Abstract
Arthrogenic Muscle Inhibition (AMI) is a phenomenon observed in individuals with joint injury or pathology, characterized by a reflexive inhibition of surrounding musculature, altered neuromuscular control, and compromised functional performance. After anterior cruciate ligament reconstruction (ACLR) one of the most obvious consequences of AMI is the lack of quadriceps activation and strength. Understanding the underlying mechanisms of AMI is crucial for developing effective therapeutic interventions. The surgical procedure needed to reconstruct the ACL has biochemical et physiological consequences such as inflammation, pain, and altered proprioception. These alterations contribute to the development of AMI. Therapeutic interventions aimed at addressing AMI encompass a multidimensional approach targeting pain reduction, inflammation management, proprioceptive training, and quadriceps activation. Early management focusing on pain modulation through modalities like ice, compression, and pharmacological agents help mitigate the inflammatory response and alleviate pain, thereby reducing the reflexive inhibition of quadriceps.
Quadriceps activation techniques such as neuromuscular electrical stimulation (NMES) and biofeedback training aid in overcoming muscle inhibition and restoring muscle strength. NMES elicits muscle contractions through electrical stimulation, bypassing the inhibitory mechanisms associated with AMI, thus facilitating muscle activation and strength gains.
Comprehensive rehabilitation programs tailored to individual needs and stage of recovery are essential for optimizing outcomes in AMI.
The objective of this clinical viewpoint is to delineate the significance of adopting a multimodal approach for the effective management of AMI, emphasizing the integration of pain modulation, proprioceptive training, muscle activation techniques, and manual therapy interventions. Highlighting the critical role of early intervention and targeted rehabilitation programs, this article aims to underscore their importance in restoring optimal function and mitigating long-term complications associated with AMI.
Keywords: arthrogenic muscle inhibition, anterior cruciate ligament reconstruction, early management, quadriceps activation
INTRODUCTION
Anterior cruciate ligament (ACL) injury is a common sports injury with an incidence rate of up to 250 000 person-years.1 The risk of ACL injuries is significant among adolescent athletes, with approximately 1 injury per 10,000 exposures in female athletes, who are 1.5 times more likely than male athletes to suffer an ACL injury. A multisport female athlete has an approximately 10% risk of ACL injuries during her high school career. Sports such as soccer, basketball, lacrosse, and football pose a particular risk of ACL injuries in adolescents, highlighting the need for targeted interventions to optimize muscle strength and activation.2 ACL injury has long-terms consequences, reducing quality of life and increasing the risk of developing early knee osteoarthritis by sevenfold .3 The most common treatment to ACL injury is a surgical reconstruction of the ligament (ACLR), with nearly 130 000 patients operated on each year in the United States.4 There is evidence that only 55% of patients return to their previous level of sports performance after ACLR.5 Alteration in neuromuscular function is one hypothesis to explain this.6 Evidence shows that quadriceps strength deficit persist for up to two years after ACLR.7 After ACL rupture, there is knee joint effusion and therefore distension which is known to increase the recruitment of type II and type Ib afferent fibers, which have an inhibitory effect on the motoneuron.8 Pain, through the activation of type III and IV nociceptors is also known to inhibit the ability to contract the quadriceps.9 This interruption of afferent input leads to reflexive inhibition at the spinal level, known as arthrogenic muscle inhibition (AMI). AMI reduces both the availability of motor neuron (motor neuron pool)10 and the ability to voluntarily activation motor neurons to a normal extent (central activation failure). This motoneuron pool inhibition is seen at the spinal level. Although AMI typically occurs after ACL rupture, and persists for weeks after surgery, central activation failure has been shown to resolve by 6 months post-surgery and can delay effective progression of rehabilitation.10,11 Persisted AMI may cause strength deficits and, in combination with change in sensorimotor input due to the ligament rupture, causes neuroplastic changes within the motor cortex.12 Several studies show a reduced corticospinal excitability of the quadriceps in the long term after ACLR and return to sport (RTS).11,13 AMI is also thought to initiate quadriceps motor unit catabolism contributing to long term muscle atrophy and strength deficits.13 Quadriceps strength deficits after ACLR are impactful and it is associated with changes in locomotion biomechanics that may initiate the onset of osteoarthritis14,15 Failure to achieve a good level of quadriceps strength symmetry may increase the risk of re-injury.16 AMI has a potential impact on the long-term reduction in quality of life, function, and return to performance rate. Therefore, it is essential to develop an evidence-based treatment strategy for our patients. Our aim is to provide a clinical perspective based on the literature to review evidence regarding therapeutic intervention for AMI after ACLR.
ASSESSMENT AND CLINICAL SIGNS
Quadriceps levels of activation
The AMI phenomenon is often misunderstood leading to delays or difficulties in rehabilitation and recurrent injuries when returning to sport. Many physiotherapists believe that reaching full active knee extension or quadricipital contraction with superior patellar glide will put an end to the AMI and allow for earlier and more intensive muscle strengthening. This is a common misconception that can lead to knee pain, swelling, and inflammation if the load and modalities of strengthening are not appropriate. Scales that allow clinicians to quantify AMI and/or quadriceps contractility (AMI classification and Rachet Scale, respectively) exist. They suggest a classification to guide therapeutic intervention. A grade 0 is defined as normal contraction, grade 1: inhibited contraction with no extension deficit, grade 2 : inhibited contraction with associated knee extension deficit due to hamstrings’ contracture and grade 3 : passive chronic extension deficit due to posterior capsular retraction .17,18 However, these scales are primarily based on clinical experience, and there is limited evidence regarding their validity and reliability. Another concern is that these scales are based on vastus medialis activation only and not on the activity being performed. Many patients can lock their knee in an unloaded state (Rachet Scale)17 or even perform a straight leg raise (SLR) without lag (AMI classification),18 indicating minimal quadriceps activation (figure 1).19–21 However, adding resistance to exercises may cause a new flexion contracture to appear highlighting inadequate quadriceps activation during the proposed movement.
Figure 1. Differentiate various level of quadriceps activity.
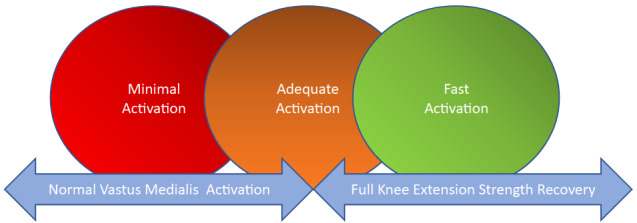
Image Credit: Inspired from Pr.Nicola Maffiuletti
To prevent this, clinicians must be able to distinguish between three levels of activation (figure 1):
Minimal activation (SLR without lag with ability to sense quadriceps contraction )
Adequate activation (full knee extension regardless of activity). It is related to patients’ ability to perform a highly qualitative quadriceps contraction (including patient feedback and sensation control) during specific movement seem to be key indicators in quadriceps activation and strengthening after ACLR
Fast activation which is related to rate of force development (RFD) is important in the later phase of rehabilitation, particularly during the return to plyometric activities.22–25
There is a linear interdependent relationship between the three levels of activation. Clinicians should be able to classify patients within these three levels to propose an appropriate treatment strategy, regarding the clinical signs, that aligns with their goal of returning to sports.
Clinical signs
AMI is a complex neurophysiological phenomenon but it is important to understand the patient’s perspective. Clinicians need to understand that a patient may regain full extension without necessarily having full quadriceps activation. The evaluation of muscle activation may be a particularly important milestone for detecting the presence of AMI, as the persistence of this problem may hinder the return to sport and increase the risk of recurrence.
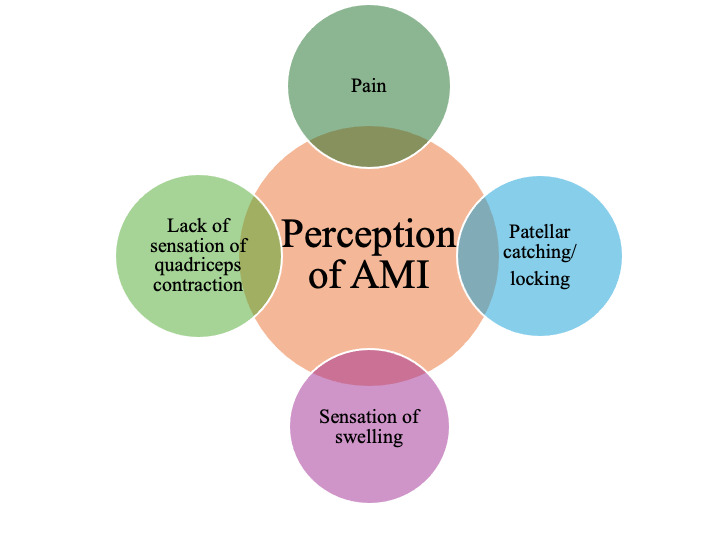
In the initial phase of rehabilitation, patients typically report experiencing pain spontaneously and during activity. Pain is commonly perceived on the medial and lateral aspects of the patella. Patients may not consciously perceive the contraction of the quadriceps and may omit it from their description. For instance, when performing exercises such as leg press/extensions or jumps, patients report experiencing painful sensations around the patella without any sensation of quadriceps engagement. Furthermore, patients frequently report experiencing patellar catching and locking, which may indicate impaired knee function (Figure 2).
Figure 2. Patient perception of AMI.
REHABILITATION INTERVENTIONS (Table 1)
Table 1. Type of intervention according to target structure.
| Interventions | Number of sessions | Sets and repetitions |
|---|---|---|
| Muscle intervention | ||
| BFR training | Minimum 2-3 times per week for 8 weeks | 4 sets of 30-15-15-15 reps of quadriceps target exercises at 30% of predicted 1RM with BFR at 40-80% when muscle exhaustion is achieved |
| Hamstring fatigue exercise | 1 time | 5-minute warm-up exercise of between 70 and 80 RPM + tempo squat until participant RPE was 15 out of 20 |
| Brain intervention | ||
| Virtual reality | 1 time per week for 3 weeks | 15 minutes of bilateral and unilateral knee flexion and extension, bilateral and unilateral quadriceps contraction, bipodal walk |
| Cross-education therapy | 3 times per week for 8-24 weeks | 3 sets of 12 repetitions at 60°/s ranging from 10° to 90° flexion |
| EMG Biofeedback with exercises | every day during the first week post ACLR | 3 sets of 10 repetitions of isometric quadriceps contraction, short arc quadriceps, heel slides, long arc quadriceps |
| Motor imagery | 8-12 times for 6-8 weeks | 5 seconds imagining and 5 seconds rest for 10-15 minutes of visualization of muscle contraction |
| Vibration | 2-3 times per week for 1-10 weeks | 10 minutes to 1 hour |
| Nerve intervention | ||
| NMES | 2-5 times per week for 3-6 weeks. Intensity is aim for as max tolerance at least 100mA (200 energy) | 60 minutes (5-minute warm-up, 50-minute stimulation session and 5-minute cool down) |
| TENS | 2-5 times per week for 3-6 weeks | 20 minutes |
| Cryotherapy | 3-4 time per week for 1-2 weeks | 20-30 minutes |
| Percutaneous electrical stimulation | 1 time | 3 sets of 6 seconds isometric contraction with electrical stimulation |
Note: BFR; blood flow restriction, NMES; neuromuscular electrical stimulation, TENS; transcutaneous electrical stimulation, RPM; revolutions per minute, RPE; rating of perceived exertion
Targeting the muscle system
Various modalities may be considered in the treatment of AMI. Sonnery-Cottet et al. conducted a comprehensive analysis of studies highlighting the effectiveness of quadriceps-based interventions for AMI. Their review encompassed multiple studies, all of which underline significant improvements in quadriceps activation attributed to exercise therapy.26 The efficacy of various modalities, such as blood flow restriction (BFR) training has been shown to have superiority addressing muscle activity.27 BFR may be relevant in the early rehabilitation stage after surgery to prevent loss of muscle mass, strength, and function with lower load on the knee , helping to reduce the consequences of AMI.28,29 Reciprocal inhibition has also been studied, using hamstring fatigue to reduce quadriceps inhibition has shown some promising results, although based on limited quality evidence.30–32
Targeting the central nervous system
It is hypothesized that AMI induces changes in the motor cortex function. Various interventions have been tested with the objective of addressing these changes. For example, training the non-involved limb has been theorized to increase bilateral motor cortices (cross-education therapy (CET)) .30Investigations into CET have been conducted but yielded conflicting findings regarding its impact on quadriceps isometric strength.33–35 Motor imagery (MI) is another intervention that has been shown to increase excitability and activity of motor neuron within the motor cortex and the spinal cord through mental practice in healthy individual. Although, it is of great interest it has yet to be tested in individuals after ACLR .30
Virtual reality (VR) may create changes in cortical circuits.36 These adaptations can be produced by different VR systems. The simplest of these systems provide sensory feedback, while more immersive systems facilitate learning by placing patients in a motivating environment or by allowing them to learn based on the observation of reality, action, and/or imitation in a modified environment. The primary outcome is an improvement in therapeutic adherence. However, it is uncertain whether there is a significant improvement in the excitability of the motor cortex function, which would reestablish cortical drive to quadriceps motor neurons.30 EMG biofeedback is another modality that has gained increasingly popularity to address motor cortex dysfunction. Real-time biofeedback may help patient better voluntarily recruit specific muscle groups. Visual or auditory guidance are two components found in the literature used to help patient perceive muscle activation during exercise. This may be useful in lack of quadriceps activation due to AMI.37 Also when combined with conventional exercise biofeedback has been shown to be more effective in improving early quadriceps muscle activation than conventional exercise alone.38 Therefore, it could be useful in restoring quadriceps activation in the early stages of rehabilitation. It is also postulated that vibration may be beneficial in the treatment of deficiencies associated with AMI.. There is evidence that vibration is a strong stimulus to modulate the central nervous system through the activation of Ia afferents fibers that project on both the spinal cord and supra-spinal structures. When local vibration is acutely applied, a decrease in force-generation capacities has been reported, principally triggered by adaptations within the central nervous system. Thus, local vibration stimuli have the potential to act as a significant neuromuscular workload by inducing some fatigue which, when repeated, could enhancing voluntary recruitment and long-term neural adaptations leading to improved muscle function.30 Indeed, Souron et al shows that local vibration training can increase motor performance through a wide range of training and vibration parameters. While there seems to be a consensus about the neural nature of the induced adaptations, the precise mechanisms of these adaptations at both the spinal and supra-spinal levels remain to be clarified .39 Sonnery-Cottet et al revealed that vibration has low-quality evidence for efficacy to treat AMI.26 A randomized controlled trial looked at the effectiveness of whole-body vibration and the results are consistent with this evidence. Another randomized controlled trial was conducted to assess the efficacy of local vibration. Local vibration allows to allocate localized stimulus to one or a group of muscles in which vibration is believed to modify the excitability, associated with muscle contraction. Despite the lack of statistical power, the results were found to be promising for increased muscle strength, countermovement jump and sprint which is encouraging.40–42
Targeting the peripheral nervous system
Some of the modalities we have already mentioned may also be included in this section. Transcutaneous electrical nerve stimulation (TENS) and neuromuscular electrical stimulation (NEMS) are two modalities that have been studied to determine if they have any effect on AMI. It is theorized that sensory TENS aims to stimulate large-diameter sensory nerves and fibers (Aβ), which results in the presynaptic inhibition of pain signals to the transmission cell (T-cell), and thereby reduce the number of sensory pain signals being transmitted to the brain. Further, excitatory afferent stimuli created by sensory TENS may override the inhibitory signals, which can increase the excitability of the quadriceps motor neuron pool. Previous evidence demonstrates that TENS plays a significant role in managing joint pain.43 When applied before or during exercises, the decreased inhibition creates a window where potential for neuron motor excitability and motor unit is temporarily restored. NMES is a clinical modality that has the potential to prevent quadriceps activation failure and associated muscle atrophy. It initiates action potentials in intramuscular nerve branches, resulting in an unvoluntary contraction of the muscle. NMES exogenously stimulates the muscle, large diameter, type II muscle fibers which are thought to be selectively recruited, resulting in a greater potential for muscle force production.11 There is some evidence to support the efficacy of NMES and TENS for AMI following ACLR.26,35,44 Published by Michael J Toth & al,45 the first study addressing the usefulness of NMES showing a significant impact on muscle contractility. The second explored the effect of stimulation of the common peroneal nerve. This intervention shows a significant impact on reducing inhibition and increasing knee extension.45,46 Findings indicate that cryotherapy also demonstrates a degree of effectiveness in treating AMI.47–50 The application of cryotherapy to the knee joint have been shown to increases EMG activity and isometric strength in the quadriceps immediately after cryotherapy.51,52 Furthermore, compressive cryotherapy has been shown to have a small effect on swelling, pain and medication reduction compared to cryotherapy alone in reduce medical use in the first three days post ACLR.35 Cooling may also reduce the effect of AMI by altering sensory input from receptors in the skin or by reducing presence of pain.10
Conclusion
The management of AMI after ACLR is multifaceted and requires a comprehensive understanding of the various levels of activation, clinical signs, and potential modalities for interventions targeting muscles, brain, and nerves. It is crucial to recognize the complexity of AMI to avoid misconceptions and ensure appropriate rehabilitation strategies.
It is of paramount importance to evaluate quadriceps activation at different levels, including minimal activation, adequate activation, and rapid activation, to tailor rehabilitation programs to the individual patient’s needs and goals, through a shared decision process related to return to sports activities. It is of significant consequence to address patients’ complaints of pain, sensation of patellar catching, and impaired knee function to manage AMI effectively and facilitate successful rehabilitation outcomes.
To resolve any early signs of AMI we propose several muscle-based treatment modalities and AMI targeted interventions, including BFR, CE therapy, hamstring fatigue, and cryotherapy, that have demonstrated promising results in the treatment of AMI. However, further research is required to establish their efficacy conclusively. Similarly, cortical interventions, including virtual reality and transcranial magnetic stimulation, have demonstrated potential in modulating cortico-spinal circuits and improving therapeutic adherence. NEMS, TENS, and vibration therapy also seem promising in addressing AMI, and warrant further investigation in order to fully appreciate their effectiveness.
Overall, a comprehensive approach integrating multiple modality interventions targeting muscles, brain, and nerves may offer the most effective management of AMI following ACLR. Future research should focus on elucidating the mechanisms underlying AMI and evaluating the long-term effectiveness of various already existing interventions to optimize functional outcomes and improve patients’ quality of life and return-to-performance rates and delays.
References
- Non-contact Anterior Cruciate Ligament Injury Epidemiology in Team-Ball Sports: A Systematic Review with Meta-analysis by Sex, Age, Sport, Participation Level, and Exposure Type. Chia L., De Oliveira Silva D., Whalan M.., et al. 2022Sports Med. 52(10):2447–2467. doi: 10.1007/s40279-022-01697-w. doi: 10.1007/s40279-022-01697-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anterior Cruciate Ligament Injury Incidence in Adolescent Athletes: A Systematic Review and Meta-analysis. Bram J. T., Magee L. C., Mehta N. N., Patel N. M., Ganley T. J. 2021Am J Sports Med. 49(7):1962–1972. doi: 10.1177/0363546520959619. [DOI] [PubMed] [Google Scholar]
- Anterior Cruciate Ligament Injury and Knee Osteoarthritis: An Umbrella Systematic Review and Meta-analysis. Webster K. E., Hewett T. E. 2022Clin J Sport Med. 32(2):145–152. doi: 10.1097/JSM.0000000000000894. [DOI] [PubMed] [Google Scholar]
- Incidence and Trends of Anterior Cruciate Ligament Reconstruction in the United States. Mall N.A., Chalmers P.N., Moric M.., et al. 2014Am J Sports Med. 42(10):2363–2370. doi: 10.1177/0363546514542796. doi: 10.1177/0363546514542796. [DOI] [PubMed] [Google Scholar]
- Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Ardern C. L., Taylor N. F., Feller J. A., Webster K. E. 2014Br J Sports Med. 48(21):1543–1552. doi: 10.1136/bjsports-2013-093398. doi: 10.1136/bjsports-2013-093398. [DOI] [PubMed] [Google Scholar]
- Neuromuscular Function of the Knee Joint Following Knee Injuries: Does It Ever Get Back to Normal? A Systematic Review with Meta-Analyses. Tayfur B., Charuphongsa C., Morrissey D., Miller S.C. 2021Sports Med. 51(2):321–338. doi: 10.1007/s40279-020-01386-6. doi: 10.1007/s40279-020-01386-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Are Muscle Strength and Function of the Uninjured Lower Limb Weakened After Anterior Cruciate Ligament Injury?: Two-Year Follow-up After Reconstruction. Chung K. S., Ha J. K., Yeom C. H.., et al. 2015Am J Sports Med. 43(12):3013–3021. doi: 10.1177/0363546515606126. [DOI] [PubMed] [Google Scholar]
- Reflex actions of knee joint afferents during contraction of the human quadriceps. Iles J. F., Stokes M., Young A. 1990Clin Physiol. 10(5):489–500. doi: 10.1111/j.1475-097X.1990.tb00828.x. [DOI] [PubMed] [Google Scholar]
- Pain and Effusion and Quadriceps Activation and Strength. Palmieri-Smith R.M., Villwock M., Downie B., Hecht G., Zernicke R. 2013J Athl Train. 48(2):186–191. doi: 10.4085/1062-6050-48.2.10. doi: 10.4085/1062-6050-48.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthrogenic Muscle Inhibition: Best Evidence, Mechanisms, and Theory for Treating the Unseen in Clinical Rehabilitation. Norte G., Rush J., Sherman D. 2022J Sport Rehabil. 31(6):717–735. doi: 10.1123/jsr.2021-0139. [DOI] [PubMed] [Google Scholar]
- Combination of eccentric exercise and neuromuscular electrical stimulation to improve quadriceps function post-ACL reconstruction. Lepley L. K., Wojtys E. M., Palmieri-Smith R. M. 2015The Knee. 22(3):270–277. doi: 10.1016/j.knee.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanisms of Arthrogenic Muscle Inhibition. Lepley A.S., Lepley L.K. 2022J Sport Rehabil. 31(6):707–716. doi: 10.1123/jsr.2020-0479. doi: 10.1123/jsr.2020-0479. [DOI] [PubMed] [Google Scholar]
- Neural drive and motor unit characteristics after anterior cruciate ligament reconstruction: implications for quadriceps weakness. Sherman D. A., Rush J., Stock M. S., Ingersoll D., Norte E. G. 2023PeerJ. 11:e16261. doi: 10.7717/peerj.16261. doi: 10.7717/peerj.16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Lewek M., Rudolph K., Axe M., Snyder-Mackler L. 2002Clin Biomech. 17(1):56–63. doi: 10.1016/S0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. Pietrosimone B., Loeser R.F., Blackburn J.T.., et al. 2017J Orthop Res. 35(10):2288–2297. doi: 10.1002/jor.23534. doi: 10.1002/jor.23534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Grindem H., Snyder-Mackler L., Moksnes H., Engebretsen L., Risberg M. A. 2016Br J Sports Med. 50(13):804–808. doi: 10.1136/bjsports-2016-096031. doi: 10.1136/bjsports-2016-096031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rééducation postopératoire des greffes du ligament croisé antérieur. Quelard B., Rachet O., Sonnery-Cottet B., Chambat P. 2010EMC - Kinésithérapie - Médecine Phys - Réadapt. 6(3):1–16. doi: 10.1016/S1283-0887(10)43946-8. [DOI] [Google Scholar]
- Arthrogenic Muscle Inhibition Following Knee Injury or Surgery: Pathophysiology, Classification, and Treatment. Sonnery-Cottet B., Hopper G. P., Gousopoulos L.., et al. 2022Video J Sports Med. 2(3):263502542210862. doi: 10.1177/26350254221086295. doi: 10.1177/26350254221086295. [DOI] [Google Scholar]
- Altered Quadriceps Control in People with Anterior Cruciate Ligament Deficiency. Williams G.N., Barrance P.J., Snyder-Mackler L., Buchanan T.S. 2004Med Sci Sports Exerc. 36(7):1089–1097. doi: 10.1249/01.MSS.0000131959.20666.11. [DOI] [PubMed] [Google Scholar]
- CLINICAL MUSCULAR EVALUATION IN PATELLOFEMORAL PAIN SYNDROME. Lobo Júnior P., Barbosa Neto I. A., Borges J. H. D. S., Tobias R. F., Boitrago M. V. D. S., Oliveira M. D. P. 2018Acta Ortopédica Bras. 26(2):91–93. doi: 10.1590/1413-785220182602187215. doi: 10.1590/1413-785220182602187215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadriceps Neuromuscular Function and Jump-Landing Sagittal-Plane Knee Biomechanics After Anterior Cruciate Ligament Reconstruction. Ward S. H., Blackburn J. T., Padua D. A.., et al. 2018J Athl Train. 53(2):135–143. doi: 10.4085/1062-6050-306-16. doi: 10.4085/1062-6050-306-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rate of Force Development as an Adjunctive Outcome Measure for Return-to-Sport Decisions After Anterior Cruciate Ligament Reconstruction. Angelozzi M., Madama M., Corsica C.., et al. 2012J Orthop Sports Phys Ther. 42(9):772–780. doi: 10.2519/jospt.2012.3780. doi: 10.2519/jospt.2012.3780. [DOI] [PubMed] [Google Scholar]
- Rate of Torque Development in the Quadriceps after Anterior Cruciate Ligament Reconstruction with Hamstring Tendon Autografts in Young Female Athletes. Suzuki M., Ishida T., Samukawa M.., et al. 2022Int J Environ Res Public Health. 19(18):11761. doi: 10.3390/ijerph191811761. doi: 10.3390/ijerph191811761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strength, rate of force development, power and reactive strength in adult male athletic populations post anterior cruciate ligament reconstruction - A systematic review and meta-analysis. Maestroni L., Read P., Turner A., Korakakis V., Papadopoulos K. 2021Phys Ther Sport. 47(24):91–104. doi: 10.1016/j.ptsp.2020.11.024. doi: 10.1016/j.ptsp.2020.11.024. [DOI] [PubMed] [Google Scholar]
- Quadriceps Neuromuscular and Physical Function After Anterior Cruciate Ligament Reconstruction. Hunnicutt J. L., McLeod M. M., Slone H. S., Gregory C. M. 2020J Athl Train. 55(3):238–245. doi: 10.4085/1062-6050-516-18. doi: 10.4085/1062-6050-516-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthrogenic muscle inhibition after ACL reconstruction: a scoping review of the efficacy of interventions. Sonnery-Cottet B., Saithna A., Quelard B.., et al. 2019Br J Sports Med. 53(5):289–298. doi: 10.1136/bjsports-2017-098401. doi: 10.1136/bjsports-2017-098401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Role of Blood Flow Restriction Therapy Following Knee Surgery: Expert Opinion. DePhillipo N. N., Kennedy M. I., Aman Z. S., Bernhardson A. S., O’Brien L. T., LaPrade R. F. 2018Arthrosc J Arthrosc Relat Surg. 34(8):2506–2510. doi: 10.1016/j.arthro.2018.05.038. doi: 10.1016/j.arthro.2018.05.038. [DOI] [PubMed] [Google Scholar]
- Blood Flow Restriction Training Applied With High-Intensity Exercise Does Not Improve Quadriceps Muscle Function After Anterior Cruciate Ligament Reconstruction: A Randomized Controlled Trial. Curran M. T., Bedi A., Mendias C. L., Wojtys E. M., Kujawa M. V., Palmieri-Smith R. M. 2020Am J Sports Med. 48(4):825–837. doi: 10.1177/0363546520904008. doi: 10.1177/0363546520904008. [DOI] [PubMed] [Google Scholar]
- Comparing the Effectiveness of Blood Flow Restriction and Traditional Heavy Load Resistance Training in the Post-Surgery Rehabilitation of Anterior Cruciate Ligament Reconstruction Patients: A UK National Health Service Randomised Controlled Trial. Hughes L., Rosenblatt B., Haddad F.., et al. 2019Sports Med Auckl NZ. 49(11):1787–1805. doi: 10.1007/s40279-019-01137-2. doi: 10.1007/s40279-019-01137-2. [DOI] [PubMed] [Google Scholar]
- Arthrogenic Muscle Inhibition: Best Evidence, Mechanisms, and Theory for Treating the Unseen in Clinical Rehabilitation. Norte G., Rush J., Sherman D. 2022J Sport Rehabil. 31(6):717–735. doi: 10.1123/jsr.2021-0139. doi: 10.1123/jsr.2021-0139. [DOI] [PubMed] [Google Scholar]
- The Use of Hamstring Fatigue to Reduce Quadriceps Inhibition After Anterior Cruciate Ligament Reconstruction. Lowe T., Dong X. N. 2018Percept Mot Skills. 125(1):81–92. doi: 10.1177/0031512517735744. [DOI] [PubMed] [Google Scholar]
- Single bout of vibration-induced hamstrings fatigue reduces quadriceps inhibition and coactivation of knee muscles after anterior cruciate ligament (ACL) reconstruction. Yu S., Lowe T., Griffin L., Dong X. N. 2020J Electromyogr Kinesiol Off J Int Soc Electrophysiol Kinesiol. 55:102464. doi: 10.1016/j.jelekin.2020.102464. doi: 10.1016/j.jelekin.2020.102464. [DOI] [PubMed] [Google Scholar]
- Cross-education improves quadriceps strength recovery after ACL reconstruction: a randomized controlled trial. Harput G., Ulusoy B., Yildiz T. I.., et al. 2019Knee Surg Sports Traumatol Arthrosc Off J ESSKA. 27(1):68–75. doi: 10.1007/s00167-018-5040-1. doi: 10.1007/s00167-018-5040-1. [DOI] [PubMed] [Google Scholar]
- Cross-education does not accelerate the rehabilitation of neuromuscular functions after ACL reconstruction: a randomized controlled clinical trial. Zult T., Gokeler A., van Raay J. J. A. M.., et al. 2018Eur J Appl Physiol. 118(8):1609–1623. doi: 10.1007/s00421-018-3892-1. doi: 10.1007/s00421-018-3892-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspetar clinical practice guideline on rehabilitation after anterior cruciate ligament reconstruction. Kotsifaki R., Korakakis V., King E.., et al. 2023Br J Sports Med. 57(9):500–514. doi: 10.1136/bjsports-2022-106158. doi: 10.1136/bjsports-2022-106158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehabilitation of Patients with Arthrogenic Muscular Inhibition in Pathologies of Knee Using Virtual Reality. Flórez Fonnegra J. P., Pino Prestan A. C., López L. L., Yepes J. C., Pérez V. Z. 2023Sensors. 23(22):9114. doi: 10.3390/s23229114. doi: 10.3390/s23229114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Use of electromyographic biofeedback in rehabilitation following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Ananías J., Vidal C., Ortiz-Muñoz L., Irarrázaval S., Besa P. 2024Physiotherapy. 123:19–29. doi: 10.1016/j.physio.2023.12.005. doi: 10.1016/j.physio.2023.12.005. [DOI] [PubMed] [Google Scholar]
- Early Activation of Quadriceps With Pressure Biofeedback for the Prevention of Arthrogenic Muscle Inhibition Following Lower Limb Orthopedic Surgeries: A Proof of Concept Clinical Trial. Achens J. T., Victor V. S. R., Joseph J. K. 2022J Chiropr Med. 21(4):296–304. doi: 10.1016/j.jcm.2022.05.005. doi: 10.1016/j.jcm.2022.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acute and chronic neuromuscular adaptations to local vibration training. Souron R., Besson T., Millet G. Y., Lapole T. 2017Eur J Appl Physiol. 117(10):1939–1964. doi: 10.1007/s00421-017-3688-8. [DOI] [PubMed] [Google Scholar]
- Local vibration training improves the recovery of quadriceps strength in early rehabilitation after anterior cruciate ligament reconstruction: A feasibility randomised controlled trial. Coulondre C., Souron R., Rambaud A.., et al. 2022Ann Phys Rehabil Med. 65(4):101441. doi: 10.1016/j.rehab.2020.08.005. doi: 10.1016/j.rehab.2020.08.005. [DOI] [PubMed] [Google Scholar]
- Whole-Body Vibration on Performance of Quadriceps After ACL Reconstruction: A Blinded Randomized Controlled Trial. Costa K. S. A. da, Borges D. T., Macedo L. de B., Lins C. A. de A., Brasileiro J. S. 2019J Sport Rehabil. 28(1):52–58. doi: 10.1123/jsr.2017-0063. doi: 10.1123/jsr.2017-0063. [DOI] [PubMed] [Google Scholar]
- Effects of focal vibration on changes in sports performance in amateur athletes: A randomized clinical trial. Canet-Vintró M., Rodríguez-Sanz J., López-de-Celis C., Campañá-Arnal E., Hidalgo-Garcia C., Pérez-Bellmunt A. Apr 10;2024 J Orthop Res Off Publ Orthop Res Soc. :42. doi: 10.1002/jor.25857. doi: 10.1002/jor.25857. [DOI] [PubMed]
- Effects of transcutaneous electrical nerve stimulation on quadriceps function in individuals with experimental knee pain. Son S.J., Kim H., Seeley M.K., Feland J.B., Hopkins J.T. 2016Scand J Med Sci Sports. 26(9):1080–1090. doi: 10.1111/sms.12539. doi: 10.1111/sms.12539. [DOI] [PubMed] [Google Scholar]
- A Comparison of Neuromuscular Electrical Stimulation Parameters for Postoperative Quadriceps Strength in Patients After Knee Surgery: A Systematic Review. Conley C. E. W., Mattacola C. G., Jochimsen K. N., Dressler E. V., Lattermann C., Howard J. S. 2021Sports Health. 13(2):116–127. doi: 10.1177/1941738120964817. doi: 10.1177/1941738120964817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utility of Neuromuscular Electrical Stimulation to Preserve Quadriceps Muscle Fiber Size and Contractility After Anterior Cruciate Ligament Injuries and Reconstruction: A Randomized, Sham-Controlled, Blinded Trial. Toth M. J., Tourville T. W., Voigt T. B.., et al. 2020Am J Sports Med. 48(10):2429–2437. doi: 10.1177/0363546520933622. doi: 10.1177/0363546520933622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immediate effect of common peroneal nerve electrical stimulation on quadriceps muscle arthrogenic inhibition in patients with knee osteoarthritis. Rafsanjani H., Khademi-Kalantari K., Rezasoltani A., Naimi S. S., Ghasemi M., Jaberzadeh S. 2017J Bodyw Mov Ther. 21(4):879–883. doi: 10.1016/j.jbmt.2017.03.003. doi: 10.1016/j.jbmt.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Cryotherapy and Transcutaneous Electric Neuromuscular Stimulation Decrease Arthrogenic Muscle Inhibition of the Vastus Medialis After Knee Joint Effusion. Hopkins J., Ingersoll C. D., Edwards J., Klootwyk T. E. 2002J Athl Train. 37(1):25–31. [PMC free article] [PubMed] [Google Scholar]
- Effects of cryotherapy on arthrogenic muscle inhibition using an experimental model of knee swelling. doi: 10.1002/art.24168. doi: 10.1002/art.24168. [DOI] [PubMed]
- Quadriceps Muscle Function After Rehabilitation With Cryotherapy in Patients With Anterior Cruciate Ligament Reconstruction. Hart J. M., Kuenze C. M., Diduch D. R., Ingersoll C. D. 2014J Athl Train. 49(6):733–739. doi: 10.4085/1062-6050-49.3.39. doi: 10.4085/1062-6050-49.3.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impact of quadriceps strengthening on response to fatiguing exercise following ACL reconstruction. Kuenze C., Eltoukhy M., Kelly A., Kim C.Y. 2017J Sci Med Sport. 20(1):6–11. doi: 10.1016/j.jsams.2016.04.015. doi: 10.1016/j.jsams.2016.04.015. [DOI] [PubMed] [Google Scholar]
- The effects of cryotherapy on quadriceps electromyographic activity and isometric strength in patient in the early phases following knee surgery. Loro W. A., Thelen M. D., Rosenthal M. D., Stoneman P. D., Ross M. D. 2019J Orthop Surg Hong Kong. 27(1):2309499019831454. doi: 10.1177/2309499019831454. doi: 10.1177/2309499019831454. [DOI] [PubMed] [Google Scholar]
- The past and future of peri-operative interventions to reduce arthrogenic quadriceps muscle inhibition after total knee arthroplasty: A narrative review. Churchill L., Bade John M., Koonce R.C., Stevens-Lapsley J.E., Bandholm T. 2024Osteoarthr Cartil Open. 6(1):100429. doi: 10.1016/j.ocarto.2023.100429. doi: 10.1016/j.ocarto.2023.100429. [DOI] [PMC free article] [PubMed] [Google Scholar]


