Abstract
A chimeric poliovirus type 1 (PV1) genome was constructed in which the 3D RNA polymerase (3Dpol) coding sequences were replaced with those from coxsackievirus B3 (CVB3). No infectious virus was produced from HeLa cells transfected with the chimeric RNA. Processing of the PV1 capsid protein precursor was incomplete, presumably due to inefficient recognition of the P1 protein substrate by the chimeric 3CD proteinase containing CVB3 3D sequences. The ability of the chimeric RNA to replicate in the absence of capsid formation was measured after replacement of the P1 region with a luciferase reporter gene. No RNA synthesis was detected, despite efficient production of enzymatically active 3Dpol from the 3D portion of the chimeric 3CD. The chimeric 3CD protein was unable to efficiently bind to the cloverleaf-like structure (CL) at the 5′ end of PV1 RNA, which has been demonstrated previously to be required for viral RNA synthesis. The CVB3 3CD protein bound the PV1 CL as well as PV1 3CD. An additional chimeric PV1 RNA that contained CVB3 3CD sequences also failed to produce virus after transfection. Since processing of PV1 capsid protein precursors by the CVB3 3CD was again incomplete, a luciferase-containing replicon was also analyzed for RNA replication. The 3CD chimera replicated at 33°C, but not at 37°C. Replacement of the PV1 5′-terminal CL with that of CVB3 did not rescue the temperature-sensitive phenotype. Thus, there is an essential interaction(s) between 3CD and other viral P2 or P3 protein products required for efficient RNA replication which is not fully achieved between proteins from the two different members of the same virus genus.
Poliovirus type 1 (PV1), the prototypic member of the picornavirus family, belongs to the genus Enteroviridae, as does coxsackievirus B3 (CVB3). CVB3 is closely related to PV1 with respect to its structure and genome organization and manifests some overlapping tissue tropism (51). Both viruses replicate primarily in enteric and respiratory tract epithelial cells; however, each can infect other target tissues that can lead to different and more severe disease. For example, PVs are able to infect nerve cells, and CVs can infect cardiomuscular tissue. In the laboratory, both viruses can infect the same cell lines (e.g., HeLa, COS, Vero, and FRhK-4) at the same optimal temperature. It is therefore likely that the RNA replication reactions of these two picornaviruses involve an equivalent set of protein-protein and protein-RNA interactions and perhaps utilize the same host cell proteins.
PV RNA replication occurs in the cytoplasm of infected cells in association with membranous complexes (3, 7, 8). These complexes contain viral polypeptides and host proteins that specifically initiate viral RNA synthesis at the 3′ ends of both plus- and minus-strand template RNAs that consist of either a homopolymeric poly(A) sequence or a heteropolymeric sequence, respectively. Once RNA synthesis is initiated, 3Dpol, the RNA-dependent RNA polymerase, catalyzes the addition of nucleotides to the growing RNA strands (12, 13). In addition to 3Dpol, other nonstructural viral proteins, RNA sequence elements, and host protein(s) are required for the initiation of synthesis of RNA; however, the mechanism by which the replication machinery recognizes the viral RNA termini and initiates RNA synthesis is obscure (44, 59).
During an infection, the replication machinery specifically amplifies viral RNAs. A complete RNA replication cycle involves the copying of genomic RNA into complementary minus-strand RNAs. Minus-strand RNAs in turn serve as templates for plus-strand synthesis. The ratio of plus to minus strands in PV-infected cells is approximately 40:1 (6, 15, 34), which suggests that there are inherent differences in the processes of the amplification of the two different strands. Towards this view, inhibitors and viral mutations exist that appear to affect one reaction and not the other (15, 20). Presumably RNA sequences within the plus- and minus-strand templates interact with the replication machinery to direct RNA synthesis catalyzed by 3Dpol.
Among members of the picornavirus family, the 3Dpol proteins share a high degree of amino acid sequence homology. Evolutionary studies suggest that the polymerase sequences of picornaviruses are relatively constrained, since this region is more highly conserved across the family than those regions coding for most other viral proteins (48). Also conserved among the picornaviruses are the RNA sequences located at the termini of the 5′ and 3′ noncoding regions (NCRs) which are believed to be involved in macromolecular interactions required for efficient RNA replication (47, 54, 56). The 5′ proximal ∼100 nucleotides of both PV and CV genomes have been predicted by computer analysis and confirmed experimentally to form a cloverleaf-like structure (CL) (2, 50). In the case of PV1 RNA, this structure has been shown to bind viral proteins 3CD, 3C, and 3AB and a host protein, poly(rC) binding protein (PCBP) (1, 2, 14, 19, 36, 37). The ability to form a ribonucleoprotein complex in vitro correlates with the ability of the virus to replicate RNA in vivo. The 3′ NCR forms an RNA structure which is also suggested to participate in RNA replication (24, 31, 32, 41). UV cross-linking studies suggest a capacity for PV1 3′ NCR to bind PV proteins containing 3Dpol sequences (19), while sequences within encephalomyocarditis virus (EMCV) 3′ NCR and the poly(A) tract bind 3Dpol directly (10, 11). Disruption or deletion of the 3′ NCR results in viruses that are debilitated in RNA replication (32, 52, 55). In addition to sequences near the genomic ends, an internal element required for RNA replication of human rhinovirus 14 was identified (29, 30). A similar element in PV1 or CVB3 has not been documented.
To dissect interactions required for RNA replication, we have generated chimeric genomes in which specific sequences of PV1 RNA were replaced with those of CVB3. A viable virus would reflect an allowable set of protein-protein and protein-RNA interactions, whereas a chimera defective in RNA replication would indicate incompatible interactions between the substituted viral protein and the rest of the viral replication machinery. By constructing genomic chimeras between two related viruses, it is possible to define interactions that are required for efficient RNA replication. Chimeric PV1 genomes were constructed that contained either the 3Dpol coding sequences and 3′ NCR of CVB3 or the 3CD coding sequences and the 3′ NCR of CVB3. Whereas neither of the chimeric genomes produced virus, the 3CD and 3′ NCR replacement was capable of RNA replication, while the 3Dpol and 3′ NCR replacement was not.
MATERIALS AND METHODS
Plasmid constructions.
Plasmid pCVB3(0) (9) contains a full-length infectious cDNA of CVB3. Plasmid pT7-PV1 (16) contains a full-length infectious cDNA of PV1, and pRLuc31 (1) contains a cDNA of a PV1-luciferase replicon. To facilitate cloning, SalI sites were introduced downstream of the poly(A) tracts in pCVB3(0), pT7-PV1, and pRLuc31. Plasmids pCVB3(0) and pRLuc31 contained preexisting SalI sites upstream of their respective promoters which would interfere with subsequent cloning. Therefore the SalI sites were eliminated by digesting each plasmid with SalI, end filling with the Klenow fragment of DNA polymerase I, and religating the DNA fragments. pCVB3 was generated by selection of a plasmid that had lost the SalI site, digestion with ClaI, end filling of the site with Klenow enzyme, and introduction of a SalI site by linker ligation. To generate pLucPV, a plasmid lacking a SalI site was digested with MluI; this site was then end filled with the Klenow enzyme, and a SalI site was introduced by linker ligation. pT7-PV1 was modified by digestion of the EcoRI site downstream of the poly(A) tract, end filling of the site with Klenow enzyme, and a SalI site was introduced by linker ligation.
Table 1 summarizes plasmids generated by PCR site-directed mutagenesis (22) to introduce or abolish restriction sites. The numbering used to describe nucleotide positions of PV1 or CVB3 genomes in expression plasmids has been adjusted to reflect the sequence numbering of the wild-type strains. All plasmids were analyzed by restriction mapping and sequencing across the PCR-generated portions to verify that no inadvertent mutations were present. Table 2 summarizes plasmids generated by conventional subcloning methods by using two-fragment ligation reactions.
TABLE 1.
Plasmids generated by PCR site-directed mutagenesisa
| Plasmid [virus] | Parent plasmid | Nucleotide change | Position | Amino acid change | Infectious status |
|---|---|---|---|---|---|
| pEXC-3D(AgeI) [PV] | pEXC-3Db | ACC | 5985–5987 | Q→T | NAc |
| pCVB3(NdeI) | pCVB3 | CATATG | 5356–5361 | V→H; Q→M | NDd |
| pT5T-3CD (AgeI) [CV] | pT5T-3CD | ACC | 5908–5910 | Q→T | NA |
| pEXC-PC-3CD [PV-CV] | pEXC-3CD(AgeI) | CAA | 5908–5910 | T→Q | NA |
| pT7PV1(Bsu36I) | pT7-PV1 | CTGA | 5417–5420 | I→L | Yes |
| pLucPV(SnaBI) | pLucPV | T,T | 109,113 | NA | NAe |
| pCVB3(SnaBI) | pCVB3 | T,C | 112,114 | NA | Yes |
TABLE 2.
Plasmids generated by two-fragment ligation reactions
| Plasmid [virus] | Vector plasmid | Insert plasmid | Restriction enzyme(s) |
|---|---|---|---|
| pT5T-3CD [CV] | pT5T-3Da | pCVB3 (NdeI) | NdeI, SalI |
| pEXC-3CD(AgeI) [PV] | pEXC-3D(AgeI) | pT5T-3CD(AgeI) | SpeIbAgeI (vector); SalIbAgeI (insert) |
| pET15-(PC)3CD(AgeI) [PV-CV] | pET15 3CDμ10c | pEXC-3CD(AgeI) | DraIII, XhoI (vector) DraIII, SalI (insert) |
| pT7PV1-CV3CD | pT7PV1(Bsu36I) | pCVB3 | Bsu36I, SalI |
| pT7PV1-CV3D | pPT7-PV1 | pEXC-PC-3CD | BglII, SalI |
| pLucPV-CV3D | pLucPV | pEXC-PC-3CD | BglII, SalI |
| pLucPV-CV3CD | pLucPV | pT7PV1-CV3CD | SpeI, SalI |
| pCVCLLucPV | pCVB3(SnaBI) | pLucPV(SnaBI) | SnaBI, SalI |
| pCVCLLucPV-CV3CD | pCVCLLucPV | pLucPV-CV3CD | SpeI, SalI |
| pLucPVΔ3Dd | pLucPV | NA | HindIII |
Preparation of PV and CV proteins.
pEXC-3D and pEXC-PC-3CD (see above) were used to transform Escherichia coli JM109 cells. Cells were grown in M9 medium at 37°C, until they reached an A600 of 0.5, at which time l-tryptophan was added to a final concentration of 20 ng/ml. Induced cells were maintained overnight with constant shaking at 25°C. Viral proteins 3CD and 3Dpol were partially purified by precipitation with 40% ammonium sulfate saturation of the soluble fraction of bacterial lysate, followed by dialysis in buffer A (0.05 M Tris [pH 8.0], 0.1% NP-40, 0.05 M KCl, 10% glycerol, 5 mM β-mercaptoethanol), and analyzed by Western blot and polymerase assays.
Recombinant (His)6-3CD fusion proteins were purified from IPTG (isopropyl-β-d-thiogalactopyranoside)-induced bacterial extracts of E. coli BL21(DE3)-pET(PC)-3CD, BL21(DE3) pET15 (CV) 3CD Age I (28a), or BL21(DE3)-pET15b 3CDμ10. Proteins were purified as previously described (37). Purified proteins were quantified by Bio-Rad protein assay and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), either by staining with Coomassie blue dye or by antibody detection.
Western blot analysis.
Samples were boiled in Laemmli sample buffer (27) and resolved by electrophoresis on SDS–12.5% polyacrylamide gels. Proteins were transferred to a nitrocellulose membrane (Schleicher & Schuell). The membrane was incubated for 1 h at 25°C with rabbit anti-PV1-3C or anti-PV1-3Dpol serum (46) and then probed with alkaline phosphatase-conjugated anti-rabbit mouse antibodies for 30 min at 25°C, followed by color detection according to the manufacturer’s protocol (Promega).
Assay of 3Dpol RNA polymerase activities.
Elongation activities of 3Dpol proteins generated from the autocatalytic cleavage of 3CD expressed in E. coli were measured as previously described (21). Reactions (50 μl) were initiated by adding 2 μl of 3D-containing extracts, and aliquots (5 μl) were removed at the indicated times. Assays were conducted in a linear range of enzyme concentrations.
In vitro transcription.
pT7-based plasmids were linearized with the appropriate enzymes. Transcriptions were performed with T7 RNA polymerase by using a MAXIscript in vitro transcription kit (Ambion, Inc.). For transfection experiments, RNA was analyzed by electrophoresis in a 1% agarose gel in 0.04 M Tris-acetate (pH 8.0)–1 mM EDTA (TAE) and stained with ethidium bromide. For translation experiments, transcription reactions were terminated by addition of DNase I. RNA was extracted with phenol-chloroform and further purified by gel filtration on a Chromaspin-1000 spin column (Clonetech) to remove unincorporated nucleoside triphosphates (NTPs). The RNA concentration was determined by A260 measurement and visualized by ethidium bromide staining in a 1% agarose–TAE gel. For electrophoretic mobility shift assays, radiolabeled RNAs were purified on a 6% sequencing gel and quantified based on radioisotope specific activity.
In vitro translation assays.
In vitro translation-RNA replication assays were performed in micrococcal nuclease-treated HeLa cell extracts as described previously (55). Reaction mixtures (50 μl) were programmed with 150 ng of full-length RNA or replicon RNA and supplemented with [35S]methionine (Amersham). Reactions mixtures were incubated at 30°C, and at indicated times, 10-μl aliquots were taken and diluted in Laemmli sample buffer and stored at −20°C. All aliquots were analyzed by SDS-PAGE. Gels were fluorographed, dried, and exposed to Kodak BioMax MR film (Kodak Corp.).
Electrophoretic mobility shift assays.
Mobility shift assays were performed as described previously (2, 4, 37). RNA probe contained the first 114 nt of PV1 sequences derived from transcription of pLucPV (SnaBI) linearized with SnaBI restriction enzyme.
Transfections and luciferase assays.
Approximately 90% confluent monolayers of HeLa cells grown in six-well plates were rinsed twice with phosphate-buffered saline (PBS). A transfection mixture (500 μl) containing ∼1 μg of crude RNA, 0.5 mg of DEAE-dextran per ml in TS buffer (137 mM NaCl, 4.4 mM KCl, 0.7 mM Na2HPO4, 25 mM Tris, 0.5 mM MgCl2, 0.68 mM CaCl2 [pH 7.4]) was applied to each well and allowed to incubate at 25°C. After 30 min, 4 ml of Dulbecco’s modified Eagle’s medium (DMEM) was added, and cultures were incubated at either 33 or 37°C for an additional hour. Medium was then replaced with MEM supplemented with 10% newborn calf serum and 1% nonessential amino acids, and plates were allowed to incubate at appropriate temperatures until harvested. At the indicated times, cells were washed twice with PBS. Cell culture lysis buffer (200 μl) (Promega) was added to each well, and cells were collected in a 1.5-ml Eppendorf tube. After pelleting of nuclei, aliquots (10 μl) of the supernatants were used in luciferase assays with the addition of 100 μl of Luciferin substrate (Promega), and light emission was measured with a Monolight 2010 luminometer (Analytical Luminescence Laboratory). In order to conduct luciferase assays in a linear range of enzyme concentrations, some extracts were diluted, and their relative light units (RLU) were calculated accordingly. Triplicate samples were analyzed for each time point, and each time course experiment was performed at least three times.
RESULTS
Construction of a PV1 genomic chimera containing CVB3 3Dpol coding sequences.
A full-length PV RNA chimera, which replaced the 3Dpol coding sequences and 3′ NCR of PV1 with those of CVB3, was engineered to determine if a closely related viral polymerase, in conjunction with PV1 proteins, could support RNA replication of an otherwise PV1 genome. A schematic diagram of this chimeric genome is displayed in Fig. 1A. When HeLa cells were transfected with RNA transcribed from the chimeric cDNA, no viable virus was recovered at either 33 or 37°C (data not shown).
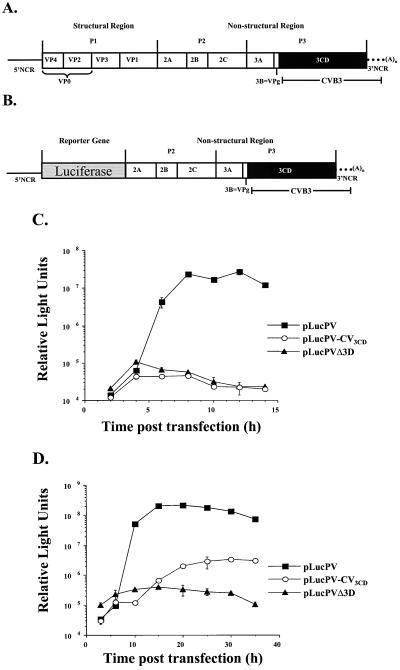
FIG. 1.
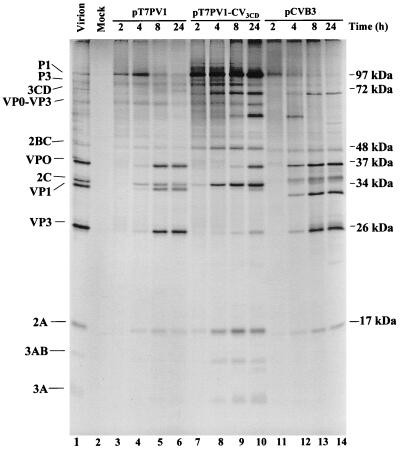
Capsid processing of PV1 chimeras containing CVB3 3Dpol. (A) Schematic representation of a full-length chimeric genome and viral polyprotein organization are shown. The viral structural proteins are derived from the P1 portion of the genome, and the nonstructural proteins are derived from the P2 and P3 portions of the genome. PV sequences are represented by solid lines and white boxes, whereas CV sequences are represented by the black box (3D coding sequences) and dotted lines (3′ NCR). (B) In vitro translation of PV1, CVB3, and chimeric T7 transcripts. Full-length transcripts from wild-type PV1 cDNA (lanes 3 to 6), chimeric PV1-CVB3 3D cDNA (lanes 7 to 10), and wild-type CVB3 cDNA (lanes 11 to 14) were used to program translation reactions in HeLa S10-ribosomal salt wash extracts. At the indicated times, aliquots were removed, diluted in Laemmli sample buffer, and subjected to electrophoresis on a 12.5% polyacrylamide–SDS gel. Lane 2 contains a mock translation mixture incubated for 8 h with no RNA added. A translation reaction mixture programmed with PV1 virion RNA was loaded in lane 1 as a marker for viral proteins. The positions of viral proteins are indicated to the left and the molecular masses are indicated to the right.
Protein processing by 3D chimera.
3D amino acid sequences constitute the C-terminal portion of the viral 3CD protein, which is responsible for proteolytic cleavage of PV1 structural and nonstructural proteins (36, 62). Introduction of the CVB3 3D sequences generated a chimeric 3CD proteinase, comprised of PV1 3Cpro sequences and CVB3 3Dpol sequences. To investigate if the RNA containing the chimeric 3CD could be efficiently translated and processed, in vitro translation reactions were programmed with the full-length chimeric RNA. Virus-specific proteins were labeled with [35S]methionine and over a time period of 2 to 24 h, aliquots were analyzed by SDS-PAGE to determine the overall pattern and kinetics of protein processing (Fig. 1B). Lanes 3 through 6 show the kinetics of processing typical for wild-type PV1 polyprotein, and lanes 11 through 14 show the characteristic protein profile resulting from wild-type CVB3 polyprotein processing. The processing pattern of the chimeric polyprotein (lanes 7 to 10) resembles that of PV, with the exception of the accumulation of a >60-kDa band (compare lanes 6 and 10) and the delayed appearance of VP0 and VP3. After 4 h, VP0 and VP3 are both evident in the wild-type PV translation (lane 4), while neither product is observed for the chimera at this time (lane 8). The >60-kDa band that accumulates at 4 h most likely represents uncleaved VP0-VP3 (also designated 1ABC) which has a predicted molecular mass of 63 kDa. VP1 is generated by 4 h, suggesting that the VP3-VP1 cleavage occurs more readily than the VP0-VP3 cleavage, consistent with previous studies (28). Efficient processing at the C terminus of VP1 is mediated by 2Apro (57). At 8 h (lane 9), cleavage of additional P1 by the chimeric 3CD produces VP0-VP3 (63 kDa) and VP1 (34 kDa), and some VP0 and VP3 are detected. By 24 h (lane 10), VP0 and VP3 accumulate, coinciding with a slight decrease of VP0-VP3 (1ABC). The results indicate that the chimeric 3CDpro cleaves the PV1 P1 less efficiently than does PV1 3CD and that even after 24 h of incubation, unprocessed capsid protein intermediates are still present. The cleavage of P2-P3 polyproteins by the chimeric 3CD in vitro appeared to occur normally.
Defective replication of a PV1 replicon containing CVB3 3Dpol.
Since failure to efficiently cleave the PV1 structural proteins may have contributed to the lack of recovery of viable virus, we employed a luciferase assay to determine the effects of CVB3 3D on RNA replication. Picornavirus replicons in which the P1 coding sequence was replaced with a reporter gene have been described previously (1, 29, 40, 43, 50, 58). Replicons replicate in cultured cells, but since they lack capsid-coding sequences, no virus is produced. RNA replication can be measured by Northern blot analysis of total cell RNA, or if a reporter gene is used, then RNA replication can be measured indirectly by the activity of the reporter gene product. As the RNA is amplified, the gene for the reporter is also amplified and translated. A complete RNA replication cycle is measured, since the exponential amplification of the reporter gene relies on the faithful copying of the input RNAs into complementary minus stands and, subsequently, the copying of the minus strands into plus strands.
The 3Dpol coding sequences of CVB3 were subcloned into a PV-luciferase replicon (Fig. 2A), which was used to measure RNA replication in cultured HeLa cells in the absence of virus production. Because input RNA from the transfection can serve as mRNA, a basal level of luciferase activity was expected, even in the absence of RNA amplification. As a measure of the basal level of luciferase activity due to translation of input RNA, a reporter PV-luciferase construct was generated which contained an internal deletion in the 3Dpol gene. The resulting RNA could be translated, but could not be replicated. Figure 2B shows that this nonreplicating RNA induced a low level of luciferase activity, which remained relatively constant and then decreased over time. The wild-type PV-luciferase replicon displayed an exponential increase in the amount of luciferase activity over time, reflecting amplification of the luciferase template due to active RNA replication. No virus-specific RNA replication was detected from the chimeric RNA at either the normal permissive temperature of 37°C (Fig. 2B) or at 33°C (Fig. 2C). Since a defect in either minus-strand synthesis or plus-strand synthesis would result in a null RNA replication phenotype, it is not possible to determine if both or only one reaction is affected by the CVB3 3D polymerase sequence. These data suggest that CVB3 3Dpol is unable to interact with other PV1 proteins or RNA sequences to support RNA replication. However, it was first necessary to demonstrate that the chimeric 3CD was able to generate a functional CVB3 3Dpol.
FIG. 2.
Lack of RNA replication of PV chimeras containing CVB3 3Dpol. (A) Schematic representation of a chimeric PV replicon in which the nonstructural genes have been replaced with a luciferase reporter gene. (B) Time course of RNA replication measured by luciferase accumulation following RNA transfection of HeLa cells at 37°C. (C) Time course of RNA replication measured by luciferase accumulation following RNA transfection of HeLa cells at 33°C.
Production of CVB3 3Dpol.
Although the overall pattern of nonstructural protein processing was observed by translation of genome-length RNAs, a detailed analysis of 3CD cleavage to 3Dpol and 3Cpro was not readily visualized. The amino acid sequence at the chimeric 3C/3D cleavage site is unlike either wild-type PV1 or CVB3 3CD junctions. We wanted to determine if the chimeric 3CD protein was capable of autocatalytic cleavage and, if so, whether a functional polymerase was produced. The 3CD cDNA was subcloned into an E. coli expression vector to generate pEXC-PC 3CD. Diagrams of pEXC-3D (which expresses PV1 3CD) and pEXC-PC-3CD (which expressed the chimeric 3CD) and their expected proteins are shown in Fig. 3A and B. Soluble E. coli extracts were analyzed by a Western blot probed with PV1 anti-3D serum (Fig. 3C). The two polymerase molecules share ∼74% amino acid identity, and both react with antiserum raised against PV1 3D. Lanes 1 to 5 contain dilutions of PV1 3CD-containing extracts. A prominent band that migrates at 72 kDa corresponds to PV1 3CD, and a second prominent band that migrates at 52 kDa identifies 3Dpol. Lanes 6 to 10 contain dilutions of extracts containing the chimeric 3CD. At lower dilutions (lanes 6 and 7), a band that migrates at 72 kDa is detected which corresponds to the fusion of PV1 3C and CVB3 3D proteins. Although the ratio of 3D to 3CD appears to be greater for the chimeric protein than for the PV protein, it is possible that the chimeric 3CD is less soluble and was therefore not recovered following the centrifugation step used to prepare the extracts. A major protein species at 52 kDa is detected which represents CVB3 3Dpol. Thus in E. coli, the chimeric 3CD is able to cleave itself to produce a protein that is the expected size of CVB3 3Dpol.
FIG. 3.
(A) Production of PV1 3Dpol and CVB3 3Dpol in E. coli. A schematic of PV1 3CD fusion protein expressed in E. coli is presented. 3CD is processed to generate active PV1 3Cpro and PV1 3Dpol. (B) Schematic of a chimeric 3CD fusion protein expressed in E. coli. The chimeric 3CD is autocleaved to generate PV1 3Cpro and CVB3 3Dpol. (C) Western blot analysis of soluble lysates of 3CD expressed in E. coli. Dilutions of extracts containing wild-type PV1 3CD (lanes 1 to 5) and chimeric 3CD (lanes 6 to 10) were resolved by SDS-PAGE (10% polyacrylamide) and transferred to nitrocellulose membrane for analysis with antiserum raised against PV1 3Dpol. The positions and sizes of viral proteins are indicated to the right. (D) Elongation activities of PV1 3Dpol and CV3 3Dpol generated from autocleavage of 3CD fusion proteins. Portions were taken at the indicated time and measured for UMP incorporation as described in Materials and Methods.
To demonstrate that the resulting CVB3 3D protein was catalytically active, we assayed preparations from the E. coli extracts for RNA chain elongation activity. Enzymatic properties of PV1 polymerase expressed in E. coli are indistinguishable from polymerase purified from PV1-infected HeLa cells (33). Therefore, polymerase activity from 3Dpol expressed in E. coli likely reflects the activity that would be expressed from a full-length polyprotein. To estimate the enzymatic activity of the CVB3 polymerase, extracts from bacteria expressing either the chimeric 3CD or wild-type PV1 3CD, both of which produce 3Dpol, were assayed for RNA polymerase activity. Poly(A) templates and oligo(U) primers were incubated with protein preparations, and the amount of [3H]UMP incorporation was measured over time (Fig. 3D). Similar levels of activity were detected for CVB3 3Dpol and PV1 3Dpol, indicating that the chimeric 3CD generates a functional polymerase in this RNA chain elongation assay. The quantitation of activity levels and protein concentrations indicates that the PV1 and CVB3 3D proteins manifest approximately the same specific activities (data not shown). Despite production of similar amounts and activities of 3D proteins in E. coli, it is possible that subtle alterations in P3 protein processing in HeLa cells might contribute to the failure of the chimeric RNA to be replicated in transfected cells.
Production of purified 3CD protein.
To examine biochemical functions of 3CD and to compare the chimeric 3CD with PV1 3CD and CVB3 3CD, we expressed and purified N-terminal histidine-tagged versions of all three proteins. CVB3 3CD and the chimeric 3CD cDNAs were recloned into a histidine expression plasmid described previously (4). 3CD is normally cleaved at a Gln-Gly junction (17) to generate 3C and 3D. The Gln-Gly amino acid sequences at the 3C-3D junction were changed to Thr-Gly for the chimeric and CVB3 proteins in order to minimize cleavage (data not shown). PV1 3CD contained a serine insertion adjacent to the 3C-3D junction, which similarly prevented cleavage (53). Proteins were purified from bacterial extracts by nickel affinity chromatography. A Coomassie blue-stained gel indicated the recovery of proteins of the expected sizes (Fig. 4A). In addition, each preparation contains faster-migrating protein species. Figure 4B shows a Western blot of the PV1 His-3CD, the chimeric His-3CD, and CVB3 His-3CD obtained with an antibody raised against PV1 3C to detect 3C-containing proteins. The conservation of amino acid sequences between PV1 3C and CVB3 3C is ∼60%. Apparently PV1 3C antiserum does not recognize epitopes in CVB3 3C (2a). Only PV1 3CD and the chimeric 3CD, which contains PV1 3C sequences, are identified in the immunoblot. The reactivity of PV1 3C antibody with the faster-migrating bands (≤30 kDa) suggests that these peptides are His-tagged, amino-terminal fragments containing 3C.
FIG. 4.
Purification and biochemical analysis of His-tagged 3CD proteins expressed in E. coli. His-tagged proteins were expressed in BL21(DE3) cells and affinity purified. (A) Coomassie blue staining of a 12.5% polyacrylamide–SDS gel following electrophoresis of fractions (lane 1, chimeric 3CD; lane 2, CVB3 3CD; lane 3, PV1 3CD) purified on a nickel column. Lane 4 contains marker proteins. (B) Western blot analysis of a gel identical to that shown in panel A. Antiserum raised against PV1 3C was used for immunoblot analysis. (C) RNA electrophoretic mobility shift analysis with an RNA probe representing the PV1 5′ CL structure. PV1 probe was incubated with buffer alone (lane 1), with recombinant PCBP2 alone, or with recombinant PCBP2 and the indicated amounts of 3CD (lanes 3 to 11). RNA-protein complexes were resolved on a 4% native polyacrylamide gel and are indicated to the left of the autoradiograph.
RNA binding by chimeric 3CD.
A previously characterized activity of PV1 3CD is the formation of a ribonucleoprotein complex with the first ∼100 nt of the 5′ end of PV1 plus-strand RNA. In the presence of a host factor, PCBP, PV1 3CD forms a stable ternary complex with 5′ proximal sequences, which fold into a CL structure (1, 2, 5, 14, 37). To test whether the chimeric 3CD could bind to the PV1 5′ proximal sequences, electrophoretic mobility shift assays were performed with PV1 CL RNA representing the 5′-terminal ∼100 nt of plus-strand RNA (Fig. 4C). Increasing amounts of purified His-3CD were incubated with probe in the presence of purified recombinant PCBP2 (rPCBP2). Only at the highest concentration does the chimeric 3CD bind the PV1 probe, while PV1 3CD forms a ternary complex with the probe and rPCBP2 at much lower concentrations. Interestingly, the authentic CVB3 3CD formed a ternary complex at the lowest concentrations of protein tested. These data indicate a significantly reduced affinity of the chimeric 3CD protein for the PV1 CL and suggest a possible cause for the failure of PV1 RNAs containing CVB3 3D sequences to support RNA replication.
RNA replication of a PV1 replicon containing CVB3 3CD.
The experiments described above showed that the replacement of PV1 3D coding sequences with those from CVB3 adversely affected 3CD RNA binding activity, while CVB3 3CD appeared to bind as well as PV1 3CD. To eliminate the defective 3CD molecule, a PV chimera that exchanged 3C sequences as well as 3D sequences for those of CVB3 was generated (Fig. 5A). RNA transcribed in vitro did not generate virus in HeLa cell transfection experiments at either 33 or 37°C (data not shown). The CVB3 3CD sequences were inserted into a luciferase-containing PV replicon to generate pLucPV-CV3CD, and this chimeric reporter replicon was used in transfection experiments to determine if the RNA could replicate in HeLa cells (Fig. 5B). Detectable luciferase activity was measured at 33°C (Fig. 5D), but not at 37°C (Fig. 5C). At 33°C, luciferase activity reached ∼5 to 20% (Fig. 5D; data not shown) of that observed with PV1 3CD, suggesting that RNA replication of the chimera was temperature sensitive and notably less efficient than the wild-type PV1 sequences.
FIG. 5.
RNA replication of a PV-replicon chimera containing CVB3 3CD. (A) A schematic representation of a full-length chimeric PV1 genome structure and viral protein organization similar to that in Fig. 1A is shown. The 3CD coding sequences and 3′ NCR of PV1 genome were replaced with those of CVB3. (B) A schematic representation of a chimeric PV replicon containing CVB3 3CD and 3′ NCR is shown. (C) Time course of RNA replication measured by luciferase accumulation following RNA transfection of HeLa cells at 37°C. (D) Time course of RNA replication measured by luciferase accumulation following RNA transfection of HeLa cells at 33°C.
In vitro translation and protein processing of 3CD chimera.
To determine the translation and protein processing efficiencies of the chimeric poliovirus genomic length RNA encoding CVB3 3CD, RNA transcribed from pT7-PV1-CVB3 3CD was used to program HeLa cell extracts competent for translation. [35S]methionine-labeled proteins were analyzed by SDS-PAGE. Products observed after 24 h of translation demonstrated that CVB3 3CDpro did not efficiently cleave the PV P1 protein (Fig. 6, lane 10). The 63-kDa band, which represents uncleaved VP0-VP3 (1ABC), is evident after 24 h of incubation. VP0 is generated to a greater extent than either VP3 or VP1, which indicates that cleavage occurs more readily at the VP0-VP3 junction than at the VP3-VP1 junction. Failure to generate VP3 and VP1 correlates with the presence of a protein of approximately 60 kDa that may represent uncleaved VP1-VP3. In vitro translations programmed with RNA transcribed from pLucPV-CV3CD were also analyzed by SDS-PAGE. CVB3 3CD processed PV1 P2 and P3 polyproteins as efficiently as did PV1 3CD (data not shown). The inability of CVB3 3CD to efficiently cleave structural proteins of PV1 most likely contributes to the inability of this chimera to produce infectious virus despite its ability to replicate RNA.
FIG. 6.
In vitro translation and processing of PV1, CVB3, and chimeric T7 transcripts. Full-length transcripts from wild-type PV1 cDNA (lanes 3 to 6), chimeric PV1-CVB3 3CD cDNA (lanes 7 to 10), and wild-type CVB3 cDNA (lanes 11 to 14) were used to program translation in HeLa S10 extracts (supplemented with a ribosomal salt wash from HeLa cells) in the presence of [35S]methionine. Reactions were analyzed as described in the legend to Fig. 2.
CL exchange in CVB3 3CD replicon.
The replacement of PV1 3CD sequences with CVB3 3CD sequences resulted in RNA replication of the chimeric RNA in HeLa cells, (Fig. 5D) albeit with low efficiency. We sought to determine whether replacement of the 5′-terminal RNA sequences with those from the cognate CVB3 CL sequence would result in more efficient RNA replication. The 5′ proximal ∼100 nt of the chimeric replicon, pLucPV-CV3CD, were replaced with those of CVB3 to generate pCVCLLucPV-CV3CD (Fig. 7A). RNA from this chimera was used to transfect HeLa cells, and samples were taken at 5-h intervals to determine luciferase activity as a measure of RNA replication (Fig. 7B). The chimeric replicon failed to replicate RNA at 37°C, demonstrating that the match between CVB3 3CD and the CVB3 CL was not sufficient to restore efficient RNA replication to the 3CD chimera at 37°C (data not shown). Interestingly, a replicon that contained only the CVB3 CL in exchange for PV1 CL replicated RNA to the same level as the wild-type PV replicon, suggesting that PV1 3CD interacted productively with CVB3 CL (Fig. 7B). At 33°C, the chimeric replicon, pCVCLLucPV-CV3CD, exhibited the same inefficient level of RNA replication as pLucPV-CV3CD, which contained only the CVB3 3CD sequences. Both genomes replicated to ∼10% of the wild-type level. These data indicate that some property or function of 3CD other than CL binding is responsible for the temperature-sensitive RNA replication phenotype.
FIG. 7.
Inability of CVB3 5′CL to rescue temperature sensitivity of PV1-CVB3 3CD replicon. (A) Schematic representation of a chimeric PV replicon in which the structural genes have been replaced with a luciferase reporter gene is presented. The first ∼100 5′ nt, 3CD coding region, and 3′ NCR of PV1 sequences were replaced with CVB3 sequences. (B) Time course of RNA replication measured by luciferase accumulation following RNA transfection of HeLa cells at 33°C. In this experiment, pRLuc31-2Cpro, which has a lethal mutation in 2C and does not replicate (25), was used as a negative RNA replication control.
DISCUSSION
Specificity of picornavirus RNA replication must, in part, rely on RNA sequences within the viral genome. It is unclear, however, whether interactions between 3Dpol and viral RNA are responsible for the specificity or whether other viral and/or host proteins are involved in directing the polymerase to its RNA target via protein-protein and protein-RNA interactions. The membranous replication complex may also contribute to the specificity of RNA replication by limiting the diffusion of competent viral replication proteins out of the complex and likewise excluding cellular mRNAs from the complex. The proximity of 3Dpol coding sequences to the 3′ end of the genome positions newly translated 3Dpol polymerase near the putative initiation site of RNA replication, increasing the likelihood that it will amplify viral RNA. The question we addressed was whether a functional RNA polymerase from a related virus could support RNA replication of a PV1 genome, or whether each RNA-dependent RNA polymerase of picornaviruses coevolved with its own RNA elements and other viral proteins within a host cell to give rise to virus-specific RNA polymerase molecules that are limited to replicating their own genome.
In order to examine the specificity of RNA replication, a series of allelic exchanges that included viral polymerase sequences were constructed between the PV1 and CVB3 genomes. Although no viable virus was recovered from any of the chimeric genomes, RNA replication was monitored in cell cultures utilizing luciferase-based PV1 replicons. A chimeric replicon that contained CVB3 3Dpol coding sequences and CVB3 3′ NCR resulted in a null RNA replication phenotype despite efficient production of 3Dpol polymerase activity from the chimeric 3CD expressed in E. coli. It is not known whether synthesis of both plus and minus strands is defective, since either would result in failure to accumulate luciferase activity in this assay.
Binding assays demonstrated that the chimeric 3CD expressed in E. coli was less efficient than either wild-type PV1 or CVB3 3CD proteins in interacting with PV1 CL in vitro. The observed affinity of CVB3 3CD for PV1 CL prompted the exchange of PV1 3CD coding sequences for those of CVB3. When the CVB3 sequences in the chimera were increased to include CVB3 3C in addition to 3D, RNA replication levels that were 5 to 20% that of wild-type PV1 levels were readily detected at 33°C, but not at 37°C. The amount of RNA generated from the PV1-CVB3 3CD chimera may not be adequate for encapsidation, suggesting that a threshold level of RNA is needed for virus production. Alternatively, a delay in the kinetics of structural protein processing was observed that may affect the coupling between RNA replication and packaging (25, 35) and contribute to the lack of virus production. The inability of CVB3 3CD to efficiently cleave PV1 P1 protein to generate capsids likely contributed to the failure of the chimeric genome to produce virus.
Measurable RNA replication by the PV1 replicon containing CVB3 3CD and 3′ NCR suggested that although CVB3 3Dpol can support RNA replication of an otherwise PV1 genome, a complete CVB3 3CD sequence is required for some step in the RNA replication reaction. The temperature sensitivity and low levels of RNA replication observed for the CVB3 3CD chimera suggest that CVB3 3CD does not interact efficiently with other PV1 viral proteins or RNA sequences. In previous studies utilizing picornavirus chimeras, it was demonstrated that incompatibility between 3CD protein and the 5′ CL structure would result in defective RNA replication (50, 61). Therefore, a possible cause for the inefficient RNA replication of the chimera described above would be an incompatible interaction between that of CVB3 3CD and the PV1 5′ CL. However, CVB3 3CD and PV1 3CD demonstrated comparable affinities for interaction with PV1 5′ CL in vitro; furthermore, there were no significant differences in RNA replication between the 3CD chimera containing PV1 5′ CL and the chimera containing CVB3 5′CL, suggesting that the 5′ CLs are interchangeable. Replacing PV1 5′ CL with CVB3 5′ CL resulted in a replicon that exhibited a wild-type RNA replication phenotype and demonstrates that all PV nonstructural proteins interact with the CVB3 5′ CL as efficiently as with PV1 5′ CL, as reported previously (26). The inability to restore RNA replication to a wild-type phenotype by introducing the CVB3 5′ CL suggests that protein-protein and/or protein-RNA interactions are required with CVB3 3CD or 3Dpol for RNA replication in addition to the 3CD-5′ CL interaction.
All chimeras utilized in this study contained the 3′ NCR of CVB3 in addition to the CVB3 3D or 3CD coding sequences. Mutations within the 3′ NCRs of picornavirus genomes lead to defects in RNA replication, suggesting a critical role for the 3′ NCR in RNA synthesis (31, 52, 55). It is not known if the exchange of the 3′ NCR contributed to the low levels of RNA replication induced by the chimeric genome. However, Rohll et al. (49) showed that exchanging the 3′ NCR of PV3 with that of CVB4 or other closely related picornavirus 3′ NCRs resulted in replicons with wild-type replication kinetics. These data suggested that sequences within the 3′ NCRs can be interchanged with minimal consequences for RNA replication despite the relatively high divergence in sequence and structure.
The process of viral RNA replication involves a series of protein-protein and protein-RNA interactions that require molecular compatibility between the interacting components. It is known that viral proteins bind or interact with one another, although not all of the functional consequences of these interactions are understood. Interactions between 3Dpol and 3AB have been demonstrated (23, 39, 42, 45, 60). Stimulation of 3Dpol polymerase activity in vitro by PV1 3AB has also been described (38, 42, 45). This observed stimulation of 3Dpol activity by 3AB occurs because 3AB increases the utilization of 3′-hydroxyl termini by 3Dpol at sites of chain elongation, most likely by interacting and recruiting 3Dpol to the sites (45). PV1 3AB similarly stimulates CVB3 3Dpol polymerase activity in vitro, indicating that PV1 3AB and CVB3 3Dpol can interact (45). Other interactions between 3B (VPg) and 3Dpol have been described (39). To determine which interactions with the CVB3 3CD protein are impaired in the PV chimera described in this report, additional exchanges that substitute the entire P3 coding region of PV1 for that of CVB3 may be informative. Such exchanges will reveal whether RNA replication is more efficient when 3Dpol interacts with the cognate 3AB protein.
ACKNOWLEDGMENTS
We thank Oliver Richards, Natalia Teterina, Mario Rinaudo, and Joanna Pyper for critical reading of the manuscript. We are also grateful to Louis E.-C. Leong for the gift of plasmid pET15(CV) 3CD-Age I, Nora Chapman and Steven Tracy for the gift of plasmid pCVB3(0), and Raul Andino for the gift of plasmid pRLuc31.
This work was supported by Public Health Service grants AI 17386 and AI 22693 from the National Institutes of Health.
REFERENCES
- 1.Andino R, Rieckhof G E, Achacoso P L, Baltimore D. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5′-end of viral RNA. EMBO J. 1993;12:3587–3598. doi: 10.1002/j.1460-2075.1993.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andino R, Rieckhof G E, Baltimore D. A functional ribonucleoprotein complex forms around the 5′ end of poliovirus RNA. Cell. 1990;63:369–380. doi: 10.1016/0092-8674(90)90170-j. [DOI] [PubMed] [Google Scholar]
- 2a.Bell, Y. C., J. H. C. Nguyen, and B. L. Semler. Unpublished observations.
- 3.Bienz K, Egger D, Pasamontes L. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology. 1987;160:220–226. doi: 10.1016/0042-6822(87)90063-8. [DOI] [PubMed] [Google Scholar]
- 4.Blair W S, Nguyen J H, Parsley T B, Semler B L. Mutations in the poliovirus 3CD proteinase S1-specificity pocket affect substrate recognition and RNA binding. Virology. 1996;218:1–13. doi: 10.1006/viro.1996.0160. [DOI] [PubMed] [Google Scholar]
- 5.Blair W S, Parsley T B, Bogerd H P, Towner J S, Semler B L, Cullen B R. Utilization of a mammalian cell-based RNA binding assay to characterize the RNA binding properties of picornavirus 3C proteinases. RNA. 1998;4:215–225. [PMC free article] [PubMed] [Google Scholar]
- 6.Burns C C, Richards O C, Ehrenfeld E. Temperature-sensitive polioviruses containing mutations in RNA polymerase. Virology. 1992;189:568–582. doi: 10.1016/0042-6822(92)90580-i. [DOI] [PubMed] [Google Scholar]
- 7.Caliguiri L A, Tamm I. Membranous structures associated with translation and transcription of poliovirus RNA. Science. 1969;166:885–886. doi: 10.1126/science.166.3907.885. [DOI] [PubMed] [Google Scholar]
- 8.Caliguiri L A, Tamm I. Characterization of poliovirus-specific structures associated with cytoplasmic membranes. Virology. 1970;42:112–122. doi: 10.1016/0042-6822(70)90243-6. [DOI] [PubMed] [Google Scholar]
- 9.Chapman N M, Tu Z, Tracy S, Gauntt C J. An infectious cDNA copy of the genome of a non-cardiovirulent coxsackievirus B3 strain: its complete sequence analysis and comparison to the genomes of cardiovirulent coxsackieviruses. Arch Virol. 1994;135:115–130. doi: 10.1007/BF01309769. [DOI] [PubMed] [Google Scholar]
- 10.Cui T, Porter A G. Localization of binding site for encephalomyocarditis virus RNA polymerase in the 3′-noncoding region of the viral RNA. Nucleic Acids Res. 1995;23:377–382. doi: 10.1093/nar/23.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui T, Sankar S, Porter A G. Binding of encephalomyocarditis virus RNA polymerase to the 3′-noncoding region of the viral RNA is specific and requires the 3′-poly(A) tail. J Biol Chem. 1993;268:26093–26098. [PubMed] [Google Scholar]
- 12.Dasgupta A, Baron M H, Baltimore D. Poliovirus replicase: a soluble enzyme able to initiate copying of poliovirus RNA. Proc Natl Acad Sci USA. 1979;76:2679–2683. doi: 10.1073/pnas.76.6.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flanegan J B, Baltimore D. Poliovirus-specific primer-dependent RNA polymerase able to copy poly(A) Proc Natl Acad Sci USA. 1977;74:3677–3680. doi: 10.1073/pnas.74.9.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamarnik A V, Andino R. Two functional complexes formed by KH domain containing proteins with the 5′ noncoding region of poliovirus RNA. RNA. 1997;3:882–892. [PMC free article] [PubMed] [Google Scholar]
- 15.Giachetti C, Semler B L. Role of a viral membrane polypeptide in strand-specific initiation of poliovirus RNA synthesis. J Virol. 1991;65:2647–2654. doi: 10.1128/jvi.65.5.2647-2654.1991. . (Errata, 65:3972 and 65:5653.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller A A, Semler B L. Linker scanning mutagenesis of the internal ribosome entry site of poliovirus RNA. J Virol. 1992;66:5075–5086. doi: 10.1128/jvi.66.8.5075-5086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanecak R, Semler B L, Anderson C W, Wimmer E. Proteolytic processing of poliovirus polypeptides: antibodies to polypeptide P3-7c inhibit cleavage at glutamine-glycine pairs. Proc Natl Acad Sci USA. 1982;79:3973–3977. doi: 10.1073/pnas.79.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen J L, Long A M, Schultz S C. Structure of the RNA dependent RNA polymerase of poliovirus. Structure. 1997;5:1109–1122. doi: 10.1016/s0969-2126(97)00261-x. [DOI] [PubMed] [Google Scholar]
- 19.Harris K S, Xiang W, Alexander L, Lane W S, Paul A V, Wimmer E. Interaction of poliovirus polypeptide 3CDpro with the 5′ and 3′ termini of the poliovirus genome. Identification of viral and cellular cofactors needed for efficient binding. J Biol Chem. 1994;269:27004–27014. [PubMed] [Google Scholar]
- 20.Heinz B A, Vance L M. The antiviral compound enviroxime targets the 3A coding region of rhinovirus and poliovirus. J Virol. 1995;69:4189–4197. doi: 10.1128/jvi.69.7.4189-4197.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hey T D, Richards O C, Ehrenfeld E. Synthesis of plus- and minus-strand RNA from poliovirion RNA template in vitro. J Virol. 1986;58:790–796. doi: 10.1128/jvi.58.3.790-796.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higuchi R. Recombinant PCR. In: Innis M A, Gelfand D H, Sninsky J J, White T J, editors. PCR protocols: a guide to methods and applications. San Diego, Calif: Academic Press, Inc.; 1990. pp. 177–196. [Google Scholar]
- 23.Hope D A, Diamond S E, Kirkegaard K. Genetic dissection of interaction between poliovirus 3D polymerase and viral protein 3AB. J Virol. 1997;71:9490–9498. doi: 10.1128/jvi.71.12.9490-9498.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson S J, Konings D A M, Sarnow P. Biochemical and genetic evidence for a pseudoknot structure at the 3′ terminus of the poliovirus RNA genome and its role in viral RNA amplification. J Virol. 1993;67:2961–2971. doi: 10.1128/jvi.67.6.2961-2971.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia X-Y, Van Eden M, Busch M G, Ehrenfeld E, Summers D F. trans-Encapsidation of a poliovirus replicon by different picornavirus capsid proteins. J Virol. 1998;72:7972–7977. doi: 10.1128/jvi.72.10.7972-7977.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson V H, Semler B L. Defined recombinants of poliovirus and coxsackievirus: sequence-specific deletions and functional substitutions in the 5′-noncoding regions of viral RNAs. Virology. 1988;162:47–57. doi: 10.1016/0042-6822(88)90393-5. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Lawson M A, Semler B L. Picornavirus protein processing—enzymes, substrates, and genetic regulation. Curr Top Microbiol Immunol. 1990;161:49–87. [PubMed] [Google Scholar]
- 28a.Leong, L. E. C., and B. L. Semler. Unpublished data.
- 29.McKnight K L, Lemon S M. Capsid coding sequence is required for efficient replication of human rhinovirus 14 RNA. J Virol. 1996;70:1941–1952. doi: 10.1128/jvi.70.3.1941-1952.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKnight K L, Lemon S M. The rhinovirus type 14 genome contains an internally located RNA structure that is required for viral replication. RNA. 1998;4:1569–1584. doi: 10.1017/s1355838298981006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melchers W J G, Hoenderop J G J, Bruins Slot H J, Pleij C W A, Pilipenko E V, Agol V I, Galama J M D. Kissing of the two predominant hairpin loops in the coxsackie B virus 3′ untranslated region is the essential structural feature of the origin of replication required for negative-strand RNA synthesis. J Virol. 1997;71:686–696. doi: 10.1128/jvi.71.1.686-696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellits K H, Meredith J M, Rohll J B, Evans D J, Almond J W. Binding of a cellular factor to the 3′ untranslated region of the RNA genomes of entero- and rhinoviruses plays a role in virus replication. J Gen Virol. 1998;79:1715–1723. doi: 10.1099/0022-1317-79-7-1715. [DOI] [PubMed] [Google Scholar]
- 33.Neufeld K L, Richards O C, Ehrenfeld E. Purification, characterization, and comparison of poliovirus RNA polymerase from native and recombinant sources. J Biol Chem. 1991;266:24212–24219. [PubMed] [Google Scholar]
- 34.Novak J E, Kirkegaard K. Improved method for detecting poliovirus negative strands used to demonstrate specificity of positive-strand encapsidation and the ratio of positive to negative strands in infected cells. J Virol. 1991;65:3384–3387. doi: 10.1128/jvi.65.6.3384-3387.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nugent C I, Johnson K L, Sarnow P, Kirkegaard K. Functional coupling between replication and packaging of poliovirus replicon RNA. J Virol. 1999;73:427–435. doi: 10.1128/jvi.73.1.427-435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsley T B, Cornell C T, Semler B L. Modulation of the RNA binding and protein processing activities of poliovirus polypeptide 3CD by the viral RNA polymerase domain. J Biol Chem. 1999;274:12867–12876. doi: 10.1074/jbc.274.18.12867. [DOI] [PubMed] [Google Scholar]
- 37.Parsley T B, Towner J S, Blyn L B, Ehrenfeld E, Semler B L. Poly (rC) binding protein 2 forms a ternary complex with the 5′-terminal sequences of poliovirus RNA and the viral 3CD proteinase. RNA. 1997;3:1124–1134. [PMC free article] [PubMed] [Google Scholar]
- 38.Paul A V, Cao X, Harris K S, Lama J, Wimmer E. Studies with poliovirus polymerase 3Dpol. Stimulation of poly(U) synthesis in vitro by purified poliovirus protein 3AB. J Biol Chem. 1994;269:29173–29181. [PubMed] [Google Scholar]
- 39.Paul A V, van Boom J H, Filippov D, Wimmer E. Protein-primed RNA synthesis by purified poliovirus RNA polymerase. Nature. 1998;393:280–284. doi: 10.1038/30529. [DOI] [PubMed] [Google Scholar]
- 40.Percy N, Barclay W S, Sullivan M, Almond J W. A poliovirus replicon containing the chloramphenicol acetyltransferase gene can be used to study the replication and encapsidation of poliovirus RNA. J Virol. 1992;66:5040–5046. doi: 10.1128/jvi.66.8.5040-5046.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilipenko E V, Maslova S V, Sinyakov A N, Agol V I. Towards identification of cis-acting elements involved in the replication of enterovirus and rhinovirus RNAs: a proposal for the existence of tRNA-like terminal structures. Nucleic Acids Res. 1992;20:1739–1745. doi: 10.1093/nar/20.7.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plotch S J, Palant O. Poliovirus protein 3AB forms a complex with and stimulates the activity of the viral RNA polymerase, 3Dpol J. Virol. 1995;69:7169–7179. doi: 10.1128/jvi.69.11.7169-7179.1995. . (Erratum, 70:682, 1996.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porter D C, Ansardi D C, Choi W S, Morrow C D. Encapsidation of genetically engineered poliovirus minireplicons which express human immunodeficiency virus type 1 Gag and Pol proteins upon infection. J Virol. 1993;67:3712–3719. doi: 10.1128/jvi.67.7.3712-3719.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richards O C, Ehrenfeld E. Poliovirus RNA replication. Curr Top Microbiol Immunol. 1990;161:89–119. doi: 10.1007/978-3-642-75602-3_4. [DOI] [PubMed] [Google Scholar]
- 45.Richards O C, Ehrenfeld E. Effects of poliovirus 3AB protein on 3D polymerase-catalyzed reaction. J Biol Chem. 1998;273:12832–12840. doi: 10.1074/jbc.273.21.12832. [DOI] [PubMed] [Google Scholar]
- 46.Richards O C, Ivanoff L A, Bienkowska-Szewczyk K, Butt B, Petteway S R J, Rothstein M A, Ehrenfeld E. Formation of poliovirus RNA polymerase 3D in Escherichia coli by cleavage of fusion proteins expressed from cloned viral cDNA. Virology. 1987;161:348–356. doi: 10.1016/0042-6822(87)90127-9. [DOI] [PubMed] [Google Scholar]
- 47.Rivera V M, Welsh J D, Maizel J V J. Comparative sequence analysis of the 5′ noncoding region of the enteroviruses and rhinoviruses. Virology. 1988;165:42–50. doi: 10.1016/0042-6822(88)90656-3. [DOI] [PubMed] [Google Scholar]
- 48.Rodrigo M J, Dopazo J. Evolutionary analysis of the picornavirus family. J Mol Evol. 1995;40:362–371. doi: 10.1007/BF00164022. [DOI] [PubMed] [Google Scholar]
- 49.Rohll J B, Moon D H, Evans D J, Almond J W. The 3′ untranslated region of picornavirus RNA: features required for efficient genome replication. J Virol. 1995;69:7835–7844. doi: 10.1128/jvi.69.12.7835-7844.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rohll J B, Percy N, Ley R, Evans D J, Almond J W, Barclay W S. The 5′-untranslated regions of picornavirus RNAs contain independent functional domains essential for RNA replication and translation. J Virol. 1994;68:4384–4391. doi: 10.1128/jvi.68.7.4384-4391.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rueckert R. Picornaviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fundamental virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 477–522. [Google Scholar]
- 52.Sarnow P, Bernstein H D, Baltimore D. A poliovirus temperature-sensitive RNA synthesis mutant located in a noncoding region of the genome. Proc Natl Acad Sci USA. 1986;83:571–575. doi: 10.1073/pnas.83.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Semler B L, Johnson V H, Dewalt P G, Ypma-Wong M F. Site-specific mutagenesis of cDNA clones expressing a poliovirus proteinase. J Cell Biochem. 1987;33:39–51. doi: 10.1002/jcb.240330105. [DOI] [PubMed] [Google Scholar]
- 54.Skinner M A, Racaniello V R, Dunn G, Cooper J, Minor P D, Almond J W. New model for the secondary structure of the 5′ non-coding RNA of poliovirus is supported by biochemical and genetic data that also show that RNA secondary structure is important in neurovirulence. J Mol Biol. 1989;207:379–392. doi: 10.1016/0022-2836(89)90261-1. [DOI] [PubMed] [Google Scholar]
- 55.Todd S, Towner J S, Brown D M, Semler B L. Replication-competent picornaviruses with complete genomic RNA 3′ noncoding region deletions. J Virol. 1997;71:8868–8874. doi: 10.1128/jvi.71.11.8868-8874.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toyoda H, Kohara M, Kataoka Y, Suganuma T, Omata T, Imura N, Nomoto A. Complete nucleotide sequences of all three poliovirus serotype genomes. Implication for genetic relationship, gene function and antigenic determinants. J Mol Biol. 1984;174:561–585. doi: 10.1016/0022-2836(84)90084-6. [DOI] [PubMed] [Google Scholar]
- 57.Toyoda H, Nicklin M J, Murray M G, Anderson C W, Dunn J J, Studier F W, Wimmer E. A second virus-encoded proteinase involved in proteolytic processing of poliovirus polyprotein. Cell. 1986;45:761–770. doi: 10.1016/0092-8674(86)90790-7. [DOI] [PubMed] [Google Scholar]
- 58.van Kuppeveld F J M, Galama J M D, Zoll J, Melchers W J G. Genetic analysis of a hydrophobic domain of coxsackie B3 virus protein 2B: a moderate degree of hydrophobicity is required for a cis-acting function in viral RNA synthesis. J Virol. 1995;69:7782–7790. doi: 10.1128/jvi.69.12.7782-7790.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wimmer E, Hellen C U, Cao X. Genetics of poliovirus. Annu Rev Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- 60.Xiang W, Cuconati A, Hope D, Kirkegaard K, Wimmer E. Complete protein linkage map of poliovirus P3 proteins: interaction of polymerase 3Dpol with VPg and with genetic variants of 3AB. J Virol. 1998;72:6732–6741. doi: 10.1128/jvi.72.8.6732-6741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiang W, Harris K S, Alexander L, Wimmer E. Interaction between the 5′-terminal cloverleaf and 3AB/3CDpro of poliovirus is essential for RNA replication. J Virol. 1995;69:3658–3667. doi: 10.1128/jvi.69.6.3658-3667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ypma-Wong M F, Dewalt P G, Johnson V H, Lamb J G, Semler B L. Protein 3CD is the major poliovirus proteinase responsible for cleavage of the P1 capsid precursor. Virology. 1988;166:265–270. doi: 10.1016/0042-6822(88)90172-9. [DOI] [PubMed] [Google Scholar]