Abstract
Purpose
To determine the physiological status of the retina by electroretinography (ERG) using skin electrodes and the RETevalTM system in eyes that had undergone pars plana vitrectomy (PPV) with silicone oil (SO) tamponade. The vitrectomy was performed for a retinal detachment and proliferative diabetic retinopathy (PDR).
Design
Retrospective case series.
Methods
ERGs were recorded with the RETevalTM system (LKC Technologies Inc. Gaithersburg, MD; Welch Allyn, Inc. Skaneateles Falls, NY) from eight eyes with PDR before and after the SO removal. The amplitudes and implicit times of the a- and b-waves of the ERGs before the SO removal were compared to that after the SO removal.
Results
ERGs were recordable in four eyes before and after the SO removal and the a- and b-amplitudes improved in three eyes and worsened in one eye after the SO removal. In the remaining four eyes, ERGs were non-recordable both before and after the SO removal.
Conclusion
These results indicate that ERGs picked up by skin electrodes can be used to assess the physiology of the retina in eyes with a SO tamponade. The flat ERGs in the SO-filled eye indicated the presence of diffuse retinal damage which was confirmed by the flat ERGs after the SO removal.
Keywords: electroretinogram, pars plana vitrectomy, proliferative diabetic retinopathy, silicone oil, skin electrode
Plain Language Summary
There has been an increasing number of reports on evaluating the retinal function using electroretinography (ERG) with skin electrodes. The main advantage of this system is the ability to record ERGs with a skin electrode that does not touch the cornea and ocular surface. This reduces the risk of infection especially in the postoperative period and in clinical situations where infection is suspected. In addition, there have been only a few reports evaluating the function of the retina by ERG in SO-filled eyes.
We recorded ERGs with the RETeval (LKC Technologies Inc. Gaithersburg, MD; Welch Allyn Inc. Skaneateles Falls, NY) device, a relatively new ERG recording system that uses skin electrodes and is less invasive. We recorded ERGs from eight SO-filled eyes with proliferative diabetic retinopathy (PDR). In 4 SO filled eyes, the amplitudes increased in three eyes after the SO removal. In the other four eyes, ERGs were non-recordable before and after the SO removal. These results suggest that the RETeval system that uses skin electrodes can be used to assess the retinal function in PDR eyes with a SO tamponade. We suggest that the absence of ERGs in the SO filled eyes was not due to the electrical non-conductive effects of SO but may indicate the presence of diffuse retinal damage which was confirmed after the SO removal.
Introduction
Silicone oil (SO) has been widely used to tamponade the retina in complicated vitreoretinal disorders such as proliferative vitreoretinopathy (PVR),1,2 giant retinal tears,1,3 traumatic injuries,2,4 proliferative diabetic retinopathy (PDR),1,3 and viral retinitis.1,5 SO is an electrically non-conductive compound, and its optical clarity allows clinicians to examine the retina by ophthalmoscopy and optical coherence tomography in eyes with a SO tamponade.
Electroretinography (ERG) has been used to determine the physiological status of the retina in eyes with suspected retinal pathology. However, ERGs are generally not recorded after intraocular surgeries especially in eyes with a filtering bleb, corneal pathology, and infectious diseases. In addition, ERGs are not recorded from eyes that have the vitreous cavity filled with air, gas, or SO because the contact lens electrodes that are used to pick up the ERGs can expose the intraocular chambers to infectious microorganisms.
The RETevalTM system (LKC Technologies Inc., Gaithersburg, MD; Welch Allyn, Inc., Skaneateles Falls, NY) is a handheld, portable ERG device that uses skin electrodes to pick up the ERGs.6 The recordings can be done rapidly, and the skin electrodes reduce the risk of corneal abrasion and extra- and intraocular infections. It has been reported that ERGs recorded with the RETeval system under both photopic and scotopic conditions according to ISCEV standards7 were comparable to the ERGs recorded with contact lens electrodes.8 In addition, the amplitudes and the implicit times of the a- and b-waves recorded with skin electrodes were significantly correlated with those components of the ERGs recorded with corneal contact lens electrodes.8 Thus, the RETeval system allows clinicians to assess the physiological status of the retina quickly and safely soon after intraocular surgery.9–11
It has been reported that the ERGs in SO filled eyes after vitrectomy for complicated retinal pathology, such as retinal detachment and giant retinal tears, were markedly reduced or even absent. Interestingly, an ERG was present after the SO removal.12–14 These ERGs were recorded using corneal contact lens electrodes or DTL electrodes, and only limited information is available on studies using skin electrodes in eyes with a SO tamponade.15 In addition, there have not been any reports on eyes with vascular occlusive diseases such as PDR that had a SO tamponade.
Thus, the purpose of this study was to determine the physiological status of the retina in eyes with a SO tamponade after vitrectomy for PDR. To accomplish this, we recorded ERGs before and after the SO removal, and determined the correlation of the amplitudes and implicit times of the a- and b-waves before and after the SO removal. The ERGs were recorded with skin electrodes.
Methods
Subjects
All the participants had undergone pars plana vitrectomy (PPV) with a SO tamponade at the Saitama Medical University Hospital in Saitama, Japan, from March 20XX to June 20XX+2 (28 months). All the patients had signed a written informed consent form that included the nature of the disorder and the possible complications of the surgery and ERG recordings. This was a retrospective study that was conducted in accordance with the tenets of Declaration of Helsinki, and the procedures were approved by the Ethics Committee of Saitama Medical University, Saitama, Japan (ID number: 18067.01).
Eight eyes of eight patients with PDR with RD that had undergone pars plana vitrectomy (PPV) with purified SO as a tamponade (SILIKON® 1000, Alcon Japan Ltd, Tokyo, Japan). There were 6 men and 2 women, and the mean ± standard deviation age was 57.6 ± 7.5 years. The SO was removed when the retinal reattachment appeared to be stable with no signs of active vitreoretinal pathology. The medical records were reviewed to determine the vitreoretinal pathology that necessitated the PPV with a SO tamponade, the ERG findings, and the best-corrected visual acuity (BCVA). In addition, information was obtained on whether the retina was attached or detached retina before and after the SO removal.
ERG Recordings
ERGs were recorded with the RETevalTM system, and the recording conditions conformed to the standards of the International Society for Clinical Electrophysiology of Vision (ISCEV).7 ERGs were recorded before and after the SO removal in all eyes. The ERGs were recorded after 20 minutes of dark-adaptation, and the combined rod-cone responses, DA 3.0, were picked up by a sensor strip skin electrode affixed to the lower eyelid. The strips contained the active, reference, and ground electrodes. A mini Ganzfeld dome was placed in front of the eye, and a 3.0 cd·s/m2 flash without background illumination was used to elicit the ERGs. The patients were instructed to fixate a point within the dome, and the fixation was monitored by an infrared camera. The amplitudes and the implicit times of the a- and b-waves of the ERGs were automatically analyzed by the software embedded in the RETevalTM system.
Statistical Analyses
The amplitudes and implicit times before and after the SO removal were compared using Wilcoxon signed rank test. The decimal BCVA was converted to the logarithm of minimum angle of resolution (logMAR) for the statistical analyses. The visual acuities of “counting fingers”, “hand movements”, “light perception”, and “no light perception” were assigned values of 2.0, 2.4, 2.7, and 3.0 logMAR units, respectively.16 The significance of the differences in the amplitudes and implicit times before and after the SO removal was determined by Wilcoxon signed rank tests. A P < 0.05 was taken to be statistically significant.
Results
The demographics and clinical information of the patients are summarized in Table 1. The vitreoretinal pathologies leading to the vitrectomy with SO tamponade were tractional retinal detachment (RD; n = 7) or combined rhegmatogenous and tractional RD involving the macula (n = 1, Table 2). The retina remained attached after the SO removal in all eight eyes. The mean duration of the SO tamponade was 210.5 ± 159.3 days. The mean interval between the first surgery and the first ERG recordings was 233.8 ± 129.9 days and that between the SO removal surgery and the second ERG recordings was 93.8 ± 96.9 days. The interval between the first surgery and the first ERG recordings was not statistically significantly correlated with the ERG amplitudes (P = 0.7190 for the a-wave and P = 0.7190 for the b-wave). The interval between the SO removal surgery and the second ERG recordings was not statistically significantly correlated with the ERG amplitudes (p = 0.461 for the a-wave and p = 0.461 for the b-wave).
Table 1.
Demographics of Patients
| Age (Years) | 57.6 ± 7.5 |
| Sex (F/M) | 2 / 6 |
| Visual acuity (log MAR units) | |
| Before SOR | 1.05 ± 0.59 |
| After SOR | 0.56 ± 0.32 |
| Duration of SO tamponade (days) | 210.5 ± 159.3 |
| Vitreoretinal pathology* | |
| TRD | 7 |
| Combined TRD and RRD | 1 |
Notes: Data is shown as mean ± standard deviation. *All eyes had proliferative diabetic retinopathy. *RD involved macula in all eyes.
Abbreviations: F, female; M, male; SOR, silicone oil removal; log MAR, logarithm of the minimum angle of resolution; TRD, tractional retinal detachment; RRD, rhegmatogeneous retinal detachment.
Table 2.
The Visual Acuity and Electroretinographic Parameters Before and After Silicone Oil Removal
| Case | Visual Acuity | ERG Parameter | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amplitude (uV) | Implicit Time (msec) | |||||||||||
| Before SOR | After SOR | Before SOR | After SOR | Before SOR | After SOR | |||||||
| Decimal VA | logMAR | Decimal VA | logMAR | a Wave | b Wave | a Wave | b Wave | a Wave | b Wave | a Wave | b Wave | |
| 1 | 0.1 | 1.00 | 0.4 | 0.40 | −7.7 | 8.2 | −10.9 | 18.7 | 12.1 | 40.1 | 19.7 | 64.9 |
| 2 | 0.2 | 0.70 | 0.3 | 0.52 | −1.6 | 2.7 | −11.8 | 15.6 | 24.3 | 60.9 | 20.5 | 51.5 |
| 3 | 0.01 | 2.00 | 0.06 | 1.22 | 0 | 0 | 0 | 0 | n.a. | n.a. | n.a. | n.a. |
| 4 | 0.4 | 0.40 | 0.6 | 0.22 | −18.6 | 19.8 | −6.4 | 19.6 | 19.6 | 50.3 | 16.5 | 59.2 |
| 5 | 0.09 | 1.05 | 0.15 | 0.82 | 0 | 0 | 0 | 0 | n.a. | n.a. | n.a. | n.a. |
| 6 | 0.01 | 2.00 | 0.2 | 0.70 | 0 | 0 | 0 | 0 | n.a. | n.a. | n.a. | n.a. |
| 7 | 0.2 | 0.70 | 0.4 | 0.40 | 0 | 0 | 0 | 0 | n.a. | n.a. | n.a. | n.a. |
| 8 | 0.3 | 0.52 | 0.6 | 0.22 | −10.8 | 10 | −17.8 | 28.9 | 22.9 | 51.5 | 20.1 | 62 |
| Average* | 0.09 | 1.05 | 0.273 | 0.56 | −4.84 | 5.09 | −5.86 | 10.35 | 19.73 | 50.70 | 19.20 | 59.40 |
| Standard deviation | 0.587 | 0.318 | 6.493 | 6.704 | 6.528 | 10.93 | 4.721 | 7.369 | 1.584 | 4.986 | ||
Notes: *Geometric mean is shown as an average for decimal VA.
Abbreviations: SOR, silicone oil removal; n.a., not applicable; VA, visual acuity; log MAR, logarithm of minimal angular resolution.
None of the eyes had a posterior synechia which would have prevented a maximum dilation of the pupils before the SO removal.
The mean ± SD of the BCVA was 1.05 ± 0.59 logMAR units with a range of 0.40 to 2.00 logMAR units before the SO removal, and it improved to 0.56 ± 0.32 logMAR units with a range of 0.22 to 1.22 logMAR units after the SO removal (P = 0.008).
Information on the time of ERG recordings and each ERG parameter of each patient is presented in Table 2. ERGs were recordable in four eyes (Group 1) and non-recordable in the other 4 eyes (Group 2) before the SO removal. In Group 1, the a- and b-wave amplitudes improved in three eyes and worsened in one eye after the SO removal. All four eyes in Group 2 still had a flat ERG after the SO removal. In total, the a-wave amplitude changed before and after the SO removal from −0.8 (−8.5, 0.0) μV [median (interquartile)] to −3.2 (−11.1, 0.0) μV (P = 0.875), respectively. The b-wave amplitude changed before the SO removal was 1.4 (0.0, 8.7) μV [median (interquartile)] to 7.8 (0.0, 18.9) μV after the SO removal (P = 0.250), respectively. The a-wave amplitude before the SO removal was significantly and positively correlated with the amplitude after the SO removal (rho = 0.8108, P = 0.015; Spearman’s rank correlation coefficient). The b-wave amplitudes before and after the SO removal were significantly and positively correlated (rho = 0.9730, P < 0.0001; Spearman’s rank correlation coefficient). All eyes were classified into those that had a detectable ERG response (Group 1) and those that had a flat ERG response (Group 2) ERGs before the SO removal (Table 2). Representative cases of Groups 1 and 2 are shown in Figures 1 and 2, respectively.
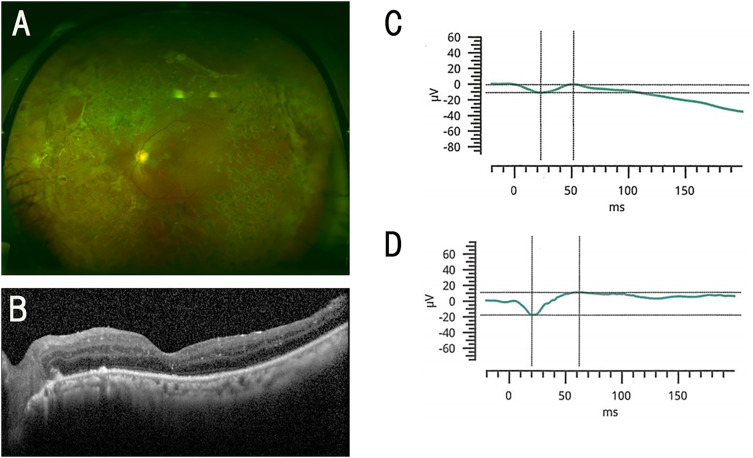
Figure 1.
Findings in a 53-year-old woman who had ERG responses in her silicone (SO) filled eye. (A) Fundus photograph of the left eye after the SO removal. (B) Optical coherence tomographic (OCT) image of the left eye after the SO removal. (C) Combined rod-cone response before SO removal. (D) Combined rod-cone response after SO removal. The decimal visual acuity improved from 0.3 to 0.6 (from 0.52 to 0.22 logarithm of the minimum angle of resolution; logMAR units) after the SO removal.
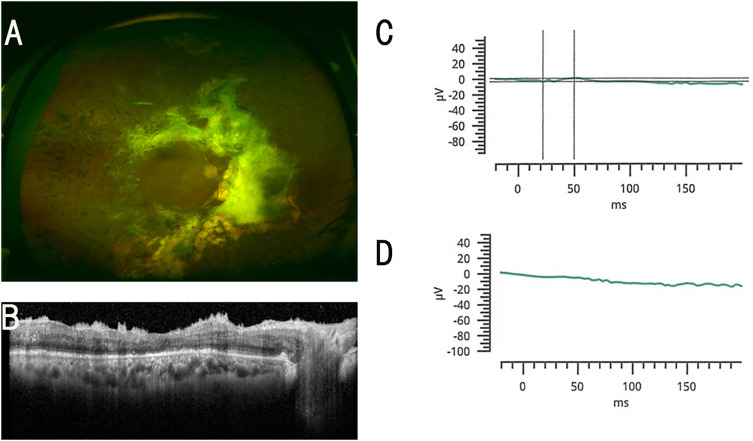
Figure 2.
Representative ERGs of a 49-year-old man whose ERG was flat in the SO filled eye. (A) Fundus photograph of the right eye after the SO removal. (B) OCT image of the right eye after the SO removal. (C) Combined rod-cone responses before the SO removal. (D) Combined rod-cone responses after the SO removal. The decimal visual acuity improved from 0.2 to 0.4 (from 0.70 to 0.40 logarithm of the minimum angle of resolution; logMAR units) after the SO removal.
Discussion
Our results showed that the amplitudes of the ERGs recorded in a SO-filled eye were significantly and positively correlated with the amplitudes after the SO removal. These findings suggested that the amplitudes of the ERGs in a SO-filled eye can be used to predict the amplitudes of the ERGs after the SO is removed. This is clinically important because the physiological status of the entire retina can be assessed objectively using the ERGs even in a SO-filled eye.
In a previous laboratory study, bilateral PPV was performed followed by an injection of SO into the vitreous cavity of one eye of rabbits. The a- and b-wave amplitudes of the ERGs of both eyes were reduced during the early postoperative period.17 With time, there was a recovery of the amplitudes to the baseline values in both eyes.17 These findings indicated that the SO was not toxic to the retina, although in clinical studies it has been reported that the ERGs were reduced or flat in SO-filled eyes.10–12,18–20 According to the results of previous studies, one possible explanation for the reduction is that the SO was toxic.18,21,22 Some authors stated that the absence of ERGs in the SO filled eyes was attributed to the electrical non-conductive property of SO.12,23,24 We used skin electrodes to record the ERGs in PDR eyes with SO tamponade. In three of four eyes where sizable response was recorded, the amplitudes increased after the SO removal. In the four eyes where no response was recorded before the SO removal had no response after the SO removal. We suggest that the absence of an ERG response before the SO removal was mainly due to a severely damaged retina by the PDR and surgery, although there was an insulation effect of the SO. Flat ERGs after the SO removal strongly suggest retinal damage. These findings are consistent with previous studies in which ERG recordings were made using contact lens electrodes in diseased eyes without vascular occlusion.12,14
Studies on the non-conducting effects of SO were performed on a theoretical model, and the results showed that the ERGs did not decrease until at least 50% of the vitreous were replaced by SO.19 When more SO was injected into the vitreous cavity, the ERG responses were reduced. If the replacement was large and the thickness of the remaining vitreous layer was reduced to 0.24-mm, the ERGs could not be detected even if the retina was functioning.19 In our patients, it was possible to record ERGs even in the presence of a large amount of SO was present which is in agreement line with previous studies.4,14,25 Frumar et al23 explained this by stating that a small but significant conducting path was present between the retinal surface and the cornea which allowed the electrical signals from the retinal neurons to pass to the pick-up electrodes. They assumed that the path is a thin film of fluid between the retina and the tamponading SO bubble. Our results agree with their findings in that ERGs can be recorded even from SO-filled eyes.
When recording ERGs with the RETevalTM, the skin electrodes are placed 2 mm below the lower margin of the eyelid. Therefore, the skin electrode is placed close to the aqueous humor when the patient is seated during recordings. In contrast, contact lens electrodes are placed relatively far from the conducting fluid and close to the SO further isolating the ERG signals.
Chen et al conducted a head-to-head comparison of the skin electrodes used for RETeval and regular ERG-jet corneal contact lens electrodes and LKC.8 They concluded that ERG waveforms recorded with skin electrodes were comparable to the contact lens electrode ERG waveforms. They further stated on a quantitative level that their analysis showed that skin electrode-based ERG implicit times were nearly identical, and amplitudes were approximately 1/3 of those obtained with traditional corneal electrodes during simultaneous recordings. Therefore, we believe that the non-recordable ERG in Group 2 was less likely to be due to the SO non-conduction and more likely to reflect severe retinal damage.
Our study has several limitations. First, this was a retrospective study with its inherent drawbacks. Second, the sample size was small, and statistical analysis on the relationship between the ERG parameters before and after SO removal could not be done. Thus, it will be necessary to verify these findings in a larger number of patients to determine whether the response always remains flat after SO removal when the eyes showed no response before SO removal. Third, no information was obtained about ERG data recorded with conventional contact lens electrode. It would be interesting to compare the ERG responses between skin electrode and corneal contact lens electrodes. Fourth, the amplitudes of the full-field ERGs are not always significantly correlated with the visual acuity. Therefore, a study on the relationship between the ERGs and visual field may be more relevant to determine if the ERGs before the SO is removed can predict the visual fields after the SO is removed.
In conclusion, the removal of SO can increase the amplitudes of the ERGs when a response is present in SO-filled eyes with PDR. The absence of the ERGs in the SO filled eyes may be due to the electrical non-conductive effects of SO. We conclude that ERG recordings with skin electrodes will allow a functional evaluation of SO-filled eye.
Acknowledgments
We would like to thank Professor Duco Hamasaki for editing the manuscript and insightful comments.
Funding Statement
This study was supported in part by a grant to KS from the Japan Society for the Promotion of Science (JSPS; KAKENHI grant number: 22K09838).
Patient Consent
Written consent for surgery and ERG recordings has been obtained. This report does not contain any personal identifying information.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Federman JL, Schubert HD. Complications associated with the use of silicone oil in 150 eyes after retina-vitreous surgery. Ophthalmology. 1988;95(7):870–875. PMID: 3174036. doi: 10.1016/S0161-6420(88)33080-0 [DOI] [PubMed] [Google Scholar]
- 2.Azen SP, Scott IU, Flynn HW, et al. Silicone oil in the repair of complex retinal detachments. A prospective observational multicenter study. Ophthalmology. 1998;105(9):1587–1597. PMID:9754162. doi: 10.1016/S0161-6420(98)99023-6 [DOI] [PubMed] [Google Scholar]
- 3.Camacho H, Bajaire B, Mejia LF. Silicone oil in the management of giant retinal tears. Ann Ophthalmol. 1992;24(2):45–49. PMID: 1562123. [PubMed] [Google Scholar]
- 4.Alexandridis E. Silicone oil tamponade in the management of severe hemorrhagic detachment of the choroid and ciliary body after surgical trauma. Ophthalmologica. 1990;200(4):189–193. PMID: 2367081. doi: 10.1159/000310105 [DOI] [PubMed] [Google Scholar]
- 5.Lim JI, Enger C, Haller JA, et al. Improved visual results after surgical repair of cytomegalovirus-related retinal detachments. Ophthalmology. 1994;101:264–269. PMID: 8115148. doi: 10.1016/S0161-6420(13)31339-6 [DOI] [PubMed] [Google Scholar]
- 6.Kato K, Kondo M, Sugimoto M, Ikesugi K, Matsubara H. Effect of pupil size on flicker ERGs recorded with RETeval system: new mydriasis-free full-field ERG system. Invest Ophthalmol Vis Sci. 2015;56(6):3684–3690. PMID:26047169. doi: 10.1167/iovs.14-16349 [DOI] [PubMed] [Google Scholar]
- 7.McCulloch DL, Marmor MF, Brigell MG, et al. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 2015;130(1):1–12. PMID: 25502644. doi: 10.1007/s10633-014-9473-7 [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Greenstein VC, Brodie SE. Qualitative and quantitative comparison of ERGs with contact lens and adhesive skin electrodes. Doc Ophthalmol. 2022;144(3):203–215. PMID: 35304683. doi: 10.1007/s10633-022-09868-w [DOI] [PubMed] [Google Scholar]
- 9.Igawa Y, Shoji T, Weinreb R, et al. Early changes in photopic negative response in eyes with glaucoma with and without choroidal detachment after filtration surgery. Br J Ophthalmol. 2023;107(9):1295–1302. PMID: 35396212. doi: 10.1136/bjophthalmol-2021-320730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terauchi G, Shinoda K, Sakai H, et al. Retinal function determined by flicker ERGs before and soon after intravitreal injection of anti-VEGF agents. BMC Ophthalmol. 2019;19(1):129. PMID: 31208350. doi: 10.1186/s12886-019-1129-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shibuya M, Yoshikawa Y, Katsumoto T, et al. Electroretinographic recordings with skin electrodes to assess effects of vitrectomy with gas tamponade on eyes with rhegmatogenous retinal detachment. Sci Rep. 2019;9(1):19948. PMID: 31882665. doi: 10.1038/s41598-019-56307-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foerster MH, Esser J, Laqua H. Silicone oil and its influence on electrophysiologic findings. Am J Ophthalmol. 1985;99(2):201–206. PMID: 3970125. doi: 10.1016/0002-9394(85)90233-8 [DOI] [PubMed] [Google Scholar]
- 13.Azarmina M, Soheilian M, Azarmina H, Hosseini B. Electroretinogram changes following silicone oil removal. J Ophthalmic Vis Res. 2011;6(2):109–113. PMID: 22454719 PMCID: PMC3306082. [PMC free article] [PubMed] [Google Scholar]
- 14.Miyake Y, Horiguchi M. Electroretinographic alterations during vitrectomy in human eyes. Graefes Arch Clin Exp Ophthalmol. 1998;236(1):13–17. PMID:9457511. doi: 10.1007/s004170050036 [DOI] [PubMed] [Google Scholar]
- 15.Ozaki K, Yoshikawa Y, Ishikawa S, et al. Electroretinograms recorded with skin electrodes in silicone oil-filled eyes. PLoS One. 2019;14(5):e0216823. PMID: 31150414. doi: 10.1371/journal.pone.0216823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moussa G, Samia-Aly E, Ch’ng SW, et al. Effect of demographics and ethnicity on laser retinopexy in preventing retinal detachment in a tertiary eye hospital in 812 eyes. Acta Ophthalmol. 2022;100(1):96–102. doi: 10.1111/aos.14899 [DOI] [PubMed] [Google Scholar]
- 17.Meredith TA, Lindsey DT, Edelhauser HF, Goldman AI. Electroretinographic studies following vitrectomy and intraocular silicone oil injection. Br J Ophthalmol. 1985;69(4):254–260. PMID:3994940. doi: 10.1136/bjo.69.4.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexandridis E, Daniel H. Results of silicone oil injection into the vitreous. Dev Ophthalmol. 1981;2:24–27. PMID; 7262406. [DOI] [PubMed] [Google Scholar]
- 19.Doslak MJ. A theoretical study of the effect of silicone oil on the electroretinogram. Invest Ophthalmol Vis Sci. 1988;29(12):1881–1884. PMID: 3192378. [PubMed] [Google Scholar]
- 20.Thaler A, Lessel MR, Gnad H, Heilig P. The influence of intravitreously injected silicone oil on electrophysiological potentials of the eye. Doc Ophthalmol. 1986;62:41–46. doi: 10.1007/BF00140545 [DOI] [PubMed] [Google Scholar]
- 21.Lee PF, Donovan RH, Mukai N, Schepens CL, Freeman HM. Intravitreal injection of silicone: an experimental study. Ann Ophthalmol. 1969;1:15–25. [PubMed] [Google Scholar]
- 22.Mukai N, Lee PF, Oguri M, Schepens CL. A long-term evaluation of silicone retinopathy in monkeys. Can J Ophthalmol. 1975;10:391–402. [PubMed] [Google Scholar]
- 23.Frumar KD, Gregor ZJ, Carter RM, Arden GB. Electroretinographic changes after vitrectomy and intraocular tamponade. Retina. 1985;5(1):16–21. PMID: 4001584. doi: 10.1097/00006982-198500510-00004 [DOI] [PubMed] [Google Scholar]
- 24.Armaly MF. Ocular tolerance to silicones. I. Replacement of aqueous and vitreous by silicone fluids. Arch Ophthalmol. 1962;68:390–395. PMID: 13862326. doi: 10.1001/archopht.1962.00960030394013 [DOI] [PubMed] [Google Scholar]
- 25.Gabel VP, Kampik A, Burkhardt J. Analysis of intraocularly applied silicone oils of various origins. Graefes Arch Clin Exp Ophthalmol. 1987;225(3):160–162. PMID: 3609755. doi: 10.1007/BF02175441 [DOI] [PubMed] [Google Scholar]