Abstract
Monitoring chronic diseases, particularly kidney disorders, in people living with HIV (PLWH) is of paramount importance. Here, a systematic search was conducted across electronic search engine and databases like PubMed, Scopus, and Google Scholar, from date of inception until December 2023, to identify pertinent studies reporting on any association between inflammation and kidney function in PLWH. Only six clinical studies in peer-reviewed journals met the inclusion criteria, involving 1467 participants aged 37 to 51, with approximately 17% being females. The report emphasizes the potential impact of highly active antiretroviral therapy (HAART) on kidney function in PLWH, highlighting the significance of monitoring inflammation markers as indicators of kidney function, even when HAART is effective. Acknowledging study limitations, particularly the scarcity of relevant research, the findings highlight a need for more research to inform on clinical guidance to optimize HIV management, particularly regarding kidney health and HAART regimens. Although very limited studies were evaluated, the study lays an important foundation for future research to uncover the complex relationship between HAART, inflammation markers, and kidney health in PLWH.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-09594-5.
Keywords: HIV, Highly active antiretroviral therapy, Inflammation, Kidney disease, Prognosis
Introduction
Highly active antiretroviral therapy (HAART) has become the cornerstone of treatment for people living with HIV (PLWH) [1]. HAART typically consists of a combination of drugs, incorporating two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) and a non-nucleoside reverse transcriptase inhibitor (NNRTI), boosted protease inhibitor, or an integrase strand transfer inhibitor [2]. Commonly, two NRTIs, tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC), together with efavirenz (EFV) are some of the prominently used HAART regimens [3]. The prominent role of HAART is to maximize viral suppression, thereby reducing HIV-associated morbidity and mortality, ultimately leading to immune function recovery, extended lifespans, and improved quality of life [1]. While the benefits of HAART on viral control and immune restoration are well-established [4], this therapeutic approach is not without its limitations and potential consequences, particularly concerning kidney function [5].
The kidneys, with their functional units called nephrons, are pivotal in maintaining the body’s homeostasis through processes like glomerular filtration, tubular secretion, and tubular reabsorption. Moreover, they are instrumental in the metabolism and excretion of drugs, rendering them vulnerable to the adverse effects of pharmaceutical agents [6]. Notably, some antiretroviral drugs, including TDF, exert their detrimental effects on the kidneys through mechanisms involving oxidative stress and inflammation [7, 8]. In the context of PLWH, chronic inflammation has been implicated in the pathogenesis of kidney dysfunction [9]. It also remains relevant to further explore the relationship between inflammation and kidney health [9, 10]. Despite the considerable body of research on HAART [11–13], there remains a notable gap in our understanding of the negative influence of this treatment regimen on kidney function, especially in the presence of inflammation.
Emerging data progressively scrutinizes available literature for prognostic value of pro-inflammatory markers for diverse medical conditions [14–16]. These include biomarkers such as tumor necrosis factor (TNF)-α, high sensitivity C-reactive protein (hs-CRP), interleukin (IL)-IL-6 and IL-1, as well as circulating adipokines like adiponectin, visfatin and resistin to detect increased risk of disease development [14–16]. In fact, pro-inflammatory markers, such as elevated levels of IL-6 or hs-CRP, can serve as early indicators of systemic inflammation, a common precursor to kidney damage in PLWH [17, 18]. Monitoring these markers can provide valuable tool into the progression of kidney disease, enabling timely interventions to mitigate the risk of renal complications. This approach can significantly improve the long-term health and quality of life for PLWH, which highlights the importance of the current systematic review. Beyond contributing to knowledge on the existing link between the pathogenesis of inflammation and the development of kidney dysfunction, this comprehensive analysis of existing literature may guide potential interventions, ultimately ensuring the long-term well-being of PLWH.
Methodology, including literature search and study selection
The Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines were followed to prepare the current systematic review [19]. However, the meta-analysis was not performed for this report. Supplementary file 1 provides a PRISMA checklist for this systematic review. The systematic review does not have a registered protocol, but online registries like International Prospective Register of Systematic Reviews (PROSPERO) were thoroughly searched to eliminate duplication of results.
Literature search strategy
In order to identify relevant clinical studies, a systematic search was conducted across major electronic search engines and database, including PubMed, Scopus, and Google Scholar. In fact, the search strategy was developed using a set of keywords and Medical Subject Headings (MeSH) such as “inflammation”, “kidney”, “Highly Active Antiretroviral Therapy” and “HIV” (Supplementary file 2). This search included the most relevant synonyms and keywords. The literature search was run from date of inception until end of December 2023. A manual search of reference lists of included was conducted to obtain any additional relevant studies. No language restrictions were applied in the search strategy, whereas EndNote version 10 (Clarivate Analytics, Philadelphia, United States of America) was used to efficiently manage the reference list, especially to remove duplicated studies.
Study inclusion and exclusion criteria
This systematic review included clinical studies reporting on the link between inflammation or pro-inflammatory markers and kidney function in adults (> 18 years) living with HIV. Importantly, included studies were those assessing the use of HAART, also containing the comparison group on placebo, and all studies reported on measurable inflammation or kidney function indices in PLWH. In vitro cell culture or animal studies were excluded, as well as studies without an accurately defined study population. The following populations, interventions/exposure, comparators, outcomes (PICO/PECO) were used:
P: PLWH on HAART
I/E: Levels of inflammatory biomarkers (in PLWH on HAART)
C: PLWH not on HAART or treatment naïve individuals
O: Markers/indicators of renal function
Data extraction and assessment of the quality of evidence
Two reviewers independently evaluated all relevant articles and carefully selected those that were appropriate. Inconsistencies were resolved by consulting a third reviewer. The primary focus of the study was to determine the prognostic value of inflammation or pro-inflammatory makers in PLWH with or at risk of developing kidney dysfunction. Mainly, to also to evaluate whether HAART contributes to deteriorated kidney dysfunction by affecting inflammatory markers in PLWH. To achieve this, relevant data items were extracted, including name of author and year of publication, type of study, the country where the study was conducted, sample number and gender distribution, the type and duration of HAART, as well as the main outcomes. Information on patient characteristics including race or ethnicity, cluster of differentiation 4 (CD4+) count and viral load as well as assessed markers of inflammation and renal function were also obtained (Supplementary file 3). Additional characteristics including information on smoking status, co-infections and cardiometabolic conditions are provided in a subsequent table (Supplementary file 3). Furthermore, two reviewers independently used the Downs and Black checklist to assess the quality of included studies, which is suitable for both randomised and non-randomised studies [20]. Any divergences were resolved by consulting the third reviewer.
Results
Study selection
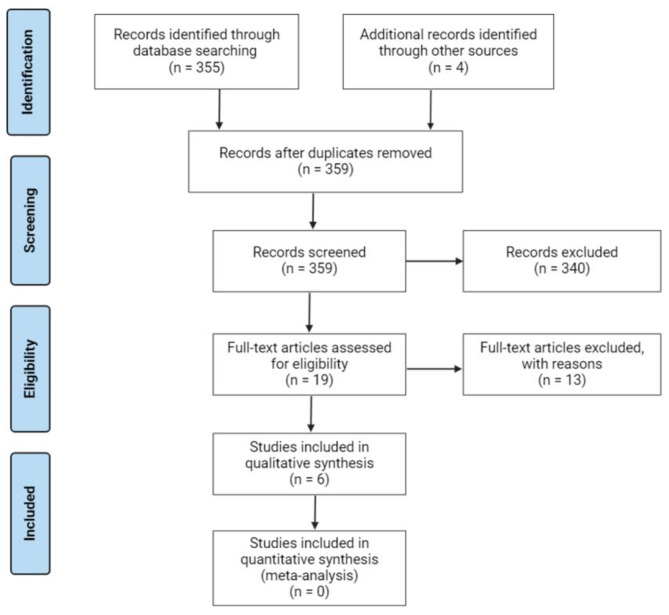
A total of 359 studies were identified and screened for suitability, with 355 records chosen through precise database searching, and four articles recovered through other sources. Only six studies met the inclusion criteria, as shown in Fig. 1. All included studies were clinical studies reporting on the potential link between modulation of inflammation and kidney function in PLWH on HAART.
Fig. 1.
A flow diagram showing study selection and inclusion criteria
Characteristic features of included studies
All included studies were published between 2010 and 2019, and the characteristic features for all articles are showed in Table 1. In total, studies contained 1467 participants with an average age between the age of 37 and 51 years, with at least 17% of participants recorded as females. Different HAART regimens ranged from the use of NNRTIs or NRTIs, specifically composing of TDF/ FTC / EFV, with some receiving it in combination with raltegravir (RAL), TDF or abacavir–lamivudine, and even sometimes switching to dolutegravir monotherapy (Table 1). Treatment duration ranged from 6, 12, 18 and up to 24 months (Table 1). In terms of country distribution, these studies were predominantly based in the United States, with each of France and the Netherlands having one study each (Table 1). There was an even distribution of racial groups among the included studies, mainly including Black and White as well as Hispanic and Latino as predominant ethnic groups (Table 1). The average CD4 + count ranged between 236 and 657 cells/µL (Supplementary Table 1). The viral load was at undetectable levels (< 50 copies/mL) in five studies and at low level (< 400 copies/mL) in one study (Supplementary Table 1). Not all studies reported basic characteristics such as cardiometabolic conditions due to the exclusion criteria [28–28, 31]. Predominant cardiometabolic conditions in other studies included diabetes (range from 3 to 14%), hypertension (range from 17 to 37%) and dyslipidemia (range from 13 to 37%) (Supplementary Table 2).
Table 1.
Clinical studies reporting on the potential association of inflammation with kidney dysfunction in people living with HIV (PLWH) on highly active antiretroviral therapy (HAART)
| Reference | Country | Type of study | Study population, including age | Race/ethnicity | Intervention, including HAART regimen and treatment duration | Main outcomes |
|---|---|---|---|---|---|---|
| Neuhaus et al., 2010 [21] | United States | Randomized Controlled Trial | PLWH on HAART (n = 287) from the Strategies for Management of Anti-Retroviral Therapy (SMART) study [22], with an average age of 40 years (28% female) | In the 33–44 years age group, the SMART study consisted mostly of Black (55.4%) and CARDIA study mostly of White (52.4%). In the 45–76 years age group, the SMART study consisted mostly of Black (49.2%), and MESA study consisted mostly of White (43.3%) | Received non-nucleoside reverse transcriptase inhibitor (NNRTIs), or reverse transcriptase inhibitors (NRTIs) for 18 months | Biomarkers of inflammation like high sensitivity-C reactive protein (hs-CRP) and interleukin (IL)-6, as well that of kidney dysfunction (cystatin C) were consistently raised in PLWH, despite HAART |
| Gupta et al., 2013 [26] | United States | Randomized Controlled Trial | PLWH on HAART (n = 30), with an average age of 38 years (13% female) | Consisted mostly of Black (53% and 67% for the continuation and switch groups, respectively) | Received tenofovir (TDF)/ emtricitabine (FTC) / efavirenz (EFV) continued versus a group that switched to TDF/FTC plus raltegravir (RAL) at 400 mg twice daily and monitored up to 6 months | The decline in renal function was associated with reduction in total cholesterol, hs-CRP, serum alkaline phosphatase, sCD14 levels in the switching group compared with the continuation group. While the sCD163 levels significantly increased in the switching group |
| Gupta et al., 2015 [23] | United States | Randomized Controlled Trial | PLWH on HAART (n = 269) which is part of previous report [27], with an average age of 38 years (14% female) | Consisted mostly of White non-Hispanics (47%) and Hispanics (33%) | Received abacavir–lamivudine/ TDF/FTC plus EFV or ritonavir-boosted atazanavir (ATV/r) for up to 24 months | Estimated glomerular filtration rate (eGFR), using cystatin C-creatinine, urine protein: creatinine ratio (uPCR), and urine albumin: creatinine ratio (uACR) was correlated with markers of systemic inflammation prior to HAART. However, uPCR and eGFR remained significantly correlated with most of the assessed inflammatory markers even after HAART |
| Shinha et al., 2015 [24] | United states | Observational study | PLWH on HAART (n = 30), with an average age of 37 years (10% females) | Consisted mostly of Black (60%). All were of non-Hispanic or Latino ethnic origin | Received TDF/FTC/EFV as their initial regimen for at least 12 months | Urine interferon gamma inducible protein 10 and Beta-2 microglobulin (B2M) were significantly after receiving HAART |
| Ozanne et al., 2017 [28] | France | Observational study | PLWH on HAART (n = 756), with mean age of 51 years (24% female) | Not reported | Received dual regimes including two NRTIs + one ritonavir boosted protease inhibitor or NNRTIs, and others for at least 24 months | Increased inflammation (high CIADIS weight score) was associated with rapid decreased in renal function in confirmed eGFR < 60 mL/min/1.73m3 |
| Wijting et al., 2019 [25] | Netherlands | Randomized Controlled Trial | PLWH on HAART (n = 95), with an average age of 46 years (12%) | Consisted mostly of Caucasian (82.1%) | Received TDF-based regimen and switched to dolutegravir monotherapy and monitored for up to 12 months | In patients on prior TDF, proteinuria improved, but proximal tubular dysfunction proportions did not change. However, serum inflammation parameters such as CRP and T-cell-ratio remained stable |
Quality assessment
All the studies were rated as having good quality evidence, with their scores ranging from 21 to 24, out of 28 total scores. Based on the different domains, overall, there was an excellent reporting bias as indicated by mean score of 11 out of 11 possible scores. The studies had overall good external validity, with a mean score of 2 out of 3 possible scores and overall good internal validity with a mean of 5 out of 7 possible scores. The overall selection bias was also rated as good, with mean score of 4 out of 6 possible scores. All studies had good power (≥ 90%) indicating that these studies had no type 2 error, as supported by a mean score of 1 (0–1) and Cohen’s Kappa value of 1.00 (Supplementary file 4).
An overview of clinical evidence linking inflammation with kidney dysfunction in PLWH on HAART
Table 1 gives an overview of clinical evidence highlighting the significance of HAART regimen selection in PLWH. Neuhaus and colleagues [21], as part of the Strategies for Management of Anti-Retroviral Therapy (SMART) trial [22], reported on the persistently elevated levels of pro-inflammatory and coagulation markers that correlated with a decline in renal indices in PLWH receiving NNRTIs, or reverse transcriptase inhibitors (NRTIs) for 18 months. Here, it was specifically shown that biomarkers like hs-CRP, IL-6, D-dimer, and cystatin C were significantly raised in PLWH, even after achieving viral suppression through antiretroviral therapy (Table 1). Furthermore, these markers were significantly elevated after adjusting for a range of confounders [26]. Ozanne and colleagues [33] further observed significant association between inflammatory marker soluble chronic immune activation and senescence (soluble- CIADIS) weighted score and eGFR < 60 mL/min/1.73m3 adjusted for age, sex, tenofovir use and having two or more non-HIV-related comorbidities in PLHIV receiving various HAART combinations. Consistently, Gupta and colleagues [23] showed that urine protein: creatinine ratio (uPCR) and eGFR correlated with inflammation in PLWH receiving abacavir–lamivudine/ TDF/ FTC plus EFV or ritonavir-boosted atazanavir (ATV/r) for up to 24 months. In a parallel effort, both studies by Shinha and co-workers [24] or Ozanne and colleagues [24] supported the notion that elevated levels inflammation is consistent with rapid decreased in renal function in PLWH on HAART. Here, inflammation was associated with raised levels of biomarkers for kidney dysfunction, including urine interferon gamma inducible protein 10 and beta-2 microglobulin (B2M), together with reduced eGFR in PLWH receiving dual regimes including two NRTIs plus one ritonavir boosted protease inhibitor or NNRTIs (Table 1).
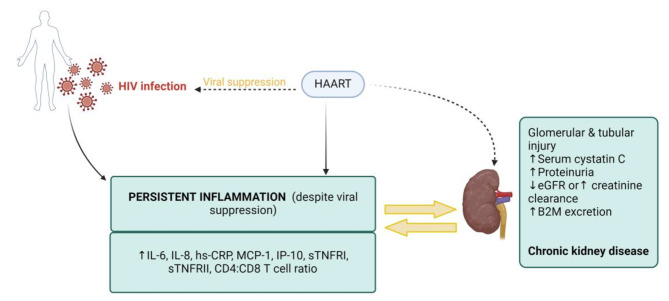
However, opposing results were reported, where an association between inflammation and kidney dysfunction in PLWH on HAART was demonstrated by Wijting and colleagues [25]. Here, they explored the consequences of switching from TDF to dolutegravir based therapy, with results indicating that switching is linked with improvements in kidney function and inflammatory markers, including CRP and the T-cell-ratio, which remained stable for these participants. The summary of the overall analysis of our data is provided in Fig. 2, below the main table. Briefly, the data shows that HIV is associated with high levels of inflammation as demonstrated by as demonstrated by elevated levels of IL-6, IL-8 or hs-CRP and these inflammatory markers have a strong association with markers of kidney dysfunction including high cystatin C, proteinuria and low eGFR, as shown in Fig. 2 below Table 1.
Fig. 2.
In people living with HIV (PLWH), inflammation persist despite viral suppression. The overall analysis of our data suggests that HIV may induce a pro-inflammatory state and treatment with highly active antiretroviral therapy (HAART) overtime exacerbates inflammation, as demonstrated by elevated levels of inflammatory markers such as interleukin-6 (IL-6), interleukin-8 (IL-8) or high-sensitivity C-reactive protein (hs-CRP). Consequently, these elevated inflammatory markers are associated with markers of renal function decline such as high cystatin C, proteinuria, reduced estimated glomerular filtration rate, beta-2 microglobulin and subsequently leading to chronic kidney disease in PLWH on HAART. The figure was created with BioRender.com.
Discussion
There is a growing interest in understanding the potential influence of HAART regimens on renal function [29], especially after prolonged use [30]. This underscores the importance of reviewing clinical evidence to inform in guiding treatment decisions for PLWH. Here, we provide a comprehensive overview of clinical evidence reporting on the association of some inflammation markers with kidney dysfunction in PLWH on HAART. The quality assessment of included studies showed a good interrater reliability which affirms the consistency and agreement between the independent reviewers, thus strengthening the overall results of the review.
Chronic inflammation understandably refers to long-lasting and persistent low-level immune activation, which can contribute to various health issues, including kidney dysfunction [31]. On other hand, systematic inflammation, can involve a more acute and systematic response by the immune system, often in response to infections or injuries. Both forms of inflammation are persistent in PLWH, with contradicting evidence reports on the effects of HAART on modulating the inflammatory status in these patients. For example, it has been suggested that viral suppression with the use of HAART improve inflammatory status and the overall human health in PLWH [32], while others have reported on the detrimental effects associated with constant exposure to HIV [33–35]. This has emphasized the importance of monitoring and addressing this persistent inflammatory response in PLWH. Data from the current systematic review supports the notion that persistently elevated pro-inflammatory response and coagulation markers like hs-CRP and IL-6 is present in PLWH, even after achieving viral suppression through HAART. Consequently, such results are correlated with elevated biomarkers signaling a decline in renal function in these patients, including cystatin C, B2M, proteinuria as well as reduced eGFR. These findings highlight the importance for continuously monitoring PLWH for levels of pro-inflammatory markers, especially hs-CRP and IL-6, as a potential correlate of the status of kidney function, irrespective of their HAART regimen. The levels of inflammation are also elevated and associated with proteinuria, albuminuria and reduced renal function prior and after HAART initiation [28]. The findings highlight the complex interplay between HIV, inflammation and renal disease with HIV having a direct effect of inducing an inflammatory state and the development of renal pathologies. These elevated levels of inflammatory markers associated with renal function decline and can have significant clinical implications, including determining the risk of kidney disease progression in PLWH. Moreover, the use of these pro-inflammatory markers may provide new diagnostic tool to predict adverse renal outcomes while serving as potential therapeutic targets for interventions. Managing inflammation is increasingly becoming a single most importance feature to mitigate the adverse effects associated with kidney dysfunction in PLWH.
The findings by Wijting and colleagues [25] provide a generally different outlook on the potential effect of some HAART regimens, indicating potentially that switching from TDF to dolutegravir could improve kidney function and inflammatory status in PLWH. These findings are of interest since ongoing research has emphasized the toxic effects of TDF on proximal tubular cell injury in different populations of PLWH [36–38], whereas dolutegravir plus lamivudine are advocated for their safety and more potency in terms of improving health outcomes in PLWH [39, 40]. Interestingly, a recent systematic review and meta-analysis did not note significant changes in inflammatory or atherogenesis biomarkers PLWH after switching to dolutegravir plus lamivudine, highlighting the intricate dynamics between specific antiretroviral drug regimens and their profound effects on inflammatory status or renal health in PLWH [41]. Certainly, the choice of HAART regimen plays a pivotal role in shaping health outcomes, significantly influencing kidney function, inflammatory markers, and other critical aspects of well-being for individuals living with HIV. These results emphasize the importance of optimizing the management of individuals living with HIV to improve their clinical outcomes, particularly concerning kidney health. Replacing TDF with dolutegravir may be an effective treatment option to improve the inflammatory status and preserve the renal function of PLWH. This will certainly improve the quality of life of PLWH and lower the burden of disease and healthcare costs, especially in resource-limited settings.
Strengths and limitations
The primary limitation of the review stems from the paucity of available literature, as only six clinical studies met the inclusion criteria. Furthermore, all included studies were conducted in Western countries with no single study from Africa, which limits the generalizability of the findings. We acknowledge the importance of evaluating the prognostic significance of inflammatory markers, however the limited data summarized makes it difficult to specifically leads to the elevated inflammation discussed, restricting a broader analysis. For example, it is acknowledged that chronic kidney disease and HIV are frequently linked to heightened inflammation within the body [42, 43]. Also, it has been reported that HAART can exacerbate this inflammatory condition [35]. Thus, future research is essential to determine whether the status of kidney disease or worsened condition of HIV could be the significant factors driving inflammation rather than HAART. We also knowledge the variation in the studied populations of included studies as a potential bias, as there are some studies which included PLWH with cardiometabolic conditions and lifestyle factors like smoking whereas others have excluded individuals with these specific conditions. This may possibly have contributed to differences in inflammation and renal outcomes among included studies. Although the current review provides a comprehensive analysis of the association of inflammation with kidney function and HAART in PLWH.
Conclusion and future perspectives
The report presents a comprehensive exploration of clinical evidence regarding the association of pro-inflammatory markers with kidney dysfunction in PLWH on HAART. The results indicated that raised pro-inflammatory markers may potentially signal kidney dysfunction in PLWH, despite the effectiveness of HAART. However, the report does acknowledge certain limitations. It recognizes the presence of conflicting evidence on the effects of HAART on inflammation in PLWH, introducing an element of uncertainty into treatment decision-making. Moreover, it is important to note that due to a scarcity of studies meeting the inclusion criteria, a quantitative analysis was unfeasible, which restrains the capacity to draw statistically significant conclusions. Despite these limitations, the report underscores the paramount importance of managing inflammation and selecting appropriate HAART regimens to improve kidney health in PLWH, urging for further research and the development of clinical guidelines in this area.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Joel Choshi was partially supported by funding from the SAMRC through its division of Research Capacity Development under the Internship Scholarship Programme. The work by Sihle E. Mabhida, reported herein was made possible through partial funding by the South African Medical Research Council through its Division of Research Capacity Development under the Researcher Development Award Programme. Ndivhuwo Muvhulawa acknowledges funding from the NRF. The content hereof is the sole responsibility of the authors and do not necessarily represent the official views of the funders.
Abbreviations
- ATV/r
Ritonavir-boosted atazanavir
- B2M
Beta-2 microglobulin
- CD4+
Cluster of differentiation 4
- CRP
C-reactive protein
- EFV
Efavirenz
- eGFR
estimated glomerular filtration rate
- FTC
Emtricitabine
- HAART
Highly Active Antiretroviral therapy
- HIV
Human immunodeficiency virus
- hsCRP
high sensitivity C-reactive protein
- IL-1
Interleukin-1
- IL-6
Interleukin-6
- IP-10
interferon-γ-induced protein-10
- NRTIs
Nucleoside reverse transcriptase inhibitor
- NNRTIs
Non-nucleoside reverse transcriptase inhibitors
- PLWH
People living with human immunodeficiency virus
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International Prospective Register for Systematic Reviews
- RAL
Raltegravir
- RTIs
Reverse transcriptase inhibitors
- sCD14
soluble cluster of differentiation-14, sCD163:soluble cluster of differentiation-163
- sTNFRI
soluble tumor necrosis factor-α receptor I
- sTNFRI
soluble tumor necrosis factor-α receptor II
- SMART
Strategy for Management of Anti-retroviral Therapy
- TAF
Tenofovir alafenamide fumarate
- TDF
Tenofovir disoproxil fumarate
- TNF-α
Tumour necrosis factor-α
- uACR
urine albumin: creatinine ratio
- uPCR
urine protein: creatinine ratio
Author contributions
Authors, J.M.C, S.H and P.V.D conceived and contributed to drafting the original manuscript. All other authors, including J.M.C, S.H, S.E.M, H.M, M.T.M, N.M, M.D.S, B.B.N, Z.J.R.M, D.N, U.N, A.P.K and P.V.D reviewed and edited the manuscript. All authors approved the final version of the manuscript.
Funding
Sidney Hanser is also funded by the National Research Foundation (NRF) (Grant number: TTK2204082828). Phiwayinkosi V. Dludla was supported in part by the NRF (Grant numbers: 117829 and 141929). The content hereof is the sole responsibility of the authors and do not necessarily represent the official views of the NRF.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Human ethics and consent to participate declarations
Not applicable.
Competing interests
The authors declare no competing interests.
Completing interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joel Choshi, Email: joel.choshi@ul.ac.za.
Phiwayinkosi V. Dludla, Email: pdludla@mrc.ac.za
References
- 1.Shafer RW, Vuitton DA. Highly active antiretroviral therapy (HAART) for the treatment of infection with human immunodeficiency virus type 1. Biomed Pharmacother. 1999;53(2):73–86. 10.1016/S0753-3322(99)80063-8 [DOI] [PubMed] [Google Scholar]
- 2.Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents: the panel on clinical practices for treatment of HIV. Ann Intern Med. 2002;137(5Part2):381–433. 10.7326/0003-4819-137-5_Part_2-200209031-00001 [DOI] [PubMed] [Google Scholar]
- 3.Achenbach CJ, Darin KM, Murphy RL, Katlama C. Atazanavir/ritonavir-based combination antiretroviral therapy for treatment of HIV-1 infection in adults. Future Virol. 2011;6(2):157–77. 10.2217/fvl.10.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson EM, Sereti I. Immune restoration after antiretroviral therapy: the pitfalls of hasty or incomplete repairs. Immunol Rev. 2013;254(1):343–54. 10.1111/imr.12064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalyesubula R, Perazella MA. Nephrotoxicity of HAART. AIDS Res Treat. 2011;2011:562790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajaj P, Chowdhury SK, Yucha R, Kelly EJ, Xiao G. Emerging kidney models to Investigate Metabolism, Transport, and toxicity of drugs and xenobiotics. Drug Metab Dispos. 2018;46(11):1692–702. 10.1124/dmd.118.082958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tourret J, Deray G, Isnard-Bagnis C. Tenofovir effect on the kidneys of HIV-infected patients: a double-edged sword? J Am Soc Nephrol. 2013;24(10):1519–27. 10.1681/ASN.2012080857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramamoorthy H, Abraham P, Isaac B, Selvakumar D. Role for NF-κB inflammatory signalling pathway in tenofovir disoproxil fumarate (TDF) induced renal damage in rats. Food Chem Toxicol. 2017;99:103–18. 10.1016/j.fct.2016.11.029 [DOI] [PubMed] [Google Scholar]
- 9.Naicker S, Rahmanian S, Kopp JB. HIV and chronic kidney disease. Clin Nephrol. 2015;83(7 Suppl 1):32–8. 10.5414/CNP83S032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyatt CM. Kidney disease and HIV infection. Top Antivir Med. 2017;25(1):13–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Pau AK, George JM. Antiretroviral therapy: current drugs. Infect Dis Clin North Am. 2014;28(3):371–402. 10.1016/j.idc.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyd MA, Boffito M, Castagna A, Estrada V. Rapid initiation of antiretroviral therapy at HIV diagnosis: definition, process, knowledge gaps. HIV Med. 2019;20(Suppl 1):3–11. 10.1111/hiv.12708 [DOI] [PubMed] [Google Scholar]
- 13.Moerman F, Van Gompel A, Nimmegeers J, Moerman J. Highly active antiretroviral therapy. BMJ. 2005;330(7504):1341–2. 10.1136/bmj.330.7504.1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitsis A, Kadoglou NPE, Lambadiari V, Alexiou S, Theodoropoulos KC, Avraamides P, et al. Prognostic role of inflammatory cytokines and novel adipokines in acute myocardial infarction: an updated and comprehensive review. Cytokine. 2022;153:155848. 10.1016/j.cyto.2022.155848 [DOI] [PubMed] [Google Scholar]
- 15.Dludla PV, Mabhida SE, Ziqubu K, Nkambule BB, Mazibuko-Mbeje SE, Hanser S, et al. Pancreatic β-cell dysfunction in type 2 diabetes: implications of inflammation and oxidative stress. World J Diabetes. 2023;14(3):130–46. 10.4239/wjd.v14.i3.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ndevahoma F, Nkambule BB, Dludla PV, Mukesi M, Natanael KN, Nyambuya TM. The effect of underlying inflammation on iron metabolism, cardiovascular risk and renal function in patients with type 2 diabetes. EJHaem. 2021;2(3):357–65. 10.1002/jha2.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su H, Lei CT, Zhang C. Interleukin-6 signaling pathway and its role in kidney disease: an update. Front Immunol. 2017;8:405. 10.3389/fimmu.2017.00405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ (Clinical Res ed). 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 20.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84. 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neuhaus J, Jacobs DR Jr., Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–95. 10.1086/652749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4 + count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–96. 10.1056/NEJMoa062360 [DOI] [PubMed] [Google Scholar]
- 23.Gupta SK, Kitch D, Tierney C, Melbourne K, Ha B, McComsey GA. Markers of renal disease and function are associated with systemic inflammation in HIV infection. HIV Med. 2015;16(10):591–8. 10.1111/hiv.12268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinha T, Mi D, Liu Z, Orschell CM, Lederman MM, Gupta SK. Relationships between renal parameters and serum and urine markers of inflammation in those with and without HIV infection. AIDS Res Hum Retroviruses. 2015;31(4):375–83. 10.1089/aid.2014.0234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijting IE, Rokx C, Zillikens MC, Smits SA, de Vries-Sluijs TE, Schurink CA, et al. Changes in renal, bone, lipid, and inflammation markers in HIV-1 patients after combination antiretroviral therapy simplification to dolutegravir monotherapy. Int J STD AIDS. 2019;30(11):1042–8. 10.1177/0956462419848962 [DOI] [PubMed] [Google Scholar]
- 26.Gupta SK, Mi D, Moe SM, Dubé MP, Liu Z. Effects of switching from efavirenz to raltegravir on endothelial function, bone mineral metabolism, inflammation, and renal function: a randomized, controlled trial. J Acquir Immune Defic Syndr. 2013;64(3):279–83. 10.1097/QAI.0b013e3182a97c39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sax PE, Tierney C, Collier AC, Fischl MA, Mollan K, Peeples L, et al. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. N Engl J Med. 2009;361(23):2230–40. 10.1056/NEJMoa0906768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozanne A, Duffau P, Dauchy FA, Rigothier C, Terrien C, Lazaro E, et al. Activation, senescence and inflammation markers in HIV patients: association with renal function. Aids. 2017;31(8):1119–28. 10.1097/QAD.0000000000001461 [DOI] [PubMed] [Google Scholar]
- 29.Wondifraw Baynes H, Tegene B, Gebremichael M, Birhane G, Kedir W, Biadgo B. Assessment of the effect of antiretroviral therapy on renal and liver functions among HIV-infected patients: a retrospective study. HIV AIDS (Auckl). 2017;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Röling J, Schmid H, Fischereder M, Draenert R, Goebel FD. HIV-associated renal diseases and highly active antiretroviral therapy-induced nephropathy. Clin Infect Dis. 2006;42(10):1488–95. 10.1086/503566 [DOI] [PubMed] [Google Scholar]
- 31.Imig JD, Ryan MJ. Immune and inflammatory role in renal disease. Compr Physiol. 2013;3(2):957–76. 10.1002/cphy.c120028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keating SM, Golub ET, Nowicki M, Young M, Anastos K, Crystal H, et al. The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of women. Aids. 2011;25(15):1823–32. 10.1097/QAD.0b013e3283489d1f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva BF, Peixoto G, da Luz SR, de Moraes S, Peres SB. Adverse effects of chronic treatment with the main subclasses of highly active antiretroviral therapy: a systematic review. HIV Med. 2019;20(7):429–38. 10.1111/hiv.12733 [DOI] [PubMed] [Google Scholar]
- 34.Nittayananta W, Talungchit S, Jaruratanasirikul S, Silpapojakul K, Chayakul P, Nilmanat A, et al. Effects of long-term use of HAART on oral health status of HIV-infected subjects. J Oral Pathol Med. 2010;39(5):397–406. 10.1111/j.1600-0714.2009.00875.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyambuya TM, Dludla PV, Mxinwa V, Nkambule BB. The effect of successful antiretroviral therapy on Immune activation and reconstitution in HIV infected adults: a systematic review and Meta-analysis. AIDS Rev. 2020;23(1):1–12. [DOI] [PubMed] [Google Scholar]
- 36.Venter WDF, Fabian J, Feldman C. An overview of tenofovir and renal disease for the HIV-treating clinician. South Afr J HIV Med. 2018;19(1):817. 10.4102/sajhivmed.v19i1.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez-Fernandez B, Montoya-Ferrer A, Sanz AB, Sanchez-Niño MD, Izquierdo MC, Poveda J, et al. Tenofovir nephrotoxicity: 2011 update. AIDS Res Treat. 2011;2011:354908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mtisi TJ, Ndhlovu CE, Maponga CC, Morse GD. Tenofovir-associated kidney disease in africans: a systematic review. AIDS Res Ther. 2019;16(1):12. 10.1186/s12981-019-0227-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel R, Evitt L, Mariolis I, Di Giambenedetto S, d’Arminio Monforte A, Casado J, et al. HIV Treatment with the two-drug Regimen Dolutegravir Plus Lamivudine in Real-world clinical practice: a systematic literature review. Infect Dis Ther. 2021;10(4):2051–70. 10.1007/s40121-021-00522-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fantauzzi A, Mezzaroma I. Dolutegravir: clinical efficacy and role in HIV therapy. Ther Adv Chronic Dis. 2014;5(4):164–77. 10.1177/2040622314530461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Llibre JM, Cahn PE, Lo J, Barber TJ, Mussini C, van Welzen BJ, et al. Changes in inflammatory and atherogenesis biomarkers with the 2-Drug Regimen Dolutegravir Plus Lamivudine in antiretroviral Therapy-Experienced, virologically suppressed people with HIV-1: a systematic literature review. Open Forum Infect Dis. 2022;9(4):ofac068. 10.1093/ofid/ofac068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rapa SF, Di Iorio BR, Campiglia P, Heidland A, Marzocco S. Inflammation and oxidative stress in chronic kidney disease-potential therapeutic role of minerals, vitamins and Plant-Derived metabolites. Int J Mol Sci. 2019;21(1). [DOI] [PMC free article] [PubMed]
- 43.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39(4):633–45. 10.1016/j.immuni.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.