Abstract
Objective
The nutritional status and inflammatory responses of patients with aneurysmal subarachnoid hemorrhage (aSAH) play a vital prognostic role. We investigated the relationship between preoperative prognostic nutritional index (PNI)、neutrophil/albumin ratio (NAR)、platelet/albumin ratio (PAR) and other factors and the clinical prognosis of patients who underwent clipping for aSAH and its predictive model.
Methods
The clinical data of 212 patients with aSAH who underwent neurosurgery at Nanyang Central Hospital between 2018 and 2023 were retrospectively analyzed. Based on the Glasgow Outcome Scale (GOS) score at 6 months postoperatively, the patients were categorized into two groups: poor (GOSI-III) and good (GOSIV-V) prognosis groups. Multivariate logistic regression analysis was performed to determine the predictive value of preoperative PNI、NAR、PAR、hyperlipidemia and Glasgow Coma Scale (GCS) for prognosis. Furthermore, nomograms and prognostic prediction models were constructed. Receiver operating characteristic curves and area under the curve (AUC) were utilized to determine the predictive values.
Results
Multivariate logistic regression analysis revealed that PNI (OR = 1.250, 95%CI 1.060 ~ 1.475, P = 0.008), NAR (OR = 0.000, 95%CI 0.000 ~ 0.004, P = 0.000), PAR(OR = 0.515, 95%CI 0.283 ~ 0.937, P = 0.030), hyperlipidemia (OR = 4.627, 95%CI 1.166 ~ 18.367, P = 0.029), and GCS(OR = 1.446, 95%CI 1.041 ~ 2.008, P = 0.028) are independent risk factors for poor postoperative prognosis. The total score of the nomogram was 200, and the AUC value was 0.972.
Conclusions
PNI and NAR can reflect the nutritional status and inflammatory responses of patients.They are significantly associated with the postoperative prognosis of patients with aSAH. Comprehensively analyzing PNI and NAR combined with other clinical indicators can more effectively guide treatment and help predict prognosis.
Keywords: Intracranial aneurysm, Subarachnoid hemorrhage, Prognostic nutritional index, Neutrophil/albumin ratio, Prognosis
Introduction
Subarachnoid hemorrhage (SAH) accounts for 3% of all strokes [1], with aneurysmal subarachnoid hemorrhage (aSAH) accounting for approximately 85% of spontaneous SAH cases [2]. Particularly in China, where the mortality rate of untreated aSAH patients is 35–40% and the disability rate of aSAH survivors is 50% [3, 4]. Although our understanding of aSAH pathogenesis and ruptured aneurysm treatments, including surgical clipping and endovascular treatment, have improved after decades of research, two treatment methods have the potential toinduce neurological deterioration. aSAH remains a serious threat to global health, 24% of endovascular treatment and 32% of surgical clamping patients still have poor prognosis at 1-year follow-up [5]. Early postoperative brain injury (EBI) and complications associated with delayed brain injury (DBI) are the primary reasons for disability and death among patients with aSAH [6]. The nutritional status and local and systemic inflammatory responses of patients are considered important mechanisms underlying brain injury.
Some studies have revealed that inflammation in the initial stage of aSAH is associated with its pathological process. Neuroinflammation and oxidative damage secondary to aSAH are essential reasons for neurological deficits. The severity of inflammatory reaction is often positively correlated with the prognosis of patients. Blood oozes into the subarachnoid space, releasing large amounts of inflammatory stimuli; these stimuli can activate microglia and trigger an inflammatory cascade [7]. Moreover, the activation of sympathetic pathway and hypothalamus-pituitary-adrenal axis and the release of catecholamines after stroke can promote systemic inflammatory response, resulting in immunosuppression after stroke, resulting in lymphopenia [8]. Furthermore, inflammatory markers, including neutrophil/albumin ratio (NAR), platelet/albumin ratio (PAR), neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and monocyte/lymphocyte ratio (MLR) are associated with aSAH prognosis [9]. In addition, a deterioration in the nutritional status and immune function of patients will affect disease prognosis.
The prognostic nutritional index (PNI) is calculated using serum albumin levels and lymphocyte count and reflects the nutritional and immune statuses. Some studies have revealed that PNI is a reliable prognostic marker for various tumors and other critical diseases; furthermore, it can be utilized to investigate the clinical prognosis of patients with aSAH after clipping [10, 11]. Multiple risk factors affect the prognosis of patients with poorly performing aSAH; however, reliable and direct predictive models to predict the prognosis of these patients undergoing surgical clipping are lacking. Therefore, in this retrospective study, we investigated the preoperative nutritional status and inflammatory responses of patients with aSAH to determine the role of multiple indicators in predicting the prognosis of these patients after clipping. Furthermore, we constructed an early prediction model for poor prognosis to manage patients after aSAH in clinical settings.
Data and methods
Patient information and grouping
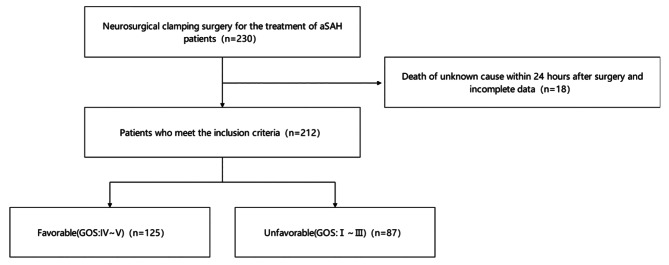
The clinical data of 212 patients with aSAH (65 men and 147 women) who underwent intracranial aneurysm clipping at the Department of Neurosurgery of Nanyang Central Hospital between January 2018 and August 2023 were reviewed (Fig. 1). The Hunt-Hess grade of the surgical patients was between I-IV grade, and the Glasgow Coma Scale (GCS) score was more than 5. Based on the Glasgow Outcome Scale (GOS) at 6 months postoperatively, the patients were categorized into two groups: poor (GOSI-III) and good (GOSIV-V) prognosis group. This study was conducted according to the principles of the Helsinki Declaration. With the approval of the hospital ethics committee. The informed consent form for surgery was signed by the families of all patients.
Fig. 1.
Flow chart for patients included in this study
The inclusion criteria were as follows: (1) age ≥ 18 years old; (2) improvements in relevant examination parameters after admission and diagnosis of SAH caused by ruptured intracranial aneurysms, as confirmed via computed tomography angiography or digital subtraction angiography; and (3) all patients who underwent intracranial aneurysm clipping within 72 h of onset.
The exclusion criteria were as follows: (1) patients with aSAH because of non-aneurysmal causes such as trauma, arteriovenous malformations, and arteriovenous fistulas; (2) Patients with acute or chronic infections, past autoimmune diseases, previous malignant tumors, uremia, liver cirrhosis, chronic heart disease and chronic lung disease; (3) those receiving anticoagulants or antiplatelet drugs for a long time; and (4) those with recurrent stroke within 6 months of postoperative follow-up; 5. Second operation.
For patients with acute hydrocephalus or intraventricular hematoma(IVH), aneurysmal clipping and contralateral ventricle drainage were performed.The patients with intracranial hypertension were treated with decompressive craniectomy (DC) according to the intraoperative conditions.All patients were transferred to ICU for treatment after operation, and head CT was reviewed within 12 h after operation.
Data extraction
The clinical data were collected from the hospital information system and follow-up records of Nanyang Central Hospital. Throughout the hospitalization period, all patients who were admitted to the hospital received routine treatment, such as controlling blood pressure, reducing cranial pressure and preventing cerebral vasospasm(CVS), etc., based on the management guidelines for aSAH [12]. Neurosurgical clipping was performed within 72 h after symptom onset. The indications and procedures for the surgery were according to the corresponding neurosurgical clipping guidelines and procedures for intracranial aneurysms [13]. Technologically mature neurosurgeons performed aneurysm clipping using the same surgical specifications and technical standards. Cerebral aneurysm clipping was performed under a microscope. After the surgery, nimodipine was administered to prevent CVS. After discharge, outpatients or telephone follow-up were divided into poor prognosis group (GOSI-III) and good prognosis group (GOSIV-V).
Basic clinical information, including age, sex, smoking history, drinking history, hypertension, diabetes, hyperlipidemia, stroke history, Hunt–Hess grade, IVH, hydrocephalus, postoperative DC, GCS, and aneurysm location, was collected. Owing to limited data, aneurysm location was divided into two types: anterior or posterior circulation.
Neutrophil, platelet, lymphocyte, and monocyte counts and albumin levels were recorded. A preoperative examination was conducted on the first day of admission to measure all blood test indexes. The indexes were determined as follows: PNI = serum albumin (g/L) + 5 × total lymphocyte count (× 109/L), NAR = neutrophils (× 109/L)/albumin (g/L), PAR = platelets (× 109/L)/albumin (g/L), NLR = neutrophils (×109/L)/lymphocytes (× 109/L), PLR = platelets (× 109/L)/lymphocytes (× 109/L), and MLR = monocytes (× 109/L)/lymphocytes (× 109/L).
Statistical analysis
SPSS25.0 software was utilized to perform statistical analysis. Comparisons between groups with measurements conforming to normal distribution were performed using t-tests, and data are expressed as mean ± standard deviation. On the other hand, the Mann–Whitney U test was performed to compare the non-normal distribution of measurement data between groups; data were expressed as median (M) and interquartile range. Categorical variables were expressed as number of instances and percentages or composition ratios, and comparisons were made using the chi-square test and Fisher’s exact probability test. Indicators that were significant in the univariate analysis were analyzed by multivariate logistic regression analysis. Furthermore, the significant indexes in multivariate analysis were considered independent risk factors for the poor prognosis of patients with aSAH after clipping. The “rms” package of R software was used to construct a column chart to obtain the scores for each indicator. The total score was obtained by adding the scores for each indicator. The higher the total score, the higher the risk of poor prognosis after aSAH. Then, the “pROC” package of R software was used to construct receiving operating characteristic (ROC) curves, and their AUC values were calculated. The larger the area, the stronger the prediction ability. Simultaneously calculate the optimal cutoff value for each indicator to obtain the relatively optimal sensitivity and specificity. The difference was considered statistically significant at a P-value of < 0.05.
When both GCS score and Hunt–Hess grade were included in multiple regression analysis, an offset was observed. The Hunt–Hess grade is recognized as a prognostic indicator, and the GCS score was included in this study. Therefore, only the GCS score was included in the final model.
Results
Baseline characteristics
In total, 212 patients with aSAH who underwent aneurysm clipping were included in this study. Among them, 125 (58.96%) had a good prognosis and 87 (41.04%) had a poor prognosis. Age, hypertension, hyperlipidemia, Hunt–Hess grade, IVH, hydrocephalus, postoperative DC, GCS score, aneurysm location, PNI, NAR, PAR, NLR, PLR, and MLR were included in univariate analysis (Table 1).
Table 1.
Univariate analysis results affecting poor prognosis in 212 patients with aSAH after surgery
| Variables | Good outcome(n = 125) | Poor outcome (n = 87) |
Test value | P-value |
|---|---|---|---|---|
| Gender | χ² =0.496 | 0.289 | ||
|
Male Female |
36 89 |
29 58 |
||
| Age (years) | 56.32 ± 10.06 | 60.78 ± 8.87 | t = 3.333 | 0.001 |
| Smoke | χ² =0.767 | 0.243 | ||
|
No Yes |
105 20 |
69 18 |
||
| Drink | χ² =1.071 | 0.203 | ||
|
No Yes |
111 14 |
73 14 |
||
| Hypertension | χ² =4.772 | 0.020 | ||
|
No Yes |
62 63 |
30 57 |
||
| Diabetes | χ² =1.159 | 0.202 | ||
|
No Yes |
116 9 |
77 10 |
||
| Hyperlipidemia | χ² =16.448 | 0.000 | ||
|
No Yes |
99 26 |
46 41 |
||
| History of stroke | χ² =0.198 | 0.411 | ||
|
No Yes |
113 12 |
77 10 |
||
| Hunt-Hess | 2(2) | 3(3) | Z = 7.473 | 0.000 |
| IVH | χ² =13.930 | 0.000 | ||
|
No Yes |
99 26 |
48 39 |
||
| Hydrocephalus | χ² =28.422 | 0.000 | ||
|
No Yes |
115 10 |
54 33 |
||
| GCS | 11(9) | 8(5) | Z = 8.512 | 0.000 |
| DC | χ² =10.549 | 0.001 | ||
|
No Yes |
109 16 |
60 27 |
||
| Location of aneurysm | χ² =9.698 | 0.002 | ||
|
Anterior circulation Posterior circulation |
92 33 |
46 41 |
||
| PNI | 48.51 ± 5.47 | 39.99 ± 5.78 | t = 10.891 | 0.000 |
| NAR | 0.15 ± 0.07 | 0.41 ± 0.18 | t = 12.664 | 0.000 |
| PAR | 5.32 ± 1.79 | 6.75 ± 2.69 | t = 4.335 | 0.000 |
| NLR | 5.54 ± 3.81 | 15.13 ± 8.78 | t = 9.581 | 0.000 |
| PLR | 172.25 ± 80.87 | 235.56 ± 106.81 | t = 4.674 | 0.000 |
| MLR | 0.35 ± 0.22 | 0.69 ± 0.41 | t = 6.990 | 0.000 |
Multivariate logistic regression analysis revealed that PNI (OR = 1.250, 95% CI: 1.060, 1.475, 0.008), NAR (OR = 0.000, 95% CI: 0.000, 0.004), PAR (OR = 0.515, 95% CI: 0.283, 0.937), hyperlipidemia (OR = 4.627, 95% CI: 1.166, 18.367, 0.029), and GCS score (OR = 1.446, 95% CI 1.041, 2.008) were independent risk factors for poor prognosis postoperatively (Table 2).
Table 2.
Multivariate logistic regression analysis results affecting postoperative poor prognosis in 212 patients with aSAH
| Influencing factors | OR value | 95%CI | P-value |
|---|---|---|---|
| PNI | 1.250 | 1.060 ~ 1.475 | 0.008 |
| NAR | 0.000 | 0.000 ~ 0.004 | 0.000 |
| PAR | 0.515 | 0.283 ~ 0.937 | 0.030 |
| Hyperlipidemia | 4.627 | 1.166 ~ 18.367 | 0.029 |
| GCS | 1.446 | 1.041 ~ 2.008 | 0.028 |
Comparison of the ROC curves of different monitoring indexes in predicting poor patient prognosis
The significance of different indexes on the poor prognosis of patients with aSAH postoperatively was analyzed. We observed that PNI (AUC = 0.865, 95% CI: 0.812, 0.908, P < 0.001), NAR (AUC = 0.954, 95% CI: 0.916, 0.978, P < 0.001), PAR (AUC = 0.675, 95% CI: 0.608, 0.738, P < 0.001), hyperlipidemia (AUC = 0.632, 95% CI: 0.563, 0.697, P < 0.001), and GCS score (AUC = 0.842, 95% CI: 0.786, 0.888, P < 0.001). Therefore, predicting the poor prognosis of patients with aSAH using a combination of several indicators is extremely vital (Table 3; Fig. 2).
Table 3.
The predictive effect of risk factor indicators on postoperative poor prognosis in aSAH patients
| Influencing factors | AUC | cutoff points | sensitivity(%) | specificity(%) | 95%CI | P-value |
|---|---|---|---|---|---|---|
| PNI | 0.865 | 43 | 78.16 | 88.00 | 0.812 ~ 0.908 | <0.001 |
| NAR | 0.954 | 0.21 | 94.25 | 87.20 | 0.916 ~ 0.978 | <0.001 |
| PAR | 0.675 | 5.6 | 63.22 | 65.60 | 0.608 ~ 0.738 | <0.001 |
| Hyperlipidemia | 0.632 | - | 47.13 | 79.20 | 0.563 ~ 0.697 | <0.001 |
| GCS | 0.842 | 9 | 75.86 | 73.60 | 0.786 ~ 0.888 | <0.001 |
| All indicators are combined | 0.972 | - | 92.00 | 94.25 | 0.940 ~ 0.990 | <0.001 |
Fig. 2.
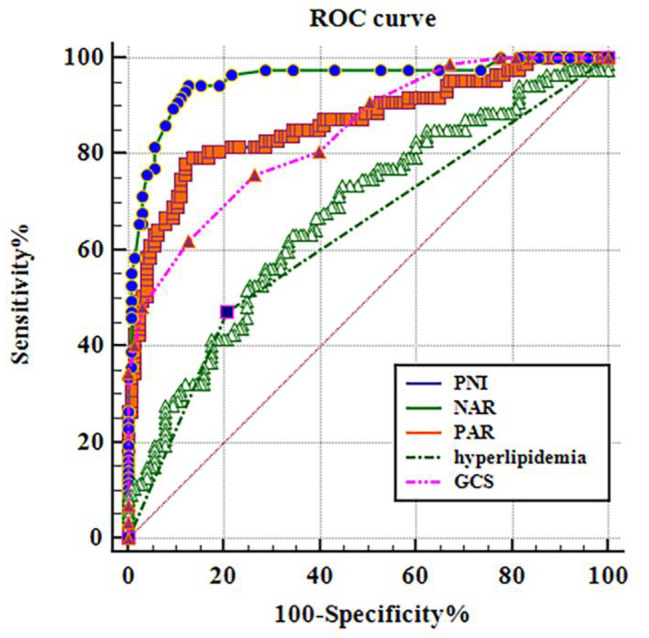
Risk factor index for predicting poor prognosis of postoperative patients with aSAH in the working characteristic curve
Risk assessment model for the poor prognosis of patients with aSAH after clipping
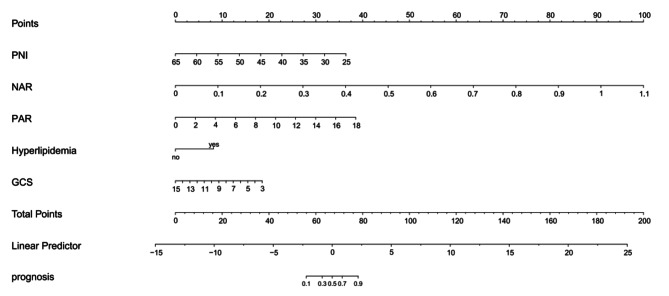
The significant indicators in logistic regression and ROC curve analyses were included in a nomogram to construct a prediction model for disease risk (Fig. 3). The total score of the nomogram was 200. When the total score was > 78, the risk of poor prognosis of patients with aSAH after clipping was > 90%. The AUC value of the nomogram was 0.972 (95% CI: 0.940, 0.990, P < 0.001). This value is significantly higher than that of other single-index models. This suggests that the constructed prediction model exhibits good accuracy and clinical application value.
Fig. 3.
A nomogram based on multiple logistic regression model for predicting poor prognosis in aSAH neurosurgical clamp patients
Discussion
aSAH is a common cerebrovascular disease requiring neurosurgery that threatens human health [14]. The management of EBI and DBI-induced postoperative complications, including CVS, delayed cerebral ischemia (DCI), Acute hydrocephalus, secondary brain swelling, and aneurysm rerupture bleeding, remains challenging [15, 16]. Multiple risk factors influence the prognosis of patients with aSAH. However, there is a lack of reliable and direct predictive models to predict the prognosis of aSAH patients undergoing surgical clamping. Therefore, it is important to focus on the prognostic outcomes of aSAH patients to improve their quality of life and survival. The aim of this retrospective observational study was to investigate the relationship between potential clinical risk factors and prognosis after aSAH clamping. Multivariate logistic regression and ROC curve analyses revealed that preoperative PNI, NAR, RAR, hyperlipidemia, and GCS score are important factors affecting the prognosis of patients with aSAH at 6 months postoperatively. Using these five risk factors, we constructed a nomogram to predict the risk of poor prognosis, facilitating the decision-making processes for management and treatment in clinical settings. The nomogram was found to be well calibrated, predictive and clinically applicable through internal validation. Therefore, our predictive model can help predict adverse outcomes and develop the best treatment strategy for patients with aSAH with poor prognosis after aneurysm clipping.
As a clinical prognostic indicator for patients, PNI is primarily used for various tumors and in major surgical research fields; however, studies on acute cerebrovascular diseases primarily focus on ischemic stroke [17]. In our study, we observed that preoperative PNI is a valuable prognostic predictor for patients with aSAH at 6 months after clipping and that high PNI functions as a protective factor (P < 0.05). PNI integrates serum albumin levels and lymphocyte count to reflect the immune responses and nutritional status of patients [18]. Serum albumin is a commonly used nutritional index, and several studies have revealed that it serves as a predictive index for the prognosis of patients with stroke. Serum albumin can increase plasma osmotic pressure, decrease brain edema, maintain blood–brain barrier (BBB) integrity, and improve the internal environment [19, 20]. Lymphocytes are essential leukocyte subsets that participate in host defense and adaptive immunity. Studies have revealed that a low lymphocyte count is a predictor of poor prognosis. Unlike neutrophils that promote inflammation, lymphocytes can inhibit inflammation [21]. As an indicator of cellular immunity, a low lymphocyte count suggests a decreased immune level. However, compared with serum albumin or lymphocytes alone, PNI is a better indicator of a patient’s nutritional and immune status than serum albumin or lymphocytes alone. A low preoperative PNI reflects a decrease in serum albumin or lymphocyte counts, demonstrates a severe inflammatory response and immunosuppression, and indicates poor general condition and reduced nutrition and immunity [22, 23]. Based on our findings, a preoperative PNI value of < 43 significantly increases the risk of adverse outcomes in patients with aSAH undergoing neurosurgical clipping.
Inflammatory cytokines reached the peak within 48 h after aSAH onset. Excessive inflammatory reactions lead to the poor prognosis of patients with aSAH [24]. In the present study, we analyzed many inflammatory indicators. Among them, NAR and PAR are significant. Elevated neutrophil counts are associated with poor prognosis and in-hospital complications of aSAH [25]. Neutrophil products such as free radicals and proteolytic enzymes mediate BBB damage. Furthermore, the release of extracellular traps by neutrophils exacerbates inflammation, destroying the BBB and aggravating damage to surrounding neurons and other brain cells [26]. Increased neutrophil counts are associated with the poor prognosis of patients with aSAH, whereas hypoproteinemia is associated with infection during hospitalization. This may be the mechanism by which NAR predicts aSAH prognosis [9].
Platelets are vital factors in the coagulation system. In addition to hemostasis and coagulation, platelets also participate in inflammation and atherosclerosis development [27]. The higher the PAR level, the higher the platelet count; this reflects the high inflammatory response state and susceptibility to thrombosis [28]. Increased the risk of DCI, resulting in a poor prognosis. Furthermore, a high PAR level indicates a low albumin count, suggesting malnutrition and high inflammation and predicting poor clinical outcomes [29].
High lipid levels increase the risk of poor aSAH prognosis. Vascular smooth muscle cells can repair the damaged aneurysm wall and delay inflammation progression. Presumably, high lipid levels promote inflammation of the aneurysm wall and lead to vascular smooth muscle cell death [30].
GCS score is a simple and effective tool to evaluate the consciousness level of patients; it can help standardize the clinical evaluation of patients with stroke and facilitate patient management. The prognosis of patients with aSAH significantly correlates with GCS score [31]. In the present study, we observed that GCS scores can effectively predict the poor prognosis of patients with aSAH after microsurgical clipping. Compared with other scoring systems, preoperative GCS can better reflect the consciousness level of patients. The GCS score should be closely monitored to reflect the severity of the patient’s condition and appropriate treatment measures should be formulated to improve the prognosis. In dynamic diseases such as aSAH, continuous GCS score monitoring may be more clinically relevant than assessing the Hunt–Hess grade at admission [32].
Neutrophils, albumin, platelets and hyperlipidemia are all indicators that are easy to intervene in clinic.In clinic, anti-inflammatory treatment methods such as Non-steroidal anti-inflammatory drugs (NSAIDs) and glucocorticoid can be selected according to the condition of patients.NSAIDs can effectively reduce vasospasm and neurological dysfunction.Glucocorticoids may help to reduce the production of vasospasm and IL-6, and promote a good prognosis of patients undergoing microsurgical clipping [33, 34]. Patients with hypoalbuminemia were treated with albumin injection, 1.25 g / kg / day. Furthermore, studies have demonstrated that albumin regulates its neuroprotective effect by decreasing brain damage and improving neurovascular remodeling. In addition, it can inhibit inflammation, scavenge free radicals, and produce free radicals, Improve the prognosis of patients [35, 36]. For patients with high platelet level, antiplatelet therapy should be given as early as possible according to clinical conditions to reduce the formation of microthrombosis, reduce the risk of DCI, and improve the prognosis [37]. Patients with hyperlipidemia can be treated with statins, Studies have revealed that statins can inhibit leukocyte migration and proliferation into blood vessels, activate cytokines, upregulate endothelial nitric oxide synthase expression and activity, improve endothelial reactivity, increase cerebral blood flow, and play an antioxidant role. Furthermore, a short statin course (lasting 2 weeks) may improve neurological prognosis. Statins are associated with decreased CVS. Although statins can increase bacteremia, based on the pathophysiological characteristics and prognosis of CVS, 2 weeks may be the best time window for statins to treat aSAH [38].
Our study has some limitations. This was a single-center retrospective cohort study with a small sample size and possibly biased results. Furthermore, we conducted patient follow-up only for 6 months. When establishing predictive models for aSAH, more attention should be paid to the nutritional status and inflammatory indicators of patients. In this study, the neutrophil count, platelet count, lymphocyte count, and albumin level were routine in-patient examinations for patients with aSAH. The calculation methods are simple, convenient to obtain, and inexpensive. Furthermore, it is easier to perform clinical interventions on the indicators and convenient to use in hospitals at all levels. In addition, we preliminarily demonstrated that the combined prediction of PNI and NAR improves the predictive performance of existing predictive models and deserves further clinical validation in additional prospective multicenter studies.
Conclusions
Using univariate and multivariate logistic regression analyses, we identified five clinical predictors, namely, PNI, NAR, PAR, hyperlipidemia, and GCS score, as well as several risk factors affecting the prognosis of patients with severe aSAH and the interactions among these factors. Furthermore, we developed a nomogram using these five predictors. Internal validation revealed the good accuracy and clinical utility of the model in helping clinicians assess the prognosis of patients with aSAH undergoing surgical clipping.
Author contributions
Zhen Sun: Conceptualization, Methodology, Formal analysis, Data curation, Resources, Writing - original draft, Writing - review and editing, Supervision, Project administration, Funding acquisition. Fei Xue: Resources, Writing - review and editing, Funding acquisition. Kunpeng Wang: Investigation, Data curation. Dongbo Zhang: Resources, Writing - review and editing, Supervision, Project administration, Funding acquisition. Mengning Dong: Writing - review and editingJiandang Zhang: Investigation, Validation, Formal analysis, Data curation, Writing - original draft. Formal analysis, Investigation. Formal analysis, Data curation, Formal analysis, Validation.
Funding
Without any financial support.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained during hospitalization from the next of kin or by the patient after recovery at follow-up.
Ethics approval
This study is in line with the principles of the Helsinki Declaration and approved by the Nanyang hospital ethics committee.The requirement for informed consent was waived in all participating hospitals because this study only involved retrospective analysis of anonymous preexisting data.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhen Sun and Fei Xue are contributed equally to this work. They are co-first authors.
References
- 1.Chai C-Z et al. Jun. Systemic Inflammation after Aneurysmal Subarachnoid Hemorrhage. International journal of molecular sciences vol. 24,13 10943. 30 2023, 10.3390/ijms241310943. [DOI] [PMC free article] [PubMed]
- 2.Macdonald R, Loch, Tom A, Schweizer. Spontaneous subarachnoid haemorrhage. Lancet (London England) vol. 2017;389(10069):655–66. 10.1016/S0140-6736(16)30668-7. 10.1016/S0140-6736(16)30668-7 [DOI] [PubMed] [Google Scholar]
- 3.Neifert SN, et al. Aneurysmal Subarachnoid Hemorrhage: the last decade. Translational Stroke Res vol. 2021;12(3):428–46. 10.1007/s12975-020-00867-0. 10.1007/s12975-020-00867-0 [DOI] [PubMed] [Google Scholar]
- 4.Li X, et al. Early Brain Injury and Neuroprotective Treatment after Aneurysmal Subarachnoid Hemorrhage: A literature review. Brain sciences 13,7 1083. 17 Jul. 2023. 10.3390/brainsci13071083. 10.3390/brainsci13071083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindgren A et al. Aug. Endovascular coiling versus neurosurgical clipping for people with aneurysmal subarachnoid haemorrhage. The Cochrane database of systematic reviews vol. 8,8 CD003085. 15 2018, 10.1002/14651858.CD003085.pub3. [DOI] [PMC free article] [PubMed]
- 6.Claassen J, Park S. Spontaneous subarachnoid haemorrhage. Lancet (London, England) vol. 400,10355 (2022): 846–862. 10.1016/S0140-6736(22)00938-2. [DOI] [PMC free article] [PubMed]
- 7.Güresir E, et al. Initial inflammatory response is an independent predictor of unfavorable outcome in patients with good-grade aneurysmal subarachnoid hemorrhage. J Crit Care. 2020;60:45–9. 10.1016/j.jcrc.2020.07.018. 10.1016/j.jcrc.2020.07.018 [DOI] [PubMed] [Google Scholar]
- 8.Attanasio L, et al. Early Lymphopenia and infections in Nontraumatic Subarachnoid Hemorrhage patients. J Neurosurgical Anesthesiology vol. 2022;34(2):243–7. 10.1097/ANA.0000000000000744. 10.1097/ANA.0000000000000744 [DOI] [PubMed] [Google Scholar]
- 9.Zhang R et al. Mar. Improving the models for prognosis of aneurysmal subarachnoid hemorrhage with the neutrophil-to-albumin ratio. Frontiers in neurology vol. 14 1078926. 24 2023, 10.3389/fneur.2023.1078926. [DOI] [PMC free article] [PubMed]
- 10.Kim YJ et al. Aug. Prognostic significance of the postoperative prognostic nutritional index in patients with glioblastoma: a retrospective study. BMC cancer vol. 21,1 942. 21 2021, 10.1186/s12885-021-08686-8. [DOI] [PMC free article] [PubMed]
- 11.Fan M-C et al. May. Preoperative prognostic nutrition index can independently predict the 6-month prognosis of elderly patients undergoing neurosurgical clipping for aneurysmal subarachnoid hemorrhage. Neurosurgical review vol. 46,1 117. 10 2023, 10.1007/s10143-023-02021-4. [DOI] [PubMed]
- 12.Hoh BL et al. 2023 Guideline for the Management of Patients With Aneurysmal Subarachnoid Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke vol. 54,7 (2023): e314-e370. 10.1161/STR.0000000000000436. [DOI] [PubMed]
- 13.Connolly ES Jr et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke vol. 43,6 (2012): 1711-37. 10.1161/STR.0b013e3182587839. [DOI] [PubMed]
- 14.van der Harst JJ, et al. The predictive value of the CTA vasospasm score on delayed cerebral ischaemia and functional outcome after aneurysmal subarachnoid hemorrhage. Eur J Neurol vol. 2022;29(2):620–5. 10.1111/ene.15139. 10.1111/ene.15139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J et al. Dec. Molecular mechanisms of neuronal death in brain injury after subarachnoid hemorrhage. Frontiers in cellular neuroscience vol. 16 1025708. 13 2022, 10.3389/fncel.2022.1025708. [DOI] [PMC free article] [PubMed]
- 16.Zhou Z et al. Mar. A nomogram for predicting the risk of poor prognosis in patients with poor-grade aneurysmal subarachnoid hemorrhage following microsurgical clipping. Frontiers in neurology vol. 14 1146106. 22 2023, 10.3389/fneur.2023.1146106. [DOI] [PMC free article] [PubMed]
- 17.Han X et al. Baseline objective Malnutritional Indices as Immune-Nutritional predictors of Long-Term recurrence in patients with Acute ischemic stroke. Nutrients 14,7 1337. 23 Mar. 2022, 10.3390/nu14071337. [DOI] [PMC free article] [PubMed]
- 18.Rigamonti A, et al. Prognostic nutritional index as a prognostic marker in glioblastoma: data from a cohort of 282 Italian patients. J Neurol Sci. 2019;400:175–9. 10.1016/j.jns.2019.04.002. 10.1016/j.jns.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 19.Zhang D et al. Nov. C-Reactive Protein/Albumin Ratio Correlates With Disease Severity and Predicts Outcome in Patients With Aneurysmal Subarachnoid Hemorrhage. Frontiers in neurology vol. 10 1186. 12 2019, 10.3389/fneur.2019.01186. [DOI] [PMC free article] [PubMed]
- 20.Abubakar S, et al. Low admission serum albumin as prognostic determinant of 30-day case fatality and adverse functional outcome following acute ischemic stroke. Pan Afr Med J. 2013;14:53. 10.11604/pamj.2013.14.53.1941. 10.11604/pamj.2013.14.53.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yun S, et al. Clinical significance of platelet to neutrophil ratio and platelet to lymphocyte ratio in patients with aneurysmal subarachnoid hemorrhage. J Clin Neuroscience: Official J Neurosurgical Soc Australasia. 2021;92:49–54. 10.1016/j.jocn.2021.07.036. 10.1016/j.jocn.2021.07.036 [DOI] [PubMed] [Google Scholar]
- 22.Zhao J et al. Oct. Prognostic nutritional index predicts clinical outcomes in patients with cerebral venous sinus thrombosis. BMC neurology vol. 21,1 404. 21 2021, 10.1186/s12883-021-02436-w. [DOI] [PMC free article] [PubMed]
- 23.Lin Y, et al. Prognostic nutritional index predicts in-hospital mortality in patients with acute type a aortic dissection. Heart lung: J Crit care vol. 2021;50(1):159–64. 10.1016/j.hrtlng.2020.06.004. 10.1016/j.hrtlng.2020.06.004 [DOI] [PubMed] [Google Scholar]
- 24.Zhang R et al. Oct. Association between neutrophil-to-albumin ratio and long-term mortality of aneurysmal subarachnoid hemorrhage. BMC neurology vol. 23,1 374. 19 2023, 10.1186/s12883-023-03433-x. [DOI] [PMC free article] [PubMed]
- 25.Zhang Y et al. Neutrophil Counts as Promising Marker for Predicting In-Hospital Mortality in Aneurysmal Subarachnoid Hemorrhage. Stroke vol. 52,10 (2021): 3266–3275. 10.1161/STROKEAHA.120.034024. [DOI] [PubMed]
- 26.Vaibhav K et al. May. Neutrophil extracellular traps exacerbate neurological deficits after traumatic brain injury. Science advances vol. 6,22 eaax8847. 29 2020, 10.1126/sciadv.aax8847. [DOI] [PMC free article] [PubMed]
- 27.Hao P, et al. Platelet to albumin ratio: a risk factor related to prognosis in patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention. Int J Cardiol. 2024;395:131588. 10.1016/j.ijcard.2023.131588. 10.1016/j.ijcard.2023.131588 [DOI] [PubMed] [Google Scholar]
- 28.Zhang T, et al. Correlation analysis of hemoglobin, albumin, lymphocyte, platelet score and platelet to albumin ratio and prognosis in patients with lung adenosquamous carcinoma. Front Oncol vol. Sep. 2023;13 1166802(7). 10.3389/fonc.2023.1166802. [DOI] [PMC free article] [PubMed]
- 29.Baba D-F et al. May. Platelet-to-Albumin Ratio: The Prognostic Utility in the Prediction of 2-Month Postoperative Heart Transplant Complications. Journal of cardiovascular development and disease vol. 10,6 241. 31 2023, 10.3390/jcdd10060241. [DOI] [PMC free article] [PubMed]
- 30.Lindbohm J et al. Adverse lipid profile elevates risk for subarachnoid hemorrhage: a prospective population-based cohort study. Atherosclerosis 274 (2018): 112–9. 10.1016/j.atherosclerosis.2018.05.011. [DOI] [PubMed]
- 31.Wu J, et al. Development of acute lung injury or acute respiratory distress syndrome after subarachnoid hemorrhage, predictive factors, and impact on prognosis. Acta Neurol Belgica vol. 2023;123(4):1331–7. 10.1007/s13760-023-02207-z. 10.1007/s13760-023-02207-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuwapattanawong K, et al. The Association between Illness Severity scores and In-hospital Mortality after Aneurysmal Subarachnoid Hemorrhage. J Neurosurgical Anesthesiology vol. 2023;35(3):299–306. 10.1097/ANA.0000000000000840. 10.1097/ANA.0000000000000840 [DOI] [PubMed] [Google Scholar]
- 33.Tso MK et al. Apr. Gene expression profiling of brain endothelial cells after experimental subarachnoid haemorrhage. Scientific reports vol. 11,1 7818. 9 2021, 10.1038/s41598-021-87301-z. [DOI] [PMC free article] [PubMed]
- 34.Miller MałgorzataM, et al. Association of Dexamethasone with Shunt Requirement, early disability, and Medical complications in Aneurysmal Subarachnoid Hemorrhage. Neurocritical care vol. 2021;34(3):760–8. 10.1007/s12028-020-01059-2. 10.1007/s12028-020-01059-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, et al. Neutrophil-to-albumin ratio as a biomarker of delayed cerebral ischemia after Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2021;147:e453–8. 10.1016/j.wneu.2020.12.084. 10.1016/j.wneu.2020.12.084 [DOI] [PubMed] [Google Scholar]
- 36.Dolapoglu A et al. Apr. The predictive value of the prognostic nutritional index for postoperative acute kidney injury in patients undergoing on-pump coronary bypass surgery. Journal of cardiothoracic surgery vol. 14,1 74. 11 2019, 10.1186/s13019-019-0898-7. [DOI] [PMC free article] [PubMed]
- 37.Garton ALA, et al. Antiplatelet therapy and outcomes after aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Clin Neurol Neurosurg. 2023;235:108025. 10.1016/j.clineuro.2023.108025. 10.1016/j.clineuro.2023.108025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu T et al. Oct. Optimal Course of Statins for Patients With Aneurysmal Subarachnoid Hemorrhage: Is Longer Treatment Better? A Meta-Analysis of Randomized Controlled Trials. Frontiers in neuroscience vol. 15 757505. 25 2021, 10.3389/fnins.2021.757505. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript or supplementary information files.