Summary
The study of vocal communication in non-human animals can uncover the roots of human languages. Recent studies of language have focused on two linguistic laws: Zipf’s law and the Menzerath-Altmann law. However, whether bats’ social vocalizations follow these linguistic laws, especially Menzerath’s law, has largely been unexplored. Here, we used Asian particolored bats, Vespertilio sinensis, to examine whether aggressive vocalizations conform to Zipf’s and Menzerath’s laws. Aggressive vocalizations of V. sinensis adhere to Zipf’s law, with the most frequent syllables being the shortest in duration. There was a negative association between the syllable number within a call and the average syllable duration, in agreement with Menzerath’s law. A decrease in the proportion of some long syllables and a decrease in the duration of several syllable types in long-duration calls explain the occurrence of this law. Our results indicate that a general compression principle organizes aspects of bat vocal communication systems.
Subject areas: Zoology, Animals, Ethology, Linguistics
Graphical abstract

Highlights
-
•
Aggressive calls of Asian particolored bats follow Zipf’s law and Menzerath’s law
-
•
Short-duration syllable types were used more frequently
-
•
Longer calls composed of shorter syllables
-
•
Menzerath’s law pattern may be driven by vocal plasticity on vocal production
Zoology; Animals; Ethology; Linguistics
Introduction
The origins of human languages have been considered to be one of the most difficult questions in science and have attracted the attention of scientists and philosophers for centuries.1 A particularly helpful approach for understanding the evolution of language is the comparison of communication systems between human and non-human animals.2 Exploring the universality of vocal organization in non-human animals can offer critical insight into the processes that shape language diversity.3,4
Recent studies have shown that human languages adhere to a battery of statistical laws, known as “linguistic laws,” which are correlated with the principles of compression (minimization of the length of a code) (reviewed in the study by Semple et al.5). Of particular significance are Zipf’s law of brevity (hereafter Zipf’s law) and the Menzerath-Altmann law (hereafter Menzerath’s law). Zipf’s law6 shows that in human language, more frequently used words tend to be shorter.7 Zipf’s law has also been shown to apply in the vocal communication system of non-human animals. The inverse association between sound duration and frequency of use is explained by the use of compression to maximize coding efficiency and reduce the cost of production of long sounds.8,9 Note that Zipf’s law is different than the Zipf-Mandelbrot law. The latter is designed to fit a specific function to a frequency distribution of ranked data.10 We address only Zipf’s law here. Menzerath’s law postulates that “the greater the whole, the smaller its constituents,” or in other words “longer communication constructs are made up with smaller parts.”11,12 This law predicts a negative relationship between constructs and constituents, such that the longer a word, the shorter the syllables that are used to construct that word. The underlying mechanisms of Menzerath’s law in vocal communication can be interpreted as selection for compression, biomechanical constraints on vocal production,13 or selection for coding efficiency.14 To date, both Zipf’s and Menzerath’s laws in vocal communication have been identified in avian, dolphin, and primate species.14,15,16,17,18
To broaden the assessment of the phylogenetic distribution and the broadscale applicability of these linguistic laws to animal vocal communication, it is essential to explore Zipf’s law and Menzerath’s law in other vocal species. Bats not only emit echolocation pulses for navigation and prey acquisition but also produce rich social vocalizations for communication.19,20 Bats are one of the few mammalian taxa that have the ability to modify vocal production through learning21,22 and the ability to exhibit vocal syntax.23,24 Adherence to Zipf’s law has been shown in social vocalizations of four species of bats (greater horseshoe bat, Rhinolophus ferrumequinum; least horseshoe bat, Rhinolophus pusillus; black-bearded tomb bat, Taphozous melanopogon; and Mexican free-tailed bat, Tadarida brasiliensis25). Whether social vocalizations in bats agree with Menzerath’s law remains uncertain.
Here, we examine whether aggressive vocalizations follow Zipf’s law or Menzerath’s law in the female Asian particolored bats, Vespertilio sinensis (family: Vespertilionidae). We only focus on aggressive vocalizations because they are one of the most common types of social vocalizations and play seminal roles in the mating and competitive activities of many bat species.26,27 Vespertilio sinensis is a nocturnal and highly social species that usually roosts in natural or artificial structures, sharing day and night roosts among hundreds of individuals.28,29 Our previous studies showed that adult females compete for a more central roosting position in the nursery colonies to increase safety and reduce energy loss.30 These aggressive interactions frequently include a vocal component (Figure 1; Table 1; see the study by Zhao et al30). First, we hypothesized that aggressive vocalizations of V. sinensis are consistent with Zipf’s law. We predicted that the duration of any specific syllable type would be negatively correlated with the number of each syllable type. Moreover, for brevity to have significance, it should be prevalent, not only when more syllables are emitted within an absolute time frame but also at a higher rate within relatively short time windows; we therefore assessed whether this pattern in the entire duration of the aggressive interactions was the same as the pattern in the shorter 4-min time window.
Figure 1.
Aggressive vocalizations of Vespertilio sinensis
(A and B) Spectrograms of syllable types.
(C) An example spectrogram of three calls. Oscillograms (above) and spectrograms (below). See Table 1 for detailed name and code adopted for each syllable.
Table 1.
Nomenclature, abbreviations, code, mean duration and total number of syllables
| Syllable abbreviation | Syllable name | Syllable code | Mean duration (ms) | Total number |
|---|---|---|---|---|
| bDFM | bent downward frequency modulation (FM) | A | 5.84 | 1,245 |
| BNBs | short broadband noise burst | Bs | 13.81 | 1,136 |
| cDFM | checked downward FM | C | 15.55 | 384 |
| QCFs | short quasi-constant frequency | D | 19.46 | 311 |
| HFM | humped FM | E | 26.59 | 328 |
| bDFM-NB | bent downward FM to noise burst | Fc | 30.13 | 508 |
| cDFM-NB-HFM | checked downward FM to noise burst to humped FM | Gc | 52.94 | 287 |
| NB-cDFM | noise burst to checked downward FM | Hc | 56.63 | 656 |
| QCFl | long quasi-constant frequency | I | 59.21 | 19 |
| BNBl | long broadband noise burst | Bl | 113.35 | 151 |
See Figure 6 for spectrograms of the syllables. Note that Fc, Gc, and Hc are combined syllables derived from concatenated simple syllables. See text.
Second, we hypothesized that aggressive vocalizations of V. sinensis adhere to Menzerath’s law. Therefore, we predicted that syllable duration would be negatively associated with the total syllable number within a call. If our data are consistent with Menzerath’s law, this would lead us to further explore three different underlying mechanisms of this law. Mechanism 1: if the mechanism is a consequence of breathing constraints, syllable duration would be negatively associated with syllable position in a call; the syllable position was represented by the position of a syllable in a sequence of syllables. For example, if there are three syllables within a call, the syllable position of the first, second, and third syllable in the call was simply 1, 2, and 3, respectively. Mechanism 2: if the mechanism is a result of the types of syllables used in a call changing with the total number of syllables of any type produced within a call, then the proportion of short syllables should increase and the proportion of long syllables should decrease with longer calls; mechanism 2 tests whether longer calls can be produced more efficiently with the use of short syllable types. Mechanism 3: if the mechanism is due to the duration of any specific syllable type changing with the number of that specific syllable used in the call, then as the number of any specific syllable within a call increases, the duration of that specific syllable should decrease. Mechanism 3 tests whether longer calls can be produced more efficiently by shortening the specific syllables produced in the call. If mechanism 3 is correct, simple syllables should also be shortened when they are concatenated into combined syllables. Testing for mechanism 3 is important because it would otherwise be difficult to conclude whether bats can reduce the duration of syllables in longer calls or alternatively use short syllables more frequently in longer calls. Together, it is important to investigate the underlying mechanisms because this helps us understand the trade-off between effectiveness of communication, and the energetic demands and breathing constraints of vocal production.
Results
Spectrographic classification scheme for vocalizations
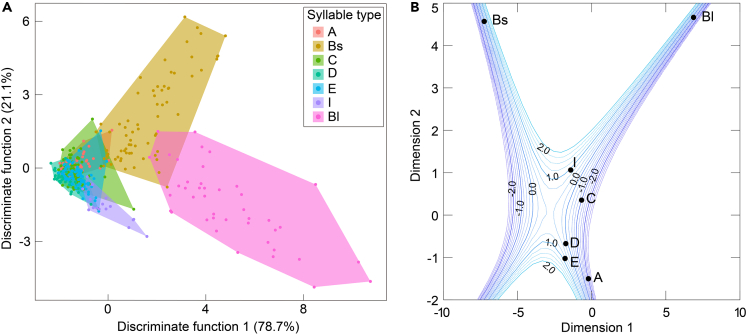
A discriminant function analysis showed that 50.4% of 361 syllables were classified to the correct syllable types (Table S1, and Figure 2A). The percent of correct classification was significantly greater than expected by chance (1/7 = 14.3%; binomial test: p < 0.001). Multidimensional scaling was used to reduce the dimensionality of distances between the different types of simple syllables along multiple dimensions in the multiparametric acoustic space. Our data show that most syllables were clearly separated along the axes of all three dimensions (Figure 2B).
Figure 2.
Differences in acoustic characteristics of seven simple syllables emitted by Vespertilio sinensis
(A) First two dimensions of a discriminant functional analysis for seven syllable types. Each color represents one syllable type. Each solid circle represents one syllable.
(B) Contour plot showing the configuration resulting from the multidimensional scaling iterations for seven syllable types. See Table 1 for detailed name and code adopted for each syllable.
Zipf’s law in aggressive vocalizations
We analyzed 5,025 syllables recorded from 15 female V. sinensis to test Zipf’s law of brevity (see Table S2, and Figure S1). The number and the duration of each syllable type are shown in Table S3 and Figure 3A. Syllable type duration correlated negatively and significantly with the number of each syllable type (linear mixed model [LMM]: estimate = −0.218, t = −3.869, p = 0.0002; Figure 3B), as predicted by Zipf’s Law.
Figure 3.
Syllable duration and test of Zipf’s law
(A) Duration of each syllable type. The median duration of syllable types in (A) increases from left to right on the X axis. Lower and upper box boundaries show the 25th and 75th percentiles, respectively, with the median inside. The lower and upper error lines depict the 10th and 90th percentiles, respectively. Outliers of the data are shown as orange dots.
(B) The relationship between syllable duration and the number of each syllable type in a sample of aggressive calls by female Vespertilio sinensis. Orange points in (B) are mean duration ± standard deviation. The line represents a linear regression (see STAR Methods). The data used for the analysis for (B) included the outliers colored orange in (A). p value is from a linear mixed model. See Table 1 for detailed name and code adopted for each syllable.
We analyzed 3,958 syllables recorded only within 4 min of the start of agonistic interactions from 15 female V. sinensis to test Zip’s law of brevity (Table S4). Our results based on the entire duration of the aggressive interactions (Figures 3A and 3B) are substantially similar to results for the subset of calls given within the shorter 4-min time window (LMM: estimate = −0.264, t = −3.629, p = 0.0004; Figure S2).
Menzerath’s law in aggressive vocalizations
We analyzed 2,568 calls produced by 15 female V. sinensis to test Menzerath’s law (see Table S2). Each call included on average 1.96 ± 1.89 (mean ± SD) syllables (range 1–24). Each bat produced on average 171.13 ± 98.01 (mean ± SD) calls and 335.00 ± 166.10 (mean ± SD) syllables. Syllable duration was negatively associated with syllable number within a call (LMM: estimate = −0.674, t = −3.100, p = 0.002), as predicted by Menzerath’s law. The top Akaike’s information criterion (AICc) model contained only one predictor variable, i.e., syllable number within a call (Table 2). Model averaging revealed that syllable number within a call was negatively and significantly associated with syllable duration (95% confidence interval: −0.951, −0.268).
Table 2.
Results of the AICc model selection procedure used to investigate the effect of syllable number within a call and syllable position on syllable duration in Vespertilio sinensis
| Model | Predictive variables | df | LogL | AICc | ΔAICc | wi |
|---|---|---|---|---|---|---|
| 1 | SyllableN (−) | 5 | −24,947.17 | 49,904.36 | 0.00 | 0.69 |
| 2 | SyllableN (−), SPC (+) | 6 | −24,947.02 | 49,906.05 | 1.69 | 0.30 |
| 3 | SPC (+) | 5 | −24,951.81 | 49,913.63 | 9.27 | <0.01 |
| 4 | Null | 4 | −24,955.40 | 49,918.81 | 14.45 | <0.01 |
Models are ranked based on the AICc values from the best to the worst model. The sign of the regression coefficient of the associations between syllable duration and syllable number within a call and syllable position is shown in parentheses (“−”: negative; “+”: positive). LogL: Log likelihoods. SyllableN: syllable number within a call. SPC: syllable position in the call.
Mechanisms of Menzerath’s law in aggressive vocalizations
-
(1)
Does syllable duration get shorter later in the call (mechanism 1)?
No significant relationship was found between syllable duration and syllable position in the call (LMM: estimate = 0.184, t = 0.328, p = 0.575). This result meant that shorter syllables failed to occur at the end of the call, which was not consistent with breathing constraints.
-
(2)
Do proportions of syllable types change with syllable number in the call (mechanism 2)?
Investigating the relationship between the syllable number within a call and the proportion and duration of different syllable types within a call can help to reveal mechanism 2 and mechanism 3, respectively (see Introduction). We found that the proportion of two short syllables (“Bs” and “D”) and two long syllables (“E” and “Fc”) was negatively associated with syllable number within a call (Table S5, and Figure 4). There were no significant relationships between the syllable number within a call and the proportion of one short syllable (“A”) and four long syllables (“Gc,” “Hc,” “I,” and “Bl”; Table S5, and Figure 4). These results suggest that the proportion of at least one long syllable type decreased in longer calls although some short syllable types also decreased in longer calls.
-
(3)
Do syllables of a particular type shorten in duration in longer calls (mechanism 3)?
Figure 4.
Test of Menzerath’s law mechanism 2
Relationship between the proportion of syllable of each type within a call as a function of the total syllable number of all types in calls of Vespertilio sinensis. Points are mean proportions ± standard error (SE). The line represents linear regression. p values are from generalized linear mixed-effects models. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See Table 1 for detailed name and code adopted for each syllable.
The duration of one short syllable type (“D”) and two long syllable types (“Hc” and “E”) was shorter in longer calls (Table S6, and Figure 5). No significant relationships were found between the duration of three short syllable types (“A,” “Bs,” and “C”) and four long syllable types (“Fc,” “Gc,” “I,” and “Bl”), and syllable number within a call (Table S6, and Figure 5). These results indicated that V. sinensis shortened specific syllable types in longer calls.
Figure 5.
Test of Menzerath’s law mechanism 3
Relationship between syllable duration and syllable number within a call for each syllable type in Vespertilio sinensis. Points are mean durations ± SE. The line represents linear regressions (see STAR Methods). p values are from generalized linear mixed-effects models. ∗∗p < 0.01, ∗∗∗p < 0.001. See Table 1 for detailed name and code adopted for each syllable. Note: The syllables of one type that are shown to have different durations can belong to the same bats and/or to different bats. The syllable duration of each bat can vary with the syllable number (see Figures S5–S14).
For mechanism 2 and 3, we also tested models that removed data points with fewer than three points per syllable number, and this did not change the conclusions drawn from the results (see Figures S3 and S4).
-
(4)
Do bats have the ability to shorten syllable duration?
There was no significant difference in the duration of “A” in simple syllables and in combined syllables (“Fc”; Figure 6A, and Table S7). No significant differences were found in the duration of “Bs” in simple syllables and in combined syllables (“Fc”; Figure 6D, and Table S7). There were also no significant differences in the duration of “Bl” in simple syllables and in combined syllables (“Fc”; “Gc”; Figure 6E, and Table S7). In contrast, the duration of the “E” syllable was significantly shorter in combined syllables (“Gc”) compared to simple syllables (Figure 6B, and Table S7). The duration of “C” in simple syllables was also significantly shorter in combined syllables compared to simple syllables (“Gc”; “H”; Figure 6C, and Table S7). The duration of the “Bs” syllable was significantly longer in combined syllables (“Hc”; “Gc”) than in simple syllables (Figure 6D, and Table S7), while the duration of “Bl” was significantly shorter in combined syllables (“Hc”) than in simple syllables (Figure 6E, and Table S7). These results suggested that V. sinensis have the capacity to shorten syllable duration.
Figure 6.
Duration of Vespertilio sinensis simple syllables compared to syllables that are combinations of two or more simple syllables
Error bars show the duration of (A) a simple “A” compared to the “A” element of an “Fc” combined syllable, (B) a simple “E” compared to the “E” element in a “Gc” combined syllable, (C) a simple “C” syllable compared to the “C” syllable in both the “Gc” and “Hc” combined syllables, (D) a simple “Bs” syllable compared to the “Bs” elements in the combined “Hc,” “Fc,” and “Gc” syllables, (E) a simple “Bl” syllable compared to the “Bl” elements in “Hc,” “Fc,” and “Gc” combined syllables. Means are shown ± SE. See Table 1 for detailed name and code adopted for each syllable. p values are from paired-sample t tests with the Bonferroni correction. ∗∗p < 0.01, ∗∗∗p < 0.001. NS: not significant (paired-sample t test with the Bonferroni correction). Note: Bats can shorten the syllables when they are combined (B, C, E) but can also elongate the syllables when they are combined (D; see Figure S15).
Discussion
We found that short-duration syllable types were used more frequently than long-duration syllable types, which supported our first hypothesis that aggressive vocalizations of V. sinensis follow Zipf’s law. Moreover, we found that longer calls comprised shorter syllables, which supported the second hypothesis that aggressive vocalizations of V. sinensis follow Menzerath’s law. Contrary to mechanism 1 that proposed that syllable duration would get shorter later in the call, our result showed that syllable duration was not correlated with syllable position in a call. We also found that as the syllable number within a call becomes longer, the proportion of some long syllables decreases, which partially supported mechanism 2. However, the proportion of some shorter syllables also decreased with an increase in syllable number within a call. Finally, we found that the duration of some syllable types shortens when used in long calls, supporting mechanism 3. To our knowledge, this is the first evidence that the vocal communication of a bat species follows Menzerath’s law.
The observed negative relationship between syllable duration and its frequency of occurrence is consistent with the observed patterns in birds,15,31 dolphins,18 and non-human primates.13,16,32,33 As the production of social vocalizations can represent a large energetic cost in many taxa34,35 including bats,36 animals may face a trade-off between the effectiveness of signal transmission and energy expenditure. Our previous study showed that “A,” “Bs,” “Bl,” and “Hc” syllables produced by female V. sinensis during aggressive interactions can contain honest information about body size and caller quality. The use of these syllables also predicts fighting success.30 In this previous study, we found that female V. sinensis preferred to use “A” and “Bs” syllables (47% of all syllables) that are relatively short instead of using “Bl” and “Hc” syllables that are relatively long. This result suggests that female V. sinensis may adopt a brevity strategy in aggressive vocalizations to increase signal transmission rate while also saving energy. Brevity may be particularly important over short time frames at the initiation of aggression. However, our results were the same when we restricted our analysis to calls given within 4 min of the first aggressive behavior pattern, suggesting that syllable duration is not subject to short-term breathing constraints.
Our results echo previous studies of social vocal patterns consistent with Zipf’s law in three bat superfamilies, namely Molossidae (T. brasiliensis), Emballonuridae (T. melanopogon), and Rhinolophidae (R. ferrumequinum and R. pusillus).25 Our findings extend the universality of this linguistic law to a fourth superfamily, the Vespertilionidae. Taken together, these results show that signal brevity is widely used in bats’ social communication.
In agreement with Menzerath’s law, we found that longer aggressive calls are made up of shorter syllables. The negative relationship between the syllable number within a call and the duration of their constituting syllables has been documented in 16 bird species,15,17 one dolphin species,18 and seven primate species.13,14,16,33,37,38 The presence of such patterns across disparate clades (e.g., birds, dolphins, non-human primates, and bats) suggests that Menzerath’s law may be a universal principle used to improve the efficiency of signal organization for signal transmission. Therefore, despite their special adaptations for ultrasound communication, echolocating bats seem to employ a taxonomically widespread strategy to enhance the efficient transfer of information.
We found that syllable duration failed to become shorter later in the call as would be expected if syllable use within calls was subject to breathing constraints.2 In other words, this result suggests that from the beginning of calls, female V. sinensis utter aggressive calls of the “suitable” duration for that call size, and syllable duration does not then decrease predictably over the course of the call. A similar result was documented in geladas’ short-range contact calls (Theropithecus gelada14).
Vocal patterns consistent with Menzerath’s law can be driven by the composition of calls changing with the syllable number.14 For example, in the western black-crested gibbon (Nomascus concolor) and cao vit gibbon (N. nasutus), the emergence of patterns in agreement with Menzerath’s law is due to an increase in the proportion of short syllable types and a decrease in the proportion of long syllable types in longer vocal sequences.16 In this study, we found that in longer calls, the proportion of certain short syllables (i.e., “Bs,” “C,” and “D”) and of certain longer syllables (i.e., “E” and “Fc”) decreased. This is at best partial support for this aspect of Menzerath’s law. One possible interpretation for the reduction in the proportion of certain short syllables in longer calls is that V. sinensis may save energy by reducing the production of syllables that contain unimportant information.
Conformity to Menzerath’s law can also be caused by a shortening of specific syllable types when used in longer calls.16 For instance, in the male geladas (T. gelada), the duration of four of six syllable types was shortened in longer calls.14 Our results showed that in long-duration calls, the duration of two longer syllables (i.e., “E” and “Hc”) and one short syllable (i.e., “D”) decreased, suggesting that the Menzerath’s law pattern in female V. sinensis may be shaped by abbreviating a specific syllable type in longer calls. We further found that when used in combination, some of the simple syllables (i.e., “E,” “C,” and “Bl”) were shortened. These results indicate that female V. sinensis have the ability to reduce the duration of syllables.
Our study suggests that compression exists in the short-distance vocal communication of female V. sinensis. The aggressive vocalizations of female V. sinensis can encode information about resource holding potential and aggressive intent, thereby playing a vital role in conflict resolution.30,39 It has been proposed that compression may be more prevalent in short-distance vocalizations than in long-distance vocalizations because the receiver may easily recognize the identity and position of the caller due to less sound attenuation for short-distance transmission.40 When trade-offs exist between signal compression and the effectiveness of signal transmission, the first concern is the effective transmission of the signal. However, signal transmission is less relevant for short-distance vocalizations, in which case efficiencies realized through the adherence to both Zipf’s law and Menzerath’s law would be more important. The aggressive vocalizations studied here commonly occur in behavioral contexts where bats are close to each other; therefore, it is reasonable to suggest that female V. sinensis may tend to select for efficiency of signal organization (minimization of signal length) to minimize energetic expenditure in vocalizations.
Limitations of the study
We acknowledge that our study has two limitations. First, because echolocation calls of bats can play a role in communication and significantly vary in signal duration, they may follow the same linguistic laws as their social calls. However, we cannot test this hypothesis with our dataset because echolocation signals of low-duty cycle echolocators emitted in a cage may give very little information about echolocation given the realities of overlap between pulse and echo. Thus, further studies are needed to examine whether echolocation calls of low-duty cycle and high-duty cycle echolocators in bats produced in an open environment conform to Zipf’s and Menzerath’s law. Second, although our results suggested that the Menzerath’s law pattern in female V. sinensis may not be due to breathing constraints on vocal production, additional physiological studies (i.e., lung capacity, respiration rate, and heart rate) should be conducted to confirm the link between energy consumption and vocal production.36
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Raw and analyzed data | This paper | N/A |
| Experimental models: Organisms/strains | ||
| Asian particoloured bats, Vespertilio sinensis | Wild-caught | N/A |
| Software and algorithms | ||
| R | R Core Team | Version 4.2.2 |
| Avisoft SASLab Pro | Avisoft Bioacoustics, Glienicke, Germany | Version 5.1 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Congnan Sun (suncongnan@hebtu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
The data supporting the results of the article are available as supplemental information.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Animals
A total of 15 adult V. sinensis females were captured using mist nets in a highway bridge (Harbin City, Heilongjiang Province, northeastern China) for this study. Bats were brought to our lab and housed in a free-flight husbandry room (length × width × height: 8 m × 5 m × 3 m).
Each bat was marked with a numbered split aluminum alloy bat ring (5.2 mm, Porzana Ltd., Icklesham, UK) for individual identification. Ambient temperature (26 ± 2°C), relative humidity (55 ± 5%) and lighting (natural photoperiod) were maintained according to ambient conditions in the natural roost. All bats were given ad libitum access to water and mealworms (Zophobas morio).
Our work adheres to the Guidelines for the Use of Animals in Research,41 to the National Natural Science Foundation of China for experiments involving vertebrate animals, and was approved by the Animal Research Ethics Board of Hebei Normal University China (approval number: HEBTU-2022LLSC044). No bat suffered any obvious injuries from capture and transport and our experiments did not result in any physical injuries or death. Bats were always treated gently. No visible physical injuries were observed, although some contests involved physical contact. All bats were released in good health at their original site of capture after the completion of the experiment.
Method details
Data collection
We reanalysed sound and video recordings of dyadic aggressive encounters between female V. sinensis originally described in Zhao et al.39 Briefly, Zhao et al.39 captured 15 adult females in July 2017 in Harbin, Heilongjiang province, China. One of the 15 females was chosen at random and placed into a temporary ‘home cage’ (50 × 50 × 50 cm) as a ‘resident’. When the resident calmed down, another bat was introduced into the home cage as an ‘intruder’. Usually, the intruder would compete for a more central roosting position by pushing the resident. The resident often produced aggressive vocalizations to defend its own private roosting territory. The aggressive encounters and aggressive vocalizations were recorded by a night-shot camera (HDR-CX 760E; Sony Corp., Tokyo, Japan) and an Avisoft UltraSoundGate116H (Avisoft Bioacoustics, Berlin, Germany) with a condenser ultrasound microphone (CM16/CMPA, Avisoft Bioacoustics), respectively. Every bat was used as the resident and interacted individually with each of the other 14 bats as intruders. The experimental procedures are described in detail in Zhao et al.39
Acoustic analysis
Following Kanwal et al.42 and Zhao et al.,39 we defined a syllable as the smallest discrete part of a vocalization and a call as the simplest emission of a whole vocalization consisting of one or more syllables. Previous work showed that the aggressive vocalizations of female V. sinensis are composed of seven simple syllables and three composite syllables43,44 (Figure 1, and Table 1). We performed statistically verifiable acoustic analyses of Asian particoloured bat vocalizations to map the acoustic boundaries of the syllables. To distinguish between syllable types, we only selected syllables with good signal-to-noise ratios and measured 22 total acoustic parameters (see Table S8). In total, we measured 361 syllables including ‘A’ (37), ‘Bs’ (69), ‘C’ (75), ‘D’ (51), ‘E’ (71), ‘I’ (16), and ‘Bl’ (42) syllables (see Table S9). Following the definitions of Luo et al. (2017), we defined ‘Bs’ as ‘B’ syllables having a duration less than 48 ms and ‘Bl’ as ‘B’ syllables having a duration greater than or equal to 48 ms. The duration of ‘B’ syllables analysed in this study indicate that these two ‘B’ syllables differ significantly in their duration (duration: 113.35 ± 99.75 ms for Bl vs. 13.81 ± 9.58 ms for Bs; U1136,151 = 0, p < 0.001). Similar results can be found in other bat species, such as greater horseshoe bats R. ferrumequinum,45 mustached bats Pteronotus parnellii,42 and greater tube-nosed bats Murina leucogaster.46
Three of the syllables described here are combinations of other syllables.43,44 The ‘Fc’ syllable is a composite of ‘A’+’B’ syllables; the ‘Gc’ syllable is a composite of ‘C’+’B’+’E’ syllables; and the ‘Hc’ syllable is a composite of ‘B’+’C’ syllables. The ‘B’ element in all three combination syllables can either be short (‘Bs’) or long (‘Bl’).
We first used the 22 acoustic parameters to construct a principal component analysis to reduce the highly correlated original variables to a smaller set of uncorrelated variables. We extracted three principal components (with eigenvalues > 1), which explained 77.46% of the total variance (Table S10). We calculated the Kaiser-Meyer-Olkin (KMO) index and conducted Bartlett's test to determine whether our data were suitable for PCA; both the KMO index (0.730) and Bartlett's criteria (=19,277.6; p < 0.001) suggested that PCA was suitable.
We next used a discriminant function analysis (DFA) on our three principal components to test for syllable types. The prior probabilities of the DFA were adjusted for unequal numbers of cases. The DFA used a leave-one-out-cross-validation procedure, which classified each syllable type based on the discriminant functions established with all syllables except the syllable being classified. The success of the DFA classification was determined with a binomial test comparing the DFA classification to a random classification.
Finally, we calculated mean values of each acoustic parameter and conducted multidimensional scaling to arrange Euclidean distances between syllables in a three-dimensional space.
Following Zhao et al.,30 we used 40 ms as a lower threshold for inter-call interval of aggressive calls for V. sinensis. Therefore, syllables were marked as belonging to the same call when the inter-syllable silence was < 40 ms. Inter-syllable silence > 40 ms indicated the beginning of a new call. We normalized each syllable to an amplitude of 0.75 V and measured the syllable duration (time between the beginning and end of a syllable; ms) from the oscillograms using a cursor.
All vocalizations were analysed using Avisoft-SASLab Pro v 5.1 (R. Specht, Avisoft Bioacoustics). Prior to acoustic measurement, we originally normalized each sound to a peak amplitude of 0.75 V. Spectrograms used to measure acoustic parameters were produced using a Hamming window and a 1024-point fast Fourier transform (75% frame size; 93.75% overlap; temporal resolution: 0.256 ms; frequency resolution: 0.244 kHz).
Statistical analysis
Test for Zipf's Law
To explore the association between syllable type duration and the frequency of use, we ran a linear mixed model (LMM) using the function ‘lmer’ in R package ‘lme4’ including the mean duration of each syllable type as the dependent variable, the number of each syllable type as an independent variable, and individual identity as a random effect. The mean duration of each syllable type per female was the sum of each specific syllable duration from all 14 trials divided by the number of this syllable type from all 14 trials. The number of specific syllable types per female was the total number of each specific syllable type per female from all 14 trials (Table S11 lists the data set).
To ensure that this pattern is not driven by vocalizations given immediately after the aggression starts, we ran a separate analysis of syllables produced only within four min of the start of aggressive interactions. The dependent variable, independent variable and random effect were the same as for the above LMM.
Test for Menzerath's law
To examine the relationship between the syllable duration and syllable number within a call, we created a LMM including syllable duration as a dependent variable, syllable number within a call and syllable position in the call as the independent variables, and bat identity and trial as random effects. Syllable number and syllable position were treated as treated as continuous variables. To obtain the optimized linear models that best explained the variation in syllable duration, we performed a model selection with normal model type using the function ‘dredge’ in R package ‘MuMIn’. The dependent and independent variables were the same as for the LMM. The model generated a set of four candidate models, including the main effects of the independent variables and all possible combinations of these main effects via ordinary least squares linear regression. The competing models were compared with the Akaike information criterion corrected for small sample size (AICc). The best models, which account for the most variation with the least predictors, have the lowest AIC values. Differences among AIC values were calculated as follows: Δi = AICi – AICmin. Furthermore, ΔAICc > 2 between the first and the second best models is considered the gold standard for model selection47; therefore, multimodel inference was performed if AIC differences were ≤ 2, using the model.avg function in the package ‘MuMIn’.48 Akaike weights (wi) were also calculated to explain the relative likelihood of a given model; these values represent the normalization of the probabilities of different models given the data.49
Underlying mechanisms of Menzerath's law
Because our result adhered to Menzerath's law (see Results section), we further investigated whether the proportion of short syllable types increased with syllable number within a call and whether the proportion of long syllable types decreased with syllable number within a call. We performed ten generalized linear mixed-effects models (GLMMs) with binomial error distributions and logit link functions with one model for each syllable type. In the GLMM, the dependent variable was the proportion of syllable types, syllable number within a call was an independent variable and bat identity and trial were random effects. The proportion of specific syllable types was calculated as (the syllable number of a specific type)/(the overall syllable number in the call). The data of the proportion of specific syllable types per individual comes from all 14 trials (Table S12). The number of calls analysed per trial per female can be found in Table S12.
To explore whether the duration of specific syllable types decreased as the call became longer, we conducted ten LMMs with one model for each syllable type. In the model, the mean duration of each syllable type was the dependent variable, syllable number within a call was an independent variable and bat identity and trial were random variables. The mean duration of each syllable type within calls of different length was calculated by dividing the sum of syllable durations by the number of this syllable type in the vocal sequence. The mean duration of each syllable type within calls per individual and the syllable number within a call per individual come from all 14 trials (Table S13). The number of calls analysed per trial per female can be found in Table S14. The level for statistical significance was set at α < 0.05 and Benjamini & Hochberg methods were used to adjust p values due to multiple testing.
To explore whether female Asian particoloured bats have the capacity to shorten syllable duration when combining simple syllables into combination syllables, we used individual mean values from all 14 trials and performed paired t tests to compare differences in duration between simple isolated syllables and combination calls that are composed of these simple syllables. Moreover, the ‘B’ component in the three combined syllables (i.e., ‘Fc’, ‘Gc’, ‘Hc’) can include either a ‘Bs’ or a ‘Bl’ component. We therefore compared differences in duration between ‘Bs’ alone and ‘Bs’ components in combined syllables and differences in duration between ‘Bl’ syllables alone and ‘Bl’ components in combined syllables. The syllable number analysed per trial per female can be found in Tables S15–S21. All statistical tests were conducted in R v. 4.2.2.50
Acknowledgments
We thank Dr. Camilla Imarisio and two anonymous reviewers for providing helpful comments on the manuscript. This research was supported by the National Natural Science Foundation of China (grant nos. 32300392 and 32371562), the Project funded by China Postdoctoral Science Foundation (grant no. 2023M730913), the Natural Science Foundation of Hebei Province (grant nos. C2023205010 and C2023205017), the Doctoral Research Foundation of Hebei Normal University (grant nos. L2022B16 and S22B048), the Innovation and Entrepreneurship Training Program for College Students (grant nos. 202310094005 and S202310094003), the Hebei Province to introduce overseas students funding project (grant no. C20230345), the Fundamental Research Funds for the Central Universities (2412023YQ002), and the Natural Science Foundation of Inner Mongolia Autonomous Region (2020LH03014).
Author contributions
Conceptualization, methodology, formal analysis, visualization, writing – original draft, and funding acquisition, C.Z. and C.S.; investigation, C.Z., C.S., Z.Z., Y.W., X.Z., and X.F.; writing – review and editing, J.R.L., J.F., and T.J.
Declaration of interests
The authors declare no competing interests.
Published: June 28, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.110401.
Contributor Information
Congnan Sun, Email: suncongnan@hebtu.edu.cn.
Tinglei Jiang, Email: jiangtl730@nenu.edu.cn.
Supplemental information
Syllable number is also given for each syllable type during each trial (column F–S), related to STAR Methods.
References
- 1.Christiansen M.H., Kirby S. In: Language evolution. Christiansen M.H., Kirby S., editors. Oxford University Press; 2003. Language evolution: the hardest problem in science? pp. 1–15. [Google Scholar]
- 2.Collier K., Bickel B., van Schaik C.P., Manser M.B., Townsend S.W. Language evolution: syntax before phonology? Proc. Biol. Sci. 2014;281 doi: 10.1098/rspb.2014.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balter M. Evolution of language. Animal communication helps reveal roots of language. Science. 2010;328:969–971. doi: 10.1126/science.328.5981.9. [DOI] [PubMed] [Google Scholar]
- 4.Freeberg T.M., Lucas J.R. Information theoretical approaches to chick-a-dee calls of Carolina chickadees (Poecile carolinensis) J. Comp. Psychol. 2012;126:68–81. doi: 10.1037/a0024906. [DOI] [PubMed] [Google Scholar]
- 5.Semple S., Ferrer-i-Cancho R., Gustison M.L. Linguistic laws in biology. Trends Ecol. Evol. 2022;37:53–66. doi: 10.1016/j.tree.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zipf G. Routledge G; 1936. The Psycho-Biology of Language: An Introduction to Dynamic Philology. [Google Scholar]
- 7.Bentz C., Ferrer-i-Cancho R. In: Proceedings of the Leiden Workshop on Capturing Phylogenetic Algorithms for Linguistics. Bentz C., Jager G., Yanovich I., editors. University of Tübingen; 2016. Zipf's law of abbreviation as a language universal; pp. 1–4. [Google Scholar]
- 8.Bezerra B.M., Souto A.S., Radford A.N., Jones G. Brevity is not always a virtue in primate communication. Biol. Lett. 2011;7:23–25. doi: 10.1098/rsbl.2010.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancho R.F.I., Solé R.V. Least effort and the origins of scaling in human language. Proc. Natl. Acad. Sci. USA. 2003;100:788–791. doi: 10.1073/pnas.0335980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandelbrot B. Structure formelle des textes et communication. Word. 1954;10:1–27. doi: 10.1080/00437956.1954.11659509. [DOI] [Google Scholar]
- 11.Altmann G. Prolegomena to Menzerath’s law. Glottometrika. 1980;2:1–10. [Google Scholar]
- 12.Teupenhayn R., Altmann G. Clause length and Menzerath's law. Glottometrika. 1984;6:127–138. [Google Scholar]
- 13.Clink D.J., Ahmad A.H., Klinck H. Brevity is not a universal in animal communication: evidence for compression depends on the unit of analysis in small ape vocalizations. R. Soc. Open Sci. 2020;7 doi: 10.1098/rsos.200151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustison M.L., Semple S., Ferrer-i-Cancho R., Bergman T.J. Gelada vocal sequences follow Menzerath’s linguistic law. Proc. Natl. Acad. Sci. USA. 2016;113:E2750–E2758. doi: 10.1073/pnas.1522072113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Favaro L., Gamba M., Cresta E., Fumagalli E., Bandoli F., Pilenga C., Isaja V., Mathevon N., Reby D. Do penguins’ vocal sequences conform to linguistic laws? Biol. Lett. 2020;16 doi: 10.1098/rsbl.2019.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang M., Ma H., Ma C., Garber P.A., Fan P.F. Male gibbon loud morning calls conform to Zipf's law of brevity and Menzerath's law: insights into the origin of human language. Anim. Behav. 2020;160:145–155. doi: 10.1016/j.anbehav.2019.11.017. [DOI] [Google Scholar]
- 17.James L.S., Mori C., Wada K., Sakata J.T. Phylogeny and mechanisms of shared hierarchical patterns in birdsong. Curr. Biol. 2021;31:2796–2808.e9. doi: 10.1016/j.cub.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Stepanov A., Zhivomirov H., Nedelchev I., Stateva P. Bottlenose dolphins' broadband clicks are structured for communication. bioRxiv. 2023 doi: 10.1101/2023.01.11.523588. Preprint at. [DOI] [Google Scholar]
- 19.Chaverri G., Ancillotto L., Russo D. Social communication in bats. Biol. Rev. 2018;93:1938–1954. doi: 10.1073/pnas.033598010. [DOI] [PubMed] [Google Scholar]
- 20.Sun C.N., Jiang T.L., Gu H., Guo X., Zhang C.M., Gong L.X., Shi B.Y., Feng J. Geographical variation of social calls and vocal discrimination in male Himalayan leaf-nosed bats. Anim. Behav. 2020;170:15–26. doi: 10.1016/j.anbehav.2020.10.003. [DOI] [Google Scholar]
- 21.Fernandez A.A., Burchardt L.S., Nagy M., Knörnschild M. Babbling in a vocal learning bat resembles human infant babbling. Science. 2021;373:923–926. doi: 10.1126/science.abf927. [DOI] [PubMed] [Google Scholar]
- 22.Prat Y., Taub M., Yovel Y. Vocal learning in a social mammal: Demonstrated by isolation and playback experiments in bats. Sci. Adv. 2015;1 doi: 10.1126/sciadv.150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohn K.M., Smarsh G.C., Smotherman M. Social context evokes rapid changes in bat song syntax. Anim. Behav. 2013;85:1485–1491. doi: 10.1016/j.anbehav.2013.04.002. [DOI] [Google Scholar]
- 24.Zhang K.K., Liu T., Liu M.X., Li A.Q., Xiao Y.H., Metzner W., Liu Y. Comparing context-dependent call sequences employing machine learning methods: an indication of syntactic structure of greater horseshoe bats. J. Exp. Biol. 2019;222 doi: 10.1242/jeb.214072. [DOI] [PubMed] [Google Scholar]
- 25.Luo B., Jiang T.L., Liu Y., Wang J., Lin A.Q., Wei X.W., Feng J. Brevity is prevalent in bat short-range communication. J. Comp. Psychol. 2013;199:325–333. doi: 10.1007/s00359-013-0793-y. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez A.A., Fasel N., Knörnschild M., Richner H. When bats are boxing: aggressive behaviour and communication in male Seba’s short-tailed fruit bat. Anim. Behav. 2014;98:149–156. doi: 10.1016/j.anbehav.2014.10.011. [DOI] [Google Scholar]
- 27.Prat Y., Taub M., Yovel Y. Everyday bat vocalizations contain information about emitter, addressee, context, and behavior. Sci. Rep. 2016;6 doi: 10.1038/srep39419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng L., Qin H.X., Li J.J., Feng J., Jiang T.L., Jiang T. Extrinsic and intrinsic factors influencing the emergence and return of the Asian particolored bat Vespertilio sinensis to the summer roost. Ecol. Evol. 2022;12 doi: 10.1002/ece3.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin L.R., Wang J., Zhang Z.Z., Sun K.P., Kanwal J.S., Feng J. Postnatal development of morphological and vocal features in Asian particolored bat, Vespertilio sinensis. Mamm. Biol. 2012;77:339–344. doi: 10.1016/j.mambio.2012.05.001. [DOI] [Google Scholar]
- 30.Zhao X., Jiang T.L., Gu H., Liu H., Sun C.N., Liu Y., Feng J. Are aggressive vocalizations the honest signals of body size and quality in female Asian particoloured bats? Behav. Ecol. Sociobiol. 2018;72:96. doi: 10.1007/s00265-018-2510-x. [DOI] [Google Scholar]
- 31.Hailman J.P., Ficken M.S., Ficken R.W. The ‘chick-a-dee’ calls of Parus atricapillus: a recombinant system of animal communication compared with written English. Semiotica. 1985;56:191–224. doi: 10.1515/semi.1985.56.3-4.191. [DOI] [Google Scholar]
- 32.Semple S., Hsu M.J., Agoramoorthy G. Efficiency of coding in macaque vocal communication. Biol. Lett. 2010;6:469–471. doi: 10.1098/rsbl.2009.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valente D.D., Gregorio C., Favaro L., Friard O., Miaretsoa L., Raimondi T., Ratsimbazafy J., Torti V., Zanoli A., Giacoma C., Gamba M. Linguistic laws of brevity: conformity in Indri indri. Anim. Cognit. 2021;24:897–906. doi: 10.1007/s10071-021-01495-3. [DOI] [PubMed] [Google Scholar]
- 34.Ryan M.J. Factors influencing the evolution of acoustic communication: biological constraints. Brain Behav. Evol. 1986;28:70–82. doi: 10.1159/000118693. [DOI] [PubMed] [Google Scholar]
- 35.Gillooly J.F., Ophir A.G. The energetic basis of acoustic communication. Proc. Biol. Sci. 2010;277:1325–1331. doi: 10.1098/rspb.2009.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaverri G., Sandoval-Herrera N.I., Iturralde-Pólit P., Romero-Vásquez A., Chaves-Ramírez S., Sagot M. The energetics of social signaling during roost location in Spix's disc-winged bats. J. Exp. Biol. 2021;224 doi: 10.1242/jeb.238279. [DOI] [PubMed] [Google Scholar]
- 37.Clink D.J., Lau A.R. Adherence to Menzerath's Law is the exception (not the rule) in three duetting primate species. R. Soc. Open Sci. 2020;7 doi: 10.1098/rsos.201557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson S.K., Heesen R., Hedwig D., Robbins M.M., Townsend S.W. An exploration of Menzerath's law in wild mountain gorilla vocal sequences. Biol. Lett. 2020;16 doi: 10.1098/rsbl.2020.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao X., Jiang T.L., Liu H., Wang Y.Z., Liu Y., Feng J. Acoustic signalling of aggressive intent in the agonistic encounters of female Asian particoloured bats. Anim. Behav. 2019;149:65–75. doi: 10.1016/j.anbehav.2019.01.012. [DOI] [Google Scholar]
- 40.Ferrer-i-Cancho R., Hernandez-Fernandez A., Lusseau D., Agoramoorthy G., Hsu M.J., Semple S. Compression as a universal principle of animal behavior. Cognit. Sci. 2013;37:1565–1578. doi: 10.1111/cogs.12061. [DOI] [PubMed] [Google Scholar]
- 41.ASAB/ABS Guidelines for the ethical treatment of nonhuman animals in behavioural research and teaching. Anim. Behav. 2023;195 https://do.org/10.1016/j.anbehav.2022.09.006 I-XI. [Google Scholar]
- 42.Kanwal J.S., Matsumura S., Ohlemiller K., Suga N. Analysis of acoustic elements and syntax in communication sounds emitted by mustached bats. J. Acoust. Soc. Am. 1994;96:1229–1254. doi: 10.1121/1.410273. [DOI] [PubMed] [Google Scholar]
- 43.Jiang T.L., Guo X., Lin A.Q., Wu H., Sun C.N., Feng J., Kanwal J.S. Bats increase vocal amplitude and decrease vocal complexity to mitigate noise interference during social communication. Anim. Cognit. 2019;22:199–212. doi: 10.1007/s10071-018-01235-0. [DOI] [PubMed] [Google Scholar]
- 44.Luo B., Lu G.J., Chen K., Guo D.G., Huang X.B., Liu Y., Feng J. Social calls honestly signal female competitive ability in Asian particoloured bats. Anim. Behav. 2017;127:101–108. doi: 10.1016/j.anbehav.2017.03.012. [DOI] [Google Scholar]
- 45.Ma J., Kobayasi K., Zhang S.Y., Metzner W. Vocal communication in adult greater horseshoe bats, Rhinolophus ferrumequinum. J. Comp. Psychol. 2006;192:535–550. doi: 10.1007/s00359-006-0094-9. [DOI] [PubMed] [Google Scholar]
- 46.Lin H.J., Kanwal J.S., Jiang T.L., Liu Y., Feng J. Social and vocal behavior in adult greater tube-nosed bats (Murina leucogaster) Zoology. 2015;118:192–202. doi: 10.1016/j.zool.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Burnham K.P., Anderson D.R. In: Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Burnham K.P., Anderson D.R., editors. Springer New York; 2004. Introduction; pp. 1–48. [Google Scholar]
- 48.Bartoń K. MuMIn: multi-model inference. 2022. https://CRAN.R-project.org/package=MuMIn R package version 1.47.1.
- 49.Lukacs P.M., Thompson W.L., Kendall W.L., Gould W.R., Doherty J.R., Burnham K.P., Anderson D.R. Concerns regarding a call for pluralism of information theory and hypothesis testing. J. Appl. Ecol. 2007;44:456–460. doi: 10.1111/j.1365-2664.2006.01267.x. [DOI] [Google Scholar]
- 50.R Core Development Team . The R Project for Statistical Computing. 4.2.2 edn. 2022. R: a language and environment for statistical computing.http://www.r-project.org [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Syllable number is also given for each syllable type during each trial (column F–S), related to STAR Methods.
Data Availability Statement
-
•
The data supporting the results of the article are available as supplemental information.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.