Abstract
Background
Obtaining intravenous access in hypotensive patients is challenging and may critically delay resuscitation. The Graduated Vascular Access for Hypotensive Patient (GAHP) protocol leverages intraosseous fluid boluses to specifically dilate proximal veins. This study aims to evaluate the efficacy of GAHP in maximizing venous targets through early distal intraosseous access and a small fluid bolus.
Methods
This was a prospective randomized cadaveric pilot study to evaluate extremity venous engorgement during intraosseous infusion. Cadavers (n = 23) had an intraosseous needle inserted into four sites: distal radius, proximal humerus, distal femur, and distal tibia. Intraosseous saline was rapidly infused, venous optimization was measured using real-time ultrasound. Primary outcome was maximum vessel circumference increase with intraosseous infusion. Secondary outcomes were: time to maximum circumference, and infusion volume required. Statistical analyses included Levene’s test for equality of variances, Wilcoxon signed-rank test, and generalized estimating equation.
Results
There was a significant mean increase of 1.03 cm (95% CI 0.86, 1.20), representing a difference of 102%. We found no significant difference in time to optimize vessel circumference across sites, but volume required significantly differed.
Conclusion
GAHP quickly and effectively increased the circumference of anatomically adjacent veins. Anatomical sites did not differ on time to reach maximum enlargement of vessels following intraosseous infusion but did differ in terms of volume required to maximize vessel circumference. Further research is needed using live, hypotensive patients.
Keywords: Graduated vascular access, Hypotensive, Intraosseous, Resuscitation, Shock
Introduction
Vascular access during resuscitation remains a critical challenge.1 In situations of profound hypotension, traditional vascular access often proves difficult, time consuming, and stressful.1, 2, 3 Traditional methods of emergent peripheral and central venous cannulation frequently carry the limitations of high failure rates, necessity of repeated attempts, protracted delays in treatment, and operator-related stress.4 Although intraosseous (IO) access has proven faster and more successful than traditional intravenous (IV) methods for hypotensive presentations, often acting as a bridge to definitive peripheral or central venous access, it has not been established as a long-term solution for vascular access.5, 6, 7, 8, 9, 10, 11 This is attributed to a lack of confidence in IO patency over prolonged cannulation, concern for dislodgement, varied flow rates, patient discomfort, and a lack of operator familiarity in settings other than austere, and emergency medicine.3, 4, 5 These challenges are magnified in dynamic environments, prolonged care scenarios, or when patient movement is required.3, 7, 12 These limitations catalyzed an exploration of an innovative approach and functional solution to address the gap between IO access and the rapid establishment of definitive intravenous access, while not interfering with initial resuscitation.
One prevalent misconception surrounding the use of IO access is that it can replace a peripheral IV or central line.13 Clinical practice continues to demonstrate that IOs are unsuitable for prolonged use; consequently, clinicians often bypass IO access, viewing it as a cumbersome procedure with limited value.4 Even when resuscitation teams are presented with a functional IO, they still expend considerable focus, effort, and time at the patient’s expense, attempting to establish more durable access.4 Substantial literature and anecdotal experience suggest that IO access provides clinicians a viable pathway to the central circulation.3, 7, 12 What is not appreciated is the relationship between fluid bolusing via IO and venous optimization (i.e., maximum venous circumference) of proximal vessels in the same extremity, potentially facilitating easier, safer, and less stressful establishment of peripheral or central venous access. This graduated process translates to quantifiably larger vessels, which may improve vascular access procedures.1, 14, 15, 16, 17, 18 This realization suggests that providers could initiate resuscitation with IO access and then utilize the established IO to sequentially step-up to definitive intravenous access. This graduated approach, which we term the Graduated Vascular Access for Hypotensive Patient (GAHP) protocol, may represent a new option in the management of patients with initially difficult vascular access.
Successful early vascular access may ultimately improve the choreography of resuscitation, decrease time-to-direct-venous-access, as well as offer a reduction or alleviation of failed venous access attempts, undue stress, and iatrogenic injury. Although other studies have evaluated maneuvers of vessel augmentation and optimization to facilitate subsequent venous access, none have involved a simultaneous commencement of resuscitation or the use of distal IO access for the expressed purpose of proximal vessel filling.12 This study aims to evaluate a protocol that simultaneously permits immediate access for resuscitation, while also optimizing venous vasculature for long-term vascular access on the same extremity or proximal junction. We hypothesized that distal IO catheter-mediated infusion would demonstrate significant, rapid expansion of proximal venous vasculature within the same extremity in cadaveric models.
Materials and methods
Study population and setting
This study was conducted at the Centre for Emergency Health Sciences in Spring Branch, Texas, between 11 October and 15 November of 2023. The study capitalized on the facility's capacity to procure recently deceased, non-frozen, non-embalmed, unaltered, adult male and female cadavers for scientific studies and procedural courses. Adult cadavers of any age, weight, sex, and race were eligible for inclusion in the study during the above time period. Exclusion criteria for cadaveric extremities included: evidence of scarring in the antecubital fossa, presence of a dialysis fistula or vascular graft, partial or complete extremity amputation, evidence of prior saphenous vein harvest, recent orthopedic surgery, or implanted hardware. Extremities were also excluded if IO catheter placement failed, indicated by fluid extravasation.
Study design
The study employed twenty-three consecutive, fresh, unembalmed cadavers of a convenience sample received by the Centre. In order to ensure that our findings would not be device dependent, the cadavers were randomized using a publicly available randomization program (https://www.random.org) to one of three commercially available IO device types: the powered EZ-IO® (TeleFlex Arrow), the manual JamshidiTM (Becton Dickinson and Company), and the mechanically-assisted SAM® IO (SAM Medical). These IO devices were selected out of convenience and available stock from the study facility. All IOs were 15-gauge in circumference and 45-mm in length. The randomization process was unblinded, with each device corresponding to a numerical assignment. Extremity selection order was also randomized. The IO needle sets were inserted into four distinct sites: distal radius (to evaluate the cephalic vein), proximal humerus (axillary vein), distal tibia (great saphenous vein) and distal femur (common femoral vein). The proximal humeral and distal femoral sites were considered to target proximal/deep vessels, while the radial and tibial sites targeted peripheral/superficial vessels. These sites were chosen to demonstrate that the technique is effective for both superficial and deep proximal venous vessels.
Once in position, the IO catheters were attached to an extension set primed with 0.9% sodium chloride (normal saline [NS]) and correct placement within the bone was confirmed by aspiration. A handheld, manually-operated syringe pump (LifeFlow®; 410 Medical, Inc., Durham, NC) was attached to the extension set, allowing investigators to precisely and rapidly deliver continuously measured fluid boluses titrated to achievement of maximum steady venous distension.19 No venous tourniquets were placed.
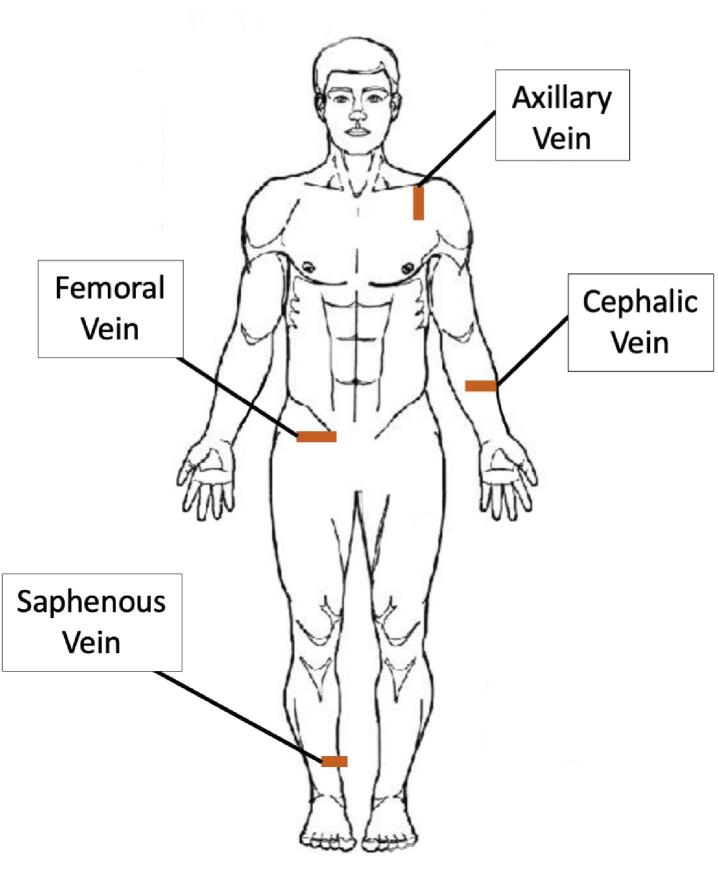
Target vessels were identified by investigators experienced with diagnostic US (Sonosite Xport, FUJIFILM SonoSite, Inc.) in the transverse plane, using non-compressive contact, at standard sites commonly used for venous cannulation in clinical practice (see Fig. 1). Measurements were taken once per extremity. Circumference change of the vessel was measured using a pre-loaded formula on the US machine, measuring from internal vessel wall to internal vessel wall. In the event that a vein of interest was unidentifiable, suggestive of a “flat” hypotensive presentation, the location of the vein was verified by co-localized anatomical structures and venous circumference was recorded as 0 cm. Video recording and US image capture was used to measure the following during bolus NS infusions at the selected IO sites: a) maximum venous circumference (measured in centimeters); b) time elapsed to reach maximum vessel circumference (measured in seconds); and c) volume of infusion required for maximal vessel circumference (measured in milliliters) as shown in Fig. 2.
Fig. 1.
Ultrasound Sites. Note: Red marks indicate ultrasound footprint achieved to visualize corresponding vessels on a cadaver. Laterality is only demonstrative of an example and not indicative of study protocol. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
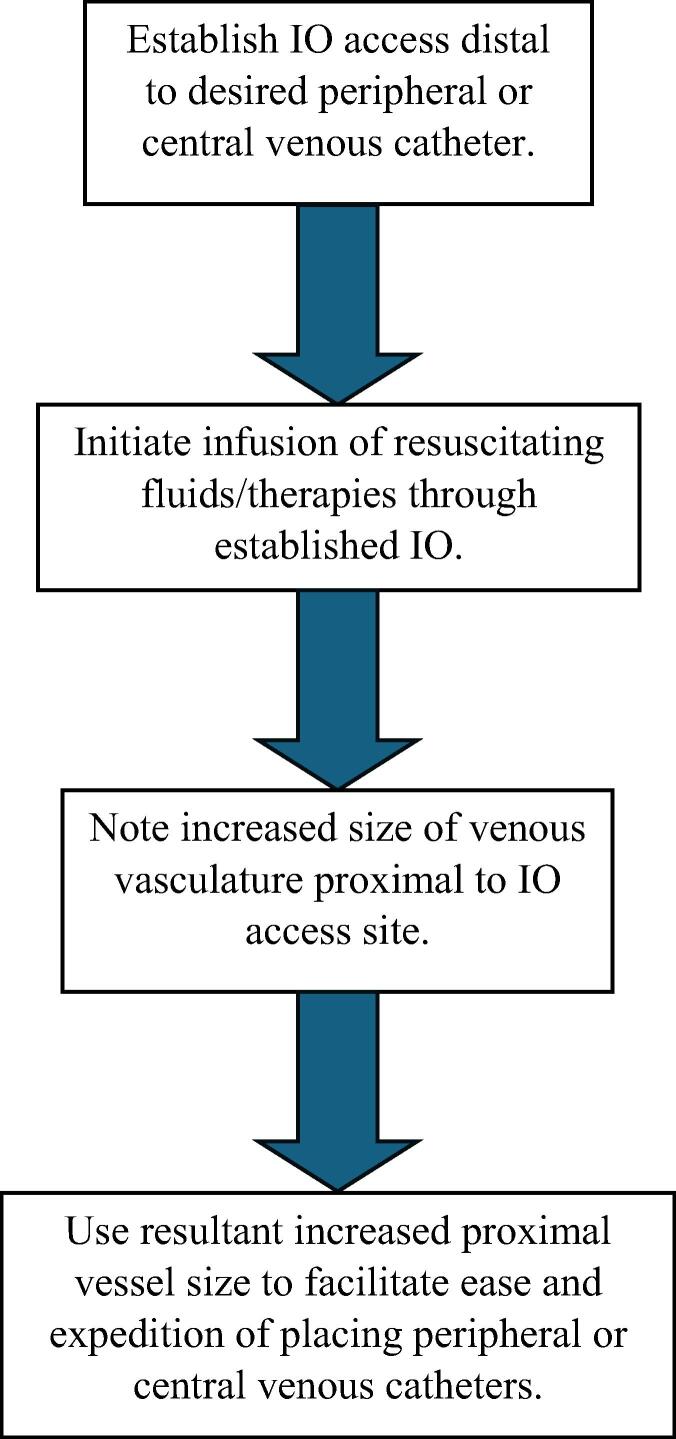
Fig. 2.
GAHP Protocol Steps.
All four extremities of each cadaver were utilized unless a limb was missing or met predetermined exclusion criteria. Within the study protocol, cadavers were numbered, and IO cannulation alternated laterality of proximal and peripheral sites (e.g., proximal left side and peripheral right side to proximal right side and peripheral left side) between odd and even cadavers.
Measures
The primary outcome was maximum change in venous circumference measured before and during IO infusion. Anatomical sites of cephalic, axillary, femoral, and saphenous veins represent independent variables (Fig. 3). Secondary outcomes included bolus volume required to achieve vessel optimization and time elapsed to achieve vessel optimization (i.e., dependent variables) in each of all four anatomical sites of investigation (i.e., independent variables).
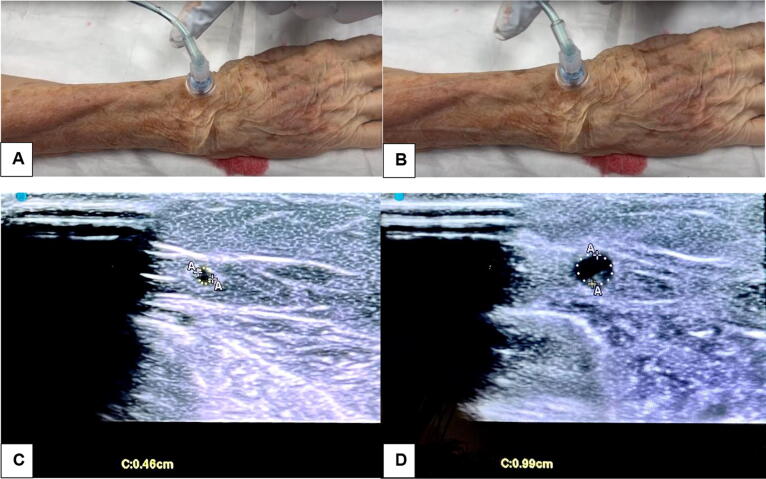
Fig. 3.
Visual Representation of Vessel Optimization. Note: Radial IO prior to fluid bolus (Image A). Radial IO with fluid bolus, with optimized vessels readily visible (Image B). Pre-bolus cephalic vein circumference of 0.46 cm (Image C). Intra-bolus cephalic vein circumference of 0.99 cm (Image D).
Statistical analysis
SAS 9.4 (SAS Institute, Cary, NC) was utilized to perform all statistical analyses. Descriptive statistics were used to characterize demographic variables. Homogeneity of data were evaluated with Levene's test for equality of variances. Wilcoxon signed-rank test was used to evaluate vessel circumference. Any cases with missing data were excluded from the analyses described in this study. To adjust for potential clustering effect, generalized estimating equation approach was used to evaluate time to maximum circumference and volume to maximum circumference between anatomical sites. Alpha was set by convention to 0.05.
Results
Twenty-three cadavers were enrolled in the study, with 85 total infusions successfully performed. Male cadavers constituted 52% (n = 12) of the specimens with mean age 81.3 years (SD 7.3 years). The mean cadaveric height was 166.1 cm (SD 8.4 cm), and mean weight was 79.5 kg (SD 16.1 kg). One humeral site was excluded for inability to identify the axillary vein under ultrasound. Three radial sites were excluded due to extravasation upon insertion of the IO, and one was excluded due to the presence of implanted hardware. Two tibial sites were excluded due to saphenous vein harvests. Data were not otherwise missing (see Table 1).
Table 1.
Cadaver Demographics and Characteristics.
| Total Participants | 23 | ||
|---|---|---|---|
| Age | |||
| Mean (SD) | 81.26 | 7.26 | |
| Gender | |||
| Male | 13 | 56.52 | |
| Female | 10 | 43.48 | |
| Weight (kg) | |||
| Mean (SD) | 79.51 | 16.08 | |
| Height (cm) | |||
| Mean (SD) | 166.08 | 8.44 | |
| IO Access Site (proximal) | |||
| Humerus | 22 | 95.65 | |
| Femur | 22 | 95.65 | |
| IO Access Site (distal) | |||
| Radius | 20 | 86.96 | |
| Tibia | 21 | 91.30 | |
| IO Device | |||
| EZ-IO® | 3 | 13.04 | |
| JamshidiTM | 9 | 39.13 | |
| SAM® IO | 11 | 47.83 | |
Note: Demographics were by investigators at the time of study.
We found a notable increase in maximum venous circumference during the IO fluid bolus when compared to baseline measurements. On average, the venous circumference measured during the fluid bolus was 1.03 cm (95% CI 0.86, 1.20) greater than that measured prior to the bolus administration across all sites, an increase of 102%. Across all sites investigated, there was a significant difference in the venous circumference measured before and during fluid bolus [S 1827.5, p <0.0001].
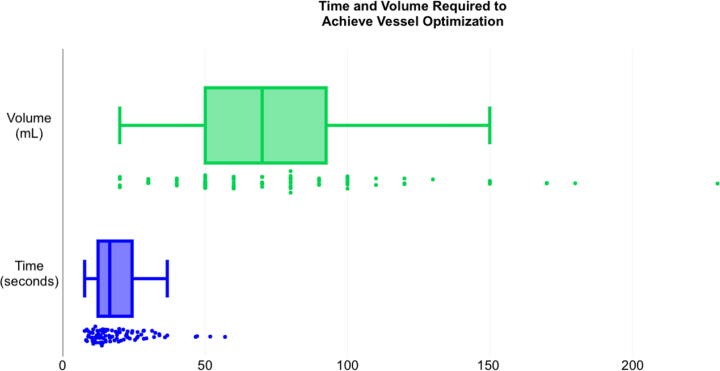
Seven measurements were excluded as above. The site of infusion had no significant effect on time elapsed to reach maximum venous circumference (p <0.053). The time of infusion ranged from 7.6 to 57.0 s (median 14.92, IQR 11.8–22.7). However, there was a significant effect of the infusion site on the volume of bolus required to reach maximum venous circumference (p <0.001). The volume of infusion required ranged from 20 to 230 mL (median 70, IQR 50–100) as shown in Fig. 4 and Table 2.
Fig. 4.
Time and Volume Required to Achieve Vessel Optimization.
Table 2.
Vessel Optimization by Site.
| IO Site | Vein | Pre-Infusion CircumferenceMean (SD) |
Optimized CircumferenceMean (SD) |
% Difference |
|---|---|---|---|---|
| Radius | Cephalic | 0.33 (0.32) | 0.71 (0.42) | 215.15 |
| Humerus | Axillary | 1.54 (1.22) | 3.04 (0.67) | 197.40 |
| Tibia | Great Saphenous | 0.10 (0.19) | 0.72 (0.18) | 720.00 |
| Femur | Common Femoral | 1.97 (1.26) | 03.26 (0.85) | 165.48 |
Note: Circumference measured in centimeters via real-time ultrasound.
Discussion
This study suggests Graduated Vascular Access for Hypotensive Patient (GAHP) protocol may improve vessel properties to benefit subsequent vascular access. The inclusion of three different types of intraosseous (IO) devices: the powered EZ-IO®, the manual JamshidiTM IO, and the mechanically assisted SAM®-IO, all 45-mm, 15 ga catheters, in a randomized cadaveric setting, is noteworthy, suggesting that various IO devices of the same length and gauge used in the study may potentially be utilized for GAHP.
The measured average increase of 1.03 cm (SD 0.80, 102%) in venous circumference during fluid bolus supports the potential utility of IO bolus infusion for optimization and subsequent venous cannulation as previous studies have suggested larger vein size is associated with improved direct cannulation attempts.1, 14, 15, 16, 17, 18 The observed engorgement of every individual vein targeted (e.g., axillary, cephalic, femoral, saphenous) by the protocol highlights the effectiveness of GAHP in facilitating rapid and considerable vessel optimization regardless of site. This finding highlights the versatility of GAHP across distal and proximal anatomical locations, and points to its potential as a standardized approach to hypotensive patients in clinical practice.
The range of optimization times provides valuable insight into the efficiency of GAHP, as the majority of veins demonstrated optimization within the 10–25 s range, indicating a consistently rapid response to the GAHP approach. This time efficiency is crucial and further illuminates that when employed in the practical sense, GAHP may yield minimal delay in resuscitation while facilitating safer, less stressful access to potentially larger, more stable peripheral or proximal deep veins. The GAHP stepped approach to expand proximal venous vasculature aligns with the need for swift intervention and subsequent longer-term venous access. This advancement in IO infusion utilization potentially enhances the accuracy of medical interventions and may also contribute to the safety and efficiency of emergent care, where rapid and reliable vascular access is paramount.
Although there was no significant difference between infusion sites for time elapsed, there was a significant difference amongst bolus volumes, suggesting that vessel location may influence bolus volume required for optimization. Particularly, the saphenous vein was augmented with the least volume. Potentially, this may be due to traditionally larger proximal deep veins requiring larger bolus amounts to reach maximal circumference, which is supported by the data demonstrating that the saphenous vein appeared to require smaller bolus volumes (see Table 2).
Limitations
Although GAHP demonstrated optimization of proximal vasculature for subsequent venous cannulation, and prior studies have shown that increased circumference of vasculature improves placement of both ultrasound-guided peripheral intravenous catheters and central venous catheters, this study was not designed to demonstrate the clinical effects of venous optimization on access time, number of attempts to cannulation, or ease of venous catheter placement.1, 14, 15, 16, 17, 18
A cadaver model does not dynamically replicate the physiologic venous changes seen in a severely hypotensive, live patient. Future studies will be needed to assess how venous drainage, collateral circulation, autonomic nervous system input, and central venous pressures are affected by this technique in live patients. Cadaveric studies show that small vessels are generally thrombosed or occluded and this can restrict outflow tracts that may otherwise contribute to less vessel filling in living subjects.20, 21 External compression by sonographers could also have influenced our data by restricting venous return, thus improving the speed and degree of optimization.
Likewise, proximal and distal insertion sites were alternated in laterality to mitigate the risk of proximal vessel filling due to prior testing, which would influence the initial assessment study outcomes. Additionally, the LifeFlow® device is a syringe-hand-pump device, which can be actuated slowly or quickly, complicating our attempts to standardize pump rates. Furthermore, this study focused on NS as the infused liquid, and results may not be generalizable to blood products, colloids, or other crystalloid solutions.
Additionally, aside from explicit limitations of this study, the GAHP protocol may have some inherent limitations in its design. In vivo, failed attempts to place an IV catheter into an augmented vein proximal to an IO needle can lead to significant complications, such as venous puncture injuries with concurrent extravasation. An occurrence like this would nullify the distal IO, which would be critical for patients in extremis, especially when the IO catheter represents their only available vascular access route. Thus, despite potentially presenting a larger target for graduated vascular access, the GAHP protocol may place users into an “all-or-nothing” scenario when it comes time for direct venous cannulation.
Clinical implications and future directions
The success of GAHP in optimizing veins within seconds may have implications for the practice of austere, emergency, and critical care. GAHP has the potential to serve as the bridge to more definitive vascular access methods for critically ill patients, especially those receiving care for out-of-hospital cardiac arrest in which administering drugs IV rather than IO may improve patient outcomes.22, 23 By simultaneously initiating resuscitation and engorging adjacent vasculature via a graduated approach to direct venous access, GAHP may be an expeditious key to enhancement of patient care in complex medical situations as well as a tool to mitigate failed vascular access attempts and iatrogenic complications. Likewise, the relatively small volumes required to optimize vessels suggests that high risk patient populations, such as those with significant cardiac or renal disease may benefit from this technique specifically because it minimizes volume overload during resuscitation and securement of definitive access. Albeit highly end-user dependent, future studies in live patient settings are essential to validate GAHP’s clinical efficacy, thereby bridging the gap between emergency vascular access needs and the achievement of successful venous cannulation.
Lastly, though we did not specifically evaluate US confirmation of IO placement in this study, we found that bolusing fluid via IO can be immediately appreciated with real-time, direct US observation of proximal venous flow. By showing that IO fluid bolusing may be immediately appreciated with real-time ultrasound observation, this study supports the feasibility of US as an avenue to confirm IO patency. This suggests the practicality of utilizing vascular US as a means to confirm the suitability and patency of IO infusions; however, further dedicated study is needed to confirm these findings.
Conclusion
The GAHP protocol significantly optimizes proximal veins in a cadaveric model; however, further research is needed to investigate whether this approach may be used to facilitate vascular access for hypotensive patients.
Disclaimer
The views expressed in this abstract reflect the results of research conducted by the author(s) and do not necessarily reflect the official policy or position of the Defense Health Agency, Department of Defense, or the U.S. Government. The Institutional Review Board for the University of Texas Health Science Center, San Antonio, reviewed and waived this study as non-human subject research (IRB #20230736NHR). Specimens were requested and approved through the Texas State Anatomical Board (Texas Funeral Commission) and the University of Texas Southwestern Willed (UTSW) Body Program for research and procedural training. No external funding was utilized or required for this study. Facilities, materials, necessary staff, and required equipment were provided by The Centre for Emergency Health Sciences.
Credit authorship contribution statement
Mathew A. Saab: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. Emily Raetz: Writing – review & editing, Validation, Investigation, Data curation. Joshua B. Lowe: Writing – review & editing, Writing – original draft, Methodology. Ian Hudson: Writing – review & editing, Writing – original draft, Formal analysis. Eric Jacobson: Writing – review & editing, Writing – original draft, Methodology. Adrianna Long: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Jennifer Achay: Writing – review & editing, Visualization, Validation, Methodology, Investigation, Data curation, Conceptualization. Scotty Bolleter: Writing – review & editing, Writing – original draft, Visualization, Validation, Resources, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. Christopher McCuller: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation. Emmanuel G. Rayas: Writing – review & editing, Formal analysis. Alexander Nunnery: Writing – review & editing, Validation, Investigation. Ryan Bierle: Writing – review & editing, Writing – original draft, Visualization, Methodology. Stephen J. Rahm: Writing – review & editing, Validation, Resources, Investigation, Funding acquisition, Data curation. Emily Epley: Writing – review & editing, Validation, Investigation, Data curation. Richard Poe: Writing – review & editing, Validation, Investigation, Data curation. Erik DeSoucy: Writing – review & editing, Writing – original draft, Visualization, Methodology. Robert De Lorenzo: Writing – review & editing, Project administration, Methodology, Conceptualization. Ryan Dumas: Writing – review & editing, Writing – original draft, Visualization, Methodology. James H. Paxton: Writing – review & editing, Writing – original draft, Visualization, Methodology. Tania Rogerson: Writing – review & editing, Writing – original draft, Visualization, Methodology. Patrick Georgoff: Writing – review & editing, Writing – original draft, Visualization, Methodology. Anne Adema: Writing – review & editing, Writing – original draft, Visualization, Methodology. Marcus Eng Hock Ong: Writing – review & editing, Writing – original draft, Visualization, Methodology. David Wampler: Writing – review & editing, Supervision, Project administration, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge the men and women who donate their remains to support medical research. Without their precious gift, untimely death and needless suffering would prevail. We would additionally like to thank the UTSW Willed Body Program and staff for their seemingly tireless efforts that enhance the medical arts.
Contributor Information
Mathew A. Saab, Email: mathew.a.saab@gmail.com.
Emily L. Raetz, Email: raetz.emily@gmail.com.
Joshua B. Lowe, Email: beaudasious12@gmail.com.
Ian L. Hudson, Email: hudsoni@uthscsa.edu.
Eric J. Jacobson, Email: ejaco211@gmail.com.
Adrianna N. Long, Email: adrianna.long.md@gmail.com.
Jennifer A. Achay, Email: jennifer.achay@hiscentre.com.
Scotty D. Bolleter, Email: scotty.bolleter@hiscentre.com.
Christopher A. McCuller, Email: mcculler@uthscsa.edu.
Emmanuel G. Rayas, Email: rayase@uthscsa.edu.
Alexander M. Nunnery, Email: nunnerya@uthscsa.edu.
Ryan P. Bierle, Email: Bierle@uthscsa.edu.
Stephen J. Rahm, Email: stephen.rahm@hiscentre.com.
Emily A. Epley, Email: emily.epley32@gmail.com.
Richard J. Poe, Email: rpoe2199@gmail.com.
Erik S. DeSoucy, Email: erikdesoucy@yahoo.com.
Robert A. De Lorenzo, Email: delorenzo@uthscsa.edu.
Ryan P. Dumas, Email: Ryan.Dumas@utsouthwestern.edu.
James H. Paxton, Email: paxtonius@hotmail.com.
Tania C. Rogerson, Email: taniarogerson@mac.com.
Patrick E. Georgoff, Email: georgoff@gmail.com.
Anne L. Adema, Email: Anne.Adema@childrenscolorado.org.
Marcus Eng Hock Ong, Email: marcus.ong@duke-nus.edu.sg.
David A. Wampler, Email: wamplerd@uthscsa.edu.
References
- 1.Blanco P. Ultrasound-guided peripheral venous cannulation in critically ill patients: a practical guideline. Ultrasound J. 2019;11:27. doi: 10.1186/s13089-019-0144-5. Published 2019 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chreiman K.M., Dumas R.P., Seamon M.J., et al. The intraosseous have it: A prospective observational study of vascular access success rates in patients in extremis using video review. J Trauma Acute Care Surg. 2018;84:558–563. doi: 10.1097/TA.0000000000001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumas R.P., Vella M.A., Maiga A.W., et al. Moving the needle on time to resuscitation: An EAST prospective multicenter study of vascular access in hypotensive injured patients using trauma video review. J Trauma Acute Care Surg. 2023;95:87–93. doi: 10.1097/TA.0000000000003958. [DOI] [PubMed] [Google Scholar]
- 4.Žunkovič M., Markota A., Lešnik A. Attitudes towards the utilization of intraosseous access in prehospital and emergency medicine nursing personnel. Medicina (Kaunas) 2022;58:1086. doi: 10.3390/medicina58081086. Published 2022 Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee P.M., Lee C., Rattner P., Wu X., Gershengorn H., Acquah S. Intraosseous versus central venous catheter utilization and performance during inpatient medical emergencies. Crit Care Med. 2015;43:1233–1238. doi: 10.1097/CCM.0000000000000942. [DOI] [PubMed] [Google Scholar]
- 6.Leidel B.A., Kirchhoff C., Bogner V., Braunstein V., Biberthaler P., Kanz K.G. Comparison of intraosseous versus central venous vascular access in adults under resuscitation in the emergency department with inaccessible peripheral veins. Resuscitation. 2012;83:40–45. doi: 10.1016/j.resuscitation.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Tan B.K.K., Chin Y.X., Koh Z.X., et al. Clinical evaluation of intravenous alone versus intravenous or intraosseous access for treatment of out-of-hospital cardiac arrest. Resuscitation. 2021;159:129–136. doi: 10.1016/j.resuscitation.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Tyler J.A., Perkins Z., De'Ath H.D. Intraosseous access in the resuscitation of trauma patients: a literature review. Eur J Trauma Emerg Surg. 2021;47:47–55. doi: 10.1007/s00068-020-01327-y. [DOI] [PubMed] [Google Scholar]
- 9.Clem M., Tierney P. Intraosseous infusions via the calcaneus. Resuscitation. 2004;62:107–112. doi: 10.1016/j.resuscitation.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh Y.L., Wu M.C., Wolfshohl J., et al. Intraosseous versus intravenous vascular access during cardiopulmonary resuscitation for out-of-hospital cardiac arrest: a systematic review and meta-analysis of observational studies. Scand J Trauma Resusc Emerg Med. 2021;29:44. doi: 10.1186/s13049-021-00858-6. Published 2021 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mody P., Brown S.P., Kudenchuk P.J., et al. Intraosseous versus intravenous access in patients with out-of-hospital cardiac arrest: Insights from the resuscitation outcomes consortium continuous chest compression trial. Resuscitation. 2019;134:69–75. doi: 10.1016/j.resuscitation.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien K., Ciccarelli B., Reiss A., Stankewicz H., Balakrishnan V. Augmentation of peripheral venous circumference for ultrasound-guided peripheral intravenous line insertion. Am J Emerg Med. 2023;73:75–78. doi: 10.1016/j.ajem.2023.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Succar B., Vella M.A., Holena D.N., Dumas R.P. Navigating the challenges of vascular access in hypotensive injured patients. Surgery. 2024;175:559–560. doi: 10.1016/j.surg.2023.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Bannon M.P., Heller S.F., Rivera M. Anatomic considerations for central venous cannulation. Risk Manag Healthc Policy. 2011;4:27–39. doi: 10.2147/RMHP.S10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields J.M., Dean A.J., Todman R.W., et al. The effect of vessel depth, circumference, and location on ultrasound-guided peripheral intravenous catheter longevity. Am J Emerg Med. 2012;30:1134–1140. doi: 10.1016/j.ajem.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Panebianco N.L., Fredette J.M., Szyld D., Sagalyn E.B., Pines J.M., Dean A.J. What you see (sonographically) is what you get: vein and patient characteristics associated with successful ultrasound-guided peripheral intravenous placement in patients with difficult access. Acad Emerg Med. 2009;16:1298–1303. doi: 10.1111/j.1553-2712.2009.00520.x. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Calero M.A., Blanco-Mavillard I., Morales-Asencio J.M., Fernández-Fernández I., Castro-Sánchez E., de Pedro-Gómez J.E. Defining risk factors associated with difficult peripheral venous Cannulation: A systematic review and meta-analysis. Heart Lung. 2020;49:273–286. doi: 10.1016/j.hrtlng.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 18.van Loon F.H.J., Korsten H.H.M., Dierick-van Daele A.T.M., Bouwman A.R.A. The impact of the catheter to vein ratio on peripheral intravenous cannulation success, a post-hoc analyses. PLoS One. 2021;16 doi: 10.1371/journal.pone.0252166. Published 2021 May 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piehl M., Smith-Ramsey C., Teeter W.A. Improving fluid resuscitation in pediatric shock with LifeFlow®: a retrospective case series and review of the literature. Open Access Emerg Med. 2019;11:87–93. doi: 10.2147/OAEM.S188110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schobinger R. Intraosseous venography. Angiology. 1960;11:283–296. doi: 10.1177/000331976001100322. [DOI] [PubMed] [Google Scholar]
- 21.Sutherland T., O'Donnell C. The artefacts of death: CT post-mortem findings. J Med Imaging Radiat Oncol. 2018;62:203–210. doi: 10.1111/1754-9485.12691. [DOI] [PubMed] [Google Scholar]
- 22.Daya M.R., Leroux B.G., Dorian P., et al. Survival after intravenous versus intraosseous amiodarone, lidocaine, or placebo in out-of-hospital shock-refractory cardiac arrest. Circulation. 2020;141:188–198. doi: 10.1161/CIRCULATIONAHA.119.042240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monaco T., Fischer M., Michael M., et al. Impact of the route of adrenaline administration in patients suffering from out-of-hospital cardiac arrest on 30-day survival with good neurological outcome (ETIVIO study) Scand J Trauma Resusc Emerg Med. 2023;31:14. doi: 10.1186/s13049-023-01079-9. Published 2023 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]