Abstract
Background:
CSF shunt infection treatment requires both surgical and antibiotic decisions. Using the Hydrocephalus Clinical Research Network (HCRN) Registry and 2004 IDSA guidelines that were not proactively distributed to HCRN providers, we previously found high adherence to surgical recommendations but poor adherence to intravenous (IV) antibiotic duration recommendations. In general, IV antibiotic duration was longer than recommended. In March 2017 new IDSA guidelines expanded upon the 2004 guidelines by including recommendations for selection of specific antibiotics.
Objectives:
To describe adherence to IDSA guideline recommendations for CSF shunt infection treatment and reinfection rates associated with adherence to guideline recommendations.
Design/Methods:
We studied a prospective cohort of children < 18 years of age undergoing treatment for first CSF shunt infection diagnosed by recovery of bacteria from CSF culture (CSF-positive infection) at one of 7 hospitals from 4/2008 to 12/2012. Adherence to 2004 and 2017 guideline recommendations was determined. Adherence to antibiotics were further classified as longer and shorter duration than guideline recommendations. Reinfection rates with 95% confidence intervals (CI) were generated.
Results:
There were 133 children with CSF-positive infections addressed by 2004 IDSA guideline recommendations, with 124 at risk for reinfection. Zero reinfections were observed among those whose treatment was fully adherent [0/14, 0% (95% CI: 0, 20)], and 15 reinfections were observed among those whose infection treatment was non-adherent [15/110, 14% (95% CI: 8, 21)]. Among the 110 first infections whose infection treatment was non-adherent, 74 first infections were treated for longer duration than recommended and 9 developed reinfection [9/74, 12% (95% CI: 6, 22)].
There were 145 children with CSF-positive infections addressed by 2017 IDSA guideline recommendations, with 135 at risk for reinfection. No reinfections were observed among children whose treatment was fully adherent [0/3, 0% (95% CI: 0, 64)], and 18 reinfections were observed among those whose infection treatment was non-adherent [18/132, 14% (95% CI: 8, 21)].
Conclusion(s):
There is no clear evidence that either adherence to IDSA guidelines or duration of treatment longer than recommended is associated with reduction in reinfection rates. Because IDSA guidelines recommend shorter IV antibiotic durations than are typically used, improvement efforts to reduce IV antibiotic duration in CSF shunt infection treatment should utilize IDSA guidelines.
Keywords: cerebrospinal, shunt, infection, treatment, antibiotic
Introduction
While life-saving and the mainstay of hydrocephalus treatment, (6) CSF shunts can cause new and chronic surgical and medical problems for children with hydrocephalus, including mechanical malfunction requiring surgical revision (11) as well as CSF shunt infection. (1, 12, 22) While numerous review articles have been written(3–6, 17), until recently no organization in the United States or elsewhere has published an official guideline for management of CSF shunt infection. Instead, embedded within 2004 guidelines for management of bacterial meningitis, the Infectious Disease Society of America (IDSA) provided recommendations for both surgical and antibiotic decisions in the treatment of CSF shunt infection.(19) Then, in March 2017 the IDSA published a set of guidelines for healthcare-associated ventriculitis and meningitis that expanded upon and provided more extensive recommendations for the treatment of CSF shunt infection than the 2004 guidelines.(20)
Surgical decisions in the treatment of CSF shunt infection include either shunt removal with external ventricular drain (EVD) placement followed by new shunt insertion, or shunt externalization followed by shunt replacement, once the CSF is sterile. While the relative benefit of each approach in preventing CSF shunt re-infection remains unclear(3–5, 10, 13, 17, 21, 26), the growing consensus within the neurosurgical community has been to remove the entire shunt at the time of the first infection surgery.(7, 25) Both the 2004 and 2017 IDSA guidelines reflect this growing consensus, suggesting shunt removal with EVD placement followed by new shunt insertion.(19)
Antibiotic decisions in the treatment of CSF shunt infection include the choice and duration of empiric and targeted intravenous (IV) antibiotics.(2, 14, 24) Here again, evidence is limited as no randomized controlled clinical trial has been conducted, with prior studies being retrospective and limited in size.(17) While the 2004 IDSA guidelines provided recommendations for the duration of IV antibiotics, (19) the 2017 IDSA guidelines expanded upon the 2004 guidelines by including recommendations for selection of specific antibiotics.(19)
The Hydrocephalus Clinical Research Network (HCRN) provides a unique opportunity to understand treatment practices for CSF shunt infection following creation of the IDSA guidelines. Using the HCRN Registry and 2004 IDSA guidelines that were not proactively distributed to HCRN providers, we previously found high adherence to surgical recommendations but poor adherence to IV antibiotic duration recommendations.(15) We did not report on reinfection rates following adherence to recommendations, nor were the 2017 guidelines available for analysis at that time, and we had an opportunity to enlarge the cohort. Therefore, the aims of this study were to describe (1) adherence to surgical and antibiotic decision recommendations in both the 2004 and 2017 IDSA guidelines for first CSF shunt infection treatment; and (2) reinfection rates associated with adherence to each set of guideline recommendations in a larger cohort of children.
Methods
We conducted a retrospective study of a longitudinal observational cohort of children recorded in the HCRN registry with first CSF shunt infection diagnosed by recovery of bacteria in CSF culture. The predictor variables of interest were adherence to the surgical and antibiotic decision recommendations in both the 2004 and 2017 IDSA guidelines, and the outcome was reinfection.
Setting
The HCRN is a collaboration of fourteen pediatric neurosurgical centers across North America, with seven active during the study period and therefore participating in this study: Children’s Hospital of Alabama, Children’s Hospital of Pittsburgh, The Hospital for Sick Children, Primary Children’s Hospital, Seattle Children’s Hospital, Texas Children’s Hospital, and St. Louis Children’s Hospital. HCRN registry data use was approved by the HCRN and the Institutional Review Boards at the University of Utah and Seattle Children’s Hospital.
Study Population and Data Collection
Within the HCRN registry, data from each neurosurgical admission for each child are collected contemporaneously. Data collection started in April 2008 and, for this study, ended on December 31, 2012, except children at The Hospital for Sick Children who were followed until December 31, 2011. To minimize misclassification bias, we included children whose initial CSF shunt placement, all subsequent shunt surgeries, and first CSF shunt infection were recorded in the HCRN registry during the study period (n=151), or whose shunt surgery histories (initial CSF shunt placement and subsequent CSF shunt revision(s)) were able to be obtained retrospectively (n=82).
The HCRN consensus definition for CSF shunt infection was used to screen for first CSF shunt infection:(9, 15, 16, 18) (a) microbiological determination of bacteria present in a culture or Gram stain of CSF, wound swab, and/or pseudocyst fluid, or (b) shunt erosion (visible hardware), or (c) abdominal pseudocyst (without positive culture); or for children with ventriculoatrial shunts, (d) presence of bacteria in a blood culture. For this study, we included only children whose first CSF shunt infection was diagnosed by recovery of bacteria in CSF culture (CSF-positive infection). Children who met the HCRN infection definition only by another criteria were excluded from further consideration because neither set of IDSA guidelines is applicable to these cases. Additionally, we excluded children who developed bacteremia (defined as any organism recovered in blood culture) or secondary ventriculitis (recovery of a different organism from that recovered initially in CSF culture) that appropriately prolonged antibiotic treatment. Finally, we focused our analysis on the children whose infecting organism was addressed by the 2004 and/or 2017 IDSA guidelines. (Figure 1)
Figure 1.
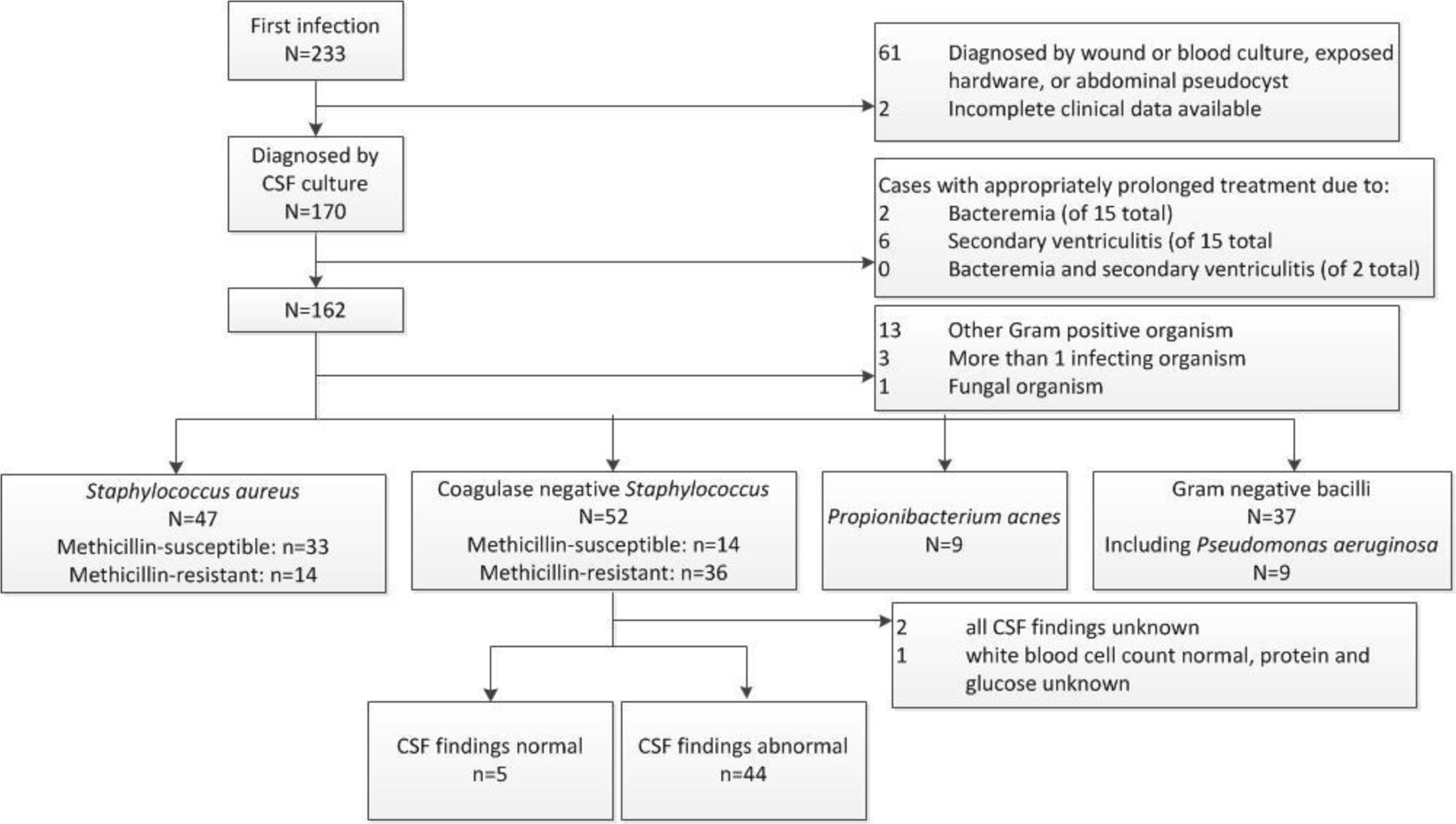
Cohort definition
Measurements
Adherence to the surgical and antibiotic decision recommendations in both the 2004 and 2017 IDSA guidelines were determined.
The 2004 IDSA guideline recommendations for management of CSF shunt infection include the surgical approach of full shunt removal with EVD placement followed by new shunt insertion once the CSF is sterile, and different antibiotic treatment durations according to the organism recovered, grouped as Staphylococcus aureus, coagulase-negative Staphylococcus, and gram-negative bacilli (Figure 2). When applicable, concurrent CSF cell counts and chemistries, and subsequent CSF culture results were also reviewed. We defined a normal CSF white blood cell count as ≤32 cells/mm3 in a child under one month of age and ≤10 cells/mm3 in a child older than one month,(23) normal CSF glucose as ≥45 mg/dL, and normal CSF protein as <200 mg/dL.
Figure 2.
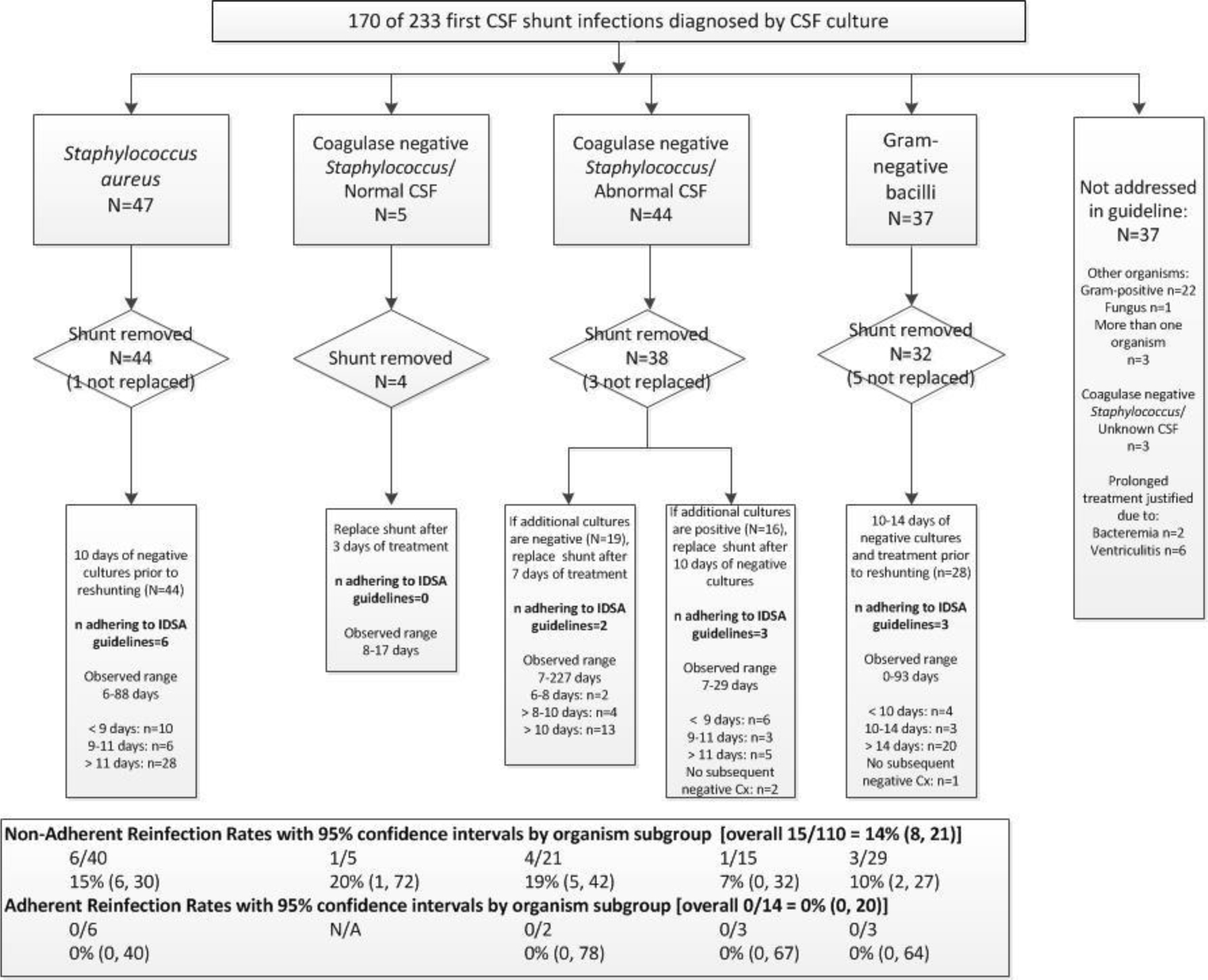
2004 IDSA guideline recommendation adherence and reinfection observed in study cohort
The 2017 IDSA guideline recommendations for management of CSF shunt infection were more detailed than 2004 guideline recommendations. (Figure 3) All relevant recommendations, grouped as Staphylococcus aureus, coagulase-negative Staphylococcus, Propionibacterium acnes, and gram-negative bacilli including Pseudomonas were considered (Table 1); only relevant recommendations for each organism were assessed. Staphylococcus aureus and coagulase-negative Staphylococcus and were further grouped as methicillin-susceptible and methicillin-resistant.
Figure 3.
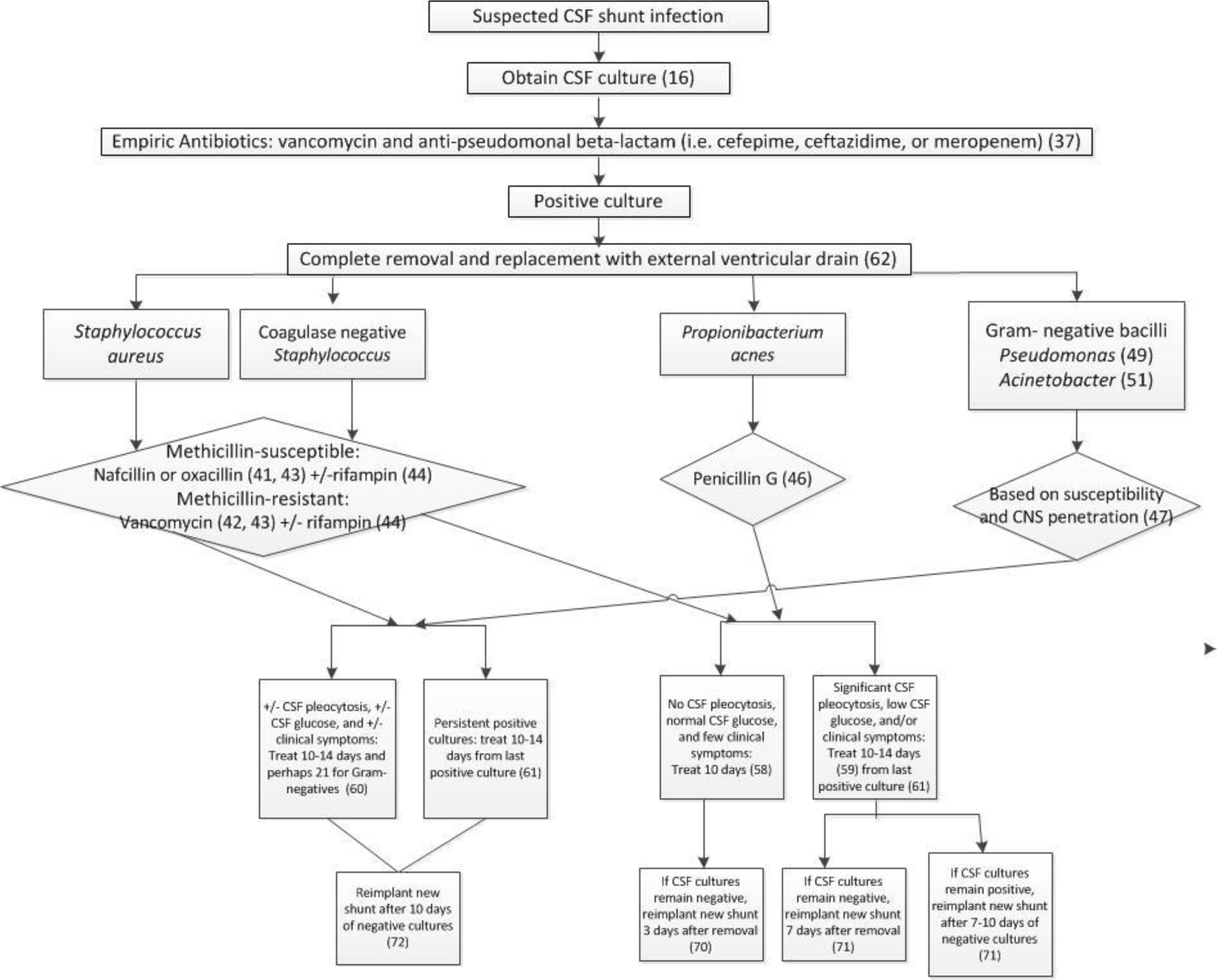
2017 IDSA guideline recommendations
Table 1.
Adherence to selected 2017 IDSA guidelines for the diagnosis and treatment of CSF shunt infection (n=145)
| Management of Staphylococcus aureus | n=47 |
|---|---|
| 41. For treatment of infection caused by methicillin-susceptible S. aureus, nafcillin or oxacillin is recommended (strong, moderate)…. | 25/33 (76%) |
| 42. For treatment of infection caused by methicillin-resistant S. aureus, vancomycin is recommended as first-line therapy (strong, moderate)….. |
13/14 (93%) |
| 44. If the staphylococcal isolate is susceptible to rifampin, this agent may be considered in combination with other antimicrobial agents for staphylococcal ventriculitis and meningitis (weak, low); rifampin is recommended as part of combination therapy for any patient with intracranial or spinal hardware such as a CSF shunt or drain (strong, low). | 13/27 (48%) |
| 60. Infections caused by S. aureus … with or without significant CSF pleocytosis, CSF hypoglycorrhachia, or clinical symptoms or systemic features should be treated for 10–14 days (strong, low)… | 12/47 (26%) |
| 61. In patients with repeatedly positive CSF cultures on appropriate antimicrobial therapy, treatment should be continued for 10–14 after the last positive culture (strong, low). | 14/32 (44%) |
| 62. Complete removal of an infected CSF shunt and replacement with an external ventricular drain combined with intravenous antimicrobial therapy is recommended in patients with infected CSF shunts (strong, moderate). | 44/47 (94%) |
| 72. In patients with infection caused by S. aureus …, a new shunt should be reimplanted 10 days after CSF cultures are negative (strong, low). | 9/47 (19%) |
| Met all relevant criteria | 2/47 (4%) |
| Management of coagulase negative Staphylococci | n=52 |
|---|---|
| 43. For treatment of infection caused by coagulase-negative staphylococci, the recommended therapy should be similar to that for S. aureus and based on in vitro susceptibility testing (strong, moderate).*
Methicillin-susceptible: nafcillin or oxacillin is recommended Methicillin-resistant: vancomycin is recommended as first-line therapy |
1/14 (7%) 35/36 (97%) |
| 44. If the staphylococcal isolate is susceptible to rifampin, this agent may be considered in combination with other antimicrobial agents for staphylococcal ventriculitis and meningitis (weak, low); rifampin is recommended as part of combination therapy for any patient with intracranial or spinal hardware such as a CSF shunt or drain (strong, low). | 11/31 (35%) |
| 58. Infections caused by a coagulase-negative staphylococcus … with no or minimal CSF pleocytosis, normal CSF glucose, and few clinical symptoms or systemic features should be treated (with nafcillin) for 10 days (strong, low). † | 4/5 (60%) |
| 59. Infections caused by a coagulase-negative staphylococcus … with significant CSF pleocytosis, CSF hypoglycorrhachia, or clinical symptoms or systemic features should be treated for 10–14 days (with nafcillin) (strong, low). † | 17/44 (39%) |
| 62. Complete removal of an infected CSF shunt and replacement with an external ventricular drain combined with intravenous antimicrobial therapy is recommended in patients with infected CSF shunts (strong, moderate). | 41/52 (79%) |
| 70. In patients with infection caused by coagulase-negative staphylococci …, with no associated CSF abnormalities and with negative CSF cultures for 48 hours after externalization, a new shunt should be reimplanted as soon as the third day after removal (strong, low). † | 0/2 (0%) |
| 71. In patients with infection caused by a coagulase-negative staphylococcus …, with associated CSF abnormalities but negative repeat CSF cultures, a new shunt should be reimplanted after 7 days of antimicrobial therapy (strong, low); †
if repeat cultures are positive, antimicrobial treatment is recommended until CSF cultures remain negative for 7–10 consecutive days before a new shunt is placed (strong, low). |
2/23 (9%) 13/24 (54%) |
| Met all relevant criteria | 1/52 (2%) |
| Management of Propionibacterium acnes | n=9 | |
|---|---|---|
| 46. For treatment of infection caused by Propionibacterium acnes, penicillin G is recommended (strong, moderate). | 0 (0%) | |
| 58. Infections caused by … P. acnes with no or minimal CSF pleocytosis, normal CSF glucose, and few clinical symptoms or systemic features should be treated for 10 days (with penicillin) (strong, low).* | 0/5 (0%) | |
| 59. Infections caused by … P. acnes with significant CSF pleocytosis, CSF hypoglycorrhachia, or clinical symptoms or systemic features should be treated for 10–14 days (with penicillin) (strong, low).* | 0/3 (0%) | |
| 62. Complete removal of an infected CSF shunt and replacement with an external ventricular drain combined with intravenous antimicrobial therapy is recommended in patients with infected CSF shunts (strong, moderate). | 6/9 (67%) | |
| 70. In patients with infection caused by … P. acnes, with no associated CSF abnormalities and with negative CSF cultures for 48 hours after externalization, a new shunt should be reimplanted as soon as the third day after removal (strong, low).* | 0/2 (0%) | |
| 71. In patients with infection caused by … P. acnes, with associated CSF abnormalities but negative repeat CSF cultures, a new shunt should be reimplanted after 7 days of antimicrobial therapy (strong, low); * if repeat cultures are positive, antimicrobial treatment is recommended until CSF cultures remain negative for 7–10 consecutive days before a new shunt is placed (strong, low). |
0/3 (0%) 1/2 (50%) |
|
| Met all relevant criteria | 0/9 (0%) | |
| Management of Gram-negative bacilli | n=28 |
|---|---|
| 47. For treatment of infection caused by gram-negative bacilli, therapy should be based on in vitro susceptibility testing with agents that achieve good CNS penetration (strong, moderate). | 22/28 (79%) |
| 48. For treatment of infection caused by gram-negative bacilli susceptible to third-generation cephalosporins, ceftriaxone or cefotaxime is recommended (strong, moderate). | 18/22 (82%) |
| 50. For treatment of infection caused by extended-spectrum beta-lactamase–producing gram-negative bacilli, meropenem should be used if this isolate demonstrates in vitro susceptibility (strong, moderate). | 1 / 2 (50%) |
| 60. Infections caused by … gram-negative bacilli with or without significant CSF pleocytosis, CSF hypoglycorrhachia, or clinical symptoms or systemic features should be treated for 10–14 days (strong, low); some experts suggest treatment of infection caused by gram-negative bacilli for 21 days (weak, low). | 10–14 days: 7/28 (25%) Up to 21 days: 10/28 (36%) |
| 61. In patients with repeatedly positive CSF cultures on appropriate antimicrobial therapy, treatment should be continued for 10–14 after the last positive culture (strong, low). | 3/15 (20%) |
| 62. Complete removal of an infected CSF shunt and replacement with an external ventricular drain combined with intravenous antimicrobial therapy is recommended in patients with infected CSF shunts (strong, moderate). | 21/28 (75%) |
| 72. In patients with infection caused by … gram-negative bacilli, a new shunt should be reimplanted 10 days after CSF cultures are negative (strong, low). | 1/28 (4%) |
| Met all relevant criteria | 0/28 (0%) |
| Management of Pseudomonas | n=9 |
|---|---|
| 49. For treatment of infection caused by Pseudomonas species, the recommended therapy is cefepime, ceftazidime, or meropenem (strong, moderate)….. | 7/9 (78%) |
| 60. Infections caused by … gram-negative bacilli with or without significant CSF pleocytosis, CSF hypoglycorrhachia, or clinical symptoms or systemic features should be treated for 10–14 days (strong, low); some experts suggest treatment of infection caused by gram-negative bacilli for 21 days (weak, low). | 10–14 days: 0/9 (0%) Up to 21 days: 4/9 (44%) |
| 61. In patients with repeatedly positive CSF cultures on appropriate antimicrobial therapy, treatment should be continued for 10–14 after the last positive culture (strong, low). | 0/5 (0%) |
| 62. Complete removal of an infected CSF shunt and replacement with an external ventricular drain combined with intravenous antimicrobial therapy is recommended in patients with infected CSF shunts (strong, moderate). | 7/9 (78%) |
| 72. In patients with infection caused by … gram-negative bacilli, a new shunt should be reimplanted 10 days after CSF cultures are negative (strong, low). | 0/9 (0%) |
| Met all relevant criteria | 0/9 (0%) |
| Management of Acinetobacter | n=0 |
|---|---|
| 51. For treatment of infection caused by Acinetobacter species, meropenem is recommended (strong, moderate),,,, | n/a |
2 children did not have methicillin susceptibility reported
3 children had no CSF studies obtained
1 child had no CSF studies obtained and 1 child had no additional cultures obtained
Each eligible child was included in each relevant guideline recommendation denominator (e.g., a child with normal CSF findings was included in the denominator of recommendations for children with normal CSF findings but was not included in the denominator of recommendations for children with abnormal CSF findings). Children who could not be evaluated for adherence due to missing data (e.g., there was no subsequent negative culture) were considered non-adherent for the recommendation for which they did not have data. Adherence to all relevant guideline recommendations within organism in aggregate was also assessed.
The reinfection outcome was defined using the HCRN consensus definition of infection above and was not limited to CSF-positive infection. The duration of follow-up for reinfection was through December 31, 2013, resulting in a minimum follow-up duration of one year. The at-risk period was defined as the period following first shunt infection during which a shunt was in place. Children who died during treatment for first infection or who did not subsequently have a shunt in place (i.e. shunt was removed without replacement) were not considered at-risk and were excluded from reinfection rates. Duration of follow-up, censored at reinfection or loss to follow-up, was not significantly different between patients whose first shunt infection treatment adhered to IDSA guidelines compared to those whose treatment was nonadherent (log rank, p=0.61)
Statistical Analyses
Guideline recommendation adherence was descriptively summarized by the number of infections for which a recommendation was relevant and the number of infections for which the recommendation was met. Reinfection rates were calculated as (Number of Children with Observed Reinfection) / (Number of Children as Risk for Reinfection). Results are presented with binomial 95% confidence intervals (CI). All statistical analyses were performed using SAS (versions 9.2 and 9.4, SAS Institute, Cary, NC).
Results
Of 3,131 children in the HCRN registry during the study period, 233 had a first CSF shunt infection, and 170 of these had CSF-positive infections for which complete clinical data were available. (Figure 1) Eight infections were excluded from further consideration as treatment was appropriately prolonged due to bacteremia or secondary ventriculitis.
2004 IDSA Guidelines
Of the remaining 162 CSF-positive infections, another 29 (18%) were not addressed by the 2004 IDSA guidelines (including 9 with Propionibacterium acnes, 6 with Enterococcus, 7 with other gram-positive organisms, 3 with more than one infecting organism, and 1 with Candida fungal infection, and 3 with coagulase-negative Staphylococcus and no CSF studies). Therefore 133 children remained with CSF-positive infections addressed by 2004 IDSA guidelines, including 47 (35%) with Staphylococcus aureus, 49 (37%) with coagulase-negative Staphylococcus and CSF indices, and 37 (28%) with gram-negative bacilli.
Figure 2 shows patterns of adherence to 2004 IDSA guideline recommendations in this cohort. Nine children were not considered at risk for reinfection due to shunt removal without replacement or death. Zero reinfections were observed among those whose treatment was fully adherent to 2004 IDSA guideline recommendations [0/14, 0% (95% CI: 0, 20)], and 15 reinfections were observed among those whose infection treatment was non-adherent [15/110, 14% (95% CI: 8, 21)]. Among the 14 infections treated in a manner adherent with 2004 IDSA guidelines, no differences in frequency were noted between HCRN sites (range 0–20%). Among the first infections whose infection treatment was non-adherent, 94 were related to duration. Among the 74 first infections treated for longer duration than recommended, 9 developed reinfection [9/74, 12% (95% CI: 6, 22)]. Among the 20 infections treated for a shorter duration than recommended in 2004 IDSA guideline recommendations, 2 developed reinfection [2/20, 10% (95% CI: 1, 32)]. Among the 29 infections not addressed by 2004 IDSA guidelines, 8 developed reinfection [8/29, 28% (95% CI: 13, 47)].
2017 IDSA Guidelines
Similarly, of the remaining 162 CSF-positive infections, 17 (10%) were not addressed by the 2017 IDSA guidelines (including 6 with Enterococcus, 7 with other gram-positive organisms, 3 with more than one infecting organism, and 1 with fungal infection). Therefore 145 children remained whose CSF-positive infections were addressed by 2017 IDSA guidelines, including 47 (32%) with Staphylococcus aureus, 52 (36%) with coagulase-negative Staphylococcus, 9 (6%) with Propionibacterium acnes, 37 (26%) gram-negative bacilli including 9 (6%) with Pseudomonas.
Figure 3 shows 2017 IDSA guideline recommendations, and Table 1 shows patterns of adherence to them in this cohort. Ten were not considered at risk for reinfection due to shunt removal without replacement or death. No reinfections were observed among children whose treatment was fully adherent to 2017 IDSA guideline recommendations [0/3, 0% (0, 64)], and 18 reinfections were observed among those whose infection treatment was non-adherent [18/132, 14% (95% CI: 8, 21)].
In most potential infection cases (n=228/231, 99%), CSF cultures were obtained to establish the diagnosis of healthcare-associated ventriculitis and meningitis (recommendation #16). In a minority of potential infection cases (n=37/231, 16%), vancomycin and an anti-pseudomonal beta-lactam were used as empiric therapy for healthcare-associated ventriculitis and meningitis (recommendation #37). The majority of CSF-positive infection cases (n=134/168, 80%) underwent complete removal of an infected CSF shunt and replacement with an external ventricular drain combined with intravenous antimicrobial therapy (recommendation #62).
Among the 8 infections with treatment that was appropriately prolonged due to bacteremia or secondary ventriculitis (not addressed by 2004 or 2017 IDSA guidelines), 2 developed reinfection [2/8, 25% (95% CI: 3, 65)].
Discussion
In this large prospective cohort of children whose infections were diagnosed by CSF culture and addressed by the 2004 and/or 2017 IDSA guideline recommendations, we observed high adherence to surgical recommendations but poor adherence to IV antibiotic duration recommendations. Zero reinfections were observed among the 15 whose treatment was fully adherent to 2004 IDSA guideline recommendations as well as the 3 whose treatment was fully adherent to 2017 IDSA guideline recommendations. Similar reinfection rates were observed among the 110 whose treatment was non-adherent to 2004 IDSA guideline recommendations as well as the 132 whose treatment was non-adherent to 2017 IDSA guideline recommendations. Due to a low reinfection event rate, we are unable to definitively conclude that either adherence to IDSA guidelines or duration of treatment longer than recommended is associated with reduction in reinfection rates.
This cohort of children who had CSF-positive infections addressed by the IDSA guidelines allows us to make the first comparisons between guideline adherence and CSF shunt reinfection, albeit limited by small number of children whose treatment was adherent. As noted earlier, evidence for the choice and duration of IV antibiotics in the treatment of CSF shunt infection is extremely limited;(17) and while the 2004 IDSA guidelines provided recommendations for the duration of IV antibiotics, (19) the 2017 IDSA guidelines included recommendations for selection of specific antibiotics.(19) Despite the lack of evidence supporting the IDSA guideline recommendations, adherence to either set of guidelines was associated with shorter IV antibiotic durations without evidence of increased reinfection rates. As such, improvement efforts to reduce IV antibiotic duration in CSF shunt infection treatment can utilize the recommendations for duration of therapy provided in the IDSA guidelines. Future efforts to improve care for this population will need to focus more on antibiotic selection and duration once an organism is identified, and less on surgical approach given the nearly uniform surgical approach to these infections we observed. Although concern for reinfection is low given this preliminary data, improvement efforts will require ongoing monitoring to ensure reinfection rates remain low.
This work has several limitations. The conduct of this study at HCRN centers that were adhering to an infection prevention bundle (8, 9) limits generalizability of findings outside the HCRN. However, the HCRN prevention bundle has been widely disseminated and implemented in the past 5 years, and it does not address recommendations from infection treatment guidelines. In addition, the multi-institutional nature of this study gives it greater generalizability than previous studies. Additional aspects of management of CSF shunt infection – including, but not limited to, the number, frequency, and method of CSF cultures obtained through EVDs, flushing of EVDs, and replacement of EVDs – is not standardized in the HCRN and was subject to variation within and between participating centers. These and other aspects of CSF shunt infection management that were addressed in the 2017 guideline recommendations (e.g. microbiologic methods) were not captured in the HCRN registry and are therefore not included in this study. For neurosurgical procedures, the assumption is that patients return to the same center for care and therefore that subsequent infections would be captured in our data. The data are also limited to treatment of first infection, and reinfection rates are higher with subsequent infections.(18) We did not limit reinfections to those within 6 months of infections so as not to further reduce our study’s power. Lastly, drug levels were not available for review in determination of concordant and appropriate IV antibiotic use.
Despite the lack of evidence supporting the IDSA guideline recommendations, adherence to either set of guidelines was associated with shorter IV antibiotic durations without evidence of increased reinfection rates. As such, because IDSA guidelines recommend shorter IV antibiotic durations than are typically used, improvement efforts to reduce IV antibiotic duration in CSF shunt infection treatment should utilize IDSA guidelines.
Acknowledgments
The authors would like to thank their colleagues for their past and ongoing support of HCRN: D Brockmeyer, M Walker, R Bollo, J Blount, J Johnston, B Rocque, L Ackacpo-Satchivi, J Oakes, P Dirks, J Rutka, M Taylor, D Curry, R Dauser, A Jea, S Lam, T Luerssen, R Ellenbogen, J Ojemann, A Lee, A Avellino, I Pollack, S Greene, E Tyler-Kabara, TS Park, J Leonard, M Smyth, N Tulipan, A Singhal, P Steinbok, D Cochrane, W Hader, C Gallagher, M Benour, E Kiehna, J G McComb, A Robison, M Handler, B O’Neill, C Wilkinson, L Governale, J Leonard, E Sribnick.
In addition, our work would not be possible without the outstanding support of the dedicated personnel at each clinical site and the data coordinating center. Special thanks goes to: J Clawson, N Tattersall, T Bach (Salt Lake City); A Arynchyna, A Bey (Birmingham); H Ashrafpour, L O’Connor (Toronto); S Martinez, SRyan (Houston); A Anderson, G Bowen (Seattle); K Diamond, A Luther (Pittsburgh); M Gabir, D Morales, D Berger, D Mercer (St. Louis); D Dawson, S Gannon (Nashville); A Cheong, R Hengel (British Columbia); S Ahmed (Calgary); A Loudermilk (Baltimore); N Rea, C Artime (Los Angeles); S Staulcup (Colorado); A Boczar (Columbus); and M Langley, V Wall, N Nunn, V Freimann, B Miller (Utah Data Coordinating Center).
The HCRN has been funded by National Institute of Neurological Disorders and Stroke (NINDS grant no. 1RC1NS068943–01), Patient Centered Outcome Research Institute (PCORI grant no. CER-1403-13857), The Gerber Foundation (reference no. 1692–3638), private philanthropy and the Hydrocephalus Association.
Funding Source:
TDS and the Nemeth family, and KBW were supported by Award K23NS062900 from the National Institute of Neurological Disorders And Stroke (NINDS) and Seattle Children’s Center for Clinical and Translational Research, and CTSA Grant Number ULI RR025014 from the National Center for Research Resources, a component of the National Institutes of Health. DL was supported by grants from NINDS, Patient Centered Outcomes Research Institute, the Hydrocephalus Association, Rudy Schulte, and research funding through Medtronic and Karl Storz. The HCRN has been funded by private philanthropy, NINDS grant 1RC1NS068943–01, a grant from The Gerber Foundation (1692–3638), Patient-Centered Outcomes Research Institute (CER-1403-13857) and the Hydrocephalus Association. None of the sponsors participated in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the funding sponsors.
Appendix 1. Hydrocephalus Clinical Research Network
Members
The HCRN currently consists of the following clinical centers and investigators: Primary Children’s Hospital, University of Utah (J Kestle); Children’s Hospital of Alabama, University of Alabama at Birmingham (C Rozzelle); Hospital for Sick Children, University of Toronto (J Drake, A Kulkarni); Texas Children’s Hospital, Baylor College of Medicine (W Whitehead); Seattle Children’s Hospital, University of Washington (S Browd, T Simon, J Hauptman); Children’s Hospital of Pittsburgh, University of Pittsburgh (I Pollack); St. Louis Children’s Hospital, Washington University in St. Louis (D Limbrick); Monroe Carell Jr. Children’s Hospital at Vanderbilt, Vanderbilt University Medical Center (J Wellons, R Naftel, C Shannon); British Columbia Children’s Hospital, University of British Columbia (M Tamber, P McDonald); Alberta Children’s Hospital, University of Calgary (J Riva-Cambrin); The Johns Hopkins Hospital (E Ahn); Children’s Hospital of Los Angeles (M Krieger); Children’s Hospital Colorado (T Hankinson); Nationwide Children’s Hospital (J Pindrik); HCRN Data Coordinating Center, Department of Pediatrics, University of Utah (R Holubkov)
Footnotes
Financial Disclosure: The authors and the Nemeth family have no financial disclosures relevant to this article to disclose.
Conflicts of Interest: The authors and the Nemeth family have no conflicts of interest relevant to this article to disclose. The study funders played no role in (1) study design; (2) the collection, analysis, and interpretation of data; (3) the writing of the report; and (4) the decision to submit the paper for publication.
Disclosure
None of the sponsors participated in design and conduct of this study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of this manuscript. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the sponsors.
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Albright AL, Pollack IF, Adelson PD, and Solot JJ. Outcome data and analysis in pediatric neurosurgery. Neurosurgery 45: 101–106, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Fan-Havard P, and Nahata MC. Treatment and prevention of infections of cerebrospinal fluid shunts. Clin Pharm 6: 866–880, 1987. [PubMed] [Google Scholar]
- 3.Gardner P, Leipzig T, and Phillips P. Infections of central nervous system shunts. Med Clin North Am 69: 297–314, 1985. [PubMed] [Google Scholar]
- 4.Gardner P, Leipzig TJ, and Sadigh M. Infections of mechanical cerebrospinal fluid shunts. Curr Clin Top Infect Dis 9: 185–214, 1988. [PubMed] [Google Scholar]
- 5.Kanev PM, and Sheehan JM. Reflections on shunt infection. Pediatr Neurosurg 39: 285–290, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Kestle JR. Pediatric hydrocephalus: current management. Neurol Clin 21: 883–895, vii, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Kestle JR, Garton HJ, Whitehead WE, Drake JM, Kulkarni AV, Cochrane DD, et al. Management of shunt infections: a multicenter pilot study. Journal of neurosurgery 105: 177–181, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Kestle JR, Holubkov R, Douglas Cochrane D, Kulkarni AV, Limbrick DD Jr., Luerssen TG, et al. A new Hydrocephalus Clinical Research Network protocol to reduce cerebrospinal fluid shunt infection. J Neurosurg Pediatr 1–6, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Kestle JR, Riva-Cambrin J, Wellons JC 3rd, Kulkarni AV, Whitehead WE, Walker ML, et al. A standardized protocol to reduce cerebrospinal fluid shunt infection: the Hydrocephalus Clinical Research Network Quality Improvement Initiative. J Neurosurg Pediatr 8: 22–29, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni AV, Rabin D, Lamberti-Pasculli M, and Drake JM. Repeat cerebrospinal fluid shunt infection in children. Pediatr Neurosurg 35: 66–71, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni AV, Riva-Cambrin J, Butler J, Browd SR, Drake JM, Holubkov R, et al. Outcomes of CSF shunting in children: comparison of Hydrocephalus Clinical Research Network cohort with historical controls: clinical article. J Neurosurg Pediatr 12: 334–338, 2013. [DOI] [PubMed] [Google Scholar]
- 12.McGirt MJ, Zaas A, Fuchs HE, George TM, Kaye K, and Sexton DJ. Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 36: 858–862, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Morissette I, Gourdeau M, and Francoeur J. CSF shunt infections: a fifteen-year experience with emphasis on management and outcome. Can J Neurol Sci 20: 118–122, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Sells CJ, Shurtleff DB, and Loeser JD. Gram-negative cerebrospinal fluid shunt-associated infections. Pediatrics 59: 614–618, 1977. [PubMed] [Google Scholar]
- 15.Simon TD, Kronman MP, Whitlock KB, Gove N, Browd SR, Holubkov R, et al. Variability in Management of First Cerebrospinal Fluid Shunt Infection: A Prospective Multi-Institutional Observational Cohort Study. The Journal of pediatrics 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon TD, Whitlock KB, Riva-Cambrin J, Kestle JR, Rosenfeld M, Dean JM, et al. Revision surgeries are associated with significant increased risk of subsequent cerebrospinal fluid shunt infection. Pediatr Infect Dis J 31: 551–556, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamber MS, Klimo P Jr., Mazzola CA, and Flannery AM. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 8: Management of cerebrospinal fluid shunt infection. J Neurosurg Pediatr 14 Suppl 1: 60–71, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Tuan TJ, Thorell EA, Hamblett NM, Kestle JR, Rosenfeld M, and Simon TD. Treatment and microbiology of repeated cerebrospinal fluid shunt infections in children. Pediatr Infect Dis J 30: 731–735, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, and Whitley RJ. Practice guidelines for the management of bacterial meningitis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 39: 1267–1284, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Michael Scheld W, et al. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venes JL. Infections of CSF shunt and intracranial pressure monitoring devices. Infect Dis Clin North Am 3: 289–299, 1989. [PubMed] [Google Scholar]
- 22.Vinchon M, and Dhellemmes P. Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery 22: 692–697, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Wallach J In: Interpretation of Diagnostic Tests. Philadelphia: Lippincott Williams & Wilkins, 2007, p. 307. [Google Scholar]
- 24.Walters BC, Hoffman HJ, Hendrick EB, and Humphreys RP. Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. Journal of neurosurgery 60: 1014–1021, 1984. [DOI] [PubMed] [Google Scholar]
- 25.Whitehead WE, and Kestle JR. The treatment of cerebrospinal fluid shunt infections. Results from a practice survey of the American Society of Pediatric Neurosurgeons. Pediatr Neurosurg 35: 205–210, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Williams MA, McAllister JP, Walker ML, Kranz DA, Bergsneider M, Del Bigio MR, Fleming L, Frim DM, Gwinn K, Kestle J, Luciano MG, Madsen JR, Molchan S, Oster-Granite ML, and Spinella G. Priorities for hydrocephalus research: report from a National Institutes of Health-sponsored workshop. Journal of neurosurgery (5 Suppl Pediatrics): 345–357, 2007. [DOI] [PubMed] [Google Scholar]


