Abstract
Alzheimer’s disease has escalated into a critical public health concern, marked by its neurodegenerative nature that progressively diminishes cognitive abilities. Recognized as a continuously advancing and presently incurable condition, AD underscores the necessity for early-stage diagnosis and interventions aimed at delaying the decline in mental function. Despite the proven efficacy of cerebrospinal fluid and positron emission tomography in diagnosing AD, their broader utility is constrained by significant costs and the invasive nature of these procedures. Consequently, the innovation of blood biomarkers such as Amyloid-beta, phosphorylated-tau, total-tau et al, distinguished by their high sensitivity, minimal invasiveness, accessibility, and cost-efficiency, emerges as a promising avenue for AD diagnosis. The advent of ultra-sensitive detection methodologies, including single-molecule enzyme-linked immunosorbent assay and immunoprecipitation-mass spectrometry, has revolutionized the detection of AD plasma biomarkers, supplanting previous low-sensitivity techniques. This rapid advancement in detection technology facilitates the more accurate quantification of pathological brain proteins and AD-associated biomarkers in the bloodstream. This manuscript meticulously reviews the landscape of current research on immunological markers for AD, anchored in the National Institute on Aging—Alzheimer’s Association AT(N) research framework. It highlights a selection of forefront ultra-sensitive detection technologies now integral to assessing AD blood immunological markers. Additionally, this review examines the crucial pre-analytical processing steps for AD blood samples that significantly impact research outcomes and addresses the practical challenges faced during clinical testing. These discussions are crucial for enhancing our comprehension and refining the diagnostic precision of AD using blood-based biomarkers. The review aims to shed light on potential avenues for innovation and improvement in the techniques employed for detecting and investigating AD, thereby contributing to the broader field of neurodegenerative disease research.
Keywords: Alzheimer’s disease, blood, biomarkers, detection technologies, pre-analytical
Introduction
Alzheimer’s disease (AD), the most prevalent form of dementia, accounts for 60–70% of all dementia cases.1 This age-related neurodegenerative disorder leads to irreversible neuronal loss, particularly in the cortex and hippocampal regions of the brain. The prevalence of AD among the elderly population is on a rising trend. In the United States, it is estimated that there are currently 6.7 million individuals aged 65 and older living with AD. Without medical breakthroughs in preventing, slowing, or treating AD, this figure is expected to grow to 13.8 million by 2060. Additionally, in 2020 and 2021, when COVID-19 entered the ranks of the top ten causes of death, Alzheimer’s was the seventh-leading cause of death. Alzheimer’s remains the fifth-leading cause of death among Americans age 65 and older.2 The core manifestations of AD-impairment of cognitive functions, memory loss, and declines in intelligence, judgment, and language skills-reflect just a fraction of the challenges posed by this neurodegenerative disorder. AD Dementia stands as a predominant cause of disability among the elderly populace, concurrently ranking as the seventh most prevalent cause of mortality across all diseases.3 Beyond the individual suffering and the immediate impact on families and caregivers, AD significantly affects socioeconomic development through various channels. From another perspective, recent analyses of cross-sectional and longitudinal data from large Alzheimer’s disease cohorts reveal that the onset of AD and the pathological changes in brain regions begin approximately 20 years before clinical symptoms emerge. Early indications include the accumulation of amyloid-beta (A-beta) protein in the brain (approximately 15–20 years before clinical onset), followed by a reduction in cortical metabolism (approximately 10–15 years before clinical onset) and brain atrophy (approximately 5–10 years before clinical onset).4,5 Therefore, early diagnosis, prevention, and intervention treatments have become key focal points of AD research, holding critical significance in reality.
The Pathological Mechanisms of Alzheimer’s Disease
The pathological mechanisms underlying AD remain elusive. Classic pathological theories include the formation of senile plaques comprised of A-beta deposition and neurofibrillary tangles (NFTs) induced by phosphorylated Tau. As research delves deeper into the pathogenesis of AD, an increasing number of pathological mechanisms and potential biomarkers have been discovered. However, regrettably, effective therapeutic interventions have yet to be identified.
The Amyloid Cascade Hypothesis
In 1985, researchers put forward a pivotal theory regarding the pathogenesis of AD, known as the “amyloid hypothesis”. This hypothesis posited that a critical pathological hallmark of AD is the abnormal accumulation of A-beta in the brain, leading to neuronal death and cognitive dysfunction.6,7 A-beta is generated from amyloid precursor protein (APP) through enzyme-mediated reactions involving α, β, and γ secretases. APP plays a crucial role in neural differentiation, cell adhesion, and signal transduction and is encoded on human chromosome 21; the processing pathways of APP can be bifurcated into two major routes: the non-amyloidogenic pathway and the amyloidogenic pathway.
In the non-amyloidogenic pathway, APP undergoes initial cleavage by α-secretase and subsequent processing by γ-secretase. This enzymatic cascade leads to the generation of soluble APPα (sAPPα) and the P3 fragment. Notably, the P3 fragment, characterized as a neurotoxic substance, is released into the extracellular milieu.8 Conversely, in the amyloidogenic pathway, APP is first cleaved by β-secretase (BACE1) at specific sites, producing sAPP β and β-CTF (C-terminal fragment), followed by γ-secretase cleavage of β-CTF to generate A-beta peptides and APP intracellular domain(AICD).9 A-beta peptides primarily exist in two forms, A-beta1-40 and A-beta1-42, naturally present in cerebrospinal fluid and brain tissue,10 composed of 39 to 43 amino acids with a molecular weight of approximately 4kDa.11 Under certain conditions, A-beta tends to aggregate into oligomers or fibrils, eventually forming extracellular amyloid plaques, which are a primary cause of cognitive decline and memory loss in AD patients.12 As one of the main pathological features of AD, extracellular amyloid plaques are mainly composed of two C-terminal variants of A-beta: A-beta1-40 and A-beta1-42. In addition, A-beta1-42 has two more hydrophobic amino acids at the C-terminal than A-beta1-40, so A-beta1-42 has A higher incidence of fibrosis and greater toxicity. The measurement of these A-beta variants has been widely applied in the early diagnosis of AD.13 Since its inception, the amyloid hypothesis has remained at the core of AD research. Despite controversies and challenges, it has substantially advanced the understanding of AD’s pathological mechanisms and the development of therapeutic strategies.14 The accumulation of A-beta protein in the brain is a primary driver of AD pathogenesis. Increased concentrations of A-beta42 are linked to the formation of insoluble amyloid fibrils in plaques.15 Because mice do not spontaneously develop amyloid pathology, transgenic mice expressing human genes associated with familial AD mutations have become crucial tools for investigating the amyloid hypothesis in AD pathogenesis.16 Early research has detailed that familial APP mutations V717F (Indiana) and APP K670N/M671L as the PDAPP and TG2576 models, represents a significant advancement in the field,17,18 under the neuron-specific platelet-derived growth factor-β promoter, lead to amyloid deposition, Additionally, the I716V (Florida) and V717I (London) mutations result in elevated A-beta42 levels. Presenilin 1 (PS1) and Presenilin 2 (PS2), subunits of the γ-secretase complex, are also implicated in familial AD through mutations that promote A-beta42 accumulation.19 Transgenic mice carrying combinations of these familial AD gene mutations exhibit common features such as A-beta plaque formation, gliosis, and synaptic degeneration. The TgCRND8 model, constructed using the PrP gene promoter to overexpress a combination of the Swedish mutation KM670/671NL and the Indiana mutation V717F, serves as a significant tool in AD research.20 The J20 mouse model utilizes the PDGF-β promoter to overexpress two well-characterized mutations: the Swedish mutation (KM670/671NL) and the Indiana mutation (V717F). This model successfully replicates diffuse A-beta protein deposition in mice, serving as an invaluable tool for AD research.21 The 5×FAD mouse model overexpresses human APP and PSEN1, incorporating the KM670/671NL (Swedish), V717I (London), and I716V (Florida) mutations, along with the PS1 gene mutations M146L and L286V, all under the control of a Thy1 promoter. Due to its stable and robust AD phenotype, this model is among the most extensively utilized in contemporary AD research.22,23 This model is widely used to study the mechanisms associated with amyloid deposition in AD.
Recently, the amyloid hypothesis has been challenged. Due to the fail of clinical trials targeting A-beta and the withdrawal of a representative paper.24 Although treatments targeting amyloid proteins for AD have consistently failed to yield satisfactory outcomes, amyloid biomarkers are increasingly validated as effective diagnostic tools.25
The Tau Hypothesis
A recent study has demonstrated that the presence of intracellular NFTs within the brain tissue of AD patients post-mortem constitutes the primary pathological hallmarks of the disease. This phenomenon is significantly correlated with the cognitive impairments observed during the progression of AD.26 Tau protein, encoded by the MAPT gene, is a microtubule-associated protein with a molecular weight ranging between 50–75kDa. It plays a crucial physiological role by binding to and stabilizing the microtubules within neurons.27 In the brains of AD patients, the tau protein is hyperphosphorylated at more than 20 residues, whereas in healthy individuals, phosphorylation is uneven and occurs at only 8–10 residues. These hyperphosphorylated tau proteins interact with each other within the neuronal cells, leading to the formation of NFTs.28 NFTs, another major pathological characteristic of AD, are observed at the ultrastructural level as insoluble, twisted fibers with a periodicity of approximately 80nm, presenting as paired helical filaments or straight filaments.29 Within this context, the tau protein consists of six isoforms composed of 352, 381, 382, 410, 412, and 441 amino acid residues, respectively.30 Therefore, mainly the phosphorylated tau protein, in connection with the cellular NFTs observed in AD patients, is closely associated with the pathology of the disease. Phosphorylated tau protein, in particular, is considered an important biomarker for the early diagnosis of AD.
The Cholinergic Hypothesis
The cholinergic hypothesis represents one of the earliest propositions regarding the pathogenesis of AD. This hypothesis emerged from seminal neurochemical findings within the brains of AD patients, revealing disturbances in acetylcholine (ACh) metabolism, closely associating acetylcholinesterase (AChE) activity with neurotoxicity.31,32 According to the classical cholinergic hypothesis, the disruption of cholinergic neurotransmission, attributed to a significant deficiency in acetylcholine, precipitates the cognitive impairments observed in AD patients. This notion suggests that a reduction in ACh metabolism could potentially ameliorate cognitive functions in AD. ACh is primarily hydrolyzed by AChE at the cholinergic synapses, Therefore, several AChE inhibitors have been approved by FDA for AD treatment. Furthermore, the deficiency in cholinergic function in AD is linked to the accumulation of A-beta and tau proteins. ACh undergoes hydrolysis catalyzed by AChE at cholinergic synapses, accelerating the accumulation of A-beta and tau proteins, and thus plays a crucial role in the pathogenesis of AD.33 Studies indicate dynamic changes in AChE activity throughout the progression of AD, with decreased levels of brain AChE detected in the early stages of the disease, suggesting its potential as an early biomarker for AD pathogenesis.34
Mitochondrial and Metabolic Dysfunctions in AD
AD is potentially driven by oxidative imbalance within the central nervous system, where reactive oxygen species (ROS) disrupt metabolic processes.35 Mitochondrial dysfunction is a hallmark of AD, with oxidative stress in the cortex and hippocampus being major features of the disease progression.36 Elevated oxidative stress in hippocampal tissues leads to cognitive decline, as ROS can damage DNA, oxidize proteins, and peroxidize lipids, impairing mitochondrial function and causing neuronal degeneration. A-beta deposition disrupts mitochondrial homeostasis and enzyme activity, damaging mitochondrial membrane potential. The disruption of the electron transport chain, coupled with ROS accumulation and reduced ATP production, exacerbates oxidative damage.37 In preclinical AD models and AD patients, elevated levels of oxidative stress biomarkers such as 4-hydroxy-2-nonenal (HNE), F2-isoprostanes, and F4-isoprostanes have been observed. Additionally, 8-hydroxy-2’-deoxyguanosine (8-OHdG) serves as a prominent biomarker indicating significant DNA oxidative damage.38–40 The lipid peroxidation markers, including 8,12-iso-iPF2a-VI, are also significantly elevated.41 These findings highlight the critical role of reactive oxygen species (ROS) and oxidative stress in the pathogenesis of AD. The mitochondrial dysfunction hypothesis posits that mitochondrial defects are primary triggers for A-beta accumulation and neurofibrillary tangle (NFT) formation, resulting in synaptic degradation and neuronal death. Energy metabolism disturbances and impaired glucose metabolism are also significant features in the pathogenesis of AD.42 Factors such as A-beta plaque formation, mitochondrial damage, and insulin resistance contribute to the metabolic dysregulation observed in AD.43 Glucose is a critical energy source for neurons, and studies have shown that brain glucose utilization decreases by nearly 45% during the progression of AD.43,44 In AD mouse models, GLUT1 deficiency accelerates A-beta deposition, leading to neurodegeneration.45 The accumulation of A-beta may impair glucose metabolism, causing oxidative damage to synapses, synaptic dysfunction, and cognitive decline. Additionally, A-beta accumulation promotes the aggregation of the translocase of the outer mitochondrial membrane (TOM) complex, damaging the activity of respiratory chain enzymes, particularly complexes III and IV.46 These disruptions highlight the significant role of impaired glucose metabolism in the pathogenesis of AD. Glucose metabolism defects in tauopathy mouse models are associated with memory impairment, long-term synaptic depression, and increased tau phosphorylation mediated by p38 mitogen-activated protein kinase (MAPK).47 Insulin resistance, a key feature of both AD and type 2 diabetes, is marked by a reduction in insulin levels by approximately 80% in AD patients compared to healthy individuals. Impaired insulin signaling affects the processing of tau and A-beta, both of which are crucial in AD pathogenesis. Elevated levels of ApoE4 contribute to reduced glucose utilization in the AD brain. ApoE plays a vital role in regulating cholesterol transport and lipid metabolism. Gene expression profiling in transgenic mice carrying human ApoE alleles indicates that those with the ApoE4 allele exhibit defective expression of insulin signaling-related genes (IRS, GLUT4, PPARG, and IDE) compared to mice with the protective ApoE2 allele.48,49 These findings underscore the significant role of disrupted glucose metabolism and insulin signaling in the development and progression of AD.
Evolving Trends in Blood Immunomarker Testing: From ATN to ATN-IVS, Single to Multiparameter Assays
Currently, the primary adjunct diagnostic approaches for AD primarily involve imaging techniques, such as Positron Emission Computed Tomography (PET) and Magnetic Resonance Imaging (MRI), alongside the analysis of cerebrospinal fluid (CSF) biomarkers. Imaging methodologies, however, only offer high reliability in the intermediate to late stages of AD, presenting limited efficacy for early-stage diagnosis and being cost-prohibitive, thus hindering their widespread application.50 CSF analysis targets AD-related biomarkers, including A-betaproteins, Tau proteins, beta-secretase, and apolipoprotein, as a crucial early diagnosis method. Nonetheless, obtaining CSF samples through lumbar puncture is an invasive procedure that carries a risk of complications, posing certain risks to patients. Furthermore, the invasiveness required to collect CSF samples adversely affects the potential for early diagnosis, disease monitoring, and the assessment of therapeutic efficacy.51 In contrast, blood-based biomarker detection, especially immunological indicators, garners increased attention as a more accessible, lower-risk, and cost-effective diagnostic option for AD. In 2018, the National Institute on Aging—Alzheimer’s Association (NIA-AA) introduced a research framework that centers on the CSF testing-based AT(N) schema as the principal diagnostic indicator.52 This framework delineates a novel classification system for AD that incorporates fluid or imaging biomarkers indicative of A-beta pathology (A), tau pathology (T), and neurodegeneration (N)—collectively termed the “ATN” classification. This system aims to categorize individuals with AD pathology into subgroups according to the presence or absence of biomarker evidence for each pathogen. Building upon the foundational 2018 framework, moreover, in 2023, the NIA-AA further refined its approach by organizing biomarkers into three broad categories: core AD biomarkers, non-specific biomarkers pivotal in AD pathogenesis yet also present in other brain disorders, and biomarkers identifying common non-AD co-pathologies. Moreover, the updated framework introduced three additional biomarker categories: “I” for inflammatory/immune mechanisms and two categories for prevalent non-AD co-pathologies, “V” for vascular brain injury and “S” for synucleinopathy (Table 1). This enhancement of the classification system underscores a comprehensive strategy to elucidate the complex pathology of AD, facilitating more precise diagnosis and enabling targeted therapeutic interventions.
Table 1.
The National Institute on Aging-Alzheimer’s Association Delineates Changes in Fluid Biomarkers 2018vs2023
| Biomarker Category | 2018 | 2023 |
|---|---|---|
| Body Fluid Samples | CSF | CSF or Plasma |
| A | Aggregated Aβ or associated pathologic state: Aβ 42, or Aβ 42/Aβ 40 ratio | Aβ42 (Core 1) |
| T | Aggregated tau (neurofibrillary tangles) or associated pathologic state: phosphorylated tau | T1(phosphorylated and secreted AD tau): p-tau 217, p-tau 181, ptau 231(Core 1) |
| T2(AD tau proteinopathy): pT205, MTBR-243, non-phosphorylated tau fragments (Core 2) | ||
| N | Neurodegeneration or neuronal injury: total tau | Injury, dysfunction, or degeneration of neuropil: NFL |
| I | / | (inflammation) Astrocytic Activation: GFAP |
| V | / | / |
| S | / | α-synuclein: αSyn-SAA |
Abbreviations: Aβ, β amyloid; CSF, cerebrospinal fluid; GFAP, glial fibrillary acidic protein.
Amyloid β
A-beta peptides, particularly A-beta1-38, A-beta1-40, and A-beta1-42, constitute one of the primary pathological hallmarks of AD, produced through the action of gamma-secretase on various cleavage sites of the precursor protein. Among these, A-beta1-40 and A-beta1-42 garner significant attention due to their propensity to aggregate into neurotoxic plaques in the brain, damaging neurons, microglia, and other neural cells tasked with clearing them.53 Over the past two decades, A-beta1-42 has emerged as a critical biomarker in the pathology of AD, with the ratio of A-beta1-42 to A-beta40 in the CSF demonstrating a strong correlation with the diagnosis and accuracy of AD.54,55 Clinical cohort studies have noted a significant decrease in the ratio of A-beta1-42 to A-beta40 in the plasma of AD patients, a trend highly consistent with biomarker results in the CSF.56 Immunoprecipitation-mass spectrometry (IP-MS) techniques have observed a declining trend in the levels of A-beta1-42 and A-beta1-40 in plasma, achieving over 90% sensitivity and specificity in distinguishing between high (A-beta+) and low (A-beta-) levels of A-beta, hence facilitating early warning and prediction of AD.57 Similarly, measurements using Single Molecule Array (Simoa) technology have indicated a downward trend in the concentrations of A-beta1-42 and A-beta1-40 in plasma, consistent with the trends observed in CSF levels. This decline is also evident in patients with mild cognitive impairment (MCI).58 However, results obtained through Magnetic Immunoassay (MIR) technology indicate an increase in A-beta1-42 levels and a decrease in A-beta40 levels in AD patients, diverging from the findings in control groups and MCI patients, likely due to lower A-beta concentrations in plasma and the insufficient sensitivity of MIR technology for A-beta detection. Moreover, traditional Enzyme-Linked Immunosorbent Assay (ELISA) techniques have shown inconsistencies in replicating the observed decrease in A-beta1-42 in CSF. Early work using Luminex xMAP technology in plasma also failed to reproduce the observed reduction of A-beta1-42 seen in CSF.59 Recent studies utilizing ELISA kits with enhanced sensitivity (EUROIMMUN) have more accurately quantified the plasma A-beta1-42/A-beta40 ratio, with results aligning with PET findings.60 In individuals carrying autosomal dominant AD mutations and patients with Down syndrome (including children), the A-beta1–42 concentrations and A-beta1-42/40 did not correlate with their CSF counterpart.61 An increase in plasma A-beta1-42 concentration and the A-beta1-42/40 ratio has been detected, possibly reflecting the overproduction of A-beta1-42 due to mutations affecting the APP.9 However, the diagnostic significance and value of changes in plasma A-beta1-42 concentration in hereditary AD patients remain unclear, necessitating further research.
Tau Protein
Tau protein, consisting of a sequence of 441 amino acids, is a neural microtubule-associated protein.62 When Tau is excessively phosphorylated, it experiences a decreased affinity for neuronal microtubules. This phosphorylated Tau (p-Tau) tends to aggregate into tangles and form amyloid deposits, causing neuron damage.63,64 p-Tau and total Tau (t-Tau) are two critical biomarkers for detection. Studies have shown that t-Tau levels in the CSF of AD patients are significantly elevated, with concentrations three times that of normal levels, demonstrating strong disease relevance, and Tau protein levels in the blood can diagnose and differentiate between normal individuals and AD patients.65 A study in plasma revealed that t-Tau levels were significantly higher in AD patients compared to the normal control group, offering a means to diagnose and distinguish between these two populations.66 Following adjustments for age and sex, an increase of one standard deviation (SD) in the logarithmic scale of plasma total tau levels was found to be associated with a 35% increase in the risk of AD dementia. Incorporating plasma t-Tau levels into a model that already accounts for age and sex significantly enhanced the stratification accuracy for the risk of AD dementia among participants. This refinement in risk assessment underscores the utility of plasma t-Tau as a potent biomarker for early detection and risk stratification in AD dementia. These findings suggest that plasma t-Tau levels may enhance the prediction of future dementia, are associated with dementia endophenotypes, and could serve as a biomarker for risk stratification in dementia prevention trials.67 This may relate to its inclusion as a marker of neurodegenerative change/neuronal injury (N) within the AT(N) biomarker framework. Phosphorylation of tau at Thr181 was the first phospho-epitope identified in biofluids and has historically been considered the gold standard for p-Tau measurements. According to the AT(N) classification, p-Tau is included as a marker for Tau pathology (T). Elevated p-tau levels at threonine 181 (T181) serve as significant indicators or predictors of neurofibrillary tangle pathology. The correlation between p-tau levels at T181 and the presence of neurofibrillary tangles underscores its utility as a biomarker for detecting and forecasting the progression of neurodegenerative conditions associated with AD. Recent research has expanded upon this foundation, revealing that phosphorylation at threonine positions 181 (pThr181), 217 (pThr217), and 231 (pThr231) of the tau protein can precisely distinguish individuals with PET findings from those with negative results. This advancement enhances the potential for p-Tau variants as diagnostic tools in the clinical assessment of AD, providing a more nuanced approach to understanding its pathophysiology.68
Neurofilament Light
Neurofilament light chain (NfL) is a cytoskeletal protein encoded by the NEFL gene, predominantly located within the intermediate filaments of neural axons and serving as a crucial component of giant myelinated axons.69 It plays a vital role in the stability and growth of axons. Upon axonal damage, NfL is released into the CSF and subsequently enters the bloodstream, which can be detected through ultra-sensitive immunoassays. As a result, NfL is categorized within the AT(N) biomarker framework as an indicator of neurodegenerative changes (N) in both CSF and blood. The levels of NfL in serum or plasma are consistent with those in CSF and are elevated in both familial and sporadic forms of AD. Notably, in familial AD, an increase in NfL concentrations can be detected up to 10 years before the onset of significant clinical symptoms.70 A recent histopathological study has revealed a negative correlation between pre-mortem plasma concentrations of NfL and post-mortem NfL staining density in the medial temporal lobe tissue samples. Specifically, individuals with higher plasma NfL concentrations exhibited lower NfL staining densities within their brain tissues. Moreover, the study demonstrated that the levels of NfL are significantly associated with cortical atrophy, correlating with the stage of disease and variations in the burden of A-beta.71 Consequently, an increase in plasma NfL concentrations could indicate the severity of brain atrophy, metabolic decline, and white matter integrity reduction, as markers for neurodegenerative changes occurring in various brain regions. Although not specific to AD diagnosis, NfL still has the potential to serve as a blood biomarker for AD and other neurodegenerative diseases.72 Utilizing ultra-sensitive detection technologies like Simoa, low concentrations of plasma NfL can be identified, making it a valuable peripheral biomarker for assessing cognitive decline and identifying individuals at risk of neurodegeneration and brain atrophy. Recent research indicates that blood levels of NfL can act as a biomarker for monitoring pre-symptomatic neurodegenerative changes and disease progression in AD, including sporadic AD, early-stage dementia, and the prodromal phase of autosomal dominant AD.73
Glial Fibrillary Acidic Protein
Glial fibrillary acidic protein (GFAP) is a crucial astrocyte cytoskeleton component and a biomarker for astrocyte activation.73 Studies have shown that, compared to cognitively unimpaired (CU) individuals, patients with AD and other related neurodegenerative diseases exhibit significantly higher levels of GFAP in the CSF.74 Furthermore, CU elderly individuals at risk for AD, as indicated by positive amyloid PET results, also show elevated levels of GFAP in their plasma.75 Given that GFAP levels rise with the accumulation of A-beta and tau proteins, GFAP is more likely to be considered a marker for amyloid aggregation rather than an indicator of neuroinflammation.76 An earlier study demonstrated a significant increase in GFAP levels among A-beta+ MCI patients and those MCI patients who progressed to dementia, compared to A-beta− MCI and stable MCI patients.77 Recent research by Pratishtha Chatterjee et al found that GFAP levels in MCI and AD patients increase over time compared to controls.61 While the longitudinal increase of NfL in the MCI stage was insignificant, a notable increase was observed in AD patients compared to controls. Recently, a study revealed that the GFAP level in the blood was higher in the A-beta-positive group than in the negative groups, and in individuals with AD or mild cognitive impairment (MCI) compared to the healthy controls.78 These findings reveal a sequential change in biomarkers during the disease progression, reflecting the underlying pathological process.77
Exosomes
Exosomes are a class of extracellular vesicles with diameters ranging from 40–160 μm (average 100 μm) containing various proteins, nucleic acids, metabolites and lipids, which play a crucial role in intercellular communication.79 While performing their physiological functions, exosomes also accelerate pathological processes of several neurological diseases, such as prion disease,80 Parkinson’s disease,81 and amyotrophic lateral sclerosis.82 As for AD, exosomes carrying cargoes including A-beta oligomers, tau proteins, and inflammatory factors lead to the progression of AD pathology through multiple pathways.83–85
Exosomes can be produced by all cells, and thus can be detected in almost all body fluids. By isolating neuronal-derived exosomes from blood samples, the levels of specific proteins and/or nucleic acids they carry can assist in the diagnosis and prediction of AD. Jia et al, showed that the concentrations of exosomal A-beta1-42, t-Tau, and p-Tau181 in peripheral blood were higher in the AD group compared to the aMCI group and controls. The diagnostic efficacy of combining these three biomarkers was generally consistent with those in cerebrospinal fluid.86 Later, the same team found that the levels of four proteins reflecting synaptic function, namely growth associated protein 43, neurogranin, synaptosome associated protein 25 and synaptotagmin 1, were lower in the AD group than in the aMCI group or controls, and these levels were significantly correlated with cognitive function. The combination of these four proteins could predict AD, further broadening the application of exosomes in the AD diagnostic model.87 Additionally, studies detecting micro-RNA (miRNA) content in blood exosomes have shown that miRNAs related to AD pathological processes, such as A-beta degradation, A-beta aggregation and tau phosphorylation, were significantly differentially expressed between AD patients and controls.88–91 Thus, a combination of miRNAs can also serve as a promising biomarker to distinguish AD from controls.
Regarding exosome detection technology, the immunomagnetic exosomal polymerase chain reaction (iMEP) platform developed by Hu et al, has enhanced operability and precision.92 Furthermore, the graphene electrolyte-gated transistor biosensor developed by Li et al, specifically detects serum neuronal-derived exosomal Abeta1-42 with 100% accuracy in identifying AD from controls.93 These development and application of new technologies provide stronger support for blood exosome detection.
Other Potential Biomarkers in Peripheral Blood
Beyond the classic ATN framework, there are various blood-derived biomarkers that are considered valuable for diagnosing and distinguishing AD. The diagnostic efficacy of these biomarkers in combination may even surpass that of blood Abate and tau proteins. Banerjee et al, found that levels of IL-6 and TNF-α in the peripheral blood of AD patients were significantly higher compared to the controls, while the level of 25-hydroxyvitamin D was significantly lower.94 Reduced vitamin D level is thought to be associated with the progression of AD,94 and IL-6 is related to neurodegeneration and apoptosis.95 Leptin can promote long-term potentiation and synaptic plasticity in the hippocampus and facilitate Abeta clearance.96 Baranowska-Bik et al, found that plasma leptin levels in female AD patients were significantly lower than the controls, while soluble leptin receptor levels were significantly higher, although these levels were not correlated with MMSE scores.97 Additionally, a prospective study showed that higher leptin levels were associated with a lower risk of AD.97 Proteomics also has extensive applications in the field of AD biomarkers.98 Jiang et al, selected 21 proteins related to neurodegeneration, immune response, inflammation, metabolism, and cardiovascular function. The combination of these proteins can accurately distinguish AD from MCI patients and assess the CNS Abeta load.98 Findings from bioinformatics research suggested that immune response, glucose metabolism, and lysosomal dysfunction may be potential targets for identifying AD.99 Overall, these biomarkers can broaden the understanding of AD from multiple perspectives. Exploring their pathogenic or protective mechanisms in the development and progression of AD can also provide new insights for the diagnosis and treatment of the disease.
Multi-Target Detection
Combining multiple biomarkers into a panel may represent a practical approach to enhance the screening capabilities for AD in clinical applications. Various studies have demonstrated that the combination of biomarkers yields high credibility and accuracy in diagnosis.57,100–102 For instance, research conducted by Oskar Hansson and his team within the BioFINDER and ADNI cohorts has confirmed that the solitary use of plasma p-Tau can accurately predict the occurrence of AD dementia within four years (AUC = 0.83). However, when combined with brief cognitive tests of memory, executive function, and APOE genotype, the accuracy of diagnosing and predicting AD significantly improved (AUC = 0.91). The accuracy was further enhanced by including cortical thickness and plasma NfL as variables. Notably, replacing plasma biomarkers with CSF biomarkers did not show a significant difference in accuracy.103
Additionally, two studies have explored the diagnostic value of combining plasma p-Tau181 and NfL. In the AD severity continuum, plasma p-Tau181 and NfL concentrations increased and showed distinct temporal dynamics and longitudinal associations with cognitive abilities, Tau-PET, and A-beta-PET. These biomarkers can provide complementary information, and their combined use may offer additional value for predicting, diagnosing, and differentiating AD from other neurodegenerative diseases.104 Another study indicated that plasma p-Tau181 has high accuracy in predicting amyloid status, potentially avoiding CSF/amyloid PET tests in approximately 60% of participants in a memory clinic setting. NfL is useful in distinguishing frontotemporal dementia (FTD) due to non-neurodegenerative causes but performed less effectively than p-Tau181 in all other comparisons. The combined use of p-Tau181 and NfL enhanced the diagnostic efficacy for FTD and non-neurodegenerative diagnoses.105 Within the AT(N) framework, being positive for both A and T (regardless of N status) classifies as AD, while being only positive for A, with T and N negative, is considered probable AD. Being positive for A and N may indicate early-stage AD. Patients negative for A are considered non-AD, whereas being positive for N, regardless of T status, is classified as non-AD neurodegenerative changes.106 Although diagnosing AD accurately using only plasma biomarkers remains challenging, the combination of various blood biomarkers can identify potential AD patients from control groups, promising to become an effective method for pre-screening AD (Table 2).
Table 2.
Combined Detection Based on A+T or A+T+N
| Year | Study | Biomarkers | Assays | Main Results |
|---|---|---|---|---|
| 2023 | Joshua Stevenson-Hoare | Aβ40, Aβ42, GFAP, NfL | Simoa | The prediction accuracy of Alzheimer’s disease clinical diagnosis by the combination of all biomarkers, APOE and polygenic risk score reached area under receiver operating AUC=0.81, with the most significant contributors being ϵ4, Aβ40 or Aβ42, GFAP and NfL. All biomarkers were significantly associated with age in cases and controls. |
| 2023 | Christophe Hirtz | Aβ42, Aβ40, t-tau APP669-711 |
IPMS AND Simoa |
The amyloid IPMS-Shim composite biomarker (combining APP669-711/Aβ42 and Aβ40/Aβ42 ratios) discriminated AD from SCI (AUC: 0.91), OND (0.89), and NDD (0.81). The IPMS-Shim Aβ42/40 ratio also discriminated AD from MCI (0.78). IPMS-Shim biomarkers have similar relevance to discriminate between amyloid-positive and amyloid-negative individuals (0.73 and 0.76 respectively) and A-T-N-/A+T+N+ profiles (0.83 and 0.85). Performances of the Simoa 3-PLEX Aβ42/40 ratio were more modest. Pilot longitudinal analysis on the progression of plasma biomarkers indicates that IPMS-Shim can detect the decrease in plasma Aβ42 that is specific to AD patients. |
| 2021 | Joel Simrén | Tau181, Aβ42, Aβ40, Tau, GFAP | Simoa | P-tau181, neurofilament light, amyloid-β (Aβ42/40), Total-tau and Glial fibrillary acidic protein were altered in AD dementia but P-tau181 significantly outperformed all biomarkers in differentiating AD dementia from CU. P-tau181 was increased in MCI converters compared to non-converters. Higher P-tau181 was associated with steeper cognitive decline and gray matter loss in temporal regions. Longitudinal change of P-tau181 was strongly associated with gray matter loss in the full sample and with Aβ measures in CU individuals. |
| 2023 | Feng Gao | P-Tau181, Aβ42, Aβ40, T-Tau, GFAP, NFL | Simoa | A combination of the APOE genotype with plasma pTau and serum GFAP demonstrated exceptional performance in distinguishing Aβ status. Furthermore, baseline GFAP levels exhibited a strong association with cognitive decline over time and brain atrophy, with higher GFAP levels predicting a faster rate of neurodegeneration. |
| 2023 | Anna Lidia Wojdała | Aβ42/40, p-tau181, p-tau231, t-tau, NFL, GFAP, UCHL-1 | Simoa | Among plasma biomarkers, we found Aβ42/Aβ40, GFAP, and p-tau231 to show the largest rate of change at the CSF biomarker-defined cut-offs for amyloidosis and tauopathy. GFAP, NF-L, and p-tau181 as the biomarkers most significantly associated with disease progression in both CSF and plasma. |
| 2023 | Zachary Winder | Aβ42, Aβ40, p-Tau-181, T-Tau, NFL GFAP, TNFa, IL-6, IL-8, IL-10, PLGF, VEGF, MMP9, TGFb |
Simoa | Included cases (N = 90) showed increased tau/amyloid beta (Aβ)42 ratio, GFAP, VEGF-A, and PlGF were positively associated with higher level of AD neuropathological change, while higher Aβ42/Aβ40 ratio was inversely associated. Higher PlGF, VEGF-A, and interleukin 6 were inversely associated with chronic cerebrovascular disease, while Aβ42/Aβ40 ratio was positively associated. |
| 2023 | Xin Wang | Aβ42, Aβ40, p-Tau-181, T-Tau | Simoa | A decrease in the Aβ42/40 ratio was associated with a faster decline in the DSB score. A higher p-tau181 concentration was associated with a faster decline in the SDMT score. |
Abbreviations: AD, Alzheimer’s disease; APOE, Apolipoprotein E; Ab40, Amyloid-beta40; Ab42, Amyloid-beta42; CSF, Cerebrospinal fluid; CU, Cognitively unimpaired; AUC, area under the curve; GFAP, Glial fibrillary acidic protein; NfL, Neurofilament light; IPMS, Immunoprecipitation-mass spectrometry; MMP, Metallo-proteinase; VEGF-A, vascular endothelial growth factor A; PlGF, placental growth factor; DSB, Digit Span Backward; SDMT, Symbol Digit Modalities Test; P-tau181, Phosphorylated-tau181; NfL, Neurofilament light.
Plasma Immunological Marker Detection: From Conventional to High Sensitivity
Traditionally, early laboratory techniques such as ELISA have been employed to detect biomarkers for AD. However, these conventional methods have limitations primarily due to their insufficient detection sensitivity and the extremely low concentration of AD biomarkers in plasma. Consequently, the research outcomes derived from these methods are often inconsistent or even contradictory, and the lack of consistency between the concentrations of biomarkers in plasma and CSF reduces their reliability for early warning of AD.
In recent years, significant advances have been made in the field of technology to achieve higher detection sensitivities. Newly developed ultra-sensitive detection platforms, such as Simoa, Immunomagnetic Reduction (IMR), Meso Scale Discovery (MSD), and Elecsys immunoassays, have successfully addressed the issues of interference by blood proteins and heterophilic antibodies, enabling effective detection of low-concentration target biomarkers. These advancements offer new possibilities for enhancing the accuracy and reliability of AD biomarker detection.
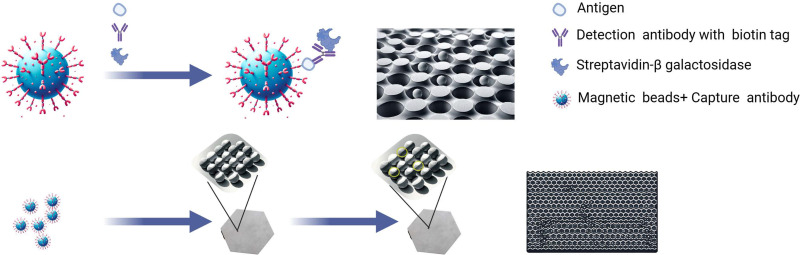
Simoa
In 2010, a significant advancement was made in the field of biomarker detection for AD with the development of a novel assay technique known as single-molecule enzyme-linked immunosorbent assay (digital ELISA).107 This method incorporates the utilization of Simoa arrays (Quanterix, Billerica, MA, USA), which consist of femtoliter-sized reaction chambers, thereby enhancing the sensitivity and specificity of AD biomarker detection.108 Central to this technology is the innovative employment of sandwich antibody complexes formed on microbeads (diameter: 2.7 μm), which are subsequently enzymatically labeled, mirroring the methodology of traditional bead-based ELISAs.
When the sample presents proteins at exceedingly low concentrations, the proportional relationship between protein molecules (and, by extension, the enzymatically labeled complexes) and the microbeads is notably diminished, typically maintaining a 1:1 ratio. This condition results in a distinctive distribution pattern where the likelihood of microbeads bearing labeled immune complexes adheres to a Poisson distribution. In the context of such low protein concentrations, the Poisson distribution reveals that microbeads are predominantly found to either harbor a singular immune complex or none. For example, the process of sequestering 50 aM of protein from a sample volume of 0.1 mL (corresponding to approximately 3000 molecules) and affixing these to 200,000 beads manifests in merely 1.5% of the beads presenting a singular protein molecule, with the remaining 98.5% devoid of protein molecules.
Subsequent steps involve loading these beads into an array configured with wells of femtoliter dimensions; the specific array utilized was 2mm in breadth, composed of approximately 50,000 wells, each with a diameter of 4.5 μm and a depth of 3.25 μm. Upon introducing fluorescent enzyme substrate droplets, the array is hermetically sealed using a rubber gasket, effectively isolating each bead within an individual femtoliter volume reaction chamber. This isolation facilitates the microbeads, each bearing a single enzyme-labeled immune complex, to generate a concentrated fluorescent signal confined solely within the 50 fl reaction chambers. Acquiring delayed fluorescence imagery of the array, facilitated by conventional microscope optics, permits the concurrent detection of myriad singular immune complexes. The quantitative protein concentration analysis within the test sample determines the ratio of wells manifesting both beads and fluorescent signals against the aggregate number of wells containing beads. This innovative platform demonstrates a superior capability in detecting protein concentrations at levels significantly lower than those detectable by traditional ELISA or multiplex assay methodologies (Figure 1).
Figure 1.
Single Molecule Array (Simoa) technology is a highly sensitive bio-detection method used to detect extremely low concentrations of biomarkers associated with Alzheimer’s Disease (AD). Prepare samples containing target AD biomarkers, like blood or cerebrospinal fluid, and employ Simoa chips or kits equipped with highly specific antibodies to capture and detect specific proteins within the samples. Enhance the signal of the antibodies bound to these proteins using signal amplification techniques, such as enzyme labeling or fluorescent tagging, allowing for the detection of proteins at the single-molecule level. Use the Simoa analyzer to read and analyze the samples, providing quantitative data on biomarker concentrations. By Figdraw.
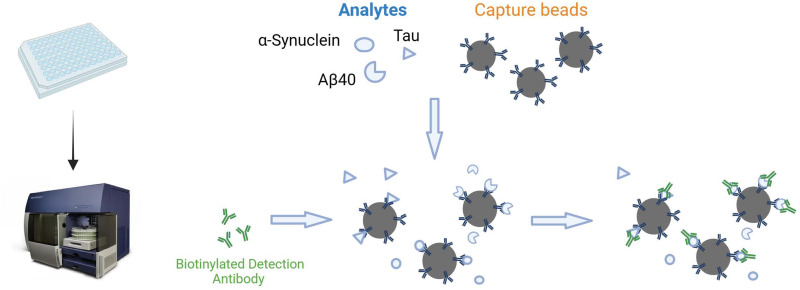
Multiplex Analyte Profiling and Cytometric Bead Array
The multiplex analyte profiling (xMAP) technology, developed by Luminex Corporation, is considered an advanced technique capable of substituting traditional ELISA methods. Characterized by its high flexibility, efficiency, and sensitivity, xMAP technology has been extensively applied in diagnosing AD and a wide range of biomedical research.109 The technique utilizes polymer beads with distinct optical properties encoded with different colors to represent various analytes. Specific antibodies or probes can be uniquely attached to each type of microbead, allowing for the capture of particular proteins or molecules in blood, saliva, or other biological samples. xMAP technology can simultaneously detect multiple biomarkers associated with AD, such as A-beta and tau proteins, making it particularly suited for the early diagnosis and monitoring of the disease’s progression.109 However, despite the potential for xMAP detection to surpass traditional ELISA methods in terms of sensitivity, current research indicates a lack of significant correlation between the concentrations of AD biomarkers measured in plasma and cerebrospinal fluid using the xMAP technique. Moreover, the reproducibility of results across different laboratories is poor.110–112 This suggests that while xMAP technology holds promise, its application in detecting AD biomarkers requires further study and validation.
The Cytometric Bead Array (CBA) method represents a sophisticated assay technique for the simultaneous quantitative analysis of multiple biomarkers. This method has been extensively applied to detect various components in food, agricultural products, and environmental samples. The technique combines flow cytometry with diverse sets of detection microspheres, each coated with antibodies specific to particular AD biomarkers. When exposed to samples containing these biomarkers, they bind to their respective antibodies on the beads. A detection antibody, typically labeled with a fluorescent tag, is added to the mixture. This antibody binds to the biomarkers, enabling the identification and quantification of each bead set (and thus each biomarker) based on its fluorescence intensity during flow cytometric analysis. The CBA methodology, distinguished by its pronounced sensitivity, specificity, and proficiency to simultaneously evaluate multiple biomarkers from an individual sample, demonstrates considerable promise for application in AD diagnostics.
Currently, methodologies for investigating neurodegenerative diseases such as Alzheimer’s and Parkinson’s, based on the ELISA experimental approach, encompass the utilization of BioLegend-developed LEGEND MAX™ Human A-beta1-42 and A-beta1-40 ELISA Kits, as well as the Tau Antibody Sampler Kit. These tools facilitate the detection of full-length APP and its various cleaved segments, including the A-beta1-40 and A-beta1-42 peptides, in addition to both phosphorylated and non-phosphorylated forms of tau. Recently, BioLegend has advanced the field by introducing a new multiplex detection system, LEGENDplex, which enables the simultaneous measurement of α-Synuclein, Tau, A-beta1-40, A-beta1-42, and NfL, thereby achieving greater sensitivity in detection. This technique facilitates an all-encompassing analysis via diverse biomarkers, significantly aiding in the early detection, continuous monitoring of disease evolution, and the formulation of tailored therapeutic interventions (Figure 2).
Figure 2.
Differentiate beads by size and fluorescence and conjugate with specific antibodies. Mix bead panel with target analyte sample, bind, then wash. Add biotinylated detection antibodies to form bead-analyte-antibody complexes. Add Streptavidin-phycoerythrin (SA-PE) for fluorescence. Use flow cytometry to segregate and quantify bead populations. Determine analyte concentrations using the assay’s standard curve. By Figdraw.
Immunoprecipitation Mass Spectrometry Assays
Immunoprecipitation-mass spectrometry (IP/MS) technology integrates immunoprecipitation (IP) and mass spectrometry (MS) analyses, primarily utilized for investigating protein interactions and functions and identifying biomarkers related to diseases. This method employs specific antibodies to capture and isolate target proteins or protein complexes for subsequent mass spectrometry analysis. For instance, in the context of AD, immunoprecipitation using magnetic beads coated with specific antibodies against A-beta enriches A-beta in plasma. These antibodies specifically target the central region of A-beta.57,113,114 Subsequently, synthetic A-beta peptides marked with stable isotopes, such as A-beta1-42 and A-beta1-40, serve as quantitative standards in mass spectrometry. In the studies conducted by Nakamura et al. Moreover, some MS-based methods can be integrated with liquid chromatography (LC/MS) to enhance the accuracy of the analysis. Recent studies, through head-to-head comparisons, have demonstrated that the LC/MS approach significantly surpasses traditional ELISA methods in terms of accuracy. Currently, the immunoprecipitation-mass spectrometry technology has been extensively applied in the research and diagnosis of A-beta, mainly due to its high specificity and sensitivity, enabling the detection of low-abundance biomarkers.101,115 However, this technology demands high-quality antibodies and involves complex sample processing and analytical procedures. As for other biomarkers in the blood, such as tau protein and NfL, precise detection methods are still lacking. Consequently, further development and validation are required to quantify different plasma biomarkers (p-Tau species).116
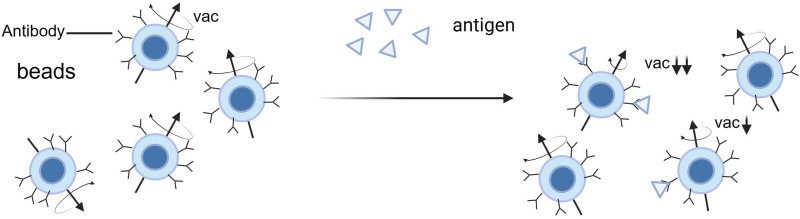
Immunomagnetic Reduction
Immunomagnetic Reduction (IMR) is a biotechnological detection method that integrates magnetic nanotechnology with immunological principles and has demonstrated significant potential for early diagnosis of AD in recent years.117 This technique is particularly adept at detecting low-concentration biomarkers in peripheral blood, offering high sensitivity while avoiding invasive procedures. The operational mechanism of IMR technology is based on specific biological probes or antibodies tagged on the surface of magnetic nanoparticles. When these antibody-laden magnetic nanoparticles bind with target biomolecules in the sample, such as AD-related A-beta or tau proteins, this leads to the aggregation or enlargement of the nanoparticles, thereby altering their original magnetic properties. More target biomolecules in the sample result in more magnetic nanoparticles participating in this binding and aggregation process, leading to a more significant reduction in magnetism (a phenomenon known as magnetic reduction). The concentration of target biomarkers in the sample can be quantitatively analyzed by measuring these changes in magnetism. IMR technology, through the precise detection of changes in magnetic nanoparticles, provides a highly sensitive detection method for various low-concentration biological markers in peripheral blood, making it particularly suitable for the early diagnosis of diseases such as AD (Figure 3).
Figure 3.
This detection method predates the high-specificity binding between select antibodies or antigens and target biomolecules. Under an applied alternating magnetic field, these magnetic particles rotate, leading to variations in the magnetic signal. The binding of target biomolecules reduces the number of magnetic particles that can rotate, thus diminishing the magnetic signal (magnetic reduction). The extent of magnetic reduction is directly proportional to the concentration of biomolecules in the sample, allowing for the quantitative analysis of biomolecules by measuring the reduction in the magnetic reagent’s signal. The functionalities of the analyzer include generating an alternating magnetic field and detecting changes in the magnetic signal to ascertain the concentration of biomolecules. By Figdraw.
The Overlooked Importance of Pre-Analytical Sample Processing
Numerous studies indicate that different sample processing procedures, from biofluid collection to analysis during the pre-analytical phase, can lead to variability in results.118 Similarly, in the detection of AD biomarkers, pre-treatment evaluations of samples demonstrate that the use of different anticoagulants results in variations in the levels of A-beta1-42, A-beta1-40, GFAP, NfL, t-Tau, and p-Tau181, with A-beta levels possibly being unstable before and after centrifugation in EDTA-treated blood, although the detected levels of t-Tau and NfL appear to be unaffected by anticoagulant factors, some contradictory results exist.119,120
Research by Teunissen et al indicates that the timing and temperature of centrifugation and storage conditions are critical pre-analytical variables. They found that A-beta detection values decrease when whole blood and separated plasma are maintained at room temperature for 24 hours but not when stored in the refrigerator, and there is no decrease when stored at −20°C for two weeks. Studies also show that GFAP, NfL, and p-Tau181 remain stable under delayed centrifugation and storage conditions, but delayed centrifugation has a significant impact on t-Tau results; t-Tau levels decrease when whole blood is maintained at room temperature for ≥3 hours, whereas they increase when whole blood is kept in the refrigerator for ≥3 hours.121 However, studies also show that t-Tau can remain stable at room temperature for 6–8 hours.119,122 Currently, there is limited research on the pre-analytical stability of foundational blood A-beta1-42, A-beta1-40, t-Tau, GFAP, NfL, and p-Tau181. Another study using the fully automatic Elecsys plasma prototype immunoassay showed similar results, recommending that whole blood and EDTA anticoagulated plasma should be placed in a 4°C refrigerator as soon as possible to avoid being left at room temperature, with results within 24 hours having strong credibility. For samples requiring longer storage, freezing at −20 or −80 °C is recommended.123 Although there is not much research on the pre-treatment of AD plasma biomarkers currently, and the detection platforms are inconsistent, in the AT(N) framework of detection indicators, most studies show that A-beta1-40 and A-beta1-42 seem to be the most unstable. However, some studies suggest that A-beta1-42/1-40 ratios can offset this downward trend; others indicate that A-beta1-42 decreases more rapidly than A-beta1-40. In summary, placing samples in a 4°C refrigerator as soon as possible and testing within 24 hours appears to be a recommended method applicable to primary healthcare units and simplifying sample processing steps.
On the other hand, studies have found that postprandial food intake can change the levels of AD blood biomarkers. Huber et al investigated the impact of pre-test food intake on the pathology of A-beta and tau, NfL, and glial cell activity biomarkers in the plasma of cognitively normal adults. Multiple blood samples were taken from 111 participants within 3 hours after a standardized meal (postprandial group, PG). In contrast, blood was drawn from a fasting subgroup within 3 hours (fasting group, FG). Results showed significant differences between FG and PG in NfL, GFAP, A-beta1-42/1-40, p-Tau181, and p-Tau231. The most remarkable changes relative to baseline for GFAP and p-Tau181 occurred within 120 minutes after the meal (p < 0.0001).124 These results suggest that AD-related plasma biomarkers may change after meals. However, more research is needed in the future using different manufacturers’ plasma biomarker immunoassay methods so that comparisons can be made between different platforms.
Conclusion
Over the past decade, the discovery of novel biomarkers has significantly advanced our understanding of the complex pathophysiology of AD, enhancing research cognition towards the disease. On the other hand, the application of ultra-sensitive detection methods, exemplified by Simoa, in the detection of plasma AD biomarkers has further propelled the utilization of blood biomarkers in the early diagnosis and prognosis assessment of AD within both specialty clinics and primary healthcare settings. The utility of these biomarkers at the individual patient level remains incomprehensive. Given the high costs associated with traditional AD detection methods such as PET-CT and the invasiveness of cerebrospinal fluid testing, blood-based biomarkers for AD are expected to enter broad clinical application in the future. New detection techniques and methodologies also bring hope for developing new neuro-specific protein biomarkers. However, the notion that biomarkers alone can more accurately predict AD still requires comprehensive validation. Longitudinal studies that combine genetics with various plasma biomarker such as A-beta1-42, p-Tau181, NFL, GFAP, et al diagnostic predictive models could provide more accurate analyses, transitioning from the diagnosis to the prediction of AD. The validation and implementation of blood biomarkers will facilitate the development of precision medicine. Another critical factor is that environmental and pre-analytical factors such as sample collection timing, storage temperature, and detection time could significantly affect the detection results of plasma biomarkers including A-beta1-40, A-beta1-42, p-Tau181, et al. Therefore, establishing a standardized AD plasma biomarker detection workflow and protocol might be an urgent issue to address for the future application of AD blood biomarkers in clinical diagnosis and prognosis.
Funding Statement
This work was supported by the Ministry of Science and Technology of the People’s Republic of China (2022YFC3602604).
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Durongbhan P, Zhao Y, Chen L, et al. A dementia classification framework using frequency and time-frequency features based on EEG signals. IEEE Trans Neural Syst Rehabil Eng. 2019;27(5):826–835. doi: 10.1109/tnsre.2019.2909100 [DOI] [PubMed] [Google Scholar]
- 2.Better MA. 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023;19(4):1598–1695. doi: 10.1002/alz.13016 [DOI] [PubMed] [Google Scholar]
- 3.Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332(7539):455–459. doi: 10.1136/bmj.38740.439664.DE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon BA, Blazey TM, Su Y, et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: a longitudinal study. Lancet Neurol. 2018;17(3):241–250. doi: 10.1016/s1474-4422(18)30028-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDade E, Wang G, Gordon BA, et al. Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology. 2018;91(14):e1295–e1306. doi: 10.1212/wnl.0000000000006277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8(6):595–608. doi: 10.15252/emmm.201606210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zolezzi JM, Bastías-Candia S, Santos MJ, Inestrosa NC. Alzheimer’s disease: relevant molecular and physiopathological events affecting amyloid-β brain balance and the putative role of PPARs. Front Aging Neurosci. 2014;6:176. doi: 10.3389/fnagi.2014.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galvão F, Grokoski KC, da Silva BB, Lamers ML, Siqueira IR. The amyloid precursor protein (APP) processing as a biological link between Alzheimer’s disease and cancer. Ageing Res Rev. 2019;49:83–91. doi: 10.1016/j.arr.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Wang Z, Chen Y, et al. FoxO mediates APP-induced AICD-dependent cell death. Cell Death Dis. 2014;5(5):e1233. doi: 10.1038/cddis.2014.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webers A, Heneka MT, Gleeson PA. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol Cell Biol. 2020;98(1):28–41. doi: 10.1111/imcb.12301 [DOI] [PubMed] [Google Scholar]
- 11.Kumar DK, Choi SH, Washicosky KJ, et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med. 2016;8(340):340ra72. doi: 10.1126/scitranslmed.aaf1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Y, Sun X, Tang D, et al. Gelsolin bound β-amyloid peptides(1-40/1-42): electrochemical evaluation of levels of soluble peptide associated with Alzheimer’s disease. Biosens Bioelectron. 2015;68:115–121. doi: 10.1016/j.bios.2014.12.041 [DOI] [PubMed] [Google Scholar]
- 13.Zhou B, Li CL, Hao YQ, Johnny MC, Liu YN, Li J. Ferrocene tripeptide Gly-Pro-Arg conjugates: synthesis and inhibitory effects on Alzheimer’s Aβ(1-42) fibrillogenesis and Aβ-induced cytotoxicity in vitro. Bioorg Med Chem. 2013;21(2):395–402. doi: 10.1016/j.bmc.2012.11.030 [DOI] [PubMed] [Google Scholar]
- 14.Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimers Res Ther. 2019;11(1):34. doi: 10.1186/s13195-019-0485-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994 [DOI] [PubMed] [Google Scholar]
- 16.Gotz J, Bodea LG, Goedert M. Rodent models for Alzheimer disease. Nat Rev Neurosci. 2018;19(10):583–598. doi: 10.1038/s41583-018-0054-8 [DOI] [PubMed] [Google Scholar]
- 17.Games D, Adams D, Alessandrini R, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373(6514):523–527. doi: 10.1038/373523a0 [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Chen KS, Knox J, et al. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature. 2000;408(6815):975–979. doi: 10.1038/35050103 [DOI] [PubMed] [Google Scholar]
- 19.Herreman A, Hartmann D, Annaert W, et al. Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc Natl Acad Sci U S A. 1999;96(21):11872–11877. doi: 10.1073/pnas.96.21.11872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chishti MA, Yang DS, Janus C, et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276(24):21562–21570. doi: 10.1074/jbc.M100710200 [DOI] [PubMed] [Google Scholar]
- 21.Mucke L, Masliah E, Yu GQ, et al. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20(11):4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oakley H, Cole SL, Logan S, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26(40):10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sturchler-Pierrat C, Abramowski D, Duke M, et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci U S A. 1997;94(24):13287–13292. doi: 10.1073/pnas.94.24.13287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piller C. Blots on a field? Science. 2022;377(6604):358–363. doi: 10.1126/science.add9993 [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Schindler SE, Bollinger JG, et al. Validation of Plasma Amyloid-β 42/40 for detecting Alzheimer disease amyloid plaques. Neurology. 2022;98(7):e688–e699. doi: 10.1212/wnl.0000000000013211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo Y, Park KS, Ha T, et al. A smart near-infrared fluorescence probe for selective detection of tau fibrils in Alzheimer’s disease. ACS Chem Neurosci. 2016;7(11):1474–1481. doi: 10.1021/acschemneuro.6b00174 [DOI] [PubMed] [Google Scholar]
- 27.Zengin A, Tamer U, Caykara T. A SERS-based sandwich assay for ultrasensitive and selective detection of Alzheimer’s tau protein. Biomacromolecules. 2013;14(9):3001–3009. doi: 10.1021/bm400968x [DOI] [PubMed] [Google Scholar]
- 28.Wang SX, Acha D, Shah AJ, et al. Detection of the tau protein in human serum by a sensitive four-electrode electrochemical biosensor. Biosens Bioelectron. 2017;92:482–488. doi: 10.1016/j.bios.2016.10.077 [DOI] [PubMed] [Google Scholar]
- 29.Kametani F, Hasegawa M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front Neurosci. 2018;12:25. doi: 10.3389/fnins.2018.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9 [DOI] [PubMed] [Google Scholar]
- 31.Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163(2):495–529. doi: 10.1006/exnr.2000.7397 [DOI] [PubMed] [Google Scholar]
- 32.Mostofi M, Mohammadi Ziarani G, Mahdavi M, et al. Synthesis and structure-activity relationship study of benzofuran-based chalconoids bearing benzylpyridinium moiety as potent acetylcholinesterase inhibitors. Eur J Med Chem. 2015;103:361–369. doi: 10.1016/j.ejmech.2015.08.061 [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Qiang X, Luo L, et al. Multitarget drug design strategy against Alzheimer’s disease: homoisoflavonoid Mannich base derivatives serve as acetylcholinesterase and monoamine oxidase B dual inhibitors with multifunctional properties. Bioorg Med Chem. 2017;25(2):714–726. doi: 10.1016/j.bmc.2016.11.048 [DOI] [PubMed] [Google Scholar]
- 34.Han SH, Park JC, Byun MS, et al. Blood acetylcholinesterase level is a potential biomarker for the early detection of cerebral amyloid deposition in cognitively normal individuals. Neurobiol Aging. 2019;73:21–29. doi: 10.1016/j.neurobiolaging.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 35.Fang EF, Hou Y, Palikaras K, et al. Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci. 2019;22(3):401–412. doi: 10.1038/s41593-018-0332-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onyango IG, Dennis J, Khan SM. Mitochondrial Dysfunction in Alzheimer’s Disease and the Rationale for Bioenergetics Based Therapies. Aging Dis. 2016;7(2):201–214. doi: 10.14336/AD.2015.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cioffi F, Adam RHI, Broersen K. Molecular mechanisms and genetics of oxidative stress in Alzheimer’s disease. J Alzheimers Dis. 2019;72(4):981–1017. doi: 10.3233/JAD-190863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J Neurosci. 1997;17(8):2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishii T, Hayakawa H, Igawa T, Sekiguchi T, Sekiguchi M. Specific binding of PCBP1 to heavily oxidized RNA to induce cell death. Proc Natl Acad Sci U S A. 2018;115(26):6715–6720. doi: 10.1073/pnas.1806912115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Domenico F, Tramutola A, Butterfield DA. Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of Alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic Biol Med. 2017;111:253–261. doi: 10.1016/j.freeradbiomed.2016.10.490 [DOI] [PubMed] [Google Scholar]
- 41.Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21(12):4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosconi L, Mistur R, Switalski R, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009;36(5):811–822. doi: 10.1007/s00259-008-1039-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neth BJ, Craft S. Insulin resistance and Alzheimer’s disease: bioenergetic linkages. Front Aging Neurosci. 2017;9:345. doi: 10.3389/fnagi.2017.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Austin BP, Nair VA, Meier TB, et al. Effects of hypoperfusion in Alzheimer’s disease. J Alzheimers Dis. 2011;26(Suppl 3):123–133. doi: 10.3233/JAD-2011-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winkler EA, Nishida Y, Sagare AP, et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci. 2015;18(4):521–530. doi: 10.1038/nn.3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li S, Shankar GM, Selkoe DJ. How do soluble oligomers of amyloid beta-protein impair hippocampal synaptic plasticity? Front Cell Neurosci. 2010;4:5. doi: 10.3389/fncel.2010.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lauretti E, Li JG, Di Meco A, Pratico D. Glucose deficit triggers tau pathology and synaptic dysfunction in a tauopathy mouse model. Transl Psychiatry. 2017;7(1):e1020. doi: 10.1038/tp.2016.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keeney JT, Ibrahimi S, Zhao L. Human ApoE isoforms differentially modulate glucose and amyloid metabolic pathways in female brain: evidence of the mechanism of neuroprotection by ApoE2 and implications for Alzheimer’s disease prevention and early intervention. J Alzheimers Dis. 2015;48(2):411–424. doi: 10.3233/JAD-150348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin YT, Seo J, Gao F, et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron. 2018;98(6):1141–1154e7. doi: 10.1016/j.neuron.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen AD, Landau SM, Snitz BE, Klunk WE, Blennow K, Zetterberg H. Fluid and PET biomarkers for amyloid pathology in Alzheimer’s disease. Mol Cell Neurosci. 2019;97:3–17. doi: 10.1016/j.mcn.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 51.Schöll M, Maass A, Mattsson N, et al. Biomarkers for tau pathology. Mol Cell Neurosci. 2019;97:18–33. doi: 10.1016/j.mcn.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jack CR, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hansra GK, Popov G, Banaczek PO, et al. The neuritic plaque in Alzheimer’s disease: perivascular degeneration of neuronal processes. Neurobiol Aging. 2019;82:88–101. doi: 10.1016/j.neurobiolaging.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 54.Niemantsverdriet E, Ottoy J, Somers C, et al. The cerebrospinal fluid Aβ1-42/Aβ1-40 ratio improves concordance with amyloid-PET for diagnosing Alzheimer’s disease in a clinical setting. J Alzheimers Dis. 2017;60(2):561–576. doi: 10.3233/jad-170327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim HJ, Park KW, Kim TE, et al. Elevation of the Plasma Aβ40/Aβ42 ratio as a diagnostic marker of sporadic early-onset Alzheimer’s disease. J Alzheimers Dis. 2015;48(4):1043–1050. doi: 10.3233/jad-143018 [DOI] [PubMed] [Google Scholar]
- 56.West T, Kirmess KM, Meyer MR, et al. A blood-based diagnostic test incorporating plasma Aβ42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: findings from a multi cohort validity analysis. Mol Neurodegener. 2021;16(1):30. doi: 10.1186/s13024-021-00451-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature. 2018;554(7691):249–254. doi: 10.1038/nature25456 [DOI] [PubMed] [Google Scholar]
- 58.Janelidze S, Stomrud E, Palmqvist S, et al. Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci Rep. 2016;6:26801. doi: 10.1038/srep26801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qu Y, Ma YH, Huang YY, et al. Blood biomarkers for the diagnosis of amnestic mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2021;128:479–486. doi: 10.1016/j.neubiorev.2021.07.007 [DOI] [PubMed] [Google Scholar]
- 60.Palmqvist S, Insel PS, Stomrud E, et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer’s disease. EMBO Mol Med. 2019;11(12):e11170. doi: 10.15252/emmm.201911170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fortea J, Vilaplana E, Carmona-Iragui M, et al. Clinical and biomarker changes of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Lancet. 2020;395(10242):1988–1997. doi: 10.1016/s0140-6736(20)30689-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wesseling H, Mair W, Kumar M, et al. Tau PTM profiles identify patient heterogeneity and stages of Alzheimer’s Disease. Cell. 2020;183(6):1699–1713.e13. doi: 10.1016/j.cell.2020.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hall S, Janelidze S, Londos E, et al. Plasma phospho-tau identifies Alzheimer’s Co-pathology in patients with Lewy body disease. Mov Disord. 2021;36(3):767–771. doi: 10.1002/mds.28370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mielke MM, Hagen CE, Xu J, et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 2018;14(8):989–997. doi: 10.1016/j.jalz.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.La Joie R, Bejanin A, Fagan AM, et al. Associations between [(18)F]AV1451 tau PET and CSF measures of tau pathology in a clinical sample. Neurology. 2018;90(4):e282–e290. doi: 10.1212/wnl.0000000000004860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15(7):673–684. doi: 10.1016/s1474-4422(16)00070-3 [DOI] [PubMed] [Google Scholar]
- 67.Pase MP, Beiser AS, Himali JJ, et al. Assessment of plasma total tau level as a predictive biomarker for dementia and related endophenotypes. JAMA Neurol. 2019;76(5):598–606. doi: 10.1001/jamaneurol.2018.4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suárez-Calvet M, Karikari TK, Ashton NJ, et al. Novel tau biomarkers phosphorylated at T181, T217 or T231 rise in the initial stages of the preclinical Alzheimer’s continuum when only subtle changes in Aβ pathology are detected. EMBO Mol Med. 2020;12(12):e12921. doi: 10.15252/emmm.202012921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Friede RL, Samorajski T. Axon caliber related to neurofilaments and microtubules in sciatic nerve fibers of rats and mice. Anat Rec. 1970;167(4):379–387. doi: 10.1002/ar.1091670402 [DOI] [PubMed] [Google Scholar]
- 70.Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90(8):870–881. doi: 10.1136/jnnp-2018-320106 [DOI] [PubMed] [Google Scholar]
- 71.Jung Y, Damoiseaux JS. The potential of blood neurofilament light as a marker of neurodegeneration for Alzheimer’s disease. Brain. 2024;147(1):12–25. doi: 10.1093/brain/awad267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577–589. doi: 10.1038/s41582-018-0058-z [DOI] [PubMed] [Google Scholar]
- 73.Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med. 2019;25(2):277–283. doi: 10.1038/s41591-018-0304-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abu-Rumeileh S, Steinacker P, Polischi B, et al. CSF biomarkers of neuroinflammation in distinct forms and subtypes of neurodegenerative dementia. Alzheimers Res Ther. 2019;12(1):2. doi: 10.1186/s13195-019-0562-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chatterjee P, Pedrini S, Stoops E, et al. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl Psychiatry. 2021;11(1):27. doi: 10.1038/s41398-020-01137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garwood CJ, Ratcliffe LE, Simpson JE, Heath PR, Ince PG, Wharton SB. Review: astrocytes in Alzheimer’s disease and other age-associated dementias: a supporting player with a central role. Neuropathol Appl Neurobiol. 2017;43(4):281–298. doi: 10.1111/nan.12338 [DOI] [PubMed] [Google Scholar]
- 77.Cicognola C, Janelidze S, Hertze J, et al. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimers Res Ther. 2021;13(1):68. doi: 10.1186/s13195-021-00804-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim KY, Shin KY, Chang KA. GFAP as a potential biomarker for Alzheimer’s disease: a systematic review and meta-analysis. Cells. 2023;12(9). doi: 10.3390/cells12091309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi: 10.1126/science.aau6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo BB, Bellingham SA, Hill AF. Stimulating the Release of Exosomes Increases the Intercellular Transfer of Prions. J Biol Chem. 2016;291(10):5128–5137. doi: 10.1074/jbc.M115.684258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stuendl A, Kunadt M, Kruse N, et al. Induction of α-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies. Brain. 2016;139(Pt 2):481–494. doi: 10.1093/brain/awv346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Basso M, Pozzi S, Tortarolo M, et al. Mutant copper-zinc superoxide dismutase (SOD1) induces protein secretion pathway alterations and exosome release in astrocytes: implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis. J Biol Chem. 2013;288(22):15699–15711. doi: 10.1074/jbc.M112.425066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bir A, Ghosh A, Chauhan A, et al. Exosomal dynamics and brain redox imbalance: implications in Alzheimer’s disease pathology and diagnosis. Antioxidants. 2024;13(3). doi: 10.3390/antiox13030316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soliman HM, Ghonaim GA, Gharib SM, et al. Exosomes in Alzheimer’s disease: from being pathological players to potential diagnostics and therapeutics. Int J Mol Sci. 2021;22(19). doi: 10.3390/ijms221910794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L, Shui X, Diao Y, Chen D, Zhou Y, Lee TH. Potential implications of miRNAs in the pathogenesis, diagnosis, and therapeutics of Alzheimer’s disease. Int J Mol Sci. 2023;24(22). doi: 10.3390/ijms242216259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jia L, Qiu Q, Zhang H, et al. Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimers Dement. 2019;15(8):1071–1080. doi: 10.1016/j.jalz.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 87.Jia L, Zhu M, Kong C, et al. Blood neuro-exosomal synaptic proteins predict Alzheimer’s disease at the asymptomatic stage. Alzheimers Dement. 2021;17(1):49–60. doi: 10.1002/alz.12166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dong Z, Gu H, Guo Q, et al. Profiling of serum exosome MiRNA reveals the potential of a MiRNA panel as diagnostic biomarker for Alzheimer’s disease. Mol Neurobiol. 2021;58(7):3084–3094. doi: 10.1007/s12035-021-02323-y [DOI] [PubMed] [Google Scholar]
- 89.Song S, Lee JU, Jeon MJ, Kim S, Sim SJ. Detection of multiplex exosomal miRNAs for clinically accurate diagnosis of Alzheimer’s disease using label-free plasmonic biosensor based on DNA-Assembled advanced plasmonic architecture. Biosens Bioelectron. 2022;199:113864. doi: 10.1016/j.bios.2021.113864 [DOI] [PubMed] [Google Scholar]
- 90.Li Y, Xia M, Meng S, et al. MicroRNA-29c-3p in dual-labeled exosome is a potential diagnostic marker of subjective cognitive decline. Neurobiol Dis. 2022;171:105800. doi: 10.1016/j.nbd.2022.105800 [DOI] [PubMed] [Google Scholar]
- 91.Cheng L, Doecke JD, Sharples RA, et al. Prognostic serum miRNA biomarkers associated with Alzheimer’s disease shows concordance with neuropsychological and neuroimaging assessment. Mol Psychiatry. 2015;20(10):1188–1196. doi: 10.1038/mp.2014.127 [DOI] [PubMed] [Google Scholar]
- 92.Hu S, Zhang L, Su Y, et al. Sensitive detection of multiple blood biomarkers via immunomagnetic exosomal PCR for the diagnosis of Alzheimer’s disease. Sci Adv. 2024;10(13):eabm3088. doi: 10.1126/sciadv.abm3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li J, Ni W, Jin D, et al. Nanosensor-driven detection of neuron-derived exosomal Aβ(42) with graphene electrolyte-gated transistor for Alzheimer’s disease diagnosis. Anal Chem. 2023;95(13):5719–5728. doi: 10.1021/acs.analchem.2c05751 [DOI] [PubMed] [Google Scholar]
- 94.Banerjee A, Khemka VK, Roy D, et al. Role of pro-inflammatory cytokines and vitamin D in probable Alzheimer’s disease with depression. Aging Dis. 2017;8(3):267–276. doi: 10.14336/ad.2016.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rani V, Verma R, Kumar K, Chawla R. Role of pro-inflammatory cytokines in Alzheimer’s disease and neuroprotective effects of pegylated self-assembled nanoscaffolds. Curr Res Pharmacol Drug Discov. 2023;4:100149. doi: 10.1016/j.crphar.2022.100149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res. 2006;45(5):369–378. doi: 10.1016/j.plipres.2006.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baranowska-Bik A, Bik W, Styczynska M, et al. Plasma leptin levels and free leptin index in women with Alzheimer’s disease. Neuropeptides. 2015;52:73–78. doi: 10.1016/j.npep.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 98.Lista S, Faltraco F, Prvulovic D, Hampel H. Blood and plasma-based proteomic biomarker research in Alzheimer’s disease. Prog Neurobiol. 2013;101–102:1–17. doi: 10.1016/j.pneurobio.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 99.Yu H, Wang F, Wu JJ, et al. Integrated transcriptomics reveals the brain and blood biomarkers in Alzheimer’s disease. CNS Neurosci Ther. 2023;29(12):3943–3951. doi: 10.1111/cns.14316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mielke MM, Dage JL, Frank RD, et al. Performance of plasma phosphorylated tau 181 and 217 in the community. Nat Med. 2022;28(7):1398–1405. doi: 10.1038/s41591-022-01822-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Janelidze S, Bali D, Ashton NJ, et al. Head-to-head comparison of 10 plasma phospho-tau assays in prodromal Alzheimer’s disease. Brain. 2023;146(4):1592–1601. doi: 10.1093/brain/awac333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Benedet AL, Milà-Alomà M, Vrillon A, et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer Disease Continuum. JAMA Neurol. 2021;78(12):1471–1483. doi: 10.1001/jamaneurol.2021.3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Palmqvist S, Tideman P, Cullen N, et al. Prediction of future Alzheimer’s disease dementia using plasma phospho-tau combined with other accessible measures. Nat Med. 2021;27(6):1034–1042. doi: 10.1038/s41591-021-01348-z [DOI] [PubMed] [Google Scholar]
- 104.Rauchmann BS, Schneider-Axmann T, Perneczky R. Associations of longitudinal plasma p-tau181 and NfL with tau-PET, Aβ-PET and cognition. J Neurol Neurosurg Psychiatry. 2021;92(12):1289–1295. doi: 10.1136/jnnp-2020-325537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sarto J, Ruiz-García R, Guillén N, et al. Diagnostic performance and clinical applicability of blood-based biomarkers in a prospective memory clinic cohort. Neurology. 2023;100(8):e860–e873. doi: 10.1212/wnl.0000000000201597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Giangrande C, Delatour V, Andreasson U, Blennow K, Gobom J, Zetterberg H. Harmonization and standardization of biofluid-based biomarker measurements for AT(N) classification in Alzheimer’s disease. Alzheimers Dement. 2023;15(3):e12465. doi: 10.1002/dad2.12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rissin DM, Kan CW, Campbell TG, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol. 2010;28(6):595–599. doi: 10.1038/nbt.1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zetterberg H, Mörtberg E, Song L, et al. Hypoxia due to cardiac arrest induces a time-dependent increase in serum amyloid β levels in humans. PLoS One. 2011;6(12):e28263. doi: 10.1371/journal.pone.0028263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Herskovits AZ, Locascio JJ, Peskind ER, Li G, Hyman BT. A Luminex assay detects amyloid β oligomers in Alzheimer’s disease cerebrospinal fluid. PLoS One. 2013;8(7):e67898. doi: 10.1371/journal.pone.0067898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mehta PD, Pirttila T, Patrick BA, Barshatzky M, Mehta SP. Amyloid beta protein 1-40 and 1-42 levels in matched cerebrospinal fluid and plasma from patients with Alzheimer disease. Neurosci Lett. 2001;304(1–2):102–106. doi: 10.1016/s0304-3940(01)01754-2 [DOI] [PubMed] [Google Scholar]
- 111.Hansson O, Zetterberg H, Vanmechelen E, et al. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol Aging. 2010;31(3):357–367. doi: 10.1016/j.neurobiolaging.2008.03.027 [DOI] [PubMed] [Google Scholar]
- 112.Song F, Poljak A, Valenzuela M, Mayeux R, Smythe GA, Sachdev PS. Meta-analysis of plasma amyloid-β levels in Alzheimer’s disease. J Alzheimers Dis. 2011;26(2):365–375. doi: 10.3233/jad-2011-101977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ovod V, Ramsey KN, Mawuenyega KG, et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13(8):841–849. doi: 10.1016/j.jalz.2017.06.2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pannee J, Törnqvist U, Westerlund A, et al. The amyloid-β degradation pattern in plasma--a possible tool for clinical trials in Alzheimer’s disease. Neurosci Lett. 2014;573:7–12. doi: 10.1016/j.neulet.2014.04.041 [DOI] [PubMed] [Google Scholar]
- 115.Janelidze S, Teunissen CE, Zetterberg H, et al. Head-to-head comparison of 8 plasma amyloid-β 42/40 assays in Alzheimer disease. JAMA Neurol. 2021;78(11):1375–1382. doi: 10.1001/jamaneurol.2021.3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimers Dement. 2022;18(12):2669–2686. doi: 10.1002/alz.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee PJ, Tsai CL, Liang CS, et al. Biomarkers with plasma amyloid β and tau protein assayed by immunomagnetic reduction in patients with amnestic mild cognitive impairment and Alzheimer’s disease. Acta Neurol Taiwan. 2022;31(2):53–60. [PubMed] [Google Scholar]
- 118.Hansson O, Mikulskis A, Fagan AM, et al. The impact of preanalytical variables on measuring cerebrospinal fluid biomarkers for Alzheimer’s disease diagnosis: a review. Alzheimers Dement. 2018;14(10):1313–1333. doi: 10.1016/j.jalz.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 119.Rózga M, Bittner T, Batrla R, Karl J. Preanalytical sample handling recommendations for Alzheimer’s disease plasma biomarkers. Alzheimers Dement. 2019;11:291–300. doi: 10.1016/j.dadm.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ashton NJ, Suárez-Calvet M, Karikari TK, et al. Effects of pre-analytical procedures on blood biomarkers for Alzheimer’s pathophysiology, glial activation, and neurodegeneration. Alzheimers Dement. 2021;13(1):e12168. doi: 10.1002/dad2.12168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Verberk IMW, Misdorp EO, Koelewijn J, et al. Characterization of pre-analytical sample handling effects on a panel of Alzheimer’s disease-related blood-based biomarkers: results from the Standardization of Alzheimer’s Blood Biomarkers (SABB) working group. Alzheimers Dement. 2022;18(8):1484–1497. doi: 10.1002/alz.12510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang JF, Liu HC, Chen HH, et al. Effect of times to blood processing on the stability of blood proteins associated with Dementia. Dement Geriatr Cognit Disord. 2020;49(3):303–311. doi: 10.1159/000509358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kurz C, Stöckl L, Schrurs I, et al. Impact of pre-analytical sample handling factors on plasma biomarkers of Alzheimer’s disease. J Neurochem. 2023;165(1):95–105. doi: 10.1111/jnc.15757 [DOI] [PubMed] [Google Scholar]
- 124.Huber H, Ashton NJ, Schieren A, et al. Levels of Alzheimer’s disease blood biomarkers are altered after food intake-A pilot intervention study in healthy adults. Alzheimers Dement. 2023;19(12):5531–5540. doi: 10.1002/alz.13163 [DOI] [PubMed] [Google Scholar]