Abstract
Background
A recent Phase 2/3 study in Japanese patients showed that caplacizumab was effective in treating immune-mediated thrombotic thrombocytopenic purpura (iTTP), with a low rate of iTTP recurrence. ADAMTS13 activity is monitored weekly during caplacizumab treatment to guide discontinuation of caplacizumab and consequently avoid exacerbations or relapse. The aim of this study was to assess changes in ADAMTS13 activity/inhibitor levels during caplacizumab treatment in this patient population.
Methods
A post hoc analysis of the Phase 2/3 study in Japanese patients was conducted. Patients ≥ 18 years old with confirmed iTTP received 10 mg of caplacizumab daily in conjunction with therapeutic plasma exchange (TPE) and immunosuppression for 30 days post-TPE. Outcomes included time to recovery of ADAMTS13 activity, ADAMTS13 activity level at treatment end, incidence of ADAMTS13 inhibitor re-elevation (ie, inhibitor boosting) during treatment, time to platelet count recovery, number of days of TPE, and safety. Outcomes according to presence of inhibitor boosting were also assessed.
Results
Nineteen patients had confirmed iTTP and were included in this analysis. Median (95% confidence interval) time to recovery of ADAMTS13 activity to ≥ 10%, ≥ 20%, and ≥ 60% was 14.6 (5.9–24.8), 18.5 (5.9–31.8), and 47.5 (18.5–60.9) days, respectively. Median (range) ADAMTS13 activity level at caplacizumab treatment end was 62.0% (29.0–101.0). Nine patients had ADAMTS13 inhibitor boosting. Delayed response of ADAMTS13 activity was observed in patients with inhibitor boosting. The median time to platelet count response and median number of TPE days were shorter in patients with inhibitor boosting compared with patients without inhibitor boosting. Rituximab was administered to almost all patients with inhibitor boosting (88.9%), after completion of TPE. Patients without inhibitor boosting who were treated with rituximab received it prior to completion of TPE. Only one patient experienced a recurrence, which occurred shortly after caplacizumab discontinuation due to an adverse event.
Conclusions
In patients with iTTP, caplacizumab with TPE and immunosuppression may reduce the risk of ADAMTS13 inhibitor boosting if rituximab is administered early in the iTTP treatment period. Early administration of rituximab in addition to caplacizumab may prevent iTTP recurrence with inhibitor boosting.
Trial registration
Keywords: Caplacizumab, ADAMTS13 inhibitors, ADAMTS13 activity, Immune-mediated thrombotic thrombocytopenic purpura, TTP
Background
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is a rare, life-threatening disorder characterized by the formation of blood clots in small blood vessels, leading to their occlusion and consequent blood flow restriction to vital organs [1]. Possible outcomes of this occlusion include microangiopathic hemolytic anemia and severe thrombocytopenia [2]. iTTP is caused by an autoantibody-mediated severe deficiency of a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13 (ADAMTS13), leading to the accumulation of ultra-large von Willebrand Factor (VWF) multimers, which bind platelets and form microvascular thrombi [1].
Until recently, the standard treatment regimen for iTTP was therapeutic plasma exchange (TPE) and immunosuppressive therapy (corticosteroids and rituximab) [3]. Caplacizumab was developed to target the A1 domain of VWF, which prevents the binding of platelets to VWF and thus reduces the risk of microvascular thrombosis [1, 3–6]. International Society on Thrombosis and Haemostasis 2020 guidance recommends caplacizumab to be added to TPE and immunosuppressive therapy in patients with iTTP [3]. Evidence from the TITAN and HERCULES clinical trials, along with real-world studies from Germany, France, Spain, and the UK indicate that the addition of caplacizumab treatment is associated with rapid recovery of platelets [4, 7–10]. Caplacizumab was approved for use in Japan in 2022, based on the primary result of the Japan Phase 2/3 study, which demonstrated that caplacizumab was effective in Japanese patients with iTTP, with a low rate of iTTP recurrence (i.e. recurrent thrombocytopenia after initial recovery if platelet count ≥ 150 × 109/L with stop of daily TPE requiring re-initiation of daily TPE) [11].
Careful monitoring of ADAMTS13 activity is required to avoid exacerbations (i.e. platelet count < 150 × 109/L following a clinical response, within 30 days of stopping TPE) or relapses (i.e. platelet count < 150 × 109/L following clinical remission, > 30 days after stopping TPE) due to early discontinuation of caplacizumab [4, 7, 10, 12]. Re-elevation of ADAMTS13 inhibitor levels (ie, inhibitor boosting) after completion of TPE may lead to a decrease in ADAMTS13 activity and subsequent recurrence of TTP [13]. A previous study in 52 Japanese patients with iTTP found that poor response to TPE was associated with ADAMTS13 inhibitor boosting [13]. Changes in ADAMTS13 activity/inhibitor levels are routinely captured while caplacizumab treatment is ongoing. Therefore, the aim of this post hoc analysis of the Japan Phase 2/3 study was to assess changes in ADAMTS13 activity and inhibitors over time during caplacizumab treatment in patients with iTTP.
Methods
Study population
This was a post hoc analysis of the Phase 2/3, prospective, single-arm, open-label study (NCT04074187) conducted at 15 centers in Japan between October 2019 and May 2021 (registration date 14th August 2019 in ClinicalTrials.gov) [11, 14]. Japanese patients (≥ 18 years old) with a clinical diagnosis of iTTP (initial or recurrent; confirmed ADAMTS13 level < 10%) who required initiation of daily TPE and had received ≤ 1 prior TPE were included. Exclusion criteria included platelet count ≥ 100,000/μL, serum creatinine level > 2.3 mg/dL (only in cases where platelet count was > 30,000/μL, so as to exclude atypical hemolytic uremic syndrome), known other causes of thrombocytopenia and known chronic treatment with anticoagulant treatment that could not be stopped.
Study design and treatment
Details of the study design have been previously described [11]. Briefly, after confirmation of eligibility, patients received 10 mg caplacizumab once daily (first dose administered intravenously at least 15 min prior to TPE and subsequent doses administered subcutaneously after TPE) with TPE and immunosuppression for a period of variable duration. Patients received 10 mg caplacizumab once daily (subcutaneously) for ≥ 30 days post-TPE, and were then followed for 4 weeks after treatment end. Patients with persistent ADAMTS13 deficiency < 10% or other symptoms of underlying disease activity were allowed to undergo treatment extension for up to 8 weeks.
Outcomes and assessments
Outcomes of this post hoc analysis included patient characteristics, time to platelet count recovery, time to normalization of all 3 organ damage markers (lactate dehydrogenase, cardiac troponin I, and serum creatinine), safety, time to recovery of ADAMTS13 activity, ADAMTS13 activity at end of treatment, and incidence of ADAMTS13 inhibitor boosting during treatment. Outcomes according to presence of inhibitor boosting were also assessed. The definition of very severe disease at baseline was French severity score ≥ 3, or severe neurological involvement (eg, coma, seizures, focal deficit), or cardiac involvement (ie, cardiac troponin level > 2.5 × upper limit of normal [ULN]). French severity score is defined as: Cerebral involvement: yes = 1; no = 0; Lactate dehydrogenase: > 10 × ULN = 1; ≤ 10 × ULN = 0; Age: > 60 years = 2; > 40– ≤ 60 years = 1; ≤ 40 years = 0 [15]. ADAMTS13 activity was assessed by enzyme immunoassay and ADAMTS13 inhibitors were assessed by the Bethesda assay [16]. ADAMTS13 activity and inhibitor titer levels were both evaluated on Day 1 of the daily TPE period, then weekly until the end of the study, and at the first and last follow-up visits. In this manuscript, inhibitor boosting was defined by inhibitor levels decreasing but then re-elevating (inhibitor titer ≥ 1 BU/mL), regardless of degree of decrease. The modified intention-to-treat (mITT) and safety population were defined as all patients who received at least one administration of caplacizumab.
Statistical analyses
No formal statistical testing was conducted due to the post hoc nature of the analysis. Data are presented descriptively.
Results
Patient disposition and baseline characteristics
Twenty-one patients were enrolled and treated with ≥ 1 dose of caplacizumab (mITT and safety population). Overall, 19 patients had confirmed iTTP and were included in these analyses (less severe iTTP, n = 8; very severe iTTP, n = 11). Median (range) age was 59.0 (22–86) years and median (range) platelet count at baseline was 23.0 (8–78) × 109/L (Table 1). The number of patients experiencing an initial TTP episode was 14 (73.7%), while 5 (26.3%) patients were experiencing a subsequent TTP episode. The median (range) of ADAMTS13 inhibitor was 1.70 (0.5–8.3) BU/mL. Seven patients had treatment extension (4 had very severe iTTP and 3 had less severe iTTP). Four patients discontinued caplacizumab treatment due to physician decision and/or adverse events, and 15 patients completed the study. At baseline, 7/19 patients had severe anemia (i.e. hemoglobin levels ≤ 80 g/L). Overall, 16/19 patients had hemoglobin data available at Weeks 4–5 after starting treatment; of these, all displayed hemoglobin values ≥ 80 g/L (range: 115–147 g/L).
Table 1.
Patient baseline characteristics
| Overall (n = 19) | |
|---|---|
| Gender, n (%) | |
| Male | 9 (47.4) |
| Female | 10 (52.6) |
| Age (years), median (range) | 59.0 (22–86) |
| Previous TTP episode, n (%) | |
| No | 14 (73.7) |
| Yes | 5 (26.3) |
| Disease severity, n (%) | |
| Very severe | 11 (57.9) |
| Less severe | 8 (42.1) |
| Platelet count (109/L), median (range) | 23.0 (8–78) |
| Serum creatinine (µmol/L), median (range) | 70.5 (49–226) |
| Lactate dehydrogenase (U/L), median (range) | 575.0 (227–1794) |
| Cardiac troponin level (µg/L), median (range) | 0.12 (0.03–7.47) |
| ADAMTS13 activity, n (%) | |
| < 10% | 18 (94.7) |
| ≥ 10% | 1 (5.3) |
| ADAMTS13 inhibitor (BU/mL), median (range) | 1.70 (0.5–8.3) |
ADAMTS13, a disintegrin and metalloproteinase with thrombospondin type 1 motif member 13; iTTP, immune-mediated thrombotic thrombocytopenic purpura
Changes to ADAMTS13 inhibitor levels
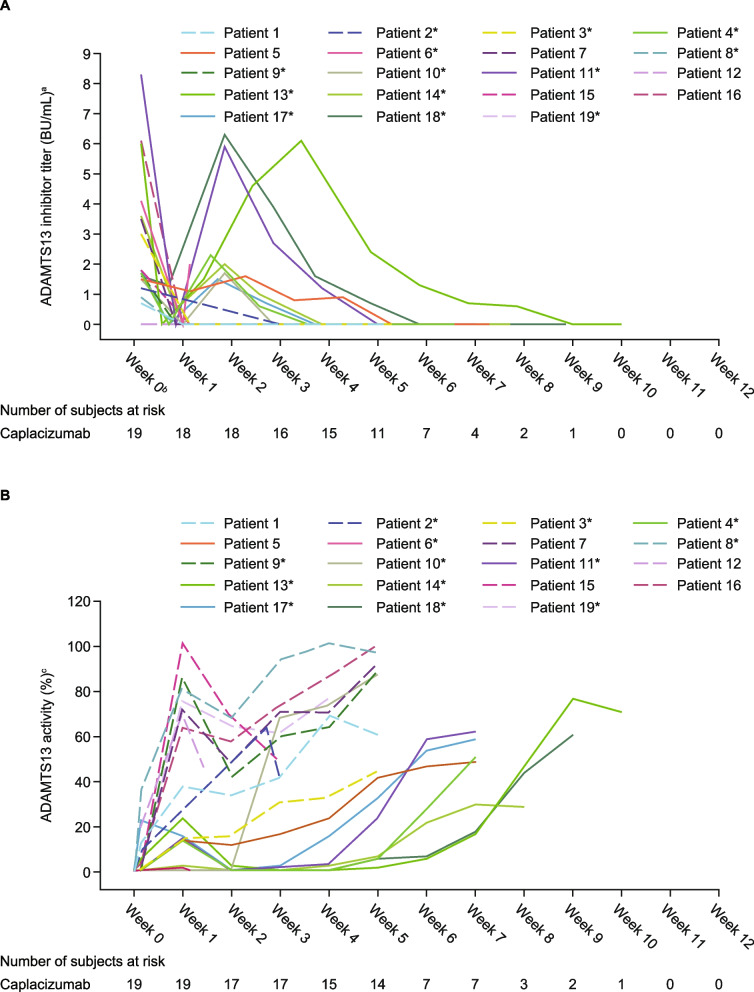
ADAMTS13 inhibitors decreased in all patients in Week 1 after the start of treatment, but inhibitor boosting was observed in 9 patients around Week 2 (Fig. 1A). Among 9 patients with inhibitor boosting, 7 patients extended caplacizumab treatment. One patient with inhibitor boosting experienced a recurrence 2 days after discontinuing caplacizumab due to an adverse event. No other patients experienced a recurrence.
Fig. 1.
ADAMTS13 (A) inhibitor levels by patient (B) activity levels by patient. Figure 1A reprinted from Blood 2023; 142(S1): 5428; Kazunori Imada et al., Post Hoc Analysis of a Phase 2/3 Study in Japanese Patients with iTTP Treated with Caplacizumab. Copyright (2023), with permission from Elsevier. aADAMTS13 inhibitor titer data points recorded as < 0.5 were plotted as 0 for all patients. bThe highest value of ADAMTS13 inhibitor titer at admission, as measured by a local laboratory if available and ADAMTS13 inhibitor titer at baseline was used. cADAMTS13 activity data points < 0.5 were plotted as 0.5 for Patient 6. ADAMTS13 activity data points recorded as < 1 were plotted as 1 for Patients 3–7, 9–11, and 13–18. Solid line: Patients with ADAMTS13 inhibitor boosting. The ‘*’ symbol indicates patients who received rituximab. Patients 2, 12, 15, 19 discontinued the study before completing. ADAMTS13, a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13; mITT, modified intention-to-treat
Time to ADAMTS13 activity recovery
Median (95% confidence interval [CI]) time to recovery of ADAMTS13 activity to ≥ 10%, ≥ 20%, and ≥ 60% was 14.6 (5.9–24.8), 18.5 (5.9–31.8), and 47.5 (18.5–60.9) days, respectively (Table 2). Median time to recovery of ADAMTS13 activity was longer in patients who received rituximab versus patients who did not receive rituximab (≥ 10%: 24.8 vs 6.3 days; ≥ 20%: 31.8 vs 6.4 days; ≥ 60%: 49.8 vs 23.9 days) and in patients experiencing an initial TTP episode versus patients experiencing a subsequent TTP episode (≥ 10%: 18.5 vs 6.9 days; ≥ 20%: 28.8 vs 13.7 days; ≥ 60%: 47.5 vs 18.6 days). Patients with ADAMTS13 inhibitor levels ≥ 2 BU/mL at baseline (n = 7) took approximately twice as long to recover ADAMTS13 activity to ≥ 10%, ≥ 20%, and ≥ 60% versus patients with baseline inhibitor levels < 2 BU/mL (≥ 10%: 33.5 vs 14.1 days; ≥ 20%: 33.5 vs 16.1 days; ≥ 60%: 54.7 vs 23.2 days). Baseline severity, platelet count or time since TTP diagnosis did not appear to influence ADAMTS13 recovery (Table 2), but presence of inhibitor boosting did have an impact (discussed in inhibitor subanalysis section). Mean ADAMTS13 activity levels increased from 11.9% at baseline to 39.4% at the end of Week 1 post-TPE. Levels decreased to 28.2% at Week 2 post-TPE and then increased to a maximum of 49.5% by the end of Week 5 post-TPE for the patients without inhibitor boosting, reflecting resolution of the underlying disease (Fig. 1B). Median (range) ADAMTS13 activity level at caplacizumab treatment end was 62.0% (29.0–101.0), and no recurrence was reported during the follow-up period.
Table 2.
Median (95% CI) time to ADAMTS13 activity ≥ 10%, ≥ 20%, and ≥ 60% overall and according to different clinical characteristics
| Overall/Clinical characteristic | Number of patients | Time to sustained ADAMTS13 activity ≥ 10%, median (95% CI), days | Time to sustained ADAMTS13 activity ≥ 20%, median (95% CI), days | Time to sustained ADAMTS13 activity ≥ 60%, median (95% CI), days |
|---|---|---|---|---|
| Overall | 19 | 14.6 (5.9–24.8) | 18.5 (5.9–31.8) | 47.5 (18.5–60.9) |
| Concomitant rituximab | ||||
| No rituximab used | 6 | 6.3 (4.7–NC) | 6.4 (4.7–NC) | 23.9 (6.9–NC) |
| Rituximab used | 13 | 24.8 (6.9–38.8) | 31.8 (13.7–38.8) | 49.8 (13.7–61.9) |
| Baseline ADAMTS13 inhibitor | ||||
| < 2 BU/mL | 12 | 14.1 (4.5–24.8) | 16.1 (4.5–31.8) | 23.2 (6.9–51.8) |
| ≥ 2 BU/mL | 7 | 33.5 (4.7–NC) | 33.5 (4.7–NC) | 54.7 (18.8–NC) |
| Time since iTTP diagnosis | ||||
| ≤ 5 days | 13 | 18.5 (5.9–37.8) | 28.8 (5.9–37.8) | 37.7 (17.6–61.9) |
| > 5 days | 6 | 10.3 (4.4–NC) | 14.1 (4.4–NC) | 39.8 (4.5–NC) |
| Disease severity at baseline | ||||
| Less severe | 8 | 10.8 (4.7–46.9) | 17.7 (4.7–53.9) | 60.9 (6.9–NC) |
| Very severe | 11 | 17.6 (4.5–24.8) | 18.5 (4.5–31.8) | 19.9 (13.7–49.8) |
| Platelet count at baseline | ||||
| < 20*109/L | 9 | 18.5 (5.9–37.8) | 28.8 (5.9–37.8) | 33.7 (6.9–NC) |
| ≥ 20*109/L | 9 | 13.7 (4.5–46.9) | 14.6 (4.5–53.9) | 49.8 (4.5–61.9) |
| Inhibitor boosting present | ||||
| No | 10 | 6.4 (4.4–13.7) | 6.4 (4.4–14.6) | 18.7 (4.5–27.8) |
| Yes | 9 | 35.7 (6.7–46.9) | 35.7 (18.5–53.9) | 56.4 (18.5–NC) |
| Previous iTTP episode | ||||
| No | 14 | 18.5 (5.9–37.8) | 28.8 (5.9–37.8) | 47.5 (18.5–60.9) |
| Yes | 5 | 6.9 (4.4–NC) | 13.7 (4.4–NC) | 18.6 (4.5–NC) |
ADAMTS13, a disintegrin and metalloproteinase with thrombospondin type 1 motif member 13; CI, confidence interval; iTTP, immune-mediated thrombotic thrombocytopenic purpura; NC, not calculated
Inhibitor boosting subanalysis
Patients with inhibitor boosting (n = 9) were generally younger than those without inhibitor boosting (n = 10; median (range) age 50.0 [23–84] years vs 66.5 [22–86] years) and had a lower median platelet count at baseline (12.0 [8–65] × 109/L vs 31.0 [9–78] × 109/L). ADAMTS13 inhibitor levels at baseline and incidence of very severe/less severe disease were similar between patients with/without inhibitor boosting (Table 3). Patients with inhibitor boosting had a delayed ADAMTS13 activity response versus patients who did not have inhibitor boosting (≥ 10%: 35.7 vs 6.4 days respectively; ≥ 20%: 35.7 vs 6.4 days respectively; ≥ 60%: 56.4 vs 18.7 days respectively; Table 2 and Fig. 1B). Median (95% CI) time to platelet count response was 2.42 (0.88–3.59) days in patients with inhibitor boosting and 3.98 (1.69–not calculated) days in patients without inhibitor boosting (Table 3). The median (range) number of days of TPE was 5.0 (3–11) and 6.5 (5–20) in patients with inhibitor boosting and without inhibitor boosting, respectively. Rituximab was used in 8/9 (88.9%) patients with inhibitor boosting and 5/10 (50.0%) patients without inhibitor boosting. Of the 8 patients with inhibitor boosting who received rituximab, 7 received it after completion of TPE. The remaining patient received rituximab treatment on the day of TPE completion; this patient had discontinued caplacizumab due to an adverse event and experienced a recurrence 2 days later. Of the 5 patients without inhibitor boosting who received rituximab, 4 (80%) received it before completion of TPE and 1 (20%) received it 2 days after completion of TPE. The median first study day of rituximab use was Day 18.5 in patients with inhibitor boosting and Day 4.0 in patients without inhibitor boosting. Two (10.5%) patients experienced recurrence, ≥ 1 major thromboembolic event, or TTP-related death. Two patients in each inhibitor boosting subgroup had ≥ 1 treatment-emergent serious adverse event.
Table 3.
Baseline characteristics and efficacy outcomes by inhibitor boosting
|
Inhibitor boosting (n= 9) |
No inhibitor boosting (n= 10) |
|
|---|---|---|
| Gender, n (%) | ||
| Male | 3 (33.3) | 6 (60) |
| Female | 6 (66.7) | 4 (40) |
| Age (years), median (range) | 50.0 (23–84) | 66.5 (22–86) |
| Previous TTP episode, n (%) | ||
| No | 9 (100) | 5 (50) |
| Yes | 0 | 5 (50) |
| Disease severity at baseline, n (%) | ||
| Very severe | 6 (66.7) | 5 (50) |
| Less severe | 3 (33.3) | 5 (50) |
| Platelet count (109/L) at baseline, median (range) | 12.0 (8–65) | 31.0 (9–78) |
| Serum creatine (µmol/L) at baseline, median (range) | 67.0 (64–226) | 87.0 (49–162) |
| Lactate dehydrogenase (U/L) at baseline, median (range) | 575.0 (227–1794) | 452.5 (234–947) |
| Cardiac troponin level (µg/L) at baseline, median (range) | 0.310 (0.03– 3.52) | 0.055 (0.03–7.47) |
| ADAMTS13 inhibitor (BU/mL) at baseline, median (range) | 1.80 (1.5–8.3) | 1.45 (0.5–6.1) |
| Time to platelet count response (days), median (95% CI) | 2.42 (0.88–3.59) | 3.98 (1.69–NC) |
| Time to normalization of all 3 organ damage marker levels (days), median (95% CI) | 4.48 (0.87–11.40) | 3.20 (0.79–4.98) |
| Number of days of TPE, median (range) | 5.0 (3–11) | 6.5 (5–20) |
| Use of concomitant rituximab, n (%) | 8 (88.9) | 5 (50.0) |
| Before completion of TPE | 0 | 4 (40.0) |
| Day of TPE completion | 1 (11.1)a | 0 |
| After completion of TPE | 7 (77.8) | 1 (10.0) |
| Time to normalization of serum creatine (days), median (95% CI) | 1.83 (0.49–NC) | 1.92 (0.98–NC) |
| Time to normalization of cardiac troponin I level (days), median (95% CI) | 6.31 ((1.49–NC) | 3.06 (1.92–NC) |
| Time to normalization of lactate dehydrogenase (days), median (95% CI) | 2.54 (0.87–NC) | 0.98 (0.46–4.98) |
CI Confidence interval, iTTP Immune-mediated thrombotic thrombocytopenic purpura, NC Not calculated, TPE Therapeutic plasma exchange, TTP Thrombotic thrombocytopenic purpura
aOne patient experienced a recurrence, 2 days after discontinuing caplacizumab due to an adverse event
Discussion
The Phase 2/3 NCT04074187 study was the first to investigate caplacizumab treatment in Japanese patients with iTTP. Our post hoc analysis of that study shows that ADAMTS13 activity and inhibitor levels were correlated with patients’ backgrounds during iTTP treatment using caplacizumab. These results suggest that early rituximab treatment during caplacizumab alongside TPE and immunosuppression treatment may reduce the risk of recurrence due to inhibitor boosting.
In this analysis, almost 50% of patients with confirmed iTTP (n = 9) experienced ADAMTS13 inhibitor boosting; of these, only 1 patient experienced a recurrence, 2 days after discontinuing caplacizumab due to an adverse event. Rituximab was administered to the majority of patients with inhibitor boosting, but in all such cases rituximab was administered after TPE was completed. In addition, patients with inhibitor boosting had earlier platelet recovery and fewer days of TPE compared with patients without inhibitor boosting. It is possible that TPE was completed earlier because of earlier platelet count recovery. None of the patients who received rituximab before completion of TPE experienced inhibitor boosting, suggesting that inhibitor boosting may be avoided if rituximab are administered early in the treatment period. Disease severity and ADAMTS13 inhibitor level at baseline did not appear to influence inhibitor boosting. Median time to recovery of ADAMTS13 activity to ≥ 10, ≥ 20, and ≥ 60% in this study was 14.6 (5.9–24.8), 18.5 (5.9–31.8), and 47.5 (18.5–60.9) days, respectively, which seems to be more rapid compared to previously reported real-world evidence data (ADAMTS13 activity > 30%: 31 days; ADAMTS13 ≥ 20%: 28 days) [8, 17].
Factors which may impact on ADAMTS13 activity recovery time include inhibitor titer at baseline; patients with inhibitor titer ≥ 2 BU/mL at baseline took longer to recover ADAMTS13 activity, and ADAMTS13 activity was shown to increase more slowly in patients with ADAMTS13 inhibitor boosting. Patients in the rituximab subgroup also took longer to achieve sustained ADAMTS13 activity (at any level), but it should be noted that administration of immunosuppressant agents was at the physician’s discretion. Therefore, rituximab may have only been administered after the physician noticed that ADAMTS13 recovery was slow or that the therapeutic effect was poor, as the TTP indication for rituximab in Japan is limited for relapsed/refractory disease [18]. Caplacizumab treatment may result in a rapid platelet recovery, thus delaying the administration of rituximab. Frontline use of rituximab and caplacizumab may prevent recurrence of iTTP and ADAMTS13 inhibitor boosting. Caplacizumab inhibits thrombus formation by preventing the binding of platelets to VWF [1], while rituximab acts as an immunosuppressant by targeting the CD20 protein marker on B cells, causing their depletion [19] and thus inhibiting ADAMTS13 inhibitor production [20, 21]. ISTH guidance recommends that patients with ADAMTS13 activity < 10% of normal receive rituximab as early as possible in order to target ADAMTS13 antibodies [22]. Limitations of the study include the small population size and that there was no control group; the authors anticipate that a further analysis with a larger sample size and more time points will be required in order to fully understand the impacts of caplacizumab and rituximab in this patient population.
Conclusion
In conclusion, these results suggest that mean time to recover ADAMTS13 activity may be more rapid than previously reported, and that time to recovery of ADAMTS13 activity is influenced by factors such as rituximab use, previous iTTP history and ADAMTS13 inhibitor level at baseline. ADAMTS13 inhibitor boosting may also be associated with longer time to recover ADAMTS13 activity. Caplacizumab in conjunction with TPE and immunosuppression may reduce the risk of inhibitor boosting if rituximab is administered early in the iTTP treatment period; therefore, these results support the use of caplacizumab in the iTTP patient population.
Acknowledgements
Medical writing support was provided by Sarah Meadows, MRes, and Hanna Mourad-Agha, PhD, CMPP, of Fishawack Communications Ltd., part of Avalere Health, and was funded by Sanofi.
Abbreviations
- ADAMTS13
Autoantibody-mediated severe deficiency of a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13
- CI
Confidence interval
- iTTP
Immune-mediated thrombotic thrombocytopenic purpura
- mITT
Modified intention-to-treat
- TPE
Therapeutic plasma exchange
- ULN
Upper limit of normal
- VWF
Von Willebrand factor
Authors’ contributions
All authors participated in data interpretation and manuscript writing/review for this work. All authors approved the submitted version.
Funding
The study was funded by Sanofi.
Availability of data and materials
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/.
Declarations
Ethics approval and consent to participate
The protocol and other relevant documents were approved by an Institutional Review Board before the study was initiated. The study was conducted in accordance with the protocol and ethical principles derived from international guidelines including the Declaration of Helsinki, and the International Conference on Harmonization Good Clinical Practice Guidelines, as well as any applicable laws, rules, and regulations. Participants or their legally authorized representative provided written informed consent.
Consent for publication
Not applicable.
Competing interests
MN, YH, and MT are employees of Sanofi and may hold shares and/or stock options in the company. ST was an employee of Sanofi at the time of this study. KI reports honoraria from Kyowa Hakko Kirin Co., Ltd., Bristol-Myers Squibb K.K., Takeda Pharmaceutical Co. Ltd., Novartis Pharma K.K, Nippon Shinyaku Co., Ltd., Chugai Pharmaceutical Co., Ltd., Otuka Pharmaceutical Co. Ltd., Astellas Pharma Inc., Meiji Seika Pharma Co. Ltd., Ono Pharmaceutical Co. Ltd., Janssen Pharmaceutical K.K., Towa Pharmaceutical Co., Ltd., AstraZeneca K.K., Nippon Kayaku Co., Ltd., Alexion Pharmaceuticals, Inc., AbbVie GK., Sanofi K.K., and Amgen K.K. YM reports consultancy from Zenyaku Kogyo, Argenx, Sanofi, and Pfizer; and research funding from Argenx, Sanofi, and Pfizer. SI and HU have no disclosures. YU reports honoraria from Sanofi, AbbVie, and Novo Nordisk; and speakers bureau from Sanofi. MM reports consultancy from Takeda, Sanofi, Alexion Pharma; research funding from Sanofi, Alexion Pharma, Chugai Pharmaceutical, and Asahikasei Pharma; and speakers bureau from Sanofi, Takeda, Alexion Pharma, and Asahikasei Pharma.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sukumar S, Lämmle B, Cataland SR. Thrombotic thrombocytopenic purpura: pathophysiology, diagnosis, and management. J Clin Med. 2021;10(3): 536. 10.3390/jcm10030536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kremer Hovinga JA, Coppo P, Lämmle B, Moake JL, Miyata T, Vanhoorelbeke K. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers. 2017;3:17020. 10.1038/nrdp.2017.20 [DOI] [PubMed] [Google Scholar]
- 3.Zheng XL, Vesely SK, Cataland SR, Coppo P, Geldziler B, Iorio A, Matsumoto M, Mustafa RA, Pai M, Rock G, Russell L, Tarawneh R, Valdes J, Peyvandi F. ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18(10):2496–502. 10.1111/jth.15010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scully M, Cataland SR, Peyvandi F, Coppo P, Knöbl P, Kremer Hovinga JA, Metjian A, de la Rubia J, Pavenski K, Callewaert F, Biswas D, De Winter H, Zeldin RK. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380(4):335–46. 10.1056/NEJMoa1806311 [DOI] [PubMed] [Google Scholar]
- 5.Cablivi (caplacizumab-yhdp). Summary of product characteristics. Ablynx NV; 2022.
- 6.Cablivi. Prescribing information. Ablynx NV; 2022.
- 7.Völker LA, Kaufeld J, Miesbach W, Brähler S, Reinhardt M, Kühne L, Mühlfeld A, Schreiber A, Gaedeke J, Tölle M, Jabs WJ, Özcan F, Markau S, Girndt M, Bauer F, Westhoff TH, Felten H, Hausberg M, Brand M, Gerth J, Bieringer M, Bommer M, Zschiedrich S, Schneider J, Elitok S, Gawlik A, Gäckler A, Kribben A, Schwenger V, Schoenermarck U, Roeder M, Radermacher J, Bramstedt J, Morgner A, Herbst R, Harth A, Potthoff SA, von Auer C, Wendt R, Christ H, Brinkkoetter PT, Menne J. Real-world data confirm the effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura. Blood Adv. 2020;4(13):3085–92. 10.1182/bloodadvances.2020001973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppo P, Bubenheim M, Azoulay E, Galicier L, Malot S, Bigé N, Poullin P, Provôt F, Martis N, Presne C, Moranne O, Benainous R, Dossier A, Seguin A, Hié M, Wynckel A, Delmas Y, Augusto JF, Perez P, Rieu V, Barbet C, Lhote F, Ulrich M, Rumpler AC, de Witte S, Krummel T, Veyradier A, Benhamou Y. A regimen with caplacizumab, immunosuppression, and plasma exchange prevents unfavorable outcomes in immune-mediated TTP. Blood. 2021;137(6):733–42. 10.1182/blood.2020008021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutt T, Shaw RJ, Stubbs M, Yong J, Bailiff B, Cranfield T, Crowley MP, Desborough M, Eyre TA, Gooding R, Grainger J, Hanley J, Haughton J, Hermans J, Hill Q, Humphrey L, Lowe G, Lyall H, Mohsin M, Nicolson PLR, Priddee N, Rampotas A, Rayment R, Rhodes S, Taylor A, Thomas W, Tomkins O, Van Veen JJ, Lane S, Toh CH, Scully M. Real-world experience with caplacizumab in the management of acute TTP. Blood. 2021;137(13):1731–40. 10.1182/blood.2020007599 [DOI] [PubMed] [Google Scholar]
- 10.Peyvandi F, Scully M, Kremer Hovinga JA, Cataland S, Knöbl P, Wu H, Artoni A, Westwood JP, Mansouri Taleghani M, Jilma B, Callewaert F, Ulrichts H, Duby C, Tersago D. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374(6):511–22. 10.1056/NEJMoa1505533 [DOI] [PubMed] [Google Scholar]
- 11.Miyakawa Y, Imada K, Ichikawa S, Uchiyama H, Ueda Y, Yonezawa A, Fujitani S, Ogawa Y, Matsushita T, Asakura H, Nishio K, Suzuki K, Hashimoto Y, Murakami H, Tahara S, Tanaka T, Matsumoto M. The efficacy and safety of caplacizumab in Japanese patients with immune-mediated thrombotic thrombocytopenic purpura: an open-label phase 2/3 study. Int J Hematol. 2023;117(3):366–77. 10.1007/s12185-022-03495-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuker A, Cataland SR, Coppo P, de la Rubia J, Friedman KD, George JN, Knoebl PN, Kremer Hovinga JA, Lämmle B, Matsumoto M, Pavenski K, Peyvandi F, Sakai K, Sarode R, Thomas MR, Tomiyama Y, Veyradier A, Westwood JP, Scully M. Redefining outcomes in immune TTP: an international working group consensus report. Blood. 2021;137(14):1855–61. 10.1182/blood.2020009150 [DOI] [PubMed] [Google Scholar]
- 13.Isonishi A, Bennett CL, Plaimauer B, Scheiflinger F, Matsumoto M, Fujimura Y. Poor responder to plasma exchange therapy in acquired thrombotic thrombocytopenic purpura is associated with ADAMTS13 inhibitor boosting: visualization of an ADAMTS13 inhibitor complex and its proteolytic clearance from plasma. Transfusion. 2015;55(10):2321–30. 10.1111/trf.13182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.A trial of caplacizumab in Japanese patients with acquired thrombotic thrombocytopenic purpura (aTTP). ClinicalTrials.gov ID NCT04074187. Available at: https://www.clinicaltrials.gov/study/NCT04074187. Accessed May 2024.
- 15.Benhamou Y, Assié C, Boelle PY, Buffet M, Grillberger R, Malot S, Wynckel A, Presne C, Choukroun G, Poullin P, Provôt F, Gruson D, Hamidou M, Bordessoule D, Pourrat J, Mira JP, Le Guern V, Pouteil-Noble C, Daubin C, Vanhille P, Rondeau E, Palcoux JB, Mousson C, Vigneau C, Bonmarchand G, Guidet B, Galicier L, Azoulay E, Rottensteiner H, Veyradier A, Coppo P. Development and validation of a predictive model for death in acquired severe ADAMTS13 deficiency-associated idiopathic thrombotic thrombocytopenic purpura: the French TMA Reference Center experience. Haematologica. 2012;97(8):1181–6. 10.3324/haematol.2011.049676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato S, Matsumoto M, Matsuyama T, Isonishi A, Hiura H, Fujimura Y. Novel monoclonal antibody-based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Transfusion. 2006;46(8):1444–52. 10.1111/j.1537-2995.2006.00914.x [DOI] [PubMed] [Google Scholar]
- 17.Prasannan N, Thomas M, Stubbs M, Westwood JP, de Groot R, Singh D, Scully M. Delayed normalization of ADAMTS13 activity in acute thrombotic thrombocytopenic purpura in the caplacizumab era. Blood. 2023;141(18):2206–13. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto M, Miyakawa Y, Kokame K, Ueda Y, Wada H, Higasa S, Yagi H, Ogawa Y, Sakai K, Miyata T, Morishita E, Fujimura Y. Diagnostic and treatment guidelines for thrombotic thrombocytopenic purpura (TTP) in Japan 2023. Int J Hematol. 2023;118(5):529–46. 10.1007/s12185-023-03657-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanif N, Anwer F. Rituximab. In: StatPearls. 2024. Treasure Island (FL) ineligible companies. Disclosure: Faiz Anwer declares no relevant financial relationships with ineligible companies.
- 20.Sargentini-Maier ML, De Decker P, Tersteeg C, Canvin J, Callewaert F, De Winter H. Clinical pharmacology of caplacizumab for the treatment of patients with acquired thrombotic thrombocytopenic purpura. Expert Rev Clin Pharmacol. 2019;12(6):537–45. 10.1080/17512433.2019.1607293 [DOI] [PubMed] [Google Scholar]
- 21.Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836–46. 10.1182/blood-2016-10-709857 [DOI] [PubMed] [Google Scholar]
- 22.Zheng XL, Vesely SK, Cataland SR, Coppo P, Geldziler B, Iorio A, Matsumoto M, Mustafa RA, Pai M, Rock G, Russell L, Tarawneh R, Valdes J, Peyvandi F. ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18(10):2486–95. 10.1111/jth.15006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/.