Abstract
Objective
Vulnerability to internet gaming disorder (IGD) has increased as internet gaming continues to grow. Cocaine- and amphetamine-regulated transcript (CART) is a hormone that plays a role in reward, anxiety, and stress. The purpose of this study was to identify the role of CART in the pathophysiology of IGD.
Methods
The serum CART levels were measured by enzyme-linked immunosorbent assay, and the associations of the serum CART level with psychological variables were analyzed in patients with IGD (n=31) and healthy controls (HC) (n=42).
Results
The serum CART level was significantly lower in the IGD than HC group. The IGD group scored significantly higher than the HC group on the psychological domains of depression, anxiety, the reward response in the Behavioral Activation System and Behavioral Inhibition System. There were no significant correlations between serum CART level and other psychological variables in the IGD group.
Conclusion
Our results indicate that a decrease in the expression of the serum CART level is associated with the vulnerability of developing IGD. This study supports the possibility that CART is a biomarker in the pathophysiology of IGD.
Keywords: Internet gaming disorder, Cocaine- and amphetamine-regulated transcript protein, Reward
INTRODUCTION
Internet gaming has become a prevalent form of leisure activity in modern society, with a significant number of Koreans actively participating in gaming. Recent statistics from 2021 indicate a game usage rate of 71.3% in Korea [1]. Notably, the younger demographic, particularly individuals in their 10s and 20s, exhibited high game usage rates of 93.7% and 85.9%, respectively. The coronavirus disease-2019 pandemic has further contributed to increased internet usage, leading to a rise in game usage during 2020, despite a decrease in previous years. While games are often seen as a means of relaxation and socialization [2], the detrimental effects of excessive gaming are becoming evident. Excessive internet gaming can negatively impact psychological and social functioning [3], leading to problems such as neglecting other activities, experiencing withdrawal and tolerance symptoms, and jeopardizing significant relationships or employment opportunities [4].
Internet gaming disorder (IGD) is characterized by excessive and uncontrolled gaming behavior, which ultimately results in functional impairment or distress [5]. Numerous studies have explored the neurophysiological and pathological factors underlying IGD. Individuals with IGD have shown altered reward sensitivity [6], deficiencies in the dopaminergic reward system [7], as well as deficits in cognitive-emotional processing, impulsivity, cue reactivity, and impaired decision-making, as observed through neuroimaging and behavioral tasks [8-10].
Neuropeptides have gained attention as biomarker in studies examining psychiatric disorders, including addiction. The cocaine- and amphetamine-regulated transcript (CART) peptide, encoded by the CARTPT gene in humans [11,12], is one such neuropeptide. CART is believed to play a role in reward processing, eating behavior, anxiety regulation, and stress responses [13]. Additionally, CART exhibits functional properties akin to an endogenous psychostimulant [14].
Immunoreactivity of CART is upregulated in various brain regions, such as the amygdala, paraventricular nucleus, periventricular area, arcuate nucleus, and ventromedial nucleus, suggesting its involvement in anxiety-like behaviors following ethanol withdrawal [15]. Studies utilizing CART-knockout mice have demonstrated the peptide’s influence on locomotor activity, conditioned place preference, and cocaine self-administration effects [16]. Notably, changes in CART expression in the ventral tegmental area of cocaine overdose victims and mutations in the CART gene have been associated with alcoholism [17]. Therefore, CART, which inhibits the rewarding effect of cocaine, has the potential for treating cocaine addiction [18].
Previous evidence indicates that low CART levels in the hypothalamus of depressed animals are linked to hyperphagia and weight gain [19,20]. CART is believed to interact with the opioid mesolimbic dopamine system, which modulates natural rewards [21]. The CART peptide, referred to as CART (55–102), CART (62–102), or their corresponding peptides found in the human brain (CART [42–89] and [49–89]) [22], exhibits a prominent association with cocaine. It is proposed that the peptide reduces the actions of cocaine or dopamine, particularly in the nucleus accumbens, serving as a significant regulatory mechanism for the physiological effects of cocaine and dopamine. For instance, cocaine administration increases locomotor activity, whereas acute injection of the CART peptide into the nucleus accumbens reduces cocaine-induced locomotor activity [22]. Given the widespread distribution of the CART peptide throughout the brain, it is evident that it plays various physiological roles. Several studies have reported associations between IGD and various psychiatric symptoms, psychological factors, and neurocognitive characteristics. IGD has been linked to stress [23-26], loneliness, depression [27], and anxiety [28]. Furthermore, recent research has demonstrated a correlation between depression/anxiety and internet addiction [29-31]. Patients with IGD also exhibit impaired inhibitory control [32] and executive functioning [24,33].
While CART is expressed in the brain, endocrine system, and neuroendocrine system, its role in the pathophysiology of IGD remains poorly understood. Moreover, the serum levels of CART have not been evaluated in individual with IGD, despite its widely recognized role in mediating substance abuse in both animals and humans. Therefore, the purpose of this study was to examine the differences in serum CART levels and clinical characteristics between patients with IGD and a healthy control (HC) group. Our hypothesis is that the CART levels in the IGD group will be lower than those in the HC group. Additionally, we aimed to investigate the correlations between serum CART levels and psychological characteristics.
METHODS
Participants
A total of 73 adults participated in this study, consisting of 31 in the IGD (age: 23.39±3.42 years) and 42 in the HC (age: 24.17±3.02 years) groups. The participants were recruited from the SMG-SNU Boramae Medical Center and the surrounding community in Seoul, Republic of Korea. The patients with IGD were seeking treatment for their addiction. The exclusion criteria for the IGD group were any history of chronic physical disease, regular use of any medication, and the presence of a comorbid psychiatric disorder, including alcohol or nicotine dependence. The IGD group included 30 males and 1 female who underwent diagnostic interviews conducted by an experienced psychiatrist to assess for IGD and any comorbid psychiatric disorders, utilizing the criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. IGD severity was evaluated using Young’s Internet Addiction Test Korean version (Y-IAT-K) [34]. Individuals with IGD spent more than 4 hours per day and more than 30 hours per week engaging in internet gaming activities, whereas HCs spent less than 2 hours per day gaming online. The HC group was recruited through advertisements and consisted of 35 healthy males and 7 healthy females who had no psychiatric history. All participants were medication-naive during the assessment. Blood sampling was conducted before the clinical functioning examination. All participants underwent the Korean version of the Wechsler Adult Intelligence Scale IV [35] to determine their intelligence quotient (IQ). Participants with an IQ below 70 were not included. The ethical committee of the SMG-SNU Boramae Medical Center approved this study protocol (IRB No. 16-2014-139), which followed the Declaration of Helsinki. All subjects were advised about the procedures and signed the written informed consent before participation.
Measures
Psychological domains
Y-IAT-K
The Y-IAT-K [34] was used to measure the severity of internet game addiction in the IGD and HC groups. In this study, a modified Y-IAT made for assessing internet games was used [36,37]. The Y-IAT consists of 20 items on a 5-point Likert scale, and the total score ranges from 20 to 100. The higher the score, the higher the likelihood of addiction. The scoring criteria are as follows: a score of 20–39 is normal, a score of 40–69 indicates a possible addiction, and a score of ≥70 indicates an addiction. The Cronbach’s alpha coefficient was 0.96.
Beck Depression Inventory-II
The Beck Depression Inventory-II (BDI-II) [38] was utilized to gauge the severity of depression. The validated Korean version of the BDI-II was used [39]. Twenty-one items are included in this questionnaire, and the answers are rated on a 4-point Likert scale. The severity of depression is determined by the overall score. A higher score means more severe depression. The Cronbach’s alpha coefficient was 0.93.
Beck Anxiety Inventory
Anxiety was measured using the Beck Anxiety Inventory (BAI), developed by Beck et al. [40] and evaluated for validity and reliability in Korea [41]. The BAI consists of 21 items, and each item asks the individual to select the degree of anxiety symptoms experienced during the past week from four sentences. A score of 0–3 points is given for each question, and a total score is obtained. The Cronbach’s alpha coefficient was 0.95.
Behavioral Inhibition System/Behavioral Activation System Scales
The Behavioral Inhibition System/Behavioral Activation System (BIS/BAS) scale is a 20-item scale developed by Carver and White [42] from Gray’s [43] theory of two general motivational systems based on behavior and emotion. It consists of the BAS and BIS subscales; the BAS subscale is divided into the three sub-factors of reward responsiveness, drive, and fun seeking [41]. The Cronbach’s alpha coefficient was 0.80.
Serum CART levels
Blood (10 mL) was collected from each subject into a serum separator tube to measure the serum CART level. The samples were allowed to clot for 30 min and were centrifuged for 15 min at approximately 1,000×g, after which the supernatant was recovered. A protease inhibitor cocktail (Roche, Manheim, Germany) was added to the serum samples, which were then stored at -80°C until use. The serum CART concentration was determined using an enzyme-linked immunosorbent assay kit (Elabscience, Houston, TX, USA) according to the manufacturer’s instructions. After the optical density (OD) 450 nm values recorded for each standard and sample were subtracted from the OD value of the zero standard, the calibration curves were prepared by plotting the corrected OD value of each standard concentration (y-axis) against the log of the respective standard concentration (x-axis).
Statistical analyses
Fisher’s exact test was performed for statistical comparisons because there was a difference in the number of males and females. The t-test was used to compare differences in demographic and psychological characteristics between the IGD and HC groups. Given the established connection between mutations in the human CART gene and depression and anxiety [44], we conducted anlysis of covariance (ANCOVA) analysis to account for potential confounding factors in group comparisons, incorporating BDI-II (depression) alone, BAI (anxiety) alone, and then both BDI-II and BAI together as covariates, sequentially. Spearman’s correlation analysis was performed to assess the correlation between the serum CART levels and the clinical characteristics. All data are presented as mean±standard deviation. A p-value <0.05 was considered significant. All statistical analyses were completed using SPSS version 23 software (IBM Corp., Armonk, NY, USA).
RESULTS
Fisher’s exact test showed that the sex ratio did not significantly affect the outcome (two-tailed p=0.071). As shown in Table 1, there was no significant difference in sex (t[71]=-1.834, p=0.071) and age (t[71]=-1.030, p=0.306) between the two groups. When the clinical characteristics of the IGD and HC groups were compared, the IGD group scored significantly higher than the HC group on the following items: the Y-IAT-K, online gaming times on weekend, BDI-II, BAI, BAS reward response, and BIS.
Table 1.
Demographic and clinical characteristics in IGD vs. HC
| Characteristic | IGD (N=31) | HC (N=42) | Test statistics | p |
|---|---|---|---|---|
| Sex (male) | 30 (96.77) | 35 (83.33) | t=-1.834 | 0.071 |
| Age (yr) | 23.387±3.422 | 24.167±3.020 | t=-1.030 | 0.306 |
| Online gaming on weekday (h/day) | 2.806±2.716 | 1.929±1.069 | t=1.908 | 0.060 |
| Online gaming on weekend (h/day) | 3.355±3.176 | 2.167±1.248 | t=2.209 | 0.030* |
| Y-IAT-K | 59.806±15.281 | 30.143±9.257 | t=10.293 | <0.001* |
| BDI-II | 13.419±9.821 | 3.476±4.301 | t=5.855 | <0.001* |
| BAI | 13.032±12.566 | 3.190±4.341 | t=4.718 | <0.001* |
| BAS reward response | 14.581±2.487 | 13.262±2.142 | t=2.428 | 0.018* |
| BAS drive | 10.484±2.448 | 10.119±2.027 | t=0.696 | 0.489 |
| BAS fun seeking | 10.161±2.570 | 9.571±2.286 | t=1.034 | 0.305 |
| BAS total | 35.226±5.948 | 32.952±5.161 | t=1.743 | 0.086 |
| BIS | 19.806±4.672 | 17.714±3.424 | t=2.209 | 0.030* |
Values are presented as number (%) or mean±standard deviation. Independent t-test was used to compare means in two groups. Online gaming in weekday/weekend: average online gaming use time per day on weekday and weekend.
p<0.05.
IGD, internet gaming disorder; HC, healthy control; Y-IAT-K, Young’s Internet Addiction Test Korean version; BDI-II, Beck Depression Inventory-II; BAI, Beck Anxiety Inventory; BAS, Behavioral Activation System; BIS, Behavioral Inhibition System
The mean serum CART level was significantly lower in the IGD group (0.967±0.803 ng/mL) than HC group (1.513±1.000 ng/mL) (Figure 1). The statistically significant differences in the corrected CART levels between the groups were observed after controlling for the influence of BDI-II alone, BAI alone, and then both BDI-II and BAI together. Upon examination of the corrected CART levels, it can be concluded that the CART levels in the IGD group are lower than those in the HC group, (F[1, 71]=5.789, p=0.019; F[1, 71]=6.749, p=0.011; F[1, 71]=5.822, p=0.018).
Figure 1.
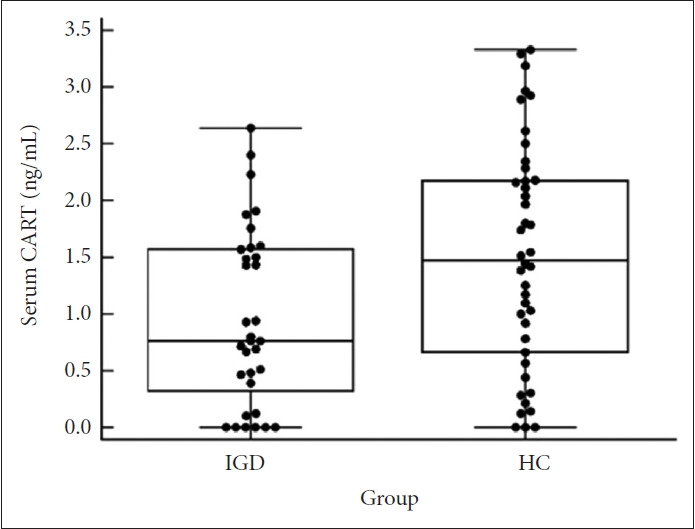
Box-and whisker plots with dots representing the serum CART concentrations of 31 IGD and 42 HC samples. The average serum CART concentrations in the IGD and HC groups were 0.967 and 1.513 ng/mL, respectively (p=0.023, Mann–Whitney test). CART, cocaine- and amphetamine-regulated transcript; IGD, internet gaming disorder; HC, healthy control.
There was no significant correlation between serum CART level and other psychological variables in the IGD group.
DISCUSSION
The present study aimed to examine differences and correlations between serum CART levels and clinical characteristics in the IGD and HC groups. Our findings are consistent with our initial hypothesis, as we observed lower CART levels in the IGD group compared to the HC group, suggesting a potential role for CART expression in the pathogenesis of IGD. For the first time, our findings revealed that the serum level of the CART peptide was significantly lower in the IGD group compared to the HC group. Additionally, the IGD group exhibited significantly higher scores than the HC group in various clinical characteristics, including online gaming times, BAS reward response scale scores (indicating higher sensitivity to pleasant reinforcers in the environment), BIS scale scores (indicating higher motivation to avoid aversive outcomes), BDI-II scores (indicating greater depression), and BAI scores (indicating greater anxiety).
The present study showed that patients with IGD had higher BAS reward response scale scores and BIS scale scores rather than the HC group. The BIS/BAS scale measures two motivational systems: BIS, which reflects the drive to avoid unpleasant consequences, and BAS, which reflects the drive to pursue goal-oriented outcomes. Specifically, the BAS scale consists of three subscales: reward responsiveness, drive, and fun-seeking. The reward responsiveness subscale assesses an individual’s sensitivity to positive reinforcement in their environment. The drive subscale measures the desire to pursue objectives, while the fun-seeking subscale evaluates the inclination to seek new rewards independently. BAS influences an individual’s sensitivity to rewards and motivates them to explore rewarding stimuli, whereas BIS affects sensitivity to punishment and leads to avoidance behavior [45]. Numerous studies have demonstrated that an imbalance in the operation of these systems is associated with reduced quality of life and various psychiatric and psychosomatic disorders. For instance, increased BAS activity is linked to substance abuse and antisocial behaviors [46], while reduced BAS activity is associated with depression [47], and an imbalance in BAS functioning is implicated in bipolar disorder [48]. High BAS sensitivity has been shown to be associated with addictive behaviors. Yen et al. [49] reported that high BAS activity and fun-seeking tendencies are among the risk factors contributing to addiction. Additionally, Kim and Lee [50] found that pathological gambling behaviors were related to low BIS scores and high BAS scores.
Furthermore, a correlation analysis was conducted to explore the relationship between the serum CART level and the mechanisms underlying IGD. The results revealed that no correlations were found in the IGD group. Since no correlations were found between the serum CART level and the severity of addiction symptoms, it is suggested that the serum CART level reflects personality traits associated with reward sensitivity and acts as a risk factor for developing IGD. A decrease in the serum CART level is associated with an increased risk of developing IGD. According to a review by Ahmadian-Moghadam et al. [51], accumulating evidence suggests that CART functions as a neurotransmitter and neuroprotective agent, primarily involved in the regulation of various processes such as feeding, addiction, stress, anxiety, innate fear, depression, learning, or memory. The CART peptide is detected in specific sets of neurons that modulate numerous activities and exists in various fragments due to post-translational processing, making the overall understanding of CART complex. Nonetheless, our study highlights the potential of the serum CART level as a candidate biomarker for IGD. Abnormal CART transmission may influence reward responsiveness, suggesting that changes in CART neurotransmission contribute to the pathophysiology of IGD.
A decrease in CART levels has several implications for addiction. First, lower CART levels may indicate issues within the central nervous system’s reward system, suggesting individuals with addiction might need more stimulation to experience rewards or show reduced responses to everyday rewards. Second, reduced CART levels could suggest a higher vulnerability to addictive behaviors or substances, potentially reflecting a lack of regulation of addictive behaviors or diminished self-control. Third, decreased CART levels might signify compromised stress coping mechanisms. Individuals with addiction often seek rewards through the reward system in response to stress, and lower CART levels may hinder this stress management process. Overall, declining CART levels reflect neurochemical and behavioral changes associated with addiction, potentially impacting its development and progression.
CART is involved in regulating the reward system of the central nervous system. Internet gaming addiction is one of the behaviors that activate the reward system, and changes in CART can influence addiction-related behaviors associated with this reward system.
This study is subject to several limitations that should be acknowledged. Firstly, the small number of female subjects compared to male subjects is a limitation. Although no significant difference was observed in the sex ratio, future studies should strive to include a more balanced representation of both genders to enhance the generalizability of the findings. In this study, in an effort to enhance the quality of the research, we attempted additional analysis by only including male participants. Firstly, the mean serum CART level was significantly lower in the IGD group (0.911±0.753 ng/mL) compared to the HC group (1.398±1.024 ng/mL). Subsequently, ANCOVA analyses were conducted using BDI (depression) alone, BAI (anxiety) alone, and both BDI and BAI together as covariates for CART levels. The results showed that in all cases, the CART levels in the IGD group were significantly lower than those in the HC group (F[1, 63]=4.013, p=0.0495; F[1, 63]=5.300, p=0.025; F[1, 63]=4.012, p=0.0496). Secondly, this study unintentionally focused solely on young adults, with an average age range of 23–24 years. A broader age distribution would have provided a more comprehensive understanding of the relationship between serum CART levels and IGD across different age groups. Therefore, future research should aim to include participants spanning a wider age range to capture potential age-related variations in the findings.
In conclusion, this study yielded two key findings. First, we found that the serum CART level was significantly lower in the IGD group compared to the HC group. Second, no significant correlations were observed between the serum CART level and the severity of addiction symptoms. These findings suggest that a decrease in serum CART level may influence the risk of developing IGD. Our results provide support for the potential involvement of serum CART in the addiction mechanism underlying IGD.
Acknowledgments
The authors would like to express our appreciation to all the individuals who gave their time to participate in this study.
Footnotes
Availability of Data and Material
The datasets generated or analyzed during the study are not publicly available due to privacy or ethical restrictions but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Dai-Jin Kim, Ji Eun Lee, Jung-Seok Choi. Data curation: Heejin Lee, Dong Huey Cheon, Arom Pyeon, Ji-Won Chun, Ji Hyun Back, Yae Eun Park. Formal analysis: Heejin Lee, Dong Huey Cheon, Ji Hyun Back, Yae Eun Park. Funding acquisition: Ji Eun Lee, Jung-Seok Choi. Investigation: So Young Yoo, Ji-Won Chun, Dai-Jin Kim, Ji Eun Lee, Jung-Seok Choi. Methodology: Heejin Lee, Ji-Won Chun, Dai-Jin Kim, Ji Eun Lee. Project administration: Ji Eun Lee, Jung-Seok Choi. Resources: Ji Eun Lee, Jung-Seok Choi. Software: Ji-Won Chun, Ji Eun Lee. Supervision: Dai-Jin Kim, Ji Eun Lee, Jung-Seok Choi. Validation: Ara Cho, Heejin Lee, Ji Eun Lee, Jung-Seok Choi. Visualization: Ara Cho, Ji Eun Lee. Writing—original draft: Ara Cho, Heejin Lee. Writing—review & editing: Ara Cho, Heejin Lee, So Young Yoo, Dai-Jin Kim, Ji Eun Lee, Jung-Seok Choi.
Funding Statement
This work was supported by Korea Mental Health R&D Project, funded by the Ministry of Health & Welfare, Republic of Korea (HI22C0404 to J-SC), and a grant from the National Research Foundation of Korea (RS- 2024-00420674 to J-SC). This research was supported by the Bio&Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (RS-2023-00223559), a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare (HI20C0013) (JE Lee), and a KIST institutional program (JE Lee).
REFERENCES
- 1. Korea Creative Content Agency. [2021 game user survey]. Naju: Korea Creative Content Agency; 2021. Korean. [Google Scholar]
- 2.King D, Delfabbro P. Internet gaming disorder: theory, assessment, treatment, and prevention. Cambridge: Academic Press; 2018. [Google Scholar]
- 3.Wang HY, Cheng C. The associations between gaming motivation and internet gaming disorder: systematic review and meta-analysis. JMIR Ment Health. 2022;9:e23700. doi: 10.2196/23700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo T, Wei D, Guo J, Hu M, Chao X, Sun Y, et al. Diagnostic contribution of the DSM-5 criteria for internet gaming disorder. Front Psychiatry. 2022;12:777397. doi: 10.3389/fpsyt.2021.777397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuss DJ. Internet gaming addiction: current perspectives. Psychol Res Behav Manag. 2013;6:125–137. doi: 10.2147/PRBM.S39476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong G, Hu Y, Lin X. Reward/punishment sensitivities among internet addicts: implications for their addictive behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:139–145. doi: 10.1016/j.pnpbp.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y, Zhang H, Tian M. Molecular and functional imaging of internet addiction. Biomed Res Int. 2015;2015:378675. doi: 10.1155/2015/378675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng Y, Deng W, Wang H, Guo W, Li T. The prefrontal dysfunction in individuals with internet gaming disorder: a meta-analysis of functional magnetic resonance imaging studies. Addict Biol. 2015;20:799–808. doi: 10.1111/adb.12154. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Ndasauka Y, Hou J, Chen J, Yang LZ, Wang Y, et al. Cue-induced behavioral and neural changes among excessive internet gamers and possible application of cue exposure therapy to internet gaming disorder. Front Psychol. 2016;7:675. doi: 10.3389/fpsyg.2016.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YJ, Kim DJ, Choi JS. The cognitive dysregulation of internet addiction and its neurobiological correlates. Front Biosci (Elite Ed) 2017;9:307–320. doi: 10.2741/e804. [DOI] [PubMed] [Google Scholar]
- 11.Douglass J, Daoud S. Characterization of the human cDNA and genomic DNA encoding CART: a cocaine- and amphetamine-regulated transcript. Gene. 1996;169:241–245. doi: 10.1016/0378-1119(96)88651-3. [DOI] [PubMed] [Google Scholar]
- 12.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Han L, Xu Y. Roles of cocaine- and amphetamine-regulated transcript in the central nervous system. Clin Exp Pharmacol Physiol. 2012;39:586–592. doi: 10.1111/j.1440-1681.2011.05642.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuhar MJ, Adams S, Dominguez G, Jaworski J, Balkan B. CART peptides. Neuropeptides. 2002;36:1–8. doi: 10.1054/npep.2002.0887. [DOI] [PubMed] [Google Scholar]
- 15.Dandekar MP, Singru PS, Kokare DM, Lechan RM, Thim L, Clausen JT, et al. Importance of cocaine- and amphetamine-regulated transcript peptide in the central nucleus of amygdala in anxiogenic responses induced by ethanol withdrawal. Neuropsychopharmacology. 2008;33:1127–1136. doi: 10.1038/sj.npp.1301516. [DOI] [PubMed] [Google Scholar]
- 16.Couceyro PR, Evans C, McKinzie A, Mitchell D, Dube M, Hagshenas L, et al. Cocaine- and amphetamine-regulated transcript (CART) peptides modulate the locomotor and motivational properties of psychostimulants. J Pharmacol Exp Ther. 2005;315:1091–1100. doi: 10.1124/jpet.105.091678. [DOI] [PubMed] [Google Scholar]
- 17.Kuhar MJ, Jaworski JN, Hubert GW, Philpot KB, Dominguez G. Cocaine- and amphetamine-regulated transcript peptides play a role in drug abuse and are potential therapeutic targets. AAPS J. 2005;7:E259–E265. doi: 10.1208/aapsj070125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu C, Zhou X, Fu Q, Peng Q, Oh KW, Hu Z. A new insight into the role of CART in cocaine reward: involvement of CaMKII and inhibitory G-protein coupled receptor signaling. Front Cell Neurosci. 2017;11:244. doi: 10.3389/fncel.2017.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dandekar MP, Singru PS, Kokare DM, Subhedar NK. Cocaine- and amphetamine-regulated transcript peptide plays a role in the manifestation of depression: social isolation and olfactory bulbectomy models reveal unifying principles. Neuropsychopharmacology. 2009;34:1288–1300. doi: 10.1038/npp.2008.201. [DOI] [PubMed] [Google Scholar]
- 20.Nakhate KT, Kokare DM, Singru PS, Subhedar NK. Central regulation of feeding behavior during social isolation of rat: evidence for the role of endogenous CART system. Int J Obes (Lond) 2011;35:773–784. doi: 10.1038/ijo.2010.231. [DOI] [PubMed] [Google Scholar]
- 21.Upadhya MA, Nakhate KT, Kokare DM, Singh U, Singru PS, Subhedar NK. CART peptide in the nucleus accumbens shell acts downstream to dopamine and mediates the reward and reinforcement actions of morphine. Neuropharmacology. 2012;62:1823–1833. doi: 10.1016/j.neuropharm.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci. 2008;9:747–758. doi: 10.1038/nrn2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Achab S, Nicolier M, Mauny F, Monnin J, Trojak B, Vandel P, et al. Massively multiplayer online role-playing games: comparing characteristics of addict vs non-addict online recruited gamers in a French adult population. BMC Psychiatry. 2011;11:144. doi: 10.1186/1471-244X-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong G, Potenza MN. A cognitive-behavioral model of internet gaming disorder: theoretical underpinnings and clinical implications. J Psychiatr Res. 2014;58:7–11. doi: 10.1016/j.jpsychires.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peltzer K, Pengpid S, Apidechkul T. Heavy internet use and its associations with health risk and health-promoting behaviours among Thai university students. Int J Adolesc Med Health. 2014;26:187–194. doi: 10.1515/ijamh-2013-0508. [DOI] [PubMed] [Google Scholar]
- 26.Spada MM. An overview of problematic internet use. Addict Behav. 2014;39:3–6. doi: 10.1016/j.addbeh.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Whang LS, Lee S, Chang G. Internet over-users’ psychological profiles: a behavior sampling analysis on internet addiction. Cyberpsychol Behav. 2003;6:143–150. doi: 10.1089/109493103321640338. [DOI] [PubMed] [Google Scholar]
- 28.Lim JA, Lee JY, Jung HY, Sohn BK, Choi SW, Kim YJ, et al. Changes of quality of life and cognitive function in individuals with internet gaming disorder: a 6-month follow-up. Medicine (Baltimore) 2016;95:e5695. doi: 10.1097/MD.0000000000005695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Younes F, Halawi G, Jabbour H, El Osta N, Karam L, Hajj A, et al. Internet addiction and relationships with insomnia, anxiety, depression, stress and self-esteem in university students: a cross-sectional designed study. PLoS One. 2016;11:e0161126. doi: 10.1371/journal.pone.0161126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rachubińska K, Cybulska AM, Grochans E. The relationship between loneliness, depression, internet and social media addiction among young Polish women. Eur Rev Med Pharmacol Sci. 2021;25:1982–1989. doi: 10.26355/eurrev_202102_25099. [DOI] [PubMed] [Google Scholar]
- 31.Teng Z, Pontes HM, Nie Q, Griffiths MD, Guo C. Depression and anxiety symptoms associated with internet gaming disorder before and during the COVID-19 pandemic: a longitudinal study. J Behav Addict. 2021;10:169–180. doi: 10.1556/2006.2021.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi JS, Park SM, Roh MS, Lee JY, Park CB, Hwang JY, et al. Dysfunctional inhibitory control and impulsivity in internet addiction. Psychiatry Res. 2014;215:424–428. doi: 10.1016/j.psychres.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 33.D’Hondt F, Billieux J, Maurage P. Electrophysiological correlates of problematic internet use: critical review and perspectives for future research. Neurosci Biobehav Rev. 2015;59:64–82. doi: 10.1016/j.neubiorev.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Young KS. Caught in the net: how to recognize the signs of internet addiction—and a winning strategy for recovery. New York: John Wiley & Sons; 1998. [Google Scholar]
- 35.Hwang ST, Kim JH, Park KB, Chey JY, Hong SH. Korean Wechsler adult intelligence scale-IV. Daegu: Korea Psychology Co; 2012. [Google Scholar]
- 36.Park M, Choi JS, Park SM, Lee JY, Jung HY, Sohn BK, et al. Dysfunctional information processing during an auditory event-related potential task in individuals with internet gaming disorder. Transl Psychiatry. 2016;6:e721. doi: 10.1038/tp.2015.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park M, Jung MH, Lee J, Choi AR, Chung SJ, Kim B, et al. Neurophysiological and cognitive correlates of error processing deficits in internet gaming disorder. Cereb Cortex. 2020;30:4914–4921. doi: 10.1093/cercor/bhaa083. [DOI] [PubMed] [Google Scholar]
- 38.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio: Psychological Corporation; 1996. [Google Scholar]
- 39.Kim MS, Lee IS, Lee CS. [The validation study I of Korean BDI-II: in female university students sample] Kor J Clin Psychol. 2007;26:997–1014. Korean. [Google Scholar]
- 40.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 41.Yook SP, Kim ZS. [A clinical study on the Korean version of Beck Anxiety Inventory: comparative study of patient and non-patient] Kor J Clin Psychol. 1997;16:185–197. Korean. [Google Scholar]
- 42.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- 43.Gray JA. Brain systems that mediate both emotion and cognition. Cogn Emot. 1990;4:269–288. [Google Scholar]
- 44.Wiehager S, Beiderbeck DI, Gruber SH, El-Khoury A, Wamsteeker J, Neumann ID, et al. Increased levels of cocaine and amphetamine regulated transcript in two animal models of depression and anxiety. Neurobiol Dis. 2009;34:375–380. doi: 10.1016/j.nbd.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Erdle S, Rushton JP. The general factor of personality, BIS–BAS, expectancies of reward and punishment, self-esteem, and positive and negative affect. Pers Individ Differ. 2010;48:762–766. [Google Scholar]
- 46.Newman JP, MacCoon DG, Vaughn LJ, Sadeh N. Validating a distinction between primary and secondary psychopathy with measures of Gray’s BIS and BAS constructs. J Abnorm Psychol. 2005;114:319–323. doi: 10.1037/0021-843X.114.2.319. [DOI] [PubMed] [Google Scholar]
- 47.McFarland BR, Shankman SA, Tenke CE, Bruder GE, Klein DN. Behavioral activation system deficits predict the six-month course of depression. J Affect Disord. 2006;91:229–234. doi: 10.1016/j.jad.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 48.Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, et al. Behavioral approach system and behavioral inhibition system sensitivities and bipolar spectrum disorders: prospective prediction of bipolar mood episodes. Bipolar Disord. 2008;10:310–322. doi: 10.1111/j.1399-5618.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 49.Yen JY, Ko CH, Yen CF, Chen CS, Chen CC. The association between harmful alcohol use and internet addiction among college students: comparison of personality. Psychiatry Clin Neurosci. 2009;63:218–224. doi: 10.1111/j.1440-1819.2009.01943.x. [DOI] [PubMed] [Google Scholar]
- 50.Kim DY, Lee JH. Effects of the BAS and BIS on decision-making in a gambling task. Pers Individ Differ. 2011;50:1131–1135. [Google Scholar]
- 51.Ahmadian-Moghadam H, Sadat-Shirazi MS, Zarrindast MR. Cocaine- and amphetamine-regulated transcript (CART): a multifaceted neuropeptide. Peptides. 2018;110:56–77. doi: 10.1016/j.peptides.2018.10.008. [DOI] [PubMed] [Google Scholar]


