Abstract
Neurotropic alphaherpesviruses have become popular tools for transynaptic analysis of neural circuitry. It has also been demonstrated that coinfection with two viruses expressing unique reporters can be used to define more complicated circuitry. However, the coinfection studies reported to date have employed nonisogenic strains that differ in their invasive properties. In the present investigation we used two antigenically distinct recombinants of the swine pathogen pseudorabies virus (PRV) in single and double infections of the rat central nervous system. Both viruses are derivatives of PRV-Bartha, a strain with reduced virulence that is widely used for circuit analysis. PRV-BaBlu expresses β-galactosidase, and PRV-D expresses the PRV membrane protein gI, the gene for which is deleted in PRV-BaBlu. Antibodies to β-galactosidase identify neurons infected with PRV-BaBlu, and antibodies monospecific for PRV gI identify neurons infected with PRV-D. The ability of these strains to establish coinfections in neurons was evaluated in visual and autonomic circuitry in which the parental virus has previously been characterized. The following conclusions can be drawn from these experiments. First, PRV-D is significantly more neuroinvasive than PRV-Bartha or PRV-BaBlu in the same circuitry. Second, PRV-D is more virulent than either PRV-Bartha or PRV-BaBlu, and PRV-BaBlu is less virulent than PRV-Bartha. Third, in every model examined, PRV-D and PRV-BaBlu coinfect some neurons, but single infections predominate. Fourth, prior infection with one virus renders neurons less permissive to infection by another virus. Fifth, prior infection by PRV-D is more effective than PRV-BaBlu in reducing invasion and spread of the second virus. Collectively, the data define important variables that must be considered in coinfection experiments and suggest that the most successful application of this approach would be accomplished by using isogenic strains of virus with equivalent virulence.
Neurotropic alphaherpesviruses can replicate within postmitotic neurons and produce infectious progeny that pass transneuronally to infect other synaptically linked neurons (20, 25). This self-amplifying spread in neurons has been exploited by a number of investigators to gain further insight into the organization of neuronal circuitry in the mammalian brain (9, 18, 27, 28, 40, 44). However, the use of a variety of different strains of the human and swine pathogens has also revealed strain-dependent differences in the invasiveness, replication, and transport of these viruses through the central nervous system (CNS) (the brain and the spinal cord). For example, selective tropism of different strains of virus has been demonstrated in a variety of systems (3, 17, 30, 32), and strain-dependent differences in the direction of transport of viruses have been reported (4, 13, 41, 47). Considerable insights into factors that influence viral virulence have also emerged from this experimental approach (1, 2, 16, 19, 21, 31, 45). However, the molecular mechanisms that direct these processes in vivo remain largely undefined.
Recombinant viruses that express unique gene products as reporters of infection are useful tools for defining connections among neurons (23, 26). In a notable application of this experimental approach, Jansen and colleagues injected two genetically modified forms of the Bartha strain of pseudorabies virus (PRV-Bartha) into peripheral targets innervated by separate populations of spinal cord neurons (23). Transynaptic infection of CNS neurons by both strains of virus was demonstrated, but the percentage of animals that exhibited dual-infected neurons was remarkably small. For example, only 20 of 256 animals exhibited productive replication of both viruses, and of those, only 8 were viewed as containing a specific pattern of infection worthy of analysis. It is likely that the low infection rate in this experiment is due, at least in part, to the use of titers of virus that were considerably below the 50% lethal dose (LD50) for PRV-Bartha in rats. However, other factors may have contributed to the low frequency of neuronal coinfection.
In the present investigation we compared the abilities of two antigenically distinct recombinants of PRV-Bartha to invade and replicate within visual and autonomic circuitry after single and double inoculations (see references 9 and 20 for reviews of the circuitry paradigms). The data demonstrate noteworthy differences in the virulence and invasiveness of these strains that influence their ability to coinfect neurons in the rat CNS.
MATERIALS AND METHODS
Animals and facilities.
Adult male Sprague-Dawley rats (n = 67) weighing 200 to 260 g at the time of inoculation were used in this analysis. All inoculations were done in a laboratory approved for use of class 2 (BSL-2) infectious agents, and the animals were housed in this facility throughout the experiment. The laboratory met the specifications of the U.S. Department of Health and Human Services (44a), and the experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Details regarding the application of these safety procedures in our laboratories have been published previously (12, 18).
Viral strains.
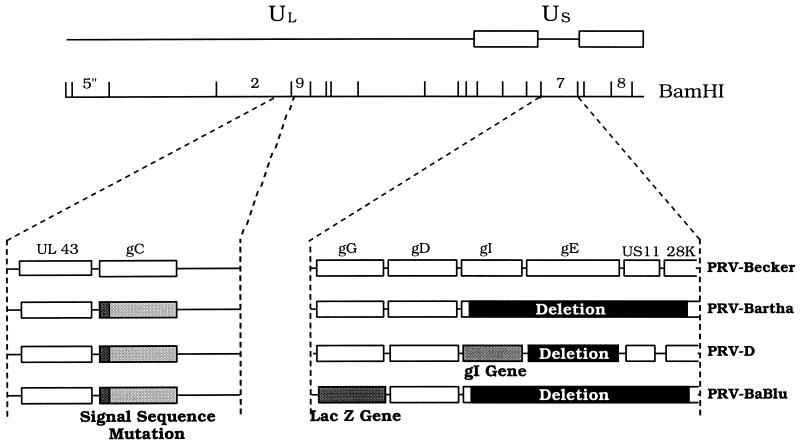
PRV-Bartha, an attenuated vaccine strain, served as the parental strain for both of the recombinants used in this investigation (5). Figure 1 illustrates the genome organizations of all of the strains used in this analysis. PRV-BaBlu contains the lacZ gene inserted into the gG gene of the unique short (Us) region of the viral genome and expresses β-galactosidase under the control of the gG promoter. Construction of this mutant was by the methods of Mettenleiter and Rauh (29) and has been described in a prior analysis of cardiac circuitry (39). PRV-D was provided by Tamar Ben-Porat and was described previously (16). PRV-D was constructed in two steps, as follows. First, the Us deletion of PRV-Bartha was repaired with wild-type PRV DNA to restore the gI, gE, Us9, and Us2 genes. The gE gene was then deleted, to reduce the virulence of this strain (16, 45). The gI protein, which is expressed by PRV-D but not by PRV-BaBlu, was used as the unique marker of neurons infected with this virus.
FIG. 1.
Schematic representation of genomic organizations of PRV mutants. Four strains of virus were used in this study. PRV-Becker (6), a wild-type laboratory strain, was used in a small group of animals to verify prior assessments of PRV virulence in visual circuitry (16). PRV-Bartha (5), an attenuated vaccine strain, was used for similar purposes and was the parental strain used for construction of PRV-BaBlu and PRV-D. Construction of PRV-BaBlu and PRV-D is described in Materials and Methods and has been reported previously (16, 38).
All strains of virus were propagated in PK-15 cells. The stock titers, in PFU per milliliter, were as follows: PRV-Becker, 5.5 × 108; PRV-Bartha, 6.25 × 108; PRV-D, 2.5 × 108; and PRV-BaBlu, 4.75 × 108. Each virus stock was aliquoted at 50 to 100 μl/tube and stored frozen at −80°C over the duration of these experiments. Aliquots were thawed immediately prior to injection, and unused portions were inactivated with Clorox bleach and discarded.
Primary antibodies.
The following reagents were used to localize infected neurons in fixed sections of brain tissue. A rabbit polyclonal antiserum (Rb133) produced against acetone-inactivated virus reacted with cells infected by all strains of virus used in this analysis (15). PRV-D-infected cells were identified with a polyvalent rabbit antiserum (Rb1544) that recognizes the glycosylated precursor of gI (formerly designated gp63). This antiserum was produced by immunizing rabbits with a polypeptide corresponding to amino acids 60 to 268 of gI expressed in Escherichia coli (45). PRV-BaBlu-infected cells were identified with mouse monoclonal antibodies specific for β-galactosidase, which were purchased from Sigma Chemical Company (St. Louis, Mo.) or 5 Prime-3 Prime, Inc. (Bolder, Colo.).
Experimental paradigms.
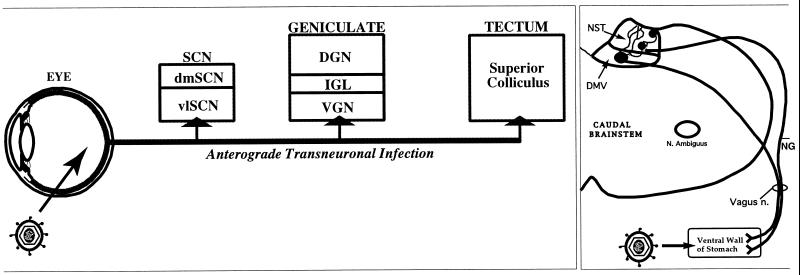
Two well-characterized models of PRV invasiveness were used in this analysis (Fig. 2). Each model has the advantage of peripheral inoculation and addresses different aspects of viral invasiveness and cell-to-cell transmission. The eye model provides a measure of spread to the brain by anterograde routes. Virus infects ganglion cell neurons in the retina (17) and spreads through axons of these neurons in the optic nerve to invade second-order CNS neurons through synaptic contacts formed between the axon terminals and target neurons (Fig. 2, left panel). Prior analysis has shown that PRV-Bartha produces a selective infection of components of this circuitry involved in the regulation of biological timing, while other functionally distinct subdivisions of the visual system involved in visual perception and reflex movement of the eyes (dorsal geniculate nucleus and tectum) are not infected (17). The caudal brain stem model provides a measure of invasiveness of the brain by retrograde transport of virus. This model involves injection of virions into the stomach wall, where they invade the peripherally projecting axons of caudal brain stem neurons in the dorsal motor vagal nucleus (DMV) that control the stomach musculature (14, 15, 46). Thus, in this model, virus particles are retrogradely transported to DMV neurons in the brain stem, where first-order replication occurs. The number of brain stem neurons infected is roughly proportional to the amount of virus injected into the stomach muscles. After replicating in the DMV neurons, virus spreads to infect other CNS neurons synaptically connected to the DMV (Fig. 2, right panel). In this report we have focused on the transynaptic spread of virus to the immediately adjacent nucleus of the solitary tract (NST) and the spatially distant paraventricular hypothalamic nucleus (PVN). CNS neurons are also infected by viral transport through sympathetic ganglia and the spinal cord in this model, but infection via these routes is delayed relative to the DMV infection (14, 15, 34).
FIG. 2.
In vivo models. The organization of neuronal circuitry in the two models used to evaluate viral invasiveness and replication in single- and dual-injection paradigms is illustrated. (Left panel) The ability of PRV to invade the nervous system via anterograde transneuronal infection was assessed in visual circuitry. In this model, virus is injected into the vitreous body of the eye, where it invades and replicates within retinal ganglion cells. Progeny virus is transported anterogradely through axons of the ganglion cells to produce transynaptic infection of neurons in the brain that receive visual input. Prior studies have demonstrated that subdivisions of this circuitry are differentially susceptible to infection by different strains of PRV, such that PRV-Bartha infects only a functionally distinct subset of retinal ganglion cells and their target neurons in the brain (11). (Right panel) Caudal brain stem neurons involved in the regulation of visceral function provided the model system for evaluating retrograde infection of the CNS. In this model, inoculation of the ventral wall of the stomach produces a retrograde infection of preganglionic parasympathetic neurons in the DMV of the caudal brain stem. Replication of virus in the DMV is followed by retrograde transynaptic infection of neurons in the adjacent NST and other synaptically linked neurons, including a population in the PVN (see Fig. 6G). It is well established that this circuitry is susceptible to all strains of PRV studied to date, including PRV-Bartha and related strains. dm, dorsomedial; vl, ventrolateral; DGN, dorsal geniculate nucleus; VGN, ventral geniculate nucleus; NG, nodose ganglion.
The LD50s of wild-type PRV (Kaplan or Becker strain) in mice and rats are similar; about 50 to 100 PFU will kill 50% of infected animals (10, 48). The LD50 of the attenuated Bartha strain in rats and mice is about 100 times greater than that of PRV-Becker (10). The LD50 of PRV-D has not been determined but appears to be less than that of PRV-Bartha and more than that of PRV-Becker (unpublished data and this report). In the experiments in this study, animals are infected with approximately the same number of PFU of each virus, because we strive to infect similar numbers of primary neurons with the same number of virions. In addition, the amount of virus used is at least 100-fold over the LD50 to ensure that sufficient virus is available to infect the first-order neurons in all animals. As a result, every animal will die, and the mean times to appearance of symptoms and to death can be quantified. Nevertheless, it is important to note that only a small number of animals actually progressed to death. Rather, animals were killed at various postinoculation intervals so that the progression of infection of each virus could be accurately gauged and compared. Further, we conducted a systematic temporal analysis to define the invasiveness of each virus in both models. The temporal range of infection of each component of a circuit was first established by killing a small number of animals at progressively longer survival intervals. Additional animals were then added at the critical time points. This approach ensured the reproducibility of the temporal sequence of infection produced by each virus. Symptoms of infection were carefully monitored, since prior studies have shown that these virulence parameters are highly predictive of the genotype of the virus and the route of infection (10, 42, 48).
Eye injections.
Two microliters of virus suspension was injected into the vitreous body of one eye (approximately 5 × 105 PFU for PRV-D and 9 × 105 PFU for PRV-BaBlu). When a mixture of both viruses was injected, a 1:1 solution of virus stock was prepared, and 2 μl of the mixture was injected into the vitreous body of one eye. For some experiments, 2 μl of one virus was injected into one eye, and 2 μl of the other virus was injected in the other eye.
Stomach injections.
A total of 4 μl of virus was injected into three sites on the ventral wall of the stomach. Three types of double-inoculation paradigms were used. In the first, a 1:1 mixture of PRV-D and PRV-BaBlu was prepared, and 4 μl of the mixture was injected into the stomach as described above. In the second, 4 μl of PRV-BaBlu was injected into three sites on the ventral stomach, followed by injection of 4 μl of PRV-D into the same sites 24 h later. In the third, the order of virus injections used in the second experiment was reversed (PRV-D followed by PRV-BaBlu 24 h later). Prior to injections, all animals were anesthetized with ketamine and xylazine as described previously (18). Further details regarding the experiments are provided in Table 1.
TABLE 1.
Experimental groups
| Expt | Virus(es) | n | Vol (μl) | PFU/ml (108) | Survival (h) |
|---|---|---|---|---|---|
| Single unilateral intravitreal injection | PRV-Bartha | 7 | 2 | 28 | 97–10 |
| PRV-D | 2 | 2 | 3.2 | 48 | |
| 4 | 2 | 3.2 | 71–77 | ||
| 2 | 2 | 3.2 | 90 | ||
| PRV-BaBlu | 1 | 2 | 4.75 | 48 | |
| 4 | 2 | 4.75 | 73 | ||
| 7 | 2 | 4.75 | 94–99 | ||
| 5 | 2 | 4.75 | 118–120 | ||
| 3 | 2 | 4.75 | 140 | ||
| Simultaneous unilateral intravitreal injection | PRV-D, PRV-BaBlu | 2 | 2 each | 3.2, 4.75 | 60 |
| 4 | 2 each | 3.2, 4.75 | 72 | ||
| 1 | 2 each | 3.2, 4.75 | 85 | ||
| 3 | 2 each | 3.2, 4.75 | 96 (died) | ||
| Simultaneous injection into different eyes | PRV-D, PRV-BaBlu | 2 | 2 each | 3.2, 4.75 | 77 |
| 2 | 2 each | 3.2, 4.75 | 85 | ||
| Single injection into ventral stomach | PRV-BaBlu | 1 | 4 | 4.75 | 48 |
| 2 | 4 | 4.75 | 73 | ||
| 3 | 4 | 4.75 | 99 | ||
| PRV-D | 3 | 4 | 3.2 | 70–72 | |
| PRV-BaBlu followed 24 h later by PRV-D into ventral stomach | PRV-D, PRV-BaBlu | 1 | 2 each | 3.2, 4.75 | 72 |
| 1 | 2 each | 3.2, 4.75 | 80 | ||
| 4 | 2 each | 3.2, 4.75 | 94 | ||
| PRV-D followed 24 h later by PRV-BaBlu into ventral stomach | PRV-D, PRV-BaBlu | 1 | 2 each | 3.2, 4.75 | 65 |
| 2 | 2 each | 3.2, 4.75 | 73 |
Tissue processing.
At specific times following infection, animals were anesthetized with an overdose of ketamine-xylazine and killed by transcardiac infusion of buffered aldehyde solutions by previously described procedures (12, 18). The brain was removed and postfixed for 1 h at 4°C prior to cryoprotection in 20% phosphate-buffered sucrose at the same temperature. Tissue was then cut in the coronal plane at 35 μm per section with a freezing microtome and stored in cryopreservant (45) at −20°C prior to immunohistochemical analysis. Immunohistochemical localizations were conducted by using immunoperoxidase or immunofluorescence procedures. In both instances, sections at a frequency of 210 μm through the brain were washed to remove cryopreservant and then transferred to primary antibodies for a 24- to 48-h incubation at 4°C. The immunoperoxidase localizations were accomplished with the avidin-biotin modification (22) of the peroxidase-antiperoxidase method with affinity-purified secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) and Vectastain Elite reagents (Vector Laboratories, Burlingame, Calif.). The immunofluorescence localizations used secondary antibodies conjugated to fluorescein isothiocyanate (FITC) or CY2 or CY3 (Jackson ImmunoResearch Laboratories, Inc.) to produce green (FITC and CY2) or red (CY3) fluorescence. These fluorescent secondary antibodies were used at a dilution of 1:500. Sections from tissue processed with FITC-conjugated immunoglobulin G were mounted on slides, and coverslips were applied with Fluoromount G (Southern Biotechnology Associates, Inc., Birmingham, Ala.). Tissues processed with the CY2- and CY3-conjugated secondary antibodies were dehydrated and cleared, and coverslips were applied with Cytoseal 60 (Stevens Scientific, River Dale, N.J.). Specific details of all of the aforementioned procedures have been published previously (12, 18).
Experimental analysis.
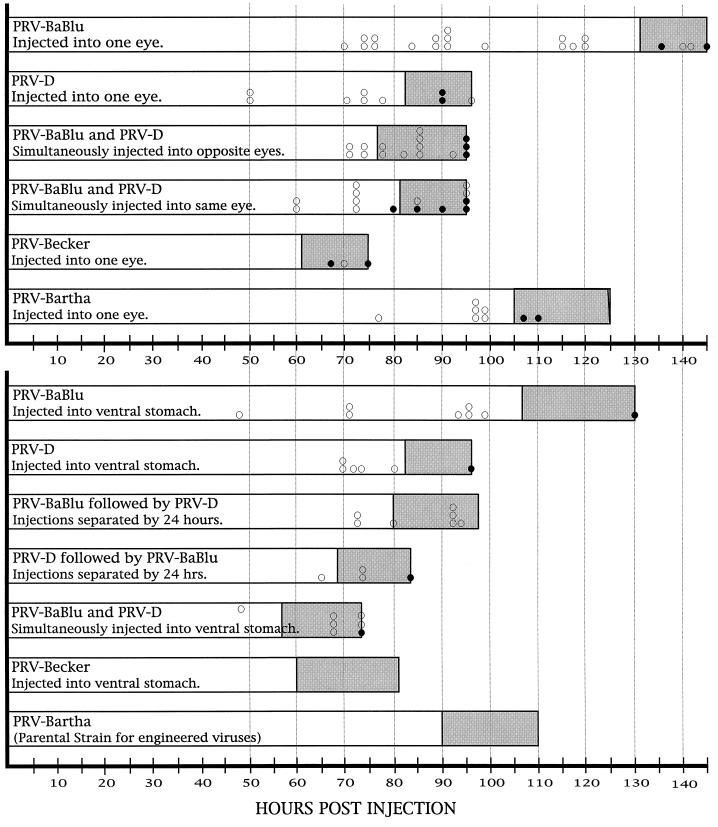
The virulence of each strain was estimated by observing and documenting symptoms of viral infection in all animals. We did not conduct a quantitative analysis of mean time to death as previously done with PRV-Becker and PRV-Bartha (10). However, a subset of animals infected with each strain succumbed naturally to infection, and these animals gave natural terminal end points. We also carefully monitored animals for overt signs of viral infection (lethargy, weight loss, or spiked coat). We combined this information with that obtained in our prior analyses of virulence (10, 16) to produce the graphic representations shown in Fig. 3.
FIG. 3.
Virulence of PRV strains. The virulence produced by single and dual infection of visual (top graph) or autonomic (bottom graph) circuitry with different strains of PRV is illustrated. The horizontal axis defines the time after infection, and each bar indicates when animals were sacrificed after inoculation with PRV. The open portion of each bar defines the time interval in which animals were free of any overt signs of viral infection. The shaded area at the right of each bar indicates the time when animals exhibited one or more of the following symptoms of infection: hunched posture, lethargy, spike coat, oronasal excretions, or weight loss. The positions of the open circles within each bar indicate the times when individual animals were killed for immunohistochemical localization of infected neurons. The closed circles within each bar mark the times that individual animals died naturally. The data for PRV-Bartha and PRV-Becker in each experimental model are largely based on those collected in three prior investigations (10, 15, 16, 34); additional animals infected with these viruses in the present study are represented by the open and closed circles.
The extent of infection in the CNS produced by each strain of virus was initially assessed with tissue processed for immunoperoxidase localization of viral antigens with the rabbit anti-PRV polyclonal antiserum that recognizes all strains of PRV used in this study. Immunoperoxidase localizations were then conducted with tissue from each animal by using the gI and β-galactosidase monoclonal antibodies. Coinfection of neurons in animals inoculated with PRV-D and PRV-BaBlu was determined by using dual-label immunofluorescence localizations. The gI glycoprotein was identified with the secondary antibody conjugated to FITC or CY2 to produce green fluorescence, and β-galactosidase was identified with the CY3-conjugated secondary antibody to produce red fluorescence. Fluorophors in each sample were excited with the appropriate filters, and infected neurons were photographed with Kodak Ektachrome 160 color film or images were digitized and analyzed with a Simple 32 image analysis system (C-Imaging Systems). Dual-labeled images were collected by dual exposure of the same frame of color slide film and by digitizing images of sections illuminated with a filter cube that excites both CY2 and CY3 (Omega). Examination of tissue with high-magnification objectives (20× to 40×) provided assurance that yellow fluorescence was due to colocalization of both fluorophors rather than to overlap of cells differentially infected with different viruses.
RESULTS
Our goal was to define basic parameters that would facilitate the use of two recombinant viruses expressing unique reporters for the definition of complex synaptic arrangements in neuronal circuitry. Accordingly, we sought to determine if prior infection of neurons with one strain of virus rendered neurons resistant to infection by a second strain. We found that differences in virulence and rate of invasion of visual and autonomic circuits by the two recombinant viruses markedly affected the ability of the two viruses to establish a double infection.
Virulence parameters.
Clear differences in the virulence of infections produced by PRV-Bartha and by the recombinant viruses were apparent in both visual and autonomic circuitry (Fig. 3). Although PRV-D and PRV-BaBlu are derived from PRV-Bartha, PRV-D was more virulent than PRV-Bartha, which, in turn, was more virulent than PRV-BaBlu. This rank order of virulence was the same for both experimental models even though they involve infection of different populations of neurons.
The reduced virulence exhibited by PRV-BaBlu was characterized by extended survival and delayed appearance of symptoms of infection. This occurred despite the fact that the titer of the parental virus was slightly higher than that of PRV-BaBlu (2.8 × 109 versus 4.75 × 108 PFU/ml), making the point that the differences in virulence could not be attributed to differences in the concentration of the injected virus. Animals with eye infections routinely survived for 120 h with no overt symptoms of infection. Four animals did not exhibit symptoms of infection prior to 132 h. Two of these rats were killed to document the extent of viral infection at 140 and 141 h postinoculation. The other two animals died without overt symptoms at 135 and 144 h. This contrasted with the appearance of symptoms and mortality in animals infected with PRV-Bartha. These animals exhibited symptoms of infection at approximately 105 h after inoculation and did not survive beyond 125 h.
A similar difference in virulence was observed when PRV-Bartha and PRV-BaBlu were injected into the stomach to produce a retrograde infection of the caudal brain stem. However, the appearance of symptoms was more rapid than that documented in animals infected through visual pathways. Animals infected with PRV-Bartha exhibited symptoms at approximately 90 h after injection and did not survive beyond 110 h. In contrast, no symptoms were observed prior to 105 h after identical injection of PRV-BaBlu. The single animal that was allowed to proceed to death in this paradigm died suddenly at 130 h with only moderate overt symptoms of infection. Considered with the visual system data, these findings indicate that insertion of the β-galactosidase gene at the gG gene locus of PRV-Bartha had the effect of delaying the onset of symptoms and extending survival by approximately 20 h. Whether this phenotype is due to the lack of gG, to effects of the lacZ insertion on adjacent genes (Us3 or gD gene), or to a direct effect of β-galactosidase expression is not clear at this time.
PRV-D was more virulent than the parental strain or PRV-BaBlu in both circuits (Fig. 3). Animals exhibited symptoms at approximately 80 h after inoculation, and no animal survived longer than 95 h, irrespective of whether virus was injected into the eye or stomach wall. Additionally, the symptoms exhibited by PRV-D-infected rats were quite similar to those produced by the virulent Becker strain of PRV. For example, oral and nasal excretions were much more pronounced in these animals than in rats inoculated with PRV-BaBlu or PRV-Bartha. It is likely that the increased virulence of PRV-D results from the expression of gI, Us9, or Us2, the genes for which are absent in PRV-Bartha and PRV-BaBlu.
As expected, when animals were coinfected with two viruses that differed in virulence, the animal exhibited the virulence parameters of the most virulent strain. This was true in every model tested (Fig. 3).
Single infections.
In single-injection paradigms, the time course of anterograde or retrograde transynaptic spread of PRV-D into the CNS was at least 24 h faster than that for PRV-Bartha or PRV-BaBlu. PRV-D-infected animals also died sooner. However, it is important to point out that recent data have shown that PRV virulence does not always correlate directly with the extent of viral invasion (46).
(i) Anterograde transneuronal infection.
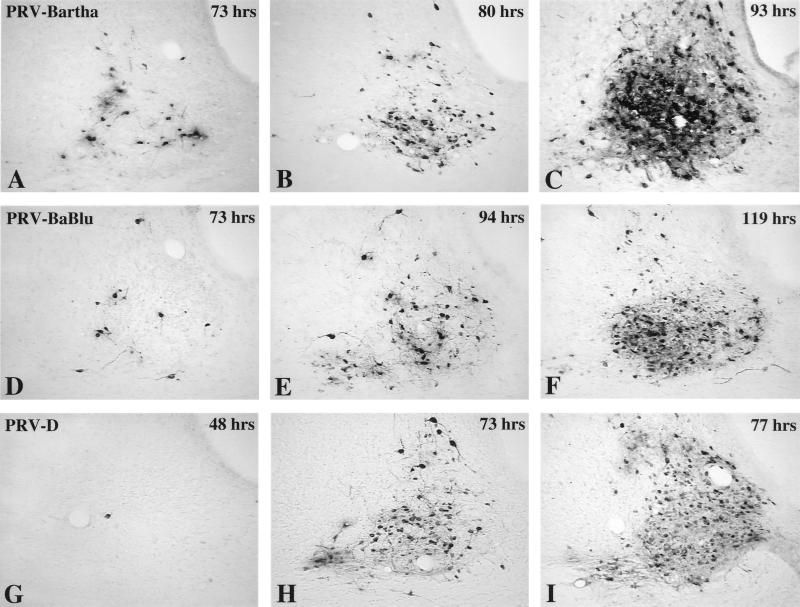
Infection of the suprachiasmatic nuclei (SCN) in the hypothalamus is dependent on the establishment of a productive infection in retinal ganglion cells in the eye, followed by anterograde transneuronal infection of a subset of SCN neurons that receive direct synaptic input from these infected ganglion cells (Fig. 2). Other SCN neurons that are not contacted by retinal axons become infected through local connections within the SCN. Therefore, three orders of viral replication and transynaptic passage can be confidently assessed in this model. Clear differences in the progression of infection in the SCN were observed following intravitreal injection of PRV-Bartha, PRV-BaBlu, and PRV-D. After injection of the parental virus (PRV-Bartha), infected neurons were first visible in the retinorecipient portion of the SCN at 73 h postinoculation (Fig. 4A) and became increasingly prevalent in this portion of the nucleus through 80 h (Fig. 4B). By 93 h, transynaptic passage of virus led to infection of neurons throughout the SCN (Fig. 4C). Replication and transynaptic passage of PRV-BaBlu was delayed relative to those produced by PRV-Bartha. Scattered infected neurons were observed in the retinorecipient SCN by 73 h, but subsequent replication and transynaptic passage of virus in the SCN were substantially delayed. For example, it took PRV-BaBlu 94 h to achieve the same extent of infection in the retinorecipient SCN that was achieved within 80 h of injection of PRV-Bartha (compare Fig. 4B and E). Furthermore, the extent of infection in the SCN at 119 h after intravitreal injection of PRV-BaBlu was less than that produced by PRV-Bartha at 93 h (compare Fig. 4C and F). This contrasted with the more rapid progression of infection produced in the same circuitry by PRV-D. Animals infected with this virus exhibited occasional infected neurons in the retinorecipient SCN as early as 48 h after injection of PRV-D (Fig. 4G), an onset of infection that was approximately a day earlier than that after injection of either PRV-Bartha or PRV-BaBlu. Thereafter, the number of infected neurons in the retinorecipient SCN increased rapidly, and there was transynaptic spread of virus throughout the SCN within 77 h of inoculation (Fig. 4I).
FIG. 4.
Anterograde transneuronal infection of the SCN. The extents of anterograde transneuronal infection of the SCN at different times following intravitreal injection of PRV-Bartha (A to C), PRV-BaBlu (D to F), and PRV-D (G to I) are illustrated. Infected neurons were detected by the avidin-biotin immunoperoxidase procedure with a rabbit polyclonal antiserum that identifies virally encoded structural and envelope proteins. The timing of anterograde transneuronal infection of SCN neurons produced by the two recombinant strains (PRV-BaBlu and PRV-D) differed substantially from that for neurons produced by the parental virus (PRV-BaBlu). Scattered PRV-Bartha-infected neurons were observed in the portion of the nucleus that receives retinal input at 73 h postinoculation, and the virus had moved through local circuit connections to infect the majority of neurons in all portions of the nucleus by 93 h. This temporal sequence was delayed in PRV-BaBlu-infected animals and accelerated in PRV-D-infected rats. The postinoculation interval is shown in the upper right corner of each micrograph.
(ii) Retrograde transneuronal infection.
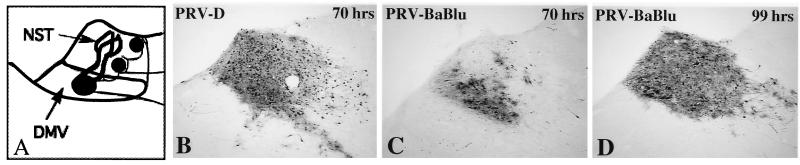
At 70 h following injection of PRV-BaBlu into the stomach, first-order replication of virus was apparent in a large number of neurons in the DMV of the caudal brain stem, and there was slight retrograde transynaptic infection of neurons in the immediately adjacent NST (Fig. 5C). By 99 h the transynaptic passage of virus had infected neurons throughout the NST (Fig. 5D) and had also spread into synaptically linked areas of the caudal brain stem, such as the area postrema and the portion of the brain stem tegmentum containing the A1 catecholamine cell group (data not shown). This temporal progression of infection was in dramatic contrast to that produced by identical inoculation of PRV-D. The same magnitude of retrograde transynaptic infection of the DMV and NST observed at 70 h after injection of PRV-D required approximately 100 h to be achieved after injection of PRV-BaBlu (compare Fig. 5B and D).
FIG. 5.
Retrograde transneuronal infection of the dorsal motor vagal complex. The temporal sequence of retrograde transynaptic infection of caudal brain stem circuitry produced by injection of PRV-D (B) and PRV-D (C and D) into the ventral wall of the stomach is illustrated. The schematic diagram in panel A illustrates the organization of neural circuits through which the neurons became infected (see also Fig. 2B). The temporal sequence of infection differed substantially for the two recombinant viruses. PRV-D produced a robust infection of the DMV and moved transynaptically to infect many neurons in the NST within 70 h of inoculation (B). In contrast, infected neurons were largely confined to the DMV at the same time after injection with PRV-BaBlu (C). An infection comparable to that produced by PRV-D at 70 h was not achieved with PRV-BaBlu until 99 h (D).
Coinfection studies. (i) PRV-D infection suppresses PRV-BaBlu invasion of visual circuitry.
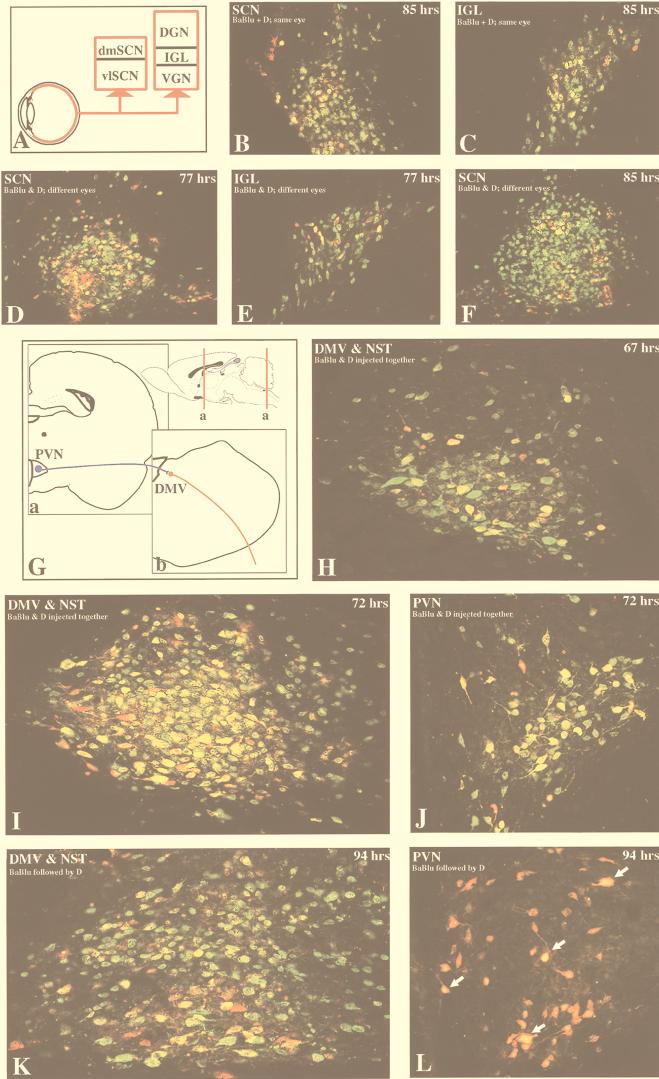
By injecting PRV-D into one eye and PRV-BaBlu into the other eye, we were able to compare the efficiencies of anterograde transynaptic infection of the two viruses under circumstances in which they did not compete with one another for the initial infection of retinal ganglion cells. Under these circumstances, PRV-D rapidly invaded second-order neurons in the SCN and then passed transynaptically to infect other neurons in the SCN by 77 h (Fig. 6D). By 95 h the virus had moved transynaptically to infect neurons throughout the SCN (Fig. 6F). Similarly, PRV-D-infected neurons were prevalent within the intergeniculate leaflet (IGL) of the thalamus (Fig. 6E), a circumscribed cell group in the geniculate complex that receives synaptic input from collaterals of the retinal axons that also synapse in the SCN (Fig. 6A). The extensive PRV-D infection in both the SCN and IGL contrasted with the restricted distribution of neurons infected with PRV-BaBlu. In both regions, the number of neurons replicating PRV-BaBlu was a small subset of the total number of infected neurons (Fig. 6D to F). Furthermore, the relative number and distribution of PRV-BaBlu-infected neurons in the longest-surviving animals were reduced from those observed at the same time when PRV-BaBlu was injected alone. Neurons replicating both viruses were observed, but single infections predominated. There are several potential explanations for this finding. PRV-BaBlu may be less efficient than PRV-D at infecting the primary retinal ganglion cells, it may be transported to the axon terminals at a lower rate, or it may be less efficient at transynaptic spread to the second-order neurons. Additionally, neurons previously infected with PRV-D may exclude PRV-BaBlu from replicating, a phenomenon that is well documented for tissue culture infections (7, 8, 24). In any case, anterograde transynaptic infection of retinorecipient neurons and subsequent spread of virus through synaptically linked neurons are dominated by PRV-D even though both viruses are injected simultaneously and do not have to compete with one another for the initial infection of retinal ganglion cells.
FIG. 6.
Coinfection of neurons in dual-injection paradigms. The extent of coinfection of neurons with the two recombinant strains (PRV-BaBlu and PRV-D) in visual (A to F) and autonomic (G to L) circuits is illustrated. Infected neurons were detected by using dual-labeling immunofluorescence techniques and antibodies that identified the unique gene products produced by each virus. Direct comparisons demonstrated that these gene products identified all neurons infected with either strain of virus. Thus, β-galactosidase and gI were efficiently expressed by the respective viruses and were a reliable marker of infection. Panels A and G illustrate the organization of the circuitry that was the subject of analysis. Analysis of the efficiency of coinfection of CNS neurons by anterograde transynaptic infection was conducted in visual circuits after simultaneous injection of both viruses into one eye (B and C) or individual injection of each strain into different eyes (D to F). The SCN and IGL were the sites of analysis, as both of these regions have been previously shown to be permissive to infection with the parental virus (PRV-Bartha; see reference 10 for a review). The ability of the two strains to establish a coinfection after retrograde transport was evaluated in caudal brain stem circuitry that innervates the viscera. Virus was injected into the ventral wall of the stomach, and retrograde transynaptic infection was evaluated in the DMV, NST, and PVN (G) (also see Fig. 2B and 5A). PRV-D infection (green fluorescence) dominated in both experimental paradigms. In visual circuitry the majority of neurons replicated only PRV-D, and a much smaller number were infected with both strains (yellow fluorescence) or selectively with PRV-BaBlu (red fluorescence). PRV-D infection also dominated in retrograde pathways, but the degree of this domination was dependent on the temporal association of the injection of the two viruses. When the two viruses were injected as a mixture, the majority of caudal brain stem neurons were infected with PRV-D alone or were coinfected with both strains; only scattered neurons were infected with only PRV-BaBlu (H and I). However, PRV-D infection also dominated when PRV-BaBlu had been injected 24 h earlier (K). Both strains of virus also passed transynaptically to infect neurons in the NST (H, I, and K) and PVN (J and L). After simultaneous stomach injection of the two viruses, PRV-D also dominated the retrograde transynaptic infection of the spatially distant PVN, but many cells replicated both strains (J). When PRV-D was injected 24 h after PRV-BaBlu, the majority of cells were infected with PRV-BaBlu (red fluorescence), but the yellow fluorescence in many of the cell nuclei indicated that they were also in early stages of replication of PRV-D. The areas, experimental paradigms, and postinoculation intervals are indicated for each panel. dm, dorsomedial; vl, ventrolateral; DGN, dorsal geniculate nucleus; VGN, ventral geniculate nucleus.
The interference effect was also apparent when PRV-D and PRV-BaBlu were injected simultaneously into the same eye. Under these circumstances, we observed extensive PRV-D replication and transynaptic infection of the SCN and IGL within 85 h. However, few neurons in either region were infected with PRV-BaBlu, either alone or in combination with PRV-D (Fig. 6B and C). This pattern of infection persisted through 95 h, the longest survival interval in this model.
(ii) PRV-D suppresses PRV-BaBlu retrograde invasion of autonomic circuitry.
PRV-D also reduced the invasiveness of PRV-BaBlu in coinjection paradigms involving autonomic circuitry. These experiments introduce the virus into the caudal brain stem through viral invasion of axon terminals and retrograde transport to the parent cell bodies (14, 15, 34, 46). Three experiments were conducted to assess neuroinvasiveness in this paradigm. The first involved simultaneous inoculation of equivalent amounts of the two strains in question. In the other two experiments, infection by each virus was separated by 24 h. One set of animals were inoculated with PRV-BaBlu followed by PRV-D, and for the other set the sequence was reversed. These manipulations allowed us to evaluate the influence of one strain on the replication and invasiveness of the other. In all three experimental models, PRV-D infected a larger population of neurons earlier in the course of infection than did PRV-BaBlu.
After injection of a mixture of both viruses, PRV-D infected a large number of DMV (first-order) and NST (second-order) neurons within 67 h (green fluorescence in Fig. 6H). A relatively small percentage of these PRV-D infected neurons were also replicating PRV-BaBlu (yellow fluorescence), while only scattered neurons were infected solely with PRV-BaBlu (red fluorescence). At longer postinoculation intervals, the number of infected neurons in both the DMV and NST increased substantially. Neurons infected with both viruses were common but were substantially reduced compared to the number of neurons replicating PRV-D. Neurons infected with only PRV-BaBlu were very rare (Fig. 6I). Often, neurons containing dense staining for β-galactosidase also exhibited more restricted staining for the gI marker of PRV-D. In some neurons the gI immunoreactivity was restricted to the cell nucleus, while in others it filled both the nucleus and the cytoplasm. It should be emphasized that the invasiveness of PRV-BaBlu in this coinjection paradigm was less than that observed at comparable survival intervals when PRV-BaBlu was injected alone. This is apparent in comparisons of the extent of PRV-BaBlu infection 70 h after individual inoculation (Fig. 5C) with that produced in the dual-inoculation paradigm at 67 and 72 h (Fig. 6H and 6I). As we observed in the eye model, the rapid invasion and subsequent spread of PRV-D interfered with infection and spread of PRV-BaBlu. Interestingly, the opposite does not appear to be true; prior infection by PRV-BaBlu does not suppress the replication of PRV-D in the same neurons. We deduced this by comparing the localizations of β-galactosidase and gI immunoreactivities in neurons replicating both viruses. We have shown previously that at early stages of infection, viral proteins are found predominantly in membranes in and around the nucleus, with little antigen present in neuronal processes. Neurons in late stages of infection exhibit robust staining of viral antigens in the cell body and processes (9, 39). The presence of gI immunoreactivity in perinuclear membranes of neurons in advanced stages of PRV-BaBlu replication (judged on the basis of the extensive distribution of β-galactosidase immunoreactivity throughout the infected neurons and their processes) indicated that prior infection with PRV-BaBlu did not suppress replication of PRV-D.
The ability of PRV-D to replicate and spread through synaptically linked neurons previously infected with PRV-BaBlu was illustrated vividly in the experiment in which PRV-BaBlu was injected into the stomach wall at 24 h prior to injection of PRV-D. When animals were killed at 94 h after the initial inoculation (an effective postinoculation interval of 70 h for PRV-D), the distribution of antigens unique to each virus in the caudal brain stem was quite similar to that observed 72 h after simultaneous injection of both strains (compare Fig. 6I and K). Thus, prior replication of PRV-BaBlu had little effect on the ability of PRV-D to replicate in the DMV and pass transynaptically to infect NST neurons.
Although prior infection with PRV-BaBlu had no apparent effect on PRV-D replication and transynaptic passage in the caudal brain stem, examination of the forebrain regions connected to the DMV and NST indicated that PRV-D replication had been delayed in these neurons. For example, the PVN projects densely to the DMV-NST complex (Fig. 6G), and neurons in this area have been shown to be infected by transynaptic passage of PRV injected into the stomach (46) and other visceral targets (see reference 27 for a review). At 72 h following simultaneous inoculation of PRV-D and PRV-BaBlu into the stomach wall, infected neurons that contained one or both viruses were observed in the PVN (Fig. 6J). As in the caudal brain stem, PRV-D infected the largest number of neurons, consistent with the previously demonstrated higher rate of replication and invasiveness. When PRV-BaBlu was injected 24 h prior to PRV-D and the animals were sacrificed 94 h after the PRV-BaBlu injection (70 h after the PRV-D injection), the extent of infection produced by PRV-D was reduced. A substantial number of PRV-BaBlu-infected PVN neurons were observed (Fig. 6L), and the relative number was essentially the same as that observed at equivalent survival in single-injection paradigms involving this virus (data not shown). However, many of the PRV-BaBlu-infected cells also exhibited gI immunoreactivity in their nuclei, indicating early stages of coinjection with PRV-D (Fig. 6J). The number of these cells was substantially reduced from the numbers observed at equivalent survival (approximately 70 h) after simultaneous injection of the two viruses (Fig. 6J) or single injection of PRV-D (data not shown). These data indicate that while invasion and transynaptic passage of PRV-D in the caudal brain stem is not compromised by prior infection with PRV-BaBlu, infection of more spatially distant regions of the brain synaptically linked to the DMV and NST is delayed.
When the temporal sequence of injection was reversed (PRV-D infection followed 24 h later by PRV-BaBlu infection), PRV-BaBlu barely invaded the CNS before the animals died. This most likely reflects the increased virulence of PRV-D and the inefficient neuroinvasiveness of PRV-BaBlu. Two animals that were sacrificed approximately 72 h after the PRV-D inoculation exhibited a pattern of PRV-D-infected neurons equivalent to that seen after single injection of this virus (data not shown). Thus, the only conclusion that we made from these data was that subsequent inoculation of PRV-BaBlu did not alter the temporal dynamics of PRV-D invasion within this time frame.
DISCUSSION
The following conclusions can be drawn from these experiments. First, the progression of invasion and spread of PRV-D in the CNS is significantly faster than that for infection by PRV-Bartha or PRV-BaBlu in the same circuitry. Second, PRV-D is more virulent than either parental PRV-Bartha or PRV-BaBlu, but PRV-BaBlu is less virulent than PRV-Bartha. Third, neurons have the capacity to replicate both recombinants under the proper circumstances. Fourth, prior infection with one recombinant reduces the ability of neurons to replicate the other recombinant strain. Fifth, prior infection by PRV-D is more effective than infection with PRV-BaBlu in reducing invasion and spread by the second virus. These findings have important implications for the design and interpretation of investigations involving the use of multiple strains of virus to define neural circuits and indicate that interference of one strain with the replication of a second strain may produce false negatives. The data also suggest that the use of isogenic strains of virus may produce more reliable co-infection.
Neurovirulence in coinfection paradigms.
The influence of virulence upon coinfection was readily apparent in all of our experimental models. The more virulent PRV-D tended to suppress the replication and spread of the less virulent PRV-BaBlu, but this attenuated strain was less efficient at reducing the replication of PRV-D. The effect could not be attributed to differences in infectious dose, since the injected concentrations of the two strains were very similar. In fact, the titer of PRV-BaBlu was slightly higher than that of the more virulent PRV-D (4.75 × 108 versus 2.5 × 108). The difference in virulence of the two PRV strains most likely reflects the presence or absence of the gI, gE, Us9, and Us2 genes in the Us region of the PRV genome. Prior analysis of PRV in visual circuitry has shown that deletion of one or both of the gE and gI envelope glycoprotein genes from this region of the wild-type genome reduces the virulence produced by intravitreal inoculation of wild-type PRV (16, 45). Similarly, selective mutations in the terminal cytoplasmic domain of gE produce a similar reduction of virulence but do not compromise viral invasiveness of visual or autonomic circuits (42, 46). We recently found that deleting the Us9 gene from PRV-Becker also resulted in a reduction of virulence (unpublished observations). The insertion of lacZ in the gG gene (PRV-BaBlu) in the present study further attenuated the virulence of PRV-Bartha and also slowed the invasiveness of the virus. The differences in virulence and invasiveness between the two strains biased the temporal course of viral replication and spread in favor of PRV-D, an aspect of invasiveness with an important influence upon the number of neurons that were coinfected with PRV-BaBlu.
Neuroinvasiveness in coinfection paradigms.
The double-infection approach has been employed with strains of herpes simplex virus and PRV engineered to express unique gene products and is dependent on the ability of two strains of virus to replicate in the same neuron. In studies of neuronal circuits that modulate the activity of the viscera and homeostatic function, Jansen and colleagues (23) and Levatte et al. (26) demonstrated that recombinant viruses expressing unique reporters can be used to dissect the synaptic organization of polysynaptic circuits. Such experiments are a powerful exploitation of the neurotropism and invasiveness of alphaherpesviruses to define principles of synaptic organization that simply cannot be defined with other techniques. Our experiments underscore the usefulness of this experimental approach but also reveal the importance of considering the biology of mixed infections by similar viruses in the nervous system of a living animal. From tissue culture experiments, we know that attachment and entry of herpesvirus particles can be blocked if the cell is already infected or if it expresses the viral membrane protein gD (7, 8, 24, 43). We do not know if such gD-mediated exclusion functions in animals. For PRV, gD is not required for cell-cell spread of infection, and thus gD-mediated exclusion may be possible only during primary infection where particles attach to infected cells. Coinjection experiments should not be subject to this type of exclusion if the rates of attachment, entry, and replication of both viruses are similar. However, any experiment in which replication of one virus takes place in a neuron prior to the attachment of the second virus particle may well be subject to gD-mediated exclusion.
The number of neurons infected by retrograde transport of PRV-D from the stomach far exceeded the number of those that were coinfected or were only replicating PRV-BaBlu. It is not clear why this should be, but one possibility is that PRV-BaBlu infects axon terminals inefficiently compared to PRV-D. Comparison of the rates of attachment, fusion, and entry for each virus has not been done. The diffusely ramifying axonal processes of DMV neurons in the stomach musculature and the environment of the inner surface of the retina provide a nonuniform surface for viral infection that we cannot mimic in the tissue culture dish. It is also possible that the presence of the gI and Us9 genes in PRV-D improves the efficiency of invasion of permissive neurons in both stomach and retina. Attachment and entry of PRV-BaBlu might be reduced by simple competition with PRV-D if the number of receptors is limiting. This possibility is supported by the predominance of PRV-D infection in both models. As we do not know the multiplicity of infection (number of virus particles per number of susceptible cells or axon terminals), we cannot say with confidence that every permissive cell is infected with both viruses. We know that while coinfection does occur, we likely are not saturating permissive cells with both viruses, because we observe an exclusive infection by PRV-BaBlu in a small number of neurons after simultaneous injection of both strains.
The differential abilities of two strains of virus with differing virulence to efficiently coinfect neurons depend on the timing of the second infection with respect to the first, the effect of the first virus on neuronal function, and the defense that the brain mounts against the infection. Even with isogenic strains where every gene is identical with the exception of a reporter, prior replication of one virus will certainly influence any subsequent replication by another virus, if only through the marked effect of herpesvirus infection on cell macromolecular metabolism (36). In addition, infection of the CNS with PRV and herpes simplex virus elicits a complex and predictable nonneuronal response that isolates infected neurons and may suppress viral replication (see reference 11 for a review). Importantly, the magnitude of this nonneuronal response is directly dependent on the virulence of the infecting virus (14, 35) and the cells that contribute to it. These cells express potent molecules such as nitric oxide in response to PRV infection (37), which have documented antiviral effects (see reference 33 for a review). Thus, the failure of PRV-BaBlu to coinfect neurons previously infected by PRV-D may be related to the nonneuronal response elicited by prior replication of PRV-D.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of Marlies Eldridge, Jen Shew Yen, Tariq Syed, and Sadiq Syed.
The contribution of J.-S. Kim was supported by a fellowship awarded by the Korea Science and Engineering Foundation. This work was supported by NIH grants RO1s MH53574 (to J. P. Card) and NINDS33506 (to L. W. Enquist).
REFERENCES
- 1.Babic N, Klupp B, Brack A, Mettenleiter T C, Ugolini G, Flamand A. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology. 1996;219:279–284. doi: 10.1006/viro.1996.0247. [DOI] [PubMed] [Google Scholar]
- 2.Babic N, Mettenleiter T C, Flamand A, Ugolini G. Role of essential glycoproteins gII and gp50 in transneuronal transfer of pseudorabies virus from the hypoglossal nerves of mice. J Virol. 1993;67:4421–4426. doi: 10.1128/jvi.67.7.4421-4426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett E M, Cassell M D, Perlman S. Two neurotropic viruses, herpes simplex virus type 1 and mouse hepatitis virus, spread along different neural pathways from the main olfactory bulb. Neuroscience. 1993;57:1007–1025. doi: 10.1016/0306-4522(93)90045-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett E M, Evans G D, Sun N, Perlman S, Cassel M D. Anterograde tracing of trigeminal afferent pathways from the murine tooth pulp to cortex using herpes simple virus type 1. J Neurosci. 1995;15:2972–2984. doi: 10.1523/JNEUROSCI.15-04-02972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartha A. Experimental reduction of virulence of Aujeszky’s disease virus. Magy Allatorv Lapja. 1961;16:42–45. [Google Scholar]
- 6.Becker C H. Zur primaren Schadingung vegetativer Ganglien nach Infektion mit dem Herpes suis Virus bei verschiedenen Tierarten. Experentia. 1967;23:209–217. doi: 10.1007/BF02136291. [DOI] [PubMed] [Google Scholar]
- 7.Campadelli-Fiume G, Arsenakis M, Farabegoli F, Roizman B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of virus. J Virol. 1988;62:159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campadelli-Fiume G, Qi S, Avitabile E, Foa-Tomasi L, Brandimarti R, Roizman B. Glycoprotein D of herpes simplex virus encodes a domain which precludes penetration of cells expressing the glycoprotein by superinfecting herpes simplex virus. J Virol. 1990;64:6070–6079. doi: 10.1128/jvi.64.12.6070-6079.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card J P. Practical considerations for the use of pseudorabies virus in transneuronal studies of neural circuitry. Neurosci Biobehavioral Rev. 1998;22:685–694. doi: 10.1016/s0149-7634(98)00007-4. [DOI] [PubMed] [Google Scholar]
- 10.Card J P, Dubin J R, Whealy M E, Enquist L W. Influence of infectious dose upon productive replication and transynaptic passage of pseudorabies virus in rat central nervous system. J Neurovirol. 1995;1:349–358. doi: 10.3109/13550289509111024. [DOI] [PubMed] [Google Scholar]
- 11.Card J P, Enquist L W. Neurovirulence of pseudorabies virus. Crit Rev Neurobiol. 1995;9:137–162. [PubMed] [Google Scholar]
- 12.Card J P, Enquist L W. Use of pseudorabies virus for definition of synaptically linked populations of neurons. In: Adolph K W, editor. Molecular virology techniques, part A. Vol. 4. San Diego, Calif: Academic Press; 1994. pp. 363–382. [Google Scholar]
- 13.Card J P, Levitt P, Enquist L W. Different patterns of neuronal infection after intracerebral injection of two strains of pseudorabies virus. J Virol. 1998;72:4434–4441. doi: 10.1128/jvi.72.5.4434-4441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Card J P, Rinaman L, Lynn R B, Lee B-H, Meade R P, Miselis R R, Enquist L W. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J Neurosci. 1993;13:2515–2539. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Card J P, Rinaman L, Schwaber J S, Miselis R R, Whealy M E, Robbins A K, Enquist L W. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990;10:1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Card J P, Whealy M E, Robbins A K, Enquist L W. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J Virol. 1992;66:3032–3041. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Card J P, Whealy M E, Robbins A K, Moore R Y, Enquist L W. Two alpha-herpesvirus strains are transported differentially in the rodent visual system. Neuron. 1991;6:957–969. doi: 10.1016/0896-6273(91)90236-s. [DOI] [PubMed] [Google Scholar]
- 18.Enquist L W, Card J P. Pseudorabies virus: a tool for tracing neuronal connections. In: Lowenstein P R, Enquist L W, editors. Protocols for gene transfer in neuroscience. Towards gene therapy of neurological disorders. Chichester, United Kingdom: John Wiley & Sons; 1996. pp. 333–348. [Google Scholar]
- 19.Enquist L W, Dubin J, Whealy M E, Card J P. Complementation analysis of pseudorabies virus gE and gI mutants in retinal ganglion cell neurotropism. J Virol. 1994;68:5275–5279. doi: 10.1128/jvi.68.8.5275-5279.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enquist L W, Husak P J, Banfield B W, Smith G A. Infection and spread of alphaherpesviruses in the nervous system. Adv Virus Res. 1999;51:237–347. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- 21.Heffner S, Kovacs F, Klupp B G, Mettenleiter T C. Glycoprotein gp50-negative pseudorabies virus: a novel approach toward a nonspreading live herpesvirus vaccine. J Virol. 1993;67:1529–1537. doi: 10.1128/jvi.67.3.1529-1537.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu S M, Raine L, Fanger H. The use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 23.Jansen A S P, Van Nguyen X, Karpitskiy V, Mettenleiter T C, Loewy A D. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science. 1995;270:253–260. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- 24.Johnson R M, Spear P. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J Virol. 1989;63:819–827. doi: 10.1128/jvi.63.2.819-827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kristensson K. Sorting signals and targeting of infectious agents through axons: an annotation to the 100 years’ birth of the name “axon.”. Brain Res Bull. 1996;41:327–333. doi: 10.1016/s0361-9230(96)00255-9. [DOI] [PubMed] [Google Scholar]
- 26.Levatte M A, Mabon P J, Weaver L C, Dekaban G A. Simultaneous identification of two populations of sympathetic preganglionic neurons using recombinant herpes simplex virus type 1 expressing different reporter genes. Neuroscience. 1998;82:1253–1267. doi: 10.1016/s0306-4522(97)00314-x. [DOI] [PubMed] [Google Scholar]
- 27.Loewy A D. Pseudorabies virus: a transneuronal tracer for neuroanatomical studies. In: Kaplitt M G, Loewy A D, editors. Viral vectors. Gene therapy and neuroscience applications. San Diego, Calif: Academic Press; 1995. pp. 349–366. [Google Scholar]
- 28.Mettenleiter T C. Molecular properties of alphaherpesviruses used in transneuronal pathway tracing. In: Kaplitt M G, Loewy A D, editors. Viral vectors. Gene therapy and neuroscience applications. San Diego, Calif: Academic Press; 1995. pp. 367–393. [Google Scholar]
- 29.Mettenleiter T C, Rauh I. A glycoprotein gX–β-galactosidase fusion gene as insertional marker for rapid identification of pseudorabies virus mutants. J Virol Methods. 1990;30:55–66. doi: 10.1016/0166-0934(90)90043-f. [DOI] [PubMed] [Google Scholar]
- 30.Norgren R B, McLean J H, Bubel H C, Wander A, Bernstein D I, Lehman M N. Anterograde transport of HSV-1 and HSV-2 in the visual system. Brain Res Bull. 1992;28:393–399. doi: 10.1016/0361-9230(92)90038-y. [DOI] [PubMed] [Google Scholar]
- 31.Peeters B, Pol J, Gielkens A, Moorman R. Envelope glycoprotein gp50 of pseudorabies virus is essential for virus entry but is not required for spread in mice. J Virol. 1993;67:170–177. doi: 10.1128/jvi.67.1.170-177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pol J M A, Gielkens A L J, van Oirschot J T. Comparative pathogenesis of three strains of pseudorabies virus in pigs. Microb Pathog. 1989;7:361–371. doi: 10.1016/0882-4010(89)90039-9. [DOI] [PubMed] [Google Scholar]
- 33.Reiss C S, Komatsu T. Does nitric oxide play a critical role in viral infections? J Virol. 1998;72:4547–4551. doi: 10.1128/jvi.72.6.4547-4551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinaman L, Card J P, Enquist L W. Spatiotemporal responses of astrocytes, ramified microglia, and brain macrophages to central neuronal infection with pseudorabies virus. J Neurosci. 1993;13:685–702. doi: 10.1523/JNEUROSCI.13-02-00685.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rinaman L, Levitt P. Establishment of vagal sensorimotor circuits during fetal development. J Neurobiol. 1993;24:641–659. doi: 10.1002/neu.480240509. [DOI] [PubMed] [Google Scholar]
- 36.Roizman B, Sears E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fundamental virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1043–1107. [Google Scholar]
- 37.Serrano, F., L. W. Enquist, and J. P. Card. Differential expression of inducible nitric oxide synthase in response to infection of the rat CNS with virulent and attenuated strains of pseudorabies virus. Submitted for publication.
- 38.Standish A, Enquist L W, Escardo J A, Schwaber J S. Central neuronal circuit innervating the rat heart defined by transneuronal transport of pseudorabies virus. J Neurosci. 1995;15:1998–2012. doi: 10.1523/JNEUROSCI.15-03-01998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Standish A, Enquist L W, Miselis R R, Schwaber J S. Dendritic morphology of cardiac related medullary neurons defined by circuit-specific infection by a recombinant pseudorabies virus expressing β-galactosidase. J Neurovirol. 1995;1:359–368. doi: 10.3109/13550289509111025. [DOI] [PubMed] [Google Scholar]
- 40.Strick P L, Card J P. Transneuronal mapping of neural circuits with alpha herpesviruses. In: Bolam J P, editor. Experimental neuroanatomy. A practical approach. Oxford, United Kingdom: IRL Press; 1992. pp. 81–101. [Google Scholar]
- 41.Sun N, Cassell M D, Perlman S. Anterograde, transneuronal transport of herpes simplex virus type 1 strain H129 in the murine visual system. J Virol. 1996;70:5405–5413. doi: 10.1128/jvi.70.8.5405-5413.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tirabassi R S, Townley R A, Eldridge M G, Enquist L W. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J Virol. 1997;71:6455–6464. doi: 10.1128/jvi.71.9.6455-6464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tognon M, Furlong D, Conley A J, Roizman B. Molecular genetics of herpes simplex virus. V. Characterization of a mutant defective in ability to form plaques at low temperatures and in a viral function which prevents accumulation of coreless capsids at nuclear pores late in infection. J Virol. 1981;40:870–880. doi: 10.1128/jvi.40.3.870-880.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ugolini G. Transneuronal tracing with alpha-herpesviruses: a review of the methodology. In: Kaplitt M G, Loewy A D, editors. Viral vectors. Gene therapy and neuroscience applications. San Diego, Calif: Academic Press; 1995. pp. 293–318. [Google Scholar]
- 44a.U.S. Department of Health and Human Services. Biosafety in microbiological and biomedical laboratories. Publication no. 88-8395. U.S. Washington, D.C: Department of Health and Human Services; 1988. [Google Scholar]
- 45.Whealy M E, Card J P, Robbins A K, Dubin J R, Rziha H-J, Enquist L W. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol. 1993;67:3786–3797. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang M, Card J P, Tirabassi R S, Miselis R R, Enquist L W. Retrograde, transneuronal spread of pseudorabies virus in defined neuronal circuitry of the rat brain is facilitated by gE mutations that reduce virulence. J Virol. 1999;73:4350–4359. doi: 10.1128/jvi.73.5.4350-4359.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zemanick M C, Strick P L, Dix R D. Direction of transneuronal transport of herpes simplex virus 1 in the primate motor system is strain-dependent. Proc Natl Acad Sci USA. 1991;88:8048–8051. doi: 10.1073/pnas.88.18.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zsak L, Zuckermann F, Sugg N, Ben-Porat T. Glycoprotein gI of pseudorabies virus promotes cell fusion and virus spread via direct cell-to-cell transmission. J Virol. 1992;66:2316–2325. doi: 10.1128/jvi.66.4.2316-2325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]