Abstract
Dilatation of the gut occurs in response to either mechanical obstruction or aperistalsis. The hallmark features are symptoms of bowel obstruction with vomiting, constipation, abdominal pain and distension. This review will primarily deal with the non-mechanical causes of gut dilatation, both intestinal and colonic, and differentiate between acute and chronic presentations.
KEYWORDS: gut dilatation, bowel obstruction, chronic intestinal pseudo-obstruction, acute colonic pseudo-obstruction
Key points
Small intestinal and colonic dilatation can result from mechanical obstruction or severe intestinal dysmotility.
The hallmark symptoms are nausea and vomiting, constipation and abdominal pain or distension.
Acute deterioration in pain with chronic intestinal pseudo-obstruction raises the possibility of perforation, ischaemia or peritonitis.
CT abdomen (with contrast, if not contraindicated) is the gold standard investigation when gut dilatation is suspected.
Introduction
The gastrointestinal tract responds to mechanical obstruction or severely disturbed motility by dilatation. In mechanical cases the segment proximal to the obstruction dilates, whereas the dilation is more diffuse in dysmotility cases.1 The dilatation compromises intestinal absorption and predisposes to sepsis and intestinal failure.
Acute intestinal obstruction
The symptoms include nausea and vomiting (of bile and gastric fluid), abdominal distention (as the intestine dilates), colicky abdominal pain (as the gut contacts to overcome the obstruction) and constipation (as content cannot progress beyond the obstruction). On examination the abdomen is distended and bowel sounds may be excessively active or reduced and high pitched. Abdominal tenderness may be diffuse or focal if there is a delayed presentation with peritonitis. While point-of care-ultrasound has emerged as a novel diagnostic modality, computed to mography is the gold standard imaging modality.2 Intravenous contrast is used if there are no renal or hypersensitivity contraindications.
Small intestinal obstruction accounts for 10–15% of surgical admissions and, in the UK, 20% of emergency laparotomies.3 Mechanical obstruction due to post-operative adhesions accounts for approximately 75% of acute small bowel obstruction presentations, followed by intestinal hernias and malignancies. Post-adhesional obstruction may occur as early as a few weeks or as late as several decades after the initial surgery. Acute medical causes include small bowel Crohn's disease, non-steroidal enteropathy, post-radiotherapy and small bowel adenocarcinomas (for example complicating coeliac disease or Lynch syndrome) or benign small bowel tumours (such as the hamartomas of Peutz-Jegher's syndrome).4 Acute episodes of non-mechanical small bowel obstruction may complicate chronic intestinal pseudo-obstruction (CIPO), representing an acute-on-chronic episode of dysmotility. Box 1 illustrates the classification of the causes of acute intestinal obstruction.
Box 1.
Classification of causes of acute intestinal obstruction
| Extrinsic |
| Adhesions |
| Hernias |
| Endometriosis |
| Haematomas |
| Neuro-endocrine tumours |
| Intrinsic |
| Crohn's disease |
| NSAID enteropathy |
| Intestinal tuberculosis |
| Intussusception |
| Intestinal neoplasia |
| Post-radiotherapy strictures |
| Ischaemic strictures |
| Intraluminal |
| Foreign bodies |
| Bezoar |
| Gallstone ileus |
Chronic intestinal pseudo-obstruction
CIPO represents a rare family of diseases characterised by obstructive symptoms in the absence of a mechanical cause. The conditions represent the most severe spectrum of gut dysmotility. A wide range of causes have been identified, which are stratified as either myopathic or neuropathic.
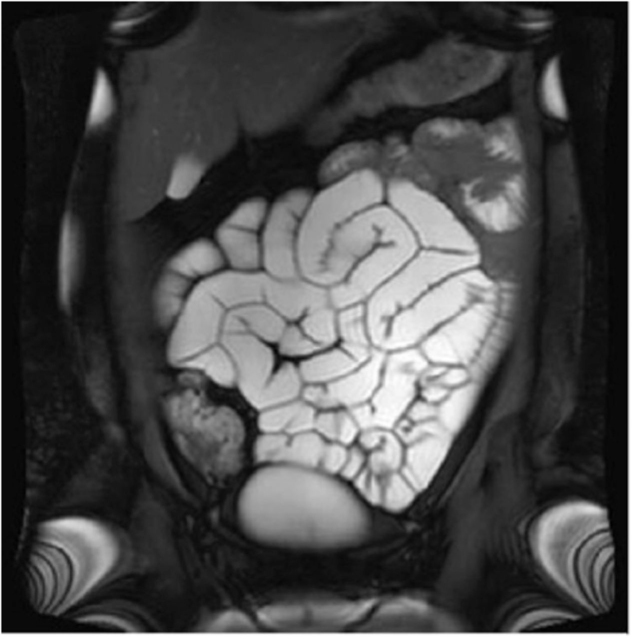
CIPO can affect people of any age. Incidence in childhood is typically associated with genetic causation5 and there is a second peak in the elderly.6 In younger patients, the presentation is typically with failure to thrive and upper gut symptoms, and neonatal diagnosis is possible in some, with non-obstructive megacystis the hallmark finding on sonography.5 By contrast, in adult patients, lower gut symptoms predominate with acute exacerbations triggered by intercurrent physical or psychological stressors.7 Diagnosis of CIPO requires that symptoms have been present for at least 6 months, and there needs to be radiological evidence of dilatation and/or air-fluid level in the gut with no evidence of mechanical cause (Fig 1).
Fig 1.
Image from dynamic small bowel MRI sequence showing generalised gut dilatation and hypomotility.
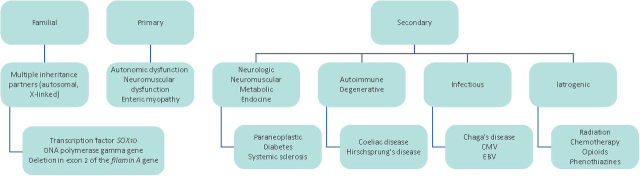
The origin of the dilatation is dysmotility rather than structural obstruction, and the aetiology of dysmotility may be primary, secondary or familial8 (Fig 2).
Fig 2.
Classification of causes of chronic intestinal pseudo-obstruction. CMV = cytomegalovirus; EBC = Epstein-Barr virus.
When primary, the pathogenesis typically relates to one or more of the following:9
-
•
decreased parasympathetic nervous activity (which in health plays an excitatory role on the colon)
-
•
altered function of interstitial cells of Cajal or neuromuscular junction
-
•
impaired enteric smooth muscle function.
Once gut dilatation is confirmed, CIPO is a diagnosis of exclusion, requiring ruling out of other causes for GI tract dysmotility. Baseline blood tests should include amylase/lipase, lactate, inflammatory markers, coeliac serology and thyroid function. Hypokalaemia may be present if there is vomiting, and a low potassium will exacerbate dysmotility.
While CT has been the standard investigation for patients presenting with abdominal pain, dynamic small bowel MRI has emerged as diagnostically valuable.10 As well as helping to exclude mechanical causes for obstruction like inflammatory bowel disease, it has increasingly been used to assess motility. Diagnostic confirmation of whether there is an enteric neuropathy or myopathy can be obtained by full thickness biopsies obtained from dilated and non-dilated gut segments when patients with suspected CIPO undergo abdominal surgery for unexplained obstructive episodes. The biopsies need examination by a pathologist with special interest in in gut pathology and access to specialist staining and possibly electron microscopy.11
Since there is no definitive curative therapy, patients with CIPO require care in a specialist service that encompasses nutrition, psychology, specialist surgery and histopathology. Prokinetic medications, in particular cholinesterase inhibitors, are the evidence-based therapies.12 Intravenous administration of neostigmine can be therapeutically helpful in the acute setting, and oral pyridostigmine can be helpful in the chronic setting.13 Endoscopic decompression can be of use in the acute setting to allow decompression, which may subsequently encourage motility.14 Avoiding surgery during acute crises is a priority since it often worsens symptoms of CIPO.11 Alternative prokinetics such as erythromycin (motilin analogue), metoclopramide (dopamine receptor antagonist) and prucalopride (5HT-4 receptor agonist) have been shown to have a limited effect in the maintenance setting.9
Small intestinal bacterial overgrowth is a frequent complication of dysmotility in CIPO patients. Confirmation is typically by hydrogen-methane breath test and treatment is with broad-spectrum antibiotics (doxycycline, metronidazole, ciprofloxacin, rifaximin) for 10-14 days.15 If nutritional support is needed, an enteral approach is preferable. Feeding may need to be into the jejunum since there is often significant gastric emptying delay.11
Acute colonic pseudo-obstruction
Acute colonic pseudo-obstruction (ACPO), which is synonymous with Ogilvie's syndrome, refers to bowel dilation in the absence of mechanical obstruction. While dilatation is primarily seen in the right colon, it may be more diffuse.14 ACPO is more common in older hospitalised patients, especially men with multiple comorbidities or post-operatively.16 The aetiology of the colonic atony is thought to involve decreased parasympathetic tone, decreased gut perfusion and metabolic factors (electrolyte disturbances, acute kidney injury) and iatrogenic causes (opioids, anticholinergics, etc).
The primary symptom is distension, but this is generally well tolerated. When pain occurs it should raise the possibility of impending ischemia or perforation, especially if it is associated with fever, guarding, leucocytosis and acidosis. With severe acute colonic dilatation, there can be accompanying colonic atonia which precipitates a volvulus (of either the sigmoid, transverse colon or caecum). In such situations, colonic decompression (via a flatus tube or at endoscopy) is critical.
Another differential is toxic megacolon which may occur in patients with inflammatory bowel disease or infectious or ischaemic colitis. These patients often have a preceding history of diarrhoea and are usually more acutely ill.
Abdominal CT scan typically shows colonic dilation proximally up to the splenic flexure in the absence of mechanical obstruction or transition point.16 The caecum being the thinnest region of the colon, caecal diameter (specifically diameter .12 cm) is an important indicator of the severity of disease.17 A gastrograffin enema is sometimes undertaken and can be therapeutic due to the resultant bowel evacuation. But such studies should not be considered if there is any suspicion of perforation or peritonitis.
Management of ACPO in the acute setting comprises keeping the patient nil by mouth and undertaking naso-gastric decompression. Maintaining hydration and electrolyte balance is part of acute conservative management of mild-to-moderate severity cases. If the dilatation does not settle, endoscopic decompression and use of prokinetics (as for CIPO above) may be necessary. The place for surgery (in the form of caecostomy or colostomy) is if there is perforation or ischaemia.
References
- 1.Hashmi SK, Ceron RH, Heuckeroth RO. Visceral myopathy: clinical syndromes, genetics, pathophysiology, and fall of the cytoskeleton. Am J Physiol Gastrointest Liver Physiol. 2021;320:G919–G935. doi: 10.1152/ajpgi.00066.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shokoohi H, Boniface KS, Loesche MA, et al. Development of a nomogram to predict small bowel obstruction using point-of-care ultrasound in the emergency department. Am J Emerg Med. 2020;38:2356–2360. doi: 10.1016/j.ajem.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Lee MJ, Sayers AE, Drake TM, et al. National prospective cohort study of the burden of acute small bowel obstruction. BJS Open. 2019;3:354–366. doi: 10.1002/bjs5.50136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tai FWD, Sidhu R. Small bowel obstruction: what a gastroenterologist needs to know. Curr Opin Gastroenterol. 2023;39:234–241. doi: 10.1097/MOG.0000000000000924. [DOI] [PubMed] [Google Scholar]
- 5.Thapar N, Saliakellis E, Benninga MA, et al. Paediatric intestinal pseudo-obstruction: evidence and consensus-based recommendations from an ESPGHAN-Led expert group. J Pediatr Gastroenterol Nutr. 2018;66:991–1019. doi: 10.1097/MPG.0000000000001982. [DOI] [PubMed] [Google Scholar]
- 6.Mousa H, Hyman PE, Cocjin J, et al. Long-term outcome of congenital intestinal pseudoobstruction. Dig Dis Sci. 2002;47:2298–2305. doi: 10.1023/a:1020199614102. [DOI] [PubMed] [Google Scholar]
- 7.Pironi L, Sasdelli AS. Management of the patient with chronic intestinal pseudo-obstruction and intestinal failure. Gastroenterol Clin North Am. 2019;48:513–524. doi: 10.1016/j.gtc.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Antonucci A, Fronzoni L, Cogliandro L, et al. Chronic intestinal pseudo-obstruction. World J Gastroenterol. 2008;14:2953–2961. doi: 10.3748/wjg.14.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alcala-Gonzalez LG, Malagelada C. Current insights on chronic intestinal dysmotility: pseudo-obstruction and enteric dysmotility. Rev Esp Enferm Dig. 2023 doi: 10.17235/reed.2023.10038/2023. in press. [DOI] [PubMed] [Google Scholar]
- 10.Menys A, Butt S, Emmanuel A, et al. Comparative quantitative assessment of global small bowel motility using magnetic resonance imaging in chronic intestinal pseudo-obstruction and healthy controls. Neurogastroenterol Motil. 2016;28:376–383. doi: 10.1111/nmo.12735. [DOI] [PubMed] [Google Scholar]
- 11.De Giorgio R, Cogliandro RF, Barbara G, et al. Chronic intestinal pseudo-obstruction: clinical features, diagnosis, and therapy. Gastroenterol Clin North Am. 2011;40:787–807. doi: 10.1016/j.gtc.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Zenzeri L, Tambucci R, Quitadamo P, et al. Update on chronic intestinal pseudo-obstruction. Curr Opin Gastroenterol. 2020;36:230–237. doi: 10.1097/MOG.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 13.Wilkie BD, Noori J, Johnston M, et al. Pyridostigmine in chronic intestinal pseudo-obstruction-a systematic review. ANZ J Surg. 2023;93:2086–2091. doi: 10.1111/ans.18478. [DOI] [PubMed] [Google Scholar]
- 14.Eisen GM, Baron TH, Dominitz JA, et al. Acute colonic pseudo-obstruction. Gastrointest Endosc. 2002;56:789–792. doi: 10.1016/s0016-5107(02)70348-9. [DOI] [PubMed] [Google Scholar]
- 15.Sachdev AH, Pimentel M. Antibiotics for irritable bowel syndrome: rationale and current evidence. Curr Gastroenterol Rep. 2012;14:439–445. doi: 10.1007/s11894-012-0284-2. [DOI] [PubMed] [Google Scholar]
- 16.Ross SW, Oommen B, Wormer BA, et al. Acute Colonic Pseudo-obstruction: Defining the Epidemiology, Treatment, and Adverse Outcomes of Ogilvie's syndrome. Am Surg. 2016;82:102–111. doi: 10.1177/000313481608200211. [DOI] [PubMed] [Google Scholar]
- 17.Underhill J, Munding E, Hayden D. Acute colonic pseudo-obstruction and volvulus: pathophysiology, evaluation, and treatment. Clin Colon Rectal Surg. 2021;34:242–250. doi: 10.1055/s-0041-1727195. [DOI] [PMC free article] [PubMed] [Google Scholar]