Abstract
Objective
Tacrolimus intrapatient variability (Tac IPV) has been considered a marker for post-graft risk. We investigated pre-transplant psychometric testing to predict Tac IPV after living kidney transplantation.
Methods
Minnesota Multiphasic Personality Inventory-2 (MMPI-2) examined during pre-transplant evaluation by 102 recipients were analyzed. Subjects were divided into two groups, low IPV (L-IPV) and high IPV (H-IPV), by cutoffs of Tac IPV: median of 24 and value of 30. T-scores of MMPI-2 scales were used to analyze difference between L-IPV and H-IPV using independent t-tests. Stepwise multiple logistic regression was used to test whether MMPI-2 scales affected Tac IPV. Confusion matrix of logistic regression was used to explain statistical power. Cutoff values of significant scales for H-IPV were analyzed by constructing receiver operating characteristic curves.
Results
Hysteria (Hy) and depression (D) scale scores and Tac IPV were associated in IPV 24 (odds ratio [OR]: 1.08, p<0.01 for Hy; OR: 0.93, p<0.01 for D) and IPV 30 models (OR: 1.09, p<0.01 for Hy; OR: 0.92, p<0.01 for D). Paranoia (Pa) scale scores were associated with Tac IPV in IPV 24 model (OR=1.10, p<0.01) and were significantly higher in H-IPV 24 (p<0.01). F1 scores of confusion matrix in IPV 24 and 30 models were 0.70 and 0.71, respectively. Cutoffs of Hy, D, and Pa scales were 51, 57, and 47, respectively.
Conclusion
MMPI-2 profile is suggested as a predictor for high Tac IPV after living kidney transplantation.
Keywords: Kidney transplantation, Recipients, Minnesota Multiphasic Personality Inventory-2, Tacrolimus, Intrapatient variability
INTRODUCTION
Tacrolimus (Tac) is widely used immunosuppressant in kidney transplantation for graft survival. Therapeutic drug monitoring is essential to help Tac management because of its high intra and interpatient variability. Although whole blood trough levels were most commonly used, they are weakly correlated with clinical outcomes in regard to immunosuppression [1]. Recently, Tac intrapatient variability (Tac IPV), the fluctuation of whole blood Tac trough levels within a patient over a period of time, has been considered as a better marker to evaluate post-graft risk [2]. High Tac IPV is a significant risk factor that can affect the prognosis of patients after solid organ transplantation. This can cause cumulative damage to the transplanted organ, including T-cell-mediated rejection, donor-specific anti-HLA antibodies, and vascular changes in kidney grafts, causing chronic ischemia and irreversible fibrotic damage [3,4].
Food and drug interactions, cytochrome P450 3A5 expression, certain comorbid illnesses, and medication non-adherence (MNA) have been known factors contributing to Tac IPV [2]. MNA obviously results in high Tac IPV, which has an impact on poor outcomes after kidney transplantation. Although MNA has been considered an important determinant of high Tac IPV, there have been rarely known other factors to be measured and accessed more easily by clinicians to predict high Tac IPV.
Psychiatric evaluation is an important step in selecting candidates for organ transplantation. Some studies indicated that neuropsychological alterations could affect the inflammatory mechanisms, which were related to graft rejection [5]. Psychological factors were shown to affect the number of immune cells in the peripheral blood [6].For instance, white blood cell counts and its subsets were increased in patients with psychiatric disorders. The patients with major depressive disorder had higher neutrophils and monocyte counts [7]. Other studies showed that personality factors were associated with differences in endocrine and immune responses after serotonergic (5-HT) stimulation, which were known to be involved in graft rejection processes. For example, the high scores of impulsivity, psychoticism, and boredom susceptibility have been identified as more responsive to the 5-HT agonist, indicating a larger reduction in T-helper cells and greater cortisol release [8]. Patients with high scores of psychoticism were found to be more responsive to a 5-HT agonist, with a considerable reduction in T-helper cells [8,9]. These imply that psychiatric property could affect graft rejection via the biological process.
During the pre-transplantation period, it is essential to assess the patient’s therapeutic adherence and understanding of the treatment. Medical compliance, including medication adherence, is the primary predictor of treatment success [10]. Significant emotional distress and certain personality traits can affect medical compliance in patients awaiting organ transplantation [11]. In a recent systematic review, factors including age <50 years, male sex, low social support, unemployment, low education, and depression were shown to be significantly associated with the MNA in kidney transplantation [12]. The Minnesota Multiphasic Personality Inventory (MMPI) and now the MMPI-2 have long been used to assess the emotional and psychological suitability of candidates for organ transplantation. It appears to be a valuable instrument for pre-transplantation evaluation of the liver and kidney [13]. The validity scales of the MMPI provide an objective clinical impression of dissimulation in the psychometric evaluation of living kidney transplantation [14]. Scale scores for defensiveness (K) and hypomania (Ma) before transplantation have been reported as risk factors for smoking reabusing after heart transplantation [15]. Taken together, the MMPI-2 can be used to assess the patient’s psychological characteristics related to medical compliance in pre-kidney transplantation evaluation alongside a psychiatric interview.
Globally, 192,307 patients were on kidney transplantation waiting lists in 2017. Given a lack of deceased donor kidneys, living kidney transplantation has become increasingly common for patients with end-stage kidney disease. It is one of the most progressive and growing areas of organ transplantation in many countries [16]. Considering the importance of managing Tac IPV to improve long-term graft survival after transplantation, it is crucial to intervene in MNA, the prominent determinant of Tac IPV. In addition, a measurable instrument to identify high Tac IPV in pre-transplant evaluation could help clinicians recognize at-risk patients and provide more intensive interventions for preventing graft failure. In the present study, we investigated the MMPI-2 profile performed by recipients during the living kidney pre-transplantation evaluation. Then, these were analyzed to test the MMPI-2 profile as a predictive factor of high Tac IPV.
METHODS
Study population
One hundred twenty-six patients who had living kidney transplantation were initially screened between February 2011 and August 2022. Inclusion criteria included: 1) age ≥19 years, 2) single kidney transplantation, 3) treated with Tac at least one year after transplantation, 4) available to calculate the coefficient of variability (CV) for blood Tac concentration between 6 and 12 months after transplantation, and 5) had performed the MMPI-2 as a pre-transplantation evaluation. Exclusion criteria were 1) death, 2) absence for follow-up or transfer to another hospital, 3) graft loss due to acute transplant rejection, 4) change in immunosuppressive drugs between 6–12 months post-transplantation, and 5) discontinued Tac use due to other infections or unknown reasons between 6–12 months post-transplantation.
Clinical measurement
Age, sex, education, occupation, family status, number of medicines, and biological relationships were assessed to examine the epidemiological characteristics of the patients. The MMPI-2 was performed as a pre-transplantation evaluation to assess the psychological factors of the recipients. The MMPI-2 is a well-standardized self-report measure designed to assess personality traits and psychopathology in an individual’s personality. Briefly, it consists of 567 statements that can be rated as “correct” or “incorrect.” Successively, statements are grouped into nine validity scales and ten clinical scales. We used the Korean version of the MMPI-2, which has been previously validated [17]. The present study was based on the results obtained from the following three validity scales: lie (L), infrequency (F), and defensiveness (K); and ten clinical scales: hypochondriasis (Hs), depression (D), hysteria (Hy), psychopathic deviate (Pd), masculinity-femininity (Mf), paranoia (Pa), psychasthenia (Pt), schizophrenia (Sc), hypomania (Ma), and social introversion (Si). MMPI-2 raw scale scores were converted to T-scores for comparison with the normal group [18].
Tac trough levels were measured between 6–12 months. All patients were tested for Tac trough levels by the same lab or using the same testing method. The estimated glomerular filtration rate (eGFR; mL/min/1.73 m2) was calculated using the modified Modification of Diet in Renal Disease equation to evaluate renal function one year after transplantation.
Tac IPV calculation
All patients were treated with a stable dose of Tac at 6 and 12 months after transplantation, with Tac plasma levels corrected for drug dosage. The standard deviation (SD) and IPV of Tac blood concentrations were confirmed for drug compliance. Patients with erroneously high Tac concentrations (>20 ng/mL) resulting from morning doses before blood sampling were excluded. Patients with extremely low Tac concentrations (<0.5 ng/mL) resulting from intentional dose reduction due to infection or disease were also excluded. The CV is the most commonly used measure of IPV. In previous retrospective studies, cutoffs for the CV have been selected as the median value for the population, ranging from 14.9% to 40% [19-21].
IPV was estimated using the following equation:
Mean Tac concentrations were calculated using outpatient Tac concentrations between 6–12 months. This time frame has been known as a stable period of post-renal transplantation to study and primarily focused time period by the current body of evidence [4,22]. Patients were divided into two groups, low IPV (L-IPV) and high IPV (H-IPV), according to the CV cutoff. We used two types of cutoff values: the median value of the study population and a value of 30 from the previous studies.
The median Tac IPV was significantly associated with an increased risk of late-onset antibody-mediated rejection and was also used as the cutoff value in previous studies [23]. The IPV 30 was selected as it aligned with the accepted pharmacokinetic definition of highly variable for the area under the concentration-time curve and Cmax, and it demonstrated poor outcomes when IPV was >30% [21].
Cutoff of median IPV value
The median IPV value of the patients was calculated and found to be 24. Patients with an IPV below the median were assigned to the L-IPV 24 group and those with a value equal to or greater than the observed median were assigned to the H-IPV 24 group.
Cutoff of IPV 30
Another cutoff value for Tac IPV (CV>30%) was used. Patients with an IPV below the cutoff were assigned to the L-IPV 30 group, and those with a value equal to or greater than 30 were assigned to the H-IPV 30 group.
Statistical analysis
Differences in the baseline characteristics and laboratory findings after transplantation were compared between the low- and high-IPV groups using independent t-tests. Explanatory variables that significantly affected Tac IPV were identified using independent t-tests. Stepwise multiple logistic regression analysis was conducted. MMPI-2 scale scores, including validity scales: L, F, K, and the 10 clinical scales, were included as independent variables influencing Tac IPV, the dependent variable. In addition, age and sex were included as independent variables for adjusting. The stepwise method was used as a variable selection method. Significant variables were selected using the minimum value of the Akaike information criterion, and the model was finally determined. The confusion matrix of logistic regression was used to explain the statistical power. To evaluate the performance of this model, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and F1-score were measured based on the confusion matrix. Cutoff values of significant variables to discriminate the H-IPV group were analyzed by constructing receiver operating characteristic (ROC) curves using moonBook package (https://github.com/cardiomoon/moonBook). Statistical significance was set at p<0.05, and the data were analyzed using R 4.2.0 (Posit PBC, Boston, MA, USA).
Ethical considerations
The study was conducted according to the guidelines of the Helsinki Declaration of 1975, as revised in 2008, and was approved by the Institutional Review Boards of Kyung Hee University Hospital (KMC 2022-07-016) and Kyung Hee University Hospital at Gangdong (KHNMC 2022-05-017). Patient consent was waived because this was a retrospective review of medical records.
RESULTS
Study population
Of the total 126 patients initially screened, 102 subjects were included in the study (Figure 1). Age, sex, duration of education, occupation, family status, and number of medicines were not significantly different between the L-IPV and H-IPV groups. The number of biologically related donors was 41 (57.7%) in L-IPV 30 and 11 (35.5%) in H-IPV 30 subgroups (p=0.04). The number of those was not significantly different between L-IPV 24 and H-IPV 24 subgroups. The L-IPV 24 and H-IPV 24 subgroups contained 49 and 53 subjects, respectively. The mean age of the L-IPV 24 subjects was 47.9±11.1 years, and 32 were men (65.3%). The mean duration of education was 13.8±2.8 years. Twenty-three (46.9%) were unemployed, 48 (98.0%) were cohabiting with family, and the mean number of medicines was 8.2±3.2. Biologically related subjects were 26 (53.1%). The mean age of the H-IPV 24 subjects was 50.3±11.6 years, with 32 men (60.4%). The mean duration of education was 13.6±2.3 years. Twenty-eight (52.8%) were unemployed, 52 (98.1%) were cohabiting with family, and the mean number of medicines was 9.0±3.0. Twenty-six (49.1%) subjects were biologically related (Table 1). The IPV 30 subgroup contained 71 L-IPV 30 and 31 high IPV 30 subjects. The mean age of the L-IPV 30 subjects was 47.7±11.2 years, and 46 were men (64.8%). The mean duration of education was 13.9±2.6 years. Thirty-seven (52.1%) were unemployed, and 69 (97.2%) were cohabiting with family. The mean number of medicines was 8.3±3.0. Biologically related subjects were 41 (57.7%). The mean age of the H-IPV 30 subjects was 52.2±11.3 years, and 21 (67.7%) were men. The mean duration of education was 13.3±2.5 years. Fourteen (45.2%) were unemployed, 31 (100.0%) were cohabiting with family, and the mean number of medicines was 9.3±3. Eleven (35.5%) subjects were biologically related (Table 2).
Figure 1.
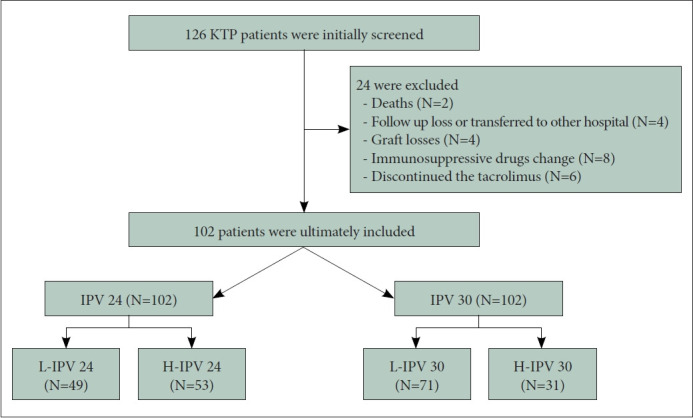
Consort diagram. KTP, living kidney transplatation; IPV 24, cutoff 24 of IPV value; IPV 30, cutoff 30 of IPV value; L-IPV, low intrapatient variability of tacrolimus; H-IPV, high intrapatient variability of tacrolimus.
Table 1.
Demographics and clinical characteristics of the group IPV 24
| Demographics | Low (N=49) | High (N=53) | p |
|---|---|---|---|
| Age (yr) | 47.9±11.1 | 50.3±11.6 | 0.30 |
| Men | 32 (65.3) | 32 (60.4) | 0.94 |
| MMPI_F | 45.78±6.90 | 47.60±8.04 | 0.22 |
| MMPI_L | 49.67±7.95 | 51.62±8.85 | 0.25 |
| MMPI_K | 51.39±10.31 | 52.87±8.81 | 0.44 |
| MMPI_D | 55.39±11.19 | 52.32±8.92 | 0.13 |
| MMPI_Hy | 51.29±7.97 | 54.58±9.73 | 0.07 |
| MMPI_Mf | 48.02±9.30 | 49.68±10.36 | 0.40 |
| MMPI_Pa | 44.63±7.06 | 49.70±8.89 | <0.01 |
| MMPI_Si | 51.88±11.41 | 49.06±9.15 | 0.17 |
| MMPI_Hs | 52.27±9.17 | 52.70±8.47 | 0.80 |
| MMPI_Pd | 46.61±12.13 | 48.19±8.39 | 0.45 |
| MMPI_Pt | 47.67±9.81 | 47.51±8.22 | 0.93 |
| MMPI_Sc | 46.10±7.69 | 46.91±8.35 | 0.62 |
| MMPI_Ma | 44.86±8.25 | 46.98±9.20 | 0.22 |
Data are presented as means±SD or number (%). Statistics: t-test.
The significance level threshold was set at p<0.05. MMPI, Minnesota Multiphasic Personality Inventory; F, infrequency; L, lie; K, defensiveness; D, depression; Hy, hysteria; Mf, masculinity-femininity; Pa, paranoia; Si, social introversion; Hs, hypochondriasis; Pd, psychopathic deviate; Pt, psychasthenia; Sc, schizophrenia; Ma, hypomania; SD, standard deviation
Table 2.
Demographics and clinical characteristics of the group IPV 30
| Demographics | Low (N=71) | High (N=31) | p |
|---|---|---|---|
| Age (yr) | 47.7±11.2 | 52.2±11.3 | 0.06 |
| Men | 46 (64.8) | 21 (67.7) | 0.77 |
| MMPI_F | 46.93±7.97 | 46.26±6.68 | 0.68 |
| MMPI_L | 50.61±8.64 | 50.87±8.11 | 0.88 |
| MMPI_K | 51.99±9.96 | 52.55±8.86 | 0.77 |
| MMPI_D | 54.82±10.74 | 51.45±8.33 | 0.12 |
| MMPI_Hy | 52.00±8.23 | 55.29±10.45 | 0.09 |
| MMPI_Mf | 49.48±10.21 | 47.52±8.99 | 0.36 |
| MMPI_Pa | 47.13±9.10 | 47.58±6.73 | 0.80 |
| MMPI_Si | 50.96±11.41 | 49.16±7.35 | 0.42 |
| MMPI_Hs | 52.38±9.19 | 49.16±7.87 | 0.85 |
| MMPI_Pd | 47.54±11.24 | 47.19±8.05 | 0.86 |
| MMPI_Pt | 47.69±9.68 | 47.35±7.25 | 0.86 |
| MMPI_Sc | 46.85±8.48 | 45.77±6.89 | 0.54 |
| MMPI_Ma | 45.30±7.90 | 47.48±10.49 | 0.25 |
Data are presented as means±SD or number (%). Statistics: t-test.
The significance level threshold was set at p<0.05. MMPI, Minnesota Multiphasic Personality Inventory; F, infrequency; L, lie; K, defensiveness; D, depression; Hy, hysteria; Mf, masculinity-femininity; Pa, paranoia; Si, social introversion; Hs, hypochondriasis; Pd, psychopathic deviate; Pt, psychasthenia; Sc, schizophrenia; Ma, hypomania; SD, standard deviation
Associations between IPV 24 and the MMPI-2 profile
The Pa scale score of the H-IPV 24 subgroup (mean±SD, 49.7±8.89) was significantly higher than that of the L-IPV 24 subgroup (44.6±7.06; t=-3.17, df=100, p<0.01) (Figure 2). Any significance was not found between L-IPV and H-IPV 24 in other mean MMPI-2 scale scores using the independent t-test (Table 1). The variables of Pa, Hy, and D were selected after the stepwise method. Significant associations were found in Pa scale scores (odds ratio [OR]: 1.10; 95% confidence interval [CI] 1.03–1.17; p<0.01), Hy scale scores (OR: 1.08; 95% CI 1.01–1.15; p<0.01), and D scale scores (OR: 0.93; 95% CI 0.88–0.98; p<0.01) with Tac IPV (Figure 3). The sensitivity, specificity, PPV, NPV, and F1 score of the model using the confusion matrix were 0.71, 0.70, 0.69, 0.73, and 0.70, respectively (Table 3). The optimal cutoff value of Pa scale scores to discriminate the H-IPV group was 47, and its area under the ROC curve (AUC) was 0.68 (95% CI 0.58–0.79). The optimal cutoff value of the Hy scale scores and the AUC were 51 and 0.61 (95% CI 0.50–0.73), respectively. The optimal cutoff value of D scale scores and the AUC were 57 and 0.57 (95% CI 0.46–0.68), respectively.
Figure 2.
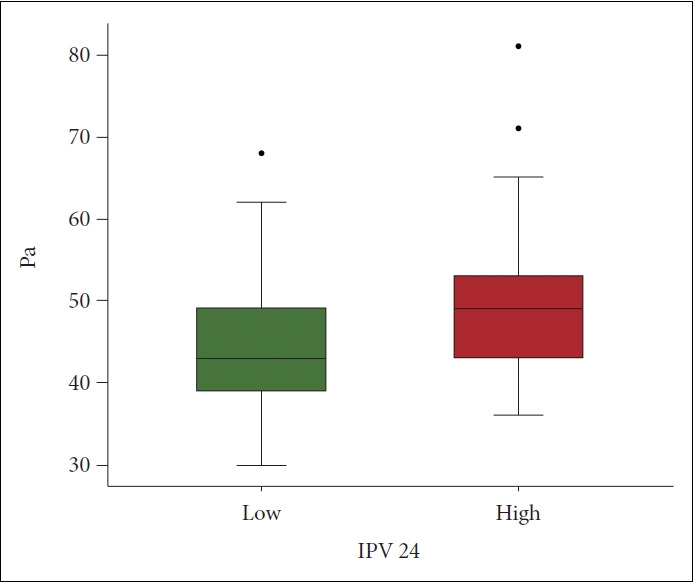
Difference of Pa scale scores between low and high IPV 24 groups. The diagram shows Pa scale scores. The difference between low and high groups were examined by the independent ttest. The Pa scale scores of high (mean±SD: 49.7±8.89) compared to low group (44.6±7.06) were significantly high (t=-3.17, df=100, p<0.01). IPV 24, cutoff 24 of IPV value; Pa, paranoia; SD, standard deviation.
Figure 3.
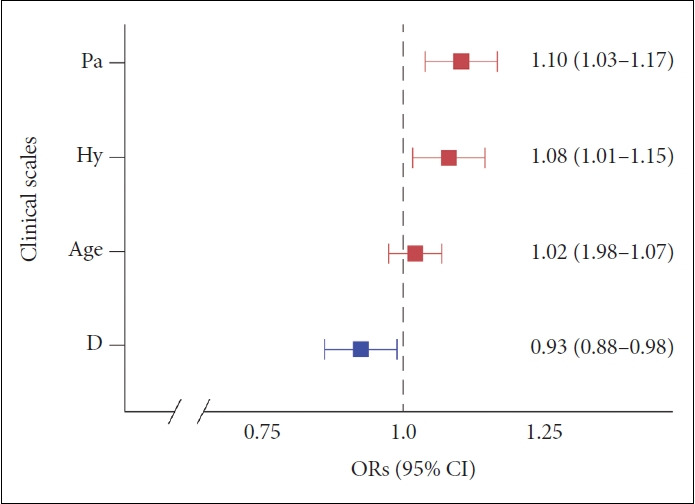
Multiple logistic regression ORs plot in IPV 24. Significant associations were found in Pa scale scores (p<0.01), Hy scale scores (p<0.01), and D scale scores (p<0.01) with Tac IPV. OR, odds ratio; CI, confidence interval; IPV 24, cutoff 24 of IPV value; Pa, paranoia; Hy, hysteria in the MMPI-2 clinical scale; D, depression; Tac IPV, tacrolimus intrapatient variability.
Table 3.
Confusion matrix of multiple logistic regression models
| Sensitivity | Specificity | PPV | NPV | F1 score | |
|---|---|---|---|---|---|
| IPV 24 | 0.71 | 0.70 | 0.69 | 0.73 | 0.70 |
| IPV 30 | 1.00 | 0.25 | 0.55 | 1.00 | 0.71 |
PPV, positive predictive value; NPV, negative predictive value; IPV 24, cutoff 24 of IPV value; IPV 30, cutoff 30 of IPV value
Associations between IPV 30 and the MMPI-2 profile
There was no significant difference between L-IPV and H-IPV 30 in their mean MMPI-2 scale scores using the independent t-test (Table 2). The variables of Hy, D, and age were selected after the stepwise method. The Hy scale scores (OR: 1.09; 95% CI 1.02–1.16; p<0.01), D scale scores (OR: 0.92; 95% CI 0.87–0.98; p<0.01), and age (OR: 1.05; 95% CI 1.00–1.11; p=0.04) were significantly associated with Tac IPV (Figure 4). The sensitivity, specificity, PPV, NPV, and F1 scores of the model using the confusion matrix were 1.00, 0.25, 0.55, 1.00, and 0.71, respectively (Table 3). The optimal cutoff value of Hy scale scores to discriminate the H-IPV group was 51, and its AUC was 0.61 (95% CI 0.48–0.73). The optimal cutoff value of D scale scores and the AUC were 57 and 0.57 (95% CI 0.45–0.69), respectively.
Figure 4.
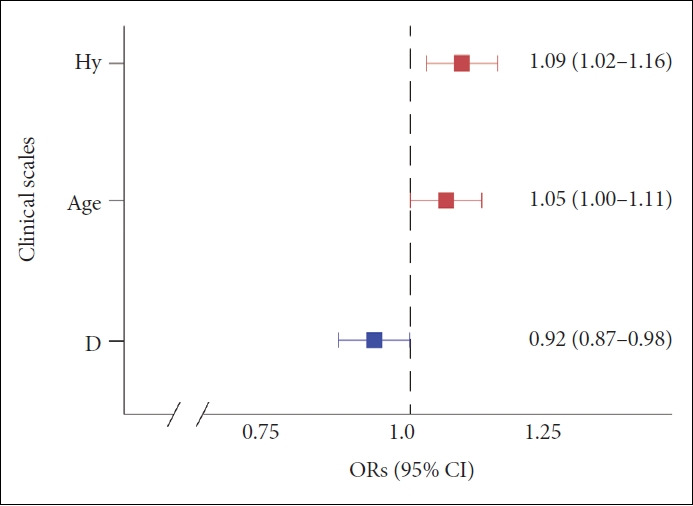
Multiple logistic regression ORs plot in the IPV 30. The Hy scale scores (p<0.01), D scale scores (p<0.01), and age (p=0.04) were significantly associated with Tac IPV. OR, odds ratio; CI, confidence interval; IPV 30, cutoff 30 of IPV value; Hy, hysteria in the MMPI-2 clinical scale; D, depression; Tac IPV, tacrolimus intrapatient variability.
Tac IPV and eGFR
Kidney function was assessed using the eGFR at one year. Differences in the mean eGFR values between the L-IPV and H-IPV groups were examined using independent t-tests. The eGFR at one year of H-IPV 30 was significantly lower than that of L-IPV 30 (mean±SD: 52.1±18.1, 61.8±14.9, respectively; df=48.4, p=0.01). There was no significant difference between L-IPV 24 and H-IPV 24 in the eGFR at one year (59.7±14.6, 58.0±18.1, respectively; df=98.2, p=0.6) (Figure 5).
Figure 5.
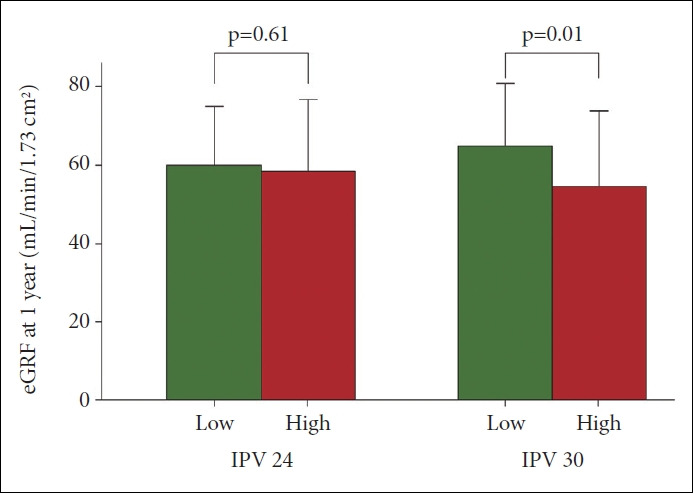
eGFR at one year after kidney transplantation between low and high groups. The difference were examined by the independent t- test. The eGFR at one year of H-IPV 30 was significantly low compared to that of L-IPV 30 (mean±SD: 52.1±18.1, 61.8±14.9, respectively; df=48.4, p=0.01). There was no significant difference between L-IPV 24 and H-IPV 24 in the eGFR at one year (59.7±14.6, 58.0±18.1, respectively; df=98.2, p=0.61). IPV 24, cutoff 24 of IPV value; IPV 30, cutoff 30 of IPV value; eGFR, estimated glomerular filtration rate; L-IPV, low intrapatient variability of tacrolimus; H-IPV, high intrapatient variability of tacrolimus; SD, standard deviation.
DISCUSSION
In this study, we found that the MMPI-2 profile performed by patients during living kidney pre-transplantation evaluation had predictive factors for Tac IPV. The scale scores of Hy, D, and Pa were predictive of Tac IPV in the IPV 24 stepwise multiple regression analysis. The cutoffs of Hy, D, and Pa scales to discriminate high Tac IPV were 51, 57, and 47, respectively. In the IPV 30 model, the scale scores of Hy and D were shown to be predictive of Tac IPV, and the cutoffs were 51 and 57, respectively.
The ORs of the Hy scale were 1.08 (95% CI 1.01–1.15, p<0.01) in the IPV 24 model and 1.09 (95% CI 1.02–1.16, p<0.01) in the IPV 30 model. It means that the risk of being included in the high IPV group is increased by 8% and 9% as the Hy scale score is increased by one, respectively. Lee et al. [24] reported significantly higher Hy scores in living kidney transplant recipients than in donors. The Hy scale scores were found to be positively correlated with laboratory findings, such as creatinine and C-reactive protein (CRP), in patients with chronic kidney disease, including those who underwent kidney transplantation [25]. High scores on the Hy scale reflect little insight into life problems, numerous somatic symptoms, denial, immaturity, and self-concentration [26]. The Hy scale constitutes the neurotic triad, which is associated with excessive concentration on physical health status, impaired attention, low self-esteem, and pessimism [27]. Taken together with these facts and our results, a higher Hy scale score during the pre-transplantation period could be considered a predictive factor for high Tac IPV after living kidney transplantation.
In the present study, the Pa scale scores of H-IPV 24 were significantly higher than L-IPV 24 groups (p<0.01). The OR was 1.10 (95% CI 1.03–1.17, p<0.01) in the IPV 24 model, implying the risk of being high IPV group increased 10% by one increment of the score. High scores on the Pa scale indicate sensitivity to others’ opinions, suspiciousness, blaming others, feeling mistreated, and hostility [26]. In a previous report on patients with chronic kidney disease [25], the Pa scale was not only significantly correlated with the Hy scale but also with eGFR, serum nitrogen, and CRP. This was considered to lead to poor medication compliance and obstacles in communicating and establishing a doctor-patient relationship. In a recent cohort of patients with apparent treatment-resistant hypertension, several psychological characteristics, including paranoid ideation, hostility, and blaming others, were significantly correlated with drug resistance, and blaming others was an independent predictor of drug resistance [28]. Considering the meaning of high Pa scale scores and its association with poor medical compliance in chronic diseases, a higher Pa scale score may be a predictor of high Tac IPV in living kidney transplants.
The mean D scale scores of this study were similar to those of a previous study in the same Korean population who had living kidney transplants (53.8±10.2 vs. 53.3±11.2) [24]. The risk of being included in the high IPV group was found to be decreased by 7% in the IPV 24 model (OR: 0.93; 95% CI 0.88–0.98; p<0.01) and 8% in the IPV 30 model (OR: 0.92; 95% CI 0.87–0.98; p<0.01) by one increment of the score. In the pre-transplantation period, manifestations of psychological exhaustion due to physical illness, such as lack of will and fatigue should be distinguished from symptoms of clinical depression [5]. In the process of pre-transplantation evaluation of this study, recipients without clinical depression were confirmed to be eligible by psychiatrists in our hospitals. Therefore, an increase in D scale scores of the present study cannot be considered as depression but as psychological characteristics in chronic kidney disease patients presenting with stress [29].
The worse renal function observed for the H-IPV 30 group compared with the L-IPV 30 group one year after transplantation concurs with recent reports of Tac IPV greater than 30% and kidney graft function [23,30,31]. Two methods have been used to determine the cutoff value for Tac IPV in previous studies, and these include the median value of the study population and a value of 30 [23,30,32,33]. The IPV value of 30 aligned with the accepted definition of a highly variable area under the concentration-time curve and Cmax [21]. Taken together, interventions to predict and reduce Tac IPV are important for long-term graft survival.
This study had several limitations. First, the sample size was relatively small because we recruited patients who completed the MMPI-2 during pre-transplant evaluation for living kidney transportation at two hospitals. Second, no other psychological instruments were used in the assessment. Given the limited time in clinical settings to evaluate candidates for kidney transplantation and the plentiful information available from the results of the MMPI-2, it could be a helpful tool with evidence in this population [13]. Third, there was a lack of long-term medical prognosis data for these patients. The observation period after transplantation was too short to achieve statistical significance. Fourth, the study findings should be interpreted with caution to the populations with different drug metabolism, health systems, and coverage for medications. Finally, other clinical outcomes, including rejections, de novo donor-specific antibodies, and chronic histologic lesions were not included. A long-term further study with these data might be more compelling.
With the increasing need for living kidney transplantation and the paucity of psychological instruments to evaluate candidates and predict Tac IPV after transplantation, this study suggested the MMPI-2 profile as a predictor for high Tac IPV, which has been known as a marker for poor outcomes after living kidney transplantation.
Acknowledgments
We would like to thank Seon Hwa Lee of the Medical Big Data Research Center of the Research Institute of Clinical Medicine in Kyung Hee University Hospital at Gangdong.
Footnotes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Ah Rah Lee, Jin Kyung Park. Data curation: Ah Rah Lee, Jin Kyung Park. Formal analysis: Ah Rah Lee, Sang Min Lee, Won Sub Kang, Jin Kyung Park. Investigation: Ah Rah Lee, Jin Kyung Park. Methodology: Ah Rah Lee, Jin Kyung Park. Project administration: Ah Rang Cho, Jong Woo Kim, Jin Kyung Park. Resources: Sang Min Lee, Won Sub Kang, Ah Rang Cho, Jong Woo Kim, Jin Kyung Park. Supervision: Sang Min Lee, Won Sub Kang, Jong Woo Kim, Ah Rang Cho. Validation: Sang Min Lee, Won Sub Kang, Ah Rang Cho, Jong Woo Kim, Jin Kyung Park. Writing— original draft: Ah Rah Lee, Jin Kyung Park. Writing—review & editing: Ah Rah Lee, Jin Kyung Park.
Funding Statement
None
REFERENCES
- 1.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623–653. doi: 10.2165/00003088-200443100-00001. [DOI] [PubMed] [Google Scholar]
- 2.Shuker N, van Gelder T, Hesselink DA. Intra-patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant Rev (Orlando) 2015;29:78–84. doi: 10.1016/j.trre.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Kuypers DRJ. Intrapatient variability of tacrolimus exposure in solid organ transplantation: a novel marker for clinical outcome. Clin Pharmacol Ther. 2020;107:347–358. doi: 10.1002/cpt.1618. [DOI] [PubMed] [Google Scholar]
- 4.Gonzales HM, McGillicuddy JW, Rohan V, Chandler JL, Nadig SN, Dubay DA, et al. A comprehensive review of the impact of tacrolimus intrapatient variability on clinical outcomes in kidney transplantation. Am J Transplant. 2020;20:1969–1983. doi: 10.1111/ajt.16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meena P, Bhargava V, Rana D, Bhalla A, Gupta A. An approach to neurological disorders in a kidney transplant recipient. Kidney360. 2020;1:837–844. doi: 10.34067/KID.0002052020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Wang Q, Zhang S, Li Z, Jiang W. The dynamic changes of cellular immunity among frontline medical workers who supported Wuhan for fighting against the COVID-19. Int Immunopharmacol. 2022;102:108392. doi: 10.1016/j.intimp.2021.108392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Euteneuer F, Dannehl K, Del Rey A, Engler H, Schedlowski M, Rief W. Peripheral immune alterations in major depression: the role of subtypes and pathogenetic characteristics. Front Psychiatry. 2017;8:250. doi: 10.3389/fpsyt.2017.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hennig J, Becker H, Netter P. 5-HT agonist-induced changes in peripheral immune cells in healthy volunteers: the impact of personality. Behav Brain Res. 1996;73:359–363. doi: 10.1016/0166-4328(96)00115-5. [DOI] [PubMed] [Google Scholar]
- 9.Shabtai M, Waltzer WC, Ayalon A, Shabtai EL, Malinowski K. Down regulation of CD45 expression on CD4 T cells during acute renal allograft rejection: evidence of a decline in T suppressor/inducer activity. Int Urol Nephrol. 2002;34:555–558. doi: 10.1023/a:1025641404743. [DOI] [PubMed] [Google Scholar]
- 10.Medved V, Medved S, Skočić Hanžek M. Transplantation psychiatry: an overview. Psychiatr Danub. 2019;31:18–25. doi: 10.24869/psyd.2019.18. [DOI] [PubMed] [Google Scholar]
- 11.Arbisi PA, Butcher JN. Relationship between personality and health symptoms: use of the MMPI-2 in medical assessments. Int J Clin Health Psychol. 2004;4:571–595. [Google Scholar]
- 12.Belaiche S, Décaudin B, Dharancy S, Noel C, Odou P, Hazzan M. Factors relevant to medication non-adherence in kidney transplant: a systematic review. Int J Clin Pharm. 2017;39:582–593. doi: 10.1007/s11096-017-0436-4. [DOI] [PubMed] [Google Scholar]
- 13.Nghiem DM, Gomez J, Gloston GF, Torres DS, Marek RJ. Psychological assessment instruments for use in liver and kidney transplant evaluations: scarcity of evidence and recommendations. J Pers Assess. 2020;102:183–195. doi: 10.1080/00223891.2019.1694527. [DOI] [PubMed] [Google Scholar]
- 14.Wutzler U, Venner M, Villmann T, Decker O, Ott U, Steiner T, et al. Recording of dissimulation and denial in the context of the psychosomatic evaluation at living kidney transplantation using the Minnesota Multiphasic Personality Inventory (MMPI) Psychosoc Med. 2009;6:Doc04. doi: 10.3205/psm000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basile A, Bernazzali S, Diciolla F, Lenzini F, Lisi G, Maccherini M, et al. Risk factors for smoking abuse after heart transplantation. Transplant Proc. 2004;36:641–642. doi: 10.1016/j.transproceed.2004.02.054. [DOI] [PubMed] [Google Scholar]
- 16.Nadjafi-Semnani M, Simforoosh N, Nadjafi-Semnani A. Living donor kidney transplantation: global and regional trend. Urol J. 2021;18:359–361. doi: 10.22037/uj.v18i.6820. [DOI] [PubMed] [Google Scholar]
- 17.Han K, Lim J, Min B, Lee J, Moon K, Kim Z. [Korean MMPI-2 standardization study] Kor J Clin Psychol. 2006;25:533–564. Korean. [Google Scholar]
- 18.Colligan RC, Offord KP. The aging MMPI: contemporary norms for contemporary teenagers. Mayo Clin Proc. 1989;64:3–27. doi: 10.1016/s0025-6196(12)65299-9. [DOI] [PubMed] [Google Scholar]
- 19.Gueta I, Markovits N, Yarden-Bilavsky H, Raichlin E, Freimark D, Lavee J, et al. High tacrolimus trough level variability is associated with rejections after heart transplant. Am J Transplant. 2018;18:2571–2578. doi: 10.1111/ajt.15016. [DOI] [PubMed] [Google Scholar]
- 20.Huang CT, Shu KH, Ho HC, Wu MJ. Higher variability of tacrolimus trough level increases risk of acute rejection in kidney transplant recipients. Transplant Proc. 2016;48:1978–1980. doi: 10.1016/j.transproceed.2016.02.081. [DOI] [PubMed] [Google Scholar]
- 21.Leino AD, King EC, Jiang W, Vinks AA, Klawitter J, Christians U, et al. Assessment of tacrolimus intrapatient variability in stable adherent transplant recipients: establishing baseline values. Am J Transplant. 2019;19:1410–1420. doi: 10.1111/ajt.15199. [DOI] [PubMed] [Google Scholar]
- 22.Whalen HR, Glen JA, Harkins V, Stevens KK, Jardine AG, Geddes CC, et al. High intrapatient tacrolimus variability is associated with worse outcomes in renal transplantation using a low-dose tacrolimus immunosuppressive regime. Transplantation. 2017;101:430–436. doi: 10.1097/TP.0000000000001129. [DOI] [PubMed] [Google Scholar]
- 23.Kim EJ, Kim SJ, Huh KH, Kim BS, Kim MS, Kim SI, et al. Clinical significance of tacrolimus intra-patient variability on kidney transplant outcomes according to pre-transplant immunological risk. Sci Rep. 2021;11:12114. doi: 10.1038/s41598-021-91630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Y, Park H, Jee HJ, Lee HJ, Gwon JG, Min H, et al. Psychological characteristics and associations between living kidney transplantation recipients and biologically related or unrelated donors. BMC Nephrol. 2020;21:355. doi: 10.1186/s12882-020-02017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai S, Mecarelli O, Pulitano P, Romanello R, Davi L, Zarabla A, et al. Neurological, psychological, and cognitive disorders in patients with chronic kidney disease on conservative and replacement therapy. Medicine (Baltimore) 2016;95:e5191. doi: 10.1097/MD.0000000000005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Locke DEC. Interpreting the MMPI-2-RF. Clin Neuropsychol. 2013;27:339–341. doi: 10.1080/13854046.2012.742291. [DOI] [PubMed] [Google Scholar]
- 27.Talarowska M, Zboralski K, Chamielec M, Gałecki P. The MMPI-2 neurotic triad subscales and depression levels after pharmacological treatment in patients with depressive disorders - clinical study. Psychiatr Danub. 2011;23:347–354. [PubMed] [Google Scholar]
- 28.Petit G, Berra E, Georges CMG, Capron A, Huang QF, Lopez-Sublet M, et al. Impact of psychological profile on drug adherence and drug resistance in patients with apparently treatment-resistant hypertension. Blood Press. 2018;27:358–367. doi: 10.1080/08037051.2018.1476058. [DOI] [PubMed] [Google Scholar]
- 29.Pereira BDS, Fernandes NDS, de Melo NP, Abrita R, Grincenkov FRDS, Fernandes NMDS. Beyond quality of life: a cross sectional study on the mental health of patients with chronic kidney disease undergoing dialysis and their caregivers. Health Qual Life Outcomes. 2017;15:74. doi: 10.1186/s12955-017-0646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigo E, Segundo DS, Fernández-Fresnedo G, López-Hoyos M, Benito A, Ruiz JC, et al. Within-patient variability in tacrolimus blood levels predicts kidney graft loss and donor-specific antibody development. Transplantation. 2016;100:2479–2485. doi: 10.1097/TP.0000000000001040. [DOI] [PubMed] [Google Scholar]
- 31.Stefanović N, Veličković-Radovanović R, Danković K, Pavlović I, CatićĐorđević A, Bašić J, et al. Effect of the interrelation between CYP3A5 genotype, concentration/dose ratio and intrapatient variability of tacrolimus on kidney graft function: Monte Carlo simulation approach. Pharmaceutics. 2021;13:1970. doi: 10.3390/pharmaceutics13111970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodall DL, Willicombe M, McLean AG, Taube D. High intrapatient variability of tacrolimus levels and outpatient clinic nonattendance are associated with inferior outcomes in renal transplant patients. Transplant Direct. 2017;3:e192. doi: 10.1097/TXD.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mo H, Kim SY, Min S, Han A, Ahn S, Min SK, et al. Association of intrapatient variability of tacrolimus concentration with early deterioration of chronic histologic lesions in kidney transplantation. Transplant Direct. 2019;5:e455. doi: 10.1097/TXD.0000000000000899. [DOI] [PMC free article] [PubMed] [Google Scholar]


