Abstract
Objectives
This study aimed to summarize the existing literature on risk factors for arrhythmias after chemotherapy in cancer patients. To provide reliable evidence for treating arrhythmias after chemotherapy in oncology patients by assessing multiple biasing factors in the literature and quantifying the risk factors.
Methods
The risk factors for arrhythmia following tumor chemotherapy were systematically collected from various reputable databases, including PubMed, Cochrane Library, MEDLINE, EMBASE, and multiple Chinese databases, covering the period from inception to May 2023. Two independent reviewers performed rigorous article screening, data extraction, and assessment of research quality. Data analysis was conducted using Review Manager 5.4 software, ensuring a standardized and robust approach to evaluate the gathered evidence.
Results
The analysis of chemotherapy-induced arrhythmias included 16 articles, encompassing 14,785 cancer patients. Among the patients, 3295 belonged to the arrhythmia group, while 11,490 were in the non-arrhythmia group. These studies identified 12 significant risk factors associated with arrhythmias following chemotherapy in cancer patients. The findings of the analysis are as follows.
General patient characteristics
The incidence of post-chemotherapy arrhythmias was 14.33 times higher in oncology patients aged ≥60 years compared to patients <60 years of age [OR = 14.33, 95%CI (8.51, 24.13), P<0.00001]. Patients with a smoking history exhibited a 1.67-fold higher risk of arrhythmia after chemotherapy [OR = 1.67, 95%CI (1.24, 2.25), P = 0.0007]. However, there was no significant correlation between gender and body mass index (BMI) with arrhythmia after chemotherapy in oncology patients (P = 0.52; P = 0.19)
Disease-related factors
Patients with a history of hypertension, diabetes, and cardiovascular disease had a 1.93-fold, 1.30-fold, and 1.76-fold increased risk of arrhythmia after chemotherapy, respectively [OR = 1.93, 95%CI (1.66, 2.24), P<0.00001; OR = 1.30, 95%CI (1.10, 2.52), P = 0.002; OR = 1.76, 95%CI (1.51, 2.05), P<0.00001]. Additionally, the incidence of arrhythmia increased 1.97 times in patients with electrolyte and acid-base balance disorders following chemotherapy [OR = 1.97, 95%CI (1.41, 2.76), P<0.00001]
Chemotherapy-related factors
Seven articles examined the association between chemotherapy drugs and post-chemotherapy arrhythmias. The results indicated that oncology patients were 3.03 times more likely to develop arrhythmias with chemotherapy drugs compared to non-chemotherapy drugs [OR = 3.03, 95%CI (2.59, 3.54), P<0.00001]. Notably, anthracyclines and fluorouracil chemotherapy demonstrated a 2.98-fold and 3.35-fold increased risk of arrhythmia after chemotherapy, respectively [OR = 2.98, 95%CI (2.51, 3.03), P<0.00001; OR = 3.35, 95%CI (2.20, 5.10), P<0.00001]. The risk of arrhythmia after chemotherapy was 1.72 times higher in patients with chemotherapy cycles longer than 4 weeks than those with cycles shorter than 4 weeks [OR = 1.72, 95%CI (1.30, 2.28), P = 0.0001]
Conclusion
The occurrence of arrhythmia after chemotherapy in cancer patients was significantly associated with the patient's age, history of smoking, history of hypertension, history of diabetes, history of cardiovascular disease, chemotherapy drug use, and cycle. However, further high-quality evidence is needed to support these results.
Keywords: Cancer, Chemotherapy, Arrhythmia, Risk factors, Meta-analysis, Systematic evaluation
1. Introduction
Cancer and cardiovascular disease are common chronic diseases threatening human health. The case fatality rates were 29 % and 32 %, respectively, ranking first and second among the natural causes of death, while the mortality rate of comorbidity was over 60 % [[1], [2], [3]]. With the emergence of anti-tumor-targeted drugs and immunotherapy, the mortality rate of various tumors has been dramatically reduced, and the survival rate has subsequently increased [4]. Cancer has become a chronic disease. However, the incidence of cardiovascular toxicity associated with antitumor therapy is increasing, affecting cancer patients' prognosis and becoming one of the leading causes of death in cancer survivors [5,6].
Multiple studies have shown that oncology patients may suffer from a wide range of arrhythmias during chemotherapy, including sinus tachycardia, bradycardia, tachyarrhythmia, and conduction defects, which can lead to severe clinical symptoms, even life-threatening and serious affect the treatment and prognosis of oncology patients [[7], [8], [9], [10]]. However, the risk factors and mortality of arrhythmia after chemotherapy in oncology patients are still unclear.
In contrast, arrhythmia, as a controllable and treatable complication, can be reduced by assessing the influencing factors after chemotherapy in patients and taking appropriate preventive measures. Therefore, this study will analyze the risk factors of post-chemotherapy arrhythmia in oncology patients using systematic evaluation and Meta-analysis. It is expected to provide the experimental basis and scientific support for clinical prevention and treatment of post-chemotherapy arrhythmia in oncology patients to guide the early screening and management of high-risk groups prone to arrhythmia after chemotherapy and provide theoretical reference for the clinical formulation of prevention, treatment, and management strategies.
2. Materials and methods
2.1. Inclusion and exclusion criteria
2.1.1. Inclusion criteria
If the study met all of the following criteria, the article was considered qualified.
-
(1)
Study type: a case-control study, cohort study, Chinese and English only;
-
(2)
Study population: ① cancer patients with clear diagnostic criteria and specific sources; ② arrhythmia including sinus bradycardia, atrioventricular block, and sick sinus node syndrome after chemotherapy in cancer patients; ③ its race, nationality, course of the disease is not limited;
-
(3)
Interventions: Interventions for chemotherapy are not limited; The control group refers to the arrhythmia group aend the comparison group refers to the non-arrhythmia group.
-
(4)
Outcome indicators: whether arrhythmia occurred after chemotherapy in cancer patients as outcome indicators (including sinus tachycardia, sinus bradycardia, atrial premature beats, ventricular premature beats, bundle branch block, atrial fibrillation, abnormal electrocardiogram, ST-T wave changes, QRS wave group low voltage abnormalities, Q-T interval extension);
-
(5)
Influence factors: The same influence factor was extracted if it was reported in 2 or more papers.
2.1.2. Exclusion criteria
Articles that met one of the following criteria were excluded from the analysis.
-
(1)
Reviews, animal studies, conference papers, and dissertations;
-
(2)
Unpublished reports, summaries, preliminary reports, and unpublished data;
-
(3)
Repeated publications and other suspiciously repeated reports;
-
(4)
The study subjects included patients without cancer and those without cancer who did not receive chemotherapy;
-
(5)
Full-text research is not available.
2.2. Search strategy
Relevant case-control and cohort studies on chemotherapy in cancer patients were collected by searching. English databases included PubMed, Cochrane Library, Embase, Medline, and Chinese databases, including CNKI (China National Knowledge Infrastructure), the VIP database (Chinese Journal of Science and Technology of VIP), and Wanfang Data. The retrieval time was from the establishment of the database to May 2023. The search terms included: Tumor, Cancer, Malignancy, Malignant Neoplasm, Neoplasia, Neoplasm, Chemotherapy, Pharmacotherapy, Drug Therapies, Arrhythmia, Atrial Fibrillation, Atrial Flutter, Bradycardia, etc. The combination of Medical Subject Heading terms and free text words. In addition, supplemented by manual retrieval, if necessary, the references of topic-related system evaluation and review literature were traced to obtain relevant literature. The PubMed retrieval strategy is as follows:
#1: "neoplasms"[MeSH Terms] OR "Tumor"[Title/Abstract] OR "Cancer"[Title/Abstract] OR "Malignancy"[Title/Abstract] OR "malignant neoplasm"[Title/Abstract] OR "Neoplasia"[Title/Abstract] OR "Neoplasm"[Title/Abstract]; #2: "arrhythmias, cardiac"[MeSH Terms] OR "atrial fibrillation"[Title/Abstract] OR "atrial flutter"[Title/Abstract] OR "Bradycardia"[Title/Abstract] OR "Arrhythmia"[Title/Abstract]; #3: "drug therapy"[MeSH Terms] OR "Chemotherapy"[Title/Abstract] OR "Pharmacotherapy"[Title/Abstract] OR "drug therapies"[Title/Abstract]; #4: #1 AND #2 AND #3.
2.3. Selection of studies and data extraction
Two researchers performed an initial screening by reading the title and abstract strictly and independently according to predefined inclusion and exclusion criteria. Further screening was performed by reading the full text to determine whether the studies met the inclusion criteria. Any disagreements on the eligibility of studies were resolved by discussion with the third reviewer. The data were organized and analyzed using Microsoft Office Excel 2007 using a pre-developed data extraction form. The extracted information included: ① General information: title, author's name, date of publication; ② Study characteristics: study type, sample size, general condition of the study population, changes in clinical indicators, influencing factors (patient baseline, history, disease history, chemotherapy characteristics), etc. After completing the data extraction, the two researchers cross-checked the extraction results and summarized the extracted data.
2.4. Quality evaluation
The methodological quality of the included literature was independently evaluated by 2 evaluators using the Newcastle-Ottawa Scale (NOS) [11]. The scale consists of two parts and applies to evaluating case-control studies and cohort studies. There are 3 primary areas included in each section, i.e., selection of study subjects (4 entries), comparability between groups (1 entry), and determination of outcome or exposure (3 entries). The NOS uses a semiquantitative star system to evaluate the risk of bias. A study can be awarded up to one star for each numbered item in the selection and outcomes categories and a maximum of two stars for comparability. A study receiving 0–4 stars was considered low quality, while a 5–9 stars study was considered high quality. After completing the quality evaluation, two reviewers cross-checked the results and discussed or negotiated with a third reviewer to resolve any inconsistent results.
2.5. Statistical analysis
We utilized the Review Manager 5.4 software, provided by the Cochrane Collaboration Network, to conduct the meta-analysis. The data were analyzed using appropriate statistical methods. The weighted mean difference (WMD) was calculated for continuous variables, while for dichotomous variables, the Mantel-Haenszel method was employed to determine the combined effect sizes. The odds ratio (OR) and its corresponding 95 % confidence interval (CI) were used to quantify the statistical effect sizes.
In cases where there was no significant heterogeneity among the studies (P ≥ 0.1, I2<50 %), a fixed-effects model was employed. Conversely, a random-effects model was utilized for the analysis in instances where significant heterogeneity was present (P < 0.1, I2 ≥50 %). Subgroup analyses were conducted to explore potential sources of heterogeneity. Sensitivity analyses were performed by systematically excluding individual studies to assess the robustness and stability of the results.
The statistical significance of the overall findings was determined using the Z-test, with a threshold of P < 0.05 considered statistically significant. Additionally, funnel plots were employed to assess publication bias and the symmetry of the data distribution.
3. Results
3.1. Literature search results
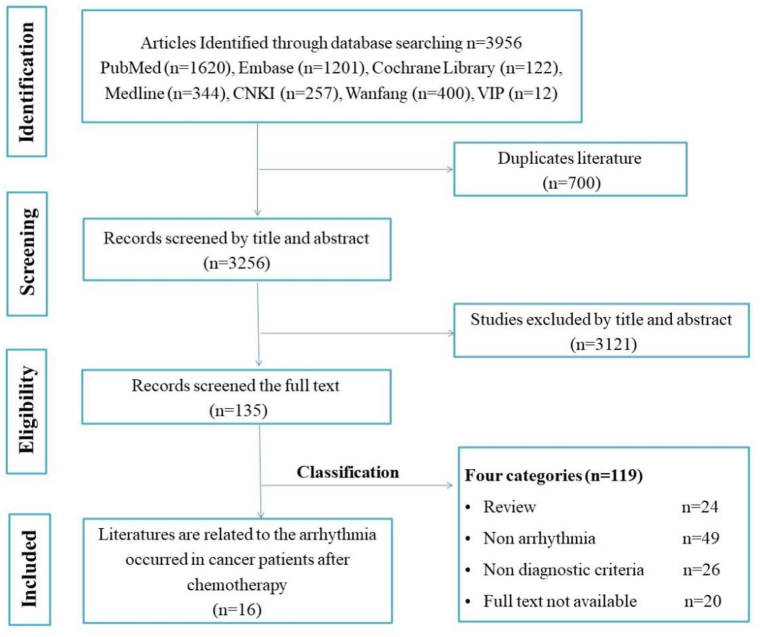
A total of 3956 articles were initially searched, of which 669 were searched in Chinese and 3287 were searched in English. After importing these articles into EndNote X9.2 and removing duplicates, 3256 articles were retained. The 3121 articles that did not meet the inclusion criteria after the title and abstract were first excluded, and then reviews, case reports, conference papers, dissertations, and duplicate publications were excluded by reading the full text. Finally, 16 eligible articles were included for analysis. The process and results of the literature screening are shown in Fig. 1.
Fig. 1.
Flowchart for the literature screening.
3.2. Basic characteristics and quality evaluation of the included studies
16 papers were eventually included, with studies published between 2000 and 2020, of which 7 were in English and 9 in Chinese; 3 were prospective studies, and 13 were retrospective studies. A total of 14785 patients were included, including 3295 in the arrhythmia group and 11490 in the non-arrhythmia group. The general characteristics of prospective and retrospective studies and the evaluation of the quality of the literature are shown in Table 1. A total of 12 influential factors associated with arrhythmia after chemotherapy in cancer patients were collected, including age, gender, BMI, smoking history, hypertension, diabetes, history of arrhythmia, history of cardiovascular disease, electrolyte acid-base balance disturbance, chemotherapy drug use and cycle, and neoadjuvant chemotherapy.
Table 1.
Basic characteristics and quality of the included studies.
| Study ID | Disease | Study Design | Arrhythmia /Non-arrhythmia |
Risk factors | NOS score | Incidence of arrhythmia |
|---|---|---|---|---|---|---|
| Ci Ren 2018 [12] | Breast cancer | CCS | 231/309 | a, j | 6 | 74.76 % |
| Wang 2011 [13] | Malignant tumor | CCS | 611/704 | a, j | 7 | 86.79 % |
| Ma 2000 [14] | Malignant tumor | CCS | 13/41 | a | 6 | 24.07 % |
| Liu 2009 [15] | Malignant tumor | CCS | 632/632 | a | 6 | 50.00 % |
| Liang 2019 [16] | Colorectal cancer | CCS | 99/55 | j, k | 7 | 55.31 % |
| Zhang 2020 [17] | Head and neck cancer | CCS | 72/70 | k | 6 | 50.70 % |
| Lu 2020 [18] | NSCLC | CCS | 40/84 | b, c, f, h, i, | 9 | 32.26 % |
| Su 2015 [19] | Breast cancer | CCS | 94/148 | a, e, g, i, j, k | 7 | 38.84 % |
| Zhou 2019 [20] | Malignant tumor | CCS | 98/226 | a, b, e, f, g, h, i, l | 9 | 30.25 % |
| Nickel AC 2018 [21] | Malignant tumor | CCS | 601/4425 | b, d, e, f, h | 8 | 11.96 % |
| Kitagawa K 2012 [22] | Breast cancer | CS | 127/4 | j | 7 | 96.95 % |
| Fradley MG 2019 [23] | B-cell malignancy | CS | 20/223 | j | 7 | 8.23 % |
| Qi 2020 [24] | Malignant tumor | CS | 152/3091 | h, j | 7 | 4.69 % |
| Rao VP 2012 [25] | Malignant tumor | CCS | 209/788 | b, c, d, e, f, l | 8 | 20.96 % |
| Peng 2018 [26] | Malignant tumor | CCS | 241/286 | e, f, h, j, k, | 8 | 27.51 % |
| Zhai 2009 [27] | Non-hodgkin lymphoma | CCS | 55/404 | j | 7 | 11.98 % |
Notes: CCS = case-control study; CS = cohort study; NSCLC=Non-small cell lung cancer; a = age; b = gender; c = smoking; d = BMI; e = hypertension; f = diabetes; g = history of arrhythmia; h = history of heart disease; i = electrolyte and acid-base balance disorders; j = chemotherapy drug; k = chemotherapy cycle; l = neo-adj chemotherapy.
3.3. Meta-analysis findings of risk factors for arrhythmia after chemotherapy in patients with cancer
The results of meta-analysis are summarized in Table 2. When significant heterogeneity was detected, sensitivity analysis was performed by excluding studies with possible sources of heterogeneity and recalculating the combined statistics.
Table 2.
Summary of meta-analysis findings.
| Risk factor | N | Heterogeneity | Test model | Combined statistics | Combined statistical value | P value |
|---|---|---|---|---|---|---|
| Age | 5 | I2 = 85 %, P<0.01 | Random | OR | 14.33 [8.51, 24.13] | 0.0001 |
| Gender | 4 | I2 = 0 %, P = 0.37 | Fixed | OR | 0.92 [0.71, 1.19] | 0.52 |
| Smoking | 2 | I2 = 0 %, P = 0.72 | Fixed | OR | 1.67 [1.24, 2.25] | 0.0007 |
| BMI≥25 kg/m2 | 2 | I2 = 0 %, P = 0.77 | Fixed | OR | 0.48 [-0.23, 1.18] | 0.19 |
| Hypertension | 5 | I2 = 47 %, P = 0.13 | Fixed | OR | 1.93 [1.66, 2.24] | 0.00001 |
| Diabetes | 5 | I2 = 0 %, P = 0.52 | Fixed | OR | 1.30 [1.10, 1.52] | 0.002 |
| History of arrhythmia | 2 | I2 = 10 %, P = 0.35 | Fixed | OR | 2.00[1.37, 2.93] | 0.0004 |
| Cardiovascular Disease | 5 | I2 = 30 %, P = 0.23 | Fixed | OR | 1.72 [1.45, 2.03] | 0.00001 |
| Electrolyte disturbance | 3 | I2 = 0 %, P = 0.39 | Fixed | OR | 1.97 [1.41, 2.76] | 0.0001 |
| Chemotherapeutics | 7 | I2 = 0 %, P = 0.62 | Fixed | OR | 3.03 [2.59, 3.54] | 0.00001 |
| Chemotherapy cycle > 4weeks | 4 | I2 = 35 %, P = 0.20 | Fixed | OR | 1.72 [1.30, 2.28] | 0.0001 |
| Neo-adj chemotherapy | 2 | I2 = 0 %,P = 1 | Fixed | OR | 1.68 [1.24, 2.27] | 0.0008 |
3.3.1. General characteristics
3.3.1.1. Age
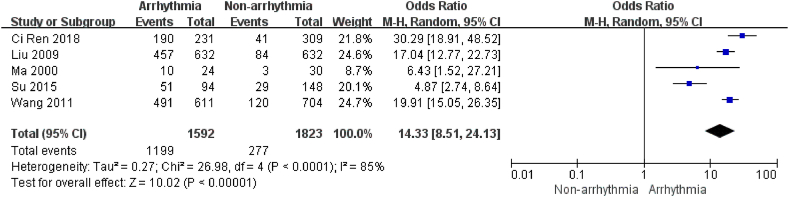
There were 5 studies [[12], [13], [14], [15],19] describing age, containing 3415 patients and 1592 patients in the arrhythmia group. There was high heterogeneity (I2 = 85 %, P < 0.01) between studies, and heterogeneity was not changed using sensitivity analysis. Therefore, a random effects model was chosen for the analysis. According to the World Health Organization's definition of middle age and old age, patients ≥60 years old were defined as senior patients, and those <60 years old were defined as junior patients in this study (http:/hwww.who.intageinglpublications/worid-eport-2015/zh/). The results showed that the risk of arrhythmia after chemotherapy was 14.33 times higher in patients of older age than in those of younger age (P < 0.0001, Fig. 2). The findings indicated that elderly age was a risk factor for arrhythmias after chemotherapy.
Fig. 2.
Forest plot for the association between age and the occurrence of arrhythmia after chemotherapy.
3.3.1.2. Gender
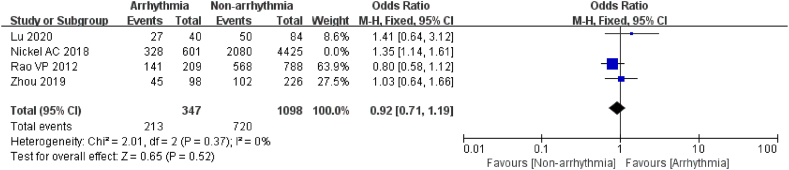
4 studies [18,20,21,25] explored the association between gender and post-chemotherapy arrhythmias. 1445 patients were enrolled, including 347 in the arrhythmia group. After sensitivity analysis to exclude significant sources of heterogeneity [21] (I2 = 0 %, P = 0.37), a fixed effect model was selected for analysis. However, there were no significant differences between the two groups (P = 0.52, Fig. 3). The research indicated that gender was not significantly related to the presence of arrhythmia after chemotherapy in oncology patients.
Fig. 3.
Forest plot for the association between gender and the occurrence of arrhythmia after chemotherapy.
3.3.1.3. Smoking
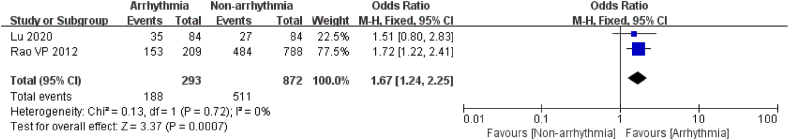
2 articles [18,25] reported the connection between smoking and the occurrence of arrhythmia after chemotherapy. A total of 1165 patients were included, of which 297 were in the arrhythmia group. Given the heterogeneity among studies (I2 = 0 %, P = 0.72), a random effects model was chosen. The outcome revealed that the risk of post-chemotherapy arrhythmia was 1.67 times higher in patients with a history of smoking than in those without a history of smoking (P = 0.0007, Fig. 4). Studies indicated that smoking is a risk factor for arrhythmia after chemotherapy in oncology patients.
Fig. 4.
Forest plot for the association between smoking and the occurrence of arrhythmia after chemotherapy.
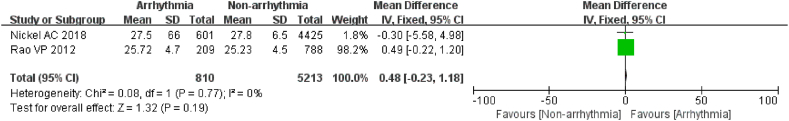
3.3.1.4. BMI
2 articles [21,25] reported the mean and standard deviation of BMI in the arrhythmia and non-arrhythmia groups, which included 6023 patients, with 810 patients in the arrhythmia group. Heterogeneity was low between studies, and a fixed effects model was preferred for analysis (I2 = 0 %, P = 0.77). The data revealed that the proportion of overweight patients with BMI >25 kg/m2 who developed arrhythmias after chemotherapy was similar in both groups and the difference was not statistically significant (P = 0.19, Fig. 5). The outcomes indicated that BMI>25 kg/m2 was not significantly associated with arrhythmia after chemotherapy in cancer patients.
Fig. 5.
Forest plot for the association between the BMI and the occurrence of arrhythmia after chemotherapy.
3.3.2. Disease-related factors
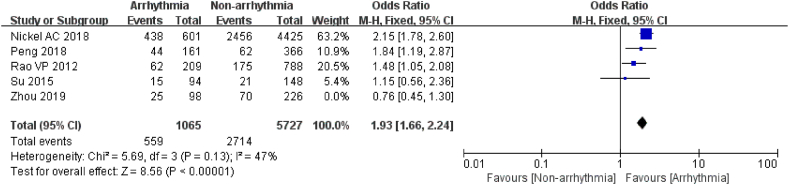
3.3.2.1. Hypertension
5 studies [[19], [20], [21],25,26] evaluated the relationship between hypertension and arrhythmia after chemotherapy with a statistically significant difference between the groups (P < 0.01). After excluding studies with a major source of heterogeneity by sensitivity analysis (I2 = 47 %, P = 0.13) [20], the results revealed that patients with a history of hypertension were 1.93 times more likely to develop arrhythmia after chemotherapy than those without a history of hypertension (P < 0.00001, Fig. 6). The findings suggested that hypertension is a risk factor for arrhythmia after chemotherapy in cancer patients.
Fig. 6.
Forest plot for the association between the hypertension and the occurrence of arrhythmia after chemotherapy.
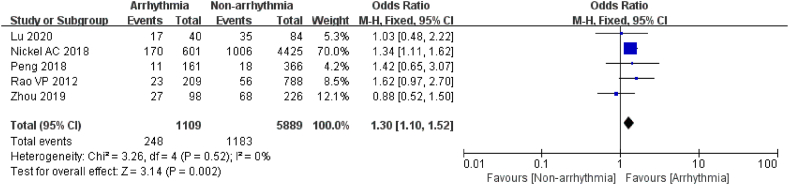
3.3.2.2. Diabetes
A total of 6998 patients were enrolled in 5 studies to assess the relationship between diabetes and arrhythmia after chemotherapy, of which 1109 were in the arrhythmia group [18,20,21,25,26]. There was a high level of homogeneity in the study, and a fixed effects model was used (I2 = 0 %, P = 0.52). The results showed that patients with a history of diabetes combined with arrhythmia after chemotherapy were 1.30 times more likely than those without a history of diabetes (P = 0.002, Fig. 7). This investigation implicated diabetes as a risk factor for arrhythmia after chemotherapy in cancer patients.
Fig. 7.
Forest plot for the association between diabetes and the occurrence of arrhythmia after chemotherapy.
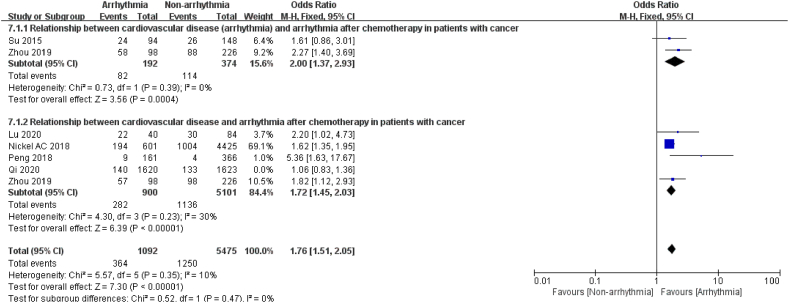
3.3.2.3. Cardiovascular disease (CVD)
6 articles [[18], [19], [20], [21],24,26] reported the relationship between cardiovascular disease and arrhythmia after chemotherapy. After sensitivity analysis to exclude significant sources of heterogeneity [24] (I2 = 10 %, P = 0.35), the pooled data revealed that the incidence of arrhythmia after chemotherapy was 1.76 times higher in patients with a history of cardiovascular disease than in patients without a history of cardiovascular disease, with similar results in the subgroup analysis, as shown in Fig. 8. Two of these studies investigated the relationship between a history of arrhythmia and post-chemotherapy arrhythmias and demonstrated that patients with a history of arrhythmia had a 2-fold increased risk of new-onset arrhythmia after chemotherapy (P = 0.0004). Another four articles observed the association of coronary artery disease, heart failure, and post-chemotherapy arrhythmias, which revealed a 1.72-fold increase in arrhythmias after chemotherapy in patients with a history of cardiac disease (P < 0.00001). It was suggested that cardiovascular disease is a risk factor for arrhythmia after chemotherapy in oncology patients.
Fig. 8.
Forest plot for the association between CVD and the occurrence of arrhythmia after chemotherapy.
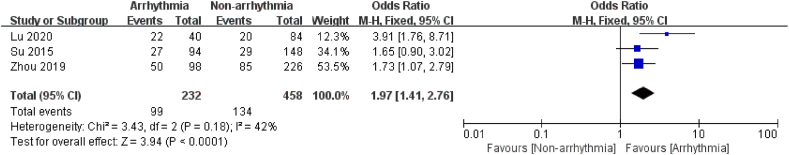
3.3.2.4. Electrolyte acid-base balance disturbance
3 Studies [[18], [19], [20]]looked at the electrolyte acid-base balance disturbance concerning arrhythmia in 690 chemotherapy patients, and 232 of them had arrhythmias after chemotherapy. Since the studies had no heterogeneity (I2 = 0 %, P = 0.39), the findings were analyzed using a fixed-effects model. The study showed a 1.97 times higher risk of arrhythmia after chemotherapy in patients with disorders of Electrolyte acid-base balance (P < 0.0001, Fig. 9). The results indicated that disturbances in electrolyte and acid-base balance are the risk factors for arrhythmia after chemotherapy in oncology patients.
Fig. 9.
Forest plot for the association between the electrolyte acid-base balance disturbance and the occurrence of arrhythmia after chemotherapy.
3.3.3. Chemotherapy-related factors
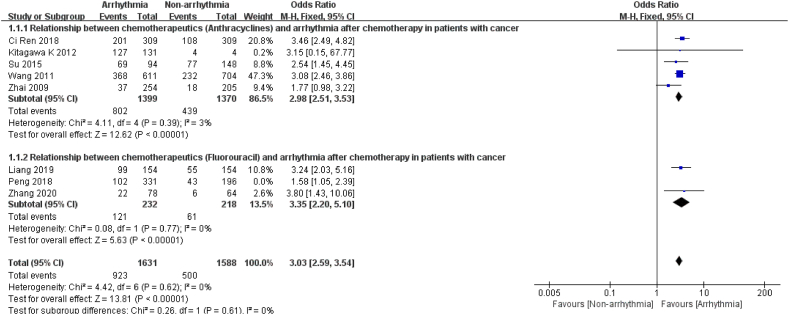
3.3.3.1. Chemotherapeutics
8 studies [12,13,16,18,19,22,26,27] described the response between the chemotherapy drugs and post-chemotherapy arrhythmia. After excluding studies with major sources of heterogeneity by sensitivity analysis [26] (I2 = 0 %, P = 0.62), the results showed significant differences between the groups, with chemotherapy drugs in oncology treatment 3.03 times more likely to trigger arrhythmia than non-chemotherapy drugs. The subgroup analysis revealed a 2.98-fold risk of arrhythmia after chemotherapy with anthracyclines (P < 0.0001) and a 3.35-fold risk of arrhythmia after chemotherapy in patients treated with fluorouracil (P < 0.0001, Fig. 10).
Fig. 10.
Forest plot for the association between the chemotherapeutics and the occurrence of arrhythmia after chemotherapy.
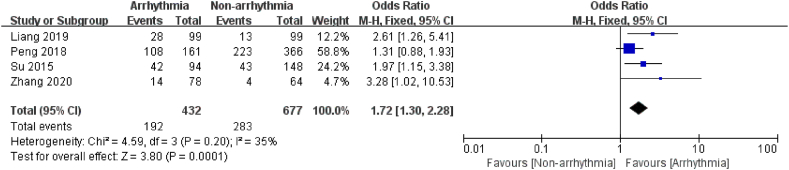
3.3.3.2. Chemotherapy cycle
4 articles [16,17,19,26] focused on the effect of the "chemotherapy cycle >4 weeks" on the occurrence of arrhythmia after chemotherapy in cancer patients. 1109 chemotherapy patients were included, with 432 in the arrhythmia group, and there was no heterogeneity within the studies (I2 = 35 %, P = 0.20). The outcomes revealed that the risk of post-chemotherapy arrhythmia was 1.72 times higher in patients with chemotherapy cycles more remarkable than 4 weeks than in those with less than 4 weeks (P = 0.0001, Fig. 11).
Fig. 11.
Forest plot for the association between the chemotherapy cycle and the occurrence of arrhythmia after chemotherapy.
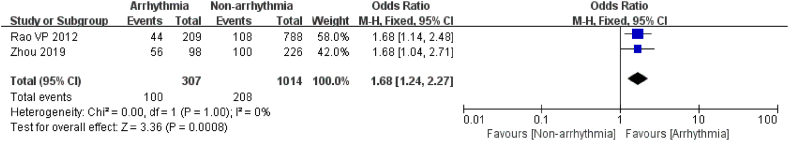
3.3.3.3. Neo-adj chemotherapy
2 studies [20,25] examined the relationship between neo-adjuvant chemotherapy and post-chemotherapy arrhythmia in 1321 patients, including 307 in the arrhythmia group. The studies had no heterogeneity (I2 = 0 %, P = 1), so the fixed effects model was selected for analysis. The study elucidated that patients with neo-adjuvant chemotherapy had a 1.68 times greater risk of developing arrhythmias after chemotherapy than unadjusted patients (P = 0.0008, Fig. 12).
Fig. 12.
Forest plot for the association between the neo-adj chemotherapy and the occurrence of arrhythmia after chemotherapy.
3.3.4. Other risk factors
Lu et al. [18] demonstrated in an observational clinical study of 124 elderly patients with non-small cell lung cancer (NSCLC) with cardiac arrhythmias during chemotherapy that Eastern Cooperative Oncology Group (ECOG) score [(OR = 3.463, 95%CI (1.579, 7.597), P<0.001], smoking index [(OR = 1.821, 95%CI (1.162, 2.854), P<0.001], disease site [(OR = 2.446, 95%CI (1.296, 4.615), P<0.001], cisplatin-containing chemotherapy regimen [(OR = 2.194, 95%CI (1.162, 4.140), P<0.001], hypoxemia [(OR = 4.152, 95%CI (1.684, 10.241), P<0.001], pulmonary infection [(OR = 2.138, 95%CI (1.187, 3.849), P = 0.002], and disturbance of electrolyte acid-base balance [(OR = 2.345, 95%CI (1.295, 4.248), P = 0.001] were risk factors for cardiac arrhythmias during chemotherapy in elderly patients with NSCLC.
Chen et al. [28] explored the relationship between cumulative dose of adriamycin and arrhythmia by observing the changes of ECG, cardiac enzymes, and echocardiography before, during, and after chemotherapy in 60 malignancy patients treated with adriamycin, and the results suggested that the incidence of arrhythmia and abnormal ECG ST-T changes increased significantly when the cumulative dose of adriamycin reached 500–600 mg/m2 or more compared with that before chemotherapy (P < 0.05). In addition, Qi et al. [24] evaluated risk factors associated with cardiotoxicity in cancer patients receiving panitumumab-containing therapy based on individual patient data from three prospectively well-controlled clinical trials. The outcomes indicated an additional risk of new arrhythmias after treatment with panitumumab in 1623 patients and an absolute incidence of arrhythmias per 100 persons per year from 3.7 % (chemotherapy alone) to 10.0 % (panitumumab-containing regimen). The results of the concurrent study revealed that previous hypertension [HR = 1.62, 95 % CI (1.04, 2.55), P = 0.033] and panitumumab-containing therapy [HR = 1.59, 95 % CI (1.01, 2.53), P = 0.046] were independent risk factors for the occurrence of an arrhythmia, which is consistent with the results of this study.
Fradley MG et al. [23] have confirmed that patients receiving treatment with ibrutinib have a higher incidence and risk of atrial arrhythmias compared to cytotoxic chemotherapy drugs [OR = 5.18, 95 % CI (1.42, 18.89), P = 0.009]. Meanwhile, Nickel AC et al. [21] analyzed the incidence of concomitant arrhythmias in 5026 oncology patients receiving chemotherapeutic agents. The results showed that 2951 patients (58.7 %) were treated with novel targeted chemotherapeutic agents (TCA) and 2075 patients (41.3 %) received anthracycline chemotherapy (A.C.), and approximately 12 % had new arrhythmias within 6 months after chemotherapy. The risk of post-chemotherapy arrhythmias was significantly higher in patients with a history of cardiovascular comorbidities such as hypertension and heart failure (P < 0.001), and TCA treatment reduced the relative risk of post-chemotherapy arrhythmia in oncology patients by 40 % compared with anthracycline-based chemotherapy.
3.4. Sensitivity analysis
This study identified the reliability of the study results by first excluding literature with high heterogeneity and comparing the before and after changes in I2 and combined effect sizes. The risk factors were also analyzed by looking at the degree of difference between the combined results of the fixed and random-effects models. The analysis showed no essential difference between the two models for each effect factor, indicating that the results were stable and reliable, as shown in Table 3.
Table 3.
Comparison of the results of the fixed-effects and random-effects model analyses.
| Risk factor | Fixed-effect model OR/MD (95%CI) | Random-effect model OR/MD (95%CI) |
|---|---|---|
| Age | 17.09 [14.37, 20.33] | 14.33 [8.51, 24.13] |
| Gender | 0.92 [0.71, 1.19] | 0.92 [0.71, 1.18] |
| Smoking | 1.67 [1.24, 2.25] | 1.67 [1.24, 2.25] |
| BMI≥25 kg/m2 | 0.48 [-0.23, 1.18] | 0.48 [-0.23, 1.18] |
| Hypertension | 1.93 [1.66, 2.24] | 1.77 [1.37, 2.27] |
| Diabetes | 1.30 [1.10, 1.52] | 1.30 [1.11, 1.53] |
| History of arrhythmia | 2.00 [1.37, 2.93] | 2.00 [1.36, 2.93] |
| Cardiovascular Disease | 1.72 [1.45, 2.03] | 1.85 [1.38, 2.47] |
| Electrolyte disturbance | 1.97 [1.41, 2.76] | 2.06 [1.30, 3.27] |
| Chemotherapeutics | 3.03 [2.59, 3.54] | 3.03 [2.59, 3.55] |
| Chemotherapy cycle > 4 weeks |
1.72 [1.30, 2.28] | 1.84 [1.26, 2.70] |
| Neo-adj chemotherapy | 1.68 [1.24, 2.27] | 1.68 [1.24, 2.27] |
3.5. Publishing bias analysis
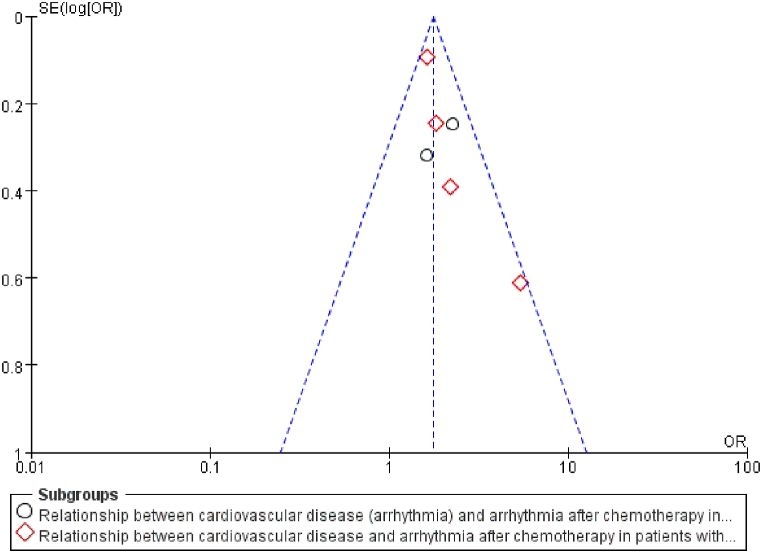
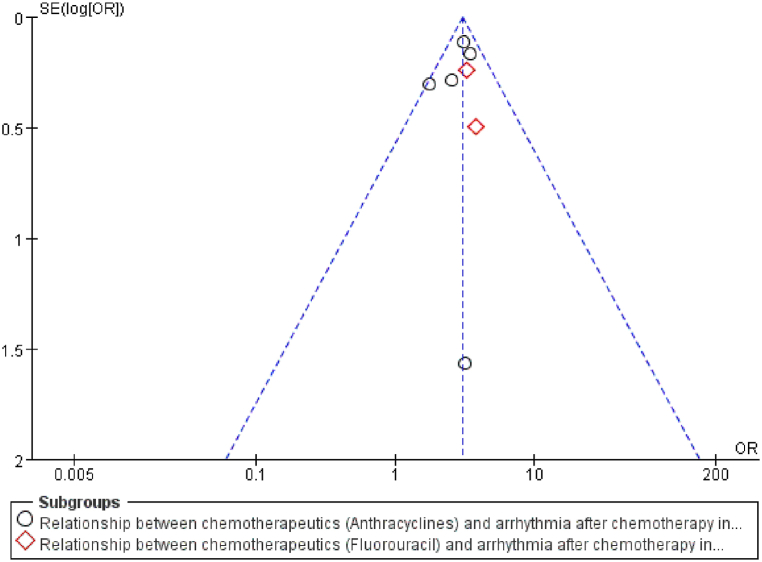
Publication bias was assessed if the analysis included more than 5 articles. The indicators with a literature number greater than 5 in this study were cardiovascular disease and chemotherapy drugs, which were tested separately for bias. The funnel plots for cardiovascular disease (Fig. 13) and chemotherapy drugs (Fig. 14) were largely symmetrical, reflecting no significant publication bias.
Fig. 13.
Funnel plot to detect publication bias for cardiovascular disease.
Fig. 14.
Funnel plot to detect publication bias for chemotherapeutics.
4. Discussion
Cancer patients may develop many types of arrhythmia after treatment, including atrial fibrillation, tachycardia, and premature ventricular contractions [9,29,30]. In contrast, patients with arrhythmia have a 5-fold increased risk of secondary ischemic stroke, a 3-fold increased risk of heart failure, and a 2-fold increased mortality rate [29]. There is limited research on the incidence of arrhythmia in cancer patients after treatment. However, related studies show that atrial fibrillation is as high as 20 % in patients with various types of tumors [31,32]. According to Y.F. et al. [31], 1.8 % of 24,125 cancer patients developed new atrial fibrillation during cancer treatment. However, the occurrence of arrhythmia in tumor patients after chemotherapy is affected by various complex factors, and there is a lack of clinical evidence to evaluate these risk factors.
Twelve risk factors associated with post-chemotherapy arrhythmia in cancer patients were evaluated, including age, gender, BMI, smoking history, hypertension, diabetes, history of arrhythmia, history of cardiovascular disease, electrolyte acid-base balance disturbance, chemotherapy drug use and cycle, and neoadjuvant chemotherapy. The results showed that 10 risk factors were significantly correlated with the incidence of arrhythmia after chemotherapy, except for gender and BMI (P<0.001). It is noteworthy that the findings of this study showed that the risk of arrhythmia after chemotherapy increases by 14.33 times in elderly patients over 60 years old, while the results of many studies also show that aging can lead to the senility of the cardiac autonomic nervous system, endothelial dysfunction, and cardiac electrophysiological activity disorder, increasing the incidence of arrhythmia after chemotherapy in oncology patients [[33], [34], [35], [36]]. In contrast, patients with a history of smoking, hypertension, diabetes, and cardiovascular disease had a 1.67-fold, 1.93-fold, 1.30-fold, and 1.76-fold increased risk of post-chemotherapy arrhythmias, respectively [37,38]. In addition, electrolyte and acid-base disorders cause an imbalance of intra-, extra-cellular, and calcium ion concentrations, altering myocardial cell excitability, conduction, and autoregulation, thus inducing arrhythmias [8]. The outcomes of this study revealed that the risk of arrhythmia after chemotherapy in patients with electrolyte and acid-base balance disturbances was 1.97 times higher than in patients with normal electrolytes.
In addition, different chemotherapeutic agents may also cause arrhythmia through various mechanisms, such as cytokine release, abnormal calcium homeostasis, and direct damage to the myocardium to produce arrhythmogenic substrates [8]. For example, treatment with anthracyclines and alkylating agents increases the sensitivity of cardiomyocytes to reactive oxygen species, which interferes with the production of endogenous antioxidants, catalase, glutathione peroxidase, and superoxide dismutase, thereby inducing arrhythmia [39,40]. Meanwhile, cyclophosphamide, melphalan, 5-fluorouracil, ibrutinib, and monoclonal antibodies can also induce arrhythmia after treatment [[41], [42], [43], [44], [45], [46]]. There are also findings showing that cisplatin is the most relevant drug for treating new-onset arrhythmia after treatment in oncology patients [[47], [48], [49]]. The results of this study showed that the risk of arrhythmias triggered by chemotherapy drugs in oncology patients was 3.03 times higher than that by non-chemotherapy drugs, with a 2.98-fold and 3.35-fold increase in the risk of arrhythmia after chemotherapy with anthracyclines and fluorouracil, respectively, and the risk of new arrhythmia increased with longer chemotherapy cycles. The findings also implied that the risk of new arrhythmias after chemotherapy was 1.72 times higher in patients with greater than 4 weeks than in those with less than 4 weeks of chemotherapy cycles and that patients using neoadjuvant chemotherapy were 1.68 times more likely to develop arrhythmias after chemotherapy than unadjusted patients.
4.1. Advantages and limitations
This research used a standardized, systematic evaluation method that included case-control studies and cohort studies, established strict inclusion and exclusion criteria, and strictly controlled the literature's quality to ensure the quality of outcome indicators. Furthermore, to ensure the reliability of the study results, this study assessed the risk of bias for potential risk of bias, compared before and after changes in I2 and combined effect sizes after excluding studies with high heterogeneity, and performed subgroup analysis for outcomes with high heterogeneity. In addition, sensitivity analysis of the random effects model and the fixed effects model in this study demonstrated that the differences between the two models were not statistically significant for each evaluation index.
In addition, some risk factors such as ECOG score, cumulative dose of chemotherapy drugs, and obstructive sleep apnea syndrome were not included because the number of articles was less than 2, which may lead to the absence of risk factors analyzed in this study. Therefore, more relevant large-scale, multicenter, prospective studies are needed to comprehensively include possible risk factors to reliably identify the risk factors for arrhythmia after chemotherapy in oncology patients.
5. Conclusion
Risk factors for arrhythmias after chemotherapy in oncology patients may include advanced age, history of smoking, history of hypertension, history of diabetes, history of cardiovascular disease (arrhythmias/heart failure/coronary artery disease), electrolyte disturbances, chemotherapy drugs, chemotherapy cycles, and neoadjuvant chemotherapy. For cancer patients receiving chemotherapy, it is essential to individualize the assessment protocol, taking into account the characteristics of each patient and the malignancy they have, followed by a targeted prevention strategy to minimize the risk of arrhythmia during oncology treatment.
Funding
The work was supported by the Chinese Medicine inheritance and innovation “thousand million” Talents Project (Qihuang Project) Qihuang Scholars and National Natural Science Foundation of China (Grant No. 81725024).
Consent for publication
Not applicable.
Code availability
All codes applied or analyzed during this study are included in this manuscript and its supplementary information files.
Data availability statement
The data supporting the findings of this study are included in the article and its supplementary materials.
CRediT authorship contribution statement
Qian-Qian Xu: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Data curation. Song-Jie Han: Writing – review & editing. Xiao-Hong Wei: Writing – review & editing. Liang-zhen You: Writing – review & editing. Li-Chao Sun: Writing – review & editing. Hong-Cai Shang: Writing – review & editing, Validation, Supervision, Methodology, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e34176.
Contributor Information
Li-Chao Sun, Email: prof_sunlichao@163.com.
Hong-Cai Shang, Email: shanghongcai@126.com.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Eyawo O., Franco-Villalobos C., Hull M.W., Nohpal A., Samji H., Sereda P., Lima V.D., Shoveller J., Moore D., Montaner J.S., Hogg R.S. Changes in mortality rates and causes of death in a population-based cohort of persons living with and without HIV from 1996 to 2012. BMC Infect. Dis. 2017;17(1):174. doi: 10.1186/s12879-017-2254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moy E., Garcia M.C., Bastian B., Rossen L.M., Ingram D.D., Faul M., Massetti G.M., Thomas C.C., Hong Y., Yoon P.W., Iademarco M.F. Leading causes of death in Nonmetropolitan and Metropolitan areas- United States, 1999-2014. Morb. Mortal. Wkly. Rep. - Surveillance Summ. 2017;66(1):1–8. doi: 10.15585/mmwr.ss6601a1. Washington, DC : 2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia M.C., Rossen L.M., Bastian B., Faul M., Dowling N.F., Thomas C.C., Schieb L., Hong Y., Yoon P.W., Iademarco M.F. Potentially excess deaths from the five leading causes of death in metropolitan and nonmetropolitan counties - United States, 2010-2017. Morb. Mortal. Wkly. Rep. - Surveillance Summ. 2019;68(10):1–11. doi: 10.15585/mmwr.ss6810a1. Washington, DC : 2002) [DOI] [PubMed] [Google Scholar]
- 4.Thuny F., Naidoo J., Neilan T.G. Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur. Heart J. 2022;43(42):4458–4468. doi: 10.1093/eurheartj/ehac456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mokdad A.H., Dwyer-Lindgren L., Fitzmaurice C., Stubbs R.W., Bertozzi-Villa A., Morozoff C., Charara R., Allen C., Naghavi M., Murray C.J. Trends and patterns of disparities in cancer mortality among US Counties, 1980-2014. JAMA. 2017;317(4):388–406. doi: 10.1001/jama.2016.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aboumsallem J.P., Moslehi J., de Boer R.A. Reverse Cardio-oncology: cancer development in patients with cardiovascular disease. J. Am. Heart Assoc. 2020;9(2) doi: 10.1161/JAHA.119.013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh E.T., Bickford C.L. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J. Am. Coll. Cardiol. 2009;53(24):2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 8.Tamargo J., Caballero R., Delpón E. Cancer chemotherapy and cardiac arrhythmias: a review. Drug Saf. 2015;38(2):129–152. doi: 10.1007/s40264-014-0258-4. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020;17(8):474–502. doi: 10.1038/s41569-020-0348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bottinor W., Parikh A., Jahangir E. Emerging cancer therapies and cardiovascular risk. J. Thromb. Thrombolysis. 2021;51(4):837–845. doi: 10.1007/s11239-020-02263-9. [DOI] [PubMed] [Google Scholar]
- 11.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 12.Zong C.R.Y. Analysis of ECG results of 103 Tibetan breast cancer patients before and after chemotherapy. Tibetan Medicine. 2018;39(3):22–23. [Google Scholar]
- 13.Wang X., Zhao B.F., Chang B.P., Shui H.F. ECG changes and clinical significance of 176 patients with malignant tumor before and after chemotherapy. Journal of Critical Care in Internal Medicine. 2011;17(6):362–371. [Google Scholar]
- 14.Ma D., Zhu H.B. Effects of adriamycin on cardiovascular system in elderly patients. S. China J. Cardiol. 2000;6(2):99–100. [Google Scholar]
- 15.Liu N., Chen X.Q., He Y.L., Shen D.L. Clinical analysis of abnormality of electrocardiography in 632 patients with neoplasms after chemotherapy. Cancer Research and Clinic. 2009;21(4):256–258. [Google Scholar]
- 16.Liang X.Q., Shen Y.Q., Huang H.X., Wei L., Yang J.F. The clinical analysis of fluorouracil induiced cardiactoxicity in colorectal cancer. Journal of Frontiers of Medicine. 2019;9(13):15–16. [Google Scholar]
- 17.Zhang Q., Shen Y.Q., Liu W.J., Huang H.S., Si T., Lin H.Y., Kong X.Y. The study of cardiotoxicity of fluorouracil on patients with head and neck cancer. Henan Medical Research. 2020;29(9):1552–1556. [Google Scholar]
- 18.Lu D., Chen J.H. Clinical characteristics and influencing factors of arrhythmia during chemotherapy in the elderly patients with non-small cell lung cancer Practical Geriatrics. 2020;34(11):1161–1164. [Google Scholar]
- 19.Su Y.J., Zhou Y. Analysis of factors affecting cardiac arrhythmias complicated by adjuvant chemotherapy after breast cancer surgery. J. Guangxi Med. Univ. 2015;32(3):419–421. [Google Scholar]
- 20.Zhou D.S., Qin L. Risk factors of arrhythmia in elderly tumor patients. Journal of Modern Oncology. 2019;29(2):311–314. [Google Scholar]
- 21.Nickel A.C., Patel A., Saba N.F., Leon A.R., El-Chami M.F., Merchant F.M. Incidence of cancer treatment-induced arrhythmia associated with novel targeted chemotherapeutic agents. J. Am. Heart Assoc. 2018;7(20) doi: 10.1161/JAHA.118.010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitagawa K., Kawada K., Morita S., Inada M., Mitsuma A., Sawaki M., Iino S., Inden Y., Murohara T., Imai T., Ando Y. Prospective evaluation of corrected QT intervals and arrhythmias after exposure to epirubicin, cyclophosphamide, and 5-fluorouracil in women with breast cancer. Ann. Oncol. : official journal of the European Society for Medical Oncology. 2012;23(3):743–747. doi: 10.1093/annonc/mdr296. [DOI] [PubMed] [Google Scholar]
- 23.Fradley M.G., Gliksman M., Emole J., Viganego F., Rhea I., Welter-Frost A., Armanious M., Lee D.H., Walko C., Shah B., Chavez J.C., McLeod H., Pinilla-Ibarz J., Schabath M.B. Rates and risk of atrial arrhythmias in patients treated with ibrutinib compared with cytotoxic chemotherapy. Am. J. Cardiol. 2019;124(4):539–544. doi: 10.1016/j.amjcard.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 24.Qi W.X., Zhao S., Chen J. Risk factors for developing cardiac toxicities in cancer patients treated with panitumumab combination therapy. Future oncology (London, England) 2020;16(19):1359–1370. doi: 10.2217/fon-2020-0050. [DOI] [PubMed] [Google Scholar]
- 25.Rao V.P., Addae-Boateng E., Barua A., Martin-Ucar A.E., Duffy J.P. Age and neo-adjuvant chemotherapy increase the risk of atrial fibrillation following oesophagectomy. Eur. J. Cardio. Thorac. Surg. : official journal of the European Association for Cardio-thoracic Surgery. 2012;42(3):438–443. doi: 10.1093/ejcts/ezs085. [DOI] [PubMed] [Google Scholar]
- 26.Peng J., Dong C., Wang C., Li W., Yu H., Zhang M., Zhao Q., Zhu B., Zhang J., Li W., Wang F., Wu Q., Zhou W., Yuan Y., Qiu M., Chen G. Cardiotoxicity of 5-fluorouracil and capecitabine in Chinese patients: a prospective study. Cancer Commun. 2018;38(1):22. doi: 10.1186/s40880-018-0292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhai L., Guo C., Cao Y., Xiao J., Fu X., Huang J., Huang H., Guan Z., Lin T. Long-term results of pirarubicin versus doxorubicin in combination chemotherapy for aggressive non-Hodgkin's lymphoma: single center, 15-year experience. Int. J. Hematol. 2010;91(1):78–86. doi: 10.1007/s12185-009-0461-8. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q., Wang H.Q., Zhu X.L., Zhou Y.W., Zhang P. Journal of Tianjin Medical University; 2004. Multiple-factor Evaluation of the Cardiac Toxicity of Epirubicin; pp. 376–384. 03. [Google Scholar]
- 29.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., Boriani G., Castella M., Dan G.A., Dilaveris P.E., Fauchier L., Filippatos G., Kalman J.M., La Meir M., Lane D.A., Lebeau J.P., Lettino M., Lip G.Y.H., Pinto F.J., Thomas G.N., Valgimigli M., Van Gelder I.C., Van Putte B.P., Watkins C.L. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612. 2020. [DOI] [PubMed] [Google Scholar]
- 30.Zamorano J.L. Specific risk of atrial fibrillation and stroke in oncology patients. Eur. Heart J. 2016;37(36):2747–2748. doi: 10.1093/eurheartj/ehw385. [DOI] [PubMed] [Google Scholar]
- 31.Hu Y.F., Liu C.J., Chang P.M., Tsao H.M., Lin Y.J., Chang S.L., Lo L.W., Tuan T.C., Li C.H., Chao T.F., Chung F.P., Liao J.N., Chen T.J., Chen S.A. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int. J. Cardiol. 2013;165(2):355–357. doi: 10.1016/j.ijcard.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Lateef N., Kapoor V., Ahsan M.J., Latif A., Ahmed U., Mirza M., Anwar F., Holmberg M. Atrial fibrillation and cancer; understanding the mysterious relationship through a systematic review. J. Community Hosp. Intern. Med. Perspect. 2020;10(2):127–132. doi: 10.1080/20009666.2020.1726571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doyle J.J., Neugut A.I., Jacobson J.S., Grann V.R., Hershman D.L. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J. Clin. Oncol. : official journal of the American Society of Clinical Oncology. 2005;23(34):8597–8605. doi: 10.1200/JCO.2005.02.5841. [DOI] [PubMed] [Google Scholar]
- 34.Strongman H., Gadd S., Matthews A., Mansfield K.E., Stanway S., Lyon A.R., Dos-Santos-Silva I., Smeeth L., Bhaskaran K. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet (London, England) 2019;394(10203):1041–1054. doi: 10.1016/S0140-6736(19)31674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chadda K.R., Ajijola O.A., Vaseghi M., Shivkumar K., Huang C.L., Jeevaratnam K. Ageing, the autonomic nervous system and arrhythmia: from brain to heart. Ageing Res. Rev. 2018;48:40–50. doi: 10.1016/j.arr.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Garner M., Routledge T., King J.E., Pilling J.E., Veres L., Harrison-Phipps K., Bille A., Harling L. New-onset atrial fibrillation after anatomic lung resection: predictive factors, treatment and follow-up in a UK thoracic centre. Interact. Cardiovasc. Thorac. Surg. 2017;24(2):260–264. doi: 10.1093/icvts/ivw348. [DOI] [PubMed] [Google Scholar]
- 37.Peters R.W., Brooks M.M., Todd L., Liebson P.R., Wilhelmsen L. Smoking cessation and arrhythmic death: the CAST experience. The cardiac arrhythmia suppression trial (CAST) investigators. J. Am. Coll. Cardiol. 1995;26(5):1287–1292. doi: 10.1016/0735-1097(95)00328-2. [DOI] [PubMed] [Google Scholar]
- 38.Banks E., Joshy G., Korda R.J., Stavreski B., Soga K., Egger S., Day C., Clarke N.E., Lewington S., Lopez A.D. Tobacco smoking and risk of 36 cardiovascular disease subtypes: fatal and non-fatal outcomes in a large prospective Australian study. BMC Med. 2019;17(1):128. doi: 10.1186/s12916-019-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farmakis D., Parissis J., Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J. Am. Coll. Cardiol. 2014;63(10):945–953. doi: 10.1016/j.jacc.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 40.Lardaro T., Self W.H., Barrett T.W. Thirty-day mortality in ED patients with new onset atrial fibrillation and actively treated cancer. Am. J. Emerg. Med. 2015;33(10):1483–1488. doi: 10.1016/j.ajem.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eskilsson J., Albertsson M., Mercke C. Adverse cardiac effects during induction chemotherapy treatment with cis-platin and 5-fluorouracil. Radiother. Oncol. : journal of the European Society for Therapeutic Radiology and Oncology. 1988;13(1):41–46. doi: 10.1016/0167-8140(88)90296-4. [DOI] [PubMed] [Google Scholar]
- 42.Kosmas C., Kallistratos M.S., Kopterides P., Syrios J., Skopelitis H., Mylonakis N., Karabelis A., Tsavaris N. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J. Cancer Res. Clin. Oncol. 2008;134(1):75–82. doi: 10.1007/s00432-007-0250-9. [DOI] [PubMed] [Google Scholar]
- 43.Foran J.M., Rohatiner A.Z., Cunningham D., Popescu R.A., Solal-Celigny P., Ghielmini M., Coiffier B., Johnson P.W., Gisselbrecht C., Reyes F., Radford J.A., Bessell E.M., Souleau B., Benzohra A., Lister T.A. European phase II study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and previously treated mantle-cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma. J. Clin. Oncol. : official journal of the American Society of Clinical Oncology. 2000;18(2):317–324. doi: 10.1200/JCO.2000.18.2.317. [DOI] [PubMed] [Google Scholar]
- 44.Quezado Z.M., Wilson W.H., Cunnion R.E., Parker M.M., Reda D., Bryant G., Ognibene F.P. High-dose ifosfamide is associated with severe, reversible cardiac dysfunction. Annals of internal medicine. 1993;118(1):31–36. doi: 10.7326/0003-4819-118-1-199301010-00006. [DOI] [PubMed] [Google Scholar]
- 45.McMullen J.R., Boey E.J., Ooi J.Y., Seymour J.F., Keating M.J., Tam C.S. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124(25):3829–3830. doi: 10.1182/blood-2014-10-604272. [DOI] [PubMed] [Google Scholar]
- 46.Feliz V., Saiyad S., Ramarao S.M., Khan H., Leonelli F., Guglin M. Melphalan-induced supraventricular tachycardia: incidence and risk factors. Clin. Cardiol. 2011;34(6):356–359. doi: 10.1002/clc.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suter T.M., Ewer M.S. Cancer drugs and the heart: importance and management. Eur. Heart J. 2013;34(15):1102–1111. doi: 10.1093/eurheartj/ehs181. [DOI] [PubMed] [Google Scholar]
- 48.Menard O., Martinet Y., Lamy P. Cisplatin-induced atrial fibrillation. J. Clin. Oncol. : official journal of the American Society of Clinical Oncology. 1991;9(1):192–193. doi: 10.1200/JCO.1991.9.1.192. [DOI] [PubMed] [Google Scholar]
- 49.Tomkowski W.Z., Wiśniewska J., Szturmowicz M., Kuca P., Burakowski J., Kober J., Fijałkowska A. Evaluation of intrapericardial cisplatin administration in cases with recurrent malignant pericardial effusion and cardiac tamponade. Support. Care Cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2004;12(1):53–57. doi: 10.1007/s00520-003-0533-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are included in the article and its supplementary materials.