Abstract
In an ageing society, the incidence of hard‐to‐heal wounds is rising. Chronic wound healing is a complex process, which requires specialised treatment. Clinical assessment of the wound is essential to establish care approaches but is usually based on visual evaluation and it remains challenging. Therefore, innovative quantitative methods for the assessment of chronic wounds are needed. We conducted a single‐centre observational study designed to assess the feasibility of a bioimpedance measurement method conducted with a multielectrode sensor array to monitor the wound healing process in patients with chronic wounds of venous, mixed venous–arterial and diabetic aetiology. In total, 104 measurements of bioimpedance were conducted in 18 ulcers during the study. Across all 7 patients analysed, the bioimpedance of the ulcers was consistently increasing as the wound surface was decreasing. The variables had significant (p < 0.001) and strong negative correlation (r = −0.86). We validated the feasibility of the bioimpedance measurement method for the monitoring of the wound healing process on the lower legs. It may be a promising quantitative method for monitoring the status of the wounds. However, long‐term measurements are needed to show the usability of the electrode dressing and bioimpedance measurement in the assessment of chronic wounds.
Keywords: bioimpedance, chronic wounds, electrode array, monitoring
1. INTRODUCTION
In an ageing society, hard‐to‐heal wounds incidence is rising and in Europe alone this condition affects around 1.5–2 million people. 1 , 2 Long‐term treatment and consumption of immense amounts of medical products create substantial costs to healthcare systems. 1
Chronic wound healing is a complex process, which requires proper and specialised treatment. 3 Clinical assessment of the wound is essential to establish care approaches, both local and systemic. Numerous parameters should be assessed including tissue types in the wound, extent, area and exudate to treat the wound according to TIMERS (T: Tissue management; I: Inflammation and Infection; M: Moisture balance; E: Epithelial Edge; R: Regeneration, Repair of tissue; S: Social factors.) principle. 4
Moreover, performing the assessment requires dressing removal, which may lead to the destruction of the regenerating epithelium. 5 The clinical assessment and management of chronic wounds remains challenging, therefore, innovative quantitative methods for assessment and monitoring chronic wounds are needed.
Many novel medical devices for monitoring wound healing progress have received much attention in recent years. A variety of sensors have been presented assessing different parameters such as temperature, pH, the concentration of C‐reactive protein, level of moisture in wounds and other physical parameters for example bioimpedance. 6 , 7 , 8 Sensors measuring bioimpedance in the wound have shown promising results for wound monitoring. 9 , 10 , 11 , 12 , 13 , 14
Bioimpedance describes the ability of a tissue to oppose the flow of alternating electrical current. The outermost layer of the epidermis (stratum corneum) consists of flattened dead keratinocytes and is characterised by higher impedance and lower conductivity. Deeper to the epidermis layers (dermis and the subcutaneous tissue), provide significantly higher electrical conductivity. 15 As the wound heals, bioimpedance gradually increases and eventually reaches the level of the intact skin, 10 therefore, it can be used as a parameter for the monitoring of the wound healing process. Even a minor abrasion induces a discernible elevation in skin conductivity. Consequently, Kekonen et al. developed a method and system based on changes in bioimpedance to evaluate the state of wound healing. 10 , 15 , 16 , 17 In 2021, the results of the first clinical proof‐of‐concept study were published. 16
In current clinical practice of chronic wounds monitoring the assessment of wounds is mainly based on visual evaluation, which can be subjective and vary depending on the experience of the medical personnel. Since wound bioimpedance has been shown to increase during the healing process, it may be proposed as a non‐interfering monitoring modality. Whilst these findings appear promising as a quantitative means of monitoring the condition of chronic ulcers, the validation of this method is needed. Thus, the primary objective of our study was to establish the feasibility of the proposed method through a clinical proof‐of‐concept conducted on patients with chronic ulcers. The secondary objective of the study was to confirm the correlation between the increase of bioimpedance in wounds and the decrease of the wound surface area during the process of wound healing.
2. MATERIALS AND METHODS
2.1. Study group
A single‐centre observational study was designed to assess the feasibility of a bioimpedance measurement method conducted with a multielectrode sensor array to monitor the wound healing process in patients with chronic wounds of venous, mixed venous–arterial or diabetic aetiology. The study was performed in compliance with the ethical principles of the Declaration of Helsinki. The Ethical Committee Approval was obtained on 22 September 2021 by the Independent Bioethics Committee for Scientific Research at the Medical University of Gdansk (number NKBBN/700/2020‐2021). Patients enrolled in the study gave informed consent prior to the inclusion.
The study included eligible participants who met the inclusion criteria. Criteria for recruitment comprised adult patients of both genders with ulcers present for more than 4 weeks, of width less than 5 cm, intact skin around the wound and moderate exudate. Patients having an infection, being pregnant or breastfeeding were excluded.
Patients' visits were held at the General Surgery Outpatient Clinic at the University Clinical Centre in Gdansk, Poland between April 2022 and April 2023. The ulcers were measured one to two times a week, every 2 weeks or less frequently, when the patient's condition was not good enough to attend a follow‐up visit to the Clinic. At each visit, clinical parameters such as wound depth, size and appearance, and the presence of fibrin or granulation tissue buds, were registered.
2.2. Measurement instrumentation
The bioimpedance measurement instrumentation consisted of a perforated multielectrode sensor array (electrode dressing manufactured by CutoSense Ltd.), and a bioimpedance measurement system for wound monitoring, which comprised a bioimpedance measurement device unit and a mobile phone application for the data collection and storage (presented in Figure 1). The sensor array is specifically designed for monitoring venous ulcers located above the ankle level. A detailed description of the device is explained elsewhere. 16 A wound dressing was made of a highly perforated, transparent and film‐like material (thermoplastic polyurethane) on which small carbon‐ink‐coated electrodes were printed and covered with a solid hydrogel. The circular‐shaped electrodes localised in the centre of the sensor array are in contact with the wound or skin and are encircled by four larger counter electrodes, which must be placed on intact skin. The measuring device was connected to the extension of the electrode dressing for the bioimpedance measurements. The impedance values measured by the electrodes were transmitted to the phone via Bluetooth and utilised to determine the Wound Status Index (WSI). The WSI is the ratio of impedances measured by electrodes of the bioimpedance sensor array and compared to a constant impedance value measured on the intact skin. Description of calculations is explained elsewhere by Kekonen et al. 16 The mobile application displayed the measurement results as the percentage value of the WSI and a graphic impedance map of the area of electrode dressing.
FIGURE 1.
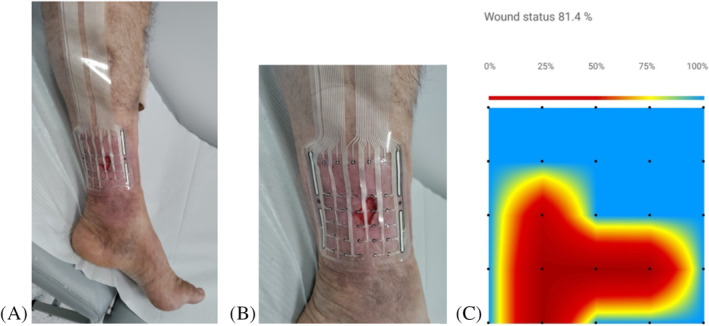
(A) Picture of a perforated multielectrode sensor array placed on the wound localised on the calf. (B) The circular shaped electrodes localised in the centre are in contact with the wound or skin, and are encircled by four larger counter electrodes, which must always be on intact skin. (C) Presentation of measurement results as a graphic impedance map: a screenshot of a mobile application. The blue colour represents intact skin, and red or yellow describes the wound area.
2.3. Procedures during visits in outpatient clinic
Prior to wound assessment and before each bioimpedance measurement, ulcers were treated according to the TIMERS strategy. 4 Antiseptic with hypochlorous acid and sodium hypochlorite was applied to the wound area. Debridement was conducted during every visit until a significant reduction of slough tissue was achieved or healthy tissue was exposed. The multielectrode sensor array was positioned directly on the wound and surrounding intact skin and covered with gauze. The contact of electrodes was ensured by either pressing gauze by researcher's hand or compression therapy, depending on clinical indications. After the measurement, the sensor array was usually taken off. To ensure a standardisation process across measured wounds black dots with markers were made on the skin in the dedicated holes at the edges of the sensor array, therefore, the sensor array was placed in the exact same place during every control visit based on the wound photographs. Wound management procedures were performed according to clinical status and TIMERS in each patient.
2.4. Wound size evaluation
Evaluation of the wound size was based on digital planimetry of wound surface. Photographs of wounds with a paper ruler next to the wound were taken with a high‐resolution mobile phone camera perpendicularly to the wound. The margins of wounds were outlined manually on the pictures by the researcher. The wound surface area [mm2] was determined by the analysis of wound surfaces in AutoCAD software.
2.5. Statistics
The number of subjects enrolled in the study was determined based on a clinical proof‐of‐concept study. 16 The primary aim of the study was to establish the feasibility of the proposed method for patients with chronic ulcerations, and thus no power calculation was performed. For the secondary outcome – correlation of WSI to wound surface – in a post‐hoc analysis with 91 measurements, at an alpha level of 0.05 and considering a coefficient of −0.7 for strong negative correlation the analysis that was performed was powered at the level of 0.98, what is sufficient to draw conclusions. GraphPad Prism software was used for conducting the statistical analyses. Pearson's linear correlation analysis was applied in the evaluation of the relationship between the wound surface area and the WSI for each wound. p‐value <0.05 was considered to indicate a statistically significant difference and the strength of the relationship was evaluated by the value of correlation coefficient.
3. RESULTS
Eleven patients were recruited to the study: three males and eight females with an age range from 37 to 93 years (mean 66.9 ± 14.8 years). Eighteen wounds were measured. The etiologies (and numbers) of the wounds were venous (9), mixed venous–arterial (4), neurotrophic (2), and acute (3), whereas the latter appeared on the skin surrounding venous ulcer during the treatment process. Two wounds were localised on the foot (lateral surface and foot stump after toe amputation), and 16 wounds were on the lower leg above the ankle. Patients' detailed descriptions including comorbidities are presented in Table 1.
TABLE 1.
Demographic characteristics of patients with chronic wounds.
| Number of wounds | Number of patients (ulcer) | Age (years), Sex | Comorbidities | Type of ulcer | Localisation of wound | Date of start | Date of end | Duration of observation (days) | Number of measurements |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 37, M | DM t.1 | N | F | 2022‐04‐11 | 2023‐06‐15 | 66 | 5 |
| 2 | 2 | 71, F | CVI | V | LL | 2022‐04‐20 | LTFU | 1 | 1 |
| 3 | 3 | 81, F | CVI, LC | M | LL | 2022‐11‐03 | 2023‐01‐05 | 65 | 10 |
| 4 | 4 | 83, F | CVI | V | LL | 2022‐11‐08 | 2022‐11‐15 | 7 | 2 |
| 5 | 5 (u1) | 93, F | CVI, HT, DM t.2, HypoT | M | LL | 2022‐11‐21 | 2023‐02‐16 | 88 | 5 |
| 6 | 5 (u2) | 93, F | CVI, HT, DM t.2, HypoT | M | LL | 2022‐11‐21 | 2023‐02‐16 | 88 | 5 |
| 7 | 6 | 72, F | CVI, HT, DM t.2, HypoT | V | LL | 2022‐11‐28 | 2022‐12‐05 | 7 | 2 |
| 8 | 7 | 76, F | CVI, HT, DM t.2 | V | LL | 2022‐12‐05 | LTFU | 1 | 1 |
| 9 | 8 | 75, F | CVI, HT, AT | M | LL | 2022‐12‐06 | 2022‐12‐14 | 8 | 2 |
| 10 | 9 | 52, M | DM t.2 | N | F | 2022‐12‐07 | LTFU | 1 | 1 |
| 11 | 10 | 51, M | CVI | V | LL | 2022‐12‐14 | 2023‐02‐14 | 63 | 4 |
| 12 | 11 (u1) | 60, M | CVI | V | LL | 2022‐12‐07 | 2023‐02‐15 | 77 | 17 |
| 13 | 11 (u2) | 60, M | CVI | V | LL | 2022‐12‐12 | 2023‐02‐15 | 70 | 16 |
| 14 | 11 (u3) | 60, M | CVI | V | LL | 2022‐12‐12 | 2023‐02‐15 | 70 | 16 |
| 15 | 11 (u4) | 60, M | CVI | Ac | LL | 2023‐01‐05 | 2023‐01‐11 | 7 | 3 |
| 16 | 11 (u5) | 60, M | CVI | Ac | LL | 2023‐01‐11 | 2023‐01‐13 | 4 | 2 |
| 17 | 11 (u6) | 60, M | CVI | Ac | LL | 2023‐01‐11 | 2023‐01‐27 | 16 | 4 |
| 18 | 11 (u7) | 60, M | CVI | V | LL | 2023‐03‐22 | 2023‐04‐19 | 28 | 8 |
Note: (a) Sex: F, female; M, male. (b) Comorbidities: CVI, chronic vein insufficiency; HT, hypertension; DM t.1/t.2, diabetes mellitus type 1/type 2; HypoT, hypothyroidism; AT, atherosclerosis; LC, lung cancer. (c) Type of ulcer: N, neuropathic; V, venous; M, mixed; Ac, acute. (d) Localisation: F, foot; LL, lower leg. (e) LTFU, lost to follow up.
In total, 104 measurements of bioimpedance were conducted during the study. Results of 4 patients were excluded from further analyses due to reasons such as too deep wound to provide good contact of electrodes to the skin and to measure bioimpedance (1 patient) or loss to follow up after the first visit to outpatient clinic and only one bioimpedance measurement (3 patients). Fourteen wounds in total having 91 measurements were statistically analysed. The time range of follow‐up duration was 4 days up to 88 days and the median duration of observation was 45.5 (7–71.75) days.
Across all 7 patients analysed, the bioimpedance (the WSI) of the ulcers was consistently increasing as the wound surface decreased. The scatter plot presented in Figure 2 contains the wound surface area and the WSI data from 14 ulcers of the study. The variables had significant and strong negative correlation, the Pearson correlation coefficient was r = −0.86, p < 0.001.
FIGURE 2.
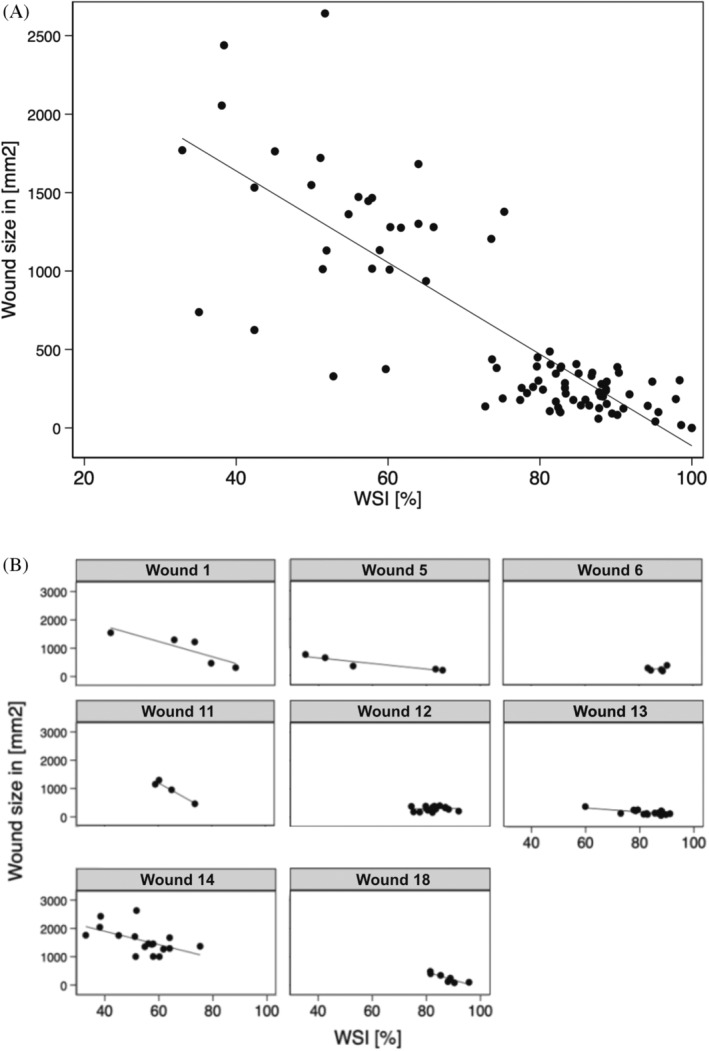
The wound surface area and the WSI data from (A) 14 ulcers in total and (B) separate results of 8 wounds, which had more than 3 measurements.
Three acute wounds, which appeared in the skin surrounding the patient's number 11 wounds during the treatment process were statistically analysed only together with chronic wounds.
In patient number 1 and patient number 11 (ulcer number 7) compression therapy was applied and electrode dressings were left underneath the primary dressing until the next dressing changed within 3 days, therefore bioimpedance measurements were conducted also at the beginning of the next control visits. In 9 patients electrode dressings were taken off from the wound right after the measurements. In patients who had electrode dressing placed on the wound underneath the primary dressing for a longer period, no adverse events were observed. In patients with ulcerations on the foot, challenges with adherence to the dressing were faced due to the geometry of the foot and due to the design of the sensor array, which was intended to be placed on a larger straight surface area. Even though bandages were applied to ensure good fixation of the dressing, the dressing was slipping off the wound surface, which made it impossible to perform a bioimpedance measurement during the next visit without changing the electrode dressing to a new one.
In patients who did not have compression therapy, it emerged that after putting an electrode sensor array on the wound, covering it with gauze and pressing only for 1–3 min, in some cases it was not enough to conduct a proper bioimpedance measurement as the electrodes did not adhere properly to the wound and skin surface, and the measurements had to be repeated at least 2 times. In patients who had sensor arrays underneath the primary dressing, and the contact of the electrodes to the wound and skin had a proper pressure, bioimpedance measurements were conducted properly and one measurement was enough to collect data.
4. DISCUSSION
Analysis of skin bioimpedance has been of interest to many researchers and a wide range of different devices measuring bioimpedance have been developed recently: a ‘wound mapping’ device based on electrical impedance spectroscopy analysis by Weber et al., 12 a bandage‐like electronic sensor was by Liao et al., 18 a custom made sensor‐sock for assessment of diabetic peripheral neuropathy using skin impedance by Tronstad et al., 11 and a flexible wireless electronic system for in situ and real‐time monitoring the bioimpedance of wounded skin by Pei. 19 Novel quantitative tools and methods aim to enable medical personnel conduct an objective assessment of the wound and make relevant adjustments in the treatment process. Moreover, these techniques move towards the analysis of wounds underneath the primary dressings, which may lead to the reduction of unnecessary dressing removal and may reduce dressing changes. It is anticipated above‐mentioned devices will make an impact on telemedicine and will enable patients to send detailed data about the status of their wounds from home, which may potentially reduce the number of visits in outpatient clinics as the clinical decisions might be made remotely.
Results presented by Kekonen et al. in a clinical proof‐of‐concept showed the feasibility of a bioimpedance‐based method for monitoring venous ulcers. 16 In our study, we have validated the feasibility of this method for the evaluation of 14 chronic wound changes of the surface in chronic ulcers in two localisations: lower leg and foot. A statistically significant strong linear correlation was found between wound surface area and the WSI. Our results support the hypothesis that bioimpedance measurements of the skin can be a promising method used as an assessment tool for the wound healing process.
The main limitation of this study is the varying number of measurements for specific patients with low numbers of measurements for some. This may have introduced bias into the calculation of the correlation coefficient. However, to mitigate this we have used analytical weights by the number of measurements for each wound. Furthermore, this situation increases the clinical generalisability of the results since a varying number of visits/measurements per wound resembles a real‐world situation in the treatment of chronic wounds.
Power analysis of the study sample was not warranted as the primary aim of the study was to establish the feasibility of the proposed method. However, as outlined in methods a post‐hoc power analysis has shown that we had sufficient power to detect strong correlation.
In patients with wounds on the lower leg electrodes adhered well to the skin, which enabled us to collect proper measurement data. However, did not adhere well to the foot region. To overcome the sensor array's placement‐related issues when measuring wounds typically located on the foot area, a specific sensor array with appropriate geometry and dimensions should be designed.
Another limitation of quantitative analyses presented in the study was the sub‐optimal measurement technique due to the medical personnel learning curve as it was one of the first implementations of the bioimpedance sensor in the clinical environment. The manual outlining of the wound margins in AutoCAD software can introduce variability, however it was conducted by a single researcher to ensure reproducibility.
Variations in pressure during the data collection process due to different techniques (pressure applied by the researcher's hand vs. pressure applied by compression therapy) may have influenced the measurements, as highlighted by Lukaski and Moore. 13 They emphasised the importance of ensuring optimal electrode contact near the wound. Interestingly, the use of compression therapy appears to enhance electrode adhesion more effectively than when gauze is held by the researcher. To ensure good electrode contact not only the pressure is essential, but also the type of wound (superficial) and its size (at least two electrodes should cover the wound to collect more valuable data).
Even though dots with marker were done on the skin in the dedicated holes at the edges of the sensor array, placement of the electrodes may vary due to the decrease of oedema and calf diameter.
The presented limitations may impair drawing conclusions from the quantitative analyses presented in the study. However, the primary aim of the study was to assess the feasibility of clinical use of the method and determine and resolve possible technical issues, and what has been done. Furthermore, since a significant strong correlation was shown it can be considered a valid clinical conclusion despite the technical limitations.
Based on the present findings, it is warranted to undertake additional studies focusing on a cohort comprising patients with wounds of identical aetiology situated on the lower extremity above the ankle. This would help demonstrate the efficacy of the method, particularly in patients with chronic vein insufficiency, where compression therapy stands as the gold standard for the treatment of lower extremity wounds.
The results presented in our paper support the feasibility of bioimpedance measurement methods for monitoring the wound healing process on the lower legs. Long‐term measurements are needed to show the usability of the electrode dressing and bioimpedance measurement in the assessment of chronic wounds.
FUNDING INFORMATION
Bioimpedance measurement devices and mobile phones were provided by CutoSense Oy. This work was supported by an EWMA research grant supported by Convatec (2021).
CONFLICT OF INTEREST STATEMENT
AK and JV collaborate with CutoSense Ltd. PS and MA took part in the study sponsored by CutoSense Ltd. No competing financial interests exist for other authors. The content of this article was expressly written by the authors listed. No ghost‐writers were used to write this article.
Antoszewska M, Spychalski P, Kekonen A, Viik J, Barańska‐Rybak W. Bioimpedance sensor array for monitoring chronic wounds: Validation of method feasibility. Int Wound J. 2024;21(8):e14899. doi: 10.1111/iwj.14899
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Olsson M, Järbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: a systematic review. Wound Repair Regen off Publ Wound Heal Soc Eur Tissue Repair Soc. 2019;27(1):114‐125. doi: 10.1111/wrr.12683 [DOI] [PubMed] [Google Scholar]
- 2. Lindholm C, Searle R. Wound management for the 21st century: combining effectiveness and efficiency. Int Wound J. 2016;13:5‐15. doi: 10.1111/iwj.12623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bowers S, Franco E. Chronic wounds: evaluation and management. Am Fam Physician. 2020;101(3):159‐166. [PubMed] [Google Scholar]
- 4. Atkin L, Bućko Z, Montero EC, et al. Implementing TIMERS: the race against hard‐to‐heal wounds. J Wound Care. 2019;23(3):S1‐S52. doi: 10.12968/jowc.2019.28.sup3a.s1 [DOI] [PubMed] [Google Scholar]
- 5. Matsumura H, Ahmatjan N, Ida Y, Imai R, Wanatabe K. A model for quantitative evaluation of skin damage at adhesive wound dressing removal. Int Wound J. 2013;10:291‐294. doi: 10.1111/j.1742-481X.2012.00975.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tessarolo M, Possanzini L, Gualandi I, et al. Wireless textile moisture sensor for wound care. Front Phys. 2021;9:9. doi: 10.3389/fphy.2021.722173 [DOI] [Google Scholar]
- 7. Salvo P, Dini V, Kirchhain A, et al. Sensors and biosensors for C‐reactive protein, temperature and pH, and their applications for monitoring wound healing: a review. Sensors (Basel). 2017;17(12):2952. doi: 10.3390/s17122952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang N, Zheng Y, Jiang X, et al. Wearable sensors and Systems for Wound Healing‐Related pH and temperature detection. Micromachines. 2021;12(4):430. doi: 10.3390/mi12040430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weber SA, Vonhoff PA, Owens FJ, Byrne JA, McAdams ET. Development of a multi‐electrode electrical stimulation device to improve chronic wound healing. 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE; 2009. Published online 2009:2145‐2148. doi: 10.1109/IEMBS.2009.5333963 [DOI] [PubMed] [Google Scholar]
- 10. Kekonen A, Bergelin M, Johansson M, Joon NK, Bobacka J, Viik J. Bioimpedance sensor array for long‐term monitoring of wound healing from beneath the primary dressings and controlled formation of H2O2 using low‐intensity direct current. Sens Switz. 2019;19(11):2505. doi: 10.3390/s19112505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tronstad C, Amini M, Olesen E, et al. Diabetic foot assessment using skin impedance in a custom made sensor‐sock. J Electr Bioimpedance. 2022;13(1):136‐142. doi: 10.2478/joeb-2022-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weber SA, Watermann N, Jossinet J, et al. Remote wound monitoring of chronic ulcers. IEEE Trans Inf Technol Biomed Publ IEEE Eng Med Biol Soc. 2010;14(2):371‐377. doi: 10.1109/TITB.2010.2042605 [DOI] [PubMed] [Google Scholar]
- 13. Lukaski HC, Moore M. Bioelectrical impedance assessment of wound healing. J Diabetes Sci Technol. 2012;6(1):209‐212. doi: 10.1177/193229681200600126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Swisher SL, Lin MC, Liao A, et al. Impedance sensing device enables early detection of pressure ulcers in vivo. Nat Commun. 2015;6:6. doi: 10.1038/ncomms7575 [DOI] [PubMed] [Google Scholar]
- 15. Kekonen A, Bergelin M, Eriksson JE, Vaalasti A, Ylänen H, Viik J. Bioimpedance measurement based evaluation of wound healing. Physiol Meas. 2017;38(7):1373‐1383. doi: 10.1088/1361-6579/aa63d6 [DOI] [PubMed] [Google Scholar]
- 16. Kekonen A, Bergelin M, Eriksson JE, et al. Bioimpedance method for monitoring venous ulcers: clinical proof‐of‐concept study. Biosens Bioelectron. 2021;178:178. doi: 10.1016/j.bios.2021.112974 [DOI] [PubMed] [Google Scholar]
- 17. Kekonen A, Bergelin M, Eriksson JE, Vesa M, Johansson M, Viik J. Long‐term monitoring of acute wound healing from beneath the primary wound dressings. Proc Bienn Balt Electron Conf BEC. 2018;2018(October):1‐4. doi: 10.1109/BEC.2018.8600956 [DOI] [Google Scholar]
- 18. Liao A, Lin MC, Ritz LC, et al. Impedance sensing device for monitoring ulcer healing in human patients. Proc Annu Int Conf IEEE Eng Med Biol Soc EMBS. 2015;2015(November):5130‐5133. doi: 10.1109/EMBC.2015.7319546 [DOI] [PubMed] [Google Scholar]
- 19. Pei X, Jin H, Dong S, et al. Flexible wireless skin impedance sensing system for wound healing assessment. Vacuum. 2019;168:168. doi: 10.1016/j.vacuum.2019.108808 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


