Abstract
Crohn's disease (CD) is a chronic, relapsing and remitting inflammatory bowel disease (IBD) that is increasing in incidence and prevalence globally. Management aims to achieve endoscopic healing, symptom resolution and improvement in quality of life. Therapeutic approaches in CD vary depending on disease phenotype. Thiopurines are important in steroid-sparing maintenance therapy, while anti-tumour necrosis factor agents play a fundamental role, especially in fistulising CD. Suboptimal response to these medications may require escalation to other immunosuppressive and biologic therapies, and surgical intervention is still required in a proportion of patients. Tailoring treatment to target specific patient phenotypes, disease severity and patient wishes is becoming more feasible with the growing array of therapeutic options in CD.
KEYWORDS: Crohn's disease, inflammatory bowel disease, biologics
Key points
-
•
Crohn's disease (CD) is common, and its incidence and prevalence are increasing.
-
•
Goals of treatment are to achieve rapid and safe symptom resolution and tissue healing and to improve quality of life.
-
•
Monitoring disease response based on clinical objective markers, alongside patient-specific disease targets, is paramount to optimising management.
-
•
Therapeutic options range depending on disease phenotype and patient choice.
-
•
Anti-tumour necrosis factor agents remain the mainstay of treatment for fistulising CD, though new drug options are being explored.
-
•
Surgical management remains a prominent therapeutic option for CD, especially in stricturing disease.
-
•
The future of CD will see a range of new medical treatments targeting immunomodulation and microbiome manipulation to try and facilitate personalised treatment approach.
Introduction
Crohn's disease (CD) is a chronic, relapsing and remitting inflammatory bowel disease (IBD) characterised by transmural inflammation and affecting variable sites along the entire gastrointestinal tract. The incidence and prevalence of CD varies across geographic regions, with the highest epidemiological burden in Europe, Oceania and North America.1 The highest incidence was reported in Oceania (29.3 per 100 000 person-years in Australia), and the highest prevalence was reported in Europe (322 per 100 000 in Germany).1 Additionally, temporal trend analyses demonstrate that newly industrialised countries in Asia, Africa and South America are facing a rising incidence, highlighting the global challenge posed by CD.1
CD has a peak incidence between 20–30 years of age, with common presenting symptoms including diarrhoea, abdominal pain and systemic symptoms such as fatigue and weight loss. Other extra-intestinal manifestations include arthropathy, dermatological (pyoderma gangrenosum, erythema nodosum), and ocular features.2, 3 In all IBD, the prevalence of at least one joint manifestation is 24%, ocular manifestation 27% and extraintestinal skin manifestation 35%.4 The widely utilised Montreal classification system classifies CD phenotype based on the age at diagnosis (<16, 17–40, >40 years old), disease location (ileal, colonic, ileocolonic, isolated upper) and disease behaviour (non-stricturing/nonpenetrating, stricturing, penetrating, perianal).5 With repeated cycles of intestinal inflammation in CD, the disease may evolve to strictures and complications of penetrating disease such as fistulas and abscess formation.6
The pathogenesis of CD continues to be elucidated and is thought to involve a complex interplay between environmental factors, immune factors, the gut microbiome and genetic disposition. Current studies propose that in a genetically susceptible individual, environmental exposures can lead to gut microbiota dysbiosis and intestinal barrier dysfunction, subsequently resulting in immune dysregulation.7, 8 Diagnosis involves a combination of clinical, biochemical, cross-sectional imaging, endoscopic, and histological investigations (Table 1).9, 10, 11
Table 1.
| Investigation | Type of investigation | Common findings in Crohn's disease | Notes |
|---|---|---|---|
| Blood tests |
|
Anaemia, thrombocytosis, elevated inflammatory markers, hypoalbuminaemia |
|
| Stool studies | Faecal calprotectin | Faecal calprotectin is elevated due to intestinal inflammation in IBD | Faecal calprotectin can aid in differentiating between IBD and functional intestinal diseases such as irritable bowel syndrome (IBS) |
|
Stool specimens should rule out infectious aetiology | Can have co-occurrence of CD and infection | |
| Endoscopy |
|
Skip lesions, aphthous ulcers, linear ulceration, cobblestone pattern, fat wrapping, strictures, fistula |
|
| Small bowel capsule endoscopy | As above |
|
|
| Histology | Biopsies obtained from endoscopy | Findings suggestive of CD include focal (discontinuous) inflammation, focal crypt irregularity, granulomas | Granulomas is suggestive of CD over ulcerative colitis |
| Imaging |
|
Mural hyperenhancement, wall thickening, strictures, fistulas, abscesses | MRE and intestinal ultrasound often preferred over CT due to reduced radiation exposure |
CD = Crohn's disease; IBD = Inflammatory bowel disease; MCS = microscopy, culture and sensitivity; OCP = ova, cysts, parasites; MRE = MR enterography
Treatment targets
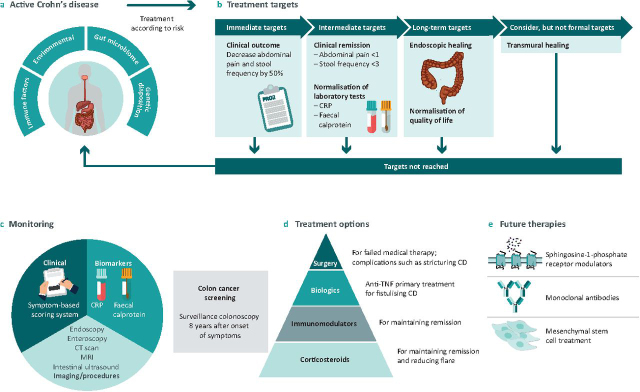
Identifying evidence-based and clinically relevant treatment targets is essential for guiding management strategies for patients with IBD. In 2021, the International Organization for the Study of IBD (IOIBD) published an update on their proposed treatment targets for IBD in the Selecting Therapeutic Targets in Inflammatory Bowel Disease II (STRIDE-II) Initiative.12 STRIDE-II recommendations for treating-to-target were classified into clinical, endoscopic and transmural assessment, biomarkers, and quality of life and disability. Targets were categorised as either immediate, intermediate or long-term.
Clinical response, defined as a decrease of at least 50% in abdominal pain and stool frequency on patient-reported outcome (PRO2), was prioritised as the immediate treatment target.12 Clinical remission (ie PRO2 abdominal pain ≤1 and stool frequency ≤3), and normalisation of C-reactive protein (CRP) and faecal calprotectin, were considered intermediate treatment targets.12 Endoscopic healing (assessed by sigmoidoscopy, colonoscopy, capsule endoscopy, or balloon enteroscopy) and normalised health related QoL were described as long-term targets.12 Histological remission and transmural healing were not included as treatment targets.
Severity of disease presentation
Methods of monitoring severity of inflammation are important to assess medication efficacy, rule out complications, and prevent progression.13 Monitoring tools include symptom-based scoring systems, biochemical markers, endoscopy, enteroscopy, and cross-sectional imaging. The benefits and weaknesses of these methods are described in Table 2.
Table 2.
Severity of Crohn's Disease
| Strengths | Weaknesses | |
|---|---|---|
|
|
Subjective interpretation, clinical symptoms poorly correlate with severity of endoscopic lesions |
| Biomarkers (C-reactive protein, calprotectin) |
|
May have normal C-reactive Protein levels in active disease |
| Endoscopic |
|
|
| Enteroscopy (including capsule endoscopy, push enteroscopy and balloon-assisted endoscopy) |
|
|
| Magnetic resonance imaging |
|
|
| CT |
|
|
| Intestinal ultrasound |
|
|
Medical therapy
Medical therapies for CD vary according to the disease phenotype and for different phases of the illness.
Corticosteroids
Steroids such as prednisolone, methylprednisolone and hydrocortisone are effective as an induction agent in CD; however, they are ineffective at maintaining remission or reducing flare frequency and disease recurrence.14 Prolonged corticosteroid therapy is associated with increased morbidity and causes numerous well-known multisystem side effects.15 Alternatively, in mild-to-moderate CD, enteric-coated budesonide is equally efficacious compared to prednisolone in inducing remission and has minimal side effects given its first pass liver metabolism and rapid elimination.16, 17 However, in cases of severe ileocaecal CD or colonic CD, prednisolone has been shown to be superior to budesonide, as evidenced by Rutgeert et al reducing mean CDAI from 279 to 136, compared to 275 to 175 (p=0.001), at the cost of increased corticosteroid-associated side effects in 48 versus 29 of the total 176 patients (p=0.003).16
Thiopurines
Unlike in ulcerative colitis (UC), 5-amino-salicylic acid (5-ASA) is deemed to be ineffective in CD.18 Instead, immunomodulation and antimetabolite activity with thiopurines is the mainstay of steroid-sparing maintenance therapy. However, thiopurines, such as azathioprine and 6-mercaptopurine, only become effective after 8–12 weeks, with the slower onset time rendering these medications ineffective as induction agents.18 Thiopurines are useful in maintaining remission and facilitating weaning of induction corticosteroid therapy. Prior to commencing thiopurines, thiopurine methyltransferase (TPMT) polymorphisms should be checked to help guide optimal dosing. TPMT is a key enzyme in thiopurine metabolism, and different polymorphisms may predispose individuals to profound myelosuppression.18 Similarly, nudix hydrolase 15 (NUDT15) deficiency also predisposes to thiopurine-induced cytotoxicities and can be tested for.18 In those that cannot tolerate thiopurines, low-dose methotrexate is an alternative option. It has been shown to induce remission in 65% of patients compared with 39% of placebo recipients (p=0.04), and reduces prednisolone requirement during relapse in 28 percent of patients compared to 58 percent of placebo recipients (p=0.01).19
Biologics
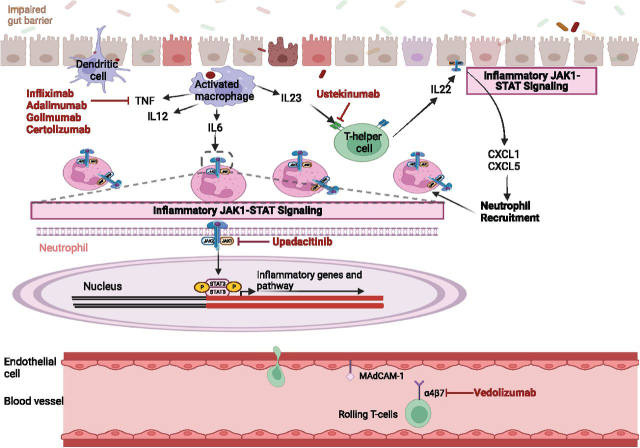
Biologic agents have facilitated landmark changes in the management of CD. The anti-tumour necrosis factor (anti-TNF) agents, namely infliximab, adalimumab, golimumab and certolizumab, work to inhibit the endotoxin-mediated shock promoted by TNF release in the mucosa and lamina propria of Crohn's patients (See Fig 1).18
Fig 1.
Mechanism of action for biologics and small molecules used in Crohn's disease. TNF = tumour necrosis factor; JAK = Janus kinase; STAT = signal transducer and activator of transcription; IL = interleukin; CXCL = chemokine.
Infliximab is the first studied anti-TNF deemed to be safe and well-tolerated in achieving and maintaining clinical remission and enabling steroid weaning.18, 20 Concurrent use of infliximab with other immunomodulatory therapies, such as azathioprine, is thought to have synergistic efficacy.21 This was demonstrated in the SONIC trial, and dual agents resulted in reduction in the formation of anti-drug antibodies (ADAs) to infliximab.21 ADAs can occur with immunologic agents such as infliximab, occurring in 7-10% of patients receiving regular infliximab, and have been shown to increase rates of infusion reactions, loss of efficacy of therapy and delayed hypersensitivity reactions.18
Adalimumab has been shown to effectively induce and maintain long-term clinical remission in moderate to severe CD, based on improvement of Crohn's Disease Activity Index (CDAI) and Inflammatory Bowel Disease Questionnaire (IBDQ) scores, and endoscopically with findings or early and sustained mucosal healing in moderate-to-severe ileocolonic CD.18 Adalimumab has similar effects on complete closure of fistulas compared to infliximab and in those who have lost response to Infliximab, Adalimumab has been shown to be effective as per the CLASSIC II trial.22
Certolizumab pegol is a pegylated Fab’ fragment of the anti-TNF monoclonal antibody (mAb) that has a high affinity for TNF-alpha. Delivered subcutaneously, certolizumab has had mixed outcomes from the PRECiSE-1 and 2 trial, being shown to be lacking in efficacy at reducing CDAI in the former, and superior to placebo in this regard in the latter.18 Owing to mixed outcomes its uptake is varied internationally in CD.
Golimumab is another anti-TNF alpha agent and in some observational studies has shown to be efficacious in CD refractory to other anti-TNF therapies, though further exploration of its clinical utility in CD is required.23
Notably, 30–50% of patients are primary non-responders to anti-TNFs, with subsequent loss of response being a significant issue.24 Primary non-response due to immunogenicity to infliximab or adalimumab may be predicted by low drug concentrations at week 14.18 Consequently, the development of newer biologic agents is a key area of immunosuppressive advancement.
T-lymphocytes have been shown to play a pivotal immunological role in CD pathogenesis. Vedolizumab inhibits the α4β7 integrin found on lymphocytes, preventing their migration into the gut wall via the mucosal addressin cell adhesion molecule-1, which is upregulated in intestinal vasculature in IBD.18 Vedolizumab has been found to successfully induce remission compared to placebo at 6 weeks in the GEMINI-II study, with significantly higher rates of remission by 52 weeks achieved in those who remitted on induction.25 They also achieved improvement in CR100 (decrease in CDAI by 100 points) response and glucocorticoid remission when given 4 or 8 times weekly.25 Vedolizumab as per the GEMINI-3 study is unlikely to be of benefit in induction, but may be a useful maintenance therapy, when bridged with co-induction agents of corticosteroids for those with moderate to severe CD who have failed previous conventional therapies.26
Ustekinumab is a monoclonal antibody against the p40 subunit of IL12 and IL-23 (See Fig 1), found in the UNITI-1, UNITI-2 and IM-UNITI trials to be superior to placebo in inducing and maintaining remission in patients with moderate-to-severe CD.27 Patients administered 6 mg/kg or 130 mg ustekinumab had higher remission rates by 6 weeks, and on 8 to 12 weekly maintenance doses were more likely to be in remission regardless of previous treatment response to an anti-TNF agent.27 Ustekinumab has been approved for use for CD following previous therapy failures and is efficacious in treating perianal disease and fistula healing.28
Janus kinase (JAK) inhibitors
In inflamed tissue in CD, especially in patients with disease refractory to anti-TNF therapy, the JAK–STAT genes and the STAT3 pathway are upregulated (Fig 1).29 Upadacitinib, a small molecular inhibitor selective for JAK1, has been shown through the U-EXCEL, U-EXCEED and U-ENDURE studies to be effective in achieving clinical remission, endoscopic response, and quality of life outcomes in the induction and maintenance treatment of moderate-to-severe CD.29 Surprisingly, however, tofacitinib, a pan-JAK inhibitor, has been found to be beneficial in UC management but not in CD.29, 30
Fistulising disease
Prior to biologics, fistulising CD was notoriously difficult to manage. ACCENT-II demonstrated that infliximab is effective in inducing closing of rectovaginal fistulas and increases duration of sustained fistula closure by 3 months compared to placebo when given as induction and maintenance therapy.31 Adalimumab has also been shown to be effective in complete closure of fistulas.22 Given JAK1 polymorphisms have been identified in fistulising disease, it would be beneficial to determine the role for upadacitinib in these patients.29
Faecal transplant
In the era of progressive understanding of the gut microbiome as a key antigenic driver in IBD, the use of multiple faecal microbiota transplants (FMT) has been explored and requires further research.32
Follow-up and surveillance
Follow-up and surveillance play an important role in the management of patients with CD, which includes the use of patient-reported outcome measures (PROMs), endoscopic evaluation and non-invasive monitoring modalities such as Intestinal Ultrasound (IUS).
PROMs are questionnaires completed by patients to assess their symptoms and overall well-being, offering valuable information on the impact of CD on patients’ lives.12 However, there is discordance between clinical symptoms and mucosal inflammation in CD, and treatment decisions focused solely on PROMs may result in over- or under-treatment.33
Objective markers of disease severity to monitor were mentioned previously, including endoscopy, faecal calprotectin and monitoring of blood tests such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).12
Non-invasive monitoring modalities such as IUS have gained relevance, adding clinical value in assessing transmural disease activity in CD.33 Kucharzik et al proposed a monitoring algorithm for CD to guide treatment decisions and assess disease activity.33 The algorithm involves an early IUS assessment in conjunction with PROMs and CRP measurement at 4–6 weeks after treatment initiation, particularly in patients with highly active disease. This approach not only detects treatment response but also excludes any possible complications. Furthermore, follow-up IUS and measurement of both CRP and calprotectin are recommended at 3 months after treatment initiation. At 6 months, these modalities are repeated, with the addition of endoscopy. Lastly, follow-up after 12 months with the possible incorporation of magnetic resonance enterography (MRE).33 While this proposed monitoring algorithm presents great potential, further studies are needed to establish its efficacy and feasibility.
Importantly, CD patients are at increased risk of colorectal cancer (CRC) and need routine screening with endoscopy. The risk of CD patients developing CRC is estimated to be 2% after 10 years, 8% after 20 years and 18% after 30 years of disease.34 This elevated risk has been theorised to arise from dysplasia, hence surveillance colonoscopy to detect dysplasia is recommended for patients 8 years after onset of symptoms.35 The intervals of surveillance vary based on the level of risk for CRC and the society issuing the guidelines, ranging from 1–5 years.36 Research efforts are ongoing to explore new technologies to improve dysplasia detection in IBD surveillance, including the use of panoramic views during colonoscopy, use of virtual chromoendoscopy with narrow band imaging and high definition iSCAN technology.
Surgical management
Typically, medical treatment is the first line of intervention for CD, however surgery becomes necessary when medical therapies fail, with some series reporting up to 75% of CD patients undergoing surgery during the course of their lives.37 Recent studies have shown a decreasing trend in the need for surgery among CD patients, attributed to changes in clinical practices, early disease detection, implementation of practice guidelines and advancements in biological therapies.38 Surgery is indicated for patients with stricturing CD with partial or complete obstructive symptoms, fistulising or perianal CD with infectious complications, failure of medical therapy, steroid dependence, high grade dysplasia and cancer.39, 40
One of the common complications in CD that may require surgery is the development of strictures, which can occur within the first 10 years after diagnosis.41 Strictures result from fibrosis of the intestines and may involve any segment of the gastrointestinal tract. There are two types of strictures: de novo strictures, typically found in the terminal ileum and ileocolonic region, and anastomotic strictures, which can develop after intestinal resection.41 The management of CD strictures involves various approaches, including medical therapy, endoscopic interventions (such as balloon dilatation), and surgical procedures.42 Up to 66% of CD patients with strictures fail medical therapy and 40% fail endoscopic dilation, thus needing surgery.43
A study by Bossuyt et al in 201814 developed a risk stratification model called the BACARDI risk model to guide treatment strategy selection for patients with stricturing ileal CD. This model assigns points based on factors, including Montreal classification, anti-TNF exposure, CARD15/NOD2 mutation, and high CRP levels. Patients are categorised into low (0–1 points), medium (2–3 points), high (4–5 points) and all risk (6 points) groups. They found that for patients with all risk factors, surgery-free survival rate is 0% after 5 years and 19% for those in the high-risk group. These patients most likely will benefit from early surgery after diagnosis of stricture instead of medical or endoscopic therapies. For medium risk group, surgery-free survival rate is 38% after 5 years, hence these patients may benefit from optimising medical therapies, performing endoscopic dilation when necessary and re-assessment of the stricture every 12 months. Lastly, the low-risk group have a surgery-free survival rate of 77% after 5 years, therefore the best clinical approach for this group is to continue medical treatment and/or endoscopic therapy if needed. This model has great potential to guide in determining the most appropriate therapeutic approach for CD-associated strictures.44
Fistulising perianal CD (CD-PAF) affects about 30–50% of CD patients during the course of their disease and about 80% of those fistulas are classified as complex.45 Medical therapy is recommended as a first line treatment however despite advances in current available therapies about two-thirds of patients with CD-PAF require perianal surgery and approximately 7% require major abdominal surgery.46 The choice of surgical procedure is based on the complexity and location of the fistula, with minimising the risk for sphincter injury in mind. About 80% of fistulas have been associated with perianal abscess, for which incision and drainage is the most common intervention.46 Adequate drainage is important since fistulas are potential sources of pelvic sepsis.47 In addition, non-cutting setons are typically used to decrease the incidence of recurrent abscess formation and the development of new fistulous tracts. Cutting setons carry a high risk of anal incontinence due to scarring of the anal canal; hence, loose setons are preferred since it can preserve the integrity of external anal sphincter.47 Surgery using setons is carried out with concomitant dual therapy with biologics to improve efficacy and healing rates, since it alone results in significant reintervention rates.48 One disadvantage on the use of setons is that the fistula tract cannot close while it is still there; currently there is no consensus on the optimal timing for seton removal. In a small prospective trial, setons were maintained for the duration of infliximab induction which resulted in an overall new abscess rate of 0%.49 Hence, this study recommended to keep the seton in place at least until the induction of the anti-TNF treatment period has been completed. After its removal, the fistula can close.
The future
Several novel therapeutic targets are being explored as treatment options for CD. These include monoclonal antibodies (risankizumab and mirikizumab) as well as sphingosine-1-phosphate receptor modulators (such as etrasimod). Furthermore, several phase I/II trials are exploring the use of mesenchymal stem cells as therapy for severe CD. These up-and-coming treatments present an exciting alternative for clinicians to consider in patients who have progressed on standard therapies.
Inflammatory cytokines play an important role in the initiation and propagation of CD. IL-23 has been identified as a key player in the maintenance of a chronic inflammatory state in the intestines. Specifically, it induces the differentiation of naïve T-helper cells, which then go on to release further pro-inflammatory molecules. Risankizumab is a monoclonal antibody targeting IL-23 that has been explored as a treatment option in 3 phase III trials. The ADVANCE, MOTIVATE, and FORTIFY studies compared risankizumab with placebo in participants with moderate to severe CD. In all three trials, risankizumab was associated with clinical remission and endoscopic improvement after 12 weeks of therapy.50 Similarly, mirikizumab is an IgG4 monoclonal antibody that binds to the p19 subunit of IL-23, inhibiting its binding to the IL-23 receptor. A recent phase II trial showed that CD patients receiving induction mirikizumab had improved endoscopic response, compared to placebo, at 12 weeks and this effect was preserved when continued as maintenance therapy.51 Both risankizumab and mirikizumab require ongoing study in phase IV and head-to-head trials to compare their effectiveness with current treatment options in CD.
Sphingosine-1-phosphate (S1P) receptor modulators have recently been explored as an oral treatment option in CD. These receptors are involved in the trafficking of lymphocytes from lymph nodes into the blood stream and are commonly known as an established treatment target for conditions such as multiple sclerosis. Early phase II data from the CULTIVATE study, demonstrated a modest effect in terms of endoscopic and clinical improvement when etrasimod was taken by patients for 14 weeks.52 These early results, although based on a small sample size, do demonstrate the potential for the use of etrasimod in the treatment of severe CD.
Mesenchymal stem cell (MSC) therapy is also currently being studied as an option to treat severe or refractory CD. These cells, derived from bone marrow, are known to inhibit T-cell reactivity as well as promote healing, and have thus been explored as a potential treatment for several autoimmune conditions. With regards to CD, most studies have looked at the use of MSC in the treatment of peri-anal fistulising disease. MSCs are theorised to downregulate immune activity and promote healing at the site of perinal fistula and reduce the need for subsequent surgery. Several phase I-III trials have studied the effect of MSC injection at perianal site on disease progression. Results have shown improved clinical as well as radiological remission at both 25 weeks and 1 year.53 Further studies with larger sample size are required to further evaluate the efficacy as well as safety of MSC as a treatment option.
The current classification system for CD is based on a combination of phenotypic manifestations of the disease as well as the use of serum biomarkers. The commonly used Montreal classification considers age of onset, location of disease, and behaviour (stricturing and/or penetrating). This, along with biomarkers such as C-reactive protein and faecal calprotectin, are currently used to inform prognostication and clinical decision making. Unfortunately, the current system is not able to account for the heterogenous nature of CD, with variable presentations, an often relapsing/remitting course, and inter-patient variability in response to treatment.
The above shortcomings are the basis for ongoing research into the molecular and genetic underpinnings of CD, which likely plays a key role in inter-patient variability with regards to disease course as well as response to treatment. A recent paper by Kamal et al54 suggests a novel ‘omics’ approach to CD classification. This approach involves looking at not only specific genes, but their subsequent transcription and translation products, to identify novel biomarkers. This can then be extrapolated to differentiate CD patients into molecular phenotypes, which would theoretically allow clinicians to estimate disease progression as well as predicted response more accurately to treatment. While promising, the implementation of this approach on a large scale is currently limited by both financial and logistical challenges. Nevertheless further study is already underway, heralding a promising future for the use of personalised medicine in CD treatment.
Fig 2 summarises the key points from this article.
Fig 2.
Crohn's disease treatment landscape. a) Risk factors leading to Crohn's Disease. b) Treatment Targets for Crohn's Disease as per STRIDE II recommendations. c) Tools to monitor severity of disease and risk for colon cancer. d) Current treatment options for CD. e) Future therapies for CD.
References
- 1.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 2.Cosnes J, Gower–Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. e4. [DOI] [PubMed] [Google Scholar]
- 3.Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal manifestations of inflammatory bowel disease: current concepts, treatment, and implications for disease management. Gastroenterology. 2021;161:1118–1132. doi: 10.1053/j.gastro.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilic Y, Kamal S, Jaffar F, et al. Prevalence of extraintestinal manifestations in inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2023;12:izad061. doi: 10.1093/ibd/izad061. [DOI] [PubMed] [Google Scholar]
- 5.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman HJ. Natural history and long-term clinical course of Crohn's disease. World J Gastroenterol. 2014;20:31. doi: 10.3748/wjg.v20.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abegunde AT, Muhammad BH, Bhatti O, Ali T. Environmental risk factors for inflammatory bowel diseases: evidence based literature review. World J Gastroenterol. 2016;22:6296–6317. doi: 10.3748/wjg.v22.i27.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–523. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 9.Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7:827–851. doi: 10.1016/j.crohns.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144–164. doi: 10.1093/ecco-jcc/jjy113. [DOI] [PubMed] [Google Scholar]
- 12.Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160:1570–1583. doi: 10.1053/j.gastro.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 13.D'incà R, Caccaro R. Measuring disease activity in Crohn's disease: what is currently available to the clinician. Clin Exper Gastroenterol. 2014;20:151–161. doi: 10.2147/CEG.S41413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezaie A, Kuenzig ME, Benchimol EI, et al. Budesonide for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2015;6:CD000296. doi: 10.1002/14651858.CD000296.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis JD, Scott FI, Brensinger CM, et al. Increased mortality rates with prolonged corticosteroid therapy when compared with antitumor necrosis factor-alpha-directed therapy for inflammatory bowel disease. Am J Gastroenterol. 2018;113:405–417. doi: 10.1038/ajg.2017.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutgeerts P, Löfberg R, Malchow H, et al. A comparison of budesonide with prednisolone for active Crohn's disease. N Engl J Med. 1994;331:842–845. doi: 10.1056/NEJM199409293311304. [DOI] [PubMed] [Google Scholar]
- 17.Rezaie A, Kuenzig ME, Benchimol EI, et al. Budesonide for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2015;6:CD000296. doi: 10.1002/14651858.CD000296.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar A, Cole A, Segal J, Smith P, Limdi JK. A review of the therapeutic management of Crohn's disease. Therap Adv Gastroenterol. 2022;15 doi: 10.1177/17562848221078456. 17562848221078456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feagan BG, Fedorak RN, Irvine EJ, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease. North American Crohn's Study Group Investigators. N Engl J Med. 2000;342:1627–1632. doi: 10.1056/NEJM200006013422202. [DOI] [PubMed] [Google Scholar]
- 20.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 21.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 22.Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn's disease: result of the CLASSIC II Trial. Gut. 2007;56:1232–1239. doi: 10.1136/gut.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greener T, Boland K, Steinhart H, Silverberg MS. The unfinished symphony: golimumab therapy for anti-tumour necrosis factor refractory crohn's disease. J Crohn's Colitis. 2018;12:458–464. doi: 10.1093/ecco-jcc/jjx176. [DOI] [PubMed] [Google Scholar]
- 24.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348:601–608. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 25.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711–721. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 26.Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn's disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618–627. doi: 10.1053/j.gastro.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2016;375:1946–1960. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 28.Honap S, Meade S, Ibraheim H, et al. Effectiveness and safety of ustekinumab in inflammatory bowel disease: a systematic review and meta-analysis. Dig Dis Sci. 2022;67:1018–1035. doi: 10.1007/s10620-021-06932-4. [DOI] [PubMed] [Google Scholar]
- 29.Abreu MT, Phimister EG. JAK1 Inhibition to Treat Crohn's Disease. N Engl J Med. 2023;388:2005–2009. doi: 10.1056/NEJMe2301147. [DOI] [PubMed] [Google Scholar]
- 30.Loftus EV, Panés J, Lacerda AP, et al. Upadacitinib induction and maintenance therapy for Crohn's disease. N Engl J Med. 2023;388:1966–1980. doi: 10.1056/NEJMoa2212728. [DOI] [PubMed] [Google Scholar]
- 31.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 32.Fehily SR, Basnayake C, Wright EK, Kamm MA. Fecal microbiota transplantation therapy in Crohn's disease: systematic review. J Gastroenterol Hepatology. 2021;36:2672–2686. doi: 10.1111/jgh.15598. [DOI] [PubMed] [Google Scholar]
- 33.Kucharzik T, Verstockt B, Maaser C. Monitoring of patients with active inflammatory bowel disease. Front Gastroenterol. 2023;2:1172318. [Google Scholar]
- 34.National Institute for Health and Care Excellence . NICE; 2022. Colorectal cancer prevention: colonoscopic surveillance in adults with ulcerative colitis, Crohn's disease or adenomas. [PubMed] [Google Scholar]
- 35.Laine L, Kalten T, Barkun A, McQuaid K, Subramanian V, Soetikno R. SCENIC International Consensus Statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology. 2015;148:639–651. doi: 10.1053/j.gastro.2015.01.031. e28. [DOI] [PubMed] [Google Scholar]
- 36.Clarke WT, Feuerstein JD. Colorectal cancer surveillance in inflammatory bowel disease: practice guidelines and recent developments. World J Gastroenterol. 2019;25:4148–4157. doi: 10.3748/wjg.v25.i30.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meima-Van Praag EM, Buskens CJ, Hompes R, Bemelman WA. Surgical management of Crohn's disease: a state of the art review. Int J Colorectal Dis. 2021;36:1133–1145. doi: 10.1007/s00384-021-03857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. doi: 10.1053/j.gastro.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 39.Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 40.Cheifetz AS. Management of Active Crohn Disease. JAMA. 2013;309:2150. doi: 10.1001/jama.2013.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan WPW, Mourad F, Leong RW. Crohn's disease associated strictures. J Gastroenterol Hepatol. 2018;33:998–1008. doi: 10.1111/jgh.14119. [DOI] [PubMed] [Google Scholar]
- 42.Pokala A, Shen B. Update of endoscopic management of Crohn's disease strictures. Intest Res. 2020;18:1–10. doi: 10.5217/ir.2019.09158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campos C, Perrey A, Lambert C, et al. Medical therapies for stricturing Crohn's disease: efficacy and cross-sectional imaging predictors of therapeutic failure. Dig Dis Sci. 2017;62:1628–1636. doi: 10.1007/s10620-017-4572-4. [DOI] [PubMed] [Google Scholar]
- 44.Bossuyt P, Debeuckelaere C, Ferrante M. Risk stratification for surgery in stricturing ileal Crohn's disease: the BACARDI risk model. J Crohns Colitis. 2018;12:32–38. doi: 10.1093/ecco-jcc/jjx110. [DOI] [PubMed] [Google Scholar]
- 45.García-Olmo D, Gómez-Barrera M, De La, Portilla F. Surgical management of complex perianal fistula revisited in a systematic review: a critical view of available scientific evidence. BMC Surg. 2023;23:29. doi: 10.1186/s12893-023-01912-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parian AM, Obi M, Fleshner P, Schwartz DA. Management of Perianal Crohn's Disease. Am J Gastroenterol. 2023;118:1323–1331. doi: 10.14309/ajg.0000000000002326. [DOI] [PubMed] [Google Scholar]
- 47.Gecse KB, Bemelman W, Kamm MA, et al. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn's disease. Gut. 2014;63:1381–1392. doi: 10.1136/gutjnl-2013-306709. [DOI] [PubMed] [Google Scholar]
- 48.Feroz SH, Ahmed A, Muralidharan A, Thirunavukarasu P. Comparison of the efficacy of the various treatment modalities in the management of perianal Crohn's fistula: a review. Cureus. 2020;12:e11882. doi: 10.7759/cureus.11882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hyder SA, Travis SPL, Jewell DP, et al. Fistulating anal Crohn's disease: results of combined surgical and infliximab treatment. Dis Colon Rectum. 2006;49:1837–1841. doi: 10.1007/s10350-006-0656-5. [DOI] [PubMed] [Google Scholar]
- 50.Choi D, Sheridan H, Bhat S. Risankizumab-rzaa: a new therapeutic option for the treatment of Crohn's disease. Ann Pharmacother. 2023;57:579–584. doi: 10.1177/10600280221130450. [DOI] [PubMed] [Google Scholar]
- 51.Sands BE, Peyrin-Biroulet L, Kierkus J, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with Crohn's disease. Gastroenterology. 2022;162:495–508. doi: 10.1053/j.gastro.2021.10.050. [DOI] [PubMed] [Google Scholar]
- 52.D'Haens G, Dubinsky MC, Peyrin-Biroulet L, et al. Etrasimod induction therapy in moderately to severely active Crohn's disease: results from a phase 2, randomised, double-blind substudy. J Crohns Colitis. 2023;17:i764–i765. [Google Scholar]
- 53.Carvello M, Lightner A, Yamamoto T, Kotze PG, Spinelli A. Mesenchymal stem cells for perianal Crohn's disease. Cells. 2019;8 doi: 10.3390/cells8070764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamal S, Parkash N, Beattie W, Christensen B, Segal JP. Are we ready to reclassify Crohn's disease using molecular classification? J Clin Med. 2023;12:5786. doi: 10.3390/jcm12185786. [DOI] [PMC free article] [PubMed] [Google Scholar]