1996, Cohn 2000, Dupont 2000, Eckhauser 1992, Gozenbach 1987, Greenberg 1994, Hopkins 1994, Investigators 1994, Jaccard 1998, Jauregui 1990, Kanellakopoulou 1993, Kempf 1996, Leal del Rosal 1995, Leaper 1987, Malangoni 1985, Paakkonen 1991, Poenaru 1990, Scandinavian 1984, Scott 1987, Shyr 1995, Smith 1980, Solomkin 2001, Study 1986, Swedish 1990, Torres 1999, Walker 1993, Yellin 1985, Zanetti 1999).
Mortality ‐ 24 studies (Angeras 1996, Brismar 1992, Brismar 1995, Brismar 1996, Busuttil 1984, Christou 1996, Cohn 2000, de Groot 1993, Dupont 2000, Eckhauser 1992, Greenberg 1994, Investigators 1994, Jaccard 1998, Kempf 1996, Malangoni 1985, Paakkonen 1991, Poenaru 1990, Scott 1987, Smith 1980, Solomkin 2001, Swedish 1990, Tornqvist 1985, Torres 1999, Zanetti 1999). The authors of this review have further sub‐classified mortality into overall mortality and mortality due to infection.
Secondary outcome: Secondary outcomes of antibiotic treatment in the form of successful eradication of infective bacteria; effectiveness at preventing wound infection, intra‐abdominal abscesses, clinical sepsis, superinfection and remote infection; development of adverse reactions; duration of therapy; post‐operative hospital stay and duration of defervescence were reported as follow:
Microbiological success was reported in 17 studies (Angeras 1996, Basoli 1997, Brismar 1992, Brismar 1995, Brismar 1996, Cohn 2000, Eckhauser 1992, Greenberg 1994, Hopkins 1994, Investigators 1994, Kempf 1996, Leal del Rosal 1995, Leaper 1987, Shyr 1995, Study 1986, Swedish 1990, Zanetti 1999).
Wound infection ‐ 18 studies (Berne 1982, Berne 1987, Berne 1993, Busuttil 1984, Cohn 2000, de Groot 1993, Gozenbach 1987, Hopkins 1994, Leal del Rosal 1995, Leaper 1987, Malangoni 1985, Paakkonen 1991, Scott 1987, Solomkin 2001, Tornqvist 1985, Torres 1999, Walker 1993, Yellin 1985).
Intra‐abdominal abscess ‐ 13 studies (Berne 1982, Berne 1993, Busuttil 1984, de Groot 1993, Gozenbach 1987, Hopkins 1994, Jaccard 1998, Malangoni 1985, Paakkonen 1991, Solomkin 2001, Tornqvist 1985, Walker 1993, Yellin 1985).
Clinical sepsis ‐ five studies (Berne 1982, Busuttil 1984, de Groot 1993, Jaccard 1998, Solomkin 2001).
Superinfection ‐ 11 studies (Brismar 1992, Brismar 1995, Brismar 1996, Cohn 2000, de Groot 1993, Greenberg 1994, Investigators 1994, Kempf 1996, Leal del Rosal 1995, Leaper 1987, Swedish 1990).
Remote infection ‐ five studies (de Groot 1993, Leaper 1987, Malangoni 1985, Paakkonen 1991, Walker 1993).
Adverse reactions ‐ 27 studies (Angeras 1996, Basoli 1997, Berne 1982, Berne 1987, Berne 1993, Brismar 1992, Brismar 1995, Brismar 1996, Cohn 2000, de Groot 1993, Dupont 2000, Eckhauser 1992, Greenberg 1994, Hopkins 1994, Kempf 1996, Leal del Rosal 1995, Leaper 1987, Paakkonen 1991, Shyr 1995, Smith 1980, Solomkin 2001, Study 1986, Swedish 1990, Torres 1999, Walker 1993, Yellin 1985, Zanetti 1999). This review further subdivided adverse reactions where possible into overall, major (for example, anaphylactic reactions, nephrotoxicity and ototoxicity where antibiotics were changed) and minor (for example, minor haematological or biochemical changes which did not necessitate change of antibiotic regimens).
Duration of therapy ‐ nine studies (Berne 1987, Berne 1993, Berne 1996, Dupont 2000, Hopkins 1994, Jaccard 1998, Shyr 1995, Yellin 1985, Zanetti 1999).
Post‐operative hospital stay ‐ six studies (Berne 1987, Berne 1993, Berne 1996, Hopkins 1994, Yellin 1985, Zanetti 1999).
Timing of defervescence ‐ five studies (Berne 1987, Berne 1993, Berne 1996, Hopkins 1994, Yellin 1985).
In 6 studies (Berne 1982, Berne 1987, Berne 1993, Berne 1996, Hopkins 1994, Yellin 1985), all of the participants had complicated appendicitis (gangrenous or perforated appendicitis) as the cause of secondary peritonitis. The other 34 included studies had peritonitis as a result of combination of different aetiological factors.
Antibiotic regimens
In this review, antibiotics belonging to the same class were grouped together for the purpose of performing meta‐analyses. There were 16 antibiotic regimens or comparators and they were listed as follows:
Aminoglycosides and antianaerobes were used in 19 studies as the comparative regimen (Berne 1982, Berne 1987, Berne 1993, Berne 1996, Busuttil 1984, Eckhauser 1992, Gozenbach 1987, Greenberg 1994, Hopkins 1994, Investigators 1994, Jauregui 1990, Malangoni 1985, Poenaru 1990, Scandinavian 1984, Shyr 1995, Study 1986, Swedish 1990, Torres 1999, Yellin 1985). Monitoring of aminoglycosides levels were explicitly mentioned in all of these studies.
However, out of the 19 studies involving aminoglycosides and antianaerobes, 14 studies further reported ranges of the peak and trough levels of the aminoglycosides (Berne 1982, Berne 1987, Berne 1993, Berne 1996, Eckhauser 1992, Gozenbach 1987, Greenberg 1994, Investigators 1994, Jauregui 1990, Malangoni 1985, Poenaru 1990, Scandinavian 1984, Shyr 1995, Yellin 1985).
Aminoglycoside plus broad spectrum penicillins with beta lactamase inhibitor ‐ one study (Dupont 2000).
Aminoglycosides, penicillins and antianaerobes ‐ one study (Scott 1987).
Broad spectrum penicillins ‐ one study (Paakkonen 1991).
Broad spectrum penicillins with beta lactamase inhibitor ‐ nine studies (Brismar 1992, Cohn 2000, Dupont 2000, Investigators 1994, Jaccard 1998, Shyr 1995, Study 1986, Walker 1993, Yellin 1985).
Broad spectrum penicillins, antianaerobes and aminoglycoside ‐ one study (Leaper 1987).
Carbapenems ‐ 13 studies (Angeras 1996, Berne 1996, Brismar 1992, Christou 1996, de Groot 1993, Eckhauser 1992, Gozenbach 1987, Jaccard 1998, Kempf 1996, Leaper 1987, Poenaru 1990, Scandinavian 1984, Solomkin 2001).
Cephalosporins alone ‐ nine studies (Berne 1982, Busuttil 1984, Christou 1996, Hopkins 1994, Malangoni 1985, Scott 1987, Tornqvist 1985, Torres 1999, Walker 1993).
Cephalosporins and antianaerobes ‐ six studies (Angeras 1996, Berne 1993, Kempf 1996, Paakkonen 1991, Scott 1987, Tornqvist 1985).
Cephalosporins and beta lactamases inhibitor ‐ two studies (Greenberg 1994, Jauregui 1990).
Clindamycin versus metronidazole regimens ‐ one study (Smith 1980).
Fluoroquinolones alone ‐ one study (Solomkin 2001).
Fluoroquinolones and antianaerobes ‐ two studies (Cohn 2000, Swedish 1990).
Monobactams and antianaerobes ‐ two studies (Berne 1987, de Groot 1993).
Imipenem/cilastatin versus other carbapenems ‐ five studies (Basoli 1997, Brismar 1995, Brismar 1996, Kanellakopoulou 1993, Zanetti 1999).
Isepamicin and antianaerobes versus amikacin and antianaerobe ‐ one study (Leal del Rosal 1995).
Risk of bias in included studies
The methodological quality of all identified studies were independently assessed by two assessors. The 40 included studies reported a total of 6832 eligible adult patients (> 16 years old). However, 1738 patients were excluded or lost to follow‐up, leaving a total of 5094 patients for analyses in this review. Details of the randomisation, numbers of centres involved in trial, adequacy of allocation concealment, blinding of assessors, power calculations, patient stratification, intention‐to‐treat analysis and duration of follow‐up were as follows:
There were 26 trials that were multicentre (Angeras 1996, Basoli 1997, Brismar 1992, Brismar 1995, Brismar 1996, Busuttil 1984, Christou 1996, Cohn 2000, Dupont 2000, Eckhauser 1992, Greenberg 1994, Investigators 1994, Jaccard 1998, Jauregui 1990, Kempf 1996, Leal del Rosal 1995, Malangoni 1985, Paakkonen 1991, Scandinavian 1984, Smith 1980, Solomkin 2001, Study 1986, Swedish 1990, Torres 1999, Walker 1993, Zanetti 1999). Concealment of allocation was
adequate (for example, use of computer generated numbered cards or sealed sequential envelopes, random table kept at pharmacy) in 24 trials (Berne 1982, Berne 1996, Brismar 1992, Brismar 1995, Brismar 1996, Christou 1996, de Groot 1993, Dupont 2000, Greenberg 1994, Hopkins 1994, Investigators 1994, Jaccard 1998, Jauregui 1990, Malangoni 1985, Scandinavian 1984, Shyr 1995, Smith 1980, Solomkin 2001, Study 1986, Swedish 1990, Torres 1999, Walker 1993, Yellin 1985, Zanetti 1999), and
unclear in 16 trials (Angeras 1996, Basoli 1997, Berne 1987, Berne 1993, Busuttil 1984, Cohn 2000, Eckhauser 1992, Gozenbach 1987, Kanellakopoulou 1993, Kempf 1996, Leal del Rosal 1995, Leaper 1987, Paakkonen 1991, Poenaru 1990, Scott 1987, Tornqvist 1985).
Patient stratification was performed using
APACHE II in 12 studies (Angeras 1996, Basoli 1997, Brismar 1995, Brismar 1996, Christou 1996, Cohn 2000, Kempf 1996, Malangoni 1985, Poenaru 1990, Solomkin 2001, Torres 1999, Zanetti 1999).
presence or absence of appendicitis in one study (Cohn 2000).
severity of infection in one study (Eckhauser 1992).
site of pathology in one study (Paakkonen 1991).
SAPS II score in one study (Dupont 2000).
MacCabe and Jackson score in two studies (Dupont 2000, Malangoni 1985).
Only one study (Basoli 1997) stratified patients prior to randomisation and in the other trials where stratification was used, this was performed after participants were allocated to their respective study arms. Despite attempts at patient stratification, results were not presented according to patient stratifications.
Power calculations were performed in 10 studies (Angeras 1996, Berne 1987, Brismar 1996, Christou 1996, Cohn 2000, Dupont 2000, Kempf 1996, Malangoni 1985, Walker 1993, Zanetti 1999).
Intention‐to‐treat analyses were numerated in 14 studies (Angeras 1996, Brismar 1992, Brismar 1995, Brismar 1995, Christou 1996, Cohn 2000, Dupont 2000, Eckhauser 1992, Greenberg 1994, Kempf 1996, Leal del Rosal 1995, Solomkin 2001, Torres 1999, Zanetti 1999). However, out of these trials, intention‐to‐treat analyses were only limited to clinical success and mortality (primary outcomes).
Nine studies (Angeras 1996, Basoli 1997, Berne 1982, Busuttil 1984, de Groot 1993, Gozenbach 1987, Kempf 1996, Study 1986, Walker 1993) performed sub‐group analysis. However, the analyses performed were inadequate to be included in this review. Follow up of participants was performed up to:
2 weeks in three studies (Scott 1987, Shyr 1995, Zanetti 1999).
more than 2 weeks in 22 studies (Angeras 1996, Brismar 1992, Brismar 1995, Brismar 1995, Busuttil 1984, Christou 1996, Cohn 2000, Dupont 2000, Gozenbach 1987, Greenberg 1994, Hopkins 1994, Investigators 1994, Jaccard 1998, Jauregui 1990, Kanellakopoulou 1993, Kempf 1996, Malangoni 1985, Paakkonen 1991, Study 1986, Swedish 1990, Tornqvist 1985, Yellin 1985).
Effects of interventions
Out of the 40 included trials, 16 different comparative antibiotic regimens were used. The commonest comparator for most of the studies was aminoglycosides and antianaerobes. Historically, gentamicin and clindamycin was considered the 'gold standard' of antibiotic treatment in peritonitis before the advent of less nephrotoxic or ototoxic antibiotic with equivalent efficacy. It is therefore not surprising to see this regimen being used frequently in the control arm of most studies.
Only results for random effects were reported for this review. Sub‐group analyses were planned for the different aetiological factors, APACHE II / POSSUM score, duration of antibiotic administration and toxicity / side‐effects. However, there was inadequate data in the studies to allow for sufficient sub‐group analyses.
The observed clinical heterogeneity amongst the trials was reflected in parameters such as study population, diagnosis, strategy of treatment, type of antibiotics, the outcome analysis and length of follow up.
Aminoglycosides and antianaerobes 19 studies used a combination of an aminoglycoside (13 studies used gentamicin as the main aminoglycoside, 1 utilised amikacin, 1 netilmicin, 3 tobramycin and 1 employed combination of either gentamicin or tobramycin) plus an antianaerobe (16 studies used clindamycin, 2 metronidazole and 1 employed combination of either clindamycin or metronidazole) as the comparative antibiotic regimen. Overall, 1956 evaluable patients were recruited and compared (845 patients in the aminoglycosides/antianaerobes and 1111 in the other regimens).
There was no significant difference in the incidence of mortality between aminoglycosides plus antianaerobes and other regimens. This was not apparent in either all causes mortality (Odds Ratio: OR: 2.03; 95% CI: 0.88, 4.71), mortality due to infection (OR: 1.51; 95% CI: 0.66, 3.43) or within the ITT analysis (OR: 2.10; 95% CI: 0.78, 5.65).
There were statistically significant differences in clinical success in favour of other regimens both in overall peritonitis and the former plus peritonitis secondary to appendicitis (OR: 0.57; 95% CI: 0.41, 0.78; p = 0.0005 and OR: 1.36; 95% CI: 0.44, 4.14; p = 0.02 respectively). There may have been an inherent bias in these results as aminoglycosides and antianaerobes were the most commonly used comparative denominators in most of the studies.
Microbiological success was significantly more effective with other regimens (OR: 0.49; 95% CI: 0.31, 0.76; p = 0.001). Data for ITT analysis was only available for one study and this was not statistically significant (OR: 0.94; 95% CI: 0.40, 2.20).
There were no differences in either the incidence of wound infection (OR: 0.84; 95% CI: 0.35, 2.02), intra‐abdominal abscesses (OR: 0.85; 95% CI: 0.40, 1.83), clinical sepsis (OR: 1.46; 95% CI: 0.07, 31.21), remote infection (OR: 1.13; 95% CI: 0.37, 3.47) and superinfection (OR: 2.15; 95% CI: 0.89, 5.17) between aminoglycosides plus antianaerobes and other regimens.
Sub‐classification of adverse reactions into overall (where sub‐classifications were not available), mainly minor and mainly major adverse reactions was not consistently reported in the studies. Within the limited data available, there were no statistically significant differences in the number of adverse events seen (OR: 1.76; 95% CI: 0.87, 3.53) even though it favoured other regimens. This is also true for the limited ITT analyses (OR: 0.69, 95% CI: 0.43, 1.11) but the latter favoured the combination of aminoglycosides and antianaerobes.
Other regimens were statistically better at reducing hospitalised stay (Weighted Mean Difference, WMD: 0.57; 95% CI: 0.06, 1.07; p = 0.03). There were no differences in duration of therapy required (WMD: 0.37; 95% CI: ‐0.05, 0.80) and time to defervescence (WMD: 0.38; 95% CI: ‐0.29, 1.05) between combination of aminoglycosides plus antianaerobes and other regimens.
Aminoglycoside plus broad spectrum penicillins with beta lactamase inhibitor One study (Dupont 2000) used a combination of amikacin plus piperacillin/tazobactam to compare against piperacillin/tazobactam. Overall, there were 159 evaluable patients in the final analysis (78 patients in the aminoglycoside/broad spectrum penicillin/beta lactamase inhibitor and 81 in piperacillin/tazobactam arm). The addition of an aminoglycoside did not confer extra benefit in either primary outcomes [mortality and clinical success (OR: 0.85; 95% CI: 0.37, 1.97 and OR: 1.03; 95% CI: 0.55, 1.91 respectively)] or in the incident of adverse reactions (OR: 1.21; 95% CI: 0.71, 2.04) and duration of therapy (WMD: 0.50; 95% CI: ‐0.47, 1.47).
Aminoglycosides, penicillins and antianaerobes One study (Scott 1987) utilised gentamicin, penicillin G and metronidazole as its comparator against cefotetan and cephradine. There were 107 evaluable patients in this study (25 patients in the gentamicin/penicillin G/metronidazole and 82 in the other regimen). The result of aminoglycoside, penicillin and antianaerobe did not differ from other regimen in terms of mortality (OR: 0.23; 95% CI: 0.01, 4.24), clinical success (OR: 1.92; 95% CI: 0.51, 7.17) or wound infection (OR: 0.80; 95% CI: 0.21, 3.08). Broad spectrum penicillins One study (Paakkonen 1991) used piperacillin to compare against a combination of cefuroxime and metronidazole. 83 evaluable patients were recruited (38 in the piperacillin arm and 45 in the cefuroxime/metronidazole arm). There were no statistically significant differences in either mortality (OR: 1.84; 95% CI: 0.29, 11.65), clinical success (OR: 1.35; 95% CI: 0.53, 3.43), wound infection (OR: 1.21; 95% CI: 0.28, 5.19), development of intra‐abdominal abscess (OR: 1.20; 95% CI: 0.23, 6.32), remote infection (OR: 0.26; 95% CI: 0.07, 1.03) or adverse reactions (OR: 1.19; 95% CI: 0.07, 19.67).
Broad spectrum penicillins with beta lactamase inhibitor Nine studies compared a combination of broad spectrum penicillin and beta‐lactamase inhibitor against other regimens. 1289 evaluable patients were recruited (687 in the broad spectrum penicillins/beta lactamase inhibitors arm and 602 in the other regimens). Out of these, six studies used piperacillin/tazobactam and three utilised ampicillin/sulbactam as their comparators.
There was no difference in the mortality (all causes and due to infection) between broad spectrum penicillin with beta lactamase inhibitor and other regimens (OR: 0.45; 95% CI: 0.09, 2.38 and OR: 0.54; 95% CI: 0.05, 6.08 respectively). ITT analyses did not show any statistically significant differences either.
Outcome for clinical success did not differ significantly between the broad spectrum penicillins plus beta‐lactamase inhibitors and other regimens (OR: 1.14; 95% CI: 0.68, 1.92). ITT analysis showed similar conclusion (OR: 1.22, 95% CI: 0.56, 2.66).
Microbiological success did not differ significantly between the regimens (OR: 1.84; 95% CI: 0.87, 3.89).
Outcome for wound infection favoured other regimens (OR: 2.15; 95% CI: 1.13, 4.11, p = 0.02).
There were no statistically significant differences in either the incidence of intra‐abdominal abscess (OR: 1.26; 95% CI: 0.40, 3.97), clinical sepsis (OR: 0.36; 95% CI: 0.01, 8.96), remote infection (OR: 0.43; 95% CI: 0.11, 1.73) or superinfection (OR: 0.88; 95% CI: 0.37, 2.12).
Adverse reactions did not show significant difference between the comparators and other regimens (OR: 0.90; 95% CI: 0.48, 1.67). Intention‐to‐treat analysis showed similar results (OR: 0.97, 95% CI: 0.70, 1.36).
Other secondary outcomes in terms of duration of therapy, days hospitalised and time to defervescence were not significantly different [(WMD: ‐0.22; 95% CI: ‐0.59, 0.15), (WMD: 0.00; 95% CI: ‐0.98, 0.98) and (WMD: 0.50; 95% CI: ‐0.21, 1.21) respectively].
Broad spectrum penicillins, antianaerobes and aminoglycoside One study (Leaper 1987) used a combination of ampicillin, metronidazole and gentamicin as their comparator. 43 evaluable patients were recruited in this study (24 patients in the broad spectrum penicillin, antianaerobe and aminoglycoside arm, and 19 in other regimen). There were no differences in either the incidence of all causes mortality (OR: 0.10; 95% CI: 0.00, 1.99) or mortality due to infection (OR: 0.14; 95% CI: 0.01, 3.16). There was no evidence that clinical (OR: 2.06; 95% CI: 0.31, 13.81) and microbiological (OR: 0.40; 95% CI: 0.02, 10.02) success were different between the two different regimens. Similarly, results for wound infection (OR: 0.37; 95% CI: 0.03, 4.42), remote infection (OR: 0.57; 95% CI: 0.14, 2.27), superinfection (OR: 0.77; 95% CI: 0.10, 6.06) and adverse reactions (OR: 0.78; 95% CI: 0.05, 13.39) did not show any difference.
Carbapenems The carbapenems were the second most commonly used antibiotics in this review ‐ 13 studies and 1591 patients (801 patients in the carbapenems arm, and 790 in the others). Out of these studies, eleven studies used imipenem/cilastatin and two, meropenem.
In assessing the mortality rate, no difference was demonstrated between carbapenems and other regimens in either all causes mortality (OR: 1.35; 95% CI: 0.40, 4.56) or mortality due to infection (OR: 0.78; 95% CI: 0.30, 2.03). Within the limited ITT analyses, the results did not differ between the former (OR: 1.04; 95% CI: 0.62, 1.76) and latter (OR: 0.75; 95%CI: 0.11, 5.03).
Primary outcome in terms of clinical success did not differ significantly (OR: 1.15; 95% CI: 0.78, 1.70). ITT analysis, similarly, did not show any differences (OR: 0.71; 95% CI: 0.47, 1.07).
Microbiological success was assessed by three studies but did not show any significant differences (OR: 1.10; 95% CI: 0.15, 8.19). ITT analysis, too, did not differ (OR: 0.78; 95% CI: 0.49, 1.24).
Results for other secondary outcomes did not differ between the carbapenems and other regimens: wound infection (OR: 0.73; 95% CI: 0.36, 1.49), intra‐abdominal abscess (OR: 1.15; 95% CI: 0.61, 2.18), clinical sepsis (OR: 0.97; 95% CI: 0.31, 3.01), remote infection (OR: 2.15; 95% CI: 0.61, 7.56), superinfection (OR: 1.01; 95% CI: 0.28, 3.64) and adverse reaction (OR: 1.28; 95% CI: 0.07, 21.86). ITT analysis for adverse reactions was performed for five studies and this did not show any significant differences (OR: 0.83; 95% CI: 0.63, 1.10).
Duration of therapy required was reported in two trials, but the results did not imply any significant difference (WMD: ‐0.49; 95% CI: ‐1.96, 0.98).
Hospitalised stay was assessed by one study and this showed significant difference in favour of carbapenems (WMD: ‐1.40; 95% CI: ‐2.47, ‐0.33; p = 0.01).
Time to defervescence was reported by one study and this significantly favoured carbapenems (WMD: ‐1.30; 95% CI: ‐1.98, ‐0.62; p = 0.0002).
Cephalosporins alone Nine studies used cephalosporins alone to compare against other regimens. 1115 evaluable patients were recruited by these studies (589 patients in the cephalosporins only arm, and 526 in the other regimens). One study (Berne 1982) used two cephalosporins (cefamandole and cefoperazone) independently to assess against other regimens in a tri‐arm study. Out of the nine studies, cefoxitin was the most frequently used cephalosporin with three studies comparing it against other regimens; two studies used cefotetan and cefamandole; and one each for cefoperazone, cefminox and cefuroxime.
Primary outcome in terms of mortality did not differ between the regimens. This was true for mortality due to all causes (OR: 0.65; 95% CI: 0.27, 1.57) and the ITT analysis (OR: 0.63; 95% CI: 0.10, 3.84), and mortality due to infection (OR: 1.21; 95% CI: 0.37, 3.89).
Clinical success (OR: 0.95; 95% CI: 0.54, 1.67) and the ITT analysis (OR: 1.25; 95% CI: 0.57, 2.74) did not differ significantly.
There were no significant differences in the secondary outcomes between cephalosporins and other regimens in terms of microbiological success (OR: 1.72; 95% CI: 0.62, 4.75), wound infection (OR: 1.08; 95% CI: 0.56, 2.05), development of intra‐abdominal abscess (OR: 1.07; 95% CI: 0.51, 2.26), clinical sepsis (OR: 1.05; 95% CI: 0.27, 4.19) and remote infection (OR: 1.31; 95% CI: 0.52, 3.30).
Adverse reactions were evaluable in two studies but did not show any significant difference (OR: 0.84; 95% CI: 0.29, 2.44). ITT analysis was performed separately in two other studies and this too did not show any difference (OR: 1.02; 95% CI: 0.65, 1.60).
There were no differences in the duration of therapy required (WMD: 0.40; 95% CI: ‐0.54, 1.34), days hospitalised (WMD: ‐0.30; 95% CI: ‐1.67, 1.07) and time to defervescence (WMD: 0.10; 95% CI: ‐0.60, 0.80) between cephalosporins alone and other regimens.
Cephalosporins and antianaerobes Six studies used a combination of cephalosporins and antianaerobes to compare against other regimens. 797 evaluable patients were recruited in these studies (372 patients in the cephalosporins / antianaerobes arm, and 425 in other regimens). Out of these, three studies used cefuroxime and one each for cefepime, cefotaxime and cephradine. All of these studies used metronidazole as the antianaerobic agent.
There were no differences in the reporting of mortality due to all causes (OR: 1.46; 95% CI: 0.57, 3.77) and the ITT analysis (OR: 0.07; 95% CI: 0.32, 2.34), and ITT analysis for mortality due to infection alone (OR: 5.45; 95% CI: 0.25, 116.63).
Five studies compared the clinical success between cephalosporins and antianaerobes versus other regimens, but the results were not significantly different (OR: 0.71; 95% CI: 0.29, 1.75). ITT analysis was only performed in one study and this did not show any difference (OR: 1.34; 95% CI: 0.83, 2.17).
Results for other secondary outcomes did not differ significantly ‐ microbiological success (OR: 0.78; 95% CI: 0.16, 3.81), wound infection (OR: 1.05; 95% CI: 0.51, 2.18), intra‐abdominal abscess (OR: 0.87; 95% CI: 0.24, 3.11), clinical sepsis (OR: 0.73; 95% CI: 0.19, 2.87), remote infection (OR: 3.77; 95% CI: 0.97, 14.72) and superinfection (OR: 3.19; 95% CI: 0.13, 81.25).
Adverse reactions (only ITT analysis results were available) did not show any significant difference (OR: 1.21; 95% CI: 067, 2.20).
Only one study reported results for duration of therapy required (WMD: ‐0.60; 95% CI:‐1.36, 0.16), days hospitalised (WMD: ‐0.90; 95% CI: ‐2.18, 0.38) and time to defervescence (WMD: ‐0.60; 95% CI: ‐1.58, 0.38), and all three outcomes were not significantly different.
Cephalosporins and beta lactamases inhibitor Two studies used a combination of cefoperazone and sulbactam to compare against other regimens (both used gentamicin and clindamycin). 176 evaluable patients were recruited (116 patients in the cefoperazone / sulbactam arm and 60 in the other regimens).
There was no difference in the outcome in terms of mortality ‐ mortality due to all causes (OR: 1.27; 95% CI: 0.29, 5.52) and mortality due to infection (OR: 1.03; 95% CI: 0.23, 4.68).
Both the studies compared the effects of cefoperazone / sulbactam against other regimens and found a significant difference in the clinical success in favour of the former regimen (OR: 3.21; 95% CI: 1.49, 6.92, p = 0.003).
Secondary outcomes in terms of microbiological success (OR: 2.51; 95% CI: 0.83, 7.57) and development of superinfection (OR: 0.45; 95% CI: 0.11, 1.82) did not differ significantly.
Adverse reactions were reported in one study and this did not show any significant difference (OR: 0.28; 95% CI: 0.05, 1.62).
Clindamycin versus metronidazole regimens One study (Smith 1984) used a combination of tobramycin and either clindamycin or metronidazole to compare the efficacy of both antianaerobic agents. 58 evaluable patients were recruited (23 patients in the clindamycin regimen and 35 in metronidazole).
There was no difference in either all causes mortality (OR: 1.60; 95% CI: 0.29, 8.71) or mortality due to infection (OR: 2.48; 95% CI: 0.38, 16.11).
Results for clinical success did not show any significant difference (OR: 0.59; 95% CI: 0.16, 2.11).
Secondary outcome in terms of adverse reactions did not differ (OR: 2.18; 95% CI: 0.34, 13.80) between the clindamycin and metronidazole regimens. The majority of adverse reactions were due to minor events.
Fluoroquinolones alone One study (Solomkin 2001) used clinafloxacin to compare against combination of imipenem and cilastatin. This study recruited 312 evaluable patients (150 in the clinafloxacin arm and 162 in the imipenem / cilastatin group).
There were no differences in the mortality rates in both regimens either in mortality due to all causes (ITT analysis) (OR: 1.69; 95% CI: 0.55, 5.23), mortality due to infection (OR: 2.18; 95% CI: 0.20, 24.24) and mortality due to infection (ITT analysis) (OR: 0.69; 95% CI: 0.11, 4.18).
Clinical success was not dissimilar between both regimens (OR: 1.12; 95% CI: 0.64, 1.98) and this is true for ITT analysis (OR: 1.28; 95% CI: 0.81, 2.01).
Secondary outcomes in terms of wound infection (OR: 1.08; 95% CI: 0.37, 3.17), development of intra‐abdominal abscess (OR: 0.80, 95% CI: 0.40, 1.60) and clinical sepsis (OR: 1.08; 95% CI: 0.21, 5.44) did not show any significant difference.
Adverse reactions (ITT analysis) was assessed and this favoured other regimens (OR: 1.47; 95% CI: 1.47, 2.14, p = 0.04). The majority of adverse reactions were due to mild events such as diarrhoea and nausea.
Fluoroquinolones and antianaerobes Two studies used a combination of fluoroquinolones and antianaerobes to compare against other regimens (piperacillin / tazobactam and gentamicin / metronidazole). One study employed ciprofloxacin and the other study used pefloxacin. Both studies used metronidazole as the antianaerobic agent. 642 evaluable patients were recruited by these studies (339 patients in the fluoroquinolones / antianaerobes arm and 303 in the other regimens).
There were no differences in the mortality rate between fluoroquinolones / antianaerobes and other regimens. This is true for either mortality due to all causes (OR: 0.73; 95% CI: 0.12, 4.50), mortality due to all causes (ITT analysis) (OR: 0.46; 95% CI: 0.04, 4.85), mortality due to infection (OR: 0.25; 95% CI: 0.01, 6.31) and mortality due to infection (ITT analysis) (OR: 0.31; 95% CI: 0.03, 3.04). For the latter two comparisons, both studies only reported either one or the other mortality, not both.
Both studies assessed the efficacy of fluoroquinolone and an antianaerobe against other regimens in terms of clinical success and showed significant difference in favour of the former (OR: 1.74; 95% CI: 1.11, 2.73, p = 0.02). However, only one of the studies used ITT analysis and this was not significantly different (OR: 1.35; 95% CI: 0.84, 2.18).
Secondary outcomes in terms of microbiological success (OR: 1.45; 95% CI: 0.85, 2.46) and superinfection (OR: 0.70; 95% CI: 0.31, 1.58) did not show any significant difference. However, the effectiveness of antibiotic regimens in preventing development of wound infection tended to favour flouroquinolones / antianaerobes (OR: 0.50; 95% CI: 0.26, 0.99, p = 0.05).
Adverse reactions (ITT analysis) did not differ significantly between flouroquinolones plus antianaerobes and other regimens (OR: 1.06; 95% CI: 0.56, 2.02)/
Monobactams and antianaerobes Two studies (Berne 1987, de Groot 1993) used a combination of aztreonam and clindamycin to compare against other regimens (gentamicin / clindamycin and imipenem / cilastatin). Both these studies recruited 164 evaluable patients (98 patients in the monobactams / antianaerobes arm and 66 in the other regimens).
Only one study (de Groot 1993) reported its mortality rate for both all causes (OR: 0.90; 95% CI: 0.12, 6.72) and mortality due to infection (OR: 0.44; 95% CI: 0.04, 5.05), both of which were not significantly different.
Outcome in terms of clinical success did not differ between monobactams plus antianaerobes and other regimens (OR: 0.69; 95% CI: 0.28, 1.71).
Secondary outcomes in the form of wound infection (OR; 1.20; 95% CI: 0.40, 3.64), development of intra‐abdominal abscess (OR: 1.85; 95% CI: 0.16, 21.26), clinical sepsis (OR: 1.38; 95% CI: 0.22, 8.77), remote infection (OR: 0.17; 95% CI: 0.01, 3.69) and superinfection (OR: 1.85; 95% CI: 0.16, 21.26) were reported by one study (de Groot 1993) and these did not show any significant difference.
Adverse reactions were reported by one study (Berne 1987) and it showed a significant difference in favour of monobactam and antianaerobe (OR: 0.19; 95% CI: 0.07, 0.54, p = 0.002). The majority of events were due to minor reactions.
Duration of therapy (WMD: ‐0.42; 95% CI: ‐1.16, 0.32), days hospitalised (WMD: ‐0.37; 95% CI: ‐1.35, 0.61) and time to defervescence were reported by one study (Berne 1987) and this did not differ significantly.
Imipenem/cilastatin versus other carbapenems Five studies used a combination of imipenem / cilastatin to compare against other carbapenems. 667 evaluable patients were recruited (326 patients in the imipenem / cilastatin arm and 341 in other regimens). Out of these studies, four studies were compared against meropenem and one biapenem.
ITT analysis was performed for both mortality due to all causes (OR: 1.450; 95% CI: 0.58, 3.88) and mortality due to infection (OR: 1.79; 95% CI: 0.50, 6.42), both of which did not differ significantly.
Clinical success (OR: 1.04; 95% CI: 0.62, 1.77) and its ITT analysis (OR: 0.61; 95% 0.22, 1.65) did not shown any significant difference.
There were no difference in either one of the measured secondary outcomes in terms of microbiological success (OR: 0.99, 95% CI: 0.53, 1.87) or superinfection (OR: 0.75; 95% CI: 0.26, 2.18).
ITT analysis for adverse reactions did not differ between monobactams / antianaerobes and other regimens (OR: 1.30; 95% CI: 0.81, 2.10).
Outcome in terms of duration of treatment required was assessed by one study (Zanetti 1999), and this tended to favour the combination of imipenem / cilastatin (WMD: ‐1.10; 95% CI: ‐2.20, 0.00, p = 0.05).
Hospitalised stay did not differ between combination of imipenem / cilastatin and other carbapenems in one single study that assessed this outcome (Zanetti 1999).
Isepamicin and antianaerobes versus amikacin and antianaerobe One study (Leal del Rosal 1995) used combination of isepamicin plus metronidazole to compare against amikacin plus metronidazole. 267 evaluable patients were recruited in this study (178 patients in the isepamicin combination and 89 in the amikacin arm).
There were no differences seen in the clinical success (OR: 0.62; 95% CI: 0.21, 1.77) and its ITT analysis (OR: 0.42; 95% CI: 0.17, 1.07).
Secondary outcomes did not significantly differ between both regimens in either the microbiological success (OR: 0.95; 95% CI: 0.47, 1.92), wound infection (OR: 0.60; 95% CI: 0.18, 2.06) and superinfection (OR: 0.77; 95% CI: 0.13, 4.74).
Adverse reactions did not show significant difference in either of the regimens (OR: 0.88; 95% CI: 0.37, 2.07).
Discussion
This review aimed to compare the efficacy of different antibiotic regimens in the capacity of an adjunctive agent in the treatment of secondary peritonitis in adults. 40 randomised controlled trials were identified from the literature that fitted the criteria for evaluability. The selected trials were heterogeneous in their patient population, underlying aetiological factors, source control and antibiotic regimens. These trials exhibited inconsistency further in the reporting of outcomes, either in the primary or secondary outcomes.
All randomised controlled trials comparing one antibiotic regimen against another were considered for evaluability. The authors did not encounter trials comparing antibiotic regimens against placebo, as the use of antibiotics for secondary peritonitis has been a well accepted practice and it would have been unethical to compare antibiotics against placebo in these circumstances. Due to the lack of well designed randomised controlled trials, this review has been unable to evaluate effectiveness of different dosing regimens and length of administration in the treatment of intra‐abdominal infection. The review therefore only focused on the post‐operative outcomes of patients treated with different antibiotic regimens in secondary peritonitis. Similarly, most studies omitted sub‐group analysis, and therefore, this was also excluded from the review.
The combination of aminoglycosides (commonly gentamicin) and clindamycin has long been a 'gold standard' regimen in the treatment of intra‐abdominal infection. Development of less nephrotoxic and ototoxic agents such as broad spectrum penicillins with beta lactamase inhibitor and carbapenems (such as the new Ertapenem) has precipitated numerous studies attempting to demonstrate the superiority of these antibiotics in the treatment of bacterial peritonitis. However, an accurate assessment of the antimicrobial therapy of intra‐abdominal infection of enteric origin is complicated by several factors, which include the patient's physiological reserves, site and cause of infection, the wide variety of pathogenic organisms involved and the effect of previous hospitalisation or antibiotic therapy.
There were huge discrepancies in the reporting of primary outcomes (mortality and clinical success) in the studies evaluated. The inconsistencies were more pronounced with the former outcome. For the purpose of this review, the authors have subdivided mortality into all cause mortality and mortality due to infection, and have utilised clinical cure as the definition of clinical success. None of the antibiotic regimens demonstrated significant difference in terms of the all cause mortality and mortality due to infection. ITT analyses similarly, showed the same conclusion. In further assessing the primary outcome in terms of clinical success, regimens utilising aminoglycoside plus an antianaerobic agent were compared against other regimens. The results were significantly different in favour of the latter (OR: 0.65; 95% CI: 0.46, 0.92) (p = 0.02). Furthermore, outcome for clinical success was highly significant in all cause peritonitis (where studies with peritonitis purely due to appendicitis were excluded) (OR: 0.57; 95% CI: 0.41, 0.78) (p = 0.0005). This perhaps may be as a result of the inherent bias in the conduct of these studies as most of the these studies were designed with the aim of comparing newer agents against the old 'gold standard' ‐ aminoglycosides and clindamycin. Clinical success was also significantly different in comparing regimens using cephalosporin plus a beta‐lactamase inhibitor (cefoperazone and sulbactam) against other regimens, in favour of the former (OR: 3.21; 95% CI: 1.49, 6.92) (p = 0.003). It perhaps, may be worth noting that, in conducting these studies, gentamicin and clindamycin were again used as the comparator. Fluoroquinolones and antianaerobes similarly showed statistically significant efficacy when compared against other regimens (OR: 1.74; 95% CI: 1.11, 2.73) (p = 0.02). Both studies (Greenberg 1994, Jauregui 1990) compared this regimen against piperacillin plus tazobactam and gentamicin plus metronidazole. Tests for heterogeneity were not significantly different. None of the other regimens had statistically significant efficacy in terms of clinical success.
Surprisingly, the outcome for microbiological success was significantly different in favour of other regimens when these were compared to regimens comprising aminoglycosides and antianaerobes (OR: 0.49; 95% CI: 0.31, 0.76) (p =0.001). Despite the effectiveness of the combination of aminoglycosides and antianaerobic agents in vitro, in reality, confounding factors such as bacterial synergism and the host response further detract its potency in vivo. Other regimens using a combination of cephalosporins plus beta‐lactamase inhibitors and fluoroquinolones plus antianaerobes appeared to have demonstrated effectiveness of both regimens when compared with other regimens in microbiological efficacy, but they did not reach statistically significant differences.
Secondary outcomes in terms of wound infection was statistically different in preference to other regimens when broad spectrum penicillins with beta‐lactamase inhibitors were compared to the former (OR:2.15; 95% CI: 1.13, 4.11) (p = 0.02). The incidence of wound infection appeared better controlled with a combination of fluoroquinolones and antianaerobes but this did not achieve a statistically significant difference. Remote infection appeared to favour regimens using cephalosporins plus antianaerobes (when compared to others), and superinfection, when compared to studies using aminoglycosides plus antianaerobes, tended to favour other antibiotic combinations. However, both these and the remaining secondary outcomes (development of intra‐abdominal abscess, clinical sepsis, remote infection and superinfection) were not statistically different between the various regimens.
Adverse reactions arising from the numerous antibiotic regimens were difficult to interpret due to the paucity or unclear data presentation. Most of the complications reported were minor, for example diarrhoea, nausea and vomiting. This may be attributed to the selection bias inherent in these studies and to the manner in which trials are governed by the different authorities to safeguard patients's safety. As a result of the highly selective and extensive exclusion criteria, adverse events were few and far between. Despite the initial concern regarding the toxic effects of aminoglycosides, there were very few reported adverse events seen in patients in the studies utilising a combination of antibiotics incorporating this group of drugs. This may be due to the stringency in which aminoglycosides levels were monitored and optimised (Fink 1989), and in careful selection of the study population. Only one study assessed the adverse effects of monobactams and antianaerobes against other regimens and this appeared to favour the former (OR: 0.19; 95% CI: 0.07, 0.54) (p = 0.002). None of the other antibiotic regimens were able to demonstrate a statistically significant difference in the incidence of adverse reactions between the arms.
The dearth of data in the studies impeded the accurate interpretation of the effectiveness of different antibiotic regimens in reducing hospital stay. Only two antibiotic regimens showed a statistically significant difference. These were in the comparisons between aminoglycosides plus antianaerobes and other regimens, favouring the latter (WMD: 0.57; 95% CI: 0.06, 1.07) (p = 0.03); and in carbapenems versus other regimens, favouring the former (WMD: ‐1.40; 95% CI: ‐2.47, ‐0.33) (p = 0.01). Similarly carbapenems appeared to be better at reducing time to defervescence when compared to other regimens (WMD: ‐1.30; 95% CI: ‐1.98, ‐0.62) (p = 0.0002). The duration of treatment required appeared to favour imipenem/cilastatin but this did not reach numerically significant levels. No other statistically significant differences were demonstrated by other regimens.
It may be prudent to mention that some well designed studies were excluded from the final meta‐analysis because of incorporation of concomitant antifungal therapy or other non‐study antibiotic such as vancomycin (Barie 1997, Scheinin 1994, Solomkin 1990, Solomkin 1996, Solomkin 2003).
Authors' conclusions
Implications for practice.
In acute life‐threatening surgical infections requiring immediate institution of antimicrobial therapy, antibiotic treatment must be empirical. The selection of empirical antibacterial therapy must take into consideration microbial factors such as the presumed spectrum of the bacterial contamination of the peritoneal cavity, as well as their pathogenicity and synergism. It must also considers drug factor, including pharmacokinetics, toxic effects, and adverse effects of the proposed regimen (Christou 1996). The current treatment options (such as carbapenems, beta lactams/beta lactamase inhibitor combinations, or a combination of antianaerobic agent with either aminoglycoside or ciprofloxacin) for complicated intra‐abdominal infections have several disadvantages. More than one agent is typically required empirically for adequate coverage of common intra‐abdominal pathogens.The initial parenterally administered therapy may not be available as an oral formulation. As the patient improves, the clinician is faced with the decision of continuing intravenous therapy of proven efficacy or prescribing an alternative oral agent, which may not be as effective or as well tolerated in that patient (Luke 1999). Another feature of most of the currently used agents for the treatment of intra‐abdominal infections is the need for multiple daily dosing to achieve acceptable efficacy. Repeated administration of intravenous infusions is time consuming and can increase indirect treatment costs. The monitoring of serum creatinine levels is an essential requirement for many renal‐excreted antibiotics, including imipenem/cilastatin. If the dose is not adjusted in patients with impaired renal function, seizures can result. Furthermore, laboratory testing adds to the cost of patientss care.
This review has shown the comparability of different antibiotic regimens in achieving clinical and microbiological success, and in reducing mortality. Within the limited and small numbered studies available for this meta‐analysis, the combination of fluoroquinolones/antianaerobes and cephalosporins/beta‐lactamase inhibitors appeared to be statistically more effective clinically.
There was no conclusive evidence to suggest that one regimen has slightly higher adverse reactions compared to another, but as previously discussed, this may be attributed to the inherent bias in which clinical trials are conducted and governed. Despite the well‐known toxicity of aminoglycosides, this group of drugs did not show significant differences in their adverse profiles. The addition of an aminoglycoside to the treatment regimen has many theoretical advantages: (i) a broader spectrum of activity, (ii) increased synergy, (iii) increased bactericidal effect and (iv) prevention of emergence of resistant strains. Results from Dupont 2000, however, do not support the routine addition of aminoglycoside such as amikacin to piperacillin/tazobactam.
No specific recommendations can be made for the first line treatment of secondary peritonitis in adults with antibiotics as all regimens showed equivocal efficacy. Other factors such as local guidelines and preferences, ease of administration, costs and availability must therefore be taken into consideration in deciding the antibiotic regimen of choice.
Implications for research.
It is often difficult to directly attribute outcome following intra‐abdominal infection to the antimicrobial regimen due to the multifactorial nature of the infection. The key determinant of outcome is the source control to deal with the site of contamination and consequence of infection. An adequate surgical procedure is generally agreed on and involves drainage of all fluid collections, closure or resection of any openings into the gastrointestinal tract and resection of inflamed or necrotic tissue.
The primary concern in the context of clinical research is that the adequacy of intervention is one of the many independent variables determining outcome. Other key factors such as patients' physiological reserves, background co‐morbidity and nutritional status also play an important role. With a small number of such patients, there is the real possibility that such patients would not be evenly distributed by randomisation and would therefore skew results. The importance of patient stratification using well established severity scoring system such APACHE II (Knaus 1985) and POSSUM (Copeland 2002) cannot be overstated as this may allow better corrrelation between the regimens and their efficacy amongst the studies.
The need for further trials comparing newer, broad spectrum agents with less toxic effects, requiring no monitoring of serum levels, single dosing and availability of oral formulation with equivalent bioavailability is warranted. Larger, multi‐centred trials in the future should therefore attempt to
stratify patients prior to randomisation
adhere to better standard of outcome definition and reporting
consider sub‐group analysis ‐ with respect to underlying presumed aetiological factors
perform intention‐to treat analysis
avoid the use of non‐study antibiotics
What's new
| Date | Event | Description |
|---|---|---|
| 26 March 2012 | Amended | Additional table linked to text. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 23 July 2008 | Amended | Converted to new review format. |
| 19 January 2005 | New citation required and conclusions have changed | Substantive amendment. |
Notes
Published protocol entitled: Antibiotics for secondary peritonitis in adults
Acknowledgements
None
Data and analyses
Comparison 1. Aminoglycosides and antianaerobes versus any other regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all causes) | 5 | 581 | Odds Ratio (M‐H, Random, 95% CI) | 2.03 [0.88, 4.71] |
| 2 Mortality (all causes ‐ ITT analysis) | 3 | 747 | Odds Ratio (M‐H, Random, 95% CI) | 2.10 [0.78, 5.65] |
| 3 Mortality (due to infection) | 5 | 541 | Odds Ratio (M‐H, Random, 95% CI) | 1.51 [0.66, 3.43] |
| 4 Clinical success | 19 | 1956 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.46, 0.92] |
| 4.1 Overall | 13 | 1336 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.41, 0.78] |
| 4.2 Appendix | 6 | 620 | Odds Ratio (M‐H, Random, 95% CI) | 1.36 [0.44, 4.14] |
| 5 Clinical success (ITT analysis) | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.15, 1.43] |
| 5.1 Overall | 1 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.15, 1.43] |
| 6 Microbiological success | 6 | 579 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.31, 0.76] |
| 7 Microbiological success (ITT analysis) | 1 | 139 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.40, 2.20] |
| 8 Wound infection | 9 | 913 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.35, 2.02] |
| 9 Intra‐abdominal abscess | 7 | 677 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.40, 1.83] |
| 10 Clinical sepsis | 2 | 195 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [0.07, 31.21] |
| 11 Remote infection | 1 | 112 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.37, 3.47] |
| 12 Superinfection | 3 | 401 | Odds Ratio (M‐H, Random, 95% CI) | 2.15 [0.89, 5.17] |
| 13 Adverse reactions | 7 | 707 | Odds Ratio (M‐H, Random, 95% CI) | 1.76 [0.87, 3.53] |
| 13.1 Overall | 3 | 340 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.31, 3.44] |
| 13.2 Major adverse reactions | 1 | 76 | Odds Ratio (M‐H, Random, 95% CI) | 3.60 [0.62, 21.06] |
| 13.3 Minor adverse reactions | 3 | 291 | Odds Ratio (M‐H, Random, 95% CI) | 2.32 [0.89, 6.06] |
| 14 Adverse reactions (ITT analysis) | 4 | 625 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.43, 1.11] |
| 14.1 Overall | 3 | 529 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.49, 1.47] |
| 14.2 Minor adverse reactions | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.16, 1.00] |
| 15 Duration of therapy | 6 | 567 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.05, 0.80] |
| 16 Days hospitalised | 5 | 490 | Mean Difference (IV, Random, 95% CI) | 0.57 [0.06, 1.07] |
| 17 TIme to defervescence | 5 | 490 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.29, 1.05] |
1.1. Analysis.
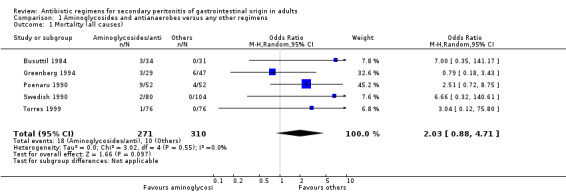
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 1 Mortality (all causes).
1.2. Analysis.
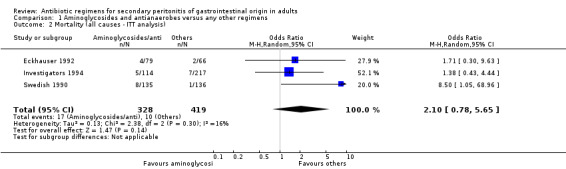
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 2 Mortality (all causes ‐ ITT analysis).
1.3. Analysis.
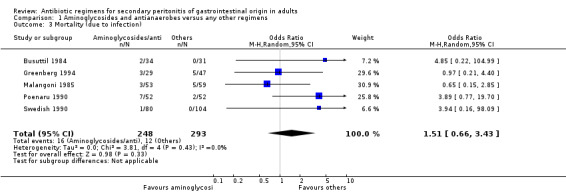
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 3 Mortality (due to infection).
1.4. Analysis.
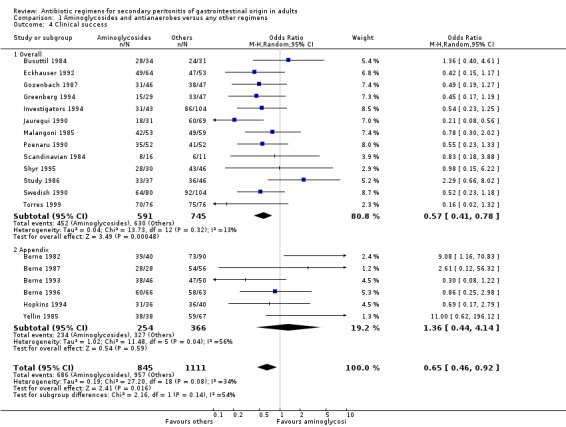
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 4 Clinical success.
1.5. Analysis.
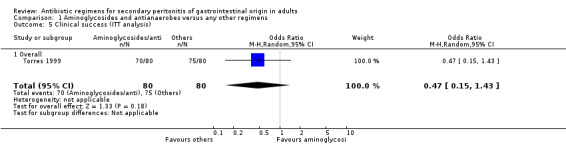
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 5 Clinical success (ITT analysis).
1.6. Analysis.
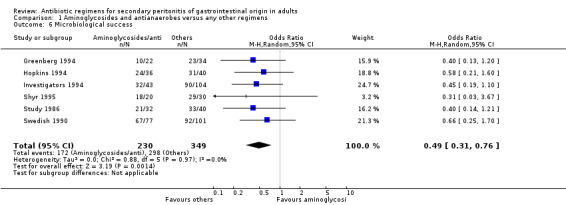
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 6 Microbiological success.
1.7. Analysis.
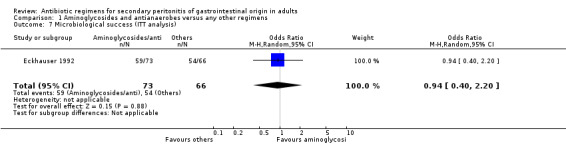
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 7 Microbiological success (ITT analysis).
1.8. Analysis.
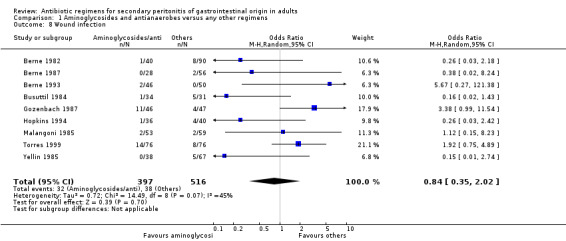
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 8 Wound infection.
1.9. Analysis.
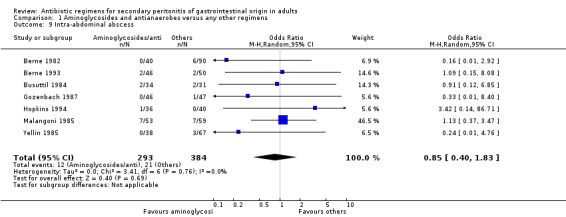
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 9 Intra‐abdominal abscess.
1.10. Analysis.
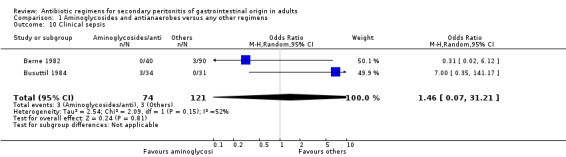
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 10 Clinical sepsis.
1.11. Analysis.
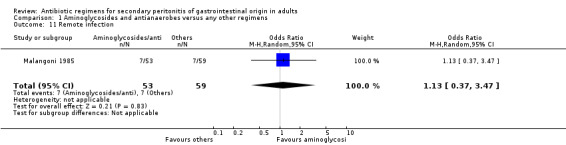
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 11 Remote infection.
1.12. Analysis.
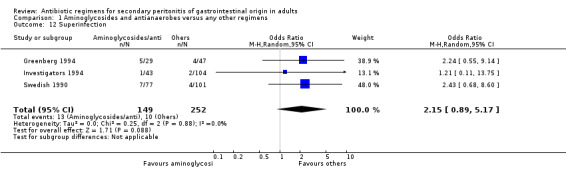
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 12 Superinfection.
1.13. Analysis.
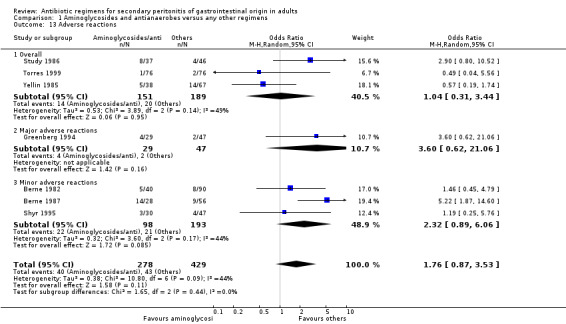
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 13 Adverse reactions.
1.14. Analysis.
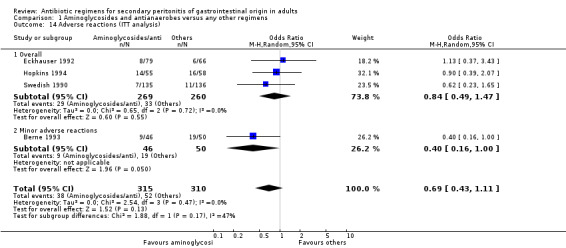
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 14 Adverse reactions (ITT analysis).
1.15. Analysis.
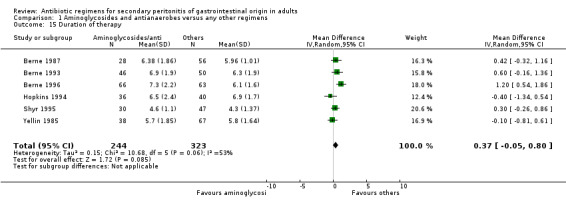
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 15 Duration of therapy.
1.16. Analysis.
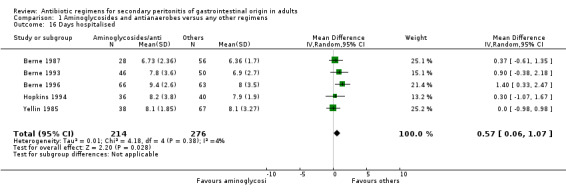
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 16 Days hospitalised.
1.17. Analysis.
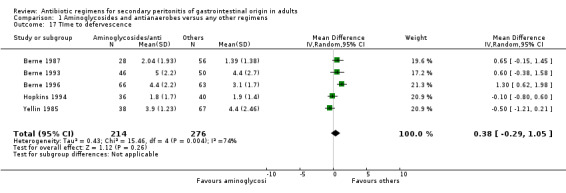
Comparison 1 Aminoglycosides and antianaerobes versus any other regimens, Outcome 17 TIme to defervescence.
Comparison 2. Aminoglycosides and broad spectrum penicillins with beta lactamase inhibitors versus other regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all causes ‐ ITT analysis) | 1 | 204 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.37, 1.97] |
| 2 Clinical success | 1 | 159 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.55, 1.91] |
| 3 Clinical success (ITT analysis) | 1 | 204 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.65, 1.97] |
| 4 Adverse reactions (ITT analysis) | 1 | 227 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.71, 2.04] |
| 4.1 Overall | 1 | 227 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.71, 2.04] |
| 5 Duration of therapy | 1 | 159 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.47, 1.47] |
2.1. Analysis.
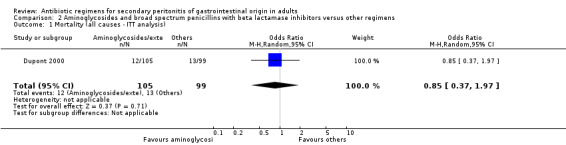
Comparison 2 Aminoglycosides and broad spectrum penicillins with beta lactamase inhibitors versus other regimens, Outcome 1 Mortality (all causes ‐ ITT analysis).
2.2. Analysis.
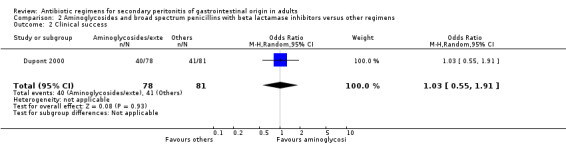
Comparison 2 Aminoglycosides and broad spectrum penicillins with beta lactamase inhibitors versus other regimens, Outcome 2 Clinical success.
2.3. Analysis.
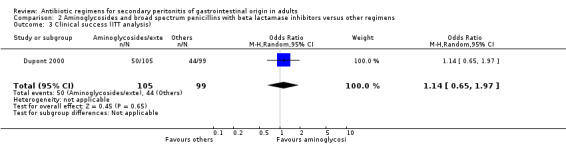
Comparison 2 Aminoglycosides and broad spectrum penicillins with beta lactamase inhibitors versus other regimens, Outcome 3 Clinical success (ITT analysis).
2.4. Analysis.
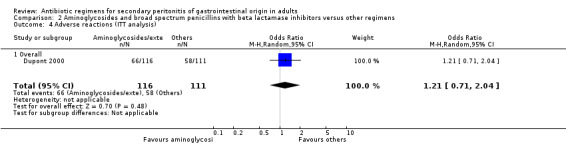
Comparison 2 Aminoglycosides and broad spectrum penicillins with beta lactamase inhibitors versus other regimens, Outcome 4 Adverse reactions (ITT analysis).
2.5. Analysis.
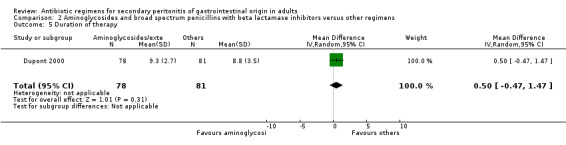
Comparison 2 Aminoglycosides and broad spectrum penicillins with beta lactamase inhibitors versus other regimens, Outcome 5 Duration of therapy.
Comparison 3. Aminoglycoside, penicillin and antianaerobes versus other regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all causes) | 1 | 107 | Odds Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.24] |
| 2 Clinical success | 1 | 107 | Odds Ratio (M‐H, Random, 95% CI) | 1.92 [0.51, 7.17] |
| 2.1 Overall | 1 | 107 | Odds Ratio (M‐H, Random, 95% CI) | 1.92 [0.51, 7.17] |
| 3 Wound infection | 1 | 107 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.21, 3.08] |
3.1. Analysis.
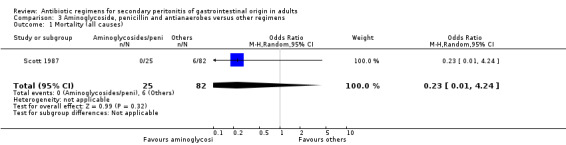
Comparison 3 Aminoglycoside, penicillin and antianaerobes versus other regimens, Outcome 1 Mortality (all causes).
3.2. Analysis.
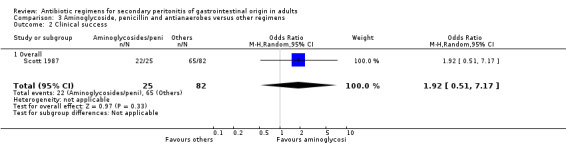
Comparison 3 Aminoglycoside, penicillin and antianaerobes versus other regimens, Outcome 2 Clinical success.
3.3. Analysis.
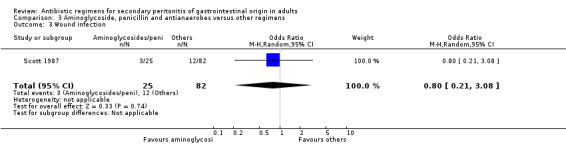
Comparison 3 Aminoglycoside, penicillin and antianaerobes versus other regimens, Outcome 3 Wound infection.
Comparison 4. Broad spectrum penicillins alone versus other regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all causes) | 1 | 83 | Odds Ratio (M‐H, Random, 95% CI) | 1.84 [0.29, 11.65] |
| 2 Clinical success | 1 | 83 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.53, 3.43] |
| 2.1 Overall | 1 | 83 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.53, 3.43] |
| 3 Wound infection | 1 | 83 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.28, 5.19] |
| 4 Intra‐abdominal abscess | 1 | 83 | Odds Ratio (M‐H, Random, 95% CI) | 1.2 [0.23, 6.32] |
| 5 Remote infection | 1 | 83 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.07, 1.03] |
| 6 Adverse reactions | 1 | 83 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.07, 19.67] |
| 6.1 Minor adverse reactions | 1 | 83 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.07, 19.67] |
4.1. Analysis.
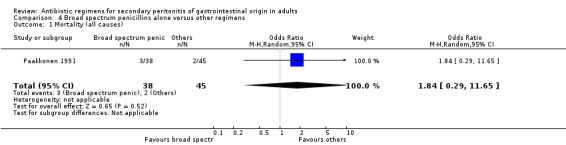
Comparison 4 Broad spectrum penicillins alone versus other regimens, Outcome 1 Mortality (all causes).
4.2. Analysis.
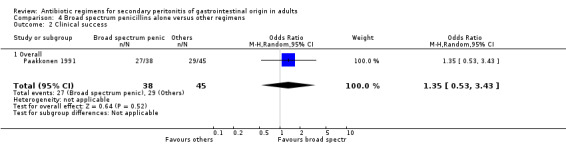
Comparison 4 Broad spectrum penicillins alone versus other regimens, Outcome 2 Clinical success.
4.3. Analysis.
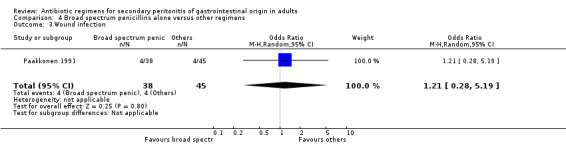
Comparison 4 Broad spectrum penicillins alone versus other regimens, Outcome 3 Wound infection.
4.4. Analysis.
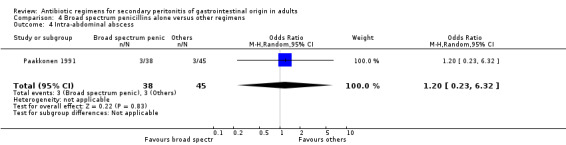
Comparison 4 Broad spectrum penicillins alone versus other regimens, Outcome 4 Intra‐abdominal abscess.
4.5. Analysis.
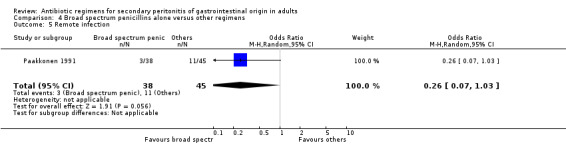
Comparison 4 Broad spectrum penicillins alone versus other regimens, Outcome 5 Remote infection.
4.6. Analysis.
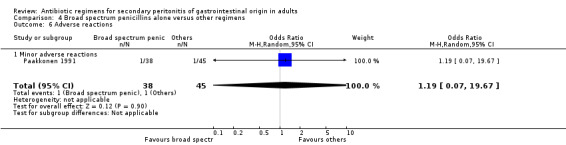
Comparison 4 Broad spectrum penicillins alone versus other regimens, Outcome 6 Adverse reactions.
Comparison 5. Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all causes) | 2 | 444 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.09, 2.38] |
| 2 Mortality (all causes ‐ ITT analysis) | 2 | 662 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.54, 1.76] |
| 3 Mortality (due to infection) | 1 | 159 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.05, 6.08] |
| 4 Mortality (due to infection ‐ ITT analysis) | 1 | 458 | Odds Ratio (M‐H, Random, 95% CI) | 3.19 [0.33, 30.91] |
| 5 Clinical success | 9 | 1289 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.68, 1.92] |
| 5.1 Overall | 8 | 1184 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.74, 2.02] |
| 5.2 Appendix | 1 | 105 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.62] |
| 6 Clinical success (ITT analysis) | 3 | 668 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.56, 2.66] |
| 7 Microbiological success | 5 | 557 | Odds Ratio (M‐H, Random, 95% CI) | 1.84 [0.87, 3.89] |
| 8 Wound infection | 3 | 584 | Odds Ratio (M‐H, Random, 95% CI) | 2.15 [1.13, 4.11] |
| 9 Intra‐abdominal abscess | 3 | 461 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [0.40, 3.97] |
| 10 Clinical sepsis | 1 | 159 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.01, 8.96] |
| 11 Remote infection | 1 | 197 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.11, 1.73] |
| 12 Superinfection | 3 | 487 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.37, 2.12] |
| 13 Adverse reactions | 4 | 378 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.48, 1.67] |
| 13.1 Overall | 3 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.39, 2.02] |
| 13.2 Minor | 1 | 77 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.17, 4.03] |
| 14 Adverse reactions (ITT analysis) | 3 | 1070 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.70, 1.36] |
| 14.1 Overall | 2 | 612 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.63, 1.33] |
| 14.2 Major | 1 | 458 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.58, 2.56] |
| 15 Duration of therapy | 4 | 500 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.59, 0.15] |
| 16 Days hospitalised | 1 | 105 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.98, 0.98] |
| 17 Time to defervescence | 1 | 105 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.21, 1.21] |
5.1. Analysis.
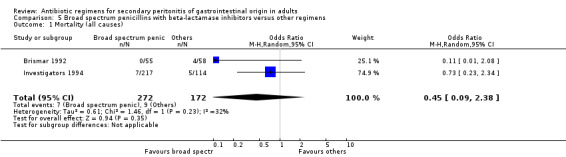
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 1 Mortality (all causes).
5.2. Analysis.
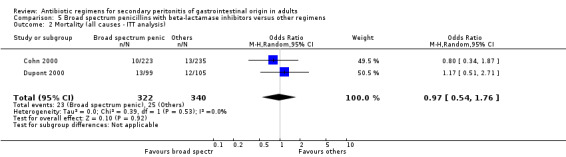
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 2 Mortality (all causes ‐ ITT analysis).
5.3. Analysis.
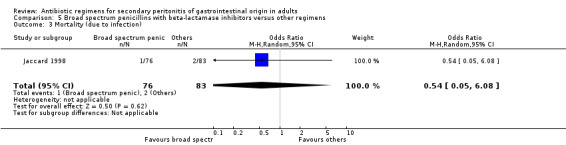
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 3 Mortality (due to infection).
5.4. Analysis.
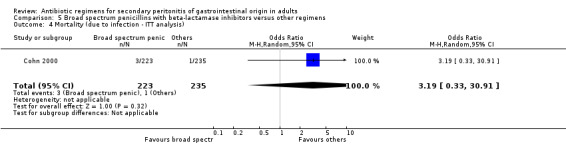
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 4 Mortality (due to infection ‐ ITT analysis).
5.5. Analysis.
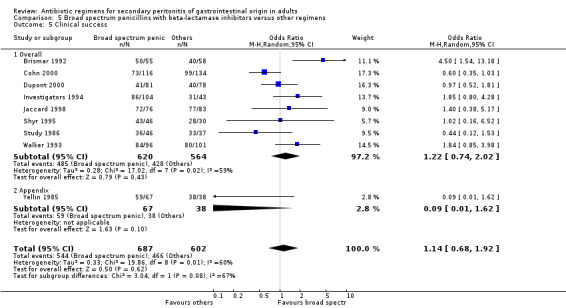
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 5 Clinical success.
5.6. Analysis.
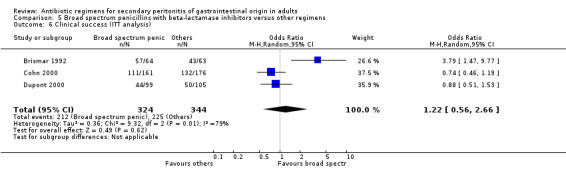
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 6 Clinical success (ITT analysis).
5.7. Analysis.
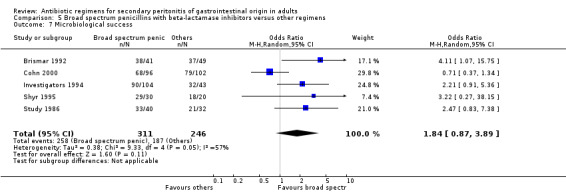
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 7 Microbiological success.
5.8. Analysis.
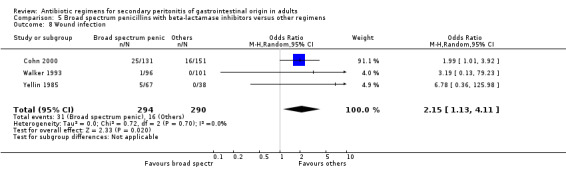
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 8 Wound infection.
5.9. Analysis.
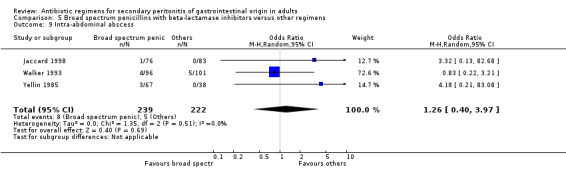
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 9 Intra‐abdominal abscess.
5.10. Analysis.
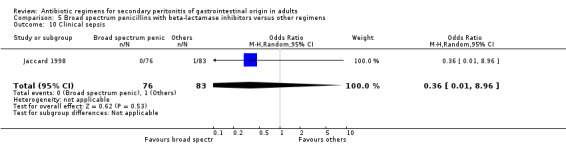
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 10 Clinical sepsis.
5.11. Analysis.
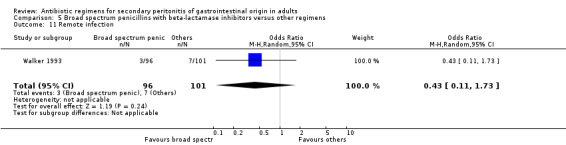
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 11 Remote infection.
5.12. Analysis.
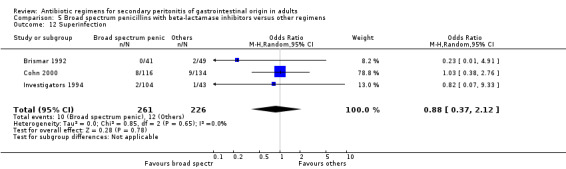
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 12 Superinfection.
5.13. Analysis.
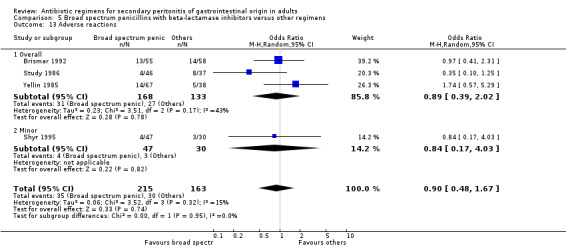
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 13 Adverse reactions.
5.14. Analysis.
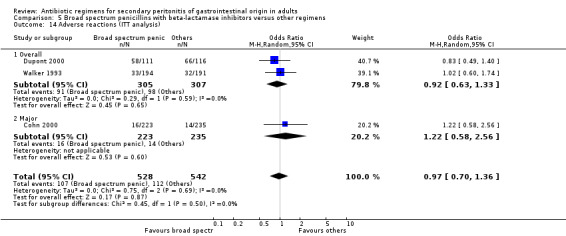
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 14 Adverse reactions (ITT analysis).
5.15. Analysis.
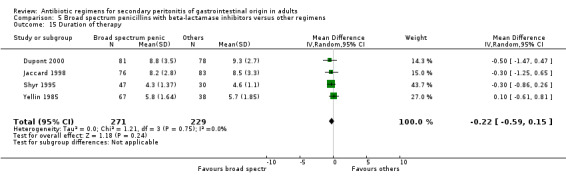
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 15 Duration of therapy.
5.16. Analysis.
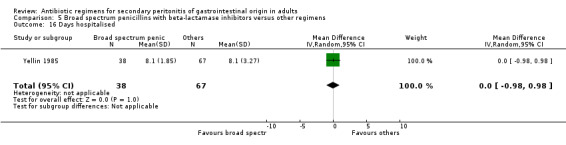
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 16 Days hospitalised.
5.17. Analysis.
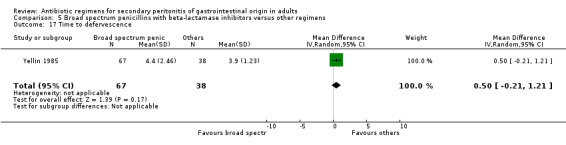
Comparison 5 Broad spectrum penicillins with beta‐lactamase inhibitors versus other regimens, Outcome 17 Time to defervescence.
Comparison 6. Broad spectrum penicillins, aminoglycosides and antianaerobes versus other regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all causes) | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.00, 1.99] |
| 2 Mortality (due to infection) | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 3.16] |
| 3 Clinical success | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 2.06 [0.31, 13.81] |
| 3.1 Overall | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 2.06 [0.31, 13.81] |
| 4 Microbiological success | 1 | 9 | Odds Ratio (M‐H, Random, 95% CI) | 0.4 [0.02, 10.02] |
| 5 Wound infection | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.03, 4.42] |
| 6 Remote infection | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.14, 2.27] |
| 7 Superinfection | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.10, 6.06] |
| 8 Adverse reactions | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.05, 13.39] |
| 8.1 Minor adverse reactions | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.05, 13.39] |
6.1. Analysis.
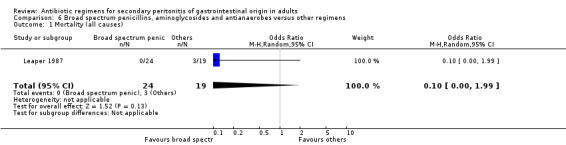
Comparison 6 Broad spectrum penicillins, aminoglycosides and antianaerobes versus other regimens, Outcome 1 Mortality (all causes).
6.2. Analysis.
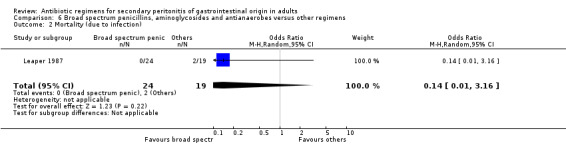
Comparison 6 Broad spectrum penicillins, aminoglycosides and antianaerobes versus other regimens, Outcome 2 Mortality (due to infection).
6.3. Analysis.
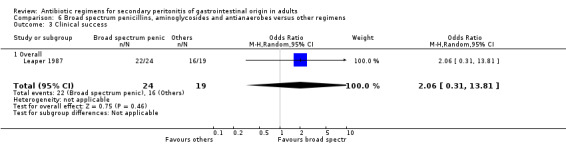
Comparison 6 Broad spectrum penicillins, aminoglycosides and antianaerobes versus other regimens, Outcome 3 Clinical success.
6.4. Analysis.
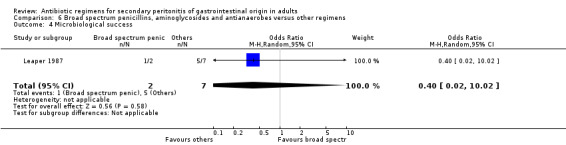
Comparison 6 Broad spectrum penicillins, aminoglycosides and antianaerobes versus other regimens, Outcome 4 Microbiological success.
6.5. Analysis.
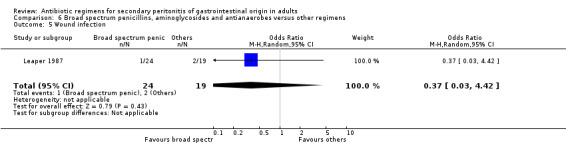
Comparison 6 Broad spectrum penicillins, aminoglycosides and antianaerobes versus other regimens, Outcome 5 Wound infection.
6.6. Analysis.
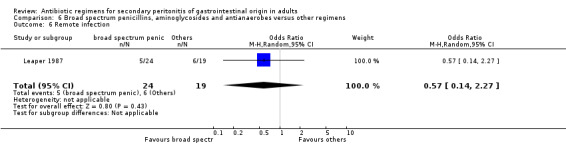
Comparison 6 Broad spectrum penicillins, aminoglycosides and antianaerobes versus other regimens, Outcome 6 Remote infection.
6.7. Analysis.
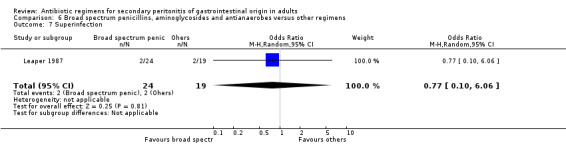
Comparison 6 Broad spectrum penicillins, aminoglycosides and antianaerobes versus other regimens, Outcome 7 Superinfection.
6.8. Analysis.
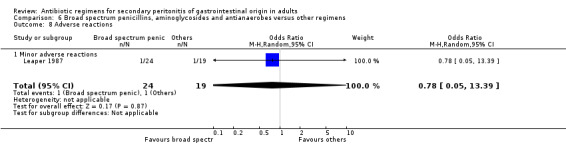
Comparison 6 Broad spectrum penicillins, aminoglycosides and antianaerobes versus other regimens, Outcome 8 Adverse reactions.
Comparison 7. Carbapenems versus other regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all causes) | 5 | 494 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.40, 4.56] |
| 2 Mortality (all causes ‐ITT analysis) | 5 | 1496 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.62, 1.76] |
| 3 Mortality (due to infection) | 6 | 852 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.30, 2.03] |
| 4 Mortality (due to infection ‐ ITT analysis) | 2 | 623 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.11, 5.03] |
| 5 Clinical success | 13 | 1720 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.78, 1.70] |
| 5.1 Overall | 12 | 1591 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.76, 1.75] |
| 5.2 Appendix | 1 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.34, 4.01] |
| 6 Clinical success (ITT analysis) | 4 | 1384 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.47, 1.07] |
| 7 Microbiological success | 3 | 164 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.15, 8.19] |
| 8 Microbiological success (ITT analysis) | 2 | 654 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.49, 1.24] |
| 9 Wound infection | 4 | 528 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.36, 1.49] |
| 10 Intra‐abdominal abscess | 4 | 644 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.61, 2.18] |
| 11 Clinical sepsis | 3 | 551 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.31, 3.01] |
| 12 Remote infection | 2 | 123 | Odds Ratio (M‐H, Random, 95% CI) | 2.15 [0.61, 7.56] |
| 13 Superinfection | 4 | 278 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.28, 3.64] |
| 14 Adverse reactions | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.07, 21.86] |
| 14.1 Overall | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.07, 21.86] |
| 15 Adverse reactions (ITT analysis) | 5 | 1396 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.10] |
| 15.1 Overall | 4 | 881 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.02] |
| 15.2 Major | 1 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.68, 2.62] |
| 16 Duration of therapy | 2 | 288 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.96, 0.98] |
| 17 Days hospitalised | 1 | 129 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐2.47, ‐0.33] |
| 18 TIme to defervescence | 1 | 129 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐1.98, ‐0.62] |
7.1. Analysis.
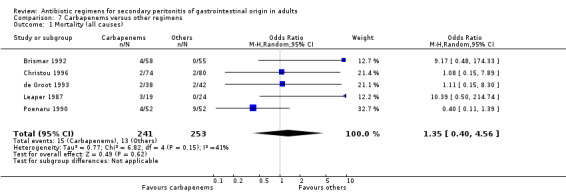
Comparison 7 Carbapenems versus other regimens, Outcome 1 Mortality (all causes).
7.2. Analysis.
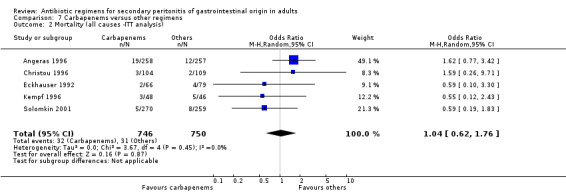
Comparison 7 Carbapenems versus other regimens, Outcome 2 Mortality (all causes ‐ITT analysis).
7.3. Analysis.
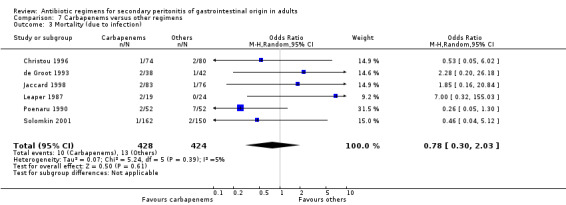
Comparison 7 Carbapenems versus other regimens, Outcome 3 Mortality (due to infection).
7.4. Analysis.
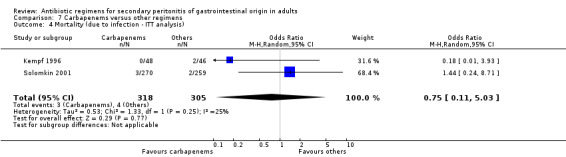
Comparison 7 Carbapenems versus other regimens, Outcome 4 Mortality (due to infection ‐ ITT analysis).
7.5. Analysis.
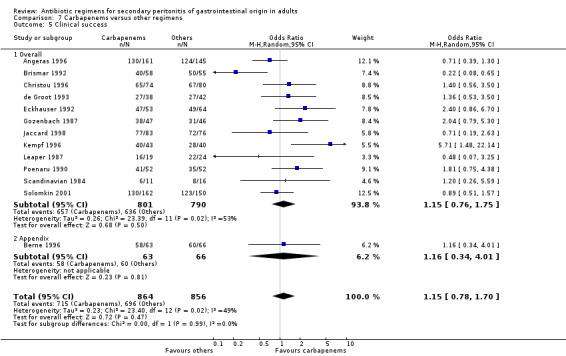
Comparison 7 Carbapenems versus other regimens, Outcome 5 Clinical success.
7.6. Analysis.
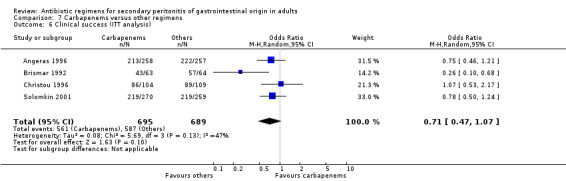
Comparison 7 Carbapenems versus other regimens, Outcome 6 Clinical success (ITT analysis).
7.7. Analysis.
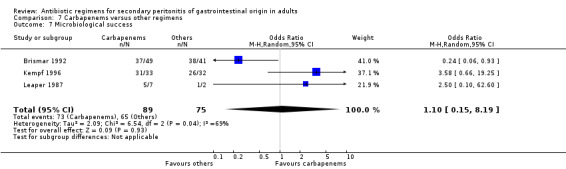
Comparison 7 Carbapenems versus other regimens, Outcome 7 Microbiological success.
7.8. Analysis.
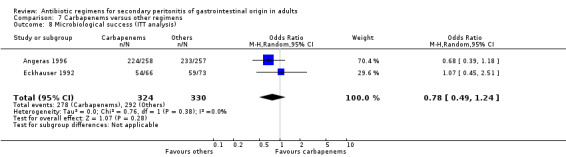
Comparison 7 Carbapenems versus other regimens, Outcome 8 Microbiological success (ITT analysis).
7.9. Analysis.
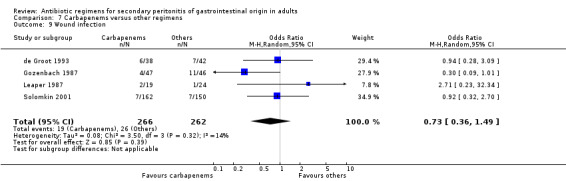
Comparison 7 Carbapenems versus other regimens, Outcome 9 Wound infection.
7.10. Analysis.
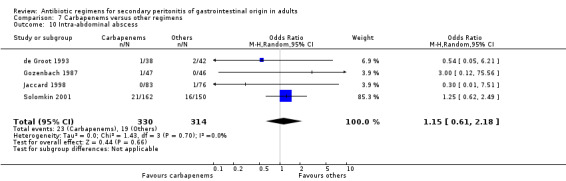
Comparison 7 Carbapenems versus other regimens, Outcome 10 Intra‐abdominal abscess.
7.11. Analysis.
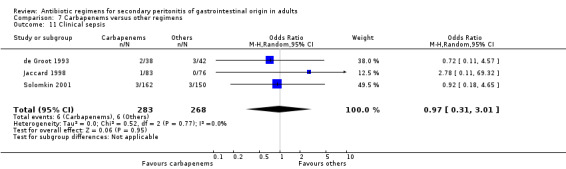
Comparison 7 Carbapenems versus other regimens, Outcome 11 Clinical sepsis.
7.12. Analysis.
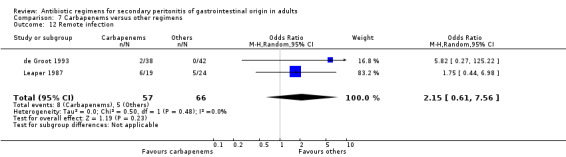
Comparison 7 Carbapenems versus other regimens, Outcome 12 Remote infection.
7.13. Analysis.
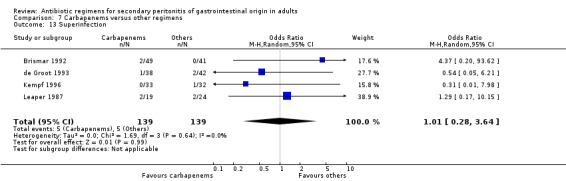
Comparison 7 Carbapenems versus other regimens, Outcome 13 Superinfection.
7.14. Analysis.
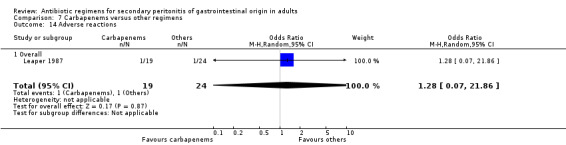
Comparison 7 Carbapenems versus other regimens, Outcome 14 Adverse reactions.
7.15. Analysis.
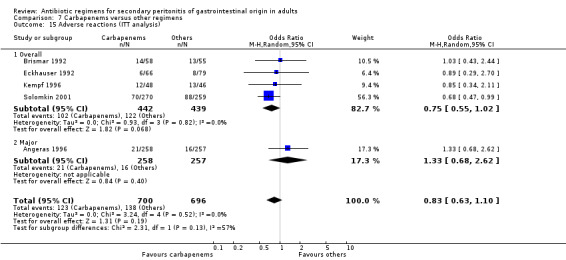
Comparison 7 Carbapenems versus other regimens, Outcome 15 Adverse reactions (ITT analysis).
7.16. Analysis.
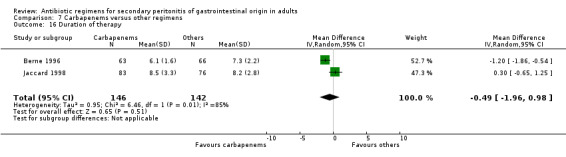
Comparison 7 Carbapenems versus other regimens, Outcome 16 Duration of therapy.
7.17. Analysis.
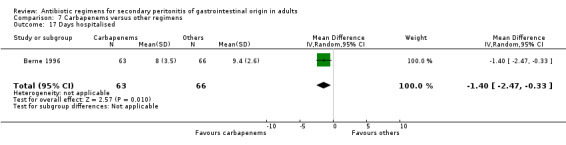
Comparison 7 Carbapenems versus other regimens, Outcome 17 Days hospitalised.
7.18. Analysis.
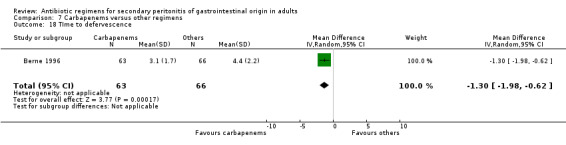
Comparison 7 Carbapenems versus other regimens, Outcome 18 TIme to defervescence.
Comparison 8. Cephalosporins alone versus other regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all causes) | 5 | 600 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.27, 1.57] |
| 2 Mortality (all causes ‐ ITT analysis) | 1 | 213 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.10, 3.84] |
| 3 Mortality (due to infection) | 3 | 331 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.37, 3.89] |
| 4 Clinical success | 8 | 993 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.54, 1.67] |
| 4.1 Overall | 6 | 787 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.59, 1.80] |
| 4.2 Appendix | 2 | 206 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.03, 6.25] |
| 5 Clinical success (ITT analysis) | 2 | 373 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.57, 2.74] |
| 5.1 Overall | 2 | 373 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.57, 2.74] |
| 6 Microbiological success | 1 | 76 | Odds Ratio (M‐H, Random, 95% CI) | 1.72 [0.62, 4.75] |
| 7 Wound infection | 8 | 961 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.56, 2.05] |
| 8 Intra‐abdominal abscess | 5 | 580 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.51, 2.26] |
| 9 Clinical sepsis | 3 | 317 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.27, 4.19] |
| 10 Remote infection | 2 | 309 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.52, 3.30] |
| 11 Adverse reactions | 2 | 282 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.29, 2.44] |
| 11.1 Overall | 1 | 152 | Odds Ratio (M‐H, Random, 95% CI) | 2.03 [0.18, 22.84] |
| 11.2 Minor adverse reactions | 1 | 130 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.21, 2.23] |
| 12 Adverse reactions (ITT analysis) | 2 | 498 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.65, 1.60] |
| 12.1 Overall | 2 | 498 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.65, 1.60] |
| 13 Duration of therapy | 1 | 76 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.54, 1.34] |
| 14 Days hospitalised | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.67, 1.07] |
| 15 Time to defervescence | 1 | 76 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.60, 0.80] |
8.1. Analysis.
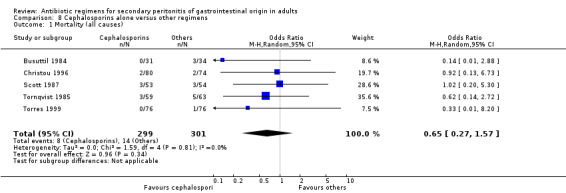
Comparison 8 Cephalosporins alone versus other regimens, Outcome 1 Mortality (all causes).
8.2. Analysis.
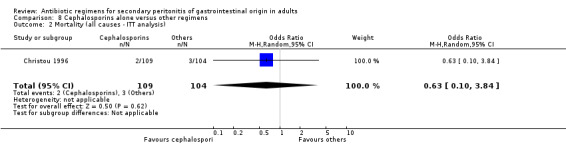
Comparison 8 Cephalosporins alone versus other regimens, Outcome 2 Mortality (all causes ‐ ITT analysis).
8.3. Analysis.
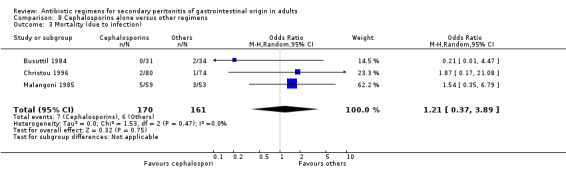
Comparison 8 Cephalosporins alone versus other regimens, Outcome 3 Mortality (due to infection).
8.4. Analysis.
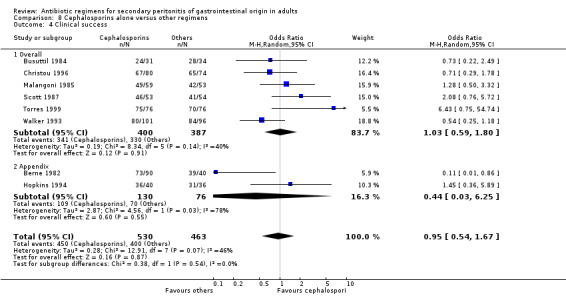
Comparison 8 Cephalosporins alone versus other regimens, Outcome 4 Clinical success.
8.5. Analysis.
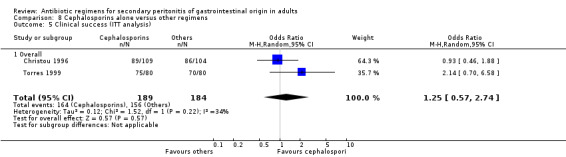
Comparison 8 Cephalosporins alone versus other regimens, Outcome 5 Clinical success (ITT analysis).
8.6. Analysis.
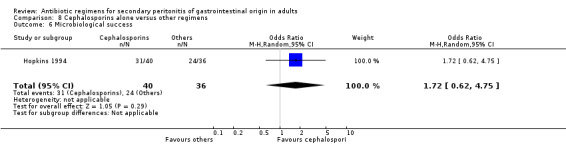
Comparison 8 Cephalosporins alone versus other regimens, Outcome 6 Microbiological success.
8.7. Analysis.
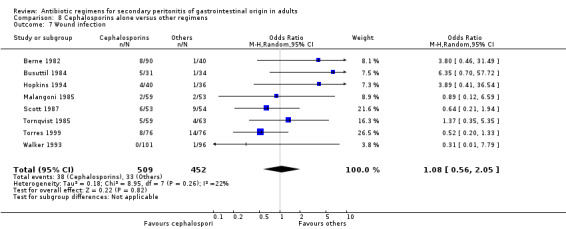
Comparison 8 Cephalosporins alone versus other regimens, Outcome 7 Wound infection.
8.8. Analysis.
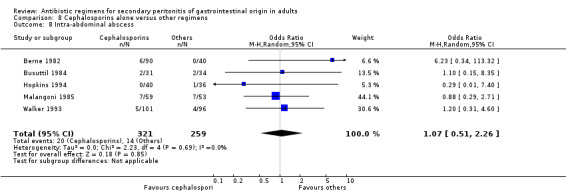
Comparison 8 Cephalosporins alone versus other regimens, Outcome 8 Intra‐abdominal abscess.
8.9. Analysis.
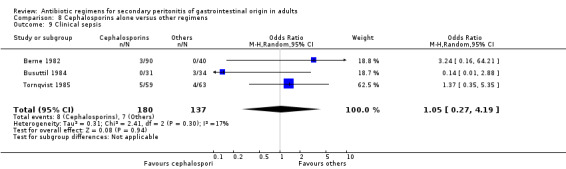
Comparison 8 Cephalosporins alone versus other regimens, Outcome 9 Clinical sepsis.
8.10. Analysis.
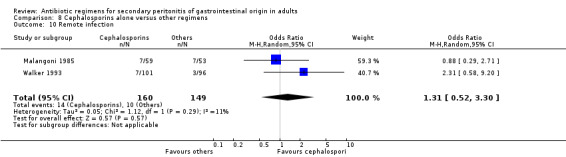
Comparison 8 Cephalosporins alone versus other regimens, Outcome 10 Remote infection.
8.11. Analysis.
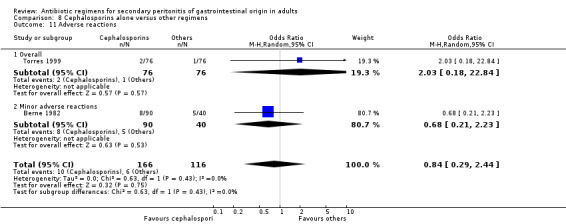
Comparison 8 Cephalosporins alone versus other regimens, Outcome 11 Adverse reactions.
8.12. Analysis.
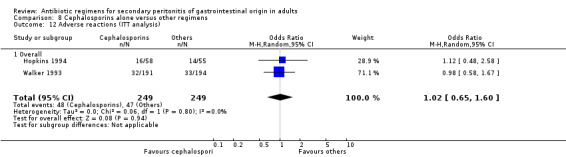
Comparison 8 Cephalosporins alone versus other regimens, Outcome 12 Adverse reactions (ITT analysis).
8.13. Analysis.
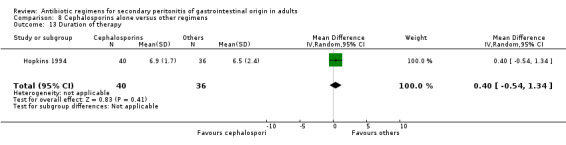
Comparison 8 Cephalosporins alone versus other regimens, Outcome 13 Duration of therapy.
8.14. Analysis.
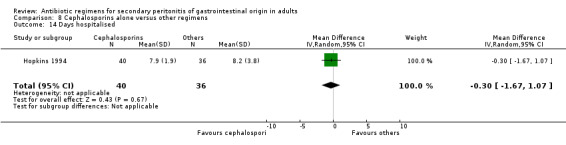
Comparison 8 Cephalosporins alone versus other regimens, Outcome 14 Days hospitalised.
8.15. Analysis.
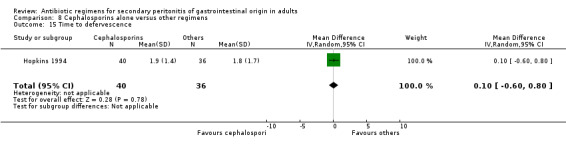
Comparison 8 Cephalosporins alone versus other regimens, Outcome 15 Time to defervescence.
Comparison 9. Cephalosporins and antianaerobes versus other regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all causes) | 3 | 312 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [0.57, 3.77] |
| 2 Mortality (all causes ‐ ITT analysis) | 2 | 609 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.32, 2.34] |
| 3 Mortality (due to infection ‐ ITT analysis) | 1 | 94 | Odds Ratio (M‐H, Random, 95% CI) | 5.45 [0.25, 116.63] |
| 4 Clinical success | 5 | 675 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.29, 1.75] |
| 4.1 Overall | 4 | 579 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.21, 1.36] |
| 4.2 Appendix | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 3.30 [0.82, 13.30] |
| 5 Clinical success (ITT analysis) | 1 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.83, 2.17] |
| 6 Microbiological success | 2 | 580 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.16, 3.81] |
| 7 Wound infection | 4 | 408 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.51, 2.18] |
| 8 Intra‐abdominal abscess | 2 | 179 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.24, 3.11] |
| 9 Clinical sepsis | 1 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.19, 2.87] |
| 10 Remote infection | 1 | 83 | Odds Ratio (M‐H, Random, 95% CI) | 3.77 [0.97, 14.72] |
| 11 Superinfection | 1 | 65 | Odds Ratio (M‐H, Random, 95% CI) | 3.19 [0.13, 81.25] |
| 12 Adverse reactions (ITT analysis) | 4 | 788 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.67, 2.20] |
| 12.1 Overall | 2 | 190 | Odds Ratio (M‐H, Random, 95% CI) | 1.72 [0.82, 3.61] |
| 12.2 Minor | 1 | 83 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.07, 19.67] |
| 12.3 Major | 1 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.38, 1.47] |
| 13 Duration of therapy | 1 | 96 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.36, 0.16] |
| 14 Days hospitalised | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐2.18, 0.38] |
| 15 TIme to defervescence | 1 | 96 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.58, 0.38] |
9.1. Analysis.
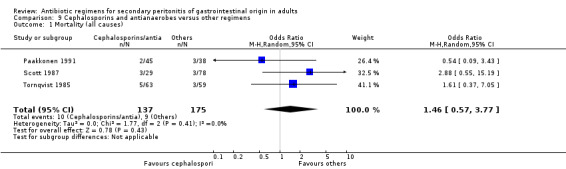
Comparison 9 Cephalosporins and antianaerobes versus other regimens, Outcome 1 Mortality (all causes).
9.2. Analysis.
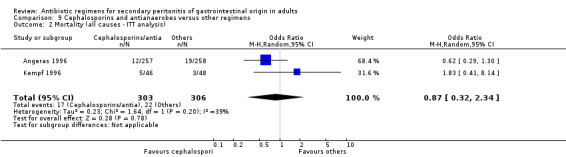
Comparison 9 Cephalosporins and antianaerobes versus other regimens, Outcome 2 Mortality (all causes ‐ ITT analysis).
9.3. Analysis.
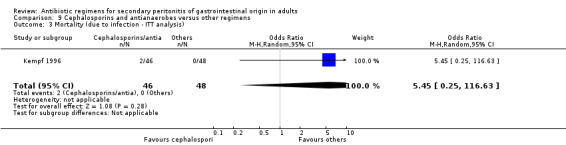
Comparison 9 Cephalosporins and antianaerobes versus other regimens, Outcome 3 Mortality (due to infection ‐ ITT analysis).
9.4. Analysis.
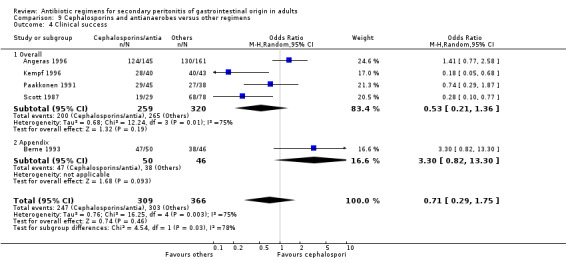
Comparison 9 Cephalosporins and antianaerobes versus other regimens, Outcome 4 Clinical success.
9.5. Analysis.
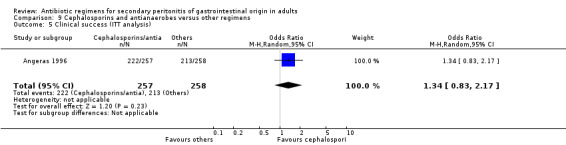
Comparison 9 Cephalosporins and antianaerobes versus other regimens, Outcome 5 Clinical success (ITT analysis).
9.6. Analysis.
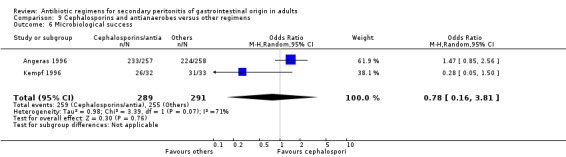
Comparison 9 Cephalosporins and antianaerobes versus other regimens, Outcome 6 Microbiological success.
9.7. Analysis.
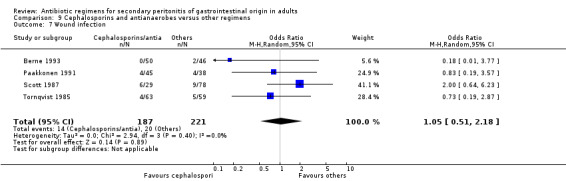
Comparison 9 Cephalosporins and antianaerobes versus other regimens, Outcome 7 Wound infection.
9.8. Analysis.
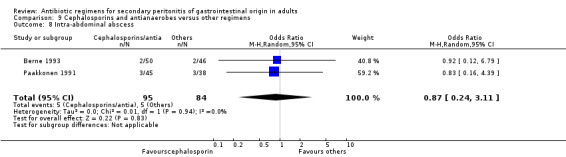
Comparison 9 Cephalosporins and antianaerobes versus other regimens, Outcome 8 Intra‐abdominal abscess.
9.9. Analysis.
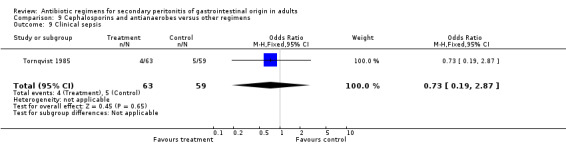
Comparison 9 Cephalosporins and antianaerobes versus other regimens, Outcome 9 Clinical sepsis.
9.10. Analysis.
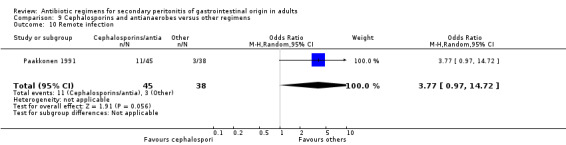
Comparison 9 Cephalosporins and antianaerobes versus other regimens, Outcome 10 Remote infection.
9.11. Analysis.
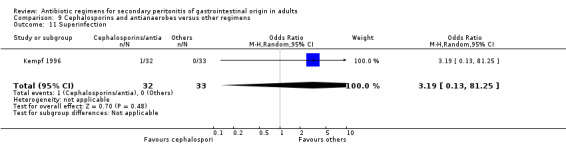
Comparison 9 Cephalosporins and antianaerobes versus other regimens, Outcome 11 Superinfection.
9.12. Analysis.
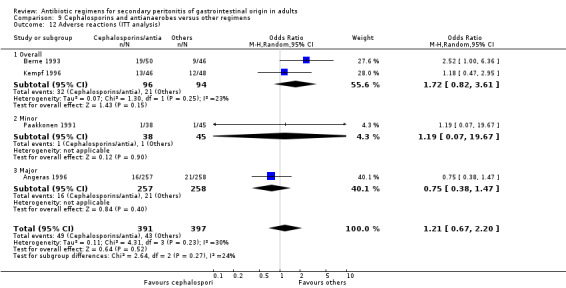
Comparison 9 Cephalosporins and antianaerobes versus other regimens, Outcome 12 Adverse reactions (ITT analysis).
9.13. Analysis.
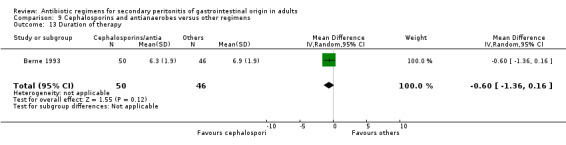
Comparison 9 Cephalosporins and antianaerobes versus other regimens, Outcome 13 Duration of therapy.
9.14. Analysis.
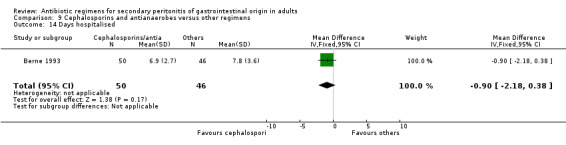
Comparison 9 Cephalosporins and antianaerobes versus other regimens, Outcome 14 Days hospitalised.
9.15. Analysis.
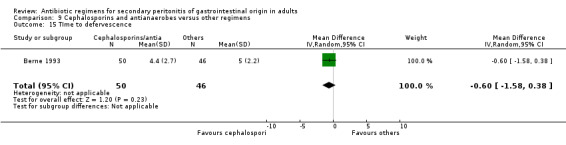
Comparison 9 Cephalosporins and antianaerobes versus other regimens, Outcome 15 TIme to defervescence.
Comparison 10. Cephalosporins and beta lactamase inhibitors versus other regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all causes) | 1 | 76 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.29, 5.52] |
| 2 Mortality (due to infection) | 1 | 76 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.23, 4.68] |
| 3 Clinical success | 2 | 176 | Odds Ratio (M‐H, Random, 95% CI) | 3.21 [1.49, 6.92] |
| 3.1 Overall | 2 | 176 | Odds Ratio (M‐H, Random, 95% CI) | 3.21 [1.49, 6.92] |
| 4 Microbiological success | 1 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 2.51 [0.83, 7.57] |
| 5 Superinfection | 1 | 76 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.11, 1.82] |
| 6 Adverse reactions | 1 | 76 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.05, 1.62] |
| 6.1 Major adverse reactions | 1 | 76 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.05, 1.62] |
10.1. Analysis.
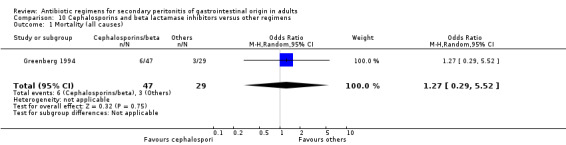
Comparison 10 Cephalosporins and beta lactamase inhibitors versus other regimens, Outcome 1 Mortality (all causes).
10.2. Analysis.
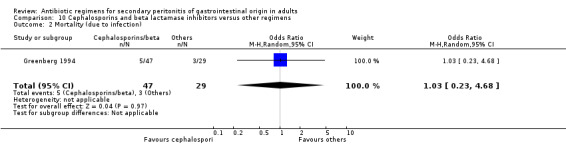
Comparison 10 Cephalosporins and beta lactamase inhibitors versus other regimens, Outcome 2 Mortality (due to infection).
10.3. Analysis.
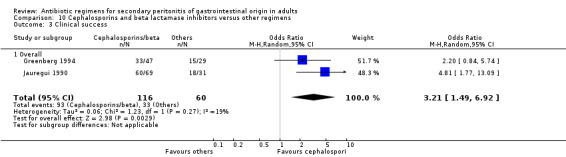
Comparison 10 Cephalosporins and beta lactamase inhibitors versus other regimens, Outcome 3 Clinical success.
10.4. Analysis.
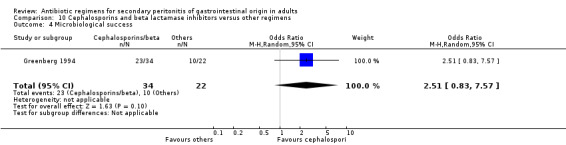
Comparison 10 Cephalosporins and beta lactamase inhibitors versus other regimens, Outcome 4 Microbiological success.
10.5. Analysis.
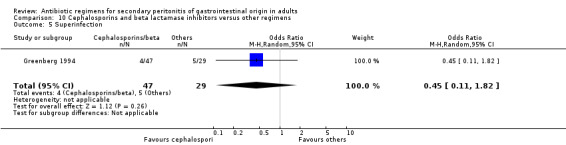
Comparison 10 Cephalosporins and beta lactamase inhibitors versus other regimens, Outcome 5 Superinfection.
10.6. Analysis.
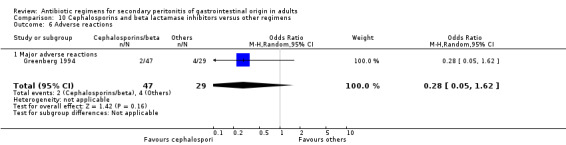
Comparison 10 Cephalosporins and beta lactamase inhibitors versus other regimens, Outcome 6 Adverse reactions.
Comparison 11. Clindamycin regimens versus nitroimidazole regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all causes) | 1 | 58 | Odds Ratio (M‐H, Random, 95% CI) | 1.6 [0.29, 8.71] |
| 2 Mortality (due to infection) | 1 | 58 | Odds Ratio (M‐H, Random, 95% CI) | 2.48 [0.38, 16.11] |
| 3 Clinical success | 1 | 58 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.16, 2.11] |
| 3.1 Overall | 1 | 58 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.16, 2.11] |
| 4 Adverse reactions | 1 | 81 | Odds Ratio (M‐H, Random, 95% CI) | 2.18 [0.34, 13.80] |
| 4.1 Minor adverse reactions | 1 | 81 | Odds Ratio (M‐H, Random, 95% CI) | 2.18 [0.34, 13.80] |
11.1. Analysis.
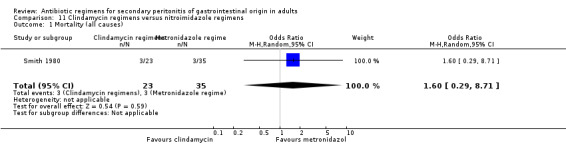
Comparison 11 Clindamycin regimens versus nitroimidazole regimens, Outcome 1 Mortality (all causes).
11.2. Analysis.
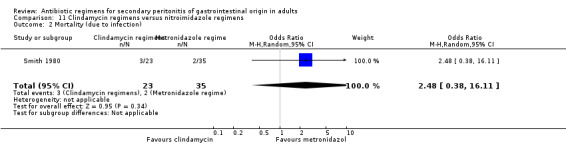
Comparison 11 Clindamycin regimens versus nitroimidazole regimens, Outcome 2 Mortality (due to infection).
11.3. Analysis.
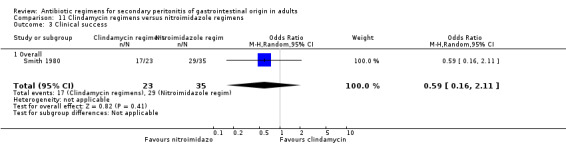
Comparison 11 Clindamycin regimens versus nitroimidazole regimens, Outcome 3 Clinical success.
11.4. Analysis.
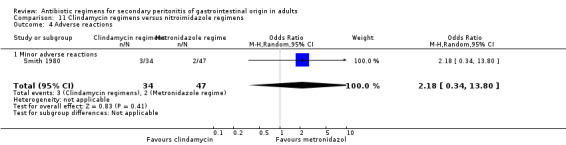
Comparison 11 Clindamycin regimens versus nitroimidazole regimens, Outcome 4 Adverse reactions.
Comparison 12. Fluoroquinolones alone versus other regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all causes ‐ ITT analysis) | 1 | 529 | Odds Ratio (M‐H, Random, 95% CI) | 1.69 [0.55, 5.23] |
| 2 Mortality (due to infection) | 1 | 312 | Odds Ratio (M‐H, Random, 95% CI) | 2.18 [0.20, 24.24] |
| 3 Mortality (due to infection ‐ ITT analysis) | 1 | 529 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.11, 4.18] |
| 4 Clinical success | 1 | 312 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.64, 1.98] |
| 5 Clinical success (ITT analysis) | 1 | 529 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.81, 2.01] |
| 6 Wound infection | 1 | 312 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.37, 3.17] |
| 7 Intra‐abdominal abscess | 1 | 312 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.40, 1.60] |
| 8 Clinical sepsis | 1 | 312 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.21, 5.44] |
| 9 Adverse reactions (ITT analysis) | 1 | 529 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [1.01, 2.14] |
| 9.1 Overall | 1 | 529 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [1.01, 2.14] |
12.1. Analysis.
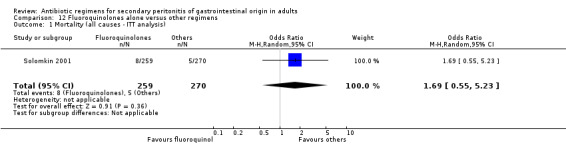
Comparison 12 Fluoroquinolones alone versus other regimens, Outcome 1 Mortality (all causes ‐ ITT analysis).
12.2. Analysis.
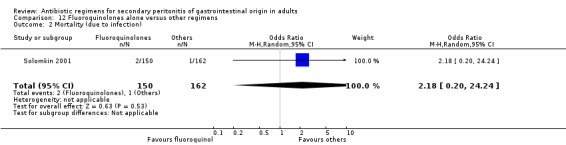
Comparison 12 Fluoroquinolones alone versus other regimens, Outcome 2 Mortality (due to infection).
12.3. Analysis.
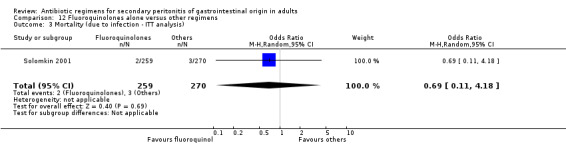
Comparison 12 Fluoroquinolones alone versus other regimens, Outcome 3 Mortality (due to infection ‐ ITT analysis).
12.4. Analysis.
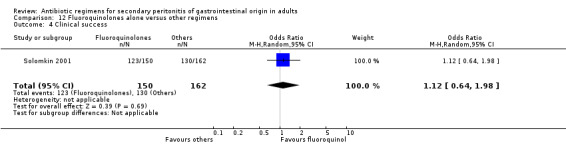
Comparison 12 Fluoroquinolones alone versus other regimens, Outcome 4 Clinical success.
12.5. Analysis.
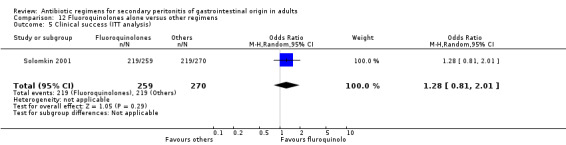
Comparison 12 Fluoroquinolones alone versus other regimens, Outcome 5 Clinical success (ITT analysis).
12.6. Analysis.
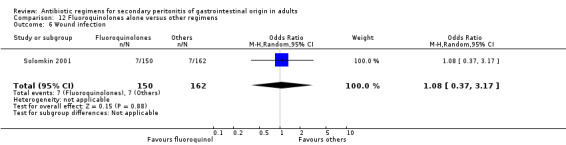
Comparison 12 Fluoroquinolones alone versus other regimens, Outcome 6 Wound infection.
12.7. Analysis.
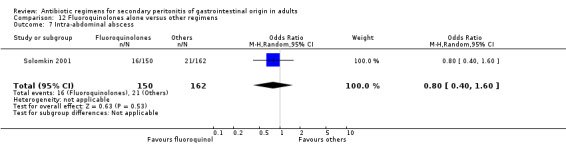
Comparison 12 Fluoroquinolones alone versus other regimens, Outcome 7 Intra‐abdominal abscess.
12.8. Analysis.
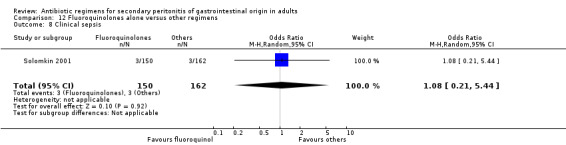
Comparison 12 Fluoroquinolones alone versus other regimens, Outcome 8 Clinical sepsis.
12.9. Analysis.
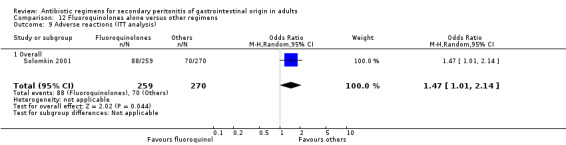
Comparison 12 Fluoroquinolones alone versus other regimens, Outcome 9 Adverse reactions (ITT analysis).
Comparison 13. Fluoroquinolones and antianaerobes versus other regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all causes) | 2 | 642 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.12, 4.50] |
| 2 Mortality (all causes ‐ ITT analysis) | 2 | 729 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.04, 4.85] |
| 3 Mortality (due to infection) | 1 | 184 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.01, 6.31] |
| 4 Mortality (due to infection ‐ ITT analysis) | 1 | 458 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.03, 3.04] |
| 5 Clinical success | 2 | 434 | Odds Ratio (M‐H, Random, 95% CI) | 1.74 [1.11, 2.73] |
| 6 Clinical success (ITT analysis) | 1 | 337 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.84, 2.18] |
| 7 Microbiological success | 2 | 376 | Odds Ratio (M‐H, Random, 95% CI) | 1.45 [0.85, 2.46] |
| 8 Wound infection | 1 | 282 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.26, 0.99] |
| 9 Superinfection | 2 | 428 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.31, 1.58] |
| 10 Adverse reactions (ITT analysis) | 2 | 729 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.56, 2.02] |
| 10.1 Overall | 1 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 1.61 [0.60, 4.28] |
| 10.2 Major | 1 | 458 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.39, 1.72] |
13.1. Analysis.
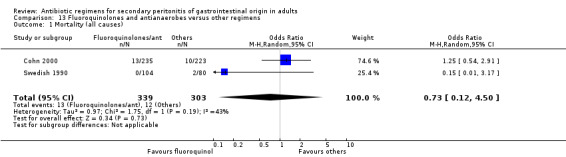
Comparison 13 Fluoroquinolones and antianaerobes versus other regimens, Outcome 1 Mortality (all causes).
13.2. Analysis.
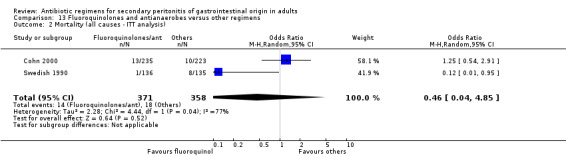
Comparison 13 Fluoroquinolones and antianaerobes versus other regimens, Outcome 2 Mortality (all causes ‐ ITT analysis).
13.3. Analysis.
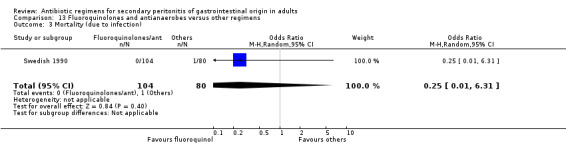
Comparison 13 Fluoroquinolones and antianaerobes versus other regimens, Outcome 3 Mortality (due to infection).
13.4. Analysis.
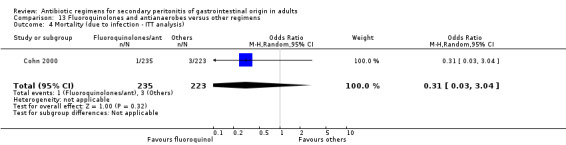
Comparison 13 Fluoroquinolones and antianaerobes versus other regimens, Outcome 4 Mortality (due to infection ‐ ITT analysis).
13.5. Analysis.
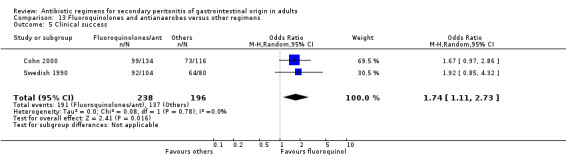
Comparison 13 Fluoroquinolones and antianaerobes versus other regimens, Outcome 5 Clinical success.
13.6. Analysis.
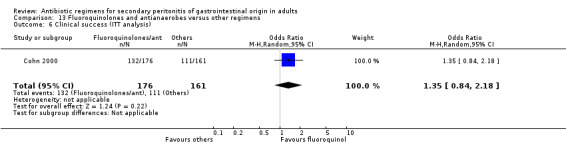
Comparison 13 Fluoroquinolones and antianaerobes versus other regimens, Outcome 6 Clinical success (ITT analysis).
13.7. Analysis.
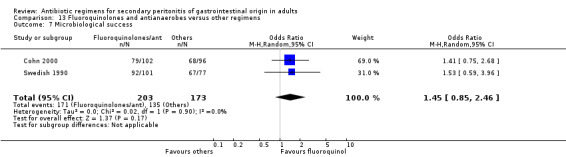
Comparison 13 Fluoroquinolones and antianaerobes versus other regimens, Outcome 7 Microbiological success.
13.8. Analysis.
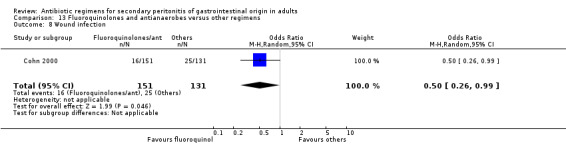
Comparison 13 Fluoroquinolones and antianaerobes versus other regimens, Outcome 8 Wound infection.
13.9. Analysis.
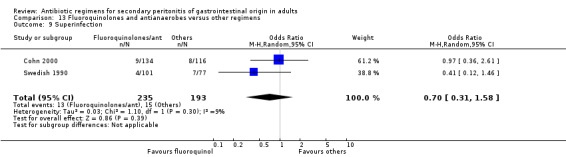
Comparison 13 Fluoroquinolones and antianaerobes versus other regimens, Outcome 9 Superinfection.
13.10. Analysis.
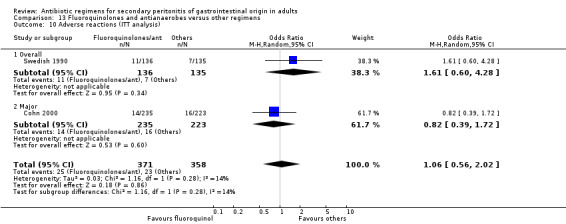
Comparison 13 Fluoroquinolones and antianaerobes versus other regimens, Outcome 10 Adverse reactions (ITT analysis).
Comparison 14. Monobactams and antianerobes versus other regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all causes) | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.9 [0.12, 6.72] |
| 2 Mortality (due to infection) | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.04, 5.05] |
| 3 Clinical success | 2 | 164 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.28, 1.71] |
| 3.1 Overall | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.29, 1.88] |
| 3.2 Appendix | 1 | 84 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.02, 8.24] |
| 4 Wound infection | 2 | 164 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.40, 3.64] |
| 5 Intra‐abdominal abscess | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 1.85 [0.16, 21.26] |
| 6 Clinical sepsis | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [0.22, 8.77] |
| 7 Remote infection | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 3.69] |
| 8 Superinfection | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 1.85 [0.16, 21.26] |
| 9 Adverse reactions | 1 | 84 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.07, 0.54] |
| 9.1 Minor | 1 | 84 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.07, 0.54] |
| 10 Duration of therapy | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.16, 0.32] |
| 11 Days hospitalised | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐1.35, 0.61] |
| 12 Time to defervescence | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.45, 0.15] |
14.1. Analysis.
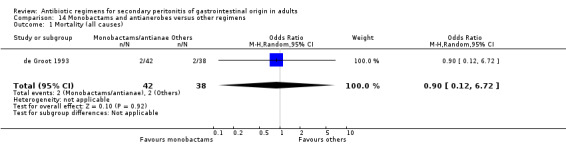
Comparison 14 Monobactams and antianerobes versus other regimens, Outcome 1 Mortality (all causes).
14.2. Analysis.
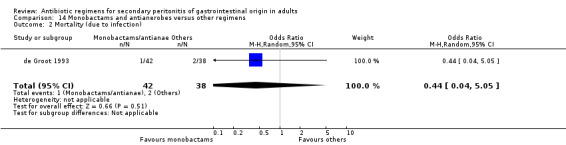
Comparison 14 Monobactams and antianerobes versus other regimens, Outcome 2 Mortality (due to infection).
14.3. Analysis.
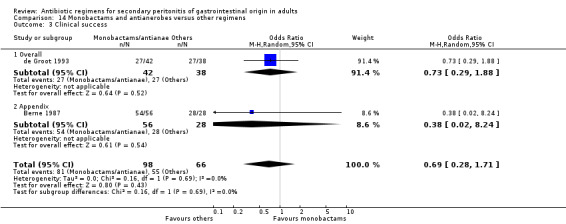
Comparison 14 Monobactams and antianerobes versus other regimens, Outcome 3 Clinical success.
14.4. Analysis.
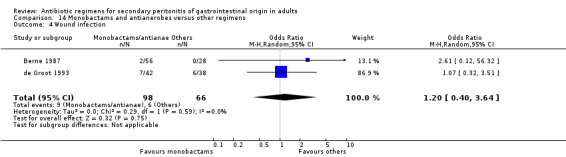
Comparison 14 Monobactams and antianerobes versus other regimens, Outcome 4 Wound infection.
14.5. Analysis.
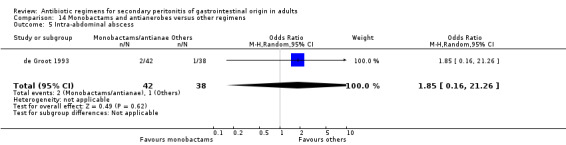
Comparison 14 Monobactams and antianerobes versus other regimens, Outcome 5 Intra‐abdominal abscess.
14.6. Analysis.
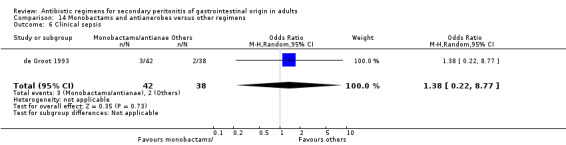
Comparison 14 Monobactams and antianerobes versus other regimens, Outcome 6 Clinical sepsis.
14.7. Analysis.
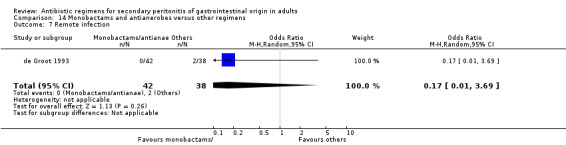
Comparison 14 Monobactams and antianerobes versus other regimens, Outcome 7 Remote infection.
14.8. Analysis.
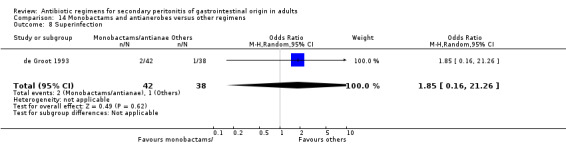
Comparison 14 Monobactams and antianerobes versus other regimens, Outcome 8 Superinfection.
14.9. Analysis.
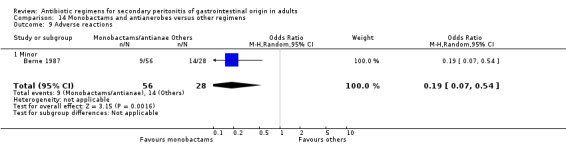
Comparison 14 Monobactams and antianerobes versus other regimens, Outcome 9 Adverse reactions.
14.10. Analysis.
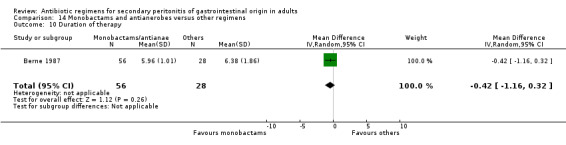
Comparison 14 Monobactams and antianerobes versus other regimens, Outcome 10 Duration of therapy.
14.11. Analysis.
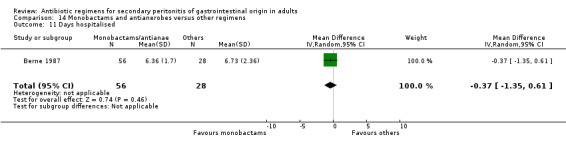
Comparison 14 Monobactams and antianerobes versus other regimens, Outcome 11 Days hospitalised.
14.12. Analysis.
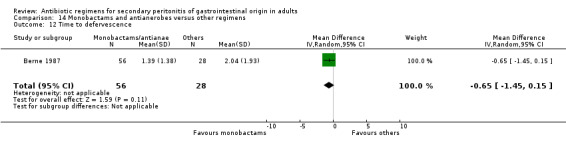
Comparison 14 Monobactams and antianerobes versus other regimens, Outcome 12 Time to defervescence.
Comparison 15. Imipenem/cilastatin versus other carbapenems.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all causes ‐ ITT analysis)) | 3 | 528 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.58, 3.88] |
| 2 Mortality (due to infection ‐ ITT analysis)) | 3 | 528 | Odds Ratio (M‐H, Random, 95% CI) | 1.79 [0.50, 6.42] |
| 3 Clinical success | 5 | 667 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.62, 1.77] |
| 4 Clinical success (ITT analysis) | 2 | 367 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.22, 1.65] |
| 5 Microbiological success | 4 | 575 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.53, 1.87] |
| 6 Superinfection | 2 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.26, 2.18] |
| 7 Adverse reactions (ITT analysis) | 4 | 810 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [0.81, 2.10] |
| 7.1 Overall | 4 | 810 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [0.81, 2.10] |
| 8 Duration of treatment | 1 | 135 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐2.20, ‐0.00] |
| 9 Days hospitalised | 1 | 135 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐4.52, 4.32] |
15.1. Analysis.
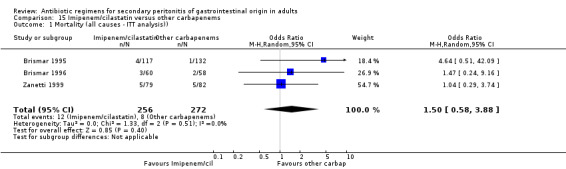
Comparison 15 Imipenem/cilastatin versus other carbapenems, Outcome 1 Mortality (all causes ‐ ITT analysis)).
15.2. Analysis.
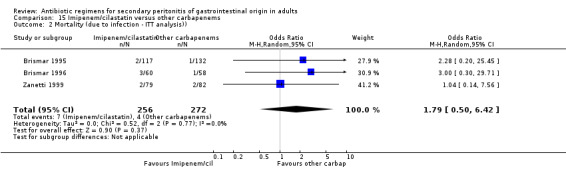
Comparison 15 Imipenem/cilastatin versus other carbapenems, Outcome 2 Mortality (due to infection ‐ ITT analysis)).
15.3. Analysis.
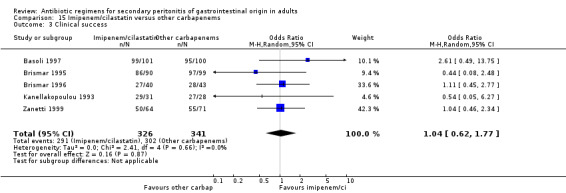
Comparison 15 Imipenem/cilastatin versus other carbapenems, Outcome 3 Clinical success.
15.4. Analysis.
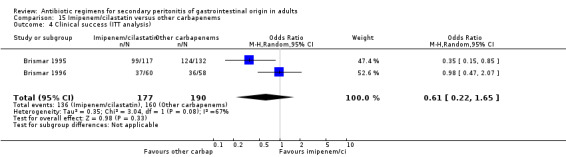
Comparison 15 Imipenem/cilastatin versus other carbapenems, Outcome 4 Clinical success (ITT analysis).
15.5. Analysis.
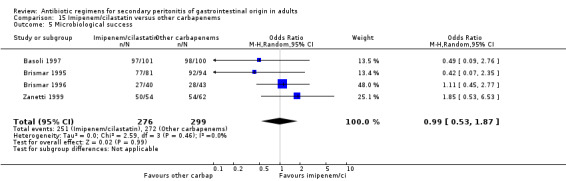
Comparison 15 Imipenem/cilastatin versus other carbapenems, Outcome 5 Microbiological success.
15.6. Analysis.
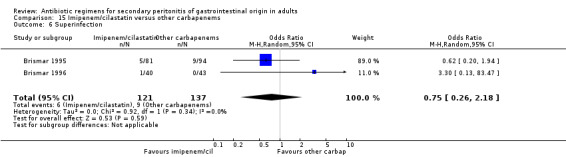
Comparison 15 Imipenem/cilastatin versus other carbapenems, Outcome 6 Superinfection.
15.7. Analysis.
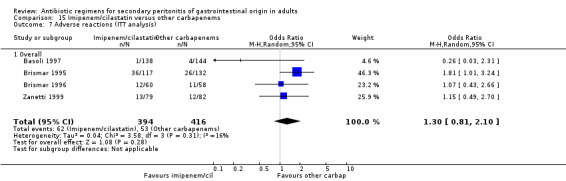
Comparison 15 Imipenem/cilastatin versus other carbapenems, Outcome 7 Adverse reactions (ITT analysis).
15.8. Analysis.
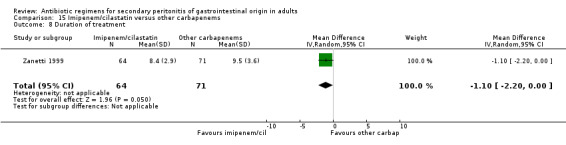
Comparison 15 Imipenem/cilastatin versus other carbapenems, Outcome 8 Duration of treatment.
15.9. Analysis.
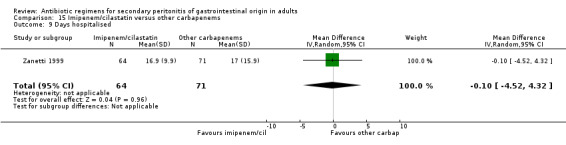
Comparison 15 Imipenem/cilastatin versus other carbapenems, Outcome 9 Days hospitalised.
Comparison 16. Isepamicin and antianaerobes versus amikacin and antianaerobes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical success | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.21, 1.77] |
| 1.1 Overall | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.21, 1.77] |
| 2 Clinical success (ITT analysis) | 1 | 267 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.17, 1.07] |
| 2.1 Overall | 1 | 267 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.17, 1.07] |
| 3 Microbiological success | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.47, 1.92] |
| 4 Wound infection | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.18, 2.06] |
| 5 Superinfection | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.13, 4.74] |
| 6 Adverse reactions (ITT analysis) | 1 | 267 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.37, 2.07] |
| 6.1 Overall | 1 | 267 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.37, 2.07] |
16.1. Analysis.
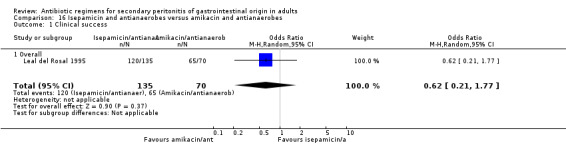
Comparison 16 Isepamicin and antianaerobes versus amikacin and antianaerobes, Outcome 1 Clinical success.
16.2. Analysis.
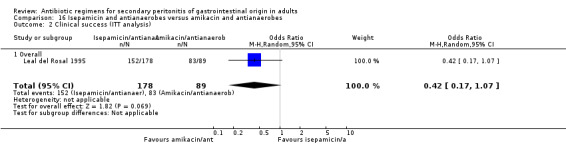
Comparison 16 Isepamicin and antianaerobes versus amikacin and antianaerobes, Outcome 2 Clinical success (ITT analysis).
16.3. Analysis.
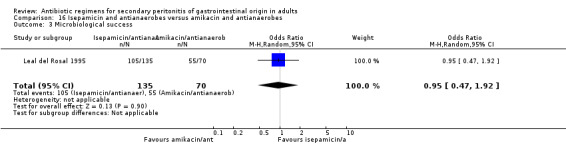
Comparison 16 Isepamicin and antianaerobes versus amikacin and antianaerobes, Outcome 3 Microbiological success.
16.4. Analysis.
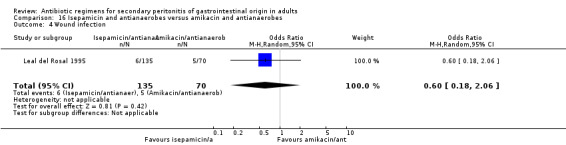
Comparison 16 Isepamicin and antianaerobes versus amikacin and antianaerobes, Outcome 4 Wound infection.
16.5. Analysis.
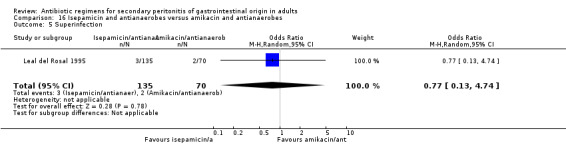
Comparison 16 Isepamicin and antianaerobes versus amikacin and antianaerobes, Outcome 5 Superinfection.
16.6. Analysis.
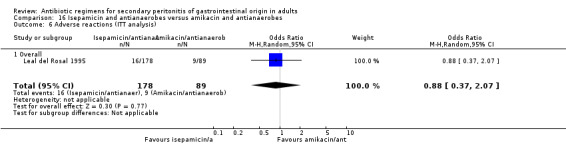
Comparison 16 Isepamicin and antianaerobes versus amikacin and antianaerobes, Outcome 6 Adverse reactions (ITT analysis).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Angeras 1996.
| Methods | Randomised controlled trial. Multi‐centre (Scandinavian) study. Randomisation method: not stated. Blinding of assessors: not stated. Patient stratification: using APACHE II score. Power calculation: performed. Intention‐to‐treat and sub‐group analysis were performed. Follow up: 30 days. | |
| Participants | Number of patients: 515. Clinically and microbiologically evaluable patients: 306. 161 (Imipenem/cilastatin, I‐C) versus 145 (Cefuroxime/metronidazole, C‐M). Male:female ratio = 1.3:1. Age range: 18‐92. APACHE II score 0‐10: n = 216 (84%) (I‐C) vs 212 (82%) (C‐M). APACHE II score 11‐20: n = 42 (16%) (I‐C) vs 45 (18%) (C‐M). Inclusion criteria: untreated or unsuccessfully treated patients with proven or suspected bacterial intra‐abdominal infection or systemic infection originating from the intra‐abdominal region. Exclusion criteria: renal failure, brain abscess or other CNS disorder, serious concomitant infection, hypersensitivity to study drugs, age < 18 years, pregnancy or breast feeding. | |
| Interventions | 2 regimens: 1) Imipenem/cilastatin 1.5‐2.0 g/day. 2) Cefuroxime 3.0‐4.5 g/day and metronidazole 1.0‐1.5 g/day. Timing of antibiotic infusion: not stated. Length: > 3 days. Median treatment time: 6 days. Median time free from fever: 4 days. Median time to be discharged from hospital: 9 days. | |
| Outcomes | Clinical and bacteriological success (ITT analysis). Mortality (ITT analysis). Adverse reactions (ITT analysis). | |
| Notes | 139/306 (45%) patients had complicated appendicitis. No statistically significant difference shown. Supported by Merck, Sharp and Dohme. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Basoli 1997.
| Methods | Randomised parallel trial. Multicentre (20 Italian centres) study. Randomisation method: not stated. Blinding of assessors: not stated. Patient stratification: using APACHE II. Power calculation: not performed. Intention‐to‐treat analysis: not performed. Sub‐group analysis: performed.Follow up: 7‐10 days after cessation of therapy. | |
| Participants | Number of patients: 287 Evaluable patients: 201. 101 (Imipenem/cilastatin, I‐C) versus 100 (Meropenem, M). Mean age: 54.4 (range: 19‐92) years. Male:female ratio = 1.3:1. Mean APACHE II score: 6.4 (I‐C), 5.9 (M). APACHE II score <11: n = 81 (80%) (I‐C) vs 85 (85%) (M). APACHE II score 11‐20: n = 20 (20%) (I‐C) vs 13 (13%) (M). APACHE II score >20: n = 0 (0%) (I‐C) vs 2 (2%) (M). Inclusion criteria: > 18 years of age, with intra‐abdominal infections extending beyond the organ wall, temperature > 38 degrees C, or a WBC > 10500/mm, with symptoms and physical findings (e.g. abdominal pain and tenderness) and radiological, ultrasonic or radionuclide (if performed) changes consistent with intra‐abdominal infection. Exclusion criteria: lactating or pregnant patients; allergy, hypersensitivity or severe reaction to study antibiotics; rapidly progressive or terminal illness; severe hepatic or renal disease; concomitant infection that would interfere with evaluation of response to study antibiotics; participation in any clinical study involving antibiotics; previous participation in this study; inability to give consent; traumatic bowel perforation requiring surgery within 12 hours; perforation or gastroduodenal ulcers requiring surgery within 24 hours, or other intra‐abdominal processes in which the primary aetiology was unlikely to be infectious. Also excluded were patients who had undergone a percutaneous drainage procedure rather than a surgical procedure. | |
| Interventions | 2 regimens: 1) Imipenem/cilastatin 500 mg (8 hourly). 2) Meropenem 1000 mg (8 hourly). Timing of antibiotic infusion: not stated.Length: > 5 days. | |
| Outcomes | Clinical and microbiological success. Adverse reactions (ITT analysis). | |
| Notes | Patients were stratified according to APACHE II score and then randomised sequentially into the 2 treatment groups. 42/201 (46%) patients had complicated appendicitis. No statistically significant difference shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Berne 1982.
| Methods | Randomised, double blinded controlled trial. Single centre (USA) study. Randomisation method: computer generated cards. Assessors were blinded. Patient stratification: not performed. Power calculation: not performed. Intention‐to‐treat analysis: not performed. Sub‐group analysis: performed. Follow up: not stated. Placebo was used to maintain double‐blinding. | |
| Participants | Number of patients: 237. Clinically evaluable patients: 130. 40 (Gentamicin/clindamycin, G‐C) versus 48 (Cefamandole) versus 42 (Cefoperazone). Mean age +/‐ standard deviation: 30 +/‐ 1.6 (G‐C), 28 +/‐ 1.4 (Cefamandole), 29 +/‐ 1.5 (Cefoperazone). Age range: 17‐64. Inclusion criteria: duration of symptoms greater than 24 hours, diffuse abdominal tenderness, temperature > 101 degrees F, white blood cell count > 13000/mm3. Exclusion criteria: < 16 or > 65 years old, pregnant or breast feeding, terminally ill, impaired renal function (serum creatinine > 1.8 mg/100 ml), allergic to study drugs or penicillin, septic shock (chills, leucopaenia and haemodynamically unstable), previously established localised periappendiceal abscess. | |
| Interventions | 3 regimens used: 1) Gentamicin 1.5 mg/kg (8 hourly) and clindamycin 600 mg (6 hourly). 2) Cefamandole 1.5 g (6 hourly) and placebo. 3) Cefoperazone 2.0 g (12 hourly) and placebo. Gentamicin serum level monitored to maintain peak serum levels at 7 +/‐ 1.5 mcg/ml and trough levels below 2 mcg/ml. Timing of antibiotic infusion: pre‐operatively. Length: > 5 days or until patient was afebrile for 48 hours. | |
| Outcomes | Clinical success. Wound infection, intra‐abdominal abscess and clinical sepsis. Adverse reactions. | |
| Notes | Adverse reactions were all minor. All patients had complicated appendicitis.Gentamicin/clindamycin regimen was statistically more effective than cephalosporin regimens alone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Berne 1987.
| Methods | Randomised, double‐blinded controlled trial. Single centre (USA) study between July 1984 and July 1985. Randomisation method: not stated. Assessors were blinded. Patient stratification: not performed. Power calculation: performed. Intention‐to‐treat analysis: not performed. Sub‐group analysis: not performed. Follow up: not stated. | |
| Participants | Number of patients: 162. Clinically evaluable patients: 84. 56 (Aztreonam/clindamycin, A‐C) versus 28 (Gentamicin/clindamycin, G‐C). Mean age: 27.4 (A‐C) versus 27.7 (G ‐C). Inclusion criteria: patients with clinical signs of gangrenous or perforated appendicitis (fever > 38 degrees C, duration of symptoms > 24 hours, diffuse abdominal tenderness, white blood cell count > 13000). Exclusion criteria: <18 or > 65 years of age. Pregnant or haemodynamically unstable (systolic blood pressure < 100 mmHg). | |
| Interventions | 2 regimens: 1) Aztreonam 1000 mg (8 hourly) and clindamycin 600 mg (6 hourly). 2) Gentamicin 1.5 mg/kg (8 hourly) and clindamycin 600 mg (6 hourly) . Gentamicin levels were adjusted to achieve peak serum levels of 6 +/‐ 1 mcg/ml. Timing of antibiotic infusion: pre‐operatively.Length of treatment > 3 days or febrile for 48 hours. | |
| Outcomes | Clinical failures. Wound infection. Adverse reactions. Duration of therapy, fever and hospitalised stay. | |
| Notes | Adverse reactions were mainly diarrhoea. No evidence of nephrotoxicity. All patients had complicated appendicitis. No statistically significant difference shown. Supported by grant from E.R. Squibb and Sons Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Berne 1993.
| Methods | Randomised, double blinded controlled trial. Single centre (USA) study between March 1989 and February 1990. Randomisation method: unstated. Assessors were blinded. Patient stratification: not used. No power calculation. Intention‐to‐treat analysis: not performed. Sub‐group analysis: not performed. Follow up: not stated. | |
| Participants | Number of patients: 96. 50 (Cefepime/metronidazole, C‐M) versus 46 (Gentamicin/clindamycin, G‐C). Mean age: 30.5 +/‐ 10.5 (C‐M), 29.0 +/‐ 9.8 (G‐C). Age range: 18‐64. Inclusion criteria: patients with clinical signs of gangrenous or perforated appendicitis (fever > 38 degrees C, diffuse abdominal tenderness, leucocyte count > 13000 ml and duration of symptoms < 24 hours). Exclusion criteria: < 18 or > 65 years old, haemodynamic instability (systolic blood pressure < 100 mmHg), pregnant or nursing women, granulocyte count < 500 /ml, serum creatinine > 2 mg/dl, active hepatic disease (ALT/AST > 3x normal), life threatening infection (including evidence of septic shock), CNS involvement, failure of more than one organ, antibiotic use in the last six weeks, and history of allergy to study drugs. | |
| Interventions | 2 regimens: 1) Cefepime 2 g (12 hourly) and metronidazole 500 mg (8 hourly). 2) Gentamicin 1.5 mg/kg (8 hourly) and clindamycin 900 mg (8 hourly). Gentamicin serum level maintained at 4.5 to 8.5 mcg/ml. Timing of antibiotic infusion: pre‐operatively.Length: febrile for 48 hours, < 14 days. | |
| Outcomes | Clinical success. Wound infection, intra‐abdominal abscess.Adverse reactions (ITT analysis). Duration of therapy, hospitalised stay and time to defervescence. | |
| Notes | All patients had complicated appendicitis. No statistically significant difference shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Berne 1996.
| Methods | Randomised, double blinded controlled trial. Single centre (USA) study between July 1990 and July 1992. Randomisation method: computerised card drawn by a blinded research pharmacist. Blinding of assessors: not stated. Patient stratification: not performed. Power calculation: not used. Intention‐to‐treat analysis: not performed.Sub‐group analysis: not performed.Follow up: not stated. | |
| Participants | Number of patients: 129. 63 (Meropenem, M) versus 66 (Tobramycin/clindamycin, T‐C). Mean age: 29.0 +/‐ 9.7 (M), 30.6 +/‐ 9.0 (T‐C). Age range: 18‐59. Inclusion criteria: patients with gangrenous or perforated appendicitis (duration of symptoms > 24 hours, temperature > 38 degrees C, white blood cell count > 13000, diffuse abdominal tenderness or rigidity and decreased or absent bowel sounds). Exclusion criteria: age > 75 years, antibiotic over last 30 days, pregnant or breast feeding women, allergy to study antibiotics, terminal illness, serum ALT or AST > 160, bilirubin > 3.0 mg/dL, ALP > 440 U, creatinine > 1.5 mg%, white blood cell count < 2000, overwhelming sepsis or septic shock, patients with chronic illness, malignancy, acquired immune deficiency syndrome, central nervous system disease, or APACHE II scores > 35. | |
| Interventions | 2 regimens: 1) Meropenem 1 g (8 hourly). 2) Tobramycin 5 mg/kg/day (divided into 3 doses) and clindamycin 900 mg (8 hourly). Tobramycin levels maintained with peaks 6 to 10 mcg/mL and troughs 0 to 2 mcg/mL.Timing of antibiotic infusion: pre‐operatively. Length: afebrile (< 38 degrees Celsius) and without physical findings or intra‐abdominal infection for 48 hours. | |
| Outcomes | Clinical success. Duration of therapy, hospitalised stay and time to defervescence. | |
| Notes | All patients had complicated appendicitis.Meropenem was statistically better at reducing post‐operative fever, duration of antibiotic treatment and hospital stay.Supported by grant from Zeneca Pharmaceuticals. Adverse reactions were all mild ‐ diarrhoea and rash. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Brismar 1992.
| Methods | Randomised controlled trial. Multicentre (Swedish and USA) study. Randomisation method: numbered sealed envelopes. Assessors were blinded. Patient stratification: not used.Power calculation: not performed. Intention‐to‐treat analysis: performed. Sub‐group analysis: not performed. Follow‐up: 1 to 2 and 4 to 6 weeks after completion of treatment. | |
| Participants | Number of patients: 134. Clinically and microbiologically evaluable patients: 113. 55 (Piperacillin/tazobactam, P‐T) versus 58 (Imipenem/cilastatin, I‐C). Mean age: 52.9 (P‐T), 54.0 (I‐C). Age range:16‐92. Inclusion criteria: > 18 years of age and suspected intra‐abdominal infections. Exclusion criteria: pregnant or lactating women; known allergy to study drugs; patients with infection resistant to study drugs; septic shock; patients treated with probenecid or other investigational drugs; antimicrobial agents within last 72 hours; impaired renal or hepatic function; serum bilirubin, transaminases or ALP greater than 3 times the upper normal limit; CNS disorders; and concomitant infection other than intra‐abdominal infection. | |
| Interventions | 2 regimens: 1) Piperacillin 4 g (8 hourly) and tazobactam 500 mg (8 hourly). 2) Imipenem 500 mg (8 hourly) and cilastatin 500 mg (8 hourly). Timing of antibiotic infusion: intra‐operatively. Length: > 3 days. | |
| Outcomes | Clinical (ITT analysis) and bacteriological success. Mortality. Superinfection. Adverse reactions (ITT analysis). | |
| Notes | 73/134 (54%) patients had complicated appendicitis. Piperacillin/tazobactam statistically more effective than imipenem/cilastatin. Majority of adverse events were mild ‐ diarrhoea and nausea. Results also published elsewhere as Eklund 1993 (excluded studies). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Brismar 1995.
| Methods | Randomised controlled trial. Multicentre (7 Swedish centre) study. Randomisation method: computer generated random envelopes. Assessors were blinded. Patient stratification: using APACHE II score. Power statement: not used. Intention‐to‐treat analysis: performed. Sub‐group analysis: not performed.Follow up: 4‐6 weeks. | |
| Participants | Number of patients: 249. Clinically and microbiologically evaluable patients: 175. 94 (Meropenem, M) versus 81 (Imipenem/cilastatin, I‐C). Mean age: 52.6. Age range: 17‐91. APACHE II score 0‐10: n = 85 (90%) (M) vs 73 (90%) (I‐C). APACHE II score 11‐20: n = 9 (10%) (M) vs 7 (9%) (I‐C). APACHE II score > 20: n = 0 (0%) (M) vs 1 (1%) (I‐C). Inclusion criteria: > 18 years old with suspected intra‐abdominal infections. Exclusion criteria: pregnant or lactating women, severe underlying disease, hepatic or renal impairment, neutropaenia, cystic fibrosis, known hypersensitivity to study drugs, antimicrobial therapy within last 72 hours, concomitant infection, another investigational drugs within last 30 days and APACHE II score > 20. | |
| Interventions | 2 regimens: 1) Meropenem 500 mg (8 hourly). 2) Imipenem 500 mg and cilastatin 500 mg (8 hourly). Timing of antibiotic infusion: pre‐ and intra‐operatively. Length: 5 ‐ 10 days. | |
| Outcomes | Clinical (ITT analysis) and microbiological success. Mortality (ITT analysis). Superinfection. Adverse reactions (ITT analysis). | |
| Notes | Majority (71%) had perforated appendicitis.No statistically significant difference shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Brismar 1996.
| Methods | Randomised parallel trial. Multicentre (9 Swedish centres) study between May 1993 and February 1994. Randomisation method: computer generated random envelopes. Blinding of assessors: not stated. Patient stratification: using APACHE II score. Power calculation: performed. Intention‐to‐treat analysis: performed. Sub‐group analysis: not performed. Follow‐up: 2 follow‐up visits ‐ 1‐2 and 4‐6 weeks after completion of treatment. | |
| Participants | Number of patients: 118. Clinically and microbiologically evaluable patients: 83. 43 (Biapenem, B) versus 40 (Imipenem/cilastatin, I‐C). Mean age: 50.7 (B), 54.2 (I‐C). Age range: 18‐84. APACHE II score 0‐10: n = 39 (91%) (B) vs 37 (93%) (I‐C). APACHE II score 11‐20: n = 4 (9%) (B) vs 3 (7%) (I‐C). Inclusion criteria: > 18 years old with complicated intra‐abdominal infections (an operative procedure or percutaneous drainage is required for diagnosis and management, and the duration of antibiotic > 5 days). Exclusion criteria: pregnant or lactating women, severe underlying disease or hepatic or renal impairment, neutropaenia, cystic fibrosis, known hypersensitivity to carbapenems, antibiotic therapy within last 72 hours, concomitant infection, patients given investigational drugs with 30 days and APACHE II score > 20. | |
| Interventions | 2 regimens: 1) Biapenem 500 mg (8 hourly). 2) Imipenem 500 mg (6 hourly) and cilastatin 500 mg (6 hourly). Timing of antibiotic infusion: pre‐ and intra‐operatively. Length: > 5 days. | |
| Outcomes | Clinical (ITT analysis) and microbiological success. Mortality (ITT analysis). Superinfection. Adverse reactions (ITT analysis). | |
| Notes | Majority (69%) patients had perforated appendicitis with peritonitis. No statistically significant difference shown. Study supported by a grant from American Synamid Co. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Busuttil 1984.
| Methods | Randomised controlled trial. Multicentre (2 USA centres) study between October 1980 and December 1981. Randomisation method: by pharmacist. Blinding of assessors: not stated. Patient stratification: not used. Power calculation: not used. Intention‐to‐treat analysis: not performed. Sub‐group analysis: performed.Follow up: 6 weeks. | |
| Participants | Number of patients: 65. 31 (Cefamandole, C) versus 34 (Gentamicin/clindamycin, G‐C).Median age: 45.Age range: 18‐89. Inclusion criteria: documented or suspected bacterial peritonitis or intra‐abdominal sepsis. Exclusion criteria: age < 18 years old; pregnancy; previous anaphylactic reactions to study drugs; renal, liver or auditory impairments; concomitant infection requiring antibiotic and significant underlying disease. | |
| Interventions | 2 regimens: 1) Cefamandole 8‐12 g per day every 4‐6 hours. 2) Gentamicin 3‐5 mg/kg/day in divided doses every 8 hours and clindamycin 600 mg (6 hourly). Gentamicin peak and trough levels were measured every 4 days but levels were not stated. Timing of antibiotic infusion: not clear. Length: not stated. | |
| Outcomes | Clinical success. Mortality. Wound infection, intra‐abdominal abscess and systemic sepsis. | |
| Notes | 25/65 (38%) of patients had complicated appendicitis. No statistically significant difference shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Christou 1996.
| Methods | Randomised, double‐blinded controlled study. Multicentre (10 Canadian centres) study. Randomisation method: sealed envelope. Assessors were blinded to randomisation. Patient stratification: APACHE II score. Power calculation: performed. Intention‐to‐treat analysis: performed. Sub‐group analysis: not performed. Follow up: 30 days. | |
| Participants | Number of patients: 213. Mean age: 48.9 (Cefoxitin, C), 52.5 (Imipenem/cilastatin, I‐C). Clinically and microbiologically evaluable patients: 154. 80 (C) versus 74 ( I‐C). Mean APACHE II score: 7.7 +/‐ 4.4 (C), 8.9 +/‐ 5.3 (I‐C). Inclusion criteria: > 16 years of age, 2 or more of the following criteria of abdominal infection (nausea and vomiting, abdominal tenderness, new mass in the abdomen, guarding and rebound tenderness, diminished bowel sounds, rigors, temperature > 38.5 degrees C, white blood cell count > 11000 x 1000000/L, or radiological evidence of intra‐abdominal infection) and planned surgical or percutaneous intervention within 48 hours of entry into study. Exclusion criteria: infections of liver or pancreas, simple acute cholecystitis or appendicitis, ascending cholangitis, duodenal or gastric perforations < 24 hours, unlikely to survive 48 hours, neutrophil count < 1000 x 1000000/L, anuria, estimated creatinine clearance < 0.33 mL/min, hypersensitivity to study drugs, > 3 doses of any antimicrobial regimen within last 72 hours, tertiary peritonitis, pregnant or breast feeding, history of seizure and participation in a concurrent study. | |
| Interventions | 2 regimens: 1) Cefoxitin 2 g (6 hourly) (C). 2) Imipenem 500 mg (6 hourly) and cilastatin (6 hourly) (I‐C). Timing of antibiotic infusion: pre‐operatively. Length: < 21 days. | |
| Outcomes | Clinical success (ITT analysis). Mortality (ITT analysis). | |
| Notes | 56/213 (26%) of patients had complicated appendicitis. Success rate in evaluable patients: 83.8% (C), 87.8% (I‐C). No statistically significant difference shown ‐ ITT analysis for success rate: 81.7% (C), 82.7% (I‐C). Study supported by Merck Frosst Canada. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Cohn 2000.
| Methods | Randomised, double‐blinded controlled trial. Multicentre (34 USA and Canada centres) study between September 1995 and May 1997. Randomisation method: not stated. Assessors were blinded. Patient stratification: by presence or absence of appendicitis and APACHE II score. Power calculation: performed. Intention‐to‐treat analysis: performed. Sub‐group analysis: not performed. Follow up: 3‐5 week post‐therapy (1‐3 weeks for appendicitis). | |
| Participants | Number of patients: 459. Clinical and microbiologically evaluable patients: 250. 134 (Ciprofloxacin/metronidazole, C‐M) versus 116 (Piperacillin/tazobactam, P‐T). Mean age: 47 (C‐M), 49 (P‐T). Mean APACHE II score: 9.6 (C‐M), 9.5 (P‐T). Inclusion criteria: patients > 18 years of age with complicated intra‐abdominal infection requiring surgical intervention or percutaneous drainage in addition to parenteral antibiotics. Exclusion criteria: pregnant or breast feeding women, allergy, renal insufficiency, an indwelling peritoneal catheter, acute pancreatitis, perforated peptic ulcer or traumatic upper gastrointestinal tract perforation < 24 hours, lower gastrointestinal perforation < 12 hours, APACHE II score > 30, not expected to survive > 48 hours and prior antibiotic therapy for last 24 hours. | |
| Interventions | 2 regimens: 1) Ciprofloxacin 400 mg (12 hourly) and metronidazole 500 mg (6 hourly). 2) Piperacillin/tazobactam 3.375 mg (6 hourly). Timing of antibiotic infusion: pre‐operative or intra‐operatively. Length: > 3 days for appendicitis, > 5 days for all other diagnoses and < 14 days. After 48 hours of therapy, patients were assessed for recovery of gastrointestinal function and patients on IV C‐M were switched to oral C‐M. | |
| Outcomes | Clinical (ITT analysis) and bacteriological success. Mortality (ITT analysis). Wound infection and superinfection. Adverse reactions (ITT analysis). | |
| Notes | Overall clinical response: 74% (C‐M) versus 63% (P‐T) (p = 0.047). ITT analysis on clinical response: 75% (C‐M) versus 69% (P‐T) ( p = 0.213). 118/282 (42%) of patients had complicated appendicitis. 90% of patients had pre‐therapy (< 2 doses of other antibiotics). Approximately 26% of patients had delayed primary closure of wounds. Supported by grant from Bayer Corp. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
de Groot 1993.
| Methods | Randomised controlled trial. Single centre (Dutch) study between August 1987 and May 1989. Randomisation method: sealed envelopes. Blinding of assessors: not used. Patient stratification: not performed. Power calculation: not used. Intention‐to‐treat analysis: not performed. Sub‐group analysis: performed. Follow up: not stated. | |
| Participants | Number of patients: 104. Clinically and microbiologically evaluable patients: N = 80. 38 (Imipenem/cilastatin, I‐C) versus 42 (Aztreonam/clindamycin, A‐C). Mean age: 58 (I‐C), 64 (A‐C). Age range: 17‐90. Inclusion criteria: adults with symptoms of local or generalised intra‐abdominal infections. Exclusion criteria: < 15 and > 90 years of age, known hypersensitivity to study drugs, immunocompromised (unless on steroids). | |
| Interventions | 2 regimens: 1) Imipenem 500 mg (6 hourly) and cilastatin 500 mg (6 hourly). 2) Aztreonam 1 g ((8 hourly) and clindamycin 600 mg (8 hourly). Timing of antibiotic infusion: intra‐operatively. Length: > 5 days. | |
| Outcomes | Clinical success. Mortality. Wound infection, intra‐abdominal abscess, clinical sepsis, superinfection and remote infection. Adverse reactions. | |
| Notes | 30/80 (38%) of patients had complicated appendicitis.No differences in outcome seen between both arms. Supported in part by grant from MSD Nederland. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Dupont 2000.
| Methods | Randomised controlled trial. Multicentre (35 French centres) study between March 1994 and July 1997. Randomisation method: computer‐generated blocks of four subjects. Assessors were blinded. Power calculation: performed. Patient stratification: SAPS II, and MacCabe and Jackson score. Intention‐to‐treat analysis: performed. Sub‐group analysis: not performed. Follow‐up: 30 days. | |
| Participants | Number of patients: 241. Number of assessable patients in intention‐to‐treat analysis: 204. 99 (Piperacillin/tazobactam, P‐T) versus 105 (Piperacillin/tazobactam and Amikacin, P‐A). Mean age: 60 (P‐T), 63 (P‐A). Mean SAPS II: 30 (P‐T), 31 (P‐T‐A). Inclusion criteria: > 18 years of age, non‐pregnant women, clinical diagnosis of complicated intra‐abdominal infection (presence of severe sepsis for patients with community acquired infections and at least SIRS for patients with postoperative or nosocomial infections). Exclusion criteria: allergy to study drugs, MacCabe and Jackson score of C or SAPS II of > 45, septic shock, neutropaenia (leucocyte count < 1000/mm3) pregnancy, non‐generalised peritonitis, and effective antimicrobial treatment given during the last 30 days prior to inclusion. | |
| Interventions | 2 regimens: 1) Piperacillin/tazobactam 4 g (6 hourly). 2) Piperacillin/tazobactam 4 g (6 hourly) and Amikacin 7.5 mg/kg (12 hourly). Timing of antibiotic infusion: not stated. Length: > 2 days, < 14 days. | |
| Outcomes | Clinical success (ITT analysis). Mortality (ITT analysis). Adverse reactions (ITT analysis). Duration of therapy. | |
| Notes | 18/204 (9%) of patients had complicated appendicitis. No statistically significant difference shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Eckhauser 1992.
| Methods | Randomised controlled trial. Multicentre (23 USA centres) study. Randomisation method: unclear. Blinding of assessors: not used. Patient stratification: according to severity of infection. Power calculation: not used. Intention‐to‐treat analysis: performed. Sub‐group analysis: not performed. Follow up: not stated. | |
| Participants | Number of patients: 145. Clinically evaluable patients: N = 117. 53 (Imipenem/cilastatin, I‐C) versus 64 (Aminoglycoside/clindamycin, A‐C). Mean age: 56.8 (I‐C), 51.9 (A‐C). Inclusion criteria: serious intra‐abdominal infections. Exclusion criteria: allergy to study drugs, previous administration of > 2 doses of another antibiotic, renal dysfunction, pregnancy or nursing, mental incapacitation, inability to give consent and concurrent participation in another clinical study. | |
| Interventions | 2 regimens: 1) Imipenem/cilastatin 500 mg (6‐8 hourly). 2) Gentamicin or tobramycin 1 mg/kg (8 hourly) and clindamycin 600 mg (6 hourly). Aminoglycoside serum level maintained at peak < 10 mcg/ml and trough > 2 mcg/ml. Timing of antibiotic infusion: not clear. Length: > 3 days. | |
| Outcomes | Clinical and bacteriological success (ITT analysis). Mortality (ITT analysis). Adverse reactions (ITT analysis). | |
| Notes | 18/117 (15%) of patients had complicated appendicitis.No statistically significant difference shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Gozenbach 1987.
| Methods | Randomised controlled trial. Single centre (Swiss) study between June 1982 and August 1985. Randomisation method: not stated. Blinding of assessors: not used. Patient stratification: not performed. Power calculation: not performed. Intention‐to‐treat analysis: not used. Sub‐group analysis: performed. Follow up: 3 weeks. | |
| Participants | Number of patients: 93. 47 (Imipenem/cilastatin, I‐C) versus 46 (Netilmicin/clindamycin, N‐C). Inclusion criteria: adult patients with an intra‐abdominal infection, localised or generalised. Exclusion criteria: intra‐abdominal infections with no bacteriological growth from primary specimen, antibiotics therapy within last 3 days and patients who died during the first 3 days after surgery. | |
| Interventions | 2 regimens: 1) Imipenem 500 mg and Cilastatin 500 mg (8 hourly). 2) Netilmicin (according to serum concentrations) and clindamycin 600 mg (8 hourly). Netilmicin level monitored to achieve peak concentrations of 4‐6 mg/L and trough of < 2 mg/L. Timing of antibiotic infusion: commenced after surgery. Length: not stated. | |
| Outcomes | Clinical success Wound infections and intra‐abdominal abscess. | |
| Notes | 53/93 (57%) of patients had complicated appendicitis.No statistically significant difference shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Greenberg 1994.
| Methods | Randomised controlled trial. Multicentre (USA) study between February 1990 and January 1993. Randomisation method: computer generated code. Blinding of assessors: not used. Patient stratification: not used. Power calculation: not performed. Intention‐to‐treat analysis: performed. Sub‐group analysis: not performed. Follow up: 4 weeks. | |
| Participants | Number of patients: 87. Clinically evaluable patients: 76. 47 (Cefoperazone/sulbactam, C‐S) versus 29 (Gentamicin/clindamycin, G‐C). Mean age: 49 (C‐S), 46 (G‐C). Age range: 18‐92. Inclusion criteria: > 18 years of age, suspected or known intra‐abdominal infection bacterial origin and either localised or generalised peritonitis. Exclusion criteria: terminally ill, pregnant or lactating women, patients with known hypersensitivity to study drugs, impaired immunological or haematological function (WBC < 500 X 1000000/L, those on immunosuppressive drugs or those with HIV infection), estimated creatinine clearance < 30 mL/min/1.73 m2 body surface area), patients unable to refrain from alcohol for 3 days after therapy, participation in another drug trial, requiring antimicrobial therapy other than study drugs, successful antibiotic therapy within last 4 days and patients with acute abdominal trauma who had not yet developed peritonitis. | |
| Interventions | 2 regimens: 1) Cefoperazone 2 g and sulbactam 1 g (12 hourly) [Interval of cefoperazone/sulbactam could be shortened to every 6‐8 hour at the discretion of the principal investigator]. 2) Gentamicin (based on body weight) and clindamycin 900 mg (8 hourly). Gentamicin levels monitored at peak 4‐8 mg/L and trough < 2 mg/L. Timing of antibiotic infusion: pre‐operatively or during the surgical procedure. Length: not stated. | |
| Outcomes | Clinical and microbiological success. Mortality. Superinfection. Adverse reactions. | |
| Notes | 24/76 (32%) of patients had complicated appendicitis. No statistically significant difference shown. Supported by grant from Pfizer Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Hopkins 1994.
| Methods | Randomised double‐blinded controlled trial. Single centre (USA) study over 4 year period. Randomisation method: computer generated randomisation table. Assessors were blinded. Patient stratification: not used. Power calculation: not performed. Intention‐to‐treat analysis: not performed. Sub‐group analysis: not performed. Follow up: 4‐6 weeks. | |
| Participants | Number of patients: 114. Clinically evaluable patients: 76. 40 (Cefotetan, C) versus 36 (Gentamicin/clindamycin, G‐C). Mean age: 29 (C), 29 (G‐C). Age range: 18‐60. Inclusion criteria: suspected complicated appendicitis (gangrenous, perforated or appendiceal abscess). Exclusion criteria: hypersensitivity to study drugs, received prior antibiotics, concomitant antibiotic for another infection focus, on other investigational drug, serum creatinine > 2.5 mg/dl, impaired immunological function or leucopaenia < 1500 mm3, CNS infection, active colitis or liver disease, pregnant or nursing. | |
| Interventions | 2 regimens: 1) Cefotetan 2 g (12 hourly). 2) Amikacin 500 mg followed by 7.5 mg/kg (12 hourly) and clindamycin 600 mg (6 hourly). Amikacin level monitored at 48 hours but levels were not stated. Timing of antibiotic infusion: prior to surgery. Length: > 5 days. | |
| Outcomes | Clinical and microbiological success. Wound infection and intra‐abdominal abscess. Adverse reactions (ITT analysis). Duration of therapy, days hospitalised and time to defervescence. | |
| Notes | All patients had complicated appendicitis. No statistically significant difference shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Investigators 1994.
| Methods | Randomised controlled trial. Multicentre (19 North American centres) study. Randomisation method: computer generated randomisation list, 2 patients were allocated to piperacillin/tazobactam group for each that was allocated to gentamicin/clindamycin. Blinding of assessors: not stated. Patient stratification: not performed. Power calculation: not performed. Intention‐to‐treat analysis: not performed. Sub‐group analysis: not performed. Follow up: 4‐6 weeks. | |
| Participants | Number of patients: 331. Mean age: 47.5 (Piperacillin/tazobactam, P‐T), 45.7 (Gentamicin/clindamycin, G‐C). Age range: 15‐89. Clinically evaluable patients: 147. 104 (P‐T) versus 43 (G‐C). Inclusion criteria: > 16 year old with clinical signs and symptoms of intra‐abdominal infection, temperature > 38 degrees C and WBC count > 1000 X 1000000/L. Exclusion criteria: known hypersensitivity to study drugs, moderate to severe renal dysfunction, liver disease, granulocyte count < 1000 X 1000000/L, or platelets count < 50000 X 1000000/L, > 2 doses of a non‐study antibacterial agent within 72 hours before enrolment (unless culture yielded resistant pathogen and patient showed no favourable response). Severely ill patients with cystic fibrosis, septic shock, active or treated leukaemia, AIDS, HIV, tuberculosis, renal dialysis and patients taking part in other investigational drugs were also excluded. | |
| Interventions | 2 regimens: 1) Piperacillin 3 g and tazobactam 375 mg (6 hourly). 2) Gentamicin 2.5 ‐ 5.0 mg/kg/24 hours in divided doses (8 hourly) and clindamycin 600 mg (6 hourly). Gentamicin level monitored at peak 4‐10 mcg/ml and trough 0.5‐2 mcg/ml. Timing of antibiotic infusion: before commencement of operation. Length: > 48 hours after resolution of symptoms and signs. | |
| Outcomes | Clinical and microbiological success. Mortality (ITT analysis). Superinfection. | |
| Notes | 73/134 (54%) patients had complicated appendicitis. No statistically significant difference shown. Majority of adverse events were mild ‐ diarrhoea and nausea. Results also published elsewhere as Polk 1993 (excluded studies). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Jaccard 1998.
| Methods | Randomised controlled trial. Multicentre (3 Swiss centres) study between December 1993 and May 1996. Randomisation method: sealed sequential envelopes. Assessors were blinded. Patient stratification: not performed. Power calculation: not performed. Intention‐to‐treat analysis: not performed. Sub‐group analysis: not performed. Follow up: 2‐4 weeks. | |
| Participants | Clinically evaluable patients: 159. 76 (Piperacillin/tazobactam, P‐T), versus 83 (Imipenem/cilastatin, I‐C). Mean age: 59.1 (P‐T), 59.1 (I‐C). Mean APACHE II score: 8.3 +/‐ 6.3 (P‐T), 7.3 +/‐ 4.9 (I‐C). Inclusion criteria: > 16 years old and peritonitis diagnosed intraoperatively. Exclusion criteria: pregnancy or lactating, expected survival < 48 hours, known allergy to study drugs, HIV, concomitant infection, infection with micro‐organism known to be resistant to study drugs, deranged LFT ( transaminases, ALP and bilirubin > 3 times upper limit of normal). | |
| Interventions | 2 regimens: 1) Piperacillin/tazobactam 4.5 g (8 hourly). 2) Imipenem/cilastatin 500 mg (6 hourly). Timing of antibiotic infusion: not stated. Length: not stated. | |
| Outcomes | Clinical success. Mortality. Intra‐abdominal abscess and clinical sepsis. Duration of therapy. | |
| Notes | Study compared both regimens in nosocomial pneumonia and peritonitis. Results were clearly illustrated for both groups of patients. % of patients with complicated appendicitis was not stated. No statistically significant difference shown. Study supported by grant from Wyeth‐Lederle and MSD‐Chibret. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Jauregui 1990.
| Methods | Randomised controlled trial. Multicentre (4 USA centres) study. Randomisation method: computer generated randomisation codes to enrol 2 patients in cefoperazone/sulbactam group for each patient assigned to gentamicin/clindamycin group. Blinding of assessors: not stated. Patient stratification: not performed. Power calculation: not used. Intention‐to‐treat analysis: not performed. Sub‐group analysis: not performed. Follow up: 4 weeks. | |
| Participants | Number of patients: 152. Clinically evaluable patients: 110. 76 (Cefoperazone/sulbactam, C‐S) versus 34 (Gentamicin/clindamycin, G‐C). Mean age: 55.1 (C‐S), 53.3 (G‐C). Age range: 20‐91. Inclusion criteria: > 18 years of age with known or suspected intra‐abdominal infection requiring both surgical and antimicrobial treatment. Exclusion criteria: pregnant or lactating, terminal illness, severe immunosuppression, any antibiotic within last 4 days and known hypersensitivity to study drugs. | |
| Interventions | 2 regimens: 1) Cefoperazone 2 g and sulbactam 1 g (12 hourly). 2) Gentamicin 4.5 ‐ 6 mg/kg/day in divided doses (8 hourly) and clindamycin 2.4 g/day (6‐8 hourly). Gentamicin level monitored at peak 6 ‐ 8 mg/l. Timing of antibiotic infusion: not stated. Length: > 5 days. | |
| Outcomes | Clinical success. | |
| Notes | Included patients with wound sepsis requiring surgery and antibiotics, but results for peritonitis were easily illustrated. % patients with complicated appendicitis was not stated. Cure rate for cefoperazone/sulbactam was statistically higher than gentamicin/clindamycin. Study partly funded by grant from Pfizer Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Kanellakopoulou 1993.
| Methods | Prospective parallel randomised controlled trial. Single centre (Greek) study. Randomisation method: unclear. Blinding of assessors: not used. Patient stratification: not performed. Power calculation: not used. Intention‐to‐treat analysis: not performed. Sub‐group analysis: not performed.Follow up: > 30 days. | |
| Participants | Number of patients: 62. Mean age: 51.3 (Meropenem, M), 45.5 (Imipenem/cilastatin, I‐C). Age range: 18‐74. Clinically evaluable patients: 59. 28 (M) versus 31 (I‐C). Inclusion criteria: > 18 years of age, presumptive diagnosis of intra‐abdominal infections diagnosed clinically on the basis of symptoms and signs of general or local peritonitis. Exclusion criteria: known allergy to study drugs,pregnancy, severe underlying disease rendering the therapeutic results non‐assessable on post‐treatment follow up, concurrent or previous antibiotic administration. | |
| Interventions | 2 regimens: 1) Imipenem/cilastatin 1 g (8 hourly). 2) Meropenem 1 g (8 hourly). Timing of antibiotic infusion: at induction of anaesthesia. Length: > 5 days. | |
| Outcomes | Clinical success. | |
| Notes | 26/62 (42%) of patients had complicated appendicitis. No statistically significant difference was shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Kempf 1996.
| Methods | Randomised controlled trial. Multicentre (5 German centres) study between December 1992 and December 1993. Randomisation method: unclear. Blinding of assessors: not used. Patient stratification: using APACHE II score. Power calculation: performed. Intention‐to‐treat analysis: performed. Sub‐group analysis: performed. Follow up: 2‐4 weeks. | |
| Participants | Number of patients: 94. Clinically evaluable patients: 83. 43 (Meropenem, M) versus 40 (Cefotaxime/metronidazole, C‐M). Mean age: 61.5 (M), 56.6 (C‐M). Age range: 20‐89. APACHE II score 0‐10: n = 26 (60%) (M) vs 28 (70%) (C‐M). APACHE II score 11‐20: n = 14 (33%) (M) vs 11(28%) (C‐M). APACHE II score > 20: n = 1 (2%) (M) vs 1 (2%) (C‐M). Inclusion criteria: > 18 years old, clinical symptoms and signs of peritonitis (abdominal tenderness, guarding and rigidity) demonstrated during surgery. Exclusion criteria: pregnant or lactating women, other investigational drugs within last 30 days, antibiotic therapy last 3 days unless organism cultured is resistant to study drugs or still present, concomitant infection, known hypersensitivity to study drugs, sever hepatic failure or neutropaenia (neutrophil < 1000 X 1000000/L), cystic fibrosis, history of seizures and severe underlying disease non expecting to survive > 48 hours. | |
| Interventions | 2 regimens: 1) Meropenem 1 g (8 hourly). 2) Cefotaxime 2 g and metronidazole 500 mg (8 hourly). Timing of antibiotic infusion: not stated. Length: 5‐10 days. | |
| Outcomes | Clinical and microbiological success. Mortality (ITT analysis). Superinfection. Adverse reactions (ITT analysis). | |
| Notes | 31/83 (37%) of patients had perforated appendicitis. Meropenem shown to be statistically significantly more successful (clinically and microbiologically) than cefotaxime/metronidazole. Study supported by grant from Zeneca GmbH. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Leal del Rosal 1995.
| Methods | Randomised controlled trial. Multi centre (multinational) study. Randomisation method: ratio 2:1, method unclear. Blinding of assessors: not used. Patient stratification: not performed. Power calculation: not performed. Intention‐to‐treat analysis: performed. Sub‐group analysis: not performed. Follow up: not stated. | |
| Participants | Number of patients: 267. Clinically evaluable patients: 205. 135 (Isepamicin/metronidazole, I‐M) versus 70 (Amikacin/metronidazole, A‐M). Mean age: 41.3 (I‐M), 43.7 (A‐M). Inclusion criteria: > 18 year old hospitalised patients with culture confirmed symptomatic intra‐abdominal infections sufficiently serious to warrant aminoglycoside plus metronidazole. Exclusion criteria: pregnant women. | |
| Interventions | 2 regimens: 1) Isepamicin 15 mg/kg (once daily) and metronidazole (dosing not stated). 2) Amikacin 7.5 mg/kg (12 hourly) and metronidazole (dosing not stated). Monitoring of aminoglycoside levels were not stated. Timing of antibiotic infusion: not stated. Length: > 5 days. | |
| Outcomes | Clinical and microbiological success (ITT analysis). Wound infection and superinfection. Adverse reactions (ITT analysis). | |
| Notes | 4% (9/205) of patients had complicated appendicitis (peri‐appendicular abscess).No statistically significant difference shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Leaper 1987.
| Methods | Randomised controlled trial. Single centre (UK) study. Randomisation method: sealed envelopes. Blinding of assessors: not stated. Patient stratification: not performed. Power calculation: not used. Intention‐to‐treat analysis: not performed. Sub‐group analysis: not performed. Follow up: until discharge or death. | |
| Participants | Number of patients: 45. Clinically evaluable patients: 43. 19 (Imipenem/cilastatin, I‐C) versus 24 (Ampicillin/metronidazole/gentamicin, A‐M‐G). Median age: 76 (I‐C), 68 (A‐M‐G). Age range: 16‐92. Inclusion criteria: clinically moderate to severe peritonitis. Exclusion criteria: < 16 years of age, known hypersensitivity to study drugs,concomitant infection, current antibiotic therapy and pregnant or lactating women. | |
| Interventions | 2 regimens: 1) Imipenem/cilastatin 500 mg (6 hourly). 2)Ampicillin 500 mg (6 hourly), metronidazole 500 mg (8 hourly) and gentamicin 80 mg (8 hourly). Gentamicin levels were monitored but ranges were not stated. Timing of antibiotic infusion: not stated. Length: > 5 days. | |
| Outcomes | Clinical and microbiological success. Wound infection, remote infection and superinfection. Adverse reactions. | |
| Notes | 5/43 (12%) patients had complicated appendicitis. No statistically significant difference was seen between both regimens. Study supported by grant from Merck, Sharpe & Dohme. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Malangoni 1985.
| Methods | Randomised controlled trial. Multi centre (3 USA centres ) study between July 1981 and January 1984. Randomisation method: computer generated random number. Blinding of assessors: double blinded. Patient stratification: using APACHE II and, McCabe and Jackson criteria. Power calculation: performed. Intention‐to‐treat analysis: not performed. Sub‐group analysis: not performed. Follow up: 1 month. Placebo was used to maintain double blinding. | |
| Participants | Number of patients: 170. Clinically evaluable patients: 112. 59 (Cefoxitin) versus 53 (Tobramycin/clindamycin). Mean age: 51 (overall). Age range: 18‐99. APACHE II score 0‐10: n = 54 (48%). APACHE II score 11‐20: n = 43 (38%). APACHE II score > 20: n = 15 (13%). Inclusion criteria: > 18 years old and suspected or known intra‐abdominal infection. Exclusion criteria: known allergy to study drugs, pregnant or lactating women, treated with other antibiotic effective against mixed infection within last 24 hours and patients with infection known to be resistant to study drugs. | |
| Interventions | 2 regimens: 1) Cefoxitin 3 g (6 hourly). 2) Tobramycin 1.5 mg/kg (8 hourly) and clindamycin 600 mg ( 6 hourly).Tobramycin level monitored at peak 4‐10 mg/ml and trough < 2 mg/ml. Timing of antibiotic infusion: unclear. Length: not stated. | |
| Outcomes | Clinical success. Mortality. Wound infection, intra‐abdominal abscess and remote infections. | |
| Notes | 22/112 (20%) of patients had complicated appendicitis.No statistically significant difference shown between both regimens. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Paakkonen 1991.
| Methods | Randomised controlled trial. Multi centre (3 Finnish centres) study. Randomisation method: unclear. Blinding of assessors: not stated. Patient stratification: according to site of pathology. Power calculation: not used. Intention‐to‐treat analysis: not performed Sub‐group analysis: not performed.Follow up: 4‐8 weeks. | |
| Participants | Number of patients: 85. Clinically evaluable patients: 83. 38 (Piperacillin, P) versus 45 (Cefuroxime/metronidazole, C‐M). Mean age: 60 (P) , 51 (C‐M).Age range: 16‐91. Inclusion criteria: > 15 years old with diagnosis of peritonitis clinically and intra‐operatively. Exclusion criteria: known allergy to study drugs, on other antibiotics within 48 hours of start of study pregnant or nursing women, severe renal impairment (creatinine > 300 mcmol/l) and infections where treatment with either regimens would be inappropriate. | |
| Interventions | 2 regimens: 1) Piperacillin 4 g (6 hourly). 2) Cefuroxime 1.5 g and metronidazole 500 mg (8 hourly). Timing of antibiotic infusion: at induction of anaesthesia. Length: > 5 days. | |
| Outcomes | Clinical success. Mortality. Wound infection, intra‐abdominal abscess and remote infections. Adverse reactions (ITT analysis). | |
| Notes | 26/83 (31%) of evaluable patients had complicated appendicitis.No statistically significant difference shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Poenaru 1990.
| Methods | Randomised controlled trial. Single centre (Canadian) study between September 1985 and October 1988. Randomisation method: unclear. Blinding of assessors: not stated. Patient stratification: using APACHE II score. Power calculation: not performed. Intention‐to‐treat analysis: not performed. Sub‐group analysis: not performed.Follow up: not stated. | |
| Participants | Number of patients: 104. 52 (Imipenem/cilastatin, I‐C) versus 52 (Tobramycin/antianaerobe, T‐A). Mean age: 52.0 (I‐C), 57.6 (T‐A). Mean APACHE II Score: 11.2 (I‐C), 13.1(T‐A). Inclusion criteria: patients admitted for emergency surgery with suspected intra‐abdominal infection. Exclusion criteria: pregnant, gynaecological and perianal infections, overt renal failure requiring dialysis, concomitant CNS infection and known allergy to study drugs, uncomplicated cholecystitis, appendicitis without perforation, traumatic bowel perforation < 12 hours and perforated peptic ulcer < 24 hours. | |
| Interventions | 2 regimens: 1) Imipenem/cilastatin 500 mg (6 hourly). 2) Tobramycin 1.5 mg/kg (8 hourly) and either clindamycin 600 mg (6 hourly) or metronidazole 500 mg (6 hourly). Tobramycin level monitored at peak 6‐10 mcg/ml and trough < 1.5 mcg/ml. Timing of antibiotic infusion: not stated. Length: not stated. | |
| Outcomes | Clinical success. Mortality. | |
| Notes | % of appendicitis patients: not stated.No statistically significant difference shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Scandinavian 1984.
| Methods | Randomised controlled trial. Multi centre (6 Scandinavian centres) study between may 1982 and October 1983. Randomisation method: sealed envelopes. Blinding of assessors: not used. Patient stratification: not used. Power calculation: not used. Intention‐to‐treat analysis: not performed. Sub‐group analysis: not performed. Follow up: not stated. | |
| Participants | Clinically evaluable patients: 27. 11 (Imipenem/cilastatin, I‐C) versus 16 (Gentamicin/clindamycin, G‐C). Inclusion criteria: 16‐80 with serious bacterial infections. Exclusion criteria: acute haematological malignant disorders, impaired renal function, infections of the CNS, infections caused by pathogens resistant to study drugs, patients who are pregnant or in shock and known allergy to study drugs. | |
| Interventions | 2 regimens: 1) Imipenem 500 mg and cilastatin 500 mg (6 hourly). 2) Gentamicin 1.5 mg/kg (8‐24 hourly) depending on serum levels and clindamycin 600 mg (6 hourly). Gentamicin levels were monitored at peak > 4 mg/l and trough < 2 mg/l. Timing of antibiotic infusion: not stated. Length: > 5 days. | |
| Outcomes | Clinical success. | |
| Notes | Patients with non‐peritonitis were included ‐ however data for primary outcome were available for peritonitis patients.115/184 (63%)of patients had complicated appendicitis.No statistically significant difference shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Scott 1987.
| Methods | Randomised controlled trial. Single centre (UK) study. Randomisation method: unclear. Blinding of assessors: not stated. Patient stratification: not used. Power calculation: not performed. Intention‐to‐treat analysis: not performed. Sub‐group analysis: not performed. Follow up: 2 weeks. | |
| Participants | Number of patients: 107. 53 (Cefotetan, C) versus 25 (Gentamicin/penicillin G/metronidazole, G‐P‐M) versus 29 (Cephradine/metronidazole, C‐M). Median age: 67. Age range: 17‐92. Inclusion criteria: > 16 years of age, judged on clinical grounds to be moderately or severely ill and to require antibiotic treatment for bacterial infections. Exclusion criteria: known allergy to study drugs, lactating or pregnant females, on any investigational drugs or antibiotics and patients unlikely to survive a course of treatment. | |
| Interventions | 3 regimens: 1) Cefotetan 2 g (12 hourly). 2) Gentamicin 80 mg (8 hourly) and penicillin G 600 mg (6 hourly) and metronidazole 500 mg (8 hourly). 3) Cephradine 1 g (6 hourly) and metronidazole 500 mg (8 hourly). Monitoring of gentamicin levels: not stated. Timing of antibiotic infusion: not stated. Length: not stated. | |
| Outcomes | Clinical success. Mortality. Wound infection. | |
| Notes | 19/107 ( 18%) of patients had complicated appendicitis. No statistically significant difference shown.Results also published elsewhere as Scott 1987a (excluded studies). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Shyr 1995.
| Methods | Open randomised controlled trial. Single centre (Taiwanese) study between November 1991 and March 1993. Randomisation method: computer generated random number in 3:2 ratio. Blinding of assessors: not performed. Patient stratification: not performed. Power calculation: not performed. Intention‐to‐treat analysis: not performed. Sub‐group analysis: not performed.Follow up: 4‐14 days. | |
| Participants | Number of patients: 77. Mean age: 60.3 (Piperacillin/tazobactam, P‐T), 61.2 (Gentamicin/clindamycin, G‐C). Clinically evaluable patients: 76. 46 (P‐T) versus 30 (G‐C). Inclusion criteria: > 16 years of age with diagnosis of peritonitis, intra‐abdominal abscess, complicated appendicitis, acute or complicated diverticulitis requiring laparotomy, acute or complicated cholecystitis and cholangitis. Exclusion criteria: known allergy to study drugs, existence of pathogen resistant to study drugs, septic shock, respiratory failure, concomitant probenecid treatment, pretreatment by more than 2 doses of antibiotics, granulocyte count < 1000 / cubic mm, platelet count < 50000 / cubic mm, serum creatinine > 2.5 mg/dl, LFTS > 3 x normal, uraemia undergoing dialysis, cystic fibrosis, pregnant or lactating women, leukaemia, concomitant infection, intra‐abdominal malignancy requiring additional chemotherapy or radiotherapy. | |
| Interventions | 2 regimens: 1) Piperacillin 4 g and tazobactam 500 mg (8 hourly). 2) Gentamicin 2.5 ‐ 5.0 mg/kg/day (given between 8 and 12 hourly) and clindamycin 600 mg (6 hourly). Gentamicin level monitored at peak 4‐10 mcg/ml and trough 0.5‐2 mcg/ml. Timing of antibiotic infusion: not stated. Length: not stated. | |
| Outcomes | Clinical and bacteriological success. Adverse reactions.Duration of therapy. | |
| Notes | 21/77 (27%) of patients had complicated appendicitis.Majority (35/77) of patients had complicated cholecystitis.No statistically significant difference shown.Partly supported by Lederle Laboratories, New York. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Smith 1980.
| Methods | Double‐blinded, randomised controlled trial. Multi centre (2 Canadian centres) study between May 1978 and September 1979. Randomisation method: schedule of random numbers kept at pharmacy. Blinding of assessors: double‐blinded. Patient stratification: not performed. Power calculation: not performed.Intention‐to‐treat analysis: not performed.Sub‐group analysis: not performed. Follow up: not stated. | |
| Participants | Number of patients: 80. Clinically evaluable patients: 57. 23 (Tobramycin/clindamycin) versus 34 (Tobramycin/metronidazole). Inclusion criteria: adults patients with intra‐abdominal infections Exclusion criteria: pregnant females, antibiotic treatment within last 30 days. | |
| Interventions | 2 regimens: 1) Tobramycin 1.5 mg/kg (8 hourly) and metronidazole 500 mg (8 hourly). 2) Tobramycin 1.5 mg/kg (8 hourly) and clindamycin 600 mg (8 hourly). Tobramycin levels were monitored but levels were not stated. Timing of antibiotic infusion: not stated. Length: > 3 days. | |
| Outcomes | Clinical success. Mortality. Adverse reactions. | |
| Notes | 24/57 (42%) of patients had complicated appendicitis.No statistically significant difference shown.Results also published elsewhere as Smith 1983 (excluded studies). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Solomkin 2001.
| Methods | Double‐blinded, randomised controlled trial. Multi centre (5 USA and Canadian centres) study. Randomisation method: randomisation with block size of four. Blinding of assessors: double‐blinded. Patient stratification: according to APACHE II score. Power calculation: no stated. Intention‐to‐treat analysis: performed.Sub‐group analysis: not performed. Follow up: not stated. Placebo was used to enable double‐blinding. | |
| Participants | Number of patients: 529. Clinically evaluable patients: 312. 150 (Clinafloxacin, C) versus 162 (Imipenem/cilastatin, I‐C). Mean age: 45.5 (C), 46.5 (I‐C). Inclusion criteria: > 18 years old with signs and symptoms of intra‐abdominal infections and if surgical or percutaneous drainage of an infectious focus was recently performed or necessary. Exclusion criteria: survival < 48 hours, APACHE II score > 30, known allergy to study drugs, other investigational therapy within last 30 days, impaired liver function, neutropaenia (< 1000 X 1000000 neutrophils/L), previous enrolment in trial, CNS disease, pregnant or breast feeding women and acute renal insufficiency. | |
| Interventions | 2 regimens: 1) Clinafloxacin 200 mg (12 hourly) with placebo.. 2) Imipenem/cilastatin 500 mg (6 hourly). Timing of antibiotic infusion: not stated. Length: > 3 days. | |
| Outcomes | Clinical success. Mortality (ITT analysis). Wound infection, intra‐abdominal abscess and clinical sepsis. Adverse reactions (ITT analysis). | |
| Notes | 167/312 (53%) of evaluable patients had complicated appendicitis. No statistically significant difference shown. Supported by grants from Parke‐Davis pharmaceutical Research. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Study 1986.
| Methods | Blinded randomised controlled trial. Multi centre (4 Swedish centres) study. Randomisation method: sealed envelopes. Blinding of assessors: double‐blinding. Patient stratification: not performed. Power calculation: not performed. Intention‐to‐treat analysis: not performed.Sub‐group analysis: performed. Follow up: 4 weeks. Placebo was used to enable double‐blinding. | |
| Participants | Number of patients: 123. Clinically evaluable patients: 83. 46 (Ampicillin/sulbactam, A‐S) versus 37 (Gentamicin/clindamycin, G‐C). Mean age: 52 (A‐S), 51 (G‐C).Age range: 17‐94. Inclusion criteria: > 17 years old with suspected serious intra‐abdominal infection requiring surgical and antibiotic treatment. Exclusion criteria: pregnant or lactating women, terminally ill patients, patients with impaired renal function, history of glycogenosis or family history of glycogen storage disease and known allergy to study drugs. | |
| Interventions | 2 regimens: 1) Ampicillin 2 g and sulbactam 1 g (6 hourly). 2) Gentamicin 1.5 mg/kg (8 hourly) and clindamycin 600 mg (6 hourly). Gentamicin level was monitored but levels were not stated.Timing of antibiotic infusion: not stated. Length: > 5 days. | |
| Outcomes | Clinical and microbiological success. Adverse reactions. | |
| Notes | % of appendicitis patients were not stated. No statistically significant difference shown.Study was supported by grant from Pfizer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Swedish 1990.
| Methods | Open randomised controlled trial. Multi centre (5 Swedish centres) study. Randomisation method: numbered sealed envelopes. Assessor was blinded. Patient stratification: not used. Power calculation: not used. Intention‐to‐treat analysis: not performed. Sub‐group analysis: not performed. Follow up: 30 days. | |
| Participants | Number of patients: 271. Clinically evaluable patients: 184. 104 (Pefloxacin/metronidazole, P‐M) versus 80 (Gentamicin/metronidazole, G‐M). Mean age: 54 (P‐M), 52 (G‐M).Age range: 18‐90. Inclusion criteria: > 17 years, suspected intra‐abdominal infection, verified by either laparotomy, drainage or puncture. Exclusion criteria: pregnant or lactating women, terminally ill patients, impaired hepatic or renal function, on another investigational drugs, known deficiency of glucose‐6‐phosphate dehydrogenase and known allergy to study drugs. | |
| Interventions | 2 regimens: 1) Pefloxacin 800 mg followed by pefloxacin 400 mg (subsequently, 12 hourly) and metronidazole 500 mg (8 hourly). 2) Gentamicin 1.5 mg/kg (8 hourly) and metronidazole 500 mg (8 hourly).Gentamicin levels were monitored but levels were not stated. Timing of antibiotic infusion: not stated. Length: > 3 days. | |
| Outcomes | Clinical and microbiological success. Mortality (ITT analysis).Superinfection. Adverse reactions (ITT analysis). | |
| Notes | 115/184 (63%) of patients had complicated appendicitis.No statistically significant difference shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Tornqvist 1985.
| Methods | Randomised controlled trial. Single centre (Swedish) study between November 1979 and December 1982. Randomisation method: not stated. Blinding of assessors: not stated. Patient stratification: not performed. Power calculation: not performed.Intention‐to‐treat analysis: not performed.Sub‐group analysis: not performed. Follow up: 3‐5 weeks. | |
| Participants | Number of patients: 148. Clinically evaluable patients: 122. 59 (Cefuroxime, C) versus 63 (Cefuroxime/metronidazole, C‐M). Median age: 66 (C), 61 (C‐M).Age range: 16‐93. Inclusion criteria: patients operated upon for diffuse peritonitis. Exclusion criteria: not stated. | |
| Interventions | 2 regimens: 1) Cefuroxime 1.5 g (8 hourly). 2) Cefuroxime 1.5 g and metronidazole 500 mg (8 hourly). Timing of antibiotic infusion: mainly pre‐operatively. Length: > 3 days. | |
| Outcomes | Mortality. Wound infection and intra‐abdominal sepsis. | |
| Notes | 42/122 (34%) of patients had perforated appendicitis.No statistically significant difference shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Torres 1999.
| Methods | Randomised controlled trial. Multi centre (2 Spanish centres) study. Randomisation method: sealed envelopes. Blinding of assessors: not used. Patient stratification: APACHE II score. Power calculation: not performed. Intention‐to‐treat analysis: performed for clinical success. Sub‐group analysis: not performed. Follow up: not stated. | |
| Participants | Number of patients: 160. Mean age: 46.6 years. Mean APACHE II score: 3.68 (Cefminox, C), 3.18 (Gentamicin/metronidazole, G‐M) Clinically evaluable patients: 152. 76 (Cefminox, C) versus 76 (Gentamicin/metronidazole, G‐M). Inclusion criteria: > 18 years old with symptoms and signs of intra‐abdominal infections. Exclusion criteria: pregnant or lactating women, known allergy to study drugs, antibiotic therapy within last 72 hours, platelet < 100000 /cubic mm, other investigational drugs within last 30 days, haemodialysis or immunosuppressive therapy, APACHE II score > 35, creatinine > 2.5 mg/dl, cirrhosis or ascites and extra‐abdominal infection. | |
| Interventions | 2 regimens: 1) Cefminox 2 g (12 hourly). 2) Gentamicin 80 mg (8 hourly) and metronidazole 500 mg (8 hourly). Gentamicin levels were monitored but ranges were not stated. Timing of antibiotic infusion: not stated. Length: not stated. | |
| Outcomes | Clinical success (ITT analysis). Mortality. Wound infection. Adverse reactions. | |
| Notes | 95/160 (59%) of patients had perforated appendicitis.No statistically significant difference shown.Supported by Tedec‐Meiji Farma, S.A., Spain. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Walker 1993.
| Methods | Double‐blinded, randomised controlled trial. Multi centre (5 USA centres) study between August 1987 and October 1990. Randomisation method: computer generated randomisation code. Blinding of assessors: double‐blinded. Patient stratification: not performed. Power calculation: performed. Intention‐to‐treat analysis: not performed. Sub‐group analysis: performed. Follow up: not stated. | |
| Participants | Number of patients: 385. Clinically evaluable patients: 197. 96 (Ampicillin/sulbactam, A‐S) versus 101 (Cefoxitin, C). Mean age: 44 (A‐S), 46 (C). Inclusion criteria: > 18 years of age with suspected bacterial intra‐abdominal infection and requiring urgent operation (visible serosal inflammation and a positive peritoneal culture). Exclusion criteria: known allergy to study drugs, concomitant antibiotics administration, successful antibiotic treatment within last 4 days, enrolment in other study, other major active infection, terminal illness, immune deficiency or neutropaenia (< 1500 neutrophils/ cubic mm), severe renal failure and pregnancy or breast‐feeding. | |
| Interventions | 2 regimens: 1) Ampicillin 2 g and sulbactam 1 g (6 hourly). 2) Cefoxitin 2 g (6 hourly). Timing of antibiotic infusion: not stated. Length: > 4 days. | |
| Outcomes | Clinical success. Wound infection, intra‐abdominal abscess and remote infection. Adverse reactions (ITT analysis). | |
| Notes | 50/197 (25%) of patients had peritonitis as a result of appendicitis. No statistically significant difference shown. Supported by grant from Roerig, a division of Pfizer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Yellin 1985.
| Methods | Double‐blinded, randomised controlled trial. Single centre (USA) study between April 1982 and June 1983. Randomisation method: computer generated random numbers. Blinding of assessors: double‐blinded. Patient stratification: not performed. Power calculation: not used. Intention‐to‐treat analysis: not performed. Sub‐group analysis: not performed.Follow up: 6 months. Placebos were used to maintain double‐blinding. | |
| Participants | Number of patients: 197. Clinically evaluable patients: 105. 67 (Ampicillin/sulbactam, A‐S) versus 38 (Gentamicin/clindamycin, G‐C). Mean age: 27.8 (A‐S), 26.7 (G‐C). Inclusion criteria: patients with perforated and gangrenous appendicitis (fever > 38 degrees C, duration of symptoms > 24 hours, diffuse abdominal tenderness and WBC count > 13000). Exclusion criteria: < 16 or > 75 years old, pregnant women and patients who had received antimicrobial therapy in the preceding 6 weeks. | |
| Interventions | 2 regimens: 1) Ampicillin 2 G and sulbactam 1 G (6 hourly). 2) Gentamicin 1.5 mg/kg (8 hourly) and clindamycin 600 mg (6 hourly). Gentamicin levels were monitored at peak 6 +/‐ 2 mcg/ml. Timing of antibiotic infusion: prior to operation. Length: afebrile for > 48 hours. | |
| Outcomes | Clinical success. Wound infection and intra‐abdominal abscess. Adverse reactions. Duration of therapy, hospital stay and defervescence.. | |
| Notes | All patients had complicated appendicitis. Trial had shown difference in clinical success rate in favour of gentamicin/clindamycin regimen. Study supported by grant from Pfizer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Zanetti 1999.
| Methods | Randomised controlled trial. Multi centre (4 Swiss centres) study. Randomisation method: sealed envelopes Blinding of assessors: not used. Patient stratification: APACHE II score. Power calculation: performed. Intention‐to‐treat analysis: performed. Sub‐group analysis: not performed. Follow up: 2 weeks. | |
| Participants | Number of patients: 161. Clinically evaluable patients: 135. 71 (Meropenem, M) versus 64 (Imipenem/cilastatin, I‐C). Mean age: 59.8 (M), 60 (I‐C). Mean APACHE II score: 5.8 (M), 6.4 (I‐C). APACHE II score 0‐10: n = 63 (89%) (M) vs 55 (86%) (I‐C). APACHE II score 11‐18: n = 8 (11%) (M) vs 9 (14%) (I‐C). Inclusion criteria: > 18 years old with moderately severe intra‐abdominal infections defined by the presence of abdominal tenderness, guarding and rigidity. Exclusion criteria: pregnancy or breastfeeding, allergy to study drugs, hepatic failure/coma, cystic fibrosis, CNS disease or history of seizures, APACHE II score > 18, severe disease rendering patient unable to complete 48 hour trial and receipt of investigational drugs within 30 days. | |
| Interventions | 2 regimens: 1) Meropenem 0.5 g (8 hourly). 2) Imipenem/cilastatin 0.5 g (6 hourly). Timing of antibiotic infusion: not stated. Length: 5‐10 days. | |
| Outcomes | Clinical and microbiological success. Mortality (ITT analysis). Adverse reactions (ITT analysis). Duration of therapy and hospitalisation. | |
| Notes | % of appendicitis patients were not stated. No statistically significant difference shown.Study was sponsored by Zeneca AG, Switzerland. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
ALT: alanine aminotransferase ALP: alkaline phosphatase APACHE: acute physiological and chronic health evaluation AST: aspartate aminotransferaseCNS: central nervous system ITT: intention‐to‐treat IV: intravenous LFT: liver function test SAPS II: simplified acute physiology score II SIRS: systemic inflammatory response syndrome WBC: white blood cell
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allo 1999 | Included patients < 16 years old. Majority of patients had appendicitis only. Concealment of allocation not stated. |
| Andaker 1987 | Majority of patients had appendicitis and other conditions with non‐generalised peritonitis. Unable to evaluate true peritonitis patients from pooled data. Certain patients received prophylactic course of antibiotics only. |
| Arguedas 1996 | No randomisation. Paediatric patients only were included in this study. No comparative regimen was used. |
| Baird 1983 | Underlying pathology was not clearly defined in the groups of patients. Included patients with traumatic wounds and conditions not requiring surgery. Poor clinical data presentation. Small number of patients in each arm of the study following high attrition rate. |
| Ball 1981 | No randomisation nor comparator. Small patient group. |
| Barboza 1994 | Method of randomisation not stated. Included patients with peritonitis secondary to trauma and gunshot wound. |
| Barie 1997 | One quarter of the patients received an additional systemic antimicrobial agent while receiving study therapy. Eight percent of patients in each group received vancomycin. |
| Bennion 1990 | Included patients < 16 years old. Only patients with gangrenous or perforated appendicitis were recruited. |
| Birolini 1985 | Included paediatric and gynaecological patients and patients not requiring surgical intervention. Inadequate concealment of allocation. Inconsistency in route of administration and dosage of antibiotics. |
| Birolini 1989 | Included paediatric patients and patients with no documented evidence of peritonitis. |
| Biron 1984 | Small patient group. Not all patients had peritonitis requiring surgical interventions. 25% of patients were treated conservatively. |
| Bubrick 1990 | Included patients with peritonitis secondary to trauma. |
| Busuttil 1982 | No randomisation nor other comparator. Moxalactam alone was the sole regimen used. |
| Cakmakci 1993 | Included non‐peritonitis patients and patients with post‐operative infections. Study drug in one arm was switched after start of trial from tobramycin to netilmycin. |
| Canadian 1983 | 31/ 141 (22%) patients had received other antibiotics within last 7 days either as treatment or prophylaxis for elective surgery and had failed therapy or developed complications. |
| Chin 1990 | This is purely a cost analysis study. |
| Christen 1987 | Included non‐peritonitis patients. Small patient group. |
| Colardyn 1996 | Included non‐peritonitis patients. Two thirds of patients in each treatment group were in intensive care unit and 53% of patients had failed to respond to antibiotic therapy before entering the study. |
| Collier 1981 | Method of randomisation not stated. Included gynaecological patients. Protocol allowed for addition of other antimicrobial therapy. |
| Cometta 1994 | Patients with nosocomial pneumonia and sepsis were included. Despite availability of data (clinical success only) for the group of patients with peritonitis, this study was excluded as APACHE II scores eventually revealed a difference in severity of illness in favour of imipenem/netilmicin group. |
| Condon 1995 | Included nosocomial intra‐abdominal infection; patients who had traumatic peritonitis, received a non‐study antibiotics and previously failed treatment of intra‐abdominal infection. |
| Danish 1984 | Included patients < 16 years of age. |
| Danziger 1988 | Small evaluable patient population, n = 27. Only 40% of patients had intra‐abdominal infections. |
| de Vries 1990 | Patients < 16 years old were included. |
| Donahue 1998 | All patients on intravenous imipenem/cilastatin were converted to oral amoxicillin/clavulanic acid at the discretion of the investigators. Patients were randomised in blocks of four. Included patients with traumatic perforation. |
| Dougherty 1995 | Included patients < 16 years of age. < 50% of patients were evaluable. |
| Drusano 1982 | Studies included patients with gynaecological infections. Even though data were available for peritonitis patients, this study was excluded as the protocol allowed for addition of aminoglycoside to the cefoxitin arm. |
| Eklund 1993 | This is a duplicate publication of study presented and published as Brismar et al 1992. |
| Fink 1989 | One arm of study included paediatric patients. < 40% of patients (n=45) were evaluable as a result of inclusion of patients with uncomplicated appendicitis and uncomplicated cholecystitis. |
| Fink 1991 | Results were derived from previous study (Fink 1989). Small evaluable patients (n=45). Despite the change of title, the results were identical to previous study and must have included paediatric patients. |
| Geroulanos 1995 | Majority of patients had only local or non‐peritonitis. Patients with uncomplicated appendicitis were also included for evaluation. |
| Harding 1982 | Included patients with gynaecological infections. Small patient population. |
| Henning 1984 | Unequal distribution of patients with complicated appendicitis in favour of tinidazole regimen. Small patient population. |
| Henry 1985 | This study utilised 2 protocols ‐ a non‐comparative study and a randomised controlled trial. Protocols allowed for addition of additional antibiotic (vancomycin, nafcillin) in the presence of organisms resistant to enterococci |
| Heseltine 1986 | Non‐randomised controlled trial. Patients were compared to historical results already included in this review (Berne 1982). |
| Hollender 1989 | In one arm of study, patients < 16 years old were included. Patients with non‐peritonitis causes of infection were included. Protocol allowed for concomitant addition of metronidazole and clindamycin at the discretion of treating physicians. |
| Holloway 1989 | Non‐randomised controlled trial. Intra‐abdominal infection was not proven. |
| Hoogkamp 1995 | Inadequate concealment of allocation ‐ patients were randomised alternately to one of the two regimens. One arm of the study received additional antibiotic (aminoglycoside) on top of the study drugs. More than 90% of patients had received 3 doses of other antibiotics. Not all patients had undergone surgery after being diagnosed with intra‐abdominal infections. |
| Huizinga 1988 | Included patients < 16 year of age and patients with peritonitis secondary to trauma. |
| Huizinga 1995 | Included patients with peritonitis as a result of trauma. About one third of the patients had received antibiotics during the three days before entry into the study. |
| Inthorn 1989 | Non‐randomised controlled trial. No comparators were used. Small patient population ‐ one fifth of patients had surgical failure. |
| Jaspers 1998 | Patients with peritonitis formed only a very small proportion of the study population (n = 10). Protocol allowed for addition of metronidazole to one arm of the trial. |
| Joshi 1986 | Patients with non‐peritonitis were included. Small number of peritonitis patients ‐ data for peritonitis only were not available. |
| Kasholm‐Tengve 1986 | Included patients with non‐peritonitis. Patient demographics were skewed in favoured of one arm. No data on clinical success was available. |
| Kirkpatrick 1983 | Included patients with non‐peritonitis. Data on patient characteristics and outcomes were limited. |
| Kooi 1990 | Only paediatric patients were included in this study. |
| Leal del Rosal 1989 | Trial included patients with peritonitis and soft tissue infections. Data for peritonitis only patients were not obtainable. Protocol allowed for addition of either clindamycin or metronidazole. |
| Lennard 1985 | Almost one‐third of patients had other antibiotics administered within last one week. Evaluable patients had |
| Levine 1989 | Majority of patients had a non‐peritonitis diagnosis. |
| Lou 1982 | Cohort study. Majority of patients had infections secondary to trauma. |
| Luke 1991 | Included patients < 16 years of age ‐ data for adults were not easily obtainable. Patients with abdominal trauma and gynaecological causes of infection were also included. |
| Luke 1999 | This was not a clinical trial but a study to assess the tolerability and safety of trovafloxacin compared to an established antibiotic regimen. Intravenous imipenem/cilastatin was switched to a completely different class of oral antibiotic (amoxycillin/clavulanic acid) at the discretion of the assessors.. |
| Marra 1998 | Patient population included non‐peritonitis infections. Data for peritonitis patients were not easily available. |
| Mehtar 1997 | Patients with non‐peritonitis cause of infections were included. Data for peritonitis patients only were not available. |
| Messick 1998 | This is only a retrospective pharmacoeconomics analysis. Clinical data had already been presented as Walker AP et al (1993). |
| Mullick 1987 | Paediatric patients were included. Different dosing regimens of clindamycin were used for adults and children. |
| Najem 1983 | Patients with peritonitis as a result of traumatic perforations were included. Results for patients with acute peritonitis only were not available. |
| Niinikoski 1993 | Patients with spontaneous post‐traumatic peritonitis were included. Patients in one arm of the study is significantly older and heavier than the other. |
| Ohlin 1999 | Patients with spontaneous and post‐traumatic peritonitis were included. |
| Polk 1993 | Duplicate publication of study conducted and published by Investigators (1994). |
| Poularas 1988 | Patients with uncomplicated appendicitis and gynaecological infections were included. Data for peritonitis patients alone were not obtainable. |
| Raahave 1970 | Study included patients < 16 years old. Study drug was administered intraperitoneally and subsequent doses were given intramuscularly. |
| Rohrborn 2000 | Discrepancy in the antibiotic regimens compared. Protocol allowed for addition of an aminoglycoside and other secondary antibiotics. No data on clinical success was obtainable. |
| Schein 1994 | This study was conducted to examine the optimal duration of courses of antibiotic therapy. No data for comparative regimens were available. |
| Scheinin 1994 | Protocol allowed for addition of aminoglycoside to treatment regimens. |
| Schentag 1983 | Included patients with operations where the bowel was not entered (abdominal aortic graft infections) or where no infection was found at laparotomy. Patients who had failed previous antimicrobial therapy were also included. Older (>40 years of age) patients were recruited. |
| Scott 1987a | Results presented elsewhere as Scott et al (1987). |
| Sirinek 1987 | Included patients < 16 years old. Different dosing regimen for different ages of patients. |
| Sirinek 1991 | Included patients < 16 years old. Different dosing regimen for different ages of patients. |
| Smith 1982 | Non randomised study with no comparator regimen. |
| Smith 1983 | Results were partly published previously as Smith et al (1980). Other part of data were from a cohort study with no comparator. |
| Smith 1984 | Included patients with non‐peritonitis. Data for patients with peritonitis were unobtainable from the pooled results. |
| Solomkin 1985 | Included patients with soft tissue infections and post‐operative pneumonia. Data for peritonitis alone were not available. |
| Solomkin 1990 | Protocol allowed for addition of vancomycin if initial cultures grew gram positive organisms believed to be resistant to study drugs. |
| Solomkin 1996 | Protocol allowed for concomitant antifungal therapy as well as vancomycin for suspected methicillin‐resistant Staphylococcus aureus or enterococcal infections. |
| Solomkin 2003 | Protocol allowed for addition of vancomycin if Enterococcus and methicillin‐resistant Staphylococcus aureus were isolated; and antifungal therapy. (See also Tellado 2002). |
| Stellato 1988 | Included patients < 16 years old. Different dosing regimens were used for different age groups. |
| Stone 1975 | Half of recruited patients were from a non‐randomised cohort study. Patients with non‐peritonitis and < 16 years old were recruited. One third of patients were given oral nystatin prophylactically. |
| Stone 1978 | No randomisation nor comparative regimen was used. |
| Stone 1981 | Included patients < 16 years old. Inadequate concealment of allocation ‐ patients were allocated based on the the last digit of their hospital numbers. |
| Stone 1982a | Included patients < 16 years old. Inadequate concealment of allocation ‐ patients were allocated based on the last digit of their hospital numbers. Almost similar cohort to Stone 1982b. |
| Stone 1982b | Included patients < 16 years old. Inadequate concealment of allocation ‐ patients were allocated based on the last digit of their hospital numbers. Almost similar cohort to Stone 1982a. |
| Stone 1983a | No descriptions were available for study. |
| Stone 1983b | Patients < 16 years of age were included. Inadequate concealment of allocation ‐ patients were randomised according to their hospital numbers. Three different cephalosporins and clindamycin dosing regimens were used. Not all patients had procedure to eradicate source of infection. |
| Stone 1984 | Patients with soft tissue infections and uncomplicated appendicitis and cholecystitis were included. Inadequate concealment of allocation. Not all patients had procedure to eradicate source of infection. |
| Tally 1981 | Patients < 12 years old were included. One arm of study allowed for the occasional addition of aminoglycoside. Not all patients had peritonitis or had undergone surgery. |
| Tally 1986 | Included patients < 16 years old, and patients with pelvic and soft tissue infections. Protocol allowed for addition of tobramycin to one arm of the study at the discretion of the treating physician. |
| Taylor 2000 | This study looked at outcome in complicated appendicitis who had been given a fixed minimum 5 day course of antibiotics versus one whose duration is purely dependent on clinical judgement. |
| Tellado 2002 | Updated results presented elsewhere as Solomkin et al (2003). |
| Teppler 2004 | Data was presented elsewhere as Solomkin et (2003). Protocol allowed for addition of vancomycin for treatment of resistant gram positive pathogens. |
| Vestweber 1994 | No randomisation nor comparator regimen was used. |
| Walters 1999 | Cost effectiveness comparison of data already presented as Solomkin et al (1996). |
| Williams 1991 | Protocol allowed for addition of vancomycin, nafcillin and metronidazole in patients with mixed infections involving gram positive organisms resistant to clindamycin. |
| Wilson 1988a | Three different cephalosporins were compared to each other. |
| Wilson 1997 | Majority of data were already presented elsewhere as Berne et al (1996). Patients who had been unsuccessfully treated with other antimicrobials were recruited. |
| Winston 1980 | Patients with non‐peritonitis source of infection were recruited. Results for peritonitis group were not obtainable. |
| Yellin 1993 | Both arms of study utilised same groups of antibiotics but only different doses of clindamycin. |
| Yellin 2002 | Patients in both arms of study were switched to oral ciprofloxacin after at least 3 days of therapy and satisfactory clinical response. |
| Yoshioka 1991 | Patients with non‐peritonitis infections were included. Data was not obtainable for patients with peritonitis only. |
Contributions of authors
Peng Wong, Andrew Gilliam, Jon Shenfine and David Leaper jointly designed and wrote the protocol. Peng Wong and Andrew Gilliam developed the search strategy and performed the relevant searches. All reviewers jointly appraised quality of trials and obtained data from trials. David Leaper provided general advice on the review.
Sources of support
Internal sources
No sources of support supplied
External sources
Merck Sharp & Dohme Limited, UK.
Declarations of interest
The Professorial Unit of Surgery of whom Professor Leaper was the lead clinician, received a one‐off limited and unconditional educational grant from Merck Sharpe & Dohme, which was meant for antibiotic trials. The reviewers can however assure that under no circumstances has this grant or Merck Sharpe & Dohme influenced the review or reviewers in any form. The company concerned certainly had no input into the wordings, opinions or conjectures of this review.
Edited (no change to conclusions)
References
References to studies included in this review
Angeras 1996 {published data only}
- Angeras MH, Darle N, Hamnstrom K, Ekelund M, Engstrom L, Takala J, et al. A comparison of imipenem/cilastatin with the combination of cefuroxime and metronidazole in the treatment of intra‐abdominal infections. Scandinavian Journal of Infectious Diseases 1996;28(5):513‐518. [DOI] [PubMed] [Google Scholar]
Basoli 1997 {published data only}
- Basoli A, Meli EZ, Mazzocchi P, Speranza V and Study Group. Imipenem/cilastatin (1.5g daily) versus meropenem (3.0g daily) in patients with intra‐abdominal infections: results of a prospective, randomized, multicentre trial. Scand J of Infect Dis 1997;29:503‐508. [DOI] [PubMed] [Google Scholar]
Berne 1982 {published data only}
- Berne TV, Yellin AW, Appleman MD, Heseltine PNR. Antibiotic management of surgically treated gangrenous or perforated appendicitis. The American Journal of Surgery 1982;144(1):8‐13. [DOI] [PubMed] [Google Scholar]
Berne 1987 {published data only}
- Berne TV, Appleman MD, Chenella FC, Yellin AE, Gill MA, Heseltine PNR. Surgically treated gangrenous or perforated appendicitis. Ann Surg 1987;205(2):133‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berne 1993 {published data only}
- Berne TV, Yellin AE, Appleman MD, Heseltine PNR, Gill MA. A clinical comparison of cefepime and metronidazole versus gentamicin and clindamycin in the antibiotic management of surgically treated advanced appendicitis. Surgery, Gynecology & Obstetrics 1993;177(Suppl):18‐22. [DOI] [PubMed] [Google Scholar]
Berne 1996 {published data only}
- Berne TV, Yellin AE, Appleman MD, Heseltine PNR, Gill MA. Meropenem versus tobramycin with clindamycin in the antibiotic management of patients with advanced appendicitis. Journal of the American College of Surgeons 1996;182(5):403‐407. [PubMed] [Google Scholar]
Brismar 1992 {published data only}
- Brismar B, Malmborg AS, Tunevall G, Wretlind B, Bergman L, Mentzing LO, et al. Piperacillin‐tazobactam versus imipenem‐cilastatin for treatment of intra‐abdominal infections. Antimicrobial Agents and Chemotherapy 1992;36(12):2766‐2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brismar 1995 {published data only}
- Brismar B, Malmborg AS, Tunevall G, Lindgren V, Bergman L, Mentzing O, et al. Meropenem versus imipenem/cilastatin in the treatment of intra‐abdominal infections. Journal of Antimicrobial Chemotherapy 1995;35(1):139‐148. [DOI] [PubMed] [Google Scholar]
Brismar 1996 {published data only}
- Brismar B, Akerlund J‐E, Sjostedt S, Johansson C, Tornqvist A, Backstrand B, et al. Biapenem versus imipenem/cilastatin in the treatment of complicated intra‐abdominal infections: Report from a Swedish study group. Scandinavian Journal of Infectious Diseases 1996;28(5):507‐512. [DOI] [PubMed] [Google Scholar]
Busuttil 1984 {published data only}
- Busuttil RW, McGrattan MA, Freischlag J. A comparative study of cefamandole versus gentamicin plus clindamycin in the treatment of documented or suspected bacterial peritonitis. Surgery, Gynecology & Obstetrics 1984;158(1):1‐8. [PubMed] [Google Scholar]
Christou 1996 {published data only}
- Christou NV, Turgeon P, Wassef R, Rotstein O, Bohnen J, Potvin M, et al. Management of intra‐abdominal infections: The case for intraoperative cultures and comprehensive broad‐spectrum antibiotic coverage. Archives of Surgery 1996;131(11):1193‐1201. [DOI] [PubMed] [Google Scholar]
Cohn 2000 {published data only}
- Cohn SM, Lipsett PA, Buchmann TG, Cheadle WG, Milsom JW, O'Marro S, et al. Comparison of intravenous/oral ciprofloxacin plus metronidazole versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections. Annals of Surgery 2000;232(2):254‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Groot 1993 {published data only}
- Groot HGW, Hustinx PA, Lampe AS, Oosterwijk WM. Comparison of imipenem/cilastatin with the combination of aztreonam and clindamycin in the treatment of intra‐abdominal infections. Journal of Antimicrobial Chemotherapy 1993;32(3):491‐500. [DOI] [PubMed] [Google Scholar]
Dupont 2000 {published data only}
- Dupont H, Carbon C, Carlet J for The Severe Generalized Peritonitis Study Group. Monotherapy with a broad‐spectrum beta‐lactam is as effective as its combination with an aminoglycoside in treatment of severe generalized peritonitis: a multicenter randomized controlled trial. Antimicrobial Agents and Chemotherapy 2000;44(8):2028‐2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eckhauser 1992 {published data only}
- Eckhauser FE, Knol JA, Raper SE, Mulholland MW, Helzerman P. Efficacy of two comparative antibiotic regimens in the treatment of serious intra‐abdominal infections: Results of a multicenter study. Clinical Therapeutics 1992;14(1):97‐109. [PubMed] [Google Scholar]
Gozenbach 1987 {published data only}
- Gozenbach HR, Simmen HP, Amgwerd R. Imipenem (N‐F‐Thienamycin) versus netilmicin plus clindamycin: A controlled and randomized comparison in intra‐abdominal infections. Annals of Surgery 1987;205(3):271‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenberg 1994 {published data only}
- Greenberg RN, Cayavec P, Danko LS, Bowen K, Montazemi R, Kearney PA, et al. Comparison of cefoperazone plus sulbactam with clindamycin plus gentamicin as treatment for intra‐abdominal infections. Journal of Antimicrobial Chemotherapy 1994;34(3):391‐401. [DOI] [PubMed] [Google Scholar]
Hopkins 1994 {published data only}
- Hopkins JA, Wilson SE, Bobey DG. Adjunctive antimicrobial therapy for complicated appendicitis: Bacterial overkill by combination therapy. World Journal of Surgery 1994;18(6):933‐938. [DOI] [PubMed] [Google Scholar]
Investigators 1994 {published data only}
- Investigators of the Piperacillin/Tazobactam Intra‐abdominal Infection Study Group. Results of the North American trial of piperacillin/tazobactam compared with clindamycin and gentamicin in the treatment of severe intra‐abdominal infections. European Journal of Surgery Supplement 1994, (573):61‐66. [PubMed] [Google Scholar]
Jaccard 1998 {published data only}
- Jaccard C, Troillet N, Harbarth S, Zanetti G, Aymon D, Schneider R, et al. Prospective randomized comparison of imipenem/cilastatin and piperacillin/tazobactam in nosocomial pneumonia and peritonitis. Antimicrobial Agents and Chemotherapy 1998;42(11):2966‐2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jauregui 1990 {published data only}
- Jauregui LE, Appelbaum PC, Fabian TC, Hageage G, Strausbaugh L, Martin LF. A randomized clinical study of cefoperazone and sulbactam versus gentamicin and clindamycin in the treatment of intra‐abdominal infections. Journal of Antimicrobial Chemotherapy 1990;25(3):423‐433. [DOI] [PubMed] [Google Scholar]
Kanellakopoulou 1993 {published data only}
- Kanellakopoulou K, Giamarellou H, Papadothomakos P, Tsipras H, Chloroyiannis J, Theakou R, et al. Meropenem versus imipenem/cilastatin in the treatment of intraabdominal infections requiring surgery. European Journal of Clinical Microbiological Infectious Diseases 1993;12(6):449‐453. [DOI] [PubMed] [Google Scholar]
Kempf 1996 {published data only}
- Kempf P, Bauernfeind A, Muller A, Blum J. Meropenem monotherapy versus cefotaxime plus metronidazole combination treatment for serious intra‐abdominal infections. Infection 1996;24(6):473‐479. [DOI] [PubMed] [Google Scholar]
Leal del Rosal 1995 {published data only}
- Leal del Rosal P. The efficacy and safety of isepamicin compared with amikacin in the treatment of intra‐abdominal infections. Journal of Chemotherapy 1995;7(2):143‐148. [PubMed] [Google Scholar]
Leaper 1987 {published data only}
- Leaper DJ, Kennedy RH, Sutton A, Johnson E, Roberts N. Treatment of acute bacterial peritonitis: A trial of imipenem/cilastatin against ampicillin‐metronidazole‐gentamicin. Scandinavian Journal of Infectious Diseases 1987;52(Suppl):7‐10. [PubMed] [Google Scholar]
Malangoni 1985 {published data only}
- Malangoni MA, Condon RE, Spiegel CA. Treatment of intra‐abdominal infections is appropriate with single‐agent or combination antibiotic therapy. Surgery 1985;98(4):648‐655. [PubMed] [Google Scholar]
Paakkonen 1991 {published data only}
- Paakkonen M, Alhava EM, Huttunen R, Karjalainen K, Lahtinen J, Miettinen P, et al. Piperacillin compared with cefuroxime plus metronidazole in diffuse peritonitis. European Journal of Surgery 1991;157(9):535‐537. [PubMed] [Google Scholar]
Poenaru 1990 {published data only}
- Poenaru D, Santis M, Christou NV. Imipenem versus tobramycin‐antianaerobe antibiotic therapy in intra‐abdominal infections. Canadian Journal of Surgery 1990;33(5):415‐422. [PubMed] [Google Scholar]
Scandinavian 1984 {published data only}
- Report from a Scandinavian Study Group. Imipenem/cilastatin versus gentamicin/clindamycin for the treatment of serious bacterial infections. The Lancet 1984;8382(1):868‐871. [PubMed] [Google Scholar]
Scott 1987 {published data only}
- Scott SD, Saddler B, Lowes JA, Karran SJ. Comparison of cefotetan versus combination therapy in peritonitis and serious intra‐abdominal sepsis. Chemioterapia 1987;6(Pt 2 S):475‐476. [PubMed] [Google Scholar]
Shyr 1995 {published data only}
- Shyr YM, Lui WY, Su CH, Wang LS, Liu CY. Piperacillin/tazobactam in comparison with clindamycin plus gentamicin in the treatment of intra‐abdominal infections. Chinese Medical Journal 1995;56(2):102‐108. [PubMed] [Google Scholar]
Smith 1980 {published data only}
- Smith JA, Skidmore AG, Forward AD, Clarke AM, Sutherland E. Prospective, randomized, double‐blinded comparison of metronidazole and tobramycin with clindamycin and tobramycin in the treatment of intra‐abdominal sepsis. Annals of Surgery 1980;192(2):213‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomkin 2001 {published data only}
- Solomkin JS, Wilson SE, Christou NV, Rotstein OD, Dellinger EP, Bennion RS, et al. Results of a clinical trial of clinafloxacin versus imipenem/cilastatin for intraabdominal infections. Annals of Surgery 2001;233(1):79‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Study 1986 {published data only}
- Study Group of Intra‐abdominal Infections. A randomized controlled trial of ampicillin plus sulbactam versus gentamicin and clindamycin in the treatment of intraabdominal infections: a preliminary report. Reviews of Infectious Diseases 1986;8(Suppl 5):S583‐S588. [PubMed] [Google Scholar]
Swedish 1990 {published data only}
- Report from a Swedish Study Group. A randomized multicentre trial of pefloxacin plus metronidazole and gentamicin plus metronidazole in the treatment of severe intra‐abdominal infections. Journal of Antimicrobial Chemotherapy 1990;26(Suppl B):173‐180. [DOI] [PubMed] [Google Scholar]
Tornqvist 1985 {published data only}
- Tornqvist A, Forsgren A, Leandoer L, Ursing J. Antibiotic treatment during surgery for diffuse peritonitis: a prospective randomized study comparing the effects of cefuroxime and of a cefuroxime and metronidazole combination. British Journal of Surgery 1985;72(4):261‐264. [DOI] [PubMed] [Google Scholar]
Torres 1999 {published data only}
- Torres AJ, Valladares LD, Jover JM, Sanchez‐Pernaute A, Frias J, Carcas AJ, et al. Cefminox versus metronidazole plus gentamicin in intra‐abdominal infections: A prospective randomized controlled clinical trial. Infections 2000;28(5):318‐322. [DOI] [PubMed] [Google Scholar]
Walker 1993 {published data only}
- Walker AP, Nichols RL, Wilson RF, Bivens BA, Trunkey DD, Edmiston CE, et al. Efficacy of a beta‐lactamase inhibitor combination for serious intra‐abdominal infections. Annals of Surgery 1993;217(2):115‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yellin 1985 {published data only}
- Yellin AE, Heseltine PNR, Berne TV, Appleman MD, Gill MA, Riggio CE, et al. The role of pseudomonas species in patients treated with ampicillin and sulbactam for gangrenous and perforated appendicitis. Surgery, Gynecology & Obstetrics 1985;161(4):303‐307. [PubMed] [Google Scholar]
Zanetti 1999 {published data only}
- Zanetti G, Harbarth SJ, Trampuz A, Ganeo M, Mosimann F, Chautemps R, et al. Meropenem (1.5 g/day) is as effective as imipenem/cilastatin (2 g/day) for the treatment of moderately severe intra‐abdominal infections. International Journal of Antimicrobial Agents 1999;11(2):107‐113. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allo 1999 {published data only}
- Allo MD, Bennion RS, Kathir K, Thompson JE, Lentz M, Meute M, et al. Ticarcillin/clavulanate versus imipenem/cilistatin for the treatment of infections associated with gangrenous and perforated appendicitis. The American Surgeon 1999;65(2):99‐104. [PubMed] [Google Scholar]
Andaker 1987 {published data only}
- Andaker L, Hojer H, Kihlstrom E, Lindagen J. Stratified duration of prophylactic antimicrobial treatment in emergency abdominal surgery. Acta Chirurgica Scandinavica 1987;153(3):185‐192. [PubMed] [Google Scholar]
Arguedas 1996 {published data only}
- Arguedas A, Sifuentes‐Osornio J, Loaiza C, Herrera M, Corrales JC, Mohs E, et al. An open, multicenter clinical trial of piperacillin/tazobactam in the treatment of pediatric patients with intra‐abdominal infections. Journal of Chemotherapy 1996;8(2):130‐136. [DOI] [PubMed] [Google Scholar]
Baird 1983 {published data only}
- Baird IM. Multicentered study of cefoperazone for treatment of intraabdominal infections and comparison of cefoperazone with cefamandole and clindamycin plus gentamicin for treatment of appendicitis and peritonitis. Reviews of Infectious Diseases 1983;5(Suppl.):S165‐S172. [DOI] [PubMed] [Google Scholar]
Ball 1981 {published data only}
- Ball P, Watson T, Mehtar S. Amoxycillin and clavulanic acid in intra‐abdominal and pelvic sepsis. Journal of Antimicrobial Chemotherapy 1981;7(4):441‐444. [DOI] [PubMed] [Google Scholar]
- Ball P, Watson T, Mehtar S. Amoxycillin and clavulanic acid in intra‐abdominal and pelvic sepsis. Journal of Antimicrobial Chemotherapy 1981;7(4):441‐444. [DOI] [PubMed] [Google Scholar]
Barboza 1994 {published data only}
- Barboza E, Castillo M, Yi A, Gotuzzo E. Clindamycin plus amikacin versus clindamycin plus aztreonam in established intraabdominal infections. Surgery 1994;116(1):28‐35. [PubMed] [Google Scholar]
Barie 1997 {published data only}
- Barie PS, Vogel SB, Dellinger EP, Rotstein OD, Solomkin JS, Yang JY, et al. A randomized, double‐blind clinical trial comparing cefepime plus metronidazole with imipenem‐cilastatin in the treatment of complicated intra‐abdominal infections. Arch Surg 1997;132(12):1294‐1302. [DOI] [PubMed] [Google Scholar]
Bennion 1990 {published data only}
- Bennion RS, Thompson JE, Baron EJ, Finegold SM. Gangrenous and perforated appendicitis with peritonitis: treatment and bacteriology. Clinical Therapeutics 1990;12(Suppl. C):31‐44. [PubMed] [Google Scholar]
Birolini 1985 {published data only}
- Birolini D, Moraes MF, Souza OS. Aztreonam plus clindamycin versus tobramycin plus clindamycin for the treatment of intraabdominal infections. Reviews of Infectious Diseases 1985;7(Suppl 4):S724‐S728. [DOI] [PubMed] [Google Scholar]
Birolini 1989 {published data only}
- Birolini D, Moraes MF, Souza OS. Comparison of aztreonam plus clindamycin with tobramycin plus clindamycin in the treatment of intra‐abdominal infections. Chemotherapy 1989;35(Suppl 1):49‐57. [DOI] [PubMed] [Google Scholar]
Biron 1984 {published data only}
- Biron S, Brochu G, Beland L, Bourque RA, Marceau P, Piche P, et al. Short‐term antibiotherapy for peritonitis: prospective, randomized trial comparing cefotaxime‐metronidazole and clindamycin‐tobramycin. Journal of Antimicrobial Chemotherapy 1984;12(Suppl B):213‐216. [DOI] [PubMed] [Google Scholar]
Bubrick 1990 {published data only}
- Bubrick MP, Heim‐Duthoy KL, Yellin AE, Berne TV, Heseltine PNR, Appleman MD, et al. Ceftazidime/clindamycin versus tobramycin/clindamycin in the treatment of intra‐abdominal infections. The American Surgeon 1990;56(10):613‐617. [PubMed] [Google Scholar]
Busuttil 1982 {published data only}
- Busuttil RW, McGrattan MA, Winston DJ. Moxalactam in the treatment of intraabdominal sepsis and other surgical infections. Review of Infectious Diseases 1982;4(Suppl):S676‐S682. [DOI] [PubMed] [Google Scholar]
Cakmakci 1993 {published data only}
- Cakmakci M, Stern A, Schilling J, Christen D, Roggo A, Geroulanos S. Randomized comparative trial of imipenem/cilastatin versus aminoglycoside plus amoxycillin plus clindamycin in the treatment of severe intra‐ and post‐operative infections. Drugs Experimental in Clinical Research 1993;19(5):223‐227. [PubMed] [Google Scholar]
Canadian 1983 {published data only}
- Canadian Metronidazole‐Clindamycin Study Group. Prospective, randomized comparison of metronidazole and clindamycin, each with gentamicin, for the treatment of serious intra‐abdominal infection. Surgery 1983;93(1 (Part 2)):221‐229. [PubMed] [Google Scholar]
Chin 1990 {published data only}
- Chin A, Gill MA, Ito MK, Yellin AE, Berne TV, Heseltine PN, et al. Treatment of intra‐abdominal infections: cost comparison of ampicillin/sulbactam and clindamycin/gentamicin. Hospital Formulary 1990;25(3):295‐296, 303‐305. [PubMed] [Google Scholar]
Christen 1987 {published data only}
- Christen D, Buchmann P, Geroulanos S. Imipenem/cilastatin versus aminoglycoside plus amoxicillin plus clindamycin in the treatment of serious postoperative infections. Scandinavian Journal of Infectious Diseases 1987;52(Suppl 1):11‐14. [PubMed] [Google Scholar]
Colardyn 1996 {published data only}
- Colardyn F and Faulkner KL on behalf of the Meropenem Serious Infection Study Group. Intravenous meropenem versus imipenem/cilastatin in the treatment of serious bacterial infections in hospitalized patients. Journal of Antimicrobial Chemotherapy 1996;38(3):523‐537. [DOI] [PubMed] [Google Scholar]
Collier 1981 {published data only}
- Collier J, Colhoun EM, Hill PL. A multicentre comparison of clindamycin and metronidazole in the treatment of anaerobic infections. Scandinavian Journal of Infectious Diseases Supplement 1981;26:96‐100. [PubMed] [Google Scholar]
Cometta 1994 {published data only}
- Cometta A, Baumgartner JD, Lew D, Zimmerli W, Pittet D, Chopart P, et al. Prospective randomized comparison of imipenem monotherapy with imipenem plus netilmicin for treatment of severe infections in nonneutropenic patients. Antimicrobial Agents and Chemotherapy 1994;38(6):1309‐1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Condon 1995 {published data only}
- Condon RE, Walker AP, Sirinek KR, White PW, Fabian TC, Nichols RL, et al. Meropenem versus tobramycin plus clindamycin for treatment of intraabdominal infections: Results of a prospective, randomized, double‐blind clinical trial. Clinical Infectious Diseases 1995;21(3):544‐550. [DOI] [PubMed] [Google Scholar]
Danish 1984 {published data only}
- The Danish Multicenter Study Group. A Danish multicenter study: cefoxitin versus ampicillin + metronidazole in perforated appendicitis. British Journal of Surgery 1984;71(2):144‐146. [DOI] [PubMed] [Google Scholar]
Danziger 1988 {published data only}
- Danziger LH, Creger RJ, Shwed JA, Stellato TA, Hau T. Randomized trial of imipenem/cilastatin versus gentamicin plus clindamycin in the treatment of polymicrobial infections. Pharmacotherapy 1988;8(6):315‐318. [DOI] [PubMed] [Google Scholar]
de Vries 1990 {published data only}
- Vries PJ, Verkooyen RP, Leguit P, Verbrugh HA. Prospective randomized study of once‐daily versus thrice‐daily netilmicin regimens in patients with intraabdominal infections. European Journal of Clinical Microbiological Infectious Diseases 1990;9(3):161‐168. [DOI] [PubMed] [Google Scholar]
Donahue 1998 {published data only}
- Donahue PE, Smith DL, Yellin AE, Mintz SJ, Bur F, Luke DR and the Trovafloxacin Surgical Group. Trovafloxacin in the treatment of intra‐abdominal infections: Results of a double‐blind, multicenter comparison with imipenem/cilastatin. American Journal of Surgery 1998;176(Suppl 6A):53S‐61S. [DOI] [PubMed] [Google Scholar]
Dougherty 1995 {published data only}
- Dougherty SH, Sirinek KR. Ticarcillin/clavulanate compared with clindamycin/gentamicin (with or without ampicillin) for the treatment of intra‐abdominal infections in pediatric and adult patients. American Surgeon 1995;61(4):297‐303. [PubMed] [Google Scholar]
Drusano 1982 {published data only}
- Drusano GL, Warren JW, Saah AJ, Caplan ES, Tenney JH, Hansen S, et al. A prospective randomized controlled trial of cefoxitin versus clindamycin‐aminoglycoside in mixed anaerobic‐aerobic infections. Surgery, Gynecology & Obstetrics 1982;154(5):715‐720. [PubMed] [Google Scholar]
Eklund 1993 {published data only}
- Eklund A‐E, Nord CE and Swedish Study Group. A randomized multicenter trial of piperacillin/tazobactam versus imipenem/cilastatin in the treatment of severe intra‐abdominal infections. Journal of Antimicrobial Chemotherapy 1993;31(Suppl A):79‐85. [DOI] [PubMed] [Google Scholar]
Fink 1989 {published data only}
- Fink MP, Helsmoortel CM, Arous EJ, Doern GV, Moriarty KP, Fairchild PG, et al. Comparison of the safety and efficacy of parenteral ticarcillin/clavulanate and clindamycin/gentamicin in serious intra‐abdominal infections. Journal of Antimicrobial Chemotherapy 1989;24(Suppl B):147‐156. [DOI] [PubMed] [Google Scholar]
Fink 1991 {published data only}
- Fink MP. Antibiotic therapy of intra‐abdominal sepsis in the elderly: Experience with ticarcillin and clavulanic acid. Surgery, Gynecology & Obstetrics 1991;172(Suppl):36‐41. [PubMed] [Google Scholar]
Geroulanos 1995 {published data only}
- Geroulanos SJ and the Meropenem Study Group. Meropenem versus imipenem/cilastatin in intra‐abdominal infections requiring surgery. Journal of Antimicrobial Chemotherapy 1995;36(Suppl A):191‐205. [DOI] [PubMed] [Google Scholar]
Harding 1982 {published data only}
- Harding G, Vincelette J, Rachlis A, Fong I, Mandell L, Feld R, et al. A preliminary report on the use of ceftizoxime vs. clindamycin/tobramycin for the therapy of intra‐abdominal and pelvic infections. Journal of Antimicrobial Chemotherapy 1982;10(Suppl C):191‐192. [DOI] [PubMed] [Google Scholar]
Henning 1984 {published data only}
- Henning C, Meden‐Britth G, Frolander F, Karlsson J, Dornbusch K. Comparative study of netilmicin/tinidazole versus netilmicin/clindamycin in the treatment of severe abdominal infections. Scandinavian Journal of Infectious Diseases 1984;16(3):297‐303. [DOI] [PubMed] [Google Scholar]
Henry 1985 {published data only}
- Henry SA. Overall clinical experience with aztreonam in the treatment of intraabdominal infections. Reviews of Infectious Diseases 1985;7(Suppl 4):S729‐S733. [DOI] [PubMed] [Google Scholar]
Heseltine 1986 {published data only}
- Heseltine PNR, Yellin AE, Appleman MD, Gill MA, Chenella FC, Berne TV, et al. Imipenem therapy for perforated and gangrenous appendicitis. Surgery, Gynecoloy & Obstetrics 1986;162(1):43‐48. [PubMed] [Google Scholar]
Hollender 1989 {published data only}
- Hollender LF, Bahnini J, Manzini N, Lau WY, Fan ST, Hermansyur K, et al. A multicentric study of netilmicin once daily versus thrice daily in patients with appendicitis and other intra‐abdominal infections. Journal of Antimicrobial Chemotherapy 1989;23(5):773‐783. [DOI] [PubMed] [Google Scholar]
Holloway 1989 {published data only}
- Holloway WJ, Winslow DL, Reinhardt JF. Cefmetazole treatment of intra‐abdominal infection. Journal of Antimicrobial Chemotherapy 1989;23(Suppl D):47‐54. [DOI] [PubMed] [Google Scholar]
Hoogkamp 1995 {published data only}
- Hoogkamp‐Korstanje JAA. Ciprofloxacin versus cefotaxime regimens for the treatment of intra‐abdominal infections. Infections 1995;23(5):278‐282. [DOI] [PubMed] [Google Scholar]
Huizinga 1988 {published data only}
- Huizinga WKJ, Baker LW, Kadwa H, Ende J, Francis AJ, Francis GM. Management of severe intra‐abdominal sepsis: Single agent antibiotic therapy with cefotetan versus combination therapy with ampicillin, gentamicin and metronidazole. British Journal of Surgery 1988;75(11):1134‐1138. [DOI] [PubMed] [Google Scholar]
Huizinga 1995 {published data only}
- Huizinga WKJ, Warren BL, Baker LW, Valleur P, Pezet DM, Hoogkamp‐Korstanjep JAA, et al. Antibiotic monotherapy with meropenem in the surgical management of intra‐abdominal infections. Journal of Antimicrobial Chemotherapy 1995;36(Suppl A):179‐189. [DOI] [PubMed] [Google Scholar]
Inthorn 1989 {published data only}
- Inthorn D, Muhlbayer D, Hartl WH. Ticarcillin/clavulanate in the treatment of severe peritonitis. Journal of Antimicrobial Chemotherapy 1989;24(Suppl B):141‐146. [DOI] [PubMed] [Google Scholar]
Jaspers 1998 {published data only}
- Jaspers CAJJ, Kieft H, Speelberg B, Buiting A, Marwijk Kooij M, Ruys GJHM, et al. Meropenem versus cefuroxime plus gentamicin for treatment of serious infections in elderly patients. Antimicrobial Agents and Chemotherapy 1998;42(5):1233‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joshi 1986 {published data only}
- Joshi M, Fitzpatrick BJ, Warren JW, Caplan ES, Tenney JH. A randomized controlled rial of moxalactam versus clindamycin/tobramycin in the treatment of mixed anaerobic/aerobic infections. The American Surgeon 1986;52(9):467‐471. [PubMed] [Google Scholar]
Kasholm‐Tengve 1986 {published data only}
- Kasholm‐Tengve B, Bartholdson P. Antibiotics in acute abdominal surgery. A clinical trial comparing the combination of ampicillin, mecillinam and metronidazole with cefoxitin alone. Acta Chirurgica Scandinavica 1986;152:267‐272. [PubMed] [Google Scholar]
Kirkpatrick 1983 {published data only}
- Kirkpatrick JR, Anderson BJ, Louie JJ, Stiver HG. Double‐blind comparison of metronidazole plus gentamicin and clindamycin plus gentamicin in intra‐abdominal infection. Surgery 1983;93(1 Pt 2):215‐216. [PubMed] [Google Scholar]
Kooi 1990 {published data only}
- Kooi GH, Pit S. Ceftazidime/metronidazole versus netilmicin/metronidazole in the treatment of perforated appendicitis in children. Clinical Therapeutics 1990;12(1):54‐60. [PubMed] [Google Scholar]
Leal del Rosal 1989 {published data only}
- Leal del Rosal P, Leal del Rosal L, Riosvelasco A, Nesbitt FC, Alanis VS. Brief report: Prospective, controlled, randomized, non‐blind comparison of intravenous/oral ciprofloxacin with intravenous ceftazidime in the treatment of severe surgical infections. The American Journal of Medicine 1989;87(Suppl 5A):183S‐184S. [DOI] [PubMed] [Google Scholar]
Lennard 1985 {published data only}
- Lennard ES, Minshew BH, Dellinger EP, Wertz MJ, Heimbach DM, Counts GW, et al. Stratified outcome comparison of clindamycin‐gentamicin versus chloramphenicol‐gentamicin for treatment of intra‐abdominal sepsis. Archives of Surgery 1985;120(8):889‐898. [DOI] [PubMed] [Google Scholar]
Levine 1989 {published data only}
- Levine DP, McNeil P, Lerner SA. Randomized, double‐blind comparative study of intravenous ciprofloxacin versus ceftazidime in the treatment of serious infections. The American Journal of Medicine 1989;87(Suppl 5A):160S‐163S. [DOI] [PubMed] [Google Scholar]
Lou 1982 {published data only}
- Lou MA, Chen DF, Bansal M, Balasubramaniam S, Thadepalli H. Evaluation of ceftizoxime in acute peritonitis. Journal of Antimicrobial Chemotherapy 1982;10(Suppl C):183‐189. [DOI] [PubMed] [Google Scholar]
Luke 1991 {published data only}
- Luke M, Iversen J, Sondergaard J, Kvist E, Lund P, Andersen F, et al. Ceftriaxone/metronidazole is more effective than ampicillin/netilmicin/metronidazole in the treatment of bacterial peritonitis. European Journal of Surgery 1991;157:397‐401. [PubMed] [Google Scholar]
Luke 1999 {published data only}
- Luke DR, Peterson J. Treatment of complicated intra‐abdominal infections: Comparison of the tolerability and safety of intravenous/oral trovafloxacin versus intravenous imipenem/cilastatin switching to oral amoxycillin/clavulanic acid. International Journal of Clinical Practice 1999;53(3):166‐173. [PubMed] [Google Scholar]
Marra 1998 {published data only}
- Marra F, Reynolds R, Stiver G, Bryce E, Sleigh K, Frighetto L, et al. Piperacillin/tazobactam versus imipenem: A double‐blind, randomized formulary feasibility study at a major teaching hospital. Diagnostic Microbiology & infectious Diseases 1998;31(2):355‐368. [DOI] [PubMed] [Google Scholar]
Mehtar 1997 {published data only}
- Mehtar S, Dewar EP, Leaper DJ, Taylor EW. A multi‐centre study to compare meropenem and cefotaxime and metronidazole in the treatment of hospitalized patients with serious infections. Journal of Antimicrobial Chemotherapy 1997;39(5):631‐638. [DOI] [PubMed] [Google Scholar]
Messick 1998 {published data only}
- Messick CR, Mamdani M, McNicholl IR, Danziger LH, Rodvold KA, Condon RE, et al. Pharmacoeconomic analysis of ampicillin‐sulbactam versus cefoxitin in the treatment of intraabdominal infections. Pharmacotherapy 1998;18(1):175‐183. [PubMed] [Google Scholar]
Mullick 1987 {published data only}
- Mullick RN, Zissis NP. Piperacillin versus gentamicin‐clindamycin combination in intra‐abdominal infections. Current Therapeutic Research ‐ Clinical and Experimental 1987;41(4):536‐541. [Google Scholar]
Najem 1983 {published data only}
- Najem AZ, Kaminski ZC, Spillert CR, Lazaro EJ. Comparative study of parenteral piperacillin and cefoxitin in the treatment of surgical infections of the abdomen. Surgery, Gynecology & Obstetrics 1983;157(5):423‐425. [PubMed] [Google Scholar]
Niinikoski 1993 {published data only}
- Niinikoski J, Havia T, Alhava E, Paakkonen M, Miettinen P, Kivilaakso E, et al. Piperacillin/tazobactam versus imipenem/cilastatin in the treatment of intra‐abdominal infections. Surgery, Gynecology & Obstetrics 1993;176(3):255‐261. [PubMed] [Google Scholar]
Ohlin 1999 {published data only}
- Ohlin B, Cederberg A, Forssell H, Solhaug JH, Tveit E. Piperacillin/tazobactam compared with cefuroxime/metronidazole in the treatment of intra‐abdominal infections. European Journal of Surgery 1999;165(9):875‐884. [DOI] [PubMed] [Google Scholar]
Polk 1993 {published data only}
- Polk HC, Fink MP, Laverdiere M, Wilson SE, Garber GE, Barie JS, et al. Prospective randomized study of piperacillin/tazobactam therapy of surgically treated intra‐abdominal infection. American Surgeon 1993;59(9):598‐605. [PubMed] [Google Scholar]
Poularas 1988 {published data only}
- Poularas J, Giamarellou H, Vlachos G, Theakou R, Patoulis J, Lekakos N, et al. The treatment of gynaecological and intra‐abdominal infections: A comparative study of cefotetan versus netilmicin plus clindamycin. Chemioterapia 1988;7(4):253‐255. [PubMed] [Google Scholar]
Raahave 1970 {published data only}
- Raahave D, Poulsen PE. Perforated appendicitis and antibiotics. A survey and a controlled clinical trial. Acta Chirurgica Scandinavica 1970;136(8):715‐723. [PubMed] [Google Scholar]
Rohrborn 2000 {published data only}
- Rohrborn A, Wacha H, Schoffel U, Billing A, Aeberhard P, Gebhard B, et al. Coverage of enterococci in community acquired secondary peritonitis: Results of a randomized trial. Surgical Infections 2000;1(2):95‐107. [DOI] [PubMed] [Google Scholar]
Schein 1994 {published data only}
- Schein M, Assalia A, Bachus H. Minimal antibiotic therapy after emergency abdominal surgery: a prospective study. British Journal of Surgery 1994;81(7):989‐991. [DOI] [PubMed] [Google Scholar]
Scheinin 1994 {published data only}
- Scheinin H, Havia T, Pekkala E, Huovinen P, Klossner J, Lehto H, et al. Aspoxicillin versus piperacillin in severe abdominal infections ‐ a comparative phase III study. Journal of Antimicrobial Chemotherapy 1994;34(5):813‐817. [DOI] [PubMed] [Google Scholar]
Schentag 1983 {published data only}
- Schentag JJ, Wels PB, Reitberg DP, Walczak P, Tyle JH, Lascola RJ. A randomized clinical trial of moxalactam alone versus tobramycin plus clindamycin in abdominal sepsis. Annals of Surgery 1983;198(1):35‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 1987a {published data only}
- Scott SD, Karran SJ. Cefotetan in the treatment of serious intra‐abdominal sepsis: a controlled clinical trial. International Journal of Clinical Pharmacological Research 1987;VII(3):229‐231. [PubMed] [Google Scholar]
Sirinek 1987 {published data only}
- Sirinek KR, Levine BA. Antimicrobial management of surgically treated gangrenous or perforated appendicitis: comparison of cefoxitin and clindamycin‐gentamicin. Clinical Therapeutics 1987;9(4):420‐428. [PubMed] [Google Scholar]
Sirinek 1991 {published data only}
- Sirinek KR, Levine BA. A randomized trial of ticarcillin and clavulanate versus gentamicin and clindamycin in patients with complicated appendicitis. Surgery, Gynecology & Obstetrics 1991;172(Suppl):30‐35. [PubMed] [Google Scholar]
Smith 1982 {published data only}
- Smith JA, Forward AD, Skidmore AG, Bell GA, Murphy JM. Metronidazole and tobramycin in intra‐abdominal sepsis. Surgery, Gynecology & Obstetrics 1982;155(2):235‐237. [PubMed] [Google Scholar]
Smith 1983 {published data only}
- Smith JA, Forward AD, Skidmore AG, Bell GA, Murphy JM, Sutherland E. Metronidazole in the treatment of intra‐abdominal sepsis. Surgery 1983;93(1 Part 2):217‐220. [PubMed] [Google Scholar]
Smith 1984 {published data only}
- Smith CR, Ambinder R, Lipsky JJ, Petty BG, Israel E, Levitt R, et al. Cefotaxime compared with nafcillin plus tobramycin for serious bacterial infections. A randomized, double‐blind trial. Annals of Internal Medicine 1984;101(4):469‐477. [DOI] [PubMed] [Google Scholar]
Solomkin 1985 {published data only}
- Solomkin JS, Fant WK, Rivera JO, Alexander JW. Randomized trial of imipenem/cilastatin versus gentamicin and clindamycin in mixed flora infections. The American Journal of Medicine 1985;78(Suppl 6A):85‐91. [DOI] [PubMed] [Google Scholar]
Solomkin 1990 {published data only}
- Solomkin JS, Dellinger EP, Christou NV, Busuttil RW. Results of a multicenter trial comparing imipenem/cilastatin to tobramycin/clindamycin for intra‐abdominal infections. Annals of Surgery 1990;212(5):581‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomkin 1996 {published data only}
- Solomkin JS, Reinhart HH, Dellinger EP, Bohnen JM, Rotstein OD, Vogel SB, et al. Results of a randomized trial comparing sequential intravenous/oral treatment with ciprofloxacin plus metronidazole to imipenem/cilastatin for intra‐abdominal infections. Annals of Surgery 1996;223(3):303‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomkin 2003 {published data only}
- Solomkin JS, Yellin AE, Rotstein OD, Christou NV, Dellinger EP, Tellado JM, et al. Ertapenem versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections. Annals of Surgery 2003;237(2):235‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stellato 1988 {published data only}
- Stellato TA, Danziger LH, Hau T, Gauderer MWL, Ferron JL, Gordon N. Moxalactam versus tobramycin‐clindamycin. A randomized trial in secondary peritonitis. Archives of Surgery 1988;123(6):714‐717. [DOI] [PubMed] [Google Scholar]
Stone 1975 {published data only}
- Stone HH, Kolb LD, Geheber CE, Currie CA. Treatment of surgical infections with tobramycin. The American Surgeon 1975;41(5):301‐308. [PubMed] [Google Scholar]
Stone 1978 {published data only}
- Stone HH, Guest BS, Geheber CE, Kolb LD. Cefamandole in treatment of peritonitis. Journal of Infectious Diseases 1978;137(Suppl):S103‐S109. [DOI] [PubMed] [Google Scholar]
Stone 1981 {published data only}
- Stone HH, Geheber CE, Kolb LD, Dunlop WE. Clinical comparison of cefotaxime versus the combination of gentamicin plus clindamycin in the treatment of peritonitis and similar polymicrobial soft‐tissue surgical sepsis. Clinical Therapeutics 1981;4(Suppl A):67‐80. [PubMed] [Google Scholar]
Stone 1982a {published data only}
- Stone HH, Geheber CE, Kolb LD, Strom PR. Clinical evaluation of cefotaxime versus gentamicin plus clindamycin in the treatment of polymicorbial peritonitis. Clinical Therapeutics 1982;5(A):1‐9. [PubMed] [Google Scholar]
Stone 1982b {published data only}
- Stone HH, Morris ES, Geheber CE, Kolb LD, Dunlop WE. Clinical comparison of cefotaxime with gentamicin plus clindamycin in the treatment of peritonitis and other soft tissue infections. Reviews of Infectious Diseases 1982;4(Suppl):S439‐S443. [DOI] [PubMed] [Google Scholar]
Stone 1983a {published data only}
- Stone HH. Metronidazole in the treatment of surgical sepsis. Surgery 1983;93(1 Pt 2):230‐234. [PubMed] [Google Scholar]
Stone 1983b {published data only}
- Stone HH, Strom PR, Fabian TC, Dunlop WE. Third generation cephalosporins for polymicrobial surgical sepsis. Archives of Surgery 1983;118(2):193‐200. [DOI] [PubMed] [Google Scholar]
Stone 1984 {published data only}
- Stone HH, Mullins RJ, Strom PR, Bourneuf AA, Geheber CE. Ceftriaxone versus combined gentamicin and clindamycin for polymicrobial surgical sepsis. The American Journal of Surgery 1984;148(4A):30‐34. [PubMed] [Google Scholar]
Tally 1981 {published data only}
- Tally FP, McGowan K, Kellum JM, Gorbach SL, O'Donnell TF. A randomized comparison of cefoxitin with or without amikacin and clindamycin plus amikacin in surgical sepsis. Annals of Surgery 1981;193(3):318‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tally 1986 {published data only}
- Tally FP, Kellum JM, Ho JL, O'Donnell TF, Barza M, Gorbach SL. Randomized prospective study comparing moxalactam and cefoxitin with or without tobramycin for the treatment of serious surgical infections. Antimicrobial Agents and Chemotherapy 1986;29(2):244‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2000 {published data only}
- Taylor E, Dev V, Shah D, Festekjian J, Gaw F. Complicated appendicitis: Is there a minimum intravenous antibiotic requirement? A prospective randomized trial. The American Surgeon 2000;66(9):887‐890. [PubMed] [Google Scholar]
Tellado 2002 {published data only}
- Tellado J, Woods GL, Gesser R, McCarroll K, Teppler H. Ertapenem versus piperacillin‐tazobactam for treatment of mixed anaerobic complicated intra‐abdominal, complicated skin and skin structure, and acute pelvic infections. Surgical Infections 2002;3(4):303‐314. [DOI] [PubMed] [Google Scholar]
Teppler 2004 {published data only}
- Teppler H, Meibohm AR, Woods GL. Management of complicated appendicitis and comparison of outcome with other primary sites of intra‐abdominal infections: Results of a trial comparing ertapenem and piperacillin‐tazobactam. Journal of Chemotherapy 2004;16(1):62‐69. [DOI] [PubMed] [Google Scholar]
Vestweber 1994 {published data only}
- Vestweber K‐H, Grundel E. Efficacy and safety of piperacillin/tazobactam in intra‐abdominal infections. European Journal of Surgery 1994;Suppl 573:57‐60. [PubMed] [Google Scholar]
Walters 1999 {published data only}
- Walters DJ, Solomkin JS, Paladino JA. Cost effectiveness of ciprofloxacin plus metronidazole versus imipenem‐cilastatin in the treatment of intra‐abdominal infections. Pharmacoeconomics 1999;16(5 Pt 2):551‐561. [DOI] [PubMed] [Google Scholar]
Williams 1991 {published data only}
- Williams RR, Hotchkin D. Aztreonam plus clindamycin versus tobramycin plus clindamycin in the treatment of intraabdominal infections. Reviews of Infectious Diseases 1991;13(Suppl 7):S629‐S633. [DOI] [PubMed] [Google Scholar]
Wilson 1988a {published data only}
- Wilson SE, Boswick JA Jr, Duma RJ, Echols RM, Jemsek JG, Lerner R, et al. Cephalosporin therapy in intraabdominal infections. A multicenter randomized, comparative study of cefotetan, moxalactam and cefoxitin. The American Journal of Surgery 1988;155(5A):61‐66. [DOI] [PubMed] [Google Scholar]
Wilson 1997 {published data only}
- Wilson SE. Results of a randomized, multicenter trial of meropenem versus clindamycin/tobramycin for the treatment of intra‐abdominal infections. Clinical Infectious Diseases 1997;24(Suppl 2):S197‐S206. [DOI] [PubMed] [Google Scholar]
Winston 1980 {published data only}
- Winston DJ, Murphy W, Young LS, Hewitt WL. Piperacillin therapy for serious bacterial infections. The American Journal of Medicine 1980;69(2):255‐261. [DOI] [PubMed] [Google Scholar]
Yellin 1993 {published data only}
- Yellin AE, Berne TV, Heseltine PNR, Appleman MD, Gill M, Chin A, et al. Prospective randomized study of two different doses of clindamycin admixed with gentamicin in the management of perforated appendicitis. The American Surgeon 1993;59(4):248‐255. [PubMed] [Google Scholar]
Yellin 2002 {published data only}
- Yellin AE, Hassett JM, Fernandez A, Geib J, Adeyi B, Woods GL, et al. Ertapenem monotherapy versus combination therapy with ceftriaxone plus metronidazole for treatment of complicated intra‐abdominal infections in adults. International Journal of Antimicrobial Agents 2002;20(3):165‐173. [DOI] [PubMed] [Google Scholar]
Yoshioka 1991 {published data only}
- Yoshioka K, Youngs DJ, Keighley MRB. A randomised prospective controlled study of ciprofloxacin with metronidazole versus amoxicillin/clavulanic acid with metronidazole in the treatment of intra‐abdominal infection. Infection 1991;19(1):25‐29. [DOI] [PubMed] [Google Scholar]
Additional references
Alderson 2004
- Alderson P, Green S, Higgins JPT, editors. Cochrane Reviewers’ Handbook 4.2.2 [updated March 2004]. In: The Cochrane Library, Issue 1, 2004. Chichester, UK: John Wiley & Sons, Ltd, 2004. [Google Scholar]
Bohnen 1983
- Bohnen J, Boulanger M, Meakins JL, McLean AP. Prognosis in generalized peritonitis. Relation to cause and risk factors. Archives of Surgery 1983;118(3):285‐290. [DOI] [PubMed] [Google Scholar]
Bohnen 1992
- Bohnen JMA, Solomkin JS, Dellinger EP, Bjornson HS, Page CP. Guidelines for clinical care: anti‐infective agents for intra‐abdominal infection. A Surgical Infection Society policy statement. Archives of Surgery 1992;127:83‐89. [DOI] [PubMed] [Google Scholar]
Bosscha 1999
- Bosscha K, Vroonhoven TJMV, Werken C. Surgical management of severe secondary peritonitis. British Journal of Surgery 1999;86(11):1371‐1377. [DOI] [PubMed] [Google Scholar]
Christou 1993
- Christou NV, Barie PS, Dellinger EP, Waymack JP, Stone HH. Surgical Infection Society intra‐abdominal infection study. Prospective evaluation of management techniques and outcome. Archives of Surgery 1993;128(2):193‐198. [DOI] [PubMed] [Google Scholar]
Copeland 2002
- Copeland GP. The POSSUM system of surgical audit. Archives of Surgery 2002;137(1):15‐19. [DOI] [PubMed] [Google Scholar]
Egger 2001
- Egger M, Smith GD, Altman DG. Systematic reviews in health care: Meta‐analysis in context. Second Edition. London: BMJ Publishing Group, 2001. [Google Scholar]
Holzheimer 2001
- Holzheimer RG, Dralle H. Antibiotic therapy in intra‐abdominal infections: a review on randomised clinical trials. European Journal of Medical Research 2001;6(7):277‐291. [PubMed] [Google Scholar]
Jones 1992
- Jones DR, Copeland GP, Cossart L. Comparison of POSSUM with APACHE II for prediction of outcome from a surgical high‐dependency unit. British Journal of Surgery 1992;79(12):1293‐1296. [DOI] [PubMed] [Google Scholar]
Knaus 1985
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical Care Medicine 1985;13(10):818‐829. [PubMed] [Google Scholar]
Mazuski 2002a
- Mazuski JE, Sawyer RG, Nathens AB, Dipiro JT, Schein M, Kudsk KA and Yowler C for The Therapeutic Agents Committee of the Surgical Infection Society. The Surgical Infection Society guidelines on antimicrobial therapy for intra‐abdominal infections: an executive summary. Surgical Infections 2002;3(3):161‐173. [DOI] [PubMed] [Google Scholar]
Mazuski 2002b
- Mazuski JE, Sawyer RG, Nathens AB, Dipiro JT, Schein M, Kudsk KA and Yowler C for The Therapeutic Agents Committee of the Surgical Infection Society. The Surgical Infection Society guidelines on antimicrobial therapy for intra‐abdominal infections: evidence for the recommendations. Surgical Infections 2002;3(3):175‐233. [DOI] [PubMed] [Google Scholar]
Mc Lauchlan 1995
- McLauchlan GJ, Anderson ID, Grant IS, Fearon KCH. Outcome of patients with abdominal sepsis treated in an intensive care unit. British Journal of Surgery 1995;82(4):524‐9. [DOI] [PubMed] [Google Scholar]
Mulier 2003
- Mulier S, Penninckx F, Verwaest C, Filez L, Aerts R, Fieuws S, Lauwers P. Factors affecting mortality in generalized postoperative peritonitis: multivariate analysis in 96 patients. World Journal of Surgery 2003;27(4):379‐384. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nichols 1992
- Nichols RL, Muzik AC. Enterococcal infection in surgical patients: the mystery continues. Clinical Infectious Diseases 1992;15(1):72‐76. [DOI] [PubMed] [Google Scholar]
Ohmann 1993
- Ohmann C, Wittmann DH, Wacha H. Prospective evaluation of prognostic scoring systems in peritonitis. Peritonitis Study Group. European Journal of Surgery 1993;159(5):267‐274. [MEDLINE: ] [PubMed] [Google Scholar]
Pacelli 1996
- Pacelli F, Doglietto GB, Alfieri S, Piccioni E, Sgadari A, Gui D, Crucitti F. Prognosis in intra‐abdominal infections. Multivariate analysis on 604 patients. Archives of Surgery 1996;131(6):641‐645. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schein 2002
- Schein M, Marshall JC. Source control: A guide to the management of surgical infections. Italy: Springer‐Verlag, 2002. [Google Scholar]
Solomkin 1984
- Solomkin JS, Meakin JL, Alo MD, Dellinger EP, Simmons RL. Antibiotics trials in intra‐abdominal infections. A critical evaluation of study design and outcome reporting. Annals of Surgery 1984;200:29‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tellado 2000
- Tellado JM, Christou NV. Intra‐abdominal infections. Madrid: Harcourt, 2000. [Google Scholar]
Wittmann 1990
- Wittmann DH, Aprahamian C, Bergstein JM. Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and Velcro analogue for temporary abdominal closure. World Journal of Surgery 1990;14(2):218‐226. [DOI] [PubMed] [Google Scholar]
Wittmann 1996
- Wittmann DH, Schein M, Condon RE. Management of secondary peritonitis. Annals of Surgery 1996;224(1):10‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]


