Abstract
Background
The WHO’s global hepatitis strategy aims to achieve viral hepatitis elimination by 2030. Migrant children and pregnant persons represent an important target group for prevention strategies. However, evidence on the burden of chronic hepatitis B (CHB) infection and the factors affecting its incidence is lacking.
Methods
EMBASE, Global Health, Global Index Medicus, Web of Science and Medline were searched for articles in any language from 1 January 2012 to 8 June 2022. Studies reporting CHB prevalence, disease severity, complications and/or prevention strategies, including vaccination, prevention of vertical transmission and access to care/treatment for migrant children and pregnant migrants, were included. Pooled estimates of CHB prevalence and hepatitis B vaccination (HBV) coverage among migrant children were calculated using random effects meta-analysis.
Findings
42 studies were included, 27 relating to migrant children and 15 to pregnant migrants across 12 European countries, involving data from 64 773 migrants. Migrants had a higher incidence of CHB than host populations. Among children, the pooled prevalence of CHB was higher for unaccompanied minors (UAM) (5%, [95% CI: 3–7%]) compared to other child migrants, including internationally adopted children (IAC) and refugees (1%, [95% CI: 1–2%]). Region of origin was identified as a risk factor for CHB, with children from Africa and pregnant migrants from Africa, Eastern Europe and China at the highest risk. Pooled estimates of HBV vaccine coverage were lower among UAM (12%, [95% CI: 3–21%]) compared to other child migrants (50%, [95% CI: 37–63%]).
Conclusion
A range of modifiable determinants of HBV prevalence in migrant children and pregnant persons were identified, including sub-optimal screening, prevention and continuum of care. There is a need to develop evidence-based approaches in hepatitis care for these groups, thereby contributing towards global viral hepatitis elimination goals.
Keywords: Migrants, chronic hepatitis B, pregnant, hepatitis, preventions, prevalences
Introduction
Chronic hepatitis B (CHB) is defined as the persistence of hepatitis B surface antigen (HBsAg) for 6 months or more after acute infection with hepatitis B virus (HBV).1 There are an estimated 296 million people living with CHB globally,2 including 6 million children younger than 5 years.3 The majority live in highly endemic areas (where the prevalence of CHB is >8% of the population), including the East Asian, Eastern Mediterranean, Western Pacific and African regions.4 In these areas, most infections occur during infancy or childhood due to vertical transmission (mostly during childbirth but occasionally during pregnancy)5 or due to horizontal household transmission between children.6 The risk of progression from acute to chronic infection is higher when infection occurs in younger age groups, compared to populations who are infected later in life. Progression rates can reach up to 90% when infection occurs in the neonatal period, compared to 20% in childhood, and <5% in immunocompetent adults.7–9 In 2019, 820 000 deaths were attributable to CHB globally, many of which were secondary to cirrhosis and hepatocellular carcinoma (HCC).10
The sustainable development goals highlighted the importance of combatting hepatitis, and in 2016, the WHO launched a global strategy for achieving viral hepatitis elimination as a public health threat by 2030,2 defined as a 90% reduction in incidence and a 65% reduction in mortality compared with the 2015 baseline.2 Paediatric and vaccine-specific targets included 90% coverage of the 3-dose vaccine regimen and reducing HBsAg in children under 5 years to <0.1%.11 It also forms part of the triple elimination initiative, which aims to synergise efforts to prevent mother-to-child transmission of HIV, HBV and syphilis.12 The success of achieving these targets is highly reliant on preventative interventions delivered during pregnancy and childhood, such as antiviral (tenofovir) prophylaxis from 28 weeks gestation in women with high viral loads (≥200 000 IU/ml),13 a timely (within 24 hours) birth dose of hepatitis B vaccination, followed by 2–6 doses in infancy, and HBV immunoglobulin (HBIG) administration at birth in high-risk cases.14–16 Global coverage with three doses of the hepatitis B vaccine is estimated to be at 84%,17 with a target rate of 90% by 2030.10
Progress towards this fast-approaching WHO global deadline has been unequal across high burden populations. In Europe, a region with low endemicity that has seen recent migration waves from high (>8%) and intermediate (2–7%) CHB endemic regions,4 migrant populations are at higher risk of CHB than the native-born population,18,19 and migrants from countries where CHB is highly prevalent (≥2%) account for 25% of all CHB infections in the EU.20 Migrant children and pregnant persons in Europe are thus an important area of focus for regional prevention and care programmes.19 However, vaccination and screening practices vary across the region, and migrant populations are often overlooked by health systems and subject to inequities in accessing healthcare (including vital preventative screening and vaccination programmes) in host countries.21–23 This has resulted in migrants being under-immunised for hepatitis B and other communicable diseases.21 Although all 31 EU/EEA countries recommend vaccination for children in high-risk groups, and 27 even recommend universal childhood HBV vaccination, only seven EU/EEA countries have a national policy for screening migrants specifically for HBV.24 With regards to antenatal screening for HBV, universal screening is national policy in seven EU/EAA countries, and opt-out screening is policy in a further 14.25
Migrant children and pregnant persons are not only a key at risk group for CHB but are also cared for within distinct healthcare pathways and services (i.e. antenatal care, paediatric clinics and childhood vaccination services) within which HBV prevention and treatment could potentially be more easily administered. This includes opportunities to deliver prevention, most notably HBV vaccination, opportunistically if flexibility exists around the commissioning of these services. A strong evidence base is therefore vital for curating informed and tailored interventions for the prevention of CHB in these specific vulnerable groups. While evidence on the prevalence of CHB in migrants has previously been the subject of systematic reviews, there is currently no evidence synthesis available on CHB in migrant children and pregnant persons specifically.26,27
Methods
We conducted a systematic review and meta-analysis, according to PRISMA guidelines,28 to determine CHB prevalence, disease severity and complications and factors affecting incidence, including primary and secondary prevention strategies (vaccination, prevention of vertical transmission, screening and access to care/treatment, respectively) among migrant children and pregnant migrant populations in Europe.
Inclusion and exclusion criteria
The inclusion and exclusion criteria were developed using the Joanna Briggs Institute methodology29 (Appendix Table AA1, p. 1). We included primary research studies with data on CHB prevalence (primary outcome) and disease severity/complications and factors affecting CHB incidence, including vaccination status, prevention of vertical transmission, screening and access to care/treatment (secondary outcomes), among migrant pregnant women and/or children aged 18 years or less born outside the country of study and living in the EU, EEA, UK and Switzerland (i.e. first generation migrants) in all migrant groups (Appendix Box 1, p. 2). Observational studies, case reports and systematic reviews were included, and comments/editorials, conference abstracts and modelling studies were excluded. Studies published before 2012 were excluded to ensure that the evidence obtained was consistent with current migration trends and CHB prevention approaches, such as global vaccination recommendations.
Search strategy and study selection
We searched EMBASE, Global Health, Global Index Medicus, Web of Science and Medline for primary research articles in any language published from 1 January 2012 to 8 June 2022 combining English-language keyword search terms and medical subject headings using Boolean operators relating to migrants, prevalence, Hepatitis B and Europe (Appendix Figure AA1, pp. 2–5). Grey literature of relevant governmental and non-governmental organisations [e.g. WHO, European Centre for Communicable Disease Control (ECDC)] was consulted. Bibliographies of the included studies were hand-searched for additional relevant studies.
Google was searched with key terms (including ‘migrant’, ‘Hepatitis B’ and ‘Europe’) and the first 50 results were reviewed. No language restrictions were applied, and Google translate and DeepL translator were used as required.30,31
Data screening, extraction and synthesis
Records were imported into EndNote and duplicates were deleted. Title, abstract and full-text screening were carried out according to the aforementioned inclusion and exclusion criteria. Data were extracted and tabulated separately for migrant children and pregnant women. Using Microsoft Excel, a standardised form was developed to extract data on the following: author and year of publication of study, study setting and location, study design, number of participants (where relevant), refugee and migrant demographics (country of origin, legal status), age group and gender (for children), CHB prevalence, disease severity or complications, HBV vaccination status/coverage, screening and access to CHB care services.
To estimate the pooled CHB prevalence and pooled HBV vaccine coverage among migrant children, we conducted meta-analyses using the random effects model available from Stata 17 metaprop function.32 This enabled the calculation of 95% confidence intervals using the statistical score and the exact binomial method, and it incorporated the Freeman–Tukey arcsine double proportions transformation.33 This method also models intra-study variability using the binomial distribution. Inter-study heterogeneity was described using the I2 statistic, which describes the percentage of variation across studies that is due to heterogeneity rather than chance. The weights of the studies were also calculated to account for the differing sample sizes of the studies. We calculated separate pooled estimates for unaccompanied minors (UAM) and children with other migration statuses, including those described as migrants, refugees, asylum seekers and international adopted children (IAC). We excluded studies where sample sizes were not specified or where prevalence data for UAM and other migration types were not disaggregated. Two studies that included data on young people aged 0–20 and 13–25 years were included.34,35
The risk of bias was assessed for all studies was carried out using the Newcastle–Ottawa scoring (NOS) system (score out of 9) and an adapted scoring system for cross-sectional studies (score out of 8). Studies that scored a total of 8 or 9 points were considered to have a low risk of bias; 7 or 6 points were considered to have a medium risk of bias; and 5 points or less were considered to have a high risk of bias.
Results
Summary of included studies
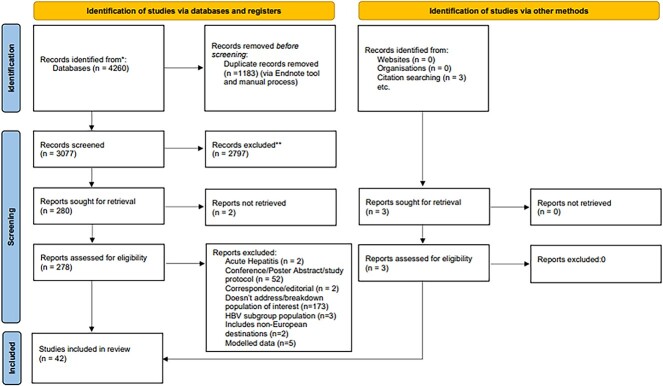
Our search returned 4260 articles, of which 3077 were screened (n = 278 full text), 42 included in the final study (Figure 1), and 23 in the meta-analyses. The studies reported on CHB prevalence (n = 35) in migrant children (n = 23) and pregnant women (n = 12), disease severity (n = 5), HBV vaccination/susceptible population (n = 26), screening (n = 5) and specialised migrant care pathways (n = 5), in a total of 64 773 migrants (Tables 1 and 2). The studies reported cases from 10 different countries (Figure 2), including Italy (n = 9), Germany (n = 8) Spain (n = 6), France (n = 4), Denmark (n = 2), United Kingdom (n = 2), Finland (n = 2), Ireland (n = 1), The Netherlands (n = 1) and Greece (n = 1).
Figure 1.
PRISMA study selection flow diagram. Adapted from the PRISMA 2020 Statement28
Table 1.
Details of all paediatric migrant hepatitis B studies
| Study | Year | Country | Study Setting | Study Purpose | Study Type | Study Population & country/region of origin | Inclusion Age Range (years) | How identified | Sample size | Prevalence Chronic HBV (%) | Vaccination Coverage (%) | Susceptible (%) | Age of Sample/at diagnosisa | Gender Split of HBV + ve (%male) | Gender Split of Sample (% male) | Screening/care pathway information | Data on complications, late severe disease |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belhassen-Garcia et al., | 2015 | Spain | Salamanca | Assess imported transmissible diseases in migrants from low income areas | Cross-sectional (medical records and physical exam) | Immigrant children and adolescents from E/W/S/C Africa, North Africa and Latin America | 0–18 | Screening in immigrants < 18 yrs | 350 | 4.3 | 39.7 | 32 | 15 | 86.7 | 53.4 | 7/15 patients with CHB had detectable HBV viral loads | |
| Bergevin et al., | 2021 | France | Dedicated migrant medical consultation service in 1 hospital, Paris | Describe health issues of newly arrived UAMs managed in a dedicated paediatric consultation service | Cross-sectional (single-centre retrospective) | UAM from Africa or Afghanistan | 0–18 | Visiting dedicated consultation service | 90 | 8.0 | 89.0 | Average time to 1st consult =3 months. UMs have free healthcare until adulthood | |||||
| Finnegan et al., | 2015 | Ireland | Specialist clinic for children born to HBV infected mothers | Describe long-term follow up of children born to HBV infected mothers | Cross-sectional (retrospective) | Children with chronic HBV infection attending specialist clinic (from Africa, Central Asia, Eastern Europe) | 0–18 | Children born to HBV infected mothers are referred | 57 | 6 m-17 yr | 60 | 60 | 69% born in Ireland received adequate prophyl. 0% born outside received adequate prophy l. 19% received vaccine alone. | 7% (n = 4) developed complication of CHB. n = 3 had fibrosis n = 3 received antivirals |
|||
| Fougere et al., | 2018 | Switzerland | Hospital reference centre for the children of asylum seekers in region. More than 90% of migrant children in Lausanne/surroundings followed in this setting. | Estimate HB vaccine protection (using post booster serology). Those with anti-HBs ≥ 100 post booster were assumed to be vaccinated. | Prospective single-centre cohort | Migrant children from Eritrea, Iraq, Syria | 1–18 | Research study | 200 | <1% | 81 | 9 yr*/ 12 yr -one case |
51 | Migrants should have 2 visits (for first dose of vaccine and serology 4–6 weeks later) | |||
| Genton et al., | 2019 | Switzerland | Migrant care facility clinic in canton of Vaud (service for asylum seekers)—but data across multiple healthcare structures primarily used by this cohort | Describe the overall clinical profile and the care pathways of unaccompanied minor asylum seekers | Cross-Sectional (retrospective study, information extracted from medical records) | UAM (asylum seekers). Afghanistan, Eritrea, Somalia, Syria | 0–18 | Routine screening of newly arrived children. | 109 | 2.8 | 87.2 | Screening of newly arrived UAMs takes place | |||||
| Giordano et al., | 2018 | Italy | University hospital of Palermo, Sicily | Examine immunisation status of IAC and compare it with vaccination certificates, focusing on measles, mumps, rubella (MMR) and hepatitis B (HBV) | Cross-sectional | Internationally adopted children (IAC). Country/region of origin not specified | 0–18 | Screening of internationally adopted children | 79 (for HBV) | 0.0 | 65.8 | 34 | 84 mon | 62 | Concordance between serology and vaccination records is 71% | ||
| Hahne et al., | 2012 | Netherlands | Population study | Assess differences in prevalence of HBV infection in The Netherlands between 1996 and 2007, and identify risk factors for HBV infection in 2007 | Cross-sectional (seroprevalence) | Representative sample of Dutch population (with oversampling or largest migrant groups from Suriname, Turkey, Morocco, Dutch Antilles, Aruba, Indonesia | 0–79 | Prevalence study (no data if already aware or not prior to study) | 2007: 6246 (0–14 = 1476, 14–29 =1002) | 2007 1st gen: 0–14: 2.3% 15–29: 22.3% 2nd gen 0–14: 1.7% 15–29: 2.4% |
|||||||
| Hampel et al., | 2016 | Germany | 5 initial refugee reception centres in Northern Germany | Assess prevalence of hepatitis B and the vaccination status of refugee population | Cross-sectional (retrospective descriptive data analysis) | Refugees. Country/region or origin not reported |
0–17 | Every refugee (newly arrived) who sought medical treatment for acute complaints offered testing | 62 (aged under 18 yrs) | 1.6 | 50 | 0% | 80.6 | Initial screening is offered but loss to follow-up and lack of joined up care is common | |||
| Hourdet et al., | 2020 | France | Health care access centre for vulnerable populations, Paris | Assess the health status of this population | Cross-sectional (retrospective, observational, monocentric) | Patients self-reporting as UAM but not recognised as such by the state from Guinea, Ivory Coast, Mali | 0–18 | Screening offered when visiting a dedicated consultation service | 301 -total 1035 consultations | 12.8 | 16 | 95 | Jurisdictional framework around this status unclear. Precarious access to care | ||||
| Hübschen et al., | 2012 | Luxembourg | Unclear | Investigate prevalence of IgG antibodies against different vaccine-preventable diseases in newcomers to Luxembourg | Cross-sectional | Refugees or asylum seekers (newly arrived) from Albania, Montenegro, Bosnia and Herzegovina, Middle East, Asia, Africa, Russia | 13–25 | Vaccine coverage study | 131 (age 13–25) | 25 | Majority of migrants lacked antibodies to one or more VPDs | ||||||
| Jablonka et al., | 2015 | Germany | single reception centre | Determine seroprevalence of antibodies against hepatitis A–E in an unselected cohort of refugees and asylum seekers during Middle East crisis | Cross-sectional | Refugees and asylum seekers from Africa, East MediterraneanEurope, SE Asia | 0–17 | On arrival screening (mandatory) | 91 | 0.0 | 40.7 | 32 | 74.7 | ||||
| Janda et al., | 2020 | Germany | Single paediatric consultation service for UMs, Municipal area Southwest Germany | Understanding frequency and clinical presentation of IDs among minor refugees, evaluate the performance and practicability of screening recommendation | Cross-sectional | UAM (refugee) from Africa (93.6%), Asia and Southern Europe | 0–18 | On arrival screening | 776 with HBV (of 890) | 7.7 | 16* | 94 | Problems with follow-up and retention in HBV service. Unable to offer antivirals | 75% of URMs with active HBV were HBeAg -ve and had low viral loads in blood (<10 IU/ml) | |||
| Kloning et al., | 2018 | Germany | 2 paediatric practices and one collective housing for refugees in region of Bavaria, Germany | Investigate the morbidity profile and the sociodemographic characteristics of unaccompanied refugee minors (URM) | Cross-sectional (retrospective data derived from medical data records of routine first medical exam) | UAM (refugee) from Afghanistan, Eritrea, Somalia, Syria | 0–18 | On arrival screening (mandatory) | 113 | 8 | 3.2 | 58 | 16* | 93.5 | No standardised pathway. Follow-up challenges due to frequent relocations | ||
| Maasen et al., | 2017 | Germany | On arrival screening | Describe microbiological screenings for infection control in unaccompanied minor refugees undertaken by the German Armed Forces Medical Service | Cross-sectional | UAM (refugee) from Afghanistan, Algeria, Benin, Egypt, Ghana, Guinea, Iran, Iraq, Libya, Morocco, Palestine, Pakistan | 0–18 | On arrival screening (mandatory) | 190 (from total sample of 219) | 1.6 | 13–18 | 100 | 100 | ||||
| Marquardt et al., | 2016 | Germany | On arrival screening in a private outpatient clinic for internal and tropical medicine, Bielefeld | Investigate the physical and mental disease burden of unaccompanied asylum-seeking adolescents | Cross-sectional | UAM (asylum-seeking adolescents) from Africa, Asia (mostly Afghanistan, Guinea, Morocco) | 12–18 | On arrival screening | 101 tested for HBV (of 102) | 7.9 | 14.9 | 16 | 100 | 76.5 | On arrival screening available | Two children required antivirals | |
| Marrone et al., | 2020 | Italy | Reception centres, Rome | Address prevalence of infectious diseases in a population of unaccompanied immigrant minors | Cross-sectional | UAM from Africa, SE Asia, Eastern Europe | 0–18 | On arrival screening | 879 | 2.5 | 18.2 | 76 | 17 | 100 | 97.6 | On arrival screening available | |
| Mockenhaupt et al., | 2016 | Germany | Berlin travel and tropical medicine clinic GeoSentinel site | Present results of screening a cohort of unaccompanied Syrian minors (UAMs) | Cross-sectional | UAM from Syrian | 0–18 | On arrival screening | 488 | 0.0 | 94 | ||||||
| Monpierre et al., | 2016 | France | Regional system for isolated foreign minors in Gironde | Describe data on overall health status obtained from a systematic medical check-up offered to URMs | Cross-sectional (data descriptive) | UAM (refugee) from Africa, Asia, Eastern Europe | 0–18 | On arrival screening (systematic) | 235 | 6.0 | 16 | 89.8 | On arrival screening available | ||||
| Norman et al., | 2021 | Spain | Migrant referral centre, Madrid | Describe seroprevalence rates for potentially transmissible viral infections in migrants attended at a referral centre in a major European city | Cross-sectional | Migrants from Africa | 0–20 | Attended for the first time (symptomatic or asymptomatic referred for a health exam) Unclear if screening was standardised. | 96 | 10.4 | 24 | 30 | |||||
| Olivan-Gonzalvo et al., | 2021 | Spain | UAM protection centres | Examine the health status and infectious diseases in a cohort of unaccompanied immigrant minors from Africa to Spain | Cross-sectional (retrospective) | UAM (Male, from Africa) | 0–18 | On arrival screening | 622 | 2.6 | 16 | 100 | 100 | Screening on admission to residential care | |||
| Pauti et al., | 2016 | France | Migrant clinics: Drs of the World Clinics in Lyon, Nice, Paris and Saint-Denis | Present degree of lack of knowledge of the HBV and HCV status of people encountered in 2014, identify socio-demographic factors related to this lack of knowledge. HBV vaccination coverage rate analysed. | Cross-sectional | Persons in precarious conditions for primary health care - 94.5% are migrants from Africa, non-EU European countries, Asia | <15 | Attendance at clinic | Unclear | 58.1 | 61.8 | Lack of systematic checking of HBV serology/ vaccination status | |||||
| Pavlopoulou et al., | 2017 | Greece | Migrant outpatient clinic of a tertiary Children’s hospital, Athens | Describe demographic, clinical and laboratory characteristics and identify possible determinants among newly arriving immigrant and refugee children | Cross-sectional | Migrant and refugee children mainly from Asia (Afghanistan, Bangladesh), Africa, Europe | 0–18 | On arrival screening | 300 | 0.0 | 57.7 | 42 | 58.7 | Health evaluation for migrant children on arrival and refugees are often referred by NGOs or social services. | |||
| Pohl et al., | 2017 | Switzerland | Tertiary health care facility in Switzerland in 2015 | Describe epidemiology and spectrum of infections of admitted paediatric refugees and asylum seekers | Cross-sectional (retrospective analysis using electronic patient records) | Paediatric refugees and asylum seekers (UAMs = 19.4%) from Africa, Eastern Europe and Asia | 0–18 | Admitted to hospital | 93 patients (105 admissions) | 5.7 | 62 | Missed opportunities to offer catch-up vaccinations during admission | |||||
| Sollai et al., | 2017 | Italy | Tertiary health care setting, Italy | Evaluate infectious diseases prevalence in a large cohort of IAC | Cross-sectional | IAC from Africa, Asia and Europe | 0–18 | On arrival screening | 1612 | 0.8 | 64.9 | 35 | 60 | IAC are screened on arrival | |||
| Theuring et al., | 2016 | Germany | Institute of Tropical Medicine and International Health, Charité- Universitätsmediz Berlin 2014–2015 | Screening for infectious diseases among unaccompanied minor refugees | Cross-sectional | UAM (refugees) from Africa, Middle East, Asia, Southern and Eastern Europe | 0–18 | On arrival screening | 1248 | 1.7 | 16 | ||||||
| Tiittala et al., 2018 | 2018 | Finland | Finland asylum seeker population study | Evaluate public health response to a large influx of asylum seekers to Finland in 2015–2016 re: national guidelines on initial health services and infectious disease screening | Cross-sectional (retrospective register-based) | Asylum Seekers – (accompanied and UAM) from Africa and Asia | 0–17 | On arrival screening (voluntary) | 9031 (3400 of which UAM) | 0.8 | Screening for Hep B, HIV, syphilis within should occur within 3 m after arrival (but 33% not reached) | ||||||
| Williams et al., | 2020 | UK | 2 paediatric infectious disease clinics, London | Evaluate a screening programme for infection in UAM children and young people against national guidance and describe rates of identified infection in cohort | Cross-sectional (retrospective, routinely collected healthcare data) | UAM (asylum seeking) | 0–18 | Voluntary screening (on the basis of an individual risk assessment) | 252 attendees, 211 (84%) tested for hepatitis B | 4.8 | 17* | 88.6 | All UAMs receive a statutory health check and are referred to infectious disease clinic for screening |
Age is expressed as mean. * = denotes median age.
UAM, unaccompanied migrant.
VPD, vaccine preventable diseases.
Table 2.
Details of all pregnant migrant hepatitis B studies
| Study | Year | Country | Study Setting | Study Purpose | Study Type | Study Population and country/region of origin | How Identified | Sample size | Overall sample prevalence (%) | Migrant prevalence (%) | Native-born prevalence (%) | Proportion Of Infected Women =Migrants | Data on complications, late severe disease | Screening/care pathway information | Transmission |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cochrane et al., | 2015 | UK | Routine antenatal care, Bristol, UK | Estimate HBV infection prevalence by region of birth in migrant populations in a large city | Cross-sectional (retrospective data linkage) | Pregnant migrant women born in regions with HBV infection prevalence > 2% from all continents. | Routine screening | 5840 | 1.7 | ||||||
| Dalmartello et al., | 2019 | Italy | Population based survey in Trento Province, Italy | Describe coverage and outcome of screening for rubella, syphilis, toxoplasmosis, CMV, HBV, HCV, HIV, & Group B Streptococcus in pregnancy | Cross-sectional | Pregnant women from No data on countries/ regions of origin. |
Routine screening/at delivery | 38 712 total women, 9237 migrant (23.8%) | 0.9 | Foreign citizenship associated with absence of screening | |||||
| Dopfer et al., | 2018 | Germany | 3 refugee reception centres in Northern Germany | Assess pregnancy rates and associated primary healthcare needs in three refugee cohorts | Cohort | Refugee women on arrival from Afghanistan, Albania, Azerbajian, Bosnia, Iraq, Montenegro, Nigeria, Syria |
Off-site mandatory check-up within their first weeks of residence. | 9 pregnant migrants (from 1533 total) | 0.0 | Variable healthcare provision between centres | |||||
| Ehmsen et al., | 2014 | Denmark | Danish NGO health clinic | Describe characteristics of undocumented migrant patients. | Cross-sectional | Undocumented migrants (including pregnant women) from Global (aggregate data) |
Voluntary attendance at NGO clinic relating to pregnancy - then screened | 96 pregnant migrants (from 1403 total) | 1.0 | Median start of ANC = 16 + 4 weeks | |||||
| Karatapanis et al., | 2012 | Greece | 1 maternity unit, Athens | Assess seroprevalence of HBV markers among parturient women escaping HBsAg prenatal testing | Cross -sectional (prospective) | Pregnant women from Africa, Albania, Asia, Eastern Europe and Roma |
Study | 53 HBsAg +ve pregnant migrants (Total 9546 pregnancies) |
5.3 | 77.9 (53/68) |
1000 women (10.6%) had no HBsAg status documented. 70.4% of these were immigrants | ||||
| Lembo et al., | 2017 | Italy | Obstetric Division of a Sicilian University Hospital, Southern Italy | Investigate prevalence of HBV and HCV serum markers in a large cohort of pregnant women | Cross-sectional | Pregnant women from Albania, China, Kazakhstan, Morocco, Poland, Romania, |
Unclear - in medical records of women delivering | 711 pregnant migrants (Total 7558 pregnancies) HBsAg status available for 6128 (81%) |
0.5 | 3.0 | 0.2 | No cases of vertical transmission in babies born to HBsAg +ve mothers. Prophylaxis given to all. |
|||
| Lo Giudice et al., | 2021 | Italy | Obstetrics and Gynaecology Operative Unit, Messina | Serological survey on blood samples from pregnant women collected during routine pregnancy screening to evaluate the rate of HBsAg and HCV antibody carriers in a low-endemic territory | Cross-sectional | Pregnant women. No data on countries/ region of origin |
Screening | 727 pregnant migrants (6169 total pregnancies) |
0.4 | 2.1 | 0.2 | 50 | |||
| Lopez-Fabal et al., | 2013 | Spain | Maternity unit in the south of Madrid | Determine prevalence and evolution of markers included in serological screening of pregnant women | Cross-sectional -retrospective | Pregnant women from Africa, Eastern Europe, South-East Asia |
Screening | 2752 pregnant migrants (8012 total pregnancies) HBV tested in 6939 | 0.8 | 1.7 | 0.4 | ||||
| Ruffini et al., | 2014 | Italy | Maternity units of the Marche region, Italy | Evaluate data on congenital and perinatal infections, excluding congenital rubella, in pregnant women in the Marche region (Italy) | Cross-sectional | Pregnant women from Albania, China, Romania, Macedonia, Nigeria, Pakistan, Senegal |
Routine screening | 397 pregnant migrants (1651 total) | 0.8 | 4.3 | 0.4 | 82.5 | |||
| Ruffini et al., | 2016 | Italy | Maternity units of the Marche region, Italy | Evaluate epidemiology of hepatitis B infection in pregnant women, according to country of origin. | Cross-sectional | Pregnant women from Albania, Africa, China, Eastern Europe, Ukraine, Western Pacific | Routine screening | 2563 pregnant migrants (10 232 total) | 0.8 | 2.7 | 0.2 | Adherence to screening in migrants: 96.7% (vs 99.4% in native population) | |||
| Ruiz-Extremera et al., | 2020 | Spain | 8 participating hospitals in Spain (Madrid, Seville, Malaga, Oviedo, Almeria, Granada) | Determine prevalence of HBV and HCV in pregnant women in Spain, focusing on country of origin, epidemiological factors and risk of vertical transmission | Multicentre open-cohort study | Pregnant women (Hep B + ve) from Africa, China (and other Asian countries), Eastern Europe, Latin America, |
‘routine clinical practice’ - assumed to mean screening but unclear | 67 HBV + ve pregnancies | 0.4 | 65.7 | 14.5% (of all pregnancies) HBeAg +ve 6 women received antivirals during pregnancy (not disaggregated by migrant status). |
Immuno-prophylaxis given correctly to all mothers. | Mothers became infected via parenteral (20.9%), sexual (4.5%) and vertical (31.3%) transmission (40.3% unknown) | ||
| Sagnelli et al., | 2016 | Italy | Delivery units at 8 Italian hospitals in different cities in Northern, Central and Southern Italy |
Estimate clinical impact of HBV infection in pregnant immigrants and their family members and to identify a clinical approach | Cross-sectional | Pregnant migrant women from Africa, Eastern Europe, East Asia, South America |
Unclear - appears routine screening of pregnant women and study screening of family members of HBV + ve | 1970 pregnant migrants | 7.3 | 20% were HBeAg +ve. Most HBsAg +ve cases had detectable HBV DNA (50% with VL ≥2000 IU/ml) |
|||||
| Santiago et al., | 2012 | Spain | A tertiary hospital in Madrid between August 2007 and October 2008 | Determine serological profile of foreign mothers against vertically transmitted infections | Cross-sectional (retrospective) | Pregnant women from Africa, Asia, Europe, Central& South America, | In hospital screening | 1214 pregnant migrants women with HBV serology. (Total 2526 migrant and 157 Spanish pregnant women) | 2 | 1.1 | |||||
| Tasa et al., | 2021 | Finland | Public maternity clinic and maternity hospital in Helsinki, Finland | Describe the use of maternal health care services and the obstetric outcomes of undocumented women and comparing results with all pregnant women in Finland | Cross-sectional (retrospective register-based) | Undocumented pregnant women from Eastern Europe and Russia, Sub-Saharan Africa, Asia, Middle East |
Routine screening | 62 undocumented pregnant migrants | 3.4 | 0.2 | 91% attended antenatal care. 70% <8visits, 40% 0–3 visits. 3 women denied access to care. |
||||
| Wendland et al., | 2016 | Denmark | 3 clinics specialising in care for UM (in Copenhagen & Jutland) | Assess screening frequency for HIV, HBV, syphilis in undocumented migrants (UM) and to compare prevalence of infection in UM with DM | Cross-sectional (retrospective) | Undocumented migrant pregnant women from Africa, Eastern Europe, Indian subcontinent Middle East/North Africa Central America, SE Asia |
Screening & birth register | 219 undocumented pregnant migrants (94 had HBV result) | 6.1 | Documented migrants have access to screening, undocumented migrants do not and rely on NGOs |
ANC, antenatal care.
Figure 2.
Number of studies by migrant destination. (N.B.: one study from Luxembourg not visible)
Most studies (39/42) were cross-sectional and described the experiences of single centres and therefore consisted of non-representative samples of migrants. Three were cohort studies, one of which undertook representative sampling.36–38 All studies reported either on paediatric or pregnant migrants except for one study that reported on pregnant UAM39 (Appendix Table A2, p. 5).
Risk of bias scores ranged from 3 to 8, with one study having a low risk of bias, 13 having a medium risk of bias and 28 having a high risk of bias (Appendix Table A3, pp. 6–7).
Child migrants
Prevalence
The prevalence of CHB among migrant children reported in 23 studies ranged from 0 to 13% (Table 1 in the article and Appendix Table A4, pp. 7–8). The prevalence varied according to several factors, including migrant type and country of origin. The pooled prevalence of CHB among UAMs was higher (5% [95% CI 3–7]) than among children with other migration statuses, including those described as migrants, refugees, asylum seekers and IAC (2% [95% CI 1–3]) (Figure 3A and B).
Figure 3.
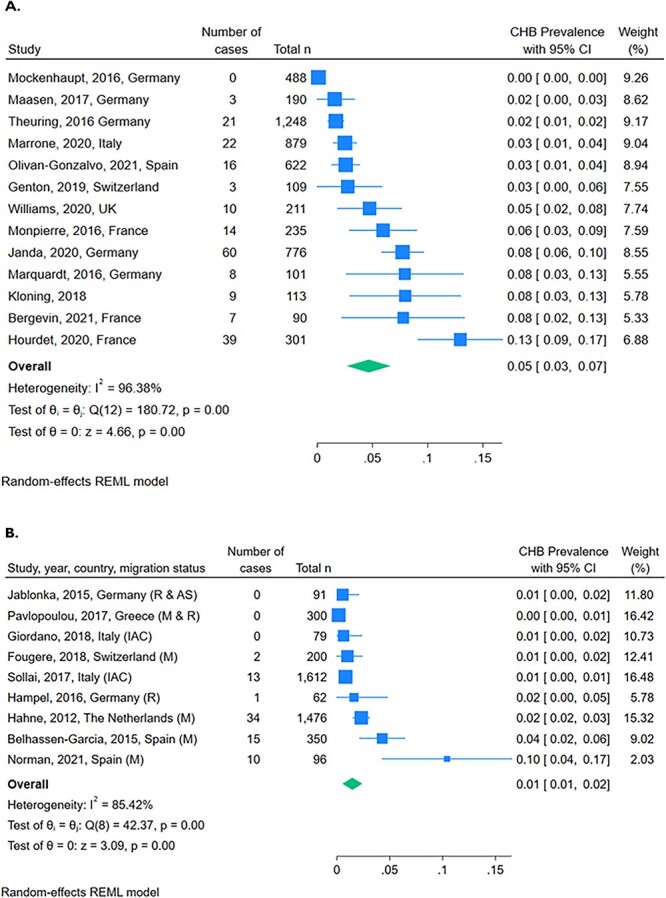
Forest plots of CHB prevalence among migrant children reported by 23 studies. (A) Unaccompanied Migrants (UAM). (B) Other migration status (M, migrants; R, refugees; AS, asylum seekers; IAC, internationally adopted children)
One study in France reported a particularly high prevalence of CHB (12.8%) among young people who self-identified as UAM, predominantly from West Africa, but not recognised by the state as such.40 By contrast, CHB was rarely identified among the IAC.41,42
In terms of country of origin, a Spanish study looking at prevalence of CHB among 350 migrant children found that all cases were from the WHO Africa Region and that there were no cases among North African and Latin American migrants.43 This finding was corroborated in a study describing CHB in UAM in Italy, where children from Gambia and Ivory Coast were found to have a higher prevalence compared to those from Libya.44 Paediatric CHB cases were also older (mean age 14.6) compared to vaccinated and non-immune cases (mean ages 11.2 and 11.5, respectively) and more likely to be male in a Spanish study of migrant children from Africa and Latin America and a German sample of UAM seeking asylum.43,45
Disease severity and complications
One small Irish study in a clinic for children born to HBV-infected mothers reported complications of CHB—finding fibrosis or inflammation in 7% (4/63) of migrant children living with HBV who originated from Africa, Central Asia and Eastern Europe.46 Two studies, one in UAM, reported that the majority (75–93%) of infected children were negative for Hepatitis B e-antigen (HBeAg)—a marker of active HBV replication and high infectivity.47,48 Most also had low viral loads, but the range of viremia differed substantially, and treatment status prior to assessment was not reported.48 Antiviral use was reported in studies from Germany in 2/8 adolescents and Ireland, in 3/57 children.45,46
Factors affecting CHB incidence
Vaccination status and susceptible population
Thirteen studies evaluated HBV immunity through vaccination and natural infection using HBV serology, although some of these did not present disaggregated paediatric data (Figure 3).34,35,41–45,49–54 Studies that did disaggregate by age group indicated that 32–76% of migrant children are Hepatitis B non-immune (no serological evidence of vaccination or prior infection).34,41–44,49,54 Vaccination coverage estimates ranged from 3.2–81% across 13 studies.34,35,41–45,49–54
Pooled vaccine coverage estimates were lower among UAMs (12% [95% CI 3–21]) than among other migration categories, including those described as migrants, refugees, asylum seekers and IAC (50% [95% CI 37–63]) (Figure 4A and B).
Figure 4.
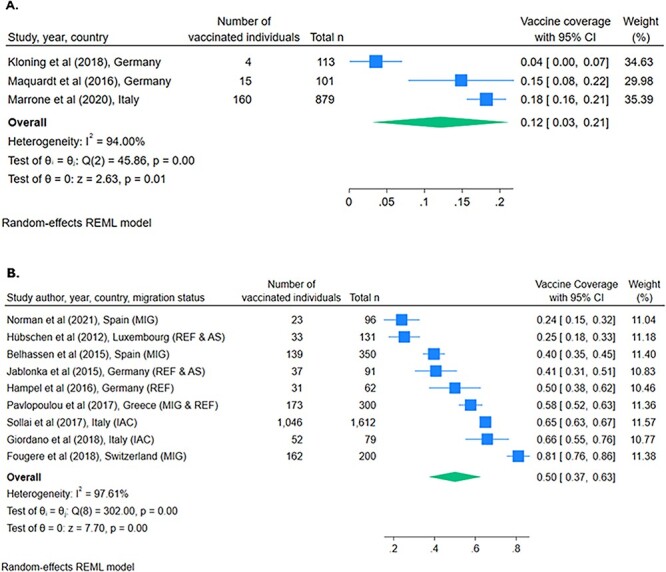
Forest plots of HBV vaccine coverage among migrant children reported by 23 studies. (A) UAM. (B) Other migration status (M, migrants; R, refugees; AS, asylum seekers; IAC, internationally adopted children)
The association between migrant type and vaccine coverage was also assessed in a Greek study, which found significantly (P = 0.015) lower coverage among refugees (defined as asylum seekers and irregular migrant children) compared to immigrants (defined as children of parents with long-term residence permits or those who entered for family reunification).49
Vaccination coverage estimates were below coverage rates across most WHO regions in all studies (Figure 2 and Appendix Table A5, pp. 10–11).35,52 Studies also noted that for most children (58.1–79.3%), vaccination records were unavailable, and when available, discrepancies frequently existed between documentation and serological evidence of HBV protection.41,42
Screening and care pathways
Eighteen studies from Germany,39,45,48,52,54–56 Spain,34,43,57 Switzerland,58 Italy,41,42,44 France,59 Greece,49 Finland47 and the UK,60 reported having paediatric migrant health screening processes on arrival. However, target populations were not always reached, with a Finnish study reporting one-third of asylum seekers not being reached and some experiencing delays in screening.47 This was also noted in a UK study, where a median delay of 6 months between arrival in the UK and infection screening was observed.60 A Swiss hospital-based study reported the absence of routine screening procedures in refugee and asylum-seeking inpatient children and the incomplete administration of catch-up immunisations.58 Retention in care was also a challenge, with six studies following UAM highlighting a high number of individuals who had tested HBV-positive being lost to follow-up due to rapid relocations,54,61 communication barriers and logistical difficulties in accessing care.48,53,60,61
Pregnant migrants
Prevalence
Estimates of CHB prevalence in pregnant migrants reported in 12 studies ranged between 0–7% (Table 2 and Appendix Table A4, pp. 7–8),38,62–72 with five of these studies reporting a significantly higher prevalence of CHB among pregnant migrants compared to native-born individuals.64,65,67,68,71
Undocumented women were also found to have a significantly higher prevalence of Hepatitis B than documented migrants (RR 2.4, 95% C.I. 1.1–5.3)72 even after adjustment for region of origin.
Prevalence variation by region of origin was described in an Italian study and found to be highest among pregnant women from the Western Pacific Region, Eastern Europe and Africa (7.0%, 4.0% and 3.3%, respectively).68 In this study, more than half (60.6%) of the HBsAg positive pregnant women originated from China and Albania.68 Prevalence differences between migrants from specific geographical areas and host populations were examined in two studies.68,70 These established CHB prevalences were significantly higher in pregnant women from China (8.1%), Albania (7.7%), Ukraine (7.2%) and Senegal (6.1%) compared to Italian women (P < 0.05)68 and in Southeast Asian (primarily Chinese) women (10.8%) compared to native Spanish women (P < 0.005).70
CHB prevalence estimates by region of origin of pregnant migrants were collated from seven studies. High prevalence estimates (>8%) were found among pregnant migrants from Eastern Europe (n = 1 study),66 Asia and Pacific (n = 1 study)70 and Africa (n = 1 study).66 Intermediate prevalence estimates (2–7%) were found among pregnant migrants from Eastern European (n = 4 studies),68,70,72,73 Asia and the Pacific (n = 3 studies)68,72,73 and Africa (n = 4 studies)62,68,72,73 and low prevalence estimates (<2%) were found among pregnant migrants from Eastern Europe (n = 2 studies),62,72 Southern Europe (n = 1 study)62 Asia and the Pacific (n = 1 study)62 and Latin America and Caribbean (n = 1 study)62 (Appendix Figure A3, p. 11).
Disease severity/complications
A fifth of CHB-infected pregnant migrants in one study were HBeAg positive, which is associated with more frequent and rapid progression towards severe liver disease and HCC, and an increased likelihood of vertical transmission.69 Most infected pregnant migrants (73.7%) also had detectable HBV DNA, and half had a viral load considered to be associated with chronic liver disease.69
Factors affecting CHB incidence
Vaccination status and susceptible population
Only one included study explored vaccination coverage in pregnancy. This study focused on women tested for HBV for the first time in the delivery room in a Greek setting, of whom 70.4% were migrants. Low vaccination-induced protection rates (mean 21.4%) were observed among these women who had missed pre-natal HBV maternal testing, but the results for vaccination coverage were not disaggregated by migrant status.73
Screening and care pathways
Pregnant migrants (including undocumented migrants) were at significantly greater risk of not being screened for HBV during pregnancy compared to native women across four studies.68,72–74 Foreign citizenship increased the odds of not being screened for HBV by 30% (OR: 1.30, 95% CI: 1.04–1.62) in Italy.74 In Greece, among women who had not had prenatal HBV screening, the majority (70.4%) were migrants, and 5.3% of these were subsequently found to have HBV, a significantly higher proportion than in the comparison group (P < 0.0001).73
Consistent findings were described in a Danish study,72 where only 43% of pregnant undocumented migrants had a screening result recorded, compared to 99.9% of pregnant people with legal Danish residency.72 Late access to antenatal care was described as an important reason for suboptimal screening coverage by a Danish study, which found that pregnant migrants first accessed antenatal care at a median gestation of 20 weeks (range 6–39 weeks).63 A Finnish study also found that 71% of undocumented migrant pregnant women received inadequate prenatal care, with 61% not receiving any antenatal care in the first trimester and 6% receiving no antenatal care at all.71 This resulted in missed opportunities to prevent mother-to-child transmission of HBV, as demonstrated by an Irish study of HBV-infected children, where 100% of foreign-born and 30% of Irish-born infected children in the study had not received HBIG or HBV vaccine prophylaxis.46 Instances of antiviral treatment not being initiated when indicated and the loss of infected women to follow-up69,72 were also documented.
Discussion
Our systematic review found intermediate to high CHB prevalence among migrant children in Europe, with a higher prevalence among those originating from the WHO African Region and a higher pooled prevalence among unaccompanied versus accompanied minors. Pooled HBV vaccination coverage estimates were also lower among UAM compared to other child migrants, and a high proportion of migrant children were found to be Hepatitis B non-immune from vaccination or prior infection across multiple geographies of origin, including those from Africa, Latin America, the Balkans, Middle East, Asia and Russia.34,41–44,49,54
Our data also show that CHB prevalence was significantly higher among migrant pregnant persons compared to the native-born pregnant population. Undocumented pregnant migrants, those declaring themselves as UAMs (but not recognised as such by the state) and those originating from Eastern Europe, China and Africa were at particularly high risk of CHB.
Based on limited data, our review showed that high HBV viral loads, and complications of CHB, including liver inflammation and fibrosis, are infrequently reported among migrant children.45–48 Among pregnant migrants, HBeAg positivity and detectable viral loads were reported among a minority of CHB cases, and the reported experience of antiviral use during pregnancy was limited.37
Both migrant children and pregnant persons experience restricted access to healthcare across Europe, leading to a reliance on ad hoc care from NGO-run clinics. This may have contributed to the paucity of data on CHB complications and antiviral use in both groups. Children with CHB are typically asymptomatic but may have high viral loads and HBeAg status, meaning they are at risk of transmission to others and also long-term at risk of developing the sequelae of CHB, such as cirrhosis and HCC in adulthood, leading to an increased risk of mortality, which is known to be significantly higher among migrants with CHB compared to native populations in European host countries.27 The long-term follow-up and monitoring of young people is therefore vital, to allow for the timely commencement of antiviral therapy coupled with regular age-appropriate counselling about viral transmissibility.27,34 Regular follow-up also provides opportunities to prevent transmission by, e.g. vaccinating close contacts.75
Among pregnant migrants, suboptimal antenatal HBV screening due to a lack of access to national pregnancy screening programmes was also reported72,73 with pregnant undocumented migrants having an even poorer chance of being screened than documented migrants, despite having a higher prevalence of HBV.73 This could possibly lead to cases of preventable vertical HBV transmission.71,73
The WHO proposes that antenatal care, including equal and timely access to HBV screening, should be easily accessible for all migrants, regardless of legal status and ability to pay.76 As part of this, the appropriate administration of antenatal antiviral prophylaxis, plus HBV vaccine and HBIG at birth for the prevention of vertical transmission should be available for all HBV positive migrants. Given the complexities in the supply and availability of these specialist treatments, this needs to be integrated into the regular healthcare system.77,78 However, the reliance on NGOs to fulfil this screening and prevention role for undocumented migrants is a reality in several European countries, leading to frequent loss of follow-up and failure to administer appropriate neonatal prophylaxis to those most at risk.46,69,72
While specific WHO guidelines exist for the prevention and treatment of CHB in migrant populations through screening, vaccination and equitable access to CHB treatments,79,80 evidence from this review suggests that there is no universal approach to HBV screening in migrant groups across Europe and that policy and practice gaps remain, specifically for children and pregnant migrant groups. Migrants from intermediate- and high-prevalence countries are not always screened,81 with some countries relying purely on symptom or patient-initiated testing approaches.82 Catch-up immunisation programmes are also not always available in European host countries,83,84 despite data indicating low HBV vaccination coverage in child migrants, below global and regional average rates.
The strengths of this study include the robust systematic methodology employed, including the meta-analyses and pooled estimates obtained. The inclusion of research studies in all languages also enabled us to capture a wide breadth of literature representative of all of Europe.
Limitations of this systematic review include the fact that most studies were cross-sectional and described the experiences of single centres, the majority of which were specialised migrant health settings. Therefore, the study populations may not necessarily have been representative of all migrant types in the reporting country. There was also a lack of consistency in the terminology used to describe different migrant populations. This has implications in the ascertainment of the true ‘at-risk’ population when estimating prevalence. In some studies, prevalence estimates in certain migrant groups may have been underestimated due to a lack of comprehensive screening programmes, language and cultural barriers, as well as stigma and discrimination that might have resulted in high-risk migrants not being captured by the studies.40,64,85 The calculation of vaccination coverage also varied between studies,49,52 and may have been potentially underestimated in cases where anti-HBS was measured prior to booster administration or in cases of co-infection with HIV (where vaccination may not induce an anti-HBS response) or in rare cases of vaccine non-responders.52 It is also worth noting that most studies originated from a small proportion of primarily Western, Northern or Central European countries, and some relied on data over a decade old.18,27,36,43,46,57,66,70,73,74 Two studies carried out in similar geographical regions and time periods may have had some overlapping cases.67,68 Caution should therefore be employed when generalising the findings to wider migrant populations.
The findings of this review highlight the need for several policy and clinical recommendations. First and foremost, national and international European policies should incorporate CHB screening and the provision of robust HBV vaccination programmes for migrant children and pregnant persons. Together, but simultaneously, flexible systems are required to enable opportunistic immunisation while still connecting to central reporting systems to enable follow-up data to be collected. This is supported by evidence that high numbers of migrants are HBV non-immune86 and that strategies to bolster screening and HBV vaccination efforts in migrant populations in Europe are cost-effective87,88 and reduce resource impact on healthcare systems.89
These interventions should be delivered alongside existing preventative health services in maternal and child health so that hepatitis B risk is addressed together with a range of other health inequalities for these populations.90 These should include robust childhood and adolescent catch-up vaccination programmes and antenatal infection screening for the prevention of congenital infections, the latter being in line with the WHO’s triple elimination programme, which aims to synergise efforts to eliminate the vertical transmission of HIV, syphilis and HBV. There is a need to capitalise on existing mobile technologies developed during the COVID-19 pandemic to develop digital migrant mobile vaccination and personal health records that are easily accessible to healthcare providers. This would improve the continuum of care for migrants with CHB and improve the efficiency and streamlining of screening procedures.48
Future research should endeavour to optimise the description of the origin of migrants, including their migration status, as well as geographical regions of origin, to facilitate evidence synthesis as well as disaggregation of age. Expansion of the research to include migrants to other high-income and low HBV prevalence contexts, such as North America and Australia, would also provide further relevant context and generalisability. Research on post-arrival transmission of CHB, the ongoing health needs of migrant children and young people living with CHB, as well as qualitative research in the field would provide valuable contextual data for quantitative findings.
Large-scale migration from high-prevalence countries has shifted the HBV landscape in Europe, potentially rendering existing elimination strategies inadequate and allowing at-risk migrant groups to go undiagnosed and untreated.91 Addressing the CHB health needs of child and pregnant migrants in Europe will require further evidence generation and advocacy in order to design equitable non-hostile health policies that are integrated into broader inclusive social policies that are responsive to the changing epidemiology and migrant profiles.89
Supplementary Material
Contributor Information
Carla Hobart, Faculty of Epidemiology and Population Health, London School of Hygiene & Tropical Medicine, Keppel Street, WC1E 7HT, London, UK.
Julia M Pescarini, Faculty of Epidemiology and Population Health, London School of Hygiene & Tropical Medicine, Keppel Street, WC1E 7HT, London, UK; Centre of Data and Knowledge Integration for Health (CIDACS), Gonçalo Moniz Institute, Oswaldo Cruz Foundation, Rua Waldemar Falcão, 121, Candeal - Salvador/BA CEP: 40296-710, Bahia, Brazil.
Laith Evans, Centre for Neonatal and Paediatric Infection, St. George’s University of London, Cranmer Terrace, London SW17 0RE, UK.
Haleema S Adil, University College London Medical School, 74 Huntley St, London WC1E 6DE, UK.
Shehzhore T Adil, University College London Medical School, 74 Huntley St, London WC1E 6DE, UK.
Anna Deal, Migrant Health Research Group, Institute for Infection and Immunity, St. George’s University London, Cranmer Terrace, London SW17 0RE, UK.
Jessica Carter, Migrant Health Research Group, Institute for Infection and Immunity, St. George’s University London, Cranmer Terrace, London SW17 0RE, UK.
Philippa C Matthews, The Francis Crick Institute, HBV Elimination Laboratory, 1 Midland Road, London NW1 1AT, UK; Division of Infection and Immunity, University College London, Gower Street, London WC1E 6BT, UK; Department of Infectious Diseases, University College London Hospital, Euston Road, London NW1 2BU, UK.
Sally Hargreaves, Migrant Health Research Group, Institute for Infection and Immunity, St. George’s University London, Cranmer Terrace, London SW17 0RE, UK.
Nuria Sanchez Clemente, Faculty of Epidemiology and Population Health, London School of Hygiene & Tropical Medicine, Keppel Street, WC1E 7HT, London, UK; Centre for Neonatal and Paediatric Infection, St. George’s University of London, Cranmer Terrace, London SW17 0RE, UK; Migrant Health Research Group, Institute for Infection and Immunity, St. George’s University London, Cranmer Terrace, London SW17 0RE, UK.
Funding
SH is funded by La Caixa (LCF/PR/SP21/52930003), the Medical Research Council (MRC/N013638/1), the National Institute for Health Research (NIHR300072; NIHR134801), the Academy of MedicalSciences (SBF005\1111), and WHO. AD is funded by an MRC-LID PhD fellowship.
Author contributions
Carla Hobart (Conceptualization [equal], Data curation [lead], Formal analysis [lead], Investigation [lead], Methodology [equal], Validation [equal], Visualisation [equal], Writing—original draft [lead]), Julia M. Pescarini (Conceptualization [equal], Methodology [equal], Supervision [equal], Validation [equal], Writing—review & editing [equal]), Laith Evans (Data curation [equal], Formal analysis [equal], Validation [equal], Visualisation [equal], Writing—review & editing [equal]), Haleema S. Adil (Formal analysis [equal], Investigation [equal], Writing—review & editing [equal]), Shehzhore T. Adil (Formal analysis [equal], Investigation [equal], Writing—review & editing [equal]), Anna Deal (Conceptualization [equal], Data curation [equal], Writing—review & editing [equal]), Jessica Carter (Validation [equal], Writing—review & editing [equal]), Philippa C. Matthews (Conceptualization [equal], Methodology [equal], Supervision [equal], Validation [equal], Writing—review & editing [equal]), Sally Hargreaves (Conceptualization [equal], Methodology [equal], Supervision [equal], Validation [equal], Visualisation [equal], Writing—review & editing [equal]), and Nuria Sanchez Clemente (Conceptualization [equal], Data curation [equal], Formal analysis [equal], Investigation [equal], Methodology [equal], Project administration [equal], Resources [equal], Software [equal], Supervision [lead], Validation [equal], Visualisation [equal], Writing—review & editing [equal]).
Data availability
All data is available upon reasonable request to the co-authors.
References
- 1. National Institute for Health and Care Excellence . NICE Guidance: Hepatitis B (chronic): diagnosis and management https://www.ncbi.nlm.nih.gov/books/NBK553697/ 2017. [PubMed]
- 2. World Health Organisation. Hepatitis B. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b#:~:text=WHO%20estimates%20that%20296%20million,carcinoma%20(primary%20liver%20cancer). World Health Organisation. Geneva. 2023.
- 3. Noubiap JJ, Ndoula ST. Ndoula ST prevention of mother-to-child transmission of hepatitis B: birth-dose vaccination is not enough. Lancet Glob Health 2022; 10:e455–6. [DOI] [PubMed] [Google Scholar]
- 4. Sharma S, Carballo M, Feld JJ, Janssen HLA. Immigration and viral hepatitis. J Hepatol 2015; 63:515–22. [DOI] [PubMed] [Google Scholar]
- 5. Sirilert S, Tongsong T. Tongsong T hepatitis B virus infection in pregnancy: immunological response, natural course and pregnancy outcomes. J Clin Med 2021; 10(13):2926. 10.3390/jcm10132926. PMID: 34210105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ansari A, Vincent JP, Moorhouse L, Shimakawa Y, Nayagam S. Risk of early horizontal transmission of hepatitis B virus in children of uninfected mothers in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health 2023; 11:e715–28. [DOI] [PubMed] [Google Scholar]
- 7. Jeng WJ, Papatheodoridis GV, Lok ASF. Hepatitis B. Lancet 2023; 401:1039–52. [DOI] [PubMed] [Google Scholar]
- 8. Beasley RP, Hwang L, Lin C et al. Hepatitis B immune globulin (HBIG) efficacy in the interruption of perinatal transmission of hepatitis B virus carrier state. Initial report of a randomised double-blind placebo-controlled trial. Lancet 1981; 2:388–93. [DOI] [PubMed] [Google Scholar]
- 9. McMahon BJ, Alward W, Hall D et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 1985; 151:599–603. [DOI] [PubMed] [Google Scholar]
- 10. Flores JE, Thompson AJ, Ryan M, Howell J. The global impact of hepatitis B vaccination on hepatocellular carcinoma. Vaccines (Basel) 2022;10(5):793. 10.3390/vaccines10050793. PMID: 35632549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The World Health Organisation . Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections for the Period 2022–2030 . https://iris.who.int/bitstream/handle/10665/360348/9789240053779-eng.pdf?sequence=1. The World Health Organisation, Geneva. 2022.
- 12. Volkov S. Elimination of mother-to-child transmission of HIV, syphilis and hepatitis B. World Health Organisation, Geneva. 2024. [Google Scholar]
- 13. World Health Organisation . Prevention of Mother-to-Child Transmission of Hepatitis B Virus: Guidelines on Antiviral Prophylaxis in Pregnancy, World Health Organisation, Geneva. 2020. [PubMed]
- 14. World Health Organisation WHO releases first-ever global guidance for country validation of viral hepatitis B and C elimination. https://www.who.int/news/item/25-06-2021-who-releases-first-ever-global-guidance-for-country-validation-of-viral-hepatitis-b-and-c-elimination. World Health Organisation, Geneva. 2021.
- 15. UK Health Security Agency . The Green Book on Immunisations: Chapter 18: Hepatitis B UK Health Security Agency. United Kingdom. Published: 20 March 2013; Last updated: 25 April 2024. https://assets.publishing.service.gov.uk/media/6655e46cd470e3279dd332cc/Hepatitis_B_Green_Book_Chapter_18_20240517.pdf.
- 16. Matthews PC, Ocama P, Wang S et al. Enhancing interventions for prevention of mother-to-child- transmission of hepatitis B virus. JHEP Reports 2023; 5:100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. The World Health Organisation. Immunization Coverage . The World Health Organisation https://www.who.int/news-room/fact-sheets/detail/immunization-coverage. 2023.
- 18.Hahné SJ, Veldhuijzen IK, Wiessing L et al. Infection with hepatitis B and C virus in Europe: a systematic review of prevalence and cost-effectiveness of screening. BMC Infect Dis. 2013;13:181. 10.1186/1471-2334-13-181. PMID: 23597411; PMCID: PMC3716892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. European Centre for Disease Prevention and Control . Epidemiological assessment of hepatitis B and C among migrants in the EU/EEA. https://www.ecdc.europa.eu/en/publications-data/epidemiological-assessment-hepatitis-b-and-c-among-migrants-eueea. European Centre for Disease Prevention and Control, Stockholm. 2016.
- 20. European Centre for Disease Prevention and Control . Hepatitis B and C Testing in the EU/EAA: Progress in Reaching the Elimination Targets, European Centre for Disease Prevention and Control, Stockholm. 2021.
- 21. Seedat F, Hargreaves S, Nellums L, Ouyang J, Brown M, Friedland JS. How effective are approaches to migrant screening for infectious diseases in Europe? A systematic review. Lancet Infect Dis 2018; 18:e259–71. [DOI] [PubMed] [Google Scholar]
- 22. Crawshaw A, Farah Y, Deal A et al. Defining the determinants of vaccine uptake and undervaccination in migrant populations in Europe to improve routine and COVID-19 vaccine uptake: a systematic review. Lancet Infect Dis 2022; 22:e254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rechel B, Mladovsky P, Ingleby D, Mackenbach JP, McKee M. Migration and health in an increasingly diverse Europe. Lancet 2013; 381:1235–45. [DOI] [PubMed] [Google Scholar]
- 24. Noori T, van der Werf M, Derrough T et al. Public Health Guidance on Screening and Vaccination for Infectious Diseases in Newly Arrived Migrants Within the EU/EEA. European Centre for Disease Prevention and Control, Stockholm. 2018.
- 25. European Centre for Disease Prevention and Control . Antenatal Screening for HIV, Hepatitis B, Syphilis and Rubella Susceptibility in the EU/EEA, European Centre for Disease Prevention and Control, Stockholm. 2016.
- 26. Lee C, Emeto T, Walsh N. Prevalence of hepatitis B virus amongst refugees, asylum seekers and internally displaced persons in low- and middle-income countries: a systematic review. J Viral Hepat 2023; 30:4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rossi C, Shrier I, Marshall L et al. Seroprevalence of chronic hepatitis B virus infection and prior immunity in immigrants and refugees: a systematic review and meta-analysis. PLoS One 2012; 7:e44611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Page M, McKenzie J, Bossuyt P et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021; 10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Joanna Briggs Institute . JBI Manual for Evidence Synthesis. JBI, Faculty of Health and Medical Sciences. The University of Adelaide, SA 5006 Adelaide, Australia. 2020. [Google Scholar]
- 30. Google . Google Translate. https://translate.google.com/. Accessed June 2022.
- 31. DeepL Translate. DeepL Translate. https://www.deepl.com/translator. Accessed June 2022.
- 32.StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC.
- 33. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014; 72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norman FF, Comeche B, Martínez-Lacalzada M et al. Seroprevalence of vaccine-preventable and non-vaccine-preventable infections in migrants in Spain. J Travel Med. 2021;28(4):taab025. 10.1093/jtm/taab025. PMID: 33611577. [DOI] [PubMed] [Google Scholar]
- 35. Hübschen J, Charpentier E, Weicherding P, Muller CP. Muller C IgG antibody prevalence suggests high immunization needs in newcomers to Luxembourg, 2012. Vaccine 2018; 36:899–905. [DOI] [PubMed] [Google Scholar]
- 36. Hahné S, De Melker H, Kretzchmar M et al. Prevalence of hepatitis B virus infection in The Netherlands in 1996 and 2007. Epidemiol Infect 2012; 140:1469–80. [DOI] [PubMed] [Google Scholar]
- 37. Ruiz-Extremera Á, Diaz-Alcazar M, Munoz-Gamez J et al. Seroprevalence and epidemiology of hepatitis B and C viruses in pregnant women in Spain. Risk factors for vertical transmission. PLoS One 2020; 15:e0233528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dopfer C, Vakilzadeh A, Happle C et al. Pregnancy Related Health Care Needs in Refugees-A Current Three Center Experience in Europe. Int J Environ Res Public Health. 2018;15(9):1934. 10.3390/ijerph15091934. PMID: 30189649; PMCID: PMC6165089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Theuring S, Friedrich-Janicke B, Portner K et al. Screening for infectious diseases among unaccompanied minor refugees in Berlin, 2014-2015. Eur J Epidemiol 2016; 31:707–10. [DOI] [PubMed] [Google Scholar]
- 40. Hourdet A, Rénier M, Van de Steeg F et al. Health condition of patients self-reporting as unaccompanied minors and not recognised as such: retrospective survey within the Hotel-Dieu health platform pass. Bulletin epidemilogique hebdomadaire 2020; 27:531–7. [Google Scholar]
- 41. Giordano D, Provenzano S, Santangelo O et al. Active immunization status against measles, mumps, rubella, hepatitis B in internationally adopted children, surveyed at the university hospital of Palermo. Sicily Ann Ig 2018; 30:431–5. [DOI] [PubMed] [Google Scholar]
- 42. Sollai S, Ghetti F, Bianchi L, de Martino M, Galli L, Chiappini E. Infectious diseases prevalence, vaccination coverage, and diagnostic challenges in a population of internationally adopted children referred to a Tertiary Care Children’s Hospital from 2009 to 2015. Medicine 2017; 96:e6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Belhassen-García M, Perez Del Villar L, Pardo-Lledias J et al. Imported transmissible diseases in minors coming to Spain from low-income areas. Clin Microbiol Infect 2015; 21:370.e5–8. [DOI] [PubMed] [Google Scholar]
- 44. Marrone R, Baglio G, Bruscino G, Costanzo G, Cavani A, Mirisola C. Prevalence of latent tuberculosis infection, hepatitis B, hepatitis C, and syphilis among newly arrived unaccompanied minors living in reception centers in Rome. Int J Infect Dis 2020; 101:126–30. [DOI] [PubMed] [Google Scholar]
- 45. Marquardt L, Krämer A, Fischer F, Prüfer-Krämer L. Health status and disease burden of unaccompanied asylum-seeking adolescents in Bielefeld, Germany: cross-sectional pilot study. Trop Med Int Health 2016; 21:210–8. [DOI] [PubMed] [Google Scholar]
- 46. Finnegan R, Tuite K, Liddy R et al. PTU-111A review of chronic hepatitis B virus infection in children in Ireland. Gut 2015; 64:A110.2–A110. [Google Scholar]
- 47. Tiittala P, Tuomisto K, Puumalainen T et al. Public health response to large influx of asylum seekers: implementation and timing of infectious disease screening. BMC Public Health 2018; 18:1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Janda A, Eder K, Fressle R et al. Comprehensive infectious disease screening in a cohort of unaccompanied refugee minors in Germany from 2016 to 2017: a cross-sectional study. PLoS Med 2020; 17:e1003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pavlopoulou I, Tanaka M, Dikalioti S et al. Clinical and laboratory evaluation of new immigrant and refugee children arriving in Greece. BMC Pediatr 2017; 17:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pauti M, Tomasino A, Mari C et al. Limiting missed opportunities for screening for hepatitis B and C among migrants in precarious situations: the doctors of the world program in France. Bulletin Epidemiologique Hebdomadaire 2016; 13:230–6. [Google Scholar]
- 51. Fougère Y, El Houss S, Suris J et al. High coverage of hepatitis B vaccination and low prevalence of chronic hepatitis B in migrant children dictate a new catch-up vaccination strategy. Vaccine 2018; 36:4501–6. [DOI] [PubMed] [Google Scholar]
- 52. Jablonka A, Solbach P, Wobse M et al. Seroprevalence of antibodies and antigens against hepatitis A-E viruses in refugees and asylum seekers in Germany in 2015. Eur J Gastroenterol Hepatol 2017; 29:939–45. [DOI] [PubMed] [Google Scholar]
- 53. Hampel A, Solbach P, Cornberg M, Schmidt RE, Behrens GMN, Jablonka A. Current seroprevalence, vaccination and predictive value of liver enzymes for hepatitis B among refugees in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2016; 59:578–83. [DOI] [PubMed] [Google Scholar]
- 54. Kloning T, Nowotny T, Alberer M, Hoelscher M, Hoffmann A, Froeschl G. Morbidity profile and sociodemographic characteristics of unaccompanied refugee minors seen by paediatric practices between October 2014 and February 2016 in Bavaria. Germany BMC Public Health 2018; 18:983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Maaßen W, Wiemer D, Frey C et al. Microbiological screenings for infection control in unaccompanied minor refugees: the German Armed Forces Medical Service’s experience. Mil Med Res 2017; 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mockenhaupt F, Barbre K, Jensenius M et al. Profile of illness in Syrian refugees: a GeoSentinel analysis, 2013 to 2015. Euro Surveill 2016; 21:30160. [DOI] [PubMed] [Google Scholar]
- 57. Oliván-Gonzalvo G. Health status and infectious diseases in male unaccompanied immigrant minors from Africa in Spain. Enfermedades infecciosas y microbiologia clinica (English ed) 2021; 39:340–4. [DOI] [PubMed] [Google Scholar]
- 58.Genton PC, Wang J, Bodenmann P et al. Clinical profile and care pathways among unaccompanied minor asylum seekers in Vaud, Switzerland. Int J Adolesc Med Health. 2019;34(3). 10.1515/ijamh-2019-0140. PMID: 32229662. [DOI] [PubMed] [Google Scholar]
- 59. Monpierre O, Baudino P, Rio-Rene P et al. Global health of unaccompanied refugee minors in Gironde (France) between 2011 and 2013. Bull Soc Pathol Exot 2016; 109:99–106. [DOI] [PubMed] [Google Scholar]
- 60. Williams B, Bacci S, Shadwick R et al. Screening for infection in unaccompanied asylum-seeking children and young people. Arch Dis Child 2020; 105:530–2. [DOI] [PubMed] [Google Scholar]
- 61. Bergevin A, Hussain M, Cruz M et al. Medical check-up of newly arrived unaccompanied minors: a dedicated pediatric consultation service in a hospital. Arch Pediatr 2021; 28:689–95. [DOI] [PubMed] [Google Scholar]
- 62. Cochrane A, Evlampidou I, Irish C, Ingle SM, Hickman M. Hepatitis B infection prevalence by country of birth in migrant populations in a large UK city. J Clin Virol 2015; 68:79–82. [DOI] [PubMed] [Google Scholar]
- 63. Ehmsen BK, Biswas D, Jensen N, Krasnik A, Norredam M. Undocumented migrants have diverse health problems. Dan Med J 2014; 61:A4897. [PubMed] [Google Scholar]
- 64. Lembo T, Saffioti F, Chiofalo B et al. Low prevalence of hepatitis B and hepatitis C virus serum markers in a cohort of pregnant women from Southern Italy. Dig Liver Dis 2017; 49:1368–72. [DOI] [PubMed] [Google Scholar]
- 65. Lo Giudice D, Calimeri S, Visalli G, Pino S, di Pietro A, Facciolà A. Prevalence of HBsAg and HCV antibodies in a cohort of pregnant women: the continuous survey as public health tool against pregnant and neonatal complications. New Microbiol 2021; 44:104–10. [PubMed] [Google Scholar]
- 66. López-Fabal F, Gómez-Garcés JL, Gómez-Garcés J. Serological markers of Spanish and immigrant pregnant women in the south of Madrid during the period 2007-2010. Rev Esp Quimioter 2013; 26:108–11. [PubMed] [Google Scholar]
- 67. Ruffini E, Compagnoni L, Tubaldi L et al. Congenital and perinatal infections in the Marche region (Italy): an epidemiological study and differences between ethnic groups. Infez Med 2014; 22:213–21. [PubMed] [Google Scholar]
- 68. Ruffini E, Gesuita R, Compagnoni L et al. Prenatal screening and the prevalence of hepatitis B infection in pregnant women in the Marche region (Central Italy): differences between ethnic groups. Epidemiol Prev 2016; 40:111–5. [DOI] [PubMed] [Google Scholar]
- 69. Sagnelli E, Taliani G, Castelli F et al. Chronic HBV infection in pregnant immigrants: a multicenter study of the Italian Society of Infectious and Tropical Diseases. New Microbiol 2016; 39:114–8. [PubMed] [Google Scholar]
- 70. Santiago B, Blazquez D, Lopez G et al. Serological profile of immigrant pregnant women against HIV, HBV, HCV, rubella, Toxoplasma gondii, Treponema pallidum, and Trypanosoma cruzi. Enferm Infecc Microbiol Clin 2012; 30:64–9. [DOI] [PubMed] [Google Scholar]
- 71. Tasa J, Holmberg V, Sainio S, Kankkunen P, Vehviläinen-Julkunen K. Maternal health care utilization and the obstetric outcomes of undocumented women in Finland - a retrospective register-based study. BMC Pregnancy Childbirth 2021; 21:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wendland A, Ehmsen B, Lenskjold V et al. Undocumented migrant women in Denmark have inadequate access to pregnancy screening and have a higher prevalence Hepatitis B virus infection compared to documented migrants in Denmark: a prevalence study. BMC Public Health 2016; 16:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Karatapanis S, Skorda L, Marinopoulos S et al. Higher rates of chronic hepatitis B infection and low vaccination-induced protection rates among parturients escaping HBsAg prenatal testing in Greece: a 2-year prospective study. Eur J Gastroenterol Hepatol 2012; 24:878–83. [DOI] [PubMed] [Google Scholar]
- 74. Dalmartello M, Parazzini F, Pedron M et al. Coverage and outcomes of antenatal tests for infections: a population based survey in the Province of Trento. Italy J Matern Fetal Neonatal Med 2019; 32:2049–55. [DOI] [PubMed] [Google Scholar]
- 75. Ayoola R, Larion S, Poppers D, Williams R. Clinical factors associated with hepatitis B screening and vaccination in high-risk adults. World J Hepatol 2019; 11:86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. World Health Organisation - Regional Office for Europe . Improving the Health Care of Pregnant Refugee and Migrant Women and Newborn Children: Technical Guidance, World Health Organisation, Geneva. 2018.
- 77. Adil H, Bazinet D, Cooke C et al. Accessing Maternal Health Care in a Hostile Environment. The Reach Alliance. The University of Toronto, Canada. 2022.
- 78. Harvey N. Pregnant women frightened away from healthcare in the UK. Doctors of the World, United Kingdom. 2017. https://www.doctorsoftheworld.org.uk/news/pregnant-women-should-never-be-frightened-away-from-antenatal-care/. Accessed June 2023. [Google Scholar]
- 79. World Health Organisation . Report on the Health of Refugees and Migrants in the WHO European Region: No Public Health without Refugee and Migrant Health. World Health Organisation, Geneva. 2018.
- 80. World Health Organisation . Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection World Health Organisation, Geneva. 2015. [PubMed]
- 81. Evlampidou I, Hickman M, Irish C et al. Low hepatitis B testing among migrants: a cross-sectional study in a UK city. Br J Gen Pract 2016; 66:e382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Piselli P, Samuilova M, Bozorgmehr K et al. Infectious-disease screening and vaccination for refugees and asylum seekers entering Europe in 2015–16: a scoping study of six European Union countries. J Refug Stud 2019; 32:i92–104. [DOI] [PubMed] [Google Scholar]
- 83. World Health Organisation. Hepatitis B Vaccination Coverage . World Health Organisation. World Health Organisation, Geneva., 2022.
- 84. Pohl C, Mack I, Schmitz T, Ritz N. The spectrum of care for pediatric refugees and asylum seekers at a tertiary health care facility in Switzerland in 2015. Eur J Pediatr 2017; 176:1681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giorgi Rossi P, Riccardo F, Pezzarossi A et al. Factors Influencing the Accuracy of Infectious Disease Reporting in Migrants: A Scoping Review. Int J Environ Res Public Health. 2017;14(7):720. 10.3390/ijerph14070720. PMID: 28678172; PMCID: PMC5551158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Serre-Delcor N, Ascaso C, Soriano-Arandes A et al. Health status of asylum seekers, Spain. Am J Trop Med Hyg 2018; 98:300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Picchio C, Nomah D, Araujo S et al. A novel model of care for simplified testing of HBV in African communities during the COVID-19 pandemic in Spain. Sci Rep 2021; 11:17063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Myran DT, Morton R, Biggs BA et al. The Effectiveness and Cost-Effectiveness of Screening for and Vaccination Against Hepatitis B Virus among Migrants in the EU/EEA: A Systematic Review. Int J Environ Res Public Health. 2018;15(9):1898. 10.3390/ijerph15091898. PMID: 30200406; PMCID: PMC6164421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. McCarthy A, Weld L, Barnett E et al. Spectrum of illness in international migrants seen at GeoSentinel clinics in 1997-2009, part 2: migrants resettled internationally and evaluated for specific health concerns. Clin Infect Dis 2013; 56:925–33. [DOI] [PubMed] [Google Scholar]
- 90. Almeida L, Caldas J, Ayres-de-Campos D, Salcedo-Barrientos D, Dias S. Maternal healthcare in migrants: a systematic review. Matern Child Health J 2013; 17:1346–54. [DOI] [PubMed] [Google Scholar]
- 91. Valles K, Prasai K, Roberts L. Sa1511 forecasting hepatitis-related liver disease among migrants to Scandinavia: estimating future disease burdens in five nations. Gastroenterology 2020; 158:S–1316. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is available upon reasonable request to the co-authors.