Abstract
The transition to adult health care (HCT, Health Care Transition), is the purposeful, planned movement of patients from paediatric to adult services. For the adolescent living with obesity (ALwO), the HCT represents a crucial window for effective intervention that can help improve body weight, adiposopathy, and metabolic complications. Nevertheless, no transition guidelines, models, and tools have been developed for these patients. The present statement of the Italian Society of Obesity examines the critical transition of ALwO from paediatric to adult healthcare. It synthesises current knowledge and identifies gaps in HCT of ALwO. Drawing on successful practices and evidence-based interventions worldwide, the paper explores challenges, including disparities and barriers, while advocating for patient and family involvement. Additionally, it discusses barriers and perspectives within the Italian health care scenario. The need for specialised training for healthcare providers and the impact of transition on healthcare policies are also addressed. The conclusions underscore the significance of well-managed transitions. The SIO recognises that without proper support during this transition, ALwOs risk facing a gap in healthcare delivery, exacerbating their condition, and increasing the likelihood of complications. Addressing this gap requires concerted efforts to develop effective transition models, enhance healthcare provider awareness, and ensure equitable access to care for all individuals affected by obesity. The document concludes by outlining avenues for future research and improvement.
Keywords: Adolescence, Youth, Health care transition, Obesity
Introduction
Obesity is a chronic, relapsing disease that can begin very early in life. Severe obesity with onset in children and particularly under 5 may have a genetic aetiology (i.e. monogenic obesities). Nonetheless, most cases are primary obesities. Secondary obesity is caused by medical conditions (i.e. hormonal imbalance in Cushing disease, use of certain medications, etc.). The WHO European Childhood Obesity Surveillance Initiative shows that nearly one in three school-aged children are living with overweight or obesity. Despite a temporary decrease in prevalence in adolescents, one quarter of them lives with overweight or obesity [1]. As children age, their risk of developing severe obesity increases, with adolescents showing a higher prevalence of severe obesity compared to children [2]. Being an adolescent living with obesity (ALwO) significantly raises the likelihood of obesity persisting into adulthood [3], along with the risk of complications [4–6] such as hypertension, dyslipidemia, hyperglycemia, and metabolic dysfunction associated steatotic liver disease (MASLD) [7, 8]. Alarmingly, both children and adolescents with severe obesity [Class II that is a body mass index (BMI) ≥ 120% of the 95th percentile, and class III obesity, a BMI ≥ 140% of the 95th percentile] can have these complications, and their prevalence rates rise as obesity severity worsens. A recent study in Italy highlighted the extent of these issues [2]. A substantial percentage of young people with severe obesity experience hypertension (42% children and 49% ALwO), low high-density lipoprotein (HDL) cholesterol levels (10% and 15%, respectively), elevated liver enzyme levels suggestive of MASLD (in about 46% for both age groups), and prediabetes, indicating a concerning trend in early metabolic disturbances (25% children and 28% ALwO) [2].
Recent projections from the World of Obesity Federation estimate a sharp rise in obesity cases among young individuals globally by 2035, highlighting the urgent need for preventive measures and effective interventions [9].
Obesity-related complications in young populations extend beyond metabolic issues to include significant health risk conditions like obstructive sleep apnoea syndrome and obesity nephropathy, emphasising the multifaceted nature of obesity-related morbidity [7].
The transition to adult health care (HCT) represents a critical opportunity for effective intervention in any chronic and potentially long-lasting condition. For ALwOs, a correct HCT can help improve body weight, adiposopathy, and metabolic complications, motivating young patients to seek profound lifestyle changes [10]. Effective anti-obesity medications and bariatric surgery support the patient struggling with appetite control, which is deemed as their main barrier to weight loss [11].
Recognising the critical nature of the transition from paediatric to adult healthcare, the Italian Society of Obesity (SIO, Società Italiana dell’Obesità) emphasises the need for tailored and structured HCT. Without proper support, ALwOs risk facing a gap in healthcare delivery, exacerbating their condition, affecting therapy compliance, and increasing the likelihood of complications. Addressing this gap requires concerted efforts to develop effective transition models, to enhance healthcare providers (HCP) awareness, and to ensure equitable access to care for all individuals affected by obesity.
The present article represents a SIO statement about the challenges, goals and practices that need implementation for an effective HCT. The document is endorsed by the Italian Society of Paediatric Endocrinology and Diabetology (SIEDP, Società Italiana di Endocrinologia e Diabetologia Pediatrica) and the Italian Society of Adolescence Medicine (SIMA, Società Italiana di Medicina dell’Adolescenza).
Transition to adult health care: theory and models
The HCT is the purposeful, planned movement of patients from paediatric to adult services [12].
Ideally occurring between the ages of 18 and 21 years [13], the process is generally long and gradual, in most cases encompassing ages 12 [14] to 26 [15–18]. During HCT, young people gain more responsibility for their health and the self-management of their disease, with parents often remaining involved ensuring the development of skills and self-management strategies [15].
Transition theory and principles inform HCT, hence it should be a collaborative and individualised effort, youth-focused, and emphasise self-management, with early and ongoing preparation. Transition preparation is recommended for all young people, with added components for people with special health care needs (CSHCN) and/or chronic conditions [15].
Lack of structured HCT results in poor outcomes, including medical complications (e.g., poor disease control, such as a suboptimal lung function in cystic fibrosis and/or asthma), treatment and self-management adherence issues, decreased quality of life, and increased healthcare costs and resource use (e.g., emergency department visits, hospitalisations) [19–33]. These outcomes highlight the need for an effective inter-professional approach to improve patient’s knowledge about therapies and skills to navigate through the national healthcare system (NHS) [19, 20, 34].
A well-structured transition plan can maximise lifelong functioning and well-being of the young patient, positively impacting retention in care, patient health, and experiences of care [33, 35, 36].
Successful transition involves the engagement and participation of the medical home team [physicians, nurses, physician assistants, care coordinators], family and caregivers, and the individual youth collaborating in a positive and mutually respectful relationship [i.e. one that honours diversity and is consistent with each family’s cultural and religious beliefs] [34]. The medical home team does not engage in transition planning alone; rather, it jointly creates and implements the plan with the other mentioned actors. The medical home team facilitates a planned, smooth, and patient- and family-centred process. Parents’ role includes active engagement, moving in and out of the decision-making position as appropriate [34]. Young people maximise their independence and centrality in decision-making. Adult HCPs must be identified and engaged to provide developmentally appropriate support during the transition [34]. After the birth of the transition concept, guidelines have been published about HCT goals and best practices, initially targeting CSHCN (“those who have or are at increased risk for a chronic physical, developmental, behavioural, or emotional condition and who also require health related services of a type or amount beyond that required by children generally”), without focusing on specific diseases. [13, 15, 34, 37, 38]. Later, diverse transition guidelines, models and tools were developed for specific diseases [see, e.g., 21, 23, 24] but not for paediatric obesity.
HCT of the patient with endocrine diseases
Research and practice models for the transition of ALWO are almost absent. Transition models exist for syndromic obesity, such as Prader–Willi syndrome (PWS), requiring a specific multidisciplinary approach, due to the intellectual disability, that compromises patient involvement in care. Effective transition models for PWS, such as the European Reference Network on Rare Endocrine Conditions (Endo-ERN), highlight the need for a multidisciplinary model with an HCT pathway coordinator linking the different intra- and extra-hospital actors and resources [39]. In the French Transend program, adolescents with PWS experience a multidisciplinary diagnostic and therapeutic work-up with periodic follow-ups in the paediatric clinic. During the transition, the coordinator fills the gap with the adult PWS specialist and territorial network of healthcare professionals, and provides an education program for the patient and his relatives [39].
In the United States the Got Transition program, a collaboration of American Academy of Family Physicians (AFP), American College of Physicians (ACP), and American Academy of Pediatrics (AAP), is established for several chronic diseases, including type 1 diabetes, Turner syndrome, and growth hormone deficiency [40]. The transition model includes three elements: 1. transition readiness assessment; 2. medical summary record; and 3. self-care assessment. The transition readiness tool assesses patients' knowledge and ability to manage their condition. It is administered by the paediatric team, ideally from age 12 [41]. Barriers to readiness include parents' reticence to lose control of the disease management and patients’ lack of motivation to learn new skills. The medical summary record, filled by the paediatrician, family, and patient, contains key medical disease-specific information. It ensures continuity of care and a strict collaboration between paediatric and adult clinicians. The self-care assessment tool supports adult care clinicians in addressing any knowledge gaps or self-care issues. A set of disease-specific assessment tools has been developed for each of the three quoted elements [40].
Other models for endocrine disease transition also focus on transition preparation, transfer and integration into adult care [42]. HCT preparation includes patient education through workshops, meetings, focus groups, and classes, generally starting around ages 12–14, according to the patient’s readiness. Transition to adult clinics requires a care coordinator who facilitates the first meeting with the adult care clinician. Joint visits with adult and paediatric endocrinologists, as well as other professionals of the multidisciplinary team, are effective in improving the efficacy of transition. The timing for HCT should be tailored to the patient’s need and readiness, though a mean age of 19 has been reported in several models [42, 43]. Integration into adult care is the last step of the transition. A critical step is that adult care physicians should be familiar with the patient’s medical history and needs, since the literature reports high drop-out rates, loss of follow-up, and return to paediatric care after the first visit in adult care settings [42]. A substantial time dedicated to previous steps and a multidisciplinary adult team have been proposed to address these issues.
The transition of ALwOs: an unmet health care need
The literature about the transition of ALwO is sparse, with the only published guidelines addressing children and adolescents with PWS [39] and ALwOs who underwent bariatric surgery [43, 44]. Endocrine societies or scientist groups disseminating transition guidelines and tools for major endocrine diseases have largely neglected the topic of transitioning youths with obesity to adult healthcare [31–33, 45–47], despite including paediatric obesity in their clinical guidelines for some time. Major international or national scientific societies for obesity, such as the European Childhood Obesity Group (ECOG), the European Society for Obesity (EASO) and the SIO, have not published specific guidelines on transition until now. Nonetheless, paediatric obesity has long been recognised as a complex chronic disease [48], and youths with obesity fit the definition of CSHCN. Only the AAP underscores the importance of a structured transition plan for youths with obesity [44].
Several hypotheses may explain why the transition of ALwOs is a neglected topic [38]. First, adolescents do not stay in treatment long enough to transition, indicating that improving long-term treatment centre attendance could be key to developing effective transition programs [38]. Second, despite obesity being recognised as a disease, it is frequently not perceived as a chronic illness, but as a self-imposed disease with an easy way-out (“eat less and move more” narrative) [35, 36, 38, 49]. This perception reduces the likelihood of obesity being included in national transition planning [38]. Third, there is no clear transition pathway [38]. Lastly, if obesity-related comorbidities are present, the youth is likely to transition to a morbidity-specific service—for hypertension/diabetes/lipids—where weight management expertise might be lacking [38]. Ideally, obesity should be managed chronically by a multidisciplinary team at specialised centres that offer integrated and coherent follow-up by several professionals. This team of HCPs (which may include clinical nutritionist, dietician, diabetologist, endocrinologist, cardiologist, surgeon, psychologist, etc.) shares a unified care project with the patient, and is coordinated by a case-manager, typically a paediatrician or internist with specialised in obesity [7, 38, 39, 44, 50]. The “obesity centres” host these specialised teams and act as spokes (secondary level) or hubs (tertiary level) within a network system that includes primary care providers. General paediatricians and practitioners should be aware of the specialised follow-up available and actively advocate for and support it when communicating with patients [7, 38, 39, 44, 50].
An effective transition plan for obesity requires the patient’s living area to have a specialised multidisciplinary adult centre that pursues the same principal health goals and adopts the same screening and management programs for complications as the paediatric centre. Moreover, the different levels of paediatric care should align with each other and follow a coherent timeline.
These requirements are challenging in real life. First, a particular area might lack either a paediatric or an adult obesity centre, complicating long-term follow-up from paediatric to adult age. For example, the SIO map of endorsed obesity centres shows a patchwork distribution, with paediatric and adult centres not always overlapping [51]. Second, even if paediatric and adult centres exist, they might not agree on the overall approach to managing obesity, leading to inconsistent clinical messages and proposals. As a complex disease, obesity may necessitate various lifestyle, psychological, pharmacological, and surgical approaches. Without a concerted transition plan, patients face a high risk of encountering conflicting and inconsistent clinical messages and recommendations as they move from one centre to another. If this inconsistency exists, it may drive patients away from specialised follow-up in favour of commercial programs or no program at all, especially if they do not require treatment for complications. Finally, the primary and secondary/tertiary level of care may not move towards transition at the same time. In Italy, for example, paediatricians—free of economic charge for families—handle prevention, treatment, and rehabilitation of children and adolescents up to age 14, with mandatory care until age 6 and optional care until age 14, while families of youths aged 6–14 can freely choose between paediatrician and family doctor. Both can ensure patients’ access to services and benefits offered by the NHS, included in the Essential Levels of Care. At the age of 14, revocation from paediatric care is an automatic step, unless there are documented chronic pathologies or situations of disability. In such selected cases, a paediatrician can follow the patients up to age 16. The most common experience for ALwOs, therefore, is that they transition to the adult primary care significantly, before they transition to an adult obesity centre. In Italy, the medical home community (“Case della Comunità”) has been foreseen by the legislative decree DM 77/2022 that describes in detail hubs and spokes, but it has not been yet implemented. Guidelines for the implementation of the organisational model of Community Hub Centres have just been issued by the National Agency for Regional Health Services (April 5, 2024) [52].
Changing the narrative of obesity in adolescents
With the increasing recognition of obesity as a chronic and relapsing disease, it is clear that its causes lie in the interaction between genes and environment, not in individual laziness. Consequently, society bears a moral responsibility to ensure the successful treatment of this condition and its associated complications. Adolescence and young adulthood are windows of opportunity for intervention that can affect obesity-related morbidity and mortality, as well as direct and indirect costs for the society.
Bariatric surgery and novel anti-obesity medications are potentially effective treatments for patients in this age group. Although sustained obesity from adolescence through midlife poses a risk of type 2 diabetes (T2D) and cardiovascular diseases-related complications, bariatric surgery is not commonly performed in adolescence and very few centres in Italy offer surgery to ALwOs [53]. The NIDDK funded Teen-LABS (Longitudinal Assessment of Bariatric Surgery) study was the first large-scale study carried out in teens with severe obesity and obesity-related complications (prediabetes, T2D, cardiovascular disease, OSAS, MASLD, and others). Two hundred and forty teens aged 13 to 19 were enrolled between 2006 and 2012 and 161 of them underwent bariatric surgery, including gastric band, gastric sleeve, and gastric bypass [54]. Teens undergoing bariatric surgery experienced significant improvement in weight, heart health, prediabetes, T2D, blood pressure, dyslipidaemia, and kidney function after the surgery [55]. Moreover, teens undergoing bariatric surgery experienced beneficial effects in adulthood in terms of weight and low prevalence of obesity-related comorbidities [56].
Beyond bariatric surgery, pharmacotherapy is recommended in combination with lifestyle changes according to consensus recommendations. The choice of an anti-obesity drug should be based on the medications’ risks and benefits [44]. In the United States, exenatide is currently approved for children aged 10 to 17 with T2D [57]. The use of liraglutide is recommended to children aged 12 or older affected by obesity unresponsive to lifestyle interventions [58]. Semaglutide has also been reported as an effective treatment for obesity in adolescents. A randomised double-blind, parallel-group, placebo-controlled trial was carried out in ALwOs: subjects were randomised in a 2:1 ratio to receive once-weekly subcutaneous semaglutide (at a dose of 2.4 mg) or placebo for 68 weeks, alongside lifestyle intervention for both groups. The treated group experienced a greater decrease in BMI compared to the placebo group [59]. The primary dietary strategy to achieve weight loss in children and teens affected by obesity and/or obesity-related comorbidities is represented by moderate-energy restriction, preferably including a cluster of food items resembling Mediterranean Diet [60]. However, recently it has been considered the use of very low-calorie diets for the treatment of obesity in children. In this regard, ketogenic diet has been already used in children due to its antiepileptic properties. Recently it has been considered for its weight-loss properties, mostly when it is set up as a very low-calorie ketogenic diet. However, the literature on this regard is scarce and although supplementation with micronutrients is of paramount importance in children, it is not known the consequences on nutritional and growth status [61].
Barriers in health care
Addressing the continuity of weight management from childhood to adulthood is challenging as the patient experiences several life changes during this period, such as increased autonomy, employment, leaving home, and self-identity development. The lack of standardised and effective transition models emphasises these difficulties. To fill this gap, research in the field of obesity transition, drawing on other disease models of transition, should be advocated [34]. The importance of coordinated HCT management is still underestimated, especially for obesity, the still poor recognition of which as a chronic disease has so far hindered its inclusion in national transition planning [13, 38, 62].
Previous reports on barriers to high-quality transition in the healthcare system have included communication gaps [10], deficits in education programs and training, inadequate time for office visits, and lack of dedicated staff, coordination, and financial resources [15, 62–65]. These issues highlight the importance of broad-based support for transition, encompassing not only medical care but also welfare. Additionally, in an epidemiological context of an ageing population with growing demand for medical care, workforce shortages for health professionals may obstruct the provision of assistance to younger adults [66]. Providing developmentally appropriate obesity care may be particularly challenging for both paediatric and adult professionals because early adulthood typically represents the chronological age extremes in their respective routine practices. Thus, adult care teams may require specific additional training to meet the needs of transition-aged patients [67] and to bridge the gap between health services based on a family-focused approach to a self-care-focused one. Conversely, paediatric ambivalence or negative beliefs about adult care must be addressed, as it may inadvertently convey to young patients and their families the message that transition is something to worry about [62]. Therefore, when obesity care is transferred from paediatric to adult medical centres, the presence of both paediatric and adult HCPs at the first transfer visit may be reassuring and help promote continuity of care [67].
Although joint care may be facilitated by the physical colocation of paediatric and adult facilities, this is not always feasible due to infrastructural and logistic factors. Nevertheless, colocation does not automatically guarantee a smooth transition. Teams should still be encouraged to work collaboratively and be actively engaged by a transition coordinator [68]. Effective HCT strategies have emphasised the need for a designated transition coordinator to ensure communication among paediatric and adult professionals, families, and patients, as well as the importance of planning a transition program with a clear timeline a few years before the transition [69].
Medical barriers to the transition process also include challenges in transferring medical information. Shared technological systems to schedule appointments and transfer electronic medical records can facilitate a seamless shift from paediatric to adult healthcare centres.
The greatest barriers reported by proxy caregivers are insufficient information regarding the transition process and inadequate access to adult HCPs with sufficient medical expertise [70].
Psychosocial factors
To understand the hurdles in obesity care transition, it is crucial to consider that adolescence and emerging adulthood represent a major life transition. This critical period implies increasing autonomy, the need for acceptance by healthy peers, self-identity construction, changes in family and societal relationships, and engagement in work or higher education [71]. During this phase of significant physical, behavioural, and psychosocial changes, the negative impact of obesity on quality of life, inter-relationships, self-acceptance, development of disordered eating behaviours, depression, and anxiety [72] may become even greater and complicate the HCT.
The complexity of HCT is compounded by the fact that it closely aligns the adolescence phase of life. While adolescence is very often defined as “the phase of life stretching between childhood and adulthood” [73], this definition fails to capture the complexity of this period of life. Adolescence is a lengthy process, starting around age 10 (early adolescence) and extending to age 20 (late adolescence) or even 24, according to some authors [74]. The early phase (10–12 years), important for rapid growth and the starting of puberty, is not typically involved in the HCT process, as youths are still under the care of the paediatrician, at this age. Therefore, this period will not be addressed here. The second period (13–15 years) is when many young people start transitioning from the paediatric to the adult health caregivers, a process that completes in the third period (16–20/24 years), regardless of their health status.
Biological, psychological, and social forces, some unprecedented like marketing and social media, interact in the evolution from youthhood to adulthood. These factors contribute to a deep rethinking of the youths’ identity, where they unknowingly oscillate, often inadequately, between carelessness and worries and uncertainties [73].
Unsurprisingly, these factors influence the HCT in different ways, depending on the specific phase of adolescence. It is common for paediatricians to observe that teens in their second period of adolescence (13–15 years) do not usually appreciate being still under paediatric care, and may openly express their disappointment [74]. They feel grown-ups and prefer adult health caregivers. Sharing waiting rooms or hospital wards with infants and younger kids conflicts with their self-perception. If they ever share aspects of their medical visits with peers, they often omit that their doctors are paediatricians to avoid teasing or bullying. This period seems ideal for transitioning to adult-tailored health services.
As their psychosocial development progresses into the third period of adolescence, the situation often changes dramatically: these new young adults begin to value the deep knowledge paediatricians have gained since childhood, sometimes knowing more details than their families. This newfound awareness can make transitioning to a new doctor challenging, especially for patients with chronic illnesses. The fear of being transitioned to an “unknown" doctor mingles with the apprehension of being perceived as “unfamiliar” by the new doctor, who might not be aware of their specific medical history, and of their critical points and frailties [68]. Another challenging aspect of HCT, for adolescents, is the sudden expectation for increased self-determination and responsibility [70, 75, 76], which many teens might not feel ready for.
Paediatric doctors or healthcare teams also face challenges during the HCT. They must persuade teenagers that the transition is not an abrupt dismissal from their care, but a comprehensive process involving the transfer of both medical information and non-medical aspects the doctor may be aware of. They must inform the young patients in detail about what to expect from the new healthcare providers and convince them that the transition is in their best interest. For instance, there might be situations and concerns that could emerge as the patients mature, which the paediatric team, due to lack of experience, might not be equipped to address. The receiving healthcare teams face mirrored challenges. Despite these patients' young age, they are entering adulthood and they need time to build trust in the new healthcare environment and personnel. Concurrently, the new team must understand and accept that these patients are grappling with the emotional impact of leaving familiar doctors.
Hence, various actors are engaged in the transition process: healthcare professionals (or healthcare teams, particularly in cases of chronic and intricate illnesses), patients, and their families. Several factors contribute to the smoothness or challenges of the transition. For doctors and teams, both technical expertise and empathic abilities, along with the capacity to collaborate seamlessly without conflicting competencies, are critical [77]. For families, their ability to embrace the transition and collaborate with the medical team plays a pivotal role. Parental involvement in the HCT is associated with better health and well-being outcomes for young adults with chronic illness, including increased self-advocacy skills, improved social functioning, and reduced hospitalisation risk. Factors like the family’s social and educational background significantly influence this aspect [76]. For patients, their response to the prospect of transitioning to different medical personnel depends on personality, character, age, education, and possible delays in psychosocial development compared to peers without long-term conditions [78], influencing their acceptance of the transition [79]. To ensure a smooth transition, it is crucial for teens to develop knowledge of their medical condition and medications, self-management skills, and an understanding of how to navigate the NHS [80].
Research strongly indicates that HCPs, from paediatric to adult care, should consider the family's social and educational context when planning the transition process. They should provide families with necessary resources and support to navigate the transition process. Continuous training and updates are essential to both paediatric and adult-care teams to address these challenges effectively [77]. There are effective programmes available that can yield positive results, though more research is needed to determine the best approaches for different groups of young people, especially those with CSHCN [81, 82]. Ultimately, the priority of any healthcare team should be the holistic well-being of patients, extending beyond their physical aspects.
Patient and family environment
The HCT represents a critical step in the shift from family-centred care to a patient-centred care model. This change increases the risk of care gap occurrences. Care gaps might arise when young adults skip office visits with the adult specialist while they still have a low ability to manage their medical condition, finally leading to worse health maintenance and adverse outcomes [76].
Parental involvement can support young adults in the transition process, reducing the risk of lower health outcomes. However, parents' attitudes may themselves represent an obstacle to transition, as they may not always fully align with adult professionals in fostering teens to take charge of their own healthcare [19]. This is particularly important for patients with intellectual disability who may struggle to reach a complete readiness for transition. At the same time, parents should avoid being overly intrusive during visits to promote the strengthening of the patient’s self-care skills [76]. Therefore, types of parental involvement may range from simple physical attendance at office visits to direct communication with the physician on the patient’s behalf.
Moreover, the change in parental roles in the management of patients’ health causes ambivalent feelings. Switching from being a full caregiver to a supportive person might induce negative feelings in parents, leading to lower collaboration during the transition. Some parents may be worried about their children’s ability to be independent in self-care, judging them as too young to take responsibility for their disease.
Involving parents during transition has been associated with an overall positive impact on patients’ adherence to medical therapy and visit attendance [83]. Moreover, young adults approaching adult care positively rate the presence of parents during the transition as “a safety net” that supports them during this important change [83].
According to the social-ecological model of readiness to transition (SMART), a feasible model is built into a social-ecological framework that includes stakeholders, multiple factors, systems, and their relationship to enhance readiness for transition [84]. The SMART model distinguishes non-modifiable and modifiable factors [84]. Non-modifiable factors include the patients’ IQ, socio-economic status, and medical conditions. Modifiable factors depend on the patient, parents, and healthcare professionals and include knowledge, expectations, motivations, skills, communication, and emotions [84]. These modifiable factors are targeted during interventions. The program includes focus groups attended by HCPs, patients, and caregivers. In the study protocol, meetings and interviews focused on long-term cancer follow-up care and were positively rated by all participants as a valid framework for transition. However, this method has not been validated for other diseases [85].
Another model, reported by Thomsen et al. in the ParTNerSTEPs (Parents in Transition—a Nurse-led Support and Transfer Educational Program) study, included adolescents with different chronic diseases [85]. The model aimed to increase parental involvement in the transition and was based on three components: a website, educational events, and transfer consultation with paediatric and adult specialists. Therefore, the program enhanced communication, education, and expectation among patients, parents, and physicians, ensuring a tailored approach that combines patient’s and physician’s needs [85]. These findings highlight the critical role of direct patient and parent involvement in transition, with planned long-term education to facilitate a smooth shift to adult care setting.
Training and education of HCPs
Ensuring a high-quality transition depends on the availability of trained and experienced HCPs. Many physicians in both the paediatric and adult sides of care feel unprepared to address the young patient’s needs during the transition process [86]. This phenomenon reflects an important gap in the knowledge and education of HCPs. To date, there are no structured and endorsed curricula and education programs that specifically address their training. Gaps in medical education are reported at undergraduate, graduate, and postgraduate levels [87]. Education goals should target the main elements and tools of transition, including patient readiness assessment, parental and patient education, communication with the social framework and services, transmission of patient medical information, and disease-specific training [88].
For paediatric and adult HCPs, the residency program should include both paediatric and adult care clinic attendance [88]. Moreover, they should practise the elaboration of electronic medical records to share the medical information with the other team members. In addition, training in clinical practice should include the elaboration of readiness assessment tools, transition goals, and patient registry. Learning activities should address the specific education needs of different learners. Residency programs in paediatric and adult care specialties (i.e. internal medicine, endocrinology, etc.) differ in terms of disease knowledge and training. For example, internal medicine and endocrinology residents who are training in adult care are likely to be more familiar with bariatric surgery and prescription of anti-obesity medications than paediatricians. Conversely, paediatric residents are used to having strict communication with parents, whereas adult care providers are not [88].
Primary HCPs are the key personnel that ensure the continuity of care for young with chronic illnesses during transition. The joint recommendations from AAP, ACP, and AFP suggest that transition-specific training should target both primary and subspecialty care. However, no formal guidelines have been released [89].
Considering the multidisciplinary nature of the transition process, education in transition should target nurses, psychologists, social workers, and other HCPs of the care team. Often, psychologists and nurses have closer contact than physicians with the family, gaining insights into patients’ needs [89].
Continuing medical education (CME) might mitigate this gap in residency education projects. Courses are available on transition care with a review of existing guidelines, models of transition, and other main themes [90–92]. However, CME lacks the practical internship activity that has been identified as an important part of healthcare providers' training.
Policy implications
Healthcare policies in the field of HCT must be flexible, evidence-based, and responsive to the evolving needs of populations, the advancements in healthcare technology, and the changing of healthcare dynamics. Collaboration among stakeholders (policymakers, HCPs, patients, and industry leaders) is essential to develop policies promoting quality, accessible, and equitable healthcare for all.
Barriers to HCT, such as poor training for transition, lack of research for best practices, discontinuity in care, and insufficient patient and parent readiness, must be addressed to improve health policies. These limitations influence the effectiveness of transitions and should be mitigated through comprehensive strategies.
The specific skills required of HCPs should be supported by health policies that fund and implement comprehensive training and education programs in the evolving healthcare landscape. This could involve initiatives to promote lifelong learning, retraining programs for existing professionals, incentives for recruitment into emerging specialties, or combined adult–paediatric residency programs.
Advancements in technology, treatments, and procedures can improve patient care but are often costly. Healthcare policies must ensure affordability and accessibility for all citizens. This might involve adjustments in reimbursement models, cost-sharing mechanisms, or budget allocations. Importantly, new technologies or treatment modalities may worsen existing disparities in access to care, if not addressed through appropriate measures. Policies aimed at promoting equity might include subsidies for underserved populations, incentives for providers to serve rural or low-income areas, or other initiatives addressing social determinants of health.
Ensuring continuity of care during transitions is crucial for patient safety, improve health outcomes, and enhance the patient experience. Effective HCT models emphasise the importance of care coordination mechanisms, such as the development of HCT plans, the assignment of care coordinators or case managers, and the implementation of communication protocols. These measures ensure relevant clinical information is shared effectively among all care team members, fostering a standardised and well-constructed framework of collaboration.
By addressing key areas such as care coordination, health information exchange, medication management, and patient engagement, policymakers can help to improve patient outcomes and experiences throughout the care continuum.
The health technologies
In this context, technological tools may support the clinicians ensuring the continuity of care and communication among the stakeholders (Table 1). Electronic medical records (EMRs) support the transmission of medical records between subspecialists and primary care physicians. EMRs, available in several but not all countries and might aid in standardising data collection. In Italy, the “Transitional Care” project uses telemedicine resources to standardise HCT [93]. The Electronic Health Record 2.0 ensures general data collection, medical information sharing and transferring [93]. However, simply transferring data is insufficient for guaranteeing an adequate transition and continuity of care; patient-centred, tailored pathways are necessary. Implementing a digital platform that includes all the actors involved in the transition process can promote continuity and personalisation of care [93].
Table 1.
Health technologies support in HCT
| Health technology type | Functionalities and role |
|---|---|
| Electrical medical records |
· Standardised data collection · Information sharing · Data transferring |
| e-Meetings |
· Health care professionals’ education · Patients’ and parents’ education · Skills development · Empowerment of paediatric-to-adult health professionals communication |
| Mobile apps |
· Self-care readiness assessment · Complete medical information availability · Medical record transmission to health care professionals |
Moreover, telemedicine can empower parental involvement and enhance patients’ readiness. Digital meetings and classes have been used to improve disease knowledge and skills acquisition for patients, as well as to present adult team and clinic to parents [86, 94]. However, these resources are only available in pilot study or research settings, and thus scientific evidence is still limited.
Finally, mobile apps can also assist in reaching and communicating with patients approaching HCT. The MyTransition app, currently being evaluated in research settings, has been developed to promote self-care and advocacy in young adults during HCT. Through the app, patients can assess their readiness, review their medical report, and transmit their health information to future HCPs using their smartphone [95].
Research needs and future direction
Although a standardised model for ALWOs’ transition has been widely advocated in international recommendations, obesity remains an orphan condition. The available models vary widely across the diverse NHSs and evidence of their effectiveness in clinical practice is very limited. Existing transition models need to be evaluated for effectiveness, safety and feasibility in large populations’ studies. This would help draw frameworks, identify best practices, and assess cost-effectiveness of HCT models, as well as their impact on clinical barriers and health outcomes in obesity transition within the Italian NHS. Additionally, strategies for patient and parental involvement during transition require further investigation. Once the best models have been identified, education and training for HCPs should be implemented.
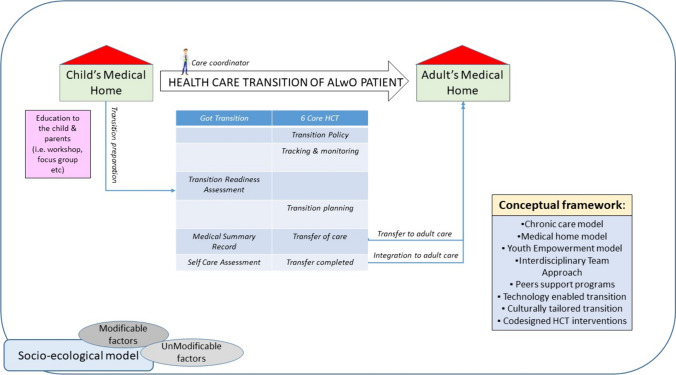
Figure 1 illustrates a theoretical framework for the HCT of ALwOs, based on current knowledge and practices in the transition of patients with endocrine diseases and chronic conditions.
Fig. 1.
A possible model of HCT for ALwO [adolescents living with obesity] puts together inputs from different models. The transition model [87] that emphasises the importance of structured transition planning and coordination between paediatric and adult healthcare providers. It provides tools and resources for healthcare professionals, youth, and families to support the transition process. The Chronic Care Model [96, 97] was originally developed for improving care for adults with chronic conditions. It emphasises proactive planning, patient education, self-management support, and coordination of care among HCPs. The Medical Home Model [98] focuses on providing patient-centred, coordinated, and comprehensive care through a medical home—a primary care practice that provides continuous, comprehensive, and coordinated care. Medical homes can facilitate transition by offering support and coordination during the transition process. The Youth Empowerment Model exploring the conceptualisation and research of empowerment in the field of youth [99] emphasises empowering young people to take an active role in managing their healthcare transition. It promotes self-advocacy, self-determination, and skill-building to help young people navigate the healthcare system and take charge of their health. The Interdisciplinary Team Approach [100] emphasises the role of the multidisciplinary team that collaborates to address the medical, psychosocial, and educational needs of transitioning youth [101]. It connects transitioning youth with peers who have already gone through the transition process. These programs provide emotional support, practical advice, and encouragement from individuals who have first-hand experience with transitioning to adult healthcare [102]. With advancements in technology, various digital tools and platforms have been developed to support healthcare transition. These include mobile apps, online resources, and telehealth services that facilitate communication, education, and coordination among healthcare providers, patients, and families. HCT models should be sensitive to the cultural, linguistic, and developmental needs of diverse populations. Culturally tailored approaches ensure that transition services are accessible, acceptable, and effective for all youth and families, regardless of their cultural backgrounds. Measuring transition readiness is important when preparing young people with chronic illness for successful transition to adult care. The Expanded Socioecological Model of Adolescent and Young Adult Readiness to Transition [Expanded SMART] offers a holistic view of factors that influence transition readiness and outcomes. A study examined the conceptual congruency of transition readiness instruments with the Expanded SMART to determine the breadth and frequency of constructs measured [103]
Conclusions
In the current statement, the SIO underscores the following key points:
Acknowledging obesity as a chronic disease: obesity, especially when it arises early in life, should be recognised as a chronic, recurring disease. Youths grappling with obesity should be recognised as CSHCN.
Significance of HCT: the purposeful transfer of patients from paediatric to adult services is a critical opportunity for effective intervention. Properly executed HCT can potentially lead to the normalisation of body weight, reversal of adiposopathy, and mitigation of metabolic complications.
Potential cost-effectiveness of proper HCT: if implemented successfully, HCT has the potential to be cost-effective in reducing morbidity and mortality associated with obesity in adulthood, which often stems from its onset during adolescence. It is emphasised that during this transition period, access to surgery, anti-obesity medications, and continued follow-up care should be ensured according to guidelines that must be developed.
Risks of inadequate support during transition: without adequate support during transition, ALwOs may face gaps in healthcare delivery, worsening their condition and increasing the likelihood of complications. Hence, there is a pressing need for the development of transition guidelines, models, and tools specifically tailored for the HCT of ALwOs.
Comprehensive approach to HCT: recognising the physical, psychological, and societal aspects of HCT is crucial, and advocates for the implementation of policies at various levels to address these aspects comprehensively.
What is already known on this subject?
Obesity is a chronic, relapsing disease that may develop very early in life.
The transition to adult health care (HCT) represents an important time window for an effective intervention in any chronic and potentially long-lasting condition.
What this study adds?
The document examines current knowledge and identifies gaps in HCT of adolescents living with obesity.
Acknowledgements
Not applicable.
Disclosure
The authors declare that no tools of artificial intelligence were used to write the present manuscript.
Abbreviations
- AAP
American Academy of Pediatrics
- AFP
American Academy of Family Physicians
- ACP
American College of Physicians
- ALwO
Adolescents living with obesity
- CSHCN
Children with special health care needs
- HCPs
Health care providers
- HCT
Health care transition
- HDL
High-density lipoprotein
- SIO
Italian Society of Obesity
- EASO
European Association for the Study of Obesity
- ECOG
European Childhood Obesity Group
- NHS
National Health Service
- PWS
Prader–Willi syndrome
- MAFLD
Metabolic associated fatty liver disease
Author contributions
Conceptualisation, methodology project administration and supervision Melania Manco; writing original draft and material preparation Giuseppina R. Umano, Anita Morandi, Andrea Vania, Valeria Guglielmi, Giovanna Muscogiuri and Melania Manco. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The study has been supported by the Italian Ministry of Health with Current Research Funds. The funder has no role in the preparation of the draft.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
Authors disclosure no competing interests.
Ethical and informed consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Anita Morandi and Giuseppina Rosaria Umano have contributed equally to this work.
Change history
10/30/2024
A Correction to this paper has been published: 10.1007/s40519-024-01700-5
References
- 1.World Health Organization. Regional Office for Europe (2022) WHO European Regional Obesity Report 2022. World Health Organization. Regional Office for Europe. https://iris.who.int/handle/10665/353747
- 2.Pedicelli S, Fintini D, Ravà L, Inzaghi E, Deodati A, Spreghini MR, Bizzarri C, Mariani M, Cianfarani S, Cappa M, Manco M (2022) Prevalence of prediabetes in children and adolescents by class of obesity. Pediatr Obes 17(7):e12900. 10.1111/ijpo.12900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman DS, Lawman HG, Galuska DA, Goodman AB, Berenson GS (2018) Tracking and variability in childhood levels of BMI: the Bogalusa Heart Study. Obesity 26(7):1197–1202. 10.1002/oby.22199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tirosh A, Shai I, Afek A, Dubnov-Raz G, Ayalon N, Gordon B, Derazne E, Tzur D, Shamis A, Vinker S, Rudich A (2011) Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med 364(14):1315–1325. 10.1056/nejmoa1006992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Sun C, Cheung M, Viikari JS, Dwyer T, Raitakari OT (2011) Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med 365(20):1876–1885. 10.1056/nejmoa1010112 [DOI] [PubMed] [Google Scholar]
- 6.Bjerregaard LG et al (2018) Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N Engl J Med 378:1302–1312. 10.1056/nejmoa1713231 [DOI] [PubMed] [Google Scholar]
- 7.Valerio G, Maffeis C, Saggese G, Ambruzzi MA, Balsamo A, Bellone S, Bergamini M, Bernasconi S, Bona G, Calcaterra V, Canali T, Caroli M, Chiarelli F, Corciulo N, Crinò A, Di Bonito P, Di Pietrantonio V, Di Pietro M, Di Sessa A, Diamanti A, Doria M, Fintini D, Franceschi R, Franzese A, Giussani M, Grugni G, Iafusco D, Iughetti L, Lamborghini A, Licenziati MR, Limauro R, Maltoni G, Manco M, Reggiani LM, Marcovecchio L, Marsciani A, Del Giudice EM, Morandi A, Morino G, Moro B, Nobili V, Perrone L, Picca M, Pietrobelli A, Privitera F, Purromuto S, Ragusa L, Ricotti R, Santamaria F, Sartori C, Stilli S, Street ME, Tanas R, Trifiró G, Umano GR, Vania A, Verduci E, Zito E (2018) Diagnosis, treatment and prevention of pediatric obesity: consensus position statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital J Pediatr 44(1):88. 10.1186/s13052-018-0525-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fornari E, Maffeis C (2019) Treatment of metabolic syndrome in children. Front Endocrinol 10:702. 10.3389/fendo.2019.00702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Obesity Atlas (2024) https://data.worldobesity.org/publications/WOF-Obesity-Atlas-v7.pdf. Accessed 25 Mar 2024
- 10.Sweeting H, Smith E, Neary J, Wright C (2016) ‘Now I care’: a qualitative study of how overweight adolescents managed their weight in the transition to adulthood. BMJ Open. 10.1136/bmjopen-2015-010774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halford JCG, Bereket A, Bin-Abbas B, Chen W, Fernández-Aranda F, Garibay Nieto N, López Siguero JP, Maffeis C, Mooney V, Osorto CK, Reynoso R, Rhie YJ, Toro-Ramos M, Baur LA (2022) Misalignment among adolescents living with obesity, caregivers, and healthcare professionals: ACTION Teens global survey study. Pediatr Obes. 10.1111/ijpo.12957 [DOI] [PubMed] [Google Scholar]
- 12.Blum RW, Garell D, Hodgman CH, Jorissen TW, Okinow NA, Orr DP, Slap GB (1993) Transition from child-centered to adult health-care systems for adolescents with chronic conditions: a position paper of the Society for Adolescent Medicine. J Adolesc Health. 10.1016/1054-139x(93)90143-d [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians, Transitions Clinical Report Authoring (2011) Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics 128(1):182–200. 10.1542/peds.2011-0969 [DOI] [PubMed] [Google Scholar]
- 14.Casado E, Gómez-Alonso C, Pintos-Morell G, Bou-Torrent R, Barreda-Bonis AC, Torregrosa JV, Broseta-Monzó JJ, Arango-Sancho P, Chocrón-de-Benzaquen S, Olmedilla-Ishishi Y, Soler-López B (2023) Transition of patients with metabolic bone disease from paediatric to adult healthcare services: current situation and proposals for improvement. Orphanet J Rare Dis 18(1):245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobart CB, Phan H (2019) Pediatric-to-adult healthcare transitions: current challenges and recommended practices. Am J Health Syst Pharm. 10.1093/ajhp/zxz165 [DOI] [PubMed] [Google Scholar]
- 16.Ragnhildstveit A, Tuteja N, Seli P, Smart L, Uzun N, Bass LC, Miranda AC, Ford TJ, Neufeld SAS (2024) Transitions from child and adolescent to adult mental health services for eating disorders: an in-depth systematic review and development of a transition framework. J Eat Disord 12(1):36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabrera Fernandez DL, Lopez KN, Bravo-Jaimes K, Mackie AS (2024) The impact of social determinants of health on transition from pediatric to adult cardiology care. Can J Cardiol 40(6):1043–1055. 10.1016/j.cjca.2024.03.023 [DOI] [PubMed] [Google Scholar]
- 18.Simon SL, Phimphasone-Brady P, McKenney KM, Gulley LD, Bonny AE, Moore JM, Torres-Zegarra C, Cree MG (2024) Comprehensive transition of care for polycystic ovary syndrome from adolescence to adulthood. Lancet Child Adolesc Health 8(6):443–455. 10.1016/S2352-4642(24)00019-1 [DOI] [PubMed] [Google Scholar]
- 19.White PH, Cooley WC, for the Transitions Clinical Report Authoring Group, American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians (2018) Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 10.1542/peds.2018-3610 [DOI] [PubMed] [Google Scholar]
- 20.Lebrun-Harris LA, McManus MA, Ilango SM et al (2018) Transition planning among US youth with and without special health care needs. Pediatrics. 10.1542/peds.2018-0194 [DOI] [PubMed] [Google Scholar]
- 21.Foster BJ (2015) Heightened graft failure risk during emerging adulthood and transition to adult care. Pediatr Nephrol. 10.1007/s00467-014-2859-7 [DOI] [PubMed] [Google Scholar]
- 22.Majumdar S (2013) The adolescent with sickle cell disease. Adolesc Med State Art Rev 24(1):295–306, xv [PubMed] [Google Scholar]
- 23.Wafa S, Nakhla M (2015) Improving the transition from pediatric to adult diabetes healthcare: a literature review. Can J Diabetes 39(6):520–528. 10.1016/j.jcjd.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 24.Chaudhry SR, Keaton M, Nasr SZ (2013) Evaluation of a cystic fibrosis transition program from pediatric to adult care. Pediatr Pulmonol 48(7):658–665. 10.1002/ppul.22647 [DOI] [PubMed] [Google Scholar]
- 25.Bohun CM, Woods P, Winter C et al (2016) Challenges of intra-institutional transfer of care from paediatric to adult congenital cardiology: the need for retention as well as transition. Cardiol Young 26(2):327–333. 10.1017/s1047951115000220 [DOI] [PubMed] [Google Scholar]
- 26.Luque Ramos A, Hoffmann F, Albrecht K et al (2017) Transition to adult rheumatology care is necessary to maintain DMARD therapy in young people with juvenile idiopathic arthritis. Semin Arthritis Rheum 47(2):269–275. 10.1016/j.semarthrit.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 27.Montano CB, Young J (2012) Discontinuity in the transition from pediatric to adult health care for patients with attention-deficit/hyperactivity disorder. Postgrad Med 124(5):23–32. 10.3810/pgm.2012.09.2591 [DOI] [PubMed] [Google Scholar]
- 28.Szymanski KM, Whittam B, Misseri R et al (2017) A case of base rate bias, or are adolescents at a higher risk of developing complications after catheterizable urinary channel surgery. J Pediatr Urol 13(2):184. 10.1016/j.jpurol.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 29.Wojciechowski EA, Hurtig A, Dorn L (2002) A natural history study of adolescents and young adults with sickle cell disease as they transfer to adult care: a need for case management services. J Pediatr Nurs 17(1):18–27. 10.1053/jpdn.2002.30930 [DOI] [PubMed] [Google Scholar]
- 30.Barr NG, Longo CJ, Embrett MG et al (2017) The transition from youth to adult mental health services and the economic impact on youth and their families. Healthc Manage Forum 30(6):283–288. 10.1177/0840470417709579 [DOI] [PubMed] [Google Scholar]
- 31.Cohen E, Gandhi S, Toulany A et al (2016) Health care use during transfer to adult care among youth with chronic conditions. Pediatrics. 10.1542/peds.2015-2734 [DOI] [PubMed] [Google Scholar]
- 32.Mosquera RA, Avritscher EB, Samuels CL et al (2014) Effect of an enhanced medical home on serious illness and cost of care among high-risk children with chronic illness: a randomized clinical trial. JAMA 312(24):2640–2648. 10.1001/jama.2014.16419 [DOI] [PubMed] [Google Scholar]
- 33.Gabriel P, McManus M, Rogers K, White P (2017) Outcome evidence for structured pediatric to adult health care transition interventions: a systematic review. J Pediatr 188:263–269. 10.1016/j.jpeds.2017.05.066 [DOI] [PubMed] [Google Scholar]
- 34.American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians-American Society of Internal Medicine (2002) A consensus statement on health care transitions for young adults with special health care needs. Pediatrics 110(6, pt 2):1304–1306 [PubMed] [Google Scholar]
- 35.Le Roux E, Menesguen F, Tejedor I et al (2021) Transition of young adults with endocrine and metabolic diseases: the ‘TRANSEND’ cohort. Endocr Connect 10:21–28. 10.1530/EC-20-0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell F, Biggs K, Aldiss SK et al (2016) Transition of care for adolescents from pediatric services to adult health services. Cochrane Database Syst Rev 4:CD009794. 10.1002/14651858.cd009794.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosen DS, Blum RW, Britto M, Sawyer SM, Siegel DM, Siegel DM Society for Adolescent Medicine (2003) Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health 33(4):309–311. 10.1016/s1054-139x(03)00208-8 [DOI] [PubMed] [Google Scholar]
- 38.Shrewsbury VA, Baur LA, Nguyen B, Steinbeck KS (2014) Transition to adult care in adolescent obesity: a systematic review and why it is a neglected topic. Int J Obes 38(4):475–479. 10.1038/ijo.2013.215 [DOI] [PubMed] [Google Scholar]
- 39.Poitou C, Holland A, Höybye C, de Graaff LCG, Bottius S, Otterlei B, Tauber M (2022) The transition from pediatric to adult care in individuals with Prader–Willi syndrome. Endocr Connect. 10.1530/erc-22-0373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American College of Pediatrics. Condition-specific tools. 10.1542/peds.2022-057267L. https://www.acponline.org/clinical-information/high-value-care/resources-for-clinicians/pediatric-to-adult-care-transitionsinitiative/condition-specific-tools. Accessed 29 Jan 2024
- 41.Wood DL, Sawicki GS, Miller MD, Smotherman C, Lukens-Bull K, Livingood WC, Ferris M, Kraemer DF (2014) The transition readiness assessment questionnaire (TRAQ): its factor structure, reliability, and validity. Acad Pediatr. 10.1016/j.acap.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 42.Vakharia JD, Stanley TL (2023) Facilitating the transition from paediatric to adult care in endocrinology: a focus on growth disorders. Curr Opin Endocrinol Diabetes Obes 30(1):32–43. 10.1097/med.0000000000000785 [DOI] [PubMed] [Google Scholar]
- 43.Cairo SB, Majumdar I, Pryor A, Posner A, Harmon CM, Rothstein DH, Delivery of Surgical Care Committee of the American Academy of Pediatrics Section on Surgery (2018) Challenges in transition of care for pediatric patients after weight-reduction surgery: a systematic review and recommendations for comprehensive care. Obes Surg 28(4):1149–1174. 10.1007/s11695-018-3138-7 [DOI] [PubMed] [Google Scholar]
- 44.Hampl SE, Hassink SG, Skinner AC, Armstrong SC, Barlow SE, Bolling CF, Avila Edwards KC, Eneli I, Hamre R, Joseph MM, Lunsford D, Mendonca E, Michalsky MP, Mirza N, Ochoa ER, Sharifi M, Staiano AE, Weedn AE, Flinn SK, Lindros J, Okechukwu K (2023) Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics 151(2):e2022060640. 10.1542/peds.2022-060640 [DOI] [PubMed] [Google Scholar]
- 45.Transitions of Care. www.endocrinetransitions.com. Accessed 15 July 2024
- 46.Consensus Statement & Guidelines. https://www.eurospe.org/clinical-practice/clinical-practice-activities/consensus-statement-guidelines/. Accessed 20 Feb 2024
- 47.Singh P, Seth A (2023) Transition of care of pediatric patients with special needs to adult care settings: children with diabetes mellitus and other endocrine disorders. Indian J Pediatr 90(11):1134–1141. 10.1007/s12098-023-04780-w [DOI] [PubMed] [Google Scholar]
- 48.Farpour-Lambert NJ, Baker JL, Hassapidou M, Holm JC, Nowicka P, O’Malley G, Weiss R (2015) Childhood obesity is a chronic disease demanding specific health care—a position statement from the Childhood Obesity Task Force (COTF) of the European Association for the Study of Obesity (EASO). Obes Facts 8:342–349. 10.1159/000441483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Busetto L, Sbraccia P, Vettor R (2022) Obesity management: at the forefront against disease stigma and therapeutic inertia. Eat Weight Disord 27(2):761–768. 10.1007/s40519-021-01217-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donini LM, Dalle Grave R, Caretto A, Lucchin L, Melchionda N, Nisoli E, Sbraccia P, Lenzi A, Cuzzolaro M (2014) From simplicity towards complexity: the Italian multidimensional approach to obesity. Eat Weight Disord 19(3):387–394. 10.1007/s40519-013-0097-9 [DOI] [PubMed] [Google Scholar]
- 51.https://sio-obesita.org/centri-accreditati-sio/. Accessed 16 May 16 2024
- 52.Agenzia Nazionale per i Servizi Sanitari Regionali (2024) Linee di indirizzo per l’attuazione del modello organizzativo delle Case della Comunità Hub. https://www.agenas.gov.it/images/agenas/PNRR/Linee_di_indirizzo_CdC.pdf
- 53.SICOB. Società Italiana di Chirurgia dell’OBesità e delle malattie metaboliche. Accessed 16 May 2024
- 54.Inge TH, Zeller MH, Jenkins TM et al (2014) Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study NIH external link. J Am Medl Assoc Pediatr 168(1):47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inge TH, Courcoulas AP, Xanthakos SA (2016) Weight loss and health status after bariatric surgery in adolescents. N Engl J Med 374(20):1989–1990. 10.1056/NEJMc1602007 [DOI] [PubMed] [Google Scholar]
- 56.Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Brandt ML, Xanthakos SA, Dixon JB, Harmon CM, Chen MK, Xie C, Evans ME, Helmrath MA, Teen–LABS Consortium (2019) Five-year outcomes of gastric bypass in adolescents as compared with adults. N Engl J Med 380(22):2136–2145. 10.1056/NEJMoa1813909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tamborlane WV, Bishai R, Geller D, Shehadeh N, Al-Abdulrazzaq D, Vazquez EM, Karoly E, Troja T, Doehring O, Carter D, Monyak J, Sjöström CD (2022) Once-weekly exenatide in youth with type 2 diabetes. Diabetes Care 45(8):1833–1840. 10.2337/dc21-2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, Mastrandrea LD, Prabhu N, Arslanian S, NN8022-4180 Trial Investigators (2020) A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med 382(22):2117–2128. 10.1056/NEJMoa1916038 [DOI] [PubMed] [Google Scholar]
- 59.Weghuber D, Barrett T, Barrientos-Pérez M, Gies I, Hesse D, Jeppesen OK, Kelly AS, Mastrandrea LD, Sørrig R, Arslanian S, STEP TEENS Investigators (2022) Once-weekly semaglutide in adolescents with obesity. N Engl J Med 387(24):2245–2257. 10.1056/NEJMoa2208601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alman KL, Lister NB, Garnett SP, Gow ML, Aldwell K, Jebeile H (2021) Dietetic management of obesity and severe obesity in children and adolescents: a scoping review of guidelines. Obes Rev 22(1):e13132. 10.1111/obr.13132 [DOI] [PubMed] [Google Scholar]
- 61.Corsello A, Trovato CM, Di Profio E, Cardile S, Campoy C, Zuccotti G, Verduci E, Diamanti A (2023) Ketogenic diet in children and adolescents: The effects on growth and nutritional status. Pharmacol Res 191:106780. 10.1016/j.phrs.2023.106780 [DOI] [PubMed] [Google Scholar]
- 62.Gray WN, Schaefer MR, Resmini-Rawlinson A, Wagoner ST (2018) Barriers to transition from pediatric to adult care: a systematic review. J Pediatr Psychol 43(5):488–502. 10.1093/jpepsy/jsx142 [DOI] [PubMed] [Google Scholar]
- 63.Ishizaki Y, Maru M, Higashino H, Katsumoto S, Egawa K, Yanagimoto Y, Nagahama T (2021) The transition of adult patients with childhood-onset chronic diseases from pediatric to adult healthcare systems: a survey of the perceptions of Japanese pediatricians and child health nurses. Biopsychosoc Med 20(6):8. 10.1186/1751-0759-6-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szalda DE, Jimenez ME, Long JE, Ni A, Shea JA, Jan S (2015) Healthcare system supports for young adult patients with pediatric onset chronic conditions: a qualitative study. J Pediatr Nurs 30(1):126–132. 10.1016/j.pedn.2014.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakurai I, Maru M, Miyamae T, Honda M (2022) Prevalence and barriers to health care transition for adolescent patients with childhood-onset chronic diseases across Japan: a nation-wide cross-sectional survey. Front Pediatr 1(10):956227. 10.1377/hlthaff.27.3.w232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colwill JM, Cultice JM, Kruse RL (2008) Will generalist physician supply meet demands of an increasing and aging population? Health Aff 27(3):w232–w241. 10.1377/hlthaff.27.3.w232 [DOI] [PubMed] [Google Scholar]
- 67.Bowen ME, Henske JA, Potter A (2010) Health care transition in adolescents and young adults with diabetes. Clin Diabetes 28(3):99–106. 10.2337/diaclin.28.3.99 [Google Scholar]
- 68.Tsamasiros J, Bartsocas CS (2002) Transition of the adolescent from the children’s to the adults’ diabetes clinic. J Pediatr Endocrinol Metab 15(4):363–367. 10.1515/jpem.2002.15.4.363 [DOI] [PubMed] [Google Scholar]
- 69.Rapley P, Davidson PM (2010) Enough of the problem: a review of time for health care transition solutions for young adults with a chronic illness. J Clin Nurs 19(3–4):313–323. 10.3233/prm-140269 [DOI] [PubMed] [Google Scholar]
- 70.Fernandes SM, O’Sullivan-Oliveira J, Landzberg MJ, Khairy P, Melvin P, Sawicki GS, Ziniel S, Kenney LB, Garvey KC, Sobota A, O’Brien R, Nigrovic PA, Sharma N, Fishman LN (2014) Transition and transfer of adolescents and young adults with pediatric onset chronic disease: the patient and parent perspective. J Pediatr Rehabil Med 7(1):43–51. 10.3233/prm-140269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nelson MC, Story M, Larson NI, Neumark-Sztainer D, Lytle LA (2008) Emerging adulthood and college-aged youth: an overlooked age for weight-related behavior change. Obesity 16(10):2205–2211. 10.1038/oby.2008.365 [DOI] [PubMed] [Google Scholar]
- 72.Lawrence JM, Yi-Frazier JP, Black MH, Anderson A, Hood K, Imperatore G, Klingensmith GJ, Naughton M, Mayer-Davis EJ, Seid M, SEARCH for Diabetes in Youth Study Group (2012) Demographic and clinical correlates of diabetes-related quality of life among youth with type 1 diabetes. J Pediatr 161(2):201–272. 10.1016/j.jpeds.2012.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC (2018) The age of adolescence. Lancet Child Adolesc Health 2(3):223–228. 10.1016/s2352-4642(18)30022-1 [DOI] [PubMed] [Google Scholar]
- 74.Bertelloni S (2011) Adolescentologia Percorsi medici e socio-educativi. Tecniche Nuove Ed., Milano [Google Scholar]
- 75.Lugasi T, Achille M, Stevenson M (2011) Patients’ perspective on factors that facilitate transition from child-centered to adult-centered health care: a theory integrated metasummary of quantitative and qualitative studies. J Adolesc Health 48(5):429–440. 10.1016/j.jadohealth.2010.10.016 [DOI] [PubMed] [Google Scholar]
- 76.Pape L, Ernst G (2022) Health care transition from pediatric to adult care: an evidence-based guideline. Eur J Pediatr 181(5):1951–1958. 10.1007/s00431-022-04385-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guidi C, Traversa C (2021) Empathy in patient care: from ‘clinical empathy’ to ‘empathic concern.’ Med Health Care Philos 24(4):573–585. 10.1007/s11019-021-10033-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Badour B, Bull A, Gupta AA et al (2023) Parental involvement in the transition from paediatric to adult care for youth with chronic illness: a scoping review of the North American Literature. Int J Pediatr. 10.1155/2023/9392040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Colver A, Rapley T, Parr JR et al (2020) Facilitating transition of young people with long-term health conditions from children’s to adults’ healthcare services—implications of a 5-year research programme. Clin Med 20(1):74–80. 10.7861/clinmed.2019-0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cassidy M, Doucet S, Luke A, Goudreau A, MacNeill L (2022) Improving the transition from paediatric to adult healthcare: a scoping review on the recommendations of young adults with lived experience. BMJ Open. 10.1136/bmjopen-2021-051314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ferris M, Wood D, Ferris M et al (2011) Toward evidence-based health care transition: the health care transition research consortium. Int J Child Adolesc Health 3(4):479–486. 10.3233/prm-140277 [Google Scholar]
- 82.Society for Adolescent Health and Medicine (2020) Transition to adulthood for youth with chronic conditions and special health care needs. J Adolesc Health 66(5):631–634. 10.1016/j.jadohealth.2020.02.006 [DOI] [PubMed] [Google Scholar]
- 83.Bert F, Camussi E, Gili R et al (2020) Transitional care: a new model of care from young age to adulthood. Health Policy 124(10):1121–1128. 10.1016/j.healthpol.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 84.Doucet S, Splane J, Luke A et al (2022) Programmes to support paediatric to adult healthcare transitions for youth with complex care needs and their families: a scoping review. Child Care Health Dev 48(5):659–692. 10.1111/cch.12984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lykkeberg B, Noergaard MW, Bjerrum M (2024) Experiences and expectations of parents when young people with congenital heart disease transfer from pediatric to adult care: a qualitative systematic review. J Child Health Care 8:13674935241231024. 10.1177/13674935241231024 [DOI] [PubMed] [Google Scholar]
- 86.Schwartz LA, Brumley LD, Tuchman LK, Barakat LP, Hobbie WL, Ginsberg JP, Daniel LC, Kazak AE, Bevans K, Deatrick JA (2013) Stakeholder validation of a model of readiness for transition to adult care. JAMA Pediatr 167(10):939–946. 10.1001/jamapediatrics.2013.2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thomsen EL, Esbensen BA, Hanghøj S, Hansson H, Boisen KA (2022) Development of a complex intervention to support parents of adolescents with chronic illness transferring from pediatrics to adult care (ParTNerSTEPs). BMC Health Serv Res 22(1):485. 10.1186/s12913-022-07888-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McManus MA, Pollack LR, Cooley WC, McAllister JW, Lotstein D, Strickland B, Mann MY (2013) Current status of transition preparation among youth with special needs in the United States. Pediatrics 131(6):1090–1097. 10.1542/peds.2012-3050 [DOI] [PubMed] [Google Scholar]
- 89.Sharma N, O’Hare K, Antonelli RC, Sawicki GS (2014) Transition care: future directions in education, health policy, and outcomes research. Acad Pediatr 14(2):120–127. 10.1016/j.acap.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Volertas SD, Rossi-Foulkes R (2017) Using quality improvement in resident education to improve transition care. Pediatr Ann 46(5):e203–e206. 10.3928/19382359-20170426-01 [DOI] [PubMed] [Google Scholar]
- 91.Kuo AA, Ciccarelli MR, Sharma N, Lotstein DS (2018) A health care transition curriculum for primary care residents: identifying goals and objectives. Pediatrics 141(Suppl 4):S346–S354. 10.1542/peds.2016-4300l [DOI] [PubMed] [Google Scholar]
- 92.Health Care Transition for Adolescents and Young Adults (2022) An Online Video CME/CEU Series Available at https://www.hscsnlearning.org/transition/. Accessed 10 Feb 2024
- 93.Education & Training for Health Care Professionals. https://www.floridahats.org/education-training-for-health-care-professionals/. Accessed 10 Feb 2024
- 94.Esposito S, Rosafio C, Antodaro F, Argentiero A, Bassi M, Becherucci P, Bonsanto F, Cagliero A, Cannata G, Capello F, Cardinale F, Chiriaco T, Consolaro A, Dessì A, Di Mauro G, Fainardi V, Fanos V, Guarino A, Li Calzi G, Lodi E, Maghnie M, Manfredini L, Malorgio E, Minuto N, Modena MG, Montori R, Moscatelli A, Patrone E, Pescio E, Poeta M, Ravelli A, Spelta M, Suppiej A, Vai S, Villa L, Zanini R, Botti R, Gaddi AV (2023) Use of telemedicine healthcare systems in children and adolescents with chronic disease or in transition stages of life: consensus document of the Italian Society of Telemedicine (SIT), of the Italian Society of Preventive and Social Pediatrics (SIPPS), of the Italian Society of Pediatric Primary Care (SICuPP), of the Italian Federation of Pediatric Doctors (FIMP) and of the Syndicate of Family Pediatrician Doctors (SIMPeF). J Pers Med 13(2):235. 10.3390/jpm13020198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Apply the (MyTransition) App In Transition (ApplyIT). https://clinicaltrials.gov/study/NCT03188224. Accessed 15 Feb 15 2024
- 96.South K, George M, Smaldone A (2022) Gaps in transition readiness measurement: a comparison of instruments to a conceptual model. J Transit Med 4(1):20220002. 10.1515/jtm-2022-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McManus M, White P, Borden C. Incorporating Pediatric-To-Adult Transition into NCQA Patient-Centered Medical Home Recognition: 2019 Update
- 98.Wagner EH, Bennett SM, Austin BT, Greene SM, Schaefer JK, Vonkorff M (2005) Finding common ground: patient-centeredness and evidence-based chronic illness care. J Altern Complement Med 11:S7–S15. 10.1016/j.acap.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 99.Grudniewicz A, Gray CS, Boeckxstaens P, De Maeseneer J, Mold J (2023) Operationalizing the chronic care model with goal-oriented care. Patient 16(6):569–578. 10.1007/s40271-023-00645-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rojas Smith L, Ashok M, Morss Dy S, Wines RC, Teixeira-Poit S (2014) Contextual frameworks for research on the implementation of complex system interventions. Report No.: 14-EHC014-EF. Agency for Healthcare Research and Quality (US), Rockville [PubMed]
- 101.Úcar Martínez X, Jiménez-Morales M, Soler Masó P, Trilla Bernet J (2017) Exploring the conceptualization and research of empowerment in the field of youth. Int J Adolesc Youth 22(4):405–418. 10.1080/02673843.2016.1209120 [Google Scholar]
- 102.Ciemins EL, Brant J, Kersten D, Mullette E, Dickerson D (2016) Why the interdisciplinary team approach works: insights from complexity science. J Palliat Med 19(7):767–770. 10.1089/jpm.2015.0398 [DOI] [PubMed] [Google Scholar]
- 103.Romkey-Sinasac C, Saunders S, Galica J (2021) Canadian resources, programs, and models of care to support cancer survivors’ transition beyond treatment: a scoping review. Curr Oncol 28(3):2134–2145. 10.3390/curroncol28030198 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.