Abstract
The biological basis for investigating dichlorodiphenyltrichloroethane (DDT) exposure and breast cancer risk stems from in vitro and animal studies indicating that DDT has estrogenic properties. The objective of this study was to update a meta-analysis from 2004 which found no association between dichlorodiphenyldichloroethylene (DDE) and breast cancer. We searched PubMed and Web of Science for studies published through June 2012 assessing DDT/DDE exposure and breast cancer. Summary Odds Ratios (ORs) with 95% confidence intervals (CIs) were calculated for the prevalence of breast cancer in the highest versus the lowest exposed groups for DDT and DDE. Difference of means of exposure for cases versus controls was analyzed for DDT and DDE. From the 500 studies screened, 46 were included in the meta-analysis. Slightly elevated, but not statistically significant summary ORs were found for DDE (1.05; 95% CI: 0.93 – 1.18) and DDT (1.02; 95% CI: 0.92 – 1.13). Lipid adjusted difference of means analysis found a significantly higher DDE concentration in cases versus controls (11.30 ng/g lipid; p = 0.01). No other difference of means analysis found significant relationships. The existing information does not support the hypothesis that exposure to DDT/DDE increases the risk of breast cancer in humans.
Keywords: DDT, DDE, Breast Cancer, Systematic Review, Meta-Analysis
1. Introduction:
In the 1940s, dichlorodiphenyltrichloroethane (DDT) became the first modern synthetic insecticide and was initially used in both military and civilian settings to combat malaria, typhus and other insect-borne human diseases, among other uses (ATSDR, 2002). By the 1960s, its use had become global. However, the United States Department of Agriculture began to implement regulations on many of DDT’s uses in response to research implicating its reduced efficacy (namely resistance in a number of insect species) and environmental and toxicological effects (ATSDR, 2002). The U.S. banned DDT in 1972, but its use continues in places such as Africa, in order to control malaria epidemics (ATSDR, 2002; Lopez-Cervantes et al., 2004; Smith, 1999).
Despite its ban in many countries, DDT remains persistent in the environment and in the food chain with a half-life of soil ranging from 2-15 years (ATSDR, 2002; Laden et al., 1999; Snedeker, 2001). DDT and its major metabolites, dichlorodiphenyldichloroethylene (DDE) and dichlorodiphenyldichloroethane (DDD), are lipophilic and therefore, have long half-lives via bioaccumulation in body fat. DDT has a half-life in serum of about 10 years; the half-life in adipose varies depending on individual fat composition and DDE has an even longer half-life, estimated at 4.2-5.6 years on a population basis (ATSDR, 2002; Smith, 1999). It has been suggested that DDT exposure may be associated with breast cancer because in vitro and animal studies have shown DDT to have estrogenic properties (Longnecker et al., 1997). Because increased estrogen levels have been linked with breast cancer, it is possible that exposure to DDT may increase breast cancer risk (Longnecker et al., 1997).
In the past twenty years, a number of epidemiological studies have been conducted to investigate the potential relationship between DDT exposure and breast cancer risk. Wolff et al. (1993) were the first to find a positive association between exposure to DDE and breast cancer risk. Many subsequent studies, however, failed to replicate these results (reviewed in Lopez-Cervantes et al., 2004). The available literature presents heterogeneous study designs, as shown by the meta-analyses conducted by Lopez-Cervantes and colleagues (2004). For example, although the biological proxies for DDT exposure have primarily been blood serum and breast adipose (Lopez-Cervantes et al., 2004), adipose obtained from biopsy samples taken from the abdomen and buttocks have been used to overcome bias associated with using benign breast disease control populations (Raaschou-Nielsen et al., 2005; van’t Veer et al., 1997). Additionally, the methods used to control for the effect of circulating lipids on serum concentrations of DDT and its metabolites varied across studies (Lopez-Cervantes et al,. 2004). This lack of standardization across studies presents a potential reason for the failure to find an association between exposure and breast cancer risk. Two meta-analysis studies have evaluated the available literature on DDT exposure and breast cancer risk (Laden et al., 2001a; Lopez-Cervantes et al., 2004). Since the 2004 meta-analysis, 12 new epidemiological studies investigating the relationship between DDT/DDE exposure and breast cancer risk were published -- including three nested case-control studies. Here we present a systematic review and meta-analysis assessing whether the most recent epidemiological studies support the hypothesis that DDT exposure increases the risk of breast cancer in the female population using both OR and mean exposure as effect size measures.
2. Methods:
2.1. Study Selection:
PubMed and Web of Science databases were searched through June 2012 for English-language publications. The following keywords search strategy were used: {"breast neoplasms"[MeSH Terms] OR ("breast"[All Fields] AND "neoplasms"[All Fields]) OR "breast neoplasms"[All Fields] OR ("breast"[All Fields] AND "cancer"[All Fields]) OR "breast cancer"[All Fields] Or "mammary glands, human"[MeSH Terms] OR { ("mammary"[All Fields] AND "glands"[All Fields] AND "human"[All Fields]) OR "human mammary glands"[All Fields] OR ("mammary"[All Fields] AND "gland"[All Fields]) OR "mammary gland"[All Fields] And ("ddt"[MeSH Terms]} OR "ddt"[All Fields] OR "dichlorodiphenyltrichloroethane"[All Fields] or (1[All Fields] AND 1-dichloro-2[All Fields] AND 2-bis[All Fields]) AND 4-chlorophenyl[All Fields] AND ("ethylene"[Supplementary Concept] OR "ethylene"[All Fields] OR "ethylenes"[MeSH Terms] OR "ethylenes"[All Fields])} or {dde[All Fields] OR dimic[All Fields] OR ("dimethylaminonaphthalene-5-sulfonaminoethylmethylamine"[Supplementary Concept] OR "dimethylaminonaphthalene-5-sulfonaminoethylmethylamine"[All Fields] OR "ddns"[All Fields]) OR (("ddt"[MeSH Terms] OR "ddt"[All Fields]) AND dehydrochloride[All Fields]) OR ("dichlorodiphenyl dichloroethylene"[MeSH Terms] OR ("dichlorodiphenyl"[All Fields] AND "dichloroethylene"[All Fields]) OR "dichlorodiphenyl dichloroethylene"[All Fields] OR "dichlorodiphenyldichloroethylene"[All Fields]) OR "dichlorodiphenyl dichloroethene"[All Fields]}
References cited in the selected articles were also reviewed for potentially eligible studies. Studies were a priori excluded according to the following criteria: 1) commentary; 2) contained duplicate data (e.g. used the same data/cohort as a study already included in the pool of eligible studies); 3) did not have date on the control group; 4) did not have exposure data; 5) did not have breast cancer risk data or that the risk could not be calculated based on the data available. Also, we excluded studies that were not published in English.
The a priori eligibility criteria of the studies to be included in the analyses are the following: (i) the paper must examine a correlation between breast cancer and DDT/DDE exposure; and (ii) the study must have primary human data (e.g. no animal studies, review papers, etc.)
2.2. Data Extraction:
The following data was extracted for each study: Study authors, publication year, country of study, study years, study design, sample type, mean exposure level and standard deviation, OR and corresponding 95% CI, and any factors used for adjustment of mean exposure levels and/or odds ratios. All data were extracted independently by two authors (F.S. and S.I.).
2.3. Statistical Analysis:
Summary odds ratio and 95% confidence intervals were estimated by comparing the number of breast cancer patients whose blood DDT/DDE concentrations fell in the highest range versus the number of patients whose samples had the lowest concentrations. ORs were pooled based on random-effects (where all studies estimate an average of a distribution of true effect size) modeling. Cut points for the different concentration groups varied from study to study. Thus, the highest versus the lowest exposure groups were determined based on the specifications within each study. We stratified according to menopausal status to look for potential differences in breast cancer risk among cases diagnosed with pre-menopausal versus post-menopausal breast cancer. The literature suggests that there is a difference between pre-menopausal and post-menopausal breast cancer due to difference in lifetime exposure to estrogen. Research done by the Collaborative Group on Hormonal Factors in Breast Cancer (2012) found that premenopausal women had a greater risk of breast cancer than postmenopausal women of similar age.
For the difference of means analysis, effect size was calculated by the difference between the mean DDT/DDE exposure of the breast cancer cases and the control group (comparison group without breast cancer). Each mean difference was weighted according to the inverse of its variance, and the average was taken (weighted mean difference, or WMD). The WMD in each study was pooled using a random-effects model. Results are given with 95% confidence intervals. Data were analyzed using the Comprehensive Meta-Analysis (CMA) software, version 2.
Between-study heterogeneity in the results of the studies was assessed using a chi-square test and the I2 measure of inconsistency based on both fixed- and random-effects modeling. Significant heterogeneity was defined as a chi-square test p-value < 0.1. I2 takes values between 0% and 100% with higher values denoting a greater degree of heterogeneity (I2 = 0-25%: no heterogeneity; I2 = 25-50%: moderate heterogeneity; I2 = 50-75%: large heterogeneity; and I2 = 75-100%: extreme heterogeneity) (Higgens et al., 2003). When heterogeneity was moderate to extreme, we stratified our analysis by study design (case-control versus nested case-control), by control group (hospital-based versus population-based), and by sample type (blood serum versus adipose tissue). In addition to stratification analyses, we conducted also meta-regression analyses against the above covariates as a comparison measure of heterogeneity.
Case control design study assesses DDT body burdens at or shortly before the time of breast cancer diagnosis. The clinical diagnosis of cancer is different than the time when cancer began to develop, and this is particularly relevant for cancers that exhibit a long latency. Furthermore, the biological monitoring of DDT presents its own potential for epidemiological bias since serum levels can also be influenced by factors that relate directly to the outcome of interest such as weight change. The weight loss experienced by cancer patients in advanced stages will mobilize the DDT/DDE stored in the adipose tissues and thus increase the serum levels. This potential bias may be overcome with the use of a nested case-control study design. A nested case-control study depends on the pre-existence of a cohort that has been followed over time. The cohort is assembled in such a way that information on exposure is collected on all subjects at baseline before the occurrence of the disease occurrence, such as blood sample taken and stored. When a case of the outcome of interest is identified, samples of the cohort who have not developed the outcome by that time are selected as controls. The advantage of the nested case-control design is that the most appropriate control group is chosen from members of the same cohort who have not developed the outcome at the time that they are chosen.
Publication bias was assessed using the methods proposed by Begg and Mazumdar (1994) and by Egger et al. (1997). All p-values are two-tailed.
3. Results:
3.1. Study Results:
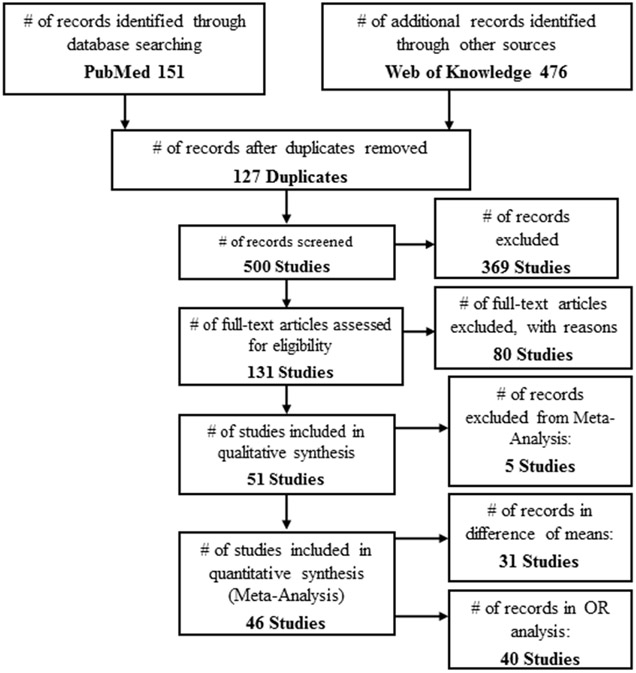
The initial search yielded 627 studies, 127 of which were duplicates. Of the 500 remaining studies screened based on title and abstract, we excluded 369 because they did not contain all of the search query terms pertaining to DDT/DDE and breast cancer. Thus leaving 131 studies for full-text screening. (Figure 1). The eligibility process excluded 80 studies, leaving 51 studies for the exclusion process. All 51 of these studies were suitable for qualitative synthesis. Table 1 presents the studies excluded from the meta-analyses – either based on whether the weighted mean difference or the OR was the effect size, or both, , leaving 46 studies for meta-analyses as detailed in Table 2 and Table 3. . Thirty-one of these studies contained enough information to be included in the difference of means analysis. Forty of the studies contained enough information for inclusion in the odds ratio meta-analysis.
Figure 1 –
Flow chart of study selection.
Table 1 -.
Studies Excluded from DDE Meta-Analysis.
| Excluded From Difference of Means Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study + Pub Date & State, Country |
Cases/ Controls (n) |
Control Group(s) |
Biologic Specimen Type + Year of Collection |
Study Design |
Case vs. Control Mean (SD) + Statistical Significance |
Units | OR (95% CI) | Reason For Exclusion |
| Aronson et al.2000; Canada | 217/213 | BBD | Adipose, 1995-1997 | CC | 693 vs. 596a; p<.05; S.S. | ng/g lipid | 1.62 (.84-3.11) | Geometric Mean & SD, No SD or SE Data |
| Bagga et al.2000; California, USA | 73/73 | HC | Adipose, 1995-1996 | CC | 800 vs. 709.1; p= .23; N.S. | ng/g lipid | 1.126 (.79-1.60) | No SD or SE Data |
| Cohn et al.2007; California, USA | 129/129 | PC | Blood Serum, 1959-1967 | nested | - | - | 1.00 (.4-2.4) | Mean Exposure Not Reported |
| Dorgan et al. 1999; Missouri, USA | 105/208 | PC | Blood Serum, 1977-1987 | nested | - | - | .8 (.4-1.5) | Mean Exposure Not Reported |
| Gammon et al.2002; New York, USA | 643/429 | PC | Blood Serum, 1996-1997 | CC | 671.96 (2.76) vs. 645.74 (2.59)a; p=.52; N.S. | ng/g lipid | 1.2 (.76-1.9) | Geometric Mean & SD |
| Hoyer et al.1998; Denmark | 240/477 | PC | Blood Serum, 1976 | nested | - | - | .88 (.56-1.38) | Mean Exposure Not Reported |
| Hoyer et al.2000; Denmark | 155/274 | PC | Blood Serum, 1976-1978 | nested | - | - | 1.4 (.7-2.8) | Mean Exposure Not Reported |
| Ibarluzea et al.2004; Spain | 198/260 | HC | Adipose, 1996-1998) | CC | 326.86 (2.78) vs. 307.24 (3.62)a; p=.57; N.S. | ng/g lipid | 1.22 (.68-2.20) | Geometric Mean & SD |
| Itoh et al.2009; Japan | 403/403 | HC | Blood Serum, 2001-2005 | CC | 360 vs. 370b; p=.1; N.S. | ng/g lipid | 1.02 (.46-2.26) | Median, No SD or SE |
| Iwasaki et al.2008; Japan | 139/278 | PC | Plasma, 1990-2002 | nested | 7.04 vs. 6.08b; p=.42; N.S. | ng/mL | 1.48 (.7-3.13) | Median, No SD or SE |
| Laden et al.2001; USA | 372/372 | PC | Blood Serum, 1989-1994 | nested | 768 (788) vs. 372 (817)b; p=.28; N.S. | ng/g lipid | .82 (.49-1.37) | Median, No SD or SE |
| Liljegren et al.1998; Sweden | 43/35 | HC (BBD) | Adipose, 1993-1995 | CC | 767 vs. 1026; N.S. | ng/g lipid | .4 (.2-1.2) | No SD or SE |
| Mendonca et al.1999; Brazil | 177/355 | HC | Blood Serum, 1995-1996 | CC | 5.1 vs. 4.8; p=.93; N.S. | ng/mL | .83 (.4-1.6) | No SD or SE |
| Moysich et al.1998; New York, USA | 154/192 | PC | Blood Serum, 1986-1991 | CC | 11.47 (10.49) vs. 10.77 (10.64); N.S. | ng/mL | 1.34 (.71-2.55) | Adjusted Mean |
| Pavuk et al.2003; Slovakia | 24/88 | PC | Blood Serum, 1998-1999 | CC | 4912.1 vs. 3129.1a; p=.04; S.S. | ng/g lipid | 3.04 (.65-14.3) | Geometric Mean, No SD or SE |
| Raaschou-Nielsen et al.2005; Denmark | 409/409 | PC | Adipose, 1993-1997 | nested | 639 vs. 686.3; statistical significance not reported, but authors noted that controls had slightly higher concentrations than cases | ng/g lipid | .7 (.5-1.2) | No SD or SE |
| Romieu et al.2000; Mexico | 120/126 | PC | Blood Serum, 1990-1995 | CC | 3840 vs. 2510; p=.02; S.S. | ng/g lipid | 3.81 (1.14-12.8) | No SD or SE |
| Rubin et al.2006; Alaska, USA | 63/63 | PC | Blood Serum, 1981-1987 | CC | 8.67 vs. 7.36a; p=.02; S.S. | ng/mL | 1.43 (.46-4.47) | Geometric Mean & SD, No SD or SE |
| Stellman et al. 2000; New York, USA | 232/323 | HC (BBD) | Adipose, Not Specified | CC | 419.2 vs. 374.1b; p=.2; N.S. | ng/g lipid | .74 (.39-1.25) | Median, No SD or SE |
| van't Veer et al. 2000; Various European Countries | 265/341 | PC & HC | Adipose, 1991-1996 | CC | 1350 vs. 1510; p=.36; N.S. | ng/g lipid | .48 (.25-.95) | No SD or SE |
| Wolff et al.2000; New York, USA h | 161/339 | HC | Blood Serum, 1994-1996 | CC | 610 (3020) vs. 660 (2730)a; N.S. | ng/g lipid | .93 (.56-1.5) | Geometric Mean & SD |
| Wolff et al.2000; New York, USA p | 110/213 | PC | Blood Serum, 1987-1992 | nested | 977 (2460) vs. 1097 (2290)a; N.S. | ng/g lipid | 1.3 (.51-3.35) | Geometric Mean & SD |
| Zheng et al.1999; Connecticut, USA | 304/186 | HC (BBD) | Adipose, 1994-1997 | CC | 736.5 vs. 784.1a; p=.41; N.S. | ng/g lipid | .9 (.5-1.5) | Geometric Mean, No SD or SE |
| Zheng et al.2000; Connecticut, USA | 475/502 | PC & HC | Blood Serum, 1995-1997 | CC | 460.1 vs. 456.2; p=.89; N.S. | ng/g lipid | .95 (.68-1.32) | Geometric Mean, No SD or SE |
| Excluded From Odds Ratio Analysis | ||||||||
| Study + Pub Date & State, Country |
Cases/ Controls (n) |
Control Group(s) |
Biologic Specimen Type + Year of Collection |
Study Design |
Case vs. Control Mean (SD) + Statistical Significance |
Units | OR (95% CI) | Reason For Exclusion |
| Ahmed et al.2002; Egypt | 43/11 (BBD)/21 (PC) | BBD & PC | Blood Serum, 1999-2000 | CC | 41 (5.2) vs. 48 (6.2), 41 (5.2) vs. 31 (2.5); P=.03; S.S. ("of borderline statistical significance") | ng/mL | - | OR Not Reported |
| Mathur et al.2002; Rajasthan, India | 135/50 | HC | Blood Serum, Not Specified | CC | 862 (154) vs. 47 (18); p<.05; S.S. | ng/mL | - | OR Not Reported |
| Ociepa-Zawal et al. 2010; Poland | 54/23 | HC | Adipose, Not Specified | CC | 956 (771) vs. 631 (371); N.S., but significant differences (p=.006) found in patients with late onset breast cancer | ng/g fat | - | OR Not Reported |
| Siddiqui et al.2005; Delhi, India | 25/25 | HC (BBD) | Blood Serum, Not Specified | CC | 11.69 (3.29) vs. 20.22 (8.24); p=.56; N.S. | ng/mL | - | OR Not Reported |
| Unger et al. 1984; Denmark | 14/21 | HC (BBD) | Adipose, Not Specified | CC | 1230 (630) vs. 1250 (760); N.S. | ng/g lipid | - | OR Not Reported |
| Waliszewski et al.2005; Mexico (Breast Cancer Cases) | 127/127 | HC | Adipose, Not Specified | CC | 980 (627) vs. 782 (282); p<.05; S.S. | ng/g lipid | - | Relative Risk |
| Waliszewski et al.2005; Mexico (BBD Cases) | 127/127 | HC | Adipose, Not Specified | CC | 1761 (1090) vs. 782 (282); p<.05; S.S. | ng/g lipid | - | Relative Risk |
| Excluded From Both Analyses | ||||||||
| Study + Pub Date & State, Country |
Cases/ Controls (n) |
Control Group(s) |
Biologic Specimen Type + Year of Collection |
Study Design |
Case vs. Control Mean (SD) + Statistical Significance |
Units | OR (95% CI) | Reason For Exclusion |
| Cassidy et al. 2005; Texas, USA | 17/17 | HC (BBD) | Adipose, 1994-1998 | CC | 975 vs. 949b; N.S. | ng/g lipid | - | Median, No SE or SD Data, OR Not Reported |
| Dewailly et al. 1994 (ER− Case Population); Quebec, Canada | 9/17 | HC (BBD) | Adipose, 1991-1992 | CC | 608.9 (338.9) vs. 765.3 (526.9); p=.63; N.S. | ng/g lipid | - | Only Stratified Data Reported, OR Not Reported |
| Dewailly et al. 1994 (ER+ Case Population); Quebec, Canada | 9/17 | HC (BBD) | Adipose, 1991-1992 | CC | 2132.2 (204.9) vs. 765.3 (526.9); p=.01; S.S. | ng/g lipid | - | Only Stratified Data Reported, OR Not Reported |
| Guttes et al. 1998; Germany | 45/20 | HC (BBD) | Adipose, 1993-1994 | CC | 838 vs. 450 | ng/g lipid | - | No SD or SE, OR Not Reported |
| Ward et al.2000; Norway | 72/72 | PC | Blood Serum, 1973-1991 | CC | 1230 vs. 1260; p=.84; N.S. | ng/g lipid | - | No SD or SE, OR Not Reported |
BBD - benign breast disease, HC - hospital control group, PC - population control group, SD - standard deviation, SE - standard error, OR - odds ratio, ER - estrogen receptor, N.S. - not statistically significant, S.S. - statistically significant. aGeometric Mean & Standard Deviation, bMedia
Table 2 –
Characteristics of studies included in DDE Meta-Analysis
| DDE Concentration, mean (SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study + Pub Date; Study Site |
Cases/ Controls (n) |
Control Group(s) |
Biologic Specimen Type, Year of Collection |
Study Design |
Cases | Controls | Units of Exposure |
p-value | Study Conclusion |
| Cohn et al.2007; California, USA | 129/129 | PC | Blood Serum, 1959-1967 | nested | - | - | - | - | - |
| Krieger et al.1994; California, USA | 150/150 | PC | Blood Serum, 1964-1971 | nested | 43.3 (25.9) | 43.1 (23.7) | ng/mL | - | - |
| Hoyer et al.1998; Denmark | 240/477 | PC | Blood Serum, 1976 | nested | - | - | - | - | - |
| Hoyer et al.2000; Denmark | 155/274 | PC | Blood Serum, 1976-1978 | nested | - | - | - | - | - |
| Dorgan et al. 1999; Missouri, USA | 105/208 | PC | Blood Serum, 1977-1987 | nested | - | - | - | - | - |
| Wolff et al.1993; New York, USA | 58/171 | PC | Blood Serum, 1985-1991 | nested | 11 (9.1) | 7.7 (6.8) | ng/mL | 0.03 | S.S. |
| Wolff et al.2000p; New York, USA | 110/213 | PC | Blood Serum, 1987-1992 | nested | 977 (2.46) | 1097 (2.29) | ng/g lipid | - | N.S. |
| Hunter et al.1997; USA | 236/236 | PC | Blood Serum, 1989-1990 | nested | 6.01 (4.56) | 6.97 (5.99) | ng/mL | 0.14 | N.S. |
| Laden et al.2001; USA | 372/372 | PC | Blood Serum, 1989-1994 | nested | 768 (788) | 372 (817) | ng/g lipid | 0.28 | N.S. |
| Iwasaki et al.2008; Japan | 139/278 | PC | Plasma, 1990-2002 | nested | 7.04 | 6.08 | ng/mL | 0.42 | N.S. |
| Raaschou-Nielsen et al.2005; Denmark | 409/409 | PC | Adipose, 1993-1997 | nested | 639 | 686.3 | ng/g lipid | - | N.R. |
| Unger et al. 1984; Denmark | 14/21 | HC (BBD) | Adipose, Not Specified | CC | 1230 (630) | 1250 (760) | ng/g lipid | - | N.S. |
| Rubin et al.2006; Alaska, USA | 63/63 | PC | Blood Serum, 1981-1987 | CC | 8.67 | 7.36 | ng/mL | 0.02 | S.S. |
| Moysich et al.1998; New York, USA | 154/192 | PC | Blood Serum, 1986-1991 | CC | 11.47 (10.49) | 10.77 (10.64) | ng/mL | - | N.S. |
| Romieu et al.2000; Mexico | 120/126 | PC | Blood Serum, 1990-1995 | CC | 3840 | 2510 | ng/g lipid | 0.02 | S.S. |
| Demers et al.2000b; Quebec, Canada | 315/305 | PC | Blood Serum, 1994-1997 | CC | 508.9 (491.1) | 480.4 (408.1) | ng/g lipid | 0.88 | N.S. |
| van't Veer et al. 1997; Various European Countries | 265/341 | PC & HC | Adipose, 1991-1996 | CC | 1350 | 1510 | ng/g lipid | 0.36 | N.S. |
| Liljegren et al.1998; Sweden | 43/35 | HC (BBD) | Adipose, 1993-1995 | CC | 767 | 1026 | ng/g lipid | - | N.S. |
| Milikan et al.2000 black; North Carolina, USA | 292/270 | PC | Blood Serum, 1993-1996 | CC | 1960 (2200) | 1690 (1700) | ng/g lipid | 0.29 | N.S. |
| Milikan et al.2000 white; North Carolina, USA | 456/389 | PC | Blood Serum, 1993-1996 | CC | 660 (800) | 760 (1200) | ng/g lipid | 0.18 | N.S. |
| Schecter et al.1997; Vietnam | 21/21 | HC | Blood Serum, 1994 | CC | 12.17 (2.41) | 16.67 (4.14) | ng/mL | - | N.S. |
| Lopez-Carrillo et al.1997; Mexico | 141/141 | HC | Blood Serum, 1994-1996 | CC | 562.48 (676.18) | 505.46 (567.22) | ng/g lipid | 0.44 | N.S. |
| Wolff et al.2000h; New York, USA | 161/339 | HC | Blood Serum, 1994-1996 | CC | 610 (3020) | 660 (2730) | ng/g lipid | - | - |
| Zheng et al.1999; Connecticut, USA | 304/186 | HC (BBD) | Adipose, 1994-1997 | CC | 736.5j | 784.1j | ng/g lipid | 0.41 | N.S. |
| Demers et al.2000a; Quebec, Canada | 315/218 | HC | Blood Serum, 1994-1997 | CC | 508.9 (491.1) | 462.7 (447.7) | ng/g lipid | 0.39 | N.S. |
| Bagga et al.2000; California, USA | 73/73 | HC | Adipose, 1995-1996 | CC | 800 | 709.1 | ng/g lipid | 0.23 | N.S. |
| Mendonca et al.1999; Brazil | 177/355 | HC | Blood Serum, 1995-1996 | CC | 5.1 | 4.8 | ng/mL | - | - |
| Olaya-Contreras et al.1998; Colombia | 153/153 | HC | Blood Serum, 1995-1996 | CC | 27.11 (61.56) | 12.18 (36.60) | ng/mL | 0.03 | S.S. |
| Aronson et al.2000; Canada | 217/213 | BBD | Adipose, 1995-1997 | CC | 693 | 596 | ng/g lipid | <.05 | S.S. |
| Zheng et al.2000; Connecticut, USA | 475/502 | PC & HC | Blood Serum, 1995-1997 | CC | 460.1j | 456.21 | ng/g lipid | 0.89 | N.S. |
| Gatto et al.2007; USA | 355/327 | PC | Blood Serum, 1995-1998 | CC | 9.9 (12.84) | 8.13 (7.78) | ng/mL | 0.14 | N.S. |
| Gammon et al.2002; New York, USA | 643/429 | PC | Blood Serum, 1996-1997 | CC | 671.96 (2.76)j | 645.74 (2.59)j | ng/g lipid | 0.52 | N.S. |
| Ibarluzea et al.2004; Spain | 198/260 | HC | Adipose, 1996-1998) | CC | 326.86 (2.78)j | 307.24 (3.62)j | ng/g lipid | 0.57 | N.S. |
| Stellman et al.2000; New York, USA | 232/323 | HC (BBD) | Adipose, Not Specified | CC | 419.2k | 374.1k | ng/g lipid | 0.2 | N.S. |
| Dello Iacovo et al.1999; Italy | 170/195 | PC | Blood Serum, 1997-1998 | CC | 9.55 (5.42) | 8.98 (5.17) | ng/mL | - | N.S. |
| Pavuk et al.2003; Slovakia | 24/88 | PC | Blood Serum, 1998-1999 | CC | 4912.1 | 3129.1 | ng/g lipid | 0.04 | S.S. |
| Ahmed et al.2002; Egypt | 43/21 | HC (BBD) | Blood Serum, 1999-2000 | CC | 41 (5.2) | 48 (6.2) | ng/mL | - | - |
| Ahmed et al.2002; Egypt | 43/11 | PC | Blood Serum, 1999-2000 | CC | 41 (5.2) | 31 (2.5) | ng/mL | - | - |
| Boada et al. 2012; Spain | 121/103 | PC | Blood Serum, 1999-2001 | CC | 357.4 (326) | 198 (207.5) | ng/g lipid | <.001 | S.S. |
| Soliman et al.2003 | 69/53 | HC | Blood Serum, Not Specified | CC | 12.7 (20.3) | 16.6 (30.1) | ng/mL | 0.6 | N.S. |
| Charlier et al.2004; Belgium | 231/290 | HC | Blood Serum, 2001-2002 | CC | 580 (580) | 310 (350) | ng/g lipid | <0.0001 | S.S. |
| Itoh et al.2009; Japan | 403/403 | HC | Blood Serum, 2001-2005 | CC | 360 | 370 | ng/g lipid | 0.1 | N.S. |
| Waliszewski et al.2005; Mexico (Breast Cancer Cases) | 127/127 | HC | Adipose, Not Specified | CC | 980 (627) | 782 (282) | ng/g lipid | < 0.05 | S.S. |
| Waliszewski et al.2005; Mexico (BBD Cases) | 127/127 | HC | Adipose, Not Specified | CC | 1761 (1090) | 782 (282) | ng/g lipid | < 0.05 | S.S. |
| Siddiqui et al.2005; Delhi, India | 25/25 | HC | Blood Serum, Not Specified | CC | 11.69 (16.45) | 20.66 (41.2) | ng/mL | 0.56 | N.S. |
| Ociepa-Zawal et al. 2010; Poland | 54/23 | HC | Adipose, Not Specified | CC | 956 (771) | 631 (371) | ng/g fat | - | - |
Table 3 –
Results of studies included in DDE Meta-Analysis
| DDE Odds Ratio (OR) | Controlled Variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study + Pub Date; Study Site |
OR (95% CI) |
p-value | Study Conclusion |
None Specified |
Age | Body Weight, Height, and/or BMI |
Pregnancy & Childbirth History |
Lactation | Family History of Breast Cancer or History of BBD |
Other Factors |
| Cohn et al.2007; California, USA | 1.00 (.4-2.4) | 0.92 | N.S. | x | xg | |||||
| Krieger et al.1994; California, USA | 1.33 (.678-2.61) | 0.43 | N.S. | x | x | x | xabc | |||
| Hoyer et al.1998; Denmark | .88 (.563-1.38) | 0.52 | N.S. | x | x | xadel | ||||
| Hoyer et al.2000; Denmark | 1.4 (.7-2.8) | - | N.S. | x | x | |||||
| Dorgan et al. 1999; Missouri, USA | .8 (.4-1.5) | 0.77 | N.S. | x | ||||||
| Wolff et al.1993; New York, USA | 3.68 (1.01-13.5) | 0.04 | S.S. | x | x | x | ||||
| Wolff et al.2000p; New York, USA | 1.3 (.51-3.35) | 0.99 | N.S. | x | x | x | x | xb | ||
| Hunter et al.1997; USA | .66 (.359-1.215) | 0.47 | N.S. | x | x | x | x | x | xbef | |
| Laden et al.2001; USA | .82 (.49-1.37) | 0.15 | N.S. | x | x | x | xb | |||
| Iwasaki et al.2008; Japan | 1.48 (.7-3.13) | 0.25 | N.S. | x | x | xabehk | ||||
| Raaschou-Nielsen et al.2005; Denmark | .7 (.5-1.2) | 0.29 | N.S. | x | x | x | x | xeg | ||
| Unger et al. 1984; Denmark | - | - | - | x | ||||||
| Rubin et al.2006; Alaska, USA | 1.43 (.46-4.47) | 0.43 | N.S. | x | x | xci | ||||
| Moysich et al.1998; New York, USA | 1.34 (.71-2.55) | 0.25 | N.S. | x | x | x | x | x | ||
| Romieu et al.2000; Mexico | 3.81 (1.14-12.8) | 0.02 | S.S. | x | x | x | xbdj | |||
| Demers et al.2000b; Quebec, Canada | 1.00 (.6-1.67) | - | N.S. | x | x | x | x | x | xdf | |
| van't Veer et al. 1997; Various European Countries | .48 (.25-.95) | 0.02 | S.S. | x | x | x | xef | |||
| Liljegren et al.1998; Sweden | .4 (.2-1.2) | - | N.S. | x | x | x | x | x | xe | |
| Milikan et al.2000 black; North Carolina, USA | 1.09 (.79-1.51) | - | N.S. | x | x | x | x | xacdg | ||
| Milikan et al.2000 white; North Carolina, USA | 1.09 (.79-1.51) | - | N.S. | x | x | x | x | xacdg | ||
| Schecter et al.1997; Vietnam | 1.14 (.23-5.68) | - | N.S. | x | ||||||
| Lopez-Carrillo et al.1997; Mexico | .76 (.41-1.42) | - | N.S. | x | x | x | x | |||
| Wolff et al.2000h; New York, USA | .93 (.56-1.5) | 0.49 | N.S. | x | xac | |||||
| Zheng et al.1999; Connecticut, USA | .9 (.5-1.5) | 0.46 | N.S. | x | x | x | x | xabci | ||
| Demers et al.2000a; Quebec, Canada | 1.36 (.71-2.63) | - | N.S. | x | x | x | x | x | xd | |
| Bagga et al.2000; California, USA | 1.13 (.79-1.60) | 0.5 | N.S. | x | ||||||
| Mendonca et al.1999; Brazil | .83 (.4-1.6) | 0.79 | N.S. | x | x | x | x | xdf | ||
| Olaya-Contreras et al.1998; Colombia | 1.95 (1.1-3.52) | 0.09 | N.S. | x | x | x | x | xa | ||
| Aronson et al.2000; Canada | 1.62 (.84-3.11) | - | N.S. | x | x | xacgh | ||||
| Zheng et al.2000; Connecticut, USA | .95 (.68-1.32) | 0.41 | N.S. | x | x | x | x | x | xbcdfh | |
| Gatto et al.2007; USA | 1.02 (.61-1.72) | 0.74 | N.S. | x | x | |||||
| Gammon et al.2002; New York, USA | 1.2 (.76-1.9) | - | N.S. | x | x | x | xc | |||
| Ibarluzea et al.2004; Spain | 1.22 (.677-2.199) | 0.4 | N.S. | |||||||
| Stellman et al.2000; New York, USA | .74 (.39-1.247) | 0.3 | N.S. | x | x | xc | ||||
| Dello Iacovo et al.1999; Italy | 1.24 (.7-2.2) | - | N.S. | x | x | x | x | xafi | ||
| Pavuk et al.2003; Slovakia | 3.04 (.65-14.3) | 0.1 | N.S. | x | xbd | |||||
| Ahmed et al.2002; Egypt | - | - | - | x | ||||||
| Ahmed et al.2002; Egypt | - | - | - | x | ||||||
| Boada et al. 2012; Spain | 0.999 (.996-1.00) | 0.284 | N.S. | x | x | x | xae | |||
| Soliman et al.2003 | 1.41 (.63-3.19) | 0.4 | N.S. | x | x | xfi | ||||
| Charlier et al.2004; Belgium | 2.21 (1.41-3.48) | 0.0006 | S.S. | x | x | x | xag | |||
| Itoh et al.2009; Japan | 1.02 (.46-2.26) | 0.46 | N.S. | x | x | x | x | x | xaehik | |
| Waliszewski et al.2005; Mexico (Breast Cancer Cases) | - | x | ||||||||
| Waliszewski et al.2005; Mexico (BBD Cases) | x | |||||||||
| Siddiqui et al.2005; Delhi, India | - | - | - | x | ||||||
| Ociepa-Zawal et al. 2010; Poland | - | - | - | x | ||||||
Menopausal status
Age at menarche
Race/Ethnicity; a Education, income, and other socioeconomic metrics; b Smoking and alcohol use; c Geography/study site
Hormone replacement therapy use
Diet
Serum lipid; triglyceride, and/or cholesterol
Presence of other pesticides in sample
Age at menopause
Physical activity
Geometric mean and/or standard deviation
Median
3.2. DDE and Breast Cancer:
Overall, the summary odds ratio evaluating the risk of breast cancer from DDE exposure was slightly elevated but not statistically significant (OR = 1.04; 95% CI: 0.94 – 1.15) (Figure 2 and Table 4). There was no publication bias among the studies (Begg p-value = 0.09, Egger’s p-value = 0.14) and heterogeneity was moderate (I2 = 31.14). To resolve this moderate heterogeneity, the results were stratified by study design and tissue sample. The OR for the nested case control studies was 0.90 (95% CI: 0.75 – 1.08). Further stratification of the nested case control by tissue type did not yield significant results (adipose tissue OR = 0.70, 95% CI: 0.45 – 1.08; and blood serum OR = 0.95, 95% CI: 0.78 – 1.17). The OR for case control studies was found not significant (OR = 1.11; 95% CI: 0.98 – 1.25), and stratification by tissue sample indicates no association (Table 4) Interestingly, stratification by blood serum case-control studies showed a statistically significant higher odds of having breast cancer in the highest exposed group compared to the lowest (OR = 1.15; 95% CI: 1.01 – 1.39). One potential explanation for this significant outcome is that case-control studies exhibited higher heterogeneity than the nested case-control studies. Heterogeneity analyses using meta-regression with random effects yielded null log-likelihood ratio p-values as well as low τ2values also indicating that there was moderate heterogeneity (Table 5).
Figure 2 –
Odds Ratio analysis for DDE exposure and Breast Cancer
Table 4 –
Overall ORs for Breast Cancer Risk and DDT/DDE levels
| Studies included | No of Studies |
OR (95% CI) | Heterogeneity χ2 test p-value |
I2 (%) | Publication bias tests (p-value) |
|
|---|---|---|---|---|---|---|
| Begg (corrected) |
Egger | |||||
| All DDE | 38 | 1.04 (0.94-1.15) | 0.020 | 31.72 | 0.09 | 0.14 |
| Case Control | 27 | 1.11 (0.98-1.23) | 0.025 | 38.26 | 0.23 | 0.06 |
| Adipose Tissue | 7 | 0.89 (0.65-1.22) | 0.090 | 52.51 | ||
| Blood Serum | 20 | 1.15 (1.01-1.32) | 0.022 | 34.68 | ||
| Nested Case Control | 11 | 0.90 (0.75-1.08) | 0.015 | 12.21 | 0.016 | 0.008 |
| Adipose Tissue | 1 | 0.7 (0.45-1.08) | 0.00 | 0.00 | ||
| Blood Serum | 10 | 0.95 (0.78-1.17) | 0.006 | 0.00 | ||
| All DDT | 18 | 1.02 (0.92-1.13) | 0.384 | 64.49 | 0.23 | 0.12 |
| Case Control | 12 | 0.99 (0.99-1.00) | 0.00 | 0.00 | 0.37 | 0.26 |
| Adipose Tissue | 3 | 1.04 (0.92-1.17) | 0.00 | 0.00 | ||
| Blood Serum | 9 | 0.99 (0.99-1.00) | 0.00 | 0.00 | ||
| Nested Case Control | 6 | 0.85 (0.56-1.28) | 0.325 | 70.76 | 0.35 | 0.26 |
| Adipose Tissue | 1 | 0.60 (0.33-1.10) | 0.00 | 0.00 | ||
| Blood Plasma | 1 | 0.99 (0.47-2.08) | 0.00 | 0.00 | ||
| Blood Serum | 4 | 1.38 (0.59-3.25) | 0.325 | 70.76 | ||
Table 5 —
DDE Meta-Regression with Random Effects - Estimates of Hetereogeneity Based on OR Effect Sizes
| Covariate | Between-study variance (τ2) |
LLR* | df | p(LLR*) |
|---|---|---|---|---|
| Study Design | 0.1183 | 24271.3 | 1 | NA |
| Study Date | 0.1183 | 24274.1 | 1 | NA |
| Tissue Type | 0.1183 | 27965 | 1 | NA |
| Control Type | 0.1182 | 13929.2 | 1 | NA |
| Design + Study Date + Tissue + Control | 0.0923 | 38445.7 | 1 | NA |
LLR = log-likelihood ratio
For the DDE and breast cancer difference of means meta-analysis, results were stratified by whether or not the study was lipid adjusted or not. In the studies that reported exposure levels unadjusted for lipids, the differences of means between cases and controls was null (0.45 ng/mL, SE = 0.85, p = 0.60) (Table 6). Heterogeneity was high in these studies, even after stratifying for design, control population, and difference of means rank. There was not a publication bias reported in these studies (Begg p-value = 0.83, Egger’s p-value = 0.58). For the lipid adjusted studies, there was a statistically significant higher DDE concentration in cases compared to controls (110.30 ng/g lipid, SE = 44.94, p = 0.01) (Table 6).
Table 6 –
Overall Difference of Means Values for Breast Cancer Risk and DDE/DDT exposure
| Studies Included | No of Studies |
Diff of Means (SE) |
p-value | Heterogeneity χ2 test p-value |
I2 (%) | Publication bias tests (p-value) |
|
|---|---|---|---|---|---|---|---|
| Begg (corrected) |
Egger | ||||||
| All DDE | |||||||
| Lipid Adjusted | 13 | 110.30 (42.94) | 0.01 | 0.092 | 89.32 | 0.012 | 0.09 |
| Adipose Tissue | 5 | 332.34 (195.02) | 0.09 | 0.249 | 88.89 | ||
| Blood Serum | 8 | 98.99 (44.02) | 0.03 | 0.045 | 85.24 | ||
| Lipid Unadjusted | 14 | 0.45 (0.85) | 0.6 | 0.080 | 85.68 | 0.28 | 0.33 |
| Case Control | 11 | 0.31 (0.98) | 0.75 | 0.096 | 87.15 | ||
| Nested Case Control | 3 | 0.86 (1.71) | 0.62 | 0.049 | 79.26 | ||
| All DDT | |||||||
| Lipid Adjusted | 7 | −0.09 (0.08) | 0.28 | 205870.69 | 96.20 | 0.50 | 0.08 |
| Adipose Tissue | 1 | −0.18 (0.25) | 0.48 | 421989.24 | 95.26 | ||
| Blood Serum | 6 | −0.08 (0.08) | 0.37 | 7884.99 | 56.76 | ||
| Lipid Unadjusted | 4 | 0.45 (0.37) | 0.23 | 609.35 | 94.28 | 0.15 | 0.29 |
3.2.1. DDE and Menopausal Status Analysis:
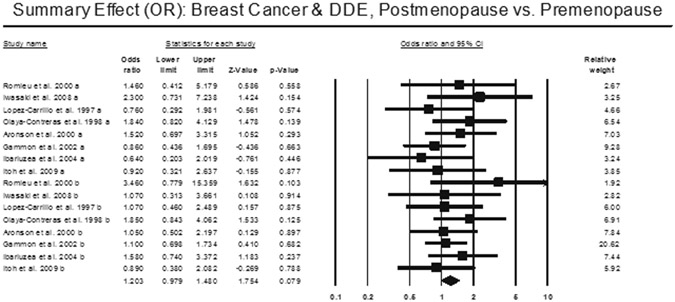
Stratification based on menopausal status found no statistically significant higher breast cancer risk for either pre- (OR = 1.165, 95% CI: 0.98 – 1.48) or post-menopausal (OR = 1.23, 95% CI: 0.94 – 1.61) stratification (Figure 3). We also found no heterogeneity (I2 = 0.00) for both pre- and post-menopausal stratification. We did not find any publication bias (Begg’s p-value – 0.44, Egger’s p-value = 0.43).
Figure 3 –
Odds Ratio analysis for breast cancer and DDE stratified by menopausal status.
3.3. DDT and Breast Cancer:
Overall, the summary odds ratio for DDT exposure and breast cancer was slightly elevated but not statistically significant (OR = 1.02, 95% CI: 0.92 – 1.13) (Table 4). There was no publication bias found (Begg’s = 0.23; Egger’s = 0.12); however, there was high heterogeneity (I2 = 64.49). Stratification by study design yielded similarly null results (Nested case control OR = 0.85, 95% CI: 0.56 – 1.28; Case Control OR = 0.99, 95% CI: 0.99 – 1.00). And further stratification by tissue sample type yielded comparable results. This stratification by study design and tissue sample showed that the high level of heterogeneity we observed was solely due to the Nested Case Control studies (Case Control I2 = 0.00; Nested Case Control I2 = 70.76). .
Similarly to the DDE and breast cancer difference of means meta-analysis, results for DDT and breast cancer difference of means analysis were stratified by whether or not the study was lipid adjusted or not. In the studies that reported exposure levels unadjusted for lipids, the differences of means between cases and controls was null (0.45 ng/mL, SE = 0.37, p = 0.23) (Table 6). In the lipid adjusted DDT studies, there was no difference in exposure between the cases and controls (−0.09 ng/g lipid, SE = 0.08, p = 0.28) (Table 6).
3. Discussion:
The results of this meta-analysis showed that the most recent body of literature does not support a relationship between DDT/DDE and breast cancer risk. We found that those exposed to the highest levels of DDT and DDE compared to those exposed to the lowest levels had a slightly elevated but not statistically significant risk of breast cancer (DDE Summary OR = 1.04, 95% CI: 0.94 – 1.15; DDT Summary OR = 1.02, 95% CI: 0.92 – 1.13). Although breast cancer patients had slightly elevated levels of DDE, the null summary OR does not indicate that DDE exposure is associated with breast cancer risk.
Overall, we observed moderate to high levels of heterogeneity among the mean exposure data even after stratification by study design and sample type. For example, even after stratifying by type of control group, tissue type and difference of means rank, the high degree of heterogeneity of the case control studies that presented mean lipid unadjusted DDE serum concentrations could not be resolved. One possible explanation for the heterogeneity among the mean exposure studies data is the fact that mean exposure represents a crude effect measure that is unadjusted for other confounders, such as exposure level and genetic markers for breast cancer. The moderate heterogeneity observed in the OR studies could be attributed to the large variety of adjustment factors. We found a lack of standardization with regard to the combination of factors accounted for in the adjustment of the crude OR among the studies used in this meta-analysis.
The quality of chemical exposure assessment may be a cause of heterogeneity. There were differences on specificity and sensitivity in the methodology used to assess DDE or DDT level in tissues. These factors may affect the results of the difference of means analyses by creating between-study heterogeneity by influencing the differences between the highest versus lowest exposure groups. However, the quality of the exposure assessments would have a minor impact on the pooled OR analyses, since this is a unit-less measure (ratio) comparing highest vs. lowest exposure group in the same cohort, and therefore enables comparison across studies.
Despite the fact that the most common methodological approach used in the studies included in this meta-analysis was the case control design, which has the disadvantage that DDT/DDE tissue levels at diagnosis may be considerably different than those at the time of cancer onset, we found little indication that the study design was related to the conflicting results in the studies analyzed. Our results support the notion that the nested case-control design, which depends on the pre-existence of a cohort that has been followed over time, is the preferred method when looking for a causal relationship between exposure and disease risk because these studies generally showed lower levels of heterogeneity than the case-control studies. However, both study designs showed low to moderate heterogeneity, supporting the idea that the study type did not affect our results. Additionally, we found only limited evidence that sample type may affect results. Stratifying the OR analysis for DDE and breast cancer by blood serum samples within the case control studies yielded a statistically significant association between DDE and breast cancer (OR = 1.15, 95% CI: 1.01-1.32). However, there was high heterogeneity among these studies indicating that there may be other factors affecting these results.
The inconsistency found among the epidemiological studies included in our meta-analysis could be due to confounders that distort the relationship between DDT/DDE exposure and breast cancer risk. Many of the studies adjusted for common variables (i.e. age, BMI, family history of breast cancer, etc.) (Table 2 and 3). However, not many of the studies adjusted for breast feeding and diet, both of which have been related to DDT/DDE body burden (Bradman et al., 2007). Lactation has been shown to help eliminate body levels of DDT/DDE in addition to helping decrease the risk of breast cancer. It is known that DDT/DDE remains in the food chain despite its ban, thus exposure through diet is a common problem (Laden et al., 1999; Snedecker, 2001). If studies do not account for these confounding variables, then the results may be skewed. Because we stratified our analysis and found generally low heterogeneity among our results, this lack of control for these variables does not seem to be significant. However, this is not enough to dismiss the possibility of differences among studies being explained by confounding variables.
Recently, there has been increasing indications that the age at which one is exposed to chemicals such as DDT may play a direct role in one’s risk for a disease. Organotins, such as tributylin, are a good example of this. There has been much evidence suggesting that exposure to organotins in utero can act as a causative agent for obesity in adulthood through epigenetic changes that alter transcription in the adipogenesis pathway (Grün et al., 2006). Because of this perceived mechanism, more research has begun to investigate how chemical exposure at different ages can differentially affect disease progression and expression. Cohn et al. (2007) reported that exposure to DDT before the age of 14 significantly increased the likelihood of developing breast cancer. They looked at a cohort of individuals who had blood samples drawn when DDT was still used in the United States (1959-1967), ensuring that the exposure level would be high among all of the individuals. They found a significant increase in the level of DDT within the cases of their cohort (those who developed breast cancer) as compared to their age-matched controls; however, this significance was found to be derived primarily from the group of cases who were younger than 14 at the time of the highest DDT exposure. This suggested that age at exposure to DDT has a direct effect on the prevalence of breast cancer within a population, providing further evidence that effects from environmental exposure may be influenced by the stage of development during exposure.
In a review paper, Cohn (2003) notes that DDT and DDE can cross into the placenta during pregnancy, thus representing a potential mechanism for fetal exposure. Moreover, a number of studies support the hypothesis that breast cancer may have fetal origins. For instance, higher birth weight and exposure to synthetic estrogen in utero appear to be associated with an increased risk of breast cancer (Michels & Xue, 2006; Palmer et al., 2006). However, fetal characteristics such as birth weight are indirect measures of the relationship between in utero development, hormonal exposures and breast cancer risk; furthermore, these studies are unable to pinpoint specific developmental events during which exposures to hormones, organochlorine pesticides, and other estrogenic substances may initiate future breast cancer development (Troisi et al., 2007). This represents a significant gap in our understanding of disease progression related to age at time of exposure and supports the need for more research to be done within this area.
The idea that exposure during specific developmental windows may affect the prevalence of a given outcome is further supported by the relationship between DDT/DDE exposure and age at menarche. Ouyang et al. (2005) used a cross sectional study of 466 women in China to show that women exposed to the highest quartile of DDT/DDE had a statistically significant lower age at the time of menarche than those exposed to the lowest quartile. This study is important to note because DDT was not banned in China until 1984; the women in this study were 21-34 years of age at the time of blood sample collections in 1998. Therefore, these women were exposed to high levels of DDT from birth for between 7 and 21 years when DDT was being used as well as exposure to remnants of the chemical after the ban since DDT is persistent in the environment. Furthermore, it has been shown that an earlier age of menarche statistically increases the risk of breast cancer (Collaborative Group on Hormonal Factors, 2012). This supports the idea that early exposure in utero and during childhood and adolescence to DDT/DDE and other environmental toxins may lead to an increased risk of disease by acting to affect normal developmental stages. Because not many of the studies incorporated into our meta-analysis controlled for age of menarche, there may have been an understatement on the risk of exposure due to confounding. Future studies should make attempts to control for this factor as well as the age of exposure in order to take into consideration the mass of new information relating developmental stage during exposure and the potential for an increased risk of adult-onset diseases.
A limitation of the OR pooled analyses is that the chemical blood burden range defining the lowest referent group, as well as the highest level group, is different across the studies. This is based on the time the study was conducted, and reflects, also, the policies regulating the use of the chemicals: for example, the level of exposure to DDT has dramatically decreased in US since the use of DDT was banned. However, the high and low group are based on the exposure level of the investigated cohort, and since the OR are unit-less measure, the pooled OR across the studies still has its merit.
Another potential limiting factor of this study is that it does not rule out the possibility that exposure to mixtures of DDT and other organochlorine pesticides and common chemicals with estrogenic properties may pose an increased breast cancer risk. Some recent in vitro studies suggest that interactions between these chemicals, endogenous and/or exogenous hormones, and their ligands and receptors may lead to homeostatic changes in hormonal concentrations in mammary tissue that can induce malignant conversion of cells (Aube et al., 2011; Valeron et al., 2009). While our systematic review yielded some studies evaluating breast cancer risk associated with exposures to other chemicals such as PCBs, HCB, beta-HCH, Dieldrin, and trans-nonachlor, they failed to examine the breast cancer risk associated with these combined chemical exposures. For example, in their population-based retrospective study, Boada et al. (2012) found that 24.3% of their breast cancer patient blood samples contained a mixture of aldrin, DDE and DDD not found in healthy subjects, but the breast cancer risk associated with exposure to the mixture was not calculated. Thus, the relationship between breast cancer and mixtures of pesticides and other chemical mixtures represents an important area in need of further investigation.
The results of our meta-analysis do not support an association between DDT and DDE exposure and the risk of breast cancer. Although we cannot completely rule out a very small effect from exposure, based on previous findings in similarly conducted meta-analyses as well as the high number of studies that we were able to analyze, we believe that this study further confirms the evidence against a relationship between DDT and breast cancer risk.
Acknowledgements:
We would like to thank Dr. Christopher Portier for his methodological insight and guidance. We thank Kirsten Bandyopadhyay for critical comments and continuous encouragement. This research was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC (SZI, MCB).
Footnotes
Disclaimer: the findings and conclusion in this report are those of the authors and do not necessarily represent the official position of ATSDR.
References:
- ATSDR. Agency for Toxic Substances and Disease Registry. 2002. Toxicological Profile for DDT, DDE, and DDD. [PubMed] [Google Scholar]
- Ahmed MT, Loutfy N, et al. (2002). "Residue levels of DDE and PCBs in the blood serum of women in the Port Said region of Egypt." J Hazard Mater 89(1): 41–48. [DOI] [PubMed] [Google Scholar]
- Aronson KJ, Miller AB, et al. (2000). "Breast adipose tissue concentrations of polychlorinated biphenyls and other organochlorines and breast cancer risk." Cancer Epidemiol Biomarkers Prev 9(1): 55–63. [PubMed] [Google Scholar]
- Aubé M, Larochelle C and Ayotte P (2011). "Differential effects of a complex organochlorine mixture on the proliferation of breast cancer cell lines." Environmental Research 111: 337–347. [DOI] [PubMed] [Google Scholar]
- Bagga D, Anders KH, et al. (2000). "Organochlorine pesticide content of breast adipose tissue from women with breast cancer and control subjects." J Natl Cancer Inst 92(9): 750–753. [DOI] [PubMed] [Google Scholar]
- Begg BC and Mazumdar M (1994). "Operating characteristics of a rank correlation test for publication bias." Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- Boada LD, Zumbado M, et al. (2012). "Complex organochlorine pesticide mixtures as determinant factor for breast cancer risk: a population-based case-control study in the Canary Islands (Spain)." Environ Health 11(1): 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Schwartz JM, et al. (2007). "Factors predicting organochlorine pesticide levels in pregnant Latina women living in a United States agricultural area." Journal of Exposure Science and Environmental Epidemiology 17: 388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy RA, Natarajan S, et al. (2005). "The link between the insecticide heptachlor epoxide, estradiol, and breast cancer." Breast Cancer Res Treat 90(1): 55–64. [DOI] [PubMed] [Google Scholar]
- Charlier C, Albert A, et al. (2003). "Breast cancer and serum organochlorine residues." Occup Environ Med 60(5): 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier C, Foidart JM, et al. (2004). "Environmental dichlorodiphenyltrichlorethane or hexachlorobenzene exposure and breast cancer: is there a risk?" Clin Chem Lab Med 42(2): 222–227. [DOI] [PubMed] [Google Scholar]
- Cohn BA, Cirillo PM, et al. (2003). "DDT and DDE exposure in mothers and time to pregnancy in daughters." Lancet 361(9376): 2205–2206. [DOI] [PubMed] [Google Scholar]
- Cohn BA, Wolff MS, et al. (2007). "DDT and breast cancer in young women: new data on the significance of age at exposure." Environ Health Perspect 115(10): 1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collarborative Grouup on Hormonal Factors in Breast Cancer (2012). "Menarch, menopause, and breast cancer risk: individual participant meta-analysis, including 118964 women with breast cancer from 117 epidemiological studies." Lancet Oncol 13(11): 1141–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Iacovo R, Celentano E, et al. (1999). "Organochlorines and breast cancer. A study on Neapolitan women." Adv Exp Med Biol 472: 57–66. [PubMed] [Google Scholar]
- Demers A, Ayotte P, et al. (2000). "Risk and aggressiveness of breast cancer in relation to plasma organochlorine concentrations." Cancer Epidemiol Biomarkers Prev 9(2): 161–166. [PubMed] [Google Scholar]
- Dewailly E, Dodin S, et al. (1994). "HIGH ORGANOCHLORINE BODY BURDEN IN WOMEN WITH ESTROGEN RECEPTOR-POSITIVE BREAST-CANCER." Journal of the National Cancer Institute 86(3): 232–234. [DOI] [PubMed] [Google Scholar]
- Dorgan JF, Brock JW, et al. (1999). "Serum organochlorine pesticides and PCBs and breast cancer risk: results from a prospective analysis (USA)." Cancer Causes & Control 10(1): 1–11. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, et al. (1997). "Bias in meta-analysis detected by a simple graphic test." BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon MD, Wolff MS, et al. (2002). "Environmental toxins and breast cancer on Long Island. II. Organochlorine compound levels in blood." Cancer Epidemiol Biomarkers Prev 11(8): 686–697. [PubMed] [Google Scholar]
- Gatto NM, Longnecker MP, et al. (2007). "Serum organochlorines and breast cancer: a case-control study among African-American women." Cancer Causes Control 18(1): 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grün F, Watanabe H, et al. (2006). "Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates." Molecular Endocrinology 20(9): 2141–2155. [DOI] [PubMed] [Google Scholar]
- Guttes S, Failing K, et al. (1998). "Chlororganic pesticides and polychlorinated biphenyls in breast tissue of women with benign and malignant breast disease." Arch Environ Contam Toxicol 35(1): 140–147. [DOI] [PubMed] [Google Scholar]
- Helzlsouer KJ, Alberg AJ, et al. (1999). "Serum concentrations of organochlorine compounds and the subsequent development of breast cancer." Cancer Epidemiol Biomarkers Prev 8(6): 525–532. [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, et al. (2003). "Measuring inconsistency in meta-analyses." BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer AP, Jorgensen T, et al. (2000). "Repeated measurements of organochlorine exposure and breast cancer risk (Denmark)." Cancer Causes & Control 11(2): 177–184. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Hankinson SE, et al. (1997). "Plasma organochlorine levels and the risk of breast cancer." N Engl J Med 337(18): 1253–1258. [DOI] [PubMed] [Google Scholar]
- Ibarluzea Jm J, Fernandez MF, et al. (2004). "Breast cancer risk and the combined effect of environmental estrogens." Cancer Causes Control 15(6): 591–600. [DOI] [PubMed] [Google Scholar]
- Itoh H, Iwasaki M, et al. (2009). "Serum organochlorines and breast cancer risk in Japanese women: a case-control study." Cancer Causes Control 20(5): 567–580. [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Inoue M, et al. (2008). "Plasma organochlorine levels and subsequent risk of breast cancer among Japanese women: a nested case-control study." Sci Total Environ 402(2-3): 176–183. [DOI] [PubMed] [Google Scholar]
- Krieger N, Wolff MS, et al. (1994). "Breast cancer and serum organochlorines: a prospective study among white, black, and Asian women." J Natl Cancer Inst 86(8): 589–599. [DOI] [PubMed] [Google Scholar]
- Laden F, Collman G, et al. (2001a). "1,1-Dichloro-2,2-bis(p-chlorophenyl)ethylene and polychlorinated biphenyls and breast cancer: combined analysis of five U.S. studies." J Natl Cancer Inst 93(10): 768–776. [DOI] [PubMed] [Google Scholar]
- Laden F, Hankinson SE, et al. (2001b). "Plasma organochlorine levels and the risk of breast cancer: an extended follow-up in the Nurses' Health Study." Int J Cancer 91(4): 568–574. [DOI] [PubMed] [Google Scholar]
- Laden F, Neas LM, et al. (1999). "Predictors of plasma concentrations of DDE and PCBs in a group of U.S. women." Environ Health Perspect 107(1): 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljegren G, Hardell L, et al. (1998). "Case-control study on breast cancer and adipose tissue concentrations of congener specific polychlorinated biphenyls, DDE and hexachlorobenzene." Eur J Cancer Prev 7(2): 135–140. [PubMed] [Google Scholar]
- Longnecker MP, Rogan WJ, and Lucier G (1997). "The human health effects of DDT (dichlorodiphenyltrichloroethanke) and PCBs (polychlorinated biphenyls) and an overview of organochlorines in public health." Annu Rev Public Health 18: 211–244. [DOI] [PubMed] [Google Scholar]
- Lopez-Carrillo L, Blair A, et al. (1997). "Dichlorodiphenyltrichloroethane serum levels and breast cancer risk: a case-control study from Mexico." Cancer Res 57(17): 3728–3732. [PubMed] [Google Scholar]
- Lopez-Cervantes M, Torres-Sanchez L, et al. (2004). "Dichlorodiphenyldichloroethane burden and breast cancer risk: A meta-analysis of the epidemiologic evidence." Environmental Health Perspectives 112(2): 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur V, Bhatnagar P, et al. (2002). "Breast cancer incidence and exposure to pesticides among women originating from Jaipur." Environ Int 28(5): 331–336. [DOI] [PubMed] [Google Scholar]
- Mendonca GA, Eluf-Neto J, et al. (1999). "Organochlorines and breast cancer: a case-control study in Brazil." Int J Cancer 83(5): 596–600. [DOI] [PubMed] [Google Scholar]
- Michels KB and Xue F (2006). "Role of birthweight in the etiology of breast cancer." Int J Cancer 119(9): 2007–2025. [DOI] [PubMed] [Google Scholar]
- Millikan R, DeVoto E, et al. (2000). "Dichlorodiphenyldichloroethene, polychlorinated biphenyls, and breast cancer among African-American and white women in North Carolina." Cancer Epidemiol Biomarkers Prev 9(11): 1233–1240. [PubMed] [Google Scholar]
- Moysich KB, Ambrosone CB, et al. (1998). "Environmental organochlorine exposure and postmenopausal breast cancer risk." Cancer Epidemiol Biomarkers Prev 7(3): 181–188. [PubMed] [Google Scholar]
- Ociepa-Zawal M, Rubis B, et al. (2010). "Accumulation of environmental estrogens in adipose tissue of breast cancer patients." J Environ Sci Health A Tox Hazard Subst Environ Eng 45(3): 305–312. [DOI] [PubMed] [Google Scholar]
- Olaya-Contreras P, Rodriguez-Villamil J, et al. (1998). "Organochlorine exposure and breast cancer risk in Colombian women." Cad Saude Publica 14 Suppl 3: 125–132. [DOI] [PubMed] [Google Scholar]
- Ouyang F, Perry MJ, et al. (2005). "Serum DDT, age at menarch, and abnormal menstrual cycle length." Occup Environ Med 62: 878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JR, Wise LA, et al. (2006). "Prenatal diethylstilbestrol exposure and risk of breast cancer." Cancer Epidemiol Biomarkers Prev 15(8): 1509–1514. [DOI] [PubMed] [Google Scholar]
- Pavuk M, Cerhan JR, et al. (2003). "Case-control study of PCBs, other organochlorines and breast cancer in Eastern Slovakia." J Expo Anal Environ Epidemiol 13(4): 267–275. [DOI] [PubMed] [Google Scholar]
- Pavuk M, Cerhan JR, et al. (2004). "Environmental exposure to PCBs and cancer incidence in eastern Slovakia." Chemosphere 54(10): 1509–1520. [DOI] [PubMed] [Google Scholar]
- Raaschou-Nielsen O, Pavuk M, et al. (2005). "Adipose organochlorine concentrations and risk of breast cancer among postmenopausal Danish women." Cancer Epidemiol Biomarkers Prev 14(1): 67–74. [PubMed] [Google Scholar]
- Recio-Vega R, Velazco-Rodriguez V, et al. (2011). "Serum levels of polychlorinated biphenyls in Mexican women and breast cancer risk." Journal of Applied Toxicology 31(3): 270–278. [DOI] [PubMed] [Google Scholar]
- Romieu I, Hernandez-Avila M, et al. (2000). "Breast cancer, lactation history, and serum organochlorines." Am J Epidemiol 152(4): 363–370. [DOI] [PubMed] [Google Scholar]
- Rubin CH, Lanier A, et al. (2006). "Breast cancer among Alaska Native women potentially exposed to environmental organochlorine chemicals." Int J Circumpolar Health 65(1): 18–27. [DOI] [PubMed] [Google Scholar]
- Schecter A, Toniolo P, et al. (1997). "Blood levels of DDT and breast cancer risk among women living in the north of Vietnam." Arch Environ Contam Toxicol 33(4): 453–456. [DOI] [PubMed] [Google Scholar]
- Siddiqui MK, Anand M, et al. (2005). "Biomonitoring of organochlorines in women with benign and malignant breast disease." Environ Res 98(2): 250–257. [DOI] [PubMed] [Google Scholar]
- Smith D. (1999). "Worldwide trends in DDT levels in human breast milk." International Journal of Epidemiology 28: 179–188. [DOI] [PubMed] [Google Scholar]
- Snedeker SM (2001). "Pesticides and breast cancer risk: a review of DDT, DDE, and dieldrin." Environ Health Perspect 109(S1): 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman AS, Wang X, et al. (2003). "Serum organochlorine levels and history of lactation in Egypt." Environ Res 92(2): 110–117. [DOI] [PubMed] [Google Scholar]
- Stellman SD, Djordjevic MV, et al. (2000). "Breast cancer risk in relation to adipose concentrations of organochlorine pesticides and polychlorinated biphenyls in Long Island, New York." Cancer Epidemiol Biomarkers Prev 9(11): 1241–1249. [PubMed] [Google Scholar]
- Stellman SD, Djordjevic MV, et al. (1998). "Relative abundance of organochlorine pesticides and polychlorinated biphenyls in adipose tissue and serum of women in Long Island, New York." Cancer Epidemiol Biomarkers Prev 7(6): 489–496. [PubMed] [Google Scholar]
- Troisi R, Potischman N and Hoover RN (2007). "Exploring the underlying hormonal mechanisms of prenatal risk factors for breast cancer: a review and commentary." Cancer Epidemiol Biomarkers Prev 16(9): 1700–1712. [DOI] [PubMed] [Google Scholar]
- Unger M, Kiaer H, et al. (1984). "Organochlorine compounds in human breast fat from deceased with and without breast cancer and in a biopsy material from newly diagnosed patients undergoing breast surgery." Environ Res 34(1): 24–28. [DOI] [PubMed] [Google Scholar]
- van't Veer P, Lobbezoo IE, et al. (1997). "DDT (dicophane) and postmenopausal breast cancer in Europe: case-control study." BMJ 315(7100): 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volerón PF, Pestano JJ, et al. (2009). "Differential effects exerted on human mmammary epithelial cells by environmentally relevant organochlorine pesticides either individually or in combination." Chemico-Biological Interactions 180: 485–491. [DOI] [PubMed] [Google Scholar]
- Waliszewski SM, Bermudez MT, et al. (2005). "Persistent organochlorine pesticide levels in breast adipose tissue in women with malignant and benign breast tumors." Bulletin of Environmental Contamination and Toxicology 75(4): 752–759. [DOI] [PubMed] [Google Scholar]
- Ward EM, Schulte P, et al. (2000). "Serum organochlorine levels and breast cancer: A nested case-control study of Norwegian women." Cancer Epidemiology Biomarkers & Prevention 9(12): 1357–1367. [PubMed] [Google Scholar]
- Wolff MS, Berkowitz GS, et al. (2000). "Organochlorine exposures and breast cancer risk in New York City women." Environ Res 84(2): 151–161. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Toniolo PG, et al. (1993). "Blood levels of organochlorine residues and risk of breast cancer." J Natl Cancer Inst 85(8): 648–652. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Zeleniuch-Jacquotte A, et al. (2000). "Risk of breast cancer and organochlorine exposure." Cancer Epidemiol Biomarkers Prev 9(3): 271–277. [PubMed] [Google Scholar]
- Zheng T, Holford TR, et al. (2000). "Risk of female breast cancer associated with serum polychlorinated biphenyls and 1,1-dichloro-2,2'-bis(p-chlorophenyl)ethylene." Cancer Epidemiol Biomarkers Prev 9(2): 167–174. [PubMed] [Google Scholar]
- Zheng T, Holford TR, et al. (1999). "DDE and DDT in breast adipose tissue and risk of female breast cancer." Am J Epidemiol 150(5): 453–458. [DOI] [PubMed] [Google Scholar]