Abstract
Background
This study explored the effects of different doses of remimazolam tosilate (RT) and propofol combined with remifentanil anesthesia on hemodynamic and inflammatory responses in patients undergoing laparoscopic surgery.
Subjects and Methods
Ninety patients with a BMI of less than 35 kg/m², classified as ASA II–III and scheduled for laparoscopic surgery, were enrolled in this study. Patients were divided into three groups: low-dose RT group (A), high-dose RT group (B), and propofol group (C). The changes in hemodynamic indices such as SBP, DBP, HR, MAP, and inflammatory response indices such as IL-6, SAA, CRP, and PCT, along with extubation time and doses of sufentanil, remifentanil, urapidil, and phenylephrine, were compared among the three groups.
Results
There were no statistically significant differences in extubation time, doses of sufentanil and remifentanil, or the usage rates and average doses of urapidil and phenylephrine between the three groups. The average dose of phenylephrine in group A was lower than in group B and group C, with a statistically significant difference. There were no statistically significant differences among the groups in SBP, DBP, HR, and MAP from T0 to T2, nor in IL-6, SAA, CRP, or PCT levels.
Conclusion
Using RT for induction and maintenance of anesthesia in laparoscopic surgery ensures stable hemodynamic and inflammatory responses in patients. Low-dose RT may reduce the usage rate and dose of vasopressors such as phenylephrine during surgery.
Keywords: remimazolam tosilate, propofol, remifentanil, hemodynamic, inflammatory, laparoscopic surgery
Introduction
With the advancement and establishment of laparoscopic minimally invasive technology alongside the formulation of guidelines for rapid rehabilitation surgery,1,2 laparoscopic surgery has been increasingly adopted in clinical practice. Compared to traditional open surgery, laparoscopic procedures offer the benefits of reduced trauma and quicker recovery. However, the stimulation from the artificial pneumoperitoneum and surgical manipulation during laparoscopic surgery can still trigger a significant stress response and postoperative pain, hindering early recovery. Sedative hypnotics, muscle relaxants, and opioids are routinely used for the induction and maintenance of general anesthesia. Propofol, the most commonly used intravenous anesthetic, is associated with injection pain and, with prolonged infusion, can lead to drug accumulation and propofol infusion syndrome. Remimazolam tosilate (RT), a novel, water-soluble, ultra-short-acting benzodiazepine, primarily acts on the γ-aminobutyric acid A (GABAA) receptor, inhibiting neuronal function and reducing neuronal excitability, which dimi nishes body activity and induces sedation and amnesia.3,4 RT effectively mitigates the adverse reactions associated with other drugs, making it an ideal candidate for anesthesia induction and maintenance.5,6 Furthermore, RT’s rapid onset, adequate sedation, safety profile, high-quality awakening, quick cognitive recovery, and good patient tolerance suggest it has significant potential for broader clinical use.7,8 Despite its promising profile, no studies have recommended specific initial and maintenance doses of RT for general anesthesia in laparoscopic patients nor examined its effects on their hemodynamic and inflammatory responses. This study investigates the impact of various doses of RT and propofol combined with remifentanil anesthesia on these parameters in laparoscopic surgery patients to provide a theoretical basis for optimizing RT’s clinical application.
Subjects and Methods
This study was approved by the ethics committee of The Third Affiliated Hospital of Sun Yat-sen University Yuedong Hospital and registered in the Chinese Clinical Trial Registration Center (ChiCTR2100042461). Informed consent was obtained from patients or their family members. Some elderly patients experiencing writing difficulties provided verbal consent after being fully informed about the purpose of the study; subsequently, consent forms were signed by the patients’ representatives.
The required sample size was estimated using reference data from experimental results at time points T1 and T2 in a pre-trial involving 30 patients (10 in each group), who were grouped according to the study protocol. As the experimental data for each index did not initially conform to a normal distribution, transformations were applied to the data to achieve normality. The formula for estimating the sample size was n=
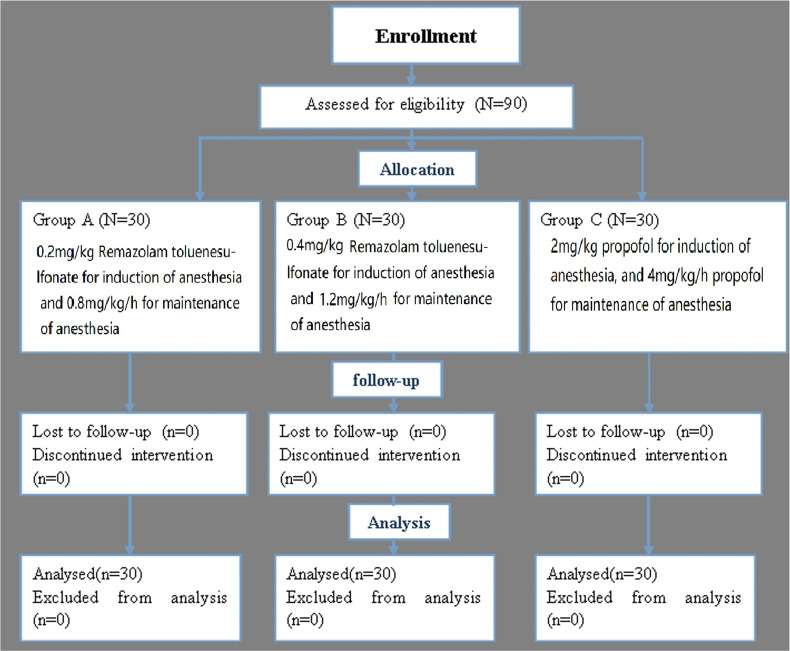
A total of 90 patients scheduled for laparoscopic surgery from December 2020 to November 2022 were included in this study, as illustrated in Figure 1. This cohort consisted of 38 patients undergoing gynecological surgery, 48 undergoing general surgery, and 4 undergoing urological surgery. The average age of the patients was (50.1±13.1) years, ranging from 23 to 79 years. The study involved 25 males and 65 females, with 58 patients classified as American Society of Anesthesiologists (ASA) Class II and 32 as ASA Class III, as detailed in Table 1. The inclusion criteria were: (1) patients older than 18 years scheduled for gynecological, general, or urological surgery under laparoscopy; (2) classified as ASA Class II–III; (3) body mass index (BMI) ≤ 35 kg/m². The exclusion criteria included individuals with known allergies to RT or a personal or familial history of severe allergies, severe circulatory, mental, or nervous system diseases, deafness, muteness, alcoholism, respiratory conditions, liver or kidney dysfunction, blood coagulation or fat metabolism disorders, or any conditions deemed unsuitable by the researchers for this clinical study.
Figure 1.
Schematic illustration of the randomized trial design, including enrollment, intervention allocation, and analysis.
Table 1.
Comparison of general data of patients in each group
| Item | Group A | Group B | Group C | F/χ2 | P |
|---|---|---|---|---|---|
| Age ( |
47.5±11.8 | 52.2±13.8 | 50.5±13.25 | 1.198 | 0.307 |
| BMI ( |
23.19±3.34 | 23.59±3.31 | 24.30±3.48 | 0.845 | 0.432 |
| Gender (male/female) | 7/23 | 8/22 | 10/20 | 0.775 | 0.679 |
| ASA Class (II/III) | 18/12 | 20/10 | 20/10 | 0.689 | 0.709 |
| Surgical type (gynecological/general/urological surgery) | 16/13/1 | 11/18/1 | 11/17/2 | 1.184 | 0.553 |
| Surgical time ( |
131.37±80.11 | 132.47±67.43 | 157.00±87.93 | 1.030 | 0.361 |
Abbreviations: BMI, Body mass index; ASA, American Society of Anesthesiologists.
Patients were randomly divided into three groups using a random number table: the low-dose RT group (0.2 mg/kg; 30 patients; Group A), the high-dose RT group (0.4 mg/kg; 30 patients; Group B), and the propofol group (30 patients; Group C).
Upon entering the operation room, all patients were administered oxygen via a mask. Monitoring included electrocardiogram (ECG), heart rate (HR), blood pressure (BP), peripheral capillary oxygen (SpO2), and Bispectral index (BIS). Peripheral venous access was established, and vital signs were recorded. Anesthesia induction was performed with low-dose RT (0.2 mg/kg) in Group A, high-dose RT (0.4 mg/kg) in Group B, and propofol (2 mg/kg) in Group C. Maintenance of anesthesia was managed with RT (0.8 mg/kg/h) in group A, RT (1.2 mg/kg/h) in group B, and propofol (4 mg/kg/h) in group C. Remifentanil (6 ng/mL) was administered using target-controlled infusion (TCI) at a plasma concentration, and cis-atracurium (0.25 mg/kg) was injected intravenously in all three groups. After ensuring adequate muscle relaxation for tracheal intubation, the procedure was performed under guidance from a visual laryngoscope. Following tracheal intubation, bilateral lung sounds were auscultated to verify symmetry. Once confirmed, the catheter was secured in the correct position and connected to the anesthesia machine for mechanical ventilation. Ventilator settings were initially set to a tidal volume of 6–8 mL/kg, a respiratory rate of 12–16 breaths/min, an inhalation/respiration ratio of 1:2, and an oxygen flow of 1 L/min, with adjustments made based on intraoperative SpO2 and PETCO2 readings. SpO2 was maintained between 98% and 100% during surgery, and PETCO2 was kept between 35 and 45 mmHg. The BIS was maintained between 40 and 60. Patients in all three groups received continuous intravenous infusions of cis-atracurium (0.1 mg/kg/h) to maintain muscle relaxation, which was discontinued 20 min before the end of the surgery. Concurrently, sufentanil (0.2μg/kg) was administered intravenously. Throughout the procedure, sufentanil, muscle relaxants, and vasoactive drugs were adjusted based on HR and BP to maintain hemodynamic stability and ensure patient safety and smooth progression of the surgery.
If hypertension occurred during the surgery [the mean arterial pressure (MAP) increased by more than 20% of the basic value], firstly, the sufentanil (0.2 μg/kg) was initially administered; if ineffective, urapidil (5–10 mg) was given intravenously. For hypotension (MAP decrease of more than 20% from baseline), phenylephrine (100 μg) was administered. If the HR dropped below 40 beats/min, atropine (0.3 mg) was administered intravenously.
At the end of the surgery, the intravenous administrations of remifentanil, RT, or propofol were stopped. Postoperatively, patients were transferred to the anesthesia recovery room. Once patients regained consciousness and could be prompted to open their eyes, the tracheal tube was removed with a spontaneous respiratory rate ≥12 breaths/min, tidal volume ≥6 mL/kg, and SpO2 ≥98%. Patients were returned to the ward when their Steward awakening score reached ≥4.
Blood samples (2 mL each) were collected from all patients in the three groups at three time points: 1 h before anesthesia (T0), 1 h after anesthesia (T1), and 1 h post-surgery (T2). These samples were analyzed using a magnetic immunoassay kit to measure inflammatory response indices, specifically plasma concentrations of interleukin 6 (IL-6), serum amyloid A (SAA), C-reactive protein (CRP), and procalcitonin (PCT), in strict accordance with the kit’s instructions. Hemodynamic indices such as systolic blood pressure (SBP), diastolic BP (DBP), HR, and MAP were monitored in T0, T1, and T2 patients. The doses of sufentanil, remifentanil, phenylephrine, urapidil, and the extubation time (from the end of surgery to tracheal extubation) for the three groups were also recorded.
SPSS21.0 was utilized for the statistical analysis of data in this study. For categorical data, differences between groups were assessed using the appropriate Chi-square test or fisher’s exact test. The Shapiro–Wilk test determined whether the data conformed to a normal distribution. Measurement data that followed a normal distribution were expressed as mean±standard deviation, and variance analysis was conducted. Data not normally distributed were presented as medians (P25, P75), and the Kruskal–Wallis test was used to evaluate these. A p-value of less than 0.05 was considered to indicate statistical significance. When the ANOVA or Kruskal–Wallis test P is less than 0.05, Holm’s method or Dunn’s test with a Bonferroni adjustment are used for pairwise comparison of post hoc tests.
Results
There were no significant differences in age, BMI, gender, ASA class, type of surgery, and surgical duration among the three groups (all P>0.05), indicating comparability across the groups, as detailed in Table 1. Similarly, no statistically significant differences were observed in SBP, DBP, HR, and MAP among the groups from T0 to T2 (all P>0.05), as shown in Table 2.
Table 2.
Effects of different doses of RT or propofol on the hemodynamics of patients (
| Item | Group A | Group B | Group C | F | P | |
|---|---|---|---|---|---|---|
| T0 | SBP (mm Hg) | 127.53±16.33 | 128.60±16.29 | 132.90±15.78 | 0.930 | 0.399 |
| DBP (mm Hg) | 74.83±9.39 | 78.13±9.06 | 77.07±10.53 | 0.907 | 0.408 | |
| HR (beats/min) | 72.77±8.96 | 76.83±10.72 | 76.60±12.23 | 1.361 | 0.262 | |
| MAP (mm Hg) | 91.90±12.04 | 97.10±11.64 | 95.38±12.23 | 1.468 | 0.236 | |
| T1 | SBP (mm Hg) | 116.20±17.14 | 115.70±26.56 | 119.40±20.12 | 0.258 | 0.773 |
| DBP (mm Hg) | 73.23±9.03 | 74.53±8.89 | 72.77±12.20 | 0.244 | 0.784 | |
| HR (beats/min) | 61.20±11.38 | 64.67±11.86 | 63.03±10.04 | 0.730 | 0.485 | |
| MAP (mm Hg) | 86.60±11.58 | 87.77±13.03 | 87.77±14.80 | 0.069 | 0.934 | |
| T2 | SBP (mm Hg) | 135.63±18.34 | 138.70±19.39 | 135.70±30.22 | 0.170 | 0.844 |
| DBP (mm Hg) | 79.27±9.99 | 79.87±13.38 | 80.57±10.89 | 0.096 | 0.909 | |
| HR (beats/min) | 80.03±15.07 | 82.50±16.00 | 78.83±12.67 | 0.489 | 0.615 | |
| MAP (mm Hg) | 96.57±14.12 | 100.10±14.75 | 101.36±13.33 | 0.936 | 0.396 | |
Abbreviations: SBP, Systolic blood pressure; DBP, Diastolic blood pressure; HR, Heart rate; MAP, Mean artery pressure.
Furthermore, there were no statistically significant differences in IL-6, SAA, CRP, and PCT levels among the three groups at T0–T2 (all P>0.05), as presented in Table 3. Similarly, no statistically significant differences were found in the extubation times and the doses of sufentanil and remifentanil among the three groups (all P>0.05). The usage rates, average doses of urapidil, and the usage rates of phenylephrine also showed no significant differences among the groups (all P>0.05). However, the average dose of phenylephrine in group A was significantly lower than in groups B and C, with these differences reaching statistical significance (P<0.05), as outlined in Table 4.
Table 3.
Effects of different doses of RT or propofol on inflammatory response indexes in patients [M (P25, P75)]
| Item | Group A | Group B | Group C | H | P | |
|---|---|---|---|---|---|---|
| T0 | IL-6 (μg/L) | <4(<4,6.75) | <4 (<4,7.23) | <4 (<4,5.94) | 4.124 | 0.121 |
| SAA (mg/L) | <5(<5,7.32) | <5 (<5,7.21) | <5 (<5,7.39) | 0.039 | 0.981 | |
| CRP (mg/L) | <5 (<5,5.63) | <5 (<5,5.85) | <5 (<5,5.98) | 1.320 | 0.517 | |
| PCT (μg/L) | 0.09(0.05,0.17) | 0.12(0.08,0.19) | 0.08(0.04,0.15) | 4.358 | 0.113 | |
| T1 | IL-6 (μg/L) | <4 (<4,6.32) | <4 (<4,6.65) | <4 (<4,5.29) | 2.523 | 0.283 |
| SAA (mg/L) | <5 (<5,6.97) | <5 (<5,7.01) | <5 (<5,7.10) | 1.134 | 0.567 | |
| CRP (mg/L) | <5 (<5,5.68) | <5 (<5,5.54) | <5 (<5,5.51) | 0.694 | 0.707 | |
| PCT (μg/L) | 0.09(0.02,0.13) | 0.11(0.05,0.16) | 0.07(0.03,0.12) | 4.867 | 0.093 | |
| T2 | IL-6 (μg/L) | 4.89(<4,9.25) | 5.23(<4,8.96) | 4.82(<4,8.84) | 1.324 | 0.516 |
| SAA (mg/L) | 6.79(<5,10.65) | 6.83(<5,10.81) | 7.01(<5,10.87) | 0.758 | 0.685 | |
| CRP (mg/L) | <5 (<5,7.54) | <5 (<5,7.86) | 5.03(<5,7.59) | 1.426 | 0.490 | |
| PCT (μg/L) | 0.12(0.07,0.16) | 0.15(0.09,0.24) | 0.09(0.05,0.17) | 5.225 | 0.077 | |
Abbreviations: RT, Remimazolam tosilate; IL-6, interleukin 6; SAA,Serum amyloid A; CRP, C-reactive protein; PCT,Procalcitonin.
Table 4.
Comparison of the extubation time among patients receiving different doses of Remimazolam tosilate or propofol, and the doses of sufentanil, remifentanil, phenylephrine, and urapidil among three groups
| Item | Group A | Group B | Group C | F/χ2 | P | |
|---|---|---|---|---|---|---|
| Extubation time ( |
24.00±14.35 | 24.23±11.65 | 22.33±10.70 | 0.194 | 0.824 | |
| Sufentanil ( |
40.95±13.14 | 41.87±11.56 | 37.7±10.13 | 1.055 | 0.353 | |
| Remifentanil ( |
2.25±1.20 | 2.27±1.01 | 2.52±1.38 | 0.466 | 0.629 | |
| Urapidil | Cases [n(%)] | 2 (6.7) | 1 (3.3) | 1 (3.3) | 0.523 | 0.770 |
| Average dose (mg) | 20 | 40 | 10 | - | - | |
| Phenylephrine | Cases [n(%)] | 2 (6.7) | 6 (20) | 7 (23.3) | 3.360 | 0.186 |
| Average dose ( |
75.00±35.36 | 1543±1112.14 | 1611.67±812.41 | 2.139 | 0.164 | |
Discussion
Propofol, a sedative drug known for its dose-dependent properties, can cause significant circulatory fluctuations and injection pain. In recent years, there has been an increased interest in identifying anesthetic agents that minimize hemodynamic fluctuations and inflammatory responses, particularly for minimally invasive procedures such as laparoscopic surgery. RT, a novel benzodiazepine, offers a compelling pharmacological profile with rapid onset and short duration of action. Its metabolism via plasma esterases, rather than hepatic pathways, makes it less reliant on organ function, an advantage in patients with compromised liver or kidney function.
Clinical studies have highlighted RT’s potential to maintain more stable hemodynamic parameters than traditional agents like Propofol and Midazolam. Additionally, the inflammatory response to surgery, as indicated by markers such as IL-6, CRP, and PCT), plays a crucial role in postoperative recovery and complications. Previous research has shown that anesthetics can differentially impact these markers, with benzodiazepines often leading to milder responses.
Our study builds on this background by rigorously comparing the effects of low and high doses of RT versus Propofol on both hemodynamic stability and inflammatory responses in laparoscopic surgery. This research addresses a significant gap in current anesthesia literature and aims to provide insights into more effective anesthesia management strategies.
Multiple clinical trials have demonstrated that procedures such as bronchoscopy, gastroduodenoscopy, and colonoscopy can be effectively performed under sedation with remimazolam. Compared to midazolam, remimazolam offers superior sedation in endoscopic procedures, with faster onset and quicker recovery, and minimally impacts circulation and respiration.9,10 Patients typically experience a slight increase in HR post-administration without injection pain or severe adverse reactions, underscoring an excellent safety profile. In this study, RT was utilized for both the induction and maintenance of anesthesia, achieving a similar anesthetic effect while exerting minimal impact on circulation.11,12
Chen Yu et al13 have further established that using remimazolam in elderly patients presents significant advantages by maintaining a stable hemodynamic state without notable changes in cardiac index and MAP, and its dosing does not significantly affect patient hemodynamics during surgery. Notably, a single injection of remimazolam (0.3 mg/kg) can achieve rapid anesthesia induction and hemodynamic stability while ensuring safety.
In this study, the depth of anesthesia was continuously monitored, with the BIS) maintained between 40 and 60 throughout the surgical procedure. Blood samples were collected twice: T1, one hour after anesthesia initiation, and T2, one-hour post-surgery. The timing of these collections allowed for a clear distinction between the two samples. IL-6 is a critical inflammatory marker that typically peaks within 2 h after inflammatory stimulation. Because the anesthesia and surgical duration exceeded 2 h, monitoring IL-6 levels was deemed clinically relevant.
Two doses of RT, 0.2 mg/kg for induction and 0.8 mg/kg/h for maintenance, and a higher regimen of 0.4 mg/kg for induction with 1.2 mg/kg/h for maintenance were compared with propofol dosing at 2 mg/kg for induction and 4 mg/kg/h for maintenance. The comparative analysis revealed no statistical differences in hemodynamic indices such as SBP, DBP, HR, and MAP (Table 2) or inflammatory response markers such as IL-6, SAA, CRP, and PCT (Table 3). This indicates that RT can match the efficacy of conventional propofol in both the induction and maintenance of anesthesia in laparoscopic surgery, concerning the stability of hemodynamic and inflammatory responses.
Due to the limitations in the sensitivity of the bedside magnetic sensitivity testing method used to measure the four inflammatory stress response indicators, results below the normal lower limit held no clinical significance. Only values like IL-6<4, SAA<5, CRP<5, and PCT<0.02 could be detected, which are qualitative and challenging to depict statistically. Thus, a tabular format was retained for data presentation. However, after consultation with statistical experts, the data description method was changed to the median (P25, P75) and the statistical method to the Kruskal–Wallis test to better handle the data’s non-parametric nature.
Wesolowski et al14 conducted a randomized controlled trial on patients undergoing cardiac surgery, revealing that the use of norepinephrine was significantly reduced during surgery for patients who received remimazolam-fentanyl anesthesia compared to those under propofol-sevoflurane anesthesia. Reflecting on the results of this study, it appears that while there was no statistical significance in the use rate of phenylephrine between the low-dose RT group (6.7%), high-dose RT group (20%) and propofol group (20.3%) due to the small sample size, an increase in sample size might demonstrate a significantly lower use rate of phenylephrine in the low-dose RT group compared to the other two groups.
Additionally, the data indicate that the dose of phenylephrine used in the low-dose RT group was significantly lower than in the high-dose RT and propofol groups (Table 4). This suggests that using RT for anesthesia induction (0.2 mg/kg) and maintenance (0.8 mg/kg/h) could reduce the usage rate and dose of vasoconstrictors such as phenylephrine in laparoscopic surgery.
Under general anesthesia, especially at high doses, drugs often cause vasodilation, leading to hypotension. This can be managed by timely blood volume replenishment or alpha-1 receptor agonists, which do not significantly affect the heart rate. Given that HR was a critical observational indicator in this study, and to avoid the influence of beta-1 receptor agonists on HR, preference was given to alpha-1 receptor agonists.
The research also confirms that high doses of RT and propofol are likely to induce hypotension, frequently necessitating the use of vasopressors to manage this condition.
Additionally, this study demonstrated that using high and low doses of RT for induction and maintenance of anesthesia in laparoscopic surgery did not result in prolonged extubation times compared to propofol. Furthermore, there were no increases in the doses of sufentanil and remifentanil or the usage rate and dose of urapidil.
Conclusion
The findings of this study confirm that RT, when used at both low and high doses for anesthesia induction and maintenance, effectively maintains hemodynamic and inflammatory stability during laparoscopic surgery. Notably, low-dose RT significantly reduced the usage and dosage of phenylephrine, suggesting a potential advantage in managing intraoperative BP without extensive reliance on additional vasopressors. As supported by prior research, these results closely align with the hypothesized benefits of RT as an anesthetic that minimally disturbs cardiovascular function.
However, the lack of significant differences in other hemodynamic and inflammatory markers across the study groups suggests that while RT is as effective as propofol, it may not offer additional benefits in all parameters measured. Future studies should, therefore, aim to explore these findings in a larger, multi-center cohort to verify the consistency of these results and better delineate the clinical scenarios most suited for RT usage. Such research could offer more definitive guidance for anesthetic choices in patients undergoing minimally invasive surgeries, potentially enhancing patient outcomes and surgical efficiencies.
Acknowledgments
We are very grateful for the support of Shenzhen Libang Precision Instrument Co., Ltd.
Funding Statement
China Guangdong Hospital Association Yiyang Health Charity Fund.
Abbreviations
RT, Remimazolam tosilate; BMI, Body Mass Index; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; HR, Heart rate; MAP, Mean artery pressure; IL-6, Interleukin 6; SAA, Serum amyloid A; CRP, C-reactive protein; PCT, Procalcitonin; GABAA, Gamma-aminobutyric acid A; ECG, Electrocardiogram; BP, Blood pressure; SpO2, Peripheral capillary oxygen saturation; TCI, Target-Controlled Infusion; ASA, American Society of Anesthesiologists; PETCO2, End expiratory carbon dioxide partial pressure; BIS, Bispectral index.
Data Sharing Statement
The corresponding author can provide the original data of this study.
Ethics Approval and Consent to Participate
This study complies with the Declaration of Helsinki. The clinical trial was registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn/index.aspx) with the registration number ChiCTR2100042461 in January 2021. The study has been approved by the Ethical Committee of The Third Affiliated Hospital of Sun Yat-sen University Yuedong Hospital under the registration number 2020H-03. Written informed consent has been collected from every subject.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Gadiya AD, Koch JEJ, Patel MS, et al. Enhanced recovery after surgery (ERAS) in adolescent idiopathic scoliosis (AIS): a meta-analysis and systematic review. Spine Deform. 2021;9(4):893–904. doi: 10.1007/s43390-021-00310-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu T, Lai T, Chen J, et al. Effect of remimazolam induction on hemodynamics in patients undergoing valve replacement surgery: a randomized, double‐blind, controlled trial. Pharmacol Res Perspect. 2021;9(5):e00851. doi: 10.1002/prp2.851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanious MK, Beutler SS, Kaye AD, et al. New hypnotic drug development and pharmacologic considerations for clinical anesthesia. Anesthesiol Clin. 2017;35(2):e95–113. doi: 10.1016/j.anclin.2017.01.017 [DOI] [PubMed] [Google Scholar]
- 4.Masui K, Stöhr T, Pesic M, et al. A population pharmacokinetic model of remimazolam for general anesthesia and consideration of remimazolam dose in clinical practice. J Anesth. 2022;36(4):493–505. doi: 10.1007/s00540-022-03079-y [DOI] [PubMed] [Google Scholar]
- 5.Lohmer LL, Schippers F, Petersen KU, et al. Time-to-event modeling for remimazolam for the indication of induction and maintenance of general anesthesia. J Clin Pharmacol. 2020;60(4):505–514. doi: 10.1002/jcph.1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pastis NJ, Yarmus LB, Schippers F, et al. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. 2019;155(1):137–146. doi: 10.1016/j.chest.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Sang N, Song K, et al. Psychomotor recovery following remimazolam-induced sedation and the effectiveness of flumazenil as an antidote. Clin Ther. 2020;42(4):614–624. doi: 10.1016/j.clinthera.2020.02.006 [DOI] [PubMed] [Google Scholar]
- 8.Sheng XY, Liang Y, Yang XY, et al. Safety, pharmacokinetic and pharmacodynamics properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol. 2020;76(3):383–391. doi: 10.1007/s00228-019-02800-3 [DOI] [PubMed] [Google Scholar]
- 9.Pamblanco D, Borkett K, Rift D, et al. A phase lib study comparing the safety and efficacy of remimazolam and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2016;83(5):984–992. doi: 10.1016/j.gie.2015.08.062 [DOI] [PubMed] [Google Scholar]
- 10.Schippers F, Pesic M, Saunders R, et al. Randomized crossover trial to compare abuse liability of intravenous remimazolam versus intravenous midazolam and placebo in recreational central nervous system depressant users. J Clin Pharmacol. 2020;60(9):1189–1197. doi: 10.1002/jcph.1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rex DK, Bhandari R, Lorch DG, et al. Safety and efficacy of remimazolam in high risk colonoscopy: a randomized trial. Digestive Liver Dis. 2021;53(1):94–101. doi: 10.1016/j.dld.2020.10.039 [DOI] [PubMed] [Google Scholar]
- 12.Petkus H, Willer BL, Tobias JD. Remimazolam in a pediatric patient with a suspected family history of malignant hyperthermia. J Med Cases. 2022;13(8):386–390. doi: 10.14740/jmc3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Cai S, Zhu XG, et al. Sedative effect of remimazolam during induction of general anesthesia in elderly patients. Chin J Anesthesiol. 2020;40(8):974–976. Chinese. [Google Scholar]
- 14.Wesolowski AM, Zaccagnino MP, Malapero RJ, et al. Remimazolam: pharmacologic considerations and clinical role in anesthesiology. Pharmacotherapy. 2016;36(9):1021–1027. doi: 10.1002/phar.1806 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author can provide the original data of this study.