Abstract
The tree shrew (Tupaia belangeri) has long been proposed as a suitable alternative to non-human primates (NHPs) in biomedical and laboratory research due to its close evolutionary relationship with primates. In recent years, significant advances have facilitated tree shrew studies, including the determination of the tree shrew genome, genetic manipulation using spermatogonial stem cells, viral vector-mediated gene delivery, and mapping of the tree shrew brain atlas. However, the limited availability of tree shrews globally remains a substantial challenge in the field. Additionally, determining the key questions best answered using tree shrews constitutes another difficulty. Tree shrew models have historically been used to study hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, myopia, and psychosocial stress-induced depression, with more recent studies focusing on developing animal models for infectious and neurodegenerative diseases. Despite these efforts, the impact of tree shrew models has not yet matched that of rodent or NHP models in biomedical research. This review summarizes the prominent advancements in tree shrew research and reflects on the key biological questions addressed using this model. We emphasize that intensive dedication and robust international collaboration are essential for achieving breakthroughs in tree shrew studies. The use of tree shrews as a unique resource is expected to gain considerable attention with the application of advanced techniques and the development of viable animal models, meeting the increasing demands of life science and biomedical research.
Keywords: Tree shrew, Animal model, Neurodegenerative diseases, Infectious diseases, Neuroscience, Phenome
INTRODUCTION
Human economic and societal development over recent decades has substantially shifted the burden of diseases (Achoki et al., 2022; GBD 2019 Diseases and Injuries Collaborators, 2020; Zhou et al., 2019). In China, for example, an analysis of the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) from 1990 to 2017 revealed marked changes in health patterns, including mortality, disability, and risk factors (GBD 2019 Cancer Risk Factors Collaborators, 2022; Zhou et al., 2019). Notably, although overall disease burden decreased, the top causes of death and disability-adjusted life-years differed significantly between 1990 and 2017, shifting from lower respiratory infections and neonatal disorders to stroke and ischemic heart disease (Zhou et al., 2019). The evolving global burden of diseases and injuries presents difficulties for biomedical research and underscores the urgent need for effective prophylactics. Consequently, establishing and applying valid disease models is essential to gain a deeper understanding of disease pathobiology and combating human diseases. Rodents, such as mice and rats, have traditionally served as the primary animal models in research. However, the genetic differences between rodents and humans, as well as disparities in comparative anatomy and disease model phenotyping and biases affecting internal and external validity, pose considerable challenges for translating murine models to human diseases (Justice & Dhillon, 2016; McGonigle & Ruggeri, 2014; Ruberte et al., 2023; van der Worp et al., 2010). Identifying experimental animals with anatomical structures and functional units similar to humans is essential for the reproducibility and translatability of human disease models. Non-human primates (NHPs) are considered the optimal models for studying human diseases and advancing biomedical research due to their high genetic, anatomical, physiological, pathological, and brain function similarities to humans (National Academies of Sciences, Engineering, and Medicine et al., 2023; Phillips et al., 2014; Roelfsema & Treue, 2014; Tarantal et al., 2022; Yao & Construction Team of the KIZ Primate Facility, 2022). The value of NHPs in biomedical research is well-established (National Academies of Sciences, Engineering, and Medicine et al., 2023), especially in modeling neurodegenerative and infectious diseases such as Alzheimer’s disease (AD; Arnsten et al., 2021; Haque & Levey, 2019; Pan et al., 2024) and COVID-19 (Ma et al., 2022; Saturday & van Doremalen, 2023; Song et al., 2020), as well as in understanding human biology (Estrada et al., 2017; Guo et al., 2023b; Wu et al., 2022; Zhang et al., 2022). However, bioethical concerns and high maintenance costs limit the widespread use of NHPs compared to rodents.
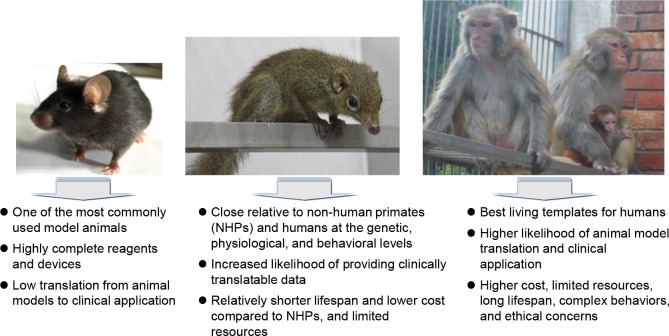
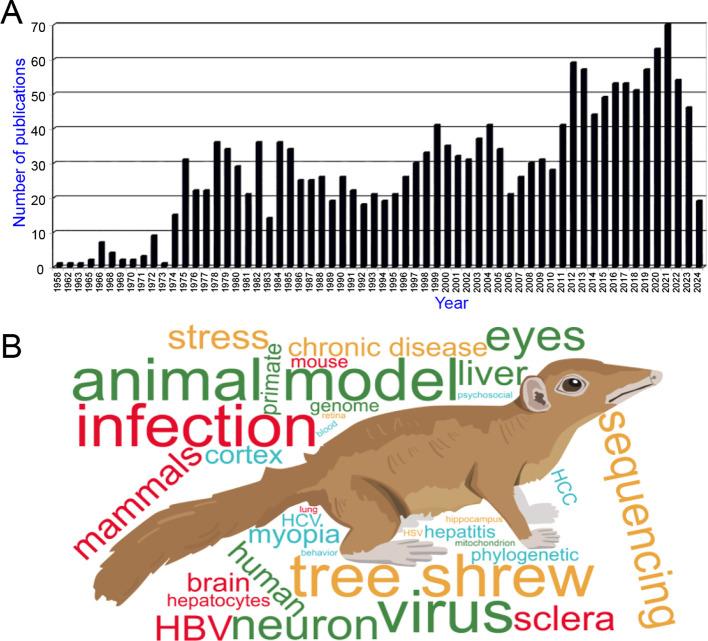
Tree shrews are genetically closer to NHPs than rodents, as evidenced by cross-species anatomical ontology, transcriptomic profiling, and comparative physiology (Dimanico et al., 2021; Fan et al., 2013, 2019; Ni et al., 2018a, 2018b; Ye et al., 2021; Zhang et al., 2020a) (Figure 1). They also offer several advantages as experimental animals, including small adult body size, brief reproductive cycle, short life span, and lower maintenance costs (Yao, 2017), with the recently launched Tree Shrew User Meeting underscoring the progress and promising future of tree shrews as animal models in neuroscience research (Savier et al., 2021). Recent reviews have highlighted the use of tree shrews in modeling human viral infections and cancer research (Kayesh et al., 2021; Li et al., 2018b; Lu et al., 2021), while earlier reviews emphasized their role as an emerging model for various human diseases (Cao et al., 2003; Xiao et al., 2017; Yao, 2017). However, research using tree shrews has not flourished as expected, as evidenced by a PubMed search using “tree shrew or Tupaia” as keywords, which yielded only 1 777 entries from 1958 to March 2024 (Figure 2). Most studies involving tree shrews over the past decades have focused on vision and neurobiology, with their use as infection models gaining increasingly attention in recent years. It is evident that fostering a larger research community dedicated to use the tree shrews as a model organism is desirable. A timely summation of recent tree shrew studies, with a comprehensive discussion of the benefits, limitations, and future directions of this animal in biomedical research, is necessary to meet the growing needs of the field and address the global burden of diseases.
Figure 1.
Advantages and disadvantages of tree shrews as experimental models compared to rodents and non-human primates
Figure 2.
Number of tree shrew publications in the field
A: Distribution of tree shrew publications since the 1950s according to a PubMed search (“tree shrews or Tupaia”) on 26 March 2024 (1 777 publications). B: Word-frequency based on abstracts of tree shrew publications in (A).
BIOLOGICAL OVERVIEW AND TAXONOMY OF TREE SHREWS
The tree shrew is a small rat-sized mammal with a high brain-to-body mass ratio, large eyes, and high mobility. It reaches sexual maturity at around 5 months and has a short gestation period of approximately 6 weeks, typically producing a litter of one to five offspring. In our Kunming breeding center (China), tree shrews have a lifespan of six to eight years. They are relatively inexpensive to maintain compared to larger experimental animals, such as monkeys and dogs. Tree shrews are widely distributed in South and Southwest China, as well as in adjacent regions of South and Southeast Asia (Fuchs & Corbach-Söhle, 2010; Peng et al., 1991). In the most recent edition of The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals (Ninth Edition), Fuchs (2024) provides an excellent biological overview of tree shrews.
Currently, the tree shrew is classified within the Order Scandentia, which comprises two recognized families, Ptilocercidae and Tupaiidae (Helgen, 2005; Xu et al., 2013). Historically, resolving the phylogeny of tree shrew species has been difficult due to limited specimens and samples from critical taxa (Roberts et al., 2011; Wang, 1987). While 15 tree shrew species are currently recognized within the genus Tupaia (Helgen, 2005), the species status of T. moellendorffi and T. palawanensis has been questioned due to their small genetic distance (Roberts et al., 2011). Tree shrews used in early biomedical research were introduced from China, Thailand, Malaysia, and nearby regions, and were likely T. belangeri and T. glis. However, confusion arose in published reports due to the differing views of taxonomists, with some regarding both species as one and others considering T. belangeri as a subspecies of T. glis (Wang, 1987), leading to the use of T. glis belangeri in some publications (e.g., McCollum & Roberts, 2014; Norton et al., 2010; Romero-Herrera & Lehmann, 1974; Siegwart & Norton, 1998). Based on the original sources and common names, both T. belangeri (e.g., Collins & Tsang, 1987; Darai et al., 1978; Fuchs & Schumacher, 1990; McBrien & Norton, 1992) and T. glis common tree shrew, e.g., (Chunhabundit et al., 1993; Kurohmaru et al., 1996; Sherman et al., 1977) have been used in biomedical studies, leading to potential ambiguity in the reproducibility of animal models when different species are not clearly identified. The NCBI taxonomy for Tupaia (ID 9394; https://www.ncbi.nlm.nih.gov/datasets/taxonomy/9394/) lists a total of 17 species. The Chinese tree shrew, initially named T. chinensis in 1879, was later reclassified as T. belangeri (Wang, 1987). The proposed reclassification of the Chinese tree shrew (T. belangeri chinensis) as a distinct species (T. chinensis) was supported by its significant genetic distance from the northern tree shrew (T. belangeri), as evidenced by our previous phylogenetic tree based on 2 117 single-copy genes (Fan et al., 2013). However, it has also been suggested that Chinese tree shrews could be classified in six subspecies of T. belangeri (T. b. chinensis, T. b. gaoligongensis, T. b. modesta, T. b. tonquinia, T. b. yunalis, T. b. yaoshanensis) based on morphological characters and body hair color (Wang, 1987). Different subspecies of Chinese tree shrews have been used to create animal models. For instance, T. b. yunalis adults have been used to establish a human rotavirus infection model (Pang et al., 1983) and test the efficacy of Valeriana jatamansi treatment (Pang et al., 1984). This subspecies has also been used to establish an infection model of Mycobacterium leprae, supporting the proliferation of this pathogen of leprosy (Wang et al., 1990). The T. b. chinensis subspecies has been used to characterize the potential roles of Tupaia immune genes in viral infection (Gu et al., 2021a, 2021b; Xu et al., 2016, 2020b) and to test aging effects on cognitive impairment and the potential for developing animal models of AD (Fan et al., 2018; Li et al., 2024). The genetic background of different tree shrew species from different geographic regions may affect their susceptibility to certain pathogens and/or naturally occurring diseases. As such, attention should be paid to the original source when selecting animals for testing.
AVAILABLE EXPERIMENTAL TREE SHREW COLONIES AND CELL LINE RESOURCES
Currently, several tree shrew colonies exist worldwide, each with specific maintenance purposes and research goals. Two primary tree shrew colonies are maintained in the United States. Of note, the University of Alabama at Birmingham (UAB) (https://www.uab.edu/research/administration/CentersCores/Pages/Tree-Shrew-Core.aspx) maintains a colony that produces 80–130 young tree shrews annually, primarily for eye and vision research as well as other studies within the United States (e.g., KhalafAllah et al., 2024; Khanal et al., 2023; Norton et al., 2010; Siegwart & Norton, 1998). The Max Planck Florida Institute for Neuroscience maintains a smaller colony of about 80–100 animals, generating young tree shrews for internal research purposes and colony maintenance.
The German Primate Center (Deutsches Primatenzentrum, DPZ) at Göttingen maintained a tree shrew colony for nearly 30 years, supplying animals to research institutes across Germany and Europe, with a peak of 200–300 animals between 2004 and 2009 (Fuchs, 2015). However, this colony was closed following the retirement of Prof. Eberhard Fuchs, and the animals were transferred to the Department of Behavioral Physiology at the Center for Behavior and Neurosciences, University of Groningen, in the Netherlands (Fuchs, 2015). Additionally, the University of Fribourg Platform for Translational Neuroscience in Switzerland maintains a tree shrew breeding facility, producing up to 40 animals per year for internal research and external users.
The largest tree shrew colony in China is maintained by the experimental animal center of the Kunming Institute of Zoology, Chinese Academy of Sciences. Established in the 1970s, this colony has faced interruptions over the past decades. Currently, it houses approximately 2 000–3 000 individuals, depending on the needs for colony expansion, and supplies around 1 000 animals annually to various institutes and universities in China and abroad. Additionally, the experimental animal centers of the Kunming Medical University and the Institute of Medical Biology of the Chinese Academy of Medical Sciences host two large colonies for research purposes, further highlighting the ample tree shrew resources in China.
The tree shrew colonies in the United States and Germany were originally sourced from Thailand. However, following the export ban of these animals from Thailand, China and Malaysia emerged as primary global suppliers. Limited access to tree shrew resources worldwide has posed a significant challenge to their widespread use in biomedical research. It is essential to introduce new tree shrews into existing colonies to prevent inbreeding and support colony expansion. However, animal rights activists have also impeded the transportation of these animals and hindered research activities involving tree shrews. It is crucial for scientists working with tree shrews to advocate for their research and emphasize the benefits of using this animal model. The tree shrew center at the Kunming Institute of Zoology is prepared to provide tree shrews to researchers globally for scientific purposes.
To minimize the use of living tree shrews and adhere to the 3Rs (replacement, reduction, and refinement) in experimental animal biology (MacArthur Clark, 2018; Sneddon et al., 2017), it is essential to develop immortalized tree shrew cells, induced pluripotent stem cells (iPSCs), and organoids. Currently, only a few immortalized tree shrew cell lines exist (e.g., Gu et al., 2019b; Yin et al., 2019; Zhang et al., 2020c), while there are no reports of iPSCs or organoids for tree shrews. The establishment of iPSCs and iPSC-derived neurons from hibernating thirteen-lined ground squirrels (Ou et al., 2018) serves as an excellent example for research on non-model organisms, including tree shrews. Developing iPSC-derived organ-on-a-chip platforms and three-dimensional (3D) organoid culture systems (Augustyniak et al., 2019; Palasantzas et al., 2023; Rauth et al., 2021; Teli et al., 2023) for tree shrews would significantly advance comparative physiology, disease modeling, and pharmacological research. To maximize the potential of tree shrew resources, it is crucial to foster international collaborations, share living tree shrews and associated cell lines, and develop innovative techniques.
DO WE NEED AN INBRED STRAIN OF TREE SHREW?
Advances in sequencing technologies (De Coster et al., 2021; Hook & Timp, 2023; Zhang et al., 2011) now allow researchers to determine the genome sequences of wild animals and non-model organisms, providing crucial insights into their genetic backgrounds, which is essential for their use as experimental animals. The application of cutting-edge gene editing techniques (Badon et al., 2024; Li et al., 2019; Luo et al., 2016; Ma et al., 1998; Pfeiffer & Stafforst, 2023; Villiger et al., 2024), especially adeno-associated virus (AAV) vector-mediated gene delivery or genome editing (Challis et al., 2022; Taha et al., 2022; Wang et al., 2019a, 2020a), has greatly accelerated the creation of genetically modified animals and gene therapy applications. These advancements in sequencing and gene editing have revolutionized the study of non-model animals. Theoretically, any wild animal could be used as an experimental animal with appropriate ethical approval, and research on non-model organisms can significantly facilitate biomedical research and our understanding of life and evolution (Cusick et al., 2021; Guo et al., 2023b; Meadows & Lindblad-Toh, 2017; Zhao et al., 2021a). However, for drug screening and other tests of new prophylactics and treatments, inbred experimental animals are preferred to ensure consistent and comparable results. This key advantage of model animals highlights the necessity of developing an inbred tree shrew strain.
In 2011, we began efforts to establish an inbred Chinese tree shrew strain at the Kunming Institute of Zoology (Chen et al., 2022a) to facilitate broader application in research, but progress has been limited. Despite over a decade of effort, we have only managed to obtain a small number of inbred offspring to the ninth filial generation (F9) (unpublished data). A significant issue is infanticide during sibling mating, which was excessively high in our inbred colonies, in contrast to regular tree shrew colonies (either closed colonies or hybrids) under the same conditions, which successfully produce offspring. The exact cause for this cannibalistic behavior is unknown (Chen et al., 2022a). Given the suggested efficacy of oxytocin in preventing infanticide (Lezama-García et al., 2019; McCarthy, 1990; Olazábal & Alsina-Llanes, 2016), we administered oxytocin to tree shrew mothers to potentially mitigate this issue. Due to the prevalence of cannibalistic behavior, we resorted to artificial rearing for inbred tree shrew pups, despite it being time-consuming. After extensive efforts, we discovered that commercially available milk substitutes for guinea pigs (Wombaroo Food Products, Australia) effectively supported the survival and growth of Chinese tree shrew pups (Chen et al., 2022a). This milk replacer simplifies the process for laboratories needing to artificially rear Chinese tree shrew pups.
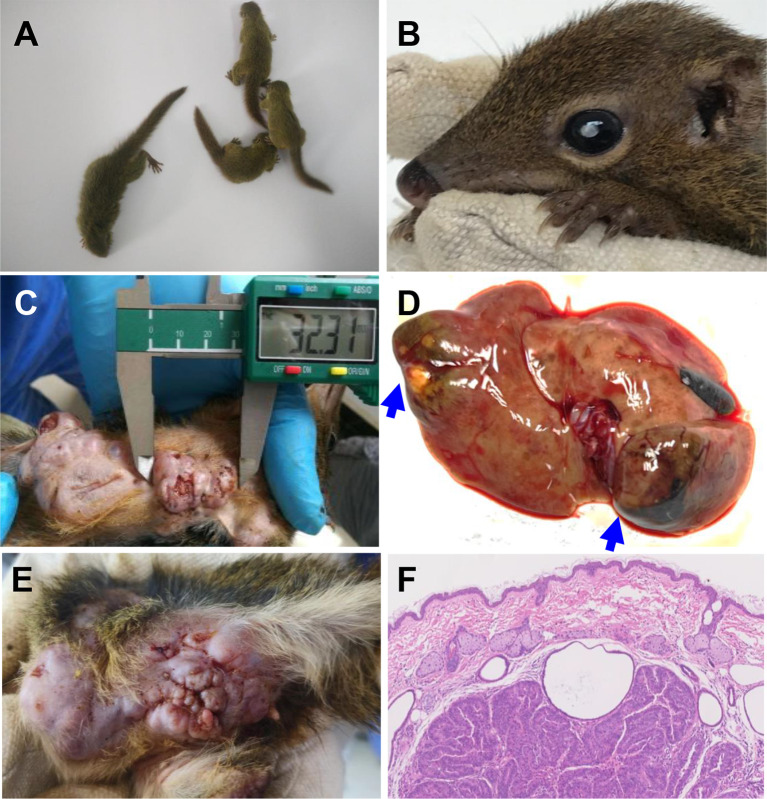
As anticipated, we observed various spontaneous diseases in the inbred tree shrews, including fetal death, idiopathic short stature, spontaneous cataracts, and spontaneous skin, breast, and liver cancers (Figure 3). Positively, the occurrence of these spontaneous diseases is currently low in our inbred tree shrew colony, suggesting that once the infanticide issue is resolved, it will not hinder the expansion of the inbred tree shrew colony. The availability of inbred tree shrew families provides a valuable resource for investigating key genetic questions, such as mutation rates in pedigrees (Bergeron et al., 2023; Wu et al., 2024), inbreeding depression (Charlesworth & Willis, 2009; Hedrick & Garcia-Dorado, 2016), and mechanisms of spontaneous disease models.
Figure 3.
Spontaneous diseases in inbred tree shrew populations
A: Idiopathic short stature (ISS). Four tree shrews reached an age of 27 days. Tree shrew on the left is a normal individual, while three individuals on the right exhibit ISS. B: Spontaneous cataracts. C: Spontaneous breast cancer. D: Spontaneous liver cancer. Tumors are indicated by blue arrows. E: Spontaneous skin cancer. F: Histological hematoxylin & eosin-stained skin section of an individual with spontaneous apocrine carcinoma cancer.
BEHAVIORAL TESTS FOR TREE SHREWS
Tree shrews exhibit skittish and agile behavior, which complicates cognitive assessments. Early behavioral tests compared learning set formation among rodents, tree shrews, and primates (Leonard et al., 1966; Ohta, 1983; Ohta et al., 1984, 1985). Correct task rates indicate the ability of an animal to learn rules, enabling intelligence comparisons across species under standardized conditions. The performance of tree shrews was intermediate between that of rodents and primates, reflecting their evolutionary phylogeny. In further comparative research, Takahashi et al. (2008) examined transitive inference, a higher-order cognitive function for logical deduction, through a spatial discrimination task involving rats and tree shrews, while Mustafar et al. (2018) compared visual cue-based decision-making discrimination tasks involving humans, monkeys, tree shrews, and rats. Both studies found that tree shrews performed better than rats, highlighting their cognitive potential. Li et al. (2022) established a response-time perceptual decision-making paradigm for tree shrews, involving a contrast-discrimination perceptual decision task to generate informative choice and response time data. Analyses suggested that in addition to choice-related evidence accumulation, time-tracking mechanisms may influence decision-making, especially when trial delays follow incorrect responses (Li et al., 2022). Tree shrews were also used as a model for analyzing cognitive flexibility (Pan et al., 2022).
At present, the repertoire of behavioral assays tailored for tree shrews is limited, often adapted from rodent-based paradigms. Ohl et al. (1998) developed a modified hole-board test for tree shrews, incorporating an associative visual task and a memory-dependent spatial task. Tree shrews rapidly learned the associative task compared to the spatial task, with their performance significantly influenced by age and chronic stress (Li et al., 2024; Keuker et al., 2004; Ohl & Fuchs, 1999). Previous object recognition tasks assessing novelty preference found that tree shrews exhibited a pronounced exploratory bias towards novel or displaced objects (Khani & Rainer, 2012; Nair et al., 2014). Shang et al. (2015) demonstrated that tree shrews effectively associated an aversive stimulus (foot shock) with lighting conditions within a light/dark box. These findings validate the suitability of tree shrews in cognitive tasks originally designed for rodents and highlight the modulatory influence of age and health status on task performance, relevant for modeling cognitive disorders.
Deficits in spatial cognition are common among patients with early AD (Coughlan et al., 2018). As such, the development of sensitive behavioral assays is imperative for evaluating AD model validity. Li et al. (2023b) subjected tree shrews to a series of rodent-derived spatial cognitive tasks, including the radial-arm maze, cheese-board maze, and water maze, to assess the sensitivity of these tasks to hippocampal lesion-induced spatial memory impairment. Their findings revealed that bilateral hippocampal lesions significantly impaired performance in all tasks, with the cheeseboard task emerging as the most sensitive indicator of spatial memory loss, potentially serving as a valuable tool for monitoring progressive cognitive decline in aging and AD models.
Furthermore, with proper equipment and behavioral paradigms, complex behaviors such as cooperation (Nowak, 2006, 2012; West et al., 2021) and social avoidance (Gellner et al., 2021; Toth & Neumann, 2013), as reported in other species, can also be investigated in tree shrews. Jiang et al. (2021b) recently designed a temporal coordination task to quantitatively assess cooperative behavior in mice, rats, and tree shrews and found that tree shrews exhibited the best cooperative abilities and strategies among the three species in the automated behavioral paradigm. Additionally, Ni et al. (2020) employed a modified social preference-avoidance test to assess social behavior towards unfamiliar conspecifics and found that male tree shrews showed social avoidance behavior while male mice showed prosocial behavior. These studies demonstrate the capability of tree shrews to serve as animal models for research on social avoidance and prosocial behaviors.
Reports on the behavioral activities of wild tree shrews remain scarce, limiting our understanding of this animal and its use in disease models. Despite having non-opposable thumbs, tree shrews are capable of grasping objects. Notably, based on forced-food grasping experiments, Maille et al. (2013) found that tree shrews exhibit paw preference at an individual level, but not at a population level, and that paw laterality can be learned. We observed an unusual restricted walking behavior in tree shrews during training sessions for various tasks (unpublished data; Supplementary Material: Video), the significance of which is unknown. By introducing artificial intelligence (AI) and deep learning-based action recognition techniques, similar to those used for NHPs and mice (Bala et al., 2020; Huang et al., 2021; Li et al., 2023c; Marks et al., 2022; Zhang et al., 2023b), and optimizing automated behavior classification systems for tree shrews, we expect to achieve better quantification of behaviors, thereby enhancing our understanding of pose estimation, inference, and behavior classification in this species.
TREE SHREW BRAIN ATLAS
Understanding the morphological structure, projections, circuits, and neural cell types of the brain is crucial for unraveling the behavioral, neural, and molecular mechanisms underlying normal brain function and neurological disorders (Qiu et al., 2024; Tian et al., 2022). With their high brain-to-body ratio, tree shrews have received considerable attention in recent decades.
Development of tree shrew brain atlas
The tree shrew nervous system has been extensively investigated, focusing on the anatomy and stereotactic localization of the brainstem, spinal cord, cortex, and thalamus (Diamond et al., 1970; Marrocco et al., 1970; Shriver & Noback, 1967; Snyder et al., 1969; Tigges & Shantha, 1969). The first version of the tree shrew brain atlas, published in the late 1970s, represented a significant milestone in neurobiological research on tree shrews (Marrocco et al., 1970; Tigges & Shantha, 1969). Marrocco et al. (1970) provided brain outlines and subfields at 10 levels using the Horsley-Clarke system of coordinates, with the horizontal zero plane passing through the ear bars and infraorbital ridge and the vertical zero plane running perpendicular to the horizontal zero and passing through the interaural line. This atlas had a profound impact on subsequent neurobiological research on tree shrews. The second version of the tree shrew brain atlas in stereotaxic coordinates, developed by Yang et al. (1990), has been crucial for studying Chinese tree shrew neurobiology. This atlas, which contains 37 brain drawings and stained figures at approximately 500 μm intervals, features the horizontal zero plane running parallel to the plane that passes through the ear bars and incisor bar at 4 mm above the interaural line, while the vertical zero plane runs perpendicular to the horizontal zero plane and passes through the interaural line.
We developed the third version of tree shrew brain atlas, providing the first systematic nomenclature and detailed mapping of the tree shrew brain, including stained sections in the coronal, sagittal, and horizontal planes using the interaural and bregma reference systems (Zhou & Ni, 2016). The atlas also features coronal, sagittal, and horizontal planes of the whole brain obtained using 9.4T magnetic resonance imaging (MRI). This version has facilitated the experiments involving tree shrew brain stereotaxic injections, tracing projections among different brain regions, and recognizing specific nuclei. Researchers have also provided detailed descriptions of the localization and comparative anatomy of various brain regions, including the amygdala, hypothalamus, thalamus, cerebellar nucleus, and piriform cortex (Airaksinen et al., 1989; Flügge et al., 1994; Maher et al., 2021; McCollum & Roberts, 2014; Ni et al., 2014; Parra et al., 2019; Rice et al., 2011). These studies have identified more than 328 subregions and clarified the boundaries between subregions, such as the olfactory bulb, basal ganglia, septal region, preoptic region, striatum, hypothalamus, thalamus, hippocampus, mesencephalon, metencephalon, and myelencephalon.
We and other researchers later characterized the distribution and anatomical localization of key proteins in different cell types within the brains of tree shrews, rats, and mice, including vasopressin, oxytocin, vasoactive intestinal polypeptide (Ni et al., 2014), neuropeptide Y (Ni et al., 2015), glutamate transporter (Balaram et al., 2015), monoamine receptor (Palchaudhuri & Flügge, 2005), corticotropin-releasing factor (Shu et al., 2015), calbindin (Ni et al., 2018a), parvalbumin (Ni et al., 2018b), nitric oxide synthase, calretinin (Rice et al., 2011), and tyrosine hydroxylase (Huang et al., 2020; Ni et al., 2021). Studies on neurotransmitters and their receptors in stress-related tree shrew brain regions during stress responses have also provided insights into the structural plasticity of the hippocampus (Fuchs & Flügge, 1998; Magariños et al., 1996), dynamic levels of serotonin receptors and adrenergic receptors (Flügge, 1995, 1996; Flügge et al., 1997), changes in glucocorticoid and mineralocorticoid receptors in the hippocampus (Meyer et al., 2001), corticotropin-releasing factor in the paraventricular nucleus of the hypothalamus and central extended amygdala (Kozicz et al., 2008), and the regulation of corticotrophin-releasing-hormone upon acute stress (Fang et al., 2016). Comparative anatomical studies have also identified differences in neuropeptide Y and its receptors in response to stress between tree shrews and rats (Zambello et al., 2010).
Doublecortin is primarily and transiently expressed in newly born neurons in the subventricular zone (SVZ) and subgranular zone (SGZ), playing a crucial role in neurogenesis within the olfactory bulb and hippocampus. In tree shrews, doublecortin-immunoreactive neurons in the SVZ and SGZ display a mix of rodent and primate-like topographic characteristics (Ai et al., 2021). Most neural progenitors in the SVZ of the tree shrew neocortex are self-amplifying intermediate progenitor cells, which contribute to brain volume expansion and the generation of a six-layered neocortex (Yin et al., 2020). Additionally, the basal radial glia and other neural stem and progenitor cells involved in tree shrew neocortex development have been identified and characterized, showing a closer relationship to NHPs than to rodents (Römer et al., 2018). Neural stem cells from tree shrews also exhibit distinct characteristics that differ from those of rats (Hu et al., 2018).
Application of single-cell and spatially resolved transcriptomics (Piwecka et al., 2023; Vandereyken et al., 2023; Wu & Zhang, 2020) in the tree shrew brain will enhance our understanding of the diversity of neural cell types and provided a comprehensive profile of their generation during brain development. Recent research on the single-cell transcriptomes of two tree shrew fetal whole brains identified 20 cell clusters, potentially comprising 14 cell types, through integrated analysis of tree shrew, rhesus macaque, and human brains (Zhuang et al., 2023). Another recent study created an integrated single-cell transcriptomic atlas of the retina from tree shrews and representative vertebrates, offering insights into the evolution and innovation of neuronal cell classes and types (Hahn et al., 2023). Furthermore, ongoing efforts are in the process of developing a fine-grained single-cell transcriptomic atlas of the tree shrew brain.
Afferent and efferent connections in tree shrew brains
Whole-brain mapping in tree shrews has revealed afferent projections to the 5th cerebellar lobule and paramedian lobule of the cerebellum (Ni et al., 2018a). Dye tracing and viral tracing have provided details into the neural circuit connections in tree shrews, highlighting the structural and functional connections between various brain regions, such as the suprachiasmatic nucleus, caudate nucleus, putamen, accumbens nucleus (Ni et al., 2021), bed nucleus of the stria terminalis (Ni et al., 2016), visual cortex (Lyon et al., 2003; Petry & Bickford, 2019), pulvinar (Day-Brown et al., 2010), temporal cortex (Chomsung et al., 2010), striate cortex (Bosking et al., 1997; Muly & Fitzpatrick, 1992), hypothalamus, and raphe (Reuss & Fuchs, 2000). These identified connections offer valuable insights into the conservation of neural pathways during mammalian brain evolution. The MRI atlas of the tree shrew provides high spatial resolution and detailed in situ brain structure data, facilitating the analysis of neuroimaging data in tree shrews (Dai et al., 2017, 2018; Huang et al., 2018; Wang et al., 2013). Additionally, the Allen Mouse Brain Common Coordinate Framework (CCF) was introduced to support the mapping and visualization of brain-wide gene expression and mesoscale connectivity (Wang et al., 2020d). Developing a standard 3D reference atlas of the tree shrew brain will be very useful for mapping and visualizing individual subregions, cell types, molecular distributions, and connections.
Currently, whole-brain imaging datasets with anatomical annotation at single-neuron resolution have been achieved in mice (Gong et al., 2016; Qiu et al., 2024) and zebrafish (Kunst et al., 2019). Recent advances in spatially resolved single-cell RNA sequencing have enabled the whole-brain mapping of molecularly defined cell types in mice (Zhang et al., 2023a), which could also be applied to the tree shrew brain. Combining single-cell tracings with detailed annotation of whole-brain regions in tree shrews will allow for the generation of a mesoscale connectome, crucial for understanding brain function and structure. Of note, current advancements provide an ideal opportunity to map large brains at subcellular resolution (Shen et al., 2022), thereby helping to uncover the intricacies of the tree shrew brain.
TREE SHREW VISUAL SYSTEM AND VISUAL CORTEX
The visual system of the tree shrew has been extensively studied over the past few decades, providing valuable insights into the anatomy, projections, circuits, and functions of the visual cortex and subcortical regions as well as the complexity of brain function (Baldwin et al., 2013; Bosking et al., 1997; Chomsung et al., 2010; Fitzpatrick, 1996; Kaas et al., 1972; Norton et al., 1985; Rockland & Lund, 1982; Ungersböck et al., 1991). Fitzpatrick (1996) reviewed the functional organization of local circuits in the visual cortex, focusing on the striate cortex, while Petry & Bickford (2019) provided a comprehensive review of the second visual system of the tree shrew. New approaches have further refined our understanding of the visual system in tree shrews.
Using intrinsic signal optical imaging and electrophysiology, Bosking et al. (2002) elucidated the spatial distribution patterns of population activity in the primary visual cortex (V1), demonstrating that thin line stimuli activate large populations of neurons in the V1, with significant overlap in activity from stimuli presented in nearby visual space. Mooser et al. (2004) depicted the morphological basis for orientation tuning in the V1 of tree shrews, revealing that the anisotropic arrangement of axon terminals is the principal source of orientation bias from feedforward connections. Further research into cross-orientation suppression has indicated that superimposed gratings affect V1 population responses through precise divisive suppression, supporting population coding in this region (MacEvoy et al., 2009). Comparisons of functional maps of orientation preference across species, including tree shrews, have shown a universal pattern in the evolution of orientation columns in the visual cortex (Kaschube et al., 2010). These orientation maps, established via moiré interference of regularly spaced ON (light-responsive)- and OFF (dark-responsive)-center retinal ganglion cell mosaics, provide a blueprint for the early development of cortical maps and receptive fields in the visual cortex of tree shrews (Paik & Ringach, 2011, 2012). Using two-photon imaging of GCaMP6 calcium signals, Lee et al. (2016) mapped the receptive fields of layer 2/3 neurons in the tree shrew visual cortex, identifying novel features of ON-OFF input convergence that support an invariant columnar architecture tailored to specific topographic constraints. Veit et al. (2014) examined receptive field structure, stimulus selectivity, response modulation, and orientation tuning for single neurons in the V1 of tree shrews, providing further evidence for the conservation of V1 functional organization between tree shrews and NHPs, and highlighting the role of intracortical recurrent processing in shaping V1 response properties (Veit et al., 2014).
Recent studies have provided more fine-grained details into neural coding in the tree shrew visual cortex. Sedigh-Sarvestani et al. (2021), using a chronic in vivo two-photon calcium imaging approach, identified a sinusoidal transformation of the visual field that forms the basis for periodic maps in the secondary visual area (V2) in the tree shrew, suggesting that cortical circuits can flexibly implement solutions for sensory surface representation. Similarly, Schumacher et al. (2022) used the same calcium imaging method to monitor V1 neurons in tree shrews during a Go/No-Go fine orientation discrimination task and observed selective and persistent learning-induced plasticity in the V1, supporting the notion that visual discrimination improves with training. Tanabe et al. (2022) discovered that tree shrew V1 neurons exhibit strong tuning to binocular disparity, comparable to that in primates, with computational modeling further suggesting that orientation-specific recurrent circuitry in the tree shrew V1 may underlie the observed tuning. These findings underscore the value of using tree shrews in studying binocular vision and demonstrating the role of cortical columns in computing stereoscopic depth.
Listing all findings from previous studies on the visual system and visual cortex of tree shrews is beyond the scope of this review. Nonetheless, research has significantly advanced our understanding of key questions about the visual system and helped reconstruct functional maps and connections. The introduction of more quantitative behavioral tests and neural modulation via optogenetic stimulation (Huang et al., 2014; Savier et al., 2021; Wang et al., 2023a) is expected to uncover the mechanisms of visual perception at multiple levels. Indeed, Wang et al. (2023a) demonstrated that optogenetic activation of CamKII projection neurons in the visual thalamus can be perceived by tree shrews. The transition from visual to optogenetic stimulus detection suggests that visual thalamus activation triggers visual percepts, paving the way for a new generation of visual prostheses based on thalamic optogenetics.
GENETIC CHARACTERIZATION OF TREE SHREW IMMUNE SYSTEM
Before the completion of tree shrew genome sequencing (e.g., Fan et al., 2013), the cloning and characterization of immune genes in the tree shrew were rather uncommon due to the lack of genetic information, with amplification primer pairs typically designed based on other species. These earlier studies provided a glimpse into tree shrew immune genes. For instance, Oppelt et al. (2010) performed the first characterization of major histocompatibility complex (MHC) class II DRB genes in tree shrews, identifying 14 DRB haplotypes in 230 individuals. Zhang et al. (2013) analyzed the genomic organization of tree shrew MHC class I genes, finding high homology between tree shrews and primates. Mu et al. (2014) characterized the tree shrew TRIM5 family, discovering that tree shrew TRIM5-cyclophilin A (TRIMCyp) fusion gene represents a new paradigm of novel gene origination.
With the release of assembled tree shrew genomes using second-generation (Fan et al., 2013) and third-generation sequencing technology (Fan et al., 2019), retrieving specific gene sequences has become more accessible via public databases, such as treeshrewDB (www.treeshrewdb.org; Fan et al., 2014; Ye et al., 2021) and TupaiaBase (http://tupaiabase.org; Sanada et al., 2019a). This genomic information has facilitated the characterization of tree shrew genes and gene families. Zhang et al. (2020b) studied the heavy chain constant region of immunoglobulin (Ig) in tree shrews and found that tree shrews possess only four classes of antibodies (IgM, IgG, IgE, and IgA), lacking IgD, with the oldest IgM antibody showing high homology with that of primates and recombinant IgG showing effective immuno-protective effects against HSV-1 infection and superior bactericidal effects compared to mouse IgG (Zhang et al., 2020b). We characterized the tree shrew guanylate-binding protein (GBP) family, which belongs to IFN-inducible GTPases and defends the host against invading pathogens, revealing five GBP genes (tGBP1, tGBP2, tGBP4, tGBP5, and tGBP7) that are ubiquitously expressed in a variety of tissues (Gu et al., 2019a). All the tGBP genes can be inducible by IFN-γ treatment or viral infections, with tGBP1 exhibiting antiviral activity against vesicular stomatitis virus (VSV) and HSV-1 (Gu et al., 2019a). Mechanistically, tGBP1 interacts with STING to initiate autophagy and restrict HSV-1 infection (Gu et al., 2021a), while it interacts with the VSV phosphoprotein and represses primary transcription of the viral genome (Gu et al., 2021b).
We also characterized 2',5'-oligoadenylate synthetase (OAS), a member of the ISG family that synthesizes 2',5'-oligoadenylate (2-5A) in tree shrews (Yao et al., 2019). Results indicated that tree shrews contain four putative OASs (tOASs, including tOAS1, tOAS2, tOASL1, and tOASL2, but no tOAS3) constitutively expressed in various tissues. These tOASs are up-regulated upon viral infection, leading to the inhibition of DNA and RNA viral replication in renal cells (Yao et al., 2019). Notably, tOASL1 knockdown impairs antiviral immune response, while its overexpression potentiates RNA virus-triggered antiviral responses (Yao et al., 2020). Mechanistically, tOASL1 promotes cellular antiviral immune responses by recruiting melanoma differentiation-associated gene 5 (MDA5), a cytoplasmic dsRNA sensor, to mitochondrial antiviral signaling gene (MAVS), and immune signaling adaptor, enhancing their interaction (Yao et al., 2020). Additionally, the tree shrew genome contains multiple copies of cyclooxygenase and lipoxygenase genes (ALOX isoforms), including four copies of ALOX15 (tupALOX15a-d), compared to the single copy found in humans and mice (Schäfer et al., 2020). The enzymatic properties of tupALOX15a and tupALOX15c show subtle differences and adhere to the “Triad Concept and Evolutionary Hypothesis of ALOX15 specificity” (Schäfer et al., 2020). Further characterization of these ALOX isoforms in tree shrews has important biological implications due to their role in the biosynthesis of inflammatory mediators.
A deeper understanding of the functions of tree shrew immune genes will provide valuable insights into their susceptibility to viral infections and their innate and adaptive immunity. Our previous research showed that the tree shrew TRIMCyp lacks antiviral activity against human immunodeficiency virus 1 (HIV-1), macaque simian immunodeficiency virus (SIVmac), and N-tropic murine leukemia virus (N-MLV) infections due to a mutation in the RING, B-box, and coiled-coil (RBCC) motif, a signature motif of the TRIM family (Mu et al., 2014). This may explain the susceptibility of tree shrews to many human viruses. We also characterized the apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3 (APOBEC3) family, which contains several antiviral factors that inhibit the replication of various human viruses, including HIV-1 and hepatitis B virus (HBV) (Sadeghpour et al., 2021; Stavrou & Ross, 2015), in tree shrews. Notably, we identified five APOBEC3 members (tsAPOBEC3s) highly expressed in immune-related tissues, which can be up-regulated by interferon-α2β treatment and edit both strands of HIV-1 DNA via deaminase-dependent mechanisms (Luo et al., 2018). The tsAPOBEC3 proteins have been shown to restrict HBV replication, possibly explaining the failure to establish a persistent HBV infection model using tree shrews (Luo & Zheng, 2020). Using tree shrew primary cells from a variety of tissues, Luo et al. (2021) showed that these cells are permissive to pseudotyped HIV-1. Tree shrew lung fibroblasts with transduction of retroviral vectors for human CD4 and CCR5 support HIV-1 entry and replication, but viral infectivity is suppressed by tsAPOBEC3 proteins, especially tsA3Z2c-Z1b (Luo et al., 2021). Similarly, the natural loss of the retinoic acid-inducible gene I (RIG-I) in tree shrews (Fan et al., 2013) results in functional replacement by MDA5, another key cytoplasmic sensor of viral pathogen-associated molecular patterns (Xu et al., 2016). The change in the RIG-I-like receptor (RLR)-mediated signaling pathway (Onomoto et al., 2021; Rehwinkel & Gack, 2020; Zheng et al., 2023) in tree shrews is accompanied by alternative splicing of tree shrew STING, with short and long STING isoforms playing different roles in modulating anti-RNA virus responses (Xu et al., 2020b). In addition, although tree shrew MAVS (tMAVS) is generally evolutionarily and functionally conserved (Xu et al., 2015), the cleavage pattern of tMAVS by the NS3/4A protease of the hepatitis C virus (HCV) differs from that of humans, leading to impaired IRF3-mediated induction of IFN-β but maintained NF-κB signaling in tree shrew primary hepatocytes upon HCV infection (Xu et al., 2020c). Consequently, tMAVS serves as a dual target for innate immune evasion and restriction of viral replication via NF-κB in tree shrew hepatocytes during HCV infection, potentially explaining why tree shrews can be acutely infected by HCV but do not develop persistent infections (Xu et al., 2020c).
Characterizing the genetic and functional aspects of tree shrew immune genes has not only enhanced our understanding of the uniqueness and evolutionary conservation of the tree shrew immune system but has also helped clarify the susceptibility of tree shrews to specific viral infections and the difficulties in establishing persistent infection models (e.g., HBV and HCV). By identifying potential restriction factors and utilizing genetic modification of target genes, it is anticipated that better tree shrew infection models will be established in the future.
GENETIC MODIFICATION OF TREE SHREWS
Unlike NHPs, tree shrews do not exhibit a menstrual cycle. Additionally, female tree shrews do not display vaginal plugs after mating, which are commonly produced by males after mating to physically obstruct the female genital tract and prevent remating (Fromhage, 2012; Stockley et al., 2020). As such, the in vitro fertilization techniques used for NHPs (e.g., Sun et al., 2008) cannot be applied to tree shrews, nor can the standard genetic modification procedures used for rats (Hirabayashi et al., 2019), mice (El Marjou et al., 2021), rhesus monkeys, and crab-eating monkeys (Luo et al., 2016; Niu et al., 2010; Sasaki et al., 2009; Zhao et al., 2019). Spermatogonial stem cells (SSCs) continuously produce spermatozoa in the testes (Kubota & Brinster, 2018) and are utilized for generating genetically modified animals (Feng et al., 2002; Wu et al., 2015). Given the difficulties of collecting tree shrew embryos (Yan et al., 2016) to establish embryonic stem cells, or the difficulties in collecting tree shrew oocytes and sperm for in vitro fertilization, we established an SSC line for successful genetic manipulation of tree shrews. We developed a culture system for tree shrew SSC expansion and created transgenic tree shrews using lentiviral transduction (Li et al., 2017). This SSC-based gene editing approach paves the way for further genome manipulation of tree shrews. To date, we have generated transgenic tree shrews overexpressing causal genes for familiar AD, such as amyloid-beta precursor protein (APP) and presenilin 1 (PSEN1) mutants (Knopman et al., 2021; Zhang et al., 2019a), with preliminary analyses, including cognitive and pathological tests, revealing neuroinflammation in brain tissues and cognitive impairment at an early age (unpublished data).
The successful culture of tree shrew SSCs also established a basis for precise genome editing using advanced gene editing techniques such as base editing (Pfeiffer & Stafforst, 2023). However, two significant obstacles remain regarding genetic modifications using SSCs. Firstly, the efficient delivery of genome editors into tree shrew SSCs has proven to be more challenging than anticipated. We are currently optimizing the vector and RNA delivery systems via electroporation. Promising in vivo delivery systems for gene therapy, especially for AAV and lipid nanoparticles (Madigan et al., 2023; Taha et al., 2022), are being evaluated for their application potential in tree shrew SSCs. Secondly, long-term culture of tree shrew SSCs (>50 passages) leads to a loss of spermatogenesis ability due to DNA damage accumulation and mitochondrial dysfunction (Li et al., 2023a). Supplementation with nicotinamide riboside has shown beneficial effects on the long-term culture of tree shrew SSCs (Li et al., 2023a). Thus, limiting culture passages should help minimize the deleterious effects of prolonged culture on gene editing.
To date, few studies have reported on the transient delivery of exogenous genes into tree shrew organs and tissues. We tested several types of AAVs and achieved consistent expression of exogenous genes in adult tree shrew brain tissues using AAV9 and AAV8 (unpublished data). Knockdown of the X-linked primate-specific gene SSX family member 1 (SSX1) in tree shrews, using Ssx1-shRNA-containing and EGFP-expressing AAV9 vectors delivered via injection into the seminiferous tubules, successfully mimics asthenoteratozoospermia phenotypes observed in human patients with SSX1 deleterious variants, providing a model for studying testis-enriched primate specific genes in spermatogenesis (Liu et al., 2023). Xu et al. (2017) explored genetic manipulation in the developing tree shrew brain using in utero electroporation (IUE) and retroviral delivery of short-hairpin RNAs, demonstrating the feasibility of gene manipulation using these methods for establishing tree shrew models of neurodevelopmental diseases.
Given the differing time and cost requirements for genetic modifications at the germline level using the SSC procedure (Li et al., 2017) versus the tissue level using transient exogenous gene delivery, researchers must balance these approaches. We advocate for the use of AAV- or lipid nanoparticle-mediated delivery of certain genes or gene editors into tree shrew tissues to create disease models due to the shorter timeframe and relatively lower cost. Nonetheless, the choice of procedure depends on the research goals and tree shrews should only be used following the 3R principles. In addition to generating genetically modified tree shrews, the establishment of SSCs and related culture systems (Li et al., 2017) provides a rapid platform for characterizing pathogenic genes causing idiopathic male infertility, including oligozoospermia, teratozoospermia, and asthenozoospermia, especially for primate-specific genes (Shao et al., 2019). Testing semen samples and testis tissues after transplantation and recovery of genetically modified SSCs with pathogenic mutations can elucidate the pathogenesis of male infertility and create valid disease models, as had been demonstrated by Liu et al. (2023).
WHY HAVE RECENT TREE SHREW DISEASE MODELS NOT LED TO BREAKTHROUGHS?
In recent years, tree shrews have been increasingly used to create animal models for different diseases, particularly infectious diseases (Kayesh et al., 2021; Li et al., 2018b; Xia et al., 2022; Xu et al., 2020a; Wang et al., 2023b; Zhang et al., 2019b; Zhao et al., 2020b) and cancer research (Jiang et al., 2017; Tu et al., 2019; Zeng et al., 2023). Various studies have also explored the effects of aging on tree shrew brains (Li et al., 2024; Rodriguez-Callejas et al., 2020; Wei et al., 2017) and the potential efficacy of natural products against AD (Wang et al., 2020c; Yang et al., 2022). Parkinson’s disease models of tree shrew were also induced by using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; Ma et al., 2013) and its oxidized product 1-methyl-4-phenylpyridinium (MPP+; Li et al., 2023d). We have provided a summary of these tree shrew models in the context of face validity, predictive validity, and construct validity (Table 1), as several excellent review papers have already been published. For instance, Cao et al. (2003) and Xiao et al. (2017) provided summaries of tree shrew models reported before 2017; Li et al. (2018b) and Kayesh et al. (2021) provided comprehensive reviews of the use of tree shrews in establishing human viral infection models; and Lu et al. (2021) reviewed tree shrew tumor models and their clinical manifestations. These reviews consistently concluded that tree shrews hold considerable promise as powerful animal models for studying human diseases.
Table 1. Summary of recent tree shrew models for human diseases.
| Disease / intervention | Modeling methods | Phenotypes and symptoms | Potential mechanism, drug efficacy, and limitations | References |
| We only compiled publications from January 2017 to May 2024, and summarized the main symptoms of each tree shrew model. Please refer to the original publication for additional details. | ||||
| Naturally aging | Adult (mean age 3.8 years), old (mean age 6 years), and aged (mean age 7.5 years) tree shrews | Aged tree shrews showed increased activated microglia labeled with ferritin and dystrophic microglia labeled with Iba1, increased oxidative RNA damage, as revealed by 8-hydroxyguanine (8-OHG) staining in all hippocampal regions, enhanced Tau hyperphosphorylation in dentate gyrus (DG), CA3, and subiculum (SUB) subregions, and phagocytic inclusions of cellular debris or neurons containing tau aggregates and/or damaged RNA in activated M2 microglia. Adult and old tree shrews harbored increased hypertrophic astrocytes in hippocampal region, while aged animals contained significant atrophic astrocytes compared to younger animals. |
Mechanism: Aged tree shrews showed increased oxidative stress, phosphorylated tau, and dystrophic microglia in hippocampus; S100A10, a protein overexpressed by neuroprotective-type astrocytes, occurred in the nucleus of hypertrophic astrocytes of young animals, but in the cytoplasmic compartment of atrophic astrocytes in aged animals. Aged tree shrews could be used to study brain and microglial alterations during aging process. Limitations: Only histochemical and immunofluorescence assays were performed, with no characterization of molecular mechanisms and potential targets. |
Rodriguez-Callejas et al., 2020; 2024 |
| Adult (about 1 year old) and aged (6 years old or older) tree shrews | Aged tree shrews showed impaired cognitive performance, as revealed by pole-board and novel object recognition tests, increased Aβ accumulation and phosphorylated Tau protein, synaptic degeneration, neuronal loss, and gliosis in cortical and hippocampal tissues. |
Mechanism: Aged tree shrews showed increased levels of Aβ40, Aβ42, total-Aβ, and phosphorylation of Tau at Ser202/Thr205 and Thr231, decreased levels of PSD95 and NeuN, and increased levels of GFAP and Iba1 in cortical and hippocampal tissues. Limitations: No characterization of natural aging pattern was performed at omics and single-cell levels. |
Li et al., 2024 | |
| Alzheimer’s disease (AD) | Adult male tree shrews (15 months old) received an intracerebroventricular (icv) injection of Aβ1-40 | Aβ1-40-treated tree shrews showed cognitive impairments, hippocampal atrophy, increased apparent diffusion coefficient (ADC) and fractional anisotropy (FA) based on diffusion tensor magnetic resonance imaging (DTI) analysis, thinner and smaller cells in hippocampal CA3 and DG subregions and reduced number of cells in DG, neurotic plaques and neurofibrillary tangles (NFTs) in hippocampus, and cell apoptosis. |
Mechanism: Expression levels of apoptosis related genes, including Bad, IAP, and Cytochrome c, were down-regulated, expression level of TNF-R1 was up-regulated, differentially expressed genes were enriched in AD pathways and other biological processes. Limitations: Sample size was too small for robust conclusions and interpretation of molecular mechanism may be biased based on transcriptome analysis alone. |
Lin et al., 2016 |
| AD and donepezil | Adult male tree shrews (1–1.5 years old) received icv injection of Aβ1-40, followed by daily treatment with donepezil (1 mg/kg) | Aβ1-40-treated tree shrews showed spatial cognitive deficits, body weight loss, hippocampal atrophy with lateral ventricle enlargement, enlarged temporal horn and increased ADC in DTI analysis, Aβ deposits, neuronal loss, and glial activation. Donepezil ameliorated all symptoms. |
Mechanism: Expression of choline acetyl transferase (ChAT) and BDNF and phosphorylation of TrkB were decreased, acetylcholinesterase (AChE) activity and fibrillary acid protein (GFAP) expression were increased, Aβ1-40 injection affected cholinergic system and BDNF/TrkB-signaling pathway. Drug efficacy: Donepezil rescued gene expression abnormalities caused by Aβ1-40, and showed a protective effect. Limitations: Detailed description of data analyses lacking, and independent validation still required. |
Zheng et al., 2018 |
| AD and Ginsenoside Rg1 (GRg1) | Adult male tree shrews (6 months old) received an Aβ25–35 injection in both hippocampal regions, with daily dose of D-galactose (D-gal) at 125 mg/kg; treatment groups included intragastric (ig) administration of donepezil daily (3 mg/kg) and GRg1 | Aβ25–35-injected group showed impaired learning and memory in Morris water maze test, increased Tau expression in hippocampal and cortical tissues, altered bacterial communities, increased oxidative stress damage, microglial activation, inflammation, and apoptosis in hippocampal and cortical tissues. Donepezil and high concentrations of GRg1 ameliorated impaired learning and memory ability and related abnormalities in tree shrews treated with Aβ25–35 and D-gal. High concentrations of GRg1 reduced Tau-positive cells and changed abundances of Proteobacteria, Verrucomicrobia, and Lactobacillus in gut microbiota |
Mechanism: Tree shrews treated with Aβ25–35 and D-gal showed increased tau expression and altered gut microbiota composition and abundance, increased Aβ1-42, BACE1, and phosphorylated Tau levels, enhanced oxidative stress injury, as revealed by increased nitrotyrosine and 8-OHG levels and decreased expression of SOD1, increased expression of Iba1 and IL-1, and decreased levels of MAP2, NeuN, β-catenin, and phosphorylated GSK-3β. Drug efficacy: Donepezil and high concentrations of GRg1 decreased Tau expression, reversed altered levels of related proteins, exerted a neuroprotective effect, and altered gut microbiota. Limitations: No detailed targets or mechanisms were tested. |
Wang et al., 2020c; Yang et al., 2022 |
| Parkinson’s disease (PD) induced by MPP+ | Male tree shrews (1.5 years old) received a unilateral nigral injection of 50 μg MPP+ | MPP+-treated tree shrews manifested PD clinical symptoms, including motor deficits (difficulties in forelimb use, loss of balance, stooped posture), bradykinesia, rest tremor, gait abnormalities, postural instability, and apomorphine-induced rotations; increased PD score based on improved Kurlan scale; marked dopaminergic neuron loss (up to 95%) in substantia nigra on lesioned side of the brain |
Mechanism: Precise injection of MPP+ into substantia nigral region depleted local dopaminergic neurons and resulted in PD-like symptoms in tree shrews. Limitations: No test for Lewy body-like pathology and related changes in circuit; no test for treatments such as stem cell transplantation and related drugs. |
Li et al., 2023d |
| Cerebral ischemia (CI) | Adult male tree shrews (6-months old) received permanent distal middle cerebral artery occlusion after anesthesia | Tree shrews in CI group exhibited a decline in aggressive behavior, seeking behavior, and voluntary activity, and showed no response to stimuli and increased neural behavior scores (higher scores represent more severe damage). Magnetic resonance imaging showed high signals at infarct area. 2,3,5-triphenyltetrazolium chloride (TTC) staining revealed a marked white infarction. Defective cortical structure and deep-stained nuclei were observed in cortex of CI group. Most neurons shrank in size, with a reduced number of NeuN- and GFAP-positive cells around lesion epicenter in the CI group relative to the sham group. |
Mechanism: Cerebral ischemia in tree shrews was demonstrated by alterations in neural behavior score and pathological analyses for neural cell morphology and infarct area. Limitations: Drug efficacy was not tested and no molecular target was identified. |
Wang et al., 2019c |
| Depression and clomipramine | Adult male tree shrews were subjected to chronic psychosocial stress induced by social defeat between dominant and subordinate animals | Socially defeated tree shrews exhibited a decrease in sucrose preference, exploration behaviors, locomotion distance, and social interaction. Oral clomipramine administration reversed almost all depression-like behaviors, but not motivational reduction. |
Mechanism: Chronic social defeat induced a broad spectrum of behavioral changes, including anhedonia, motivation reduction, and social avoidance, with no report of potential mechanism. Drug efficacy: Clomipramine had a therapeutic effect on socially defeated animals, but no effect on rescuing motivational reduction. Limitations: No detailed target for clomipramine was analyzed and no molecular mechanism regarding why clomipramine had no effect on reversing reduced motivation in socially defeated tree shrews was provided. |
Shen et al., 2018 |
| Cocaine motivation | Adult male tree shrews were subjected to cocaine self-administration (SA) | Tree shrews were trained to acquire cocaine SA. Cocaine craving (as measured by number of active nose-pokes) gradually increased, then declined along cocaine withdrawal timeline; incubation of cue-induced cocaine craving occurred on withdrawal day (WD) 45. Knockdown of Cav1.2 inhibited cocaine craving on WD45. |
Mechanism: Striatal dopamine D1 receptor (D1R), but not D2R, mediated up-regulation of Cav1.2, showing a time-dependent increase in nucleus accumbens (NAc) during incubation of cocaine craving. Drug efficacy: Injection of L-type calcium channels antagonist verapamil in NAc decreased cocaine-seeking behaviors. Limitations: D1R-Cav1.2 signaling underlying incubation of cocaine craving needs validation, especially in the context of cocaine relapse. |
Duan et al., 2021 |
| Cocaine-seeking habit | Adult male tree shrews were subjected to cocaine and sucrose SA, compared to sucrose-habitual behavior and cocaine-seeking habitual behavior | Habitual cocaine-seeking behaviors (HCSB) and habitual sucrose-seeking behaviors (HSSB) were established in tree shrews. |
Mechanism: Animals with HCSB exhibited higher D1Rs and Cav1.2 expression but lower D2Rs and Cav1.3 expression in the putamen, while animals with HSSB exhibited lower membrane expression of D2R in the putamen than animals with non-habitual behavior. Limitations: Imbalanced function between putamen and nucleus caudate and the neural circuits need to be further determined. |
Duan et al., 2022 |
| Glaucoma / ocular hypertension | Adult tree shrews (1.7-7.7 years old) were injected with ferromagnetic beads (50 μL of a 25 mg/mL solution) into anterior chamber, which were then directed into iridocorneal angle by a magnet to block aqueous outflow | Tree shrews injected with ferromagnetic beads (treated group) showed significantly increased intraocular pressure (IOP) relative to control group during 12-week follow-up period, average of 22.7±3.6 mm Hg. Significant retinal nerve fiber layer (RNFL) was thinner and optic nerve axon counts and density were reduced in treated group, along with progressive bowing of lamina cribrosa and posterior displacement of anterior laminar surface in tree shrews with increasing severity of experimental glaucoma |
Mechanism: Reductions in RNFL thickness and optic nerve axon counts and density were associated with IOP elevation. Optic nerves in the treated group showed histopathology consistent with glaucomatous optic neuropathy Limitations: No drug efficacy was tested |
Samuels et al., 2018 |
| Hyperopia | Starting at 24±1 days of visual experience, juvenile tree shrews (6 weeks old) received light treatment through amber filters (BPI 500/550 dyed acrylic) either atop cage (Filter group, 300–400 lux) or through amber-dyed lenses fitted into a goggle frame (Goggle group). Age-matched animals raised in standard lighting (100–300 lux) were used as the control group | Animals in Filter group became progressively more hyperopic, whereas animals in the Goggle and control groups showed continued normal emmetropization during 11-day treatment period. Significant hyperopia (mean [SE] = 3.5 [0.6] D) was observed in Filter group compared to Goggle (0.2 [0.8] D) and control groups (1.0 [0.2] D). Filter group had significantly thicker choroid at the end of 11-day treatment. Vitreous chamber depth in Filter group decreased with increasing hyperopia, whereas that in the Goggle and control groups was similar. End-of-treatment vitreous chamber depth was significantly smaller than that in the other two groups. |
Mechanism: Exposure to ambient amber light caused substantial hyperopia in tree shrews but had no effect on refractive error in Goggle group. Limitations: No drug efficacy or prophylactics was tested. |
Khanal et al., 2021 |
| Choroidal neovascularization (CNV) | Adult tree shrews (2 years old) were subjected to CNV by laser photocoagulation | Retinal edema and damaged Haller’s layer in choroid occurred 7 days after photocoagulation; after day 14, neovascularization in retina destroyed ganglion cell layer, disrupted nerve fiber layer, loosened Haller’s and Sattler’s layers, with neovascularization surrounded by neutrophils, macrophages, and fibroblasts; microvessel density increased. |
Mechanism: Choroid and retinal transcriptomic data were used to identify differentially expressed genes during CNV; signaling pathways, including fatty acid metabolic pathway and cell adhesion molecular signaling, and genes in ribosomal protein family and complement system were involved in tree shrew CNV. Limitations: No drug efficacy was tested and no molecular target was verified. |
Jia et al., 2021 |
| Chronic experimental autoimmune uveitis (EAU) | Adult tree shrews (4–8 months old) were subcutaneously immunized at tail base and both thighs with six inter-photoreceptor retinoid-binding proteins (IRBPs) of tree shrews (R14, IRBP1197-1211, R16 and IRBP1041-1071) and bovines (R14 and R16) | IRBP1197–1211 and R14 induced ocular inflammation and chronic EAU (including conjunctival hyperemia, ciliary injections, whitish hypopyon spots, and corneal ulcers and edema), with subretinal deposits and retinal damage. Inflammatory infiltration of innate immune cells and adaptive immune cells into conjunctiva, cornea, anterior chamber, ciliary processes, choroid, and retina, and retinal lesions were main histopathological changes. Subretinal deposits positively expressed Aβ, CD8, and P2RY12. RGS4 inhibitor CCG203769 and dihydroartemisinin significantly alleviated retinal pathological damage of IRBP1197-1211-induced EAU by decreasing expression of CD4 T-cells. |
Mechanism: Chronic EAU in tree shrews was elicited by bovine R14 and tree shrew IRBP1197-1211, characterized by retinal degeneration, retinal damage with subretinal Aβ deposits and microglial/macrophage infiltration, and T-cell response, likely by altering multiple signaling pathways including mitogen-activated protein kinase (MAPK) signaling. Limitations: No molecular target was experimentally verified. |
Hu et al., 2022 |
| Diabetic retinopathy | Male adult tree shrews received a single intraperitoneal injection of streptozotocin (STZ; 175 mg/kg) | In total, 75% of animals developed type 1 diabetes with sustained hyperglycemia; islet β cells were almost lost in the pancreas of diabetic animals; serum levels of glycated hemoglobin, free fatty acids, total cholesterol, high- and low-density lipoproteins, and triglycerides were increased; cone photoreceptor function was compromised as shown by diminished A- and B-wave photopic electroretinogram amplitudes; retinal function was lost; retinal ganglion cell (RGC) function was compromised, with loss of RGCs and peripheral short-wave sensitivity (SWS) cones. |
Mechanism: SWS cones and RGCs were lost after STZ injection, with increased expression of TRIB3, VEGF, and p-AKT/p-mTOR axis, but reduced levels of ISR-1 protein in diabetic retinas. Limitations: No drug efficacy was tested. |
Gorbatyuk et al., 2022 |
| Diabetic cerebral ischemia | Adult male tree shrews fed a high-fat diet for 8 weeks received an intravenous injection of 2% STZ (100 mg/kg body weight), with photochemically induced cortical thrombotic cerebral ischemia | STZ application resulted in a relatively high ratio (up to 77%) of successful diabetes modeling. Diabetic animals showed polydipsia, polyphagia, polyuria, thinned retina layers with disordered structures, with pathological retinal changes aggravated after cerebral ischemia. Ischemic postconditioning reduced pathological changes in diabetic retinopathy and alleviated retinopathy. |
Mechanism: Diabetes and cerebral ischemia increased retinal VEGF expression, which was reduced by ischemic postconditioning. Limitations: No drug efficacy was tested for diabetic retinopathy; molecular mechanisms of the ameliorating effects of ischemic postconditioning were not determined. |
Zhao et al., 2020a |
| Ischemia stroke and diabetes mellitus | Adult male tree shrews were treated with STZ (Zhao et al., 2020a), and thrombotic cerebral ischemic stroke was induced using photochemical treatment | Tree shrews with diabetes mellitus (DM) and DM ischemic stroke (DMIS) showed increased water intake, urine volume, serum glucose levels, altered biochemical indicators, and abnormal ultrastructure of cellular organelles; DMIS group exhibited a larger infarct size. Transcriptomic analysis of infarct tissues identified differentially expressed genes and enriched pathways. |
Mechanism: DM tree shrews showed increased IL-8 expression and activation of inflammatory status relative to healthy controls; DMIS group showed increased expression of CCL7, ATP-binding cassette sub-family A member 12, and adhesion G protein-coupled receptor E2 relative to DM group. Limitations: No drug efficacy was tested and no molecular target was verified. |
Zhao et al., 2021b |
| Ischemic postconditioning reduced infarct size and reduced nerve cell injury in tree shrews with DMIS. Transcriptomic analysis of ischemic cerebral tissues identified differentially expressed genes and enriched pathways. |
Mechanism: Ischemic postconditioning reduced inflammation and stress responses by decreasing activity of TNF and NF-κB signaling, and Fc gamma R-mediated phagocytosis. Limitations: No drug efficacy was tested and no molecular target was experimentally verified. |
Zhao et al., 2021c | ||
| Diabetes and regeneration of islet β-cells | Male tree shrews (8 weeks old) were intraperitoneally injected with STZ (150 mg/kg) | Increased fasting blood glucose and mean serum insulin levels after STZ treatment for 3 days; islets were smaller and deformed, with visible cell necrosis and degeneration, and number of islets was reduced; intra-islet cell regeneration was only scattered at day 7 after STZ treatment, with no regeneration of centroacinar cells |
Mechanism: Proportion and expression of insulin-positive cells in islets were decreased after STZ treatment; positive PDX-1 expression was observed in pancreatic interstitial cells and dendritic cells of pancreatic lymph; repair mechanism of STZ-injured islet β-cells in tree shrews was similar to that of humans. Limitations: No drug efficacy or stem cell transplantation therapy was tested. |
Zhao et al., 2018 |
| Diabetes mellitus and mesenchymal stem cell (MSC) transplantation | Male tree shrews were intraperitoneally injected (lower right quadrant of abdomen) with STZ (170 mg/kg), with intraportal transplantation of insulin-producing cells (IPCs) derived from human MSCs | Tree shrews showed increased blood glucose levels, loss of body weight, and no intact islets in the pancreas after STZ treatment; after IPC transplantation (which expressed islet-related genes and secreted high levels of insulin) one week after STZ treatment, blood glucose levels decreased to normal levels in diabetic animals and body weight recovered; liver tissues of diabetic animals with IPC transplantation showed extensive and diffuse edema and scattered insulin-positive cells in hepatic sinusoids. |
Mechanism: Transplantation of IPCs expressing insulin, NGN3, and PDX1, derived from MSCs, reversed hyperglycemia and weight loss in diabetic tree shrews induced by STZ. Limitations: Recovery of pancreatic islets in STZ-induced diabetes model was not examined, with a different mechanism compared to human diabetes; no follow-up study was conducted to monitor the fate and potential risk of malignancy of transplanted cells. |
Zhu et al., 2023 |
| Chronic stomach mucosal injury | Adult tree shrews (2-3 years old) were administered a daily intraperitoneal injection of MPTP (2 mg/kg/day) for 13 weeks | Tree shrews showed local congestion or diffuse hemorrhagic spots on the gastric mucosa, multiple regions of redness and bleeding on inner wall covered with white moss, or partial slight shedding of gastric mucosa; H&E staining of gastric mucosa showed immune cell infiltrations. |
Mechanism: Integration of transcriptomic and proteomic data revealed changes in mRNA and proteins in gastric mucosa, and identified several genes (e.g., RPL4, ANXA1, GAST, and DDC) involved in MPTP-induced mucosal injury. Limitations: No experimental assays to characterize the highlighted genes in MPTP-induced mucosal injury and affected signaling pathways were conducted. |
Wang et al., 2024a |
| Asthenoteratozoospermia | Adult male tree shrews (6 months old or older) were injected with TR-AAV9-Ssx1-shRNA (each 80 μL, dose of 1.5×1012 vector genome/mL) into seminiferous tubules of bilateral testes via rete testis for 60 days | Levels of Ssx1 mRNA and protein were significantly reduced in the testes of tree shrews with TR-AAV9-Ssx1-shRNA injection. Tree shrews with Ssx1 knockdown displayed reduced testis weight, impaired spermatogenesis or loss of germ cells in seminiferous tubules, abnormal sperm morphologies (absent, coiled, or angulated flagella; absence of peripheral or central microtubules at midpiece and principal piece of sperm flagella), and reduced sperm motility. |
Mechanism: Expression of multiple spermatogenesis-associated factors was dysregulated in testes of tree shrews with Ssx1 deficiency, and these factors were enriched in multiple biological processes involved in spermatogenesis Limitations: No detailed mechanism regarding SSX1 deficiency in spermatogenesis was determined and no drug efficacy was tested. |
Liu et al., 2023 |
| Breast cancer | Female tree shrews (6 months to 1 year old) were administered an intraductal injection of PI3KCA-H1047R or H-RAS-Q61L lentiviruses at different titers (3×107 TU/mL and 1.4×106 TU/mL, respectively) into 3rd pair of mammary glands | Incidence of breast tumor in tree shrews injected with PI3KCA-H1047R lentivirus was 41.7%–58.3% within 3–10 weeks after infection, but no breast tumors were detected in animals injected with H-RAS-Q61L lentivirus within 10 weeks. Dominant pathological type was intraductal papilloma and atypical hyperplasia, with only one case of invasive ductal carcinoma. Tumor sections were positive for progesterone receptor (PR) but negative for human epidermal growth factor receptor-2 (HER-2) staining and had weak or no staining for estrogen receptor-α (ERα). PI3K inhibitor alpelisib inhibited growth of transplanted tumors in NOD-SCID mice. |
Mechanism: Common PI3KCA-H1047R mutation caused a high incidence of breast tumor in tree shrews, which were hormone receptor-positive and HER2 negative. PI3K pathway was involved in development of breast tumors. Limitations: Precise reason why PI3KCA-H1047R and H-RAS-Q61L lentiviruses behaved differently in mammary tumor induction in tree shrews and FVB mice was not determined, with this species-specific pattern having important implications regarding development of breast cancer in humans. |
Zeng et al., 2023 |
| Female tree shrews (10–12-months old) were injected with DMBA (10 mg/kg) in one side of lumbar mammary fatty pad four times (once per week for 2 weeks and suspended for one week), with or without intramuscularly injection of MPA (100 mg/kg) once per 2 weeks five times from 1st DMBA injection | Around 10% of tree shrews with DMBA treatment and 50% of tree shrews with DMPA and MPA developed breast lesions. All induced breast lesions were intraductal papilloma and atypical ductal hyperplasia, with almost equal incidence. Breast lesions were highly vascular and had regular morphology, clear margins, and heterogeneous enhancement. Breast lesions were positive for ERα, PR, and cytokeratin 5/6 (CK5/6) staining, but negative for HER-2 staining. |
Mechanism: Combination of DMBA and MPA effectively established breast precancerous lesions including intraductal papilloma and atypical ductal hyperplasia in tree shrews. Tissues of atypical ductal hyperplasia showed a higher expression of B cell lymphoma-extra-large (Bcl-xl) and lower expression of B cell lymphoma 2 associated X protein (Bax) than those with intraductal papilloma. Limitations: No detailed mechanism was studied for induced breast lesions and no drug effects were tested. |
Chen et al., 2019 | |
| Spontaneous mammary gland tumor | Female tree shrews of different ages | Spontaneous mammary tumors usually occurred in virgin tree shrews at 2–3 years old, with an incidence rate of 24.6% (15/61). Simultaneous or metachronous multiplex tumor rate was 60%. Familial mammary gland tumor incidence was observed for some pedigrees. Intraductal papillary adenomas were predominant and tubulopapillary carcinomas were also found but at a lower frequency. All tumors were PR-positive, nearly all were negative for HER-2 staining (4.3%), and 91.3% were positive for ERα staining. |
Mechanism: No PTEN and PIK3CA mutations were observed in mammary tumors after sequencing. Most simultaneous or metachronous multiplex tumors exhibited possible origins from multiple sites. More than 25% of mammary tumor cells expressed Nectin-4. Limitations: Genetic aspects of familial inheritance of mammary tumors were not determined and no drug effects were tested. |
Chi et al., 2020 |
| Glioblastoma | Adult tree shrews (1–2 years old) were intracranially injected with pTomo-H-RasV12-shp53 lentivirus (3×1011/ml titer, expressing HRasV12 and shRNA targeting tree shrew Tp53) into hippocampus | Tree shrews showed slight neurological symptoms, including ataxia, imbalance, and emaciation after lentivirus delivery for 4 weeks. Induced gliomas exhibited aggressive behavior and relatively short latency, with reduced animal survival. Gliomas showed increased cell density, necrosis and pseudopalisades, vascular hyperplasia, and active cellular proliferation, and resembled human high-grade gliomas. |
Mechanism: Transcriptomic profiling of tree shrew gliomas indicated that tumors belonged to mesenchymal subgroup of human glioblastoma multiforme. Limitations: No drug effects were tested. |
Tong et al., 2017 |
| Pancreatic cancer | Adult male tree shrews (2–3 years old) were injected with lentiviral vectors containing mutant oncogene KRASG12D and shRNAs targeting Tp53, Cdkn2a, Cdkn2b, and Cdkn2a/b in head of pancreas | Pancreatic tumors with full penetrance were observed at 3–7 weeks after lentivirus injection. Tumors were moderately differentiated ductal adenocarcinomas and expressed several well-established markers of human pancreatic cancer, including CK19, Muc5, MMP7, and Hes1. Tree shrews showed gross morphological changes associated with pancreatic cancer, including intestinal darkening, colorectal inflation, gall bladder dilatation, and blockage of bile overflow. Induced pancreatic cancer originated from malignant transformation of acinar cells. |
Mechanism: Induced pancreatic cancer resembled human pancreatic ductal adenocarcinoma based on transcriptomic profiling; expression of oncogenic Kras along with loss of Tp53, Cdkn2a, and Cdkn2b expression were essential for transformation of acinar cells and induction of tree shrew pancreatic cancer. Rb1 signaling pathway was actively involved in this process. Limitations: No drug effects were tested. |
Tu et al., 2019 |
| Basal cell carcinoma (BCC) | Adult male tree shrews were intracutaneously injected with pCDH-mSmoA1 lentivirus (5.6×105 TU) and shRNA targeting tree shrew Tp53 lentivirus (shp53, 2×105 TU) in dorsum and tail skin | Tree shrews with pCDH-SmoA1 lentivirus injection exhibited human BCC-like pathological characteristics, including hyperplasia of skin cells with hair follicle disruption, pigmentation, and nuclear explosion expansion. Around 40% of tree shrews presented with BCC-like phenotypes after 4 weeks following delivery of pCDH-SmoA1 lentivirus, which reached 60% after 6–8 weeks. Injection of pCDH-mSmoA1 and shp53 lentiviruses resulted in BCC-like phenotypes in 70% of tree shrews at 2 weeks, which reached 100% after 4 weeks. |
Mechanism: Activation of Hh signaling pathway by SmoA1 overexpression induced tree shrew BCC, which was further accelerated by knockdown of tumor suppressor p53. Limitations: No characterization of related molecular markers of BCC was conducted and no drug effects were tested. |
Jiang et al., 2017 |
| Acute respiratory distress syndrome (ARDS) | Lipopolysaccharide (LPS, 180–200 mg/kg) was administered to tree shrews via intratracheal instillation | LPS-treated tree shrews demonstrated symptoms of respiratory failure between days 3 and 5, and exhibited behavioral abnormalities (less activity, piloerection, and tachypnea) from day 3. LPS-treated animals showed a PaO2 to FiO2 (P/F) ratio of 160–200 mmHg, suggesting moderate ARDS. LPS treatment caused bilateral patchy infiltrates, typical diffuse alveolar damage, pulmonary inflammation, and lung tissue injury (e.g., thickened alveolar septum, hemorrhage, edema, proteinaceous debris, massive neutrophils accumulation, and infiltration). |
Mechanism: One-hit intratracheal instillation of LPS led to symptoms resembling acute phase of human ARDS. Limitations: No detailed mechanism of this LPS-induced model was determined and no drug effects were tested. |
He et al., 2024 |
| Chronic pulmonary inflammation (CPI) | Adult male tree shrews (4–6 months old) were subcutaneously injected with 0.25 mL of solution (2 mg/mL of bovine type II collagen, 0.1 mol/L acetic acid, emulsified in complete Freund’s adjuvant) to induce arthritis | Joint inflammation severity worsened over two weeks and peaked at day 21, followed by remission and slow recovery. Swollen soft tissue, narrowed joint spaces, marginal erosions, denser and abnormal lymphoid infiltrates, and cartilage damage were observed in bone joints, including forelimb joint. Diffused lymphoid infiltrates in lung tissue were detected at day 21. Typical CPI but no fibrosis developed pathologically in the collagen-induced arthritis associated CPI model. |
Mechanism: Abnormal up-regulation of pulmonary chemokine CXCL10 was associated with lung damage, and CXCL10-CXCR3 chemotaxis mediated joint inflammation in this model. Treatment with CXCR3 antagonist NBI74330 down-regulated inflammatory infiltrates and expression of inflammatory factors and chemokines. Limitations: No experimental assays were conducted to reveal the initiation and role of CXCL10-CXCR3 chemotaxis. |
Gao et al., 2018 |
| Idiopathic pulmonary fibrosis | Tree shrews (3–5 months old) were administered bleomycin (1.75 U/kg body weight) via intratracheal catheter | After bleomycin exposure for 21 days, fibrotic responses were observed in a significant portion of the lungs, with increased levels of fibrotic collagen area and hydroxyproline relative to control animals. Bleomycin exposure induced myofibroblast differentiation, increased extracellular matrix proteins, and activated FAK signaling in lung tissues, indicating increased pro-fibrotic responses and fibrotic remodeling. During fibrosis, monocyte-derived macrophages were significantly recruited and polarized to a profibrotic phenotype. Macrophage-derived profibrotic mediators from bleomycin-exposed tree shrews promoted fibroblast activation. |
Mechanism: FAK signaling was actively mediated pro-fibrotic responses in lungs of bleomycin-exposed tree shrews. Monocyte-derived macrophage-fibroblast crosstalk induced fibrosis. Limitations: No drug effects were tested. |
Che et al., 2021; Larson-Casey et al., 2020 |
| Systemic sclerosis | Adult tree shrews (10–12 months old) were subcutaneously injected with different doses of bleomycin (0.4, 2, and 4 ng/mL; 100 µL of solution) for 21 days | Long-term injection of bleomycin caused ulcers in the skin injection site of tree shrews and induced inflammation and/or fibrosis in skin and internal organs. Medium to high doses of bleomycin caused markedly thickened dermis, infiltration of inflammatory cells, increased collagen fibers and collagen volume fraction in skin, pulmonary septal thickening, and fibrosis and inflammatory cells infiltration in the lung. Increased expression of α-SMA in skin and presence of serum autoantibodies (antinuclear antibodies and anti-scleroderma-70) were observed in bleomycin-treated animals. |
Mechanism: Bleomycin-treated tree shrews showed similar pathological and serological changes to human systemic sclerosis. Ten hub genes (KIF20A, KIF11, UBE2C, BUB1B, CDK1, CCNB2, AURKB, TOP2A, PLK1, and NCAPG) were identified based on transcriptomic profiling; immune cell infiltrations in the skin may play a key role. Limitations: No drug effects were tested. |
Zheng et al., 2024 |
| Periodontitis | Adult male tree shrews (2–6 months old) were modeled using a 2-0 ligature through the mandibular space between the first and second molars, with tightening on the subgingival surface of first molar crown | Tree shrews showed ligature-induced inflammatory reactions, e.g., swelling, redness, and tissue softening in the gingiva at week 2. Redness and swelling of the gums continued in weeks 3 and 4, and spontaneous bleeding of the gingiva was observed at week 4, followed by gradual reduction of swelling and redness and disappearance of hemorrhaging at weeks 5–8. Severe bone destruction and significantly increased molar root exposure were observed after induction of periodontitis, along with substantial inflammatory infiltrates, apical migration of junctional epithelium, and alveolar bone loss. |
Mechanism: Inflammatory infiltrates and transition from acute to chronic inflammation were involved in development of gingival swelling, redness, spontaneous bleeding, and bone loss after induction of periodontitis Limitations: No drug effects were tested. |
Ma et al., 2023 |
| Osteonecrosis | Adult male tree shrews (6 months old) were intravenously injected with LPS (300 μg/kg), followed by three intraperitoneal injections of methylprednisolone (MPS; 130 mg/kg) over a 24 h interval, with subsequent delivery of MPS two times per week until 12 weeks | Treated tree shrews showed increased levels of bone alkaline phosphatase, bone GLA protein, N-terminal propeptide of type I collagen, and C-terminal propeptide of type I collagen relative to control animals, with subchondral trabecular bone deterioration of femoral heads, including cortical bone partial collapse, trabecular fracture, trabecular sparseness, thinning, and increased intercellular spacing. Increased cell apoptosis and fused adipose cells into vacuoles, and decreased bone quality were observed. |
Mechanism: Administration of low-dose LPS combined with high-dose MPS resulted in femoral head necrosis in tree shrews. Limitations: No drug efficacy was tested and no detailed target or mechanism was studied. |
Chen et al., 2020 |
| Osteoporosis | Bilateral ovaries were removed in healthy female tree shrews (6 months old; ovariectomy (OVX) group) after anesthesia, with animals euthanized at 6 months after surgery | Animals in the OVX group showed a significantly low level of serum estradiol, high bone turnover and bone loss, increased body weight, and reduced uterus weight and uterus coefficient compared to the sham group. OVX group exhibited a decreased in number and connections of trabeculae in the third lumbar vertebra, decreased structural stiffness, and fewer osteoblasts but more osteoclasts relative to the sham group. |
Mechanism: Osteoporosis was induced in tree shrews by ovariectomy. Transcriptomic analysis of first lumbar vertebra identified several differentially expressed genes associated with osteoporosis. Limitations: No drug efficacy was tested and no detailed target or mechanism was studied. |
Wang et al., 2019b |
| SARS-CoV-2 infection | Adult (1-year old) and aged (5–6 years old) tree shrews were infected with 107 TCID50 SARS-CoV-2 strain 107 by oral, intranasal, and ocular routes | Lung infiltrates were visible from 3 days post-infection (dpi) in 85% of infected animals. Most lung lobes had high viral RNA copies, which peaked at 3 dpi and decreased to undetectable levels at 14 dpi. Sporadic or massive pulmonary punctate hemorrhage, thickened alveolar septa, and interstitial hemorrhage were observed. Viral antigens were present in a small number of pneumocytes in infected lung tissues. Elevated white blood cell, lymphocyte, monocyte, and granulocyte counts were detected, as well as increased aspartate aminotransferase after infection in the adult group and decreased monocytes in the old group. |
Mechanism: Tree shrews were susceptible to SARS-CoV-2 infection, displaying viral shedding, lung lesions, and alterations in blood biochemical indices. Limitations: No vaccine or drug efficacy was tested, no data about host immune response were provided, and no intra- or inter-species transmission study was performed. |
Xu et al., 2020a |
| Young (6–12 months old), adult (2–4 years old), and old (5–7 years old) tree shrews were infected nasally with 106 PFU SARS-CoV-2 | Some infected animals showed an increased body temperature. Low viral shedding and replication in tissues occurred in all three groups. Pathological changes included pulmonary abnormalities, widened pulmonary septum, interstitial hyperemia, airway obstruction, consolidation of lung margin, local hemorrhagic necrosis, and infiltration of inflammatory cells. Mild histopathological changes were observed in brain, heart, liver, and pancreas. |
Mechanism: Tree shrews were less susceptible to SARS-CoV-2 infection but may be a potential intermediate host. Limitations: No data about host immune response were provided and no intra- or inter-species transmission study was performed. |
Zhao et al., 2020b | |
| Zika virus (ZIKV) infection |
Tree shrews (5 months old) were infected with 105 or 106 PFU of ZIKV strain GZ01 via subcutaneous injection; neonatal tree shrews (1-day old) were inoculated via the intracerebral route with 105 PFU of ZIKV | Tree shrews showed ZIKV infection and replication in primary kidney, testis, and peripheral blood cells. ZIKV-infected tree shrews developed transient viremia and skin rash at 2 dpi (which disappeared at 3 dpi), with the abdomen and chest being the main sites of dermatological manifestations. Apparent cutaneous lesions characterized by massive hemorrhage and inflammatory cell infiltration were observed in the hypodermis, along with in situ viral replication. ZIKV caused systemic infection involving multiple organs, including the brain. Testes had the highest viral load. ZIKV infection had a protection against secondary homologous infection. ZIKV was neurovirulent and replicated in neonatal animals, leading to a 75% mortality rate within 21 dpi. |
Mechanism: Genes with antiviral immunity and inflammatory response were up-regulated by ZIKV infection in tree shrews. Both innate and adaptive immune responses were induced by ZIKV infection. Limitations: Exact mechanism underlying dermatological manifestations was not examined and no vaccine or drug efficacy was tested. |
Zhang et al., 2019b |
| Sexually mature tree shrews were infected with 105 or 106 PFU ZIKV GZ01 strain via the vaginal route | No dermatological manifestations were observed in tree shrews after ZIKV vaginal infection. Viral RNA loads were detected in blood, saliva, urine, and vaginal douching, with ZIKV viremia in vaginal lavage peaking at 1 dpi and persisting until 10 dpi in several animals. ZIKV vaginal infection led to systemic multi-tissue and multi-organ infections. |
Mechanism: Female tree shrews were infected with ZIKV via the vaginal route. Expression levels of key inflammatory genes, including IL6, IL8, TNFα, CCL5, and CXCL9, were increased in the spleen of ZIKV-infected tree shrews. Limitations: No vaccine or drug efficacy was tested. |
Baloch et al., 2021 | |
| Dengue virus (DENV) infection | Adult tree shrews were infected with 1×104 PFU DENV-2 or DENV-3 through intravenous injection or multisite subcutaneous injection | DENV-3 productively proliferated in tree shrew bone marrow mesenchymal stem cells at 4 dpi. Half of the animals presented with viremia, and most presented with high neutralizing antibody titers at 7 and 15 dpi. Alterations in some hematological and biochemical parameters, including modest thrombocytopenia, slight decrease in white blood cell count, and increased levels of aspartate transaminase, alanine aminotransferase, and alkaline phosphatase were observed in tree shrews after DENV infection. Viral RNA was barely detectable in the liver at 48 dpi but was detectable in the brain. Intra-brain bleeding lesions were more severe in animals with an intravenous injection of DENV than those with subcutaneous infection. |
Mechanism: Tree shrews displayed fever, viremia, abnormal aminotransferase levels, and death after DENV infection, resembling clinical manifestations in humans. Limitations: Potential mechanism regarding vascular leakage and central nervous system impairment after DENV infection was not determined and no drug efficacy was tested. |
Jiang et al., 2021a |
| Tree shrew fibroblast cells were infected with DENV-1, DENV-2, DENV-3, and DENV-4 | DENV serotypes 1–4 replicated in tree shrew fibroblast cells, with a linear increase in viral load at 24–96 h post-infection in both cells and culture supernatants. DENV-2 had the highest viral growth among all serotypes. Different DENV serotypes showed variable viral replication kinetics and TLR and cytokine expression profiles in fibroblast cells. |
Mechanism: TLR8 mRNA was induced during DENV infection. Knockdown of TLR8 led to an increase in DENV-1 viral load. Limitations: No drug efficacy was tested, no detailed mechanism was studied, and no in vivo infection of DENV was performed. |
Kayesh et al., 2017c | |
| Kaposi’s sarcoma-associated herpesvirus (KSHV) | Tree shrews (4–5 months old) were infected with 5×107 GFU rKSHV.219 via the intravenous route | Tree shrew kidney epithelial cells were the most susceptible cells to KSHV infection, with KSHV genomic DNA, mRNA, and KSHV-specific proteins detected in this cell type up to 32 dpi. KSHV DNA and mRNA were detected in peripheral blood mononuclear cells and various tissues of KSHV-infected tree shrews. Lymphocyte infiltration, lymphoid tissue focal aggregation, alveolar wall thickening, hepatocyte edema, and hepatic necrosis were observed in the spleen, lung, and liver of a certain proportion of KSHV-infected animals. |
Mechanism: Tree shrews exhibited a robust innate immune response against KSHV infection. Limitations: No transcriptomic profiling was performed in tissues upon KSHV infection and no drug efficacy was tested. |
Li et al., 2021a |
| Herpes simplex virus-1 (HSV-1) | Adult female tree shrews (6 months old) were infected with 106 PFU HSV-1 strain McKrae by dropping on each eye without ocular scarification (McKrae group) and HSV-1 17+ strain with ocular scarification (17+ group). Trigeminal ganglia (TG) were collected from 17+ group at 58 dpi | Herpes simplex keratitis was induced in McKrae group, and eyes recovered at 33 dpi. The 17+ group exhibited more pronounced scarring and opaqueness in cornea and inflammation in eye lids during acute infection stage between 5 and 15 dpi compared to McKrae group. Thickening cornea, inflammatory infiltration, weakened luminousness, rough cornea surface, and deciduous corneal epithelial cells were observed during weeks 1 and 3 post-infection, with corneas showing recovery by 33 dpi. Titer of active virus peaked at 5 and 10 dpi, then reduced to the lowest level at 13 dpi, with persistent infection or spontaneous reactivation. Viral protein was detected in the cornea epithelial layer and retina neuronal ganglion cells at 5–20 dpi. Cornea supernatants and ciliary ganglion homogenates from the McKrae group at 46 dpi induced a cytopathic effect at 3 days post co-cultivation in RS1 cells. HSV-1 efficiently infected tree shrew eyes and persisted during latency period. |
Mechanism: Decreased transcript levels of ICP0, ICP4, and LAT were detected after the acute infection stage, but LAT increased at 46 dpi, indicating latency of HSV-1 in tree shrew eyes (ciliary ganglion). Comparison of transcriptomes of infected TGs from tree shrews, mice, and humans showed that HSV-1 transcription in acutely infected TGs differed dramatically between mice and tree shrews. HSV-1 transcripts were detected in mouse TGs during acute infection but had an abortive infectious cycle in tree shrew TGs. During latency, LAT was detected in mouse, tree shrew, and human TGs but ICP0 transcripts were only found in tree shrew and human TGs. Infected human and tree shrew TGs exhibited a more similar LAT region transcription peak. Limitations: No detailed molecular mechanism regarding HSV-1 latency in tree shrews was uncovered and no drug efficacy was tested. |
Li et al., 2020; Wang et al., 2020b |
| Influenza A viruses (IAVs) | Healthy adult tree shrews were infected with 105 TCID50/mL swine influenza virus subtype H3N2 (SW2783 group) and avian influenza virus subtype H6N6 (ZZ346 group) through nasal drip, conjunctiva drip, and pharyngeal tonsil drip | Tree shrews presented with decreased appetite, reduced activity, and increased nasopharyngeal secretions at 1 dpi which lasted 8 days, with severe symptoms in the SW2783 group. Both viruses replicated and spread, with SW2783 showing stronger replication capacity than ZZ346. Infection was restricted to the upper respiratory tract, and nucleoprotein expression peaked at 3–5 dpi and gradually recovered at 7–14 dpi. Various histopathological changes were observed in respiratory tract tissues, with limited cell necrosis and infiltration of inflammatory cells in mucosal epithelium of nasal turbinate. Both viruses were transmissible in tree shrews, and SW2783 could transmit from tree shrews to guinea pigs. |
Mechanism: Swine SW2783 and avian ZZ346 strains infected tree shrews, induced effective innate and adaptive immune responses, and regulated response degrees. In total, 14 differentially expressed miRNAs were identified in turbinate tissues from SW2783-infected tree shrews, including some miRNAs involved in viral replication and proliferation by regulating signal transduction, and others playing an antiviral role via regulating immune response. Limitations: No vaccine or drug efficacy was tested. |
Wang et al., 2023b, 2024c |
| Adult tree shrews were infected with 106 PFU avian H5N1 (highly virulent) and H7N9 (lowly pathogenic) into nostrils and trachea by aerosolized administration and onto tonsils and conjunctivae with pipette | H5N1 group showed elevated body temperature, body weight loss, and decreased locomotor activity starting from 2 dpi, with one of four infected animals dying. Tree shrews infected with H7N9 exhibited no clinical symptoms. Both viruses were continuously detected in nasal, oral, tracheal, and conjunctival swab samples and lung tissues, peaking at 1–2 dpi and replicating in lung tissues. Animals infected with H5N1, but not H7N9, displayed severe diffuse pneumonia, with focal inflammation around bronchioles. Antibody responses were detected in infected animals. |
Mechanism: H5N1-infected tree shrews had higher expression levels of cytokine genes (IFNG, IL6, TNFA) compared to those without infection or with H7N9 infection, and severity of pneumonia was correlated with mRNA expression of proinflammatory cytokines. Limitations: Reasons for significant differences in symptoms between H5N1 infection and H7N9 infection were not determined and no vaccine or drug efficacy was tested. |
Sanada et al., 2019c | |
| Tree shrews were infected intranasally with 106 TCID50 H9N2 viruses (Y280-wt and Y280 virus with mammalian adaptation mutation PB2-E627K). For ex vivo cultures of nasal turbinate, trachea, and lung tissues, tissue blocks were infected with 106 TCID50 H9N2 (Y280-wt and Y280-PB2-E627K) for 2 h, then cultured with fresh medium to 72 h dpi | Viral shedding was found in nasal washes of tree shrews, with longer persistence of PB2-E627K mutant virus up to 6 dpi. Tree shrews infected by PB2-E627K exhibited increased body temperature and body weight loss. H9N2 virus replicated in nasal turbinate and lungs. Animals with Y208-wt infection showed infiltration of inflammatory cells and focal edema on submucosal layer of nasal turbinate at 2 dpi, while those infected with PB2-E627K showed increased severity of lesions characterized by necrotic and sloughed epithelial cells and increased lymphocytic infiltration. Lung tissues showed similar lesion patterns. PB2-E627K virus replicated more efficiently than Y280-wt virus in ex vivo culture model of nasal turbinate, trachea, and lung tissues. |
Mechanism: mRNA expression levels of cytokines, including TNF-α, IFN-β, IL-8, IL-13, and IL-6, were increased in infected tree shrews and respiratory tissues. PB2-E627K virus mutant had a higher induction effect than Y280-wt virus. Limitations: No transcriptomic profiling was performed for tissues infected with two viruses of different virulence and no vaccine or drug efficacy was tested. |
Li et al., 2018a | |
| Tree shrews (1–4 months old) were infected intranasally with 106 EID50 pandemic H1N1 (pdmH1N1), avian H5N1, and human H7N9, respectively. Contact infection between tree shrews and guinea pigs was also tested | Three influenza viruses efficiently replicated in tree shrew primary renal cells and lung cells, and induced expression of IFNβ, IFIT2, and OASL. Tree shrews showed subclinical symptoms after infection with three viruses, with viral tropism in respiratory tract. Lung lesions, including obvious alveolar edema, interstitial edema, hemorrhage, and inflammatory cell infiltration, were observed at 3 dpi, with pdmH1N1 infection showing more severe lesions than H5N1 and G7N9. Influenza viruses induced protective responses against homologous challenge in tree shrews. PdmH1N1 and H7N9, but not H5N1, were transmissible in tree shrews. H7N9, but not pdmH1N1, was transmissible from tree shrews to guinea pigs. |
Mechanism: Tree shrews were infected by different subtypes of influenza viruses, which induced a humoral immune response upon infection. Limitations: No detailed mechanisms were studied and no vaccine or drug efficacy was tested. |
Xu et al., 2019 | |
| Influenza B viruses (IBVs) | Male tree shrews were intranasally inoculated with 106 TCID50 IBV V0215 or Y12. Infected tree shrews were compared with infected ferrets and mice | IBVs replicated in the respiratory tract of tree shrews, ferrets, and mice, with peak viral titers in nasal washes at 2 dpi. Infected tree shrews, but not ferrets, exhibited weight loss. Seroconversion was observed in tree shrews, with higher antibody titer in animals inoculated with V0215 compared to Y12. Clinical signs and pathological changes were mild. Elevated levels of cytokines and inflammation were detected in respiratory tract of all three infected animals. Infected tree shrews presented with rhinitis and pneumonia, and Y12 infection caused more disease severity relative to V0215 infection in tree shrews. |
Mechanism: Peak and period of respiratory IBV viral shedding in tree shrews was similar to those in humans. IBV infection led to elevated levels of cytokines in respiratory tract of tree shrews. Limitations: No mechanism was reported for different virulence and tissue tropism of V0215 and Y12 in tree shrews and between tree shrews and ferrets or mice. No intra- or inter-species transmission was performed and no vaccine or drug efficacy was tested. |
Yuan et al., 2019 |
| Human adenovirus (HAdV) species B | Male tree shrews were intranasally infected with 5×106 TCID50 HAdV-55. For HAdV-55 vaccine evaluation, tree shrews were intramuscularly vaccinated with 2×1010 of inactivated HAdV-55 VPs, and two booster vaccinations on days 14 and 28 | HAdV species B (HAdV-55, HAdV-7, HAdV-14) replicated in tree shrew primary cells from the kidney, lung, and trachea and caused the cytopathic effect (CPE). HAdV-55 infection led to rapid weight loss and increased body temperature. HAdV-55 was detected in respiratory tract and lung of infected tree shrews with interstitial pneumonia. Pre-vaccination provided a protective effect against homologous infection. HAdV-55 transmitted among tree shrews. |
Mechanism: HAdV-55 infection induced cytokine gene expression in peripheral blood mononuclear cells (PBMCs) of tree shrews. Pre-vaccination inhibited HAdV-55 replication in vivo. Limitations: No mechanisms for pathological alterations and HAdV-55 distribution in respiratory tract and other organs were investigated. |
Li et al., 2021c |
| Epstein-Barr virus (EBV) | Adult tree shrews (1–1.5 years old) were inoculated with 3.9×108 copies of EBV by intravenous injection | Most tree shrews (8/10) showed evidence of EBV infection, with intermittent or transient increases in EBV copy number and expression of EBV genes in PBMCs post-infection. Anti-EBV capsid antigen IgG increased by varying degrees in tree shrews with increasing EBV copy number. Splenic corpuscle hyperplasia, inflammatory cell infiltration in liver, and extensive lymphocyte proliferation in mesenteric lymph node enlargement were observed in some infected animals, but none had obvious abnormalities in lungs or nasopharynx. |
Mechanism: Tree shrews were infected by EBV via intravenous infusion and presented with different patterns concerning EBV copy number. EBV-related gene expression was detected after infection. Lymphocytes and spleen were primary targets of EBV infection Limitations: No study on the mechanisms of EBV entry into the nasopharynx was conducted and no drug or vaccine efficacy was tested. |
Wang et al., 2017 |
| Primary tree shrew PBMCs were infected with 1×107 copies of EBV. Adult tree shrews were injected with 2×107 copies of EBV via femoral vein. Daily intraperitoneal injection of cyclosporine A (CsA, 25 mg/kg) was used for immunosuppression at 5 weeks post-infection after two consecutive negative results of EBV-DNA | Tree shrew PBMCs supported EBV replication and proliferation. EBV-infected tree shrews displayed fever and weight loss, increased white blood cell count and total cell counts, and decreased neutrophils after infection. Immune organs (spleen and lymph nodes) had higher viral loads. Tree shrews acted as asymptomatic carriers of EBV, with viral protein detectable in blood and tissues. EBV infection increased percentage of immature neutrophils (rod-shaped nucleus) and neutrophils containing specific cytoplasmic intoxication particles. EBV infection altered gut microflora in composition and metabolome profile, peaking at 7 dpi. Autophagy and ferroptosis signaling pathways were activated by EBV infection, along with alterations in the competing endogenous RNA (ceRNA) network that regulates EBV-host interactions. CsA increased the proliferation ability of EBV-infected PBMCs, promoted transformation into immortalized cells, and caused EBV reactivation in infected animals. |
Mechanism: Tree shrews possessed the same key residues in the complement C3d receptor 2 (CR2) that binds to the gp350 protein as humans, which facilitates viral entry. EBV infection led to dynamic transcriptomic changes in tree shrew blood cells. Neutropenia in EBV-infected animals at early infection may affect EBV immune escape and long-term latency. Changes in gut microflora during EBV infection were correlated with fecal metabolic profile. EBV infection caused alterations in ceRNA networks that remodel the immune microenvironment. Immunosuppression caused EBV reactivation in latency. Limitations: No study on the mechanisms underlying the proposed neutrophil suppression hypothesis or alterations in gut microbiota and ceRNA network during EBV infection was conducted and no drug or vaccine efficacy was tested. |
Xia et al., 2022, 2023, 2024; Shi et al., 2023 | |
| Hepatitis B virus (HBV) | Adult tree shrews (>2 months old) were treated with triamcinolone steroid (5 mg/kg) for 2 days, followed by intraperitoneal inoculation with 108 copies of HBV in 1 mL of human serum | Infected animals displayed enhanced serum HBV and viral proteins were synthesized de novo in hepatocytes, with hepatitis B surface antigen (HBsAg) and hepatitis Be antigen (HBeAg) titers, and HBV DNA and covalently closed circular DNA (cccDNA) copies peaking at day 9, declining at days 15 and 21, and becoming undetectable at day 42. HBV infection led to elevated alanine transaminase levels and inflammatory infiltration and hepatocyte ballooning degeneration in liver. |
Mechanism: A complete and natural process from HBV infection, replication, to elimination was modulated in tree shrews, with a noncytopathic-mediated control of cccDNA content during acute viral hepatitis Limitations: No characterization for host immune response during HBV infection and elimination was performed and no vaccine efficacy was tested. |
Li et al., 2021b |
| Newborn tree shrews were subcutaneously administered with 106 copies of HBV genotype A (HBV-A) or C (HBV-C) per animal; Adult tree shrews (1-year old) were intraperitoneally injected with 106-7 copies of HBV-A | HBV-A showed higher intrahepatic replication than HBV-C in newborn tree shrews, leading to abnormal architecture of liver cell cords and mitotic figures at 8 dpi. HBV-DNA and HBsAg were detected in liver tissues of adult animals at 28 dpi. HBV infection caused expression alterations in IFN-β, TLR1, TLR3, TLR9, and cGAS, and suppression of NTCP expression during chronic phase of infection. |
Mechanism: Suppression of IFN-β may have contributed to establishment of chronic HBV infection in tree shrews. Limitations: No precise mechanisms for signaling pathways activated by HBVinfection and host immune response during chronic HBV infection were determined and no vaccine efficacy was tested. |
Kayesh et al., 2017a | |
| Hepatitis C virus (HCV) | Tree shrew bone marrow-derived mesenchymal stem cells (BM-MSCs) overexpressing human CD81, OCLN, and miR-122 were infected with 0.5 MOI HCVcc derived from J6/JFH1 (HCV2a) | HCV viral copies and core protein were detected in culture supernatants of infected BM-MSCs overexpressing human CD81/OCLN or CD81/OCLN/miR-122. VEGF treatment increased BM-MSC infectivity to HCV. |
Mechanism: BM-MSCs overexpressing human CD81, OCLN, and miR-122 supported HCV replication and infectious virus production, and VEGF enhanced HCV infectivity in these cells. Limitations: No data on host immune response or the exact mechanisms underlying HCV replication and production of infectious particles were provided and no drug efficacy was tested. |
Lu et al., 2020 |
| Adult tree shrews (1-year old) were infected intraperitoneally with HCV (genotypes 1a, 1b, 4a, and 2a (JFH1)) for 41 weeks | All HCV genotypes established infections and showed intermittent HCV propagation. Infected animals produced anti-core and anti-NS3 antibodies and had increased levels of reactive oxygen species (ROS) in serum and liver. Pathological changes, including lymphocytic infiltration, disturbance of hepatic cords, and initiation of fibrosis, were observed in livers of infected tree shrews. |
Mechanism: Intrahepatic levels of TLR3, TLR7, and TLR9 were significantly increased upon HCV infection. Increased levels of IFNβ in tree shrews with HCV genotypes 1a and 2a infection were detected, along with a decrease in NTCP. Humoral and innate immune responses and ROS were involved in HCV infection. Limitations: No detailed mechanism was studied and no drug or vaccine efficacy was tested. |
Kayesh et al., 2017b | |
| Tree shrews (6 months old) were infected intravenously with 1×107 copies of HCVcc (J6/JFH1) in the tail | Nearly half of the tree shrews were infected, with intermittent HCV viremia and mild hepatitis. HCV-specific proteins (Core, E2, NS3/4, and NS5A) were detected in hepatocytes of infected tree shrews. Various degrees of microvesicular fat accumulation, vacuolar degeneration, hepatic edema, and lymphocytic infiltration were observed in the livers of infected animals. |
Mechanism: HCV infection led to mild hepatitis in tree shrews. Limitations: No validation for sample infectivity of infected tree shrews was conducted, no data on host immune response was provided, and no drug or vaccine efficacy was tested. |
Feng et al., 2017 | |
Based on the disease models listed in Table 1, most recently reported tree shrew models pertain to infectious diseases, including Epstein Barr virus (Xia et al., 2022, 2024), Zika virus (Baloch et al., 2021; Zhang et al., 2019b, 2019c), human, avian, and swine influenza viruses (Li et al., 2018a; Sanada et al., 2019c; Wang et al., 2023b, 2024c; Xu et al., 2019; Yuan et al., 2019), human adenovirus species B (Li et al., 2021c; Liu et al., 2024), HBV (Kayesh et al., 2017a; Li et al., 2021b; Sanada et al., 2019a), HCV (Feng et al., 2017; Kayesh et al., 2017b; Lu et al., 2020), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; Xu et al., 2020a; Zhao et al., 2020b). The successful establishment of these viral infection models in tree shrews offers a valuable platform for studying host-virus interactions, cross-species infection mechanisms (Wang et al., 2023b), and testing multiple anti-viral treatments, including neutralizing monoclonal antibodies (Liu et al., 2024), ionizable lipid SM-102-based CRISPR nanoparticles (Yi et al., 2023), and hepatitis B surface-small antigen and hepatitis B core antigen vaccinations (Sanada et al., 2019b). With advancements in detecting viruses carried by tree shrews using meta-transcriptomic sequencing and other high-throughput cutting-edge techniques, it may be possible to identify natural viral infections in wild and artificially reared tree shrews (Zhou et al., 2024), thereby broadening our knowledge about the susceptibility of tree shrews to viral infections and aiding the establishment of tree shrew infection models.
Tree shrews can be used to model lung lesions during viral infections (Li et al., 2021c; Xu et al., 2020a), as well as pulmonary fibrosis induced by bleomycin and pro-fibrotic mediators (Che et al., 2021) and acute respiratory distress syndrome induced by intratracheal instillation of lipopolysaccharide (He et al., 2024). These tree shrew lung disease models provide a valuable opportunity for understanding the pathogenesis of lung diseases and for preclinical drug development. Several studies have also reported the development of spontaneous breast tumors (Elliot et al., 1966; Sun et al., 2021; Xia et al., 2012) and mammary gland cancer (Chi et al., 2020) in tree shrews, suggesting their potential as a valid model for breast cancer research. Indeed, intraductal injection of lentivirus expressing the PIK3CA p.H1047R mutation has been shown to induce a high incidence of breast cancer in tree shrews (Zeng et al., 2023), while administration of 7,12-dimethylbenz(a)anthracene (DMBA) and medroxyprogesterone acetate (MPA) in combination can also induce breast tumor (Xia et al., 2014) and precancerous lesions (Chen et al., 2019).
Tree shrews have also been used to develop models for various other human diseases, such as systemic sclerosis induced by subcutaneous injection of bleomycin (Zheng et al., 2024), periodontitis induced by nylon thread ligatures around the lower first molars (Ma et al., 2023), steroid-associated osteonecrosis induced by low-dose lipopolysaccharide combined with high-dose methylprednisolone (Chen et al., 2020), radiation (20-Gy)-induced tensor veli palatini muscle injury (Zhao et al., 2022), kidney oxalate calculi disease induced by intraperitoneal injection of glyoxylic acid (Wang et al., 2024b), chronic autoimmune uveitis with subretinal deposits and retinal damage induced by immunization with inter-photoreceptor retinoid-binding proteins (Hu et al., 2022), diabetic retinopathy induced by sustained hyperglycemia following a single streptozotocin dose (Gorbatyuk et al., 2022), and cocaine-seeking (Duan et al., 2021). Although the therapeutic and prophylactic applications of these models await further validation, the existing data are promising, demonstrating a high degree of similarity between tree shrew pathobiology and human diseases.
The characterization of spontaneous disease models in tree shrew breeding colonies offers crucial insights into the establishment of related disease models. For instance, Klein et al. (2022) reported a high prevalence of AA-amyloidosis (up to 72%) in their tree shrew colony. Combined with previous reports of age-related accumulation of amyloid deposits in the brain (Fan et al., 2018; Li et al., 2024; Yamashita et al., 2012), this suggests that tree shrews may possess genetic and physiological predispositions to developing AD, although further validation is needed. Intracerebroventricular injection of Aβ1-40 has been shown to induce cognitive impairment and neuronal apoptosis in tree shrews, resembling AD symptoms (Lin et al., 2016), with donepezil providing a protective effect in this model (Zheng et al., 2018). Similarly, Yang et al. (2022) claimed that intracerebral injection of Aβ25-35 can lead to AD-like symptoms, which can be ameliorated by ginsenoside Rg1 treatment through regulation of oxidative stress, apoptosis, and neuroinflammation. These studies again highlight the potential of using tree shrews to create AD models.
With their large eyes and high structural similarity to human eyes, tree shrews are an excellent model for studying eye diseases, such as refractive development (Gawne et al., 2017; Norton & McBrien, 1992), myopia (Abbott et al., 2011; Khanal et al., 2023; Marsh-Tootle & Norton, 1989; Norton et al., 2006), and glaucoma (Samuels et al., 2018). Noninvasive in vivo imaging techniques, such as optical coherence tomography (OCT) and scanning laser ophthalmoscopy, allow for detailed examination of the anatomical features of the tree shrew retina (Abbott et al., 2011; Grannonico et al., 2024; Miller et al., 2024; Sajdak et al., 2019; Samuels et al., 2018), facilitating the study of related diseases. For instance, Samuels et al. (2018) injected ferromagnetic beads into the anterior chamber of tree shrews, inducing sustained elevation of intraocular pressure and optic nerve damage akin to human glaucoma, and monitored changes in retinal nerve fiber layer thickness using near-infrared OCT. Researchers optimized visible-light OCT fibergraphy (vis-OCTF) (Grannonico et al., 2021; Miller et al., 2020) to non-invasively visualize individual retinal ganglion cell axon bundles and their surrounding microvasculature in living tree shrew retinas, demonstrating a dense retinal nerve fiber layer, a thick ganglion cell layer, and distinct sublayer structures within the inner plexiform layer (Grannonico et al., 2024; Miller et al., 2024). These studies provide direct evidence for the use of tree shrews in investigating optic neuropathies and retinal ganglion cell injury. Similarly, tree shrews have also been utilized as models for myopia. Notably, limited bandwidth short-wavelength light can produce slowly developing myopia in tree shrews, resembling human juvenile-onset myopia (Khanal et al., 2023). She et al. (2023) explored the therapeutic effects of ambient narrowband long-wavelength light on myopia in lens-induced and form-deprivation myopia tree shrew models and found that repeated red light can reduce myopia, providing a potential reference for red-light therapy in humans. In contrast to rodents, tree shrews are diurnal, and their sleeping patterns are similar to humans (Coolen et al., 2012; Dimanico et al., 2021), making them a suitable model for studying sleep regulation and function, e.g., cold exposure effects on rapid-eye-movement sleep (van Hasselt et al., 2023).
The genome-based approach for creating animal models (Yao, 2017) is particularly beneficial for tree shrews, especially for characterizing primate-specific genes also found in tree shrews, as exemplified by the study on the primate-specific SSX1 gene in asthenoteratozoospermia (Liu et al., 2023). As we deepen our understanding of the tree shrew’s unique genetic features and common genes and pathways at the genome and transcriptome levels (Fan et al., 2018; Ye et al., 2021), we will gain better insights into the validity and feasibility of using tree shrews to model certain human diseases.
However, it must be acknowledged that, compared to earlier studies on the susceptibility of tree shrews to HBV (Su et al., 1987; Walter et al., 1996; Yan et al., 1996), HCV (Amako et al., 2010; Xie et al., 1998; Xu et al., 2007; Zhao et al., 2002), HDV (Li et al., 1995), and HEV (Yu et al., 2016) infections, many recent reports on tree shrew disease models have not garnered sufficient attention and have rarely been replicated in multiple research groups. This lack of interest and replication limits their use in evaluating drug efficacy and new treatments. One reason for limited global interest is the inaccessibility of tree shrew resources. Another reason is the challenge of meeting all three types of validity for certain human diseases with a tree shrew model. The utility of these models is limited because they have not yet led to landmark discoveries, such as the identification of sodium taurocholate cotransporting polypeptide (NTCP) as the HBV receptor (Yan et al., 2012), nor have they been used successfully to test potential drugs and vaccines. Despite the challenges in making important progress, scientific discovery is a gradual process, and multidisciplinary perspectives and international collaborations can enhance the translational potential of these models. To increase awareness and use of tree shrews in research, tree shrew studies should be promoted at scientific meetings through workshops, posters, and lectures. In addition, training courses, videos, guidelines, and protocols (e.g., housing, handling, substance application, blood collection, anesthesia) should be made available to ensure new users can rapidly learn how to handle these experimental animals.
KEY QUESTIONS FOR FUTURE TREE SHREW STUDIES
The study of tree shrew biology is driven by both curiosity about the animal and the demands of biomedical research. From an evolutionary perspective, every species is unique, providing insights into natural history and evolution. For instance, investigating paw preference (Maille et al., 2013) and cooperation (Jiang et al., 2021b) in tree shrews has deepened our understanding of the evolution of manual laterality and cooperative behavior. The functional dissection of spatial coding, orientation maps, and visual learning in the tree shrew V1 system (Bosking et al., 2002; MacEvoy et al., 2009; Schumacher et al., 2022; Tanabe et al., 2022) has helped clarify the fundamental activities and mechanisms of the visual cortex. Due to the homology between their visual system and that of primates, tree shrews are a valuable model for testing and refining novel therapeutic approaches for visual disorders, with their similar eye structure to humans also facilitating our understanding of myopia (Abbott et al., 2011; Khanal et al., 2023; Marsh-Tootle & Norton, 1989; Norton et al., 2006). Creating tree shrew models of human diseases is highly desirable (Table 1) and a primary reason for securing financial support for their study. We believe that addressing the following key questions with tree shrews in biomedical research is crucial for understanding the scientific value of this species in the long term. These questions are not limited to tree shrew studies but have broader implications in the era of big data.
Question #1. Can the main biological traits of tree shrews be genetically decoded?
Our current knowledge of tree shrew phenotypes, including reproduction, physiology, behavior, and aging, are quite limited. To fully decode the biological traits of tree shrews and establish disease models, comprehensive data on their complete phenome, transcriptome, epigenome, and genome are needed. This data-driven personalized approach has been proposed for human populations (Yurkovich et al., 2024), which integrates genetics and environmental factors to elucidate the development of various phenotypes, including normal traits and diseases. Longitudinal measurements of related phenotypes during growth and ageing are critical for precisely defining life status, necessitating the use of multiple phenomic profiling technologies. Notably, templates established for human populations (c.f. Liu & Crawford, 2022; Yurkovich et al., 2024; and references therein) and model animals such as mice (Bogue et al., 2023; Brown et al., 2018) and rats (Zinski et al., 2021) can be adapted for tree shrew genome-phenome studies. Algorithms and software programs developed for rodent behavioral tests should also be optimized to accommodate the specific characteristics of tree shrews. A user-friendly and publicly available integrated databank and biobank would also help attract researchers to this field. By gaining a deeper understanding of tree shrews, we can fully leverage this experimental resource.
Question #2. Can a digitized tree shrew life be created through precise modeling?
The recent advancements in AI have opened new avenues for basic sciences and biomedical research, significantly benefiting health management and clinical practices (Agu & Obulose, 2024; Ong et al., 2024; Thirunavukarasu et al., 2023; Voigtlaender et al., 2024; Zhang et al., 2023b). With the application of large language models and the accumulation of big data on tree shrews at the cellular level—including DNA alterations, RNA regulation and expression, protein expression and degradation, and metabolite production—alongside meso- and macro-scale phenotypic data and longitudinal phenome dynamics, it may be possible to reconstruct a digitized life of tree shrews. Such an approach would integrate computational models linking the genome, epigenome, environment, and lifestyle with phenotypic information, including behaviors. This may provide predictive information regarding the development and onset of diseases in tree shrews, enhancing their use in modeling human diseases.
Question #3. Can an ideal tree shrew model be established for human disease prediction and precision medicine?
Our understanding of human diseases has significantly evolved over the past decades. In addition to common diseases, such as diabetes, stroke, hypertension, and AD, there are more than 7 000 rare diseases in humans (100,000 Genomes Project Pilot Investigators et al., 2021; Kernohan & Boycott, 2024; Tambuyzer et al., 2020; Zhu et al., 2022). The pathobiology of these rare diseases is highly complex, requiring long-term efforts to establish models using appropriate experimental animals. Similar to rodents, tree shrews will not be the sole solution for this problem. Identifying which common or rare diseases can be best modeled in tree shrews remains an open question. While existing studies (e.g., Table 1) are promising, they have not yet provided sufficient evidence that an ideal tree shrew model for certain human disease can be established. Answers from the previous two questions will hopefully guide future efforts in developing valid tree shrew disease models. Genetically modified tree shrews or viral vector-mediated gene delivery offer excellent approaches for characterizing gene functions and mechanisms. However, this requires detailed analyses at the levels of specific cell types, cell clusters, cell communications, and networks. In tree shrew brain research, it is critical to accurately define neuronal cell types, connections, circuits, and projections. Researchers must fully consider the unique and dominant position of tree shrews in terms of behavior or face validity when establishing disease models. This also applies to cross-species studies, as tree shrews offer distinct advantages compared to rodents, such as their visual system and social attributes. An equally important question is how studying the tree shrew brain can enhance our knowledge of the human brain and related disorders. Under these conditions, tree shrews may serve as a useful tool to verify the effectiveness and safety of related disease research. The high face validity and predictive validity of tree shrews in certain disease models are crucial for analyzing disease mechanisms. Addressing the lack of specific research reagents and toolkits, common to all non-model organisms, remains an urgent need. To solve these issues, we must undergo a paradigm shift now and, in the future, utilizing cutting-edge technologies.
CONCLUSIONS
Due to the urgent need for animal models to meet the changing global burden of diseases, ethical considerations, cost efficiency, resource availability, and suitability as viable animal models, tree shrews have emerged as a promising model in the field. Tree shrews, being more closely related to humans than mice and rats, are more likely to yield translational insights to humans (Yao, 2017). Indeed, over the past decades, several key findings in tree shrews have broadened our knowledge about life and health. Notably, the discovery of tree shrew susceptibility to HBV (Su et al., 1987; Walter et al., 1996; Yan et al., 1996) and HCV infections (Amako et al., 2010; Xie et al., 1998; Xu et al., 2007; Zhao et al., 2002) led to the identification of NTCP as a functional receptor for HBV and HDV (Li, 2015; Yan et al., 2012). This landmark discovery resolved a long-standing mystery regarding HBV entry, spurring subsequent studies to characterize the NTCP structure (Qi & Li, 2022; and references therein), establish in vitro HBV infection models (Guo et al., 2023a), and develop potent and selective inhibitors (Chen et al., 2022b; Liu et al., 2021), all contributing to the prevention and treatment of these infections. Similarly, the initial establishment of the tree shrew model for myopia (Marsh-Tootle & Norton, 1989; Norton et al., 2006; Sherman et al., 1977) opened new avenues for studying the pathobiology of myopia and evaluating prophylactics. The use of tree shrews in visual cortex research has also yielded rich insights regarding the working theory of the visual system (Fitzpatrick, 1996; Lee et al., 2016; MacEvoy et al., 2009; Sedigh-Sarvestani et al., 2021; Tanabe et al., 2022). The development of a chronic psychosocial stress model in tree shrews (Fuchs, 2005; Fuchs & Flügge, 2002; Kramer et al., 1999; Magariños et al., 1996) has also facilitated the discovery of potential treatments (Czéh et al., 2001; Fuchs et al., 1996; Parésys et al., 2016; Schmelting et al., 2014; van der Hart et al., 2002). The decoding of the tree shrew genome (Fan et al., 2013, 2019; Ye et al., 2021) and genetic modifications using SSCs (Li et al., 2017) have further paved the way for uncovering more secrets of this animal and broadening its applications in biomedical research.
By summarizing the key findings and animal models, we believe that tree shrews can serve as an adjunct and alternative to NHPs (Cao et al., 2003; Savier et al., 2021; Xiao et al., 2017; Yao, 2017), and as an essential complement to studies of model organisms for both basic and biomedical research (Lehner, 2013; Yamamoto et al., 2024). Researchers have consistently endeavored to establish tree shrews as viable laboratory animals, focusing on resource sharing, inbred strain development, and behavioral, genetic, and animal model optimization. Tree shrews are particularly appealing to investigators seeking models for testing potential drug therapies for infectious and neurodegenerative diseases, given their close relationship to NHPs and lower cost. Addressing the longstanding low acceptance of tree shrews in biomedical research is crucial. A bright future for tree shrew research is anticipated, especially with increased international collaborations and the sharing of resources and technologies.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
Acknowledgments
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Y.G.Y. conceived the idea and designed the study. Y.G.Y. wrote the entire draft, L.L. wrote the draft section “Behavioral tests for tree shrews”, and R.J.N. and J.N.Z. wrote the draft section “Tree shrew brain atlas”. Y.G.Y., H.L., L.X., and D.Y. compiled Table 1. All authors provided valuable discussion, edited the manuscript, and read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Prof. Jianhua JC Cang and Dr. Xiaorong Liu from the University of Virginia, and Prof. David Fitzpatrick from the Max Planck Florida Institute for Neuroscience for their helpful comments. We thank Mr. Weibo Kang for help with the preparation of Figure 2 and Prof. Xiaoyong Man for making Figure 3H.
Biography
Authors are listed in alphabetic order from the fourth author onwards
Funding Statement
This work was supported by the STI2030-Major Projects (2021ZD0200900 to Y.G.Y.) and "Light of West China" Program of the Chinese Academy of Sciences (xbzg-zdsys-202302 to Y.G.Y.)
References
- 100, 000 Genomes Project Pilot Investigators, Smedley D, Smith KR, et al 100, 000 genomes pilot on rare-disease diagnosis in health care - preliminary report. The New England Journal of Medicine. 2021;385(20):1868–1880. doi: 10.1056/NEJMoa2035790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott CJ, Grünert U, Pianta MJ, et al Retinal thinning in tree shrews with induced high myopia: optical coherence tomography and histological assessment. Vision Research. 2011;51(3):376–385. doi: 10.1016/j.visres.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Achoki T, Sartorius B, Watkins D, et al Health trends, inequalities and opportunities in South Africa's provinces, 1990–2019: findings from the Global Burden of Disease 2019 Study. Journal of Epidemiology & Community Health. 2022;76(5):471–481. doi: 10.1136/jech-2021-217480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agu PC, Obulose CN Piquing artificial intelligence towards drug discovery: tools, techniques, and applications. Drug Development Research. 2024;85(2):e22159. doi: 10.1002/ddr.22159. [DOI] [PubMed] [Google Scholar]
- Ai JQ, Luo RC, Tu T, et al Doublecortin-expressing neurons in Chinese tree shrew forebrain exhibit mixed rodent and primate-like topographic characteristics. Frontiers in Neuroanatomy. 2021;15:727883. doi: 10.3389/fnana.2021.727883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen MS, Flügge G, Fuchs E, et al Histaminergic system in the tree shrew brain. The Journal of Comparative Neurology. 1989;286(3):289–310. doi: 10.1002/cne.902860302. [DOI] [PubMed] [Google Scholar]
- Amako Y, Tsukiyama-Kohara K, Katsume A, et al. 2010. Pathogenesis of hepatitis C virus infection in Tupaia belangeri. Journal of Virology, 84 (1): 303–311.
- Arnsten AFT, Datta D, Preuss TM Studies of aging nonhuman primates illuminate the etiology of early-stage Alzheimer's-like neuropathology: an evolutionary perspective. American Journal of Primatology. 2021;83(11):e23254. doi: 10.1002/ajp.23254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustyniak J, Bertero A, Coccini T, et al Organoids are promising tools for species-specific in vitro toxicological studies. Journal of Applied Toxicology. 2019;39(12):1610–1622. doi: 10.1002/jat.3815. [DOI] [PubMed] [Google Scholar]
- Badon IW, Oh Y, Kim HJ, et al Recent application of CRISPR-Cas12 and OMEGA system for genome editing. Molecular Therapy. 2024;32(1):32–43. doi: 10.1016/j.ymthe.2023.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala PC, Eisenreich BR, Yoo SBM, et al Automated markerless pose estimation in freely moving macaques with OpenMonkeyStudio. Nature Communications. 2020;11(1):4560. doi: 10.1038/s41467-020-18441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaram P, Isaamullah M, Petry HM, et al Distributions of vesicular glutamate transporters 1 and 2 in the visual system of tree shrews (Tupaia belangeri) The Journal of Comparative Neurology. 2015;523(12):1792–1808. doi: 10.1002/cne.23727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MKL, Wei HY, Reed JL, et al Cortical projections to the superior colliculus in tree shrews (Tupaia belangeri) The Journal of Comparative Neurology. 2013;521(7):1614–1632. doi: 10.1002/cne.23249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloch Z, Shen ZL, Zhang L, et al Recapitulating Zika virus infection in vagina of tree shrew (Tupaia belangeri) Frontiers in Cellular and Infection Microbiology. 2021;11:687338. doi: 10.3389/fcimb.2021.687338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron LA, Besenbacher S, Zheng J, et al Evolution of the germline mutation rate across vertebrates. Nature. 2023;615(7951):285–291. doi: 10.1038/s41586-023-05752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogue MA, Ball RL, Walton DO, et al Mouse phenome database: curated data repository with interactive multi-population and multi-trait analyses. Mammalian Genome. 2023;34(4):509–519. doi: 10.1007/s00335-023-10014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosking WH, Crowley JC, Fitzpatrick D Spatial coding of position and orientation in primary visual cortex. Nature Neuroscience. 2002;5(9):874–882. doi: 10.1038/nn908. [DOI] [PubMed] [Google Scholar]
- Bosking WH, Zhang Y, Schofield B, et al Orientation selectivity and the arrangement of horizontal connections in tree shrew striate cortex. Journal of Neuroscience. 1997;17(6):2112–2127. doi: 10.1523/JNEUROSCI.17-06-02112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SDM, Holmes CC, Mallon AM, et al High-throughput mouse phenomics for characterizing mammalian gene function. Nature Reviews Genetics. 2018;19(6):357–370. doi: 10.1038/s41576-018-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Yang EB, Su JJ, et al The tree shrews: adjuncts and alternatives to primates as models for biomedical research. Journal of Medical Primatology. 2003;32(3):123–130. doi: 10.1034/j.1600-0684.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- Challis RC, Ravindra Kumar S, Chen XH, et al Adeno-associated virus toolkit to target diverse brain cells. Annual Review of Neuroscience. 2022;45:447–469. doi: 10.1146/annurev-neuro-111020-100834. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Willis JH The genetics of inbreeding depression. Nature Reviews Genetics. 2009;10(11):783–796. doi: 10.1038/nrg2664. [DOI] [PubMed] [Google Scholar]
- Che PL, Wang MM, Larson-Casey JL, et al A novel tree shrew model of pulmonary fibrosis. Laboratory Investigation. 2021;101(1):116–124. doi: 10.1038/s41374-020-00476-3. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Zhang QY, Yu DD, et al Optimization of milk substitutes for the artificial rearing of Chinese tree shrews (Tupaia belangeri chinensis) Animals. 2022a;12(13):1655. doi: 10.3390/ani12131655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Ou C, Yang C, et al A novel animal model of induced breast precancerous lesion in tree shrew. Biological and Pharmaceutical Bulletin. 2019;42(4):580–585. doi: 10.1248/bpb.b18-00688. [DOI] [PubMed] [Google Scholar]
- Chen Q, Ma ZX, Xia LB, et al A tree shrew model for steroid-associated osteonecrosis. Zoological Research. 2020;41(5):564–568. doi: 10.24272/j.issn.2095-8137.2020.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SW, Zhang L, Chen Y, et al Inhibiting sodium taurocholate cotransporting polypeptide in HBV-related diseases: from biological function to therapeutic potential. Journal of Medicinal Chemistry. 2022b;65(19):12546–12561. doi: 10.1021/acs.jmedchem.2c01097. [DOI] [PubMed] [Google Scholar]
- Chi HY, Tanaka Y, Hifumi T, et al Pathological and genetic aspects of spontaneous mammary gland tumor in Tupaia belangeri (tree shrew) PLoS One. 2020;15(5):e0233232. doi: 10.1371/journal.pone.0233232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomsung RD, Wei HY, Day-Brown JD, et al Synaptic organization of connections between the temporal cortex and pulvinar nucleus of the tree shrew. Cerebral Cortex. 2010;20(4):997–1011. doi: 10.1093/cercor/bhp162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunhabundit P, Thongpila S, Mingsakul T, et al Microvascularization of the common tree shrew (Tupaia glis) superior cervical ganglion studied by vascular corrosion cast with scanning electron microscopy. Acta Anatomica. 1993;148(1):49–56. doi: 10.1159/000147522. [DOI] [PubMed] [Google Scholar]
- Collins PM, Tsang WN Growth and reproductive development in the male tree shrew (Tupaia belangeri) from birth to sexual maturity. Biology of Reproduction. 1987;37(2):261–267. doi: 10.1095/biolreprod37.2.261. [DOI] [PubMed] [Google Scholar]
- Coolen A, Hoffmann K, Barf RP, et al. 2012. Telemetric study of sleep architecture and sleep homeostasis in the day-active tree shrew Tupaia belangeri. Sleep, 35(6): 879–888.
- Coughlan G, Laczó J, Hort J, et al Spatial navigation deficits-overlooked cognitive marker for preclinical Alzheimer disease? Nature Reviews Neurology. 2018;14(8):496–506. doi: 10.1038/s41582-018-0031-x. [DOI] [PubMed] [Google Scholar]
- Cusick JA, Wellman CL, Demas GE The call of the wild: using non-model systems to investigate microbiome-behaviour relationships. Journal of Experimental Biology. 2021;224(10):jeb224485. doi: 10.1242/jeb.224485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh B, Michaelis T, Watanabe T, et al Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(22):12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai JK, Wang SX, Shan D, et al A diffusion tensor imaging atlas of white matter in tree shrew. Brain Structure and Function. 2017;222(4):1733–1751. doi: 10.1007/s00429-016-1304-z. [DOI] [PubMed] [Google Scholar]
- Dai JK, Wang SX, Shan D, et al Super-resolution track-density imaging reveals fine anatomical features in tree shrew primary visual cortex and hippocampus. Neuroscience Bulletin. 2018;34(3):438–448. doi: 10.1007/s12264-017-0199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darai G, Schwaier A, Komitowski D, et al Experimental infection of Tupaia belangeri (tree shrews) with herpes simplex virus types 1 and 2. The Journal of Infectious Diseases. 1978;137(3):221–226. doi: 10.1093/infdis/137.3.221. [DOI] [PubMed] [Google Scholar]
- Day-Brown JD, Wei H, Chomsung RD, et al Pulvinar projections to the striatum and amygdala in the tree shrew. Frontiers in Neuroanatomy. 2010;4:143. doi: 10.3389/fnana.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coster W, Weissensteiner MH, Sedlazeck FJ Towards population-scale long-read sequencing. Nature Reviews Genetics. 2021;22(9):572–587. doi: 10.1038/s41576-021-00367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond IT, Snyder M, Killackey H, et al Thalamo-cortical projections in the tree shrew (Tupaia glis) The Journal of Comparative Neurology. 1970;139(3):273–306. doi: 10.1002/cne.901390303. [DOI] [PubMed] [Google Scholar]
- Dimanico MM, Klaassen AL, Wang J, et al Aspects of tree shrew consolidated sleep structure resemble human sleep. Communications Biology. 2021;4(1):722. doi: 10.1038/s42003-021-02234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Jin LT, Du WJ, et al Alterations of dopamine receptors and the adaptive changes of L-type calcium channel subtypes regulate cocaine-seeking habit in tree shrew. Life. 2022;12(7):984. doi: 10.3390/life12070984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Meng YM, Du WJ, et al Increased cocaine motivation in tree shrews is modulated by striatal dopamine D1 receptor-mediated upregulation of Cav 1.2. Addiction Biology. 2021;26(6):e13053. doi: 10.1111/adb.13053. [DOI] [PubMed] [Google Scholar]
- El Marjou F, Jouhanneau C, Krndija D Targeted transgenic mice using CRISPR/Cas9 technology. Methods in Molecular Biology. 2021;2214:125–141. doi: 10.1007/978-1-0716-0958-3_9. [DOI] [PubMed] [Google Scholar]
- Elliot OS, Elliot MW, Lisco H Breast cancer in a tree shrew (Tupaia glis) Nature. 1966;211(5053):1105. doi: 10.1038/2111105a0. [DOI] [PubMed] [Google Scholar]
- Estrada A, Garber PA, Rylands AB, et al Impending extinction crisis of the world's primates: why primates matter. Science Advances. 2017;3(1):e1600946. doi: 10.1126/sciadv.1600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Huang ZY, Cao CC, et al Genome of the Chinese tree shrew. Nature Communications. 2013;4:1426. doi: 10.1038/ncomms2416. [DOI] [PubMed] [Google Scholar]
- Fan Y, Luo RC, Su LY, et al Does the Genetic feature of the Chinese tree shrew (Tupaia belangeri chinensis) support its potential as a viable model for Alzheimer's disease research? Journal of Alzheimer's Disease. 2018;61(3):1015–1028. doi: 10.3233/JAD-170594. [DOI] [PubMed] [Google Scholar]
- Fan Y, Ye MS, Zhang JY, et al Chromosomal level assembly and population sequencing of the Chinese tree shrew genome. Zoological Research. 2019;40(6):506–521. doi: 10.24272/j.issn.2095-8137.2019.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Yu DD, Yao YG Tree shrew database (TreeshrewDB): a genomic knowledge base for the Chinese tree shrew. Scientific Reports. 2014;4:7145. doi: 10.1038/srep07145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Sun YJ, Lv YH, et al High activity of the stress promoter contributes to susceptibility to stress in the tree shrew. Scientific Reports. 2016;6:24905. doi: 10.1038/srep24905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng LX, Chen YL, Dettin L, et al Generation and in vitro differentiation of a spermatogonial cell line. Science. 2002;297(5580):392–395. doi: 10.1126/science.1073162. [DOI] [PubMed] [Google Scholar]
- Feng Y, Feng YM, Lu CX, et al. 2017. Tree shrew, a potential animal model for hepatitis C, supports the infection and replication of HCV in vitro and in vivo. Journal of General Virology, 98 (8): 2069–2078.
- Fitzpatrick D The functional organization of local circuits in visual cortex: insights from the study of tree shrew striate cortex. Cerebral Cortex. 1996;6(3):329–341. doi: 10.1093/cercor/6.3.329. [DOI] [PubMed] [Google Scholar]
- Flügge G Dynamics of central nervous 5-HT1A-receptors under psychosocial stress. Journal of Neuroscience. 1995;15(11):7132–7140. doi: 10.1523/JNEUROSCI.15-11-07132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge G Alterations in the central nervous alpha 2-adrenoceptor system under chronic psychosocial stress. Neuroscience. 1996;75(1):187–196. doi: 10.1016/0306-4522(96)00292-8. [DOI] [PubMed] [Google Scholar]
- Flügge G, Ahrens O, Fuchs E Monoamine receptors in the amygdaloid complex of the tree shrew (Tupaia belangeri) The Journal of Comparative Neurology. 1994;343(4):597–608. doi: 10.1002/cne.903430409. [DOI] [PubMed] [Google Scholar]
- Flügge G, Ahrens O, Fuchs E β-adrenoceptors in the tree shrew brain. II. Time-dependent effects of chronic psychosocial stress on [125I]iodocyanopindolol binding sites. Cellular and Molecular Neurobiology. 1997;17(4):417–432. doi: 10.1023/A:1026387311220. [DOI] [PubMed] [Google Scholar]
- Fromhage L Mating unplugged: a model for the evolution of mating plug (dis-)placement. Evolution. 2012;66(1):31–39. doi: 10.1111/j.1558-5646.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- Fuchs E Social stress in tree shrews as an animal model of depression: an example of a behavioral model of a CNS disorder. CNS Spectrums. 2005;10(3):182–190. doi: 10.1017/S1092852900010038. [DOI] [PubMed] [Google Scholar]
- Fuchs E Tree shrews at the German Primate Center. Primate Biology. 2015;2(1):111–118. doi: 10.5194/pb-2-111-2015. [DOI] [Google Scholar]
- Fuchs E. 2024. Tree shrews. In: Golledge H, Richardson C. The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals. 9th ed. Chichester: Wiley-Blackwell, 324–339.
- Fuchs E, Corbach-Söhle S. 2010. Tree shrews. In: Hubrecht R, Kirkwood J. The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals. 8th ed. Chichester: Wiley-Blackwell, 262–275.
- Fuchs E, Flügge G Stress, glucocorticoids and structural plasticity of the hippocampus. Neuroscience & Biobehavioral Reviews. 1998;23(2):295–300. doi: 10.1016/s0149-7634(98)00031-1. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Flügge G. 2002. Social stress in tree shrews: effects on physiology, brain function, and behavior of subordinate individuals. Pharmacology, Biochemistry & Behavior, 73 (1): 247–258.
- Fuchs E, Kramer M, Hermes B, et al. 1996. Psychosocial stress in tree shrews: clomipramine counteracts behavioral and endocrine changes. Pharmacology, Biochemistry & Behavior, 54 (1): 219–228.
- Fuchs E, Schumacher M Psychosocial stress affects pineal function in the tree shrew (Tupaia belangeri) Physiology & Behavior. 1990;47(4):713–717. doi: 10.1016/0031-9384(90)90083-g. [DOI] [PubMed] [Google Scholar]
- Gao B, Lin J, Jiang ZM, et al Upregulation of chemokine CXCL10 enhances chronic pulmonary inflammation in tree shrew collagen-induced arthritis. Scientific Reports. 2018;8(1):9993. doi: 10.1038/s41598-018-28404-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne TJ, Siegwart JT Jr, Ward AH, et al The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Experimental Eye Research. 2017;155:75–84. doi: 10.1016/j.exer.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2019 Cancer Risk Factors Collaborators The global burden of cancer attributable to risk factors, 2010–19: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2022;400(10352):563–591. doi: 10.1016/S0140-6736(22)01438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellner AK, Voelter J, Schmidt U, et al Molecular and neurocircuitry mechanisms of social avoidance. Cellular and Molecular Life Sciences. 2021;78(4):1163–1189. doi: 10.1007/s00018-020-03649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Xu DL, Yuan J, et al High-throughput dual-colour precision imaging for brain-wide connectome with cytoarchitectonic landmarks at the cellular level. Nature Communications. 2016;7:12142. doi: 10.1038/ncomms12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk OS, Pitale PM, Saltykova IV, et al A novel tree shrew model of diabetic retinopathy. Frontiers in Endocrinology. 2022;12:799711. doi: 10.3389/fendo.2021.799711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grannonico M, Miller DA, Liu MN, et al Global and regional damages in retinal ganglion cell axon bundles monitored non-invasively by visible-light optical coherence tomography fibergraphy. Journal of Neuroscience. 2021;41(49):10179–10193. doi: 10.1523/JNEUROSCI.0844-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grannonico M, Miller DA, Liu MN, et al Comparative in vivo imaging of retinal structures in tree shrews, humans, and mice. eNeuro. 2024;11(3):ENEURO.0373–23.2024. doi: 10.1523/ENEURO.0373-23.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu TL, Yu DD, Fan Y, et al Molecular identification and antiviral function of the guanylate-binding protein (GBP) genes in the Chinese tree shrew (Tupaia belangeri chinesis) Developmental & Comparative Immunology. 2019a;96:27–36. doi: 10.1016/j.dci.2019.02.014. [DOI] [PubMed] [Google Scholar]
- Gu TL, Yu DD, Li Y, et al Establishment and characterization of an immortalized renal cell line of the Chinese tree shrew (Tupaia belangeri chinesis) Applied Microbiology and Biotechnology. 2019b;103(5):2171–2180. doi: 10.1007/s00253-019-09615-3. [DOI] [PubMed] [Google Scholar]
- Gu TL, Yu DD, Xu L, et al Tupaia GBP1 interacts with STING to initiate autophagy and restrict herpes simplex virus type 1 infection. The Journal of Immunology. 2021a;207(11):2673–2680. doi: 10.4049/jimmunol.2100325. [DOI] [PubMed] [Google Scholar]
- Gu TL, Yu DD, Xu L, et al Tupaia guanylate-binding protein 1 interacts with vesicular stomatitis virus phosphoprotein and represses primary transcription of the viral genome. Cytokine. 2021b;138:155388. doi: 10.1016/j.cyto.2020.155388. [DOI] [PubMed] [Google Scholar]
- Guo HB, Urban S, Wang WS In vitro cell culture models to study hepatitis B and D virus infection. Frontiers in Microbiology. 2023a;14:1169770. doi: 10.3389/fmicb.2023.1169770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YT, Shao Y, Bi XP, et al Harvesting the fruits of the first stage of the Primate Genome Project. Zoological Research. 2023b;44(4):725–728. doi: 10.24272/j.issn.2095-8137.2023.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J, Monavarfeshani A, Qiao M, et al Evolution of neuronal cell classes and types in the vertebrate retina. Nature. 2023;624(7991):415–424. doi: 10.1038/s41586-023-06638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque RU, Levey AI Alzheimer's disease: a clinical perspective and future nonhuman primate research opportunities. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(52):26224–26229. doi: 10.1073/pnas.1912954116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Zhao Y, Fu ZL, et al A novel tree shrew model of lipopolysaccharide-induced acute respiratory distress syndrome. Journal of Advanced Research. 2024;56:157–165. doi: 10.1016/j.jare.2023.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick PW, Garcia-Dorado A Understanding inbreeding depression, purging, and genetic rescue. Trends in Ecology & Evolution. 2016;31(12):940–952. doi: 10.1016/j.tree.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Helgen KM. 2005. Order scandentia. In: Wilson DE, Reeder DM. Mammal Species of the World: A Taxonomic and Geographic Reference. 3rd ed. Baltimore: John Hopkins University Press, 104–109.
- Hirabayashi M, Takizawa A, Hochi S Embryonic stem cells and gene manipulation in rat. Methods in Molecular Biology. 2019;2018:115–130. doi: 10.1007/978-1-4939-9581-3_5. [DOI] [PubMed] [Google Scholar]
- Hook PW, Timp W Beyond assembly: the increasing flexibility of single-molecule sequencing technology. Nature Reviews Genetics. 2023;24(9):627–641. doi: 10.1038/s41576-023-00600-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu KJ, Lv LB, Huang H, et al A novel tree shrew model of chronic experimental autoimmune uveitis and its disruptive application. Frontiers in Immunology. 2022;13:889596. doi: 10.3389/fimmu.2022.889596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YD, Zhao Q, Zhang XR, et al. 2018. Comparison of the properties of neural stem cells of the hippocampus in the tree shrew and rat in vitro. Molecular Medicine Reports, 17 (4): 5676–5683.
- Huang K, Han YN, Chen K, et al A hierarchical 3D-motion learning framework for animal spontaneous behavior mapping. Nature Communications. 2021;12(1):2784. doi: 10.1038/s41467-021-22970-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Nie BB, Ma C, et al Stereotaxic 18F-FDG PET and MRI templates with three-dimensional digital atlas for statistical parametric mapping analysis of tree shrew brain. Journal of Neuroscience Methods. 2018;293:105–116. doi: 10.1016/j.jneumeth.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Huang XY, Elyada YM, Bosking WH, et al Optogenetic assessment of horizontal interactions in primary visual cortex. Journal of Neuroscience. 2014;34(14):4976–4990. doi: 10.1523/JNEUROSCI.4116-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZH, Ni RJ, Luo PH, et al Distribution of tyrosine-hydroxylase-immunoreactive neurons in the hypothalamus of tree shrews. The Journal of Comparative Neurology. 2020;528(6):935–952. doi: 10.1002/cne.24803. [DOI] [PubMed] [Google Scholar]
- Jia J, Qiu DD, Lu CX, et al Transcriptome analysis of choroid and retina from tree shrew with choroidal neovascularization reveals key signaling moieties. Frontiers in Genetics. 2021;12:654955. doi: 10.3389/fgene.2021.654955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LM, Lu CX, Sun QM Tree shrew as a new animal model for the study of Dengue virus. Frontiers in Immunology. 2021a;12:621164. doi: 10.3389/fimmu.2021.621164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LP, Shen QS, Yang CP, et al Establishment of basal cell carcinoma animal model in Chinese tree shrew (Tupaia belangeri chinensis) Zoological Research. 2017;38(4):180–190. doi: 10.24272/j.issn.2095-8137.2017.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang MP, Wang MYX, Shi QQ, et al Evolution and neural representation of mammalian cooperative behavior. Cell Reports. 2021b;37(7):110029. doi: 10.1016/j.celrep.2021.110029. [DOI] [PubMed] [Google Scholar]
- Justice MJ, Dhillon P Using the mouse to model human disease: increasing validity and reproducibility. Disease Models & Mechanisms. 2016;9(2):101–103. doi: 10.1242/dmm.024547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Hall WC, Killackey H, et al Visual cortex of the tree shrew (Tupaia glis): architectonic subdivisions and representations of the visual field. Brain Research. 1972;42(2):491–496. doi: 10.1016/0006-8993(72)90548-3. [DOI] [PubMed] [Google Scholar]
- Kaschube M, Schnabel M, Löwel S, et al Universality in the evolution of orientation columns in the visual cortex. Science. 2010;330(6007):1113–1116. doi: 10.1126/science.1194869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayesh MEH, Ezzikouri S, Chi HY, et al. 2017a. Interferon-β response is impaired by hepatitis B virus infection in Tupaia belangeri. Virus Research, 237 : 47–57.
- Kayesh MEH, Ezzikouri S, Sanada T, et al. 2017b. Oxidative stress and immune responses during hepatitis C virus infection in Tupaia belangeri. Scientific Reports, 7 (1): 9848.
- Kayesh MEH, Kitab B, Sanada T, et al Susceptibility and initial immune response of Tupaia belangeri cells to dengue virus infection. Infection, Genetics and Evolution. 2017c;51:203–210. doi: 10.1016/j.meegid.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Kayesh MEH, Sanada T, Kohara M, et al Tree shrew as an emerging small animal model for human viral infection: a recent overview. Viruses. 2021;13(8):1641. doi: 10.3390/v13081641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernohan KD, Boycott KM The expanding diagnostic toolbox for rare genetic diseases. Nature Reviews Genetics. 2024;25(6):401–415. doi: 10.1038/s41576-023-00683-w. [DOI] [PubMed] [Google Scholar]
- Keuker JIH, de Biurrun G, Luiten PGM, et al Preservation of hippocampal neuron numbers and hippocampal subfield volumes in behaviorally characterized aged tree shrews. The Journal of Comparative Neurology. 2004;468(4):509–517. doi: 10.1002/cne.10996. [DOI] [PubMed] [Google Scholar]
- KhalafAllah MT, Fuchs PA, Nugen F, et al Heterogenous thinning of peripapillary tissues occurs early during high myopia development in juvenile tree shrews. Experimental Eye Research. 2024;240:109824. doi: 10.1016/j.exer.2024.109824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal S, Norton TT, Gawne TJ Amber light treatment produces hyperopia in tree shrews. Ophthalmic & Physiological Optics. 2021;41(5):1076–1086. doi: 10.1111/opo.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal S, Norton TT, Gawne TJ Limited bandwidth short-wavelength light produces slowly-developing myopia in tree shrews similar to human juvenile-onset myopia. Vision Research. 2023;204:108161. doi: 10.1016/j.visres.2022.108161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khani A, Rainer G Recognition memory in tree shrew (Tupaia belangeri) after repeated familiarization sessions. Behavioural Processes. 2012;90(3):364–371. doi: 10.1016/j.beproc.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Klein A, Radespiel U, Felmy F, et al AA-amyloidosis in captive northern tree shrews (Tupaia belangeri) Veterinary Pathology. 2022;59(2):340–347. doi: 10.1177/03009858211066847. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Amieva H, Petersen RC, et al Alzheimer disease. Nature Reviews Disease Primers. 2021;7(1):33. doi: 10.1038/s41572-021-00269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozicz T, Bordewin LAP, Czéh B, et al Chronic psychosocial stress affects corticotropin-releasing factor in the paraventricular nucleus and central extended amygdala as well as urocortin 1 in the non-preganglionic Edinger-Westphal nucleus of the tree shrew. Psychoneuroendocrinology. 2008;33(6):741–754. doi: 10.1016/j.psyneuen.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Kramer M, Hiemke C, Fuchs E Chronic psychosocial stress and antidepressant treatment in tree shrews: time-dependent behavioral and endocrine effects. Neuroscience & Biobehavioral Reviews. 1999;23(7):937–947. doi: 10.1016/s0149-7634(99)00027-5. [DOI] [PubMed] [Google Scholar]
- Kubota H, Brinster RL Spermatogonial stem cells. Biology of Reproduction. 2018;99(1):52–74. doi: 10.1093/biolre/ioy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst M, Laurell E, Mokayes N, et al. 2019. A cellular-resolution atlas of the larval zebrafish brain. Neuron, 103 (1): 21–38. e5.
- Kurohmaru M, Maeda S, Suda A, et al. 1996. An ultrastructural and lectin-histochemical study on the seminiferous epithelium of the common tree shrew (Tupaia glis). Journal of Anatomy, 189 (Pt 1): 87–95.
- Larson-Casey JL, He C, Che PL, et al Technical advance: the use of tree shrews as a model of pulmonary fibrosis. PLoS One. 2020;15(11):e0241323. doi: 10.1371/journal.pone.0241323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Huang XY, Fitzpatrick D Topology of ON and OFF inputs in visual cortex enables an invariant columnar architecture. Nature. 2016;533(7601):90–94. doi: 10.1038/nature17941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner B Genotype to phenotype: lessons from model organisms for human genetics. Nature Reviews Genetics. 2013;14(3):168–178. doi: 10.1038/nrg3404. [DOI] [PubMed] [Google Scholar]
- Leonard C, Schneider GE, Gross CG Performance on learning set and delayed-response tasks by tree shrews (Tupaia glis) Journal of Comparative and Psychology. 1966;62(3):501–504. [Google Scholar]
- Lezama-García K, Mariti C, Mota-Rojas D, et al Maternal behaviour in domestic dogs. International Journal of Veterinary Science and Medicine. 2019;7(1):20–30. doi: 10.1080/23144599.2019.1641899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Bi R, Wang L, et al Characterization of long-term ex vivo expansion of tree shrew spermatogonial stem cells. Zoological Research. 2023a;44(6):1080–1094. doi: 10.24272/j.issn.2095-8137.2023.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CH, Yan LZ, Ban WZ, et al Long-term propagation of tree shrew spermatogonial stem cells in culture and successful generation of transgenic offspring. Cell Research. 2017;27(2):241–252. doi: 10.1038/cr.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CJ, Hui YQ, Zhang R, et al A comparison of behavioral paradigms assessing spatial memory in tree shrews. Cerebral Cortex. 2023b;33(19):10303–10321. doi: 10.1093/cercor/bhad283. [DOI] [PubMed] [Google Scholar]
- Li CW, McHaney KM, Sederberg PB, et al Tree shrews as an animal model for studying perceptual decision-making reveal a critical role of stimulus-independent processes in guiding behavior. eNeuro. 2022;9(6):ENEURO.0419–22.2022. doi: 10.1523/ENEURO.0419-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CX, Xiao ZF, Li YR, et al Deep learning-based activity recognition and fine motor identification using 2D skeletons of cynomolgus monkeys. Zoological Research. 2023c;44(5):967–980. doi: 10.24272/j.issn.2095-8137.2022.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, Baloch Z, Zhao Y, et al Establishment of tree shrew animal model for Kaposi's sarcoma-associated herpesvirus (HHV-8) infection. Frontiers in Microbiology. 2021a;12:710067. doi: 10.3389/fmicb.2021.710067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Mei LY, Nie XP, et al. 2023d. New tree shrew Parkinson’s model: a cost-effective alternative to monkey models. bioRxiv.
- Li HL, Xiang BL, Li X, et al Cognitive deficits and Alzheimer's disease-like pathologies in the aged Chinese tree shrew. Molecular Neurobiology. 2024;61(4):1892–1906. doi: 10.1007/s12035-023-03663-7. [DOI] [PubMed] [Google Scholar]
- Li J, Shi TD, Han JF, et al A systematic study of Tupaia as a model for human acute hepatitis B infection. The Journal of Veterinary Medical Science. 2021b;83(6):1004–1011. doi: 10.1292/jvms.21-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Hong SY, Chen WJ, et al Advances in detecting and reducing off-target effects generated by CRISPR-mediated genome editing. Journal of Genetics and Genomics. 2019;46(11):513–521. doi: 10.1016/j.jgg.2019.11.002. [DOI] [PubMed] [Google Scholar]
- Li LH, Li Y, Li X, et al HSV-1 infection and pathogenesis in the tree shrew eye following corneal inoculation. Journal of Neurovirology. 2020;26(3):391–403. doi: 10.1007/s13365-020-00837-0. [DOI] [PubMed] [Google Scholar]
- Li QF, Ding MQ, Wang H, et al The infection of hepatitis D virus in adult tupaia. National Medical Journal of China. 1995;75(10):611–613. [PubMed] [Google Scholar]
- Li RF, Yuan B, Xia XS, et al Tree shrew as a new animal model to study the pathogenesis of avian influenza (H9N2) virus infection. Emerging Microbes & Infections. 2018a;7(1):166. doi: 10.1038/s41426-018-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RF, Zanin M, Xia XS, et al The tree shrew as a model for infectious diseases research. Journal of Thoracic Disease. 2018b;10(S19):S2272–S2279. doi: 10.21037/jtd.2017.12.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WH The hepatitis B virus receptor. Annual Review of Cell and Developmental Biology. 2015;31:125–147. doi: 10.1146/annurev-cellbio-100814-125241. [DOI] [PubMed] [Google Scholar]
- Li X, Zhou ZC, Liu WK, et al Chinese tree shrew: a permissive model for in vitro and in vivo replication of human adenovirus species B. Emerging Microbes & Infections. 2021c;10(1):424–438. doi: 10.1080/22221751.2021.1895679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N, Xiong LL, Zhang RP, et al Injection of Aβ1–40 into hippocampus induced cognitive lesion associated with neuronal apoptosis and multiple gene expressions in the tree shrew. Apoptosis. 2016;21(5):621–640. doi: 10.1007/s10495-016-1227-4. [DOI] [PubMed] [Google Scholar]
- Liu CY, Si W, Tu CF, et al Deficiency of primate-specific SSX1 induced asthenoteratozoospermia in infertile men and cynomolgus monkey and tree shrew models. The American Journal of Human Genetics. 2023;110(3):516–530. doi: 10.1016/j.ajhg.2023.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SY, Crawford DC Maturation and application of phenome-wide association studies. Trends in Genetics. 2022;38(4):353–363. doi: 10.1016/j.tig.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XL, Li ZF, Li X, et al Neutralizing monoclonal antibodies protect against human adenovirus type 55 infection in transgenic mice and tree shrews. Emerging Microbes & Infections. 2024;13(1):2307513. doi: 10.1080/22221751.2024.2307513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang L, Yan H, et al Design of dimeric bile acid derivatives as potent and selective human NTCP inhibitors. Journal of Medicinal Chemistry. 2021;64(9):5973–6007. doi: 10.1021/acs.jmedchem.1c00078. [DOI] [PubMed] [Google Scholar]
- Lu CX, Feng Y, Sun XM, et al Tree shrew bone marrow-derived mesenchymal stem cells express CD81, OCLN, and miR-122, facilitating the entire hepatitis C virus life cycle. Journal of Medical Virology. 2020;92(12):3465–3474. doi: 10.1002/jmv.25710. [DOI] [PubMed] [Google Scholar]
- Lu T, Peng HM, Zhong LP, et al The tree shrew as a model for cancer research. Frontiers in Oncology. 2021;11:653236. doi: 10.3389/fonc.2021.653236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo MT, Fan Y, Mu D, et al Molecular cloning and characterization of APOBEC3 family in tree shrew. Gene. 2018;646:143–152. doi: 10.1016/j.gene.2017.12.060. [DOI] [PubMed] [Google Scholar]
- Luo MT, Mu D, Yang X, et al Tree shrew cells transduced with human CD4 and CCR5 support early steps of HIV-1 replication, but viral infectivity is restricted by APOBEC3. Journal of Virology. 2021;95(16):e0002021. doi: 10.1128/JVI.00020-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo MT, Zheng YT The tsAPOBEC3 proteins restrict HBV replication and may limit the establishment of persistent infection in tree shrews. Cellular & Molecular Immunology. 2020;17(10):1107–1108. doi: 10.1038/s41423-020-0369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Li M, Su B Application of the genome editing tool CRISPR/Cas9 in non-human primates. Zoological Research. 2016;37(4):214–219. doi: 10.13918/j.issn.2095-8137.2016.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon DC, Jain N, Kaas JH The visual pulvinar in tree shrews II. Projections of four nuclei to areas of visual cortex. The Journal of Comparative Neurology. 2003;467(4):607–627. doi: 10.1002/cne.10940. [DOI] [PubMed] [Google Scholar]
- Ma KL, Gao JH, Huang ZQ, et al Motor function in MPTP-treated tree shrews (Tupaia belangeri chinensis) Neurochemical Research. 2013;38(9):1935–1940. doi: 10.1007/s11064-013-1099-8. [DOI] [PubMed] [Google Scholar]
- Ma LY, Chen R, Zhang YL, et al The tree shrew as a new animal model for the study of periodontitis. Journal of Clinical Periodontology. 2023;50(8):1075–1088. doi: 10.1111/jcpe.13842. [DOI] [PubMed] [Google Scholar]
- Ma Q, Ma WJ, Song TZ, et al Single-nucleus transcriptomic profiling of multiple organs in a rhesus macaque model of SARS-CoV-2 infection. Zoological Research. 2022;43(6):1041–1062. doi: 10.24272/j.issn.2095-8137.2022.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Wong ASY, Tam HY, et al In vivo genome editing thrives with diversified CRISPR technologies. Zoological Research. 1998;39(2):58–71. doi: 10.24272/j.issn.2095-8137.2017.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur Clark J The 3Rs in research: a contemporary approach to replacement, reduction and refinement. British Journal of Nutrition. 2018;120(S1):S1–S7. doi: 10.1017/S0007114517002227. [DOI] [PubMed] [Google Scholar]
- MacEvoy SP, Tucker TR, Fitzpatrick D A precise form of divisive suppression supports population coding in the primary visual cortex. Nature Neuroscience. 2009;12(5):637–645. doi: 10.1038/nn.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan V, Zhang F, Dahlman JE Drug delivery systems for CRISPR-based genome editors. Nature Reviews Drug Discovery. 2023;22(11):875–894. doi: 10.1038/s41573-023-00762-x. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS, Flügge G, et al Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. Journal of Neuroscience. 1996;16(10):3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher EE, Prillaman ME, Keskinoz EN, et al Immunocytochemical and ultrastructural organization of the taste thalamus of the tree shrew (Tupaia belangeri) The Journal of Comparative Neurology. 2021;529(10):2558–2575. doi: 10.1002/cne.25109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maille A, Jäschke N, Joly M, et al Does a nonprimate mammal, the northern tree shrew (Tupaia belangeri), exhibit paw preference in two forms of a grasping task? Journal of Comparative Psychology. 2013;127(1):14–23. doi: 10.1037/a0029238. [DOI] [PubMed] [Google Scholar]
- Marks M, Jin QH, Sturman O, et al Deep-learning based identification, tracking, pose estimation, and behavior classification of interacting primates and mice in complex environments. Nature Machine Intelligence. 2022;4(4):331–340. doi: 10.1038/s42256-022-00477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco RT, De Valois RL, Boles JI A stereotaxic atlas of the brain of the tree shrew (Tupaia glis) Journal für Hirnforschung. 1970;12(4):307–312. [PubMed] [Google Scholar]
- Marsh-Tootle WL, Norton TT Refractive and structural measures of lid-suture myopia in tree shrew. Investigative Ophthalmology & Visual Science. 1989;30(10):2245–2257. [PubMed] [Google Scholar]
- McBrien NA, Norton TT The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri) Vision Research. 1992;32(5):843–852. doi: 10.1016/0042-6989(92)90027-G. [DOI] [PubMed] [Google Scholar]
- McCarthy MM Oxytocin inhibits infanticide in female house mice (Mus domesticus) Hormones and Behavior. 1990;24(3):365–375. doi: 10.1016/0018-506X(90)90015-P. [DOI] [PubMed] [Google Scholar]
- McCollum LA, Roberts RC Ultrastructural localization of tyrosine hydroxylase in tree shrew nucleus accumbens core and shell. Neuroscience. 2014;271:23–34. doi: 10.1016/j.neuroscience.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonigle P, Ruggeri B Animal models of human disease: challenges in enabling translation. Biochemical Pharmacology. 2014;87(1):162–171. doi: 10.1016/j.bcp.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Meadows JRS, Lindblad-Toh K Dissecting evolution and disease using comparative vertebrate genomics. Nature Reviews Genetics. 2017;18(10):624–636. doi: 10.1038/nrg.2017.51. [DOI] [PubMed] [Google Scholar]
- Meyer U, van Kampen M, Isovich E, et al Chronic psychosocial stress regulates the expression of both GR and MR mRNA in the hippocampal formation of tree shrews. Hippocampus. 2001;11(3):329–336. doi: 10.1002/hipo.1047. [DOI] [PubMed] [Google Scholar]
- Miller DA, Grannonico M, Liu MN, et al Visible-light optical coherence tomography fibergraphy for quantitative imaging of retinal ganglion cell axon bundles. Translational Vision Science & Technology. 2020;9(11):11. doi: 10.1167/tvst.9.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DA, Grannonico M, Liu MN, et al. 2024. Visible-light optical coherence tomography fibergraphy of the tree shrew retinal ganglion cell axon bundles. IEEE Transactions on Medical Imaging.
- Mooser F, Bosking WH, Fitzpatrick D A morphological basis for orientation tuning in primary visual cortex. Nature Neuroscience. 2004;7(8):872–879. doi: 10.1038/nn1287. [DOI] [PubMed] [Google Scholar]
- Mu D, Yang H, Zhu JW, et al. 2014. Independent birth of a novel TRIMCyp in Tupaia belangeri with a divergent function from its paralog TRIM5. Molecular Biology and Evolution, 31 (11): 2985–2997.
- Muly EC, Fitzpatrick D The morphological basis for binocular and ON/OFF convergence in tree shrew striate cortex. Journal of Neuroscience. 1992;12(4):1319–1334. doi: 10.1523/JNEUROSCI.12-04-01319.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafar F, Harvey MA, Khani A, et al Divergent solutions to visual problem solving across mammalian species. eNeuro. 2018;5(4):ENEURO.0167–18.2018. doi: 10.1523/ENEURO.0167-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair J, Topka M, Khani A, et al Tree shrews (Tupaia belangeri) exhibit novelty preference in the novel location memory task with 24-h retention periods. Frontiers in Psychology. 2014;5:303. doi: 10.3389/fpsyg.2014.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine, Division on Earth and Life Studies, Health and Medicine Division, et al. 2023. Nonhuman Primate Models in Biomedical Research: State of the Science and Future Needs. Washington: National Academies Press.
- Ni RJ, Huang ZH, Luo PH, et al The tree shrew cerebellum atlas: systematic nomenclature, neurochemical characterization, and afferent projections. The Journal of Comparative Neurology. 2018a;526(17):2744–2775. doi: 10.1002/cne.24526. [DOI] [PubMed] [Google Scholar]
- Ni RJ, Huang ZH, Shu YM, et al Atlas of the striatum and Globus pallidus in the tree shrew: comparison with rat and mouse. Neuroscience Bulletin. 2018b;34(3):405–418. doi: 10.1007/s12264-018-0212-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni RJ, Luo PH, Shu YM, et al Whole-brain mapping of afferent projections to the bed nucleus of the stria terminalis in tree shrews. Neuroscience. 2016;333:162–180. doi: 10.1016/j.neuroscience.2016.07.017. [DOI] [PubMed] [Google Scholar]
- Ni RJ, Shu YM, Li T, et al Whole-brain afferent inputs to the caudate nucleus, putamen, and accumbens nucleus in the tree shrew striatum. Frontiers in Neuroanatomy. 2021;15:763298. doi: 10.3389/fnana.2021.763298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni RJ, Shu YM, Luo PH, et al Immunohistochemical mapping of neuropeptide Y in the tree shrew brain. The Journal of Comparative Neurology. 2015;523(3):495–529. doi: 10.1002/cne.23696. [DOI] [PubMed] [Google Scholar]
- Ni RJ, Shu YM, Wang J, et al Distribution of vasopressin, oxytocin and vasoactive intestinal polypeptide in the hypothalamus and extrahypothalamic regions of tree shrews. Neuroscience. 2014;265:124–136. doi: 10.1016/j.neuroscience.2014.01.034. [DOI] [PubMed] [Google Scholar]
- Ni RJ, Tian Y, Dai XY, et al Social avoidance behavior in male tree shrews and prosocial behavior in male mice toward unfamiliar conspecifics in the laboratory. Zoological Research. 2020;41(3):258–272. doi: 10.24272/j.issn.2095-8137.2020.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu YY, Yu Y, Bernat A, et al Transgenic rhesus monkeys produced by gene transfer into early-cleavage-stage embryos using a simian immunodeficiency virus-based vector. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(41):17663–17667. doi: 10.1073/pnas.1006563107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Amedo AO, Siegwart JT Jr Darkness causes myopia in visually experienced tree shrews. Investigative Ophthalmology & Visual Science. 2006;47(11):4700–4707. doi: 10.1167/iovs.05-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Amedo AO, Siegwart JT Jr The effect of age on compensation for a negative lens and recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri) Vision Research. 2010;50(6):564–576. doi: 10.1016/j.visres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, McBrien NA Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Research. 1992;32(5):833–842. doi: 10.1016/0042-6989(92)90026-F. [DOI] [PubMed] [Google Scholar]
- Norton TT, Rager G, Kretz R ON and OFF regions in layer IV of striate cortex. Brain Research. 1985;327(1-2):319–323. doi: 10.1016/0006-8993(85)91527-6. [DOI] [PubMed] [Google Scholar]
- Nowak MA Five rules for the evolution of cooperation. Science. 2006;314(5805):1560–1563. doi: 10.1126/science.1133755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MA Evolving cooperation. Journal of Theoretical Biology. 2012;299:1–8. doi: 10.1016/j.jtbi.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Ohl F, Fuchs E Differential effects of chronic stress on memory processes in the tree shrew. Cognitive Brain Research. 1999;7(3):379–387. doi: 10.1016/S0926-6410(98)00042-1. [DOI] [PubMed] [Google Scholar]
- Ohl F, Oitzl MS, Fuchs E Assessing cognitive functions in tree shrews: visuo-spatial and spatial learning in the home cage. Journal of Neuroscience Methods. 1998;81(1-2):35–40. doi: 10.1016/S0165-0270(98)00011-9. [DOI] [PubMed] [Google Scholar]
- Ohta H Learning set formation in slow lorises (Nycticebus coucang) Folia Primatologica. 1983;40(4):256–267. doi: 10.1159/000156108. [DOI] [PubMed] [Google Scholar]
- Ohta H, Ishida H, Matano S Learning set formation in ring-tailed lemurs (Lemur catta) Folia Primatologica. 1984;43(1):53–58. doi: 10.1159/000156170. [DOI] [Google Scholar]
- Ohta H, Matsutani S, Ishida H, et al Learning set formation in common tree shrews (Tupaia glis) Folia Primatologica. 1985;44(3-4):204–209. doi: 10.1159/000156213. [DOI] [Google Scholar]
- Olazábal DE, Alsina-Llanes M Are age and sex differences in brain oxytocin receptors related to maternal and infanticidal behavior in naïve mice? Hormones and Behavior. 2016;77:132–140. doi: 10.1016/j.yhbeh.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Ong JCL, Seng BJJ, Law JZF, et al Artificial intelligence, ChatGPT, and other large language models for social determinants of health: current state and future directions. Cell Reports Medicine. 2024;5(1):101356. doi: 10.1016/j.xcrm.2023.101356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onomoto K, Onoguchi K, Yoneyama M Regulation of RIG-I-like receptor-mediated signaling: interaction between host and viral factors. Cellular & Molecular Immunology. 2021;18(3):539–555. doi: 10.1038/s41423-020-00602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppelt C, Wutzler R, von Holst D Characterisation of MHC class II DRB genes in the northern tree shrew (Tupaia belangeri) Immunogenetics. 2010;62(9):613–622. doi: 10.1007/s00251-010-0466-8. [DOI] [PubMed] [Google Scholar]
- Ou JX, Ball JM, Luan YZ, et al. 2018. iPSCs from a hibernator provide a platform for studying cold adaptation and its potential medical applications. Cell, 173 (4): 851–863. e16.
- Paik SB, Ringach DL Retinal origin of orientation maps in visual cortex. Nature Neuroscience. 2011;14(7):919–925. doi: 10.1038/nn.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik SB, Ringach DL Link between orientation and retinotopic maps in primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(18):7091–7096. doi: 10.1073/pnas.1118926109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palasantzas VEJM, Tamargo-Rubio I, Le K, et al iPSC-derived organ-on-a-chip models for personalized human genetics and pharmacogenomics studies. Trends in Genetics. 2023;39(4):268–284. doi: 10.1016/j.tig.2023.01.002. [DOI] [PubMed] [Google Scholar]
- Palchaudhuri M, Flügge G 5-HT1A receptor expression in pyramidal neurons of cortical and limbic brain regions. Cell and Tissue Research. 2005;321(2):159–172. doi: 10.1007/s00441-005-1112-x. [DOI] [PubMed] [Google Scholar]
- Pan MT, Zhang H, Li XJ, et al Genetically modified non-human primate models for research on neurodegenerative diseases. Zoological Research. 2024;45(2):263–274. doi: 10.24272/j.issn.2095-8137.2023.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan TT, Liu C, Li DM, et al Nucleus accumbens-linked executive control networks mediating reversal learning in tree shrew brain. Zoological Research. 2022;43(4):528–531. doi: 10.24272/j.issn.2095-8137.2022.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang QF, Liu JC, Wan XB, et al Experimental infection of adult Tupaia belangeri yunalis with human rotavirus. Chinese Medical Journal. 1983;96(2):85–94. [PubMed] [Google Scholar]
- Pang QF, Wan XB, Chen SD, et al Treatment of rotavirus infection in tree shrews (Tupaia belangeri yunalis) with herbal Valeriana jatamansi (VJ) Journal of Traditional Chinese Medicine. 1984;4(4):301–306. [PubMed] [Google Scholar]
- Parésys L, Hoffmann K, Froger N, et al Effects of the synthetic neurosteroid: 3β-methoxypregnenolone (MAP4343) on behavioral and physiological alterations provoked by chronic psychosocial stress in tree shrews. International Journal of Neuropsychopharmacology. 2016;19(4):pyv119. doi: 10.1093/ijnp/pyv119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra A, Baker CA, Bolton MM Regional specialization of pyramidal neuron morphology and physiology in the tree shrew neocortex. Cerebral Cortex. 2019;29(11):4488–4505. doi: 10.1093/cercor/bhy326. [DOI] [PubMed] [Google Scholar]
- Peng YZ, Ye ZZ, Zou RJ, et al. 1991. Biology of Chinese Tree Shrews. Kunming: Yunnan Science and Technology Press. (in Chinese)
- Petry HM, Bickford ME The second visual system of the tree shrew. The Journal of Comparative Neurology. 2019;527(3):679–693. doi: 10.1002/cne.24413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer LS, Stafforst T Precision RNA base editing with engineered and endogenous effectors. Nature Biotechnology. 2023;41(11):1526–1542. doi: 10.1038/s41587-023-01927-0. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, et al Why primate models matter. American Journal of Primatology. 2014;76(9):801–827. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwecka M, Rajewsky N, Rybak-Wolf A Single-cell and spatial transcriptomics: deciphering brain complexity in health and disease. Nature Reviews Neurology. 2023;19(6):346–362. doi: 10.1038/s41582-023-00809-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XB, Li WH Unlocking the secrets to human NTCP structure. The Innovation. 2022;3(5):100294. doi: 10.1016/j.xinn.2022.100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Hu YC, Huang YM, et al Whole-brain spatial organization of hippocampal single-neuron projectomes. Science. 2024;383(6682):eadj9198. doi: 10.1126/science.adj9198. [DOI] [PubMed] [Google Scholar]
- Rauth S, Karmakar S, Batra SK, et al. 2021. Recent advances in organoid development and applications in disease modeling. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer, 1875 (2): 188527.
- Rehwinkel J, Gack MU RIG-I-like receptors: their regulation and roles in RNA sensing. Nature Reviews Immunology. 2020;20(9):537–551. doi: 10.1038/s41577-020-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss S, Fuchs E Anterograde tracing of retinal afferents to the tree shrew hypothalamus and raphe. Brain Research. 2000;874(1):66–74. doi: 10.1016/S0006-8993(00)02578-6. [DOI] [PubMed] [Google Scholar]
- Rice MW, Roberts RC, Melendez-Ferro M, et al Neurochemical characterization of the tree shrew dorsal striatum. Frontiers in Neuroanatomy. 2011;5:53. doi: 10.3389/fnana.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TE, Lanier HC, Sargis EJ, et al Molecular phylogeny of treeshrews (Mammalia: Scandentia) and the timescale of diversification in Southeast Asia. Molecular Phylogenetics and Evolution. 2011;60(3):358–372. doi: 10.1016/j.ympev.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Lund JS Widespread periodic intrinsic connections in the tree shrew visual cortex. Science. 1982;215(4539):1532–1534. doi: 10.1126/science.7063863. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Callejas JD, Fuchs E, Perez-Cruz C. 2020. Increased oxidative stress, hyperphosphorylation of tau, and dystrophic microglia in the hippocampus of aged Tupaia belangeri. Glia, 68 (9): 1775–1793.
- Rodriguez-Callejas JD, Irene-Fierro M, Aguilar-Navarro S, et al. 2024. Astroglial atrophy associates with loss of nuclear S100A10 in the hippocampus of aged male tree shrews. bioRxiv.
- Roelfsema PR, Treue S Basic neuroscience research with nonhuman primates: a small but indispensable component of biomedical research. Neuron. 2014;82(6):1200–1204. doi: 10.1016/j.neuron.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Römer S, Bender H, Knabe W, et al Neural progenitors in the developing neocortex of the northern tree shrew (Tupaia belangeri) show a closer relationship to gyrencephalic primates than to lissencephalic rodents. Frontiers in Neuroanatomy. 2018;12:29. doi: 10.3389/fnana.2018.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Herrera AE, Lehmann H. 1974. The myoglobin of primates: VI. Tupaia glis belangeri (Common treeshrew). Biochimica et Biophysica Acta (BBA) - Protein Structure, 359 (2): 236–241.
- Ruberte J, Schofield PN, Sundberg JP, et al Bridging mouse and human anatomies; a knowledge-based approach to comparative anatomy for disease model phenotyping. Mammalian Genome. 2023;34(3):389–407. doi: 10.1007/s00335-023-10005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghpour S, Khodaee S, Rahnama M, et al Human APOBEC3 variations and viral infection. Viruses. 2021;13(7):1366. doi: 10.3390/v13071366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdak BS, Salmon AE, Cava JA, et al Noninvasive imaging of the tree shrew eye: wavefront analysis and retinal imaging with correlative histology. Experimental Eye Research. 2019;185:107683. doi: 10.1016/j.exer.2019.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BC, Siegwart JT, Zhan WJ, et al A novel tree shrew (Tupaia belangeri) model of glaucoma. Investigative Ophthalmology & Visual Science. 2018;59(7):3136–3143. doi: 10.1167/iovs.18-24261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada T, Tsukiyama-Kohara K, Shin-I T, et al Construction of complete Tupaia belangeri transcriptome database by whole-genome and comprehensive RNA sequencing. Scientific Reports. 2019a;9(1):12372. doi: 10.1038/s41598-019-48867-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada T, Yamamoto N, Kayesh MEH, et al Intranasal vaccination with HBs and HBc protein combined with carboxyl vinyl polymer induces strong neutralizing antibody, anti-HBs IgA, and IFNG response. Biochemical and Biophysical Research Communications. 2019b;520(1):86–92. doi: 10.1016/j.bbrc.2019.09.072. [DOI] [PubMed] [Google Scholar]
- Sanada T, Yasui F, Honda T, et al Avian H5N1 influenza virus infection causes severe pneumonia in the Northern tree shrew (Tupaia belangeri) Virology. 2019c;529:101–110. doi: 10.1016/j.virol.2019.01.015. [DOI] [PubMed] [Google Scholar]
- Sasaki E, Suemizu H, Shimada A, et al Generation of transgenic non-human primates with germline transmission. Nature. 2009;459(7246):523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- Saturday T, van Doremalen N Pathogenesis of severe acute respiratory syndrome coronavirus-2 in nonhuman primates. Current Opinion in Virology. 2023;63:101375. doi: 10.1016/j.coviro.2023.101375. [DOI] [PubMed] [Google Scholar]
- Savier E, Sedigh-Sarvestani M, Wimmer R, et al A bright future for the tree shrew in neuroscience research: summary from the inaugural Tree Shrew Users Meeting. Zoological Research. 2021;42(4):478–481. doi: 10.24272/j.issn.2095-8137.2021.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer M, Fan Y, Gu TL, et al. 2020. The lipoxygenase pathway of Tupaia belangeri representing Scandentia. Genomic multiplicity and functional characterization of the ALOX15 orthologs in the tree shrew. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 1865 (2): 158550.
- Schmelting B, Corbach-Söhle S, Kohlhause S, et al Agomelatine in the tree shrew model of depression: effects on stress-induced nocturnal hyperthermia and hormonal status. European Neuropsychopharmacology. 2014;24(3):437–447. doi: 10.1016/j.euroneuro.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Schumacher JW, McCann MK, Maximov KJ, et al. 2022. Selective enhancement of neural coding in V1 underlies fine-discrimination learning in tree shrew. Current Biology, 32 (15): 3245–3260. e5.
- Sedigh-Sarvestani M, Lee KS, Jaepel J, et al. 2021. A sinusoidal transformation of the visual field is the basis for periodic maps in area V2. Neuron, 109 (24): 4068–4079. e6.
- Shang SJ, Wang C, Guo CB, et al The formation and extinction of fear memory in tree shrews. Frontiers in Behavioral Neuroscience. 2015;9:204. doi: 10.3389/fnbeh.2015.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Chen CY, Shen H, et al GenTree, an integrated resource for analyzing the evolution and function of primate-specific coding genes. Genome Research. 2019;29(4):682–696. doi: 10.1101/gr.238733.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She ZH, Ward AH, Gawne TJ The effects of ambient narrowband long-wavelength light on lens-induced myopia and form-deprivation myopia in tree shrews. Experimental Eye Research. 2023;234:109593. doi: 10.1016/j.exer.2023.109593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Qi KK, Duan Y, et al Differential effects of clomipramine on depression-like behaviors induced by the chronic social defeat paradigm in tree shrews. Journal of Psychopharmacology. 2018;32(10):1141–1149. doi: 10.1177/0269881118793560. [DOI] [PubMed] [Google Scholar]
- Shen Y, Ding LF, Yang CY, et al Mapping big brains at subcellular resolution in the era of big data in zoology. Zoological Research. 2022;43(4):597–599. doi: 10.24272/j.issn.2095-8137.2022.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Norton TT, Casagrande VA Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Research. 1977;124(1):154–157. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- Shi N, Chen HL, Lai YJ, et al Cyclosporine A induces Epstein-Barr virus reactivation in tree shrew (Tupaia belangeri chinensis) model. Microbes and Infection. 2023;25(8):105212. doi: 10.1016/j.micinf.2023.105212. [DOI] [PubMed] [Google Scholar]
- Shriver JE, Noback CR Cortical projections to the lower brain stem and spinal cord in the tree shrew (Tupaia glis) The Journal of Comparative Neurology. 1967;130(1):25–53. doi: 10.1002/cne.901300103. [DOI] [PubMed] [Google Scholar]
- Shu YM, Ni RJ, Sun YJ, et al Distribution of corticotropin-releasing factor in the tree shrew brain. Brain Research. 2015;1618:270–285. doi: 10.1016/j.brainres.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Siegwart JT Jr, Norton TT The susceptible period for deprivation-induced myopia in tree shrew. Vision Research. 1998;38(22):3505–3515. doi: 10.1016/S0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- Sneddon LU, Halsey LG, Bury NR. 2017. Considering aspects of the 3Rs principles within experimental animal biology. Journal of Experimental Biology, 220 (Pt 17): 3007–3016.
- Snyder M, Killackey H, Diamond IT Color vision in the tree shrew after removal of posterior neocortex. Journal of Neurophysiology. 1969;32(4):554–563. doi: 10.1152/jn.1969.32.4.554. [DOI] [PubMed] [Google Scholar]
- Song TZ, Zheng HY, Han JB, et al Delayed severe cytokine storm and immune cell infiltration in SARS-CoV-2-infected aged Chinese rhesus macaques. Zoological Research. 2020;41(5):503–516. doi: 10.24272/j.issn.2095-8137.2020.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrou S, Ross SR APOBEC3 proteins in viral immunity. The Journal of Immunology. 2015;195(10):4565–4570. doi: 10.4049/jimmunol.1501504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley P, Franco C, Claydon AJ, et al Revealing mechanisms of mating plug function under sexual selection. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(44):27465–27473. doi: 10.1073/pnas.1920526117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JJ, Yan RQ, Gan YQ, et al Experimental infection of human hepatitis B virus (HBV) in adult tree shrews. Chinese Journal of Pathology. 1987;16(2):103–106,166. [PubMed] [Google Scholar]
- Sun J, Liu WJ, Guo YB, et al Characterization of tree shrew telomeres and telomerase. Journal of Genetics and Genomics. 2021;48(7):631–639. doi: 10.1016/j.jgg.2021.06.004. [DOI] [PubMed] [Google Scholar]
- Sun Q, Dong J, Yang WT, et al Efficient reproduction of cynomolgus monkey using pronuclear embryo transfer technique. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(35):12956–12960. doi: 10.1073/pnas.0805639105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha EA, Lee J, Hotta A Delivery of CRISPR-Cas tools for in vivo genome editing therapy: trends and challenges. Journal of Controlled Release. 2022;342:345–361. doi: 10.1016/j.jconrel.2022.01.013. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ushitani T, Fujita K Inference based on transitive relation in tree shrews (Tupaia belangeri) and rats (Rattus norvegicus) on a spatial discrimination task. The Psychological Record. 2008;58(2):215–227. doi: 10.1007/BF03395612. [DOI] [Google Scholar]
- Tambuyzer E, Vandendriessche B, Austin CP, et al Therapies for rare diseases: therapeutic modalities, progress and challenges ahead. Nature Reviews Drug Discovery. 2020;19(2):93–111. doi: 10.1038/s41573-019-0049-9. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Fu JM, Cang JH. 2022. Strong tuning for stereoscopic depth indicates orientation-specific recurrent circuitry in tree shrew V1. Current Biology, 32 (24): 5274–5284. e6.
- Tarantal AF, Noctor SC, Hartigan-O'Connor DJ Nonhuman primates in translational research. Annual Review of Animal Biosciences. 2022;10:441–468. doi: 10.1146/annurev-animal-021419-083813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teli P, Kale V, Vaidya A Beyond animal models: revolutionizing neurodegenerative disease modeling using 3D in vitro organoids, microfluidic chips, and bioprinting. Cell and Tissue Research. 2023;394(1):75–91. doi: 10.1007/s00441-023-03821-2. [DOI] [PubMed] [Google Scholar]
- Tian JJ, Ren M, Zhao PL, et al Dissection of the long-range projections of specific neurons at the synaptic level in the whole mouse brain. Proceedings of the National Academy of Sciences of the United States of America. 2022;119(40):e2202536119. doi: 10.1073/pnas.2202536119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges J, Shantha TR. 1969. A Stereotaxic Brain Atlas of the Tree Shrew: (Tupaia glis). Baltimore: Williams & Wilkins Co.
- Tong Y, Hao J, Tu Q, et al A tree shrew glioblastoma model recapitulates features of human glioblastoma. Oncotarget. 2017;8(11):17897–17907. doi: 10.18632/oncotarget.15225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth I, Neumann ID Animal models of social avoidance and social fear. Cell and Tissue Research. 2013;354(1):107–118. doi: 10.1007/s00441-013-1636-4. [DOI] [PubMed] [Google Scholar]
- Tu Q, Yang D, Zhang XN, et al A novel pancreatic cancer model originated from transformation of acinar cells in adult tree shrew, a primate-like animal. Disease Models & Mechanisms. 2019;12(4):dmm038703. doi: 10.1242/dmm.038703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungersböck A, Kretz R, Rager G Synaptogenesis in the primary visual cortex of the tree shrew (Tupaia belangeri) The Journal of Comparative Neurology. 1991;308(3):491–504. doi: 10.1002/cne.903080313. [DOI] [PubMed] [Google Scholar]
- van der Hart MGC, Czéh B, de Biurrun G, et al Substance P receptor antagonist and clomipramine prevent stress-induced alterations in cerebral metabolites, cytogenesis in the dentate gyrus and hippocampal volume. Molecular Psychiatry. 2002;7(9):933–941. doi: 10.1038/sj.mp.4001130. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Howells DW, Sena ES, et al Can animal models of disease reliably inform human studies? PLoS Medicine. 2010;7(3):e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hasselt SJ, Epifani L, Zantinge D, et al A study on REM sleep homeostasis in the day-active tree shrew (Tupaia belangeri): cold-induced suppression of REM sleep is not followed by a rebound. Biology. 2023;12(4):614. doi: 10.3390/biology12040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandereyken K, Sifrim A, Thienpont B, et al Methods and applications for single-cell and spatial multi-omics. Nature Reviews Genetics. 2023;24(8):494–515. doi: 10.1038/s41576-023-00580-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit J, Bhattacharyya A, Kretz R, et al On the relation between receptive field structure and stimulus selectivity in the tree shrew primary visual cortex. Cerebral Cortex. 2014;24(10):2761–2771. doi: 10.1093/cercor/bht133. [DOI] [PubMed] [Google Scholar]
- Villiger L, Joung J, Koblan L, et al CRISPR technologies for genome, epigenome and transcriptome editing. Nature Reviews Molecular Cell Biology. 2024;25(6):464–487. doi: 10.1038/s41580-023-00697-6. [DOI] [PubMed] [Google Scholar]
- Voigtlaender S, Pawelczyk J, Geiger M, et al Artificial intelligence in neurology: opportunities, challenges, and policy implications. Journal of Neurology. 2024;271(5):2258–2273. doi: 10.1007/s00415-024-12220-8. [DOI] [PubMed] [Google Scholar]
- Walter E, Keist R, Niederöst B, et al. 1996. Hepatitis B virus infection of tupaia hepatocytes in vitro and in vivo. Hepatology, 24 (1): 1–5.
- Wang CY, Ye YS, Long WH, et al RNA sequencing and proteomic profiling reveal alterations by MPTP in chronic stomach mucosal injury in tree shrew Chinese (Tupaia belangeri chinensis) Scientific Reports. 2024a;14(1):74. doi: 10.1038/s41598-023-50820-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Tai PWL, Gao GP Adeno-associated virus vector as a platform for gene therapy delivery. Nature Reviews Drug Discovery. 2019a;18(5):358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Zhang F, Gao GP CRISPR-based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell. 2020a;181(1):136–150. doi: 10.1016/j.cell.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EL, Ye YS, Zhang K, et al Longitudinal transcriptomic characterization of viral genes in HSV-1 infected tree shrew trigeminal ganglia. Virology Journal. 2020b;17(1):95. doi: 10.1186/s12985-020-01344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Huang ZY, Wu YY, et al Primate-like tree shrew will be the preferred animal for investigating nephrolithiasis. Urolithiasis. 2024b;52(1):3. doi: 10.1007/s00240-023-01507-6. [DOI] [PubMed] [Google Scholar]
- Wang HY, Liu JH, Ye SZ, et al Preliminary observations on experimental leprosy in tupaias (Tupaia belangeri yunalis) Leprosy Review. 1990;61(1):12–18. [PubMed] [Google Scholar]
- Wang J, Azimi H, Zhao YL, et al Optogenetic activation of visual thalamus generates artificial visual percepts. eLife. 2023a;12:e90431. doi: 10.7554/eLife.90431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LM, Lu JL, Zeng YQ, et al Improving Alzheimer's disease by altering gut microbiota in tree shrews with ginsenoside Rg1. FEMS Microbiology Letters. 2020c;367(4):fnaa011. doi: 10.1093/femsle/fnaa011. [DOI] [PubMed] [Google Scholar]
- Wang QH, Liu ZH, Zeng X, et al Integrated analysis of miRNA-mRNA expression of newly emerging swine H3N2 influenza virus cross-species infection with tree shrews. Virology Journal. 2024c;21(1):4. doi: 10.1186/s12985-023-02260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QH, Zeng X, Tang S, et al Pathogenicity and anti-infection immunity of animal H3N2 and H6N6 subtype influenza virus cross-species infection with tree shrews. Virus Research. 2023b;324:199027. doi: 10.1016/j.virusres.2022.199027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QX, Ding SL, Li Y, et al. 2020d. The Allen mouse brain common coordinate framework: a 3D reference atlas. Cell, 181 (4): 936–953. e20.
- Wang SX, Shan D, Dai JK, et al Anatomical MRI templates of tree shrew brain for volumetric analysis and voxel-based morphometry. Journal of Neuroscience Methods. 2013;220(1):9–17. doi: 10.1016/j.jneumeth.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Wang YL, Ma ZX, Zheng YY, et al Establishment of an osteoporosis model in tree shrews by bilateral ovariectomy and comprehensive evaluation. Experimental and Therapeutic Medicine. 2019b;17(5):3644–3654. doi: 10.3892/etm.2019.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX Taxonomic research on Burma-Chinese tree shrew, Tupaia belangeri (Wagner), from Southern China. Zoological Research. 1987;8(3):213–230. [Google Scholar]
- Wang YY, Niu RZ, Wang JD, et al Establishment of brain ischemia model in tree shrew. Brain Research. 2019c;1718:194–200. doi: 10.1016/j.brainres.2019.05.011. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yi X, Du L, et al A study of Epstein-Barr virus infection in the Chinese tree shrew (Tupaia belangeri chinensis) Virology Journal. 2017;14(1):193. doi: 10.1186/s12985-017-0859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Hua HR, Chen QQ, et al Dynamic changes in DNA demethylation in the tree shrew (Tupaia belangeri chinensis) brain during postnatal development and aging. Zoological Research. 2017;38(2):96–102. doi: 10.24272/j.issn.2095-8137.2017.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West SA, Cooper GA, Ghoul MB, et al Ten recent insights for our understanding of cooperation. Nature Ecology & Evolution. 2021;5(4):419–430. doi: 10.1038/s41559-020-01384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DD, Qi XG, Yu L, et al Initiation of the primate genome project. Zoological Research. 2022;43(2):147–149. doi: 10.24272/j.issn.2095-8137.2022.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Qin D, Qian Y, et al A new era of mutation rate analyses: concepts and methods. Zoological Research. 2024;45(4):767–780. doi: 10.24272/j.issn.2095-8137.2024.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhang K Tools for the analysis of high-dimensional single-cell RNA sequencing data. Nature Reviews Nephrology. 2020;16(7):408–421. doi: 10.1038/s41581-020-0262-0. [DOI] [PubMed] [Google Scholar]
- Wu YX, Zhou H, Fan XY, et al Correction of a genetic disease by CRISPR-Cas9-mediated gene editing in mouse spermatogonial stem cells. Cell Research. 2015;25(1):67–79. doi: 10.1038/cr.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia HJ, He BL, Wang CY, et al PTEN/PIK3CA genes are frequently mutated in spontaneous and medroxyprogesterone acetate-accelerated 7, 12-dimethylbenz(a)anthracene-induced mammary tumours of tree shrews. European Journal of Cancer. 2014;50(18):3230–3242. doi: 10.1016/j.ejca.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Xia HJ, Wang CY, Zhang HL, et al Characterization of spontaneous breast tumor in tree shrews (Tupaia belangeri chinenesis) Zoological Research. 2012;33(1):55–59. doi: 10.3724/SP.J.1141.2012.01055. [DOI] [PubMed] [Google Scholar]
- Xia W, Chen HL, Feng YW, et al Tree shrew is a suitable animal model for the study of Epstein Barr virus. Frontiers in Immunology. 2022;12:789604. doi: 10.3389/fimmu.2021.789604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Liu L, Shi N, et al Epstein Barr virus infection in tree shrews alters the composition of gut microbiota and metabolome profile. Virology Journal. 2023;20(1):177. doi: 10.1186/s12985-023-02147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Shi N, Li CQ, et al RNA-Seq and miRNA-Seq data from Epstein-Barr virus-infected tree shrews reveal a ceRNA network contributing to immune microenvironment regulation. Virulence. 2024;15(1):2306795. doi: 10.1080/21505594.2024.2306795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Liu R, Chen CS Tree shrew (Tupaia belangeri) as a novel laboratory disease animal model. Zoological Research. 2017;38(3):127–137. doi: 10.24272/j.issn.2095-8137.2017.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie ZC, Riezu-Boj JI, Lasarte JJ, et al Transmission of hepatitis C virus infection to tree shrews. Virology. 1998;244(2):513–520. doi: 10.1006/viro.1998.9127. [DOI] [PubMed] [Google Scholar]
- Xu D, Zhu YG, Xu ZH Efficient genetic manipulation in the developing brain of tree shrew using in utero electroporation and virus infection. Journal of Genetics and Genomics. 2017;44(10):507–509. doi: 10.1016/j.jgg.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Xu L, Fan Y, Jiang XL, et al Molecular evidence on the phylogenetic position of tree shrews. Zoological Research. 2013;34(2):70–76. doi: 10.3724/SP.J.1141.2013.02070. [DOI] [PubMed] [Google Scholar]
- Xu L, Yu DD, Fan Y, et al Loss of RIG-I leads to a functional replacement with MDA5 in the Chinese tree shrew. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(39):10950–10955. doi: 10.1073/pnas.1604939113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Yu DD, Ma YH, et al COVID-19-like symptoms observed in Chinese tree shrews infected with SARS-CoV-2. Zoological Research. 2020a;41(5):517–526. doi: 10.24272/j.issn.2095-8137.2020.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Yu DD, Peng L, et al Characterization of a MAVS ortholog from the Chinese tree shrew (Tupaia belangeri chinensis) Developmental & Comparative Immunology. 2015;52(1):58–68. doi: 10.1016/j.dci.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Xu L, Yu DD, Peng L, et al An alternative splicing of Tupaia STING modulated anti-RNA virus responses by targeting MDA5-LGP2 and IRF3. The Journal of Immunology. 2020b;204(12):3191–3204. doi: 10.4049/jimmunol.1901320. [DOI] [PubMed] [Google Scholar]
- Xu L, Yu DD, Yao YL, et al Tupaia MAVS is a dual target during hepatitis C virus infection for innate immune evasion and viral replication via NF-κB. The Journal of Immunology. 2020c;205(8):2091–2099. doi: 10.4049/jimmunol.2000376. [DOI] [PubMed] [Google Scholar]
- Xu S, Li XY, Yang JY, et al Comparative pathogenicity and transmissibility of pandemic H1N1, avian H5N1, and human H7N9 influenza viruses in tree shrews. Frontiers in Microbiology. 2019;10:2955. doi: 10.3389/fmicb.2019.02955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XP, Chen HB, Cao XM, et al. 2007. Efficient infection of tree shrew (Tupaia belangeri) with hepatitis C virus grown in cell culture or from patient plasma. Journal of General Virology, 88 (Pt 9): 2504–2512.
- Yamamoto S, Kanca O, Wangler MF, et al Integrating non-mammalian model organisms in the diagnosis of rare genetic diseases in humans. Nature Reviews Genetics. 2024;25(1):46–60. doi: 10.1038/s41576-023-00633-6. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Fuchs E, Taira M, et al Somatostatin-immunoreactive senile plaque-like structures in the frontal cortex and nucleus accumbens of aged tree shrews and Japanese macaques. Journal of Medical Primatology. 2012;41(3):147–157. doi: 10.1111/j.1600-0684.2012.00540.x. [DOI] [PubMed] [Google Scholar]
- Yan H, Zhong GC, Xu GW, et al Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife. 2012;1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LZ, Sun B, Lyu LB, et al Early embryonic development and transplantation in tree shrews. Zoological Research. 2016;37(4):252–258. doi: 10.13918/j.issn.2095-8137.2016.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan RQ, Su JJ, Huang DR, et al Human hepatitis B virus and hepatocellular carcinoma I. Experimental infection of tree shrews with hepatitis B virus. Journal of Cancer Research and Clinical Oncology. 1996;122(5):283–288. doi: 10.1007/BF01261404. [DOI] [PubMed] [Google Scholar]
- Yang WG, Liu JL, Luo YQ, et al. 1990. A stereotaxic atlas of the brain of Tupaia belangeri and macaque monkey living in Guangxi. Nanning: Guangxi Science & Technology Publishing House. (in Chinese)
- Yang Y, Wang LM, Zhang CJ, et al Ginsenoside Rg1 improves Alzheimer's disease by regulating oxidative stress, apoptosis, and neuroinflammation through Wnt/GSK-3β/β-catenin signaling pathway. Chemical Biology & Drug Design. 2022;99(6):884–896. doi: 10.1111/cbdd.14041. [DOI] [PubMed] [Google Scholar]
- Yao YG Creating animal models, why not use the Chinese tree shrew (Tupaia belangeri chinensis)? Zoological Research. 2017;38(3):118–126. doi: 10.24272/j.issn.2095-8137.2017.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YG, Construction Team of the KIZ Primate Facility Towards the peak: the 10-year journey of the National Research Facility for Phenotypic and Genetic Analysis of Model Animals (Primate Facility) and a call for international collaboration in non-human primate research. Zoological Research. 2022;43(2):237–240. doi: 10.24272/j.issn.2095-8137.2022.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YL, Yu DD, Xu L, et al Molecular characterization of the 2', 5'-oligoadenylate synthetase family in the Chinese tree shrew (Tupaia belangeri chinensis) Cytokine. 2019;114:106–114. doi: 10.1016/j.cyto.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Yao YL, Yu DD, Xu L, et al Tupaia OASL1 promotes cellular antiviral immune responses by recruiting MDA5 to MAVS. The Journal of Immunology. 2020;205(12):3419–3428. doi: 10.4049/jimmunol.2000740. [DOI] [PubMed] [Google Scholar]
- Ye MS, Zhang JY, Yu DD, et al Comprehensive annotation of the Chinese tree shrew genome by large-scale RNA sequencing and long-read isoform sequencing. Zoological Research. 2021;42(6):692–709. doi: 10.24272/j.issn.2095-8137.2021.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JZ, Lei XL, Guo FT, et al Co-delivery of Cas9 mRNA and guide RNAs edits hepatitis B virus episomal and integration DNA in mouse and tree shrew models. Antiviral Research. 2023;215:105618. doi: 10.1016/j.antiviral.2023.105618. [DOI] [PubMed] [Google Scholar]
- Yin BW, Song QK, Chen LX, et al Establishment of an immortalized intestinal epithelial cell line from tree shrews by lentivirus-mediated hTERT gene transduction. Cytotechnology. 2019;71(1):107–116. doi: 10.1007/s10616-018-0270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin CH, Zhou X, Yao YG, et al Abundant self-amplifying intermediate progenitors in the subventricular zone of the Chinese tree shrew neocortex. Cerebral Cortex. 2020;30(5):3370–3380. doi: 10.1093/cercor/bhz315. [DOI] [PubMed] [Google Scholar]
- Yu WH, Yang CC, Bi YH, et al Characterization of hepatitis E virus infection in tree shrew (Tupaia belangeri chinensis) BMC Infectious Diseases. 2016;16:80. doi: 10.1186/s12879-016-1418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B, Yang CG, Xia XS, et al The tree shrew is a promising model for the study of influenza B virus infection. Virology Journal. 2019;16(1):77. doi: 10.1186/s12985-019-1171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkovich JT, Evans SJ, Rappaport N, et al The transition from genomics to phenomics in personalized population health. Nature Reviews Genetics. 2024;25(4):286–302. doi: 10.1038/s41576-023-00674-x. [DOI] [PubMed] [Google Scholar]
- Zambello E, Fuchs E, Abumaria N, et al Chronic psychosocial stress alters NPY system: different effects in rat and tree shrew. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34(1):122–130. doi: 10.1016/j.pnpbp.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Zeng L, Zhang HY, Yang CY, et al. 2023. Generation of a tree shrew breast cancer model using lentivirus expressing PIK3CA-H1047R. Zoological Research, 44 (1): 94–97.
- Zhang DF, Xu M, Bi R, et al Genetic analyses of Alzheimer's disease in China: achievements and perspectives. ACS Chemical Neuroscience. 2019a;10(2):890–901. doi: 10.1021/acschemneuro.8b00435. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chiodini R, Badr A, et al The impact of next-generation sequencing on genomics. Journal of Genetics and Genomics. 2011;38(3):95–109. doi: 10.1016/j.jgg.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Luo RC, Man XY, et al The anatomy of the skin of the Chinese tree shrew is very similar to that of human skin. Zoological Research. 2020a;41(2):208–212. doi: 10.24272/j.issn.2095-8137.2020.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JJ, Xiao HJ, Bi YW, et al Characteristics of the tree shrew humoral immune system. Molecular Immunology. 2020b;127:175–185. doi: 10.1016/j.molimm.2020.09.009. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shen ZL, Feng Y, et al Infectivity of Zika virus on primary cells support tree shrew as animal model. Emerging Microbes & Infections. 2019b;8(1):232–241. doi: 10.1080/22221751.2018.1559707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Pan XJ, Jung W, et al Molecularly defined and spatially resolved cell atlas of the whole mouse brain. Nature. 2023a;624(7991):343–354. doi: 10.1038/s41586-023-06808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang NN, Zhang L, Deng YQ, et al Zika virus infection in Tupaia belangeri causes dermatological manifestations and confers protection against secondary infection. Journal of Virology. 2019c;93(8):e01982–18. doi: 10.1128/JVI.01982-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lai GY, Volpe G, et al Towards a primate single-cell atlas. Zoological Research. 2022;43(4):691–694. doi: 10.24272/j.issn.2095-8137.2022.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XH, Dai ZX, Zhang GH, et al Molecular characterization, balancing selection, and genomic organization of the tree shrew (Tupaia belangeri) MHC class I gene. Gene. 2013;522(2):147–155. doi: 10.1016/j.gene.2013.03.113. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Yu DD, Wu Y, et al Establishment and transcriptomic features of an immortalized hepatic cell line of the Chinese tree shrew. Applied Microbiology and Biotechnology. 2020c;104(20):8813–8823. doi: 10.1007/s00253-020-10855-x. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Luo ZY, Sun YW, et al From beasts to bytes: revolutionizing zoological research with artificial intelligence. Zoological Research. 2023b;44(6):1115–1131. doi: 10.24272/j.issn.2095-8137.2023.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JG, Lai LX, Ji WZ, et al Genome editing in large animals: current status and future prospects. National Science Review. 2019;6(3):402–420. doi: 10.1093/nsr/nwz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Gao F, Gao S, et al Biodiversity-based development and evolution: the emerging research systems in model and non-model organisms. Science China Life Sciences. 2021a;64(8):1236–1280. doi: 10.1007/s11427-020-1915-y. [DOI] [PubMed] [Google Scholar]
- Zhao L, Huang SY, Liao QW, et al RNA-seq analysis of ischemia stroke and normal brain in a tree shrew model with or without type 2 diabetes mellitus. Metabolic Brain Disease. 2021b;36(7):1889–1901. doi: 10.1007/s11011-021-00813-5. [DOI] [PubMed] [Google Scholar]
- Zhao L, Liao QW, Zhang YT, et al Ischemic postconditioning mitigates retinopathy in tree shrews with diabetic cerebral ischemia. Journal of Diabetes Research. 2020a;2020:6286571. doi: 10.1155/2020/6286571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Tan SF, Liao QW, et al The neuroprotective effect and RNA-sequence analysis of postconditioning on the ischemic stroke with diabetes mellitus tree shrew model. Brain and Behavior. 2021c;11(11):e2354. doi: 10.1002/brb3.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao PC, Xia W, Wei JL, et al. 2022. An investigation of the mechanisms of radiation-induced muscle injury in a tree shrew (Tupaia belangeri) model. Dose-Response, 20 (1): 15593258221082878.
- Zhao XP, Tang ZY, Klumpp B, et al Primary hepatocytes of Tupaia belangeri as a potential model for hepatitis C virus infection. Journal of Clinical Investigation. 2002;109(2):221–232. doi: 10.1172/JCI0213011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wang JB, Kuang DX, et al Susceptibility of tree shrew to SARS-CoV-2 infection. Scientific Reports. 2020b;10(1):16007. doi: 10.1038/s41598-020-72563-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YQ, Liu YQ, Yuan JF, et al Regeneration of islet β-cells in tree shrews and rats. Animal Models and Experimental Medicine. 2018;1(2):152–161. doi: 10.1002/ame2.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Niu SW, Zhao HB, et al Donepezil improves the cognitive impairment in a tree shrew model of Alzheimer's disease induced by amyloid-β1–40 via activating the BDNF/TrkB signal pathway. Metabolic Brain Disease. 2018;33(6):1961–1974. doi: 10.1007/s11011-018-0303-6. [DOI] [PubMed] [Google Scholar]
- Zheng J, Shi WJ, Yang ZQ, et al RIG-I-like receptors: molecular mechanism of activation and signaling. Advances in Immunology. 2023;158:1–74. doi: 10.1016/bs.ai.2023.03.001. [DOI] [PubMed] [Google Scholar]
- Zheng LT, Chen SY, Wu QL, et al Tree shrews as a new animal model for systemic sclerosis research. Frontiers in Immunology. 2024;15:1315198. doi: 10.3389/fimmu.2024.1315198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Tian RR, Wang XR, et al Identification of novel mammalian viruses in tree shrews (Tupaia belangeri chinensis) Zoological Research. 2024;45(2):429–438. doi: 10.24272/j.issn.2095-8137.2023.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JN, Ni RJ. 2016. The tree shrew (Tupaia belangeri chinensis) brain in stereotaxic coordinates. Singapore: Springer.
- Zhou MG, Wang HD, Zeng XY, et al Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2019;394(10204):1145–1158. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CY, Hong W, Wang L, et al Towards key scientific questions in the diagnosis and treatment of rare diseases: summary from the 297th Meeting of the Shuangqing Forum. Zoological Research. 2022;43(2):234–236. doi: 10.24272/j.issn.2095-8137.2022.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZJ, Feng BZ, Yang YW, et al Transplantation of insulin-producing cells derived from human MSCs to treat diabetes in a non-human primate model. Artificial Organs. 2023;47(8):1298–1308. doi: 10.1111/aor.14538. [DOI] [PubMed] [Google Scholar]
- Zhuang XL, Zhang JJ, Shao Y, et al Integrative omics reveals rapidly evolving regulatory sequences driving primate brain evolution. Molecular Biology and Evolution. 2023;40(8):msad173. doi: 10.1093/molbev/msad173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinski AL, Carrion S, Michal JJ, et al Genome-to-phenome research in rats: progress and perspectives. International Journal of Biological Sciences. 2021;17(1):119–133. doi: 10.7150/ijbs.51628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.