Abstract
Introduction
The venous thromboembolism (VTE) risk screening forms were developed to allow for recording identified risk factors for VTE including thrombophilia, history of VTE, postpartum hemorrhage, and cesarean delivery, and documentation of specific actions taken to mitigate these risks and reduce complications due to VTE.
Methods
Compliance with hospital guidelines in assessing VTE risk and appropriate prescribing of thromboprophylaxis was evaluated prior to the introduction of VTE risk screening forms (March 2022). Efficacy of the new VTE risk screening forms was also assessed (April 2023). Patient discharge summaries and patient medical records including medication charts were used to review the documentation of VTE risk assessments and details of thromboprophylaxis prescribing.
Results
Of 74 postnatal patients, 37.8% had VTE risk assessment documented prior to the introduction of VTE risk screening forms. Of 37 patients identified to be at moderate to high risk of VTE requiring pharmacological prophylaxis, 70.3% (n = 26) were appropriately prescribed pharmacological prophylaxis. After the risk screening forms were introduced, a total of 67 antenatal, postnatal, and gynecologic patients were studied. Of these, 32.8% (n = 22) of patients had all required fields completed appropriately. When using the forms, 26.9% (n = 7) of postnatal and 88% (n = 22) of gynecological patients were rated as medium or high risk, and all received medical review within 24 hours. Pharmacological prophylaxis was indicated in 88% (n = 22) of gynecological, 43.8% (n = 7) of antenatal, and 38.5% (n = 10) of postnatal patients, and all were appropriately prescribed.
Conclusion
The guideline review and introduction of VTE risk screening forms was valuable to provide guidance in the risk assessment for VTE and to identify patients requiring prophylaxis.
Keywords: VTE prophylaxis, medication safety, pharmacological prophylaxis, pregnancy, women’s health
INTRODUCTION
Venous thromboembolism (VTE), which includes deep vein thrombosis and pulmonary embolism, is a major cause of morbidity and mortality and is associated with approximately 7% of all deaths in Australian hospitals.[1] Pregnancy is a major risk factor for VTE, with pregnant women up to 10 times more likely to develop VTE compared with the general population.[2–5] Other risk factors for pregnancy-related VTE include hereditary or acquired thrombophilia, personal or family history of VTE, superficial venous thrombosis, obesity, postpartum hemorrhage, and assisted reproduction.[6] Cesarean delivery, especially emergency cesarean delivery, increases the risk for postnatal women.[5,6] Thromboprophylaxis with low molecular weight heparin (LMWH) is the anticoagulant of choice for pregnant women, as LMWH does not cross the placenta and thus, presents a low risk to the developing fetus.[6] It has also been proven effective in many postoperative settings and has been adopted for use in the period after cesarean delivery.[5] Therefore it is important to perform VTE risk assessment in obstetrics patients and initiate prophylaxis management for high-risk patients.[5]
The Australian Commission on Safety and Quality in Health Care (ACSQHC) provides clinical care standards indicators to assess compliance with the recommendations of VTE prophylaxis for patients admitted to Australian hospitals.[7] The indicators include assessing (1) proportion of patients admitted to hospital assessed for VTE risk within 24 hours of admission, (2) proportion of patients prescribed appropriate VTE prophylaxis, and (3) proportion of patients separated from hospital on VTE prophylaxis with a care plan documenting prescribed medicine(s), dosage, and duration of treatment.[7]
In the study hospital, the lack of a formal process to ensure VTE screening was performed and documented consistently has resulted in difficulties in carrying out audits on VTE risk screening. The screening was documented inconsistently in different sections in the patient medical records, including nursing/midwifery care plan, hospital medication chart, and surgical planning documents. This was reflected in the poor compliance at the study hospital in the national hospital medication chart audit with regard to VTE risk assessment documentation.[8,9] Following discussions at the hospital Clinical Governance Committee, the need to review the VTE assessment process was identified to ensure consistent practice for all patients. A working party led by the Safety, Quality, and Performance Directorate, consisting of multidisciplinary team members, was formed to improve the VTE prophylaxis clinical practice and documentation.
The VTE guideline[10] was updated to supersede previous VTE guidelines and to align with the ACSQHC and other national standards.[5–7] The clinical guideline is currently used across two hospital sites within Women and Newborn Health Services in Western Australia. The VTE Risk Screening forms were developed to allow for the recording of risks identified and documentation of specific actions taken to minimize these risks. Risk Screening forms were compiled following extensive benchmarking with national and international standards and practices, including the risk assessment for VTE checklist by the Royal College of Obstetricians and Gynaecologists.[11] Every woman’s VTE risk status is to be assessed by a nurse, midwife or medical officer at the preadmission clinic, antenatal booking visit, or on admission to the hospital as per the updated hospital guideline.[10] Risk factors identified are documented, and if screening indicates moderate or high risk of VTE, the medical officer completes the bleeding risk and outcome section of the form. The recommended prophylaxis according to the woman’s risk status is to be determined by the medical officer assessing the contraindications to both pharmacological and mechanical prophylaxis before prescribing prophylaxis.[10]
The objectives of the study were (1) to evaluate compliance of VTE risk screening, thromboprophylaxis prescribing, and documentation of risk assessment prior to the introduction of the new risk screening forms; and (2) evaluate efficacy of the new forms in the management of VTE prophylaxis.
METHODS
Human Research Ethics approval was obtained from the Women and Newborn Health Service Quality Improvement Committee. Informed consent was waived as the quality improvement activity was determined to be an internal, health delivery-focused, negligible-risk project. Standards for quality improvement reporting excellence (SQUIRE) guidelines were followed in reporting and publishing the findings.[12]
Following baseline assessment of VTE risk screening, three VTE risk screening paper forms (one for gynecology, one for antenatal obstetrics, and one for postnatal obstetrics) were implemented across two hospital sites within Women and Newborn Health Services to improve screening and prescribing practices for obstetrics and gynecology patients. An education package for the newly implemented forms were prepared by a clinical practice improvement coordinator and pharmacist and disseminated to clinicians in the hospital by email and in person few weeks before implementation of the form.
Study Setting
The primary study location was King Edward Memorial Hospital (KEMH), which is a 300-bed (including 100 neonatal cots) hospital and the only tertiary maternity and gynecological center in Western Australia. More than 6000 births take place annually, and it is the only major referral center in the state for high-risk pregnancies. The hospital also provides services to approximately 5000 women with gynecological conditions each year, including malignant and nonmalignant urological problems, sexually transmitted diseases, and reproductive disorders.
The Women and Newborn Service in Osborne Park Hospital, Western Australia was also included in the evaluation of the efficacy of the VTE risk screening forms. This site provides convenience for women with low- to moderate-risk pregnancies while receiving equally specialized care at a small, suburban site. This secondary site shares the same clinical guideline[10] with the primary study site and was included in the implementation of VTE risk screening forms.
Baseline Data Collection and Analysis
For data collection before the use of VTE risk screening forms, the audit involved a 1-week retrospective study of consecutive postnatal inpatients discharged from KEMH. A list of inpatients discharged between March 14 and March 20, 2022 was obtained from Stork discharge summaries, a clinical perinatal database used by the state’s public health maternity services. Two clinical pharmacists were involved in data collection. Compliance with the hospital guideline including VTE risk assessment, prescribing of prophylaxis, and management on discharge were assessed. For patients without risk assessment documented, the auditors evaluated the VTE risk independently using information obtained from discharge summaries and patient medical records. No audit training was conducted; the auditors were experienced clinical pharmacists who were familiar with hospital medical record or discharge summaries. Medication charts were reviewed to assess the VTE pharmacological thromboprophylaxis prescribing. Hospital discharge summaries were examined for patient counseling and prescription documented on discharge. A data collection tool, created using Microsoft Excel, was used to record relevant data such as documentation of VTE risk assessments and details of thromboprophylaxis prescribing. Data collected were analyzed using Microsoft Excel.
Intervention Data Collection and Analysis
Data collection commenced at least 6 months after introduction of the forms to allow time for hospital-wide communication, education, and change in practice. Data were collected between April 1st and 30th, 2023 using random sampling of patients receiving obstetrics care (antenatal and postnatal) and gynecology care at either hospital. The study sample consisted of 25 antenatal, 25 postnatal, and 25 gynecology patients across the two hospital sites. One clinical practice improvement coordinator was involved in the data collection. Medical records including medication charts, medical notes, and VTE risk screening forms were audited to evaluate use of the screening tool and the management of VTE prophylaxis. The forms require the four sections to be completed for all patients, as appropriate: risk rating, referral and documentation, assessment of bleeding risk, and outcome. Risks for VTE were classified as low, medium, or high by the clinician completing the screening process. The referral and documentation sections outline the appropriate VTE prophylaxis options and duration for the clinician to consider. Medication charts were reviewed to assess the VTE pharmacological thromboprophylaxis prescribing. Hospital discharge summaries were examined for patient counselling and prescription documented on discharge. The data collected were also recorded and analyzed using Microsoft Excel.
RESULTS
Baseline Assessment
Before the VTE risk screening form was implemented, compliance with the VTE guideline was deemed satisfactory if the prescriber had documented VTE risk assessment, prescribed mechanical prophylaxis (i.e., graduated compression stocking) on the hospital medication chart, appropriately prescribed pharmacological prophylaxis at the correct dose and duration, and documented thromboprophylaxis plan in the patient’s discharge summary.
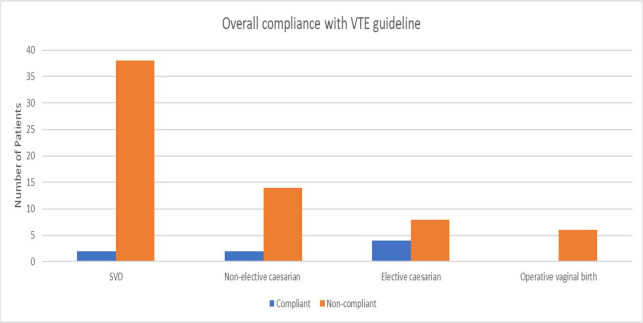
A total of 114 postnatal patients were discharged from KEMH between March 14 and March 20, 2022. Medical records were obtained, and data were collected for 74 of these patients, the remaining patients were unable to be followed up due to difficulties obtaining paper medical records. Of these 74 patients, only 10.8% (n = 8) were deemed compliant with the hospital’s VTE prophylaxis guideline. Among those who were deemed noncompliant, 57.6% (n = 38) were spontaneous vaginal deliveries, 21.2% (n = 14) were nonelective lower uterine segment cesarean deliveries, 12.1% (n = 8) were elective lower uterine segment cesarean deliveries, and 9.1% (n = 6) were operative vaginal births. Figure 1 illustrates compliance with VTE guidelines by birth type.
Figure 1.
Compliance with VTE guideline prior to introduction of VTE Risk Screening forms. SVD, spontaneous vaginal deliveries; VTE, venous thromboembolism.
Risk assessment
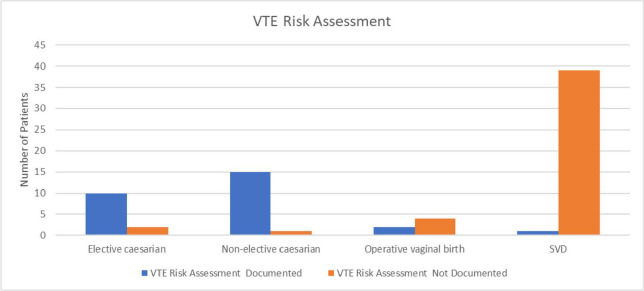
The ACSQHC recommends measuring the proportion of patients assessed within 24 hours of admission.[7] However, for the purposes of the audit, it was not deemed feasible as the lack of documentation in most patients’ medical records did not allow the time and date of assessment to be determined. It was noted that 37.8% (n = 28) of patients had an assessment of VTE risk documented in either their progress notes, operation report, or on the medication chart. The remaining had no documentation and therefore it was unknown if they were assessed for VTE risk at admission. Figure 2 represents risk assessments for different modes of birth.
Figure 2.
Documentation of VTE risk assessment before introduction of VTE Risk Screening forms. SVD, spontaneous vaginal deliveries; VTE, venous thromboembolism.
VTE prophylaxis
A total of 37 patients (50.0%) were identified to be at moderate to high risk of VTE and therefore required pharmacological prophylaxis. Of these patients, 70.3% (n = 26) were appropriately prescribed pharmacological prophylaxis. Figure 3 shows the proportion of patients who were clinically indicated for mechanical or pharmacological prophylaxis versus those who were prescribed mechanical or pharmacological prophylaxis. The reasons for pharmacological prophylaxis omission were not documented for the remaining patients who were clinically indicated but did not receive pharmacological prophylaxis.
Figure 3.
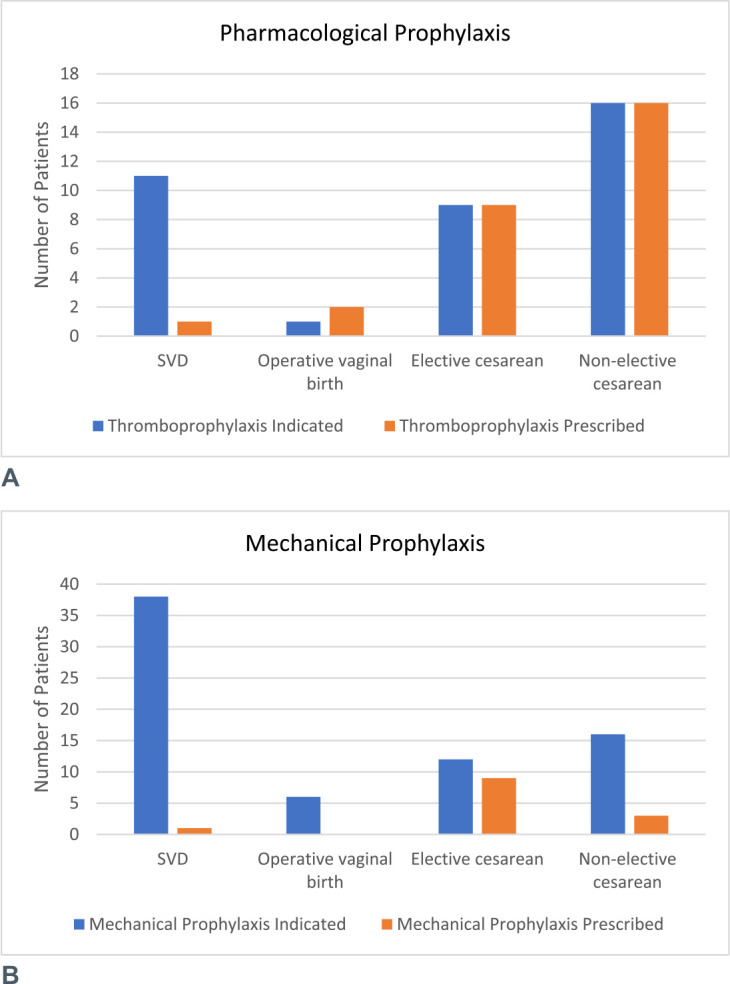
Pharmacological (A) and mechanical (B) prophylaxis before introduction of VTE Risk Screening forms. SVD, spontaneous vaginal deliveries; VTE, venous thromboembolism.
Discharge
Continuity of care is essential when patients are discharged from hospital with a high-risk medication such as enoxaparin.[4,5] The proportion of patients discharged with a care plan documenting prescribed medicine(s) for VTE prophylaxis, dose, and duration of treatment was assessed. During the audit period, 39.2% (n = 29) were prescribed enoxaparin to reduce the risk of VTE on discharge. Of the patients who were prescribed pharmacological VTE prophylaxis, 62.1% (n = 18) were discharged from hospital with the appropriate documentation in their medical discharge summary.
Improvement With VTE Risk Screening Forms
A total of 67 patients, comprised of 16 antenatal, 26 postnatal, and 25 gynecological patients from both sites were included in the study (Table 1).
Table 1.
Breakdown of the medical records audited across two study sites
| Area | Number of Records |
||
|---|---|---|---|
| Gynecological | Antenatal | Postnatal | |
| Primary study site | 25 | 5 | 13 |
| Secondary study site | 0 | 11 | 13 |
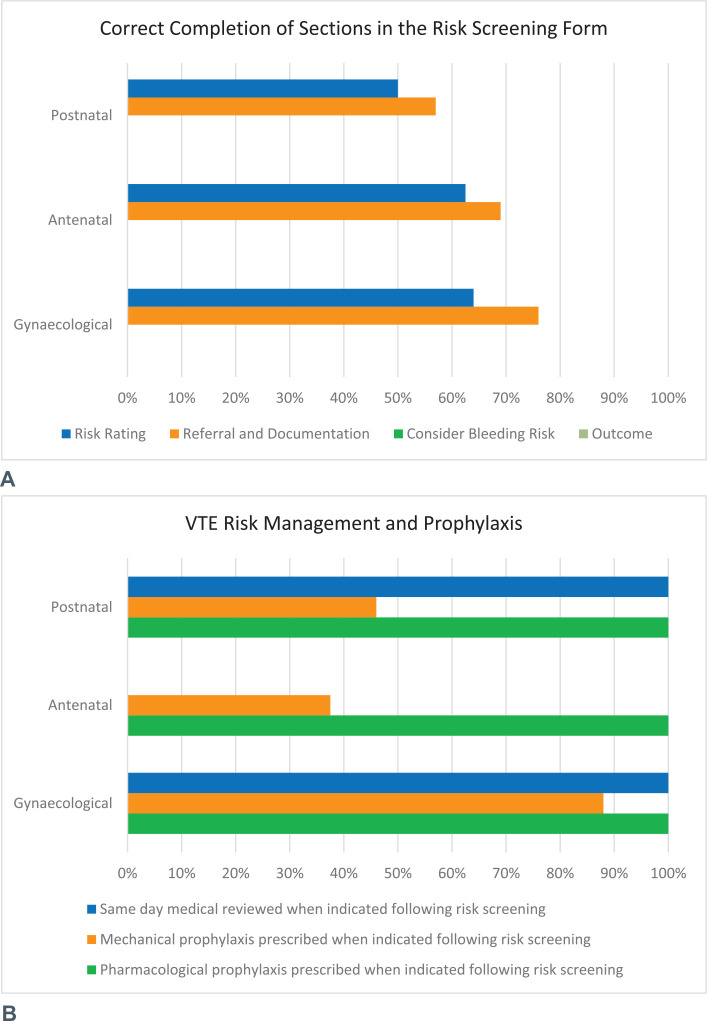
All patients who were audited had VTE risk screening forms documented and filed in the medical record. The forms were completed appropriately for 32.8% (n = 22) patients, including 62% (n = 10) of antenatal, 46% (n = 12) of postnatal, and 0% of gynecological patients had all the required fields of the risk screening forms completed appropriately.
Section 1 of the form requires risk screening and referrals. Section 2 requires management and prophylaxis duration, if indicated. Both of these sections should be completed for all patients. Sections 3 and 4 require assessment of bleeding risk and outcomes to be completed by medical staff only if the patient is deemed as moderate or high risk, as these sections outline the appropriate VTE prophylaxis options and duration for the clinician to consider. None of the audited forms had both sections 3 and 4 completed. Although these sections were not required for low-risk patients, these sections were also not completed for patients who were deemed as moderate or high risk. Figure 4 provides an overview of compliance with correctly completing each section of the form.
Figure 4.
Compliance of VTE prophylaxis (A) after the introduction of VTE Risk Screening forms (B). VTE, venous thromboembolism.
Risk Assessment
Patients who were classified as medium or high risk for VTE require a medical review within 24 hours. A total of 26.9% (n = 7) postnatal and 88% (n = 22) gynecological patients were rated as medium and high risk following the risk screening, and all received medical review within 24 hours. No antenatal cases audited required a medical review within 24 hours from a VTE prophylaxis perspective.
VTE Prophylaxis
Mechanical VTE prophylaxis (graduated compression stocking) was indicated for all patients audited in the study, and it was correctly documented and initiated for 60% (n = 40) of all patients (88% [n = 22] of gynecological, 37.5% [n = 6] of antenatal, and 46% [n = 12] of postnatal patients). Pharmacological prophylaxis (enoxaparin) was also indicated for 58% (n = 39) of all patients (88% [n = 22] of gynecological, 43.8% [n = 7] of antenatal, and 38.5% [n = 10] of postnatal patients) following risk screening, and all were appropriately documented and prescribed as per the hospital VTE clinical guideline.
Discharge
There were 12 patients total (17.9%) (16.7% [n = 2] postnatal and 83.3% [n = 10] gynecology) requiring VTE pharmacological prophylaxis on discharge. All of these patients were counseled and had the prescription documented in the relevant discharge summary. No antenatal cases audited required pharmacological VTE prophylaxis upon discharge.
DISCUSSION
The study assessed the impact of introducing VTE risk screening forms in the hospital on VTE risk assessment and documentation. Analysis of compliance with VTE prophylaxis guidelines (per ACSQHC) prior to use of the VTE Risk Screening form required significant effort and challenging for the auditors to find relevant information in the medical record. When screening did occur, it was documented inconsistently in different sections of the patient medical records including nursing/midwifery care plan, hospital medication chart, and surgical planning documents. The lack of a dedicated documentation tool for VTE risk assessment was associated with the lack of a formal process to ensure that screening was performed and documented consistently. As a result of missing or inconsistent documentation, some patients were not assessed within 24 hours of admission for VTE risk.
The designated screening form enables the risk factors for VTE to be assessed according to the hospital guidelines and documented consistently for clinicians. Following the introduction of the VTE risk screening form, all patients had the forms documented and filed in the patient medical record (versus only 10.8% compliance previously); however, only 32.8% (n = 22) of the forms were completed appropriately. Compliance with form completion, particularly section 3 (bleeding risk assessment) and section 4 (outcome), should be improved by additional education of staff members.
Before the risk assessment form introduction, 70.3% (26 of 37) of patients identified to be at moderate to high risk of VTE were appropriately prescribed pharmacological prophylaxis. Following risk assessment using VTE risk screening forms, pharmacological prophylaxis was indicated for 58.2% (39 of 67) of patients, and all were appropriately prescribed enoxaparin. This reflected that both the guideline and risk assessment form were useful for the identification of women requiring appropriate enoxaparin therapy for VTE prophylaxis. Education following the guideline update and implementation of the risk assessment form may have increased awareness of VTE assessment and appropriate anticoagulation therapies for patients.[12] This is consistent with a recent study on pharmacist interventions in KEMH,[13] which reported that the number of pharmacy interventions involving enoxaparin was higher prior to the implementation of VTE risk assessment forms.[13]
It is important for patients who require pharmacological VTE prophylaxis on discharge and their families to receive counseling on its use, and documentation of this requirement is to be recorded in the relevant discharge summary to ensure appropriate transition of care. Of the patients who were prescribed pharmacological VTE prophylaxis on discharge, appropriate documentation in the patients’ medical discharge summary was observed.
The introduction of risk assessment forms encouraged consistent performance and documentation of VTE risk assessment for obstetrics and gynecology patients, improved the documentation of risk assessment and prescribing of risk-appropriate VTE prophylaxis, thereby improving the management of VTE prophylaxis.
Limitations
Limitations of the study include the variability that lies within individual clinicians in the assessment and documentation of risk factors with the lack of a dedicated documentation tool for the assessment before the implementation of the VTE risk assessment form. Another limitation of the study is that the compliance of VTE management before and after the introduction of the risk assessment forms cannot be directly compared because of different audit criteria and methodology used. The difference in sample size and patient mix also introduces limitation and bias. Finally, the small sample size of the audit for each patient category of antenatal, postnatal, and gynecology limits the generalization on the effectiveness of the VTE risk assessment form implementation.
CONCLUSION
The multifaceted strategies including the guideline review, the introduction of VTE risk screening forms, and education were valuable to provide guidance on the risk assessment for VTE and assists with the identification of patients requiring VTE prophylaxis. The forms also allow the consistent documentation of VTE risk assessment in the patient medical record.
Acknowledgment
The authors acknowledge Ms. Breanna Hill (KEMH) for her work in the implementation of the VTE Risk Assessment forms.
Footnotes
Sources of Support: None. Conflicts of Interest: None.
References
- 1.Maternal deaths in Australia . Australian Institute of Health and Welfare. Updated Dec 14, 2020. Accessed Oct 14, 2023. www.aihw.gov.au/reports/mothers-babies/maternal-deaths-in-australia/contents/summary
- 2.Nichols KM, Henkin S, Creager MA. Venous thromboembolism associated with pregnancy. J Am Coll Cardiol. 2020;76:2128–2141. [DOI] [PubMed] [Google Scholar]
- 3.McColl MD Ramsay JE Tait RC et al.. Risk factors for pregnancy associated venous thromboembolism. Thromb Haemost. 1997;78:1183–1188. [PubMed] [Google Scholar]
- 4.Simpson EL, Lawrenson RA, Nightingale AL, Farmer RD. Venous thromboembolism in pregnancy and the puerperium: incidence and additional risk factors from a London perinatal database. BJOG. 2001;108:56–60. [DOI] [PubMed] [Google Scholar]
- 5.Clinical Practice Guideline for the Prevention of Venous Thromboembolism in Patients Admitted to Australian Hospitals. Commonwealth of Australia; National Health and Medical Research Council; 2009. [DOI] [PubMed] [Google Scholar]
- 6.Merriman E. Thromboembolism in pregnancy. Blood. 2022;24:28–30. [Google Scholar]
- 7.Venous Thromboembolism Prevention Clinical Care Standard . Australian Commission on Safety and Quality in Health Care. Accessed Oct 14, 2023. www.safetyandquality.gov.au/standards/clinical-care-standards/venous-thromboembolism-prevention-clinical-care-standard
- 8.National Standard Medication Chart (NSMC) audit. Australian Commission on Safety and Quality in Health Care; 2020. Accessed Oct 14, 2023. www.safetyandquality.gov.au/our-work/medication-safety/national-standard-medication-chart-nsmc-audit
- 9.King Edward Memorial Hospital Audit Results , 2022. National Standard Medication Chart (NSMC) audit; Australian Commission on Safety and Quality in Health Care, 2022. [Google Scholar]
- 10.Venous thromboembolism (VTE): Prevention and management, version 3.1; Obstetrics and Gynaecology Clinical Practice Guideline. Government of Western Australia; North Metropolitan Health Service; Women and Newborn Health Service; 2023. Accessed April 2023. [Google Scholar]
- 11.Reducing the risk of venous thromboembolism during pregnancy and the pueroerium; Green-top Guideline No. 37a. Royal College of Obstetricians and Gynaecologists; 2015. [Google Scholar]
- 12.Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for quality improvement reporting excellence): Revised publication guidelines from a detailed consensus process. J Nurs Care Qual. 2016;31:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frestel J Teoh SWK Broderick C et al.. A health integrated platform for pharmacy clinical intervention data management and intelligent visual analytics and reporting. Explor Res Clin Soc Pharm. 2023;12:100332. [DOI] [PMC free article] [PubMed] [Google Scholar]