Abstract
In this study, various constraints of Cd toxicity on growth, morpho-anatomical characters along with physiological and biochemical metabolic processes of Solanum melongena L. plants were analyzed. Conversely, ameliorative role of iron oxide nanoparticles (FeONPs) was examined against Cd stress. For this purpose, the following treatments were applied in completely randomized fashion; 3 mM CdCl2 solution applied with irrigation water, 40 and 80 ppm solutions of FeONPs applied via foliar spray. Regarding the results, Cd caused oxidative damage to plants’ photosynthetic machinery, resulting in elevated levels of stress-markers like malondialdehyde (MDA), hydrogen peroxide (H2O2), and electrolytic leakage (EL) along with slight increase in antioxidants activities, including glutathione (GsH), ascorbate (AsA), catalases (CAT), peroxidases (POD), superoxide dismutase (SOD), and ascorbate peroxidases (APX). Also, high Cd level in plants disturb ions homeostasis and reduced essential minerals uptake, including Ca and K. This ultimately reduced growth and development of S. melongena plants. In contrast, FeONPs supplementations improved antioxidants (enzymatic and non-enzymatic) defenses which in turn limited ROS generation and lowered the oxidative damage to photosynthetic machinery. Furthermore, it maintained ionic balance resulting in enhanced uptake of Ca and K nutrients which are necessary for photosynthesis, hence also improved photosynthesis rate of S. melongena plants. Overall, FeONPs foliar spray effectively mitigated Cd toxicity imposed on S. melongena plants.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05464-z.
Keywords: Abiotic stress, Heavy metals, Iron oxide, Nanoparticles, Oxidative stress, Soil contamination
Introduction
Heavy metals are environmental contaminants that also cause critical pollution issues in water and soil and pose a threat to plants and animals. Heavy metals in soil are accumulating from mining, industry, and agricultural sources. Industrial and sewage waste deposited various heavy-metals including Fe, Mn, Cu, Ni, Co, Cd, Zn, and Hg in soil which persist there for a long time [1]. Despite these are essential micronutrients, required for plants’ regular processes, their excess concentration negatively regulates their physiology, metabolism, senescence, and growth [2, 3].
Cadmium is highly toxic for biota even at low level. Its toxic effects are detrimental for the flora and fauna [3]. Cd is exceedingly poisonous for humans and other living organisms, highly toxic for terrestrial and aquatic life [4]. Various natural systems released cadmium including forest fires, volcanic eruptions, rocks weathering, wastewater treatment and anthropogenic activities also contaminated to environment [5, 6]. Cadmium pollution originates from anthropogenic activities, which include mining, metallurgical operations, electroplating, paint production, combustion emissions, and overuse of pesticides and fertilizers [7]. In agricultural soil Cd concentration increased due to industries development and agronomy [8].
Cd is readily absorbed by plants due to its higher solubility and mobility in comparison to other metals [9]. After absorption, it is mainly deposited in edible plant tissues [10]. In plants Cd is absorbed through roots, accumulated in aerial parts and thus affected normal plant growth. In addition to oxidative impairment and disrupted uptake of nutrients, cadmium stress results in a decrease in biomass and photosynthetic rate in plants [11, 12]. Cd stress disrupted redox balance and resulted in enhanced reactive oxygen species (ROS) accumulation and peroxidation of lipid bilayer in cell membrane, decreasing the photosynthetic efficiency and retarding the photosynthetic pigments [13]. Osmolytes, chelating agents, enzymatic and non-enzymatic antioxidants produced by plants are highly effective agents to reduce the stress brought by heavy metals [14]. Furthermore, according to Tanveer & Shabala, [15], Cd directly affects the regulation of Zn and Fe through Zrt/Irt-like Protein (ZIP) and Natural Resistance-Associated Macrophage Protein (NRAMP).
In the crust Iron is fourth most profuse element of the Earth and necessary micronutrient for plant growth. Despite the fact that it is widespread in earth’s crust, its availability to plant roots is extremely limited. Because iron oxides are very less soluble and are present in soil solution at very low concentration. Large amount of iron is entangled with soil particles [16] but the plants can’t intake this iron directly [17]. Plants have developed various mechanism to uptake Fe from the soil solution. For example release root exudates and chemicals that convert insoluble Fe into soluble forms. Besides this, plants make association with microbes that are capable of Fe chelation. According to studies, graminaceous and non-graminaceous plants have different strategies for absorbing iron. The phytosiderophores (PS) secreted by graminaceous plants, like Setaria italica, are capable of binding Fe3+ with ease. Fe-PS associations are subsequently reabsorbed into the roots. While, non-graminaceous plants like tomato absorb iron in form of ferrous (Fe2+). These plants reduced ferric (Fe3+) to ferrous (Fe2+) at root cell membrane and then absorb ferrous ions [18].
Still these strategies are not fulfilling the requirements of efficient growth and development of plants [19]. For improved agronomic production, metabolism of plants and vitality, iron (Fe) is recognized as an essential element. Food crops having iron deficiency showed poor agronomic quality and decreased yield. Staple crops like rice are highly vulnerable to Fe deficiency [20]. Thus, exogenous supplementations of Fe could be the solution for fulfilling this deficiency.
Nanotechnology has been widely used in various industries. This technology has also grabbed the attention of agriculturists [21, 22]. However, nanoparticles (NPs) are made in a variety of sizes, kinds, shapes and are utilized in a wide range of fields, including electronics, agriculture, and medicine [23, 24]. For an objective of environmental restoration, the potential of NPs has been thoroughly investigated [25, 26]. To mitigate abiotic stresses in plants, NPs with a size of 100 nm have been potentially used for agricultural practices [27, 28]. These unique properties are high surface action, unique magnetic characteristics, elevated reaction sites, better catalytic effectiveness [29]. Numerous studies have shown that NPs enhance the crops quality, rhizome development, and seed germination grown under conditions of stress [30–32].
Use of nano fertilizers have introduced new innovations in agricultural sustainability. In conventional method of using chemical fertilizers, plants can have only small amounts fertilizers and the remaining proportion runoff from the fields cause serious environmental hazards. Use of nano fertilizers is a good alternative environment friendly and cost effective approach [33–35].
Exogenous application of NPs can strengthen plants’ defense mechanisms against oxidative stress, which lowers the amount of ROS that plants bioaccumulate [36, 37]. Investigation has shown that NPs reduce the toxicity of Cd and Pb in rice seedlings when they are sprayed foliarly [38] or applied in soil [39, 40]. According to Chen et al. [41], silicon increased K+ level in xylem sap, which in turn improved xylem hydraulic conduction and osmotic gradient in plants. Similar to this, supplementation of Fe influenced K+ uptake and its translocation in plants [42]. Therefore, in this particular context, nano-fertilizers or nano-encapsulated nutrients might be capable of releasing nutrients properly and on demand, hence regulate plant growth [43].
The application of metallic NPs, such as FeONPs, in the agricultural industry has gained attention recently [44]. For example, ferromagnetic Fe3O4 NPs were found to improve wheat growth while reducing the plants’ uptake of metals. Increasing biomass, nutrients and also increasing wheat’s resistance to Cd are key benefits of using these NPs [45]. Research on paramagnetic (Fe2O3) NPs effects on soil aggregates and plant accumulation of heavy metals is still relatively unexplored [46].
Many studies have been carried out on response of NPs against abiotic stresses in plants, in recent years [35]. For exapmle, plants grown in As (V) contaminated soil may be able to acquire tolerance to strong adsorbent qualities of FeONPs. According to Afzal et al. [47] they are essential for promoting plant growth, development, stress tolerance, and targeted nutrient availability. For instance, prior studies have demonstrated that FeONPs increase plant growth and productivity while mitigating the destructive effects of Cd in wheat [48, 49]. By reducing arsenic accumulation and metal phytotoxicity, FeONPs also improve iron uptake and ensure tolerance toward oxidative stress in rice crops [50].
Eggplant is being cultivated worldwide, with China, India, and Turkey ranking among the leading producers [51]. It is also called as brinjal, aubergine, melongene, garden egg, and guinea squash in various places. According to recent Food and Agriculture Organization (FAO) figures, eggplant ranks sixth in total global output, trailing only tomatoes, onions, cucumbers and gherkins, and cabbages, with 52.3 million tons produced in 2017. It is the third most consumed vegetable in China, India, and Bangladesh, which together account for nearly 40% of the world’s population. Despite eggplant production is increased in recent years, its productivity is limited due to abiotic stresses [52]. Especially, heavy metals pollution dropped its growth and output [53]. Heavy metals such as lead, cadmium, and mercury can accumulate in soil and inhibit eggplant growth, lowering yields and potentially deteriorate consumer health [54, 55]. Efficient soil management and remediation strategies are essential for mitigating these consequences and ensuring sustainable eggplant production. In this study, toxic effects of Cd on morpho-anatomical features, physiological and biochemical metabolic processes, and nutrient uptake in S. melongena plants, were analyzed along with ameliorative role of FeONPs applied via foliar spray.
Materials and methods
Experimental layout
In this study, a completely randomized design was used that contained two factors and three replicates. The experiment was conducted at botanical garden, University of the Punjab, Lahore, Pakistan. Two factor variables studied in this research were cadmium (Cd toxicity) and FeONPs. FeONPs of 30 nm particle size and 5 mg mL− 1 density in water were purchased from Sigma-Aldrich. In a pilot experiment various concentrations for Cd (1, 2, 3, 4 and 5 mM) and FeONPs (20, 40, 80 and 120 ppm) were tested. Cd imposed significant stress over 3 mM concentration. Higher concentrations were more critical for survival of the plants. Whereas FeONPs showed best results at 40 and 80 ppm. In this study, two concentrations for FeONPs (i.e. 40 and 80 ppm) were prepared in 2% Tween-20 solution and applied via foliar spray until all the leaves of plant get fully wet. Conversely, for Cd toxicity, 3 mM CdCl2 solution at rate of 1 L per pot was applied along with irrigation water. The following treatments were applied at one week interval. S. melongena seeds were procured from Punjab seeds, Lahore. Garden soil was firstly air dried and filled in the pots, 5.5-6 Kg soil per pot. Seeds were sown, after germination thinning was done and maximum of 4 equal sized plants were maintained in each pot. Aforementioned treatments were applied at 4 leaves stage. After 3 weeks of treatment, one replica from each treatment was uprooted and growth indices (shoot length and root length) were analyzed. The remaining plants from other replicas were maintained till the end of experiment and used for further physiological and biochemical analysis as listed below.
Analyzing the effect of cd stress on stem anatomy
To study the effect of Cd stress on anatomical features of stem, width of xylem, cambium and pith rays tissues was observed under the light microscope. For this purpose, stem under observation for anatomical features was kept in mixture of 95% ethyl alcohol and anhydrous glycerine with 1:1 (V/V). Safranine was used as dyeing agent. Photographs were taken on mobile camera.
Estimation of photosynthetic pigments and photosynthesis related parameters
0.5 g of fresh leaf sample from each replicate was crushed in liquid nitrogen using pestle and mortar. This paste was homogenized in 80% acetone, the mixture was centrifuged, 4500 rpm at 20 °C, for 5 min. Using a spectrophotometer, the filtrate was analyzed at wavelengths of 470, 652, 665, and 750 nm in order to estimate the amounts of carotenoids, chlorophyll-a, and chlorophyll-b followed by Vernon, [56].
Infrared gas analyzer (LCpro SD, ADC BioScientific, UK) with broad chamber was used to analyze photosynthesis rate (Pn), transpiration rate (Tr), gas exchange (gs) and intercellular CO2 (Ci). Readings were taken using PAR from the leaf chamber light source at 900 µmol m− 2 s− 1 and ambient CO2 averaging 400 ppm all-round the observation time. Before taking the readings, waited for three minutes after inserting the leaf into the leaf chamber to allow the leaf to adapt to the pre-set conditions [57].
Analyzing stress markers
Malondialdehyde (MDA)
The method described by Heath & Packer, [58] was used to measure malondialdehyde (MDA). Fresh leaf (0.5 g) sample from each replicate was taken and homogenized in phosphate buffer (50 mM, 25 ml) having pH 7.8, containing 1% polyethene pyrrole, using pestle and mortar. Homogenate was centrifuged at 8,000 × g for twenty minutes. After being heated to 100 oC for half hour, the mixture was rapidly cooled on ice. A (Hitachi U-200, Tokyo, Japan) spectrophotometer was used to measure the absorbance of the obtained supernatant at wavelengths of 532 and 600 nm.
The MDA content was mounted using the following equation.
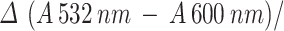 |
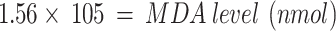 |
Hydrogen peroxide (H2O2) measurment
Hydrogen peroxide was extracted from plants followed by Patterson et al. [59]. Fresh leaves of each replicate were taken grinded to make paste, following that 0.15 g charcoal and 5 ml of 5% trichloroacetic acid (TCA) added in it. After 20 min at 4 oC, the mixture was centrifuged at 7,000 × g. With the addition of 17 M ammonia, the pH was maintained at 8.4 and then filtered. Filtrate was separated into 1 mL aliquots following that catalase (0.1 g) was added in this aliquot and left for 10 min at room temperature. Only reaction mixture (containing all aforementioned chemicals without plant material) was considered for blank. 1 ml of colorimetric reagent was added to both the non-aliquot (blank) and aliquot (sample) solutions. The solution was incubated at 30 oC for ten minutes. A spectrophotometer was used to determine the absorbance, which was at 505 nm. The calorimetric reagent contains 10 mg of phenol, 5 mg of peroxidase (150 U mg− 1), and 10 mg of 4-aminoantipyrine, all diluted in 50 ml of 100 mM acetic acid buffer (pH 5.6).
Electrolyte leakage
Three-week-old aerial portions of plants were harvested. Then rinsed with deionized water and incubated for different periods of time in solution (5 × 10 − 3% (v/v) Silwet L-77) control with 50 µM MV. Following the incubation period, conductivity was measured using a conductor meter. It was then autoclaved at 120 oC for 30 min to remove the electrolytes. The total percentage of electrolytes was indicated as electrolyte leakage. Measurement was determined in triplicate.
Determination of non-enzymatic antioxidants
Glutathione (GSH)
Ellman’s [60] approach was used to quantify the total GSH content. The method is based on the reaction of 5,5-dithiobis-2-nitrobenzoic acid (DTNB) with sulfhydryl compounds to generate a yellow hue. An aliquot (3 mL) of 4% sulfosalicylic acid was mixed with 0.5 mL of the filtrate produced by macerating the fresh leaf tissue in phosphate buffer. Following that Ellman’s reagent was added in this mixture. The OD readings were obtained at 412 nm. The intensity of the yellow color is directly proportional to GSH concentration.
Ascorbate (AsA)
The ascorbate was determined using 2, 2’-dipyridyl. 0.5 g fresh leaves for every replicate were pulverized into a fine powder using liquid nitrogen in a mortar. This homogenized frozen powder was taken falcon conical tubes containing 2% metaphosphoric acid (2 mL). This mixture was centrifuged for 15 min at 4 oC at 22,000 g. Supernatant was obtained in separate tubes. Reaction mixture contained supernatant 0.2 mL, 0.6 mL of 0.3 M potassium (K) phosphate buffer (pH 7.4), 5 mM EDTA, and 0.2 mL of Milli Q water. At 525 nm, the absorbance was measured. A reference curve spanning from 0 to 25 nmol of ascorbic acid was employed. Measurements were made in triplicate for every sample [61].
Determination of antioxidant enzymes
For this purpose, fresh leaves (0.5 g) from each replicate were obtained and homogenized in 10 mL of precooled phosphate buffer (50 mM) having pH 7.8. The mixture was taken in conical flask and centrifuged at 6000 rpm (20 min at 4 oC). The supernatant was taken in separate tubes and stored in freezer. This supernatant (enzyme extract) was then used for subsequent analysis of CAT, POD, APX and SOD activities [62].
Catalase (CAT) activity
To find the catalase activity, the previously prepared enzyme extract was mixed in reaction mixture. The reaction mixture contained 0.001% Antifoam 204 and 100 mM H2O2 with (50 mM K2HPO4/KH2PO4 buffer pH 7) solution. For quantification of the CAT enzymatic activity the coefficient 43.6 [mM− 1 cm− 1] was used. Hydrogen peroxide disappearance was determined by formation of curve of lope during absorbance for forty minutes at 30 °C with 240 nm range [63].
Superoxide dismutase (SOD) activity
Reaction mixture for analyzing SOD activities contained 0.3 mL for each of 130 mM methionine, 50 µM nitro blue tetra-zolium, 100 µM EDTA-Na2, and 20 µM riboflavin. Enzyme extract (0.05 mL) was added in this reaction mixture and then subjected to high intensity light (4000 lx) for 20 min. Following illumination, optical density (OD) was read at 560 nm [64].
Peroxidase (POD) activity
To determine POD, 4-methylcatechol used as substrate. The oxidation of 4-methylcatechol increased with the absorption of hydrogen peroxide. This was measured by reading OD at 420 nm using UV/VIS spectrophotometer. Sodium phosphate buffer (pH 7.0), 5 mM H2O2, 5 mM 4-methylcatechol, 500 µL of enzyme extract (as mentioned in Sect. 2.4) in total volume of 3.0 mL reaction mixture was maintained at room temperature. One unit of enzyme activity was defined as 0.01 change in absorbance per minute, under assay conditions [65].
Ascorbate peroxidase (APX) activity
The reaction was investigated in a 1 mL solution containing 2.5 mM H2O2, 1 M sodium ascorbate, and 80 nM potassium phosphate buffer. To estimate the oxidation ratio of ascorbate, H2O2 was added to commence the reaction, and the reduction of absorbance was observed for 1 min at 290 nm [66, 67].
Estimation of mineral nutrients and heavy metal uptake
The sample digestion procedure used by Paul et al. [68] involved a 9:4 ratio of HNO3 and HClO4. For this, 0.1 g dried shoot tissues were grinded using pestle and mortar. This powder was then taken in 100 ml digestion flask with 10 ml of HNO¬3 and left overnight. Following that, 8 mL of HClO4 was added to the mixture, which was heated on a hot plate until fume formation stopped. The digestion is complete when the solution turns colorless. Cool the solution and add 20 mL of distilled water. After filtration, the solution was tested for mineral nutrients (Ca and K) using a flame photometer (Sherwood model 360). This prepared sample solution was also analyzed for Cd and Fe heavy metals using an atomic absorption spectrophotometer (Thermo Scientific iCE 3000 Series) with wavelength range of 180–900 nm.
Statistical analysis
R software was used for 2-way completely randomized design ANOVA followed by least significant difference mean compare test at p < 0.05 significance level. Graphs presenting mean values for three replicates ± SE and letters for significant variance in mean values were also plotted on R. Similarly, Pearson’s correlation and principle component analysis were performed using R-software.
Results
Effect of FeONPs on growth of S. melongena plants under cd toxicity
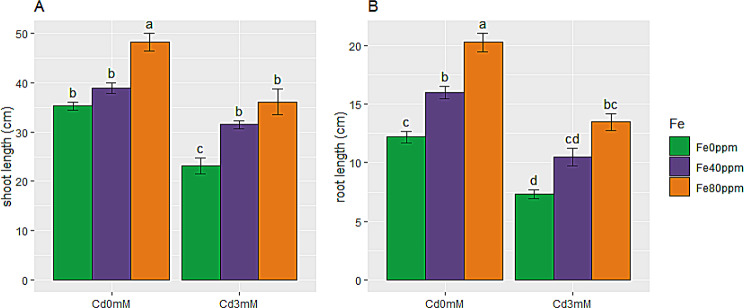
The Growth of S. melongena was greatly impeded by Cd toxicity as evident by Fig. 1A and B. Shoot and root length were reduced (34 and 40%) greatly over 3 mM Cd stress. In contrast to this, FeONPs foliar application enhanced growth of S. melongena plants at both levels (40 and 80 ppm). FeONPs treatments, incremented shoot length by 10 and 37%, while root length by 31 and 66%, respectively, as compared to control plants. The same trend was also observed for plants given Cd stress. FeONPs supplementations successfully masked the hindrance in growth of plants due to Cd stress. Overall, maximum growth was observed in plants treated with 80 ppm FeONPs foliar spray, without Cd stress, while minimum growth was observed in Cd-stress only plants.
Fig. 1.
Growth attributes of S. melongena plants, exposed to Cd toxicity, along with FeONPs foliar spray. (A) Shoot length and (B) Root length. Graph bars represent mean value of three replicates, while the error bars represent standard error. Different letters obtained after LSD test, showed that mean values are significantly different at p < 0.05
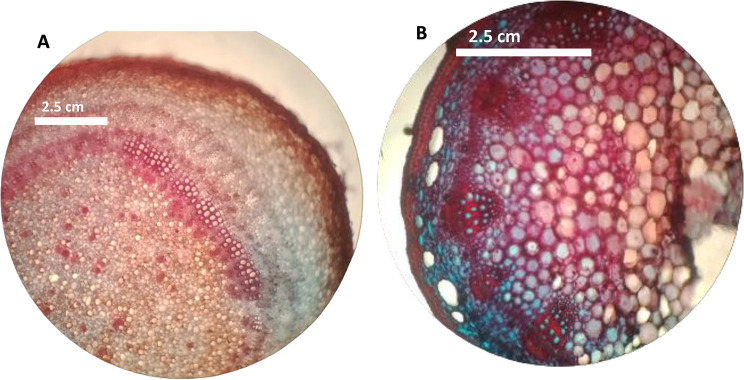
Effect of cd stress on stem anatomy of S. melongena plants
Figure 2A and B depicted significant changes in stem anatomy under Cd stress, including changes in vascular tissue shape and structural integrity. Cd exposure disrupted vascular bundles inside the stem, resulting in decreased xylem and phloem differentiation. These changes in stem anatomy may be due to Cd-induced cell wall destruction, as well as changes in cell division and differentiation processes. Furthermore, Cd stress most likely harmed the vascular transport system, impairing the efficient uptake and distribution of water, nutrients, and photosynthesis inside the plant.
Fig. 2.
Stem anatomy of S. melongena plants, (A) control plant and (B) Cd stressed plant
Effect of FeONPs on photosynthetic pigments and photosynthesis related attributes of S. melongena plants under Cd toxicity
Chlorophyll a, b and carotenoids
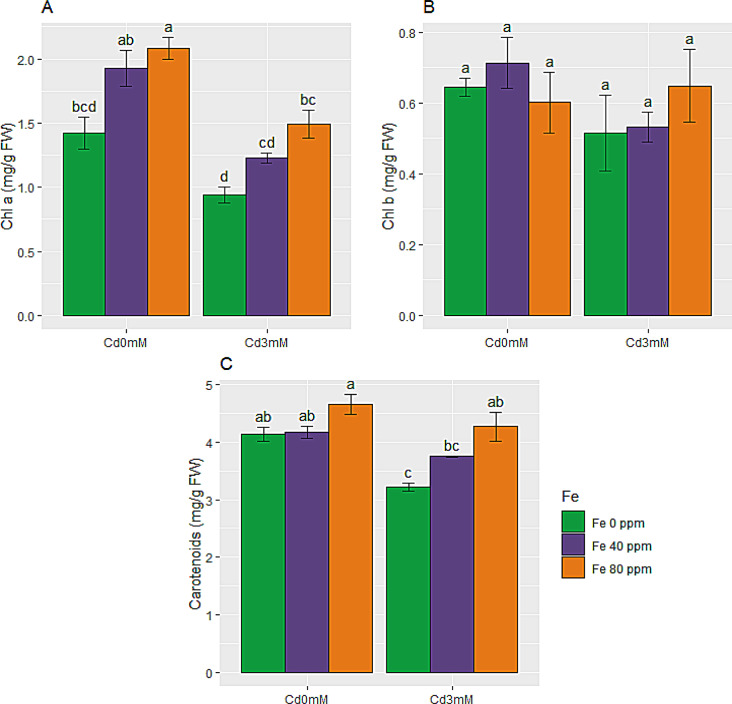
Results for chlorophyll a, b and carotenoids are shown in Fig. 3A-C. It is revealed that Cd toxicity readily declined carotenoids photosynthetic pigments in S. melongena plants. But the decrease in chl a and b was not statistically significant. Chl a, b and carotenoids were decreased by 34, 20 and 22% under the effect of Cd stress. Conversely, the damaging constrains of Cd stress were reversed successfully by FeONPs supplementations via foliar spray. A significant increase in chl a, b and carotenoids content is evident from Fig. 3A-C with FeONPs treatments under stress conditions as equated to stress only plants. FeONPs also effectively improved photosynthetic pigmentation in control plants. Combinedly, maximum photosynthetic pigmentation was observed under high level of FeONPs supplements while minimum values were observed for Cd stress treatment.
Fig. 3.
Photosynthetic pigmentation of S. melongena plants, exposed to Cd toxicity, along with FeONPs foliar spray. (A) Chlorophyll a, (B) Chlorophyll b, and (C) Carotenoids. Graph bars represent mean value of three replicates, while the error bars represent standard error. Different letters obtained after LSD test, showed that mean values are significantly different at p < 0.05
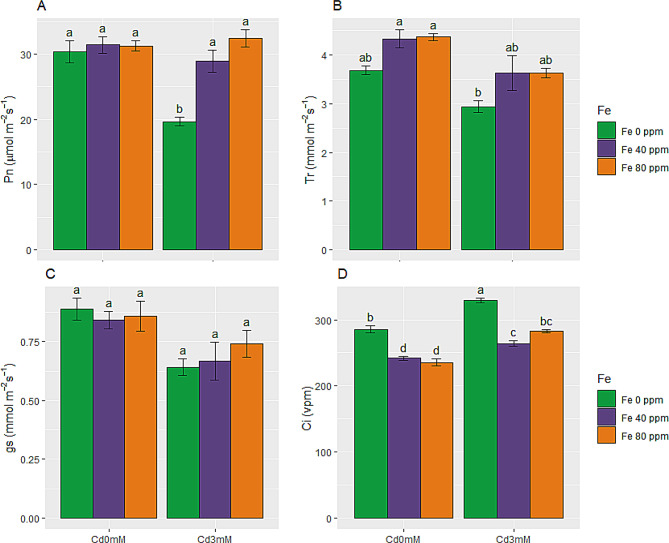
Photosynthesis rate (pn), transpiration rate (tr), gas exchange (gs) and intercellular CO2 (ci)
Photosynthesis rate relied on results of photosynthetic pigmentation i.e. Cd toxicity halted Pn promptly. Similarly, Tr and gs were also lowered under Cd stress. But this decrease in Tr and gs was not statistically significant. Pn, Tr and gs were reduced by 35, 20 and 28%, respectively, under Cd toxicity, compared to control. On the other hand, Ci level was elevated (15%) under Cd stress. In comparison to Cd stress only, stressed plants also given FeONPs supplementations had somewhat improved Pn, Tr and gs by while lowered Ci. Overall, maximum photosynthesis rate (32.39 µmol m− 2 s− 1) was observed in Cd stress plants with 80 ppm FeONPs treatment. FeONPs supplementations restored (to the level of control plants) photosynthesis related attributes in Cd stress conditions as shown in Fig. 4A-D.
Fig. 4.
Photosynthetic attributes of S. melongena plants, exposed to Cd toxicity, along with FeONPs foliar spray. (A) Net photosynthesis rate, (B) Transpiration rate, (C) Stomatal conductance and (D) Intercellular CO2. Graph bars represent mean value of three replicates, while the error bars represent standard error. Different letters obtained after LSD test, showed that mean values are significantly different at p < 0.05
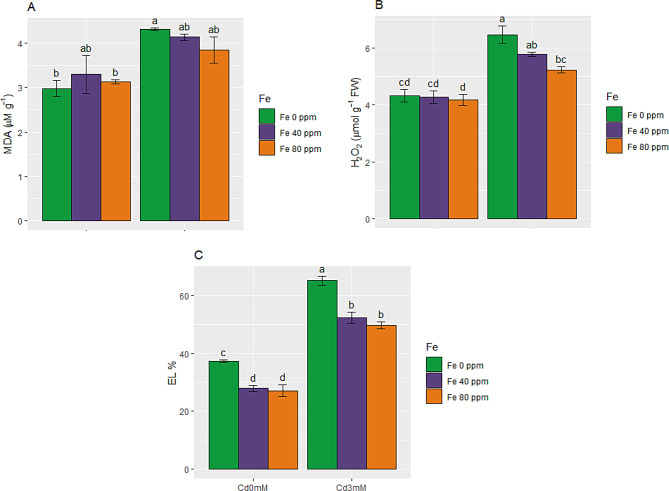
Effect of FeONPs on stress marker (MDA, H2O2 and EL) in S. melongena plants under Cd toxicity
Malondialdehyde (MDA) and hydrogen peroxide (H2O2) contents found in control plants 2.98 and 4.33 µmol g− 1 FW, respectively. While in plants subjected to Cd toxicity nearly half fold increase was observed under Cd stress. This is clear indication of lipid peroxidation in S. melongena cells due to oxidative damage caused by Cd stress. While in plants grown in Cd toxicity FeONPs reduced both MDA (39 and 29%) and H2O2 (34 and 21%) at low as well as high level (40 ppm and 80 ppm), as shown in Fig. 5A and B. This reduction was only statistically significant for H2O2.
Fig. 5.
Stress markers in S. melongena plants, exposed to Cd toxicity, along with FeONPs foliar spray. (A) Malondialdehyde, (B) Hydrogen peroxide, and (C) Electrolytic leakage. Graph bars represent mean value of three replicates, while the error bars represent standard error. Different letters obtained after LSD test, showed that mean values are significantly different at p < 0.05
EL enhanced readily (75%) in plants exposed to 3 mM Cd stress. In contrast, FeONPs treatments at both levels tended to reduce EL in control as well as stressed plants. FeONPs 40 and 80 ppm supplementations reduced EL from 65% in Cd-stressed plants to 52 and 49%, respectively (Fig. 5C).
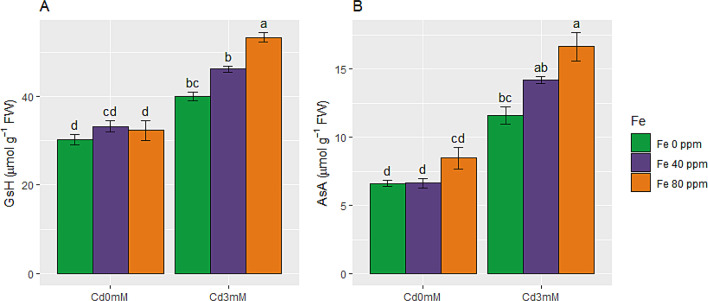
Effect of FeONPs on non-enzymatic antioxidants (GsH and AsA) in S. melongena plants under Cd toxicity
Glutathione (GsH) and ascorbate (AsA) contents were enhanced significantly when plants were exposed to Cd toxicity as compared to control conditions. In Cd stress treated plants GsH and AsA were enhanced by 32 and 75%, respectively, as compared to control group. The effect of FeONPs supplementation was more prominent in stressed plants as compared to non-stressed plants. So, maximum values for GsH and AsA were found in plants exposed to Cd toxicity along with 80 ppm FeONPs foliar spray while minimum values were observed in control plants, as depicted in Fig. 6A and B.
Fig. 6.
Non-enzymatic antioxidants of S. melongena plants, exposed to Cd toxicity, along with FeONPs foliar spray. (A) Glutathione and (B) Ascorbate. Graph bars represent mean value of three replicates, while the error bars represent standard error. Different letters obtained after LSD test, showed that mean values are significantly different at p < 0.05
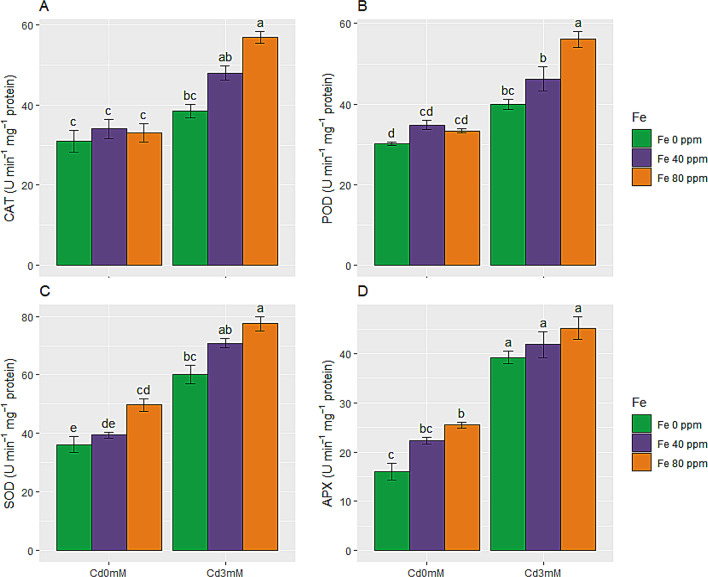
Effect of FeONPs on enzymatic antioxidants in S. melongena plants under cd toxicity
Antioxidant enzymes were rapidly enhanced under Cd stress. FeONPs supplementations, further added in this increment under stress conditions. FeONPs also improved SOD and APX enzymes in control plants. CAT enzyme activity was incremented by 24% in stress only plants. This change was not statistically significant. SOD activity was enhanced by 37% in stress only plants. FeONPs treatments at both levels (40 and 80 ppm) significantly enhanced activity of CAT and SOD enzymes in control and stress conditions.
POD and APX enzymes activity were also readily enhanced (33 and 145%, respectively) due to Cd toxicity as equated to control plants. FeONPs (40 and 80 ppm) foliar spray significantly enhanced POD enzyme activity in Cd stress conditions. While, increase in APX activity due to foliar application of FeONPs was only statistically significant in control conditions. Overall, maximum antioxidant enzymes activities were found in plants given 3 mM Cd stress along with foliar spray of 80 ppm FeONPs (as shown in Fig. 7A-D). This depicted, defense system of S. melongena plants activated in response to Cd toxicity and FeONPs supplements to ensure survivability and improved growth.
Fig. 7.
Enzymatic antioxidants of S. melongena plants, exposed to Cd toxicity, along with FeONPs foliar spray. (A) Catalases, (B) Peroxidases, (C) Superoxide dismutase and (D) Ascorbate peroxidase. Graph bars represent mean value of three replicates, while the error bars represent standard error. Different letters obtained after LSD test, showed that mean values are significantly different at p < 0.05
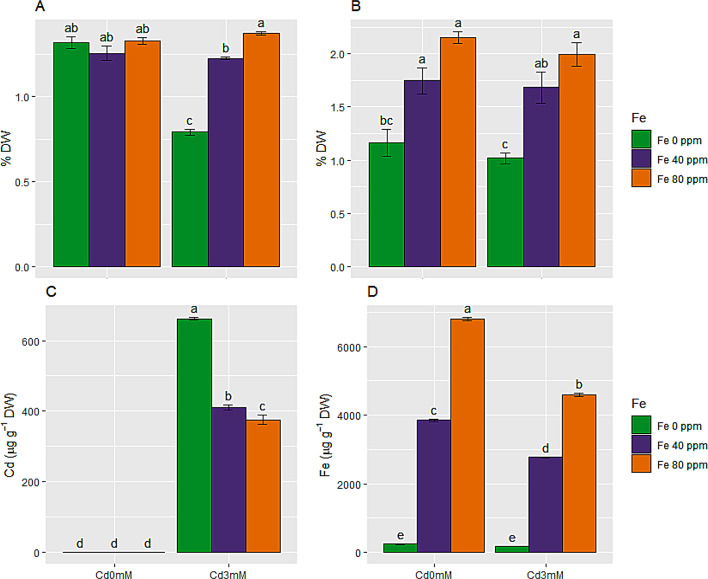
Effect of FeONPs on mineral nutrients in S. melongena plants under cd toxicity
Ca2+ and K+ were reduced by 40 and 13%, respectively, in shoot of S. melongena plants exposed to Cd toxicity. While, FeONPs treatments at both low and high levels (40 and 80 ppm) greatly improved these mineral nutrients in stressed plants as compared to stress only plants. Ca2+ and K+ were reduced to 0.79 and 1.02%DW from 1.32 and 1.16%DW, respectively, under Cd stress. High level of FeONPs supplement was more effective in improving these mineral nutrients in control as well as stress conditions, as depicted from results in Fig. 8A and B.
Fig. 8.
Minerals and heavy metal uptake in S. melongena plants, exposed to Cd toxicity, along with FeONPs foliar spray. (A) Calcium, (B) Potassium, (C) Cadmium and (D) Iron. Graph bars represent mean value of three replicates, while the error bars represent standard error. Different letters obtained after LSD test, showed that mean values are significantly different at p < 0.05
Effect of FeONPs on Cd and Fe heavy metals in S. melongena plants under Cd toxicity
Cadmium and iron metal ions were not traced in the control group of plants. Maximum Cd metal (661.7 µg g− 1 DW) was traced in plants given 3 mM Cd stress. FeONPs foliar application at both levels significantly reduced Cd metal ions in S. melongena plants (Fig. 8C) depicting heavy metal tolerance through avoidance mechanism. On the other hand, Cd stress significantly reduced Fe metal ions in S. melongena plants, as compared to control plants. The highest value for Fe metal ions was traced in plants supplied with 80 ppm FeONPs (Fig. 8D).
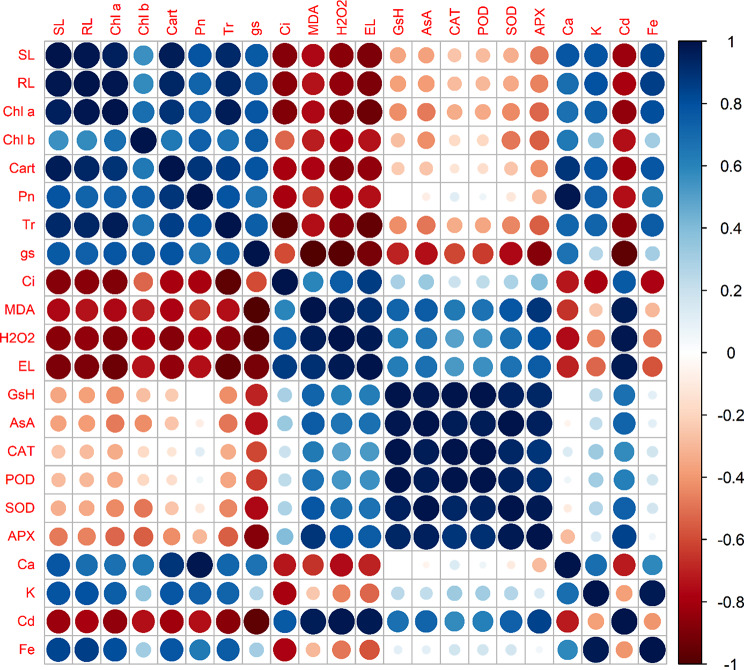
Pearson’s correlation and principle component analysis (PCA)
Pearson’s correlation and PCA were mounted to assess the relation among various parameters studied in this research. The relation between individual treatment applied in this work was also analyzed. Correlation in Fig. 9 revealed that Fe content and the growth, physiological attributes including photosynthetic pigmentation, photosynthesis rate, transpiration and stomatal conductance along with nutritional ions (Ca and K) are in significantly positive correlation with each other. This depicted that, FeONPs supplementation might enhanced Fe content in S. melongena plants, which is an essential component of various physiological and metabolic processes, also assist in metals’ chelation sequestrations and exclusion processes. This enrichment of Fe in might have sequestered or excluded Cd metal under stress conditions. Thus, halted the oxidative damages caused by Cd toxicity. As Fig. 9 showed, Cd concentration in S. melongena plants is in significant positive correlation with stress markers (MDA, H2O2, EL) and antioxidants (GsH, AsA, CAT, POD, SOD and APX). This revealed that Cd entry in the plants made such changes in cellular process that resulted in ROS generation and caused lipid peroxidation. Also, it stimulated the antioxidant defense system of the plants.
Fig. 9.
Pearson’s correlation for various studied parameters of S. melongena plants subjected to Cd stress along with FeONPs foliar spray. (Various abbreviations used are as follows, SL; shoot length, RL; root length, Chl a; chlorophyll a, Chl b; chlorophyll b, Pn; net photosynthesis rate, Tr; transpiration rate, gs; stomatal conductance, Ci; intercellular CO2, MDA; malondialdehyde, H2O2; hydrogen peroxide, EL; electrolytic leakage, GsH; glutathione, AsA; ascorbate, CAT; catalase, POD; peroxidase, SOD; superoxide dismutase, APX; ascorbate peroxidase, Ca; calcium, K; potassium, Cd; cadmium and Fe; Ferrous)
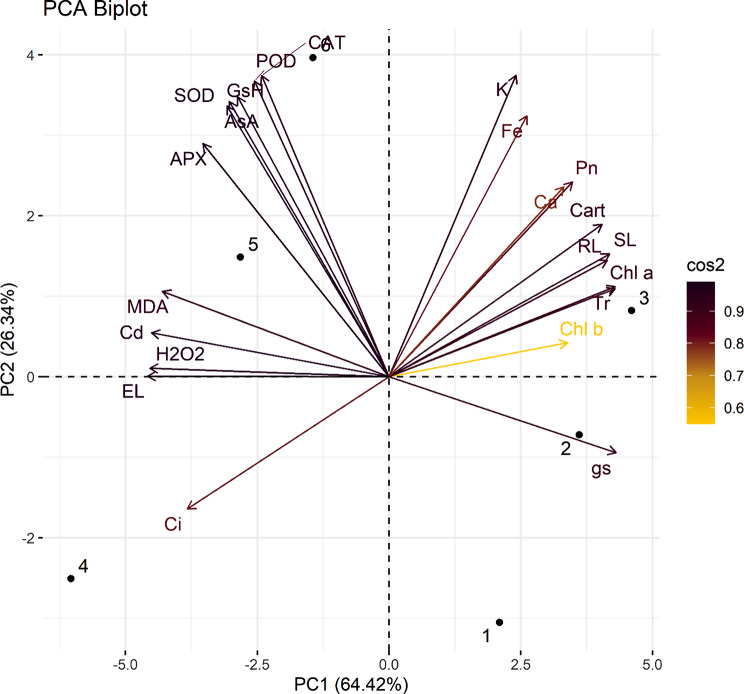
PCA biplot (Fig. 10) also supports Pearson’s correlation results. This revealed that all studied treatments (1, 2, 3 up to 6) were successfully distributed in the first two components of PCA i.e. PC1 and PC2. This also showed that various parameters studied can be grouped in to two. Those that are positively aligned with PC1 and include growth, nutrients, and photosynthesis related parameters. While others which are positively aligned with PC2 and include stress markers as well as antioxidants studied in this research. Arrow length from the origin point reflects the contribution of that specific parameter in this PCA result. Out of all the components exhibited in PCA, PC1 (64.42%) and PC2 (26.34%) had maximum contribution with total of 90.76%.
Fig. 10.
PCA Biplot for various parameters studied and individual treatments applied in this experiment. (Various abbreviations used are same as in Fig. 10. The numbers 1, 2, 3 up to 6 represents treatments applied and are as follows: 1; control, 2; FeONPs 40 ppm, 3; FeONPs 80 ppm, 4; CdCl2 3 mM, 5; 2 + 4 and 6; 3 + 4)
Discussion
Cadmium toxicity is a serious hazard to eggplant production and agricultural sustainability. However, the use of FeONPs is a promising approach for reducing Cd toxicity and increasing crop productivity in Cd-contaminated soil. FeONPs successfully squester Cd ions via a variety of ways, lowering their bioavailability to plants [69]. Furthermore, FeONPs-mediated changes in soil fertility help to promote sustainable agriculture practices. Further research and implementation of FeONPs-based remediation solutions are critical for improving soil health, crop productivity, and food security in Cd-affected agricultural systems. Studies have demonstrated that Cd exposure suppresses root extension and alters cell division and elongation processes, resulting in lower root growth. Furthermore, Cd toxicity can impede shoot development by interfering with photosynthetic activity and nutrient uptake [70].
Ur Rahman et al. [71] observed that Cd exposure significantly reduced shoot and root length in wheat seedlings. Similarly, Zhang et al. [72] discovered that Cd stress reduced shoot and root growth in rice plants by altering root cell elongation and lowering shoot biomass accumulation. These findings are congruent with the results of our investigation, which found that 3 mM Cd stress dramatically reduced shoot and root length in eggplants. However, in our experiment, the use of FeONPs resulted in better eggplant development despite Cd stress. This aligned with the findings of other research that have shown the ability of FeONPs to reduce Cd toxicity and promote plant development in wheat plants [49].
Cd stress caused a decrease in Chl a, Chl b, and carotenoid contents, all of which are necessary components of the photosynthetic machinery. These findings were coined with Waris et al. [73], who found reduction in photosynthetic pigmentations in L. sativa under Cd toxicity. Furthermore, Cd-induced oxidative stress might impede photosynthetic processes by interrupting electron transport chains and preventing carbon fixation. Metwali et al. [74] estimated that Cd exposure resulted in a considerable drop in chlorophyll content and carotenoid levels in wheat and maize. Furthermore, Cd stress has been demonstrated to reduce photosynthetic indices like Pn, Tr, and gs, limiting carbon uptake and biomass production in plants [75].
In our experiment, applying 3 mM Cd stress reduced photosynthetic pigments and other factors in eggplants, affecting photosynthetic ability and growth. However, the following application of FeONPs increased eggplant photosynthetic pigmentation and related indices, minimizing negative impacts of Cd stress. Various factors contribute to the improvement in photosynthetic performance after FeONPs treatment. FeONPs may reduce Cd-induced ROS generation in plants by directly scavenging ROS and improving antioxidant defense systems [76]. FeONPs significantly boosted (39%) chlorophyll contents in wheat plants grown under Cd and salinity stress [49]. In another study, FeONPs at rate of 100 mg Kg− 1 improved total chlorophyll yield by 39.04% in wheat grown in As spiked soil [77]. Also, seed priming with 70 ppm FeONPs effectively increase chlorophyll contents (28.7%) in P. sativum grown in drought conditions [78]. These findings along with results of this study revealed that FeONPs had a significant role in chlorophyll synthetic machinery of plants. FeONPs supplements either via foliar spray or by seed priming improved photosynthesis and related attributes. This indeed helps plant to ameliorate abiotic stresses.
Cadmium (Cd) stress causes oxidative stress in plants, resulting in the formation of ROS and an increase in lipid peroxidation, H2O2, and EL, all of which are commonly used stress markers. Cd disturbs cellular homeostasis and produces ROS, which damage lipids, proteins, and nucleic acids, hence limiting plant growth and development. [79] found that Cd exposure increases levels of MDA, a result of lipid peroxidation, in diverse plant species, indicating oxidative damage to cellular membranes. Furthermore, Cd stress causes the buildup of H2O2, a highly reactive ROS that can oxidize cellular components and disturb cellular functions [80]. In our experiment, 3 mM Cd stress caused elevated levels of MDA, H2O2, and electrolyte leakage in eggplants, which is consistent with earlier studies. For instance, lead toxic effects elevated these stress markers in A. esculentus [81]. These enhanced stress markers show that Cd exposure causes oxidative stress and cellular damage, which most likely contributed to the observed reduction in plant growth and physiological activities. However, the subsequent application of FeONPs boosted eggplants’ antioxidant defense system, resulting in increased levels of non-enzymatic antioxidants including glutathione (GSH) and ascorbate (AsA), as well as enzymatic antioxidants like CAT, POD, SOD, and APX. Such findings are also reported in studies reviewed by Maqsood et al. [82]. The application of FeONPs successfully scavenged ROS and increased activity of antioxidant enzymes, lowering oxidative stress and the levels of MDA, H2O2, and EL in eggplant. Besides, FeONPs enhanced tolerance against oxidative damage caused by Cd and salt stress in wheat. Especially, it reduced MDA, H2O2 and EL in stressed plants. Furthermore, FeONPs improved SOD, POD and total phenolics that scavenged ROS and maintained a redox balance [49]. Similar results were also coined in wheat plants exposed to As polluted soil. Where FeONPs application stimulated antioxidative enzymes and reduced ROS [77].
Cd inhibits nutrient uptake mechanism and affects ion homeostasis, resulting in Ca and K deficits that are essential for different physiological processes and plant growth. Cd exposure lowers Ca and K uptake and translocation in rice [83]. Cd competes with calcium and potassium for uptake by roots and can impair the activity of transport proteins involved in nutrient uptake and translocation. As a result, Cd stress causes lower Ca and K concentrations in plant tissues, disrupting vital physiological functions and jeopardizing plant growth and development [84]. In this study, applying 3 mM Cd stress lowered Ca and K levels in eggplants, which is consistent with earlier findings. Ca and K deficits are likely to have contributed to the reported decline in plant growth and physiological processes, as these nutrients are essential for cell wall integrity, osmoregulation, enzyme activation, and photosynthesis. However, subsequent treatment of FeONPs increased Ca and K levels in eggplants, alleviating the deleterious effects of Cd stress on nutrient absorption and accumulation.
Manzoor et al. [49] found that treatment of FeONPs dramatically reduced Cd uptake and accumulation in wheat plants growing in Cd-polluted soils. Similarly, Chatterjee et al. [85] discovered that FeONPs foliar spray efficiently reduced As uptake in rice plants subjected to arsenic stress, resulting in less As buildup in plant. In this study, foliar treatment of FeONPs reduced Cd absorption in eggplants exposed to 3 mM Cd stress, as previously observed. This reduction in Cd uptake can be due to FeONPs’ conjugation with Cd ions, preventing their transportation inside plants. FeONPs help protect plant tissues from Cd-induced toxicity by reducing Cd uptake, as well as adverse effects of Cd stress on their growth and development.
Conclusion
Cadmium (Cd) stress poses a significant threat to crop yield and agricultural sustainability. Various anthropogenic activities cause soil Cd pollution, either directly or indirectly. Cd heavy-metal inhibits plant metabolism and stunts development. This study revealed that Cd produced oxidative damage to plants’ photosynthetic machinery, resulting in elevated levels of stress indicators (MDA, H2O2, and EL) and a modest increase in antioxidant defenses, both non-enzymatic (GsH and AsA) and enzymatic (CAT, POD, SOD, and APX). In addition, elevated Cd levels in plants disrupt ion homeostasis and limit the uptake of important minerals such as Ca and K. This finally slowed the growth and development of S. melongena plants. In contrast, FeONPs treatment regulated the antioxidant defense system, which reduced ROS production and oxidative damage to photosynthetic machinery. Furthermore, it maintained ionic equilibrium, resulting in increased uptake of Ca and K nutrients required for photosynthesis, and hence boosted the photosynthesis rate of S. melongena plants. Overall, FeONPs foliar spray effectively reduced Cd toxicity on S. melongena plants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors are thankful to Researchers Supporting Project number (RSP2024R393), King Saud University, Riyadh, Saudi Arabia.
Author contributions
MKG; Conceptualization, writing-original draft preparation, AAS & ZN; Validation, Resource acquisition and Investigation, SU; Experimentation, Methodology and Statistical analysis, SS; writing-review and editing. All authors read and approved the final manuscript.
Funding
Researchers Supporting Project number (RSP2024R393), King Saud University, Riyadh, Saudi Arabia.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
We declare that the manuscript reporting studies do not involve any human participants, human data or human tissues. So, it is not applicable. We declare that the manuscript reporting studies do not involve any human participants, human data or human tissues. So, it is not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anis Ali Shah, Email: anisalibot@gmail.com.
Sheeraz Usman, Email: miansheerazusman@gmail.com.
References
- 1.Rahimzadeh MR, Rahimzadeh MR, Kazemi S, Moghadamnia AA. Cadmium toxicity and treatment: an update. Caspian J Intern Med. 2017;8(3):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone SL. Role of the ubiquitin proteasome system in plant response to abiotic stress. Int Rev cell Mol Biology. 2019;343:65–110. 10.1016/bs.ircmb.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 3.Peng H, Shahidi F. Cannabis and cannabis edibles: a review. J Agric Food Chem. 2021;69(6):1751–74. 10.1021/acs.jafc.0c07472 [DOI] [PubMed] [Google Scholar]
- 4.Chellaiah ER. Cadmium (heavy metals) bioremediation by Pseudomonas aeruginosa: a minireview. Appl Water Sci. 2018;8(6):154. 10.1007/s13201-018-0796-5 [DOI] [Google Scholar]
- 5.Liu X, Chen S, Chen M, Zheng G, Peng Y, Shi X, Teng S. Association study reveals genetic loci responsible for arsenic, cadmium and lead accumulation in rice grain in contaminated farmlands. Front Plant Sci. 2019;10:61. 10.3389/fpls.2019.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manzoor M, Gul I, Kallerhoff J, Arshad M. Fungi-assisted phytoextraction of lead: tolerance, plant growth–promoting activities and phytoavailability. Environ Sci Pollut Res. 2019;26:23788–97. 10.1007/s11356-019-05656-3 [DOI] [PubMed] [Google Scholar]
- 7.Haider FU, Liqun C, Coulter JA, Cheema SA, Wu J, Zhang R, Farooq M. Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol Environ Saf. 2021;211:111887. 10.1016/j.ecoenv.2020.111887 [DOI] [PubMed] [Google Scholar]
- 8.Abbas SZ, Rafatullah M, Ismail N, Lalung J. Isolation, identification, and characterization of cadmium resistant Pseudomonas sp. M3 from industrial wastewater. J Waste Manage. 2014;2014:1–6. 10.1155/2014/160398 [DOI] [Google Scholar]
- 9.Song WE, Chen SB, Liu JF, Li CHEN, Song NN, Ning LI, Bin LIU. Variation of cd concentration in various rice cultivars and derivation of cadmium toxicity thresholds for paddy soil by species-sensitivity distribution. J Integr Agric. 2015;14(9):1845–54. 10.1016/S2095-3119(14)60926-6 [DOI] [Google Scholar]
- 10.Adil MF, Sehar S, Chen G, Chen ZH, Jilani G, Chaudhry AN, Shamsi IH. Cadmium-zinc cross-talk delineates toxicity tolerance in rice via differential genes expression and physiological/ultrastructural adjustments. Ecotoxicol Environ Saf. 2020;190:110076. 10.1016/j.ecoenv.2019.110076 [DOI] [PubMed] [Google Scholar]
- 11.Ecotoxicology and environmental safety, 130, 43–53.
- 12.Zou J, Wang G, Ji J, Wang J, Wu H, Ou Y, Li B. Transcriptional, physiological and cytological analysis validated the roles of some key genes linked cd stress in Salix matsudana Koidz. Environ Exp Bot. 2017;134:116–29. 10.1016/j.envexpbot.2016.11.005 [DOI] [Google Scholar]
- 13.Ecotoxicology and Environmental Safety, 187, 109790.
- 14.Haisel D, Cyrusová T, Vaněk T, Podlipná R. The effect of nanoparticles on the photosynthetic pigments in cadmium—zinc interactions. Environ Sci Pollut Res. 2019;26:4147–51. 10.1007/s11356-018-04060-7 [DOI] [PubMed] [Google Scholar]
- 15.Tanveer M, Shabala S. Entangling the interaction between essential and nonessential nutrients: implications for global food security. Plant Nutrition and Food Security in the era of Climate Change. Academic; 2022. pp. 1–25.
- 16.Bindraban PS, Dimkpa C, Nagarajan L, Roy A, Rabbinge R. Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants. Biol Fertil Soils. 2015;51(8):897–911. 10.1007/s00374-015-1039-7 [DOI] [Google Scholar]
- 17.Mimmo, T., Del Buono, D., Terzano, R., Tomasi, N., Vigani, G., Crecchio, C., ... & Cesco, S. (2014). Rhizospheric organic compounds in the soil–microorganism–plant system: their role in iron availability. European Journal of Soil Science, 65(5), 629–642.
- 18.Ariga T, Hazama K, Yanagisawa S, Yoneyama T. Chemical forms of iron in xylem sap from graminaceous and non-graminaceous plants. Soil Sci Plant Nutr. 2014;60(4):460–9. 10.1080/00380768.2014.922406 [DOI] [Google Scholar]
- 19.Colombo C, Palumbo G, He JZ, Pinton R, Cesco S. Review on iron availability in soil: interaction of Fe minerals, plants, and microbes. J Soils Sediments. 2014;14:538–48. 10.1007/s11368-013-0814-z [DOI] [Google Scholar]
- 20.Singh A, Singh NB, Hussain I, Singh H, Singh SC. Plant-nanoparticle interaction: an approach to improve agricultural practices and plant productivity. Int J Pharm Sci Invent. 2015;4(8):25–40. [Google Scholar]
- 21.Hamzah Saleem M, Usman K, Rizwan M, Jabri A, H., Alsafran M. Functions and strategies for enhancing zinc availability in plants for sustainable agriculture. Front Plant Sci. 2022;13:1033092. 10.3389/fpls.2022.1033092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frontiers in plant science, 13, 992535.
- 23.Ma C, White JC, Dhankher OP, Xing B. Metal-based nanotoxicity and detoxification pathways in higher plants. Environ Sci Technol. 2015;49(12):7109–22. 10.1021/acs.est.5b00685 [DOI] [PubMed] [Google Scholar]
- 24.Journal of hazardous materials, 322, 2–16.
- 25.Mohammadi H, Hatami M, Feghezadeh K, Ghorbanpour M. Mitigating effect of nano-zerovalent iron, iron sulfate and EDTA against oxidative stress induced by chromium in Helianthus annuus L. Acta Physiol Plant. 2018;40:1–15. 10.1007/s11738-018-2647-2 [DOI] [Google Scholar]
- 26.Sebastian A, Nangia A, Prasad MNV. A green synthetic route to phenolics fabricated magnetite nanoparticles from coconut husk extract: implications to treat metal contaminated water and heavy metal stress in Oryza sativa L. J Clean Prod. 2018;174:355–66. 10.1016/j.jclepro.2017.10.343 [DOI] [Google Scholar]
- 27.Yang J, Cao W, Rui Y. Interactions between nanoparticles and plants: phytotoxicity and defense mechanisms. J Plant Interact. 2017;12(1):158–69. 10.1080/17429145.2017.1310944 [DOI] [Google Scholar]
- 28.Wang Y, Jiang F, Ma C, Rui Y, Tsang DC, Xing B. Effect of metal oxide nanoparticles on amino acids in wheat grains (Triticum aestivum) in a life cycle study. J Environ Manage. 2019;241:319–27. 10.1016/j.jenvman.2019.04.041 [DOI] [PubMed] [Google Scholar]
- 29.Environmental Pollution, 254, 113032.
- 30.Ghafariyan MH, Malakouti MJ, Dadpour MR, Stroeve P, Mahmoudi M. Effects of magnetite nanoparticles on soybean chlorophyll. Environ Sci Technol. 2013;47(18):10645–52. [DOI] [PubMed] [Google Scholar]
- 31.α-Fe 2 O 3 nanoparticle fertilizer. RSC advances, 8(43), 24075–24083. [DOI] [PMC free article] [PubMed]
- 32.Kah M, Tufenkji N, White JC. Nano-enabled strategies to enhance crop nutrition and protection. Nat Nanotechnol. 2019;14(6):532–40. 10.1038/s41565-019-0439-5 [DOI] [PubMed] [Google Scholar]
- 33.Ecotoxicology and Environmental Safety, 230, 113142.
- 34.Microorganisms, 10(9), 1837.
- 35.Frontiers in Plant Science, 13, 973782.
- 36.Science of the Total Environment, 721, 137778.
- 37.Wang Z, Yue L, Dhankher OP, Xing B. Nano-enabled improvements of growth and nutritional quality in food plants driven by rhizosphere processes. Environ Int. 2020;142:105831. 10.1016/j.envint.2020.105831 [DOI] [PubMed] [Google Scholar]
- 38.Toumey C. (2020). Notes on Environmental Nanoscience. Doctoral dissertation. Berlin: Nature Portfolio. [DOI] [PubMed]
- 39.Environmental Pollution, 269, 116134.
- 40.Science of the Total Environment, 712, 136497.
- 41.Chen D, Cao B, Wang S, Liu P, Deng X, Yin L, Zhang S. Silicon moderated the K deficiency by improving the plant-water status in sorghum. Sci Rep. 2016;6(1):22882. 10.1038/srep22882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okturen Asri F, Sonmez S. (2014, September). Effects of potassium and iron applications on nutrient concentrations of tomato plants grown in soilless culture. In VI Balkan Symposium on Vegetables and Potatoes 1142 (pp. 329–334).
- 43.Iannone MF, Groppa MD, de Sousa ME, van Raap MBF, Benavides MP. Impact of magnetite iron oxide nanoparticles on wheat (Triticum aestivum L.) development: evaluation of oxidative damage. Environ Exp Bot. 2016;131:77–88. 10.1016/j.envexpbot.2016.07.004 [DOI] [Google Scholar]
- 44.Tanveer Y, Yasmin H, Nosheen A, Ali S, Ahmad A. Ameliorative effects of plant growth promoting bacteria, zinc oxide nanoparticles and oxalic acid on Luffa acutangula grown on arsenic enriched soil. Environ Pollut. 2022;300:118889. 10.1016/j.envpol.2022.118889 [DOI] [PubMed] [Google Scholar]
- 45.Chemosphere, 214, 269–277.
- 46.Konate K, Abdourahime GAYE. A proposal analytical model and simulation of the attacks in routing protocols of MANETs: implementation of a secure model of mobility. Int J Appl Graph Theory Wirel Ad Hoc Netw Sens Netw. 2017;9(2):1–8. [Google Scholar]
- 47.Afzal S, Sharma D, Singh NK. Eco-friendly synthesis of phytochemical-capped iron oxide nanoparticles as nano-priming agent for boosting seed germination in rice (Oryza sativa L). Environ Sci Pollut Res. 2021;28:40275–87. 10.1007/s11356-020-12056-5 [DOI] [PubMed] [Google Scholar]
- 48.Hussain A, Ali S, Rizwan M, ur Rehman MZ, Qayyum MF, Wang H, Rinklebe J. Responses of wheat (Triticum aestivum) plants grown in a cd contaminated soil to the application of iron oxide nanoparticles. Ecotoxicol Environ Saf. 2019;173:156–64. 10.1016/j.ecoenv.2019.01.118 [DOI] [PubMed] [Google Scholar]
- 49.Manzoor, N., Ahmed, T., Noman, M., Shahid, M., Nazir, M. M., Ali, L., ... & Wang, G. (2021). Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Science of the Total Environment, 769, 145221. [DOI] [PubMed]
- 50.Bidi H, Fallah H, Niknejad Y, Tari DB. Iron oxide nanoparticles alleviate arsenic phytotoxicity in rice by improving iron uptake, oxidative stress tolerance and diminishing arsenic accumulation. Plant Physiol Biochem. 2021;163:348–57. 10.1016/j.plaphy.2021.04.020 [DOI] [PubMed] [Google Scholar]
- 51.Taher D, Solberg SØ, Prohens J, Chou YY, Wu TH. World vegetable center eggplant collection: origin, composition, seed dissemination and utilization in breeding. Front Plant Sci. 2017;8:279838. 10.3389/fpls.2017.01484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alam I, Salimullah M. Genetic engineering of eggplant (Solanum melongena L.): Progress, controversy and potential. Horticulturae. 2021;7(4):78. 10.3390/horticulturae7040078 [DOI] [Google Scholar]
- 53.Özkay F, Kıran S, Taş İ, Kuşvuran Ş. Effects of copper, zinc, lead and cadmium applied with irrigation water on some eggplant plant growth parameters and soil properties. Türk Tarım ve Doğa Bilimleri Dergisi. 2014;1(3):377–83. [Google Scholar]
- 54.Othman YA, Al-Assaf A, Tadros MJ, Albalawneh A. Heavy metals and microbes accumulation in soil and food crops irrigated with wastewater and the potential human health risk: a metadata analysis. Water. 2021;13(23):3405. 10.3390/w13233405 [DOI] [Google Scholar]
- 55.Atamaleki A, Yazdanbakhsh A, Gholizadeh A, Naimi N, Karimi P, Thai VN, Fakhri Y. Concentration of potentially harmful elements (PHEs) in eggplant vegetable (Solanum melongena) irrigated with wastewater: a systematic review and meta-analysis and probabilistic health risk assessment. Int J Environ Health Res. 2022;32(7):1419–31. 10.1080/09603123.2021.1887461 [DOI] [PubMed] [Google Scholar]
- 56.Vernon LP. Spectrophotometric determination of chlorophylls and pheophytins in Plant extracts. Anal Chem. 1960;32:1144–50. 10.1021/ac60165a029 [DOI] [Google Scholar]
- 57.Environmental Pollution, 252, 1377–1387.
- 58.Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125(1):189–98. 10.1016/0003-9861(68)90654-1 [DOI] [PubMed] [Google Scholar]
- 59.Patterson BD, MacRae EA, Ferguson IB. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem. 1984;139(2):487–92. 10.1016/0003-2697(84)90039-3 [DOI] [PubMed] [Google Scholar]
- 60.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–7. 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- 61.Mansour R, Dakhlaouia S, Msahli W, Ksouri R, Ksouri MW. Differential responses of Cakile maritima at two development stages to salinity: changes on phenolic metabolites and related enzymes and antioxidant activity. Med Chem. 2018;8:100–8. 10.4172/2161-0444.1000500 [DOI] [Google Scholar]
- 62.Usman S, Yaseen G, Noreen Z, Rizwan M, Noor H, Elansary HO. Melatonin and arginine combined supplementation alleviate salt stress through physiochemical adjustments and improved antioxidant enzymes activity in Capsicum annuum L. Sci Hort. 2023;321:112270. 10.1016/j.scienta.2023.112270 [DOI] [Google Scholar]
- 63.Chance B, Maehly A. (1955). [136] Assay of catalases and peroxidases. [DOI] [PubMed]
- 64.Khanom F, Kayahara H, Tadasa K. Superoxide-scavenging and prolyl endopeptidase inhibitory activities of Bangladeshi indigenous medicinal plants. Biosci Biotechnol Biochem. 2000;64(4):837–40. 10.1271/bbb.64.837 [DOI] [PubMed] [Google Scholar]
- 65.Onsa GH, bin Saari N, Selamat J, Bakar J. Purification and characterization of membrane-bound peroxidases from Metroxylon sagu. Food Chem. 2004;85(3):365–76. 10.1016/j.foodchem.2003.07.013 [DOI] [Google Scholar]
- 66.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22(5):867–80. [Google Scholar]
- 67.Amako K, Chen GX, Asada K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994;35(3):497–504. [Google Scholar]
- 68.Paul V, Ramesh KV, Pandey R. (2017). Analysis of Mineral Nutrients: Sampling Techniques and Methods of Digestion for Plant Samples. Manual of ICAR Sponsored Training Programme for Technical Staff of ICAR Institutes on Physiological Techniques to Analyze the Impact of Climate Change on Crop Plants, 77.
- 69.Ahmad B, Zaid A, Zulfiqar F, Bovand F, Dar TA. Nanotechnology: a novel and sustainable approach towards heavy metal stress alleviation in plants. Nanatechnol Environ Eng. 2023;8(1):27–40. 10.1007/s41204-022-00230-8 [DOI] [Google Scholar]
- 70.Pérez Chaca MV, Vigliocco A, Reinoso H, Molina A, Abdala G, Zirulnik F, Pedranzani H. Effects of cadmium stress on growth, anatomy and hormone contents in Glycine max (L.) Merr. Acta Physiol Plant. 2014;36:2815–26. 10.1007/s11738-014-1656-z [DOI] [Google Scholar]
- 71.Acta Physiologiae Plantarum, 43, 1–13.
- 72.Environmental Science and Pollution Research, 26, 23119–23128. [DOI] [PubMed]
- 73.γ-aminobutyric acid (GABA) on physio-biochemical attributes of lettuce (Lactuca sativa L.) under cadmium toxicity. Journal of Plant Growth Regulation, 42(8), 5041–5057.
- 74.Metwali MR, Gowayed SM, Al-Maghrabi OA, Mosleh YY. Evaluation of toxic effect of copper and cadmium on growth, physiological traits and protein profile of wheat (Triticum Aestivium L.), maize (Zea mays L.) and sorghum (Sorghum bicolor L). World Appl Sci J. 2013;21(3):301–4. [Google Scholar]
- 75.Yang S, Zhang J, Chen L. Growth and physiological responses of Pennisetum sp. to cadmium stress under three different soils. Environ Sci Pollut Res. 2021;28:14867–81. 10.1007/s11356-020-11701-3 [DOI] [PubMed] [Google Scholar]
- 76.Rai P, Singh VP, Sharma S, Tripathi DK, Sharma S. Iron oxide nanoparticles impart cross tolerance to arsenate stress in rice roots through involvement of nitric oxide. Environ Pollut. 2022;307:119320. 10.1016/j.envpol.2022.119320 [DOI] [PubMed] [Google Scholar]
- 77.Manzoor, N., Ali, L., Al-Huqail, A. A., Alghanem, S. M. S., Al-Haithloul, H. A. S., Abbas, T., ... & Wang, G. (2023). Comparative efficacy of silicon and iron oxide nanoparticles towards improving the plant growth and mitigating arsenic toxicity in wheat (Triticum aestivum L.). Ecotoxicology and Environmental Safety, 264, 115382. [DOI] [PubMed]
- 78.Mazhar MW, Ishtiaq M, Maqbool M, Ullah F, Sayed SR, Mahmoud EA. Seed priming with iron oxide nanoparticles improves yield and antioxidant status of garden pea (Pisum sativum L.) grown under drought stress. South Afr J Bot. 2023;162:577–87. 10.1016/j.sajb.2023.09.047 [DOI] [Google Scholar]
- 79.Jawad Hassan, M., Ali Raza, M., Ur Rehman, S., Ansar, M., Gitari, H., Khan, I., ... & Li, Z. (2020). Effect of cadmium toxicity on growth, oxidative damage, antioxidant defense system and cadmium accumulation in two sorghum cultivars. Plants, 9(11), 1575. [DOI] [PMC free article] [PubMed]
- 80.Farid M, Shakoor MB, Ehsan S, Ali S, Zubair M, Hanif MS. Morphological, physiological and biochemical responses of different plant species to cd stress. Int J Chem Biochem Sci. 2013;3:53–60. [Google Scholar]
- 81.Raza, H. Z., Shah, A. A., Noreen, Z., Usman, S., Zafar, S., Yasin, N. A., ... & Aslam, M. (2024). Calcium oxide nanoparticles mitigate lead stress in Abelmoschus esculentus though improving the key antioxidative enzymes, nutritional content and modulation of stress markers. Plant Physiology and Biochemistry, 206, 108171. [DOI] [PubMed]
- 82.Maqsood, M. F., Shahbaz, M., Khalid, F., Rasheed, Y., Asif, K., Naz, N., ... & Attia, H. (2023). Biogenic nanoparticles application in agriculture for ROS mitigation and abiotic stress tolerance: A review. Plant Stress, 10, 100281.
- 83.Li, S., Yu, J., Zhu, M., Zhao, F., & Luan, S. (2012). Cadmium impairs ion homeostasis by altering K + and Ca2 + channel activities in rice root hair cells. Plant, cell & environment, 35(11), 1998–2013. 10.1111/j.1365-3040.2012.02532.x [DOI] [PubMed] [Google Scholar]
- 84.Adil, M. F., Sehar, S., Han, Z., Lwalaba, J. L. W., Jilani, G., Zeng, F., ... & Shamsi, I. H. (2020). Zinc alleviates cadmium toxicity by modulating photosynthesis, ROS homeostasis, and cation flux kinetics in rice. Environmental Pollution, 265, 114979. [DOI] [PubMed]
- 85.Chatterjee, A., Mridha, D., Banerjee, J., Chanda, S., Ray, K., Acharya, K., ... & Sarkar, J. (2021). Green synthesis of iron oxide nanoparticles and their ameliorative effect on arsenic stress relief in Oryza sativa seedlings. Biocatalysis and Agricultural Biotechnology, 38, 102207.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.