Abstract
Background
Hyposalivation is treated using oral cholinergic drugs; however, systemic side effects occasionally lead to discontinuation of treatment. We aimed to investigate the effects of transdermal pilocarpine on the salivary gland skin on saliva secretion and safety in rats.
Methods
Pilocarpine was administered to rats orally (0.5 mg/kg) or topically on the salivary gland skin (5 mg/body). Saliva volume, the number of sweat dots, and fecal weight were measured along with pilocarpine concentration in plasma and submandibular gland tissues.
Results
Saliva volume significantly increased 0.5 h after oral administration and 0.5, 3, and 12 h after topical administration. Fecal weight and sweat dots increased significantly 1 h after oral administration; however, no changes were observed after topical application. The pilocarpine concentration in the submandibular gland tissues of the topical group was higher than that in the oral group at 0.5, 3, and 12 h of administration.
Conclusions
Pilocarpine application to salivary gland skin persistently increased salivary volume in rats without inducing sweating or diarrhea. Transdermal pilocarpine applied to the skin over the salivary glands may be an effective and safe treatment option for hyposalivation.
Keywords: Cholinergic agonist, Hyposalivation, Saliva, Safety, Sweating, Transdermal formulation
Background
Saliva plays an important role in oral health [1, 2]. It prompts digestion through starch breakdown with amylase, prevents cavities by regulating pH, and defends against bacteria and viruses. Saliva also plays a role in lubricating the oral cavity and protecting the oral mucosa. Normal, unstimulated salivary secretory rates vary between 800 and 1500 mL per day [3], and a reduction in an unstimulated whole saliva flow rate of less than 0.1 mL/min or a stimulated flow rate of less than 0.7 mL/min is typically defined as hyposalivation [4]. The causes of decreased saliva secretion are diverse and include systemic, neurological, or drug-related factors or dysfunction of the salivary glands [5–8]. Hyposalivation causes oral diseases, such as oral candidiasis, bad breath, alveolar pyorrhea, and periodontal disease, along with dysphagia and taste disorders [9–13]. Moreover, the subjective feeling of having a dry mouth (xerostomia) reduces the quality of life (QOL). Hence, hyposalivation is a condition that warrants treatment.
The treatment strategies for hyposalivation include non-drug therapy, such as salivary gland massage and oral care, and drug therapy [14–16]. Cholinergic agonists, including pilocarpine hydrochloride and cevimeline hydrochloride, are administered orally. However, oral cholinergic agonists often cause systemic side effects, such as sweating, diarrhea, nausea, and vomiting [17–21], and a study focusing on patients with primary Sjögren’s syndrome reported a high rate of treatment discontinuation owing to these side effects [22]. Moreover, oral cholinergic agonists cannot be administered to patients with peptic ulcer disease or asthma and may cause cardiovascular compromise [23, 24]. Therefore, topical formulations that promote saliva secretion while minimizing systemic side effects have been investigated. Mouthwashes [25–27], lozenges [28, 29], mouth sprays [30, 31], and oral patches [32] of pilocarpine, constituting topical formulations administered through the oral mucosa, have been studied; however, they are limited by their short duration of action and because they cause discomfort in the oral cavity. Moreover, their safety has not yet been sufficiently investigated.
Previously, we demonstrated that applying scopolamine ointment, a transdermal preparation for drooling, to the skin over the salivary glands of rats resulted in its topical delivery to the salivary glands without passing through the vascular system and sustainably suppressed salivary secretions [33]. Therefore, based on this observation, we hypothesized that the application of pilocarpine to the skin over the salivary glands could be a treatment option for hyposalivation with fewer systemic side effects.
In this study, we aimed to evaluate the effect of transdermal administration of pilocarpine to the skin over the salivary glands on salivary secretion, its safety, and drug distribution properties in salivary glands in rats.
Materials and methods
Experimental animals
Adult male Sprague–Dawley rats (250–400 g) were obtained from Japan SLC (Hamamatsu, Shizuoka, Japan). The rats were housed in the institutional animal facility in a controlled environment (temperature 25 ± 1 °C and 12 h light/dark cycle) with access to solid feed and water ad libitum and acclimatized to the study environment for at least one week. Animals were maintained, and experiments were performed following the Guidelines for the Care and Use of Laboratory Animals at Kanazawa University. This study is reported in accordance with the ARRIVE Statement for animal studies and was approved by the Committee on Animal Experimentation of Kanazawa University (Kanazawa, Japan; approval no. AP-224382). Adequate measures were taken to minimize pain and discomfort in the experimental animals. Rats were classified into four groups: application on the skin over submandibular and sublingual glands that were part of salivary glands (SSG); application on the skin on the back of the neck (SBN), i.e., the dorsal side of SSG; peroral administration (PO); and non-treated (NT). We used a total of 72 rats assigned to four groups (six rats per group) for each measurement (saliva volume, sweating, and defecation), and 54 rats assigned to three groups other than the NT group (six rats for each of the three collection points per group) for the measurement of drug concentration.
Preparation and administration of drugs
Pilocarpine hydrochloride was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan), and hydrophilic cream was purchased from Viatris Inc. (Tokyo, Japan). Pilocarpine concentration in the ointment was selected for maximum salivary volume, similar to oral administration, based on a preliminary experiment performed in a previous study on scopolamine ointments [33].
Rat body hair at the application site was carefully removed completely after applying a depilatory cream (Kracie, Ltd.; Tokyo, Japan). We applied 0.05 g of 10% pilocarpine ointment (total dose of 5 mg/rat) in the SSG and SBN groups [33]. In the PO group, pilocarpine hydrochloride was dissolved in 0.9% (w/v) NaCl and administered orally via a gastric tube at a concentration of 0.5 mg/kg body weight.
Measurements
Saliva volume was measured based on our previous study [33, 34] before pilocarpine application and at 0.5, 3, 6, 9, and 12 h after application in the NT, SSG, and SBN groups. In the PO group, measurements were performed before administration and at 0.5, 1, 2, and 3 h after administration.
The iodine-starch sweat test, a method used to assess sweating, was performed based on a previous study [35], and sweat was assessed before application and 1, 3, 6, 9, and 12 h after application of the pilocarpine ointment in all groups. At each point, the animals were anesthetized using an intraperitoneal injection of medetomidine hydrochloride (0.15 mg/kg body weight), midazolam (2.0 mg/kg body weight), and butorphanol (2.5 mg/kg body weight), and a 2% (w/v) iodine/ethanol solution was applied to the volar surface of the hind feet. After the surface was dried, a 1 g/mL starch/castor suspension was applied. Fine black dots appeared on the volar surface within 3–5 min. At 15 min after applying the starch/castor suspension, representative images were captured using an electronic camera when the black dots were stable, and no new dots appeared. The researcher counted the black dots on the hindfoot, representing the number of sweat dots.
Rats used for the measurement of defecation weight were acclimatized in metabolic cages for at least 48 h, and defecation was assessed before application and at 1, 2, 3, 6, 9, and 12 h after application in all groups. In addition to body weight at the start of the test, food intake, water intake, and urine volume were measured over the 12 h of the experiment, and fecal weight was measured at each point [36].
Blood and submandibular glands were collected at 0.5, 3, and 12 h following ointment application, and were pretreated as our previous study [33]. Drug concentration was determined using the triple quadrupole LC-MS-8050 (Shimadzu; Kyoto, Kyoto, Japan) coupled with an LC-30 A system (Shimadzu). Chromatography was performed on an InertSustain Amide (ID 2.1 mm × 50 mm; GL Sciences; Tokyo, Tokyo, Japan) at 40 °C using step-gradient elution (flow rate, 0.4 mL/min) as follows: 92% B to 2 min, 92 to 10% 2.5 min, 10% 4.5 min, 10 to 92% 5 min, and 92% 7 min (A, water containing 0.1% formic acid; B, 0.1% formic acid containing acetonitrile). D3-pilocarpine was used as the internal standard. The mass numbers of the molecular and product ions for each compound were as follows: pilocarpine (209.1 > 95.1, CE − 32 eV) and D3-pilocarpine (212.1 > 98.1, CE -32 eV). LabSolution LCMS Software version 5.89 (Shimadzu; Kyoto, Kyoto, Japan) was used for data manipulation. The limit of quantitation for each compound was 1 nM, and values below the limit of quantitation were excluded from the analysis. The tissue-to-plasma partition coefficients for the submandibular gland (Kp, SG) were determined using the following formula:
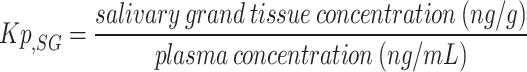 |
Statistical analysis
Data are presented as mean ± standard deviation. Statistical analyses were performed using one-way repeated-measures ANOVA with post-hoc Dunnett’s test, one-way ANOVA with post-hoc Tukey’s honest significant difference (HSD) test, and Student’s t-test. Differences were considered significant at p < 0.05. All statistical analyses were performed using IBM SPSS Statistics for Windows version 27 (IBM).
Results
Temporal variations in saliva volume after Pilocarpine Administration
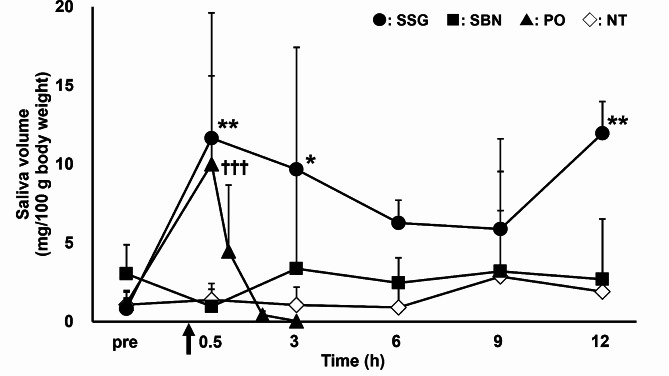
Figure 1 shows the changes in the saliva volume measured before and 0.5, 3, 6, 9, and 12 h after the application of pilocarpine ointment in the SSG and SBN groups and before and 0.5, 1, 2, and 3 h after oral administration of pilocarpine in the PO group. In the NT and SBN groups, the saliva volumes before and at any time point after application were not different. Saliva volume increased significantly at 0.5, 3, and 12 h after application compared to that before application in the SSG group. In the PO group, the saliva volume increased significantly only 0.5 h after administration compared to that before administration.
Fig. 1.
Time course of saliva volume before and after the topical or oral pilocarpine administration to rats. Circles, squares, triangles, and open rhombuses represent SSG, SBN, PO, and NT groups, respectively (n = 6 per group). Each point represents mean ± standard deviation. *p < 0.05, **p < 0.01, and ***p < 0.001 for each point versus before application in the SSG group. †p < 0.05, ††p < 0.01, and †††p < 0.001 for each point versus before administration in the PO group. NT, non-treated; PO, pilocarpine administered orally; SBN, pilocarpine ointment applied to the skin on the back of the neck; SSG, pilocarpine ointment applied to the skin over the salivary glands
Effect of pilocarpine administration on sweating
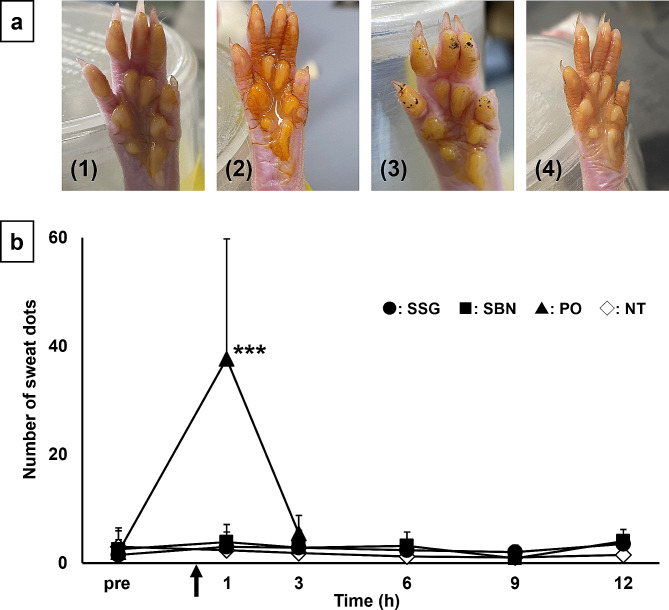
In the iodine-starch sweat test (Fig. 2), the number of sweat dots increased significantly 1 h after pilocarpine administration compared with that before administration in the PO group. In contrast, no change was observed in the number of sweat dots in SSG and SBN groups at any time point after pilocarpine application compared to that before application, similar to the NT group.
Fig. 2.
(a) Representative images of the iodine-starch sweat test on rat hind feet at 1 h after the topical and oral administration. Black dots represent sweating spots. a-1: SSG, a-2: SBN, a-3: PO, a-4: NT. (b) Time course of sweat dots before and after the topical and oral administration of pilocarpine in rats. Circles, squares, triangles, and open rhombuses represent SSG, SBN, PO, and NT groups, respectively (n = 6 per group). Each point represents the mean ± standard deviation. *p < 0.05, **p < 0.01, and ***p < 0.001 for each point versus before administration in the PO group. NT, non-treated; PO, pilocarpine administered orally; SBN, pilocarpine ointment applied to the skin on the back of neck; SSG, pilocarpine ointment applied to the skin over the salivary glands
Effect of pilocarpine administration on defecation
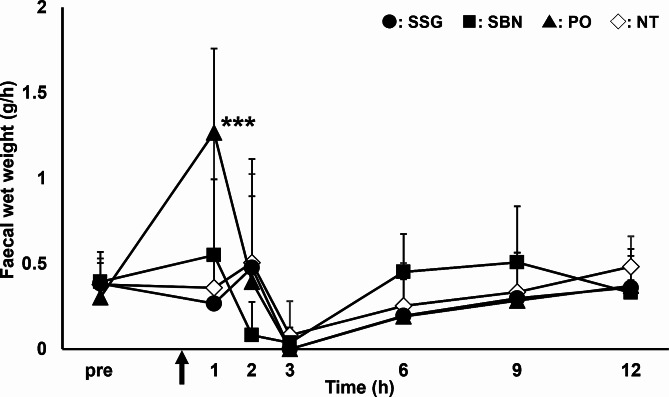
The changes in the fecal wet weight per hour are shown in Fig. 3. In the PO group, fecal weight increased significantly 1 h after pilocarpine administration compared to that before administration. All rats in the PO group had loose or muddy stools 1–2 h after pilocarpine administration. In contrast, in the SSG and SBN groups, no significant changes were observed in fecal weight after pilocarpine administration compared to that before administration at any time point and all stools were normal, similar to that in the NT group. There were no significant differences in body weight, water intake, food intake, or urine volume between the groups.
Fig. 3.
Time course of fecal weight before and after topical and oral pilocarpine administration to rats. Circles, squares, triangles, and open rhombuses represent SSG, SBN, PO, and NT groups, respectively (n = 6 per group). Each point represents mean ± standard deviation. *p < 0.05, **p < 0.01, and ***p < 0.001 for each point versus before administration in the PO group. NT, non-treated; PO, pilocarpine administered orally; SBN, pilocarpine ointment applied to the skin on the back of neck; SSG, pilocarpine ointment applied to the skin over the salivary glands
Drug concentration after pilocarpine administration
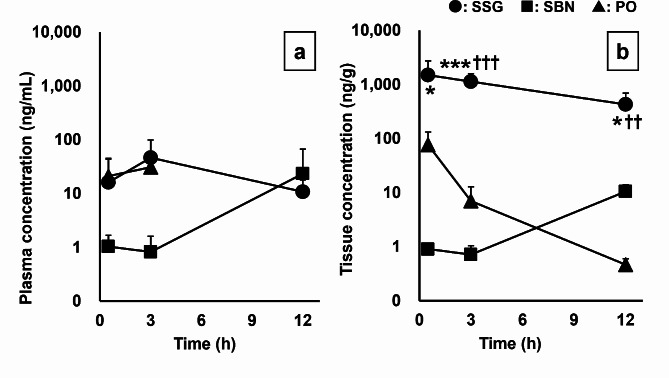
Figure 4 shows the changes in drug concentrations in the plasma (a) and submandibular gland tissue (b) after pilocarpine administration in SSG, SBN, and PO groups (SSG, SBN: 5 mg/body, PO: 0.5 mg/kg body weight). There was no significant difference in pilocarpine plasma concentrations in the PO, SSG, and SBN groups. The drug concentrations in all plasma samples at 12 h in the PO group were below the limit of quantitation. The pilocarpine concentration in submandibular gland tissues of the SSG group was significantly higher than that in the PO and SBN groups at all time points. The drug concentrations in more than half of the tissue samples at 3 and 12 h in the PO group were below the limit of quantitation. The Kp,SG values of the SSG group were significantly higher than those of the PO group at 3 h and the SBN group at 12 h (Table 1).
Fig. 4.
Time course of pilocarpine concentrations after topical application or oral administration of pilocarpine. (a) Pilocarpine concentrations in the plasma (SSG: n = 6 at each collection point. SBN: 0.5 h, n = 4; 3 h, n = 5; 12 h, n = 6. PO: 0.5 h, n = 6; 3 h, n = 6, excluding values below the limit of quantitation). (b) Pilocarpine concentrations in the submandibular gland tissue (SSG: n = 6 at each point. SBN: 0.5 h, n = 1; 3 h, n = 3; 12 h, n = 6. PO: 0.5 h, n = 6; 3 h, n = 6; 12 h, n = 2, excluding values below the limit of quantitation). Circles, squares, and triangles represent SSG, SBN, and PO groups, respectively. Each point indicates the mean ± standard deviation. *p < 0.05, **p < 0.01, and ***p < 0.001 for the SSG group versus the PO group. †p < 0.05 and ††p < 0.01, and †††p < 0.001 for the SSG group versus the SBN group. PO, pilocarpine administered orally; SBN, pilocarpine ointment applied to the skin on the back of the neck; SSG, pilocarpine ointment applied to the skin over the salivary glands
Table 1.
Tissue-to-plasma partition coefficients of pilocarpine in the rat salivary glands (Kp, SG) after pilocarpine ointment application
| 0.5 h | 3 h | 12 h | |
|---|---|---|---|
| PO | 7.5 ± 5.2 | 4.7 ± 9.9 | ― |
| SBN | ― | 1.9 ± 0.7 | 2.4 ± 1.9 |
| SSG | 690.6 ± 1072.5 | 60.0 ± 49.7* | 45.5 ± 28.5†† |
n = 2–6 (excluding values below the limit of quantitation). Data are shown as mean ± standard deviation. PO, orally administered pilocarpine; SBN, pilocarpine ointment applied to the skin on the back of the neck; SSG, pilocarpine ointment applied to the skin over the salivary glands. *p < 0.05 compared with the PO group, ††p < 0.01 compared with the SBN group
Discussion
Our findings indicated that applying pilocarpine to the skin over the salivary glands in rats increased saliva volume, and this effect lasted for at least 12 h. We showed that transdermal administration of pilocarpine caused fewer systemic side effects, such as sweating and diarrhea, than did oral administration.
An increase in pilocarpine ointment–induced saliva volume was not observed in the SBN group but only in the SSG group. This result suggests that the site of application influences the enhancing effect of pilocarpine on salivary secretion, similar to that observed with scopolamine ointment [33], and that the application of pilocarpine to the skin closer to the salivary glands is important to ensure effectiveness. Moreover, the increase in saliva volume in the SSG group after applying pilocarpine was comparable to that in the PO group and almost mirrored changes in saliva volume caused by oral topical preparations of pilocarpine in previous reports [32]. The effect of the pilocarpine ointment on salivary secretion in the SSG group began 30 min after application, similar to that in the PO group. This effect disappeared within 1 h in the PO group and lasted for 12 h in the SSG group. The duration of action of intraorally administered pilocarpine, such as sprays and oral mucosa patches, was previously reported to be approximately 1 h [25, 26, 32]; therefore, we believe that the effect of pilocarpine applied to the skin over the salivary glands can last longer than that of oral or intraoral administration. However, between the oral and transdermal routes of administration, the transdermal dose (10% pilocarpine in ointment) was considerably higher than the oral dose (0.5 mg/kg). Future studies should investigate dose-normalization between these routes to allow for more direct efficacy and safety comparisons.
A major problem with the treatment of hyposalivation using oral cholinergic drugs is the associated systemic side effects, such as sweating, diarrhea, and nausea. However, most studies on the administration of cholinergic drugs via the oral mucosa have focused only on saliva secretion, and systemic side effects have rarely been investigated in detail. Therefore, we investigated the effects of pilocarpine ointment on sweat and defecation to evaluate its systemic side effects. In the iodine-starch sweat test, the number of sweat dots increased 1 h after oral administration of pilocarpine, but no sweating was observed after the application of the pilocarpine ointment. Sweating is a side effect that can occur at any time while using oral cholinergic drugs for hyposalivation and is the most common cause for discontinuing treatment [22, 37]. This study indicates that the use of the pilocarpine ointment may not cause sweating. Although fecal weight increased 1 h after the oral administration of pilocarpine, it did not change after applying pilocarpine ointment. Thus, transdermal pilocarpine preparations are less likely to cause diarrhea as a side effect. Based on these findings, we believe that transdermal administration of pilocarpine to the skin over the salivary glands may reduce the treatment discontinuation rate owing to systemic side effects and lead to improved QOL in patients who require treatment for hyposalivation.
After application, we measured pilocarpine concentrations in the blood and submandibular gland to evaluate the effect of drug tissue distribution on the efficacy and safety of the pilocarpine ointment. The pilocarpine concentration in the submandibular gland tissues of the SSG group was significantly higher than that in the PO and SBN groups, and the Kp, SG values of the SSG group were extremely high. Thus, applying a pilocarpine ointment to the skin over the salivary glands led to higher concentrations of pilocarpine in the salivary glands than application at other sites or oral administration. Our results suggest that pilocarpine applied to the skin over the salivary glands reaches the salivary glands without passing through the vascular system. The pilocarpine concentration in the salivary gland tissues and the Kp, SG values in the SSG group remained high for 12 h after application. We believe that these results were related to the sustained increase in salivary volume. A previous study showed that the application of topical pilocarpine to the eyelid skin resulted in pupil constriction and high pilocarpine concentrations in the conjunctiva and eyeball that lasted for 8 h [38]. Therefore, a sustained increase in salivary volume observed up to 12 h after transdermal application of pilocarpine may be due to the slow release or the prolonged presence of pilocarpine in the salivary gland tissue or skin. However, the exact mechanisms underlying this extended effect require further investigation. In contrast, there were no statistically significant differences in the plasma concentrations of pilocarpine, which were associated with systemic side effects, among the three groups. Because the plasma concentration of pilocarpine in rats decreases relatively quickly, it is possible that we did not collect blood at the point when the plasma concentration was the highest [39]. Moreover, we also speculate that one of the reasons was the large variation in pilocarpine concentrations resulting from the use of data from different individuals at each measurement point. Future studies will require measuring drug concentrations over time for the same individuals.
Our study has some limitations that warrant further consideration. Our experiments were performed in rats but not in humans. The muscarinic receptor subtypes of the salivary glands are similar in rats and humans; however, the structures of the salivary glands differ. Skin permeability to drugs is higher in rat skin than human skin, and highly polar drugs have particularly high skin permeability in rats. Therefore, it is important to acknowledge that the findings from this rat model may not directly translate to human hyposalivation, given the differences in salivary gland anatomy and physiology between species. Because the distribution of eccrine sweat glands differs between humans and rats, the same results cannot be guaranteed in humans. Further studies in human subjects are necessary to validate the efficacy and safety of transdermal pilocarpine for hyposalivation management. Second, the focus was on the submandibular gland, neglecting the parotid gland’s significant contribution to stimulated saliva secretion in this study. A comprehensive evaluation of both major salivary glands may provide a more complete understanding of pilocarpine’s effects on salivary flow. Finally, a dose-response curve was not evaluated in this study, a limitation that should be addressed in future investigations.
Conclusions
In conclusion, the results of our in vivo study suggest that pilocarpine, when applied to the skin over the salivary glands of rats, resulted in a sustained increment of salivary secretion. Applying pilocarpine to the skin over the salivary glands effectively increases salivary secretion because pilocarpine is translocated into the salivary gland tissue. We hypothesized that transdermal administration of pilocarpine is less likely to cause systemic side effects than oral administration due to the relatively lower blood concentration of pilocarpine. Therefore, transdermal pilocarpine application to the skin over the salivary glands may be an effective and safe treatment option for hyposalivation, and further studies on human subjects are necessary.
Author contributions
N.I.: Conceptualization, writing the original draft, writing the review and editing, methodology, investigation, formal analysis, and funding acquisition. A.K.: Methodology, investigation, writing the original draft, formal analysis, and data curation. K.T.: Methodology and investigation. H.A.: Investigation, resources, and supervision. T.S.: Methodology and supervision. S.M.: Methodology. Y.S.: Methodology and supervision. Y.K.: Resources and supervision. H.N.: Methodology and supervision. Y.S.: Methodology and supervision. R.M.: Resources and supervision. All authors reviewed the manuscript.
Funding
This study was financially supported by JSPS KAKENHI (grant numbers JP20K16072 and JP23K06232).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Animals were maintained, and experiments were performed following the Guidelines for the Care and Use of Laboratory Animals at Kanazawa University. This study is reported in accordance with the ARRIVE Statement for animal studies and was approved by the Committee on Animal Experimentation of Kanazawa University (Kanazawa, Japan; approval no. AP-224382).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Melo JLMA, Coelho CPES, Nunes FPES, Heller D, Grisi DC, Guimarães MDCM, et al. A scoping review on hyposalivation associated with systemic conditions: the role of physical stimulation in the treatment approaches. BMC Oral Health. 2023;23:505. 10.1186/s12903-023-03192-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assy Z, Brand HS. A systematic review of the effects of acupuncture on xerostomia and hyposalivation. BMC Complement Altern Med. 2018;18:57. 10.1186/s12906-018-2124-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner MD. Hyposalivation and xerostomia: etiology, complications, and medical management. Dent Clin North Am. 2016;60:435–43. 10.1016/j.cden.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 4.Navazesh M, Kumar SK. Measuring salivary flow: challenges and opportunities. J Am Dent Assoc. 2008;139Suppl:s35–40. 10.14219/jada.archive.2008.0353 [DOI] [PubMed] [Google Scholar]
- 5.Epstein JB, Beier Jensen S. Management of hyposalivation and xerostomia: Criteria for treatment strategies. Compend Contin Educ Dent. 2015;36:600–3. [PubMed] [Google Scholar]
- 6.Kapourani A, Kontogiannopoulos KN, Manioudaki AE, Poulopoulos AK, Tsalikis L, Assimopoulou AN et al. A review on xerostomia and its various management strategies: the role of advanced polymeric materials in the treatment approaches. Polym (Basel) 2022;14. [DOI] [PMC free article] [PubMed]
- 7.Napeñas JJ, Brennan MT, Fox PC. Diagnosis and treatment of xerostomia (dry mouth). Odontology. 2009;97:76–83. 10.1007/s10266-008-0099-7 [DOI] [PubMed] [Google Scholar]
- 8.Soutome S, Yanamoto S, Nishii M, Kojima Y, Hasegawa T, Funahara M, et al. Risk factors for severe radiation-induced oral mucositis in patients with oral cancer. J Dent Sci. 2021;16:1241–6. 10.1016/j.jds.2021.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming M, Craigs CL, Bennett MI. Palliative care assessment of dry mouth: what matters most to patients with advanced disease? Support Care Cancer. 2020;28:1121–9. 10.1007/s00520-019-04908-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadig SD, Ashwathappa DT, Manjunath M, Krishna S, Annaji AG, Shivaprakash PK. A relationship between salivary flow rates and Candida counts in patients with xerostomia. J Oral Maxillofac Pathol. 2017;21:316. 10.4103/jomfp.JOMFP_231_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quilici D, Zech KN. Prevention and treatment options for medication-induced xerostomia. Gen Dent. 2019;67:52–7. [PubMed] [Google Scholar]
- 12.Salum FG, Medella-Junior FAC, Figueiredo MAZ, Cherubini K. Salivary hypofunction: an update on therapeutic strategies. Gerodontology. 2018;35:305–16. 10.1111/ger.12353 [DOI] [PubMed] [Google Scholar]
- 13.Seo K, Kim HN. Effects of oral health programmes on xerostomia in community-dwelling elderly: a systematic review and meta-analysis. Int J Dent Hyg. 2020;18:52–61. 10.1111/idh.12418 [DOI] [PubMed] [Google Scholar]
- 14.Berk L. Systemic pilocarpine for treatment of xerostomia. Expert Opin Drug Metab Toxicol. 2008;4:1333–40. 10.1517/17425255.4.10.1333 [DOI] [PubMed] [Google Scholar]
- 15.Riley P, Glenny AM, Hua F, Worthington HV. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database Syst Rev. 2017;7:CD012744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navazesh M, Ship II, Xerostomia. Diagnosis and treatment. Am J Otolaryngol. 1983;4:283–92. 10.1016/S0196-0709(83)80072-6 [DOI] [PubMed] [Google Scholar]
- 17.Atkinson JC, Grisius M, Massey W. Salivary hypofunction and xerostomia: diagnosis and treatment. Dent Clin North Am. 2005;49:309–26. 10.1016/j.cden.2004.10.002 [DOI] [PubMed] [Google Scholar]
- 18.Braga MA, Tarzia O, Bergamaschi CC, Santos FA, Andrade ED, Groppo FC. Comparison of the effects of pilocarpine and cevimeline on salivary flow. Int J Dent Hyg. 2009;7:126–30. 10.1111/j.1601-5037.2008.00326.x [DOI] [PubMed] [Google Scholar]
- 19.Casson RJ. Medical therapy for glaucoma: a review. Clin Exp Ophthalmol. 2022;50:198–212. 10.1111/ceo.13989 [DOI] [PubMed] [Google Scholar]
- 20.Heiskanen V, Zadik Y, Elad S. Photobiomodulation therapy for cancer treatment-related salivary gland dysfunction: a systematic review. Photobiomodul Photomed Laser Surg. 2020;38:340–7. [DOI] [PubMed] [Google Scholar]
- 21.Porter SR, Scully C, Hegarty AM. An update of the etiology and management of xerostomia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:28–46. 10.1016/j.tripleo.2003.07.010 [DOI] [PubMed] [Google Scholar]
- 22.Noaiseh G, Baker JF, Vivino FB. Comparison of the discontinuation rates and side-effect profiles of pilocarpine and cevimeline for xerostomia in primary Sjögren’s syndrome. Clin Exp Rheumatol. 2014;32:575–7. [PubMed] [Google Scholar]
- 23.Barbe AG. Medication-induced xerostomia and hyposalivation in the elderly: culprits, complications, and management. Drugs Aging. 2018;35:877–85. 10.1007/s40266-018-0588-5 [DOI] [PubMed] [Google Scholar]
- 24.Pakala RS, Brown KN, Preuss CV. Cholinergic medications. In: StatPearls edn. Treasure Island (FL): StatPearls Publishingcopyright ©2024. Pearls Publishing LLC. 2024. [PubMed]
- 25.Bernardi R, Perin C, Becker FL, Ramos GZ, Gheno GZ, Lopes LR, et al. Effect of pilocarpine mouthwash on salivary flow. Braz J Med Biol Res. 2002;35:105–10. 10.1590/S0100-879X2002000100015 [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Ahn HJ, Choi JH, Jung DW, Kwon JS. Effect of 0.1% pilocarpine mouthwash on xerostomia: Double-blind, randomised controlled trial. J Oral Rehabil. 2014;41:226–35. 10.1111/joor.12127 [DOI] [PubMed] [Google Scholar]
- 27.Motamed B, Alaee A, Azizi A, Jahandar H, Fard MJK, Jafari A. Comparison of the 1 and 2% pilocarpine mouthwash in a xerostomic population: a randomized clinical trial. BMC Oral Health. 2022;22:548. 10.1186/s12903-022-02576-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamlar DD, Schuller DE, Gahbauer RA, Buerki RA, Staubus AE, Hall J, et al. Determination of the efficacy of topical oral pilocarpine for postirradiation xerostomia in patients with head and neck carcinoma. Laryngoscope. 1996;106:972–6. 10.1097/00005537-199608000-00011 [DOI] [PubMed] [Google Scholar]
- 29.Taweechaisupapong S, Pesee M, Aromdee C, Laopaiboon M, Khunkitti W. Efficacy of pilocarpine lozenge for post-radiation xerostomia in patients with head and neck cancer. Aust Dent J. 2006;51:333–7. 10.1111/j.1834-7819.2006.tb00453.x [DOI] [PubMed] [Google Scholar]
- 30.Frydrych AM, Davies GR, Slack-Smith LM, Heywood J. An investigation into the use of pilocarpine as a sialagogue in patients with radiation induced xerostomia. Aust Dent J. 2002;47:249–53. 10.1111/j.1834-7819.2002.tb00337.x [DOI] [PubMed] [Google Scholar]
- 31.Pereira RMS, Bastos MDR, Ferreira MP, de Freitas O, de Macedo LD, de Oliveira HF, et al. Topical pilocarpine for xerostomia in patients with head and neck cancer treated with radiotherapy. Oral Dis. 2020;26:1209–18. 10.1111/odi.13343 [DOI] [PubMed] [Google Scholar]
- 32.Tanaka A, Nakano H, Yoneto K, Yoneto C, Furubayashi T, Suzuki K, et al. Topical xerostomia treatment with hyaluronate sheets containing pilocarpine. Biol Pharm Bull. 2022;45:403–8. 10.1248/bpb.b21-00763 [DOI] [PubMed] [Google Scholar]
- 33.Ishida N, Oshima Y, Katsura A, Imamura R, Arakawa H, Shimada T, et al. Application site of Transdermal scopolamine influences efficacy and drug concentration in salivary glands in rats. Biol Pharm Bull. 2023;46:1805–9. 10.1248/bpb.b23-00561 [DOI] [PubMed] [Google Scholar]
- 34.Bagavant H, Trzeciak M, Papinska J, Biswas I, Dunkleberger ML, Sosnowska A et al. A method for the measurement of salivary gland function in mice. J Vis Exp 2018;(131). [DOI] [PMC free article] [PubMed]
- 35.Chen Z, Zhao J, Yan Y, Zhang L, Du L, Liu X, et al. Differential distribution and genetic determination of eccrine sweat glands and hair follicles in the volar skin of C57BL/6 mice and SD rats. BMC Vet Res. 2022;18:316. 10.1186/s12917-022-03416-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narita Y, Fukumoto K, Fukunaga M, Kondo Y, Ishitsuka Y, Jono H, et al. Comparative study of Constipation Exacerbation by Potassium Binders using a Loperamide-Induced Constipation Model. Int J Mol Sci. 2020;21:2491. 10.3390/ijms21072491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng CQ, Xu H, Liu L, Wang RN, Liu YT, Li J, et al. Efficacy and safety of pilocarpine for radiation-induced xerostomia in patients with head and neck cancer: a systematic review and meta-analysis. J Am Dent Assoc. 2016;147:236–43. 10.1016/j.adaj.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 38.See GL, Sagesaka A, Todo H, Wierzba K, Sugibayashi K. Pharmacokinetics and tissue distribution of pilocarpine after application to eyelid skin of rats. J Pharm Sci. 2019;108:2942–8. 10.1016/j.xphs.2019.04.012 [DOI] [PubMed] [Google Scholar]
- 39.Omori Y, Endo T, Hara Y, Nishiyama M, Midgley I, Smart CI, et al. Absorption, distribution and excretion of 14C-pilocarpine following oral administration to rats. Arzneimittelforschung. 2004;54:171–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.