Abstract
Background
Up to now several studies estimate the prevalence of HBV, HCV, and TB among people living with HIV (PLWH) in Iran; however, their results are inconsistent. This study aimed to estimate the overall prevalence of HBV, HVC, and TB among Iranian PLWH.
Methods
In this systematic review and meta-analysis six databases including Medline, Web of Science, Scopus, MagIran, Scientific Information Database (SID), and Barakat Knowledge network system were searched up to October 2023 with no language restriction. All studies estimated the prevalence of HBV, HCV, and TB among PLWH in Iran were included. The random-effects model was used to report the study estimates. Results were reported at a 95% confidence interval (CI).
Results
Out of 1050 retrieved references, 58 articles met the eligibility criteria. Overall among PLWH, HBV prevalence was 13.0% (95% CI: 11.0, 15.0), HCV prevalence was 54% (95% CI: 45.0, 64.0), and TB prevalence was 19% (95% CI: 13.0, 24.0). The results from multivariate meta-regression analysis showed no statistically significant association between HBV and TB prevalence with the year of study, quality of studies, age, gender, and persons who inject drugs (PWID). HCV prevalence was significantly associated with PWID.
Conclusion
We found HBV, HCV, and TB infections are common among PLWH in Iran and required to be screened and treated with effective and timely services.
Keywords: HIV, Hepatitis B, Hepatitis C, Tuberculosis, Systematic review, Iran
Key messages
The prevalence of HBV, HCV, and TB was high among Iranian PLWH
The prevalence was higher among at-risk groups such as PWIDs and homeless people
The prevalence of HCV was more than HBV and TB among PLWH
Introduction
Human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) are blood born viruses. HIV and HBV are primarily transmitted via injection drug use and unprotected sexual contact. HCV mainly transmitted via sharing the needles or other equipment for drug injection. Because of the same transmission routes of these viruses, the HIV infected people are at a higher risk of HBV and HCV than the general population [1]. By suppressing the immune system, HIV provides the basis for opportunistic infections such as tuberculosis (TB). The risk of TB among HIV-infected people is about 20 times more than the others and TB remains the main cause of death among people living with HIV (PLHW) [2]. HBV increases the risk of the progression of disease and mortality among HIV infected patients [3, 4].
The global burden of HIV-HCV co-infection is 2.75 million [1]. The prevalence of HIV-HCV co-infection is estimated from 2.4%, 4.0%, 6.4%, and 82.4% among the general population, heterosexually exposed people, men who have sex with men, and people who inject drugs respectively [5]. The prevalence of HCV infection among prisoners in Iran estimated from 19%, 25%, and 53% among general prisoners, prisoners who use drugs, and prisoners who inject drugs respectively [6]. The global prevalence of HBV among HIV infected people estimated approximately 5–10% [7]. It is estimated that 900,000 new cases of TB has occurred among people living with HIV (PLWH) in 2017, and about 300,000 people died of HIV associated TB [8]. The overall prevalence of TB/HIV co-infection is estimated at 14% in Iran [9].
In Iran, HIV infection is concentrated among people who inject drugs [10], in addition, injecting drugs is a major risk factor for HCV, so the coinfection of HIV with viral hepatitis B and C complicate the health status of these people and increase the risk of liver cancer [11]. According to the results of conducted systematic reviews, the prevalence of HCV among Iranian prisoners and TB/HIV coinfection in Iran were 28.0% [6] and 14.0% [9] respectively.
Several studies have estimated the prevalence of HBV, HCV, and TB among PLWH in Iran from 2003 to 2022; however, their results are inconsistent [12–19]. Although there are systematic reviews regarding the co-infection of TB/HIV [9] and prevalence of HCV among high-risk groups [6] in Iran, there are not sufficient evidence regarding the overall prevalence of TB, HCV, and HIV among PLWH in Iran. Our study simultaneously summarized the evidence regarding the prevalence of three important infectious diseases among PLWH. In addition, studies regarding the coinfection of HIV, HBV, HCV, and TB are in priority for research in Iran especially among high risk groups [20]. In this systematic review and meta-analysis, we aimed to estimate the overall prevalence of HBV, HVC, and TB among Iranian PLWH.
Methods
Searching
A search strategy using the following keywords was designed: hepatitis C, hepatitis B, tuberculosis, HIV, acquired immunodeficiency syndrome, prevalence, and incidence. The international and national databases including Medline, Web of Science, Scopus, MagIran, Scientific Information Database (SID), and Barakat Knowledge network system were searched up to October 2023. In addition, the reference lists of selected studies were scanned in order to obtain more related studies.
Two investigators (ADI, BE) were independently responsible for screening the retrieved studies. The obtained references from the databases were included in the EndNote software. In the first step, duplicated studies were removed, and in the next step, the mentioned investigators based on the eligibility criterion screened the title and abstract of reminded studies. Any disagreement was resolved by the discussion and judgment of the third author (AM).
Inclusion criteria
All cross-sectional studies and studies that reviewed the patients' records retrospectively which had estimated the prevalence of HBV, HCV, and TB among Iranian HIV infected patients, regardless of sex and age, were included in this systematic review. There were no restrictions in date of publication and language of studies. The definition for HIV positive was two positive ELISA tests which confirmed by Western Blot. Positive HBV surface antigen (HBsAg) was defined as HBV, and positive anti-HCV antibody was considered as HCV infection. Diagnosis of TB was based on at least two positive acid-fast bacilli (AFB) in three sputum sample or one positive AFB plus radiographic evidence and clinical symptoms.
Risk of bias assessment and data extraction
Eight items from the Joanna Briggs Institute (JBI) critical appraisal checklist for prevalence studies were selected for risk of bias assessment [21]. These items included: 1) the appropriateness of the sampling frame to address the target population, 2) the appropriateness of the sampling method, 3) the sample size was adequate. 4) The subjects and the setting of the study described in detail? 5) The use of a valid method for the identification of the outcome? 6) Were the outcomes (HBV, HCV, and TB) measured in a standard, reliable way for all participants? 7) The appropriateness of statistical analysis? 8) Was the response rate adequate, and if not, was the low response rate managed appropriately? Each item scored as follow: meet the criteria equal two, unclear equal one, and not meet the criteria equal zero. The range of score for each study was 0–16. Studied that scored 15 or more were considered as the high quality, studies with scores 11–14 considered as intermediate quality and studies with lower than 11 scores were considered as low quality. Two authors (ADI, BE) were responsible for quality assessment independently. The Kappa statistic for the agreement between two authors was 71.0%.
The following variables were extracted from included studies: the name of first author, year of publication, year of study conduction, type of study design, the location of study, characteristics of study population, mean age of participants, gender, sample size, the number of PLWH, and the number of people with HBV, HCV, and TB.
Assessment of heterogeneity
The statistical heterogeneity was assessed using the chi-squared (Chi2) test at the 5% statistical level. In addition, the heterogeneity across included studies was quantified by I2 statistics, and the between-study variance was assessed using tua-squared (Tua2) statistic [22].
Data analysis
The prevalence of HBV, HCV, and TB was calculated by dividing the number of people with HBV, HCV, and TB to the number of HIV patients. Meta-analysis was used to obtain the pooled estimate of the prevalence of HBV, HCV, and TB among HIV patients. The included studies were conducted in different provinces of Iran and by various researchers. In addition, the study populations of the included studies were different, so in addition to differences among the results of studies due to sampling variation, there is a random variation in the prevalence in various studies. Therefore, the random-effects model was used to the report of summary measures [23].
The meta-regression was used to identify the sources of heterogeneity in the results of the included studies. Subgroup analysis was conducted based on the gender, provinces of Iran, quality of studies, and date of study conduction (≤ 2005, 2006–2010 and after 2011). Both Stata 11 (Stata Corp, College Station, TX, USA) and Review Manager [Review Manager (RevMan) Version 5.3 were used for data analysis. The pooled prevalence of HBV, HCV, and TB was reported at a 95% confidence interval (CI).
Results
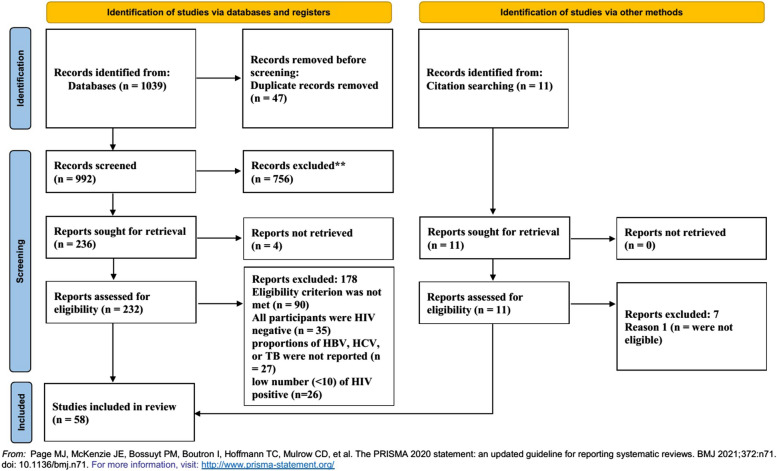
Out of 1050 retrieved references from the six databases, 58 articles [12–17, 19, 24–74] met the eligibility criteria (Fig. 1). Among these articles 40, 42, and 14 have been reported the prevalence of HBV, HCV, and Tuberculosis among PLWH, respectively. These studies involved 17,905 PLWH. The characteristics of the included studies are reported in Table 1. The results of risk of bias assessment are shown in Fig. 2.
Fig. 1.
A flow chart showing the stages of retrieving articles and assessing the eligibility criteria for meta-analysis of prevalence of HBV, HCV, and TB among HIV-POSITIVE patients
Table 1.
Characteristics of included studies and prevalence of hepatitis B and C and tuberculosis infections among people living with HIV
| Author (year) | Location | Sudy design | Study population (Sampling Method) | Number of HIV-Positive participants | % Male | Mean Age | % PWID | HBV Positive (%) | HCV-positive (%) | TB-positive (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Moradmand (2011) [58] | Tehran | Cross-sectional | HIV-POSITIVE patients in counseling center (Patients records) | 365 | 80.00 | 37 | 50.96 | 90 (24.66) | 129 (35.34) | |
| Aminzadeh (2007) [28] | Tehran | Cross-sectional | iHospitalized PWIDs (Convenience sample) | 21 | 100.00 | 37 | 100.00 | 3 (14.29) | 13 (61.9) | |
| Tayeri (2008) [71] | Isfahan | Cross-sectional | PWIDs patients referring to counseling center | 106 | 100.00 | 51 | 100.00 | 2 (1.89) | 80 (75.47) | |
| Hajiabdolbaghi (2014) [44] | Tehran | Cross-sectional | Hospitalized HIV-POSITIVE patients (Records of patients) | 498 | 84.30 | 76.51 | 137 (27.51) | |||
| Daryazadeh (2016) [34] | Isfahan | Cross-sectional | HIV-POSITIVE patients in counseling center (Records of patients) | 241 | 0.00 | 39 | 0.00 | 3 (1.24) | 79 (32.78) | 16 (6.64) |
| Hosseinirad (2017) [46] | Tehran | Cross-sectional | HIV-POSITIVE patients referring to counseling center | 481 | 85.00 | 42 | 77.75 | 32 (6.65) | 331 (68.81) | |
| Vaziri (2008) [73] | Kermanshah | Cross-sectional | HIV-POSITIVE patients referring to counseling center | 887 | 98.00 | 31 | 13.98 | 19 (2.14) | 35 (3.95) | |
| Aghakhani (2016) [24] | Tehran | Cross-sectional | HIV-POSITIVE patients referring to counseling center | 92 | 54.30 | 38 | 41.30 | 30 (32.61) | ||
| Aghasadeghi (2013) [25] | Tehran | Cross-sectional | HIV-POSITIVE patients counseling center | 600 | 69.60 | 36.9 | 53.60 | 9 (1.5) | ||
| Alavi SM (2012) [12] | Ahvaz | Cross-sectional | Hospitalized HIV-POSITIVE patients (Records of patients) | 123 | 100.00 | 32.4 | 80.49 | 81 (65.85) | ||
| Alavi (2007) [26] | Ahvaz | Cross-sectional | Hospitalized PWID patients hospital | 104 | 100.00 | 28 | 67.53 | 46 (44.23) | 77 (74.04) | |
| Ataei (2010) [30] | Isfahan | Cross-sectional | HIV-POSITIVE patients referring to counseling center | 130 | 98.00 | 50.23 | 81.54 | 15 (11.54) | 100 (76.92) | |
| Davarpanah (2013) [13] | Shiraz | Cross-sectional | HIV-POSITIVE patients referring to counseling center | 226 | 95.00 | 35.6 | 74.78 | 196 (86.73) | ||
| Davarpanah (2007) [35] | Shiraz | Cross-sectional | HIV-POSITIVE patients referring to counseling center | 228 | 90.00 | 35.5 | 0.00 | 17 (7.46) | ||
| Davarpanah (2015) [36] | Shiraz | Cross-sectional | HIV-POSITIVE patients referring to counseling center (Random sampling) | 840 | 0.00 | 37 | 0.00 | 29 (3.45) | ||
| Davarpanah (2015) [37] | Shiraz | Cross-sectional | HIV-POSITIVE patients referring to counseling center | 186 | 88.00 | 37 | 73.12 | 66 (35.48) | ||
| Davarpanah (2009) [38] | Shiraz | Cross-sectional | HIV-POSITIVE patients referring to counseling center | 459 | 85.00 | 0.00 | 14 (3.05) | |||
| Davoodian (2009) [14] | Bandar Abbas | Cross-sectional | Prisoners PWIDs (Random sampling) | 38 | 100.00 | 35.4 | 100.00 | 3 (7.89) | 35 (92.11) | |
| Doosti-Irani (2017) [75] | Khoramabad | Cross-sectional | Male homeless people (Convenience sample) | 20 | 100.00 | 35.86 | 38.76 | 18 (90) | ||
| Farhoudi (2016) [15] | Tehran | Cross-sectional | Prisoners HIV positive patients (Active case finding) | 85 | 100.00 | 34.55 | 0.00 | 3 (3.53) | 50 (58.82) | 5 (5.88) |
| Foroughi (2017) [43] | Tehran | Cross-sectional | Street and labour children (Biobehavioural surveillance survey) | 45 | 95.00 | 15.9 | 0.00 | 2 (4.44) | 5 (11.11) | |
| Hashemi-Shahri (2016) [16] | Zahedan | Retrospective | Hospitalized patients With HIV/AIDS | 41 | 73.00 | 43.9 | 0.00 | 11 (26.83) | 13 (31.71) | |
| Javad Zahedi (2014) [49] | Kerman | Cross-sectional | HIV-POSITIVE patients referring to counseling center | 165 | 82.00 | 40.4 | 76.36 | 6 (3.64) | 122 (73.94) | |
| Keramat (2011) [17] | Hamadan | Cross-sectional | HIV-POSITIVE patients referring to counseling center | 15 | 72.00 | 29.7 | 52.51 | 1 (6.67) | 13 (86.67) | |
| Khazaei (2016) [51] | Abadan | Cross-sectional | HIV-POSITIVE patients in counseling center (Records of patients) | 366 | 86.00 | 33.5 | 73.50 | 20 (5.46) | 126 (34.43) | 80 (21.86) |
| Khorvash (2014) [52] | Isfahan | Cross-sectional | HIV-POSITIVE patients referring to counseling center | 64 | 0.00 | 33.64 | 10.94 | 12 (18.75) | 8 (12.5) | |
| Khosravi (2010) [53] | Shiraz | Cross-sectional | HIV-POSITIVE patients referring to counseling center | 101 | 88.00 | 54.9 | 35.64 | 87 (86.14) | ||
| Khosravi (2012) [54] | Ahvaz | Cross-sectional | Hospitalized HIV-POSITIVE patients | 50 | 90.00 | 30 | 0.00 | 16 (32) | 8 (16) | 9 (18) |
| Koochak (2017) [55] | Tehran | Cross-sectional | HIV-POSITIVE patients referring to counseling center | 200 | 72.00 | 0.00 | 121 (60.5) | |||
| Mohammad Nejad (2013) [56] | Tehran | Cross-sectional | Hospitalized HIV-POSITIVE patients | 213 | 92.00 | 35 | 46.48 | 24 (11.27) | ||
| Mohammadi (2009) [57] | Tehran | Cross-sectional | HIV-POSITIVE patients referring to counseling center | 391 | 51.66 | 57 (14.58) | 282 (72.12) | |||
| Motamedifar (a) (2015) [59] | Shiraz | Retrospective | HIV-POSITIVE patients referring to counseling center | 765 | 70.00 | 39 | 64.71 | 59 (7.71) | ||
| Motamedifar (b) (2015) [60] | Shiraz | Retrospective | HIV-POSITIVE patients referring to counseling center | 1480 | 98.00 | 37.4 | 0.00 | 178 (12.03) | ||
| Rabirad (2012) [62] | Tehran | Cross-sectional | Hospitalized HIV-POSITIVE patients (Records of patients) | 71 | 92.00 | 35 | 0.00 | 20 (28.17) | ||
| Rahimi-Movaghar (2010) [63] | Tehran | Cross-sectional | PWIDs who were selected from drug treatment centers and community (Volunteer) | 96 | 96.00 | 33.87 | 100.00 | 69 (71.88) | 77 (80.21) | |
| Ramezani (2014) [64] | Arak | Cross-sectional | PWID attending methadone maintenance clinics | 19 | 100.00 | 33.3 | 100.00 | 5 (26.32) | 15 (78.95) | |
| Ramezani (2009) [65] | Tehran | Cross-sectional | HIV-POSITIVE patients referring to counseling center | 92 | 72.00 | 36.7 | 47.83 | 4 (4.35) | 63 (68.48) | |
| Rezaianzadeh (2012) [67] | Shiraz | Retrospective | HIV-POSITIVE patients referring to counseling center | 1339 | 85.00 | 36 | 73.71 | 1044 (77.97) | ||
| SeyedAlinaghi (2011) [68] | Tehran | Cross-sectional | Hospitalized HIV-POSITIVE patients | 201 | 86.00 | 36 | 48.76 | 89 (44.28) | 135 (67.16) | |
| Tabarsi (2015) [69] | Tehran | Cross-sectional | HIV-POSITIVE ptients referring to counseling center | 154 | 82.00 | 36 | 0.00 | 58 (37.66) | ||
| Amiri (2014) [29] | Tehran | Cross-sectional | Homeless individuals (Convenience sampling) | 20 | 87.00 | 41 | 27.49 | 3 (15) | 17 (85) | 1 (5) |
| Azadmanesh (2008) [31] | Tehran | Cross-sectional | HIV-POSITIVE patients referring to counseling center | 106 | 75.00 | 36.4 | 15.09 | 22 (20.75) | ||
| Bokharaei-Salim (2016) [33] | Tehran | Cross-sectional | Hospitalized HIV-POSITIVE patients | 109 | 62.00 | 35.2 | 41.28 | 50 (45.87) | ||
| Kasraiyan (2003) [18] | Shiraz | Cross-sectional | Blood donors (Volunteers) | 34 | 94.00 | 33.4 | 0.00 | 2 (5.88) | 4 (11.76) | |
| Amini (2020) [27] | Sanandaj | Cross-sectional | HIV positive individuals referring to Consultation Center | 185 | 76.00 | 39.26 | 0.00 | 99 (53.51) | ||
| Azmoudeh-Ardalan (2021) [32] | Sanandaj | Cross-sectional | HIV positive individuals referring to Consultation Center | 184 | 76.00 | 39.26 | 0.00 | 24 (13.04) | ||
| Dehghani-Dehej (2020) [39] | Tehran | Cross-sectional | HIV-positive individuals who were referred to hospitals | 72 | 0.00 | 33.6 | 0.00 | 7 (9.72) | 6 (8.33) | |
| Dehghani-Dehej (2020) [39] | Tehran | Cross-sectional | HIV-positive individuals who were referred to hospitals | 126 | 63.60 | 35.3 | 0.00 | 18 (14.29) | 79 (62.7) | |
| Dehghani-Dehej (2018) [40] | Tehran | Cross-sectional | HIV-positive individuals who were referred to HIV laboratory of national centre | 140 | 64.00 | 35.7 | 0.00 | 62 (44.29) | ||
| Donyavi (2019) [41] | Tehran | Cross-sectional | IUD participants with established HIV infection who were referred to hospitals | 161 | 95.00 | 38.9 | 100.00 | 20 (12.42) | 134 (83.23) | |
| Hatami (2018) [45] | National | Cross-sectional | HIV-positive individuals | 908 | 67.00 | 36.9 | 34.00 | 22 (2.42) | 350 (38.55) | |
| Jamshidi (2020) [47] | Tehran | Cross-sectional | HIV-positive individuals who were referred to hospitals | 190 | 63.00 | 36.5 | 43.00 | 23 (12.11) | 85 (44.74) | |
| Janbakhsh (2017) [48] | Kermanshah | Cross-sectional | HIV-positive individuals (random sampling) | 200 | 0.00 | 0.00 | 77 (38.5) | |||
| Lavaee (2022) [19] | Shiraz | Cross-sectional | HIV-positive individuals who were referred to Shiraz HIV research center for routine dental treatment | 73 | 53.00 | 39.12 | 0.00 | 4 (5.48) | 27 (36.99) | |
| Naghibifar (2021) [61] | Tehran | Cross-sectional | HIV positive individuals referring to Consultation Center | 3047 | 77.00 | 44.24 | 44.00 | 98 (3.22) | 961 (31.54) | 415 (13.62) |
| Rastegarian (2020) [66] | Shiraz | Cross-sectional | HIV positive individuals referring to Consultation Center | 251 | 63.00 | 40.8 | 0.00 | 15 (5.98) | ||
| Talebi-Taher (2017) [70] | Tehran | Retrospective | HIV-positive individuals (Records of patients) | 194 | 51.00 | 37.82 | 0.00 | 23 (11.86) | ||
| Teimoori (2019) [72] | Ahvaz | Cross-sectional | HIV positive individuals referring to Consultation Center | 229 | 98.00 | 32 | 0.00 | 135 (58.95) | ||
| Zayedi (2020) [74] | Ahvaz | Cross-sectional | HIV-positive individuals who were referred to hospitals | 78 | 86.00 | 29 | 83.00 | 25 (32.05) |
Fig. 2.
Results of risk of bias assessment
Prevalence of HBV among PLWH
The pooled prevalence of HBV among PLWH was 13.0% (95% CI: 11.0, 15.0) (Fig. 3). Results of studies that reported the prevalence of HBV among PLWH were high heterogeneous (I2 = 96.20%). HBV prevalence was higher among men (15.3%), intermediate quality studies (14.8%), and studies conducted 2005 or earlier (19.2%) (Table 2).
Fig. 3.
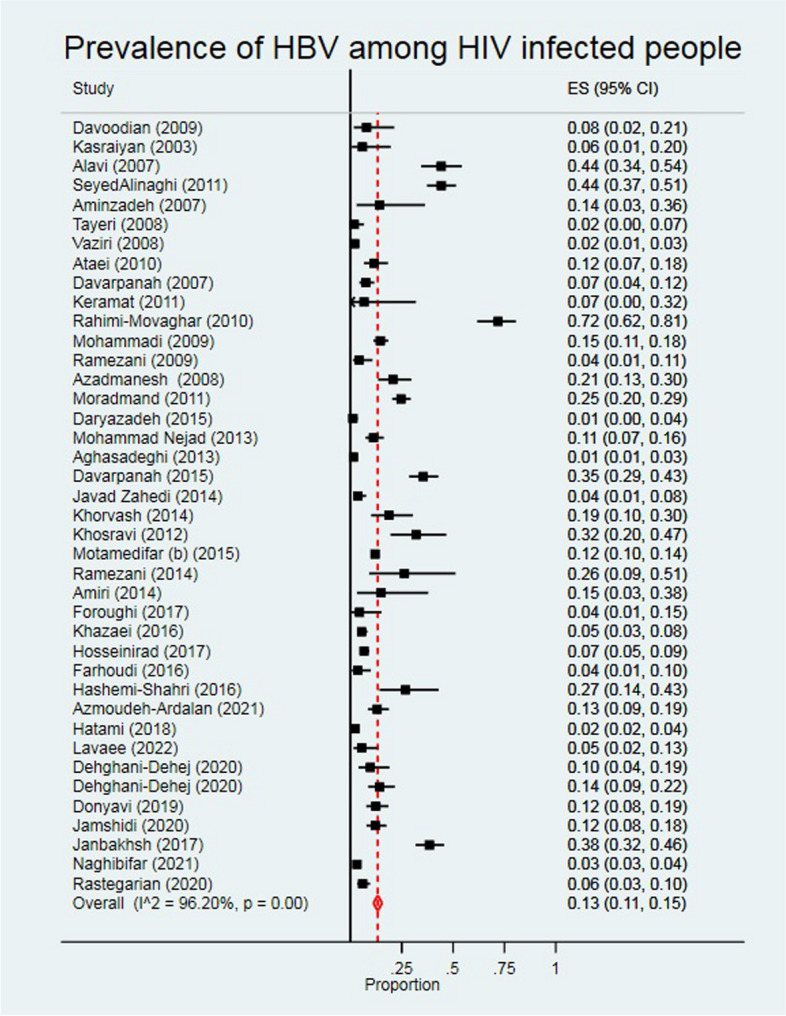
The individual and pooled prevalence of HBV among people diagnosed with HIV
Table 2.
Prevalence of hepatitis B, C and tuberculosis infections among people living with HIV by gender, quality of papers, and years of study
| Variables | HBV | HCV | TB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P% | 95% CI | I2% | N | P% | 95% CI | I2% | N | P% | 95% CI | I2% | |
| Gender | ||||||||||||
| Men | 8 | 15.3 | 7.0, 23.7 | 96.76 | 9 | 67.3 | 49.2, 85.3 | 96.50 | 2 | 21.6 | 17.3, 25.9 | 0.00 |
| Women | 1 | 9.7 | 4.0, 19.0 | 0.00 | 1 | 8.3 | 3.1, 17.3 | 0.00 | - | - | - | - |
| Both | 31 | 13.3 | 10.9, 15.6 | 92.37 | 32 | 52.2 | 41.1, 63.2 | 99.49 | 12 | 14.7 | 10.3, 19.1 | 97.13 |
| Quality of papers | ||||||||||||
| High | 14 | 13.9 | 10.2, 17.5 | 96.73 | 16 | 51.6 | 32.7, 70.5 | 99.65 | 4 | 26.1 | 5.9, 46.2 | 97.80 |
| Intermediate | 17 | 14.8 | 10.6, 18.9 | 94.47 | 16 | 65.7 | 58.1, 73.3 | 99.43 | 5 | 13.0 | 7.7, 18.4 | 96.31 |
| Low | 9 | 10.3 | 6.4, 14.3 | 94.53 | 10 | 40.7 | 23.1, 58.3 | 98.90 | 5 | 14.1 | 7.3, 20.9 | 94.80 |
| Year of study | ||||||||||||
| 2002–2005 | 3 | 19.2 | 0.04, 42.4 | 0.00 | 3 | 59.4 | 15.7, 99.99 | 0.00 | - | - | - | - |
| 2006–2010 | 14 | 16.3 | 11.0, 21.6 | 97.60 | 14 | 63.8 | 41.3, 86.3 | 99.63 | 5 | 22.4 | 9.1, 35.7 | 98.28 |
| 2011–2015 | 13 | 12.1 | 8.0, 16.1 | 94.97 | 14 | 51.0 | 37.1, 64.9 | 98.33 | 8 | 15.7 | 8.7, 22.7 | 97.20 |
| 2016–2020 | 10 | 10.7 | 7.5, 13.9 | 94.53 | 11 | 45.0 | 34.5, 55.6 | 97.90 | 1 | 13.6 | 12.4, 14.9 | 0.00 |
| Geographical region | ||||||||||||
| Tehran | 18 | 15.1 | 11.3, 18.9 | 97.04 | 19 | 53.6 | 43.2, 64.1 | 98.38 | 7 | 18.3 | 11.4, 25.2 | 94.14 |
| Center | 5 | 7.7 | 2.6, 12.8 | 86.74 | 5 | 55.0 | 28.2, 81.8 | 98.13 | 1 | 6.6 | 3.8, 10.6 | 0.00 |
| West | 4 | 15.1 | 0.04, 30.7 | 97.62 | 4 | 58.1 | 14.3, 99.99 | 99.28 | - | - | - | - |
| Southwest | 3 | 26.9 | 0.09, 54.7 | 0.00 | 3 | 41.5 | 12.2, 70.9 | 0.00 | 3 | 35.2 | 6.4, 64.1 | 0.00 |
| South | 6 | 11.7 | 6.0, 17.3 | 92.96 | 7 | 56.5 | 4.10, 7.19 | 99.07 | 3 | 4.7 | 2.1, 7.3 | 0.00 |
| Southeast | 3 | 10.9 | 0.02, 21.7 | 0.00 | 3 | 66.6 | 39.6, 93.6 | 0.00 | - | - | - | - |
| National | 1 | 2.4 | 1.5, 3.6 | 0.00 | 1 | 38.5 | 35.4, 41.8 | 0.00 | - | - | - | - |
| Study population | ||||||||||||
| HIV-positive patients referring to counseling center | 25 | 10.2 | 8.1, 12.2 | 95.61 | 26 | 50.8 | 38.7, 62.8 | 99.56 | 8 | 12.3 | 7.7, 16.9 | 97.45 |
| HIV-positive PWIDs | 7 | 25.5 | 7.6, 43.3 | 97.80 | 7 | 79.8 | 74.3, 85.4 | 59.18 | - | - | - | - |
| Hospitalized HIV-positive patients | 4 | 28.5 | 9.3, 47.6 | 95.57 | 4 | 40.5 | 16.4, 64.5 | 96.09 | 4 | 35.0 | 15.5, 54.4 | 95.99 |
| HIV-positive Homeless people | 1 | 15.0 | 3.2, 37.9 | 0.00 | 2 | 87.9 | 77.9, 98.0 | 0.00 | 1 | 5.0 | 0.01, 24.9 | - |
| HIV-positive Prisoners | 1 | 3.5 | 0.07, 10.0 | 0.00 | 1 | 58.8 | 47.6, 69.4 | 0.00 | 1 | 5.0 | 1.9, 13.2 | 0.00 |
| HIV-positive Street and labour children | 1 | 4.4 | 0.05, 15.1 | 0.00 | 1 | 11.1 | 3.7, 24.1 | 0.00 | - | - | - | - |
| Volunteers for blood doner | 1 | 5.9 | 0.07, 19.7 | 0.00 | 1 | 11.8 | 3.3, 27.5 | 0.00 | - | - | - | - |
| Overall | 40 | 13.3 | 11.2, 15.5 | 96.20 | 42 | 54.3 | 44.6, 64.0 | 99.38 | 14 | 17.5 | 12.7, 22.3 | 97.69 |
Most studies were conducted in Tehran (18 studies) with an overall HBV prevalence of 15.1% (95% CI: 11.3, 18.9).
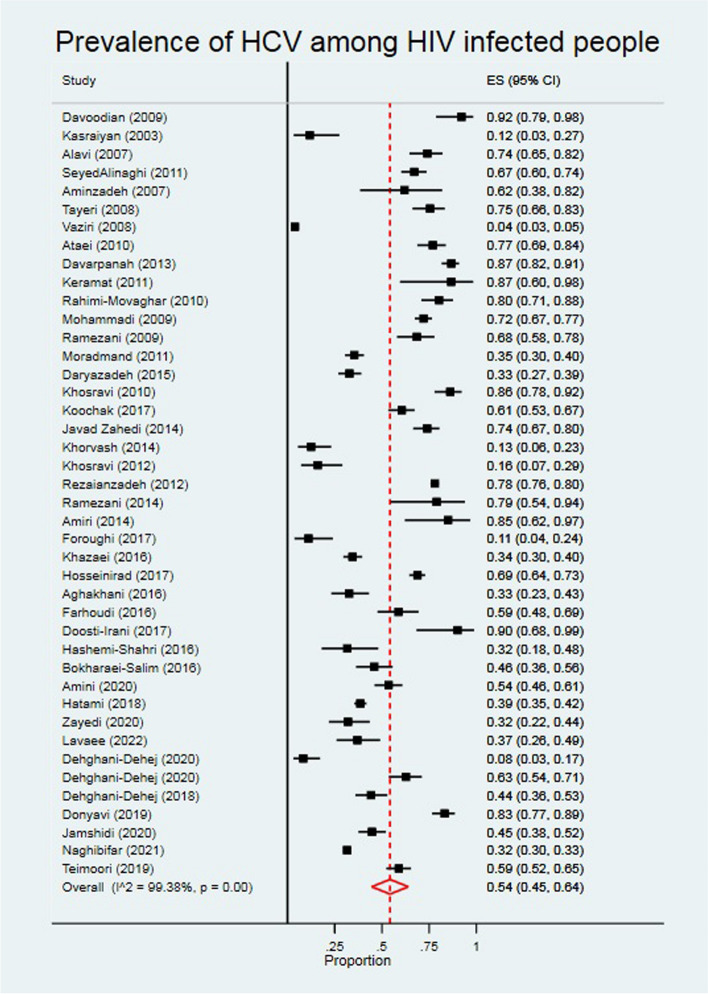
Prevalence of HCV among PLWH
The pooled prevalence of HCV among PLWH was 54.0% (95% CI: 45.0, 64.0) (Fig. 4). According to the results of the heterogeneity test, the I2 was 99.38%. HCV prevalence was higher among men, (67.3%), intermediate quality studies (65.7%) studies conducted from 2006 to 2010 (63.8%) (Table 2). Most of the included studies (19 studies) were related to Tehran province. The overall prevalence of HCV among PLWH in Tehran was 53.6 (95% CI: 432.2, 64.1). The prevalence of HCV in other regions of Iran is shown in Table 2.
Fig. 4.
The individual and pooled prevalence of HCV among people diagnosed with HIV
Prevalence of TB among PLWH
The overall prevalence of TB among PLWH was 17.0% (95% CI: 13.0, 22.0) (Fig. 5). The I2 for the results of studies that have been reported the prevalence of TB among PLWH was 97.69%. TB prevalence was higher among men (21.6%), high quality studies (26.1%), and studies conducted from 2006 to 2010 (22.4%) (Table 2). The highest prevalence of TB among PLWH was related to southwest of Iran with 35.2% (95% CI: 6.4, 64.1) (Table 2).
Fig. 5.
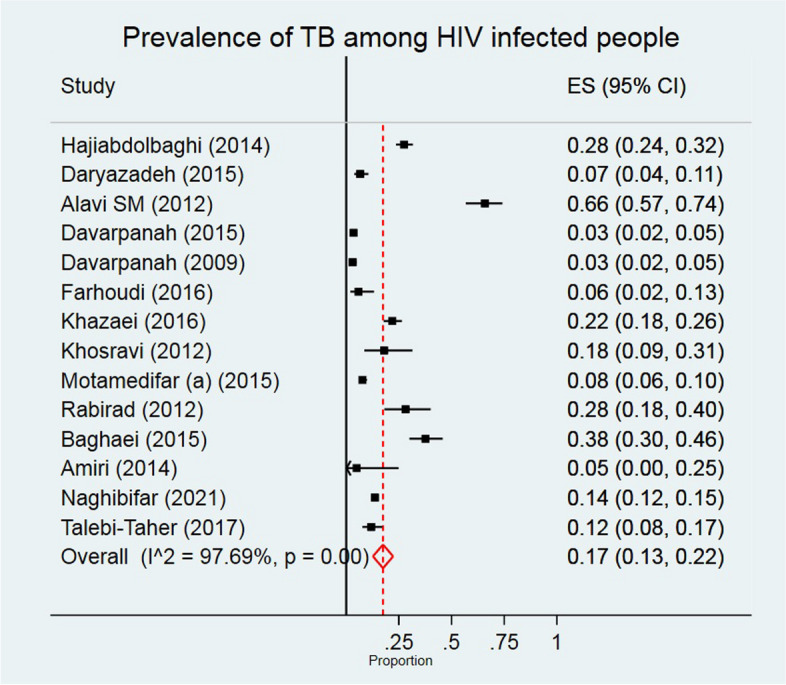
The individual and pooled prevalence of TB among people diagnosed with HIV
Meta-regression analysis
The results from multivariate meta-regression analysis showed no statistically significant association between HBV prevalence and year of study, quality of studies, age, gender, and PWID (Table 3). HCV prevalence was significantly associated with PWID (crud. Beta = 0.48, p-value 0.004). TB prevalence was associated with gender (crude Beta = 48) and PWID (crude Beta 0.72 but these associations were not statistically significant.
Table 3.
Crude and adjusted meta-regression to identify the source of heterogeneity
| HBV | ||||||
| Variables | Crude | Adjusted | ||||
| Coeff | p-value | 95% CI | Coeff | p-value | 95% CI | |
| Year of study (for every year increase) | -0.005 | 0.274 | -0.15, 0.004 | -0.008 | 0.468 | -0.03, 0.02 |
| Quality (for every score increase) | 0.004 | 0.556 | -0.01, 0.2 | 0.02 | 0.453 | -0.04, 0.08 |
| Mean age (for every year increase) | -0.004 | 0.317 | -0.01, 0.004 | -0.01 | 0.158 | -0.03, 0.004 |
| Proportion of male (for every 1% increase) | 0.24 | 0.216 | -0.14, 0.62 | 0.17 | 0.707 | -0.75, 1.09 |
| Proportion of PWID (for every 1% increase) | 0.11 | 0.359 | -0.13, 0.35 | 0.15 | 0.402 | -0.21, 0.50 |
| HCV | ||||||
| Variables | Coeff | p-value | 95% CI | Coefficient | p-value | 95% CI |
| Year of study (for every year increase) | -0.01 | 0.168 | -0.03, 0.005 | -0.003 | 0.814 | -0.03, 0.02 |
| Quality (for every score increase) | 0.004 | 0.716 | -0.02, 0.03 | -0.02 | 0.458 | 0.08, 0.04 |
| Mean age (for e very year increase) | 0.01 | 0.021 | 0.002, 0.03 | 0.006 | 0.401 | -0.008, 0.02 |
| Proportion of male (for every 1% increase) | 0.51 | 0.068 | -0.04, 1.06 | 0.49 | 0.310 | -0.49, 1.47 |
| Proportion of PWID (for every 1% increase) | 0.48 | 0.004 | 0.17, 0.79 | 0.28 | 0.172 | -0.13, 0.68 |
| TB | ||||||
| Variables | Coeff | p-value | 95% CI | - | - | - |
| Year of study (for every year increase) | -0.01 | 0.517 | -0.05, 0.03 | - | - | - |
| Quality (for every score increase) | 0.007 | 0.623 | -0.02, 0.08 | - | - | - |
| Mean age (for every year increase) | -0.02 | 0.118 | -0.05, 0.006 | - | - | - |
| Proportion of male (for every 1% increase) | 0.48 | 0.248 | -0.39, 1.34 | - | - | - |
| Proportion of PWID (for every 1% increase) | 0.72 | 0.141 | -0.37, 1.80 | - | - | - |
Discussion
Our findings suggest that among people living with HIV, one in eight infected with HBV, one in two infected with HCV and one in six infected with Tuberculosis. Men who live with HIV have a higher prevalence of HBV, HCV, and Tuberculosis infection; while injection was the only significant predictor for high HCV prevalence. We observed no significant change in prevalence of HBV, HCV and Tuberculosis overtime among people living with HIV.
Overall, the results of this study indicated a considerable prevalence of Iranian PLWH affected by HBV, HCV, and TB. In addition, the prevalence of these infectious diseases among higher at risk PLWH groups such as PWIDs, homeless people, and hospitalized patients was more than other groups such as HIV patients referring to the counseling center. Due to the same transmission routes of HIV, HBV, and HCV, it is expected that the prevalence of HBV and HCV be higher among HIV infected people. In Iran, injecting drug use (IDU) is one of the main risk factors of HIV [76]. On the other hand, sharing syringe is the main risk factor for HCV [75], so the high prevalence of HCV among PLWH can be justified.
In a review article in 2014, the prevalence of HBV co-infection among Iranian HIV positive people was ranged from 1.8–26.8% [77], similar to what we found. In India, this figure was 7.28% to 10.7%, and in Europe, was from 3.6% to 59.0% [77], similar to our results. According to the findings of a meta-analysis, the combined prevalence of HBsAg among people living with HIV in Latin America was 7.0% with a confidence interval of 95% (7.0–7.0%) [78]. According to the results of a systematic review in Iran, the prevalence of coinfection with HIV and HBV among injecting drug users and prisoners was 1.88% and 0.13%, respectively. In addition, 10.95% of injecting drug users (95% CI: 2.82–19.08%) were positive for both HIV and HCV [79].
We found an overall HCV prevalence of 54%, the prevalence was different based on the study population, and the highest HCV prevalence was related to PWIDs which was from 74.0% to 85.0%. This finding was similar to the global median HCV prevalence (82%; IQR: 55–84) among PWIDs [5].
Tuberculosis among PLWH is a major health issue. Immunodeficiency in PLWH leads to opportunistic infectious diseases such as TB. The pooled prevalence of TB among Iranian PLWH in the included studies was ranged from 12.7% to 22.3%. Results of a systematic review and meta-analysis showed that the global prevalence of TB among HIV-positive prisoners was 31% (95% CI: 22–39), the prevalence in Africa, America, Asia, and Europe was 13%, 37%, 40%, and 28% respectively [80]. The pooled prevalence in Iran was 19.0% and the highest prevalence was related to southwest of Iran (35.2%) that nearly same to the two America countries included United States and Brazil [80]. A reason for the high prevalence of TB in our meta-analysis may be due to more detection and case finding of TB among PLWH. According to the Iranian guideline for management and treatment of the co-infection of HIV and TB, all PLWH in the time of diagnosis and subsequent visits are evaluated for active TB.
Based on the results of a study in Iran adherence to Highly active antiretroviral therapy (HAART) is between 60.0 to 70.0%, although the adherence in Iranian PLWH is acceptable [81] but, about 30.0% of these patients have not adherence to HAART, so this issue may increase the risk of coinfection with TB. On the other hand, based on the report of the World Bank the coverage of antiretroviral therapy in Iranian PLWH was increased from 2000 to 2022, this value in 2022 was 37.0% [82].
Based on the results of meta-regression analysis HCV prevalence was significantly associated with PWID and age in the crude analysis. TB prevalence was associated with gender and PWID, however, these associations were not statistically significant. In addition, we could not run the multivariable meta-regression and adjust other variables for TB prevalence, because a total number of studies that reported the TB prevalence was 12, and there was missing data regarding the proportion of PWID and male gender. Therefore, we only reported the crud estimates.
The strength point of current study is that we conducted a comprehensive systematic review and estimated the prevalence of HCV, HBV, and TB prevalence as three important infectious diseases among PLWH. However, our systemic review had two major limitations. First, the high heterogeneity between the findings of included studies limited us to identify the source of heterogeneity. Last, studies with low and intermediate quality may also affected our findings.
Despite the mentioned limitations, our results may be useful in planning for better management of PLWH and treatment of HCV, HBV, and TB coinfection with HIV. In addition, these findings may be useful evidence for health policymakers' decision-making.
Conclusions
The results of this systematic review showed that the prevalence of HBV, HCV, and TB was high among Iranian PLWH especially higher at-risk groups such as PWIDs and homeless people. Therefore, it seems the strength of the surveillance system is necessary for increase the screening and therapeutic activities for viral hepatitis and TB among PLWH in Iran.
Acknowledgements
Not applicable.
Authors’ contributions
A.DI. and B.E. conducted the database search, screening, and quality assessment. ADI, AM, and EM contributions to the analysis of data, and drafting. ADI, AM, EM, and BE revising the article.
Funding
None
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. HIV and hepatitis coinfections. World Health Organization [updated June 2017; cited 31 December, 2023]. Available from: http://www.who.int/hiv/topics/hepatitis/en/.
- 2.World Health Organization. Tuberculosis & HIV: World Health Organization; [cited 29 May 2024]. Available from: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/treatment/tuberculosis-hiv#:~:text=Tuberculosis%20(TB)%20remains%20the%20leading,million%20TB%20deaths%20that%20year.
- 3.Kouame GM, Boyd A, Moh R, Badje A, Gabillard D, Ouattara E, et al. Higher mortality despite early antiretroviral therapy in human immunodeficiency virus and hepatitis B virus (HBV)-coinfected patients with high HBV replication. Clin Infect Dis. 2018;66(1):112–20. 10.1093/cid/cix747 [DOI] [PubMed] [Google Scholar]
- 4.Thio CL, Seaberg EC, Skolasky R, Phair J, Visscher B, Muñoz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet. 2002;360(9349):1921–6. 10.1016/S0140-6736(02)11913-1 [DOI] [PubMed] [Google Scholar]
- 5.Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):797–808. 10.1016/S1473-3099(15)00485-5 [DOI] [PubMed] [Google Scholar]
- 6.Behzadifar M, Gorji HA, Rezapour A, Bragazzi NL. Prevalence of hepatitis C virus infection among prisoners in Iran: a systematic review and meta-analysis. Harm Reduct J. 2018;15(1):24. 10.1186/s12954-018-0231-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Utsumi T, Lusida MI. Viral hepatitis and human immunodeficiency virus co-infections in Asia. World J Virol. 2015;4(2):96–104. 10.5501/wjv.v4.i2.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Tuberculosis [updated 18 September 2018; cited 14 October 2018]. Available from: http://www.who.int/news-room/fact-sheets/detail/tuberculosis.
- 9.Pourakbari B, Mamishi S, Banar M, Keshtkar AA, Mahmoudi S. Prevalence of TB/ HIV co-infection in Iran: a systematic review and meta-analysis. Ann Ig. 2019;31(4):333–48. [DOI] [PubMed] [Google Scholar]
- 10.Rahimi-Movaghar A, Amin-Esmaeili M, Haghdoost AA, Sadeghirad B, Mohraz M. HIV prevalence amongst injecting drug users in Iran: a systematic review of studies conducted during the decade 1998–2007. Int J Drug Policy. 2012;23(4):271–8. 10.1016/j.drugpo.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 11.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 12.Alavi SM, Talebi Z, Bakhtiarinia PP. Pulmonary tuberculosis risk factors in hospitalized HIV positive patients in Ahvaz, Iran (2001–09). J Gorgan Univ Med Sci. 2012;14(3):82–6. [Google Scholar]
- 13.Davarpanah M, Khademolhosseini F, Rajaeefard A, Tavassoli A, Yazdanfar S, Rezaianzadeh A. Hepatitis C virus infection in HIV positive attendees of Shiraz behavioral diseases consultation center in southern Iran. Indian J Community Med. 2013;38(2):86–91. 10.4103/0970-0218.112437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davoodian P, Dadvand H, Mahoori K, Amoozandeh A, Salavati A. Prevalence of selected sexually and blood-borne infections in injecting drug abuser inmates of bandar abbas and roodan correction facilities, Iran, 2002. Braz J Infect Dis. 2009;13(5):356–8. 10.1590/S1413-86702009000500008 [DOI] [PubMed] [Google Scholar]
- 15.Farhoudi B, SeyedAlinaghi S, Mohraz M, Hosseini M, Farnia M. Tuberculosis, hepatitis C and hepatitis B co-infections in patients with HIV in the Great Tehran Prison, Iran. Asian Pac J Trop Dis. 2016;6(1):82–3. 10.1016/S2222-1808(15)60989-6 [DOI] [Google Scholar]
- 16.Hashemi-Shahri SM, Sharifi-Mood B, Kouhpayeh HR, Moazen J, Farrokhian M, Salehi M. Sexually transmitted infections among hospitalized patients with human immunodeficiency virus infection and acquired immune deficiency syndrome (HIV/AIDS) in Zahedan, Southeastern Iran. Int J High Risk Behav Addict. 2016;5(3):e28028. 10.5812/ijhrba.28028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keramat F, Eini P, Majzoobi MM. Seroprevalence of HIV, HBV and HCV in persons referred to hamadan behavioral counseling center, west of Iran. Iran Red Crescent Med J. 2011;13(1):42–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Kasraiyan L, Torab JS. The frequency of HIV infection in blood donors in Shiraz blood transfusion organization from 1998 to 2002. 2003. [Google Scholar]
- 19.Lavaee F, Modarresi F, Amookhteh S, Amiri MA. Evaluation of association of oral bacterial profile with HBV and HCV infection and T lymphocyte level in HIV-positive patients. Int J Dent. 2022;2022(1):8622181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doosti-Irani A, Holakouie-Naieni K. Determination the research priorities in the field of HIV/AIDS in Iran: a systematic review article. Iran J Public Health. 2016;45(9):1149–58. [PMC free article] [PubMed] [Google Scholar]
- 21.Joanna Briggs Institute. JBI critical appraisal checklist for prevalence studies 2017 [updated 2017; cited 23 October 2018]. Available from: http://joannabriggs.org/assets/docs/critical-appraisal-tools/JBI_Critical_Appraisal-Checklist_for_Analytical_Cross_Sectional_Studies2017.pdf.
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin Res Ed). 2003;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ (Clin Res Ed). 2011;342:d549. 10.1136/bmj.d549 [DOI] [PubMed] [Google Scholar]
- 24.Aghakhani A, Mohraz M, Aghasadeghi MR, Banifazl M, Vahabpour R, Karami A, et al. Occult hepatitis B virus infection and S gene escape mutants in HIV-infected patients after hepatitis B virus vaccination. Int J STD AIDS. 2016;27(11):967–72. 10.1177/0956462415602419 [DOI] [PubMed] [Google Scholar]
- 25.Aghasadeghi MR, Mohraz M, Bahramali G, Aghakhani A, Banifazl M, Foroughi M, et al. Frequency and genotype of hepatitis D virus infection in patients infected with HIV and those undergoing hemodialysis. Hepat Mon. 2013;13(5):e7481. 10.5812/hepatmon.7481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alavi SM, Etemadi A. HIV/HBV, HIV/HCV and HIV/HTLV-1 co infection among injecting drug user patients hospitalized at the infectious disease ward of a training hospital in Iran. Pak J Med Sci. 2007;23(4):510–3. [Google Scholar]
- 27.Amini S, Khodabandehloo M. Prevalence of hepatitis c virus genotypes in HIV positive patients referring to the consultation center for behavioral diseases, Sanandaj, Iran. Iran J Microbiol. 2020;12(6):650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aminzadehv Z, Aghazadeh SK. Seroepidemiology of HIV, syphilis, hepatitis B and C in intravenous drug users at Loghman Hakim hospital. Iran J Med Microbiol. 2007;1(3):53–6. [Google Scholar]
- 29.Amiri FB, Gouya MM, Saifi M, Rohani M, Tabarsi P, Sedaghat A, et al. Vulnerability of homeless people in Tehran, Iran, to HIV, tuberculosis and viral hepatitis. PLoS One. 2014;9(6):e98742. 10.1371/journal.pone.0098742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ataei B, Tayeri K, Kassaian N, Farajzadegan Z, Babak A. Hepatitis B and C among patients infected with human immunodeficiency virus in Isfahan, Iran: seroprevalence and associated factors. Hepat Mon. 2010;10(3):188–92. [PMC free article] [PubMed] [Google Scholar]
- 31.Azadmanesh K, Mohraz M, Aghakhani A, Edalat R, Jam S, Eslamifar A, et al. Occult hepatitis B virus infection in HIV-infected patients with isolated hepatitis B core antibody. Intervirology. 2008;51(4):270–4. 10.1159/000160217 [DOI] [PubMed] [Google Scholar]
- 32.Azmoudeh-Ardalan F, Khodabandehloo M. Prevalence of overt and occult hepatitis b virus infection among HIV-positive people referring to consultation center for behavioral diseases, Kurdistan province, Iran. Iran Biomed J. 2021;25(6):434–40. 10.52547/ibj.25.6.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bokharaei-Salim F, Keyvani H, Esghaei M, Zare-Karizi S, Dermenaki-Farahani SS, Hesami-Zadeh K, et al. Prevalence of occult hepatitis C virus infection in the Iranian patients with human immunodeficiency virus infection. J Med Virol. 2016;88(11):1960–6. 10.1002/jmv.24474 [DOI] [PubMed] [Google Scholar]
- 34.Daryazadeh S. Prevalence of simultaneous infections and causes of death of positive-HIV patients in incubation and AIDS phases. Razi J Med Sci. 2016;22(139):46–55. [Google Scholar]
- 35.Davarpanah MA, Darvishi M, Mehrabani D. The prevalence of HBS antigenemia in patients with HIV infection in Shiraz, southern Iran. Iran Red Crescent Med J. 2007;9(4):224–5. [Google Scholar]
- 36.Davarpanah MA, Hoseini SM. Incidence of active tuberculosis among human immunodeficiency virus (HIV)-positive patients and evaluation of their responses to usual anti-tuberculosis medications in Shiraz, South West of Iran. Galen Med J. 2015;4(2):115–20. 10.31661/gmj.v4i2.260 [DOI] [Google Scholar]
- 37.Davarpanah MA, Motazedian N, Fallahzadeh E, Rasti M, Rahmati H, Motazedian N. Hepatitis B virus infection serology and the associated risk factors among patients with HIV in Shiraz, Iran. Shiraz E Med J. 2015;16(4):e24676.
- 38.Davarpanah MA, Rafiee GH, Mehrabani D. The prevalence of M. tuberculosis infection and disease in HIV positive individuals in Shiraz, Southern Iran. Iran Red Crescent Med J. 2009;11(2):199–202. [Google Scholar]
- 39.Dehghani-Dehej F, Hosseini Z, Mortazkar P, Khanaliha K, Esghaei M, Fakhim A, et al. Prevalence of HCV and/or HBV coinfection in Iranian HIV-infected patients. Futur Virol. 2020;15(3):155–63. 10.2217/fvl-2019-0066 [DOI] [Google Scholar]
- 40.Dehghani-Dehej F, Sarvari J, Esghaei M, Hosseini SY, Garshasbi S, Kalantari S, et al. Presence of different hepatitis C virus genotypes in plasma and peripheral blood mononuclear cell samples of Iranian patients with HIV infection. J Med Virol. 2018;90(8):1343–51. 10.1002/jmv.24925 [DOI] [PubMed] [Google Scholar]
- 41.Donyavi T, Bokharaei-Salim F, Khanaliha K, Sheikh M, Bastani MN, Moradi N, et al. High prevalence of occult hepatitis C virus infection in injection drug users with HIV infection. Adv Virol. 2019;164(10):2493–504. [DOI] [PubMed] [Google Scholar]
- 42.Ghafari M, Cheraghi Z, Doosti-Irani A. Occupational risk factors among Iranian farmworkers: a review of the available evidence. Epidemiol Health. 2017;39:e2017027. 10.4178/epih.e2017027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foroughi M, Moayedi-Nia S, Shoghli A, Bayanolhagh S, Sedaghat A, Mohajeri M, et al. Prevalence of HIV, HBV and HCV among street and labour children in Tehran. Iran Sex Transm Infect. 2017;93(6):421–3. 10.1136/sextrans-2016-052557 [DOI] [PubMed] [Google Scholar]
- 44.Hajiabdolbaghi M, Jafari S, Alijani N, Hedayat-Yaghoobi M. Prevalence of opportunistic infections among hospitalized patients with HIV/AIDS in Tehran Imam Khomeini Hospital (Iran), during 2009–2012. J Isfahan Med Sch. 2014;31(266):2149–53. [Google Scholar]
- 45.Hatami H, Namdaritabar H, Lotfaliany M, Akbarpour S, Zafari N. Occurrence of HBV and HCV and their probable risk factors in the newly registered HIV/AIDS patients in the center for control of communicable diseases in 2016. Iran J Epidemiol. 2018;14(3):225–33. [Google Scholar]
- 46.Hosseini Rad M, JamshidiMakiani M, Kalantari S, Sohrabi S, Ahmadniya H, Zanganeh M. The study of seroprevalence of hepatitis B and hepatitis C and behavioral factors in HIV patients referred to the consultation clinics of Tehran Behavioral Diseases Health Center. Med Sci J. 2017;27(1):53–61. [Google Scholar]
- 47.Jamshidi S, Bokharaei-Salim F, Esghaei M, Bastani MN, Garshasbi S, Chavoshpour S, et al. Occult HCV and occult HBV coinfection in Iranian human immunodeficiency virus-infected individuals. J Med Virol. 2020;92(12):3354–64. 10.1002/jmv.25808 [DOI] [PubMed] [Google Scholar]
- 48.Janbakhsh A, Farzinpoor J, Mansouri F, Vaziri S, Sayad B, Afsharian M, et al. Prevalence of occult hepatitis B in HIV positive patients (adolescents and adults) in Kermanshah-Iran. Int J Pediatr. 2017;5(9):5797–803. [Google Scholar]
- 49.Javad Zahedi M, Darvish Moghaddam S, HayatbakhshAbasi M, Parnian M, Shokoohi M. Hepatitis B, C virus co-infection and behavioral risks in HIV-positive patients in southern Iran. J Pak Med Assoc. 2014;64(2):134–7. [PubMed] [Google Scholar]
- 50.Kasraeian L, Torab-Jahromi S. The frequency of HIV infection in blood donors in Shiraz blood transfusion organization from 1998 to 2002. J Zanjan Univ Med Sci. 2003;11(42):49–52. [Google Scholar]
- 51.Khazaei S, Molaeipoor L, Rezaeian S, Ayubi E, Yari M, Valipour AA, et al. Predictors of tuberculosis in HIV/AIDS patients referred to behavioral diseases consultation center: a registry-based study in Abadan, Southwest of Iran. Shiraz E Med J. 2016;17(10):e41542.
- 52.Khorvash F, Javadi A, Tayeri K, Ataei B. Occult hepatitis B virus infection among human immunodeficiency virus-infected patients with isolated hepatitis B core antibody in Isfahan, Iran. J Res Med Sci. 2014;19(SPEC. ISSUE):S64–6. [PMC free article] [PubMed] [Google Scholar]
- 53.Khosravi A, Bahmani M, Ghezel-Sofla I. Co-infection by hepatitis C virus in human immunodeficiency virus infected patients in southwest of Iran. Iran J Clin Infect Dis. 2010;5(4):223–7. [Google Scholar]
- 54.Khosravi AD, Alavi SM, Hashemzade M, Abasi E, Seghatoleslami S. The relative frequency of Mycobacterium tuberculosis and Mycobacterium avium infections in HIV positive patients, Ahvaz, Iran. Asian Pac J Trop Med. 2012;5(1):71–4. 10.1016/S1995-7645(11)60249-6 [DOI] [PubMed] [Google Scholar]
- 55.Koochak HE, Babaei A, Pourdast A, Golrokhy R, Ra-Soolinejad M, Khodaei S, et al. Prevalence of adverse drug reactions to highly active antiretroviral therapy (HAART) among HIV positive patients in Imam Khomeini Hospital of Tehran, Iran. Infect Disord Drug Targets. 2017;17(2):116–9. 10.2174/1871526517666170117111350 [DOI] [PubMed] [Google Scholar]
- 56.Mohammad Nejad E, Ehsani SR, Rabirad N, Deljo R, Ranjbarn S, Rezaee S, et al. Prevalence of HBV in HIV patients referred to Imam Khomeini Hospital, Tehran, Iran from 2008–2010. Iran Red Crescent Med J. 2013;15(4):379–80. 10.5812/ircmj.4883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohammadi M, Talei G, Sheikhian A, Ebrahimzade F, Pournia Y, Ghasemi E, et al. Survey of both hepatitis B Virus (HBsAg) and hepatitis C virus (HCV-Ab) coinfection among HIV positive patients. Virol J. 2009;6:1–5. 10.1186/1743-422X-6-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moradmand BB, Seyedalinaghi S, Chaman R, Hosseini M, Hasibi M, Moharrami B, et al. Frequency and correlates of co-infection hepatitis C and hepatitis B with HIV. Knowl Health. 2011;6(3):40–3. [Google Scholar]
- 59.Motamedifar M, Ebrahim-Saraie HS, Abadi ARH, Moghadam MN. First outcome of MDR-TB among co-infected HIV/TB patients from south-west Iran. Tuberc Respir Dis. 2015;78(3):253–7. 10.4046/trd.2015.78.3.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Motamedifar M, Taheri M, Lankarani KB, Gholami M, Lari MA, Faramarzi H, et al. The prevalence and risk factors of hepatitis delta virus in HIV/HBV co-infected patients in Shiraz, Iran, 2012. Iran J Med Sci. 2015;40(5):448–53. [PMC free article] [PubMed] [Google Scholar]
- 61.Naghibifar Z, Eskandari S, Sajjadipour M, Kavousi A, Etemad K. Evaluation of the prevalence of HIV co-infections and the related risk factors in HIV-positive cases in Imam Khomeini hospital, Tehran during 2004–2018. Iran J Epidemiol. 2021;16(4):285–95. [Google Scholar]
- 62.Rabirad N, Nejad EM, Hadizadeh R, Begjani J, Ehsani SR. The prevalence of TB in HIV patients and risk factor with frequent referral (Iran, 2009–10). Iran Red Crescent Med J. 2012;15(1):58–61. 10.5812/ircmj.4401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahimi-Movaghar A, Razaghi EM, Sahimi-Izadian E, Amin-Esmaeili M. HIV, hepatitis C virus, and hepatitis B virus co-infections among injecting drug users in Tehran. Iran Int J Infect Dis. 2010;14(1):e28-33. 10.1016/j.ijid.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 64.Ramezani A, Amirmoezi R, Volk JE, Aghakhani A, Zarinfar N, McFarland W, et al. HCV, HBV, and HIV seroprevalence, coinfections, and related behaviors among male injection drug users in Arak, Iran. AIDS Care. 2014;26(9):1122–6. 10.1080/09540121.2014.882485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramezani A, Mohraz M, Aghakhani A, Banifazl M, Eslamifar A, Khadem-Sadegh A, et al. Frequency of isolated hepatitis B core antibody in HIV-hepatitis C virus co-infected individuals. Int J STD AIDS. 2009;20(5):336–8. 10.1258/ijsa.2008.008377 [DOI] [PubMed] [Google Scholar]
- 66.Rastegarian M, Zeighami A, Shahriarirad R, Erfani A, Arefkhah N, Ghorbani F, et al. Serosurvey of HBV surface antigen and anti-HBV surface antibody among HIV-infected patients in Fars province, Southern Iran. Infez Med. 2020;28(4):572–5. [PubMed] [Google Scholar]
- 67.Rezaianzadeh A, Hasanzadeh J, Alipour A, Davarpanah MA, Rajaeifard A, Tabatabaee SHR. Impact of hepatitis C on survival of HIV-infected individuals in Shiraz; South of Iran. Hepat Mon. 2012;12(2):106–11. 10.5812/hepatmon.4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.SeyedAlinaghi S, Jam S, Mehrkhani F, Fattahi F, Sabzvari D, Kourorian Z, et al. Hepatitis-C and hepatitis-B co-infections in patients with human immunodeficiency virus in Tehran, Iran. Acta Med Iran. 2011;49(4):252–7. [PubMed] [Google Scholar]
- 69.Tabarsi P, Baghaei P, Moniri A, Marjani M, Velayati AA. Detection of latent and active tuberculosis among HIV-positive patients at the North of Tehran. Int J Mycobacteriol. 2015;4:64. 10.1016/j.ijmyco.2014.11.020 [DOI] [Google Scholar]
- 70.Talebi-Taher M, Abbasian L, Alavi-Niakou SN, Javad-Moosavi SA, Pahlavani S. Tuberculin skin test conversion among individuals with human immunodeficiency virus infection on antiretroviral therapy in a referral teaching hospital, Tehran, Iran. Tanaffos. 2017;16(3):201–6. [PMC free article] [PubMed] [Google Scholar]
- 71.Tayeri K, Kasaeian N, Fadaei NR, Ataei B. The prevalence of hepatitis B, hepatitis C and associated risk factors in intravenous drug addicts (IVDA) with HIV in Isfahan. J Isfahan Med Sch. 2008;26(90):273–8. [Google Scholar]
- 72.Teimoori A, Ebrahimi S, Keshtkar N, Khaghani S, Salmanzadeh S, Ghafari S. Prevalence and genetic diversity of HCV among HIV-1 infected individuals living in Ahvaz, Iran. BMC Infect Dis. 2019;19(1):1–7. 10.1186/s12879-019-4052-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vaziri S, Mansouri F, Sayad B, Afsharian M, Janbakhsh A, Karami M. Hepatitis D virus infection among HIV-HBV co-infected patients in Kermanshah, West of Iran. Hepat Mon. 2008;8(4):252–7. [Google Scholar]
- 74.Zayedi E, Makvandi M, Teimoori A, Samarbaf-Zadeh AR, Ghafari S, Seyedian SS, et al. Prevalence of hepatitis C virus among HIV-infected patients. Iran J Microbiol. 2020;12(2):156–63. [PMC free article] [PubMed] [Google Scholar]
- 75.Doosti-Irani A, Mokhaeri H, CheginiSharafi A, Aghasadeghi MR, Hajimiragha M, Saki M, et al. Prevalence of HIV, HBV, and HCV and related risk factors amongst male homeless people in Lorestan Province, the West of Iran. J Res Health Sci. 2017;17(1):e00373. [PubMed] [Google Scholar]
- 76.Nasirian M, Doroudi F, Gooya MM, Sedaghat A, Haghdoost AA. Modeling of human immunodeficiency virus modes of transmission in Iran. J Res Health Sci. 2012;12(2):81–7. [PubMed] [Google Scholar]
- 77.Askari A, Hakimi H, NasiriAhmadabadi B, Hassanshahi G, Kazemi AM. Prevalence of hepatitis B co-infection among HIV positive patients: narrative review article. Iran J Public Health. 2014;43(6):705–12. [PMC free article] [PubMed] [Google Scholar]
- 78.Tengan FM, Abdala E, Nascimento M, Bernardo WM, Barone AA. Prevalence of hepatitis B in people living with HIV/AIDS in Latin America and the Caribbean: a systematic review and meta-analysis. BMC Infect Dis. 2017;17(1):587. 10.1186/s12879-017-2695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.BagheriAmiri F, Mostafavi E, Mirzazadeh A. HIV, HBV and HCV coinfection prevalence in Iran–a systematic review and meta-analysis. PLoS One. 2016;11(3):e0151946. 10.1371/journal.pone.0151946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dianatinasab M, Joulaei H, Ghorbani M, Zarei N, Rezaeian S, Fararouei M, et al. Prevalence of tuberculosis in HIV-positive prisoners: a systematic review and meta-analysis. AIDS Rev. 2018;20(2):114–24. [DOI] [PubMed] [Google Scholar]
- 81.Khalili H, Rohani R, Seyedalinaghi S, Hajiabdolbaghi M, Dashti-Khavidaki S, Talasaz AH. Adherence to antiretroviral therapy among Iranian HIV/AIDS patients. Curr Clin Pharmacol. 2012;7(2):111–5. 10.2174/157488412800228910 [DOI] [PubMed] [Google Scholar]
- 82.The World Bank. Antiretroviral therapy coverage (% of people living with HIV) - Iran, Islamic Rep [cited June 02, 2024]. Available from: https://data.worldbank.org/indicator/SH.HIV.ARTC.ZS?locations=IR.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.