Abstract
The granule cells (GCs) of the dentate gyrus transiently express markers of the GABAergic phenotype early during development. However, GCs are generated throughout life, posing the question of whether the newborn neurons in the adult rodent recapitulate the development of the neurotransmitter phenotype of GCs generated during embryonic and early postnatal development. In this work we asked whether newborn GCs transiently express a GABAergic phenotype during their development in the adult rat. Using retroviral infection, we labeled dividing cells in the dorsal hippocampus with GFP, identified them as granule cells, and determined their expression of GABAergic markers at different developmental stages. We found that GFP-positive cells express Prox-1 and calbindin, identifying them as GCs. GABA or GAD67 was expressed in 13% of GFP-positive cells at 7 dpi, in 16% at 10 dpi and in 20% at 15 dpi. At 30 dpi, however, no GFP-positive cell somata containing GABAergic markers were detected, but their mossy fiber boutons did contain GAD67. Interestingly, developing GCs detected with doublecortin and PSA-NCAM in non-injected adult rats, did not express GABAergic markers, suggesting that retroviral injection/infection stimulates their transient expression. However, in non-injected rats, a number of mossy fiber boutons of newborn granule cells detected with PSA-NCAM did express GAD67. Our findings reveal that developing GCs born in the adult are able to transiently up-regulate the expression of GABAergic markers to be detected in their soma in response to insults, while they constitutively express GAD67 in their mossy fibers.
Keywords: Neurogenesis, Granule cells, Dentate gyrus, GAD, GABA, Prox-1, GFP, Doublecortin, PSA-NCAM
Introduction
The granule cells (GCs) of the dentate gyrus (DG), which constitute a glutamatergic projection to the hippocampus in the adult rodent, are known to transiently express markers of the GABAergic phenotype during their postnatal development (Gutiérrez et al., 2003; Safiulina et al., 2006). These markers disappear on completion of development but can be reexpressed by enhanced excitability (Gutiérrez, 2005). Therefore, besides expressing the markers of the glutamatergic phenotype (glutamate, VGlut-1), they express GABA, GAD67, VGAT (Gómez-Lira et al., 2005; Gutiérrez et al., 2003; Lamas et al., 2001; Zander et al., 2010). Interestingly, during the first 4–6 days of life, their activation produces only GABAA-R mediated responses in their post-synaptic target cells (Safiulina et al., 2006), after which time the same activation produces simultaneous glutamate-R- and GABA-R mediated responses until completion of development (Gutiérrez et al., 2003; Walker et al., 2001). Thus, in the GCs of adult rats, the GABAergic markers are down-regulated and GABAergic transmission disappears, and their stimulation produces exclusively glutamatergic responses (Gómez-Lira et al., 2005; Gutiérrez et al., 2003; Lamas et al., 2001; Safiulina et al., 2006).
Granule cells are generated throughout life, i.e., they are born in the adult rat and their physiological characteristics during their development are parallel to those described for GCs born in the embryonic period or early in the post-natal rat (Espósito et al., 2005). Although evidence has appeared suggesting that newborn GCs in the adult rat do not release GABA (Toni et al., 2008), there is no systematic analysis of their neurotransmitter phenotype to date. It is known that epileptic seizures up-regulate GAD67 and GABA in the GCs and their mossy fibers (for a review see Gutiérrez, 2005), and that neurogenesis is also up-regulated (Parent et al., 1997). Interestingly, single seizures produce a strong up-regulation of GAD67 in cells located in the infragranular layer of the DG, where most of GCs born in the adult are located (Ramírez and Gutiérrez, 2001), which suggested that GCs born in the adult can express a GABAergic phenotype.
The fact that many GCs express GABAergic markers during development, and that seizures up-regulate them in the adult, suggests that developing GCs born in the adult rat may express them as well. In this work we assessed the expression of GAD67 and GABA in newborn GCs, identified by their expression of GFP, which was induced by retroviral infection. This analysis was carried out at 7, 10, and 15 days post-injection (dpi), as this is the critical period at which many GCs express the markers of the GABAergic phenotype (Gutiérrez et al., 2003) and at 30–35 dpi, when the newborn GCs are fully developed and integrated to the circuit (Ge et al., 2008).
Materials and methods
Viral vector production
We used a retroviral vector based on the Moloney murine leukemia virus to express enhanced GFP driven by a CAG promoter. Retroviral particles were assembled using three separate plasmids containing the capside (CMV-vsvg), viral proteins (CMV-gag/pol) and transgene (CAG-GFP) (Tashiro et al., 2006). These were a gift from F.H. Gage (Salk Institute, La Jolla, CA). Plasmids were transfected to 293T cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The virus-containing supernatant was harvested 48 h after transfection, passed through a 0.22 μm filter and concentrated by two rounds of ultracentrifugation (Tashiro et al., 2006). Expression titers were estimated on 293T cells by serial dilutions. Titers ranged from 5×107 cfu/ml to 3×108 cfu/ml.
Animals and stereotaxic surgery
For our studies we used 30 male Sprague Dawley rats (150–170 g) of 6 to 8 weeks of age; 23 of them were injected with the retrovirus, 3 were injected with PBS (at dpi 15) and 4 were used as control, non-injected rats. They were anesthetized (100 mg/kg ketamine and 5 mg/kg xylazine) and the virus was infused (1 μl at 0.1 μl/min) into the right dorsal DG (anteroposterior, −2.7; mediolateral, −1.7; dorsoventral, −3.4; Fig. 1A). A Hamilton syringe (26s-gauge needle with point style 4; Hamilton Co., Reno NV, USA) was used for all injections.
Fig. 1.
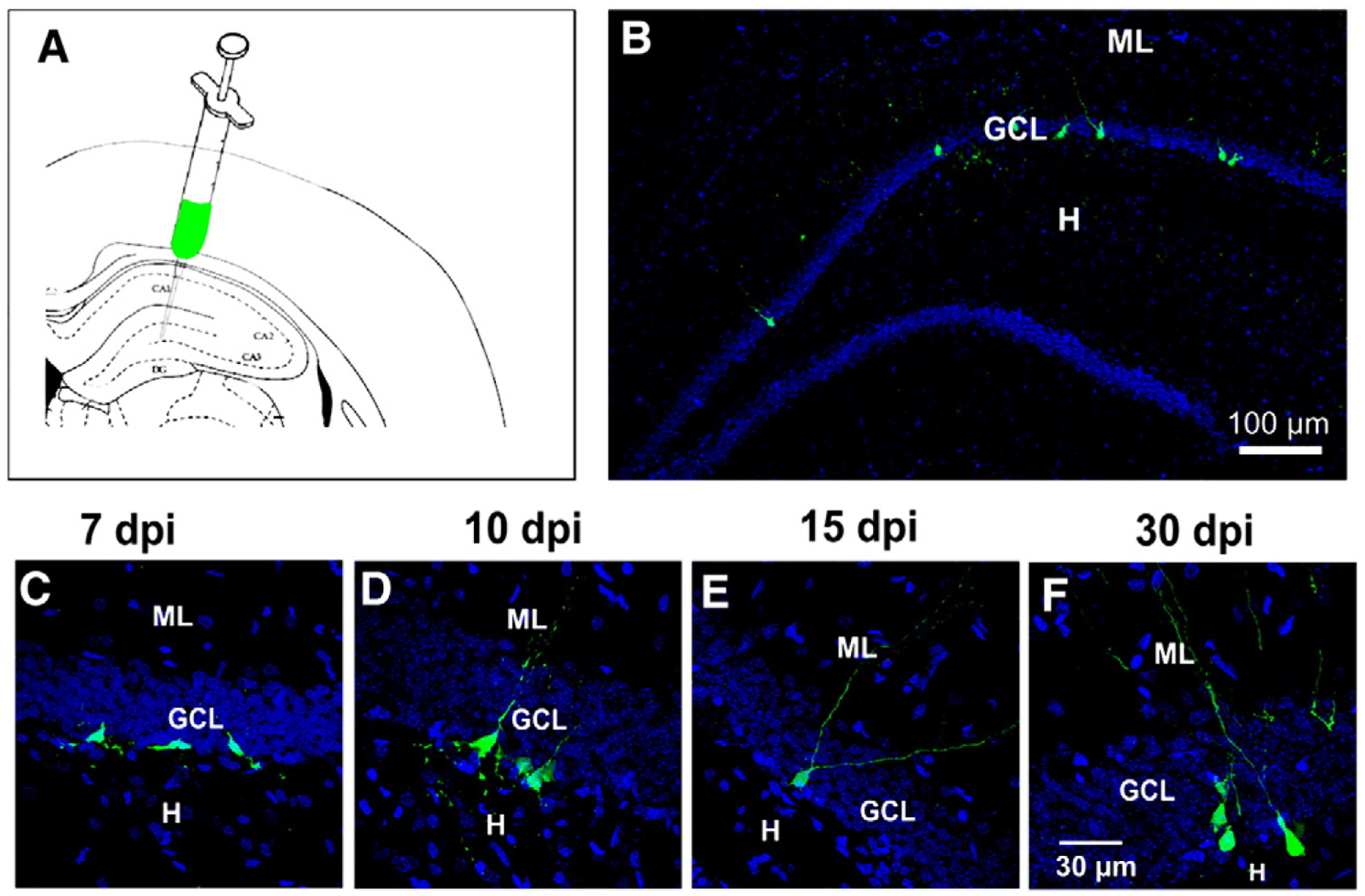
Newborn cells were detected in adult rats by retroviral infection. A) Schematic view of the hippocampus depicting the site of injection of the retrovirus. B) Panoramic view of a dentate gyrus with GFP+ cells in the inner granule cell layer at 15 dpi. Confocal images showing the morphology of GFP+ cells at the post-injection times analyzed: C) at 7, D) at 10, E) at 15 and F) at 30 dpi. DAPI is shown in blue. ML, molecular layer; GCL, granule cell layer; H, hilus. Calibration in F applies to panels C–E.
Immunohistochemistry
The animals were anesthetized and transcardially perfused with phosphate buffer (PB) 0.1 M, pH 7.4 followed by 4% paraformalde-hyde (PFA). Rats injected with the retrovirus were prepared at 7, 10, 15 and 30 dpi. Brains were post-fixed overnight in 4% PFA and then transferred into 30% sucrose solution. Serial 30 μm coronal sections of the brain were cut using a cryostat (Leica CM1510, Leica Microsystems, Wetzlar, Germany), and for our immunostaining analysis we selected one every 3 sectioned slices. Sections were incubated with BSA 5% in PBS Triton X-100 0.3%, for 2 h at room temperature and further incubated with primary antibodies at 4 °C overnight in PBS triton X-100 0.3% and BSA 1%. We used the following antibodies: chicken anti-GFP (1:1000, Abcam, Cambridge, USA), rabbit anti-Prox1 (1:1000, Chemicon, Temecula, CA), mouse anti-GAD67 (1:1000, Chemicon), rabbit anti-GABA (1:1000, Sigma Aldrich, St Louis, MO, USA), rabbit anti-Calbindin D-28K (1:200, Chemicon), rabbit anti-doublecortin (DCX; 1:1000, Abcam) and mouse anti-PSA-NCAM (1:500, Chemicon). As negative controls, slices were simultaneously processed in the absence of the primary antibody. After rinsing 3 times in PBS Triton X-100 0.3%, the slices were incubated with secondary antibodies for 2 h at room temperature. They were: donkey anti-chicken FITC (1:100), goat anti-mouse TRITC (1:100), goat anti-rabbit TRITC (1:100), goat anti-rabbit Cy5 (1:100) and goat anti-mouse CY5 (1:100; all from Jackson Immunoresearch, West Grove, PA). Finally, the slices were rinsed 3 times in PBS and mounted in slides using Vectashield with DAPI mounting medium (Vector, Burlingame, CA). All analyses were performed in double or triple-stained sections (GFP, Prox-1 and GAD67/GABA; GFP, Calbindin D-28K and GAD67/GABA; GFP and DCX; GFP, DCX and GAD67; DCX and GAD67; GFP and PSA-NCAM; PSA-NCAM and GAD67) using an epifluorescent microscope (Axio Scope, Carl Zeiss) and analyzed with the software Axio Vision V4.8 (Carl Zeiss, Goettingen, Germany). Selected sections were analyzed with a confocal microscope (Leica TCS-SPE) and the software Leica LAS AF Lite (Leica Microsystems Wetzlar, Germany). Colocalization was assessed in three-dimensional reconstructions through the entire Z-axis of each cell GFP+, using consecutive optical slices at 0.5–1 μm intervals. Cells were counted by eye in one every 3 slices from the ipsilateral side of the injection at 7, 10, 15 and 30 days post-retrovirus injection. Each experiment was performed at least in three different animals.
Electrophysiology
For electrophysiological recordings, 3 rats were injected with the virus in the dorsal hippocampus, as previously described. At 14 dpi, rats were decapitated under profound pentobarbital anesthesia and 300 μm thick slices were cut in a vibroslicer (Leica VT 1200, Wetzlar, Germany) submerged in sucrose-based cutting solution containing (in mM): 87 NaCl, 25 NaHCO3, 25 glucose, 75 sucrose, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2 and 4 MgCl2 equilibrated with 95% O2–5% CO2, at 4 °C. Recordings were conducted with the patch clamp technique in the whole-cell mode, on the stage of a bright field/fluorescence microscope (Eclipse E600FN; Nikon, Tokyo). Putative fluorescent newborn GCs and adult GCs were recorded in the whole cell patch-clamp configuration in current clamp using borosilicate pipettes pulled to yield 4–6 MΩ (Flaming-Brown P-97 puller; Sutter Instrument Company, Novato, CA) and filled with K-gluconate intracellular solution (in mM: 133 K-gluconate, 7 KCl, 1.5 MgCl2, 4 ATP-Mg, 0.5 GTP-Na, 0.5 EGTA, 10 HEPES, pH 7.2 with KOH). The artificial cerebrospinal fluid used contained (in mM): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 1 MgCl2, 2 CaCl2, and 25 glucose, and was constantly oxygenated. The whole-cell signals were recorded with a Multiclamp 700B amplifier (Molecular Devices, Palo Alto, CA), filtered at 5 kHz, digitized, acquired (Digidata 1440A; Molecular Devices), and off-line analyzed with the pClamp10.1 program (Molecular Devices).
Results
We determined the number of GFP-expressing (GFP+) newborn cells at 7, 10 and 15 dpi (Fig. 1B), which constitutes the critical period during which GABAergic markers are expressed in GCs of developing rats (Gutiérrez et al., 2003), and after 30–35 dpi, when newborn cells are completely developed. Therefore, as markers of the maturity of the newborn cells, DCX, calbindin and PSA-NCAM cells were determined in the GFP+ cells (Rao and Shetty, 2004; Snyder et al., 2009). Newborn, GFP+ cells were located in the subgranular and inner third of the granular layer of the DG (Fig. 1B), with morphological (Figs. 1C–F) and electrophysiological characteristics (Fig. 4I) that correspond to the developmental stages studied, as reported in Espósito et al. (2005).
Fig. 4.
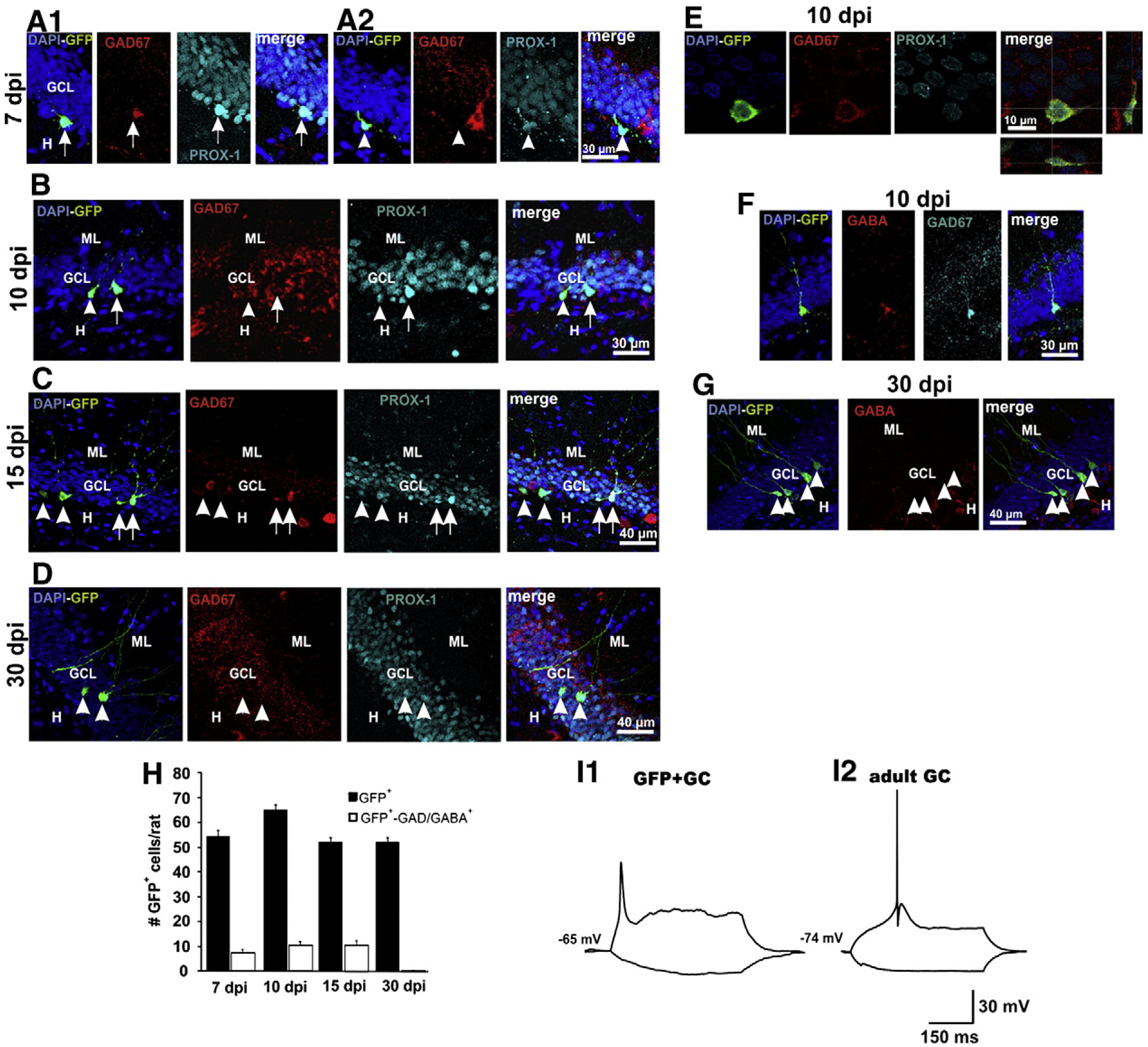
Expression of GABAergic markers in GCs born in the adult rat. A1) Confocal images at 7 dpi showing a GFP+ granule cell expressing GAD67 (arrow) and A2) a granule cell that does not express GAD67 (arrowhead). A interneuron can be observed beside the GFP+ granule cell. B and C) Images depicting newborn GCs at 10 and 15 dpi, respectively, which express (arrows) and do not express (arrowheads). D) At 30 dpi, no GFP+ granule cell expressed . E) Three-dimensional reconstructions through the Z-axis of GFP+ cells (fifty optical slices at 0.5 μm intervals) were used to verify the co-localization of GFP, GAD67 and Prox1. DAPI, blue; GAD67 Red; Overlay with Prox1, in light blue, and orthogonal projections are shown on the right panel. F) Confocal images depicting the coexistence of GAD67 and GABA in a GFP+ granule cell at 10 dpi. G) At 30 dpi, no GFP+ granule cell expressed GABA. Calibrations in the rightmost panels apply for all panels in the corresponding row. ML, molecular layer; GCL, granule cell layer; H, hilus. H) Mean number of GFP+ GCs per rat (black bars) and number of GFP+ GCs expressing GAD67/GABA at the different postinjection times (n=5/bar). I) Electrophysiological recordings of a developing GFP+ granule cell at 14 dpi and of an adult granule cell.
We counted the number of GFP+ cells in one every 3 slices along the hippocampus (n=5 rats at each post-infection time) and found 54.7±2.3, 65.3±2.1, 52.4±1.7 and 52.7±1.7 cells per rat, at 7, 10, 15 and 30 dpi, respectively. We confirmed the expression of DCX/PSA-NCAM and calbindin in 70% and 30% of the GFP+ cells (at 10 dpi), respectively, identifying them as neurons (Figs. 2A–C). These results agree with previous reports (Espósito et al., 2005; Piatti et al., 2011; Snyder et al., 2009). Eight GFP+ cells at 12–14 dpi were randomly selected to electro-physiologically verify their neuronal phenotype. Contrary to mature, embryonically generated GCs, their high Rinput, low Cm and the low amplitude and wide action potentials, evoked by depolarizing the membrane potential, identify the recorded cells as immature GCs (Fig. 4I; Espósito et al., 2005). The electrophysiological characteristics of the GFP+ and adult GCs are summarized in Table 1.
Fig. 2.
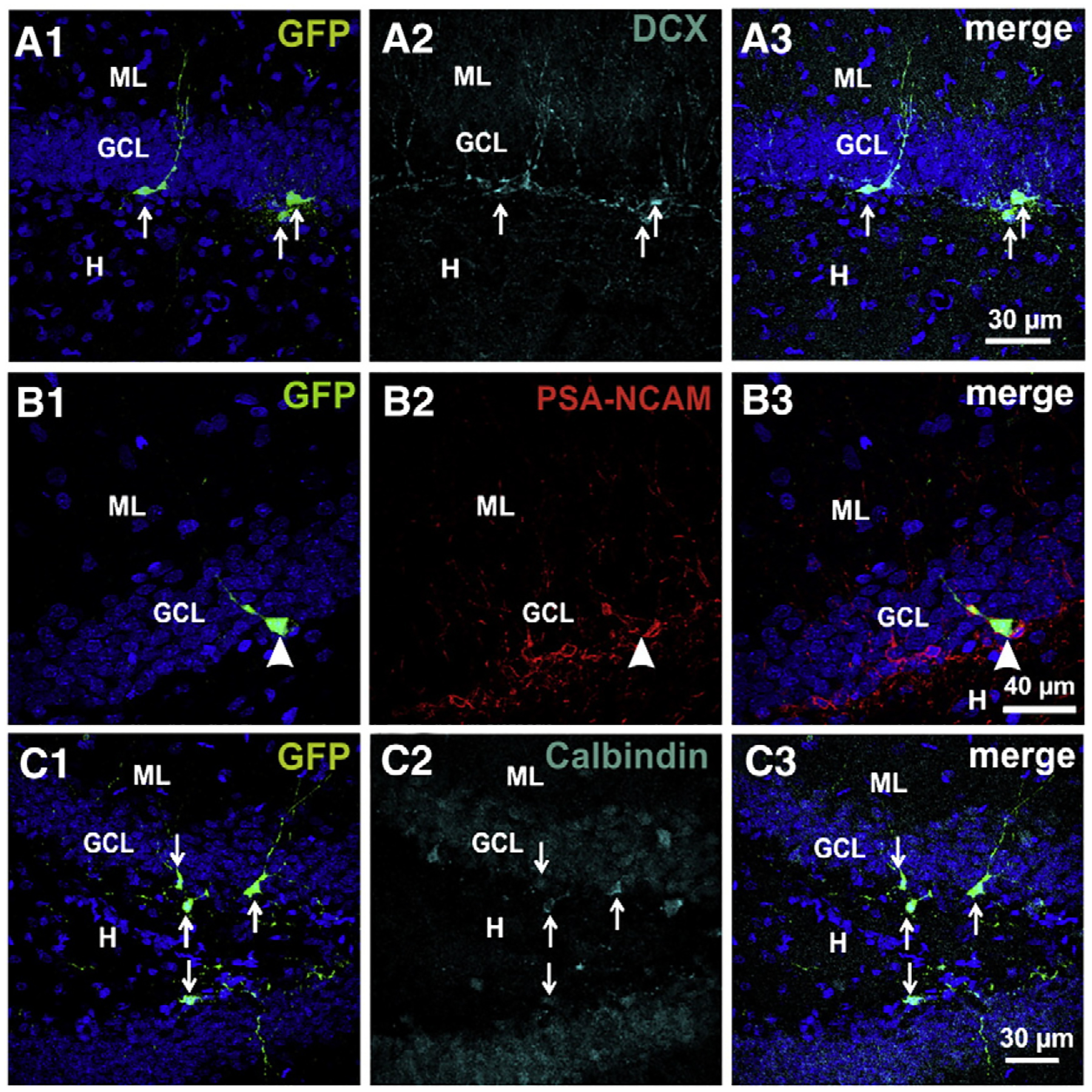
GFP+ cells are newborn granule cells. Confocal images showing that developing GCs (all examples at 10 dpi) express DCX (A), PSA-NCAM (B) and calbindin (C).
Table 1.
Electrophysiological properties of newborn and adult granule cells.
| GFP+ | Adult | |
|---|---|---|
| V rest (mV) | −64.7±1.0 | −74.5±1.4 |
| R input (GΩ) | 2.8±0.1 | 0.43±0.01 |
| C m (pF) | 23.6±1.6 | 43.6±1.9 |
| AP amp (mV) | 40.4±1.6 | 80.4±2.9 |
| AP slope (mV/ms) | 18.4±4.2 | 171.8±36.8 |
| AP half duration (ms) | 5.5±1.3 | 1.32±0.1 |
| Tau | 74.7±4.4 | 31.1±3.5 |
| Morphological classa | C | D |
| Position in the GCLa | 1–2 | 1–3 |
Values are mean±s.e.m.; n=8 cells.
Classification of Espósito et al. (2005).
Because our goal was to assess the expression of GABAergic markers in developing newborn GCs, and because these GABAergic markers could be expressed in interneurons containing GFP, we first sought to identify the GFP+ cells as GCs by their expression of prospero-related homeobox 1 gene (Prox1), a transcription factor specifically expressed in these cells and that controls its generation (Karalay et al., 2011; Pleasure et al., 2000). With this analysis, we determined that all GFP+ cells were Prox1-positive at the different post-infection times, identifying them as GCs (Figs. 3A–D). Therefore, we next determined the expression of the GABAergic markers GAD67 and/or GABA in identified GFP+–Prox1+ GCs. By conducting a double fluorescent labeling of already-labeled GFP+ cells, against Prox1 and GAD67 and/or GABA, we could confidently determine that from the total of identified GFP+ GCs analyzed from 5 rats at each post-infection time, 30 out of 219 (13%) at 7 dpi, 64 out of 392 (16%) at 10 dpi, and 54 out of 262 (20%) at 15 dpi, expressed GAD67/GABA (Fig. 4H). Figs. 4(A–E) depict examples of GFP+–Prox1+ cells that express and that do not express the GABAergic markers at all post-infection times. To corroborate the correct determination of the GABAergic phenotype, we verified in 3 preparations that GFP+ cells that expressed GAD67 also expressed GABA, which was the case (Fig. 4F). Interestingly, at 30 dpi no GFP+ granule cell (out of 265) was found to express either GAD67 or GABA (Figs. 4G, H). However, because the mossy fibers normally express GAD67 (for a review see Gutiérrez, 2005), we analyzed the expression of the enzyme in the mossy fibers of preparations at 15 and 30 dpi, when GFP+ cells already extend their axons to CA3 area. In agreement with previous reports, the stratum lucidum of CA3 was immunoreactive to GAD67 (Fig. 5A1). We were able to find 7 GFP+ axons with giant boutons, identifying them as mossy fibers (Fig. 5A2), all of which had GAD67 in their characteristic giant boutons (Fig. 5B).
Fig. 3.
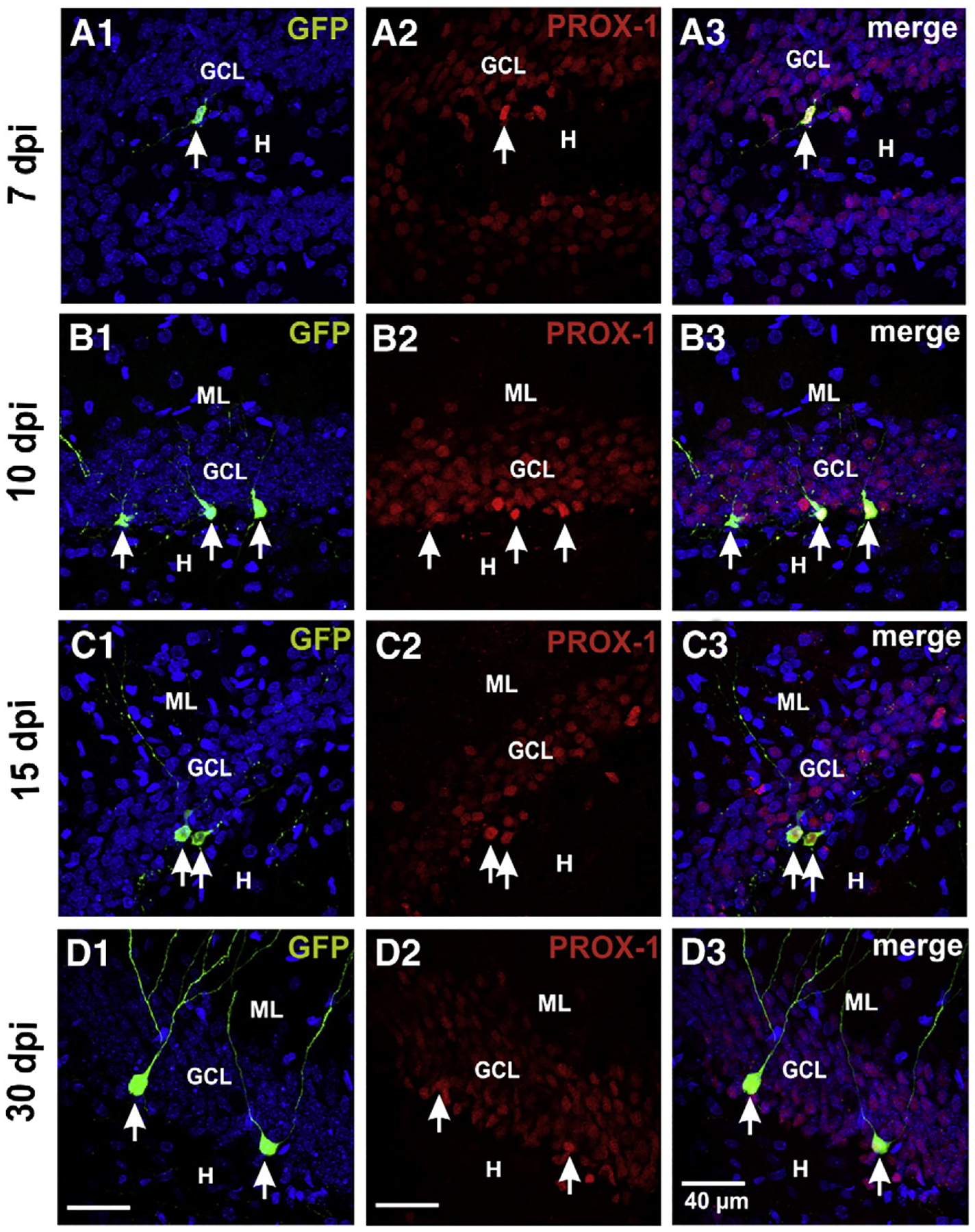
Newborn cells were positively identified as GCs by their expression of Prox1, at 7 (C), 10 (D), 15 (E) and 30 dpi (F). ML, molecular layer; GCL, granule cell layer; H, hilus. Arrows signal the newborn GCs.
Fig. 5.
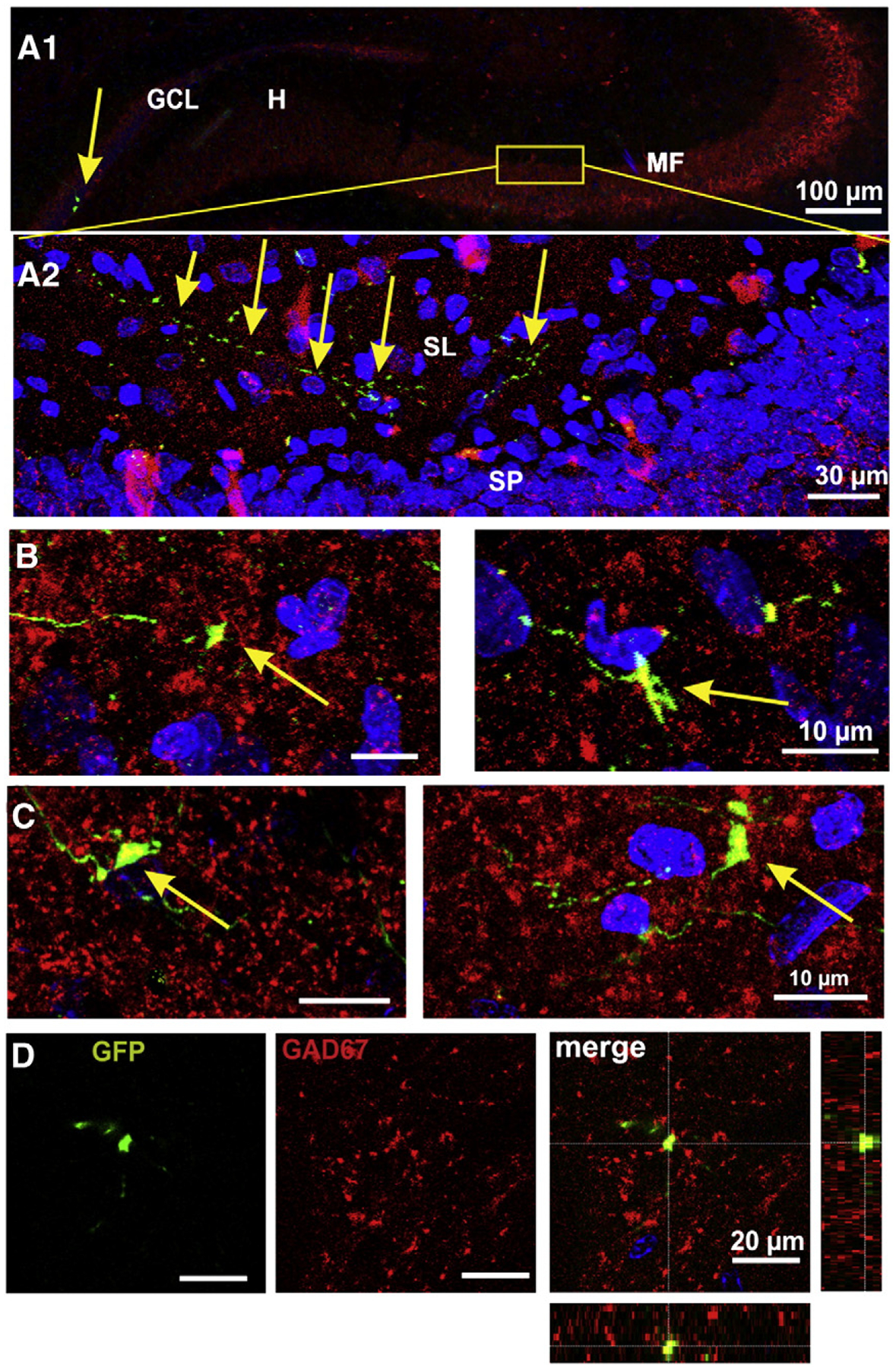
The mossy fibers express GAD67. A1) Panoramic view of the mossy fiber projection showing, on the left hand, two GFP+ GCs (arrow) and mossy fibers. The area shown at high magnification in A2 is signaled. A2) Confocal image of an axon of a GFP+ granule cell is shown along the stratum lucidum of CA3. B) Confocal images (50 optical slices of 0.5 μm) of mossy fiber giant boutons expressing GAD67 at 15 dpi. C) Mossy fiber boutons of GFP+ GCs at 30 dpi, did express GAD67 enzyme. D) Three-dimensional reconstructions through the Z-axis of GFP+ boutons (fifty optical slices at 0.5 μm intervals) were used to verify the co-localization of GFP and GAD67. Orthogonal projections are shown on the right most panel.
The transient somatic expression of GAD67/GABA in the aforementioned experiments could be due to either a developmental trait or to the insult that constitutes the injection/infection of the dividing cells with the retrovirus. Therefore, in 4 non-injected rats we conducted double labeling against GAD67 or GABA, and DCX and PSA-NCAM, which are known to identify developing cells. Surprisingly, contrary to DCX+ cells from injected rats, where co-localization of DCX and GAD was evidenced (Fig. 6A), in non-injected rats no DCX+ or PSA-NCAM+ cell expressed GABAergic markers (Fig. 6B), suggesting that the injection of the retrovirus induced the expression of GABAergic markers in developing newborn cells. However, although the somata of GFP+ cells at 30 dpi did not express GABAergic markers, we confirmed that their giant mossy fiber boutons contained GAD67 (Figs. 5C, D). Because the injection of the retrovirus implies a mechanical damage and possibly inflammation of the tissue (Breunig et al., 2008; Zhao, 2008), we investigated whether the injection of PBS alone was able to induce the expression of GABAergic markers. In rats injected with PBS, as with the retroviral infection, we found some PSA-NCAM+ positive cells expressing GAD67 (Fig. 6C), suggesting that the injection itself is able to activate the expression of GABAergic markers. Further, because PSA-NCAM also stains mossy fibers from developing GCs, we could corroborate that some boutons also expressed GAD67, as shown to happen in all the other experimental conditions (Fig. 6D).
Fig. 6.
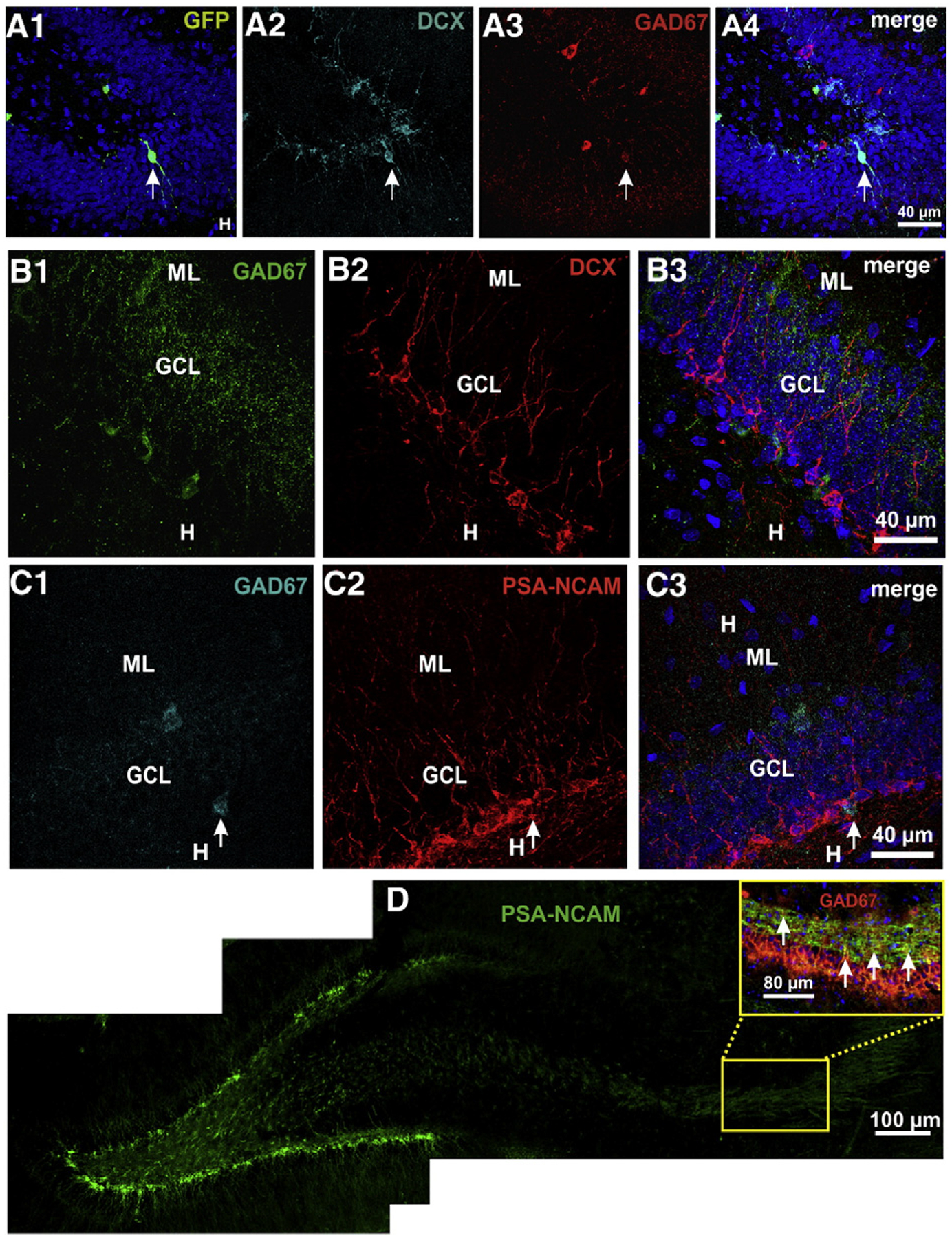
Newborn GCs of injected rats, both with retrovirus and PBS, but not of control rats express GAD in their somata, while all express GAD67 in their mossy fibers. A) GFP+ cell co-expressing DCX and GAD67. B) Developing cells in non-injected adult rats, detected by their expression of doublecortin (DCX), did not express GAD67. C) Some developing cells, detected by their expression of PSA-NCAM, expressed GAD67 in PBS injected rats. D) In control, non injected rats, a number of PSA-NCAM+ mossy fibers expressed GAD67.
Discussion
It has been described that the GCs of the DG transiently express a GABAergic phenotype during development, whereby the markers of this phenotype, GAD67, GABA and the vesicular GABA transporter can be detected at varying degrees throughout the development (Gómez-Lira et al., 2005; Gutiérrez et al., 2003; Zander et al., 2010). Moreover, during this period, stimulation of the mossy fibers produces monosynaptic GABA-R-mediated fast responses in their target cells (Gutiérrez et al., 2003; Kasyanov et al., 2004; Walker et al., 2001). Further, recent evidence demonstrates that some mossy fiber boutons co-release glutamate and GABA, while others only release glutamate or GABA. In the adult rat, however, boutons release exclusively glutamate (Beltran and Gutierrez, 2012). Considering this, we hypothesized that developing GCs born in the adult rat may recapitulate the transient expression of the GABAergic phenotype of GCs that occurs in the developing rat. This is important because the integration of GCs born in the adult rat impact on the modulation of hippocampal circuits, which participate in cognitive hippocampal-dependent tasks (reviewed by Marín-Burgin et al., 2012; Ming and Song, 2011).
In this work, we analyzed the expression of GAD67 and GABA in newborn GCs identified by their expression of GFP after retroviral infection. The number of GFP+ cells that we detected is in agreement with the efficiency of the viral infection reported by several studies (Espósito et al., 2005; Piatti et al., 2011). The granule cell phenotype of the GFP+ cells was confirmed by their expression of Prox1, which is a transcription factor specifically expressed in GCs but not in interneurons (Pleasure et al., 2000); moreover, they had the morphological and electrophysiological characteristics of developing GCs (Espósito et al., 2005).
We determined that 15–20% of developing GFP+ GCs expressed GAD67/GABA, while the adult, GFP+ GCs did not express these markers at 30 dpi. However, while these results refer to the somatic expression of the GABAergic markers, we observed that GFP+ mossy fiber boutons expressed GAD67 at all ages. There may be several possible reasons why only this percentage of developing GFP+ cells expresses GAD67, while adult GFP+ cells do not. First: a developmental trait. It has been shown that many, but not all GCs born perinatally express the GABAergic markers in their somata (Gutiérrez et al., 2003; Maqueda et al., 2003). Despite this, their stimulation provoke almost exclusively GABA-mediated responses in their post-synaptic target cells, during the first postnatal days (Safiulina et al., 2006), and mixed glutamatergic–GABAergic responses until completion of development (Gutiérrez et al., 2003; Walker et al., 2001), when they become exclusively GABAergic (Gutiérrez, 2000; Gutiérrez et al., 2003). Second, GCs are born continuously but different cohorts of newborn can be found at a given time (Mathews et al., 2010; Piatti et al., 2011). It is possible that some cohorts do express GAD67 (as found in developing rats; Gutiérrez et al., 2003) and they may be differentially integrated to circuits. Indeed, Mathews et al. (2010) proposed that although early and adult born cells are not different from each other, they might be recruited to different networks. We can hypothesize, that the GAD67-expressing population may be distributed into such networks to serve a distinctive function, which is still to be determined. In this regard, the possible existence of reserve pools of neurons that can respecify their neurotransmitter phenotype (Dulcis and Spitzer, 2011), could apply to newborn GCs that can be recruited into dynamic, existing circuits.
Noteworthy, while GFP+ granule cell somata may not express GAD67/GABA, we found that their mossy fibers did express GAD67, as has been shown to occur normally in developing and adult mossy fibers (see also Gutiérrez et al., 2003; Ramírez and Gutiérrez, 2001; Sloviter et al., 1996). Third: injection/infection induced expression of GABAergic markers. In our experiments using DCX and PSA-NCAM to identify developing GCs in non-injected rats, we found that none of these cells expressed GABAergic markers in their somata, while GAD was present in their mossy fiber boutons.
This may suggest that the injection constitutes an insult to which a number of GCs react by transiently expressing the GABAergic. This insult-induced expression, however, was not observed in the surrounding adult GCs, which did not express GAD67 or GABA. Therefore, it can be hypothesized that developing GCs in the adult rat are more prone to express markers of the GABAergic phenotype after an insult. Indeed, it is recognized that the injection of retrovirus per se causes a lesion and inflammation (Breunig et al., 2008; Zhao, 2008). Moreover, it has been shown that newborn GCs overexpress GAD67 after seizures (Jiang et al., 2004) and that newborn GCs in culture conditions can express a GABAergic phenotype in a manner dependent on activity, and by kainate or BDNF exposure (Babu et al., 2007), as previously described to occur in adult GCs (Gómez-Lira et al., 2005). Therefore, our data suggest that the retroviral infection is not the cause of the expression of GABAergic markers, as a number of newborn GCs from rats injected with PBS also expressed GAD67 or GABA. On the other hand, newborn GCs in non-injected rats that were detected by DCX or PSA-NCAM did not express the GABAregic markers. Thus, provided that appropriate injection controls are considered, the use of the retroviral infection is well suited for the study of the neurotransmitter phenotype of the infected neurons.
Whether GCs containing GAD67 in their mossy fibers are able to release GABA is still unknown. There is evidence showing that newborn GCs do not release GABA (Toni et al., 2008), but recent evidence has established that some identified mossy fiber boutons of developing GCs do co-release glutamate and GABA, while others release only glutamate (Beltran and Gutierrez, 2012), opening the possibility of having some cells that can release GABA. Different cohorts of cells participate in the integration of incoming inputs from the cortex in a differential manner (Marín-Burgin et al., 2012). Insults, like seizures, besides increasing the number of newborn GCs (Parent et al., 1997), can up-regulate the expression of a GABAergic phenotype in existing ones or turn on the GABA-releasing machinery (they constitutively express GAD67 in their mossy fibers) converting them into GABA-releasing cells. In this way, they may contribute to dampen excitability in the microcircuit to which they belong, as it has been described that newborn GCs receive enhanced excitatory drive after seizures (Wood et al., 2011).
Acknowledgments
The authors wish to thank Paula Vergara, Beatriz Osorio, and Benjamín Munoz for excellent technical assistance, Dr. F.H. Gage for providing us with the plasmids for retroviral construction, and A. Schinder and S. Jessberger for constructive discussions. This study was supported by grants 45754 and I020/193/10 FON. INST.-29-10 to R.G. from the Consejo Nacional de Ciencia y Tecnología.
References
- Babu H, Cheung G, Kettenmann H, Palmer TD, Kempermann G, 2007. Enriched monolayer precursor cell cultures from micro-dissected adult mouse dentate gyrus yield functional granule cell-like neurons. PLoS One 4 (e388), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran JQ, Gutierrez R, 2012. Co-release of glutamate and GABA from single, identified mossy fiber giant boutons. J. Physiol 10.1113/jphysiol.2012.236372 (Published online before print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig JJ, Rakic P, Mackls JD, 2008. Evolving methods for the labeling and mutation of postnatal neuronal precursor cells: a critical review. In: Gage FH, Kempermann G, Song H (Eds.), Adult Neurogenesis. Cold Spring Harbor Laboratory Press, New York, pp. 49–80. [Google Scholar]
- Dulcis D, Spitzer NC, 2011. Reserve pool neuron transmitter respecification: novel neuroplasticity. Dev. Neurobiol 72, 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF, 2005. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J. Neurosci 25, 10074–10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Sailor KA, Ming G, Song H, 2008. Synaptic integration and plasticity of new neurons in the adult hippocampus. J. Physiol 586, 3759–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Lira G, Lamas M, Romo-Parra H, Gutiérrez R, 2005. Programmed and induced phenotype of the hippocampal granule cells. J. Neurosci 25, 6939–6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez R, 2000. Seizures induce simultaneous GABAergic and glutamatergic neuro-transmission in the dentate gyrus-CA3 system. J. Neurophysiol 84, 3088–3090. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R, 2005. The dual glutamatergic–GABAergic phenotype of the hippocampal granule cells. Trends Neurosci. 28, 297–303. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R, Romo-Parra R, Maqueda J, Vivar C, Ramírez M, Morales MA, Lamas M, 2003. Plasticity of the GABAergic phenotype of the “glutamatergic” granule cells of the rat dentate gyrus. J. Neurosci 23, 5594–5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Wang J-C, Zhang Z, Sheerin AH, Zhang X, 2004. Response of seizure-induced newborn neurons in the dentate gyrus of adult rats to second episode of seizures. Brain Res. 1007, 248–252. [DOI] [PubMed] [Google Scholar]
- Karalay O, Doberauer K, Vadodaria KC, Knobloch M, Berti L, Miquelajauregui A, Schwark M, Jagasia R, Taketo MM, Tarabykin V, Lie DC, Jessberger S, 2011. Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc. Natl. Acad. Sci 108, 5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasyanov AM, Safiulina VF, Voronin LL, Cherubini E, 2004. GABA-mediated giant depolarizing potentials as coincidence detectors for enhancing synaptic efficacy in the developing hippocampus. Proc. Natl. Acad. Sci 16, 3967–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas M, Gómez-Lira G, Gutiérrez R, 2001. Vesicular GABA transporter mRNA expression in the dentate gyrus and in mossy fibre synaptosomes. Mol. Brain Res 93, 209–214. [DOI] [PubMed] [Google Scholar]
- Maqueda J, Ramírez M, Lamas M, Gutiérrez R, 2003. Glutamic acid decarboxylase (GAD)67, but not GAD65, is constitutively expressed during development and transiently overexpressed by activity in the granule cells of the rat. Neurosci. Lett 353, 69–71. [DOI] [PubMed] [Google Scholar]
- Marín-Burgin A, Mongiat LA, Pardi MB, Schinder AF, 2012. Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science 335, 1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews EA, Morgenstern NA, Piatti VC, Zhao C, Jessberger S, Schinder AF, Gage FH, 2010. A distinctive layering pattern of mouse dentate granule cells is generated by developmental and adult neurogenesis. J. Comp. Neurol 518, 4479–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G-L, Song H, 2011. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH, 1997. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci 17, 3727–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti VC, Davies-Sala MG, Espósito MS, Mongiat LA, Trinchero MF, Schinder AF, 2011. The timing of neuronal maturation in the adult hippocampus is modulated by local network activity. J. Neurosci 31, 7715–7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasure SJ, Collins AE, Lowenstein DH, 2000. Unique expression patterns of cell fate molecules delineate sequential stages of dentate gyrus development. J. Neurosci 20, 6095–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez M, Gutiérrez R, 2001. Activity-dependent expression of GAD67 in the granule cells of the rat hippocampus. Brain Res. 917, 139–146. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK, 2004. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur. J. Neurosci 19, 234–246. [DOI] [PubMed] [Google Scholar]
- Safiulina VF, Fattorini G, Conti F, Cherubini E, 2006. GABAergic signaling at mossy fiber synapses in neonatal rat hippocampus. J. Neurosci 26, 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS, Dichter MA, Rachinsky TL, Dean E, Goodman JH, Sollas AL, Martin DL, 1996. Basal expression and induction of glutamate decarboxylase and GABA in excitatory granule cells of the rat and monkey hippocampal dentate gyrus. J. Comp. Neurol 373, 593–618. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA, 2009. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J. Neurosci 18, 14484–14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Zhao C, Gage FH, 2006. Retrovirus-mediated single-cell gene knockout technique in adult newborn neurons in vivo. Nat. Protoc 1, 3049–3055. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF, 2008. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat. Neurosci 11, 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MC, Ruiz A, Kullmann DM, 2001. Monosynaptic GABAergic signaling from dentate to CA3 with a pharmacological and physiological profile typical of mossy fiber synapses. Neuron 29, 703–715. [DOI] [PubMed] [Google Scholar]
- Wood JC, Jackson JS, Jakubs K, Chapman KZ, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O, 2011. Functional integration of new hippocampal neurons following insults to the adult brain is determined by characteristics of pathological environment. Exp. Neurol 229, 484–493. [DOI] [PubMed] [Google Scholar]
- Zander JF, Münster-Wandowski A, Brunk I, Pahner I, Gómez-Lira G, Heinemann U, Gutiérrez R, Laube G, Ahnert-Hilger G, 2010. Synaptic and vesicular coexistence of VGLUT and VGAT in selected excitatory and inhibitory synapses. J. Neurosci 30, 7634–7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, 2008. Retrovirus-mediated cell labeling. In: Gage FH, Kempermann G, Song H (Eds.), Adult Neurogenesis. Cold Spring Harbor Laboratory Press, New York, pp. 101–117. [Google Scholar]


