Abstract
Background –
Bruton’s tyrosine kinase (BTK) is important in B-cell signalling. Efficacy has been reported for BTK inhibitors (BTKi) in human autoimmune diseases. Canine pemphigus foliaceus (cPF) is one of the most common canine autoimmune skin diseases.
Objectives –
To determine the safety and efficacy of the BTKi PRN1008 in the treatment of cPF.
Animals –
Four privately owned dogs.
Materials and methods –
Four dogs diagnosed with PF were administered BTKi PRN1008. Initial dosages approximated to 15 mg/kg once daily, increased to twice daily if inadequate response was seen. Treatment continued for 20 weeks, attempting to decrease to every other day. Dogs were monitored with complete blood counts, serum biochemistry panels, urinalyses and evaluated with a modified version of a validated human Pemphigus Disease Activity Index (cPDAI). Serum anti-desmocollin-1 (DSC-1) and desmoglein-1 (DSG-1) immunoglobulin (Ig)G titres were performed before and after the treatment period. Drug bound to target was measured in peripheral blood mononuclear cells (PBMC).
Results –
All four dogs showed reduction in lesions and cPDAI score during the first two weeks of treatment. Three dogs continued to improve and sustained near complete remission by 20 weeks, at which point three responses were considered “good” and one “fair”. Final daily dosages were in the range 17–33 mg/kg. Anti-DSC-1 IgG titre decreased dramatically in one dog, was undetectable in two and was uninterpretable in one dog. No dogs had detectable IgG to DSG1. A possible adverse event occurred in one dog.
Conclusions and clinical importance –
BTKi PRN1008 monotherapy may have some beneficial effects in some cases of cPF.
Résumé
Contexte –
La BTK (Bruton’s tyrosine kinase) est une importante voie de signal des cellules B. L’efficacité des inhibiteurs de BTK (BTKi) a été rapportée dans les maladies auto-immunes chez l’homme. Le pemphigus foliacé (cPF) est la dermatose auto-immune la plus fréquente chez le chien.
Objectifs –
Déterminer l’innocuité et l’efficacité de BTKi PRN 1008 dans le traitement de cPF.
Sujets –
Quatre chiens de propriétaires.
Matériels et méthodes –
Quatre chiens diagnostiqués PF ont reçus BTKi PRN 1008. Les doses initiales étaient approximativement de 15 mg/kg une fois par jour, augmenté à deux fois par jour si une réponse inadéquate était observée. Le traitement a été poursuivi pendant 20 semaines, avec essai de diminution à un jour sur deux. Les chiens ont été suivis avec une numération formule, biochimie complète et analyses urinaires et évalués avec une version modifiée de cPDAI (human Pemphigus Disease Activity Index). Les titres d’immunoglobulines (Ig) G anti-desmocolline 1 (DSC-1) et desmogléine-1 (DSG-1) ont été réalisés avant et après traitement. Le médicament lié à la cible a été mesuré dans les cellules mononuclées sanguines périphériques (PBMC).
Résultats –
Les quatre chiens ont montré une diminution des lésions et du score cPDAI au cours des deux premières semaines de traitement. Trois chiens ont continués à s’améliorer jusqu’à guérison presque complète à la semaine 20, à chaque point, trois réponses ont été considérées “bonne” et une “faible”. Les dosages quotidiens finaux àtaient dans les valeurs 17–33 mg/kg. Les titres d’IgG anti-DSC-1 ont diminués drastiquement chez un chien, étaient non détectable pour deux et étaient non interprétable pour un chien. Aucun chien n’avait d’IgG détectable à DSG1. Un effet indésirable possible a été observé chez un chien.
Conclusions et importance clinique –
BTK1 PRN 1008 en monothérapie pourrait avoir des effets bénéfiques dans certains cas de cPF.
Resumen
Introducción –
la tirosina quinasa de Bruton (BTK) es importante en las señales celulares de linfocitos B. Se ha publicado acerca de la eficacia de los inhibidores de BTK (BTKi) en enfermedades autoinmunes humanas. El pénfigo foliáceo canino (cPF) es una de las enfermedades cutáneas autoinmunes caninas más comunes.
Objetivos –
Determinar la seguridad y eficacia del BTKi PRN1008 en el tratamiento de cPF.
Animales –
cuatro perros de propietarios particulares.
Métodos y materiales –
cuatro perros diagnosticados con PF recibieron BTKi PRN1008. Las dosis iniciales fueron de aproximadamente 15 mg/kg una vez al día, que se aumentaron a dos veces al día si se observaba una respuesta inadecuada. El tratamiento continuó durante 20 semanas, intentando disminuir a cada dos días. Los perros fueron evaluados con recuentos sanguíneos completos, paneles de bioquímica sérica y análisis de orina, y valorados con una versión modificada de un índice de actividad de la enfermedad del penfigo humano validado (cPDAI). Los t ítulos sericos de inmunoglobulina (Ig)G anti-desmocolina-1 (DSC-1) y desmogleina-1 (DSG-1) se realizaron antes y después del período de tratamiento. El fármaco unido a la proteína diana se midió en células mononucleares de sangre periférica (PBMC).
Resultados –
los cuatro perros mostraron una reducción en las lesiones y del valor de cPDAI durante las primeras dos semanas de tratamiento. Tres perros continuaron mejorando y mantuvieron una remisión casi completa a las 20 semanas, momento en el que tres respuestas se consideraron “buenas” y una “regular”. Las dosis diarias finales estuvieron en el rango de 17–33 mg/kg. El título de IgG anti-DSC-1 disminuyó drásticamente en un perro, fue indetectable en dos y no fue interpretable en un perro. Ningún perro tenía IgG detectable a DSG1. Un posible evento adverso ocurrió en un perro.
Conclusiones e importancia clínica –
la monoterapia con BTKi PRN1008 puede tener algunos efectos beneficiosos en algunos casos de cPF.
Zusammenfassung
Hintergrund –
Die Bruton Tyrosinkinase (BTK) ist wichtig bei der Signalgebung von B-Zellen. Bei Autoimmunerkrankungen des Menschen wurde bereits eine Wirksamkeit für BTK Inhibitoren (BTKi) beschrieben. Der Pemphigus foliaceus (cPF) des Hundes ist eine der häufigsten caninen Autoimmunerkrankungen.
Ziele –
Eine Bestimmung der Sicherheit und der Wirksamkeit der BTKi PRN1008 bei der Behandlung des cPF.
Tiere –
Vier Hunde in Privatbesitz.
Methoden und Materialien –
Vier Hunden, die mit PF diagnostiziert worden waren, wurde BTKi PRN1008 verabreicht. Anfangsdosen waren annähernd 15 mg/kg einmal täglich, wurden allerdings bei unzureichender Verbesserung auf zweimal täglich erhöht. Die Behandlung wurde 20 Wochen lang fortgesetzt, wobei versucht wurde, auf jeden zweiten Tag zu reduzieren. Die Hunde wurden mittels Blutbild, Serumbiochemie und Urinanalyse kontrolliert, sowie mit einer modifizierten Version eines validierten humanen Pemphigus Disease Activity Index (cPDAI) evaluiert. Es wurden Serum Anti-Desmocollin-1 (DSC-1) und Desmoglein-1 (DSG-1) Immunglobulin (Ig)G Titer vor und nach der Behandlungsperiode bestimmt. Mittels peripherer Blutmononuklearzellen (PBMC) wurde das ans Zielmolekül gebundene Medikament bestimmt.
Ergebnisse –
Alle vier Hunde zeigten weniger Veränderungen und einen verringerten cPDAI Wert während der ersten zwei Wochen der Behandlung. Drei Hunde verbesserten sich weiterhin und blieben nach 20 Wochen nahezu in Remission, wodurch zu diesem Zeitpunkt die Verbesserung bei drei als „gut“ und einem als „fair“ eingestuft wurde. Am Ende lagen die Dosierungen im Bereich von 17–33 mg/kg. Anti-DSC-1 IgG Titer verringerten sich bei einem Hund drastisch, waren bei zwei anderen nicht feststellbar und bei einem Hund nicht interpretierbar. Keiner der Hunde hatte messbare IgG auf DSG-1. Eine mögliche Neben-wirkungsreaktion trat bei einem Hund auf.
Schlussfolgerungen und klinische Bedeutung –
BTKi PRN1008 Monotherapie könnte in einigen Fällen von cPF eine günstige Auswirkung haben.
要約
背景 –
Bruton型チロシンキナーゼ (BTK) は、B細胞シグナル伝達において重要である。ヒト自己免疫疾患におけるBTK阻害剤 (BTKi) の有効性が報告されている。犬の天疱瘡 (cPF) は、最も一般的な犬の自己免疫性皮膚疾患の1つである。
目的 –
本研究の目的は、cPF治療におけるBTKi PRN1008の安全性および有効性を判断することであった。
供試動物–
4頭の飼育犬。
材料と方法 –
PFと診断した4頭の犬にBTKi PRN1008を投与した。初期投与量は1日1回15 mg/kgと概算され、不十分な反応が見られた場合は1日2回に増量した。治療は20週間継続し、投与量を隔日に減量するよう試みた。供試犬を全血球計算、血清生化学パネルおよび尿検査でモニターし、修正されたヒト天疱瘡疾患活動指数 (cPDAI) で評価した。血清抗デスモコリン−1 (DSC-1) およびデスモグレイン−1 (DSG-1) 免疫グロブリン (Ig) G力価を、治療期間の前後で測定した。標的に結合した薬物を末梢血単核細胞 (PBMC) で測定した。
結果 –
治療開始2週間で、4頭の犬すべてが病変とcPDAIスコアの低下を示した。 3頭の犬は改善を続け、 20週間までにほぼ完全寛解を維持した。その時点で、3頭の反応は「良」、1頭は「可」と見なされた。最終的な1日投与量は17〜33 mg/kgの範囲であった。抗DSC-1 IgG力価は1頭の犬で劇的に減少し、2頭では検出できず、1頭の犬では解釈できなかった。 DSG1に対するIgGが検出された犬はいなかった。 1頭の犬で有害事象の可能性が生じた。
結論と臨床的重要性 –
BTKi PRN1008単剤療法は、cPFの一部の症例でいくつかの有益な効果をもたらす可能性がある。
摘要
背景–
Bruton酪氨酸激酶(BTK)在B细胞信号转导中很重要。已报告BTK抑制剂(BTKi)在人类自身免疫性疾病中的疗效。犬落叶型天疱疮(cPF)是最常见的犬自身免疫性皮肤病之一。
目的–
确定BTKi PRN1008治疗cPF的安全性和疗效
动物–
四只私家犬。
方法和材料 –
对诊断为PF的4只犬给予BTKi PRN1008。初始剂量约为15mg/kg, 每日一次, 如果观察到反应不足, 则增加至每日两次。治疗持续20周, 尝试降至隔日一次。通过全血细胞计数、血清生化全项和尿分析对犬进行监测, 并使用经验证的人天疱疮疾病活动指数 (cPDAI) 的改良版进行评价。在治疗期前后进行血清抗桥粒胶糖蛋白−1(DSC-1)和桥粒芯糖蛋白−1(DSG-1)免疫球蛋白(Ig)G滴度检测。在外周血单核细胞(PBMC)中测定与靶标结合的药物。
结果 –
在治疗的前两周, 所有4只犬均显示病变减轻和cPDAI评分下降。3只犬持续改善并在20周时维持接近完全缓解, 此时认为3只效果为“良好”, 1只为“一般”。最终日剂量范围为17–33mg/kg。在1只犬中, 抗 DSC-1 IgG滴度显著降低, 在2只犬中检测不到, 在1只犬中无法判断。所有犬均未检出DSG1 IgG。一只犬可能发生了不良事件。
结论和临床重要性–
BTKi PRN1008单药治疗在某些cPF病例中可能有一定效果。
Resumo
Histórico –
A tirosina quinase de Bruton (BTK) é importante na sinalização de células B. A eficácia de inibidores da BTK (BTKi) em doenças autoimunes humanas já foi relatada. O pênfigo foliáceo canino (cPF) é uma das doenças cutâneas autoimunes caninas mais comuns.
Objetivos –
Determinar a segurança e a eficácia do BTKi PRN1008 no tratamento de cPF.
Animais –
Quatro cães de proprietarios.
Métodos e materiais –
Quatro cães diagnosticados com PF receberam BTKi PRN1008.As dosagens iniciais foram aproximadamente 15 mg / kg uma vez ao dia, aumentadas para duas vezes ao dia se houvesse resposta inadequada. O tratamento continuou por 20 semanas, com a tentativa de diminuir a dose para dias alternados. Os cães foram monitorados por hemograma completo, perfis bioquímicos séricos e urinálise, e avaliados utilizando uma versão modificada de um Índice validado de Atividade de Doença para o Pênfigo humano (cPDAI). Os títulos séricos de anticorpos (Ig) G anti-desmocolina-1 (DSC-1) e anti-desmogleina-1 (DSG-1) foram realizados antes e após o período de tratamento. A ligação do fármaco ao alvo foi mensurada em células mononucleares do sangue periférico (PBMC).
Resultados –
Todos os quatro cães apresentaram redução nas lesões e escore de cPDAI durante as duas primeiras semanas de tratamento. Três cães continuaram a melhorar e mantiveram a remissão quase completa em 20 semanas. Neste momento, três respostas foram consideradas “boas” e uma “razoável”. As dosagens diárias finais ficaram na faixa de 17 a 33 mg / kg. O título de IgG anti-DSC-1 diminuiu drasticamente em um cão, foi indetectável em dois e não foi interpretável em um cão. Nenhum cão apresentou IgG detectável para DSG1. Um possível efeito adverso ocorreu em um cão.
Conclusões e importância clínica –
A monoterapia com BTKi PRN1008 pode ter efeitos benéficos em alguns casos de cPF.
Introduction
Canine pemphigus foliaceus (cPF) is one of the most common cutaneous autoimmune diseases in the dog.1 The presumed mechanism is a type II immune response, mediated by antibodies directed against the transmembrane proteins desmocollin-1 (DSC-1) and, rarely, desmoglein-1 (DSG-1).1,2 The treatment of cPF generally includes glucocorticoids often with other immunosuppressive therapies, all having adverse effects.3 Identifying additional effective and safe treatments for cPF is important in veterinary dermatology.
Bruton’s tyrosine kinase (BTK) is a protein that supports humoral immunity. Autoreactive B cells are dependent upon BTK for survival to a greater degree than normal B cells, reflected as loss of autoantibodies with maintenance of total antibody levels when BTK is absent.4 BTK inhibitor (BTKi) drugs act by inhibiting BTK in non-T white blood cells, including B cells, neutrophils and mast cells, reducing downstream signalling from the B-cell receptor and the surface immunoglobulin (Ig)G receptor on B and other non-T white blood cells, plus inhibiting neutrophil migration.4,5 Previous reports using BTKi in the treatment of cPF have included two posters, one of which presented findings of Dog 1 in the present study,6 and our previous report on BTKi PRN473.7
PRN1008 is an oral, reversible covalent BTKi developed by Principia Biopharma for the treatment of human autoimmune disease including pemphigus foliaceus; it has been given the generic name rilzabrutinib.5,8 PRN1008 has undergone toxicological testing in healthy beagle dogs, including a 12 week Good Laboratory Practice study by Principia Biopharma.
Our hypothesis was that PRN1008 would be effective in the treatment of cPF. The primary objective of this study was to determine efficacy and safety in naturally occurring cPF. A secondary objective was to evaluate serum levels of anti-DSC-1 and DSG-1 IgG titres, before and after the treatment period. The hypothesis was that as B-cell signalling was decreased, less anti-DSC-1 and anti-DSG-1 IgG would be produced.
Methods and materials
Study design and animals
This was a pilot, nonblinded, noncontrolled study; the protocol was approved by the Institutional Animal Care and Use Committee, Protocol #19200.
Inclusion criteria
Cases were enrolled if they fulfilled the following criteria: (i) clinical features compatible with subcorneal pustular disease (pustules, erosions and crusts); (ii) histological demonstration of subcorneal pustular dermatitis with acantholysis; (iii) no dermatophytes seen on periodic acid Schiff-stained histological slides and/or growth on fungal culture; (iv) no or minimal improvement with appropriate antibiotic treatment; and (v) no previous treatment with, or failure of, appropriate immunosuppressive medication. Any immunosuppressive medication was stopped before the trial.
Exclusion criteria
Pregnancy, or active systemic disease, including neoplasia.
Laboratory procedures
Blood sampling was performed 4 h post medication at two, four, eight, 12, 16 and 20 weeks and also at 0 and 24 h after the first administration. The BTK probe PRN933 and BTK detection antibody (Clone 53, BD Biosciences; San Jose, CA, USA) used in this study were used as reported in a similar study of cPF and PRN473.7 PRN933 was synthesized in-house and validated to show similar binding affinity to those studies. Further details of processing of the dogs’ B cells and measuring BTK occupancy [% of the BTK enzyme in peripheral blood mononuclear cells (PBMC) bound by PRN1008] were as described previously.7 Determination of IgG titres to DSC-1 and DSG-1 was performed as described previously, utilizing transfected 293T cells expressing canine DSC-1 or DSG-1 on their surface and nontransfected 293T cells without these recombinant proteins.4,7 Sera were tested at 1:20, 1:50, 1:100, 1:200, 1:400, 1:800, 1:1,600 and 1:3,200, with the last positive dilution recorded. Desmocollin-1 and DSG-1 (IgG) titres were performed before and at the end of treatment for each dog.
Medication
PRN1008 was provided as both 100 and 300 mg tablets. The medication was given once daily with food. This dose was based on previous studies with PRN473,7 and pharmacological data (Francis D. Expert Pharmacology/Toxicology Review – PRN1008; v.2.0; dated 21 July 2014. Principia Biopharma, data on file). The frequency was increased to twice daily if lesions did not diminish after two to four weeks of treatment, or if BTK occupancy data were considered suboptimal (<70%).9 Treatment was maintained for 20 weeks with attempts to decrease to every other day after 12 weeks if the dog’s response was judged as “good” (as described in the following subsection).
Monitoring
Dogs were monitored with complete blood count (CBC), serum biochemistry panel and urinalysis at each visit. They were evaluated semiquantitatively with a modified canine version of a validated human Pemphigus Disease Activity Index (cPDAI; Figure S1).10 A dog’s response to treatment was evaluated qualitatively at the end of treatment as good (>90% of lesions resolved on the drug alone), fair (≥50% of lesions resolved; after 8–16 weeks the clinician decided to add corticosteroids to the treatment regimen) or poor (no improvement in lesions following the first two weeks, stopping BTKi and instituting other medication intervention). Dogs were monitored/evaluated at two, four, eight, 12, 16 and 20 weeks while receiving the BTKi. Evaluation via the cPDAI was performed by two veterinarians blinded to the other’s scoring.
Results
Four dogs were treated with PRN1008; their details are given in Table 1. All four dogs had positive clinical responses, showing lesion reduction in the first two weeks (see Figure S2). Owners reported improvement or resolution of the following signs: lethargy, impaired appetite and/or pyrexia. All four dogs continued to improve and three (dogs 1, 3 and 4) sustained near-complete remission by the end of the study (Figure 1). Dog 2 required intervention with corticosteroid as mometasone ointment applied to the affected area. Initial daily dosages of PRN1008 ranged from 15 to 20 mg/kg (median 17.5, mean 17 mg/kg) and final daily dosages from 17 to 33 mg/kg (median 31.5, mean 28 mg/kg). Doses administered to dogs 1 to 3 were increased to twice daily at four weeks. No dog was able to have dosing frequency reduced to every other day.
Table 1.
Summary of details of four dogs receiving therapy for pemphigus foliaceus with PRN1008
| Dog | Age years) | Breed | Sex | Lesion distribution | Duration of therapy weeks | Response to therapy* | Desmocollin 1 serum titre | Treatment emergent adverse effects |
|---|---|---|---|---|---|---|---|---|
| 1 | 12 | Labrador retriever | Female spayed | Generalized | 20 | Good | 1:800 to 1:40 | None |
| 2 | 8 | Rough coated collie | Female | Facial dominant | 20 | Fair | † | ‡pyometra |
| 3 | 5 | Rat terrier | Female spayed | Generalized | 20 | Good | Not detected | None |
| 4 | 12 | Labrador retriever | Male castrated | Generalized | 20 | Good | Not detected | None |
| Dose of treatment (daily) – dose only increased after two to four weeks of therapy | Dose (mg/kg) | Condition deteriorated or worsened after the treatment trial? | Treatment given every two days? | Median BTK occupancy (%) at 4 h | |
|---|---|---|---|---|---|
| 1 | 500–1,000 mg | 16–32 | Yes | No | 73 |
| 2 | 450–1,000 mg | 15–33 | Yes | No | 71.5 |
| 3 | 200–300 mg | 20–30 | Yes | No | 75 |
| 4 | 600 mg | 17 | Yes | No | 75.5 |
BTK, Bruton’s tyrosine kinase occupancy measured at 4 h after oral medication (median value for six sampling points during the 20 week trial therapy)
Good, 90% of lesions resolved; Fair, 50% of lesions resolved and acceptable to owner.
All cells took up fluorescence which was independent of transfection status.
Treatment was stopped for two weeks when pyometra was diagnosed. An increase in blood levels of alanine aminotransferase and aspartate aminotransferase returned to normal after the dose of PRN1008 was reduced.
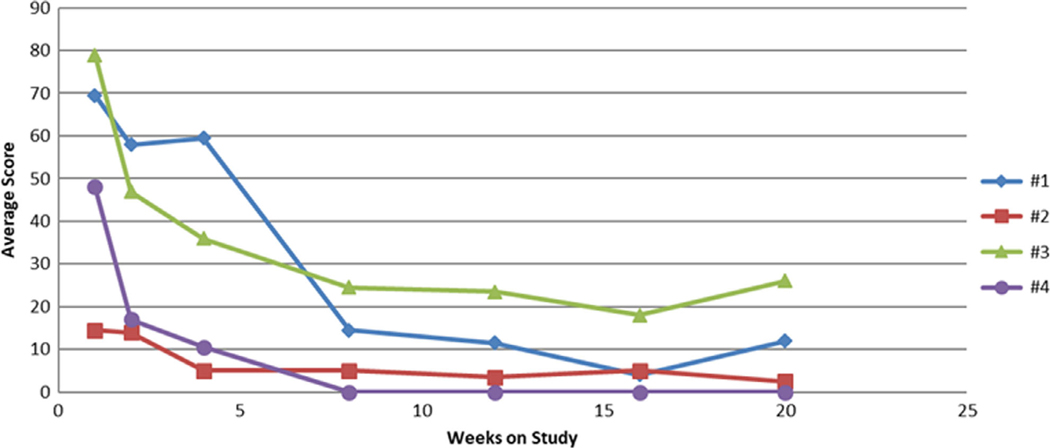
Figure 1.
Canine Pemphigus Disease Activity Index (cPDAI) scores over time for four dogs receiving PRN1008.
The cPDAI indicated that all four dogs continued to respond to treatment up to 20 weeks (Figure 1). Qualitatively, the responses of three dogs (1, 3 and 4) were considered good and that of Dog 2 was fair.
The anti-DSC-1 IgG titre was decreased in Dog 1 and was undetectable in both pre- and post-treatment sera in dogs 3 and 4. Pre- and post-treatment sera from Dog 2 stained positively in both transfected and nontransfected cells. The target(s) of serum IgG in this dog remain unknown. No dog had a positive titre to DSG-1.
The median 4 h post-medication BTK median occupancy over the treatment period ranged from 71.5% to 75.5% (Table 1). Although the CD21 results showed variability in the percentage of B cells between examinations, there was no sustained depletion of B-cell counts over the course of treatment; the percentage of CD21 positive-staining cells at the end of the study was greater than, or equal to, the percentage before receiving the BTKi (data not shown).
Discussion
It is presumed that PRN1008 affects cPF through interference with neutrophil migration and B-lymphocyte function, and by decreasing the production of the autoantibodies targeting transmembrane proteins such as DSC-1.6,11 This may be supported by the decrease in anti-DSC-1 antibody titres in Dog 1 observed herein.
Adverse effects were uncommon. Dog 2 developed pyometra; given that it was an 8-year-old intact female, it is unclear whether PRN1008 was the direct cause. In Dog 2, PRN1008 was likely to have been responsible for increases in serum alanine aminotransferase and aspartate aminotransferase values that returned to normal upon decreasing the dose.
In our previous report, five of nine dogs (55%) treated with PRN473 had a good response, compared to the 75% of dogs in this report.7 Two of the dogs treated with PRN473 concurrently developed immune-mediated pol yarthritis, compared to no dogs treated with PRN1008. Occupancy data suggest that PRN1008 binds to BTK, limiting its functionality. Dog 2 with fair response had the lowest occupancy data, although this was still above the 70% desired range. Occupancy was not correlated with clinical efficacy in our previous study.7
We previously noted the cPDAI presented some difficulties in evaluating the clinical findings.7 This may have accounted for the discrepancy between qualitative evaluation and cPDAI: although Dog 2 was qualitatively assessed with a fair response, its cPDAI was judged better than those of dogs 1 and 3, which were assessed as good.
No dog had anti-DSG-1 antibodies, which is not surprising as this antigen is a target in a minority of dogs with cPF.3 Not all dogs having detectable anti-DSC-1 antibodies has been demonstrated previously;2,7 this may be a consequence of inherently lower sensitivity of immunofluorescence techniques, differences between dogs with classic facial PF and those with a more truncal/generalized distribution,12 and the possibility that some dogs have autoantibodies against unknown antigens or a combination of these factors.
The limitations to this study are the small number of dogs treated and the lack of a control group. As in our previous report using PRN473, potential pharmacogenetic differences between breeds could not be accounted for in such a small cohort and this could account for the clinical response variability.
Supplementary Material
Figure S1. Scoring sheet for Canine Pemphigus Disease Activity Index.
Figure S2. Clinical images of Dog 1 before and after treatment with PRN1008.
Acknowledgements
Verena Affolter for help in formatting Figure S2 and Lisa Mamo for performing the immunofluorescence studies.
Sources of Funding:
This study was funded by Principia Biopharma which donated the medication, co-developed the study design, and paid for both case monitoring expenses and client participation fees.
Footnotes
Conflicts of Interest: A. Bisconte, M. Francesco, R. Hill, Masjedizadeh, P. Nunn and S. Gourlay were, or are, employees of Principia Biopharma.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- 1.Bizikova P, Dean GA, Hashimoto T, et al. Cloning and establishment of canine desmocollin-1 as a major autoantigen in canine pemphigus foliaceus. Vet Immunol Immunopathol 2012; 149: 197–207. [DOI] [PubMed] [Google Scholar]
- 2.Olivry T, LaVoy A, Dunston SM, et al. Desmoglein-1 is a minorautoantigen in dogs with pemphigus foliaceus. Vet Immunol Immunopathol 2006; 110: 245–255. [DOI] [PubMed] [Google Scholar]
- 3.Bizikova P, Olivry T. Oral glucocorticoid pulse therapy for induction of treatment of canine pemphigus foliaceus - a comparative study. Vet Dermatol 2015; 26: 354–358. [DOI] [PubMed] [Google Scholar]
- 4.Crofford LJ, Nyhoff LE, Sheehan JH, et al. The role of Bruton’styrosine kinase in autoimmunity and implications for therapy. Expert Rev Clin Immunol 2016; 12: 76–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith PF, Krishnarajah J, Nunn PA, et al. A phase I trial ofPRN1008, a novel reversible covalent inhibitor of Bruton’s tyrosine kinase, in healthy volunteers. Br J Clin Pharmacol 2017; 83: 2,367–2,376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murrell DF, Gourlay S, Francesco M, et al.A pilot study of the efficacy of a Bruton’s tyrosine kinase inhibitor in the treatment of dogs with pemphigus foliaceus. Poster presented at The Australasian College of Dermatologists – 2017 Annual Scientific Meeting, May 2017. Available at https://www.principiabio.com/technology/presentations-publications/page/2/?publication_category=prn1008 Accessed May 23, 2020. [Google Scholar]
- 7.Goodale EC, Varjonen EK, Outerbridge CA, et al. Efficacy of aBruton’s tyrosine kinase inhibitor [PRN-473] in the treatment of canine pemphigus foliaceus. Vet Dermatol 2020. 10.1111/vde.12841. [DOI] [PubMed] [Google Scholar]
- 8.Langrish CL, Bradshaw JM, Owens TD, et al. PRN1008, a reversible covalent BTK inhibitor in clinical development for immune thrombocytopenic purpura. Blood 2017; 130 (Suppl 1): 1,052 (abstract). [Google Scholar]
- 9.Bisconte A, Hill R, Bradshaw M, et al. Efficacy in collagen induced arthritis models with a selective, reversible covalent Bruton’s tyrosine kinase inhibitor PRN473 is driven by durable target occupancy rather than extended plasma exposure (THER5P.904). J Immunol 2015; 194 (Suppl 1): 139.6 (abstract). [Google Scholar]
- 10.Hebert V, Boulard C, Houivet E, et al. Large international validation of ABSIS and PDAI pemphigus severity scores. J Invest Dermatol 2019; 139: 31–33. [DOI] [PubMed] [Google Scholar]
- 11.Yabuzoe A, Nishifuji K, Sekiguchi M, et al. Neutrophils contact to plasma membrane of keratinocytes including desmosomal structures in canine pemphigus foliaceus. J Vet Med Sci 2008; 70: 807–812. [DOI] [PubMed] [Google Scholar]
- 12.Bizikova P, Mamo L. Detection of anti-desmocollin-1 and anti-desmoglein-1 autoantibodies in dogs with pemphigus foliaceus with or without the classic facial involvement. Vet Dermatol 2019; 30: 291–292 (abstract). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Scoring sheet for Canine Pemphigus Disease Activity Index.
Figure S2. Clinical images of Dog 1 before and after treatment with PRN1008.