Abstract
Background
Small-cell lung cancer (SCLC) is a leading cause of cancer-related death. However, the prognostic value of the tumor shrinkage rate (TSR) after chemotherapy for SCLC is still unknown.
Methods
We performed a retrospective analysis of 235 patients with SCLC. The TSR cutoff was determined based on receiver-operating characteristic curve analysis. The associations of TSR with progression-free survival (PFS) and overall survival (OS) were assessed using univariate and multivariate Cox proportional hazards models. Survival curves were obtained by the Kaplan–Meier method and compared using the log-rank test. Recurrence patterns after first-line treatment were summarized in a pie chart. A nomogram was constructed to validate the predictive role of the TSR in SCLC.
Results
The TSR cutoff was identified to be − 6.6%. Median PFS and OS were longer in the group with a TSR < –6.6% than in the group with a TSR ≥ − 6.6%. PFS and OS were also longer in patients with extensive SCLC when the TSR was < − 6.6% than when it was > − 6.6%. Brain metastasis-free survival was better in the group with a TSR < − 6.6%. There was a significant positive correlation between TSR and PFS. Furthermore, univariate and multivariate regression analyses showed that the TSR, patient age, and previous radiotherapy were independent prognostic factors for OS while TSR and M stage were independent prognostic factors for PFS.
Conclusions
The TSR may prove to be a good indicator of OS and PFS in patients receiving chemotherapy-based first-line treatment for SCLC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-024-02001-4.
Keywords: Small-cell lung cancer, Tumor shrinkage rate, Chemotherapy, Prognosis, Radiomics
Background
Lung cancer is the second most common type of cancer and one of the leading causes of cancer-related mortality worldwide, with an annual death toll of 1.8 million [1, 2]. Histologically, about 15–20% of cases are small-cell lung cancer (SCLC) [3]. SCLC is a highly aggressive primary tumor and one of the most lethal human cancers, causing about 250,000 deaths each year [4]. According to the Veterans Administration Lung Cancer Study Group, SCLC can be classified as limited-disease small-cell lung cancer (LD-SCLC) or extensive-disease small-cell lung cancer (ED-SCLC). Approximately 70% of cases of SCLC are ED-SCLC at the time of diagnosis, with a median survival time of 7–12 months. Furthermore, the 5-year overall survival (OS) rate has been reported to be less than 2% for patients with ED-SCLC [4] and 12–17% for those with LD-SCLC, with a median survival of 18–23 months [5].
Tumor size is a key indicator of the effectiveness of therapy. Reduction in tumor size and the sum of the longest diameter (SLD) are the main targets of cytotoxic anticancer drugs and are considered prerequisites for clinical benefit from chemotherapy [6]. Hence, decreasing tumor size and the SLD of all target lesions are listed among the essential criteria for assessing the effectiveness of therapy in the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines developed by the World Health Organization. Based on RECIST, the objective response rate (ORR) is proposed as the standard for assessing the response of solid tumors to anticancer therapy. However, the ORR has limitations in terms of assessing changes in dynamic tumor burden. In the first-line trials carried out in colorectal cancer (CRC), Tange et al. [7] and Buyse et al. [8] found that the ORR could not be validated as an accurate surrogate of OS. When used to assess the clinical outcomes of anticancer therapy, the World Health Organization and RECIST guidelines for calculating the ORR have low efficiency [9, 10]. Furthermore, there are no reports of a correlation of increased ORR with improved progression-free survival (PFS) or OS [11]. Therefore, new parameters with improved precision for reflecting the efficacy of anticancer treatment are needed. Novel response parameters, including early tumor shrinkage (ETS) [12–17] and tumor shrinkage rate (TSR) [10, 18, 19], have recently been investigated in the treatment of some cancers. Both the ETS and TSR reflect the percentage change in tumor size at a defined time point in relation to baseline. ETS has been reported to be associated with survival time in some patients treated with anticancer regimens [12–20]. However, some patients have not shown ETS, which has hindered its further use [21]. Recently, increased a TSR was observed to correlate significantly with post-progression survival and OS in patients with CRC in large Phase III trials [22, 23]. Almansour et al. identified a reduction in tumor size of at least 10% after treatment to be one of the indicators of improved OS in patients with advanced malignant melanoma [24], and He et al. reported that tumor shrinkage of more than 8.32% could predict the long-term outcome in patients with non-small-cell lung cancer (NSCLC) receiving targeted therapies [25]. These findings suggest that indicators representing the percentage change in tumor size, such as the TSR, may be good parameters for reflecting the efficacy of anticancer regimens. Therefore, we hypothesized that the TSR may be a useful parameter for predicting survival in patients with SCLC receiving chemotherapy.
The purpose of this study was to investigate the clinical value of the TSR in prediction of the long-term outcome of chemotherapy in patients with SCLC. We also sought to use the TSR as a surrogate marker and identify the optimal cutoff value that dichotomizes patients with SCLC in the hope of designing more individualized treatment plans.
Methods
Clinical data and eligibility criteria
The study was supported by the First Affiliated Hospital of Wenzhou Medical University (Wenzhou, China). A total of 688 patients identified in the hospital’s electronic medical records system to have been diagnosed with SCLC between January 1, 2006 and December 31, 2016 were initially enrolled in the study.
All patients were required to meet the following criteria: a pathologically confirmed diagnosis; an Eastern Cooperative Oncology group (ECOG) score of 0–3; systemic treatment received before enrollment; imaging assessment within 2 weeks before treatment and after at least two cycles of systemic therapy for up to 4 months; lesions evaluable on two images; and a diagnosis of locally advanced SCLC or advanced unresectable SCLC.
The following exclusion criteria were applied: pathological findings suggesting a mixed cancer, such as small-cell mixed squamous cell carcinoma; missing prognostic data; no history of treatment for SCLC; a history of surgery; and lesions not assessable by imaging. Finally, 235 patients with SCLC were included in the study. The study was approved by our institutional ethics committee (permit number: 2018140). All procedures were performed in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki. Informed consent was obtained from all study participants.
Study design
The TSR was calculated for each patient using the following formula:
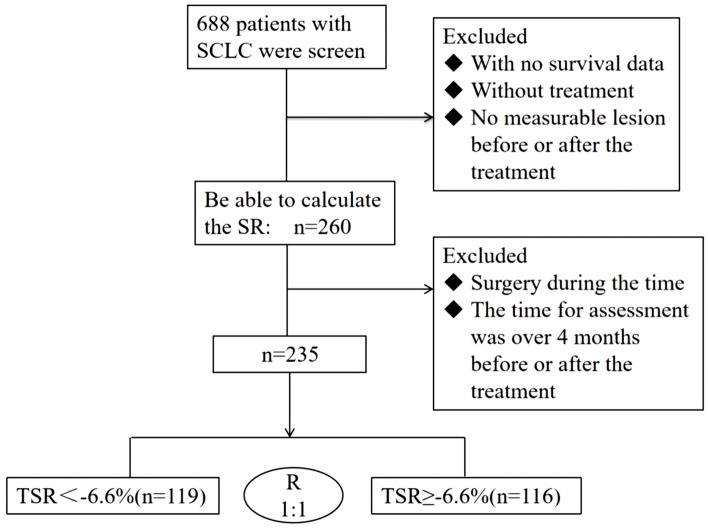
TSR = (tumor burden after treatment – tumor burden before treatment)/(evaluation time after treatment – evaluation time before treatment) × 100%. According to the receiver-operating characteristic (ROC) curve analysis, the TSR value with the highest sensitivity and specificity was –6.6%. Therefore, this value was used as the cutoff. The eligible patients were divided into a TSR < –6.6% (responder) group (n = 119) and a TSR ≥ –6.6% (non-responder) group (n = 116) (Fig. 2). Except for response to initial chemotherapy and history of radiotherapy, there was no significant between-group difference in baseline characteristics (Table 1).
Fig. 2.
Flowchart showing the patient selection process. R, ratio; SCLC, small-cell lung cancer; SR: shrinkage rate; TSR: tumor shrinkage rate
Table 1.
Patient characteristics
| Characteristic | TSR < − 6.6% | TSR ≥ − 6.6% | P value |
|---|---|---|---|
| No. (%) | No. (%) | ||
| (n = 119) | (n = 116) | ||
| Age, years | 64 (27–82) | 63 (31–85) | 0.149 |
| ≥ 70 | 27 (23%) | 36 (31%) | |
| < 70 | 92 (77%) | 80 (69%) | |
| Sex | 0.318 | ||
| Male | 104 (87%) | 96 (83%) | |
| Female | 15 (13%) | 20 (17%) | |
| ECOG score | 0.228 | ||
| 0–1 | 111 (93%) | 103 (89%) | |
| ≥ 2 | 8 (7%) | 13 (11%) | |
| Smoking status | 0.910 | ||
| ≥ 20 pack/year | 89 (75%) | 84 (72%) | |
| < 20 pack/year | 27 (23%) | 31 (27%) | |
| Missing | 3 (2%) | 1 (1%) | |
| CNS metastasis at baseline | 12 (10%) | 8 (7%) | 0.381 |
| Body mass index (BMI) | 0.891 | ||
| ≥ 24 | 37 (31%) | 37 (32%) | |
| 18.5–23.9 | 69 (58%) | 65 (56%) | |
| < 18.5 | 9 (8%) | 9 (8%) | |
| Missing | 4 (3%) | 5 (4%) | |
| Stage at initial diagnosis | 0.858 | ||
| ED | 65 (55%) | 63 (54%) | |
| LD | 54 (45%) | 54 (46%) | |
| Response to initial chemotherapy | < 0.001* | ||
| CR | 8 (7%) | 0 (0) | |
| PR | 106 (89%) | 68 (59) | |
| SD | 4 (3%) | 25 (21) | |
| PD | 1 (1%) | 23 (20) | |
| Regimen of initial chemotherapy | 0.193 | ||
| Cisplatin plus etoposide | 95 (80%) | 86 (74%) | |
| Carboplatin plus etoposide | 16 (13%) | 17 (15%) | |
| Cisplatin plus irinotecan | 3 (3%) | 4 (3%) | |
| Other* | 5 (4%) | 9 (8%) | |
| Patients with prior radiotherapy | 62 (52%) | 42 (36%) | 0.014* |
BMI: body mass index; CNS: central nervous system; CR: complete response; ECOG: Eastern Cooperative Oncology Group; ED: extensive disease; LD: limited disease; PD: progressive disease; PR: partial response; TSR: tumor shrinkage rate; SD: stable disease
Assessment of tumor load
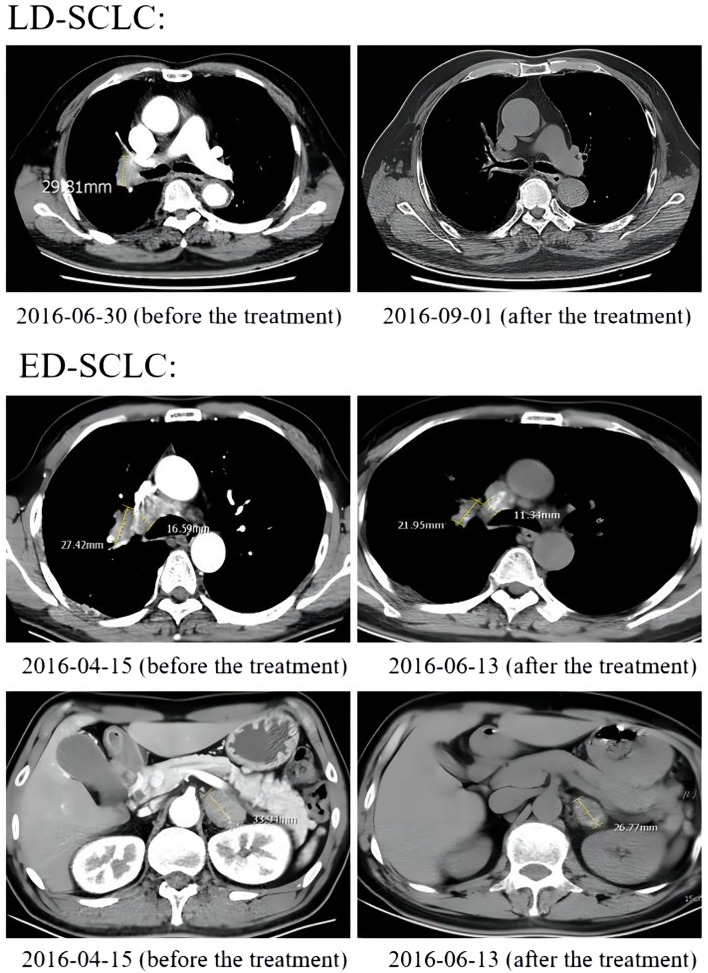
According to RECIST version 1.1, the sum of the maximum diameter of the target lesion was assessed at the same level on the medical images before and after treatment. For patients who had multiple organ metastases or multiple mediastinal lymph node metastases (LNM), the three largest lesions were measured to determine the sum of their diameters. Any metastatic lesion present in the image before treatment without the measuring after treatment would not be assessed after treatment (Fig. 1).
Fig. 1.
CT imaging findings in representative patients with LD-SCLC and ED-SCLC before and after treatment. ED-SCLC: extensive-disease small-cell lung cancer; LD-SCLC: limited-disease small-cell lung cancer
Staging
TNM staging was performed according to the 8th edition of the International Association for Lung Cancer staging system. According to the Veterans Administration Lung Cancer Study Group classification, the enrolled patients were divided into an LD-SCLC group and an ED-SCLC group. The ED-SCLC group included patients with lesions involving more than 50% of the chest, those with malignant pleural or pericardial effusion, and those with a tumor too large to be covered by the radiation field. The LD-SCLC group included patients with lesions that were limited to one side of the chest only, those with or without ipsilateral hilar LNM, those with ipsilateral mediastinal LNM, and those with ipsilateral supraclavicular LNM. A small pleural effusion and a mild superior vena cava compression were allowed.
ECOG score
The ECOG scoring system is as follows: 0, fully active, able to perform all pre-disease activities without restriction; 1, restricted in physically strenuous activity but ambulatory and able to perform work of a light or sedentary nature (e.g., light housework, office work); 2, ambulatory and capable of self-care but unable to carry out any work activities or up and about more than 50% of waking hours; 3, capable of only limited self-care or confined to bed or chair for more than 50% of waking hours; 4, completely disabled, incapable of self-care, totally confined to bed or chair; or 5, deceased.
Treatment options
The cisplatin + etoposide regimen was administered in 77% of patients, the carboplatin + etoposide regimen in 14%, the irinotecan + cisplatin regimen in 3%, the irinotecan + carboplatin regimen in 1%, and other regimens, including paclitaxel + cisplatin, paclitaxel + carboplatin, or oral etoposide + radiotherapy, in 5%. Radiotherapy fields included the lung, mediastinum, spinal cord, and brain.
Follow-up procedures
Follow-up information about therapeutic efficacy and the prognosis was collected through to October 31, 2017 by telephone interviews and according to the hospital’s electronic medical records system. The primary outcomes were PFS and OS. The secondary outcome was brain metastasis-free survival (BFS). In brief, OS was defined as the interval between the date of diagnosis and either the date of death or the date of the last follow-up. PFS was calculated as the interval between the date of diagnosis and the date of disease progression, and BFS as the interval between the date of diagnosis and detection of brain metastasis. Patients who were censored at the last follow-up date or who had died without evidence of brain metastasis were censored for incidence of brain metastasis [26].
Nomogram
The predictive model based on traditional diagnostic factors and the TSR was constructed based on univariate and multivariate Cox regression analyses. To construct a scoring system able to evaluate OS of < 6, < 12, and < 18 months and PFS of < 3, < 6, and < 12 months in patients with SCLC, we established a nomogram based on age, tumor burden, radiotherapy, and shrinkage rate and a nomogram based on M stage and shrinkage rate using the “regplot” package. A calibration curve was also established to compare the probabilities of nomogram-predicted and observed outcomes. The clinical value of the nomograms was evaluated by decision curve analysis.
Statistical analysis
Kaplan–Meier survival curves were constructed for PFS, OS, and BFS and compared between groups using the log-rank test. The 3-month, 6-month, 12-month, and 18-month survival rates were evaluated using ROC curves, and the area under the curve (AUC) was calculated to evaluate the diagnostic efficacy of the model. A Cox proportional hazards model was used for univariate and multivariate analyses of age, sex, smoking status, treatment regimen, TNM stage, history of radiotherapy, body mass index, ECOG score, and tumor burden. Categorical variables were compared between groups using the Chi-squared test, and continuous variables were compared using the independent-samples t test and Wilcoxon test. All statistical analyses were performed using R software (version 4.1.1 R; Foundation for Statistical Computing, Vienna, Austria). A P value < 0.05 was considered statistically significant.
Results
Patient characteristics
The clinical data for the 688 patients diagnosed to have SCLC during the study period were collected retrospectively. According to the enrollment and preliminary exclusion criteria, 260 patients with data sufficient to determine TSR were included, 25 of whom were excluded because their history of surgery and assessment had exceeded 4 months. Finally, data for 235 patients were included. Based on ROC curve analysis, the TSR cutoff was set as − 6.6%. Accordingly, the 235 patients were assigned to a responder group (TSR < − 6.6%, n = 119) or a non-responder group (TSR ≥ − 6.6%, n = 116) (Fig. 2).
The baseline characteristics of the two groups are described in Table 1. There were no significant between-group differences in baseline characteristics, except for initial response to treatment and history of radiotherapy. Most of the enrolled patients were younger than 70 years and 85% were men. Approximately 91% of the patients had an ECOG score of 0–1. Most patients were heavy smokers, and 54% had ED-SCLC. For most of the eligible patients (77%), the first-line treatment was based mainly on the cisplatin + etoposide regimen, with a minority receiving a cisplatin + etoposide or irinotecan + cisplatin regimen. There was a significant between-group difference in the initial response to treatment (P < 0.001).
PFS and OS
According to their disease stage, the 235 patients were divided into an ED-SCLC group and an LD-SCLC group for survival analysis (Supplementary Fig. 1A, B). Median OS was 15.80 months in both groups. PFS was approximately 1 month longer in the LD-SCLC group than in the ED-SCLC group (7.67 months vs. 6.87 months, P = 0.01) (Supplementary Fig. 1B).
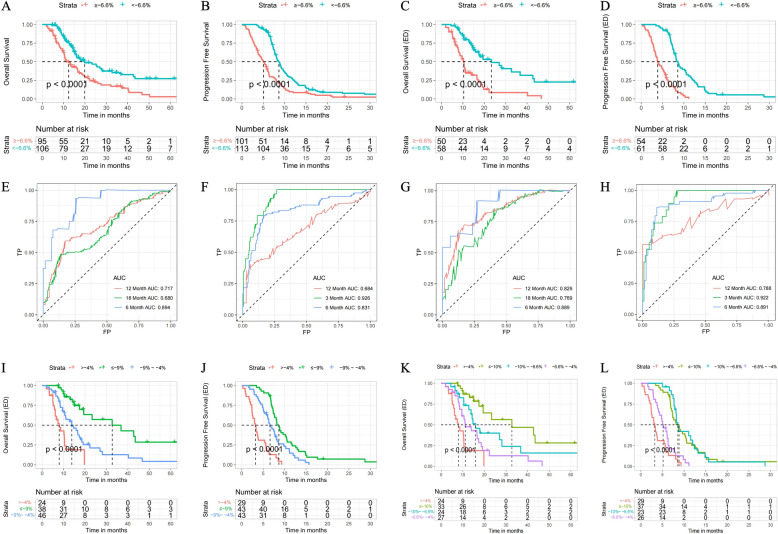
Kaplan–Meier survival curve analysis showed that the median OS was 7.7 months longer in the responder group than in the non-responder group (20.00 months vs. 12.33 months; hazard ratio [HR] 0.55, 95% confidence interval [CI) 0.38–0.78, P < 0.0001) (Fig. 3A), as was median PFS (8.57 months vs. 5.07 months, HR 0.47, 95% CI 0.35–0.63, P < 0.0001) (Fig. 3B), indicating that the prognosis of the enrolled patients was predicted more accurately by our research method than by the traditional method.
Fig. 3.
Kaplan–Meier curves showing A OS and B PFS in the group with a TSR of < − 6.6% and the group with a TSR of ≥ − 6.6%. C Kaplan–Meier curves showing C OS and D PFS in patients with ED-SCLC in the group with a TSR of < − 6.6% and the group with a TSR of ≥ − 6.6%. D Kaplan–Meier curves showing PFS in patients with ED-SCLC in the group with a TSR of < − 6.6% and the group with a TSR of ≥ − 6.6%. E Time-dependent ROC curves for OS and the TSR in patients with SCLC. F Time-dependent ROC curves for PFS and the TSR in patients with SCLC. G Time-dependent ROC curves for OS and TSR in patients with ED-SCLC. H Time-dependent ROC curves for PFS and the TSR in patients with ED-SCLC. Kaplan–Meier curves for I OS and J PFS in patients with ED-SCLC in groups with a TSR of ≤ − 9%, a TSR of − 9% to − 4%, and a TSR of > − 4%. Kaplan–Meier curves for K OS and L PFS in patients with ED-SCLC in groups with a TSR of ≤ − 10%, a TSR of − 10% to − 6.6%, a TSR of − 6.6% to − 4%, and a TSR of > − 4%. ED-SCLC: extensive-disease small-cell lung cancer; OS: overall survival; PFS: progression-free survival; ROC:receiver-operating characteristic; SCLC: small-cell lung cancer; TSR: tumor shrinkage rate
Kaplan–Meier analysis was also used to examine differences in survival between responders and non-responders in the LD-SCLC group and the ED-SCLC group. In the LD-SCLC group, the OS curves for the responder and non-responder groups intersected, with a median OS of 16.03 months and 16.14 months, respectively (P = 0.17) (Supplementary Fig. 1C); however, there was a significant difference in PFS between the two groups (8.3 months vs. 6.2 months, P = 0.04) (Supplementary Fig. 1D), suggesting that our research method was more effective for prediction of PFS than for OS in patients with LD-SCLC. In the ED-SCLC group, median OS was approximately 13 months longer in the responder group than in the non-responder group (23.33 months vs. 10.20 months, P < 0.0001) (Fig. 3C). Median PFS was 8.57 months in the responder group and 3.90 months in the non-responder group (hazard ratio 0.28, 95% CI 0.17–0.44, P < 0.0001) (Fig. 3D).
To evaluate the prognostic value of our method, we then calculated the AUC for OS of < 6, < 12 and < 18 months and PFS of < 3, < 6 and < 12 months using ROC curves. For OS, the 12-month and 18-month AUCs were significantly lower than the 6-month AUC in patients with SCLC and in those with LD-SCLC (0.717 and 0.679 vs 0.895, Fig. 3E; 0.581 and 0.580 vs 0.915, Supplementary Fig. 1E). There was no obvious difference in the AUC for OS at 6, 12, or 18 months in patients with ED-SCLC (Fig. 3G). For PFS, the AUCs for all patients with SCLC and those with LD-SCLC decreased gradually between 3 and 12 months (0.927 vs 0.831 vs 0.679, Fig. 3F; 0.941 vs 0.753 vs 0.592, Supplementary Fig. 1F). In contrast, there were little difference in the AUCs between 3 and 12 months in patients with ED-SCLC (Fig. 3H). Overall, the ROC curves confirmed that our research method was well able to estimate the probabilities of OS and PFS, especially comparatively short survival and when applied to patients with ED-SCLC.
The above findings established the value of the TSR in patients with ED-SCLC and indicated that for these patients, stratification using − 6.6% as the TSR cutoff could better predict the prognosis than dividing patients into LD-SCLC and ED-SCLC groups. A smaller TSR value was associated with a better prognosis. Next, the patients with ED-SCLC were sub-grouped further according to various TSR values. Taking TSRs of − 9% and − 4% as the cutoffs, the patients were divided into three groups. The three survival curves were well separated for these patients. Median OS was 32.7 months for patients with a TSR of ≤ − 9%, 13.9 months for those with a TRS of 9% to − 4%, and 8 months for those with a TSR > − 4% (Fig. 3I); median PFS was 8.7, 6.6, and 3.2 months, respectively (Fig. 3J). Next, taking TSR values of − 10%, − 6.6%, and − 4% as the cutoffs, the patients with ED-SCLC were divided into four groups for survival analysis. Median OS was 32.7 months for a TSR ≤ − 10%, 15.9 months for a TSR of 10% to − 6.6%, 11.1 months for a TSR of − 6.6% to − 4%, and 8 months for a TSR > − 4%. The four OS curves were significantly different (Fig. 3K). In contrast, there was no significant difference in median PFS among the four groups, the value being 8.8 months for a TSR of ≤ − 10%, 8.6 months for a TSR of − 10% to − 6.6%, 5.1 months for a TSR of − 6.6% to − 4%, and 3.2 months or a TSR of > − 4%. This finding suggested that for PFS, there was no need to divide the patients with TSR values < − 6.6% into groups (Fig. 3L).
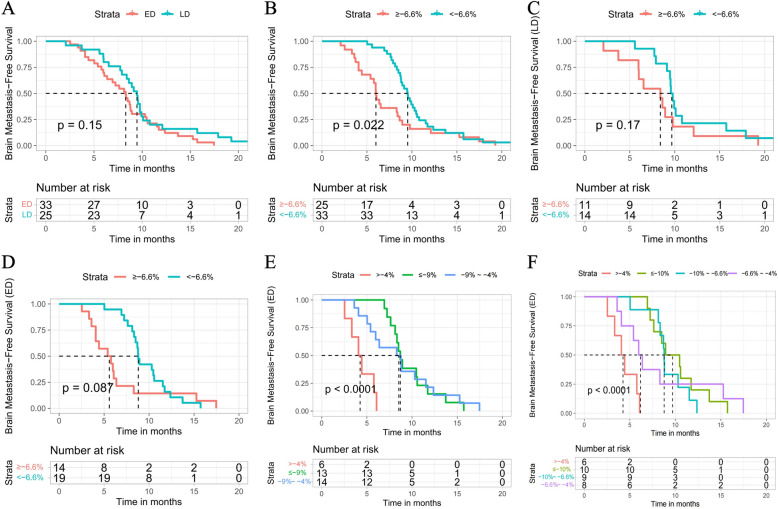
We then investigated the patients who had SCLC without brain metastasis. Fifty-nine of the 235 study participants did not have brain metastasis at diagnosis based on computed tomography and magnetic resonance scans but developed brain metastasis during treatment. The 59 patients were divided into LD-SCLC and ED-SCLC groups. However, as shown in Fig. 4A, the curves intersected, so we were unable to use this classification method to predict the prognosis of patients with SCLC and brain metastasis. Next, we used our nomogram method to analyze the relationship between the various TSRs and the prognosis of these patients. According to the survival analysis, the median BFS was 9.50 months in the responder group and 5.97 months in the non-responder group (P = 0.022) (Fig. 4B). We also grouped patients with LD-SCLC or ED-SCLC and brain metastasis by various TSR values and found that the survival curves of the two groups intersected (Fig. 4C, D). Median BFS in patients with LD-SCLC and brain metastasis was 9.67 months in the responder group and 8.63 months in the non-responder group, and was 8.79 months and 5.45 months, respectively, in patients with ED-SCLC and brain metastasis.
Fig. 4.
A Kaplan–Meier curves for BFS in patients with LD-SCLC and ED-SCLC. B Kaplan–Meier curves for BFS in patients with SCLC in a group with a TSR of < − 6.6% and a group with a TSR of ≥ − 6.6%. C Kaplan–Meier curves for BFS in patients with LD-SCLC in a group with a TSR of < − 6.6% and a group with a TSR of ≥ − 6.6%. D Kaplan–Meier curves for BFS in patients with ED-SCLC in a group with a TSR of < –6.6% and a group with a TSR of ≥ − 6.6%. E Kaplan–Meier curves for BFS in patients with ED-SCLC in a group with a TSR of ≤ − 9%, a group with a TSR of − 9% to − 4% and a TSR of > − 4%. F Kaplan–Meier curves for BFS in patients with ED-SCLC in a group with a TSR of < − 10%, a group with a TSR of − 10% to − 6.6%, a group with a TSR of − 6.6% to − 4%, and a group with a TSR of > − 4%. BFS, brain metastasis-free survival; ED-SCLC: extensive-disease small-cell lung cancer; LD-SCLC: limited-disease small-cell lung cancer; SCLC: small-cell lung cancer; TSR: tumor shrinkage rate
Using TSR values of − 9% and − 4% as the cutoffs, we then divided the patients with ED-SCLC and brain metastasis into three groups. Median BFS was 8.67 months for a TSR of ≤ − 9%, 8.6 months for a TSR of − 9% to − 4%, and 4.2 months for a TSR of > − 4%. As shown in Fig. 4E, the curves for the TSR ≤ − 4% and TSR > − 4% groups were clearly separated. However, when we divided the same patients into four groups, as shown in Fig. 4F, there was a difference in median BFS between the TSR ≤ − 4% and TSR > − 4% groups. Therefore, for patients with ED-SCLC and brain metastasis, stratification using a TSR cutoff of − 4% could better predict the prognosis than stratification using a TSR cutoff of − 6.6%.
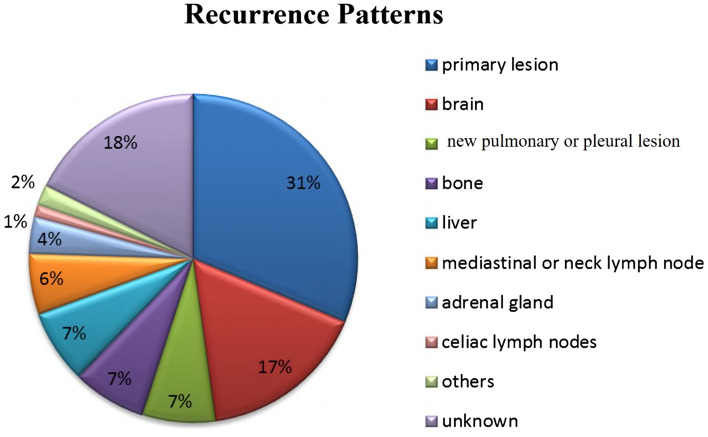
Recurrence patterns
The recurrence pattern after failure of the first-line treatment of SCLC was mainly relapse of the primary lesion (31%), followed by brain metastasis (17%), lung or pleural metastasis (7%), bone metastasis (7%), liver metastasis (7%), mediastinal or cervical LNM (6%), adrenal metastasis (4%), intra-abdominal LNM (1%), pancreatic and intestinal metastasis (2%), and unknown (18%) (Fig. 5).
Fig. 5.
Disease recurrence patterns
Analysis of survival risk factors
Using the univariate and multivariate Cox proportional hazards models, we analyzed the associations of PFS and OS with the patients’ clinical characteristics, including age, sex, smoking status, body mass index, ECOG score, TNM stage, treatment regimen, history of radiotherapy, and tumor burden. The univariate Cox proportional hazards model showed a correlation of smoking ≥ 20 packs/year with PFS, with an increase in risk of 44% (HR 1.44, 95% CI 1.03–2.02, P = 0.035), and a correlation of M1 stage with PFS, with an increase in risk of 46% (HR 1.46, 95% CI 1.09–1.95, P = 0.01). Furthermore, age older than 70 years was an indicator of poor OS, with an increase in risk of 58% (HR 1.58, 95% CI 1.10–2.29, P = 0.015). Previous radiotherapy was an indicator of favorable OS, with a decrease in risk of 33% (HR 0.67, 95% CI 0.47–0.96, P = 0.03). No other patient factor had a significant correlation with PFS or OS (Table 2). The multivariate Cox proportional hazards model revealed that M stage had an impact on PFS, while age, prior radiotherapy, and tumor burden had an impact on OS (Table 3).
Table 2.
Results of univariate analysis of PFS and OS
| Covariate | PFS, years | OS, years | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, years | ||||
| ≥ 70 | 1.03 (0.74–1.44) | 0.844 | 1.58 (1.10–2.29) | 0.015* |
| < 70 | 1 (Ref) | 1 (Ref) | ||
| Sex | ||||
| Male | 1.30 (0.85–1.98) | 0.224 | 1.06 (0.64–1.74) | 0.826 |
| Female | 1 (Ref) | 1 (Ref) | ||
| Smoking status | ||||
| ≥ 20 pack/year | 1.44 (1.03–2.02) | 0.035* | 1.36 (0.90–2.07) | 0.145 |
| < 20 pack/year | 1 (Ref) | 1 (Ref) | ||
| BMI | ||||
| ≥ 24 | 0.96 (0.70–1.31) | 0.793 | 0.71 (0.48–1.04) | 0.075 |
| < 18.5 | 0.91 (0.51–1.60) | 0.747 | 0.93 (0.45–1.93) | 0.848 |
| 18.5–23.9 | 1 (Ref) | 1 (Ref) | ||
| ECOG score | ||||
| ≥ 2 | 0.82 (0.49–1.37) | 0.445 | 1.05 (0.59–1.88) | 0.860 |
| 0–1 | 1 (Ref) | 1 (Ref) | ||
| T | ||||
| T3–4 | 1.04 (0.78–1.38) | 0.802 | 1.01 (0.71–1.44) | 0.941 |
| T1–2 | 1 (Ref) | 1 (Ref) | ||
| N | ||||
| N1–3 | 1.53 (0.85–2.76) | 0.161 | 1.27 (0.65–2.51) | 0.484 |
| N0 | 1 (Ref) | 1 (Ref) | ||
| M | ||||
| M1 | 1.46 (1.09–1.95) | 0.010* | 1.31 (0.92–1.86) | 0.128 |
| M0 | 1 (Ref) | 1 (Ref) | ||
| First-line CT | ||||
| EP/EC | 0.80 (0.35–1.82) | 0.597 | 0.43 (0.16–1.17) | 0.163 |
| IP/IC | 0.82 (0.27–2.44) | 0.717 | 0.34 (0.08–1.54) | |
| Others | 1 (Ref) | 1 (Ref) | ||
| Prior radiotherapy | ||||
| Yes | 0.75 (0.57–1.01) | 0.054 | 0.67 (0.47–0.96) | 0.030* |
| No | 1 (Ref) | 1 (Ref) | ||
| Tumor burden | ||||
| > 8 cm | 0.98 (0.94–1.02) | 0.233 | 0.98 (0.93–1.02) | 0.308 |
| ≤ 8 cm | 1 (Ref) | 1 (Ref) | ||
| TSR | ||||
| ≥ − 6.6% | 1.10 (1.06–1.13) | 1.25E−08* | 1.11 (1.07–1.15) | 1.14E−07* |
| < − 6.6% | 1 (Ref) | 1 (Ref) | ||
BMI: body mass index; CI: confidence interval; ECOG: Eastern Cooperative Oncology Group; EP: cisplatin + etoposide; HR: hazard ratio; IC: irinotecan + cisplatin; IP: irinotecan + carboplatin; OS: overall survival; PFS: progression-free survival; TSR: tumor shrinkage rate
Table 3.
Results of multivariate analysis of PFS and OS
| Covariate | PFS, years | OS, years | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, years | ||||
| ≥ 70 | 1.01 (0.72–1.41) | 0.974 | 1.75 (1.21–2.57) | 0.003* |
| < 70 | 1 (Ref) | 1 (Ref) | ||
| Sex | ||||
| Male | 1.322 (0.73–2.40) | 0.358 | 1.63 (0.78–3.40) | 0.193 |
| Female | 1 (Ref) | 1 (Ref) | ||
| Smoking status | ||||
| ≥ 20 pack/year | 1.42 (0.89–2.26) | 0.144 | 1.45 (0.78–2.67) | 0.240 |
| < 20 pack/year | 1 (Ref) | 1 (Ref) | ||
| ECOG | ||||
| ≥ 2 | 1.23 (0.98–2.13) | 0.061 | 1.33 (0.69–2.56) | 0.401 |
| 0–1 | 1 (Ref) | 1 (Ref) | ||
| T | ||||
| T3–4 | 1.09 (0.81–1.47) | 0.574 | 1.11 (0.76–1.61) | 0.594 |
| T1–2 | 1 (Ref) | 1 (Ref) | ||
| N | ||||
| N1–3 | 1.71 (0.93–3.15) | 0.086 | 1.47 (0.75–2.88) | 0.267 |
| N0 | 1 (Ref) | 1 (Ref) | ||
| M | ||||
| M1 | 1.73 (1.27–2.34) | 0.0005* | 1.42 (0.99–2.04) | 0.060 |
| M0 | 1 (Ref) | 1 (Ref) | ||
| Prior radiotherapy | ||||
| Yes | 0.77 (0.57–1.05) | 0.093 | 0.69 (0.48–0.99) | 0.046* |
| No | 1 (Ref) | 1 (Ref) | ||
| Tumor burden | ||||
| > 8 cm | 1.04 (1.00–1.09) | 0.054 | 1.09 (1.03–1.15) | 0.002* |
| ≤ 8 cm | 1 (Ref) | 1 (Ref) | ||
| TSR | ||||
| ≥ − 6.6% | 1.13 (1.09–1.17) | 1.24E−12* | 1.14 (1.09–1.18) | 2.87E−10* |
| < − 6.6% | 1 (Ref) | 1 (Ref) | ||
CI: confidence interval; ECOG: Eastern Cooperative Oncology Group; HR: hazard ratio; OS: overall survival; PFS: progression-free survival; TSR: tumor shrinkage rate
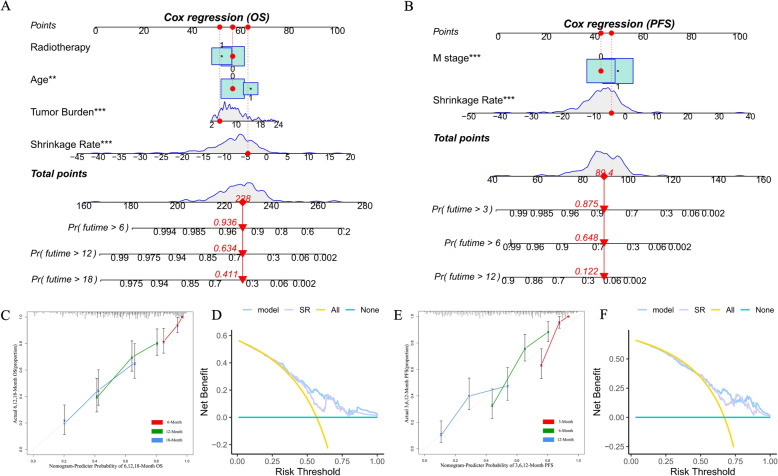
Construction of nomograms and evaluation of their performance
Based on previous work, we constructed a nomogram combining age, radiotherapy, tumor burden, and the TSR that was able to predict OS of > 6 months, > 12 months and > 18 months (Fig. 6A) and a nomogram combining M stage and TSR that was able to predict PFS > 3 months, > 6 months, and > 12 months (Fig. 6B). We evaluated the predictive performance of the nomogram using a calibration curve, which showed good agreement between the predicted probability of the nomogram and the actual probability (Fig. 6C, E). Next, decision curve analysis was used to explore the clinical utilization of the nomograms. Figure 6D, F shows the results of the decision curve analysis for the two models (nomogram model and shrinkage rate) when applied to OS and PFS. In comparison with the shrinkage rate, the nomogram model had higher net benefit in prediction of which patients should receive more aggressive treatment. This finding suggested that the nomogram combining traditional diagnostic factors and the shrinkage rate has better predictive power than the shrinkage rate alone.
Fig. 6.
A Nomogram to determine the probability of OS for > 6 months, > 12 months, and > 18 months. B A nomogram to determine the probability of PFS for > 6 months, > 12 months, and > 18 months. B Value of each risk factor can be converted into a corresponding score according to the “points”. After adding up the individual risk scores for these risk factors, draw a line descending from the axis labeled “Total points” until it intercepts each clinical predictive score for SCLC. C Calibration curves for the OS nomogram at 6, 12, and 18 months. E Calibration curves for the PFS nomogram at 3, 6, and 12 months. Results of DCA for two models (the nomogram model and shrinkage rate) applied to OS (D) and PFS (F), respectively. The DCA curves measure the net benefit (y-axis) versus the model’s high risk threshold (x-axis) for the different models. DCA: decision curve analysis; OS: overall survival; PFS: progression-free survival; SCLC: small-cell lung cancer
Discussion
Chemotherapy is beneficial for patients with advanced SCLC. However, there are controversies regarding the optimal method for evaluation of this benefit. To the best of our knowledge, this study is the first to report an association between the TSR and the clinical outcomes in patients with SCLC receiving chemotherapy. In this study, we first calculated the optimal TSR cutoff using ROC curve analysis. Next, we analyzed the correlations of various clinical indicators with PFS and OS. Based on the ROC curve analysis, we set -6.6% as the tumor shrinkage threshold and used this cutoff value to identify responders and non-responders to chemotherapy. We found that the median PFS and OS in responders were 8.57 months and 20.00 months, respectively, both of which were significantly longer than the 5.07 months and 12.33 months in non-responders (both P < 0.0001). Cox regression and Kaplan–Meier curve analyses showed that tumor shrinkage of 6.6% was an independent prognostic factor for PFS and OS (both P < 0.0001). Further analyses of PFS and OS in patients with ED-SCLC and those with LD-SCLC showed that a TSR of ≥ 6.6% was a valid prognostic factor for PFS and OS (both P < 0.0001) in the ED-SCLC group but not in the LD-SCLC group.
There are considerable differences in baseline characteristics between patients with LD-SCLC and those with ED-SCLC, including tumor burden and performance status. Given the advanced nature of ED-SCLC, OS is poor; however, both PFS and OS could potentially be improved if patients with ED-SCLC are responsive to chemotherapy. However, patients with LD-SCLC have an earlier tumor stage, lower tumor burden, a higher ECOG score, and slower disease progression, so have a more favorable prognosis. Therefore, the impact of tumor regression in patients with LD-SCLC may not be as obvious as in those with ED-SCLC. Moreover, the lack of consistency between the TSR and OS outcome in patients with LD-SCLC may be attributed to a relationship similar to that observed between the ORR and OS. The potential adverse reactions associated with agents that improve the TSR may also affect OS in patients with LD-SCLC. Furthermore, this was a single-center study, and its conclusions may be insufficient to fully explain why the TSR performs poorly in evaluation of OS in patients with LD-SCLC.
In patients with brain metastasis, the BFS curve for the group with a TSR < 6.6% intersected with that for the group with a TSR ≥ 6.6%, indicating relatively weak prognostic efficacy. We then validated our nomogram for prediction of PFS and OS of > 6 months, > 12 months, and > 18 months. These findings suggest that tumor shrinkage by 6.6% is a valid evaluation marker for patients with SCLC, those with ED-SCLC, and those who are free of brain metastasis.
The TSR cutoff was a key factor in analysis of survival. Thiam et al. reported that a TSR of 10% was a reliable predictor of the clinical outcome in patients with metastatic renal cell carcinoma who received vascular endothelial growth factor-targeted therapies [27]. Furthermore, a recent meta-analysis of patients with CRC showed that a 20% reduction in SLD in responders was associated with improved OS and PFS (P < 0.001) in comparison with non-responders [6]. He et al. reported a threshold of 8.23% tumor shrinkage based on the SLD according to ROC curve analysis [25]. In our study, ROC curve analysis indicated that the TSR cutoff that yielded the highest sensitivity and specificity was 6.6%, which is similar to the 8.23% reported by He et al. Therefore, we considered a TSR cutoff of 6.6% to be acceptable for identification of responders and non-responders to our therapeutic regimen.
Tumor burden is important in clinical assessment of anticancer therapies. However, the role of the TSR in prognostic assessment is unknown. Birchard et al. [28] and Grothey et al. [11] showed that tumor shrinkage did not correlate with survival in patients with NSCLC or in patients with CRC. However, other studies have found that the TSR is correlated with the prognosis [29, 30]. Wang et al. assessed the TSR after topotecan plus bispecific 6 phosphatase mononucleotide peptide therapy in patients with lung cancer receiving radiotherapy, and found that the prognosis in a group with a TSR > 34% was better than that in a group with a TSR < 34% [29]. Nougaret et al. also found that PFS was significantly better in patients with low rectal cancer and a TSR > 70% than in their counterparts with a TSR < 70% after chemotherapy or radiotherapy (P < 0.001) [30]. In another study, the TSR was found to have a predictive role in patients with pancreatic cancer receiving chemotherapy [31]. The authors reported that TSR was weakly but significantly associated with OS. Similarly, another recent study in patients with NSCLC receiving gefitinib demonstrated that the TSR was positively correlated with PFS and an independent prognostic factor for PFS and also had prognostic value in terms of PFS and OS in patients with NSCLC receiving epidermal growth factor receptor–tyrosine kinase inhibitor (EGFR–TKI) therapy [32]. Consistent with these findings, we believe that TSR might also be useful as an additional predictor of OS and PFS. Overall, our results and those of previous studies suggest that the TSR may be useful as a prognostic tool in patients with malignant tumors, including lung cancer.
There are several reasons for the discrepancies in the results between these studies. First, the pharmacological aim of most anticancer agents is to inhibit cell proliferation, mediate arrest of tumor cell growth, and shrink the tumor [33]. However, some anticancer agents only prolong arrest of tumor growth and slow proliferation of cancer cells [34], leading to a limited TSR after anticancer treatment. Therefore, different studies with different anticancer agents may not produce the same TSR or survival outcomes. Second, acquisition of further genetic changes is another important reason for development of resistance to anticancer therapeutics [35–37]. Takeda et al. [38] showed that EGFR–TKI-treated patients with NSCLC who had an EGFR-positive mutation eventually developed resistance to TKIs by acquiring additional genetic changes. Third, intratumoral heterogeneity of clones may lead to different survival outcomes and TSRs. The TSR may be associated with intratumoral heterogeneity of clones sensitive to treatment [32]. If the percentage of tumor cells sensitive to anticancer treatment is high, the TSR will be high after treatment. Therefore, differences in the percentage of sensitive tumor cells and treatment regimens will lead to differences in the TSR and survival. Michor et al. [39] demonstrated that reduction of tumor size upon treatment with an EGFR–TKI depends on the fractions of cells responsive to these agents.
This study had several limitations. First, it had a retrospective design, and the sample size was relatively small. Prospective investigations in larger cohorts are needed to confirm the reproducibility of our findings. Second, all TSR data were based solely on morphological features measured by anatomical imaging. Although we were able to show the value of TSR, other molecularly targeted drugs (e.g., bevacizumab) might lead to slowing of tumor growth or changes in tumor density rather than tumor shrinkage and be unlikely to show a prognostic role of TSR in clinical settings [40, 41]. For such drugs, a negative relationship between the TSR and PFS or OS might be observed. Third, the value of the TSR was assessed at a single center, so multicenter cooperation for TSR is required. Fourth, although we found that the TSR could be used as a predictor of survival outcomes in patients with SCLC who received chemotherapy, the molecular mechanism remains to be clarified in further studies. As mentioned above, intratumoral heterogeneity, acquisition of additional genetic changes, differences in the mechanisms of action of anticancer drugs, and recurrence can lead to minor or slow tumor shrinkage and different TSR values, including no change in tumor diameter. Under these conditions, TSR might not be related to survival nor act as a prognostic marker of PFS or OS. The optimal TSR cutoff should be investigated further, as should the impact of the TSR when combined with other prognostic indicators.
Conclusions
The results of this study indicate that the TSR can be used as an independent predictor of the survival outcome in patients with SCLC treated with chemotherapy, especially those with ED-SCLC and those who have SCLC without brain metastasis. This research may also contribute to refinement of the prognostic criteria and even development of novel targeted therapeutic evaluation criteria.
Supplementary Information
Supplementary file 1. Supplementary Fig. 1. Kaplan–Meier curves for (A) OS and (B) PFS in the LD-SCLC and ED-SCLC groups. Kaplan–Meier curves for (C) OS and (D) PFS for patients with LD-SCLC in a group with a TSR of < − 6.6% and a group with a TSR of ≥ − 6.6%. Time-dependent ROC curves for (E) OS and (F) PFS and the TSR in patients with LD-SCLC. ED-SCLC, extensive-disease small-cell lung cancer; LD-SCLC, limited-disease small-cell lung cancer; OS, overall survival; PFS, progression-free survival; TSR, tumor shrinkage rate.
Acknowledgements
We thank Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Author contributions
All authors were involved in the preparation of this manuscript. JJ, XL, and YZ conceived and designed the experiments. YZ, ZW, and HW analyzed the data and wrote the manuscript. KZ, HC, SZ, AS, SY, and YW revised the manuscript. All the authors discussed the results and approved submission of the manuscript.
Funding
The study was funded by the National Natural Science Foundation of China (81871966) and the Natural Science Foundation of Zhejiang Province, China (LY15H160062).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
All procedures were approved by our institutional ethics committee (permit number 2018140) and performed in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki. All study participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuchen Zhou, Zhonghan Wu and Haowen Wang contributed equally to this work.
Contributor Information
Xiaoming Lin, Email: sheermanlin@126.com.
Jingjing Jin, Email: AshleyJin0831@163.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;713:209–49. 10.3322/caac.21660. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2022. J Natl Cancer Center. 2024;41:47–53. 10.1016/j.jncc.2024.01.006. 10.1016/j.jncc.2024.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021;71:3. 10.1038/s41572-020-00235-0. 10.1038/s41572-020-00235-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;1712:725–37. 10.1038/nrc.2017.87. 10.1038/nrc.2017.87 [DOI] [PubMed] [Google Scholar]
- 5.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;3789804:1741–55. 10.1016/s0140-6736(11)60165-7. 10.1016/s0140-6736(11)60165-7 [DOI] [PubMed] [Google Scholar]
- 6.Petrelli F, Pietrantonio F, Cremolini C, Di Bartolomeo M, Coinu A, Lonati V, de Braud F, Barni S. Early tumour shrinkage as a prognostic factor and surrogate end-point in colorectal cancer: a systematic review and pooled-analysis. Eur J Cancer. 2015;517:800–7. 10.1016/j.ejca.2015.02.011. 10.1016/j.ejca.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 7.Tang PA, Bentzen SM, Chen EX, Siu LL. Surrogate end points for median overall survival in metastatic colorectal cancer: literature-based analysis from 39 randomized controlled trials of first-line chemotherapy. J Clin Oncol. 2007;2529:4562–8. 10.1200/jco.2006.08.1935. 10.1200/jco.2006.08.1935 [DOI] [PubMed] [Google Scholar]
- 8.Buyse M, Thirion P, Carlson RW, Burzykowski T, Molenberghs G, Piedbois P. Relation between tumour response to first-line chemotherapy and survival in advanced colorectal cancer: a meta-analysis. Meta-analysis group in cancer. Lancet. 2000;3569227:373–8. 10.1016/s0140-6736(00)02528-9. 10.1016/s0140-6736(00)02528-9 [DOI] [PubMed] [Google Scholar]
- 9.Oxnard GRSL. Response phenotype as a predictive biomarker to guide treatment with targeted therapies. J Clin Oncol. 2013;3130:3739–41. 10.1200/JCO.2013.51.8365 [DOI] [PubMed] [Google Scholar]
- 10.Hu J, Zhang Z, Zheng R, Cheng L, Yang M, Li L, Liu B, Qian X. On-treatment markers as predictors to guide anti-EGFR MoAb treatment in metastatic colorectal cancer: a systematic review with meta-analysis. Cancer Chemother Pharmacol. 2017;792:275–85. 10.1007/s00280-016-3196-2. 10.1007/s00280-016-3196-2 [DOI] [PubMed] [Google Scholar]
- 11.Grothey A, Hedrick EE, Mass RD, Sarkar S, Suzuki S, Ramanathan RK, Hurwitz HI, Goldberg RM, Sargent DJ. Response-independent survival benefit in metastatic colorectal cancer: a comparative analysis of N9741 and AVF2107. J Clin Oncol. 2008;262:183–9. 10.1200/jco.2007.13.8099. 10.1200/jco.2007.13.8099 [DOI] [PubMed] [Google Scholar]
- 12.Piessevaux H, Buyse M, Schlichting M, Van Cutsem E, Bokemeyer C, Heeger S, Tejpar S. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2013;3130:3764–75. 10.1200/jco.2012.42.8532. 10.1200/jco.2012.42.8532 [DOI] [PubMed] [Google Scholar]
- 13.Modest DP, Laubender RP, Stintzing S, Giessen C, Schulz C, Haas M, Mansmann U, Heinemann V. Early tumor shrinkage in patients with metastatic colorectal cancer receiving first-line treatment with cetuximab combined with either CAPIRI or CAPOX: an analysis of the German AIO KRK 0104 trial. Acta Oncol. 2013;525:956–62. 10.3109/0284186x.2012.752580. 10.3109/0284186x.2012.752580 [DOI] [PubMed] [Google Scholar]
- 14.Öcal O, Schinner R, Schütte K, de Toni EN, Loewe C, van Delden O, Vandecaveye V, Gebauer B, Zech CJ, Sengel C, et al. Early tumor shrinkage and response assessment according to mRECIST predict overall survival in hepatocellular carcinoma patients under sorafenib. Cancer Imaging. 2022;221:1. 10.1186/s40644-021-00439-x. 10.1186/s40644-021-00439-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ura T, Hironaka S, Tsubosa Y, Mizusawa J, Kato K, Tsushima T, Fushiki K, Chin K, Tomori A, Okuno T, et al. Early tumor shrinkage and depth of response in patients with metastatic esophageal cancer treated with 2-weekly docetaxel combined with cisplatin plus fluorouracil: an exploratory analysis of the JCOG0807. Esophagus. 2023;202:272–80. 10.1007/s10388-022-00968-9. 10.1007/s10388-022-00968-9 [DOI] [PubMed] [Google Scholar]
- 16.Okano N, Morizane C, Okusaka T, Sadachi R, Kataoka T, Kobayashi S, Ikeda M, Ozaka M, Mizutani T, Sugimori K, et al. Early tumor shrinkage and depth of response as predictors of survival for advanced biliary tract cancer: an exploratory analysis of JCOG1113. Oncologist. 2024;291:e97–107. 10.1093/oncolo/oyad220. 10.1093/oncolo/oyad220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller L, Gairing SJ, Kloeckner R, Foerster F, Schleicher EM, Weinmann A, Mittler J, Stoehr F, Halfmann MC, Düber C, et al. The prognostic role of early tumor shrinkage in patients with hepatocellular carcinoma undergoing immunotherapy. Cancer Imaging. 2022;221:54. 10.1186/s40644-022-00487-x. 10.1186/s40644-022-00487-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seidel C, Busch J, Weikert S, Steffens S, Bokemeyer C, Grünwald V. Tumour shrinkage measured with first treatment evaluation under VEGF-targeted therapy as prognostic marker in metastatic renal cell carcinoma (mRCC). Br J Cancer. 2013;10912:2998–3004. 10.1038/bjc.2013.662. 10.1038/bjc.2013.662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Chen X, Bao Y, Zhang W, Jiang L, Zhu J, Wang Y, Wu L, Wan G, Peng L, et al. EUS-derived maximum tumor thickness and tumor shrinkage rate as independent prognostic factors in locally advanced esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy. Endosc Ultrasound. 2023;124:369–76. 10.1097/eus.0000000000000008. 10.1097/eus.0000000000000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Sung C, Dartois C, Ramchandani R, Booth BP, Rock E, Gobburu J. Elucidation of relationship between tumor size and survival in non-small-cell lung cancer patients can aid early decision making in clinical drug development. Clin Pharmacol Ther. 2009;862:167–74. 10.1038/clpt.2009.64. 10.1038/clpt.2009.64 [DOI] [PubMed] [Google Scholar]
- 21.Stintzing S, Modest DP, Rossius L, Lerch MM, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016;1710:1426–34. 10.1016/S1470-2045(16)30269-8. 10.1016/S1470-2045(16)30269-8 [DOI] [PubMed] [Google Scholar]
- 22.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;1510:1065–75. 10.1016/s1470-2045(14)70330-4. 10.1016/s1470-2045(14)70330-4 [DOI] [PubMed] [Google Scholar]
- 23.Cremolini C, Loupakis F, Antoniotti C, Lonardi S, Masi G, Salvatore L, Cortesi E, Tomasello G, Spadi R, Zaniboni A, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015;266:1188–94. 10.1093/annonc/mdv112. 10.1093/annonc/mdv112 [DOI] [PubMed] [Google Scholar]
- 24.Almansour H, Afat S, Serna-Higuita LM, Amaral T, Schraag A, Peisen F, Brendlin A, Seith F, Klumpp B, Eigentler TK, et al. Early tumor size reduction of at least 10% at the first follow-up computed tomography can predict survival in the setting of advanced melanoma and immunotherapy. Acad Radiol. 2022;294:514–22. 10.1016/j.acra.2021.04.015. 10.1016/j.acra.2021.04.015 [DOI] [PubMed] [Google Scholar]
- 25.He X, Zhang Y, Ma Y, Zhou T, Zhang J, Hong S, Sheng J, Zhang Z, Yang Y, Huang Y, et al. Optimal tumor shrinkage predicts long-term outcome in advanced nonsmall cell lung cancer (NSCLC) treated with target therapy: result from 3 clinical trials of advanced NSCLC by 1 institution. Medicine. 2016;9531: e4176. 10.1097/md.0000000000004176. 10.1097/md.0000000000004176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu S, Wang J, Zhang W, Li J, Wu H, Huang Z, Zhou G, Pan J, Chen M. Analysis of factors affecting brain metastasis in limited-stage small-cell lung cancer treated with definitive thoracic irradiation. Front Oncol. 2020;10: 556634. 10.3389/fonc.2020.556634. 10.3389/fonc.2020.556634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiam R, Fournier LS, Trinquart L, Medioni J, Chatellier G, Balvay D, Escudier B, Dromain C, Cuenod CA, Oudard S. Optimizing the size variation threshold for the CT evaluation of response in metastatic renal cell carcinoma treated with sunitinib. Ann Oncol. 2010;215:936–41. 10.1093/annonc/mdp466. 10.1093/annonc/mdp466 [DOI] [PubMed] [Google Scholar]
- 28.Birchard KR, Hoang JK, Herndon JE, Patz EF. Early changes in tumor size in patients treated for advanced stage nonsmall cell lung cancer do not correlate with survival. Cancer. 2009;1153:581–6. 10.1002/cncr.24060. 10.1002/cncr.24060 [DOI] [PubMed] [Google Scholar]
- 29.Wang TL, Ren YW, Wang HT, Yu H, Zhao YX. Association of topoisomerase II (TOP2A) and dual-specificity phosphatase 6 (DUSP6) single nucleotide polymorphisms with radiation treatment response and prognosis of lung cancer in Han Chinese. Med Sci Monit. 2017;23:984–93. 10.12659/msm.899060. 10.12659/msm.899060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephanie N, Philippe R, Nicolas M, Marie A, Frederic B, David A, Claire L. MR volumetric measurement of low rectal cancer helps predict tumor response and outcome after combined chemotherapy and radiation therapy. Radiology. 2012;2632:409–18. [DOI] [PubMed] [Google Scholar]
- 31.Kaga YSY, Kubota Y, Tagawa T, Yamamoto T, Ikusue T. Early tumor shrinkage as a predictor of favorable outcomes in patients with advanced pancreatic cancer treated with FOLFIRINOX. Oncotarget. 2016;741:67314–20. 10.18632/oncotarget.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park DI, Kim SY, Kim JO, Jung SS, Park HS, Moon JY, Chung CU, Kim SS, Seo JH, Lee JE. The prognostic value of the tumor shrinkage rate for progression-free survival in patients with non-small cell lung cancer receiving gefitinib. Tuberc Respir Dis. 2015;784:315–20. 10.4046/trd.2015.78.4.315. 10.4046/trd.2015.78.4.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leyton JLJ, Perumal M, Dhaliwal H, He Q, Aboagye EO. Early detection of tumor response to chemotherapy by 3’ deoxy-3’-[18F fluorothymidine positron emission tomography: the effect of cisplatin on a fibrosarcoma tumor model in vivo. Can Res. 2005;6510:4202–10. 10.1158/0008-5472.CAN-04-4008 [DOI] [PubMed] [Google Scholar]
- 34.Cirkel GAWF, Bins S, Gadellaa-van Hooijdonk CG, van Werkhoven E, Willems SM. The time to progression ratio: a new individualized volumetric parameter for the early detection of clinical benefit of targeted therapies. Ann Oncol. 2016;278:1638–43. 10.1093/annonc/mdw223 [DOI] [PubMed] [Google Scholar]
- 35.Kjeldsen E, Nielsen CJF, Roy A, Tesauro C, Jakobsen AK, Stougaard M, Knudsen BR. Characterization of camptothecin-induced genomic changes in the camptothecin-resistant T-ALL-derived cell line CPT-K5. Cancer Genomics Proteomics. 2018;152:91–114. 10.21873/cgp.20068. 10.21873/cgp.20068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutta AK, Hewett DR, Fink JL, Grady JP, Zannettino ACW. Cutting edge genomics reveal new insights into tumour development, disease progression and therapeutic impacts in multiple myeloma. Br J Haematol. 2017;1782:196–208. 10.1111/bjh.14649. 10.1111/bjh.14649 [DOI] [PubMed] [Google Scholar]
- 37.Takahashi Y, Hori T, Cooper TK, Liao J, Desai N, Serfass JM, Young MM, Park S, Izu Y, Wang HG. Bif-1 haploinsufficiency promotes chromosomal instability and accelerates Myc-driven lymphomagenesis via suppression of mitophagy. Blood. 2013;1219:1622–32. 10.1182/blood-2012-10-459826. 10.1182/blood-2012-10-459826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeda MOI, Nakagawa K. Survival outcome assessed according to tumor response and shrinkage pattern in patients with EGFR mutation-positive non-small-cell lung cancer treated with gefitinib or erlotinib. J Thorac Oncol. 2014;92:200–4. 10.1097/JTO.0000000000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michor F, Polyak K. The origins and implications of intratumor heterogeneity. Cancer Prev Res. 2010;311:1361–4. 10.1158/1940-6207.Capr-10-0234. 10.1158/1940-6207.Capr-10-0234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Bruyne S, Van Damme N, Smeets P, Ferdinande L, Ceelen W, Mertens J, Van de Wiele C, Troisi R, Libbrecht L, Laurent S, et al. Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br J Cancer. 2012;10612:1926–33. 10.1038/bjc.2012.184. 10.1038/bjc.2012.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung WS, Park MS, Shin SJ, Baek SE, Kim YE, Choi JY, Kim MJ. Response evaluation in patients with colorectal liver metastases: RECIST version 1.1 versus modified CT criteria. AJR Am J Roentgenol. 2012;1994:809–15. 10.2214/ajr.11.7910. 10.2214/ajr.11.7910 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1. Supplementary Fig. 1. Kaplan–Meier curves for (A) OS and (B) PFS in the LD-SCLC and ED-SCLC groups. Kaplan–Meier curves for (C) OS and (D) PFS for patients with LD-SCLC in a group with a TSR of < − 6.6% and a group with a TSR of ≥ − 6.6%. Time-dependent ROC curves for (E) OS and (F) PFS and the TSR in patients with LD-SCLC. ED-SCLC, extensive-disease small-cell lung cancer; LD-SCLC, limited-disease small-cell lung cancer; OS, overall survival; PFS, progression-free survival; TSR, tumor shrinkage rate.
Data Availability Statement
No datasets were generated or analysed during the current study.